- 1Health Through Evidence, Athens, Greece
- 2Pfizer Hellas, Athens, Greece
- 3First Department of Pediatrics, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece
- 4University General Hospital of Patras, University of Patras, Patras, Greece
- 5Neonatal Department and Neonatal Intensive Care Unit, Rea Maternity Hospital, Athens, Greece
- 6Pfizer Ltd, Tadworth, United Kingdom
Objective: The objective was to assess the burden of respiratory syncytial virus (RSV) and evaluate the cost-effectiveness of maternal vaccination using the bivalent RSV prefusion F-protein (RSVpreF) vaccine to prevent RSV infections among Greek infants.
Methods: A Markov model was adapted from the perspective of a public payer to simulate the health and economic outcomes of RSV from birth to 1 year of age. Key inputs for the model, including vaccine efficacy, utility values, epidemiological data, and direct medical costs [prices in euros (€), 2024], were obtained from official sources. Model main outcomes were medically attended RSV cases, RSV-related deaths, quality-adjusted life-years (QALY) gained, direct medical costs and incremental cost-effectiveness ratios (ICER).
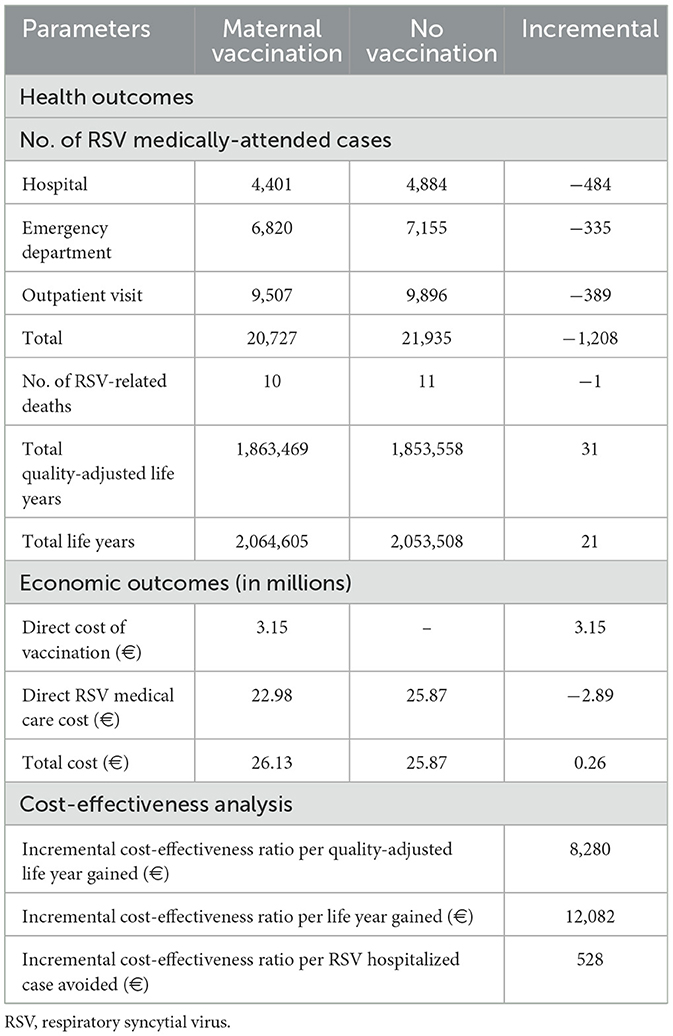
Results: The model analysis estimated that the annual number of RSV medically attended cases would be 21,935, with 22% requiring hospitalization, 32% managed in the emergency department (ED), and the remaining cases treated in outpatient settings. Furthermore, 11 RSV-related deaths were estimated. These cases represent a significant economic burden, with direct medical costs of ~€26 million. With a year-round maternal RSVpreF vaccination coverage of 19.5%, over 1,200 RSV medically-attended cases could be prevented annually. Vaccination benefits translated to 31 additional QALYs compared with no vaccination. Thus, the model analysis indicated that RSVpreF vaccination is a cost-effective strategy, resulting in an ICER of €8,280 per QALY gained compared to no vaccination.
Conclusion: Administering maternal RSVpreF vaccination year-round can provide protection to infants against RSV from birth. From a payer perspective, maternal RSVpreF vaccination has been evaluated as a cost-effective alternative compared to no intervention, underscoring its value as a preventive strategy against RSV in Greece.
Introduction
Respiratory syncytial virus (RSV) is a common pathogen that causes recurrent infections throughout a person's lifetime, with infants and chronically ill older adults most at risk of developing severe disease (1). RSV usually causes upper respiratory tract infections, but can also progress to acute lower respiratory tract infections (ALRTIs) (1). RSV is transmitted to infants primarily from their parents and other children, with the majority of cases acquired in a community setting (2–4).
RSV is the leading cause of viral bronchiolitis and pneumonia in children under 5 years of age, with infants under 6 months being at the highest risk for severe disease, including bronchiolitis and pneumonia, where respiratory distress is a key feature (5, 6). Globally, in 2019, RSV accounted for 33 million ALRTIs in children under 5 years, with 6.6 million cases occurring in infants under 6 months, equating to an incidence rate of 96.3 per 1,000 infants. The burden is particularly high in low- and middle-income countries (7). Furthermore, 45,700 ALRTI-related deaths in children under 6 months were attributed to RSV in 2019, with infants under 6 months accounting for over 50% of in-hospital RSV-related deaths among children under 5 years (7). Mortality and case fatality rates are highest in the first 3 months of life, making this a critical period of vulnerability (7–9). RSV causes more respiratory-related deaths than influenza and has a higher pneumonia-related case fatality ratio in children under 5 years (7, 10–12).
RSV leads to substantial clinical burden due to the high incidence of hospitalization, particularly in younger infants (7, 13, 14). Approximately 40% of the 3.6 million global RSV-associated hospitalizations are among children < 6 months, with infants in their first 3 months of life being most vulnerable and accounting for over 60% of hospitalizations in the first 6 months (7). Though prematurity and other chronic diseases increase risk of RSV-associated hospitalization, ~80% of hospitalizations are in healthy, term infants, emphasizing the need to protect all infants from RSV (15, 16). The burden of RSV is not limited to hospitalization and includes outpatient services as well as the management of recurrent respiratory infections; ~20% of infants hospitalized for RSV require readmission to hospital or Intensive Care Unit (ICU) with infants < 6 months accounting for up to 50% of all readmissions (17–19).
Moreover, RSV is associated with considerable direct healthcare use and costs, which has been estimated at ~€4.8 billion globally per year in children < 5 years of age (20). Direct health costs for RSV-associated ALRTI are particularly high in those aged < 6 months or with severe disease (18, 21). Hospitalization is the primary driver of RSV healthcare costs, with the cost of inpatient care influenced by disease severity and patient age, with the greatest burden incurred in those < 6 months of age (9, 18, 20–22). Caring for a child with RSV-associated ALRTI impacts a caregiver's ability to work to a greater extent than other respiratory infections (23).
The recently licensed bivalent RSV prefusion F protein vaccine (RSVpreF) shows promise in addressing an unmet medical need (24). This potential stems from its demonstrated efficacy, safety, and ability to provide protection against RSV in infants through the transfer of maternal antibodies generated in response to the vaccine, as demonstrated in a pivotal Phase III placebo-controlled clinical trial, the “MATernal Immunization Study for Safety and Efficacy” (MATISSE) (25).
Decision makers need to understand the potential health and economic outcomes of a maternal RSVpreF vaccination strategy to maximize the effective use of health care resources. Hence, the objective of this study was to evaluate the health and economic burden of RSV disease and the cost-effectiveness of maternal vaccination with RSVpreF for the prevention of RSV among infants in Greece.
Materials and methods
Target population
The model population included liveborn infants (n = 76,541) born to 76,500 women during a 1-year period. The liveborn infants were characterized by gestational age in weeks (wGA) at birth, defined as: full term (≥37 wGA), late preterm (32–36 wGA), early preterm (28–31 wGA), and extreme preterm (≤ 27 wGA). Estimates of born infants (live and stillbirths), number of women giving birth and the distribution of births by term status (Figure 1) in a single year (2023) was provided by the Hellenic Statistical Authority (26).
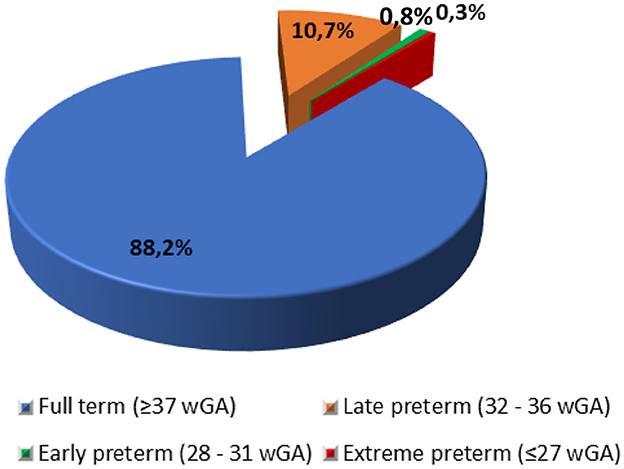
Figure 1. Distribution of births by term status. wGA, Weeks of gestational age. Source: Hellenic Statistical Authority (26).
Model overview
The published model (27) utilizes a cohort-based framework and a Markov process to simulate health outcomes and economic costs associated with RSV infections in newborn infants from birth to 1 year of age. The model population was initially stratified based on weeks of gestational age (wGA) at birth. Infants were assumed to either receive protection against RSV through maternal vaccination or remain unvaccinated, representing the no-intervention scenario.
Expected clinical outcomes for infants were projected on a monthly basis (with a model cycle length of 1 month) using factors such as age, gestational age at birth (wGA), disease and fatality rates (which vary by age, wGA, and calendar month), and the mother's vaccination status, through the 1-year modeling horizon. Clinical outcomes included medically attended RSV cases, categorized by care setting [hospitalization, emergency department (ED), or outpatient visit (OV)], as well as RSV-related deaths requiring hospitalization. Infants whose mothers received RSVpreF were assumed to have a reduced risk of developing RSV, with the extent of risk reduction (including initial effectiveness and waning) influenced by the clinical setting (hospital vs. ED/OV), timing of maternal vaccination relative to birth, and the infant's wGA at birth. The risk of death from RSV and other causes (non-RSV) was modeled as age- and wGA-dependent. Moreover, the model incorporates a lifetime horizon to account for the long-term impact of premature RSV-related mortality on life expectancy, allowing for the estimation of total life-years (LYs) and quality-adjusted life years (QALYs) for the modeled cohort. The expected costs of medical treatment for RSV-LRTI were calculated based on unit costs associated with hospital, ED and OV, while vaccination costs, including vaccine unit cost and administration.
The base-case analysis was conducted from the perspective of the Greek public payer and incorporated only direct medical costs. A cost-effectiveness threshold of €44,000 per outcome gained or avoided was applied in the analysis [two times the Greek Gross Domestic Product (GDP) per capita]. A widely accepted assumption, drawn from various published studies, suggests that a health intervention can be deemed cost-effective if its incremental cost-effectiveness ratio (ICER) falls within the range of one to three times the country's GDP per capita (28–30). Additionally, a 3.5% annual discount rate was applied to both costs and outcomes in the analysis (31, 32).
Model inputs and parameters
Disease incidence and mortality
In the absence of comprehensive national surveillance data on RSV burden in Greece, the annual incidence rates of RSV by month of age and by care setting (i.e., hospital, ED, and OV) were derived from a multidimensional real-world evidence study (33) (Table 1). This study was selected for its alignment with hospitalization patterns observed in Greece and was further validated by local clinical experts (neonatal pediatricians) from three major pediatric hospitals in Greece. These local clinical experts confirmed that RSV hospitalization rates extracted from the real-world evidence study (33) better reflect their real-world clinical experience than aggregated Europe estimates (7). A key consideration in our data selection was the variation in hospitalization practices across Europe. In many central European countries, hospital admissions for RSV require prior approval from general practitioners, whereas in Greece, infants are more readily admitted due to the absence of such a requirement and a lower threshold for hospitalization. A similar pattern has been observed in influenza-related hospitalizations, where Greek infants are hospitalized at rates above the Europe average (34, 35). Given these contextual factors, we determined that RSV hospitalization rates obtained from the real-world evidence study (33) serve as a reasonable proxy for Greece, as hospitalization rates in Greece are expected to be higher than the EU mean (14). To further validate our assumptions, we used the EU mean and explored the impact of alternative incidence estimates in a scenario analysis using RSV hospitalization rates from Del Riccio et al. (14).
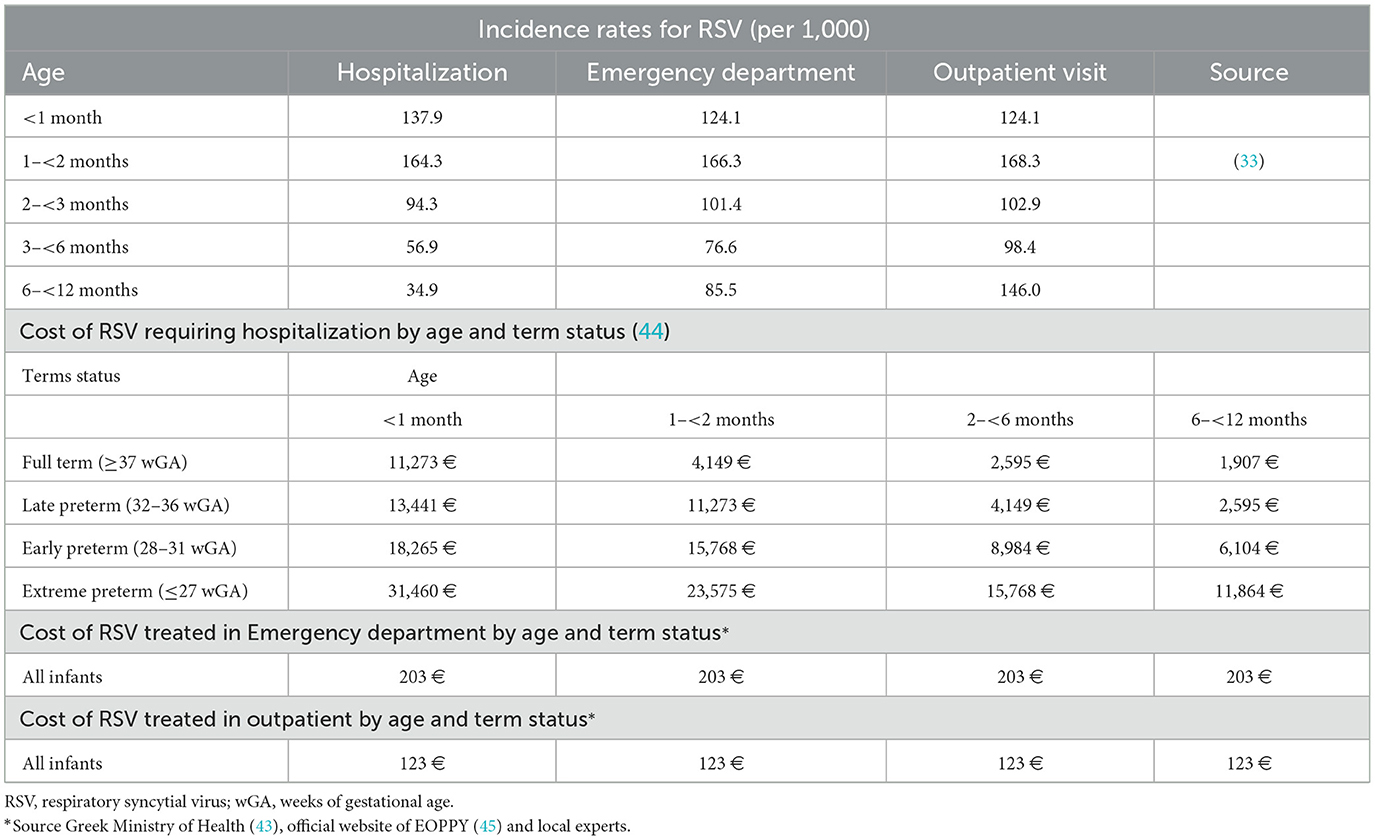
Table 1. Incidence rates of respiratory syncytial virus by care setting and age, along with associated direct medical costs.
Age-specific relative rates of RSV based on term status (late, early, and extreme preterm compared to full-term infants) were calculated using data from the study by Rha et al. (36) and were assumed to be applicable across all care settings (Table 1). Due to the limited number of early and extreme preterm infants included in Rha et al.'s study, a combined relative rate of RSV was determined for these groups. The distribution of RSV cases across calendar months was determined using insights provided by local experts (Supplementary Figure S1).
RSV-associated mortality was based on hospitalization case-fatality rate (CFR), ~0.20–0.25 per 100 cases as reported in a published study (37) and estimates from local clinical experts. Furthermore, due to limited research on CFR related to RSV, the CFR for preterm infants was derived by applying a relative risk of death for preterm infants, as informed by a published study (11) and local clinical experts' estimation. At this point, it should be mentioned that it was assumed infants with RSV who were treated in the outpatient setting were not considered to be at risk of disease-related death. Moreover, the general infant mortality rate (per 1,000 live births, death due to non-RSV causes) was obtained from the Hellenic Statistical Authority (26).
Maternal vaccination effectiveness and coverage
Setting-specific vaccine effectiveness (VE) estimates were derived from the cumulative efficacy data for the primary endpoints of the MATISSE trial (25). The efficacy of the MATISSE study against severe RSV-positive medically attended lower respiratory tract illness has been comprehensively presented in previous related published studies (27, 38, 39). At this point, it is important to emphasize that vaccine efficacy (VE) was assumed to decline linearly from 6 months post-vaccination to 0% by 9 months, as illustrated in Figure 2.
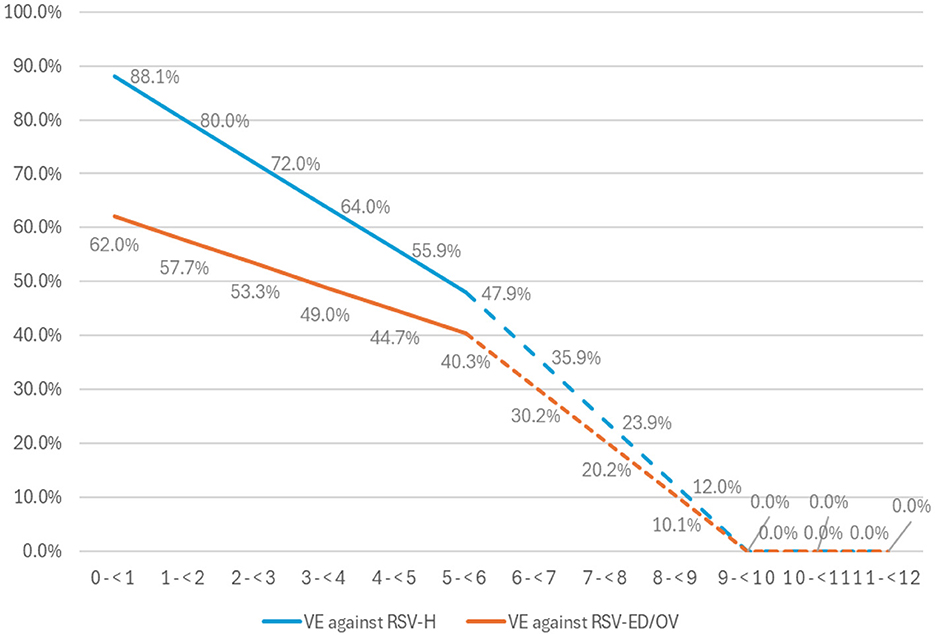
Figure 2. The effectiveness of the vaccine (VE) against RSV-LRTI requiring hospitalization (RSV-H) and RSV-LRTI treated in the emergency department or outpatient visit for full term and late preterm infants born at least 2 weeks after maternal vaccination. ED, emergency department; OV, outpatient visit; RSV, for respiratory syncytial virus. Observed RCT VE data in solid lines and extrapolated data in dashed lines.
Moreover, the coverage of maternal vaccination (RSVpreF) was assumed to be 19.5% based on values for influenza vaccination of pregnant women as reported in a recent Greek study (40) and was assumed to be constant across calendar months. The vaccine administration window was assumed to be between 24 and 36 weeks consistent with EMA's regulatory approval, and the distribution of RSVpreF administration by fetal wGA was informed by local experts.
Utilities data
A utility value of 1 was assumed for infants without RSV-LRTI. For those with RSV-LRTI, utility values during a 14-day illness period were set at 0.59 for RSV-related hospitalization and 0.84 for RSV cases treated in ED or OV settings (41). The corresponding QALY losses were estimated to be 0.0157 for infants experiencing RSV-related hospitalization and 0.0061 for those treated in ED/OV settings (41). These QALY losses were assumed to remain consistent across all term statuses (41). For individuals aged ≥1 year, utility values were derived from reference population norms in a published study (42) or children aged 1–17 years, utility values were estimated through linear interpolation between values for 1-year-olds and adults aged ≥18 years.
Costs inputs
Cost inputs included vaccination expenses and direct medical costs attributable to RSV for episodes of RSV-related hospitalization (RSV-Hospital), RSV-ED, and RSV-OV, categorized by age and term status.
The bivalent RSVpreF unit cost per dose of €205.98 was extracted from the Greek Ministry of Health (43). Following local clinical practice, it was assumed that all pregnant women would need a healthcare professional for the administration of vaccine, hence, the cost of a physician visit was charged (€10 per visit).
The age- and term-specific direct medical costs of RSV hospitalizations were derived from DRG tariffs set by the Greek Ministry of Health (44). Costs for non-hospitalized RSV cases, including ED and OV, were estimated based on resource use reported by local experts and unit costs from the National Organization For Health Care Services (EOPYY) official website (45), and Greek Ministry of Health (43). All unit costs correspond to the year of the analysis (2024, €; Table 1).
Model sensitivity and scenario analyses
To address statistical uncertainties in several key parameters, both deterministic sensitivity analyses (DSA) and probabilistic sensitivity analyses (PSA) were conducted. A one-way DSA was performed to identify the key drivers of the model and assess areas of uncertainty. For variables where sensitivity parameters were unavailable, upper and lower bounds were tested using a ±25% variation from the mean value. DSA were performed for the following variables: vaccination effectiveness, disutilities, RSV hospitalization rate, and cost input. The impact of joint parameter uncertainty was explored by PSA. Typical probability distributions were used in the analyses (46). A total of 1,000 simulations were conducted to generate a distribution of incremental results, providing an estimate of the overall parametric uncertainty surrounding the cost-effectiveness findings.
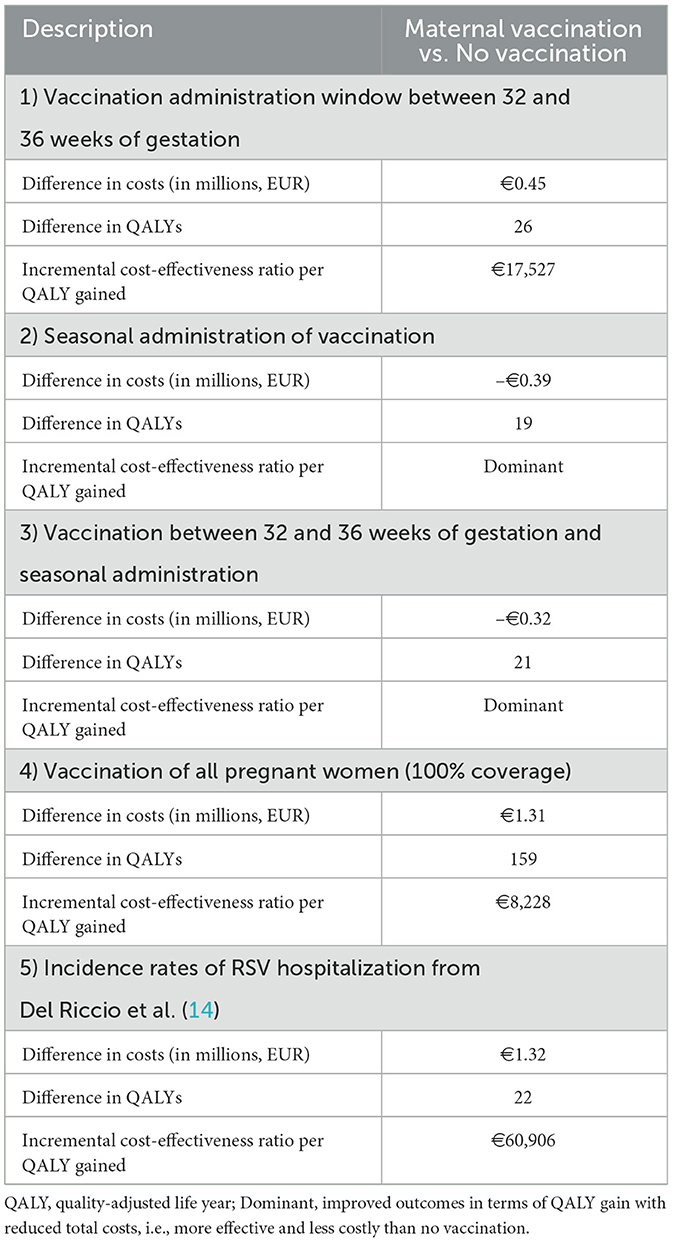
In order to test key uncertainty not addressed in either the DSA or PSA, several specific scenario analyses were conducted. More specifically, (1) vaccination administration window between 32 and 36 weeks of gestation, (2) seasonal administration of vaccination (September to January), (3) vaccination between 32 and 36 weeks of gestation and seasonal administration (September to January), (4) vaccination of all pregnant women (vaccine coverage of 100%), and (5) Incidence rates of RSV hospitalization from Del Riccio et al. (14) were applied to assess any impact on the overall results.
Results
Base case results
The model analysis estimated that the annual number of RSV medically attended cases in Greece would be 21,935, with 22% requiring hospitalization, 32% managed in the ED, and the remaining cases treated in OV. Furthermore, 11 RSV-related deaths were estimated. These RSV-cases represent a significant economic burden, with direct medical costs of ~€26 million (Table 2).
Year-round RSVpreF vaccination with 19.5% coverage was projected to reduce hospitalizations by 484 cases, ED encounters by 335 cases, OV by 389 cases, and RSV-related deaths by 1 over a 1-year period. The effectiveness benefits associated with RSVpreF vaccination translate into this strategy accruing 31 QALYs compared to no vaccination (Table 2). The model analysis demonstrated that RSVpreF vaccination is a cost-effective strategy. It estimated ICERs of €12,082 per LY gained, €8,280 per QALY gained, and €528 per RSV-related hospitalization avoided, compared to no vaccination (Table 2).
Sensitivity and scenario analyses results
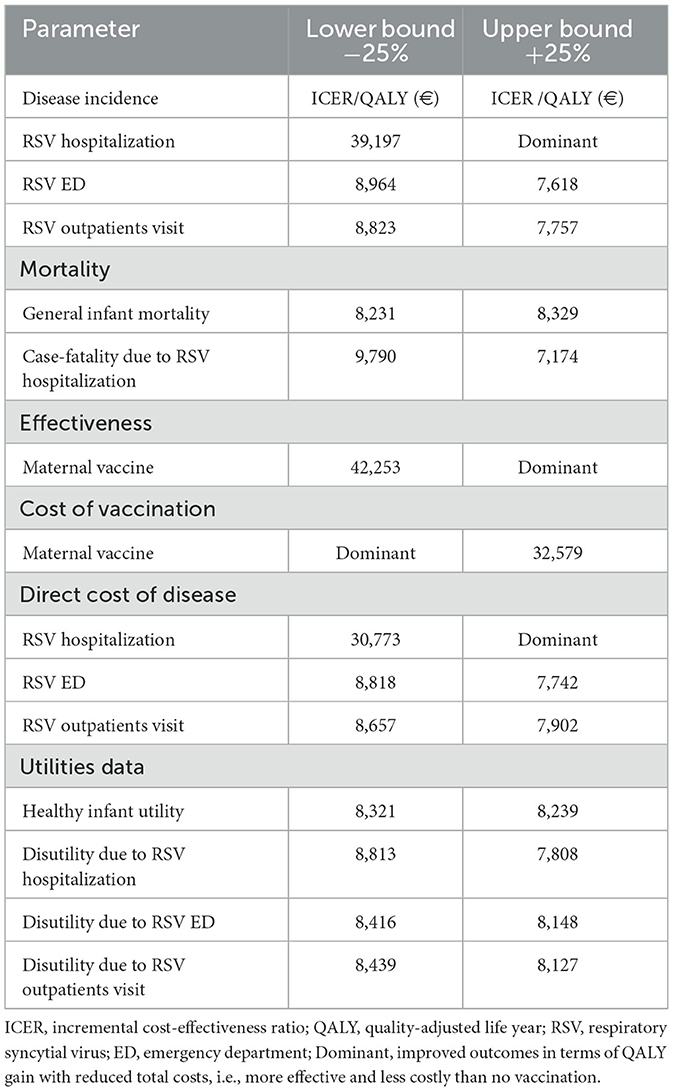
The DSA results demonstrated that the base case model outcomes were robust to variations in clinically reasonable parameter inputs. The model was most sensitive to changes in the efficacy of the RSVpreF vaccine, the hospitalization rate associated with RSV, and the cost of the RSVpreF vaccine (Table 3).
Furthermore, the PSA results indicated that, at an assumed cost-effectiveness threshold of €44,000 per QALY gained, the maternal vaccination had a 98% probability of being a cost-effective option compared to no vaccination (Figure 3). Additionally, across all scenario analyses, the maternal vaccination consistently remained a cost-effective strategy compared to no vaccination staying below the cost-effectiveness threshold, defined as one (€22,000) to three (€66,000) times the GDP per capita of Greece (Table 4).
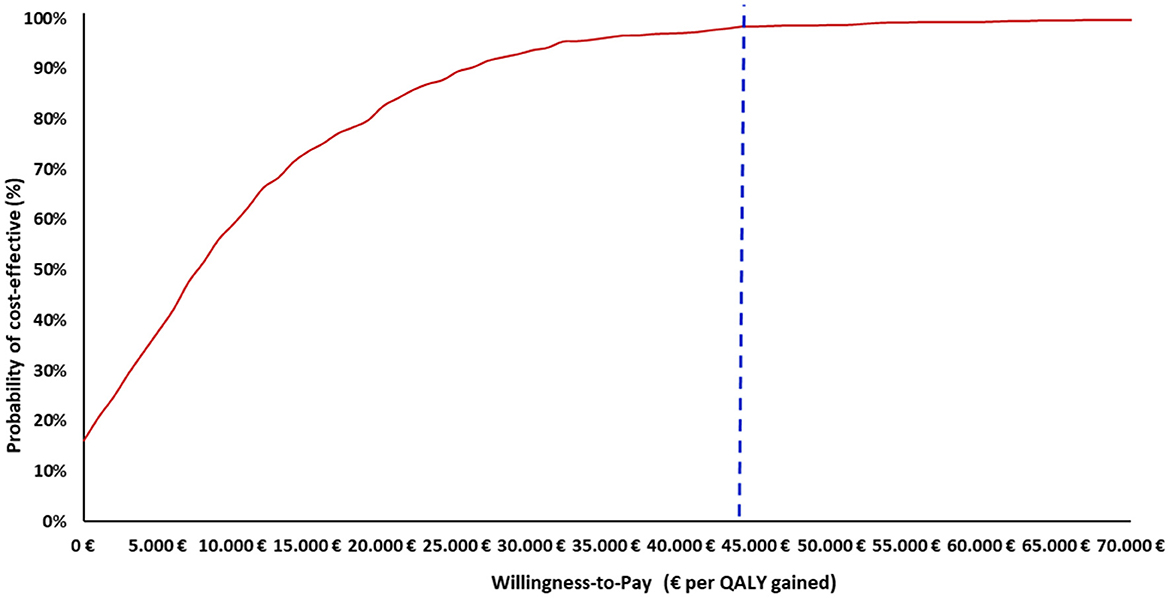
Figure 3. Cost–effectiveness acceptability curve of maternal vaccination strategy vs. no vaccination strategy. QALY, Quality-adjusted life year. The x-axis indicates the WTP thresholds (€), while the y-axis represents the probability that the vaccination strategy is cost-effective at each threshold level. The vertical dashed line marks the cost-effectiveness threshold of €44,000 per QALY gained.
Discussion
This study suggests that maternal vaccination with RSVpreF would be a cost-effective intervention to prevent RSV infections among infants, reduce hospitalizations and alleviate strain on medical resources. Assuming a year-round RSVpreF maternal vaccination coverage of 19.5%, >1,200 RSV medically-attended cases could be prevented annually with 31 additional QALYs gained and ICERs of €12,082 per LY gained, €8,280 per QALY gained and €528 per RSV hospitalized case avoided compared to no vaccination. Sensitivity analysis revealed robustness of the base-case findings to changes in input parameters and assumptions with maternal RSVpreF vaccination being cost-effective (and even cost saving) vs. no vaccination in all sensitivity and scenario analyses.
Our findings are consistent with those presented in the previously conducted studies of maternal vaccination with RSVpreF. More specifically, a cost-effectiveness study conducted in Spain (27) from a payer perspective showed that maternal immunization with RSVpreF vaccine was a dominant alternative (more effective and less costly) compared with a no vaccination strategy. Similar findings were reported for Canada (47), which showed that from a societal and payer perspective the maternal vaccination with RSVpreF was cost-effective compared to no vaccination at the cost-effectiveness threshold of $50,000 per QALY gained. Moreover, a multi-country study that was conducted in Europe (48) showed that from the payer perspective, maternal vaccination with RSVpreF compared to no vaccination strategy was cost-saving in Scotland, and cost-effective at threshold of €20,000–30,000 per QALY gained in England, Finland and Denmark.
It should be emphasized that the value of maternal vaccination to protect infants from RSV suggests multifaceted benefits, from direct health protection to the mother and infant to long-term societal impacts (49, 50). The RSV vaccine may offer protection to infants during their most vulnerable developmental stages and has the potential to reduce RSV-related long-term consequences (50). Additionally, it could contribute to herd immunity, potentially offering some level of protection to those who cannot be vaccinated (51, 52). Apart from the humanistic and clinical benefit, vaccination may reduce healthcare costs by preventing disease outbreaks and reduce the need for medical treatments and hospitalizations (51–53). Furthermore, protection against RSV in infancy ensures healthier childhood development, reduces long-term health complications, and promotes a better quality of life (51, 53).
Overall, vaccination is not only a successful health intervention, but also an effective investment in healthcare system. Recent studies showed that for every €1 invested in routine childhood immunization resulted €3 in cost savings from a societal perspective for national pediatric immunization programs (54, 55). Hence, it becomes clear that vaccination intervention such as RSV immunization is a sound decision, promoting public health by reducing the overall incidence of the virus and alleviating the burden on healthcare systems (56). Thus, RSV maternal vaccination to protect infants is a vital strategy for improving health outcomes and optimizing healthcare delivery in Greece.
Even though an established methodology was used in this study, some potential limitations should be acknowledged. First, in the absence of local RSV-specific incidence data for Greece, incidence rates were derived from a published study and validated by local clinical experts with extensive experience in managing respiratory infections. While we selected published study real-world data as the primary source due to its alignment with hospitalization patterns observed in Greek clinical settings, we recognize that using data from another country introduces potential generalizability concerns. This selection was validated by local clinical experts (neonatal pediatricians), who confirmed that Spanish hospitalization rates closely mirror their clinical experience. Additionally, differences in hospitalization criteria between Greece and central Europe—where Greek infants are admitted more readily—support the rationale that Spanish hospitalization rates may better approximate the burden in Greece compared to aggregated EU estimates. To address concerns about potential overestimation, we conducted a sensitivity analysis using hospitalization estimates from Del Riccio et al. (14), a systematic review providing RSV hospitalization rates for multiple EU countries, including Greece. This additional analysis allowed us to test more conservative assumptions and assess the robustness of our cost-effectiveness findings under different hospitalization scenarios. Moreover, to account for potential variability, a sensitivity analysis was conducted, exploring variations of ±25% in disease base case incidence rates. The results demonstrated that maternal vaccination remained a cost-effective option, underscoring the robustness of the model outputs. The primary findings were consistent across a wide range of parameter values. However, our findings should be interpreted with caution, and we emphasize the urgent need for high-quality, Greece-specific RSV surveillance data to strengthen future evaluations. Second, the current model does not capture the potential direct effects of vaccination against RSV among vaccinated pregnant individuals either before or after giving birth since maternal vaccination efficacy among pregnant individuals was not an outcome in the MATISSE phase 3 trial. Future research should explore this dimension, as it may further enhance the overall value proposition of maternal RSV vaccination Third, in the absence of local data, RSV-associated mortality in our model was estimated based on published studies and input from local clinical experts. However, a series of sensitivity analyses demonstrated that the model outcomes are robust, as the main results remained consistent across a wide range of parameter values. Fourth, our analysis was conducted from the perspective of the public payer and, therefore did not incorporate indirect societal costs such as caregiver absenteeism, loss of productivity, or long-term respiratory sequelae associated with severe RSV infections (e.g., wheezing, asthma). While not modeled explicitly, the inclusion of these indirect costs in future studies may further strengthen the economic case for maternal RSV vaccination, given the well-documented burden of RSV on families and the healthcare system.
Conclusions
The present study found that administering maternal RSVpreF vaccination year-round can provide protection to infants against RSV from birth. From a payer perspective, maternal RSVpreF vaccination has been evaluated as a cost-effective alternative compared to no intervention, underscoring its value as a preventive strategy against RSV in Greece.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was an economic evaluation analysis based on previously publicly available data and does not involve any new studies of human or animal subjects performed by any of the authors.
Author contributions
GG: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. AS: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Investigation, Project administration, Resources. EM: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. MD: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. TS: Conceptualization, Data curation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. GD: Conceptualization, Data curation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. AC: Conceptualization, Data curation, Validation, Visualization, Writing – original draft, Writing – review & editing. CT: Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. DM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. MB: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was sponsored by Pfizer. The study sponsor, Pfizer Inc., reviewed the study research plan and study manuscript; data analyses were conducted by Health Through Evidence. All final analytic decisions and the decision to submit for publication were made solely by study investigators.
Conflict of interest
AS, MB, EM, MD, and DM are employees of Pfizer and may own stocks. GG and CT are owners of Health Through Evidence, which received funding from Pfizer in connection with the development of this manuscript and study. TS, GD and AC are HCP experts, which were paid consultants to Pfizer in connection with the development of this manuscript.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1611483/full#supplementary-material
References
1. Chatterjee A, Mavunda K. Krilov LR. Current state of respiratory syncytial virus disease and management. Infect Dis Ther. (2021) 10:5–16. doi: 10.1007/s40121-020-00387-2
2. Anderson EJ, DeVincenzo JP, Simões EAF, Krilov LR, Forbes ML, Pannaraj PS, et al. SENTINEL1: two-season study of respiratory syncytial virus hospitalizations among U.S. infants born at 29 to 35 weeks' gestational age not receiving immunoprophylaxis. Am J Perinatol. (2020) 37:421–9. doi: 10.1055/s-0039-1681014
3. Frange P, Toubiana J, Parize P, Moulin F, Scemla A, Leruez-Ville M. Preventing respiratory syncytial virus infections in hospitalized children and adults: should we do better? Infect Prev Pract. (2020) 2:100041. doi: 10.1016/j.infpip.2020.100041
4. Jacoby P, Glass K, Moore HC. Characterizing the risk of respiratory syncytial virus in infants with older siblings: a population-based birth cohort study. Epidemiol Infect. (2017) 145:266–71. doi: 10.1017/S0950268816002545
5. Feikin DR, Fu W, Park DE, Shi Q, Higdon MM, Baggett HC, et al. Is higher viral load in the upper respiratory tract associated with severe pneumonia? Findings from the PERCH Study. Clin Infect Dis. (2017) 64(suppl_3): S337–46. doi: 10.1093/cid/cix148
6. Meissner HC. Viral bronchiolitis in children. N Engl J Med. (2016) 374:62–72. doi: 10.1056/NEJMra1413456
7. Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. (2022) 399:2047–64. doi: 10.1016/S0140-6736(22)00478-0
8. Scheltema NM, Gentile A, Lucion F, Nokes DJ, Munywoki PK, Madhi SA, et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob Health. (2017) 5:e984–91. doi: 10.26226/morressier.5ad774e1d462b80296ca6e1d
9. Cromer D, van Hoek AJ, Newall AT, Pollard AJ, Jit M. Burden of paediatric respiratory syncytial virus disease and potential effect of different immunisation strategies: a modelling and cost-effectiveness analysis for England. Lancet Public Health. (2017) 2:e367–74. doi: 10.1016/S2468-2667(17)30103-2
10. Wang X, Li Y, O'Brien KL, Madhi SA, Widdowson MA, Byass P, et al. Global burden of respiratory infections associated with seasonal influenza in children under 5 years in 2018: a systematic review and modelling study. Lancet Glob Health. (2020) 8:e497–510. doi: 10.1016/S2214-109X(19)30545-5
11. Hansen CL, Chaves SS, Demont C, Viboud C. Mortality associated with influenza and respiratory syncytial virus in the US, 1999-2018. JAMA Netw Open. (2022) 5:e220527. doi: 10.1001/jamanetworkopen.2022.0527
12. Wei JS. How lethal is SARS-CoV-2 pneumonia when compared with respiratory syncytial virus and influenza in young children? Aust J Gen Pract. (2020) 49:683–6. doi: 10.31128/AJGP-04-20-5357
13. Li Y, Johnson EK, Shi T, Campbell H, Chaves SS, Commaille-Chapus C, et al. National burden estimates of hospitalisations for acute lower respiratory infections due to respiratory syncytial virus in young children in 2019 among 58 countries: a modelling study. Lancet Respir Med. (2021) 9:175–85. doi: 10.1016/S2213-2600(20)30322-2
14. Del Riccio M, Spreeuwenberg P, Osei-Yeboah R, Johannesen CK, Fernandez LV, Teirlinck AC, et al. Burden of respiratory syncytial virus in the European union: estimation of RSV-associated hospitalizations in children under 5 years. J Infect Dis. (2023) 228:1528–38. doi: 10.1093/infdis/jiad188
15. Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. (2013) 132:e341–8. doi: 10.1542/peds.2013-0303
16. Doucette A, Jiang X, Fryzek J, Coalson J, McLaurin K, Ambrose CS. Trends in respiratory syncytial virus and bronchiolitis hospitalization rates in high-risk infants in a United States Nationally Representative Database, 1997-2012. PLoS ONE. (2016) 11:e0152208. doi: 10.1371/journal.pone.0152208
17. Hartmann K, Liese JG, Kemmling D, Prifert C, Weißbrich B, Thilakarathne P, et al. Clinical burden of respiratory syncytial virus in hospitalized children aged < =5 years (INSPIRE Study). J Infect Dis. (2022) 226:386–95. doi: 10.1093/infdis/jiac137
18. Demont C, Petrica N, Bardoulat I, Duret S, Watier L, Chosidow A, et al. Economic and disease burden of RSV-associated hospitalizations in young children in France, from 2010 through 2018. BMC Infect Dis. (2021) 21:730. doi: 10.1186/s12879-021-06399-8
19. Thwaites R, Buchan S, Fullarton J, Morris C, Grubb E, Rodgers-Gray B, et al. Clinical burden of severe respiratory syncytial virus infection during the first 2 years of life in children born between 2000 and 2011 in Scotland. Eur J Pediatr. (2020) 179:791–9. doi: 10.1007/s00431-019-03564-9
20. Zhang S, Akmar LZ, Bailey F, Rath BA, Alchikh M, Schweiger B, et al. Cost of respiratory syncytial virus-associated acute lower respiratory infection management in young children at the regional and global level: a systematic review and meta-analysis. J Infect Dis. (2020) 222:S680–7. doi: 10.1093/infdis/jiz683
21. McLaurin KK, Farr AM, Wade SW, Diakun DR, Stewart DL. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol. (2016) 36:990–6. doi: 10.1038/jp.2016.113
22. Paramore LC, Ciuryla V, Ciesla G, Liu L. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. Pharmacoeconomics. (2004) 22:275–84. doi: 10.2165/00019053-200422050-00001
23. Pokrzywinski RM, Swett LL, Pannaraj PS, Yi J, Pavilack MS, Kumar VR, et al. Impact of respiratory syncytial virus-confirmed hospitalizations on caregivers of US preterm infants. Clin Pediatr. (2019) 58:837–50. doi: 10.1177/0009922819843639
24. Agency EM. Abrysvo. European Public Assessment Report. Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/abrysvo (Accessed May 7, 2024).
25. Kampmann B, Madhi SA, Munjal I, Simões EAF, Pahud BA, Llapur C, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. (2023) 388:1451–64. doi: 10.1056/NEJMoa2216480
26. Hellenic Statistical Authority (EL. STAT.). Estimated Population. (2023). Available online at: www.statistics.gr/en/home/ (Accessed May 7, 2024).
27. Álvarez Aldean J, Rivero Calle I, Rodríguez Fernández R, Aceituno Mata S, Bellmunt A, Prades M, et al. Cost-effectiveness analysis of maternal immunization with RSVpreF vaccine for the prevention of respiratory syncytial virus among infants in Spain. Infect Dis Ther. (2024) 13:1315–31. doi: 10.1007/s40121-024-00975-6
28. Thokala P, Ochalek J, Leech AA, Tong T. Cost-effectiveness thresholds: the past, the present and the future. Pharmacoeconomics. (2018) 36:509–22. doi: 10.1007/s40273-017-0606-1
29. Cameron D, Ubels J. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: a systematic review. Glob Health Action. (2018) 11:1447828. doi: 10.1080/16549716.2018.1447828
30. Tzanetakos C, Gourzoulidis G. Does a standard cost-effectiveness threshold exist? The Case of Greece. Value Health Reg Issues. (2023) 36:18–26. doi: 10.1016/j.vhri.2023.02.006
31. Gourzoulidis G, Barmpouni M, Kossyvaki V, Vietri J, Tzanetakos C. Health and economic outcomes of 20-valent pneumococcal conjugate vaccine compared to 15-valent pneumococcal conjugate vaccine strategies for adults in Greece. Front Public Health. (2023) 11:1229524. doi: 10.3389/fpubh.2023.1229524
32. Warren S, Barmpouni M, Kossyvaki V, Gourzoulidis G, Perdrizet J. Estimating the clinical and economic impact of switching from the 13-valent pneumococcal conjugate vaccine (PCV13) to higher-valent options in Greek infants. Vaccines. (2023) 11:1369. doi: 10.3390/vaccines11081369
33. Martinón-Torres F, Carmo M, Platero L, Drago G, López-Belmonte JL, Bangert M, et al. Clinical and economic burden of respiratory syncytial virus in Spanish children: the BARI study. BMC Infect Dis. (2022) 22:759. doi: 10.1186/s12879-022-07745-0
34. Sakkou Z, Stripeli F, Papadopoulos NG, Critselis E, Georgiou V, Mavrikou M, et al. Impact of influenza infection on children's hospital admissions during two seasons in Athens, Greece. Vaccine. (2011) 29:1167–72. doi: 10.1016/j.vaccine.2010.12.014
35. Kohns Vasconcelos M, Loens K, Sigfrid L, Iosifidis E, Epalza C, Donà D, et al. Aetiology of acute respiratory infection in preschool children requiring hospitalisation in Europe-results from the PED-MERMAIDS multicentre case-control study. BMJ Open Respir Res. (2021) 8:e000887. doi: 10.1136/bmjresp-2021-000887
36. Rha B, Curns AT, Lively JY, Campbell AP, Englund JA, Boom JA, et al. Respiratory syncytial virus-associated hospitalizations among young children: 2015-2016. Pediatrics. (2020) 146:e20193611. doi: 10.1542/peds.2019-3611
37. Li X, Hodgson D, Flaig J, Kieffer A, Herring WL, Beyhaghi H, et al. Cost-effectiveness of respiratory syncytial virus preventive interventions in children: a model comparison study. Value Health. (2023) 26:508–18. doi: 10.1016/j.jval.2022.11.014
38. Huerta JL, Kendall R, Ivkovic L, Molina C, Law AW, Mendes D. Economic and clinical benefits of bivalent respiratory syncytial virus Prefusion F (RSVpreF) maternal vaccine for prevention of RSV in infants: a cost-effectiveness analysis for Mexico. Vaccines. (2025) 13:77. doi: 10.3390/vaccines13010077
39. Nazareno AL, Wood JG, Muscatello DJ, Homaira N, Hogan AB, Newall AT. Estimating the cost-effectiveness of maternal respiratory syncytial virus (RSV) vaccination in Australia: a dynamic and economic modelling analysis. Vaccine. (2025) 46:126651. doi: 10.1016/j.vaccine.2024.126651
40. Maltezou HC, Pelopidas Koutroumanis P, Kritikopoulou C, Theodoridou K, Katerelos P, Tsiaousi I, et al. Knowledge about influenza and adherence to the recommendations for influenza vaccination of pregnant women after an educational intervention in Greece. Hum Vaccin Immunother. (2019) 15:1070–4. doi: 10.1080/21645515.2019.1568158
41. Roy LMC. Deriving Health Utility Weights for Infants with Respiratory Syncytial Virus (RSV) (2013).
42. Janssen B, Szende A. Population norms for the EQ-5D. In:Szende A, Janssen B, Cabases J, , editors. Self-Reported Population Health: An International Perspective Based on EQ-5D. Dordrecht: Springer Netherlands (2014). p. 19–30. doi: 10.1007/978-94-007-7596-1_3
43. Greek Ministry of Health (2024). Drug Price Bulletin. Available online at: http://www.moh.gov.gr (Accessed December 7, 2024).
44. Greek Ministry of Health (2024). Diagnosis-related group (DRG). Available online at: http://www.moh.gov.gr (Accessed December 7, 2024).
45. National Organisation for Healthcare Services Provision (2024). Official web site of EOPYY. Available online at: https://www.eopyy.gov.gr/ (Accessed December 17, 2024).
46. Briggs AH, Schulpher CK. Decision Modelling for Health Economic Evaluation. UK: Oxford University Press (2006). doi: 10.1093/oso/9780198526629.001.0001
47. Shoukat A, Abdollahi E, Galvani AP, Halperin SA, Langley JM, Moghadas SM. Cost-effectiveness analysis of nirsevimab and maternal RSVpreF vaccine strategies for prevention of respiratory syncytial virus disease among infants in Canada: a simulation study. Lancet Reg Health Am. (2023) 28:100629. doi: 10.1016/j.lana.2023.100629
48. Getaneh AM, Li X, Mao Z, Johannesen CK, Barbieri E, van Summeren J, et al. Cost-effectiveness of monoclonal antibody and maternal immunization against respiratory syncytial virus (RSV) in infants: evaluation for six European countries. Vaccine. (2023) 41:1623–31. doi: 10.1016/j.vaccine.2023.01.058
49. Simões EAF, Pahud BA, Madhi SA, Kampmann B, Shittu E, Radley D, et al. Efficacy, safety, and immunogenicity of the MATISSE (Maternal Immunization Study for Safety and Efficacy) maternal respiratory syncytial virus prefusion F protein vaccine trial. Obstet Gynecol. (2025) 145:157–67. doi: 10.1097/AOG.0000000000005817
50. Kenmoe S, Chu HY, Dawood FS, et al. Burden of respiratory syncytial virus-associated acute respiratory infections during pregnancy. J Infect Dis. (2024) 229(Supplement_1): S51–60. doi: 10.2139/ssrn.4557035
51. Shattock AJ, Johnson HC, Sim SY, Carter A, Lambach P, Hutubessy RCW, et al. Contribution of vaccination to improved survival and health: modelling 50 years of the expanded programme on immunization. Lancet. (2024) 403:2307–16. doi: 10.1016/S0140-6736(24)00850-X
52. Rodrigues CMC, Plotkin SA. Impact of vaccines; health, economic and social perspectives. Front Microbiol. (2020) 11:1526. doi: 10.3389/fmicb.2020.01526
53. Talbird SE, Carrico J, La EM, Carias C, Marshall GS, Roberts CS, et al. Impact of routine childhood immunization in reducing vaccine-preventable diseases in the United States. Pediatrics. (2022) 150:e2021056013. doi: 10.1542/peds.2021-056013
54. Carrico J, Mellott CE, Talbird SE, Bento-Abreu A, Merckx B, Vandenhaute J, et al. Public health impact and return on investment of Belgium's pediatric immunization program. Front Public Health. (2023) 11:1032385. doi: 10.3389/fpubh.2023.1032385
55. Cook C, CJ, Talbird S, Gountas I, Skroumpelos A, Boutselakou E, et al. Public Health and Economic Impact of Pediatric Immunization Program In Greece. (2024). Available online at: https://www.rtihs.org/publications/public-health-and-economic-impact-pediatric-immunization-program-greece (Accessed December 1, 2024).
Keywords: respiratory syncytial virus, maternal vaccination, Greece, infants, preventive strategy
Citation: Gourzoulidis G, Solakidi A, Markatis E, Detsis M, Siahanidou T, Dimitriou G, Charitou A, Tzanetakos C, Mendes D and Barmpouni M (2025) Burden of respiratory syncytial virus disease in infants and the potential value of maternal immunization in Greece. Front. Public Health 13:1611483. doi: 10.3389/fpubh.2025.1611483
Received: 14 April 2025; Accepted: 30 June 2025;
Published: 16 July 2025.
Edited by:
Xiaozhen Lai, Peking University, ChinaReviewed by:
Ana Afonso, NOVA University of Lisbon, PortugalEva Piano Mortari, Bambino Gesù Children's Hospital (IRCCS), Italy
Copyright © 2025 Gourzoulidis, Solakidi, Markatis, Detsis, Siahanidou, Dimitriou, Charitou, Tzanetakos, Mendes and Barmpouni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: George Gourzoulidis, Z291cnpvdWxpZGlzQGh0ZS5ncg==; Z291cnpvdWxpZGlzLmdAZ21haWwuY29t
†ORCID: George Gourzoulidis orcid.org/0000-0002-7239-9829
 George Gourzoulidis
George Gourzoulidis Argyro Solakidi
Argyro Solakidi Eleftherios Markatis2
Eleftherios Markatis2 Tania Siahanidou
Tania Siahanidou Gabriel Dimitriou
Gabriel Dimitriou Charalampos Tzanetakos
Charalampos Tzanetakos