- 1Department of Clinical Laboratory, Aerospace Center Hospital, Beijing, China
- 2Peking University Fifth School of Clinical Medicine, Beijing, China
- 3Department of Pathology, Aerospace Center Hospital, Beijing, China
- 4Department of Literature and Science, University of Wisconsin-Madison, Madison, WI, United States
- 5Department of Gynecology, Aerospace Center Hospital, Beijing, China
Background: The present study aimed to assess the prevalence and genotype distribution of Human papillomavirus (HPV) in Beijing females.
Methods: A total of 73,697 subjects [both Department of Gynecology (n = 35,666) and Physical Examination center (n = 38,031)] were retrieved between June 2014 and December 2023. HPV testing was performed by Tellgenplex® HPV-27 DNA genotyping Test system.
Results: An overall HPV infection rate of 17.28% (12,736/73,697) over the past decade (2014 to 2023), with a significant decrease in annual incidence (p < 0.001). HPV-52, 16, and 58 were predominant in high-risk types, while HPV-61, 81, and 43 were the most prevalent in low-risk HPV types. Age-specific patterns revealed bimodal infection peaks in individuals under 21 and between 51 ~ 56 years. Furthermore, a significant increase in HPV infection rates was observed with the progression of pathological severity (p < 0.001).
Conclusion: This study emphasizes the importance of HPV infection rate from real word and developing vaccines specifically for the Chinese population. In the future, it is necessary to design more suitable vaccines based on Chinese HPV type ranking, especially for HR-HPV (HPV-39, HPV-53, and HPV-56) and LR-HPV (HPV-43, HPV-61, and HPV-81).
1 Introduction
Cervical cancer continues to rank among the top gynecologic cancers worldwide. According to current data, it is ranked as the 4th most common cancer among women worldwide (1). In 2022, cervical cancer was the eighth most prevalent cancer, with 661,021 new cases reported globally (2). In China, cervical cancer ranked sixth among malignant tumors in 2022, with 150,700 incident cases (3). Persistent infection by high-risk human papillomavirus (HR-HPV) served as the major risk factors (4, 5). While low-risk (LR-HPV) infection has been associated with conditions like plantar warts, genital warts, and hand lesions (6, 7).
Vaccination is a primary preventive measure against cervical cancer (8). The World Health Organization (WHO) aims to eliminate cervical cancer by 2030 through the 90–70-90 strategy (9). The bivalent vaccine has shown 90% efficacy in protecting against HPV-16 and HPV-18 (5). The quadrivalent HPV vaccine showed an 88% reduction in cervical cancer incidence among those vaccinated before age 17 (10). Additionally, the nine-valent HPV vaccine could potentially provide broader coverage and prevent 90% of cervical cancer cases (11). By now, the available HPV vaccines in China were mainly invented using epidemiological data only from western countries (12). However, the prevalence of HPV infection and genotype distribution varies between countries and regions (13). Which highlighting the importance of analyzing baseline HPV infection data in different regions to evaluate vaccine effectiveness and adjust cancer prevention strategies.
Through literature search, in China, most studies on HPV infection rates mainly focused on the gynecological patients (14, 15), while the HPV infection data in healthy individuals is relatively lacking, as a consequence, we believe that this will significantly overestimate HPV infection rates in China. Similarly, we found that HPV infection survey in the Beijing also mostly come from gynecological patients (4, 16), which also be confront of the selection bias problem. Therefore, it is of great necessary to conduct a new epidemiological survey on HPV infection in Beijing, which should include both gynecological and health personnel. Secondary prevention measure for Cervical cancer including HPV screening and cytological examinations (8). Most screening and diagnostic efforts are directed toward the early identification of HR-HPV and Papanicolaou (Pap) smears (1).
The present study aimed to achieve the following work. First, the prevalence and genotype distribution of HPV will be surveyed, importantly, all participants not only from Department of Gynecology but also Physical Examination (PE) center, therefore, the prevalence estimation about HPV is closer to the real world in general population. Second, to compare of HPV positive rates in different pathological grades based on ThinPrep cytologic test (TCT) results.
2 Methods
2.1 Patients and sample collection
This retrospective study was approved by the Institutional Review Board (IRB) of Aerospace Center Hospital. A total of 127,363 HPV results were retrospectively retrieved from laboratory information system (LIS) of Aerospace Center Hospital (monocentric) between June 2014 and December 2023. The first time HPV detection of all participants in our center were included, while the second or more HPV tests for the same participants were excluded (n = 53,166). Besides, the male subjects were also excluded (n = 500). Finally, a total of 73,697 female subjects from both the Department of Gynecology (n = 35,666) and PE center (n = 38,031) were included. Patients with menstrual problems, abnormal leucorrhea, etc. visited Gynecology Department, while participants only for health check-up were admitted to PE center (Figure 1).
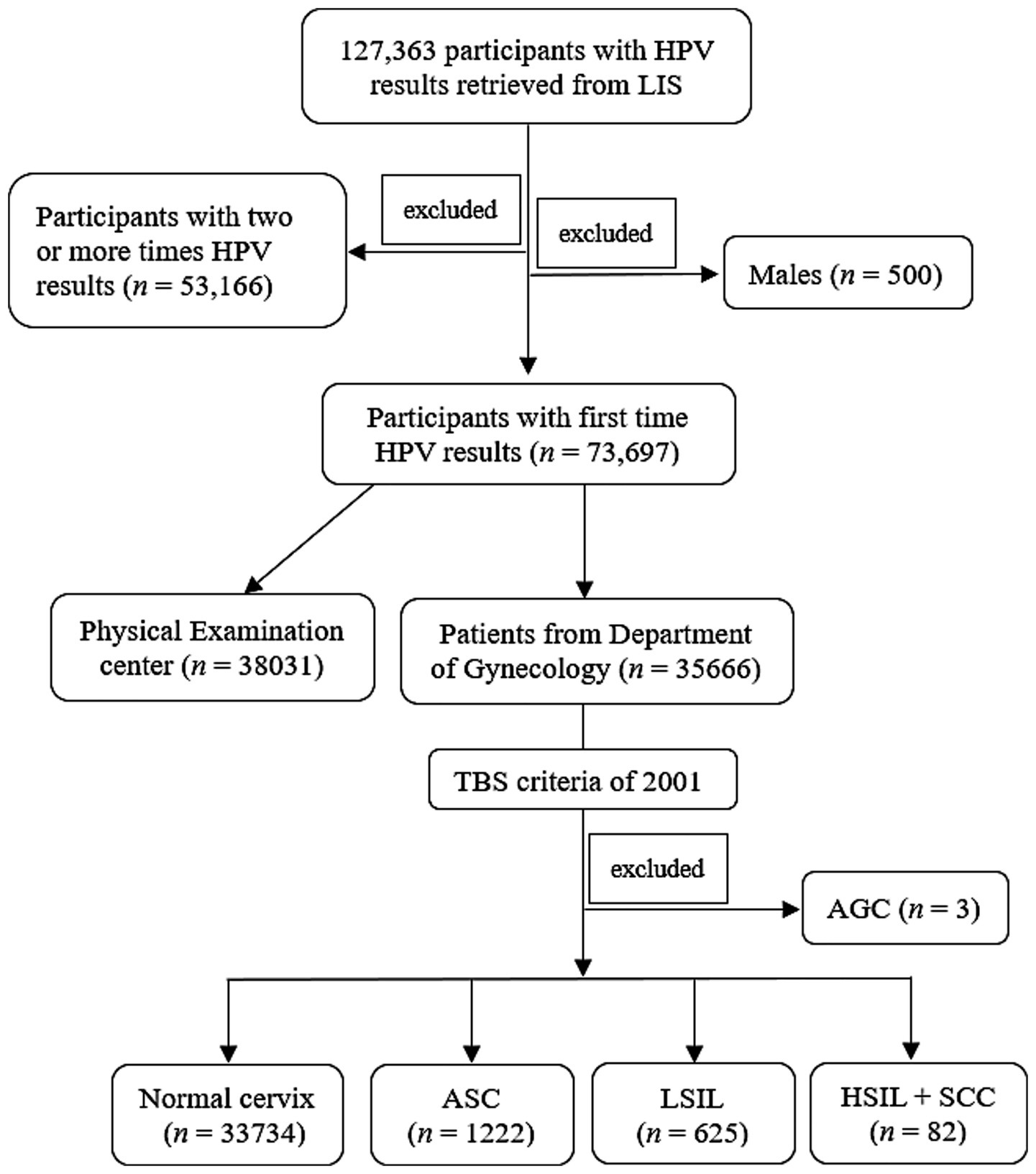
Figure 1. Study schematic. LIS, laboratory information system; TBS, The Bethesda System; AGC, atypical glandular cells; ASC, atypical squamous cells; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; SCC, squamous cell carcinoma.
To ensure the accuracy of the test results, all participants were advised to refrain from using vaginal medications or engaging in sexual intercourse within 3 days prior to testing. During specimen collection, clinical physicians use a specialized cervical brush to collect exfoliated cells, which were then placed into an elution tube containing cell preservation solution and sealed for storage. For gynecological patients, two sets of specimens were routinely collected, one for HPV testing, and another set for TCT.
2.2 HPV-DNA genotype testing
HPV testing was performed by Tellgenplex® HPV27 DNA genotyping Test system (Tellgen Corporation, Shanghai). The experimental protocol includes DNA extraction, multiple PCR amplification, bead-coated hybridization, and digital signal processing (Luminex 200TM, Thermo Fisher) (12). The totaling detectable HPV types was 27, including LR-HPV types (6, 11, 40, 42, 43, 44, 55, 61, 81, 83) and HR-HPV types (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 82). The mixed HPV infection means co-infection with both LR-HPV and HR-HPV.
2.3 TCT determination
According to The Bethesda System (TBS) of 2001 (17), TCT results were mainly divided into two major parts: Negative for Intraepithelial Lesion or Malignancy and Epithelial Cell Abnormalities, subsequently, the abnormal part was further classified according to the TBS criteria. In the present research, there were only three patients with atypical glandular cells (AGC), who were then excluded when performing TCT analysis. Finally, TCT was divided into five categories: (a) Negative for Intraepithelial Lesion or Malignancy; (b) Atypical squamous cells (ASC) of undetermined significance (ASC-US) or cannot exclude HSIL (ASC-H); (c) low-grade squamous intraepithelial lesion (LSIL), encompassing cervical intraepithelial neoplasia (CIN) 1; (d) High-grade squamous intraepithelial lesion (HSIL), encompassing CIN II and CIN III, and (e) Squamous cell carcinoma (SCC). Due to the small number of HSIL and SCC subgroup, which were then incorporated into the HSIL+SCC group (Figure 1). All TCT results were independently reviewed by at least two experienced pathologists. In case of discordance between the two pathologists’ interpretations, the joint review will be performed to resolved discrepancies.
2.4 Statistical analysis
Statistical analyses were conducted using SPSS software (version 20.0; IBM Corporation, Armonk, NY, USA). Chi-square tests or Fisher’s exact tests were applied when comparing HPV infection positivity rates across different years or age groups, as appropriate. The linear-by-linear association test and gamma value were utilized to evaluate the changes in HPV prevalence across the years and age groups. GraphPad Prism 16 was employed for data visualization, with cumulative frequencies used for calculating HPV types. Statistical significance was set at p < 0.05.
3 Results
3.1 The overall prevalence of HPV infection
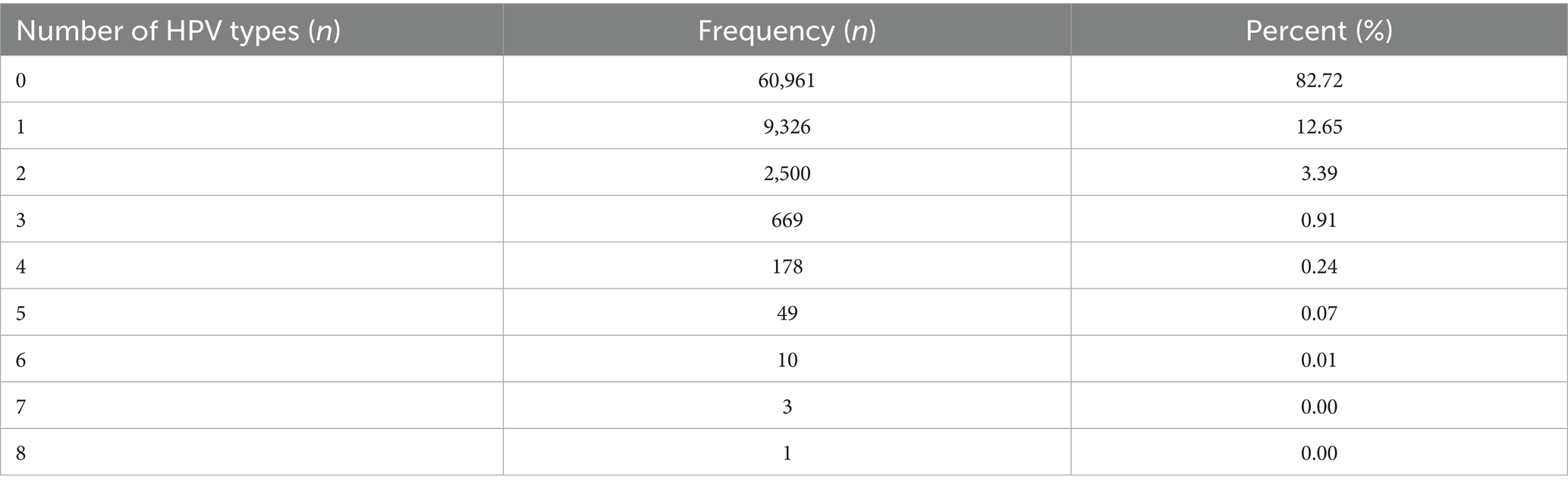
For the included 73,697 participants, the average age is 42.88 ± 12.88 years. There were 12,736 female subjects confirmed to be infected by HPV with the infection rate of 17.28% (95% CI: 16.89–17.67%). The single and multiple HPV infection rate were 12.65% (95% CI: 12.39–12.91%) (9,326/73697) and 4.63% (95% CI: 4.47–4.79%) (3,410/73697), respectively (Table 1).
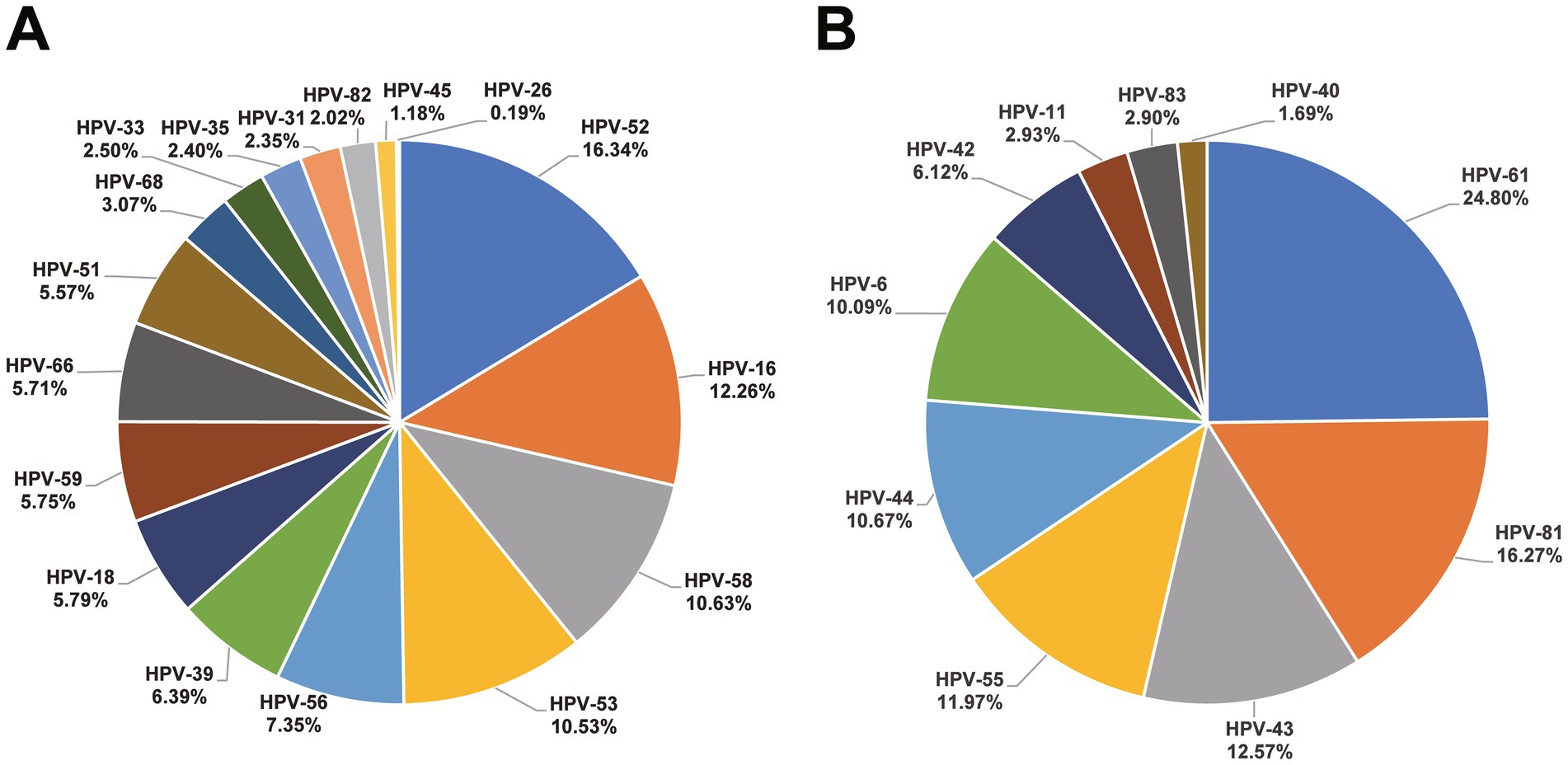
A total of positive 17,379 HPV results (including 12,233 HR-HPV and 5,146 LR-HPV) were detected among these 12,736 HPV infected subjects. HPV-52 (16.34%), HPV-16 (12.26%), and HPV-58 (10.63%) were the most common in HR-HPV, as for LR-HPV, HPV-61 (24.80%), HPV-81 (16.27%), and HPV-43 (12.57%) were predominant. The distribution of HPV types is depicted in Figure 2. Additionally, in the HR-HPV group, the proportion of single infections (7,910/9852, 80.29%) exceeds that of multiple infections (infected by two or more HPV types) (1942/9852, 19.71%). As for LR-HPV group, single infections (4,032/4561, 88.40%) surpass multiple infections in the same way (529/4561, 11.60%). A total of 1,677 subjects infected were infected with both HR-HPV and LR-HPV, the mixed infection rate was 2.28%.
Separately, in the PE group, the infection rate was 13.17% (95% CI: 12.81–13.53%) (5,007/38031), while in the patient group, it was 21.67% (95% CI: 21.19–22.15%) (7,729/35666), the HPV infection rate between the two groups reached a statistical significance (χ2 = 931.317, p < 0.001).
3.2 Distribution of HPV infection rates among different years
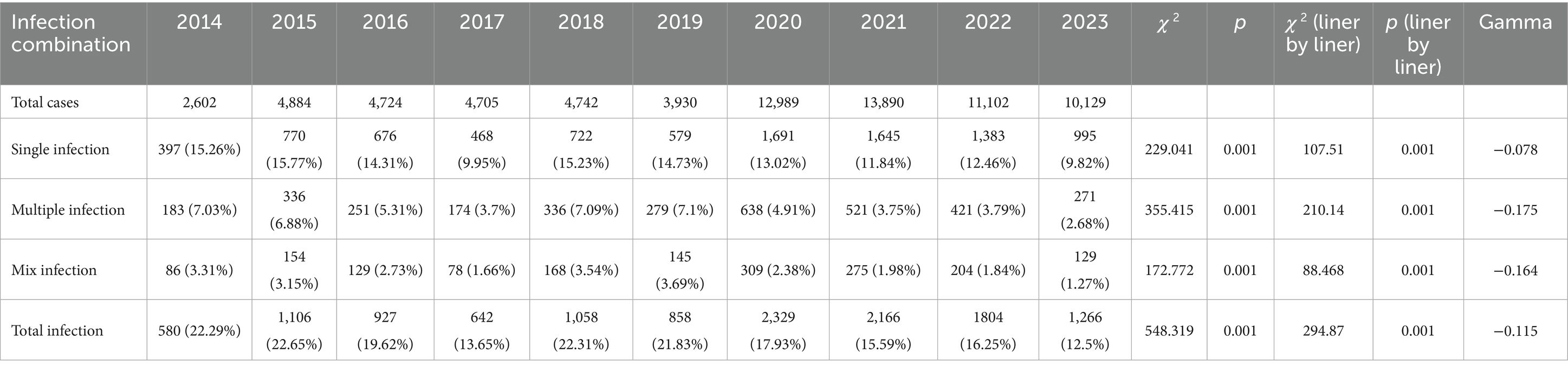
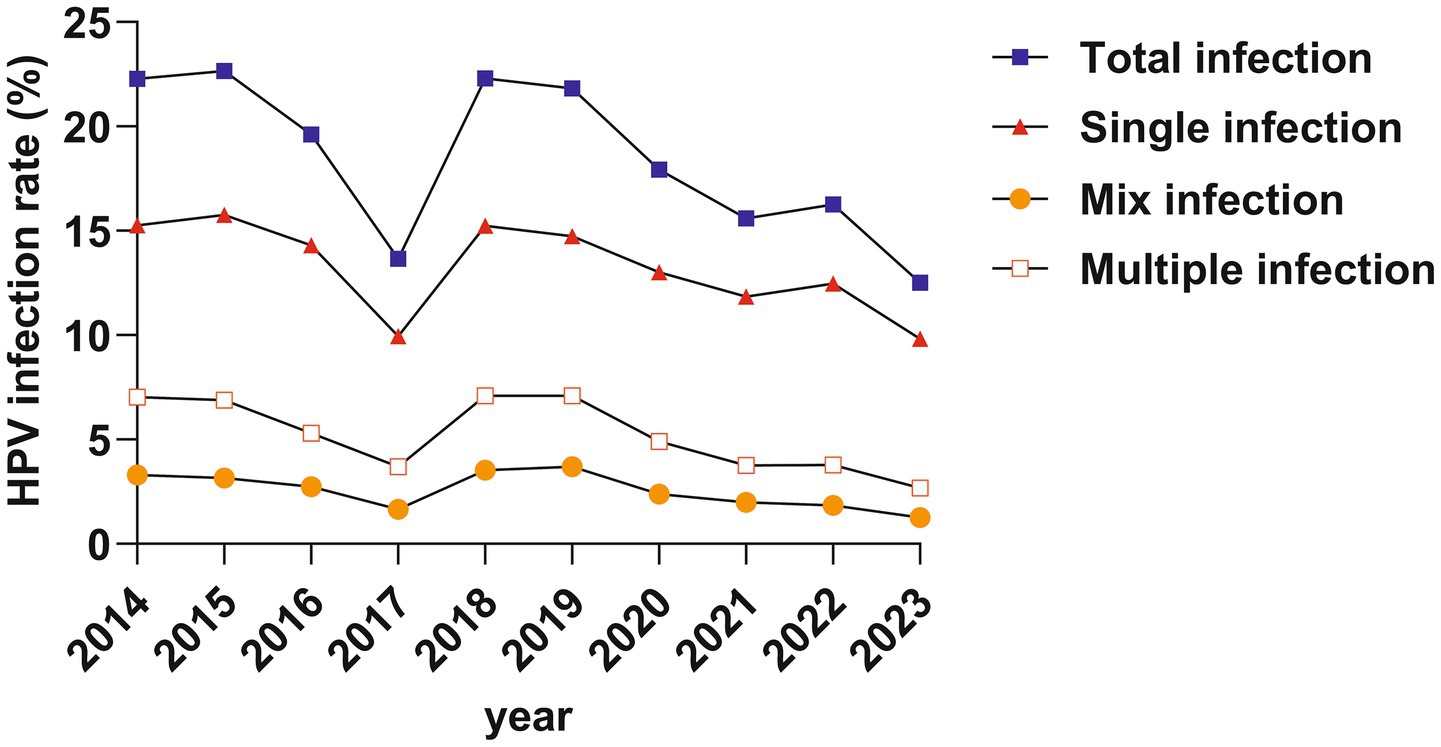
The infection rates varied significantly over the years (all p < 0.001). Over the past decade, there has been a downward trend (All gamma values are negative) in total infection, single infection, multiple infection, and mixed infection (infected by both LR-HPV and HR-HPV) (all p < 0.001) (Table 2; Figure 3).
3.3 Prevalence of HPV infection in different age groups
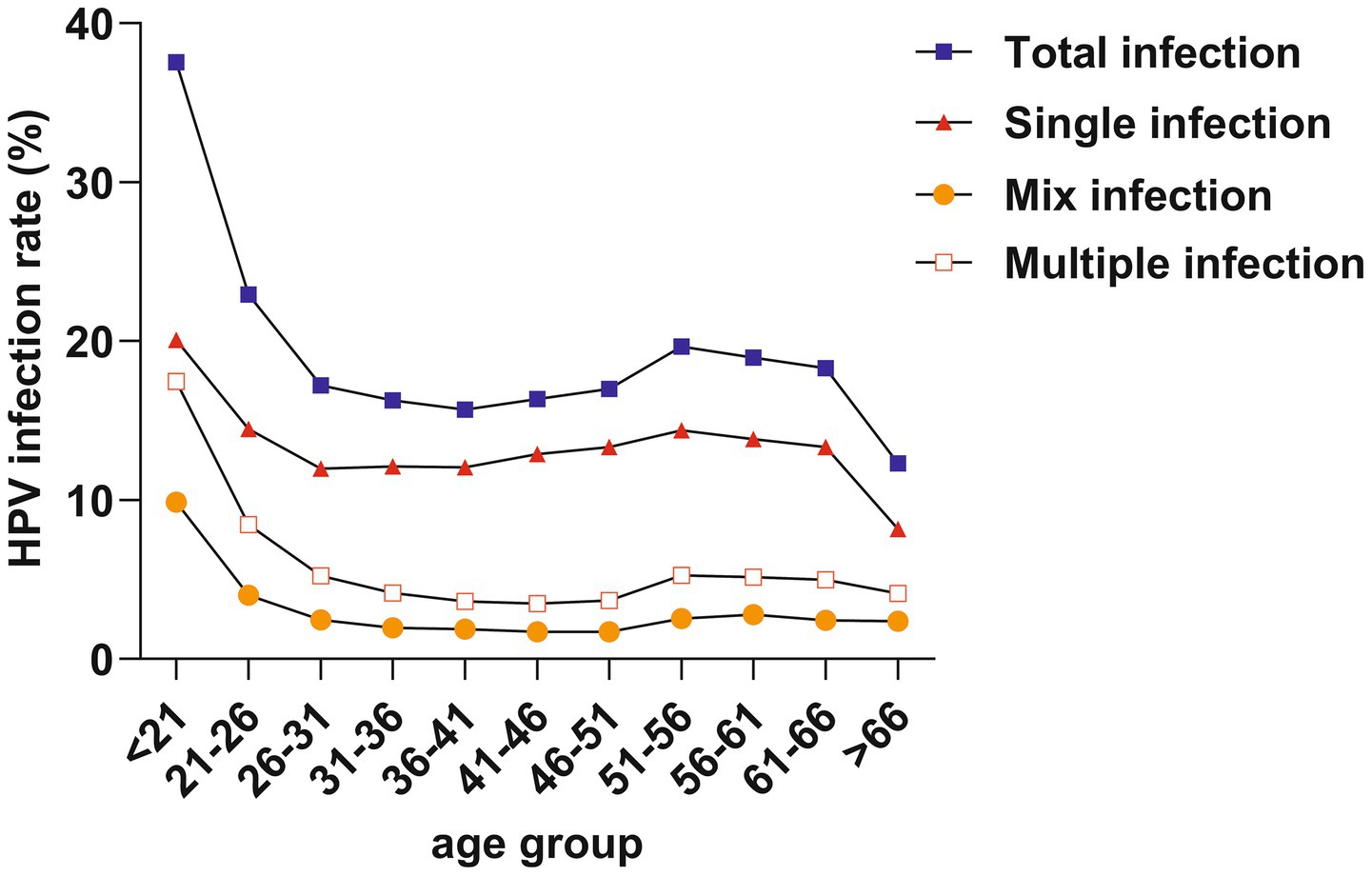
We divided the ages into 11 subgroups, starting from 21 years old, with each group spanning 5 years. The total infection rate exhibited a bimodal distribution, with the first peak at < 21 years (37.55%) and a gradual decline followed by a second peak at 51–56 years (19.64%). Furthermore, trends in single infection, multiple infection, and mixed infection mirrored those of total infection, with the first peaks at < 21 years old and a second peak at 51–56 years old (Table 3; Figure 4).
3.4 Prevalence of HPV infection rate among different TCT groups
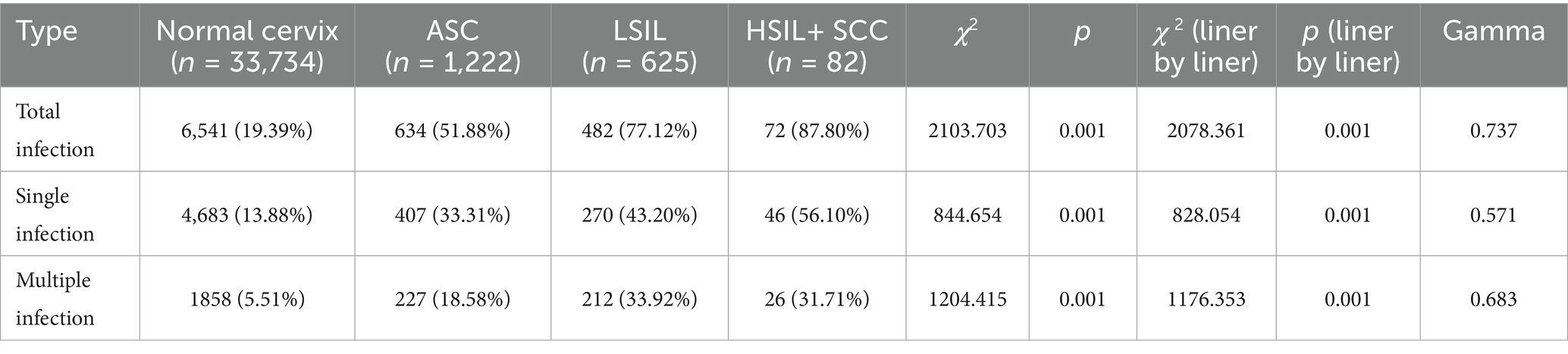
The total HPV infection rate among Normal cervix, ASC, LSIL, and HSIL+SCC subgroups were 19.39% (6,541/33734), 51.88% (634/1222), 77.12% (482/625), and 87.80% (72/82), respectively (χ2 = 2103.703, p < 0.001), the linear-by-linear association test also disappeared an evident rising trend (gamma = 0.737). The single and multiple HPV infection rates across these cytological classifications also demonstrated analogous statistical findings (Table 4).
4 Discussion
This retrospective study, investigated the overall HPV infection rate in Beijing, with an observed decline in annual incidence rates. Besides, the age distribution of HPV infections exhibits a bimodal pattern, and HPV infection rates showed an increasing trend with the severity of pathological classification. Importantly, the current nine-valent vaccine does not cover all the relatively high proportion HPV types in Beijing females.
Over the past decade, the average HPV infection rate was 17.28% in present study. Comparatively, data from other regions and central studies in China differed (4, 14, 18–20): the HPV detection positive rate ranged from 15.54% (21) to 45.57% (22). The variation can be attributed to several factors. Firstly, the majority of studies only included the gynecological outpatient or inpatient individuals, where infection rates are higher compared to the general population. Therefore, it cannot truly reflect the HPV infection rate among Chinese women in the real world. Our study included both gynecological patients and individuals undergoing routine health checks, similar to the study in Nanning, which also included both outpatient and health examination participants (20), providing a more accurate reflection of HPV infection rates among Chinese women in Beijing. Secondly, different economic conditions, health infrastructure, and vaccine uptake might also contribute to the variability in HPV prevalence, as observed in comparisons across different cities in China (23). The last reason cannot be ignored is that different testing reagents may lead to different HPV positivity rates. Our study utilized reagents that detected 27 types of HPV, suggesting that the more types tested, the higher the HPV positivity rate increases. Thereby, we suggest that all future literature must report the number of determined HPV types. Due to the variation in HPV infection rates across different regions worldwide, there is an urgent need to carried out a nationwide epidemiological investigation of HPV infection rate in China.
The most frequently occurring HR-HPV types in our study were HPV-52, HPV-16, and HPV-58, similar to data from Northern China (4), Sichuan (24), and Nanning (20). The prominence of HPV-52 in Beijing aligns with previous study (4), although not as strongly associated with cervical cancer as HPV-16 and HPV-18, HPV-52 is highly related to cervical lesions, emphasizing the importance of promoting the nine-valent vaccine in Beijing. However, HPV-18 (rank 9, 0.96%) did not represent a high proportion, differing from descriptions in some literature from the Asian region (rank 2, 1.4%) (25). Besides, the proportion of several HR-HPV (HPV-39, HPV-53, and HPV-56) are relatively high. However, the existing nine-valent HPV vaccines do not cover the relatively high-proportion HPV types listed above. As for LR-HPV, the proportion of HPV-43, HPV-61, and HPV-81 were significantly high than that of HPV-6 and HPV-11, unfortunately, these high-proportion types are also not included in the HPV vaccine by now. The above findings might have important implications for national vaccine strategy in China, such as the future vaccine formulation or expansion of genotype coverage domestic vaccines. It is highly necessary to develop vaccines specifically tailored for the Chinese population based on this epidemiological data.
Over the years, there has been a declining trend in HPV infection rates. This trend reflects the control of HPV transmission due to increased vaccine coverage and public health awareness. This trend is consistent with results from Southern China (18), but inconsistent with the rising trend observed in Guangzhou (15). This inconsistency may be attributed to Beijing’s advantages in vaccination and healthcare.
A bimodal distribution phenomenon in age groups has been observed in present research, which peaks in the < 21 and 51 ~ 56 years age groups, consistent with other studies (4, 18, 23). Moreover, a declining trend was observed in all age groups except those over 66 years, which may be attributed to the health conditions and public awareness. It is possible that older adults women face challenges in clearing prior infections and are at higher risk of new infections (26). The occurrence of bimodal peaks may be attributed to immature immune protection and high-risk behaviors in younger individuals (27), leading to a higher likelihood of progression to LSIL. In menopausal women, significant hormonal changes may alter the immune system, increasing susceptibility to HPV infections and reducing HPV clearance rates (26). A cervical cancer modeling study indicated that screening every 3 years for women aged 25–69 was associated with a lifetime risk of cervical cancer of 1 in 532 (28). In contrast, for unvaccinated and unscreened women, the lifetime risk of cervical cancer was 1 in 45. These results underscore the importance of government initiatives to promote HPV vaccination prior to sexual debut in young women and advocate for HPV screening in menopausal women.
As the primary cause of cervical lesions, HPV infection is almost universally present in patients with any cervical cancer pathology (29). The present study revealed a notable escalation in HPV infection rates concomitant with the progression of pathological severity, consistent with the results of a former systematic review (30), which reported overall infection rates of 59.6% for CIN I and 84.8% for CIN II+. Similarly, a meta-analysis in 2020 (31) indicated HPV infection rates of 79.56% for CIN I and 87.00% for CIN II/III. Besides, Previous research has reported that HPV-52, HPV-16, and HPV-58 was the top three prevalent types in China for CIN (32), which is consisted with our study.
The study is subject to limitations. First, it is a single-center retrospective study, which may affect generalizability to the broader Chinese population. Second, although we attempted to investigate the prevalence of HPV in the real world, the proportion of participants from the PE center and the Gynecological Department is close to 1:1, underestimating the proportion of the healthy population. Third, vaccination status was not obtained in the 73,697 female subjects, therefore, we could not assess the protection effectiveness of HPV vaccine in present study.
To conclude, present study investigated the prevalence and genotypes distribution of different HPV types from both healthy populations and Gynecological patients. The overall HPV infection rate has been decreasing year by year, appeared as a pronounced bimodal phenomenon grouped by age, and showed a significant upward trend with the progression of pathological severity. In the future, it is necessary to design more suitable vaccines based on Chinese HPV type ranking, especially for HR-HPV (HPV-39, HPV-53, and HPV-56) and LR-HPV (HPV-43, HPV-61, and HPV-81).
Data availability statement
All relevant data is contained within the article. The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Institution Review Board (IRB) of Aerospace Center Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this is a retrospective study, and all the participants’ results was retrospectively collected. Therefore, none written informed consent was acquired.
Author contributions
MB: Methodology, Writing – original draft, Investigation, Formal analysis, Data curation, Funding acquisition, Writing – review & editing, Conceptualization. YL: Methodology, Data curation, Investigation, Software, Conceptualization, Visualization, Writing – original draft. FM: Project administration, Writing – review & editing. QG: Project administration, Writing – review & editing. ZL: Resources, Writing – review & editing. YX: Writing – review & editing. JF: Resources, Funding acquisition, Writing – review & editing. FF: Resources, Writing – review & editing. GL: Resources, Project administration, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by scientific research and cultivation project of Aerospace Center Hospital (2022PY003), and Hygiene and Health Development Scientific Research Fostering Plan of Haidian District Beijing (HP2022-30-507001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fowler, JR, Maani, EV, Dunton, CJ, Gasalberti, DP, and Jack, BW. Cervical Cancer. Treasure Island, FL: StatPearls Publishing (2024).
2. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
3. Zheng, RS, Chen, R, Han, BF, Wang, SM, Li, L, Sun, KX, et al. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi. (2024) 46:221–31. doi: 10.3760/cma.j.cn112152-20240119-00035
4. Zhu, X, Wang, Y, Lv, Z, and Su, J. Prevalence and genotype distribution of high-risk HPV infection among women in Beijing, China. J Med Virol. (2021) 93:5103–9. doi: 10.1002/jmv.27013
5. Crosbie, EJ, Einstein, MH, Franceschi, S, and Kitchener, HC. Human papillomavirus and cervical cancer. Lancet. (2013) 382:889–99. doi: 10.1016/S0140-6736(13)60022-7
6. de Martel, C, Georges, D, Bray, F, Ferlay, J, and Clifford, GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. (2020) 8:e180–90. doi: 10.1016/S2214-109X(19)30488-7
7. Ginsburg, O, Bray, F, Coleman, MP, Vanderpuye, V, Eniu, A, Kotha, SR, et al. The global burden of women's cancers: a grand challenge in global health. Lancet. (2017) 389:847–60. doi: 10.1016/S0140-6736(16)31392-7
8. Cohen, PA, Jhingran, A, Oaknin, A, and Denny, L. Cervical cancer. Lancet. (2019) 393:169–82. doi: 10.1016/S0140-6736(18)32470-X
9. Gultekin, M, Ramirez, PT, Broutet, N, and Hutubessy, R. World Health Organization call for action to eliminate cervical cancer globally. Int J Gynecol Cancer. (2020) 30:426–7. doi: 10.1136/ijgc-2020-001285
10. Lei, J, Ploner, A, Elfstrom, KM, et al. HPV vaccination and the risk of invasive cervical Cancer. N Engl J Med. (2020) 383:1340–8. doi: 10.1056/NEJMoa1917338
11. Huh, WK, Joura, EA, Giuliano, AR, Iversen, OE, de Andrade, RP, Ault, KA, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomised, double-blind trial. Lancet. (2017) 390:2143–59. doi: 10.1016/S0140-6736(17)31821-4
12. Liao, G, Jiang, X, She, B, Tang, H, Wang, Z, Zhou, H, et al. Multi-infection patterns and co-infection preference of 27 human papillomavirus types among 137,943 gynecological outpatients across China. Front Oncol. (2020) 10:449. doi: 10.3389/fonc.2020.00449
13. De Sanjose, S, Diaz, M, Castellsague, X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. (2007) 7:453–9. doi: 10.1016/S1473-3099(07)70158-5
14. Zhang, H, and Zhang, S. Prevalence and genotype distribution of human papillomavirus infection among female outpatients in Northeast China: a population-based survey of 110,927 women. Arch Gynecol Obstet. (2023) 308:35–41. doi: 10.1007/s00404-022-06653-7
15. Yang, X, Li, Y, Tang, Y, Li, Z, Wang, S, Luo, X, et al. Cervical HPV infection in Guangzhou, China: an epidemiological study of 198,111 women from 2015 to 2021. Emerg Microbes Infect. (2023) 12:e2176009. doi: 10.1080/22221751.2023.2176009
16. Wang, J, Li, H, Zhang, J, Wang, H, Li, Y, Liu, Z, et al. Epidemiology and genotypes analysis of human papillomavirus infection in Beijing, China. Virol J. (2024) 21:19. doi: 10.1186/s12985-024-02292-3
17. Solomon, D, Davey, D, Kurman, R, Moriarty, A, O'Connor, D, Prey, M, et al. The 2001 Bethesda system: terminology for reporting results of cervical cytology. JAMA. (2002) 287:2114–9. doi: 10.1001/jama.287.16.2114
18. Luo, LP, He, P, Liu, QT, Jiang, YH, Zhang, YN, Li, QZ, et al. Prevalence and genotype distribution of HPV infection among 214,715 women from southern China, 2012-2018: baseline measures prior to mass HPV vaccination. BMC Infect Dis. (2021) 21:328. doi: 10.1186/s12879-021-06019-5
19. Mai, Q, Yang, X, Cheng, H, Wu, G, and Wu, Z. Prevalence and genotype distribution of human papillomavirus among women with cervical lesions in Shenzhen city, China. Hum Vaccin Immunother. (2021) 17:965–71. doi: 10.1080/21645515.2020.1805993
20. Wei, X, Lu, Q, and Wang, S. Prevalence characteristics of cervical human papillomavirus genotypes in Nanning, China: a 10-year survey of 77,756 women from one medical center. J Med Virol. (2022) 94:2787–95. doi: 10.1002/jmv.27498
21. Zhou, HL, Zhang, W, Zhang, CJ, Wang, SM, Duan, YC, Wang, JX, et al. Prevalence and distribution of human papillomavirus genotypes in Chinese women between 1991 and 2016: a systematic review. J Infect. (2018) 76:522–8. doi: 10.1016/j.jinf.2018.02.008
22. Ruan, Y, Li, H, Liu, M, Cao, G, Xu, X, Han, L, et al. A retrospective analysis of human papillomavirus (HPV) prevalence and genotype distribution among 25,238 women in Shanghai, China revealed the limitations of current HPV-based screening and HPV vaccine. Cancer Epidemiol. (2023) 84:102372. doi: 10.1016/j.canep.2023.102372
23. Wang, R, Guo, XL, Wisman, GB, Wisman, GBA, Schuuring, E, Wang, W-f, et al. Nationwide prevalence of human papillomavirus infection and viral genotype distribution in 37 cities in China. BMC Infect Dis. (2015) 15:257. doi: 10.1186/s12879-015-0998-5
24. Long, W, Yang, Z, Li, X, Chen, M, Liu, J, Zhang, Y, et al. HPV-16, HPV-58, and HPV-33 are the most carcinogenic HPV genotypes in southwestern China and their viral loads are associated with severity of premalignant lesions in the cervix. Virol J. (2018) 15:94. doi: 10.1186/s12985-018-1003-x
25. Bruni, L, Diaz, M, Castellsague, X, et al. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. (2010) 202:1789–99. doi: 10.1086/657321
26. Gameiro, CM, Romao, F, and Castelo-Branco, C. Menopause and aging: changes in the immune system--a review. Maturitas. (2010) 67:316–20. doi: 10.1016/j.maturitas.2010.08.003
27. Moscicki, AB, Hills, N, Shiboski, S, Powell, K, Jay, N, Hanson, E, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. (2001) 285:2995–3002. doi: 10.1001/jama.285.23.2995
28. Malagon, T, Kulasingam, S, Mayrand, MH, et al. Age at last screening and remaining lifetime risk of cervical cancer in older, unvaccinated, HPV-negative women: a modelling study. Lancet Oncol. (2018) 19:1569–78. doi: 10.1016/S1470-2045(18)30536-9
29. Walboomers, JM, Jacobs, MV, Manos, MM, Bosch, FX, Kummer, JA, Shah, KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. (1999) 189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<>3.0.CO;2-F
30. Zhu, B, Liu, Y, Zuo, T, Cui, X, Li, M, Zhang, J, et al. The prevalence, trends, and geographical distribution of human papillomavirus infection in China: the pooled analysis of 1.7 million women. Cancer Med. (2019) 8:5373–85. doi: 10.1002/cam4.2017
31. Wei, L, Xie, X, Liu, J, Zhao, Y, Chen, W, Zhao, C, et al. Efficacy of quadrivalent human papillomavirus vaccine against persistent infection and genital disease in Chinese women: a randomized, placebo-controlled trial with 78-month follow-up. Vaccine. (2019) 37:3617–24. doi: 10.1016/j.vaccine.2018.08.009
Keywords: human papillomavirus, prevalence, genotype, pathology, vaccination
Citation: Bai M, Li Y, Ma F, Gao Q, Lv Z, Xu Y, Feng J, Fu F and Liang G (2025) Prevalence and genotype assessment of HPV in 73,697 females from Beijing, China. Front. Public Health. 13:1623627. doi: 10.3389/fpubh.2025.1623627
Edited by:
Eleftherios Paschalis Ilios, Harvard University, United StatesReviewed by:
Abdoul Karim Ouattara, University Norbert Zongo, Burkina FasoJing Li, Sichuan University, China
Copyright © 2025 Bai, Li, Ma, Gao, Lv, Xu, Feng, Fu and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Feng, ZmVuZ2ppbmcwMDgwQDEyNi5jb20=; Fengxian Fu, ZnVmeDFAMTI2LmNvbQ==; Guowei Liang, bGlhbmdndW93ZWk3MjFAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Mingjian Bai
Mingjian Bai Yunxiang Li2†
Yunxiang Li2† Fucun Ma
Fucun Ma Qian Gao
Qian Gao Zhiyong Lv
Zhiyong Lv Fengxian Fu
Fengxian Fu Guowei Liang
Guowei Liang