- 1Local Health Unit 3, Department of Prevention, Food Hygiene and Nutrition Service, Genoa, Italy
- 2Department of Infectious Diseases, Galliera Hospital, Genoa, Italy
- 3Department of Health Sciences, University of Genoa, Genoa, Italy
- 4Operating Unit Hospital Hygiene, Galliera Hospital, Genoa, Italy
Introduction: Hepatitis A virus (HAV) remains a significant foodborne pathogen, particularly when food handlers serve as the source of contamination. Its high infectivity and environmental persistence allow the virus to survive on hands, surfaces, and food, facilitating widespread transmission even from a single distribution point.
Methods: This systematic review, Prospero registration number: CRD420250651930, analyzed 32 studies reporting HAV outbreaks linked to food handlers to assess whether vaccination could be an effective preventive strategy.
Results: Most outbreaks occurred in North America and Europe, with index cases almost exclusively identified among food workers. Outbreak sizes varied, though the majority involved fewer than 50 cases.
Discussion: Studies highlighted critical challenges, including underreporting, asymptomatic cases, and delayed interventions. Control measures largely relied on immunoglobulin administration, while vaccination was rarely implemented and showed poor adherence among food service staff. Although economic analyses were limited and sometimes inconclusive, some evidence suggested potential healthcare savings from prevention efforts. Considering HAV’s high transmissibility and the difficulty of timely outbreak detection, targeted vaccination of food handlers—especially those in high-risk settings or seasonal employment—emerges as a promising method of biological risk management in food industries. These considerations could support food industries in considering vaccination as a tool to prevent foodborne HAV transmission.
1 Introduction
Hepatitis A, caused by the hepatitis A virus (HAV), is the most common form of acute viral hepatitis worldwide among types A, B, C, and E (1). HAV infection is globally widespread. According to the World Health Organization (WHO), there are approximately 159 million new HAV infections each year, resulting in around 1.5 million clinical cases and 39,000 deaths (2–4).
Hepatitis A may present as isolated (sporadic) cases or occur in the form of epidemics (5). Its incidence varies greatly between countries and is closely linked to factors such as socio-demographic index, hygiene, and sanitary conditions (6). While high-income countries generally maintain good hygiene standards, these remain insufficient in many low-and middle-income regions (3, 7, 8).
In developed countries, most adults are susceptible to HAV infection. Here, outbreaks are typically driven by interpersonal transmission within high-risk groups, whereas foodborne infections are more likely to cause sporadic cases (9). However several outbreaks in these regions have been associated with contaminated food (10, 11). This shift is likely influenced by increasing international travel and global food imports, which may alter the epidemiology of hepatitis A by facilitating both outbreaks in developed countries and global transmission of the virus (12, 13).
HAV is primarily transmitted through the ingestion of food or water contaminated with feces from an infected individual, or via direct contact with an infected person (14).
Even minimal quantities—such as 1,300 infectious units per gram of food—are sufficient to cause infection. This high transmission potential is partly due to the virus’s remarkable environmental stability. HAV can remain infectious in water, soil, and on contaminated surfaces (fomites).
Its persistence is further enhanced at low temperatures, which allows it to survive for extended periods in various food matrices, including leafy greens, carrots, fennel, green onions, spinach, berries, aromatic herbs, and shellfish. For instance, HAV has been found to survive for months on frozen berries and remain infectious on surfaces depending on temperature and humidity conditions (15, 16). Furthermore, under low humidity, it can persist on foods like lettuce, bell peppers, melon, and dried tomatoes.
To inactivate HAV thermally, cooking or boiling at a minimum of 85°C (185°F) for at least one minute is required. These characteristics allow HAV to remain viable throughout the entire food chain—from production to consumption—posing a risk of fecal contamination at any stage in what is known as the “farm to fork” pathway (17).
These findings confirm that HAV can persist on food long enough to threaten consumer health. While some hygiene treatments may help reduce viral load, none have proven completely effective in eliminating the virus (18).
The virus’s resistance to acidic pH enables it to reach the intestinal tract in an infectious form; it’s incubation period range from 15 to 50 days (19).
During this time, the hepatitis A virus (HAV) is excreted in large quantities in feces, reaching concentrations up to 1011 genome copies per gram just before symptom onset (20). After the appearance of jaundice, viral shedding decreases rapidly as anti-HAV antibodies develop. Nonetheless, infants and young children can continue shedding the virus for up to six months post-infection (21).
Food handlers play a crucial role in preventing HAV transmission. If infected, they can transmit the virus to susceptible individuals through the food they prepare (22) and have been identified as a major source of foodborne hepatitis A outbreaks (23). A single infected food handler can transmit the virus to dozens or even hundreds of individuals during food harvesting, handling, preparation, or distribution, significantly impacting public health and healthcare costs (24–26). The primary transmission route through food handlers is direct hand-to-food contact.
Throughout the food production chain, agricultural products undergo multiple stages of handling, increasing the risk of cross-contamination by infected workers or contaminated surfaces (20). Experimental studies have shown that HAV can maintain its infectivity on hands for at least four hours (27). Simply rinsing hands with water may reduce the viral load by 10 to 100 times but is insufficient for complete removal (18). Although HAV cannot replicate outside a host, such as in food and water, its low infectious dose poses a significant risk to consumers regardless of the contamination level (28).
Despite wide variation in the number of food handlers worldwide, their role in ensuring food safety is universally essential. Nevertheless, food safety strategies have generally prioritized environmental hygiene and sanitation over direct preventive measures such as vaccination. As of now, mandatory HAV vaccination for food handlers is enforced only in a few countries, such as Germany, while in most others it remains voluntary or merely recommended.
Establishing clear epidemiological links between foodborne HAV infections and specific contamination sources remains a major challenge. This difficulty stems from the global nature of food supply chains, where ingredients can originate from distant locations, be incorporated into numerous products, and become contaminated at very low levels. As a result, outbreaks are often detected too late to trace the source effectively. The foods most commonly implicated in outbreaks include shellfish, leafy greens, and both fresh and frozen fruits—particularly berries. However, due to the possibility of cross-contamination, virtually any food can be involved (20).
Therefore, understanding HAV endemicity and identifying the main sources of infection—both human and food-related—are crucial for developing targeted prevention strategies. In line with this, a recent ECDC report on hepatitis A prevention emphasizes the importance of collaboration between public health authorities and the food safety sector to help reduce the burden of foodborne infections (29).
Other narrative reviews from recent literature have examined foodborne HAV outbreaks and the central role of food handlers in transmission, highlighting the potential benefit of immunizing food workers. Such measures could enhance food safety in compliance with HACCP (Hazard Analysis and Critical Control Points) principles while also mitigating biological risk among food industry personnel (17).
The objective of this systematic review is to assess the impact of food handlers on foodborne hepatitis A (HAV) infections, examining the crucial role they play in transmitting the virus through food.
In line with PROSPERO registration standards, this review focuses on identifying and evaluating studies that explore the role of HAV vaccination in reducing the risk of transmission via food handlers. The main outcome of the review is to establish that HAV vaccination plays a significant role in preventing foodborne HAV infections transmitted through food handlers, in light of the current epidemiology of the virus.
2 Materials and methods
The study protocol was registered in PROSPERO (registration number: CRD420250651930). This review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, ensuring compliance with current standards for systematic review reporting. The research question was formulated using the PICO framework. The study population included individuals exposed to HAV infection, with a particular focus on food handlers. The primary outcomes assessed were the prevalence of HAV infection and its association with food handling practices.
2.1 Data sources and search strategy
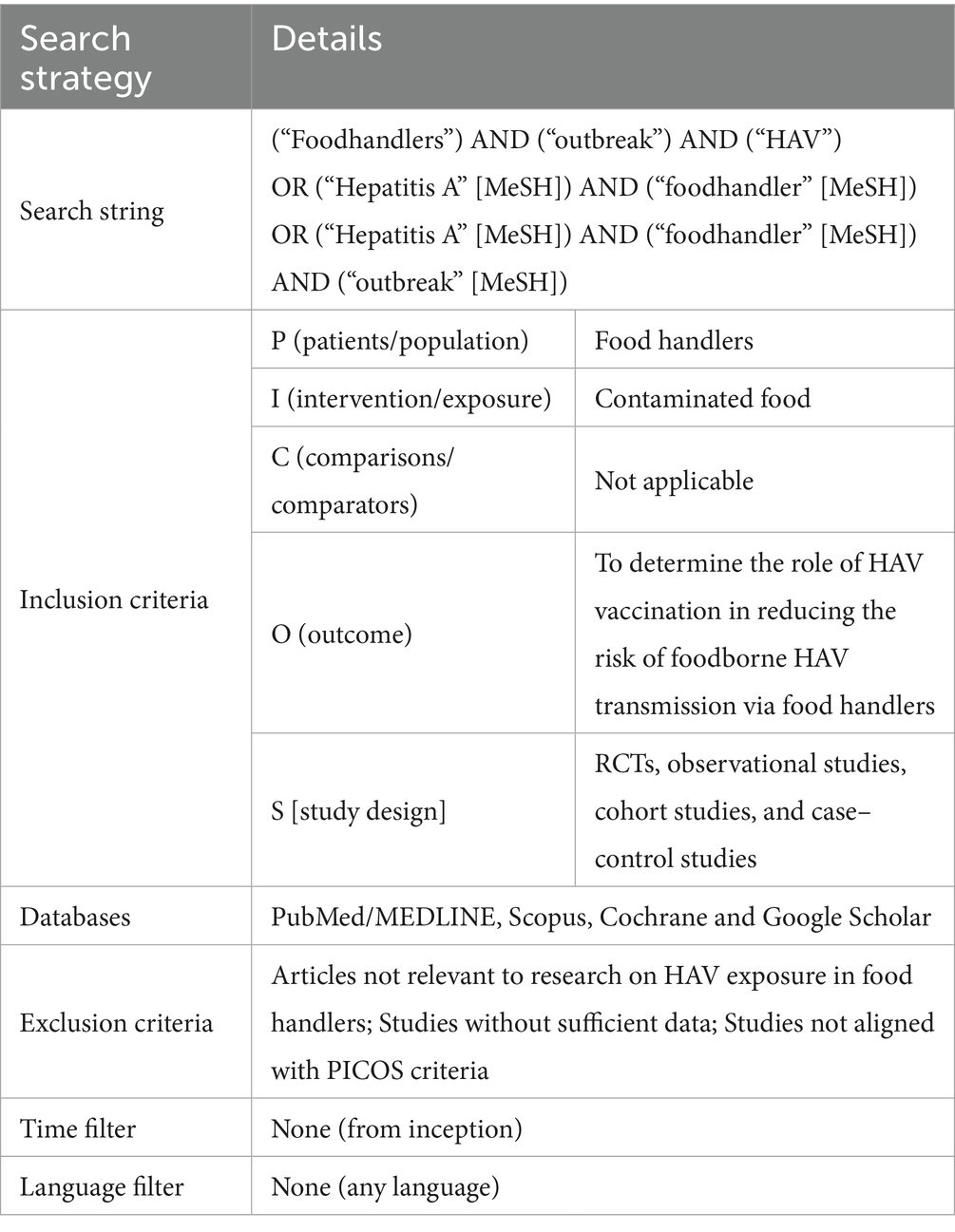
A comprehensive literature search was performed in the PubMed/MEDLINE, Scopus, Cochrane, and Google Scholar databases from inception up to December 2025 without language restriction. The search strategy included a combination of Medical Subject Headings [MeSH] terms and keywords: (“Hepatitis A” [MeSH] OR HAV) AND (“food handlers” [MeSH] OR “food contamination”) AND (“outbreak” [MeSH] OR “infection” OR “prevalence” OR “diagnosis”). MeSH terms were applied following the nomenclature and guidelines of the National Center for Biotechnology Information (NCBI).
2.2 Study selection
The inclusion criteria were:
1. Studies identifying a food handler as the index case and describing subsequent infection cases related to exposure to that individual.
2. Studies explicitly linking secondary infections to food handlers, emphasizing the risk of foodborne transmission.
The exclusion criteria were:
1. Studies not directly related to HAV exposure in food handlers.
2. Studies lacking sufficient data on HAV infection, prevalence, or diagnosis.
3. Studies not meeting the PICOS criteria
Studies not meeting these criteria were excluded. No restrictions were placed on publication date or language. For further details on the search strategy, see Table 1.
2.3 Data extraction and risk of bias assessment
The articles will initially be selected by screening the abstracts. Articles deemed relevant in this first phase will be read in full and further evaluated based on inclusion/exclusion criteria, study design, population characteristics, nutritional intervention, and primary outcomes. The selection process will be carried out independently by two reviewers. Their decisions will be recorded separately and compared. Any disagreements will be resolved through consensus; if necessary, a third reviewer will be consulted. A standardized data extraction form will be used to collect information on study design, population characteristics, type and dosage of supplements, primary and secondary outcomes, and follow-up duration.
The quality and potential risk of bias of the included studies will be assessed independently by four researchers using tools tailored to study type: Observational cohort and cross-sectional studies: NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Only one study was fair quality while the others were of medium quality.
In order to ensure scientific accuracy, a PRISMA flowchart was used to document the overall selection process (Figure 1).
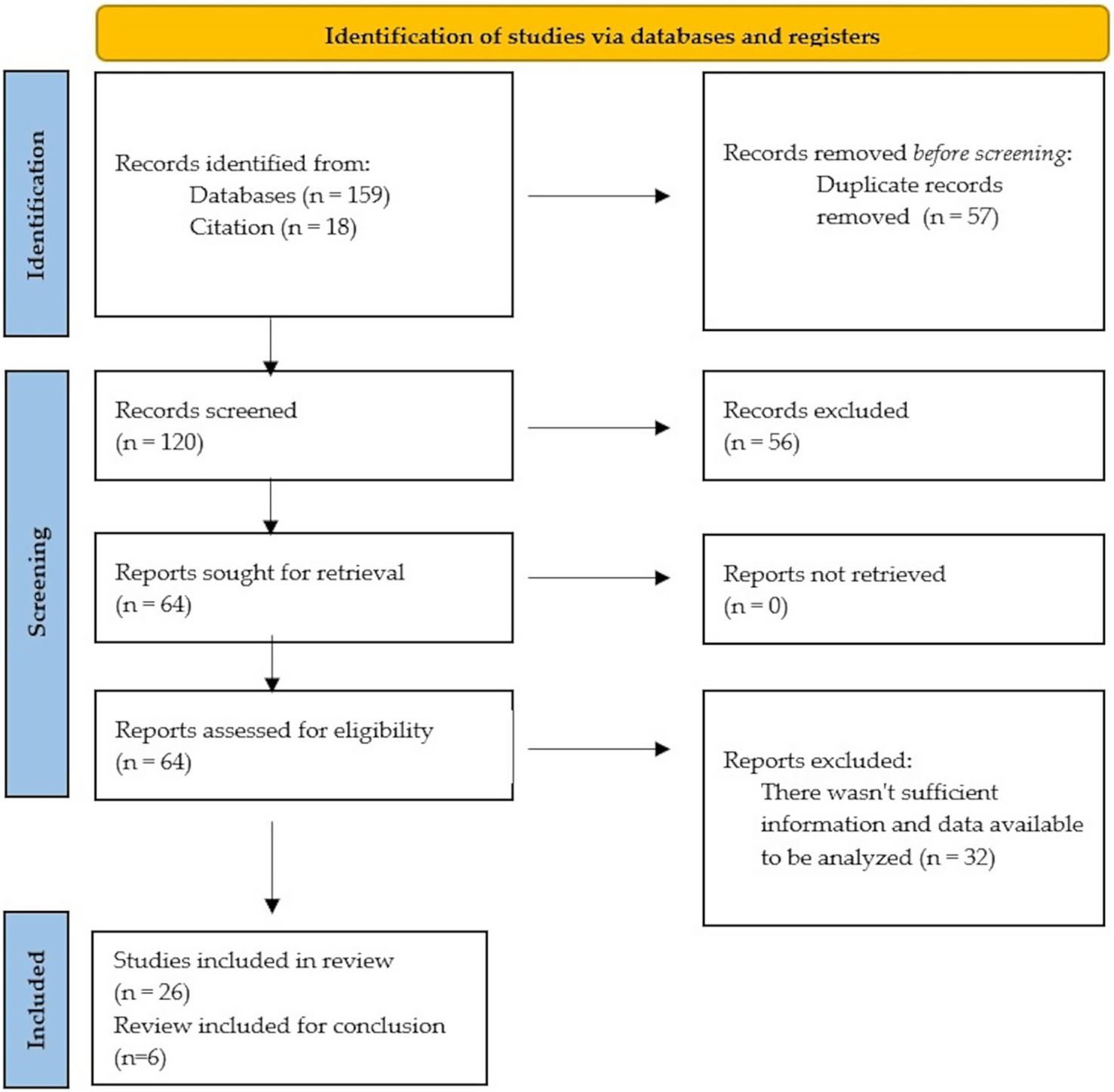
Figure 1. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only.
3 Results
3.1 Study selection and characteristics
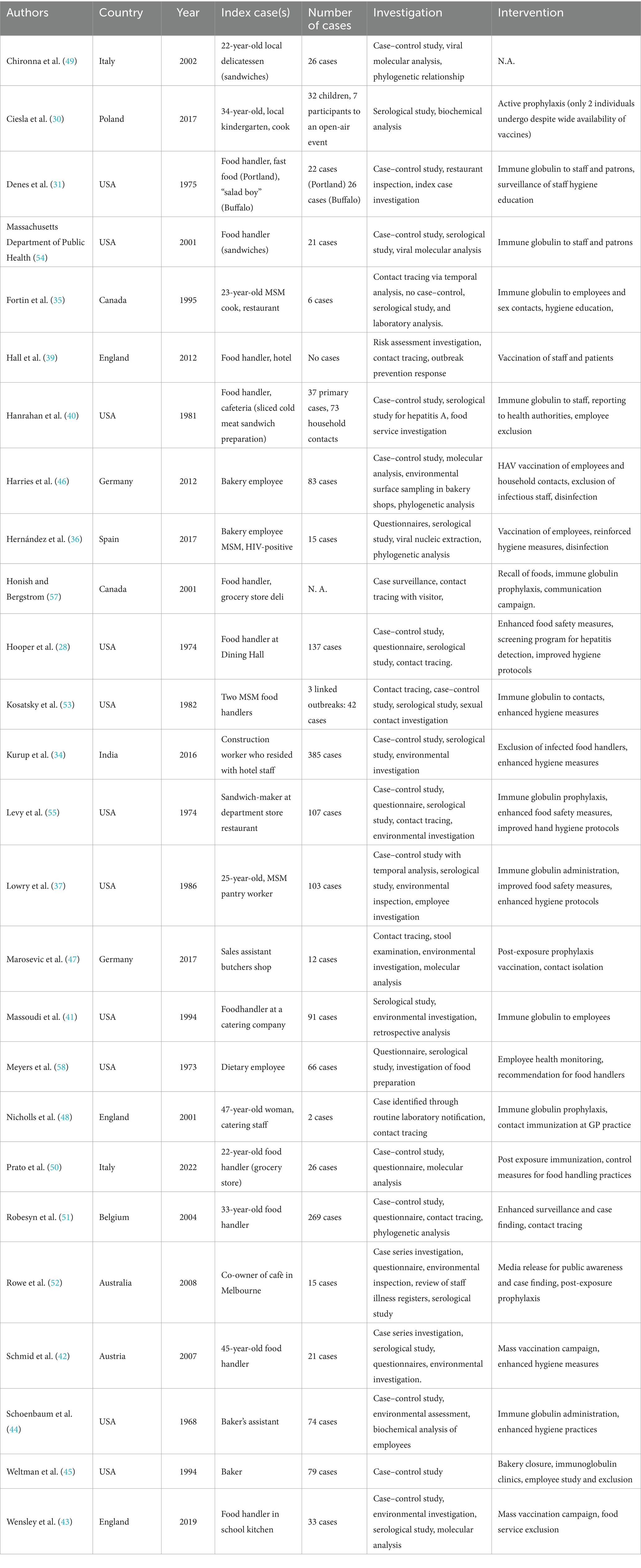
Of the 32 studies reviewed, 26 focus on specific outbreaks, while 6 are reviews. Most of the outbreak studies concentrated on single outbreaks, with only a few authors reporting consecutive outbreaks (30, 31). Among the 28 remaining studies, Tricco and Shenoy (24, 32) conducted reviews of multiple outbreaks (in Canada and India, respectively), Bidawid produced an experimental study (18), and Todd conducted a literature review (33).
Publication dates, which typically corresponded to the outbreak year or the following year, ranged from 1973 to 2020. Regarding geographical distribution, most studies were conducted in North America (15 papers) and Europe (11), with a minority in India (2) and Australia (1). Notably, studies reporting outbreaks in North America were predominantly published before 2000 (13 of 15), while all studies reporting European outbreaks were published after 2000 (11 of 11).
3.2 Index case characteristics
In almost all cases, the source patient was a food handler or someone who came into direct contact with food. In only one case (34), the index case was a construction worker who had nonetheless come into contact with food despite not being directly involved in the food production or distribution chain. Only three studies (35–37) reported the index case as MSM (men who have sex with men), and only Hernandez’s study reported HIV co-infection. However, it remains unclear whether other studies actually investigated these aspects of the source patient (comorbidities or sexual orientation), and therefore whether the relatively low rate of HIV co-infection or homosexuality reflects an actual absence of these factors in the “source” population or results from underreporting by the authors.
A summary of the studies reporting individual outbreaks with available data on the number of cases, country, and year is presented in Table 2.
3.3 Outbreak magnitude and reporting challenges
The number of direct or indirect infections and primary and secondary cases was reported by most authors, ranging from 0 (38) to 385 cases (34). In most published works, however, the number of infections was fewer than 50 (14 of the 26 studies reporting this data). It should be noted that many authors clearly identified potential underestimation of the number of exposed individuals among their studies’ limitations (31, 38–43), related to diagnostic challenges in asymptomatic or paucisymptomatic patients (44, 45) or difficulties in case tracking (36, 46).
3.4 Intervention strategies
Interventions primarily focused on administering immunoglobulins directed against HAV, with few cases favoring a vaccination-based approach.
Regarding vaccination, an interesting finding was that the acceptance and administration rates, when reported (30, 43, 47) were subt-optimal. Specifically, Wensley reported that the percentage of vaccinated school staff was between 56 and 60.6%, while the number of vaccinated students in primary and secondary schools was 94 and 74.5% respectively, despite a school outbreak of 33 confirmed HAV cases (43). While this may partly be attributed to the outbreak progression and not solely to anti-vaccination sentiment among staff, it is worth noting in a review context since adherence to protective measures is fundamental for containing HAV infections.
3.5 Immunoglobulin prophylaxis
Regarding immunoglobulin prophylaxis, this was administered in the study by Nicholls (to 486 of 725 exposed subjects) after a delay (3 weeks post-exposure), leading the author to suggest that vaccination would have been preferable (48). Fortin also reported delays between notification to health services and the start of immunoglobulin administration (35). Conversely, Hanrahan reported administering immunoglobulins relatively quickly (an average of 12.3 days after the onset of primary cases) (49). Denes reported that immunoglobulins were administered to 11,500 people across two outbreaks but noted that effectiveness was questionable due to the extended period between outbreak onset and the immunoglobulin administration campaign (31).
Delayed reporting was also highlighted by Prato (who argued that passive surveillance would have identified only a reduced number of secondary cases—16 of the 26 actually found) (50) and Lowry, who also reported issues related to MSM among the infected individuals, potentially “masking” other transmission routes (37).
In contrast, Honish reported the possibility of rapid identification and prompt immunoglobulin administration through the establishment of a telephone hotline, although the study’s limitations included difficulty in analyzing the campaign’s effectiveness (40).
3.6 Hygiene measures and multimodal approaches
Regarding inspection and strengthening of hygiene measures, many studies reported this system as an essential approach to pursue in combination with other control methods (28, 31, 35, 36, 39, 42) or as a standalone measure (37, 44, 45, 51, 52).
Nearly all authors employed a multimodal approach to their respective outbreaks, often focusing on a case–control methodology that enabled identification of the infection source in a relatively timely manner. In several cases (43, 47, 50, 51, 53, 54), authors utilized genomic typing to trace the outbreak progression. Massoudi and the Foodborne USA study reported that approaches based on interviews and self-assessment of hygiene practices might be ineffective due to subjectivity, leading to recall bias and data loss, thus implying the need for a multimodal approach (41, 55).
4 Discussion
The prevention of HAV infection through vaccination of food handlers remains an important question for public health protection. Epidemiological studies in literature are increasing in number and quality, with access to more data and information and the ability to utilize genetic sequencing techniques. Alternative but valid systems, such as computerized databases to analyze food consumption and processing, allow us to obtain valuable, important, and potentially real-time information on epidemic trends.
However, significant caveats remain in outbreak management: numerical underestimation (both in the number of outbreaks and in the number of cases within a single episode), the risk of recall bias for past events, and the time factor, which is fundamental in the epidemiological approach.
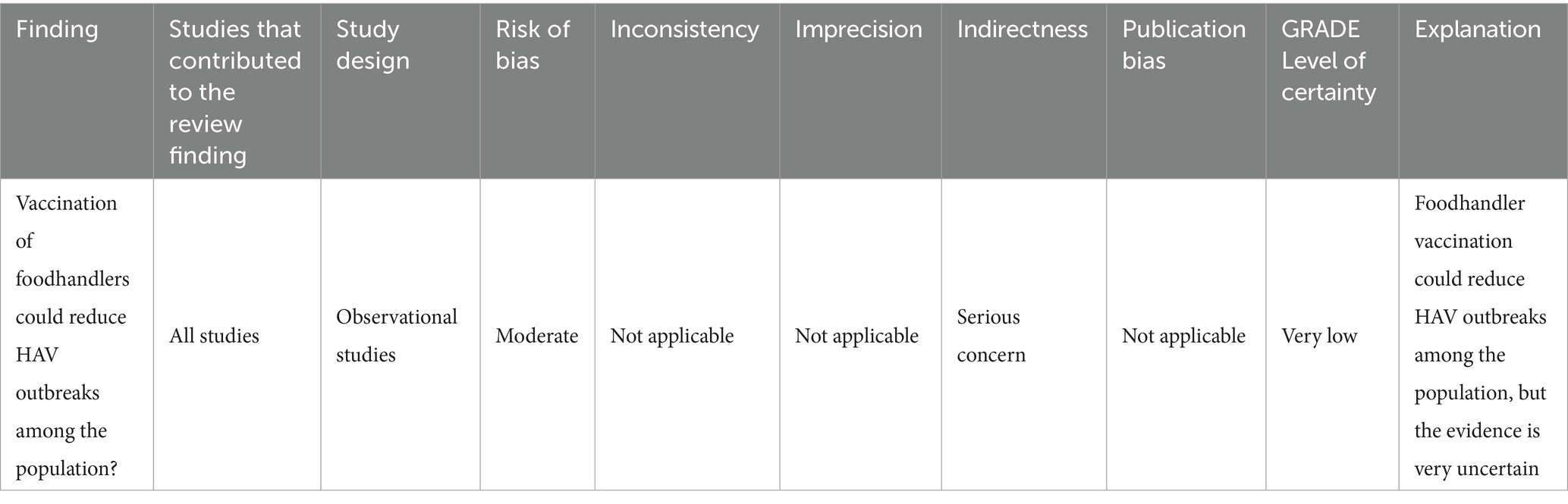
Observational studies dominate the literature and exhibit a moderate risk of bias. These studies often fail to systematically report vaccination data. Based on the GRADE methodology, these limitations translate into a very low certainty of evidence about recommendation for vaccination of food handlers against HAV. This rating reflects the overall quality of evidence, which is considered very low, and the presence of significant methodological and practical limitations that hinder a robust assessment of the intervention’s effectiveness (see Table 3).
Also noteworthy is adherence to control and prevention measures, which remains difficult to evaluate and often subject to biases related to staff self-assessment, and finally, food service personnel’s willingness to undergo vaccination.
The CDC suggests a cautious approach to vaccination, strongly recommending it for personnel at risk for other reasons (staff returning from travel to endemic areas or MSM). Finally, it should be emphasized that the current social climate leads many people to find temporary or even seasonal jobs in food service, thereby exponentially expanding the pool of individuals who should undergo HAV vaccination and making monitoring virtually impossible. It is therefore crucial to implement the education about food safety among both permanent and temporary food handlers.
The public health “one-size-fits-all” approach is therefore potentially burdened with problems, as is the approach tied to profiling MSM individuals or those who have traveled from endemic countries.
From the perspective of individual food business owners, the direct economic benefits of vaccinating their employees, although not immediately apparent—especially in regions with a low incidence of HAV—could be considered both to prevent business disruptions resulting from infectious episodes and to comply with major food safety regulations.
Specifically, the European Regulation EC852/2004 (Annex 2) requires that “…No person suffering from or being a carrier of a disease likely to be transmitted through food or afflicted, for example, with diarrhea is to be permitted to handle food or enter any food-handling area in any capacity if there is any likelihood of direct or indirect contamination. Any person so affected and employed in a food business and who is likely to come into contact with food is to report immediately the illness or symptoms, and if possible, their causes, to the food business operator (56).
In conclusion, although the literature suggests that HAV vaccination can be an effective tool for preventing outbreaks, employer and employee participation in vaccination within the food industry could be encouraged through public incentives or subsidies.
Further, the improvement of surveillance and reporting of HAV cases would be invaluable in order to provide better data for assessing the risk associated with food handlers and for informing targeted prevention strategies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
CT: Conceptualization, Validation, Writing – review & editing. FP: Validation, Writing – original draft, Writing – review & editing. CP: Validation, Writing – review & editing, Writing – original draft, Data curation. MR: Validation, Writing – original draft, Investigation. MS: Methodology, Data curation, Validation, Writing – original draft. MC: Writing – review & editing, Writing – original draft, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. World Health Organization. Global alert and response (GAR): hepatitis A. Available online at: http://www.who.int/csr/disease/hepatitis/whocdscsredc2007/en/index4.html#estimated (accessed January 13, 2025).
3. World Health Organization. WHO immunological basis for immunization series, module 18: hepatitis A update. Geneva: WHO (2019).
4. World Health Organization. WHO position paper on hepatitis a vaccines – October 2022. Wkly Epidemiol Rec (2022) 97: 493–512. Availalbe online at: https://www.who.int/publications/i/item/who-wer9740-493-512 (accessed January 13, 2025).
5. Cuthbert, JA. Hepatitis a: old and new. Clin Microbiol Rev. (2001) 14:38–58. doi: 10.1128/CMR.14.1.38-58.2001
6. Lai, M, and Chopra, S. Hepatitis A virus infection in adults: epidemiology, clinical manifestations, and diagnosis. UpToDate. Available online at: https://www.uptodate.com/contents/hepatitis-a-virus-infection-in-adults-epidemiology-clinical-manifestations-and-diagnosis (accessed January 13, 2025).
7. Cao, G, Jing, W, Liu, J, and Liu, M. The global trends and regional differences in incidence and mortality of hepatitis a from 1990 to 2019 and implications for its prevention. Hepatol Int. (2021) 15:1068–82. doi: 10.1007/s12072-021-10232-4
8. Zeng, D-Y, Li, J-M, Lin, S, Dong, X, You, J, Xing, Q-Q, et al. Global burden of acute viral hepatitis and its association with socioeconomic development status, 1990–2019. J Hepatol. (2021) 75:547–56. doi: 10.1016/j.jhep.2021.04.035
9. Migueres, M, Lhomme, S, and Izopet, J. Hepatitis a: epidemiology, high-risk groups, prevention and research on antiviral treatment. Viruses. (2021) 13:1900. doi: 10.3390/v13101900
10. Severi, E, Verhoef, L, Thornton, L, Guzman-Herrador, BR, Faber, M, Sundqvist, L, et al. Large and prolonged food-borne multistate Hepatitis a outbreak in Europe associated with consumption of frozen berries, 2013 to 2014. Euro Surveill. (2015) 20:21192. doi: 10.2807/1560-7917.es2015.20.29.21192
11. Garcia Vilaplana, T, Leeman, D, Balogun, K, Ngui, SL, Phipps, E, Khan, WM, et al. Hepatitis a outbreak associated with consumption of dates, England and Wales, January 2021 to April 2021. Euro Surveill. (2021) 26:2100432. doi: 10.2807/1560-7917.ES.2021.26.20.2100432
12. Jacobsen, KH. Globalization and the changing epidemiology of Hepatitis a virus. Cold Spring Harb Perspect Med. (2018) 8:a031716. doi: 10.1101/cshperspect.a031716
13. Gandhi, AP, Al-Mohaithef, M, Aparnavi, P, Bansal, M, Satapathy, P, Kukreti, N, et al. Global outbreaks of foodborne hepatitis a: systematic review and meta-analysis. Heliyon. (2024) 10:e28810. doi: 10.1016/j.heliyon.2024.e28810
14. Wenzel, JJ, and Allerberger, F. Hepatitis a as a foodborne infection. Lancet Infect Dis. (2014) 14:907–8. doi: 10.1016/S1473-3099(14)70897-7
15. Cook, N, Bertrand, I, Gantzer, C, Pinto, RM, and Bosch, A. Persistence of Hepatitis a virus in fresh produce and production environments, and the effect of disinfection procedures: a review. Food Environ Virol. (2018) 10:253–62. doi: 10.1007/s12560-018-9349-1
16. Roos, YH. Water and pathogenic viruses inactivation—food engineering perspectives. Food Eng Rev. (2020) 12:251–67. doi: 10.1007/s12393-020-09234-z
17. Fallucca, A, Restivo, V, Sgariglia, MC, Roveta, M, and Trucchi, C. Hepatitis a vaccine as opportunity of primary prevention for food handlers: a narrative review. Vaccine. (2023) 11:1271. doi: 10.3390/vaccines11071271
18. Bidawid, S, Farber, JM, and Sattar, SA. Contamination of foods by food handlers: experiments on Hepatitis a virus transfer to food and its interruption. Appl Environ Microbiol. (2000) 66:2759–63. doi: 10.1128/AEM.66.7.2759-2763.2000
19. European Centre for Disease Prevention and Control. Factsheet about hepatitis A (2017). Available online at: https://www.ecdc.europa.eu/en/hepatitis-A/facts (accessed January 13, 2025).
20. Randazzo, W, and Sánchez, G. Hepatitis a infections from food. J Appl Microbiol. (2020) 129:1120–32. doi: 10.1111/jam.14727
21. Centers for Disease Control and Prevention (CDC). Hepatitis A. In: CDC yellow book 2024: Health information for international travel. Available online at: https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/hepatitis-a#transmission (accessed January 13, 2025).
22. Sinha, A, and Dutta, S. Waterborne & foodborne viral hepatitis: a public health perspective. Indian J Med Res. (2019) 150:432–5. doi: 10.4103/ijmr.IJMR_1430_18
23. Fiore, AE. Hepatitis a transmitted by food. Clin Infect Dis. (2004) 38:705–15. doi: 10.1086/381671
24. Tricco, AC, Pham, B, Duval, B, De Serres, G, Gilca, V, Vrbova, L, et al. A review of interventions triggered by hepatitis a infected food-handlers in Canada. BMC Health Serv Res. (2006) 6:151. doi: 10.1186/1472-6963-6-157
25. Dalton, CB, Haddix, A, Hoffman, RE, and Mast, EE. The cost of a foodborne outbreak of hepatitis a in Denver, Colo. Arch Intern Med. (1996) 156:1013–6.
26. Lucioni, C, Cipriani, V, Mazzi, S, and Panunzio, M. Cost of an outbreak of hepatitis a in Puglia, Italy. Pharmacoeconomics. (1998) 13:257–66. doi: 10.2165/00019053-199813020-00008
27. Mbithi, JN, Springthorpe, VS, Boulet, JR, and Sattar, SA. Survival of hepatitis a virus on human hands and its transfer on contact with animate and inanimate surfaces. J Clin Microbiol. (1992) 30:757–63. doi: 10.1128/jcm.30.4.757-763.1992
28. Hooper, RR, Juels, CW, Routenberg, JA, Harrison, WO, Kilpatrick, ME, Kendra, SJ, et al. An outbreak of type a viral hepatitis at the naval training center, San Diego: epidemiologic evaluation. Am J Epidemiol. (1977) 105:148–55. doi: 10.1093/oxfordjournals.aje.a112367
29. European Centre for Disease Prevention and Control. Annual epidemiological report for 2022: hepatitis A. Stockholm: ECDC (2024).
30. Cieśla, A, Bociąga-Jasik, M, Sieklucki, J, and Pleśniak, R. Epidemiological investigation on hepatitis a virus infection outbreak in the area of Rzeszow City during the years 2017/18. Clin Exp Hepatol. (2020) 6:321–6. doi: 10.5114/ceh.2020.102176
31. Denes, AE, Smith, JL, Hindman, SH, Fleissner, ML, Judelsohn, R, Englender, SJ, et al. Foodborne hepatitis a infection: a report of two urban restaurant-associated outbreaks. Am J Epidemiol. (1977) 105:156–62. doi: 10.1093/oxfordjournals.aje.a112368
32. Shenoy, B, Andani, A, Kolhapure, S, Agrawal, A, and Mazumdar, J. Endemicity change of hepatitis a infection necessitates vaccination in food handlers: an Indian perspective. Hum Vaccin Immunother. (2022) 18:1868820. doi: 10.1080/21645515.2020.1868820
33. Todd, EC, Greig, JD, Bartleson, CA, and Michaels, BS. Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 4. Infective doses and pathogen carriage. J Food Prot. (2008) 71:2339–73. doi: 10.4315/0362-028x-71.11.2339
34. Kurup, KK, Manickama, P, and Gurav, Y. Infected food handlers led to an outbreak of hepatitis a in Ernakulam district, Kerala, southern India, 2016. Clin Epidemiol Glob Health. (2020) 8:308–12. doi: 10.1016/j.cegh.2019.08.001
35. Fortin, A, and Milord, F. Hepatitis a in restaurant clientele and staff—Quebec. Can Commun Dis Rep. (1998) 24:53–9.
36. Hernández, E, De Castro, V, Avellón, A, González, I, Muniozgurena, N, Vázquez, S, et al. Hepatitis a outbreak associated with a food handler in Bizkaia, 2017. Enferm Infecc Microbiol Clin. (2019) 37:569–73. doi: 10.1016/j.eimc.2019.01.011
37. Lowry, PW, Levine, R, Stroup, DF, Gunn, RA, Wilder, MH, and Konigsberg, C. Hepatitis a outbreak on a floating restaurant in Florida, 1986. Am J Epidemiol. (1989) 129:155–64. doi: 10.1093/oxfordjournals.aje.a115104
38. Centers for Disease Control and Prevention (CDC). Foodborne hepatitis A—Missouri, Wisconsin, and Alaska, 1990–1992. MMWR Morb Mortal Wkly Rep. (1993) 42:526–34.
39. Hall, V, Abrahams, A, Turbitt, D, Cathcart, S, Maguire, H, and Balasegaram, S. No evidence of transmission from an acute case of hepatitis a in a food handler: follow-up of almost 1,000 potentially exposed individuals, London, United Kingdom, April 2012. Euro Surveill. (2014) 19:20865. doi: 10.2807/1560-7917.es2014.19.30.20865
40. Hanrahan, JP, Zimmerman, KL, Toly, MH, Prowda, RL, Grabau, JC, and Morse, DL. An outbreak of hepatitis a linked to a food handler in a cafeteria. N Y State J Med. (1984) 84:10–3.
41. Massoudi, MS, Bell, BP, Paredes, V, Insko, J, Evans, K, and Shapiro, CN. An outbreak of hepatitis a associated with an infected food handler. Public Health Rep. (1999) 114:157–64. doi: 10.1093/phr/114.2.157
42. Schmid, D, Fretz, R, Buchner, G, König, C, Perner, H, Sollak, R, et al. Foodborne outbreak of hepatitis a, November 2007–January 2008, Austria. Eur J Clin Microbiol Infect Dis. (2009) 28:385–91. doi: 10.1007/s10096-008-0633-0
43. Wensley, A, Smout, E, Ngui, SL, Balogun, K, Blomquist, P, Edelstein, M, et al. An outbreak of hepatitis a virus infection in a secondary school in England with no undetected asymptomatic transmission among students. Epidemiol Infect. (2022) 151:e6. doi: 10.1017/S095026882200190X
44. Schoenbaum, SC, Baker, O, and Jezekgand, Z. Common-source epidemic of hepatitis due to glazed and iced pastries. Am J Epidemiol. (1976) 104:74–80. doi: 10.1093/oxfordjournals.aje.a112275
45. Weltman, AC, Bennett, NM, Ackman, DA, Misage, JK, Campana, JJ, Fine, LS, et al. An outbreak of hepatitis a associated with a bakery. New York, 1994: the 1968 west branch, Michigan outbreak repeated. Epidemiol Infect. 117:333–41. doi: 10.1017/s0950268800001515
46. Harries, M, Monazahian, M, Wenzel, J, Jilg, W, Weber, M, Ehlers, J, et al. Foodborne hepatitis a outbreak associated with bakery products in northern Germany, 2012. Euro Surveill. (2014) 19:20992. doi: 10.2807/1560-7917.es2014.19.50.20992
47. Marosevic, D, Belting, A, Schönberger, K, Carl, A, Wenzel, JJ, and Brey, R. Hepatitis a outbreak in the general population due to a MSM-associated HAV genotype linked to a food handler, November 2017–February 2018, Germany. Food Environ Virol. (2019) 11:149–56. doi: 10.1007/s12560-019-09375-3
48. Nicholls, M, Bruce, M, and Thomas, J. Management of hepatitis a in a food handler at a London secondary school. Commun Dis Public Health. (2003) 6:26–9.
49. Chironna, M, Lopalco, P, Prato, R, Germinario, C, Barbuti, S, and Quarto, M. Outbreak of infection with hepatitis a virus (HAV) associated with a food handler and confirmed by sequence analysis reveals a new HAV genotype IB variant. J Clin Microbiol. (2004) 42:2825–8. doi: 10.1128/JCM.42.6.2825-2828.2004
50. Prato, R, Lopalco, PL, Chironna, M, Germinario, C, and Quarto, M. An outbreak of hepatitis a in southern Italy: the case for vaccinating food handlers. Epidemiol Infect. (2006) 134:799–802. doi: 10.1017/S0950268805005388
51. Robesyn, E, De Schrijver, K, Wollants, E, Top, G, Verbeeck, J, and Van Ranst, M. An outbreak of hepatitis a associated with the consumption of raw beef. J Clin Virol. (2009) 44:207–10. doi: 10.1016/j.jcv.2008.12.012
52. Rowe, SL, Tanner, K, and Gregory, JE. Hepatitis a outbreak epidemiologically linked to a food handler in Melbourne, Victoria. Commun Dis Intell Q Rep. (2009) 33:46–8. doi: 10.33321/cdi.2009.33.10
53. Kosatsky, T, and Middaugh, JP. Linked outbreaks of hepatitis a in homosexual men and in food service patrons and employees. West J Med. (1986) 144:307–10.
54. Centers for Disease Control and Prevention (CDC). Foodborne transmission of hepatitis A—Massachusetts, 2001. MMWR Morb Mortal Wkly Rep. (2003) 52:565–7.
55. Levy, BS, Fontaine, RE, Smith, CA, Brinda, J, Hirman, G, Nelson, DB, et al. A large food-borne outbreak of hepatitis a. possible transmission via oropharyngeal secretions. JAMA. (1975) 234:289–94.
56. European Parliament and Council Regulation (EC). No 852/2004 of 29 April 2004 on the hygiene of foodstuffs. (2006). Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:02004R0852-20210324 (Accessed January 13, 2025).
57. Honish, L, and Bergstrom, K. Hepatitis a infected food handler at an Edmonton, Alberta retail food facility: public health protection strategies. Can Commun Dis Rep. (2001) 27:177–80.
Keywords: hepatitis A, outbreak, food, food handlers, vaccination, public health
Citation: Trucchi C, Del Puente F, Piccinini C, Roveta M, Sartini M and Cristina ML (2025) Determining the burden of foodborne hepatitis A spread by food handlers: suggestions for a targeted vaccination? Front. Public Health. 13:1617004. doi: 10.3389/fpubh.2025.1617004
Edited by:
Silvio Tafuri, University of Bari Aldo Moro, ItalyReviewed by:
Andreu Comas-Garcia, Universidad Cuauhtémoc San Luis Potosí, MexicoAshenafi Berhanu, Haramaya University, Ethiopia
Copyright © 2025 Trucchi, Del Puente, Piccinini, Roveta, Sartini and Cristina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Filippo Del Puente, ZmlsaXBwby5kZWwucHVlbnRlQGdhbGxpZXJhLml0
†These authors have contributed equally to this work and share first authorship
 Cecilia Trucchi1†
Cecilia Trucchi1† Filippo Del Puente
Filippo Del Puente Carolina Piccinini
Carolina Piccinini Marina Sartini
Marina Sartini Maria Luisa Cristina
Maria Luisa Cristina