- 1Guangxi Key Laboratory of Environmental Exposomics and Entire Lifecycle Heath, Guilin Medical University, Guilin, China
- 2School of Public Health, Guilin Medical University, Guilin, China
- 3School of Humanities and Management, Guilin Medical University, Guilin, China
Objective: The relationship between insulin resistance and cognitive function has long been a subject of interest, but the association between the metabolic syndrome-insulin resistance (METS-IR) index and cognitive impairment remains unclear.
Methods: We utilized data from the 2015 China Health and Retirement Longitudinal Study (CHARLS) national survey, which, after screening, included a final sample of 12,307 participants. Cognitive function was assessed through face–to–face interviews via the MMSE scale. Multivariate logistic regression was used to evaluate the correlation between the METS-IR index and cognitive impairment. Using regression analysis results from fully adjusted models, we subsequently explored the nonlinear relationship between the METS-IR index and cognitive impairment via smooth curve fitting with constrained cubic splines and sought potential inflection points. Additionally, we executed a battery of sensitivity and subgroup analyses to validate the robustness of our findings.
Results: The study included 12,307 participants, of whom 49.02% were aged 45–60 years and 52.89% were female. The results revealed that for each unit increase in the METS-IR index, the risk of cognitive impairment increased by 1.4% (OR = 1.014, 95% CI: 1.004–1.023; p < 0.01). When the METS-IR index was used as a categorical variable, compared with Q1, the odds of cognitive impairment increased by 17.1, 38.7, and 49.5% for each unit increase in the METS-IR index in the Q2, Q3, and Q4 groups, respectively. In addition, a nonlinear pattern was found in the analysis, and the endpoint of the METS-IR index was determined to be 38.1. On the left side of the endpoint, a one-unit increase in the METS-IR index was associated with a 3.1% increase in the risk of cognitive impairment. On the right side of the endpoint, the risk of cognitive impairment increased by 1.0% for each unit increase in the METS-IR index (all p < 0.05).
Conclusion: This study highlighted the significant association between high METS-IR and the risk of cognitive impairment in Chinese middle-aged and older adult individuals. In addition, there was a specific nonlinear relationship between the METS-IR index and cognitive impairment (the inflection point was 38.1). Lowering the METS-IR index below 38.1 through lifestyle changes and diet control can significantly reduce the risk of cognitive impairment and may decrease the incidence of dementia.
1 Introduction
The worldwide phenomenon of population aging presents numerous challenges, particularly with respect to the growing concern over cognitive decline (1–3). Cognitive impairment typically signifies a reduction in an individual’s functional capacity and is considered a transitional phase between normal cognitive health and dementia (4). According to the latest World Alzheimer’s Disease Report, the number of people living with dementia globally is expected to double from 55 million in 2019 to 139 million by 2050. In China, the incidence and mortality rates of Alzheimer’s disease are increasing rapidly, making it the fifth leading cause of death for both urban and rural residents (5). Currently, there is a lack of treatments that can effectively slow the progression of dementia, which further highlights the importance of early recognition of cognitive impairment. Prompt interventions, such as cognitive rehabilitation therapy, dietary modification, and moderate-intensity exercise, can substantially reduce the risk of dementia (6).
Metabolic syndrome poses a significant risk for cardiovascular disease, affecting nearly one-third of adults in China and often cooccurring with obesity and type 2 diabetes (7). Insulin resistance, characterized by the reduced effectiveness of insulin in target organs, is regarded as the central mechanism underlying metabolic syndrome. Recent research has identified infrared heat not only as an early indicator of type 2 diabetes but also as a novel risk factor for cognitive decline, impacting both diabetic and nondiabetic patients (8). The existing evidence indicates that insulin not only crosses the blood–brain barrier via relevant receptors to play a key role in regulating behavior and metabolic processes but also influences memory formation by modulating the β-amyloid burden and synaptic plasticity (9). There are various methods for clinically assessing insulin resistance (IR), with the insulin clamp and glucose tolerance tests widely recognized as frequently used standards for measuring peripheral insulin sensitivity. Nevertheless, its application in routine clinical practice is limited because of its complex procedures and invasive nature (10). Therefore, there is an urgent need for an efficient and convenient diagnostic method to assess IR levels in patients.
Research has demonstrated that the METS-IR index, which integrates body mass index (BMI), triglyceride (TG), fasting plasma glucose (FPG), and high-density lipoprotein cholesterol (HDL-C) levels, offers a straightforward, reproducible, and reliable approach for assessing IR (11–13). Compared with traditional indicators such as fasting glucose and HOMA-IR, the METS-IR index has superior predictive performance because of its ability to capture multiple integrated clinical variables, including BMI, fasting blood glucose, and lipid profiles, simultaneously. This suggests that it may provide a more comprehensive assessment of IR. In patients with type 2 diabetes, METS-IR performs better than HOMA-IR does, and in the risk assessment of nonalcoholic fatty liver disease, METS-IR is superior to HOMA-IR in predicting incident cases (11, 14). Numerous epidemiological studies have consistently shown a strong association between METS-IR and various health conditions, such as cardiovascular disease, cancer, and other disorders (15–17). In a community-based population survey, the METS-IR index was used to identify patients with early mild cognitive impairment but only in patients without diabetes (18). However, the utility of the METS-IR as a proxy for IR in evaluating its link with cognitive decline remains limited, and no studies have explored its nonlinear relationship. Additionally, variations in study design, including differences in timing, METS-IR range, sex ratios, and adjustment factors, have led to an unclear understanding of the relationship between METS-IR and cognitive impairment in Chinese populations.
In this context, we hypothesized that the increase in the METS-IR index may be associated with cognitive impairment in middle-aged and older adult Chinese individuals. To verify this hypothesis, this study utilizes the China Health and Retirement Longitudinal Study (CHARLS) database—a nationwide, representative high-quality dataset on health status among middle-aged and older adult individuals. It focuses on relevant data from cognitive function surveys with the aim of exploring the relationship between the METS-IR index and cognitive impairment in this population.
2 Materials and methods
2.1 Study population
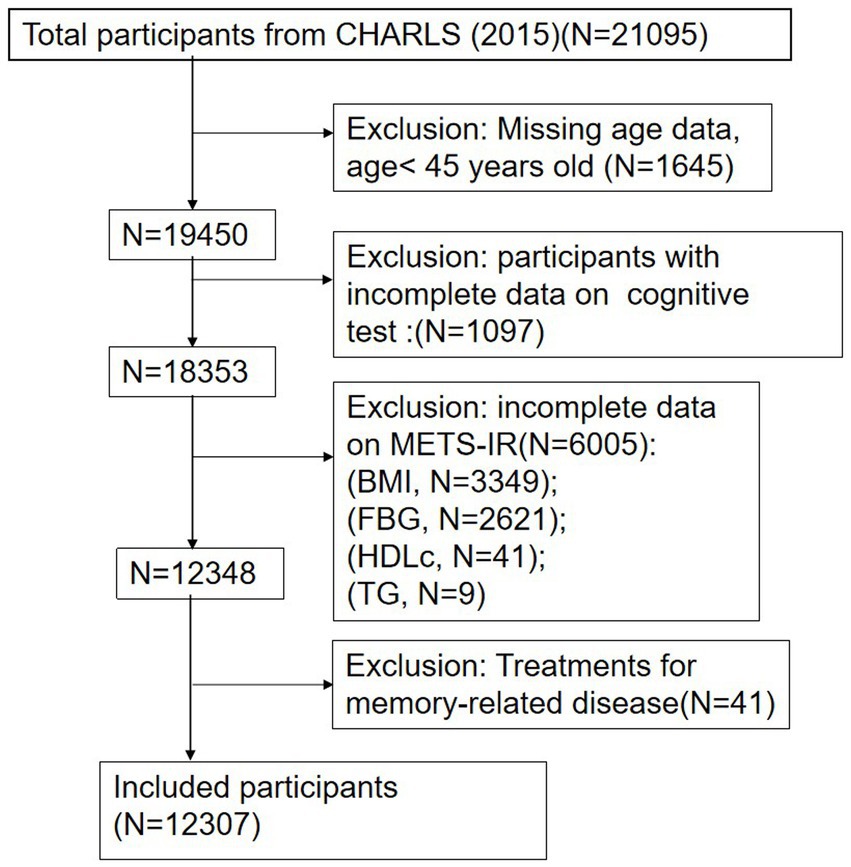
The cross-sectional data for this study were derived from CHARLS, a national longitudinal survey that employs a multistage probability proportional to the size sampling method. This method selected residents aged 45 years and older from 450 villages across 150 counties in 28 provinces of China for ongoing follow-up. The CHARLS comprehensively covers family, health, and various healthcare-related information, providing valuable insights into the health and well-being of China’s older adult population. The baseline survey commenced in 2011 with 17,708 participants, and follow-up surveys were conducted biennially, resulting in five waves of available data (2011, 2013, 2015, 2018, and 2020). Blood samples were collected during the 2011 and 2015 surveys. This study utilized data from the third wave of the 2015 survey, with a total sample size of 21,095 participants. During the data preprocessing phase, 1,645 participants younger than 45 years old and 1,097 individuals with incomplete cognitive data were excluded. Further screening excluded 6,005 participants lacking relevant indicators of the METS-IR index and 41 participants who had taken psychotropic medications related to memory. Ultimately, a total of 12,307 participants were included in the analysis. The selection process for participants is detailed in Figure 1. All participants provided informed consent prior to their involvement, and the study received ethical approval from the Peking University Biomedical Ethics Review Board (IRB 00001052-11015).
2.2 Assessment of cognitive function
In the CHARLS, cognitive function was assessed via the Mini-Mental State Examination (MMSE) questionnaire, a well-established tool that evaluates cognitive performance through tests of episodic memory and mental integrity. Episodic memory was assessed via two methods: immediate recall and delayed recall. For the immediate word recall test, participants were asked to memorize a list of 10 words and recall as many as possible. After a 5-min interval, they were then given a delayed word recall test, where they were asked to repeat the same words. Each correctly recalled word was scored, with a total possible score of 20 for overall situational memory.
The assessment of mental integrity included tests of temporal orientation, numerical ability, and figure-drawing skills. For temporal orientation, participants were asked to provide the survey date (including month, day, and year), day of the week, and season. The digit span test required participants to serially subtract 7 from 100 five times. Additionally, participants were asked to copy an image of two overlapping pentagrams. Like in the episodic memory test, each correctly answered question or successfully copied image earned one point, with a total score for mental integrity ranging from 0 to 11. The scores for episodic memory and mental integrity were combined to form an overall cognitive score, which ranged from 0 to 31. Previous research has confirmed that the use of the MMSE scale to assess overall cognitive function in middle-aged and older adult Chinese individuals has satisfactory validity and reliability (19). Cognitive impairment is typically defined as an overall cognitive score less than 11 (20, 21).
2.3 Definition of METS-IR
In this nationwide survey, blood samples from participants were collected by the Chinese Center for Disease Control and Prevention. All personnel involved in sample collection underwent rigorous training, passed examinations, and met strict qualification standards. The collected blood samples were transported to a local laboratory and stored at 4°C. Venous blood was separated into plasma and red–brown layers, with each component stored in separate 0.5 mL cryotubes. The cryotubes were immediately refrigerated at −20°C and shipped to the Chinese Center for Disease Control and Prevention headquarters in Beijing within 2 weeks. Upon arrival, they were placed in deep freezers and maintained at −80°C until analysis at the CMU laboratory. Notably, in the third wave of the CHARLS, the target sample for blood samples was a total of 21,100 interviewed individuals. In practice, blood samples were collected from 13,420 participants, resulting in a response rate of 64%. Data on FPG, TG, BMI, and HDLc were collected to calculate the METS-IR index as follows: METS-IR = (ln [(2 × FPG) + TG] × BMI)/(ln [HDLc]) (16). The participants were divided into four groups according to the quartile of the METS-IR index (Q1, Q2, Q3, and Q4).
2.4 Covariates
In accordance with previous studies (6, 17), demographics and health-related factors were considered common variables in the analysis of this study. The demographic variables included gender (male and female), age (45–60 years, 60 years and older), marital status (married and cohabiting, others including divorced, widowed, unmarried and separated), education (illiterate, primary school, secondary and above) and place of residence (urban, rural). The health-related variables included smoking status, alcohol consumption, body mass index, hypertension, diabetes, depression, etc. Smoking status was classified as never smoked, current smoker, or former smoker. Drinking conditions are defined as drinking at least once a month and are classified as never drinking alcohol, drinking less than once a month, and drinking more than once a month. BMI was calculated as weight (in kilograms) divided by the square of height (in meters), with units of kg/m2. BMI falls into three categories: less than or equal to 18.5, 18.5–24, and more than or equal to 24. Hypertension was defined as blood pressure above 140/90 mm Hg (based on the average of three measurements), self-reported hypertension or the use of antihypertensive medication by participants. Diabetes was defined as a fasting blood glucose greater than or equal to 7.0 mmol/L or self-reported diabetes, with the use of antidiabetic medications. The CHARLS assesses participants’ depression symptoms via the 10-item Center for Epidemiologic Studies Depression Scale (CESD-10), which has proven to be an effective, reliable, and practical tool for assessing mental health (22). In accordance with previous studies, participants who scored 10 or more on this scale were defined as having depression (23).
2.5 Statistical analysis
Continuous variables are shown as the means ± standard deviations (SDs), whereas categorical variables are presented as counts (percentages). Differences across the various quartile groups of METS-IR were evaluated via the chi-square test or the Kruskal–Wallis H test. The participants were categorized on the basis of METS-IR quartile, and the baseline characteristics of each group of individuals were compared.
First, a multivariate logistic regression model was used to assess the correlation between METS-IR and cognitive impairment. Three models were developed: Model I was not adjusted; Model II was adjusted by sex and age; and Model III was fully adjusted to include factors such as sex, age, marital status, education, location, smoking status, drinking status, BMI, hypertension, diabetes, and depression.
Second, the potential nonlinear relationship between METS-IR and cognitive impairment was investigated via a constrained cubic spline model based on the Model III adjustment variable. Likelihood ratio tests were also used to determine threshold effects between the METS-IR index and cognitive impairment and to identify potential inflection points in the case of nonlinear associations. Moreover, saturation threshold effect analysis was employed to identify potential inflection points.
Subsequently, subgroup analysis and interaction analysis were performed according to sex, age, marital status, education level, smoking status, alcohol consumption, BMI, and depression status to investigate whether these factors affect the relationship between METS-IR and the risk of cognitive impairment.
Furthermore, previous studies have shown a significant association between hypertension, diabetes, overweight and obesity, and cognitive impairment (24–26). Several sensitivity analyses were conducted to verify the robustness of these results. First, participants with no hypertension were analysed. Second, the association between METS-IR and cognitive impairment was explored in participants without diabetes. Finally, participants with a body mass index above 24 were excluded from the sensitivity analyses.
We used R software (version 4.3.3) and SPSS (version 26.0) for all the statistical analyses, and p < 0.05 (two-tailed) was considered statistically significant.
3 Results
3.1 Baseline characteristics of participants based on MetS-IR quartiles
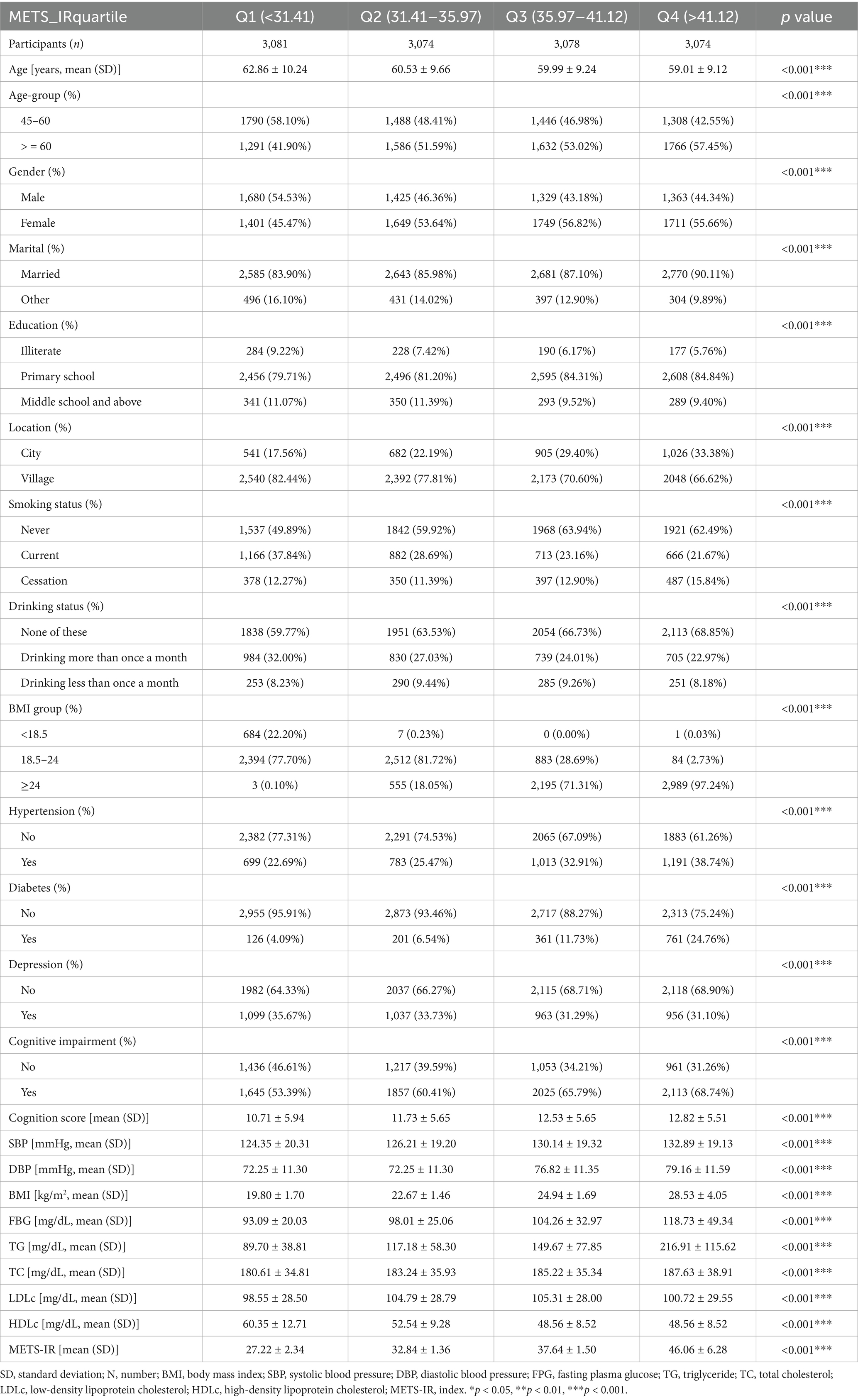
This study included a total of 12,307 participants, 52.89% of whom were female and 47.11% of whom were male. Participants aged 45–60 years accounted for 49.02%, whereas those aged 60 years and above accounted for 50.98%. The participants were divided into four groups based on the METS-IR quartile: Q1 (< 30.41 mg/dL), Q2 (30.41–35.97 mg/dL), Q3 (35.97–41.12 mg/dL), and Q4 (> 41.12 mg/dL). The means ± standard deviations of each quartile were 27.22 ± 2.34, 32.84 ± 1.36, 37.64 ± 1.50, and 46.06 ± 6.28, respectively. In the highest quartile (Q4), there was a greater proportion of female participants with hypertension, diabetes, overweight, and obesity than in the other groups. In contrast, the proportions of men in this group of smokers and alcohol consumers were lower. Additionally, participants with higher METS-IR levels tended to have elevated BMIs, TG levels, and TC levels. Furthermore, compared with those in the lowest quartile (Q1), participants in the other quartiles presented lower cognitive scores and tended to have a greater proportion of participants with cognitive impairment. Baseline characteristics are shown in Table 1.
3.2 Multiple regression analysis of the METS-IR index with cognitive impairment
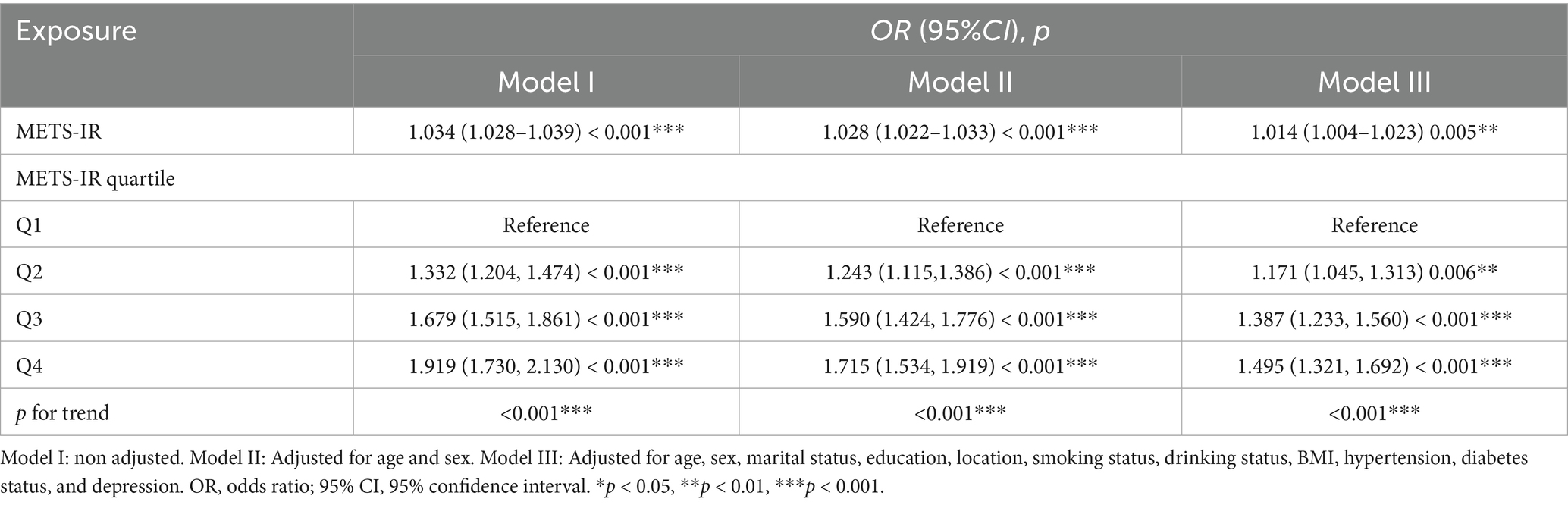
The results of the multiple linear regression analysis of the correlation between the METS-IR index and cognitive impairment are shown in Table 2. METS-IR was significantly correlated with cognitive impairment, and subjects with a higher METS-IR index tended to have worse cognitive function. After full adjustment, the odds ratio suggests that for every unit increase in the METS-IR index, there is a 1.4% increase in the odds of cognitive impairment (OR = 1.014, 95% CI: 1.004–1.023; p < 0.01). Furthermore, when the METS-IR index was used as a classification variable, a significant association was found between the quartile of the METS-IR index and cognitive impairment after sufficient adjustment of the variable. Compared with the first quarter, the risk of cognitive impairment was greater in the other quarters of participants (OR = 1.171, 95% CI: 1.045–1.313, p < 0.01), (OR = 1.387, 95% CI: 1.233–1.560, p < 0.001), and (OR = 1.495, 95% CI: 1.321–1.692, p < 0.001), respectively. Furthermore, these values indicate that for each unit increase in METS-IR levels, the odds of cognitive impairment were increased by 17.1, 38.7, and 49.5% in the Q2, Q3, and Q4 groups, respectively, compared with those in the Q1 group.
3.3 Nonlinear relationship between the METS-IR index and the risk of cognitive impairment
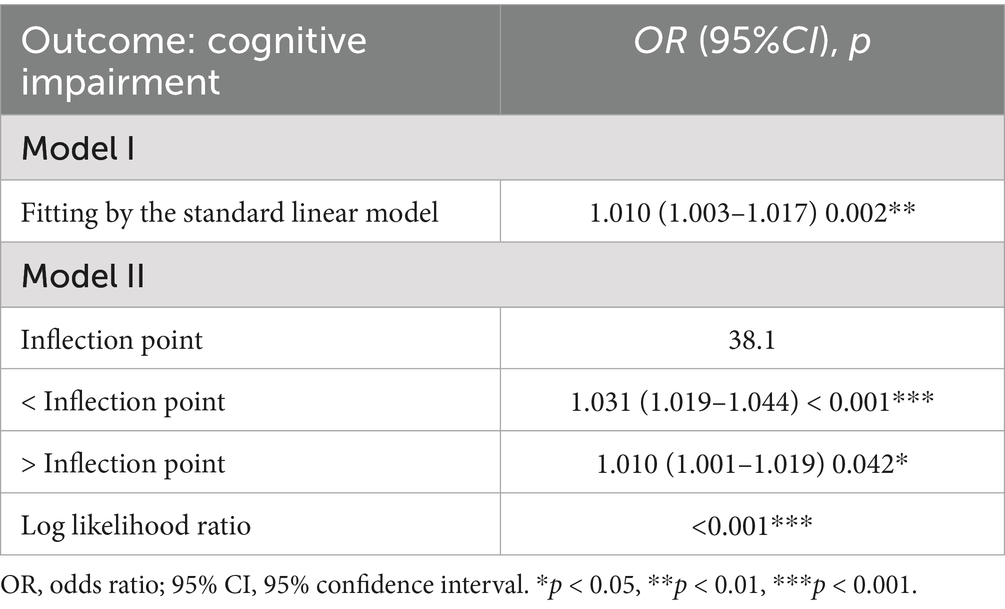
The nonlinear relationship between METS-IR and cognitive impairment risk is shown in Table 3. After fully adjusting for confounding factors, there was a special positive association between METS-IR and the risk of cognitive impairment. The inflection point is 38.1 according to saturation threshold effect analysis. Before the inflection point, (OR = 1.031, 95% CI: 1.019–1.044, p < 0.001), and after the inflection point, (OR = 1.010, 95% CI: 1.001–1.019, p < 0.05) are shown in Figure 2. To further validate the robustness of our findings, we included MMSE scores in our analysis. The results of the cognitive score were consistent with those of the cognitive impairment group, further demonstrating the reliability of the results (see Additional file 1).
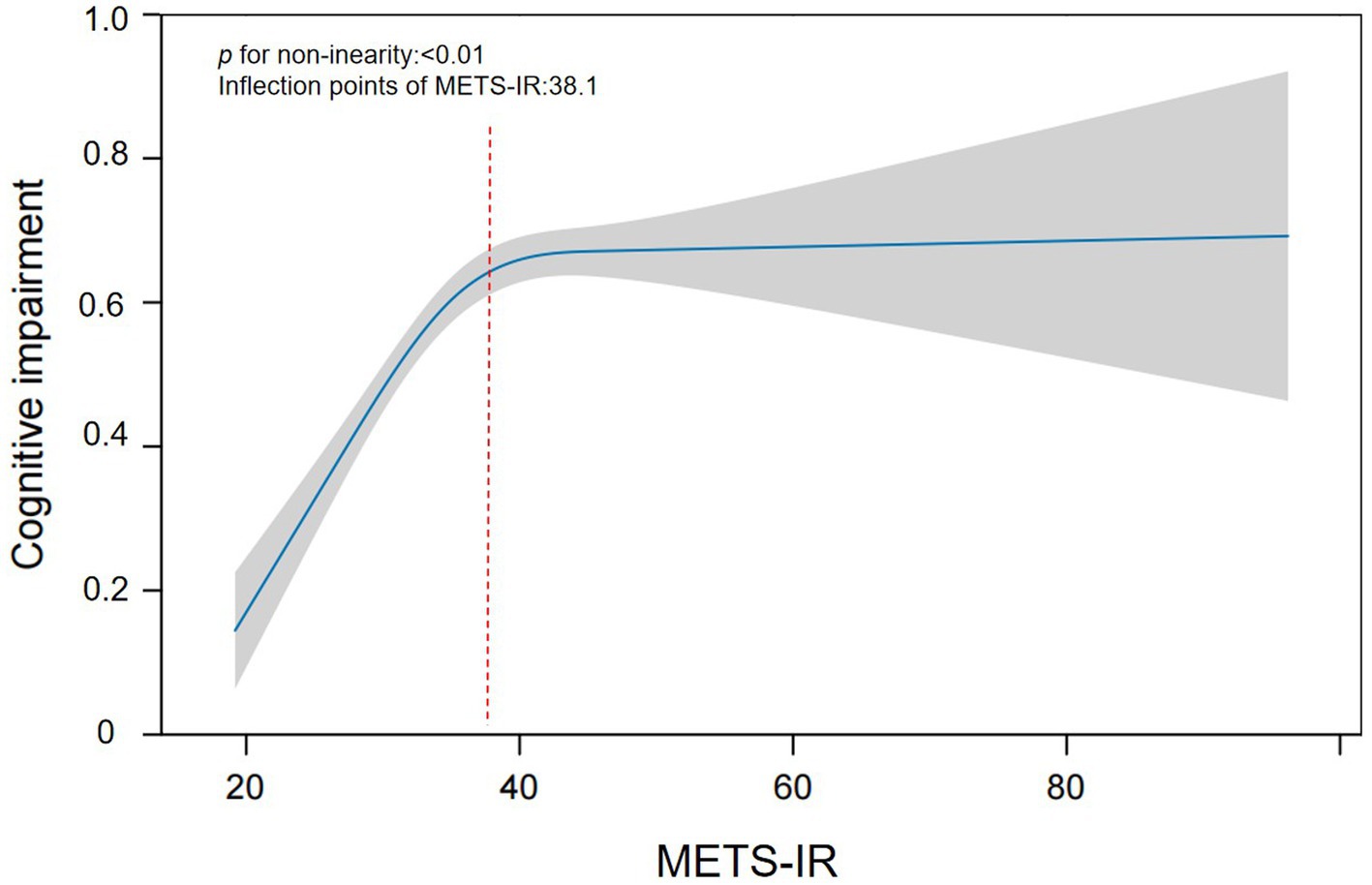
Figure 2. Smoothing curve fitting was used to assess the nonlinear relationship between the METS-IR index and cognitive impairment.
3.4 Subgroup and sensitivity analysis
The associations between METS-IR scores and cognitive impairment risk were not affected by age, marital status, education, smoking status, alcohol consumption, BMI, or depression, except for sex (p > 0.05 for interactions). In addition, female participants had a greater risk of cognitive impairment than male participants did (p = 0.016 for interaction), as shown in Figure 3. Sensitivity analyses were also conducted to ensure the reliability of the findings. We focused on specific groups and adjusted for various health factors to verify the strength and consistency of the relationship between the METS-IR score and cognitive impairment. First, the analysis was conducted in a nonhypertensive group of 8,621 participants, adjusting for age, sex, marital status, education, BMI, diabetes, depression, and other factors. The results still revealed a positive association between the METS-IR quartile and cognitive impairment (OR = 1.405, 95% CI: 1.215–1.634; p < 0.001). Second, after excluding participants with diabetes, the results were similar after adjusting for confounding variables, and the association between the METS-IR quartile and cognitive impairment remained positive and significant (OR = 1.431, 95% CI: 1.297–1.691, p < 0.001). Finally, the analysis was narrowed to participants with a BMI of less than 24 and was adjusted for all the previously mentioned factors. The results also revealed an association between the METS-IR quartile and cognitive impairment (OR = 1.342, 95% CI: 1.106–1.904, p < 0.01) (see Additional file 2).
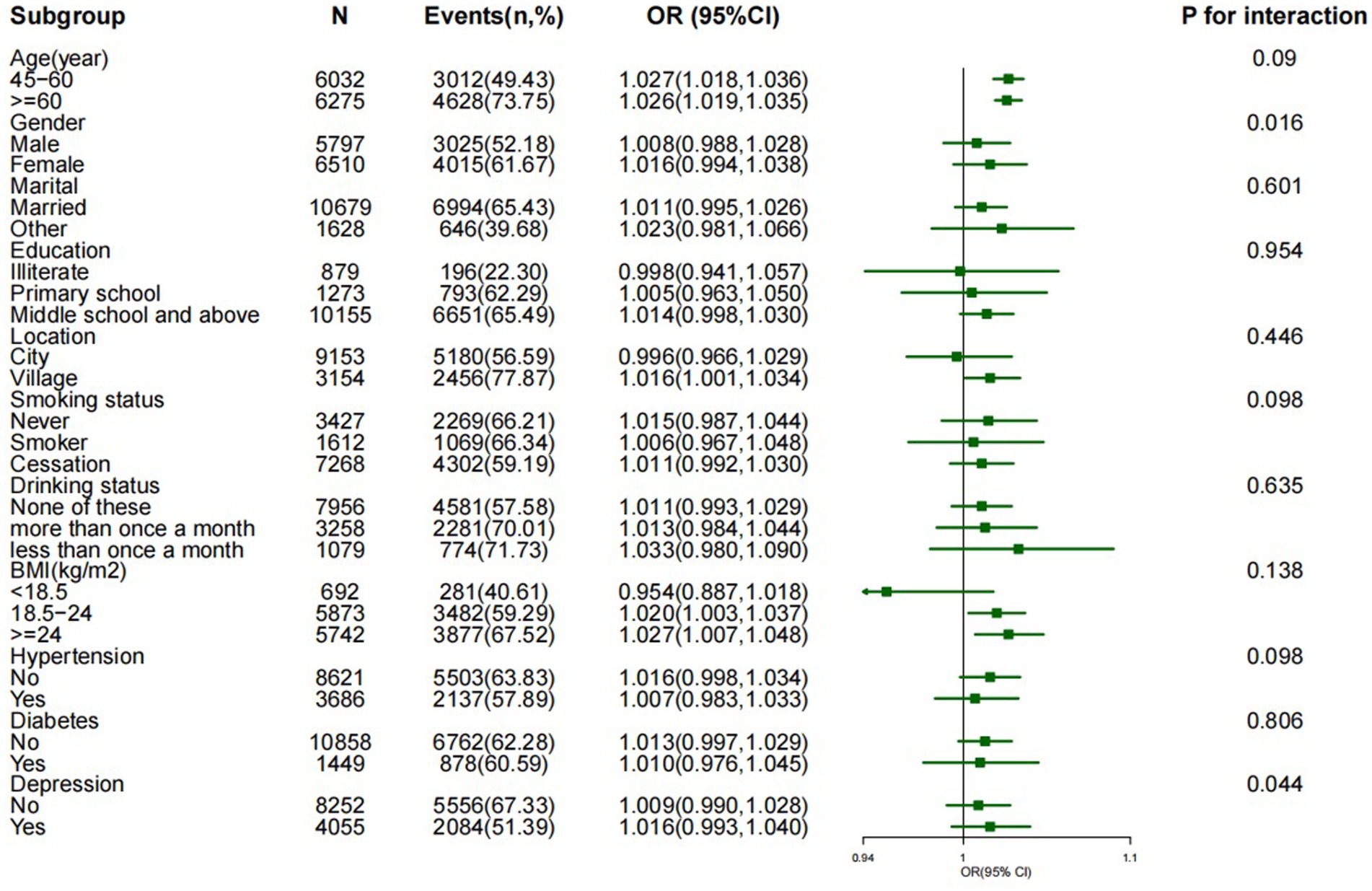
Figure 3. Effect size of the METS-IR index on cognitive impairment in the subgroup. Notes: adjusted for age, sex, marital status, education, location, smoking status, drinking status, BMI, hypertension, diabetes status, and depression.
4 Discussion
This study was a cross-sectional study involving 12,307 participants to investigate the association between METS-IR and cognitive impairment in middle-aged and older Chinese individuals. The results revealed that elevated METS-IR was associated with a significantly increased risk of cognitive impairment. Furthermore, inflection points were determined by observing saturation threshold effects, and the relationship between METS-IR scores and cognitive impairment differed across tests. The findings remained uniform across all clusters and sensitivity examinations, particularly those focused on females and people aged 60 years and above.
As the world’s population ages, cognitive deficits intensify, making the related decline an acute public health issue. Symptoms of cognitive impairment usually occur several years before dementia begins, highlighting the importance of investigating the process and developing methods to prevent risk factors (27, 28). Previous studies have shown that genetic predisposition, cardiovascular disease, and other factors contribute to cognitive deterioration (29–31).
Insulin resistance is characterized by reduced sensitivity and responsiveness to insulin, which is at the core of various health problems, including diabetes, cardiovascular disease, and cognitive decline. Although the insulin clamp and fasting glucose have been considered the best standards for assessing IR, most studies tend to use alternative indicators for evaluation (32–34). A large cohort study revealed a significant association between the triglyceride glucose index, a simple measure of IR, and cognitive impairment among older adult individuals in China (35). Another study using HOMA-IR as a surrogate indicator for IR indicated that this index is associated with an increased risk of cognitive decline in obese adolescents and older individuals (36). In recent years, the METS-IR index has gained increasing interest as an important indicator for assessing IR due to its simplicity, reliability, and practicality compared with the HOMA-IR and insulin clamp techniques. Unfortunately, only one study has addressed this topic, which identified METS-IR as an effective predictor of mild cognitive impairment in nondiabetic individuals (18). Therefore, the current evidence regarding the relationship between METS-IR and cognitive impairment is quite limited, and further investigation is necessary to elucidate their association.
In this study, we revealed that there are sex differences in the risk of cognitive impairment, with further subgroup analysis revealing that women have a greater risk of cognitive impairment than men do. Research has indicated that women are more susceptible to cognitive impairment than men are, and the incidence of dementia is also higher among women across all age groups (2, 37). Research has suggested that estrogen is critical for memory function and that estrogen rehabilitation therapy can have a protective effect on neurons while improving cognitive performance (38). In this study, most female participants were in the perimenopausal or postmenopausal stage, which corresponds to a physiological phase characterized by a significant decline in estrogen levels within the body. This specific biological state can reasonably explain the more pronounced decline in cognitive ability observed in women than in men during our analysis. Therefore, the lack of protective effects of estrogen may play a dominant role in cognitive decline, while the relative importance of insulin resistance (IR) may be lower (2). However, the specific mechanisms by which estrogen affects cognitive function still require further investigation.
To further investigate the association between the METS-IR index and cognitive impairment in greater depth, we employed quartile classification of the METS-IR index prior to analysis. In Model III, after adjusting for potential confounding variables, we observed that an elevated METS-IR index was significantly associated with an increased risk of cognitive impairment compared with the lowest quartile (Q1) (OR = 1.495, 95% CI: 1.321–1.692, p < 0.001). Previous studies have identified blood pressure, diabetes, and obesity as risk factors for cognitive impairment (24–26). Therefore, we conducted a systematic sensitivity analysis by focusing on specific subgroups and adjusting for various health factors. The results confirmed a significant correlation between METS-IR and the risk of cognitive dysfunction. When we use smooth curve fitting to study the possible nonlinear relationship between two variables, we obtain interesting results. This study revealed a nonlinear relationship between METS-IR and cognitive impairment for the first time. The METS-IR inflection point was 38.1. When the METS-IR exceeded 38.1, each unit decrease in the METS-IR was associated with a 1.0% reduction in the risk of cognitive impairment. Conversely, when the METS-IR was less than 38.1, each unit decrease in the METS-IR corresponded to a 3.1% reduction in the risk of cognitive impairment. This suggests that as the METS-IR decreases, the risk of cognitive impairment gradually diminishes. The nonlinear relationship depicted in the curve-fitting plot aligns with these findings. For individuals with METS-IR values less than 38.1, the impact of other cognitive impairment risk factors is relatively diminished, thereby enhancing the effect of METS-IR. Upon examining the data, we observed that categorizing METS-IR, with the cut-off at Q4 (>38.1), places this threshold beyond the inflection point of the smoothed curve. When analysing the association between METS-IR and cognitive impairment risk, dividing the index into quartiles might diminish the statistical power and compromise result stability, primarily because very few participants present high METS-IR levels. Therefore, excessively high METS-IR values may weaken the direct impact on the increased risk of cognitive impairment when the model is constructed. This explains the observed nonlinear relationship between METS-IR and cognitive impairment risk. The discovery of this curve-like relationship has important clinical implications and can serve as a surrogate marker of IR to manage cognitive decline in middle-aged and older adult individuals. Reducing BMI, TG, and FPG levels through dietary modification, cognitive rehabilitation, and other comprehensive measures can significantly reduce the risk of cognitive impairment, particularly by maintaining the METS-IR below 38.1.
While the precise connection between cognitive decline and IR remains somewhat obscure, multiple elements account for these findings. Insulin passes through the blood–brain barrier via distinct receptors, affecting metabolic activity and behavior in the body (11, 39). IR often signifies a particular metabolic disorder characterized by elevated insulin levels, which may lead to neurodegenerative changes and persistent memory decline due to the long-term exposure of brain neurons to an environmental state of elevated insulin levels (40). Previous studies have suggested that peripheral insulin and brain insulin regulate glucose metabolism in the brain. Most individuals with IR/compensatory hyperinsulinemia exhibit metabolic abnormalities, which may decrease glucose metabolism in specific regions of the brain, subsequently affecting learning and memory by modulating synaptic plasticity, synapse density, synaptic transmission, and neurogenesis (41). In addition, impaired insulin signalling and neuroinflammation are both associated with cognitive function, and all of these pathological features are essential contributors to the decline in cognitive ability. Research has revealed elevated levels of inflammatory markers, particularly cytokines/chemokines involved in affected areas, in the brain tissue of patients with cognitive impairments (42). Animal experiments have revealed that in obese mouse models, the mechanism through which TNF-α is inhibited can effectively halt its interference in the insulin signalling process, thereby significantly improving insulin sensitivity and glucose homeostasis (43). In overweight and obese individuals, high levels of TG are more likely to cross the blood–brain barrier and may interfere with nerve transmission and lead to neurological dysfunction by inducing insulin receptor resistance (44). In the stratified analysis, the association between the METS-IR index and cognitive impairment was observed only in overweight and obese individuals, suggesting an association between traditional IR and obesity. Components of the METS-IR index, including FPG, TG, BMI, and HDLc, play a vital role in these mechanisms. Therefore, investigating these potential mechanisms simultaneously reveals a potential pathway between the METS-IR index and cognitive function.
With increasing age, the issue of cognitive decline has become increasingly prominent. Cognitive deficits, often seen as initial signs of dementia, are linked to significantly poor prognosis, impacting individual quality of life and imposing a burden on families and society (45). Therefore, early prevention of cognitive impairment is crucial and urgent. Our research revealed that the METS-IR index can be used as an alternative marker to predict cognitive impairment in middle-aged and older adult Chinese individuals and that there is a significant relationship between a high METS-IR index and cognitive impairment. Targeted interventions could help alleviate cognitive impairment, which may reduce the incidence of dementia. Additionally, investigating predictive risk is critical for early prevention to detect cognitive impairment.
This cross-sectional study is based on a representative sample from China and supports the hypothesis that there is a significant correlation between the METS-IR index and cognitive impairment. Unlike previous studies, our survey analysed the METS-IR index as a categorical and continuous variable related to cognitive impairment, reducing information loss and quantifying their relationship after adjusting for numerous confounding factors. In addition, the sensitivity study focused on participants without hypertension, with diabetes, or with a BMI less than 24 kg/m2. Further validation of the subgroup analysis confirmed that this relationship persisted in participants. In summary, we identified METS-IR as a risk factor for cognitive impairment and elucidated the relationship between the two, providing new perspectives for the prevention and management of cognitive impairment.
This study also has several limitations. First, given the cross-sectional nature of the study, the direct cause of the association between METS-IR levels and cognitive deficits is unclear. In addition, our analysis is based on existing databases, and there are minor data omissions in the calculation of the METS-IR index that may lead to selection bias in the results. Finally, as with all observational studies, despite adjustments for known potential confounding factors, residual confounding caused by unmeasured or uncontrolled confounding factors may still exist, so some unconsidered confounding variables may affect the results.
5 Conclusion
The study was conducted via the Chinese CHARLS database, which contains a nationally representative sample of Chinese middle-aged and older adult individuals. The study revealed a significant link between higher METS-IR levels and an increased risk of cognitive impairment. Notably, there is a nonlinear relationship between METS-IR and the risk of cognitive impairment. A further reduction in METS-IR was associated with a significantly reduced risk of cognitive impairment when the METS-IR value was less than 38.1. In addition, gender differences in the research findings were observed. This study provides additional insights into how cognitive function can facilitate clinical counselling and optimize prevention decisions. In the future, further prospective research is needed to gain a deeper understanding of the impact of insulin resistance on the risk of cognitive impairment and provide more effective guidance for the prevention and management of cognitive disorders.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: China Health and Retirement Longitudinal Study (CHARLS).
Ethics statement
The studies involving humans were approved by the Biomedical Ethics Review Committee of Peking University (IRB approval number IRB00001052-11015). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NJ: Writing – original draft, Data curation, Methodology, Writing – review & editing. CM: Investigation, Software, Writing – original draft. ZF: Supervision, Conceptualization, Writing – original draft. YT: Writing – original draft, Supervision, Formal analysis. XC: Visualization, Writing – original draft, Project administration. YH: Writing – original draft, Validation, Formal analysis. WP: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the Guangxi Science and Technology Base and Talent Special Project (grant number AD18050005) and the Major Science and Technology Projects in Guangxi (GKAA22096026).
Acknowledgments
We would like to thank the communities and data collection teams for their support in carrying out the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1607228/full#supplementary-material
References
1. Apostolou, A, Kennedy, JL, Person, MK, Jackson, EMJ, Finke, B, McGuire, LC, et al. Alzheimer's disease and related dementia diagnoses among American Indian and Alaska native adults aged ≥45 years, Indian Health Service system, 2016-2020. J Am Geriatr Soc. (2024) 72:2834–41. doi: 10.1111/jgs.19058
2. Jia, L, Du, Y, Chu, L, Zhang, Z, Li, F, Lyu, D, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. (2020) 5:e661–71. doi: 10.1016/S2468-2667(20)30185-7
3. Sehar, U, Kopel, J, and Reddy, PH. Alzheimer's disease and its related dementias in US native Americans: a major public health concern. Ageing Res Rev. (2023) 90:102027. doi: 10.1016/j.arr.2023.102027
4. Zaciragic, A, Dervisevic, A, Valjevac, A, Fajkic, A, Spahic, S, Hasanbegovic, I, et al. Difference in the standard and novel lipid profile parameters between patients with Alzheimer's disease and vascular dementia stratified by the degree of cognitive impairment. Mater Socio Med. (2022) 34:100–6. doi: 10.5455/msm.2022.34.100-106
5. Li, R, Qi, J, Yang, Y, Wu, Y, Yin, P, Zhou, M, et al. Disease burden and attributable risk factors of Alzheimer's disease and dementia in China from 1990 to 2019. J Prev Alzheimers Dis. (2022) 9:306–14. doi: 10.14283/jpad.2021.69
6. Wang, J, Li, L, Zhang, Z, Zhang, X, Zhu, Y, Zhang, C, et al. Extracellular vesicles mediate the communication of adipose tissue with the brain and promote cognitive impairment associated with insulin resistance. Cell Metab. (2022) 34:1264–1279.e8. doi: 10.1016/j.cmet.2022.08.004
7. Thomas, MS, Calle, M, and Fernandez, ML. Healthy plant-based diets improve dyslipidemias, insulin resistance, and inflammation in metabolic syndrome. A narrative review. Adv Nutr. (2023) 14:44–54. doi: 10.1016/j.advnut.2022.10.002
8. Izuo, N, Watanabe, N, Noda, Y, Saito, T, Saido, TC, Yokote, K, et al. Insulin resistance induces earlier initiation of cognitive dysfunction mediated by cholinergic deregulation in a mouse model of Alzheimer's disease. Aging Cell. (2023) 22:e13994. doi: 10.1111/acel.13994
9. Kullmann, S, Heni, M, Hallschmid, M, Fritsche, A, Preissl, H, and Häring, HU. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev. (2016) 96:1169–209. doi: 10.1152/physrev.00032
10. Minh, HV, Tien, HA, Sinh, CT, Thang, DC, Chen, CH, Tay, JC, et al. Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J Clin Hypertens (Greenwich). (2021) 23:529–37. doi: 10.1111/jch.14155
11. Bello-Chavolla, OY, Almeda-Valdes, P, Gomez-Velasco, D, Viveros-Ruiz, T, Cruz-Bautista, I, Romo-Romo, A, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. (2018) 178:533–44. doi: 10.1530/EJE-17-0883
12. Song, K, Lee, E, Lee, HS, Lee, H, Lee, JW, Chae, HW, et al. Comparison of SPISE and METS-IR and other markers to predict insulin resistance and elevated liver transaminases in children and adolescents. Diabetes Metab J. (2025) 49:264–74. doi: 10.4093/dmj.2024.0302
13. Tsai, KZ, Chu, CC, Huang, WC, Sui, X, Lavie, CJ, and Lin, GM. Prediction of various insulin resistance indices for the risk of hypertension among military young adults: the CHIEF cohort study, 2014-2020. Cardiovasc Diabetol. (2024) 23:141. doi: 10.1186/s12933-024-02229-8
14. Peng, H, Xiang, J, Pan, L, Zhao, M, Chen, B, Huang, S, et al. METS-IR/HOMA-IR and MAFLD in U.S. adults: dose–response correlation and the effect mediated by physical activity. BMC Endocr Disord. (2024) 24:132. doi: 10.1186/s12902-024-01646-w
15. Wang, T, Yi, Z, Tan, Y, Huang, Y, Li, T, Gao, S, et al. Changes in the metabolic score for insulin resistance index for risk prediction of stroke in middle-aged and older Chinese population. EPMA J. (2024) 15:599–610. doi: 10.1007/s13167-024-00388-y
16. Wang, G, Zhu, Z, Wang, Y, Zhang, Q, Sun, Y, Pang, G, et al. The association between METS-IR, an indirect index for insulin resistance, and lung cancer risk. Eur J Pub Health. (2024) 34:800–5. doi: 10.1093/eurpub/ckad234
17. Sloten, TT, Sedaghat, S, Carnethon, MR, Launer, LJ, and Stehouwer, CDA. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. (2020) 8:325–36. doi: 10.1016/S2213-8587(19)30405-X
18. Cui, Y, Xu, Z, Cui, Z, Guo, Y, Wu, P, and Zhou, X. Comparative study of insulin resistance surrogate indices to predict mild cognitive impairment among Chinese nondiabetic adults. Lipids Health Dis. (2024) 23:357. doi: 10.1186/s12944-024-02353-0
19. Li, L, Zhuang, L, Xu, Z, Jiang, L, Zhai, Y, Liu, D, et al. U-shaped relationship between non-high-density lipoprotein cholesterol and cognitive impairment in Chinese middle-aged and elderly: a cross-sectional study. BMC Public Health. (2024) 24:1624. doi: 10.1186/s12889-024-19164-8
20. Liu, H, Zou, L, Zhou, R, Zhang, M, Gu, S, Zheng, J, et al. Long-term increase in cholesterol is associated with better cognitive function: evidence from a longitudinal study. Front Aging Neurosci. (2021) 13:691423. doi: 10.3389/fnagi.2021.691423
21. Zhou, S, Song, S, Jin, Y, and Zheng, ZJ. Prospective association between social engagement and cognitive impairment among middle-aged and older adults: evidence from the China health and retirement longitudinal study. BMJ Open. (2020) 10:e040936. doi: 10.1136/bmjopen-2020-040936
22. Chen, H, and Mui, AC. Factorial validity of the Center for Epidemiologic Studies Depression Scale short form in older population in China. Int Psychogeriatr. (2014) 26:49–57. doi: 10.1017/S1041610213001701
23. Jiang Hong, C, Zhu, F, and Qin, T. Relationships between chronic diseases and depression among middle-aged and elderly people in China: a prospective study from CHARLS. Curr Med Sci. (2020) 40:858–70. doi: 10.1007/s11596-020-2270-5
24. Wu, M, Lu, C, Chen, F, Fan, Y, Li, G, and Zhou, L. Age of hypertension onset and cognitive function in elderly individuals: an observational study from the NHANES 2011-2014. Eur Geriatr Med. (2024) 15:561–70. doi: 10.1007/s41999-023-00920-9
25. Casagrande, SS, Lee, C, Stoeckel, LE, Menke, A, and Cowie, CC. Cognitive function among older adults with diabetes and prediabetes, NHANES 2011-2014. Diabetes Res Clin Pract. (2021) 178:108939. doi: 10.1016/j.diabres.2021.108939
26. Zhang, Y, Zhang, P, and Yin, D. Association between a body shape index and cognitive impairment among us older adults from a cross-sectional survey of the NHANES 2011-2014. Lipids Health Dis. (2024) 23:169. doi: 10.1186/s12944-024-02165-2
27. Javitt, DC. Cognitive impairment associated with schizophrenia: from pathophysiology to treatment. Annu Rev Pharmacol Toxicol. (2023) 63:119–41. doi: 10.1146/annurev-pharmtox-051921-093250
28. Mian, M, Tahiri, J, Eldin, R, Altabaa, M, Sehar, U, and Reddy, PH. Overlooked cases of mild cognitive impairment: implications to early Alzheimer's disease. Ageing Res Rev. (2024) 98:102335. doi: 10.1016/j.arr.2024.102335
29. Goyal, P, Didomenico, RJ, Pressler, SJ, Ibeh, C, White-Williams, C, Allen, LA, et al. Cognitive impairment in heart failure: a Heart Failure Society of America scientific statement. J Card Fail. (2024) 30:488–504. doi: 10.1016/j.cardfail.2024.01.003
30. Koh, K, Ishiura, H, Beppu, M, Shimazaki, H, Ichinose, Y, Mitsui, J, et al. Novel mutations in the ALDH18A1 gene in complicated hereditary spastic paraplegia with cerebellar ataxia and cognitive impairment. J Hum Genet. (2018) 63:1009–13. doi: 10.1038/s10038-018-0477-0
31. Adams, D, Sekijima, Y, Conceição, I, Waddington-Cruz, M, Polydefkis, M, Echaniz-Laguna, A, et al. Hereditary transthyretin amyloid neuropathies: advances in pathophysiology, biomarkers, and treatment. Lancet Neurol. (2023) 22:1061–74. doi: 10.1016/S1474-4422(23)00334-4
32. Hong, S, Han, K, and Park, CY. The insulin resistance by triglyceride glucose index and risk for dementia: population-based study. Alzheimer's Res Ther. (2021) 13:9. doi: 10.1186/s13195-020-00758-4
33. Cui, Y, Tang, TY, Lu, CQ, and Ju, S. Insulin resistance, and cognitive impairment: evidence from neuroimaging. J Magn Reson Imaging. (2022) 56:1621–49. doi: 10.1002/jmri.28358
34. Rhea, EM, Leclerc, M, Yassine, HN, Capuano, AW, Tong, H, Petyuk, VA, et al. State of the science on brain insulin resistance and cognitive decline due to Alzheimer's disease. Aging Dis. (2024) 15:1688–725. doi: 10.14336/AD.2023.0814
35. Bai, W, An, S, Jia, H, Xu, J, and Qin, L. Relationship between triglyceride-glucose index and cognitive function among community-dwelling older adults: a population-based cohort study. Front Endocrinol (Lausanne). (2024) 15:1398235. doi: 10.3389/fendo.2024.1398235
36. Luciano, R, Barraco, GM, Muraca, M, Ottino, S, Spreghini, MR, Sforza, RW, et al. Biomarkers of Alzheimer disease, insulin resistance, and obesity in childhood. Pediatrics. (2015) 135:1074–81. doi: 10.1542/peds.2014-2391
37. Wang, K, Xu, L, Liu, L, Zhan, S, Wang, S, and Song, Y. Sex differences in the association between the change in triglyceride-glucose index and cognitive decline: a population-based cohort study. J Affect Disord. (2022) 316:42–9. doi: 10.1016/j.jad.2022.08.014
38. Engler-Chiurazzi, EB, Singh, M, and Simpkins, JW. From the 90's to now: a brief historical perspective on more than two decades of estrogen neuroprotection. Brain Res. (2016) 1633:96–100. doi: 10.1016/j.brainres.2015.12.044
39. Soto, M, Cai, W, Konishi, M, and Kahn, CR. Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. Proc Natl Acad Sci USA. (2019) 116:6379–84. doi: 10.1073/pnas.1817391116
40. Tian, N, Song, L, Hou, T, Fa, W, Dong, Y, Liu, R, et al. Association of Triglyceride-Glucose Index with Cognitive Function and Brain Atrophy: a population-based study. Am J Geriatr Psychiatry. (2024) 32:151–62. doi: 10.1016/j.jagp.2023.09.007
41. Ott, V, Benedict, C, Schultes, B, Born, J, and Hallschmid, M. Intranasal administration of insulin to the brain impacts cognitive function and peripheral metabolism. Diabetes Obes Metab. (2012) 14:214–21. doi: 10.1111/j.1463-1326.2011.01490
42. Ferreira, ST, Clarke, JR, Bomfim, TR, and De Felice, FG. Inflammation, defective insulin signalling, and neuronal dysfunction in Alzheimer's disease. Alzheimers Dement. (2014) 10:S76–83. doi: 10.1016/j.jalz.2013.12.010
43. Hotamisligil, GS, Shargill, NS, and Spiegelman, BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. (1993) 259:87–91. doi: 10.1126/science.7678183
44. Li, J, Xue, C, Yang, H, Zhang, J, Li, G, Li, J, et al. Simulated weightlessness induces hippocampal insulin resistance and cognitive impairment. Life Sci. (2023) 333:122112. doi: 10.1016/j.lfs.2023.122112
Keywords: METS-IR, cognitive impairment, middle-aged and older adult, insulin resistance, CHARLS
Citation: Jiang N, Ma C, Feng Z, Tang Y, Chen X, He Y and Pang W (2025) Associations between the METS-IR index and cognitive function in community-dwelling Chinese middle-aged and older adult individuals: a cross-sectional study. Front. Public Health. 13:1607228. doi: 10.3389/fpubh.2025.1607228
Edited by:
Ben Nephew, Worcester Polytechnic Institute, United StatesReviewed by:
Yifan Li, Guangzhou University of Chinese Medicine, ChinaEdgardo Molina-Sotomayor, Metropolitan University of Educational Sciences, Chile
Copyright © 2025 Jiang, Ma, Feng, Tang, Chen, He and Pang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiyi Pang, cC53ZWl5aUBsaXZlLmNu
 Nian Jiang
Nian Jiang Chenlu Ma1,2
Chenlu Ma1,2 Weiyi Pang
Weiyi Pang