- 1Department of Cardiology, The First People’s Hospital of Xiaoshan District, Xiaoshan Affiliated Hospital of Wenzhou Medical University, Hangzhou, Zhejiang, China
- 2Department of Oncology, The People's Hospital of Jiangshan, Quzhou, Zhejiang, China
- 3Department of Breast Surgery, Hangzhou TCM Hospital Affiliated to Zhejiang Chinese Medical University, Hangzhou Hospital of Traditional Chinese Medicine, Hangzhou, Zhejiang, China
- 4Department of Oncology, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, Fujian, China
Background: To evaluate the spatiotemporal variation in ischemic stroke attributed to particulate matter 2.5 (PM2.5) on global, regional, and national scales from 1990 to 2021 is essential for mitigating air pollution and controlling ischemic stroke.
Methods: The death and disability-adjusted life years (DALYs) were extracted from the Global Burden and Disease Study (GBD) 2021. We utilized joinpoint regression and decomposition analysis to assess PM2.5 exposure and pinpoint high-risk areas.
Results: In 2021, PM2.5 caused approximately 0.90 million mortality and 18.29 million DALYs due to ischemic stroke worldwide. The age-standardized rates (ASRs) of ischemic stroke linked to ambient PM2.5 slightly declined, while those associated with household PM2.5 significantly decreased over the past 32 years. The burden of ischemic stroke attributable to ambient and household PM2.5 exhibited considerable heterogeneity across 204 countries. Household PM2.5 significantly affected ischemic stroke burdens in low Socio-demographic indices (SDI) regions, whereas ambient PM2.5 had a greater impact in middle, high-middle, and high SDI regions. In the regions with an SDI below 0.7, including Southern Sub-Saharan Africa and East, South, and Southeast Asia, there was a positive correlation between SDI and ASRs linked with ambient PM2.5. Notably, in the 65–95 age group, the age-specific rates associated with ambient PM2.5 showed a substantial decline among females, while the rates for males remained relatively stable.
Conclusion: Our results presented that PM2.5 significantly affects global ischemic stroke burden, particularly among the male population and in low SDI regions. It highlighted the urgency of integrating PM2.5 reduction strategies with ischemic stroke prevention programs.
Introduction
Stroke is the second leading cause of death worldwide, which is categorized into two primary types: ischemic stroke and hemorrhagic stroke (1). Ischemic stroke, accounting for approximately 87% of all stroke cases, is a critical cerebrovascular disease characterized by the abrupt disruption of blood flow to the brain (2). It is typically caused by thrombosis, embolism, and systemic hypoperfusion. Ischemic stroke is influenced by modifiable risk factors like hypertension, diabetes, physical inactivity, and tobacco use, as well as non-modifiable risk factors such as age, gender, and genetics (3, 4). The substantial disease burden necessitates comprehensive strategies to optimize stroke risk factor management.
Air pollution is an important modifiable risk factors, with particulate matter (PM) being one of the most detrimental pollutants to global health (5, 6). Numerous studies have explored the relationship between PM2.5 exposure (particulate matter with an aerodynamic diameter of less than 2.5 μm) and a spectrum of adverse health outcomes, including respiratory and cardiovascular diseases, primarily through mechanisms such as systemic inflammation and oxidative stress (7, 8). Long-term exposure to PM2.5 is significantly linked to increased incidence of ischemic stroke, with a 0.0091% elevation in disease risk observed for each 1 μg/m3 augmentation in PM2.5 concentration (9, 10). Consequently, a thorough evaluation and analysis of ischemic stroke attributable to PM2.5 is imperative for formulating public health strategies to reduce the impact of air pollution on the incidence and burden of ischemic stroke.
Previous studies on the spatiotemporal trend of ischemic stroke linked to PM2.5 have been conducted based on the Global Burden of Disease Study (GBD) 2019 (11–13). The COVID-19 pandemic that erupted at the end of 2019 led to unprecedented challenges, including overwhelming healthcare facilities, profound economic disruptions, and extensive social transformations (14). Meanwhile, the COVID-19 lockdown resulted in a decrease in PM2.5 concentrations while concurrently postponing the disease diagnosis and treatment, potentially altering epidemiological dynamics of ischemic stroke associated with PM2.5 (15). Ischemic stroke also represented a potential complication in the COVID-19 survivors within 9 months post-infection (16). Hence, to assess the influence of the COVID-19 pandemic on disease burden, we evaluated the spatiotemporal trend of PM2.5-related ischemic stroke based on the latest GBD 2021 study.
Methods
Data sources and collection
Our analysis was based on the most recent data sourced from the GBD 2021,1 which offered a comprehensive and comparative assessment of 288 estimates related to death causes, along with 87 risk factors, across 21 regions and 204 countries and territories during 1990–2021 (17). Our study specifically concentrated on ischemic strokes occurring in individuals aged 25 years and older. The participants were categorized into consecutive five-year age brackets (20–24, 25–29, 30–34… 90–94, and 95 + years). We also extracted the socio-demographic index (SDI) from the GBD 2021 study, a composite measure made for each country according to education, income per capita, and fertility rate. To classify all countries and territories, the SDI values in 2021 were utilized to divide them into five regions: low, low-middle, middle, high-middle, and high SDI regions.
The latest data comprised information on deaths, Disability-adjusted life year (DALYs) and corresponding age-standardized rate (ASR) for ischemic stroke, categorized by country, region, year, and age groups. DALYs were derived by aggregating the years lived with disability (YLDs) and the years of life lost (YLLs). YLDs were computed by multiplying the prevalence by the respective standardized disability weights for each health state. Additionally, YLLs were estimated based on a reference maximum observed life expectancy. ASRs were widely utilized to enhance the accuracy of disease burden comparisons, as they considered the age variation across populations.
Particulate matter pollution encompassed both ambient PM2.5 and household PM2.5. The former aspect, known as ambient particulate matter pollution, referred to as the annual mean concentration of particles with a diameter less than 2.5 micrometers, weighted by population (18). The latter aspect, commonly described as household air pollution stemming from solid fuels like coal, wood, dung, and charcoal, was estimated using both the proportion model and the PM2.5 mapping model (19). The impact of PM2.5 exposure on ischemic stroke was assessed using the proportional population attributable fraction, while simultaneously considering the potential effects of geography, year sex, and age.
Statistical analyses
To evaluate the temporal trends, we implemented two approaches, including the estimated annual percentage change (EAPC) and the average annual percentage change (AAPC). A linear regression model was applied to compute the EAPC, and two formulas were set as followed.
In these equation, X denoted the calendar year, ε represented the error term, β referred to the coefficient. We also employed joinpoint analysis to compute annual percentage change (APC) along with its associated 95% confidence interval (CI). The AAPC was measured as the weighted average of APCs to succinctly describe the trends over a predetermined fixed period. The equation employed to compute AAPC was set as followed.
where b symbolized the coefficient for the segment, wi signified the length of the segment, i referred to the specific ith segment.
Decomposition analysis, motivated by Das Gupta’s method, was applied to gain insights into the disparities in disease burden across different regions (20). This approach attributed DALYs difference to the combined influences of three factors, such as population size, population aging, and epidemiological changes. We carried out the pearson’s correlation analysis to evaluate the relationship between ASRs and SDI. All statistics and visualization were executed via the R software (version 4.2.2). The establishment of statistical significance was predicated on a p value less than 0.05.
Results
Global ischemic stroke burden caused by particulate matter pollution
In 2021, the global impact of PM2.5 air pollution resulted in approximately 0.90 million ischemic stroke deaths and 18.29 million DALYs, representing an ASMR of 11.01 (95% UI 8.44–13.96) per 100,000 population and an ASDR of 215.64 (95% UI 168.84–266.03) per 100,000 population (Table 1). The ASRs in 2021 were less than those recorded in 1990, suggesting a downward trend as indicated by negative values for both EAPC (−2.19, 95%CI: −2.36 to −2.03 for ASMR; −2.04, 95%CI: −2.20 to −1.88 for ASDR) and AAPC (−2.13, 95%CI: −2.2 to −2.05 for ASMR; −1.98, 95%CI: −2.05 to −1.90 for ASDR) (Table 1; Supplementary Tables S1, S2; Supplementary Figures S1A,B). It indicated two periods of continuous decline in the ASMR and ASDR based on the joinpoint analysis (Figures 1A,D; Supplementary Tables S1, S2).
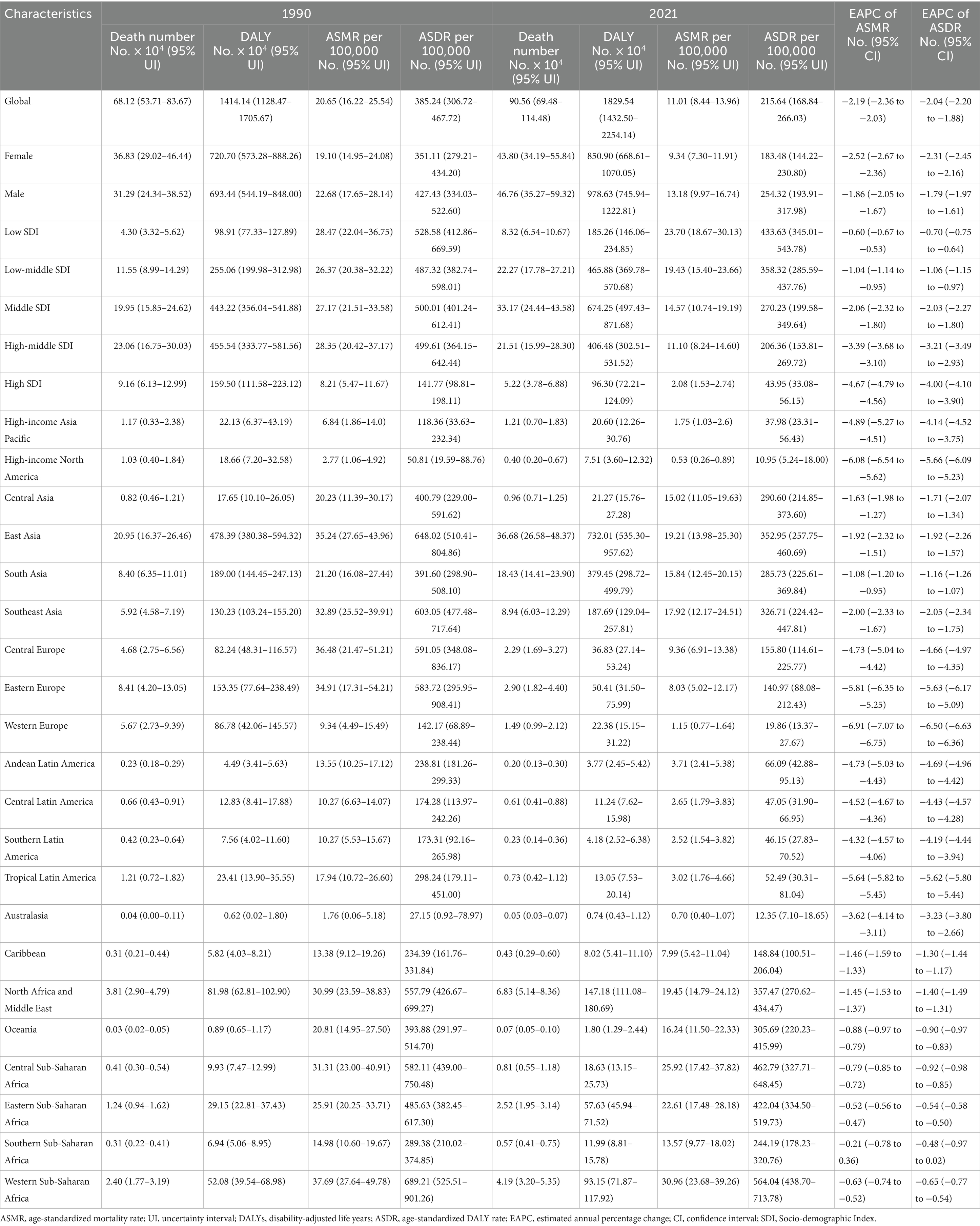
Table 1. The global burden of ischemic stroke attributable to PM2.5 air pollution in 1990 and 2021 and the temporal trends during 1990–2021.
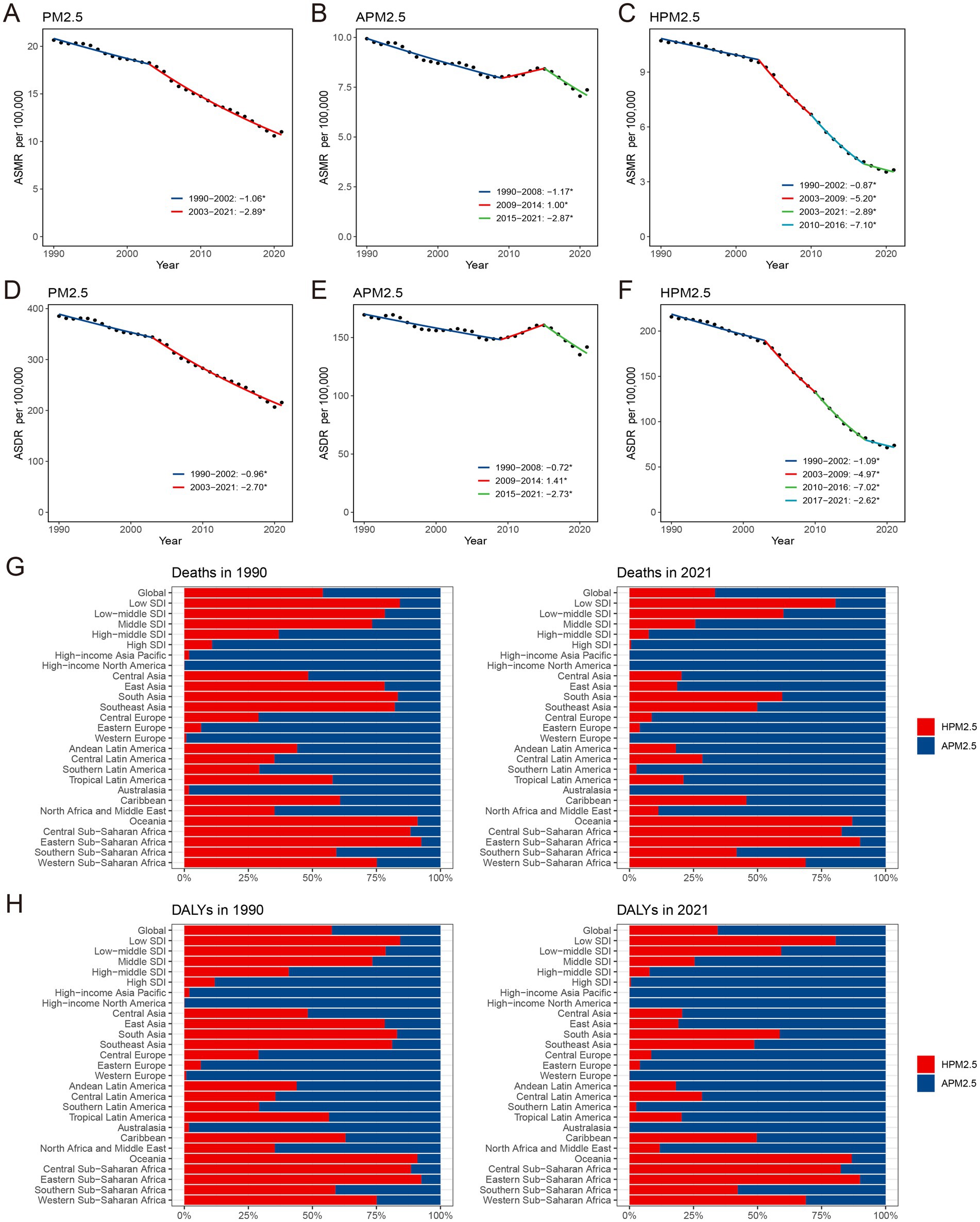
Figure 1. The global APCs in the age-standardized mortality and DALYs rate for ischemic stroke attributable to PM2.5 (A,D), ambient PM2.5 (B,E) and household PM2.5 (C,F), 1990–2021. Effect of ambient PM2.5 and household PM2.5 on the deaths (G) and DALYs (H) burden of PM2.5-related ischemic stroke. *p < 0.05, APC, annual percentage change; DALYs, disability-adjusted life-years; PM, particulate matter.
The ASMR and ASDR of ambient PM2.5-related ischemic stroke exhibited a slight downward trend, as evidenced by the negative EAPC and AAPC (Supplementary Tables S3–S5). It indicated a similar change pattern for both ASMR and ASDR according to the joinpoint analysis (Figures 1B,E). Specifically, the ASR decreased from 1990 to 2008, increased from 2009 to 2014, and then declined again from 2015 to 2021. The ASRs of household PM2.5-associated ischemic stroke decreased staggeringly over past three decades (Figures 1C,F; Supplementary Table S6). In 2021, household PM2.5 contributed a notably lower proportion to the overall air pollution-related ischemic stroke burden compared to ambient PM2.5 (Figures 1G,H; Supplementary Figures S1C,D).
Regional ischemic stroke burden caused by particulate matter pollution
With respect to the SDI region, household PM2.5 had a more pronounced impact on the ischemic stroke burden in low SDI region, whereas ambient PM2.5 made a more dominant contribution in middle, high-middle, and high SDI regions (Figures 1G,H). In all five SDI regions, the proportion of ischemic stroke death and DALYs attributed to household PM2.5 showed a decrease in 2021 compared to those recorded in 1990, while ambient PM2.5-related percentage rose (Supplementary Figures S1C,D). For ischemic stroke attributed to PM2.5 or household PM2.5, the ASRs tended to be lower in regions with higher SDI (Table 1; Figure 2). Both ASMR and ASDR of household PM2.5-associated ischemic stroke approached zero in high SDI region (Supplementary Table S6). In the low, low-middle, and middle SDI regions, the ASRs of ambient PM2.5-related ischemic stroke increased slowly over time, supported by positive values for both AAPC and EAPC (Figure 2; Supplementary Tables S3–S5).
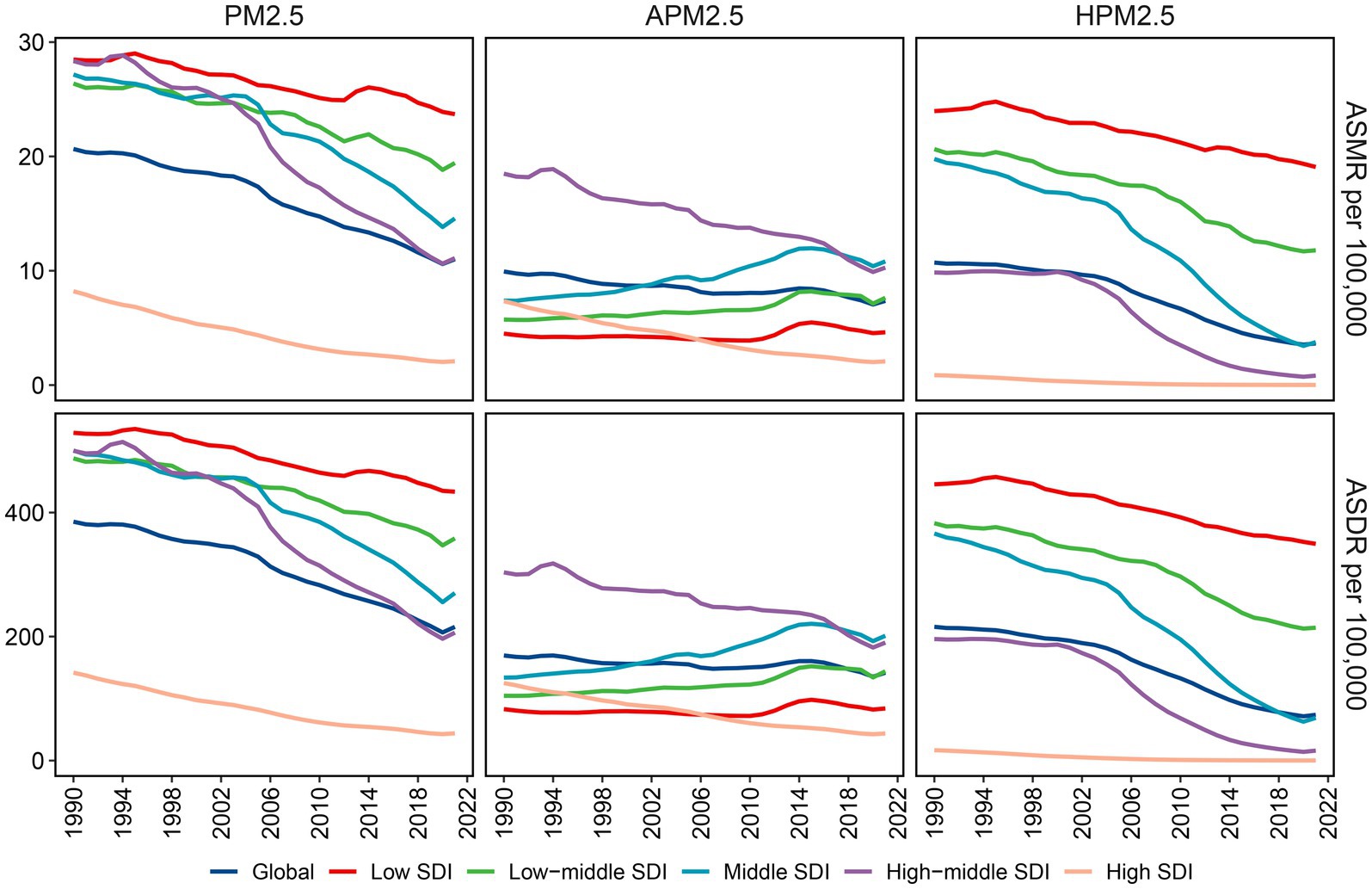
Figure 2. Variations in ischemic stroke ASMR and ASDR attributable to PM2.5, ambient PM2.5 and household PM2.5 across different SDI regions. ASMR: age-standardized mortality rate; ASDR: age-standardized DALYs rate; PM, particulate matter; SDI, socio-demographic index.
With respect to the GBD region, Africa suffered the heaviest ischemic stroke burden attributable to PM2.5 in 2021 (Table 1). While the highest ASMR and ASDR were manifested in the Africa, East Asia, and North Africa and Middle East. The distribution of ischemic stroke burden attributable to ambient PM2.5 and household PM2.5 varied among the GBD regions (Supplementary Figures S1C,D). Ambient PM2.5-related ischemic stroke exceeded 95% of the total burden in some high-income regions, namely High-income Asia Pacific, High-income North America, Western Europe, and Australasia (Figures 1G,H). A substantial proportion of the disease burden was attributed to household PM2.5 in Central, Eastern, and Western Sub-Saharan Africa, as well as Oceania. The contribution of household PM2.5 to both ischemic stroke mortality and DALYs declined across all GBD regions from 1990 to 2021.
In addition, there was a notable decline in both ASMR and ASDR for ischemic stroke associated with PM2.5 and household PM2.5 in almost all GBD regions (Table 1; Supplementary Tables S1–S4, S6). However, both ASMR and ASDR for ambient PM2.5-related ischemic stroke significantly increased in Oceania, Sub-Saharan Africa, Central Asia, East Asia, South Asia, and Southeast Asia (Supplementary Tables S3–S5).
National ischemic stroke burden caused by particulate matter pollution
In the context of national level, the burden of PM2.5-related ischemic stroke displayed heterogeneity across 204 countries. ASMR and ASDR showed an upward trend in 15 and 12 countries, respectively. A downward trend was recorded for ASMR in 177 countries, and for ASDR in 179 countries (Supplementary Table S7). India, China, Bangladesh, and Indonesia ranked as the top four countries with the highest PM2.5-related ischemic stroke burden. The highest ASMR and ASDR were observed in several countries, such as Guinea-Bissau, Ghana, Gambia, Sierra Leone, Togo, Senegal, Benin, Liberia (West Africa), Egypt, Yemen (North Africa and Middle East), Haiti and Afghanistan (Figure 3A; Supplementary Figure S2A). Estonia and Portugal had the most minimal EAPC value, all less than −9.0 (Figure 4A; Supplementary Figures S3A, S4A, S5A). In contrast, the highest EAPC for both ASMR and ASDR were observed in Lesotho, Zimbabwe, Libya and Mozambique.
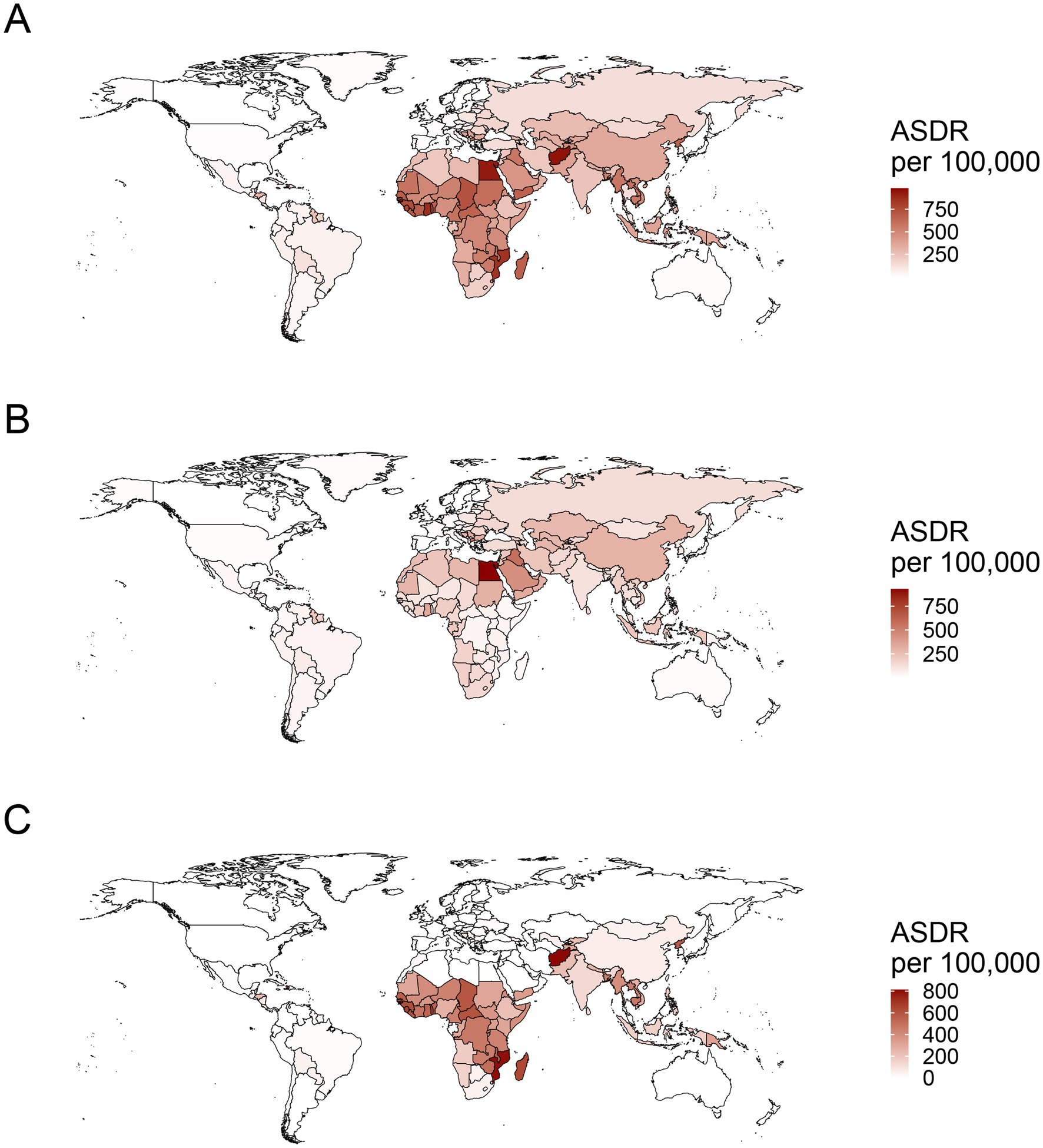
Figure 3. The worldwide distribution of ASDR for ischemic stroke attributed to PM2.5 (A), ambient PM2.5 (B), and household PM2.5 (C) air pollution in 2021. ASDR, age-standardized DALYs rate; PM, particulate matter.

Figure 4. Temporal trends of ASDR for ischemic stroke attributed to PM2.5 (A), ambient PM2.5 (B), and household PM2.5 (C) air pollution during 1990–2021. ASDR, age-standardized DALYs rate; PM, particulate matter.
Ambient PM2.5-related ASMR decreased in 114 countries, increased in 69, and remained stable in 21 (Supplementary Table S8). ASDR declined in 116 countries, rose in 64, and stayed unchanged in 24. China, India, and Egypt were the top three countries with the highest burden of ambient PM2.5-related ischemic stroke. The maximal ASRs of ambient PM2.5-associated ischemic stroke were observed in the countries in North Africa and the Middle East, including Egypt, Iraq, Saudi Arabia, United Arab Emirates, Oman and Bahrain, along with those in Eastern Europe such as Bulgaria, North Macedonia, and Bosnia and Herzegovina (Figure 3B; Supplementary Figure S2B). Eastern and Southern Asia (Viet Nam, Mongolia, Bhutan, Timor-Leste, China, and India) and Africa (Cabo Verde, Tanzania, Lesotho, Angola, Sudan, Ghana, Kenya) exhibited the highest EAPC for both ASMR and ASDR (Figure 4B; Supplementary Figures S3B, S4B, S5B). More specifically, Viet Nam, Mongolia, and Equatorial Guinea emerged as the top three countries with the highest EAPC values above 4.0.
Over past 32 years, a declining trajectory was identified in household PM2.5-related ASMR across 184 countries and in household PM2.5-related ASDR across 185 countries (Supplementary Table S9). India, China, and Bangladesh were recognized as the top three countries bearing the highest cases of household PM2.5-related ischemic stroke. The majority of the top ten countries with the high ASRs consisted of Africa nations, such as Guinea-Bissau, Mozambique, Gambia, Sierra Leone, Madagascar, Togo, and Guinea (Figure 3C; Supplementary Figure S2C). Among the top ten countries with the lowest EAPC in ASRs, the majority were situated in Europe, encompassing Estonia, Portugal, Norway, Luxembourg, Austria, Finland, Sweden, United Kingdom, and Ireland (Figure 4C; Supplementary Figures S3C, S4C, S5C). The countries of Viet Nam, Mongolia, and Equatorial Guinea stood out as the top three with the highest EAPC in both ASMR and ASDR.
Global ischemic stroke burden attributable to particulate matter pollution by age, gender, and SDI
The age-specific death and DALYs rate for ischemic stroke associated with PM2.5 or ambient PM2.5 escalated with age increase (Figures 5A,B; Supplementary Figures S6A,B). For ischemic stroke associated with household PM2.5 exposure, the age-specific DALYs rate peaked in the 80–84 age group, while the age-specific mortality rate reached its highest point in the 90–94 age group (Figure 5C; Supplementary Figures S6C). Furthermore, the EAPCs of both death and DALYs rates exhibited negative values across all age groups for ischemic stroke attributable to PM2.5 or household PM2.5, signifying a notable declining trend (Figures 5D,F; Supplementary Figures S6D,F). For ambient PM2.5-associated ischemic stroke, the age-specific death and DALYs rate exhibited an upward trend among individual aged below 50, while demonstrating a decline among those aged above 60 (Figure 5E; Supplementary Figures S6E).
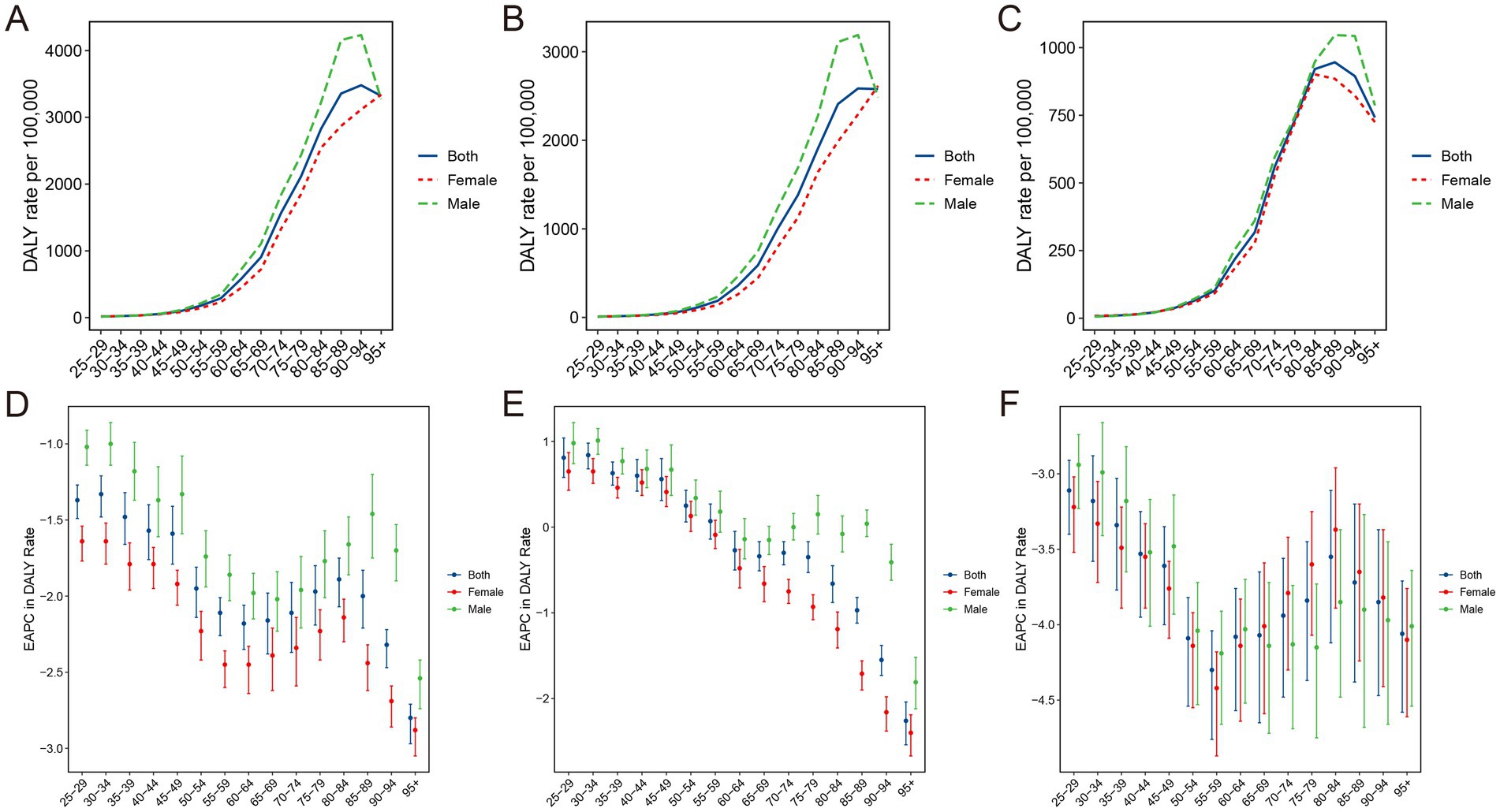
Figure 5. Age-specific DALYs rate of ischemic stroke attributed to PM2.5 (A), ambient PM2.5 (B), and household PM2.5 (C) air pollution and the corresponding changes in EAPC (D–F), by sex, in 2021. DALYs, disability-adjusted life-years; PM, particulate matter; EAPC, estimated annual percentage change.
The findings of joinpoint analysis for ASMR and ASDR, stratified by gender, were shown in Supplementary Figure S7. Contrary to the dramatic drop in ASRs for ischemic stroke attributed to PM2.5 or household PM2.5, the ASRs trend for ambient PM2.5-related ischemic stroke remained relatively constant in the male populations. Additionally, the second segment of the ASRs temporal trends increased significantly in ischemic stroke associated with ambient PM2.5 for both female and male populations. For both ambient and household PM2.5, the mortality and DALYs rates were significantly higher in males than in females (Figures 5B,C; Supplementary Figures S6B,C). Interestingly, among individuals aged below 80, the mortality and DALYs rates for ischemic stroke attributed to household PM2.5 were nearly identical between males and females (Figure 5C; Supplementary Figure S6C). In the 65–95 age group, the age-specific rates of ambient PM2.5-related ischemic stroke exhibited a significant decline among females, whereas males demonstrated a relatively stable trend.
For ischemic stroke associated with PM2.5 or household PM2.5, both ASMR and ASDR decreased with increased SDI (Figures 6A,B). These observations were consistent that in the high-middle and high SDI regions for ischemic stroke associated with ambient PM2.5. However, in the regions with low, low-middle, and middle SDI, the ASRs related to ambient PM2.5 demonstrated an upward trend corresponding to the SDI rise. In the GBD regions with an SDI below 0.7, including Southern Sub-Saharan Africa and East, South, and Southeast Asia, a positive correlation was identified between SDI and ASMR linked with ambient PM2.5 (Supplementary Figure S8). The pattern of ASDR based on SDI at the regional level was consistent with that of ASMR (Supplementary Figure S9).
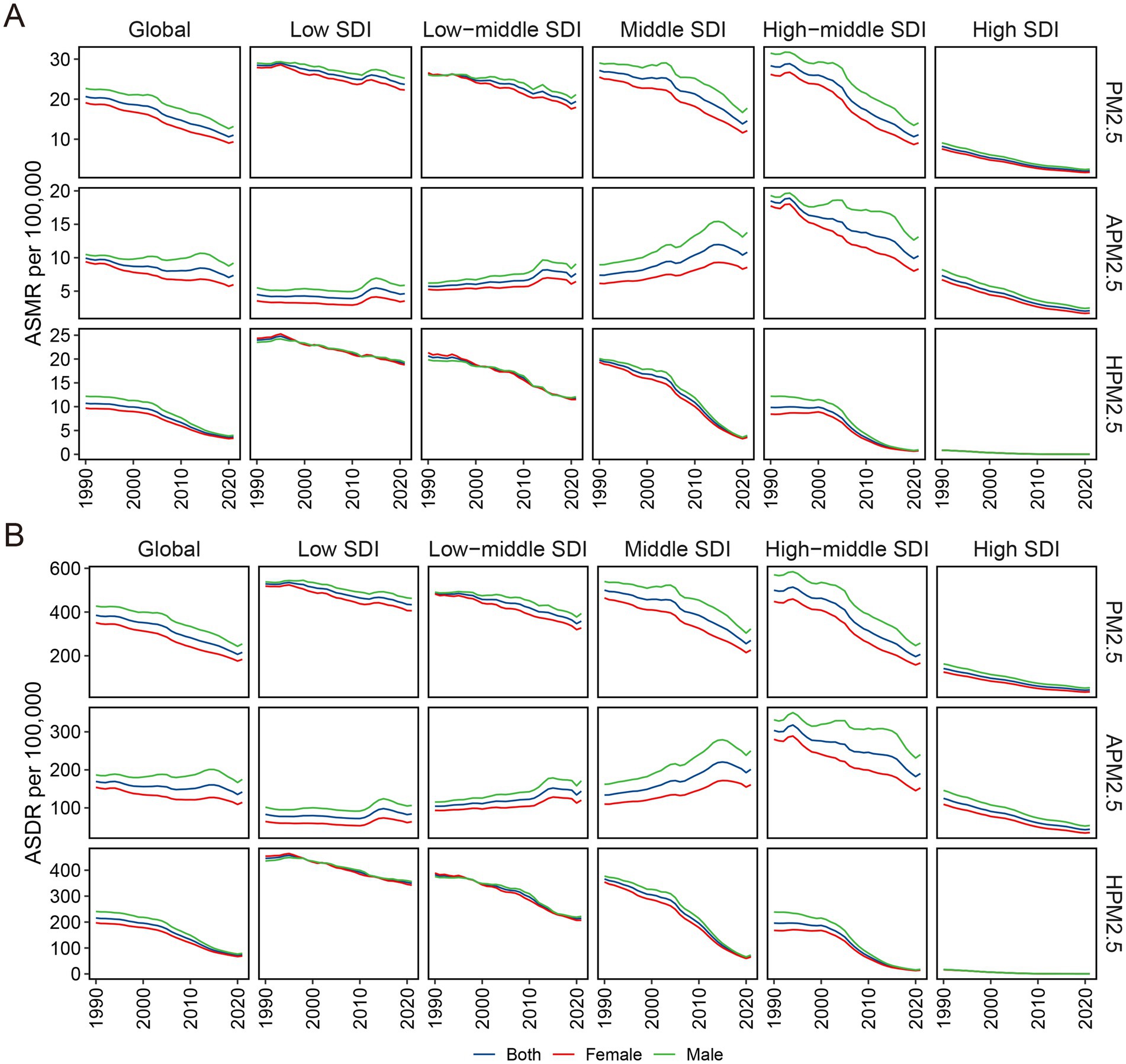
Figure 6. Sex disparity in ASMR (A) and ASDR (B) for ischemic stroke attributable to PM2.5, ambient PM2.5, and household PM2.5 air pollution across SDI regions. ASMR, age-standardized mortality rate; ASDR, age-standardized DALYs rate; PM, particulate matter; SDI, socio-demographic index.
There was an inverse relationship between ASRs and SDI across 204 nations for both ischemic stroke attributed to PM2.5 (R = −0.51, p < 0.01 for ASMR in 1990; R = −0.82, p < 0.01 for ASMR in 2021; R = −0.57, p < 0.01 for ASDR in 1990; R = −0.75, p < 0.01 for ASDR in 2021) and household PM2.5 (R = −0.75, p < 0.01 for ASMR in 1990; R = −0.82, p < 0.01 for ASMR in 2021; R = −0.77, p < 0.01 for ASDR in 1990; R = −0.82, p < 0.01 for ASDR in 2021) (Supplementary Figures S10A,C, S11A,C, S12A,C, S13A,C). Both in 1990 and 2021, a unimodal curve was perceptible when examining the correlation between SDI and ASRs for ischemic stroke associated with ambient PM2.5. It is noteworthy that over this period, the peak of the correlation curve underwent a transition, moving from 0.8 down to 0.7 (Supplementary Figures S10B, S11B, S12B, S13B).
The decomposition of the change in ischemic stroke attributed to particulate matter pollution
It presented a substantial worldwide escalation in DALYs attributed to PM2.5 and ambient PM2.5, but a significant reduction in DALYs linked to household PM2.5 (Figure 7; Supplementary Table S10). In the low and low-middle SDI regions, the DALYs increase of ischemic stroke related to PM2.5 and household PM2.5 was primarily driven by population growth and aging. The effect of epidemiological changes on DALYs growth showed a negative impact. All epidemiological changes, population growth and aging led to a considerable DALYs escalation linked to ambient PM2.5 across the low, low-middle, and middle SDI regions. In the high SDI region, the epidemiological changes led to a considerable DALYs reduction.
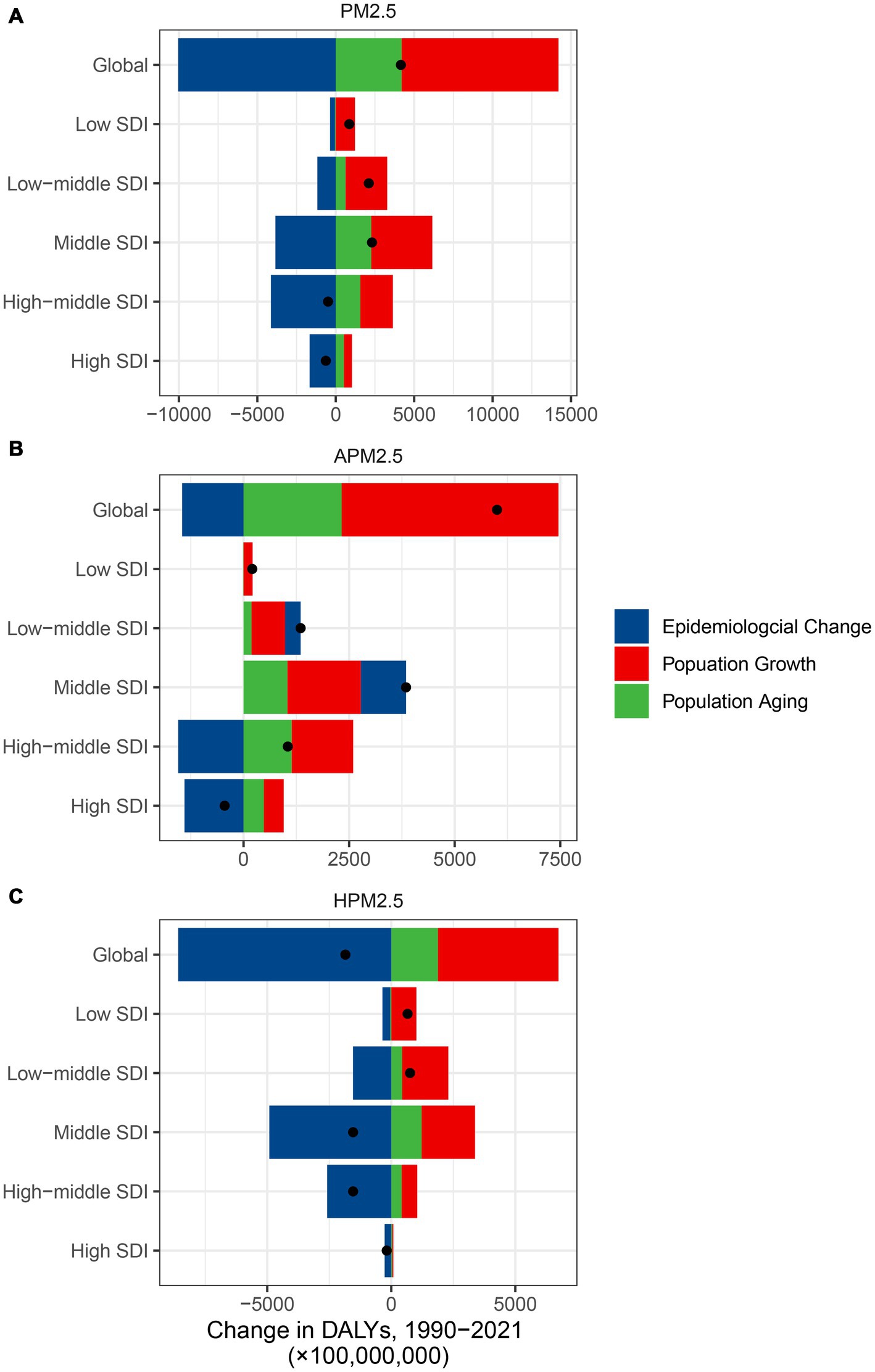
Figure 7. The contributions of three population-level factors to the DALYs change for ischemic stroke attributed to PM2.5 (A), ambient PM2.5 (B), and household PM2.5 (C) air pollution from 1990 to 2021 according to the decomposition analysis DALYs, disability-adjusted life years; PM, particulate matter pollution; SDI, sociodemographic index.
Discussion
In the study, we conducted a comprehensive evaluation of the global, regional, and national burden and temporal trends of PM2.5-related ischemic stroke, stratifying the analysis by SDI, age, and sex. The findings highlighted the critical impact of particulate matter pollution on ischemic stroke, offering valuable scientific insights to guide policymakers in formulating effective preventive and remedial approaches.
In contrast to the dramatic decline in household PM2.5-related ASRs, it presented a more gradual decrease of ambient PM2.5-related ASRs over the past 32 years. According to the decomposition analysis, population growth and aging contributed to a measurable increase of DALYs associated with ambient PM2.5, whereas the epidemiological changes resulted in a considerable household PM2.5-associated DALYs reduction. Several underlying factors may elucidate this discrepancy. Firstly, the widespread adoption of cleaner fuels in household, such as ethanol, liquefied petroleum gas, electrification, and solar energy, could markedly reduce indoor particulate matter levels (21). Cookstove intervention programs, such as clean fuels, proper ventilation, and permeable construction material, may serve as another potential approach in reducing the disease burden associated with household PM2.5, especially in low SDI regions (22, 23). Secondly, the Environmental Kuznets Curve hypothesis suggested that environmental degradation escalated with industrialization and urbanization initially but declined as economies reach a certain income level, forming an inverted U-shape (24). The increase in population, road density and trip activity during urbanization had a significantly positive impact on PM2.5 concentrations (25). The emissions of sulfur dioxide, elemental carbon, and nitrates comprised the principal components of particulate matter, which proved to be detrimental (26). A reduction in these pollutants originating from mobile, point, area, and non-road sources could serve as a guiding principle for improving air quality management and reducing ischemic stroke mortality. During the COVID-19 pandemic, the enhancement of air quality was regarded as a benefit of the lockdown, resulting from the cessation of anthropogenic emissions (27). As the pandemic came to an end and society resumed its customary rhythm, there was a potential for a rise in air pollution. An economically sustainable “green recovery” plan should be more effectively implemented in the post-COVID-19 era, given the observed slight increase in the ASRs linked to both ambient and household PM2.5 during 2020–2021. The extensive adoption of telemedicine prompted by COVID-19 may be a crucial strategy for treating patients with PM2.5-related ischemic strokes and minimizing our carbon footprint (28).
SDI discrepancies led to varying disease burdens. There was a notable correlation between ASRs and SDI in the study. The ASRs associated with PM2.5 in the low SDI regions exceeded those in the high SDI regions. We also presented a modest upward trend of ambient PM2.5-related ASRs across the regions with low, low-middle, and middle SDI. It may be attributed to insufficient medical resource, limited health awareness, and low economic affordability in the low SDI regions (29, 30). In addition, economies in low SDI regions tended to depend more heavily on polluting industries and technologies, which may be one potential explanation (31). Low-income and middle-income countries allocated fewer resources to pollution control compared to high-income countries (32). Formulating tailored policies meticulously for various SDI regions to combat air pollution may be a more rational and influential strategy. Historical experiences from high-income countries could also help to inform potential evidence-based solutions, including medical specialization policy, green infrastructure, emissions-based air control measures, and zoning laws (33, 34). Nations across the world must collaborate closely, for air pollution in one country can yield far-reaching effects on its neighboring states (35).
Regarding the ASRs associated with ambient PM2.5, a stable trend was noted in males compared with a dramatic downward trend in females. The EAPCs of age-specific rates attributable to ambient PM2.5 in males were higher than those observed in females. The differences in time-activity patterns may be one explanation. It was reported that women spent the majority of their time at home, dedicating 83% of their daytime, in contrast to 57% for men (36). The sex-specific differences could also be attributed to variations in social functioning and biological distinctions between females and males (37). The biological factors related to sex, including hormonal profiles, lung capacity, bronchial hyperresponsiveness, and tobacco exposure, were distinct between males and females (38). However, the definitive cause of discrepancies by sex remained ambiguous, necessitating further investigation.
An approximate 10% increase in stroke mortality was observed with the rise in PM2.5 levels (39). There were some molecular biological mechanisms linking stroke and PM2.5. Diabetes, hypertension, hyperlipidemia, and atrial fibrillation, which were common risk factors for ischemic stroke, exhibited a significant association with PM2.5 exposure (10). The elevation of platelet count and DNA methylation induced by PM2.5 exposure increased the thrombosis risk through the activation of megakaryocytes, potentially representing one fundamental pathogenesis of ischemic stroke associated with PM2.5 (40). Additionally, the detrimental effects of PM2.5 on endothelial cells were mediated by inflammatory responses, oxidative stress, coagulation activation, autophagy, and ferroptosis (10). Some inflammatory factors, including IL-6 and IL-17, exacerbated leukocyte proliferation and inflammatory responses, thereby increasing the cerebral infarct area by activating the nuclear factor-kappa B and mitogen-activated protein kinase signaling pathways (41, 42). Inhaled particulate matter also can provoke inflammation in the lung, gut, and brain through the generation of reactive oxygen species production, neuroinflammation, microglial activation, and neuronal damage (43). Furthermore, the blood–brain barrier and the nasal mucosa-olfactory bulb pathway were two primary routes by which PM2.5 can infiltrate the brain (44, 45). PM2.5 can induce dysfunction in brain endothelial cells and exacerbate neurovascular injury through the mediation of perivascular macrophages (46). The definite mechanism in molecular gene level required further verification in future researches.
In this study, we conducted a thorough investigation into the spatiotemporal burden of ischemic strokes associated with PM2.5 based on the latest GBD 2021 study. However, there were several inevitable limitations. First, the composition of PM2.5 is intricate, encompassing diverse physical structures, chemical compositions, and sources of its constituents (47). The GBD 2021 study did not provide the detailed components of PM2.5, thus we did not incorporate the various constituents of PM2.5 into the analysis. Second, the considerable heterogeneity in data quality across regions may lead to uncertainty and bias of burden estimates, thereby impacting the generalizability of the findings. But two essential enhancements—the non-zero floor and the revised Bayesian algorithm—were implemented to alleviate stochastic variation in the GBD 2021 study (17). Third, several long-term cardiovascular and neurological complications were observed in survivors of COVID-19 (48, 49). The long-term impact of COVID-19 on ischemic stroke should be addressed in the forthcoming GBD 2023 study.
In conclusion, while the global burden of ischemic stroke attributable to both ambient and household PM2.5 declined from 1990 to 2021, there was still a notable increase of ischemic stroke associated with ambient PM2.5 in some countries, such as Viet Nam, Mongolia, and Equatorial Guinea. We explored the profound influence of ambient and household PM2.5 on ischemic stroke, reinforcing the urgent need of mitigating PM2.5 levels and enhancing public awareness. The results further furnished a robust epidemiological foundation for the prevention, control, and management of ambient PM2.5 pollution.
Data availability statement
Publicly available datasets were analyzed in this study. The data can be found here: https://ghdx.healthdata.org/gbd-results-tool. The R scripts of this study can be accessed on the website (https://github.com/lantian1986/ischemic_stroke_PM2.5).
Author contributions
YL: Formal analysis, Funding acquisition, Visualization, Writing – original draft, Writing – review & editing. YM: Data curation, Formal analysis, Writing – review & editing. WL: Data curation, Formal analysis, Writing – review & editing. TL: Conceptualization, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. GL: Conceptualization, Methodology, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research received grants from Medical Scientific Research Foundation of Zhejiang Province (2023XY002), National Natural Science Foundation of China (82405069), Natural Science Foundation of Fujian Province (2024 J08185), Fujian Province Health and Wellness Science and Technology Program (2022QNA065). The funding body had no involvement in the study’s design, data collection, analysis, interpretation, or manuscript preparation.
Acknowledgments
We acknowledge the Global Burden of Disease, Injuries, and Risk Study (GBD) 2021, which presented detailed information.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1608086/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | EAPC of ASMR (A) and ASDR (B) of PM2.5-associated ischemic stroke from 1990 to 2021. ASMR (C) and ASDR (D) of ischemic stroke associated with ambient PM2.5 and household PM2.5 in 1990 and 2021. EAPC, estimated annual percentage change; ASMR, age-standardized mortality rate; ASDR, age-standardized DALYs rate; PM, particulate matter.
SUPPLEMENTARY FIGURE S2 | The worldwide distribution of ASMR for ischemic stroke attributed to PM2.5 (A), ambient PM2.5 (B), and household PM2.5 (C) air pollution in 2021. ASMR, age-standardized mortality rate; PM, particulate matter.
SUPPLEMENTARY FIGURE S3 | The worldwide distribution of ASMR for ischemic stroke attributed to PM2.5 (A), ambient PM2.5 (B), and household PM2.5 (C) air pollution in 1990. ASMR, age-standardized mortality rate; PM, particulate matter.
SUPPLEMENTARY FIGURE S4 | The worldwide distribution of ASDR for ischemic stroke attributed to PM2.5 (A), ambient PM2.5 (B), and household PM2.5 (C) air pollution in 1990. ASDR, age-standardized DALYs rate; PM, particulate matter.
SUPPLEMENTARY FIGURE S5 | Temporal trends of ASMR for ischemic stroke attributed to PM2.5 (A), ambient PM2.5 (B), and household PM2.5 (C) air pollution during 1990-2021. ASMR, age-standardized mortality rate; PM, particulate matter.
SUPPLEMENTARY FIGURE S6 | Age-specific death rate of ischemic stroke attributed to PM2.5 (A), ambient PM2.5 (B), and household PM2.5 (C) air pollution and the corresponding changes in EAPC (D–F), by sex, in 2021. PM, particulate matter; EAPC, estimated annual percentage change.
SUPPLEMENTARY FIGURE S7 | The global APCs of the ASMR and ASDR for ischemic stroke attributable to PM2.5, ambient PM2.5, and household PM2.5 air pollution, 1990-2021. Female ASMR (A–C) and ASDR (D–F); male ASMR (G–I) and ASDR (J–L) *p<0.05, APC: annual percentage change; ASMR, age-standardized mortality rate; ASDR, age-standardized DALYs rate; PM, particulate matter.
SUPPLEMENTARY FIGURE S8 | The correlation between socio-demographic index and ASMR for ischemic stroke attributed to PM2.5, ambient PM2.5, and household PM2.5 air pollution across the GBD regions. ASMR, age-standardized mortality rate; PM, particulate matter; GBD, Global Burden of Disease.
SUPPLEMENTARY FIGURE S9 | The correlation between socio-demographic index and ASDR for ischemic stroke attributed to PM2.5, ambient PM2.5, and household PM2.5 air pollution across the GBD regions. ASDR, age-standardized DALYs rate; PM, particulate matter; GBD, Global Burden of Disease.
SUPPLEMENTARY FIGURE S10 | The relationship between socio-demographic index in 2021 and ASMR in 1990 for ischemic stroke attributed to PM2.5 (A), ambient PM2.5 (B), and household PM2.5 (C) air pollution across 204 countries or territories. ASMR, age-standardized mortality rate; PM, particulate matter.
SUPPLEMENTARY FIGURE S11 | The relationship between socio-demographic index in 2021 and ASMR in 2021 for ischemic stroke attributed to PM2.5 (A), ambient PM2.5 (B), and household PM2.5 (C) air pollution across 204 countries or territories. ASMR, age-standardized mortality rate; PM, particulate matter.
SUPPLEMENTARY FIGURE S12 | The relationship between socio-demographic index in 2021 and ASDR in 1990 for ischemic stroke attributed to PM2.5 (A), ambient PM2.5 (B), and household PM2.5 (C) air pollution across 204 countries or territories. ASDR, age-standardized DALYs rate; PM, particulate matter.
SUPPLEMENTARY FIGURE S13 | The relationship between socio-demographic index in 2021 and ASDR in 2021 for ischemic stroke attributed to PM2.5 (A), ambient PM2.5 (B), and household PM2.5 (C) air pollution across 204 countries or territories. ASDR, age-standardized DALYs rate; PM, particulate matter.
Footnotes
References
1. Ajoolabady, A, Wang, S, Kroemer, G, Penninger, JM, Uversky, VN, Pratico, D, et al. Targeting autophagy in ischemic stroke: from molecular mechanisms to clinical therapeutics. Pharmacol Ther. (2021) 225:107848. doi: 10.1016/j.pharmthera.2021.107848
3. Towfighi, A, Boden-Albala, B, Cruz-Flores, S, El Husseini, N, Odonkor, CA, Ovbiagele, B, et al. Strategies to reduce racial and ethnic inequities in stroke preparedness, care, recovery, and risk factor control: a scientific statement from the American Heart Association. Stroke. (2023) 54:e371–88. doi: 10.1161/STR.0000000000000437
4. Boehme, AK, Esenwa, C, and Elkind, MS. Stroke risk factors, genetics, and prevention. Circ Res. (2017) 120:472–95. doi: 10.1161/CIRCRESAHA.116.308398
5. Hayes, RB, Lim, C, Zhang, Y, Cromar, K, Shao, Y, Reynolds, HR, et al. PM2.5 air pollution and cause-specific cardiovascular disease mortality. Int J Epidemiol. (2020) 49:25–35. doi: 10.1093/ije/dyz114
6. Kamarehei, B, Farhadi, M, Soleimani, F, Dolati, M, Sepahvand, A, Bayat, M, et al. The level, source, and health outcome of PM(2.5) exposure in Southwest Iran. Toxicol Rep. (2024) 13:101730. doi: 10.1016/j.toxrep.2024.101730
7. Chen, CY, Huang, KY, Chen, CC, Chang, YH, Li, HJ, Wang, TH, et al. The role of PM2.5 exposure in lung cancer: mechanisms, genetic factors, and clinical implications. EMBO Mol Med. (2025) 17:31–40. doi: 10.1038/s44321-024-00175-2
8. Ding, R, Huang, L, Yan, K, Sun, Z, and Duan, J. New insight into air pollution-related cardiovascular disease: an adverse outcome pathway framework of PM2.5-associated vascular calcification. Cardiovasc Res. (2024) 120:699–707. doi: 10.1093/cvr/cvae082
9. Swieczkowski, M, Lip, GYH, Kurasz, A, Dabrowski, EJ, Tomaszuk-Kazberuk, A, Kaminski, JW, et al. Association between exposure to air pollution and increased ischemic stroke incidence: a retrospective population-based cohort study (EP-PARTICLES study). Eur J Prev Cardiol. (2024) 32:276–287. doi: 10.1093/eurjpc/zwae301
10. Chen, Z, Liu, P, Xia, X, Wang, L, and Li, X. The underlying mechanism of PM2.5-induced ischemic stroke. Environ Pollut. (2022) 310:119827. doi: 10.1016/j.envpol.2022.119827
11. Liu, S, Lv, Y, Zhang, Y, Suo, H, Wang, F, and Gao, S. Global trends and burden of stroke attributable to particulate matter pollution from 1990 to 2019. Ecotoxicol Environ Saf. (2024) 274:116205. doi: 10.1016/j.ecoenv.2024.116205
12. Guo, LH, Lin, LZ, Zhou, Y, Jalaludin, B, Morawska, L, Dharmage, SC, et al. Global, regional, and national burden of ischemic heart disease attributable to ambient PM(2.5) from 1990 to 2019: an analysis for the global burden of disease study 2019. Environ Res. (2024) 241:117635. doi: 10.1016/j.envres.2023.117635
13. Fan, J. The burden of ischemic heart disease attributable to ambient and household particulate matter pollution, 1990-2019: a global analysis. Environ Sci Pollut Res Int. (2023) 30:114514–24. doi: 10.1007/s11356-023-30336-8
14. Wiersinga, WJ, Rhodes, A, Cheng, AC, Peacock, SJ, and Prescott, HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. (2020) 324:782–93. doi: 10.1001/jama.2020.12839
15. Zhang, Y, Wu, W, Li, Y, and Li, Y. An investigation of PM2.5 concentration changes in mid-eastern China before and after COVID-19 outbreak. Environ Int. (2023) 175:107941. doi: 10.1016/j.envint.2023.107941
16. Zuin, M, Mazzitelli, M, Rigatelli, G, Bilato, C, and Cattelan, AM. Risk of ischemic stroke in patients recovered from COVID-19 infection: a systematic review and meta-analysis. Eur Stroke J. (2023) 8:915–22. doi: 10.1177/23969873231190432
17. Collaborators GBDCoD. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2100–32. doi: 10.1016/S0140-6736(24)00367-2
18. Collaborators GBDRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1923–94. doi: 10.1016/S0140-6736(18)32225-6
19. Diabetes, GBD, and Air, PC. Estimates, trends, and drivers of the global burden of type 2 diabetes attributable to PM(2.5) air pollution, 1990-2019: an analysis of data from the global burden of disease study 2019. Lancet Planet Health. (2022) 6:e586–600. doi: 10.1016/S2542-5196(22)00122-X
20. Cheng, X, Tan, L, Gao, Y, Yang, Y, Schwebel, DC, and Hu, G. A new method to attribute differences in total deaths between groups to population size, age structure and age-specific mortality rate. PLoS One. (2019) 14:e0216613. doi: 10.1371/journal.pone.0216613
21. Quansah, R, Semple, S, Ochieng, CA, Juvekar, S, Armah, FA, Luginaah, I, et al. Effectiveness of interventions to reduce household air pollution and/or improve health in homes using solid fuel in low-and-middle income countries: a systematic review and meta-analysis. Environ Int. (2017) 103:73–90. doi: 10.1016/j.envint.2017.03.010
22. Thomas, E, Wickramasinghe, K, Mendis, S, Roberts, N, and Foster, C. Improved stove interventions to reduce household air pollution in low and middle income countries: a descriptive systematic review. BMC Public Health. (2015) 15:650. doi: 10.1186/s12889-015-2024-7
23. Dasgupta, S, Wheeler, D, Huq, M, and Khaliquzzaman, M. Improving indoor air quality for poor families: a controlled experiment in Bangladesh. Indoor Air. (2009) 19:22–32. doi: 10.1111/j.1600-0668.2008.00558.x
24. Destek, MA, and Sarkodie, SA. Investigation of environmental Kuznets curve for ecological footprint: the role of energy and financial development. Sci Total Environ. (2019) 650:2483–9. doi: 10.1016/j.scitotenv.2018.10.017
25. Zhou, C, Chen, J, and Wang, S. Examining the effects of socioeconomic development on fine particulate matter (PM(2.5)) in China's cities using spatial regression and the geographical detector technique. Sci Total Environ. (2018) 619-620:436–45. doi: 10.1016/j.scitotenv.2017.11.124
26. Peterson, GCL, Hogrefe, C, Corrigan, AE, Neas, LM, Mathur, R, and Rappold, AG. Impact of reductions in emissions from major source sectors on fine particulate matter-related cardiovascular mortality. Environ Health Perspect. (2020) 128:17005. doi: 10.1289/EHP5692
27. Kumar, P, Hama, S, Omidvarborna, H, Sharma, A, Sahani, J, Abhijith, KV, et al. Temporary reduction in fine particulate matter due to 'anthropogenic emissions switch-off' during COVID-19 lockdown in Indian cities. Sustain Cities Soc. (2020) 62:102382. doi: 10.1016/j.scs.2020.102382
28. Davies, B, Kenia, P, Nagakumar, P, and Gupta, A. Paediatric and adolescent asthma: a narrative review of telemedicine and emerging technologies for the post-COVID-19 era. Clin Exp Allergy. (2021) 51:393–401. doi: 10.1111/cea.13836
29. Xie, D, Shen, Z, Yang, L, Zhou, D, Li, C, and Liu, F. Global, regional, and national burden of type 2 diabetes mellitus attributable to particulate matter pollution from 1990 to 2021: an analysis of the global burden of disease study 2021. Diabetes Res Clin Pract. (2024) 218:111934. doi: 10.1016/j.diabres.2024.111934
30. Lan, T, Lu, Y, He, J, Zhan, C, Wang, X, Shao, X, et al. Global, reginal, national burden and risk factors in female breast cancer from 1990 to 2021. iScience. (2024) 27:111045. doi: 10.1016/j.isci.2024.111045
31. Rentschler, J, and Leonova, N. Global air pollution exposure and poverty. Nat Commun. (2023) 14:4432. doi: 10.1038/s41467-023-39797-4
32. Fuller, R, Landrigan, PJ, Balakrishnan, K, Bathan, G, Bose-O'Reilly, S, Brauer, M, et al. Pollution and health: a progress update. Lancet Planet Health. (2022) 6:e535–47. doi: 10.1016/S2542-5196(22)00090-0
33. Sriram, V, and Bennett, S. Strengthening medical specialisation policy in low-income and middle-income countries. BMJ Glob Health. (2020) 5:e002053. doi: 10.1136/bmjgh-2019-002053
34. Baumgartner, J, Brauer, M, and Ezzati, M. The role of cities in reducing the cardiovascular impacts of environmental pollution in low- and middle-income countries. BMC Med. (2020) 18:39. doi: 10.1186/s12916-020-1499-y
35. Seihei, N, Farhadi, M, Takdastan, A, Asban, P, Kiani, F, and Mohammadi, MJ. Short-term and long-term effects of exposure to PM10. Clin Epidemiol Glob Health. (2024) 27:101611. doi: 10.1016/j.cegh.2024.101611
36. Curto, A, Wellenius, GA, Mila, C, Sanchez, M, Ranzani, O, Marshall, JD, et al. Ambient particulate Air pollution and blood pressure in Peri-urban India. Epidemiology. (2019) 30:492–500. doi: 10.1097/EDE.0000000000001014
37. Liao, M, Braunstein, Z, and Rao, X. Sex differences in particulate air pollution-related cardiovascular diseases: a review of human and animal evidence. Sci Total Environ. (2023) 884:163803. doi: 10.1016/j.scitotenv.2023.163803
38. Clougherty, JE. A growing role for gender analysis in air pollution epidemiology. Environ Health Perspect. (2010) 118:167–76. doi: 10.1289/ehp.0900994
39. Stafoggia, M, Cesaroni, G, Peters, A, Andersen, ZJ, Badaloni, C, Beelen, R, et al. Long-term exposure to ambient air pollution and incidence of cerebrovascular events: results from 11 European cohorts within the ESCAPE project. Environ Health Perspect. (2014) 122:919–25. doi: 10.1289/ehp.1307301
40. Jin, X, Yu, H, Wang, B, Sun, Z, Zhang, Z, Liu, QS, et al. Airborne particulate matters induce thrombopoiesis from megakaryocytes through regulating mitochondrial oxidative phosphorylation. Part Fibre Toxicol. (2021) 18:19. doi: 10.1186/s12989-021-00411-4
41. Zhang, Q, Liao, Y, Liu, Z, Dai, Y, Li, Y, Li, Y, et al. Interleukin-17 and ischaemic stroke. Immunology. (2021) 162:179–93. doi: 10.1111/imm.13265
42. del Zoppo, G, Ginis, I, Hallenbeck, JM, Iadecola, C, Wang, X, and Feuerstein, GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. (2000) 10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x
43. Ruggles, A, and Benakis, C. Exposure to environmental toxins: potential implications for stroke risk via the gut- and lung-brain Axis. Cells. (2024) 13:803. doi: 10.3390/cells13100803
44. Kang, N, Chen, G, Tu, R, Liao, W, Liu, X, Dong, X, et al. Adverse associations of different obesity measures and the interactions with long-term exposure to air pollutants with prevalent type 2 diabetes mellitus: the Henan rural cohort study. Environ Res. (2022) 207:112640. doi: 10.1016/j.envres.2021.112640
45. Cristaldi, A, Fiore, M, Oliveri Conti, G, Pulvirenti, E, Favara, C, Grasso, A, et al. Possible association between PM(2.5) and neurodegenerative diseases: a systematic review. Environ Res. (2022) 208:112581. doi: 10.1016/j.envres.2021.112581
46. Kim, D, Gil, J, and Bae, ON. PM2.5 potentiates oxygen glucose deprivation-induced neurovascular unit damage via inhibition of the Akt/beta-catenin pathway and autophagy dysregulation. Environ Pollut. (2024) 359:124728. doi: 10.1016/j.envpol.2024.124728
47. Li, B, Ma, Y, Zhou, Y, and Chai, E. Research progress of different components of PM(2.5) and ischemic stroke. Sci Rep. (2023) 13:15965. doi: 10.1038/s41598-023-43119-5
48. Gadsden, T, Downey, LE, Vilas, VDR, Peiris, D, and Jan, S. The impact of COVID-19 on essential health service provision for noncommunicable diseases in the South-East Asia region: a systematic review. Lancet Reg Health Southeast Asia. (2022) 1:100010. doi: 10.1016/j.lansea.2022.04.006
Keywords: ischemic stroke, air pollution, particulate matter, Global Burden of Disease, disability-adjusted life years
Citation: Lu Y, Mao Y, Liu W, Lan T and Lan G (2025) Spatiotemporal trends of ischemic stroke burden attributable to PM2.5 from 1990 to 2021. Front. Public Health. 13:1608086. doi: 10.3389/fpubh.2025.1608086
Edited by:
Kaijian Hou, Shantou University, ChinaReviewed by:
Yang Wang, Yunnan Normal University, ChinaMirmajid Farhadi, Consultant, Khorramabad, Iran
Xiaoyi Zhang, Jacobi Medical Center, United States
Copyright © 2025 Lu, Mao, Liu, Lan and Lan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaochen Lan, ZnlkZmV5ODg4QGZqbXUuZWR1LmNu; Tian Lan, bGFuX3RpYW5fbHRAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yunyan Lu1†
Yunyan Lu1† Tian Lan
Tian Lan Gaochen Lan
Gaochen Lan