- 1Department of Pharmacy and Social Pharmacy, University of Veterinary Medicine and Pharmacy in Kosice, Kosice, Slovakia
- 2Biomedical Centre Martin, Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, Martin, Slovakia
- 3Department of Morphological Disciplines, University of Veterinary Medicine and Pharmacy in Kosice, Kosice, Slovakia
- 41st Department of Psychiatry, Faculty of Medicine, Pavol Jozef Safarik University and University Hospital, Kosice, Slovakia
- 5Department of Psychology, Faculty of Arts, Comenius University in Bratislava, Bratislava, Slovakia
- 6Department of Languages, Faculty of Pharmacy, Comenius University in Bratislava, Bratislava, Slovakia
- 7Department of Pharmacology and Toxicology, Faculty of Pharmacy, Comenius University in Bratislava, Bratislava, Slovakia
- 8Department of Organisation and Management of Pharmacy, Faculty of Pharmacy, Comenius University in Bratislava, Bratislava, Slovakia
Introduction: Cognitive impairment (CI) is a growing public health problem. Our study is based on the fact that cognitive assessment in community pharmacy focused on early identification of undiagnosed CI has received limited attention. As pharmacists are the most accessible health professionals due to the availability of community pharmacies to the public, they have the potential to bring improvement in this area. Early identification of at-risk patients with CI by performing cognitive testing within advanced pharmaceutical care may improve the availability of targeted physician-indicated treatment.
Methods and analysis: The study is a multicentric study that will include cognitive screening within pharmaceutical care. We will use the Slovak version of the short form of the Montreal Cognitive Assessment (s-MoCA) test. Study participants will be at-risk patients undergoing cognitive screening in community pharmacies. Secondarily, we will evaluate the risk factors related to CI, such as at-risk medication use and modifiable dementia risk factors (e.g., cardiovascular and mental comorbidities, aging, and lifestyle habits).
Ethics and dissemination: This study was approved by the Ethics Committee of the Faculty of Pharmacy, Comenius University Bratislava (Ethics Committee Statement 01/2024). All procedures follow the relevant guidelines and regulations and the Declaration of Helsinki.
1 Introduction
1.1 Epidemiology and cognitive screening
Cognitive disorders represent a growing public health concern given the increasing number of seniors reaching an at-risk age (1, 2), associated with a subjectively or objectively measurable decline in at least one of the domains of cognitive functions, such as verbal abilities, spatial orientation, episodic memory, processing speed and executive functions (3). Only limited attention has been given to cognitive assessment in the community pharmacy targeting the early identification of undiagnosed cognitive impairment (CI) (4–9). As pharmacists are the most accessible health professionals due to the availability of community pharmacies to the public, they have the potential to be involved in early identification of at-risk patients who have not yet received a professional examination by a physician (4). Dementia and Alzheimer’s disease are the most common cognitive disorders affecting older people aged 60+ (1, 10), with a globally devastating impact on society with a disproportionate burden on health, economic and social care (11–13). Therefore, prevention, diagnosis, and pharmacological and non-pharmacological treatment of dementia are among the international priorities of the World Health Organization (WHO) (1, 2). It is essential to recognize CI at a reversible stage, and early intervention and appropriate interventions can prevent the development of dementia. This stage called mild cognitive impairment (MCI), is a transitional phase between cognitive ability decline due to age and the terminal stage of CI, dementia. Mental health and memory problems are crucial issues with significant impact on life quality. They can also affect patients’ level of medication adherence and self-care ability, which can result in poor long-term health outcomes (5, 8). Secondarily, cognitive decline may also be accelerated by the use of at-risk medications, especially when taking them for a long time (14, 15). These drugs suppress the cholinergic neurotransmitter pathways affecting brain structures such as the hippocampus and the neocortex, which are already vulnerable to age-related CI (16). Analysis of chronic pharmacotherapy can help identify at-risk medications and suggest a safe alternative, if possible. Anticholinergics, sedatives and benzodiazepines are the commonly used drugs with adverse effects on cognitive function (15–18). When they are combined, their adverse effect is cumulative (15, 19). A well-used tool is an assessment of the anticholinergic burden (ACB) scale (17). Implementing cognitive screening for early identification of patients with CI into pharmaceutical care can forward these patients to a doctor without undue delay and allow early initiation of their treatment and monitoring of further cognitive deterioration. Moreover, treatment of CI could reduce the risk of non-adherence to pharmacotherapy for already diagnosed chronic diseases (5, 20). Community pharmacists are suitable for preventing dementia by implementing cognitive screening within advanced pharmaceutical care (5, 20). Early identification of at-risk patients with CI could improve the availability of physician-indicated targeted treatment.
Establishing a diagnosis for CI requires a comprehensive approach and an overall assessment of the patient’s cognitive performance using multiple diagnostic tools and is strictly the physician’s responsibility (21). Brief screening tests such as the Mini-Mental State Examination (MMSE) (22), the Clock Drawing Test (CDT) (23), and the Montreal Cognitive Assessment (MoCA) are used for an indicative examination of cognitive abilities. The MoCA test is a gold standard, one of the most commonly used cognitive screening tools worldwide. It is characterized by a high sensitivity and specificity for MCI (21), and short and standard versions of the MoCA appear to be effective in identifying CI (24). It was translated into many languages, including Slovak (25, 26). Its time-saving short version for pharmacy practice is suitable (27–29). If poorer cognitive abilities are noted in an orientation cognitive screening within pharmaceutical care, it does not mean the patient suffers from CI. A suspected CI is suggested. A physician’s assessment of the patient’s cognitive status is needed to confirm the CI (21).
1.2 CI risk factors
The development of CI and dementia is related to the interaction of many factors. Nowadays, potentially modifiable factors are well-known (12) (Figure 1). Cardiovascular risk factors are together with Aging and the Incidence of Dementia (CAIDE) expressed by the CAIDE Dementia Risk Score (30). They can evaluate a patient’s risk of developing dementia (31). Most of these factors of CI, such as hypertension, obesity, diabetes and high cholesterol, can be effectively influenced by pharmacists’ interventions. At present, pharmacists’ competencies are expanding and include the management of chronic diseases and medication safety (32), assessment of potentially inappropriate medication use in the older adult (33), monitoring biochemical parameters, blood pressure measurement, obesity management, smoking cessation and others, all of which contribute to the individualisation of pharmaceutical care that is more patient-centered (34). Chronic cardiovascular disease and metabolic syndrome (MetS) are among the modifiable risk factors for dementia. Therefore, attention should be paid to monitoring cognitive abilities in patients suffering from these conditions. CI may lead to non-adherence to treatment, which in turn may result in the development of dementia (20, 35). Advanced pharmaceutical care now routinely includes the management of most modifiable risk factors for CI and dementia. This is a relevant basis for the subsequent expansion of pharmaceutical care targeting early recognition of CI through simple cognitive screening as a pharmaceutical service.
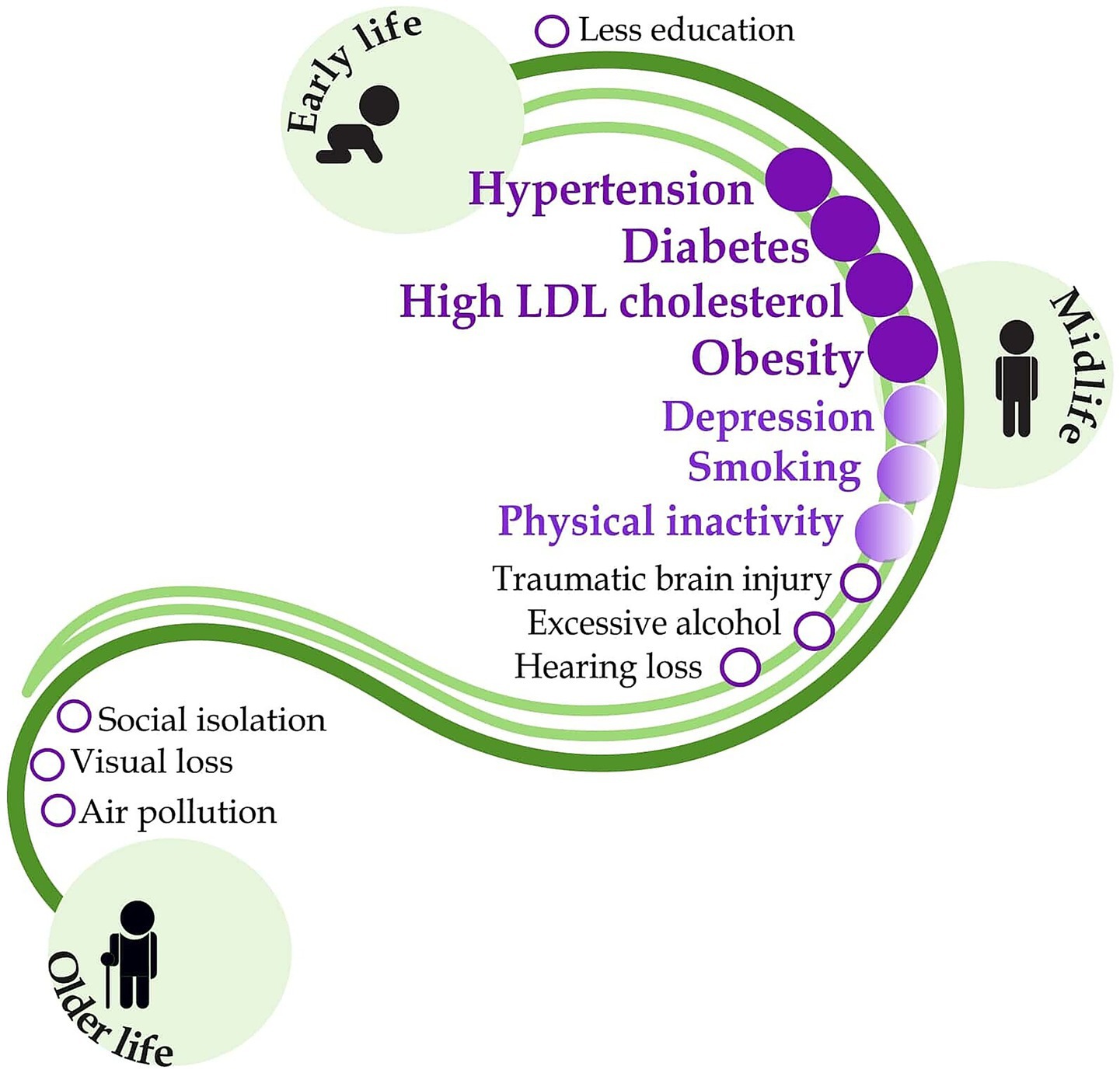
Figure 1. Modifiable risk factors for dementia and the potential role of pharmacists in their management. Dark purple indicates routinely performed pharmaceutical interventions with a well-documented patient outcome. Light purple shows interventions with poor outcome evidence, and the black letters indicate risk factors outside the pharmacist’s competence. Figure was adapted from the original figure by Livingston et al. (12) with permission from Elsevier LTD., which has been expanded to include information regarding the potential role of pharmacists in their management according to Macekova et al. (34), using Inkscape graphics editor.
1.3 Role of pharmacists
Pharmacists can support the management of most patients suffering from diseases that do not require a visit to the doctor, thus reducing the burden of medical care. Consequently, physicians can focus on patients with severe disorders (36). In addition, pharmacists can play a role in managing patients suffering from chronic diseases (37), including cognitive disorders (6, 8, 9, 38). Nevertheless, it is still uncertain whether these pharmaceutical interventions bring adequate benefits to the patients and improve their health status. The patient’s cognitive state may be the limitation for achieving satisfactory results, as in the case of CI, the patient needs an individual, specific approach in the provision of health care, including pharmaceutical care. For this reason, our study aimed to develop a standard protocol for assessing cognitive screening of patients aged 50 years and over in community pharmacies so that the pharmacist can adapt this approach to the patient in the provision of pharmacy care.
2 Methods and analysis
2.1 Design and setting
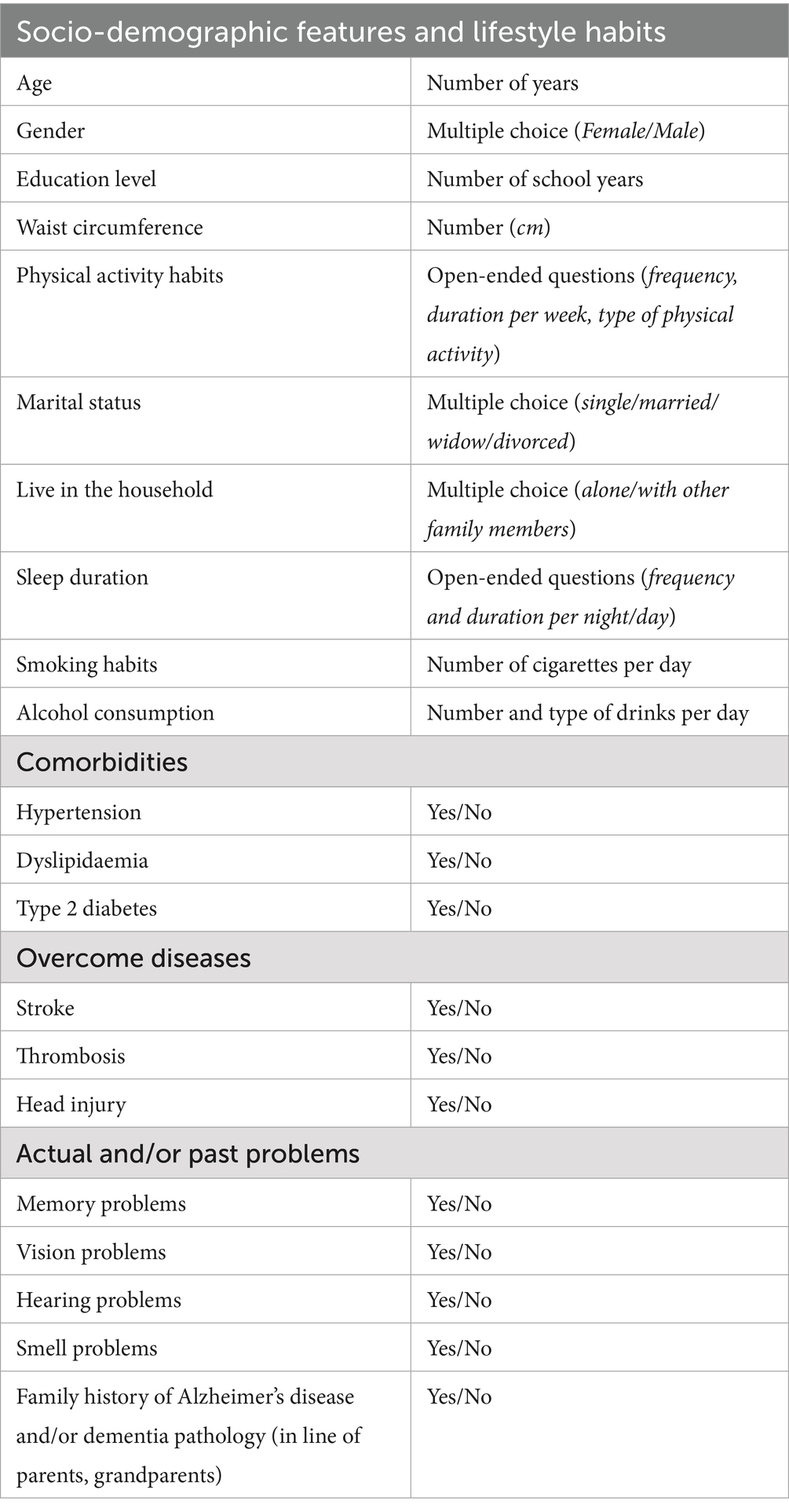
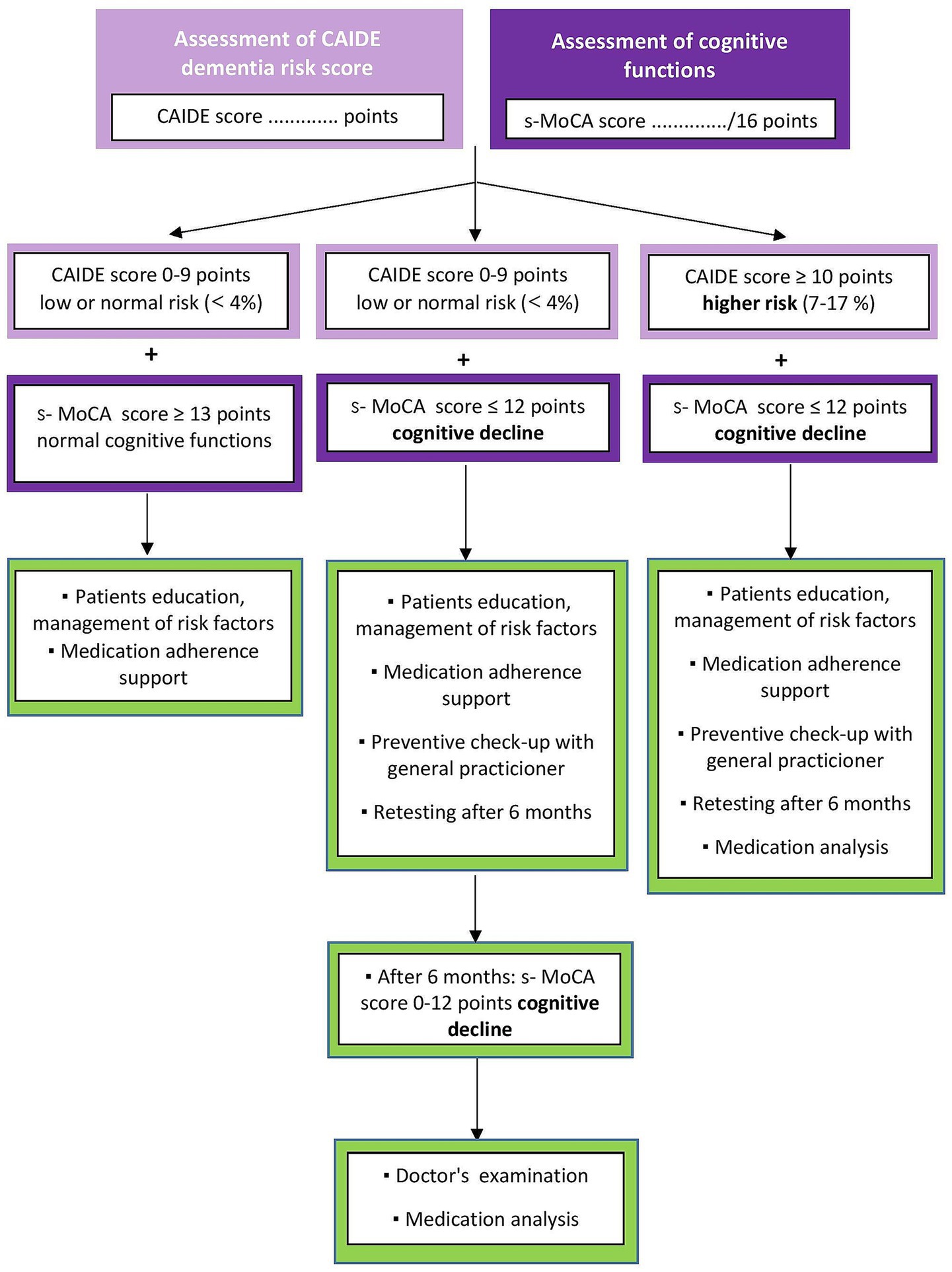
We designed a study protocol for a prospective observational clinical cohort study in Slovakia to evaluate multifactorial and specific risk factors for CI in the adult population aged 50+ as a part of advanced pharmaceutical care in community pharmacies (Figure 2). The current study protocol is developed based on our previous findings (39, 40) and offers an advanced method of identifying patients at risk for CI by pharmacist intervention. Firstly, we focus on performing a cognitive screening using a short version of the Montreal Cognitive Assessment (21, 28). Secondly, we will assess the risk of developing dementia according to the CAIDE Dementia Risk Score, which includes lifestyle factors, age, and comorbidities altogether expressed as the CAIDE score (31). Third, we will assess the risk of medication use by impacting cognitive health according to the ACB scale using the free available online ACB calculator (41).
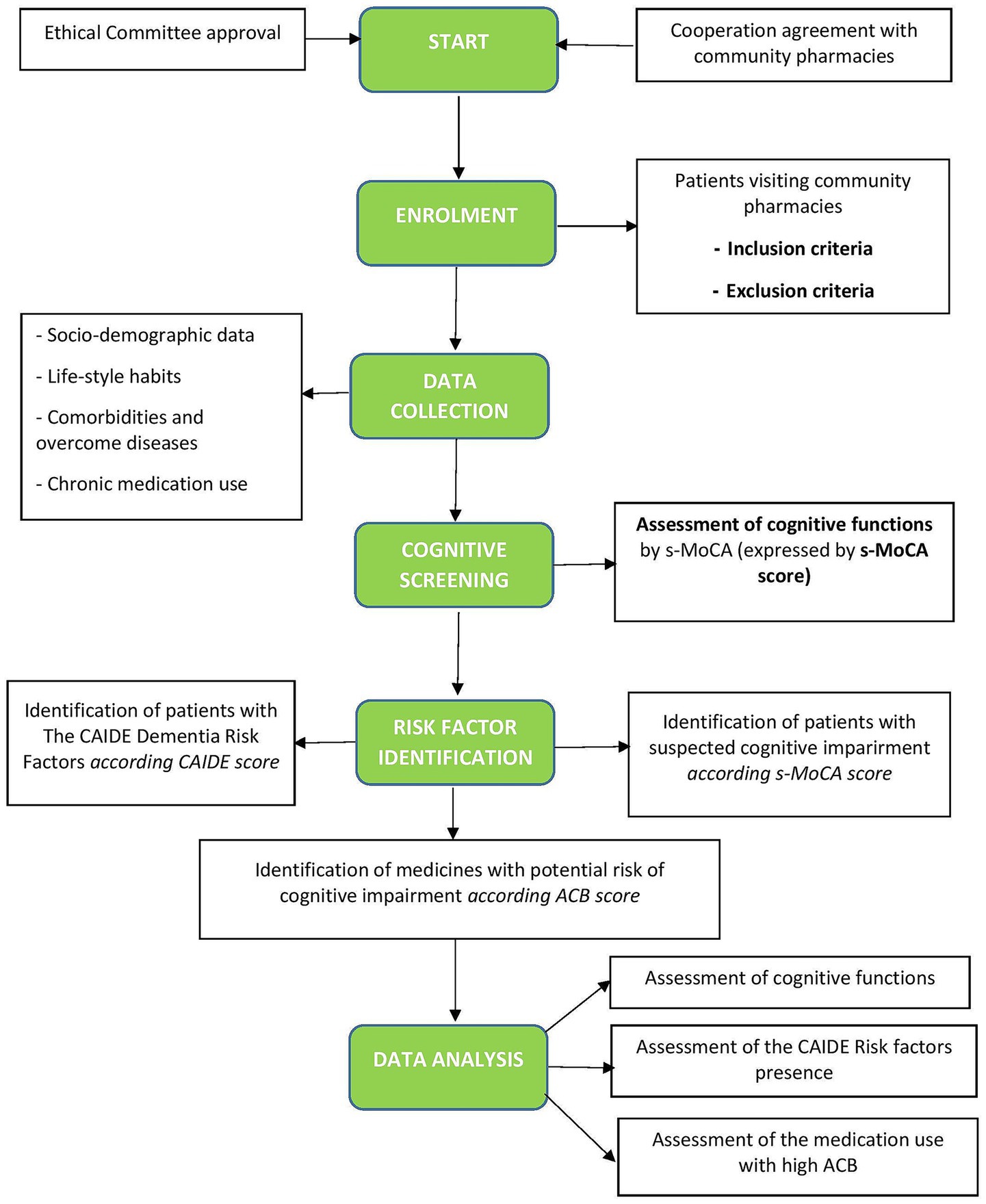
Figure 2. Flowchart of the study. CAIDE, Cardiovascular Risk Factors, Aging, and Incidence of Dementia (The CAIDE Dementia Risk Score), s-MoCA, short version of the Montreal Cognitive Assessment Screening Tool; ACB, Anticholinergic Burden Scale.
2.2 Cohort and sample size calculation
Adult patients aged 50 years or older receiving pharmaceutical care (patients who visit community pharmacy for different reasons, e.g., to collect a medication with/without prescription, pharmaceutical counseling, etc.) in community pharmacies in Slovakia who can complete the questionnaires and are willing to participate in the study will be included. Inclusion and exclusion criteria are shown in Table 1. Exclusion criteria also include disorders which can be related to cognitive dysfunction (e.g., schizophrenia). Pharmacy clients will receive written information about the study objectives, and consent will be obtained to confirm participation. By their signature, they will also agree to participate only once (to avoid duplicities). Individuals will be informed that participation is voluntary and can be terminated at any time. Also, they will be ensured that access to the collected data will be restricted to the study investigators only. The minimum sample size for our analysis was based on the estimated proportion. The sample size was set using an online sample size calculator for a 95% confidence interval and a 5% margin of error (42), according to previously used in Kosirova et al. (33). The minimum number of included subjects was estimated to be 385 according to the total number of adults aged 50 and over in 2023 (N = 2,033,555); (43).
2.3 Data collection
Data will be collected by trained pharmacists working in community pharmacies. They will be recorded on a pre-printed study form (socio-demographic data, lifestyle habits, evidence of comorbidities, and family history of Alzheimer’s disease and dementia). Table 2 summarizes all items that will be recorded.
2.4 Place and staffing requirements
The screening site will be community pharmacies with suitable cognitive screening space. A suitable space may be a separate room in the pharmacy or a separate part of another area to guarantee confidentiality for the patient. Only trained pharmacists or pharmacy students under the supervision of a trained pharmacist may perform the screening. The pharmacist enrolled in the study must also provide the name and address of the pharmacy where the screening will be conducted.
Participating pharmacists will be educated through a 2-h webinar followed by a workshop with a pharmacists´ competency assessment, according to Rickles and colleagues (8), which will be prepared and implemented with the cooperation of the Slovak Chamber of Pharmacists, the Faculty of Pharmacy of Comenius University Bratislava, the University of Veterinary Medicine and Pharmacy in Kosice and medical societies. Trained pharmacists will be trained in using cognitive screening tools and the MoCA Test, receive information on modifiable risk factors for dementia and early warning signs, and gain practical experience in strategies to identify at-risk patients within pharmaceutical care. The training program will also provide a detailed overview of study consent forms and documents, an overview of the patient care process, dementia risk assessment, and an overview of protocols for patient follow-up, data collection, and reporting.
2.5 Personal competence
1. Collecting data and performing cognitive screening – trained pharmacists or pharmacy students under the supervision of a trained pharmacist.
2. Interpretation of results – trained pharmacists only.
3. Patient outcomes should be communicated to the physician–pharmacists only.
2.5.1 The target group of individuals for which the screening is recommended
1. Aged 50+.
2. Suffering from cardiovascular disease and/or 2 type diabetes mellitus (in case of DM2 without age limitation).
3. With dementia risk factors according to the CAIDE score.
4. With a family history of cognitive disorders (MCI, dementia, Alzheimer’s disease).
5. With at least one of the Alzheimer’s disease international early warning signs.
2.6 Cognitive screening
The gold standard screening tool, the Montreal Cognitive Assessment (MoCA), one of the available cognitive screening instruments, scans seven cognitive domains: executive functioning; visuospatial abilities; language; attention, concentration and working memory; abstract reasoning; memory and orientation, will be used. We use the short variant (s-MoCA) (28, 29) in the Slovak language, presenting a comparable alternative for detecting MCI and dementia. This short variant consists of 8 items. These items measure visuospatial and executive functions (trail making and clock drawing), language abilities (animal naming – rhinoceros), attention (serial 7 s – counting by subtracting seven), verbal fluency (naming for 1 min), abstraction (watch), delayed recall of words, orientation (place) (29). According to our previous outcomes, this simple, shortened version is an easy-to-use cognitive screening. It is an applicable compound of pharmaceutical care for adult patients aged 50 and older with cardiovascular disease and/or suspected metabolic syndrome (sMetS) to explore their cognitive state. This shortened form of the MoCA scale has a range of 0–16 points; the time of completion is 5–7 min (which is only one-third the length of the original MoCA), and a cut-off of ≤12 represents a cognitive impairment (29).
2.7 Assessment of dementia risk factors
The CAIDE scoring system will be applied to the occurrence of modifiable risk factors (31). It was designed to determine the risk of developing dementia in cognitively intact individuals aged 40 to 65 years. This tool assesses common, easy-to-obtain, measurable data such as age, sex, education, blood pressure, cholesterol level, BMI, and physical activity. Each item is scored, and the resulting score represents the level of risk of developing cognitive impairment or dementia. The cut-off score represents 8–9 points for low and normal risk. Higher scores (10–15) indicate an increased risk of developing cognitive impairment (31).
We will also assess an occurrence of the suspected MetS (sMetS) as it can be easily identified in community pharmacy and may affect cognitive function (40, 44). The presence of sMetS will be estimated according to the International Diabetes Federation Worldwide Definition of MetS (45), 2005, modified for the European population (46). Accordingly, patients will be divided into groups according to the presence or absence of sMetS (sMetS+; sMetS-), and we will compare cognitive abilities in the test s-MoCA between two subpopulations (sMetS+/sMetS-) (40).
2.8 Analysis of chronic medication
Medication analysis will be focused on the identification of at-risk medication use with an anticholinergic burden risk used in included participants. We will assess the cumulative effect of medication with anticholinergic properties taking long-term, which can adversely impact cognitive performance and physical abilities and increase the mortality risk (15). We will use an expert opinion-derived risk scale, the ACB Scale, which helps quantify the risk of anticholinergic burden (17). The list of at-risk medications is summarized in Supplementary Table 1. The scale ranks the anticholinergic activity of drugs into four categories, according to their anticholinergic effect and potential for impairment of cognition: (i) no anticholinergic activity, ACB = 0; (ii) possible anticholinergic activity, ACB = 1; (iii) definite anticholinergic activity, ACB = 2; and (iv) definite high anticholinergic activity, ACB = 3 (15). Medication with the scores 1, 2 and 3 can be found in Supplementary Table 1. If the medication is not displayed in the list, its ACB score equals 0. In patients taking more than one medication from the list, scores are cumulative, and a total score ≥3 means a high risk for cognitive impairment. Each one-point increase in the ACB total score is associated with an apparent decline in the MMSE score (16).
2.9 Patient counseling
Pharmaceutical counseling will be provided to patients concerning their needs, family and personal history and comorbidities or even physician’s recommendation (Supplementary Table 2). The analysis plan is shown in Figure 3. During the interpretation of the results of cognitive screening (following initial screening or, if necessary, after retesting 6 months later, by the patient’s consent and an agreed appointment with the pharmacist based on a call), it is essential to explain to the patients that this simple cognitive screening is not a substitute for a medical examination and that the result of the screening is not a physician’s diagnosis. Next, the pharmacist will provide a patient’s education about other factors that may influence cognitive decline (such as stress, lifestyle, sleep disturbances, depression and certain medications or substance use, e.g., caffeine, alcohol). Patients will get printed educational materials about risk factors for cognitive impairment and early warning signs of dementia.
According to results of screening and pharmacist’s consideration, patients may be referred to a physician if their condition requires it. In such cases, the pharmacist will use the uniform information form for the physician (Supplementary Table 3). The patient receives an examination report informing the physician of the findings.
2.10 Physician referral
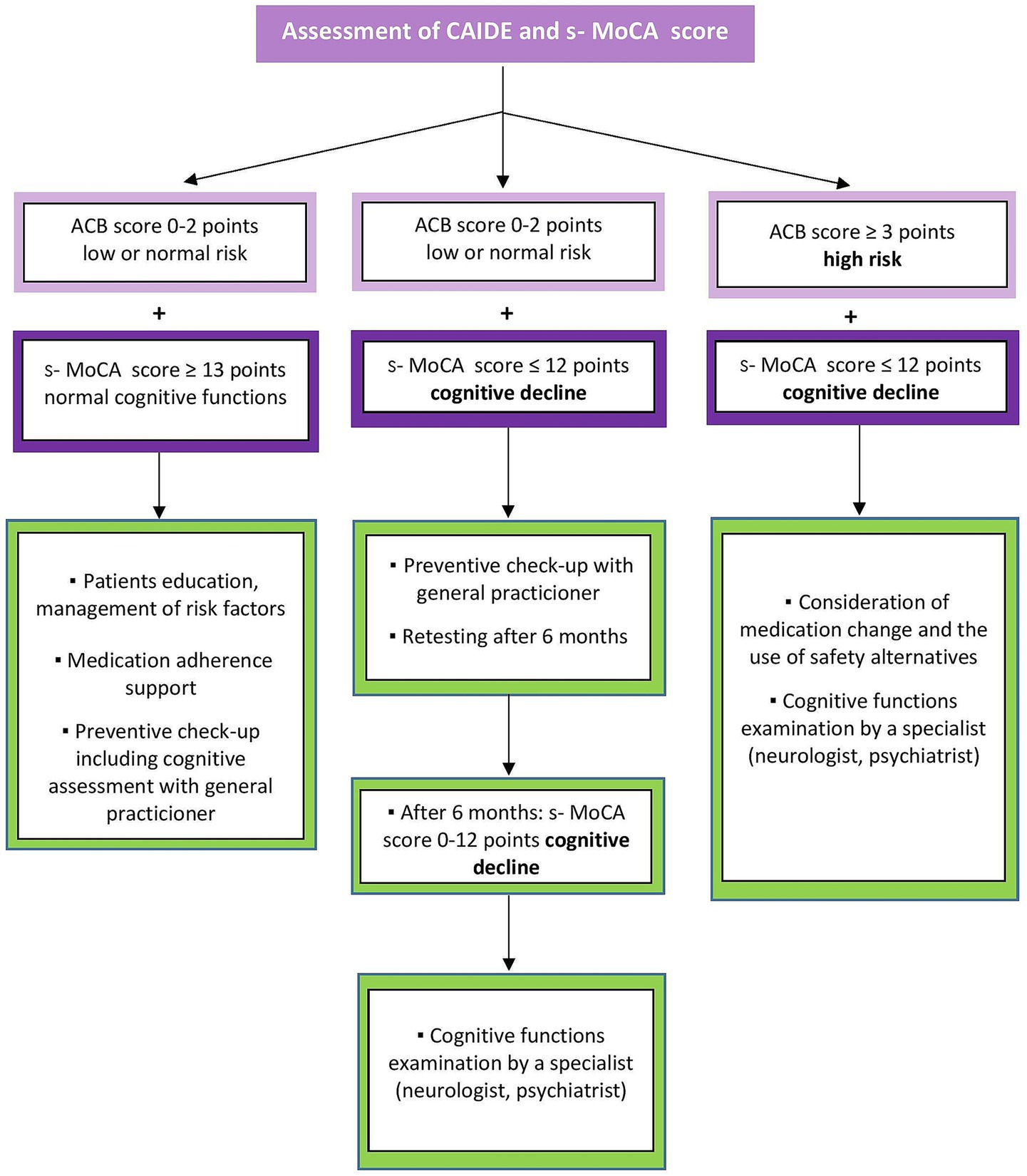
For each patient, the pharmacist will specifically consider whether their condition requires a visit to the doctor or a pharmacy consultation is sufficient. The analysis plan is shown in Figure 4. Specific groups of patients are referred to a physician if they score decreased cognitive abilities on the s-MoCA test. Patients:
• with memory problems,
• with at least one of the early signals of dementia,
• at risk of developing dementia, according to CAIDE,
• that do not have regular preventive check-ups,
• that are long-term users of at-risk medication (Supplementary Table 1),
• with long-term use of over-the-counter memory aids.
The result of the pharmacotherapy analysis will be received only by a doctor. The patient will not be given the results to maintain adherence. Every patient in the study should be referred to a general practitioner for a preventive check-up if they have not had one in the last 2 years. During follow-up, the pharmacist will contact patients electronically to obtain feedback on whether they have addressed their condition with a doctor and whether the presence of CI has been confirmed.
3 Outcomes
In this study, we focus on a cohort of adults aged 50+ with lower cognitive abilities expressed by s-MoCA scores (≤ 12 points). These patients will be defined as those with suspected CI (CI+) who will be referred for medical examination. We will then record whether a physician has confirmed our suspicion of CI.
In the CI+ group, we will collect data on the prevalence of CI risk factors (expressed by CAIDE score) and the use of risk medications (expressed by ACB score) and then compare them with a control group.
4 Statistical analyses and mitigation of bias
The obtained data will be analyzed using GraphPad, version 8.0.1 (GraphPad Prism, San Diego, CA, USA) and the SAS Education Analytical Suite for Microsoft Windows, version 9.3 (SAS Institute Inc., Cary, NC, USA). We will perform fundamental descriptive analyses (calculation of mean and SD), normality tests for the included variables, correlation tests, t-tests for two independent variables, and one-way analysis of variance (ANOVA) in the case of more independent groups. Multivariate regression models will be used to compare cognitive outcomes across subgroups.
Since there is no randomization or intervention control in this study, several types of bias can affect validity. In the study, we will mitigate selection bias through design-stage (broad and inclusive recruitment, clear criteria, consistent enrolment) and analysis-stage strategies (statistical adjustment, sensitivity analyses). Observer bias will be mitigated through standardization, pharmacist training, objective measurements, and data monitoring strategies. Social desirability bias will be mitigated through use of questionnaire design (e.g., using neutral wording and validated scales) and through pharmacist training with a focus on his/her empathy and neutrality.
5 Discussion
5.1 Objectives and hypotheses
5.1.1 Study objectives
1. To perform a standardized cognitive screening by a trained pharmacist within advanced pharmaceutical care in a community pharmacy.
2. To test the association between the presence of modifiable Cardiovascular Risk Factors, Aging, and Incidence of Dementia (according to The CAIDE Dementia Risk Score) and poorer cognitive abilities (expressed s-MoCA score) in the adult population aged 50+.
3. To assess the medication use related to CI by medication analysis as a part of pharmaceutical counseling in a community pharmacy.
5.1.2 Hypotheses
According to our previous results, we hypothesize:
1. Short cognitive screening, a part of advanced pharmaceutical care, can help identify patients who need further cognitive evaluation by a general practitioner or a specialist.
2. Analysis of pharmacotherapy can help to identify at-risk medication use related to CI.
Pharmacists in a community pharmacy could help monitor modifiable risk factors of CI.
5.2 Strengths and limitations of this study
• The study focuses on at-risk patients with CIs within pharmaceutical care in a community pharmacy. Implementing cognitive screening in the pharmacy setting may contribute to the early identification of patients with CI, reducing the pressure on ambulatory care associated with CI prevention.
• Data will be collected using easy-to-use cognitive screening by a pharmacist.
• The realization of cognitive screening, correct evaluation of results and interpretation, and the pharmacist’s final decision about referring a patient to a physician will depend on their experiences and critical judgment. Hence, the education of pharmacists in their training program is a crucial stage of this study. It is also important to consider the patient’s other comorbidities, for instance, whether there exists a bidirectional relationship between depressive disorder and cognitive deficits, which may affect the outcome of screening tests. The exclusion of institutionalized older adults may limit the generability of the study cohort.
• Pharmacists must be able to communicate outcomes concerning patients’ conditions. Collaboration between pharmacists and physicians is important because a patient with suspected CI identified by a pharmacist cannot be diagnosed with CI without the doctor’s confirmation.
• Furthermore, various tools can characterize the anticholinergic burden score differently. While one tool can classify drugs such as clopidogrel, furosemide, and zolpidem as potentially anticholinergic, according to another tool (e.g., the ACB Calculator), they have no increased risk.
Ethics statement
This study was approved by the Ethics Committee of the Faculty of Pharmacy, Comenius University Bratislava (Ethics Committee Statement 01/2024). The relevant guidelines and regulations and the Declaration of Helsinki will perform all procedures. Although the data will not be anonymised to allow future follow-ups according to the algorithm, only the investigators can access the collected data. This study's results will be published with full respect for privacy and personal data protection. All procedures will comply with the General Data Protection Regulation (GDPR). The study does not anticipate any adverse reactions resulting from participation.
Author contributions
ZM: Funding acquisition, Writing – original draft, Conceptualization, Methodology. MK: Writing – original draft, Conceptualization, Methodology. NH: Writing – original draft. JD: Writing – review & editing. MH: Writing – review & editing. VZ: Writing – review & editing. JK: Writing – review & editing, Supervision, Funding acquisition, Writing – original draft. MS: Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. All this work is supported by the SLeK/01/2024 grant from the Slovak Chamber of Pharmacists (Zuzana Macekova) and VEGA 1/0707/25 from the Science Grant Agency (Jan Klimas).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1606381/full#supplementary-material
References
1. Livingston, G, Huntley, J, Sommerlad, A, Ames, D, Ballard, C, Banerjee, S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. (2020) 396:413–46. doi: 10.1016/S0140-6736(20)30367-6
2. WHO. (2019) Risk reduction of cognitive decline and dementia: WHO guidelines. Available online at: https://www.who.int/publications/i/item/9789241550543 [Accessed January 9, 2025]
3. Tarawneh, R, and Holtzman, DM. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harb Perspect Med. (2012) 2:a006148. doi: 10.1101/cshperspect.a006148
4. Abubakar, A, Sinclair, J, Kabisatpathy, S, and Lutz, S. Community pharmacists expand access to cognitive function assessments with Cognivue clarity™ device. Alzheimers Dement. (2023) 19:e082979. doi: 10.1002/alz.082979
5. Arikawe, O, Morrissey, H, and Ball, P. Community pharmacy brief screening intervention to improve health outcomes for patients diagnosed with chronic diseases. J Adv Pharm Educ Res. (2022) 12:1–8. doi: 10.51847/bmamIaRVB8
6. Climent, MT, Pardo, J, Muñoz-Almaraz, FJ, Guerrero, MD, and Moreno, L. Decision tree for early detection of cognitive impairment by community pharmacists. Front Pharmacol. (2018) 9:1232. doi: 10.3389/fphar.2018.01232
7. Harasani, K, Xhafaj, D, Lekaj, A, Veshi, L, and Olvera-Porcel, MDC. Screening for mild cognitive impairment among older Albanian patients by clinical pharmacists. Int J Pharm Pract. (2021) 29:189–91. doi: 10.1093/ijpp/riab001
8. Rickles, NM, Skelton, JB, Davis, J, and Hopson, J. Cognitive memory screening and referral program in community pharmacies in the United States. Int J Clin Pharm. (2014) 36:360–7. doi: 10.1007/s11096-013-9904-7
9. Tuula, A, Merks, P, Waszyk-Nowaczyk, M, Drozd, M, Petrova, G, Viola, R, et al. Evaluation of medication safety assessment tools for pharmacist-led medication reviews: the eastern European pilot project. Front Pharmacol. (2024) 15:1348400. doi: 10.3389/fphar.2024.1348400
10. Tsai, C-L, Pai, M-C, Ukropec, J, and Ukropcová, B. Distinctive effects of aerobic and resistance exercise modes on neurocognitive and biochemical changes in individuals with mild cognitive impairment. Curr Alzheimer Res. (2019) 16:316–32. doi: 10.2174/1567205016666190228125429
11. Jones, E, Aigbogun, MS, Pike, J, Berry, M, Houle, CR, and Husbands, J. Agitation in dementia: real-world impact and burden on patients and the healthcare system. J Alzheimer's Dis. (2021) 83:89–101. doi: 10.3233/JAD-210105
12. Livingston, G, Huntley, J, Liu, KY, Costafreda, SG, Selbæk, G, Alladi, S, et al. Dementia prevention, intervention, and care: 2024 report of the lancet standing commission. Lancet. (2024) 404:572–628. doi: 10.1016/S0140-6736(24)01296-0
13. Nemcikova, M, Katreniakova, Z, and Nagyova, I. Social support, positive caregiving experience, and caregiver burden in informal caregivers of older adults with dementia. Front Public Health. (2023) 11:1104250. doi: 10.3389/fpubh.2023.1104250
14. Lisibach, A, Benelli, V, Ceppi, MG, Waldner-Knogler, K, Csajka, C, and Lutters, M. Quality of anticholinergic burden scales and their impact on clinical outcomes: a systematic review. Eur J Clin Pharmacol. (2021) 77:147–62. doi: 10.1007/s00228-020-02994-x
15. Salahudeen, MS, Duffull, SB, and Nishtala, PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. (2015) 15:31. doi: 10.1186/s12877-015-0029-9
16. Lepkowsky, CM. Medications linked to cognitive impairment in older adults. Pract Innov. (2016) 1:253–64. doi: 10.1037/pri0000033
17. Boustani, M, Campbell, N, Munger, S, Maidment, I, and Fox, C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. (2008) 4:311–20. doi: 10.2217/1745509X.4.3.311
18. Tanaka, T, Akishita, M, Kojima, T, Son, B-K, and Iijima, K. Anticholinergic burden quantified using the Japanese risk scale as a predictor of frailty and sarcopenia among community-dwelling older adults: a 9-year Kashiwa cohort study. Geriatr Gerontol Int. (2025) 25:520–7. doi: 10.1111/ggi.70012
19. Ramos, H, Moreno, L, Perez-Tur, J, Chafer-Pericas, C, Garcia-Lluch, G, and Pardo, J. CRIDECO anticholinergic load scale: an updated anticholinergic burden scale. Comparison with the ACB scale in Spanish individuals with subjective memory complaints. J Pers Med. (2022) 12:207. doi: 10.3390/jpm12020207
20. Martin, P, Tamblyn, R, Benedetti, A, Ahmed, S, and Tannenbaum, C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. (2018) 320:1889–98. doi: 10.1001/jama.2018.16131
21. Nasreddine, ZS, Phillips, NA, Bédirian, V, Charbonneau, S, Whitehead, V, Collin, I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
22. Folstein, MF, Folstein, SE, and McHugh, PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
23. Herrmann, N, Kidron, D, Shulman, KI, Kaplan, E, Binns, M, Leach, L, et al. Clock tests in depression, Alzheimer’s disease, and elderly controls. Int J Psychiatry Med. (1998) 28:437–47. doi: 10.2190/5QA5-PHUN-1Q9F-C0PB
24. Abzhandadze, T, Berg, OI, Mavridis, A, Lindvall, E, Quinn, T, Sunnerhagen, KS, et al. The prognostic test accuracy of the short and standard forms of the Montreal cognitive assessment. Cerebrovasc Dis. (2024) 54:1–7. doi: 10.1159/000540372
25. Csefalvay, Z. Montreal screening of cognitive functions (MoCA) in complex rehabilitation of patients with brain damage. Rehabilitacia. (2011) 48:116–9.
26. Csefalvay, Z, and Marková, J. (2005) Montreal cognitive assessment (MoCA). Available online at: http://www.mocatest.org/ (Accessed February 10, 2025).
27. Borson, S, Scanlan, J, Brush, M, Vitaliano, P, and Dokmak, A. The mini-cog: a cognitive “vital signs” measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. (2000) 15:1021–7. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6
28. Bezdicek, O, Cervenkova, M, Moore, TM, Stepankova Georgi, H, Sulc, Z, Wolk, DA, et al. Determining a short form Montreal cognitive assessment (s-MoCA) Czech version: validity in mild cognitive impairment Parkinson’s disease and cross-cultural comparison. Assessment. (2020) 27:1960–70. doi: 10.1177/1073191118778896
29. Roalf, DR, Moore, TM, Wolk, DA, Arnold, SE, Mechanic-Hamilton, D, Rick, J, et al. Defining and validating a short form Montreal cognitive assessment (s-MoCA) for use in neurodegenerative disease. J Neurol Neurosurg Psychiatry. (2016) 87:1303–10. doi: 10.1136/jnnp-2015-312723
30. Manuel, AR, Ribeiro, P, Silva, G, Rodrigues, PM, and Nunes, MVS. Exploring the relationship between CAIDE dementia risk and EEG signal activity in a healthy population. Brain Sci. (2024) 14:1120. doi: 10.3390/brainsci14111120
31. Sindi, S, Calov, E, Fokkens, J, Ngandu, T, Soininen, H, Tuomilehto, J, et al. The CAIDE dementia risk score app: the development of an evidence-based mobile application to predict the risk of dementia. Alzheimers Dement. (2015) 1:328–33. doi: 10.1016/j.dadm.2015.06.005
32. Simpson, SH, Johnson, JA, and Tsuyuki, RT. Economic impact of community pharmacist intervention in cholesterol risk management: an evaluation of the study of cardiovascular risk intervention by pharmacists. Pharmacotherapy. (2001) 21:627–35. doi: 10.1592/phco.21.6.627.34538
33. Kosirova, S, Urbankova, J, Klimas, J, and Foltanova, T. Assessment of potentially inappropriate medication use among geriatric outpatients in the Slovak Republic. BMC Geriatr. (2023) 23:567. doi: 10.1186/s12877-023-04260-y
34. Macekova, Z, Krivosova, M, Viola, R, Zufkova, V, Klimas, J, and Snopkova, M. Advanced pharmaceutical services in community pharmacies. Pharm Pract. (2025) 23:1–17. doi: 10.18549/PharmPract.2025.1.3073
35. Willis, A, Rivers, P, Gray, LJ, Davies, M, and Khunti, K. The effectiveness of screening for diabetes and cardiovascular disease risk factors in a community pharmacy setting. PLoS One. (2014) 9:e91157. doi: 10.1371/journal.pone.0091157
36. Steed, L, Sohanpal, R, Todd, A, Madurasinghe, VW, Rivas, C, Edwards, EA, et al. Community pharmacy interventions for health promotion: effects on professional practice and health outcomes. Cochrane Database Syst Rev. (2019) 12:CD011207. doi: 10.1002/14651858.CD011207.pub2
37. Roncal-Belzunce, V, Gutiérrez-Valencia, M, Leache, L, Saiz, LC, Bell, JS, Erviti, J, et al. Systematic review and meta-analysis on the effectiveness of multidisciplinary interventions to address polypharmacy in community-dwelling older adults. Ageing Res Rev. (2024) 98:102317. doi: 10.1016/j.arr.2024.102317
38. Ng, R, El-Den, S, Stewart, V, Collins, JC, Roennfeldt, H, McMillan, SS, et al. Pharmacist-led interventions for people living with severe and persistent mental illness: a systematic review. Aust N Z J Psychiatry. (2022) 56:1080–103. doi: 10.1177/00048674211048410
39. Macekova, Z, Fazekas, T, Krivosova, M, Dragasek, J, Zufkova, V, Klimas, J, et al. Identification of a link between suspected metabolic syndrome and cognitive impairment within Pharmaceutical Care in Adults over 75 years of age. Healthcare. (2023) 11:718. doi: 10.3390/healthcare11050718
40. Macekova, Z, Krivosova, M, Fazekas, T, Snopkova, M, and Klimas, J. Short cognitive screening in elderlies as a part of advanced pharmaceutical care in Slovak community pharmacies - The pilot study KOGNIMET-SK. Eur Pharm J. (2022) 69:37–42. doi: 10.2478/afpuc-2022-0005
41. ACB Calculator. (2024) ACB calculator. Available online at: https://acbcalc.com/ [Accessed December 9, 2024]
42. Sample Size Calculator. (2025) Sample size calculator. Available online at: https://www.calculator.net/sample-size-calculator.html [Accessed January 29, 2025]
43. Slovak Statistics. (2024) Dataset. Available online at: https://slovak.statistics.sk/wps/portal/ext/Databases/!ut/p/z1/jcxBDoIwEIXhs3iCvlqwdTkYKTUNsdUidGNYmSaKLoznF4lbibN7yfcPi6xlcehf6dI_033or-Pu4upMzrmDbRroZlnCCK5RhwCUkp0m4KRRRcEJqvZbmCPttd9lHFnO4j_9DPj0-HGEsY8T2WiqMmkBZXUOQ1XwaycESHzBzI_HLbRItHgDLV4J0A!!/dz/d5/L2dBISEvZ0FBIS9nQSEh/ [Accessed January 17, 2025]
44. Macekova, Z, Fazekas, T, Stanko, P, Vyhnalek, M, Dragasek, J, Krivosova, M, et al. Cognitive screening within advanced pharmaceutical care in elderly patients with suspected metabolic syndrome. Int J Gerontol. (2022) 16:355–60. doi: 10.6890/IJGE.202210_16(4).0008
45. Alberti, KGMM, Zimmet, P, and Shaw, J. Metabolic syndrome a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. (2006) 23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x
Keywords: clinical pharmacy services, cognitive impairment, pharmacist-led screening, community pharmacy, dementia prevention in primary care, physician-pharmacist cooperation
Citation: Macekova Z, Krivosova M, Hudakova N, Dragasek J, Hajduk M, Zufkova V, Klimas J and Snopkova M (2025) Study protocol for identification of patients with risk of cognitive impairment in advanced pharmaceutical care in a community pharmacy. Front. Public Health. 13:1606381. doi: 10.3389/fpubh.2025.1606381
Edited by:
Ana Pires, Universidade Atlântica, PortugalReviewed by:
Claudia Oliveira, University of Algarve, PortugalYufeng Wang, The University of Auckland, New Zealand
Copyright © 2025 Macekova, Krivosova, Hudakova, Dragasek, Hajduk, Zufkova, Klimas and Snopkova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Klimas, amFuLmtsaW1hc0B1bmliYS5zaw==
 Zuzana Macekova
Zuzana Macekova Michaela Krivosova
Michaela Krivosova Nikola Hudakova
Nikola Hudakova Jozef Dragasek
Jozef Dragasek Michal Hajduk
Michal Hajduk Viera Zufkova6
Viera Zufkova6 Jan Klimas
Jan Klimas Miroslava Snopkova
Miroslava Snopkova