- 1Department of Health Service Management, Faculty of Health Service, Naval Medical University, Shanghai, China
- 2Department of Mathematics and Physics, Naval Medical University, Shanghai, China
Introduction: Chronic kidney disease (CKD) has a high prevalence, poor prognosis, and high medical costs, and awareness of the disease is low. Therefore, in this study, we aimed to simulate and analyze the evolution of CKD burden among different groups at high risk of CKD in Shanghai, with or without screening intervention, and provide a quantifiable basis for the selection of screening intervention strategies for CKD.
Methods: A micro-simulation model was constructed to analyze the evolution of CKD burden using data from CKD screening of the population in the Jing’an and Minhang Districts of Shanghai, China, from January 2015 to December 2020. SAS Statistical Software 9.4 was used to simulate and analyze the evolution of disease burden under different screening intervention strategies.
Results: By 2033, screening interventions for high-risk groups with hypertension, diabetes, and an age of 65 years and older would be associated with 6,250 fewer patients with end-stage renal disease. Furthermore, the number of patients with end-stage renal disease would be reduced to only 41.64% of the projected number of patients without screening intervention, leading to a general improvement in the quality of life of the population, better quality-adjusted life-years, and a reduction in the economic burden of disease.
Discussion: The results of this study highlight the importance of combining the concepts of integrated prevention and treatment of chronic diseases to improve screening and intervention of CKD for people with hypertension, diabetes, and those aged 65 years and older, thereby effectively reducing the number of patients with end-stage renal disease, lowering the cost of treatment and intervention, and improving the quality of life of the population.
1 Introduction
Chronic kidney disease (CKD) refers to structural or functional impairments in the kidneys caused by various factors. Due to the high prevalence, poor prognosis, high medical costs, and low awareness of the disease, CKD has become a serious threat to human health, following cardiovascular diseases, diabetes, and malignant tumors (1–7). According to relevant statistics, the global prevalence of CKD is 10.1–13.3%. It is projected that by 2040, CKD will become the fifth leading cause of mortality worldwide (8). In China, a cross-sectional epidemiological study conducted in 2012 showed a CKD prevalence of 10.8% among individuals aged 18 years and older (9). In 2015, Shanghai included the prevention and control of CKD in its 3-year public health action plan. The Jing’an and Minhang districts were selected as screening bases for high-risk groups with CKD, the high-risk population for CKD refers to individuals who have at least one of the following risk factors: ① older adult(s) aged 65 and above; ② Hypertension; ③ Diabetes; ④ Hyperuricemia; ⑤ Has a family history of kidney disease, and the results revealed a detection rate of 25.75%. Specifically, the detection rates were 23.50% for males and 27.29% for females (10, 11). CKD often has a subtle onset; however, most patients only seek medical attention when they have symptoms, which typically occur in the middle to late stages of the disease. When CKD progresses to the end stage, it poses a serious threat to patients’ lives.
To alleviate the heavy social burden caused by CKD and the considerable medical expenses associated with dialysis, health system needs to prioritize measures to better manage patients with early-stage CKD. Although there is consensus on the early prevention and treatment of CKD (11), the practical evaluation of prevention and treatment strategies in real society often faces many constraints. This is mainly manifested in the difficulty of evaluating the effectiveness of the early prevention and treatment of CKD. CKD has a long course of development and often requires years of follow-up, making it difficult to obtain a health economic evaluation of the impact of changes in disease burden on the population in the presence or absence of intervention through prevention and control strategies in real society.
Furthermore, the traditional “intervention control” method for evaluating prevention and control strategies requires high implementation conditions and is difficult to implement. For example, problems such as high resource consumption, lengthy cycles, and a complex intervention and comparison policy environment during evaluation implementation are encountered. Moreover, the comparison of disease treatment involves ethical issues that are difficult to address. During the research process, there are many human interventions that research participants would not easily accept, resulting in a limited quantity and quality of evidence.
Therefore, in this study, we aimed to construct a micro-simulation model for the evolution of CKD in patients. The micro-level data files, containing demographic information and CKD prevalence, were integrated to simulate changes in individual disease occurrence and progression due to different screening intervention strategies for CKD. Based on this, the progression of disease burden in the population was analyzed and recommendations were proposed for the selection of prevention and treatment strategies.
2 Materials and methods
2.1 Data sources
2.1.1 Literature collection
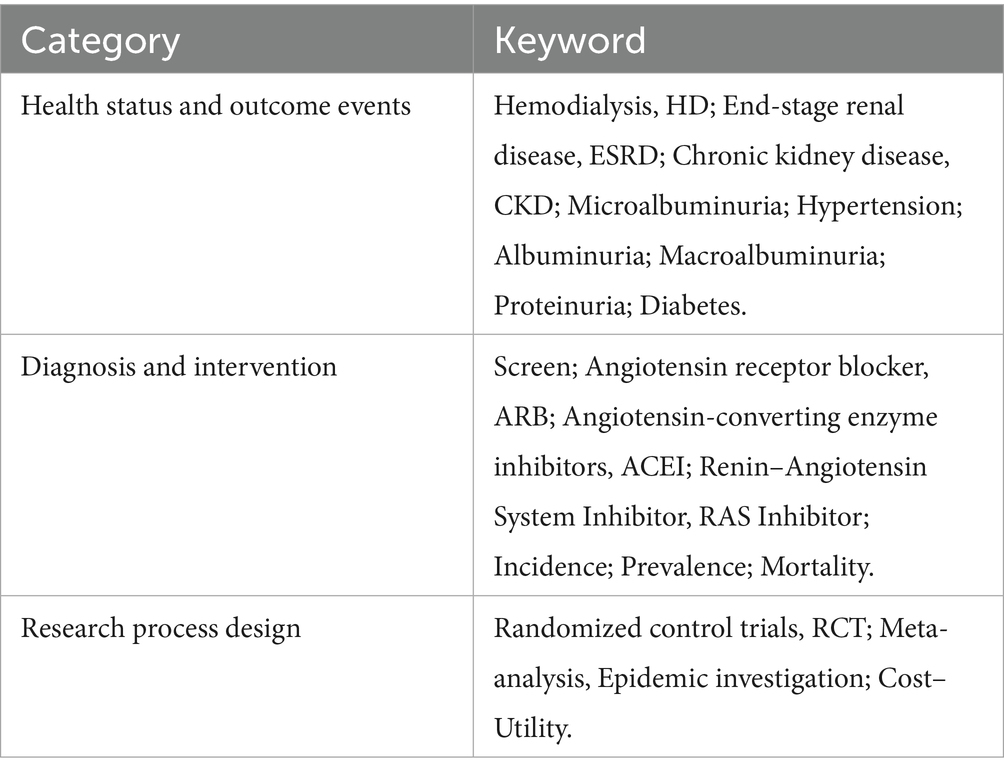
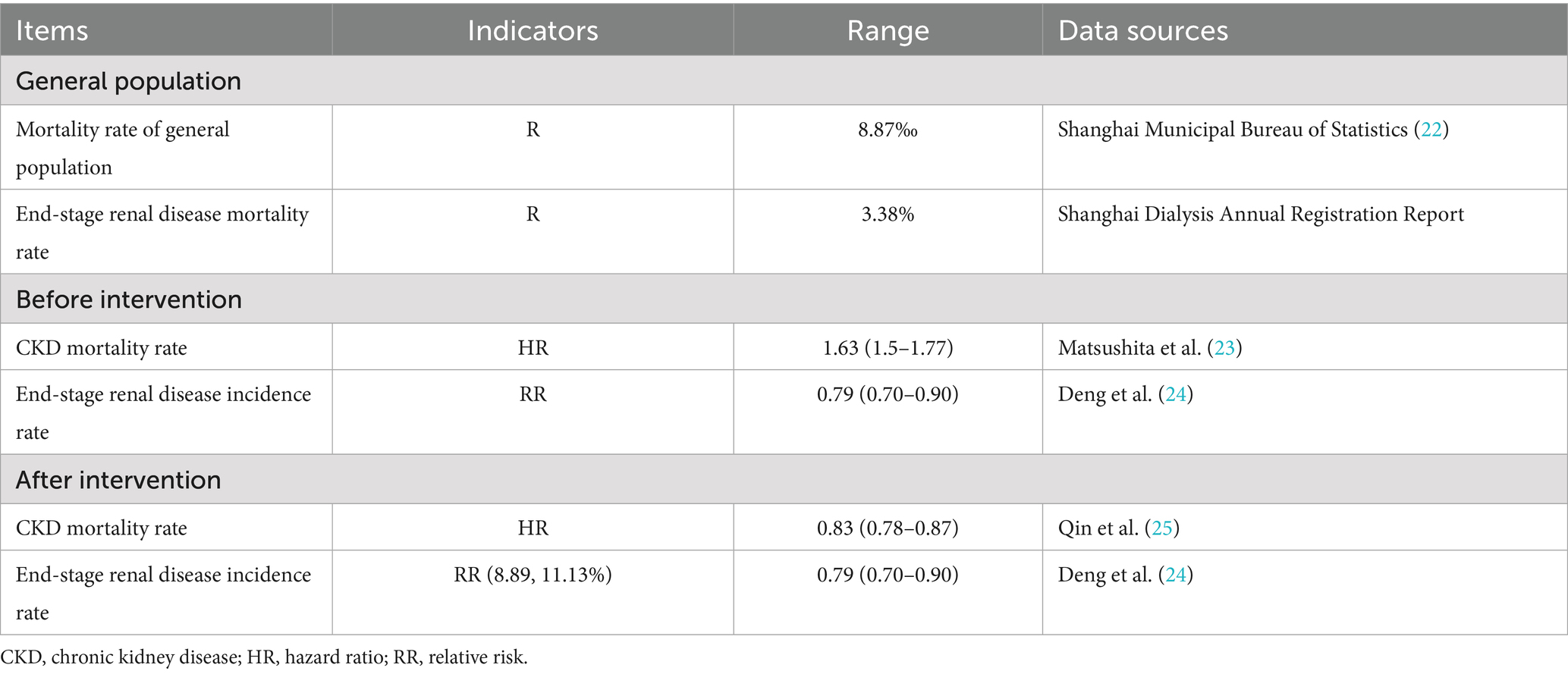
The probability of disease state transition and utility data related to disease burden were collected through systematic literature searches. We primarily analyzed disease state transitions, with or without screening and intervention, which were mainly obtained from epidemiological surveys. Probability parameters for disease state transitions in CKD with screening intervention were obtained with a focus on relevant intervention studies. Studies have shown that angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) reduce urinary protein levels. The relative effectiveness of ACEIs and ARBs compared with a placebo or no treatment was obtained from the latest relevant meta-analyzes. The literature search databases included MEDLINE, the Cochrane Library, and EMBASE. The model parameters retrieved through the search terms are shown in Table 1.
The collected indicators included the probability of death, incidence rate of end-stage renal disease, mortality of end-stage renal disease, mortality of diagnosed CKD, incidence rate of end-stage renal disease in diagnosed CKD, mortality of end-stage renal disease in diagnosed CKD, mortality of undetected CKD, incidence rate of undetected CKD, mortality of CKD, morbidity of end-stage renal disease of CKD, probability of death due to diagnosed and intervened CKD, probability of end-stage renal disease onset due to diagnosed and intervened CKD, and the probability of death due to diagnosed and intervened end-stage renal disease in CKD.
We calculated the probability of state transition based on the collected event occurrence rate.
2.1.2 Survey data collection
The data in this study were gathered from a CKD screening population in the Jing’an and Minhang Districts of Shanghai, China, from January 1, 2015, to December 22, 2020. The collected information included demographic and sociological characteristics, height, weight, diastolic and systolic blood pressure, health insurance type, screening date, urinary protein concentration, urinary albumin–creatinine ratio, blood creatinine concentration, estimated glomerular filtration rate, and screening results. The total number of individuals screened was 104,593. Among them, 28,931 were known to have CKD before screening, and the remaining 75,662 individuals had no signs of CKD. The prevalence of CKD in the population and medical costs were derived from this database.
Data on the prevalence of CKD and its direct medical costs were collected from the CKD screening database.
2.2 Research methods
2.2.1 Module for initial data file
Based on the changes in the registered population by age in Shanghai, this study carried out predictions until the beginning of 2023. The number of individuals in the population by age and disease stage in the starting year was estimated considering the distribution of high-risk factors (hypertension, diabetes) related to CKD and the prevalence of CKD among high-risk groups. Accordingly, microdata files were constructed to serve as the foundation for the simulation.
2.2.2 Module for evaluating disease state
Based on the natural progression of CKD combined with survey data and literature search results, we obtained the transition probabilities among different CKD states. The parameters were mainly derived from large-scale queue analysis and meta-analysis results, combined with sensitivity analysis results, to synchronize transition probabilities from multiple sources. The model simulated the annual progression of the disease for individual patients. Figure 1 illustrates the disease state transitions in CKD.
2.2.3 Module for annual adjustment and result analysis
This module was used to make necessary adjustments and prepare data for the next year’s simulation. Subsequently, a comprehensive analysis of the simulation results was performed. The model simulated CKD-related mortality events in the population based on the mortality probabilities that were obtained for different CKD states. After the simulation, the model generated results, which were then comprehensively analyzed.
2.3 Data analysis
SAS statistical software 9.4 was used to simulate and analyze the evolution of disease burden under different screening intervention strategies.
3 Results
3.1 Construction of micro-simulation data files for CKD
Using data from CKD screening results and estimated new cases identified through screening, initial microdata files for individuals with CKD were constructed. The construction process was as follows:
1. It was assumed that the distribution of high-risk groups for CKD (hypertension and diabetes) in Shanghai’s registered population matched the distribution of high-risk groups participating in the screening. The age of the screened patients followed a normal data distribution, with a mean age of 71.45 years and a standard deviation of 8.18 years. Based on the proportion of the population with a high risk of CKD (10%), microdata with age characteristics for high-risk individuals with CKD in Shanghai’s registered population were randomly generated. In this paper, we considered the minimum age to be 45 years.
2. A new micro-database of the screened patients with CKD was randomly generated for age and CKD stage characteristics. The screening data for patients with CKD, including the prevalence and proportion of newly identified patients, were combined with the generated microdata containing age and CKD stage characteristics. Table 2 presents the constructed microdata for newly screened patients with CKD in Shanghai by age and CKD stage.
Table 2. Microdata constructed for newly screened patients with CKD in Shanghai by age and CKD stage.
3.2 Obtained model simulation parameters
3.2.1 Disease state transition parameters
Transition probabilities or event occurrence probabilities are typically not directly available in the literature and are usually calculated from event incidence rates. Transition probabilities, or event occurrence probabilities, were calculated based on event incidence rates. Table 3 shows the query results.
3.2.2 Utility parameters
Health utility in the model was measured by quality-adjusted life years (QALYs), where perfect health had a QALY value of 1, and death had a QALY value of 0. Data on the disease state in CKD were obtained from relevant literature. An analysis in Japan showed that the QALY values for CKD stages 1–4 were approximately 0.81. The QALY value of 0.658 for end-stage renal disease was obtained from the Chronic Renal Insufficiency Cohort study.
3.2.3 Medical cost data
Table 4 presents the outpatient medical expenditures for patients with CKD. The data indicate that outpatient medical expenses for patients with end-stage disease were significantly higher than those for patients with pre-end-stage disease. The discount rate for costs during the simulation process was set at 5%.
CKD community screening mainly relies on urinalysis and blood creatinine tests for assessment. Therefore, the screening cost mainly comprises these two parts. Both costs were determined based on the Shanghai healthcare service prices, which were 4 and 8 yuan, respectively. The cost of treatment primarily includes ACEI and ARB medication expenses, which, based on market price research, amounts to 30 yuan per week.
3.3 Results of CKD burden estimation
By constructing the micro-simulation model, we focused on simulating the evolution of disease burden over the next 10 years for patients identified in the year 2023 under scenarios with and without screening and management interventions. A total of five situations were simulated: first, screening and intervention in the hypertension group; second, screening and intervention in the diabetes group; third, screening and intervention in both the hypertension and diabetes groups; fourth, screening and intervention for individuals aged 65 years and older; and fifth, screening and intervention for individuals with hypertension, diabetes, and aged 65 years and older. We analyzed the data of individuals aged 45 years and older.
Table 5 presents the evolution of CKD disease burden in the presence and absence of screening intervention. From the perspective of the change in the number of patients with end-stage disease each year, it is evident that the screening intervention significantly reduces this number in different screened groups compared with those without intervention. The growth rate of patients with end-stage renal disease each year is effectively controlled through screening interventions. Judging by the simulation results of 2024, without intervention, the increase in patients with end-stage renal disease in the older adult, hypertension, and diabetes groups roughly aligns with the reported annual increase of 2000 cases in Shanghai (12, 13), signifying the accuracy of the simulation results.

Table 5. Evolution of patients with end-stage renal disease under different screening intervention strategies.
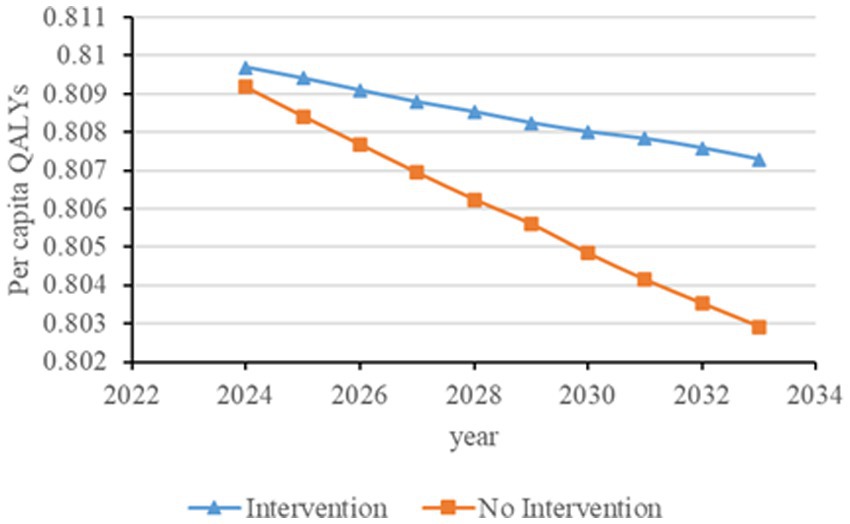
Figures 2–6 illustrate the changes in annual QALYs per capita resulting from screening interventions in different population groups. It can be observed that, over time, the quality of life in both the groups with and without screening interventions decreases annually. However, compared with the group without screening intervention every year, the group with screening intervention consistently exhibited a significantly higher quality of life.

Figure 2. Changes in per capita quality-adjusted life-years before and after hypertension screening and intervention. QALYs, quality-adjusted life-years.
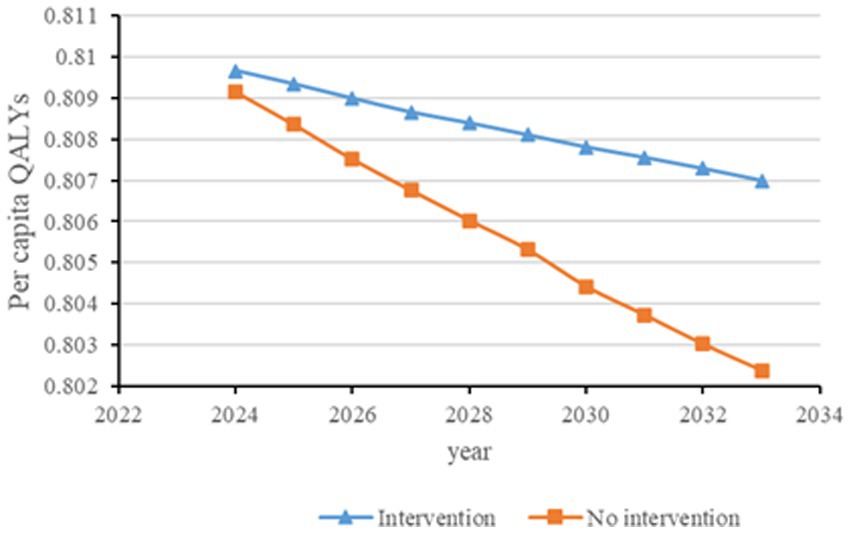
Figure 3. Changes in per capita quality-adjusted life-years before and after diabetes screening and intervention. QALYs, quality-adjusted life-years.
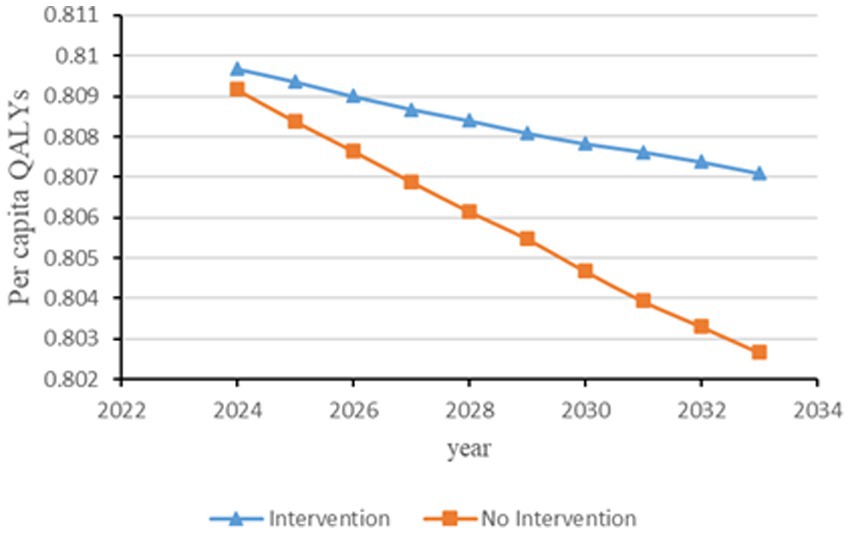
Figure 4. Changes in per capita quality-adjusted life-years before and after hypertension and diabetes screening and intervention. QALYs, quality-adjusted life-years.

Figure 5. Changes in per capita quality-adjusted life-years before and after screening and intervention for individuals aged 65 years and older. QALYs, quality-adjusted life-years.
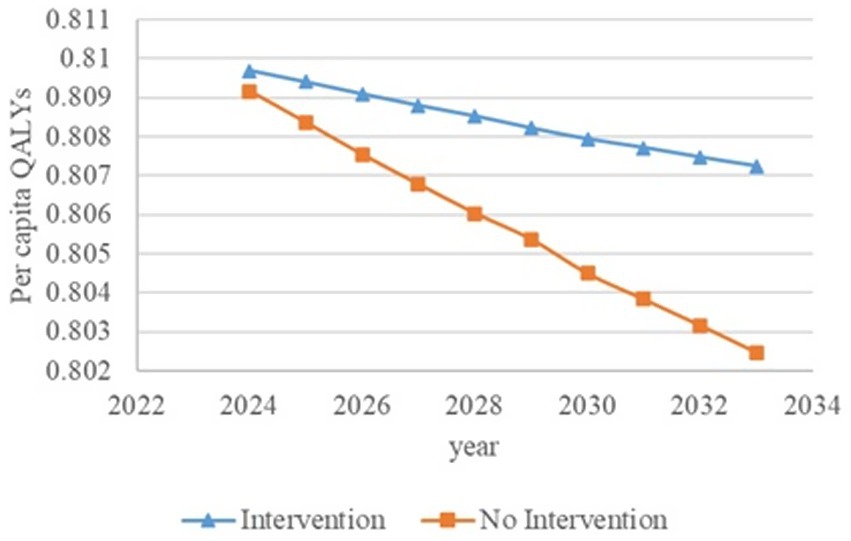
Figure 6. Changes in per capita quality-adjusted life-years before and after screening and intervention for individuals with hypertension, diabetes, and an age of 65 years and older. QALYs, quality-adjusted life-years.
Table 6 shows variations in the economic burden of disease for patients with end-stage renal disease in the hypertension, diabetes, and older adult groups under CKD screening intervention. According to the results, screening intervention effectively reduces the overall economic burden of patients with end-stage renal disease. By 2033, the annual cost savings for the screened and intervened group are predicted to amount to 454 million yuan.

Table 6. Changes in the economic burden of end-stage renal disease for hypertension, diabetes, and older adult groups.
3.4 Results of sensitivity analysis
The parameters influencing the simulation analysis included the transition probabilities of CKD, costs, and utility values. These parameters were adjusted, and a single-factor sensitivity analysis was conducted.
Figure 7 illustrates the changes in the annual average disease economic burden of the older adult, hypertension, and diabetes groups when the CKD transition probability and cost increase or decrease by 10%. The single-factor sensitivity analysis results show that cost changes have a greater impact on the economic burden of CKD, and the disease economic burden fluctuates within a certain range. Meanwhile, the economic burden of disease in the intervention group was lower than that in the non-intervention group.

Figure 7. Single-factor sensitivity analysis results of the impact of disease state transition probability and cost changes on the economic burden of diseases (Older Adult, Hypertension, and Diabetes Groups).
Table 7 illustrates the impact of a 10% increase or decrease in transfer probability and utility value on the per capita QALYs values of the older adult, hypertension, and diabetes cohorts in 2033. Changes in utility values show a greater impact on per capita QALYs.

Table 7. Single-factor sensitivity analysis results of the impact of disease state transition probability and utility value changes on QALYs.
4 Discussion
4.1 Early screening intervention for CKD effectively reduces disease burden
First, we found that screening interventions for high-risk groups with CKD can significantly reduce the number of patients with end-stage renal disease. As indicated by the simulation results, the number of patients with end-stage renal disease gradually increases from 2024 to 2033 when considering changes in the scope of screening interventions for various groups. However, the number of patients with end-stage disease subjected to intervention is significantly lower than that without intervention. Expanding the scope of screening intervention for high-risk groups further decreases the number of patients with end-stage renal disease. By 2033, screening intervention for high-risk groups, including individuals with hypertension, diabetes, and an age of 65 years and older, can reduce the number of patients with end-stage disease by 6,250 individuals. This would substantially reduce the disease burden in these groups.
Second, screening interventions for groups at a high risk of CKD effectively improve the quality of life of the population. Our simulation results show that, compared with no screening intervention, screening intervention for groups with hypertension, diabetes, and those aged 65 years and older can enhance the QALYs of the population, reflecting an improvement in their overall quality of life. When analyzing these high-risk groups individually, the greatest increase in QALYs occurs when screening intervention is implemented for individuals aged 65 years and older, with an increase of 0.21 years. This is closely followed by the diabetes group, which shows an increase of 0.20 years, while the hypertension group shows the lowest increase at 0.19 years. This suggests that screening interventions for individuals aged 65 years and older can significantly enhance the overall quality of life among the population.
Third, screening interventions for high-risk groups with CKD effectively reduce the economic burden of end-stage renal disease treatment. End-stage renal disease treatment incurs high costs, imposing a substantial economic burden on both society and families. From the simulation results, it can be observed that screening interventions for high-risk groups can effectively reduce the number of patients with end-stage disease. In 2033, when screening interventions are applied to high-risk groups comprising individuals with hypertension, diabetes, and an age of 65 years or older, the number of patients with end-stage renal disease is only 41.64% of the count without screening interventions. Therefore, implementing screening interventions for all these high-risk groups may annually reduce the end-stage renal disease intervention expenses by 454 million yuan.
4.2 Integrated prevention and treatment for chronic diseases provides guidance for effectively utilizing limited healthcare resources to address the high burden of CKD
In the 1970s, Western countries began establishing integrated systems for the prevention and treatment of chronic diseases. Countries such as the United States, the United Kingdom, and Japan adjusted and restructured their healthcare service systems to improve the quality and efficiency of healthcare services, forming integrated healthcare service systems. Research in foreign countries covers the outcomes, management, and quality of integrated healthcare services, the analysis of factors and mechanisms affecting these services, and the evaluations of methods and effectiveness for special groups (14–17). Since 2009, research on integrated prevention and treatment in China has gradually increased, with a significant rise in research papers published after 2015. Research has focused on the construction, modeling, and evaluation of integrated healthcare service systems, as well as on analyzing the construction of integrated models in developed countries and their implications for China. Both domestic and international research results generally support the idea that integrated healthcare service models can reduce hospitalization rates and medical expenses for chronic diseases, improve the health and quality of life of patients, enhance service quality, and reduce diagnosis and treatment time.
In accordance with the hierarchical diagnosis and treatment system, Shanghai has piloted the construction of a comprehensive CKD screening, diagnosis, and treatment system in the Jing’an District. The district has established a three-tier prevention and treatment network that includes early screening for high-risk groups, along with follow-up, diagnosis, treatment, and intervention for patients. These measures align with the concept of integrated prevention and treatment. Judging by the results of this micro-simulation experiment on screening interventions, regular screening of individuals aged 65 years and older, as well as those with hypertension and diabetes, and the provision of timely and effective interventions for identified patients can improve patients’ quality of life and reduce their economic burden. Based on Shanghai’s practices, public health outcomes could be improved by implementing integrated prevention and treatment measures for these high-risk groups. However, in practice, healthcare institutions at all levels must effectively implement their responsibilities and tasks within the diagnosis and treatment system in order to realize the goals of the screening and diagnosis system.
4.3 Exploring integrated prevention and treatment of CKD provides practical experience in reducing disease burden
Integrated prevention and treatment refers to a patient-centered approach that integrates the management and provision of various healthcare services, including health promotion, disease prevention, treatment, and end-of-life care. It involves the coordination of various healthcare institutions to provide lifelong continuous services to the population based on their health needs. According to the concept of integrated prevention and treatment, the functions of healthcare institutions at all levels are effectively integrated to prevent and control diseases. From the pilot practice of integrated prevention and treatment of CKD in Shanghai, community-level healthcare institutions mainly engage in early screening of groups at a high risk of CKD, while secondary and tertiary healthcare institutions primarily focus on the treatment of the disease. Therefore, the integrated prevention and treatment process in Shanghai includes both prevention (screening) and treatment (intervention), effectively integrating these aspects of care.
Shanghai has piloted a “27 + 8 + 3” CKD specialty referral mechanism, involving 27 community health service centers, eight district-level hospitals, and three municipal-level hospitals. The city has built up a three-tier prevention and treatment network, with each level of healthcare institution responsible for specific tasks. Early screening of high-risk groups, along with follow-up, diagnosis, treatment, and patient intervention, is carried out effectively as part of the system (18). Results from the pilot program show that the patients’ loss-to-follow-up rate was maintained below 10%, the screening rate for renal damage indicators among high-risk groups reached 70%, the patient filing rate reached 80%, compliance with bidirectional referrals reached 70%, the incidence of end-stage CKD was reduced by 10%, and the incidence of CKD combined with cardiovascular events was reduced by 15%. These results demonstrate the effectiveness of this system’s construction as an integrated prevention and treatment approach for CKD in Shanghai (19).
Moreover, guided by the concept of integrated prevention and treatment, China has gradually established a national Chronic Kidney Disease Management Center (CKDMC) since 2020 (21). Its purpose is to build China’s capacity to prevent and treat CKD and construct a national research network for kidney disease prevention, diagnosis, and treatment. For example, the First Affiliated Hospital of Xi’an Jiaotong University relies on the CKDMC to promote the standardization of the whole-process management, diagnosis, and treatment of CKD throughout the region (20). Both models emphasize the importance of integrated prevention and treatment. Comparatively, Shanghai’s integrated prevention and treatment system has clearer responsibilities and closer connections between institutions within the system, effectively implementing policies. This is the key to Shanghai’s success in achieving integrated prevention and treatment of CKD. However, the effectiveness of prevention and treatment needs to be further improved by enhancing the screening and management capabilities at the community level during the implementation process. Apart from clarifying the responsibilities at each level, building effective collaborative mechanisms among different levels within the integrated prevention and treatment system and strengthening screening and management capabilities at the community level are essential not only for achieving integrated prevention and treatment of CKD, but also the core issues that need to be addressed in the process of system construction. Table 8 lists the core content of the two modes.
5 Conclusion
According to our results, it is recommended to incorporate the concept of integrated prevention and treatment of chronic diseases and continue to explore the construction of the Shanghai CKD screening and prevention system. It is necessary to strengthen collaboration among institutions within the system, effectively implement the key measures of the three-tier CKD prevention and treatment network, and conduct screening and intervention for CKD in individuals with hypertension, diabetes, and an age of 65 years and older. This approach has the potential to effectively reduce the number of patients with end-stage disease, reduce the cost of treatment and intervention, and improve the quality of life of the population.
The single-factor sensitivity analysis results show that the factors affecting the economic burden of CKD are mainly the probability of disease state transition and the cost of disease intervention. In case of a 10% increase or decrease in both, except for the non-intervention group, the change in the probability of disease state transition has a greater impact on the economic burden of disease, whereas the rest are more affected by cost changes. Regarding quality of life, the main factors affecting the annual average QALYs are utility value and probability of disease state transition. When both factors increase or decrease by 10%, changes in utility value have a greater impact on the results.
This study had a few limitations. First, we assumed that the distribution of high-risk groups participating in the screening of CKD is consistent with the distribution of the registered residence population in Shanghai, which may have led to certain errors in the simulation results. Second, the disease state transition probability, mortality, and prevalence of population high-risk factors (hypertension, diabetes) for CKD in the research process are from relevant literature reports or meta-analysis results; therefore, the accuracy of their parameters may also lead to simulation errors to a certain extent. Third, we analyzed the risk of disease onset with and without intervention; therefore, compared with the actual situation, it may, to some extent, expand the screening intervention effect. Fourth, this model assumed full participation in the screening but did not consider the impact of changes in population participation in screening interventions on the results. However, the targeted implementation of prevention and control strategies affects the distribution of screening and treatment intervention behaviors among the affected population, thereby influencing the distribution of screening results and the probability of disease state transition. The probability of disease state transition affects the outcome of disease burden in the population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Committee on ethics of Medicine, Naval Medical University, PLA. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YL: Writing – original draft, Project administration. YM: Data curation, Writing – review & editing. PL: Investigation, Writing – review & editing. PX: Writing – review & editing, Resources. GD: Writing – review & editing, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This publication was funded by Key Project of National Social Science Fund (22AGL034). The funder had no role in the study’s design, data collection, analysis, decision to publish, or the preparation of the manuscript.
Acknowledgments
We are grateful for the enthusiastic cooperation of the Health Commission of Shanghai.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CKD, Chronic Kidney Disease; ACEI, Angiotensin-Converting Enzyme Inhibitor; ARB, Angiotensin II Receptor Blocker; CKDMC, Chronic Kidney Disease Management Center; QALYs, Quality-adjusted life years.
References
1. Rajapurkar, MM, John, GT, and Kirpalani, AL. What do we know about chronic kidney disease in India: first report of the Indian CKD registry. BMC Nephrol. (2012) 13:10. doi: 10.1186/1471-2369-13-10
2. Imai, E, Horio, M, and Watanabe, T. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. (2009) 13:621–30. doi: 10.1007/s10157-009-0199-x
3. Bruck, K, Stel, VS, and Gambaro, G. CKD prevalence varies across the European general population. J Am Soc Nephrol. (2016) 27:2135–47. doi: 10.1681/ASN.2015050542
4. Barreto, SM, Ladeira, RM, and Duncan, BB. Chronic kidney disease among adult participants of the ELSA-Brasil cohort: association with race and socioeconomic position. J Epidemiol Community Health. (2016) 70:380–9. doi: 10.1136/jech-2015-205834
5. United States Renal Data System. 2016 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda: National Institutes of Health (2016).
6. Ene-Iordache, B, Perico, N, and Bikbov, B. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health. (2016) 4:307–19. doi: 10.1016/S2214-109X(16)00071-1
7. Couser, WG, Remuzzi, G, Mendis, S, and Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. (2011) 80:1258–70. doi: 10.1038/ki.2011.368
8. Bikbov, B, Purcell, CA, Levey, AS, Smith, M, Abdoli, A, Abebe, M, et al. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2020) 395:709–33. doi: 10.1016/S0140-6736(20)30045-3
9. Zhang, L, Wang, F, Wang, L, Wang, W, Liu, B, Liu, J, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. (2012) 379:815–22. doi: 10.1016/S0140-6736(12)60033-6
10. Chanjuan, S. Analysis of the prevalence and influencing factors of chronic kidney disease in Shanghai. Shanghai: Naval Medical University (2019).
11. Zhao, L, Mei, C, Wu, B, and Xiong, L. Community screening analysis of high-risk groups of chronic kidney disease in Jing'an district of Shanghai. Chin J Nephrol. (2020) 12:1–5. doi: 10.3760/cma.j.issn.1001-7097.2020.01.001
12. Pengpai News. (2018). There are nearly 14000 uremic dialysis patients in Shanghai, with the oldest being a 101 year old peritoneal dialysis patient. Available online at: https://m.thepaper.cn/newsDetail_forward_1524247. (Accessed September 05, 2016).
13. Health Tips. A Brief Discussion on Prevention and Treatment of Uremia (2018). Shanghai Jianyao Lu.
14. Chunlin, J, and Fen, L. Empirical study on integrated prevention and treatment of stroke. Beijing: Science Press (2021).
15. Lilley, EK, and Jogee, A. The future of chronic conditions management in NSW: integrated care for people with chronic conditions. Int J Integr Care. (2017) 17:A86. doi: 10.5334/ijic.3198
16. Uittenbroek, RJ, Reijneveld, SA, Stewart, RE, Spoorenberg, SL, Kremer, HP, and Wynia, K. Development and psychometric evaluation of a measure to evaluate the quality of integrated care: the patient assessment of integrated elderly care. Health Expect. (2016) 19:962–72. doi: 10.1111/hex.12391
17. Rawlinson, C, Lesage, S, Gilles, I, and Peytremann-Bridevaux, I. Healthcare stakeholders' perspective on barriers to integrated care in Switzerland: results from the open-ended question of a nationwide survey. J Eval Clin Pract. (2022) 28:129–34. doi: 10.1111/jep.13605
18. Wang, JS. Advantages and significance of a three-level prevention and treatment system for chronic kidney disease in Shanghai. Acad J Second Mil Med. (2018) 39:24–8. doi: 10.16781/j.0258-879x.2018.01.0024
19. Pengpai News. “Three year action” for chronic nephropathy prevention and treatment in Shanghai: reduce the incidence rate of the end stage by 10%. (2018). Available online at: https://baijiahao.baidu.com/s?id=1603671809574474940&wfr=spider&for=pc,2018-06-19 (Accessed March 15, 2018).
20. Sohu Available online at: https://m.sohu.com/a/276027424_100281680/ (Accessed October 22, 2018).
21. Xia, W, and Song, J. Introduction to the core units of the National Chronic Kidney Disease Management Center (CKDMC). Chin J Nephrol Dial Transplant. (2021) 30:97–8. doi: 10.3969/j.issn.1006-298X.2021.01.017
22. Shanghai Municipal Bureau of Statistics. (2021). Shanghai Statistical Yearbook 2021. China Statistics Press. Available online at: https://tjj.sh.gov.cn/tjnj/tjnj2021e.htm
23. Matsushita, K, van der Velde, M, Astor, BC, Woodward, M, Levey, AS, de Jong, PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. (2010). 375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5
24. Deng, X, Li, D, Tang, Q, and Chen, Y. ACEI and ARB lower the incidence of end-stage renal disease among patients with diabetic nephropathy: a meta-analysis. Comput. Math. Methods. Med. (2022) 1–6. doi: 10.1155/2022/6962654
25. Qin, Y, Chen, T, Chen, Q, Lv, JL, Qi, N, Wu, C, et al. The effect of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use on mortality in patients with chronic kidney disease: a meta-analysis of observational studies. Pharmacoepidemiol Drug Saf. (2016) 25:503–511. doi: 10.1002/pds.3941
Keywords: micro-simulation, screening interventions, disease burden, chronic renal insufficiency, cost of illness
Citation: Li Y, Ma Y, Liu P, Xu P and Duan G (2025) Evaluating disease burden in chronic kidney disease screening using a micro-simulation model. Front. Public Health. 13:1608445. doi: 10.3389/fpubh.2025.1608445
Edited by:
Munkhtuya Tumurkhuu, Wake Forest Baptist Medical Center, United StatesReviewed by:
Chala Kenenisa Edae, Jimma University, EthiopiaBecky Ness, Mayo Clinic Health System in Mankato, United States
Copyright © 2025 Li, Ma, Liu, Xu and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangfeng Duan, ZGdmX2plcnJ5QDE2My5jb20=; Ping Xu, eHVwaW5nc2hAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Yang Li
Yang Li Yuqin Ma1†
Yuqin Ma1† Ping Xu
Ping Xu Guangfeng Duan
Guangfeng Duan