- 1Department of Nursing, Tri-Service General Hospital, National Defense Medical University, Taipei, Taiwan
- 2College of Nursing, National Defense Medical University, Taipei, Taiwan
- 3Department of Computer Science and Information Engineering, National Taitung University, Taitung, Taiwan
- 4Department of Nursing, Tri-Service General Hospital Songshan Branch, Taipei, Taiwan
Metabolic syndrome is a critical predictor of future cardiometabolic disease and an emerging public health concern, particularly in high-demand populations such as military personnel. This study aimed to develop and evaluate sex-specific machine learning models for the early detection of metabolic syndrome using annual health check data. We analyzed records from 179,620 Taiwanese Air Force personnel between 2014 and 2022, incorporating demographic, anthropometric, clinical, lifestyle, mental health, and biochemical variables. Six machine learning algorithms—including logistic regression, random forest, K-nearest neighbor, support vector machine, neural network, and naïve Bayes—were trained separately for men and women. Among these models, logistic regression outperformed the others, achieving an accuracy and area under the curve (AUC) of 0.89. Body mass index, age, and alanine aminotransferase levels were consistent predictors across sexes. For men, total cholesterol and uric acid contributed significantly, while hemoglobin and hematocrit were more predictive in women. These findings demonstrate that sex-specific predictive models can support early identification of individuals at high risk for metabolic syndrome, enabling targeted prevention strategies and strengthening population health efforts in military populations and other young to middle-aged adult groups.
1 Introduction
Metabolic syndrome (MetS) is a cluster of interrelated conditions including obesity, elevated blood glucose, dyslipidemia, and hypertension. These conditions frequently co-occur in individuals at increased risk of cardiovascular disease and type 2 diabetes and are strong predictors of morbidity and mortality (1). As of 2018, the global prevalence of MetS was estimated at 25% and has since continued to rise, making it a growing public health concern (2). Among Air Force personnel, high stress levels increase susceptibility to metabolic disorders (3), underscoring the need for early risk detection and targeted prevention strategies in this population.
Numerous factors contribute to MetS, including age, sex (3, 4), chronic disease history (5), family history (5), and behaviors such as smoking (6), alcohol consumption (7), betel nut use (5), and physical inactivity (8). Betel nut chewing is particularly relevant in Asian populations, where its high prevalence and established associations with central obesity, dyslipidemia, and impaired glucose regulation make it a culturally specific conditioning factor in the development of MetS (9). Although body mass index (BMI) is frequently used in population screening to identify individuals at risk (10), it is not itself a causal determinant of MetS. Instead, the pathophysiological link between MetS and cardiometabolic disease arises from the quantity, distribution, and functionality of adipose tissue, with visceral fat and dysfunctional adipose compartments playing a more critical role than overall body weight (11). This distinction underscores the need to interpret BMI cautiously and to consider more direct indicators of body fat and adiposity function in risk assessments. Mental health status (12) and biochemical indicators—such as white blood cell count, hemoglobin, total cholesterol, alanine aminotransferase (ALT), and uric acid level (13, 14)—also show strong associations with MetS risk. While many predictive models have been developed, most rely on traditional statistical methods such as logistic regression and may not fully capture complex interactions among variables.
Machine learning offers a data-driven alternative capable of analyzing high-dimensional health data and identifying nonlinear patterns (15). It has shown promise in disease prediction across various health domains. This study focuses on six widely used machine learning algorithms: logistic regression (LR), random forest (RF), K-nearest neighbor (KNN), support vector machine (SVM), neural network (NN), and naïve Bayes (NB) (16, 17). Each algorithm varies in structure and learning mechanism, providing different strengths in predictive modeling (18–20).
Despite increasing interest in machine learning for health prediction (21), its application to MetS risk detection in military populations remains underexplored. This study aims to evaluate the predictive accuracy of multiple machine learning models for MetS using annual health check data from Taiwanese Air Force personnel. We further examine how sex-specific models may enhance prediction by identifying distinct risk profiles in men and women. Our goal is to inform early detection and personalized prevention strategies, contributing to improved metabolic health and operational readiness in high-demand populations.
2 Materials and methods
2.1 Study design and cohort
This population-based study used data from the Taiwanese Military Health Management Information System, which collects annual worksite health examination data from active-duty personnel. We included Air Force members aged 18 to 58 years who underwent health screenings between 2014 and 2022. Data included demographic characteristics, anthropometric measures, medical history, lifestyle behaviors, mental health indicators, and biochemical parameters. All procedures followed the ethical standards of the 1975 Declaration of Helsinki and were approved by the Institutional Review Board of Tri-Service General Hospital, Taiwan (approval number: A202305142).
2.2 Measures
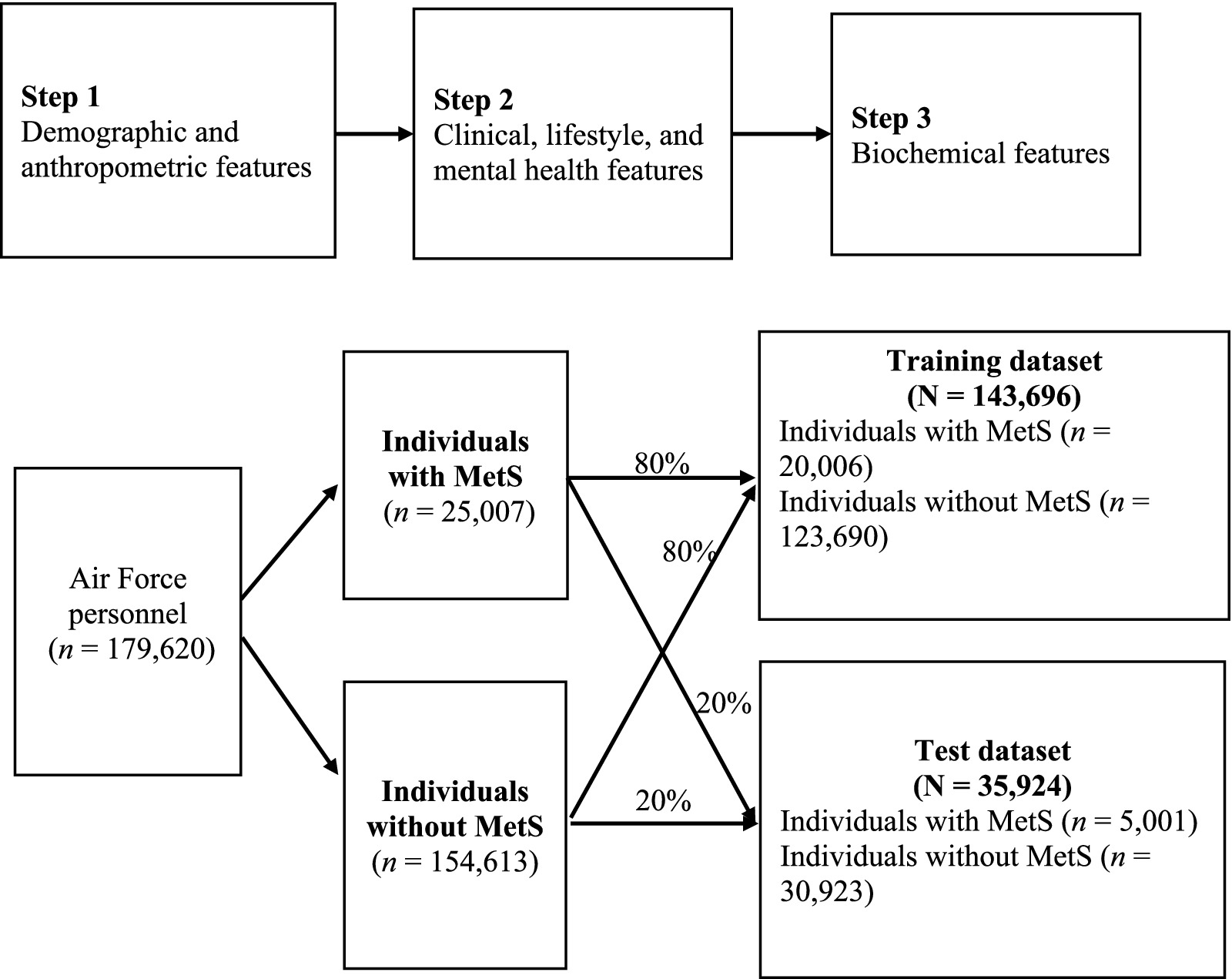
We initially examined 36 features potentially associated with MetS (22), grouped into three categories: (1) demographic and anthropometric; (2) clinical, lifestyle, and mental health; and (3) biochemical.
2.2.1 Step 1: demographic and anthropometric features
Age, sex, waist circumference, and BMI were included. BMI was calculated as weight (kg) divided by height squared (m2).
2.2.2 Step 2: clinical, lifestyle, and mental health features
Clinical features included history of chronic disease, family history, and blood pressure (systolic and diastolic). Lifestyle features included smoking, betel nut use, alcohol consumption, physical activity, rapid fatigue during exercise, infection within 1 month, and regular medication use. The mental health features—insomnia, depression, hostility, anxiety, interpersonal sensitivity, and suicidal ideation—were assessed using the Brief Symptom Rating Scale-5 (BSRS-5), a validated five-item scale scored on a 5-point Likert scale (0–4) (23, 24). Higher scores indicated poorer psychological well-being. The Cronbach’s α value for the BSRS-5 ranges from 0.77 to 0.90 (23).
2.2.3 Step 3: biochemical features
Biochemical features included liver function markers—aspartate aminotransferase (AST) and alanine aminotransferase (ALT); renal function markers—blood urea nitrogen (BUN) and creatinine; hematological parameters—red blood cell (RBC) count, white blood cell (WBC) count, hemoglobin, hematocrit, and platelet count; and cardiovascular indicators—total cholesterol (TC), uric acid (UA), triglycerides, high-density lipoprotein cholesterol level (HDL-C), low-density lipoprotein cholesterol (LDL-C), and fasting plasma glucose. The Air Force personnel fasted overnight before venous blood collection. Samples were processed using a clinical chemistry analyzer (ADVIA1800, Siemens, United States).
2.3 Study outcomes
The study outcome was the occurrence of MetS during a 9-year surveillance period. MetS diagnosis followed the modified National Cholesterol Education Program Adult Treatment Panel III criteria, with modifications from the International Diabetes Federation, which accounts for waist circumference norms in the Asian population (25). Individuals meeting three or more of the following criteria were diagnosed with MetS (4): triglycerides ≥150 mg/dL, fasting plasma glucose ≥100 mg/dL, HDL-C < 40 mg/dL (men) or <50 mg/dL (women), systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg, and waist circumference ≥90 cm (men) or ≥80 cm (women).
Because triglycerides, fasting plasma glucose, HDL-C, blood pressure, and waist circumference were used to define the MetS outcome, these variables were not included as predictors in the machine learning models to avoid circularity and redundancy. In addition, the BSRS total score overlapped with its five individual items, which were retained to provide more granular information. Therefore, seven features were excluded, and the remaining 29 features were retained for model development using the sequential three-step process described above (Figure 1).
2.4 Machine learning
A total of 29 key features of MetS were included in the models in three sequential steps (Figure 1). The contribution of each feature to model performance was evaluated. The dataset was divided into MetS and non-MetS groups at an 80:20 ratio, yielding training and test datasets of 143,696 and 35,924 Air Force personnel, respectively. To address class imbalance, the synthetic minority oversampling technique (SMOTE) was applied exclusively to the training dataset prior to model development, while the test dataset was left unchanged to ensure unbiased evaluation (26). For comparison, we also assessed two other approaches, adaptive synthetic sampling (ADASYN) and class weighting, both of which produced area under the receiver operating characteristic curve (AUC) values similar to those obtained with SMOTE (see Supplementary Table S1 for detailed results). Because SMOTE is well validated and widely applied in biomedical prediction research, it was selected as the primary method for handling class imbalance in this study.
Data preprocessing included standardization to ensure compatibility across models. Six machine learning algorithms—KNN, RF, LR, SVM, NN, and NB—were implemented via Python (version 3.8) with libraries including scikit-learn, imbalanced-learn, and SHapley Additive exPlanations (SHAP). Each model was initially trained with default hyperparameters, followed by optimization through grid search.
Hyperparameter optimization was conducted using grid search with 5-fold cross-validation for the RF, SVM, and NN models, whereas KNN, LR, and NB were implemented with standard or default configurations. For RF, the grid included variations in the number of trees, maximum depth, and minimum split size; for SVM, the penalty parameter (C) and kernel coefficient (gamma) were tuned with the RBF kernel; and for NN, hidden layer sizes, regularization (alpha), and learning rate were explored. The detailed parameter ranges for all models are provided in Supplementary Table S2.
Model performance was evaluated via multiple metrics, including accuracy, F1 score, precision, recall, specificity, and area under the receiver operating characteristic curve (AUC). Accuracy was calculated via the following equation: true positives (TP) + true negatives (TN) / (TP + false negatives [FN] + false positives [FP] + TN) = (TP + TN) / total sample count. The F1 score is the harmonic mean of precision and sensitivity. The precision was calculated as follows: TP / (TP + FP). Recall was calculated as follows: TP / (TP + FN). The specificity was calculated as follows: TN / (FP + TN).
The discriminatory ability of the models was visualized via receiver operating characteristic (ROC) curves. Feature importance was assessed via SHAP values, which provided insights into the top predictors for MetS in the male and female subgroups. These analyses identified the 10 most influential features for each subgroup, providing tailored insights into risk patterns.
2.5 Statistical analysis
Continuous variables are presented as means and standard deviations; categorical variables are shown as frequencies and percentages. Group comparisons between individuals with and without MetS were conducted using independent t-tests for continuous data and chi-square tests for categorical data. A p-value <0.05 indicated statistical significance.
3 Results
3.1 Cohort characteristics
The study included 179,620 active-duty Air Force personnel, of whom 83.5% were men. Table 1 summarizes the cohort characteristics. A total of 25,007 individuals (13.9%) met the criteria for MetS, whereas 154,613 (86.1%) did not. Individuals in the MetS group were significantly older than those in the non-MetS group (mean age: 34.36 ± 5.94 vs. 29.88 ± 6.79 years; p < 0.001). The proportion of men was also greater in the MetS group than in the non-MetS group (95.3% vs. 81.5%; p < 0.001). Mental health scores, excluding those for suicide attempts, were significantly higher in the MetS group. In addition, all biochemical measurements were significantly greater in the MetS group than in the non-MetS group.
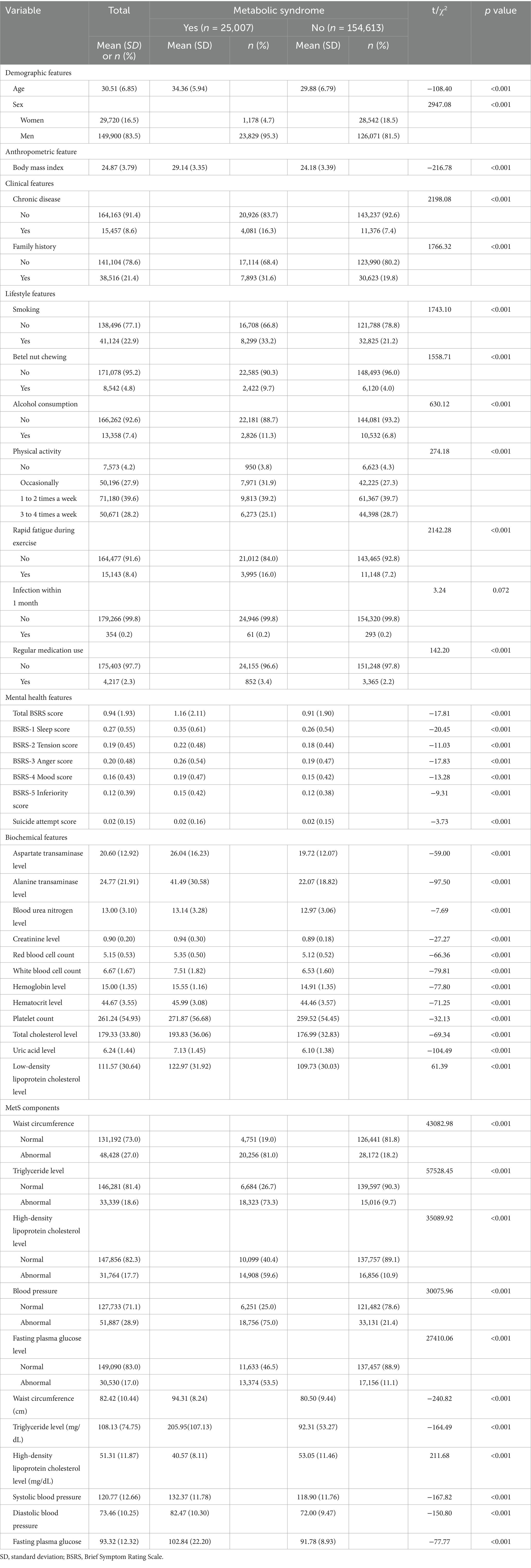
Table 1. Demographic, anthropometric, disease, lifestyle, mental health, and biochemical features of the study cohort (N = 179,620).
The chi-square test revealed sex-specific patterns in the prevalence of MetS and its component abnormalities (Table 2). Among men, the most common abnormality was elevated blood pressure (32.9%), followed by increased waist circumference (28.4%), elevated triglycerides (21.3%), hyperglycemia (19.0%), and reduced HDL-C (17.6%). In women, increased waist circumference was most prevalent (19.9%), followed by reduced HDL-C (17.9%), elevated blood pressure (8.9%), hyperglycemia (7.0%), and elevated triglycerides (5.0%).
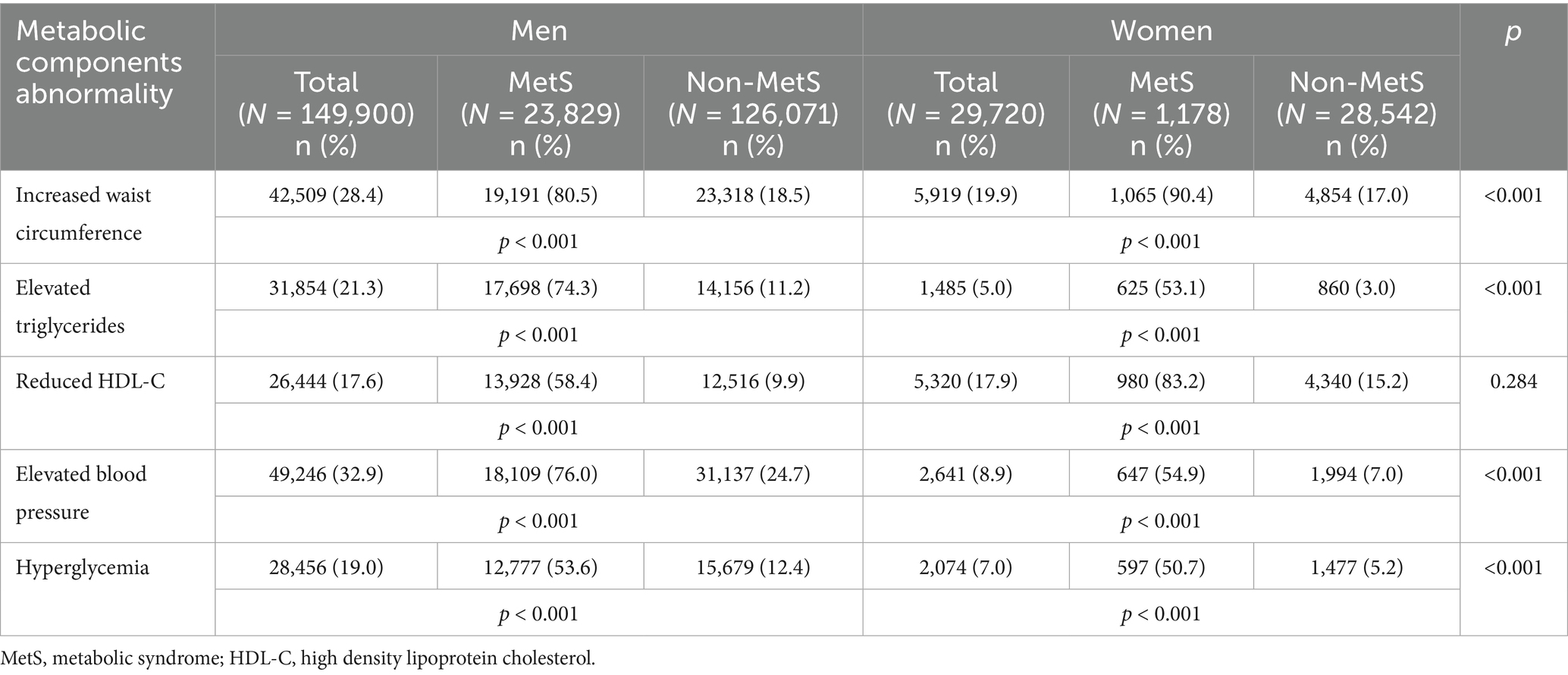
Table 2. Sex-specific prevalence of metabolic syndrome by its component abnormality of the cohort study (N = 179,620).
When examining the prevalence of MetS within each abnormality, men with increased waist circumference (80.5%), elevated blood pressure (76.0%), or elevated triglycerides (74.3%) were most likely to meet MetS criteria, followed by reduced HDL-C (58.4%) and hyperglycemia (53.6%). In women, increased waist circumference (90.4%) and reduced HDL-C (83.2%) were the strongest correlates of MetS, followed by elevated blood pressure (54.9%), elevated triglycerides (53.1%), and hyperglycemia (50.7%).
Overall, 17.6% of men and 17.9% of women presented with reduced HDL-C (p = 0.284), indicating no significant sex difference in overall prevalence. However, when stratified by MetS status, reduced HDL-C was observed in 58.4% of men with MetS versus 9.9% without MetS, and in 83.2% of women with MetS versus 15.2% without MetS. These findings suggest that while the overall prevalence of reduced HDL-C was comparable between sexes, within the MetS subgroup, women were disproportionately more likely than men to exhibit reduced HDL-C.
3.2 Model performance
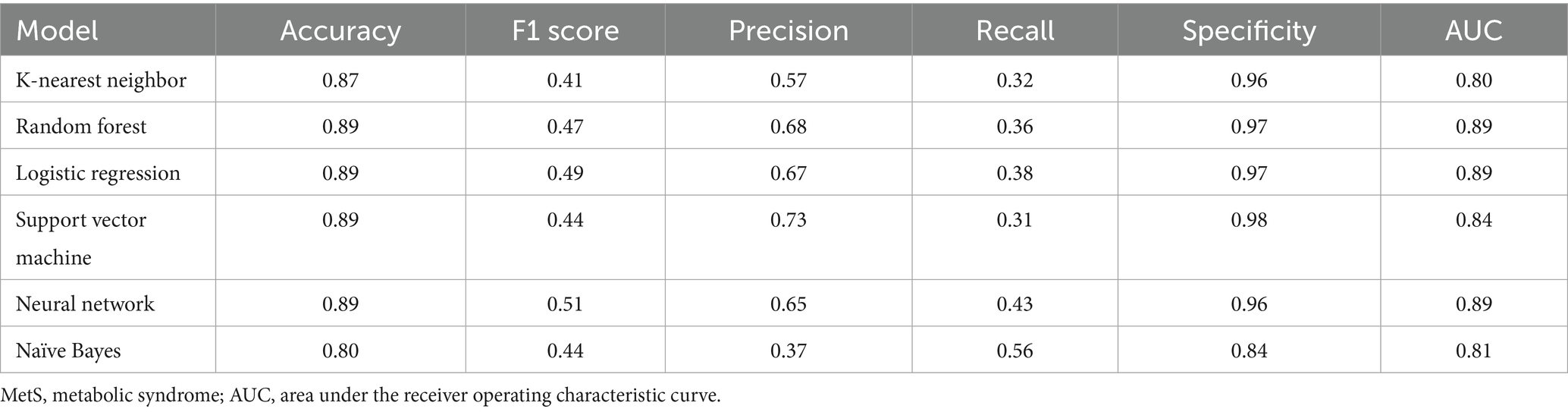
The six machine learning models demonstrated accuracies ranging from 0.80 to 0.89. Among them, the RF, LR, SVM, and NN models achieved the highest overall accuracy (0.89). The NN model had the highest F1 score (0.51), indicating balanced performance in precision and recall. The SVM model had the highest precision (0.73), followed by RF (0.68), LR (0.67), NN (0.65), KNN (0.57), and NB (0.37) models. The model recall values ranged from 0.31 to 0.56, and the specificity values ranged from 0.84 to 0.98 (Table 3).
For predicting MetS events, the LR, RF, and NN models yielded the highest AUC values (all rounded to 0.89), followed by SVM (0.84), NB (0.81), and KNN (0.80; Figure 2). Pairwise comparisons using DeLong’s test indicated that LR achieved the highest AUC (0.894), significantly exceeding RF (0.890; ΔAUC = 0.004; p_FDR < 0.001) and NN (0.873; ΔAUC = 0.021; p_FDR < 0.001; Table 4). RF also significantly outperformed NN (ΔAUC = 0.017; p_FDR < 0.001). The overall performance ranking was LR > RF > SVM > NN, with all four significantly outperformed NN and NB (all p_FDR < 0.001). Although the absolute AUC differences among LR, RF, and NN were small (≤0.021), they remained statistically significant after multiple-comparison adjustment.
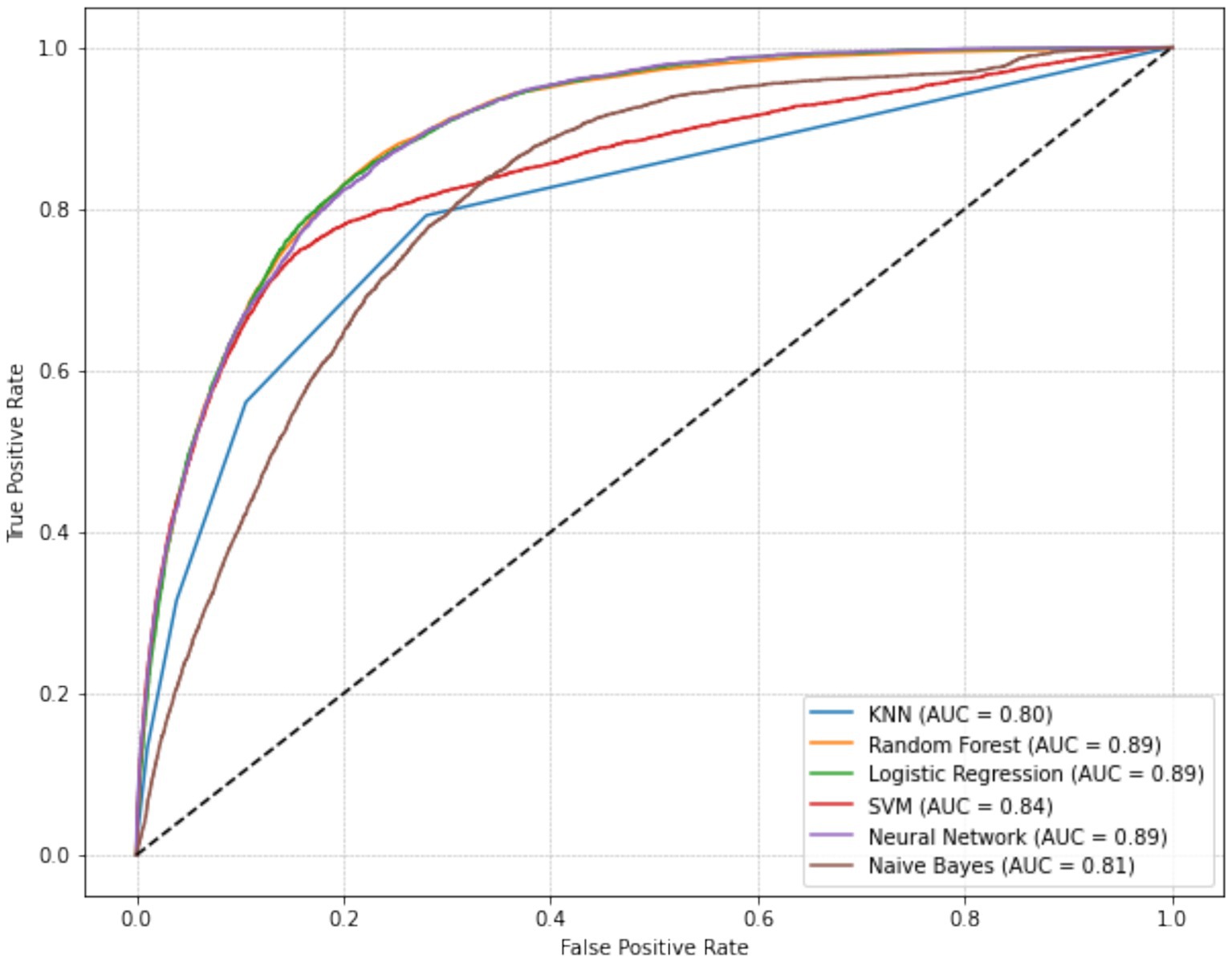
Figure 2. Receiver operating characteristic (ROC) curves of six machine learning models for predicting metabolic syndrome, with Logistic Regression achieving the highest AUC (0.894), followed by Random Forest, SVM, and Neural Network.
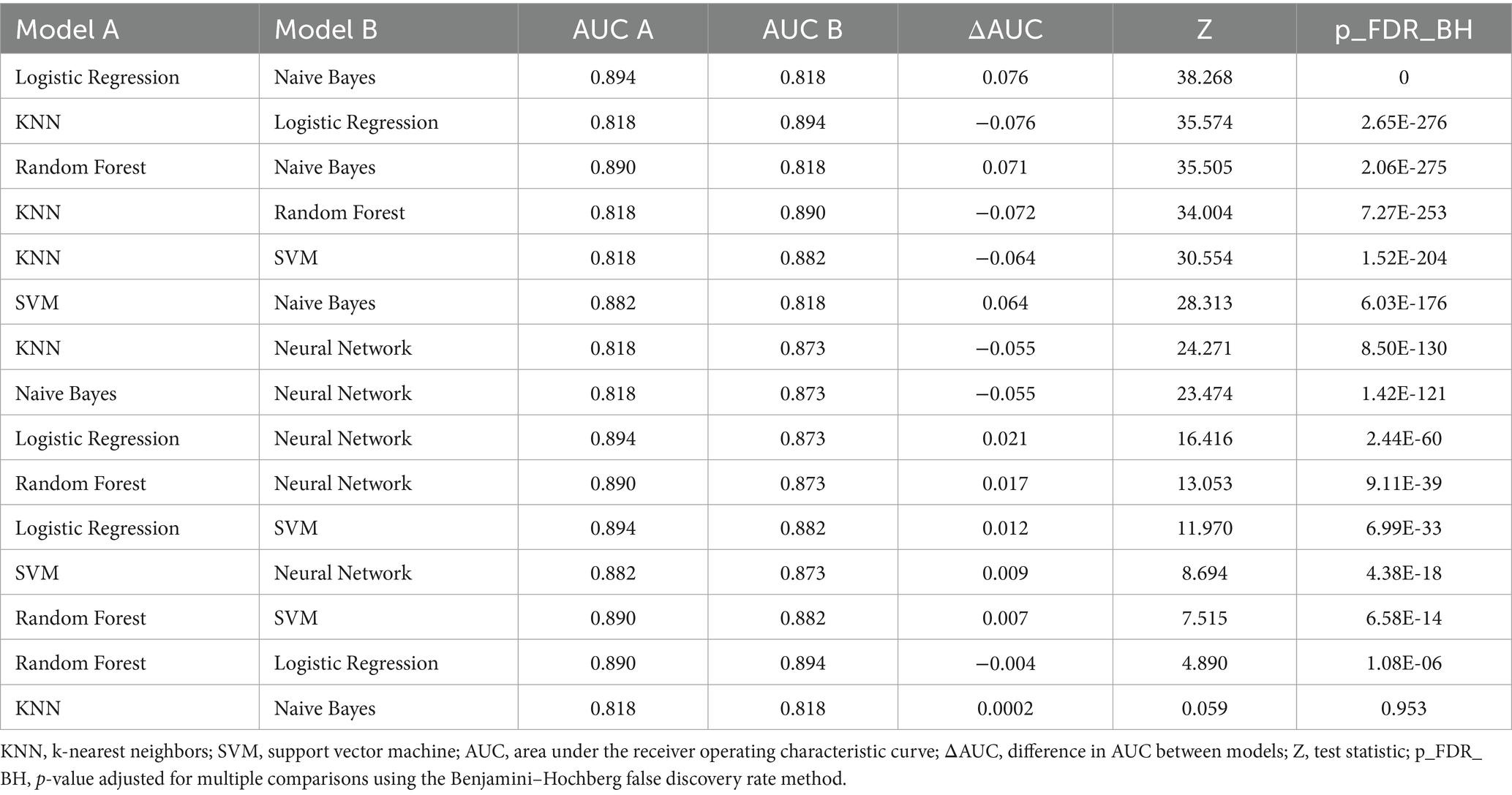
Table 4. Pairwise comparison of area under the receiver operating characteristic curve values among six machine learning models for predicting metabolic syndrome using DeLong’s test.
3.3 Sex-specific MetS predictors
All six models passed the Hosmer–Lemeshow test, indicating good model fit. The top 10 predictive features for MetS identified by SHAP analysis are shown in Figure 3. Across the entire cohort, BMI, age, ALT level, hemoglobin, and uric acid were the five most strongly predictive features (Figures 3a,b). Features related to clinical, lifestyle, and mental health contributed minimally to the prediction. For men, BMI, age, ALT level, total cholesterol, and uric acid level were the most influential predictors (Figures 3c,d). For women, BMI, age, hemoglobin, ALT level, and hematocrit were the most predictive features (Figures 3e,f). Across both sexes, BMI, age, and ALT levels were consistently identified as the most influential predictors.
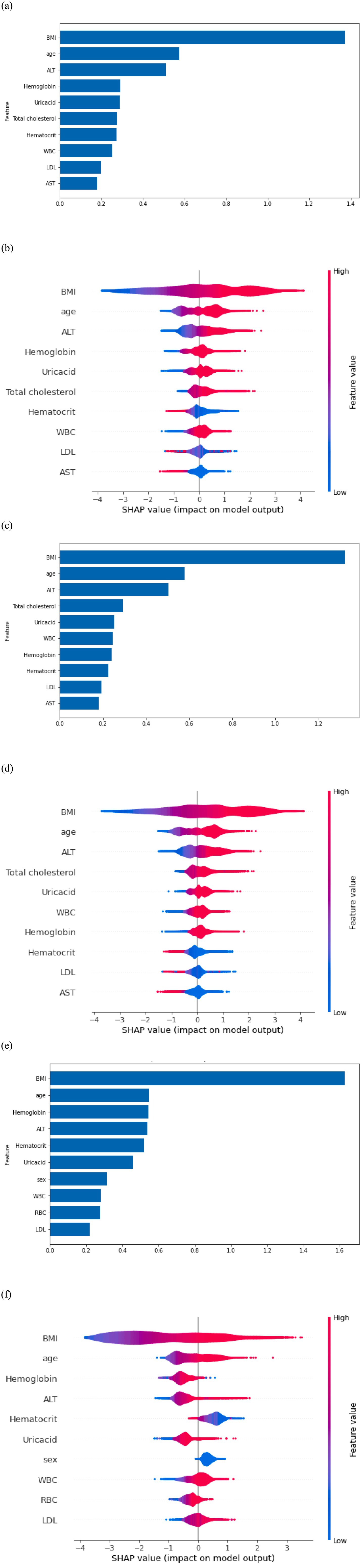
Figure 3. (a) Feature importance in the overall model. (b) Shapley Additive exPlanations (SHAP) on the overall model output. (c) Feature importance in the model for men. (d) SHAP on model output for men. (e) Feature importance in the model for women. (f) SHAP on model output for women.
4 Discussion
Maintaining optimal health in military personnel is crucial as poor health can hinder their ability to fully dedicate themselves to national security (3). This study represents a novel attempt to explore sex-based differences in early MetS detection among Air Force personnel via machine learning models. We systematically developed prediction models by sequentially incorporating demographic, anthropometric, clinical, lifestyle, mental health, and biochemical features. Six machine learning algorithms were employed, and the models were tailored by sex. Model performance and feature importance were subsequently evaluated.
Among the models, the LR model exhibited the most robust performance in predicting MetS, achieving an accuracy and AUC of 0.89. The RF model also performed well. The findings revealed that BMI, age, and ALT level were the three strongest predictors of MetS in Air Force personnel. For men, total cholesterol and uric acid were also key predictors, whereas hemoglobin and hematocrit were influential for women. These results emphasize the importance of tailoring early MetS risk detection models to account for sex-based differences.
Beyond model-derived predictors, our analysis of individual MetS components revealed a noteworthy sex-specific pattern in HDL-C abnormalities. Although the overall prevalence of reduced HDL-C was similar between men and women, within the MetS subgroup women were disproportionately more likely than men to exhibit reduced HDL-C. This observation aligns with findings from Ramezankhani et al., who reported that HDL-C decline was more strongly associated with MetS progression in women than in men (27). While HDL-C was not included as a predictor variable in our models due to its role in defining the MetS outcome, the sex-specific distribution observed in our study underscores the importance of developing tailored prediction models that reflect distinct risk profiles in men and women.
Elevated ALT levels were consistently identified as a significant predictor of MetS in both sexes. ALT is a specific marker of liver function, as it is predominantly localized in hepatocytes and is released into the bloodstream following liver cell damage (28). Its elevation is strongly linked to fatty liver disease, a condition that leads to lipid accumulation in the liver and other organs, ultimately promoting insulin resistance—a key factor in the development and progression of MetS (29). In contrast, AST reflects systemic enzymatic activity, as it is found in multiple tissues, including the heart, brain, and skeletal muscles, making it less specific to liver function. The liver-specific role of ALT in MetS development explains its stronger association with MetS than that of AST. Our findings align with those of previous studies conducted among military personnel, which reported the significance of ALT in MetS prediction (30). Additionally, elevated ALT levels have been associated with occupational stress and fatigue, which are factors prevalent among military personnel (31).
In our study, biochemical parameters exhibited greater specificity for MetS prediction than did disease-related and lifestyle factors. This may be attributed to the gradual emergence of biochemical abnormalities during the pathophysiological progression of MetS. Prioritizing these biochemical markers in screening processes can enhance early detection and improve intervention strategies for MetS. In contrast, disease-related and lifestyle factors, such as smoking and alcohol consumption, are well-established risks that indirectly contribute to biochemical changes leading to MetS (32). This highlights the need for an integrated approach that combines biochemical monitoring with lifestyle interventions to provide comprehensive strategies for preventing and managing MetS.
Interestingly, mental health indicators were not identified as key predictors of MetS in this cohort. One possible explanation is the unique resilience and coping mechanisms instilled by military training, which may mitigate the impact of psychological stress on metabolic health (33, 34). The strong emphasis on discipline, physical fitness, and mental toughness in military culture likely contributes to this resilience (35). However, two alternative explanations should also be considered. First, military recruitment and retention policies generally exclude individuals with severe mental health conditions, leading to a more homogeneous cohort with less variability in psychological indicators. Secondly, reliance on self-reported measures of psychological distress may limit the ability to fully capture real-world mental health conditions. In particular, social desirability bias may have led participants to underreport BSRS-5 symptoms, potentially attenuating the observed associations with MetS and highlighting the need for further research and targeted intervention strategies.
This study has several limitations. First, the lack of genetic and dietary data limits the comprehensiveness of prediction models. The incorporation of such data in future studies may increase model accuracy. Second, because the study was conducted on Air Force personnel, the generalizability of the findings to other populations remains uncertain. Finally, feature importance was analyzed via cross-sectional data, which hindered the investigation of causal relationships between the features and MetS. Large-scale longitudinal studies must be conducted in the future.
5 Conclusion
We developed sex-specific MetS prediction models for military personnel that incorporate demographic, anthropometric, clinical, lifestyle, mental, and biochemical features. The LR model achieved the highest accuracy and AUC for MetS prediction, followed closely by the RF model. BMI, age, and ALT level emerged as the most important predictors of MetS in both sexes. For men, total cholesterol and uric acid were also significant, whereas hemoglobin and hematocrit were influential for women. These findings highlight the importance of sex-based differences in early MetS risk detection and the utility of early prediction models in routine health screenings.
Based on these results, population-level interventions should emphasize structured weight management, education on liver health (e.g., reducing alcohol consumption and unhealthy diets), and age-specific health screenings for all personnel. For men, targeted strategies should include dietary modifications to lower total cholesterol and uric acid levels, combined with regular monitoring to enable early management of these risks. For women, interventions should prioritize nutritional support to maintain adequate hemoglobin and hematocrit levels, along with routine screening to detect and address underlying causes of deficiencies. Tailoring preventive strategies to sex-specific risk profiles may enhance early detection and optimize the management of MetS in military populations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Tri-Service General Hospital, Taiwan. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because written informed consent was waived by the Institutional Review Board because the study involved secondary analysis of anonymized health data collected for routine occupational health surveillance, posed minimal risk to participants, and did not involve any direct interaction or intervention.
Author contributions
W-YW: Visualization, Methodology, Conceptualization, Writing – original draft, Validation. Y-SW: Software, Data curation, Methodology, Writing – original draft, Formal analysis, Visualization. YH: Conceptualization, Funding acquisition, Writing – original draft, Project administration, Validation, Methodology. W-CT: Conceptualization, Supervision, Writing – review & editing, Methodology, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the Medical Affairs Bureau, Ministry of National Defense (MND), Taiwan (grant numbers: MND-MAB-D-112092, MND-MAB-D-113126, and MND-MAB-E-114228). The funder was not involved in the study design, data collection, data analysis and interpretation, writing of the manuscript, or decision to submit this article for publication.
Acknowledgments
The authors thank Shin Hong Lin, MD, previous Air Force Colonel and Chief, the Medical Affairs Section, the Office of the Inspector General, and Min-Hui Hsieh, Air Force Command Headquarters, the Ministry of National Defense, Taiwan, Wu-Chien Chien and Nien-Ting Kuo, National Defense Medical Center, and Hao-Yi Wu, Tri-Service General Hospital for their help throughout the research process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1625461/full#supplementary-material
References
1. Hayden, MR. Overview and new insights into the metabolic syndrome: risk factors and emerging variables in the development of type 2 diabetes and cerebrocardiovascular disease. Medicina. (2023) 59:e561. doi: 10.3390/medicina59030561
2. Neeland, IJ, Lim, S, Tchernof, A, Gastaldelli, A, Rangaswami, J, Ndumele, CE, et al. Metabolic syndrome. Nat Rev Dis Primers. (2024) 10:77. doi: 10.1038/s41572-024-00563-5
3. Wang, WY, Li, CH, Wu, YS, Chien, WC, Wang, KY, and Tzeng, WC. Gender differences in the prevalence of metabolic syndrome among taiwanese air force personnel: a population-based study. J Cardiovasc Nurs. (2020) 35:502–11. doi: 10.1097/JCN.0000000000000714
4. Ying, X, Yang, S, Li, S, Su, M, Wang, N, Chen, Y, et al. Prevalences of metabolic syndrome and its sex-specific association with socioeconomic status in rural China: a cross-sectional study. BMC Public Health. (2021) 21:2033. doi: 10.1186/s12889-021-12074-z
5. Chang, HC, Wu, YS, Tzeng, WC, Wu, HY, Lee, PC, and Wang, WY. Sex differences in risk factors for metabolic syndrome in middle-aged and senior hospital employees: a population-based cohort study. BMC Public Health. (2023) 23:587. doi: 10.1186/s12889-023-15491-4
6. Kim, JH, Kim, BJ, Kang, JG, Kim, BS, and Kang, JH. Association between cigarette smoking and diabetes mellitus using two different smoking stratifications in 145 040 Korean individuals: self-reported questionnaire and urine cotinine concentrations. J Diabetes. (2019) 11:232–41. doi: 10.1111/1753-0407.12837
7. Lin, Y, Ying, YY, Li, SX, Wang, SJ, Gong, QH, and Li, H. Association between alcohol consumption and metabolic syndrome among Chinese adults. Public Health Nutr. (2021) 24:4582–90. doi: 10.1017/S1368980020004449
8. Sheng, J, Abshire, DA, Heiney, SP, and Wirth, MD. Acculturation, physical activity, and metabolic syndrome in Asian American adults. J Transcult Nurs. (2022) 33:675–84. doi: 10.1177/10436596221114150
9. Huang, YC, Geng, JH, Wu, PY, Huang, JC, Chen, SC, Chang, JM, et al. Betel nut chewing increases the risk of metabolic syndrome and its components in a large Taiwanese population follow-up study category: original investigation. Nutrients. (2022) 14:1018. doi: 10.3390/nu14051018
10. Carabello, M, and Wolfson, JA. Mexican immigrant health advantage in metabolic syndrome? Examining the contributions of demographic, socioeconomic, and health behavior characteristics. SSM Popul Health. (2021) 16:100932. doi: 10.1016/j.ssmph.2021.100932
11. Chait, A, and den Hartigh, LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. (2020) 7:22. doi: 10.3389/fcvm.2020.00022
12. Tzeng, WC, Chiang, YS, Feng, HP, Chien, WC, Tai, YM, and Chen, MJ. Gender differences in metabolic syndrome risk factors among patients with serious mental illness. Int J Ment Health Nurs. (2020) 29:254–65. doi: 10.1111/inm.12670
13. Gesteiro, E, Megía, A, Guadalupe-Grau, A, Fernandez-Veledo, S, Vendrell, J, and González-Gross, M. Early identification of metabolic syndrome risk: a review of reviews and proposal for defining pre-metabolic syndrome status. Nutr Metab Cardiovasc Dis. (2021) 31:2557–74. doi: 10.1016/j.numecd.2021.05.022
14. Wu, YS, Tzeng, WC, Chu, CM, and Wang, WY. Metabolic syndrome and its related factors among hospital employees: a population-based cohort study. Int J Environ Res Public Health. (2021) 18:9826. doi: 10.3390/ijerph18189826
15. Moradi, M, and Ghadiri, N. Different approaches for identifying important concepts in probabilistic biomedical text summarization. Artif Intell Med. (2018) 84:101–16. doi: 10.1016/j.artmed.2017.11.004
17. Popchev, I, and Daniela, O. Multi-step methods for machine learning models with web metrics. Proceed Bulgarian Acad Sci. (2023) 76:1707–16. doi: 10.7546/CRABS.2023.11.08
18. Choe, EK, Rhee, H, Lee, S, Shin, E, Oh, SW, Lee, JE, et al. Metabolic syndrome prediction using machine learning models with genetic and clinical information from a nonobese healthy population. Genomics Inform. (2018) 16:e31. doi: 10.5808/GI.2018.16.4.e31
19. Lee, S, Lee, H, Choi, JR, and Koh, SB. Development and validation of prediction model for risk reduction of metabolic syndrome by body weight control: a prospective population-based study. Sci Rep. (2020) 10:10006. doi: 10.1038/s41598-020-67238-5
20. Park, JE, Mun, S, and Lee, S. Metabolic syndrome prediction models using machine learning and sasang constitution type. Evid Based Complement Alternat Med. (2021) 2021:8315047. doi: 10.1155/2021/8315047
21. Eyvazlou, M, Hosseinpouri, M, Mokarami, H, Gharibi, V, Jahangiri, M, Cousins, R, et al. Prediction of metabolic syndrome based on sleep and work-related risk factors using an artificial neural network. BMC Endocr Disord. (2020) 20:169. doi: 10.1186/s12902-020-00645-x
22. Grundy, SM, Cleeman, JI, Daniels, SR, Donato, KA, Eckel, RH, Franklin, BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. (2005) 112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404
23. Lee, MB, Liao, SC, Lee, YJ, Wu, CH, Tseng, MC, Gau, SF, et al. Development and verification of validity and reliability of a short screening instrument to identify psychiatric morbidity. J Formos Med Assoc. (2003) 102:687–94.
24. Lung, FW, and Lee, MB. The five-item brief-symptom rating scale as a suicide ideation screening instrument for psychiatric inpatients and community residents. BMC Psychiatry. (2008) 8:53. doi: 10.1186/1471-244X-8-53
25. Health Promotion Administration, Taiwan. Diagnostic criteria of metabolic syndrome in Taiwan. (2007) Available online at: https://www.hpa.gov.tw/Pages/EBook.aspx?nodeid=1175 (accessed May 12, 2023).
26. Fernandez, A, Garcia, S, Herrera, F, and Chawla, NV. Smote for learning from imbalanced data: progress and challenges, marking the 15-year anniversary. J Artif Intell Res. (2018) 61:863–905. doi: 10.1613/jair.1.11192
27. Ramezankhani, A, Azizi, F, and Hadaegh, F. Gender differences in changes in metabolic syndrome status and its components and risk of cardiovascular disease: a longitudinal cohort study. Cardiovasc Diabetol. (2022) 21:227. doi: 10.1186/s12933-022-01665-8
28. Thakur, S, Kumar, V, Das, R, Sharma, V, and Mehta, DK. Biomarkers of hepatic toxicity: an overview. Curr Ther Res Clin Exp. (2024) 100:100737. doi: 10.1016/j.curtheres.2024.100737
29. Zohara, Z, Adelekun, A, Seffah, KD, Salib, K, Dardari, L, Taha, M, et al. The prospect of non-alcoholic fatty liver disease in adult patients with metabolic syndrome: a systematic review. Cureus. (2023) 15:e41959. doi: 10.7759/cureus.41959
30. Rhee, C, Kim, J, Kim, JY, Chang, E, Park, SY, Lee, W, et al. Clinical markers associated with metabolic syndrome among military aviators. Aerosp Med Hum Perform. (2015) 86:970–5. doi: 10.3357/AMHP.4362.2015
31. Chen, KW, Meng, FC, Shih, YL, Su, FY, Lin, YP, Lin, F, et al. Sex-specific association between metabolic abnormalities and elevated alanine aminotransferase levels in a military cohort: the CHIEF study. Int J Environ Res Public Health. (2018) 15:545. doi: 10.3390/ijerph15030545
32. Zhang, N, Liu, X, Wang, L, Zhang, Y, Xiang, Y, Cai, J, et al. Lifestyle factors and their relative contributions to longitudinal progression of cardio-renal-metabolic multimorbidity: a prospective cohort study. Cardiovasc Diabetol. (2024) 23:265. doi: 10.1186/s12933-024-02347-3
33. Gifford, RM, O'Leary, TJ, Double, RL, Wardle, SL, Wilson, K, Boyle, LD, et al. Positive adaptation of HPA axis function in women during 44 weeks of infantry-based military training. Psychoneuroendocrinology. (2019) 110:104432. doi: 10.1016/j.psyneuen.2019.104432
34. Westphal, RJ, and Convoy, SP. Military culture implications for mental health and nursing care. Online J Issues Nurs. (2015) 20:4. doi: 10.3912/OJIN.Vol20No01Man04
Keywords: metabolic syndrome, machine learning, predictive model, sex differences, military personnel, precision prevention
Citation: Wang W-Y, Wu Y-S, Huang Y and Tzeng W-C (2025) Early risk detection of metabolic syndrome using sex-specific machine learning models in military personnel. Front. Public Health. 13:1625461. doi: 10.3389/fpubh.2025.1625461
Edited by:
Mohammad Hossein Ebrahimi, Shahroud University of Medical Sciences, IranReviewed by:
TaChen Chen, Nihon Pharmaceutical University, JapanGabriela Carrasco, University of Chile, Chile
Copyright © 2025 Wang, Wu, Huang and Tzeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Chii Tzeng, d2N0emVuZ0BtYWlsLm5kbWN0c2doLmVkdS50dw==
†These authors have contributed equally to this work
‡ORCID: Yen Huang, orcid.org/0009-0006-0744-0254
 Wei-Yun Wang
Wei-Yun Wang Yi-Syuan Wu
Yi-Syuan Wu Yen Huang4‡
Yen Huang4‡ Wen-Chii Tzeng
Wen-Chii Tzeng