- 1Menzies School of Health Research, Charles Darwin University, Alice Springs, NT, Australia
- 2School of Biotechnology, Faculty of Applied Sciences and Biotechnology, Shoolini University, Solan, India
- 3Australian e-Health Research Centre, CSIRO, Brisbane, QLD, Australia
Introduction
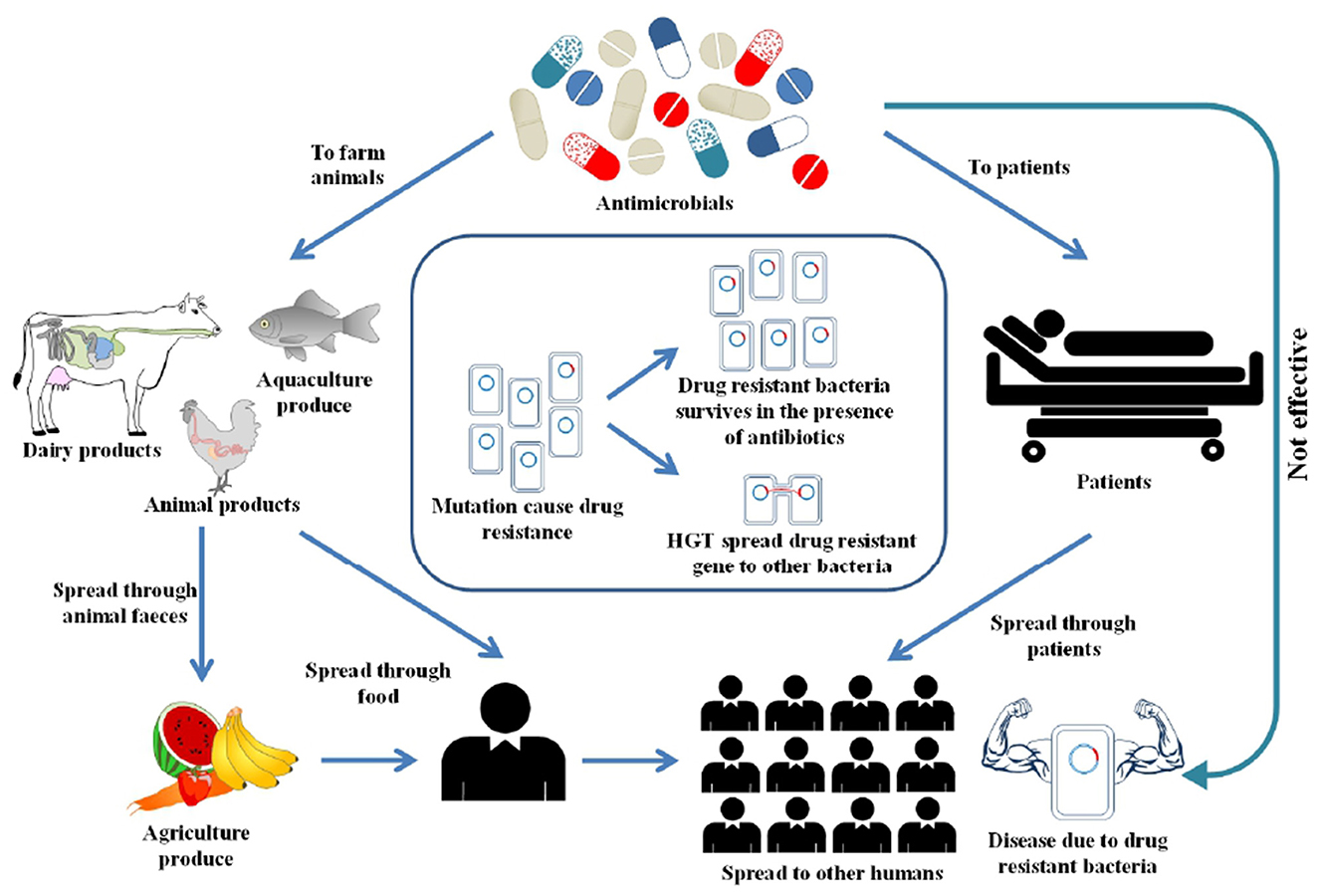
Antimicrobial Resistance (AMR) has been declared as one of the top ten global public health threats (1). There were an estimated 4.95 million deaths globally due to AMR in 2019, in which bacterial AMR alone was directly responsible for killing up to 1.27 million people (2). A World Bank report projected that a high AMR impact scenario could lead to a 3.8% decline in global annual GDP by 2050 (3). Humans are exposed to AMR through interconnected pathways, including healthcare, agriculture, animals and environment (4). Figure 1 illustrates the interlinked factors that drive AMR in the environment. However, the reality is more complex than depicted in the figure. AMR predominantly occurs from overuse of antimicrobials, that creates selective pressure, leading to the development of resistance against antimicrobials (5). Recent COVID-19 pandemic has significantly increased the use of antibiotics (6). This rise was influenced by several factors, including uncertainty in early diagnosis of COVID, limited treatment guidelines, concern over secondary bacterial infections, and overwhelmed healthcare systems (7). Besides the overuse of antibiotics, other factors such as socio-cultural and economic factors also influence the AMR spread (8). Vulnerable groups with pre-existing health conditions and high burden of diseases are most susceptible to AMR. For instance, remote Indigenous communities in Australia, who experience a high disease burden recorded high rates of azithromycin resistance, methicillin resistant Staphylococcus aureus and gram-negative resistance (9–11). Behavioral factors such as unnecessary antibiotic use, over the counter access to antibiotics without a prescription in low and middle-income countries (LMICs) and inappropriate disposal of antibiotics, can also affect the spread of AMR (12).
Overuse of antimicrobials is not exclusive to humans as large quantities of antimicrobials are use in food-producing animals (13). In healthcare settings, AMR surveillance is mainly used to guide immediate actions such as selecting the right antimicrobials or changing how antimicrobials are used (14). However, for environmental AMR, it is harder to link any results directly to immediate actions. One of the main reasons is that antimicrobial resistance genes (ARGs) naturally exist in the environment, such as in soil even with no known anthropogenic activity (15). The extent to which anthropogenic activities, such as the release of antimicrobials into the environment influences the naturally occurring AMR in environmental settings is largely unknown. There is no baseline data for AMR in environmental settings where antimicrobial residues accumulate through runoff. Next, how ARGs and antibiotic residues changes over time due to anthropogenic activities is largely unexplored. Estimating the level of AMR in these environments is difficult without sufficient baseline data that serve as a proper reference point for effective monitoring and evaluation of interventions.
AMR in the face of climate change
According to the Lancet Countdown on Health and Climate Change, climate change has gradually increased disease risks which is expected to have significant impacts on the emergence and severity of AMR (16). Temperature variability and extreme weather events, such as heatwaves are increasing in frequency (17). Warmer temperatures have led to the emergence of climate-sensitive infections and are also associated with accelerating bacterial reproduction, which enhances the possibilities of horizontal gene transfer (18, 19). Elevated temperatures also influence heavy metal concentrations in the environment and their uptake by bacteria which can contribute to the proliferation of AMR (20). Climate uncertainties increase farmers reliance on antimicrobials as a defense against disease outbreaks and reduced crop yields (21). Other climate-mediated consequences, such as droughts exacerbate water and food insecurity, can lead to weakened immune systems, malnutrition, and increased susceptibility to infections (22). Floods also increase the risks of displacing populations and poor sanitation, which make ideal circumstances for infection spread (23). These challenges are further worsened by poor living conditions, overcrowded spaces and substandard housing, limited access to clean water and healthcare in low resource settings. Thus climate change is an underappreciated but critical driver of AMR, and hence, public health strategies must account for these emerging risks.
Global efforts in AMR surveillance
The World Health Assembly initiated global action plans to tackle AMR, which vary for LMICs and high-income countries (HICs) (24). In LMICs, the focus is on issues like poor regulatory enforcement, and the unrestricted use of antibiotics; while in HICs, such as the European Union, action plans aim to strengthen AMR knowledge through surveillance, improve infection control and raise public awareness. Nevertheless, antibiotic usage is well monitored in healthcare settings among HICs; environmental settings need to be monitored to get a clear picture of AMR occurrence and propagation.
The World Health Organization (WHO) has made global efforts to enhance the AMR data collection through Global Antimicrobial Resistance and Use Surveillance System (GLASS) (25). GLASS collaborates with many regional AMR networks such as the Central Asian and European Surveillance of Antimicrobial Resistance (CAESAR), the European Antimicrobial Resistance Surveillance Network (EARS-Net), the Latin American Network for Antimicrobial Resistance Surveillance (ReLAVRA), and the Western Pacific Regional Antimicrobial Consumption Surveillance System (WPRACSS). Over 100 countries have participated in this surveillance till date. Despite the wealth of data collected through GLASS, some challenges continue in understanding and interpreting this data due to variable data quality across diverse regions and healthcare. GLASS report also indicated that many countries lack sufficient surveillance data on AMR, particularly LMICs. Another initiative, Global Antibiotic Research & Development Partnership (GARDP) provides data on AMR surveillance in LMICs (26). Recently, the G7 compliance report on AMR also emphasized on the AMR data collection across human, animal, and environmental health sectors in alignment with the “One Health” framework (27).
Limited research on environmental AMR
Although global efforts have been made to improve AMR surveillance, research studies are more focused on clinical settings. Substantial efforts have been made toward monitoring AMR in clinical and veterinary settings (28). At present, many studies have investigated AMR in relation to humans, largely from the health perspective, followed by slightly fewer studies showing interest in the spread of AMR among animals and significantly less focusing on environmental factors contributing to AMR emergence. Given that the environment plays a significant role in the emergence and spread of AMR, it is important to develop more holistic approaches that comprise both healthcare and environmental. The absence of environmental surveillance is a critical gap in AMR research and limits the ability to provide evidence-based recommendations to policymakers. This imbalance emphasizes the pressing need for research aimed at elucidating the mechanisms underlying AMR development in environmental contexts.
One significant challenge is the lack of understanding of which environmental settings are most susceptible to AMR transmission. Such understanding is essential for targeted surveillance and development of interventions. High-risk environments where AMR transmission is most likely to occur need to be clearly defined. Another major obstacle is the research funding. Generally, clinical research, such as hospital infections related AMR receives more funding than research in environmental settings. Funding is generally allocated to antimicrobial stewardship in the healthcare sector. Funding for the One Health initiative is crucial to address the environmental AMR. A recent One Health initiative SAAFE AMR surveillance program funded by Australian Government recognizes the interconnection between people, animals, plants and their shared environments (29). More programs like this are needed to tackle this complex AMR issue.
Future directions
Local AMR surveillance through a multi-disciplinary approach
Global efforts are essential for AMR mitigation, but local solutions that can address the root causes of resistance in specific settings are also equally important. Several local factors can influence the AMR emergence. For instance, social determinants of health such as limited access to healthcare, poor housing and sanitation and geographical remoteness are well-known AMR contributors (8). Traditional farming practices, such as the use of fertilizers and economic pressure to increase production, could impact antimicrobial use. Such factors can be monitored through local surveillance that can provide insight into the micro-dynamics of AMR at ground levels. Local surveillance enables early detection of AMR reservoirs. This can be achieved by establishing local AMR monitoring networks among local/place-based researchers, health workers, farmers, community members and stakeholders which can help in identifying high-risk settings and prioritize local needs.
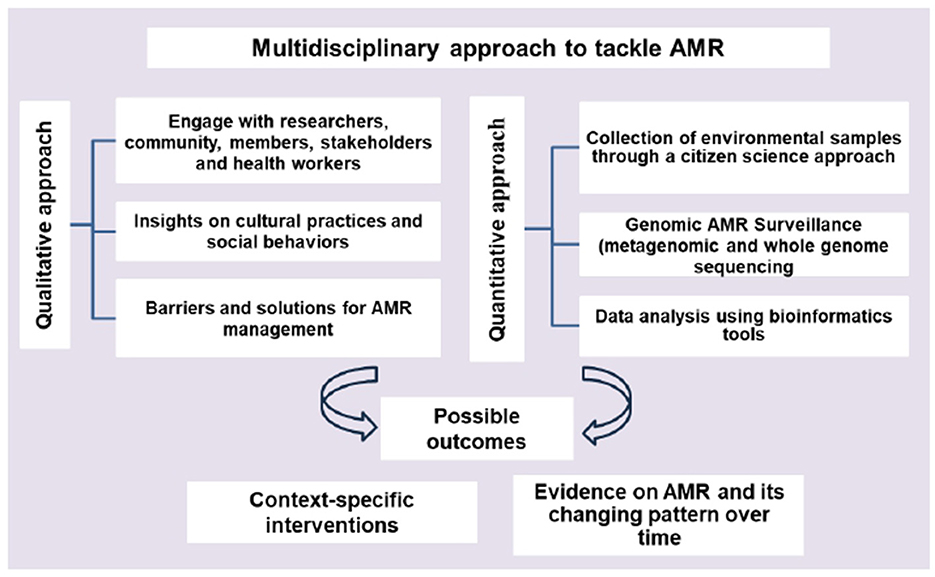
A combined qualitative and quantitative approach (Figure 2) can offer a comprehensive understanding, context and depth of the problem. Qualitative approaches can provide insights into how cultural practices and social behaviors influence the use of antimicrobials (30). Knowing the concerns of the communities and collecting environmental data through a citizen science approach can unlock many benefits, such as improving data quantity and quality, engaging communities and stakeholders and increasing awareness. Samples that citizen scientists can gather might come from a broader range of locations. Metagenomics including whole genome sequencing (WGS) can provide useful information about how resistance spreads within and between different reservoirs (31). Recently, many studies showed the potential of machine learning methods in predicting AMR with WGS methods (32). Using these techniques in local settings could facilitate rapid data collection and analysis of resistant genes and strains, with outcomes that support implementation of context specific interventions. However, the application of genomic approaches has been mostly restricted to research, and a lack of awareness, global co-ordination and political will to invest in using these technologies in active monitoring is a major obstacle that must be overcome.
Key recommendations
• Stable funding is critical for long-term AMR monitoring. More funding should be allocated towards multi-disciplinary and One Health approaches.
• Continuous monitoring of antimicrobial use, and AMR awareness among the public and stakeholders could help in AMR surveillance and stewardship.
• Investing in scalable and locally informed AMR surveillance strategies is essential to achieve long-term AMR control at both the community and global levels.
• Capacity building among researchers, stakeholders, general practitioners, nurses and community members is needed.
• Parallel efforts should be carried out to reduce antimicrobial use and spread of infections in humans and animals.
Author contributions
RT: Writing – original draft, Writing – review & editing, Conceptualization. HD: Writing – review & editing. TW: Writing – review & editing. SM: Writing – review & editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to acknowledge NHMRC TCR grant 2039696 to support this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO. Antimicrobial resistance (2023) Available online at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (Accessed April 25, 2025).
2. Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. (2022) 399:629–55. doi: 10.1016/S0140-6736(21)02724-0
3. Jonas OB, Irwin A, Berthe FCJ, Le Gall FG, Marquez PV. Drug-resistant infections: a threat to our economic future (Vol. 2 of 2): final report (English) HNP/Agriculture Global Antimicrobial Resistance Initiative Washington, DC: World Bank Group. (2017).
4. Ukhurebor KE, Athar H, Adetunji CO, Aigbe UO, Onyancha RB, Abifarin O. Environmental implications of petroleum spillages in the Niger Delta region of Nigeria: a review. J Environ Manage. (2021) 293:112872. doi: 10.1016/j.jenvman.2021.112872
5. Endale H, Mathewos M, Abdeta D. Potential causes of spread of antimicrobial resistance and preventive measures in one health perspective-a review. Infect Drug Resist. (2023) 16:7515–45. doi: 10.2147/IDR.S428837
6. Fukushige M, Ngo NH, Lukmanto D, Fukuda S, Ohneda O. Effect of the COVID-19 pandemic on antibiotic consumption: a systematic review comparing 2019 and 2020 data. Front Public Health. (2022) 10:946077. doi: 10.3389/fpubh.2022.946077
7. Subramanya SH, Czyż DM, Acharya KP, Humphreys H. The potential impact of the COVID-19 pandemic on antimicrobial resistance and antibiotic stewardship. VirusDisease. (2021) 32:330–7. doi: 10.1007/s13337-021-00695-2
8. Wozniak TM, Cuningham W, Ledingham K, McCulloch K. Contribution of socio-economic factors in the spread of antimicrobial resistant infections in Australian primary healthcare clinics. J Glob Antimicrob Resist. (2022) 30:294–301. doi: 10.1016/j.jgar.2022.06.005
9. Hare KM, Grimwood K, Chang AB, Chatfield MD, Valery PC, Leach AJ, et al. Nasopharyngeal carriage and macrolide resistance in Indigenous children with bronchiectasis randomized to long-term azithromycin or placebo. Eur J Clin Microbiol Infect Dis. (2015) 34:2275–85. doi: 10.1007/s10096-015-2480-0
10. Macmorran E, Harch S, Athan E, Lane S, Tong S, Crawford L, et al. The rise of methicillin resistant Staphylococcus aureus: now the dominant cause of skin and soft tissue infection in Central Australia. Epidemiol Infect. (2017) 145:2817–26. doi: 10.1017/S0950268817001716
11. Turnidge JD, Gottlieb T, Mitchell DH, Coombs GW, Daly DA, Bell JM. Community-onset Gram-negative Surveillance Program annual report, 2012. Commun Dis Intell Q Rep. (2014) 38:E54–8. doi: 10.33321/cdi.2014.38.11
12. Kanan M, Ramadan M, Haif H, Abdullah B, Mubarak J, Ahmad W, et al. Empowering low- and middle-income countries to combat amr by minimal use of antibiotics: a way forward. Antibiotics. (2023) 12:1504. doi: 10.3390/antibiotics12101504
13. Allel K, Day L, Hamilton A, Lin L, Furuya-Kanamori L, Moore CE, et al. Global antimicrobial-resistance drivers: an ecological country-level study at the human-animal interface. Lancet Planet Health. (2023) 7:e291–303. doi: 10.1016/S2542-5196(23)00026-8
14. Salam MA, Al-Amin MY, Salam MT, Pawar JS, Akhter N, Rabaan AA, et al. Antimicrobial resistance: a growing serious threat for global public health. Healthcare. (2023) 11:1946. doi: 10.3390/healthcare11131946
15. Larsson DGJ, Flach CF. Antibiotic resistance in the environment. Nat Rev Microbiol. (2022) 20:257–69. doi: 10.1038/s41579-021-00649-x
16. Beggs PJ, Trueck S, Linnenluecke MK, Bambrick H, Capon AG, Hanigan IC, et al. The 2023 report of the MJA-Lancet Countdown on health and climate change: sustainability needed in Australia's health care sector. Med J Aust. (2024) 220:282–303. doi: 10.5694/mja2.52245
17. Ibáñez A, Garrido-Chamorro S, Barreiro C. Microorganisms and Climate Change: A Not So Invisible Effect. Microbiol Res. (2023) 14:918–47. doi: 10.3390/microbiolres14030064
18. Omazic A, Bylund H, Boqvist S, Högberg A, Björkman C, Tryland M, et al. Identifying climate-sensitive infectious diseases in animals and humans in Northern regions. Acta Vet Scand. (2019) 61:53. doi: 10.1186/s13028-019-0490-0
19. Awad DA, Masoud HA, Hamad A. Climate changes and food-borne pathogens: the impact on human health and mitigation strategy. Clim Change. (2024) 177:92. doi: 10.1007/s10584-024-03748-9
20. Magnano San Lio R, Favara G, Maugeri A, Barchitta M, Agodi A. How antimicrobial resistance is linked to climate change: an overview of two intertwined global challenges. Int J Environ Res Public Health. (2023) 20:1681. doi: 10.3390/ijerph20031681
21. Singh BK, Delgado-Baquerizo M, Egidi E, Guirado E, Leach JE, Liu H, et al. Climate change impacts on plant pathogens, food security and paths forward. Nat Rev Microbiol. (2023) 21:640–56. doi: 10.1038/s41579-023-00900-7
22. Agostoni C, Baglioni M, La Vecchia A, Molari G, Berti C. Interlinkages between climate change and food systems: the impact on child malnutrition-narrative review. Nutrients. (2023) 15:416. doi: 10.3390/nu15020416
23. Yusuff SI, Tajudeen YA, Oladunjoye IO, Oladipo HJ, Bolarinwa OV, Popoola OT, et al. The need to increase antimicrobial resistance surveillance among forcibly displaced persons (FDPs). Trop Dis Travel Med Vaccin. (2023) 9:12. doi: 10.1186/s40794-023-00198-6
24. WHO. Monitoring and evaluation of the global action plan on antimicrobial resistance (2019) Available online at: https://iris.who.int/bitstream/handle/10665/325006/9789241515665-eng.pdf.
25. WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) (2015) Available online at: https://www.who.int/initiatives/glass (Accessed April 25, 2025).
26. GARDP. New partnership to improve access to new antibiotics in low- and middle-income countries to boost efforts to combat TB and AMR (2024). Available online at: https://gardp.org/new-partnership-to-improve-access-to-new-antibiotics-in-low-and-middle-income-countries-to-boost-efforts-to-combat-tb-and-amr/ (Accessed April 30, 2025)
28. Bengtsson-Palme J, Abramova A, Berendonk TU, Coelho LP, Forslund SK, Gschwind R, et al. Towards monitoring of antimicrobial resistance in the environment: For what reasons, how to implement it, and what are the data needs? Environ Int. (2023) 178:108089. doi: 10.1016/j.envint.2023.108089
29. SAAFE. Australia's Cooperative Research Centre for solving Antimicrobila Resistance 2024 Available online at: https://www.crcsaafe.com.au/ (Accessed April 30, 2025)
30. Nardulli P, Ballini A, Zamparella M, De Vito D. The role of stakeholders' understandings in Emerging antimicrobial resistance: a one health approach. Microorganisms. (2023) 11:2797. doi: 10.3390/microorganisms11112797
31. Díaz-Torres O, Los Cobos EOV, Kreft JU, Loge FJ, Díaz-Vázquez D, Mahlknecht J, et al. A metagenomic study of antibiotic resistance genes in a hypereutrophic subtropical lake contaminated by anthropogenic sources. Sci Total Environ. (2024) 927:172216. doi: 10.1016/j.scitotenv.2024.172216
Keywords: antimicrobial use, climate change, environment surveillance, multi-disciplinary approach, metagenomics
Citation: Thakur R, Dhar H, Wozniak TM and Mathew S (2025) Addressing the overlooked frontier in AMR research and surveillance. Front. Public Health 13:1625515. doi: 10.3389/fpubh.2025.1625515
Received: 13 May 2025; Accepted: 29 July 2025;
Published: 02 September 2025.
Edited by:
Paolo Lauriola, International Society Doctors for the Environment (ISDE), ItalyReviewed by:
Giovanna Liguori, Azienda Sanitaria Locale di Foggia, ItalyStefano Zona, Local Health Unit of Modena, Italy
Copyright © 2025 Thakur, Dhar, Wozniak and Mathew. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rishu Thakur, cmlzaHUudGhha3VyQG1lbnppZXMuZWR1LmF1
 Rishu Thakur
Rishu Thakur Hena Dhar
Hena Dhar Teresa M. Wozniak3
Teresa M. Wozniak3