- 1Department of Respiratory and Critical Care Medicine, Zhejiang University School of Medicine Second Affiliated Hospital Linping Campus, Hangzhou, Zhejiang, China
- 2Department of General Practice, Yunhe Street Community Health Service Center, Hangzhou, Zhejiang, China
Background: Klebsiella pneumoniae (KP), a prominent member of the Enterobacteriaceae family, is recognized as an opportunistic pathogen responsible for a variety of diseases. Despite its significant threat to public health, there is a lack of epidemiological information concerning the burden of KP infection in the lower respiratory tract.
Methods: Age-standardized rates (ASR) of disability-adjusted life-years (DALYs) and deaths rates (ASDRs) attributed to KP infection were obtained from Global Burden of Disease (GBD) 2021, stratified by sex, age, socio-demographic Index (SDI) quintiles and seven super regions. We also calculated the average annual percentage changes (AAPCs) of ASR-DALYs and ASDRs for KP infection using the Joinpoint regression analysis to evaluate the trend of disease burden.
Results: In 2021, the global ASR-DALYs and ASDRs attributable to KP infection were 124.4 and 2.68 per 100,000, with AAPCs of −3.23% and −2.42%, respectively. The highest burden of ASR-DALYs was observed in children under 5 years of age, with a rate of 775.75 per 100,000 (95% uncertainty interval [UI]: 601.07 to 973.76), while the highest ASDRs were found in individuals over 70 years of age, with a rate of 18.05 per 100,000 (95% UI: 15.84–19.70). Notably, there were significant increasing trends in DALYs and death rates due to KP infection in Central Europe, Eastern Europe, and Central Asia across all age groups above 15 years, with the most pronounced increase observed in individuals over 70 years of age, characterized by AAPCs of 0.85% (95% confidence interval [CI]: 0.64 to 1.05) and 1.00% (95% CI: 0.85–1.17), respectively.
Conclusion: Over the past 32 years, the global burden of KP infection in the lower respiratory tract has generally declined, but it has increased among the older population in Central/Eastern Europe and Central Asia. This rise is likely due to inappropriate antibiotic use, widespread antimicrobial resistance, emerging virulent and multidrug-resistant strains, and an aging population, highlighting the need for vigilant monitoring and intervention measures.
Introduction
Klebsiella pneumoniae (KP), a Gram-negative rod-shaped member of the Enterobacteriaceae family, colonizes the human gut and oropharynx asymptomatically but transforms into a formidable pathogen under immunosuppressed conditions (1, 2). KP infection in the lower respiratory tract was initially associated with community-acquired pneumonia (CAP) in populations of diabetics and alcoholics. However, since the advent of the antibiotic era, it has evolved into a major healthcare-associated pathogen (HAP). Currently, KP is the causative agent of severe nosocomial infections, including pneumonia, urinary tract infections (UTIs), cystitis, surgical wound infections and life-threatening infections like endocarditis and septicemia. These infections predominantly affect inpatients and immunocompromised individuals, especially those who have been using antibiotics for an extended period or are undergoing invasive medical procedures (3).
KP infection presents a significant public health threat due to its strong pathogenic potential and close association with multidrug resistance. The bacterium rapidly disseminates in healthcare settings through the production of antibiotic resistance genes, particularly carbapenemases (e.g., KPC, NDM, VIM, OXA-48-like), leading to difficult-to-treat infections (3, 4). In China, KP infection accounts for 11.9% of ventilator-associated and ICU-acquired pneumonia cases, with severe infection rates in neonatal units ranging from 18% to 68% (5, 6). The escalating threat posed by KP infection is further intensified by the global rise in extended-spectrum β-lactamase (ESBL)-producing and carbapenem-resistant (CR) strains (7–9). ESBL-producing Klebsiella pneumoniae (ESBL-KP) are relatively prevalent worldwide, with an average prevalence rate in humans of 32.7% (10). Furthermore, carbapenem-resistant Klebsiella pneumoniae (CRKP) also has been increasingly reported in both healthcare associated infection and environment during recent years worldwide (11, 12). In GBD 2019 Antimicrobial Resistance study, KP was associated with a greater number of deaths (1,105,000) and a burden of years of life losts (YLLs) of 31.4 million (13). The burden of KP infection is associated with more deaths and YLLs burden than Streptococcus Pneumoniae or tuberculosis (14).
However, despite the significant clinical impact of KP infection, comprehensive global data on the burden and trends of KP infection in the lower respiratory tract remain fragmented (15). Using the GBD 2021 database, we examined the DALYs and death burdens associated with KP infection in the lower respiratory tract. This study provides a comprehensive analysis of KP infection trends and variations in both temporal and spatial dimensions. Specifically, our study has the following objectives: (1) a descriptive and trend analysis of KP infection burden at global and regional levels, and (2) an investigation of spatial and temporal variations in KP infection patterns. This approach offers a more complete understanding of the global burden of KP infection and their trends over the last three decades.
Materials and methods
Data sources and disease definition
This research is a retrospective observational study that analyzes the disease burden using secondary data from the GBD 2021 database, a comprehensive collaborative initiative led by the Institute for Health Metrics and Evaluation (IHME) at the University of Washington (16, 17). As an open-access health data repository, the GBD database systematically quantifies the health loss attributed to 371 diseases and injuries and 88 risk factors across 204 countries and territories from 1990 to 2021. It serves as a critical resource for global health research and evidence-based policymaking, with continuous updates since its inception in 1990. Our analysis specifically utilized KP infection in the lower respiratory tract epidemiology metrics from this global dataset, accessible via the IHME official portal.1 Estimates of these metrics were calculated using the Bayesian hierarchical meta-regression tools including the Cause of Death Ensemble model (CODEm) for estimating fatal outcomes and YLLs, and DisMod-MR 2.1, a Bayesian meta-regression tool for evaluating nonfatal health loss (16). Detailed data sources and model methods were reported in GBD 2021 (16). In addition, GBD study employs a multi-tiered geographic classification system comprising seven super-regions: (1) Sub-Saharan Africa; (2) North Africa and Middle East; (3) South Asia; (4) Southeast Asia, East Asia and Oceania; (5) Latin America and Caribbean; (6) Central Europe, Eastern Europe and Central Asia; and (7) High-income regions. The SDI serves as a composite index of development status, showing a strong correlation with health outcomes. The 204 countries and territories were grouped into five SDI quintiles: low-SDI, low-middle-SDI, middle-SDI, high-middle-SDI, and high SDI regions (18). The age groups were divided into five categories: under 5, 5–14, 15–49, 50–69, and over 70 years.
The KP infection in the lower respiratory tract is identified by the International Classification of Diseases, Ninth Revision (ICD-9) codes (482.0) and ICD-10 codes (J15.0) for diagnosis (13).
Statistical analysis
The data used Age-standardized rates of disability-adjusted life-years (ASR-DALYs, per 100,000 population) and age-standardized death rates (ASDRs, per 100,000 population) as main indicators to measure the disease burden. The metric of disability-adjusted life years (DALYs) is calculated through dual components: years of life lost (YLLs) derived from premature mortality (calculated as deaths multiplied by standard life expectancy at death age) and years lived with disability (YLDs) determined by multiplying case numbers with condition-specific disability weights and duration of impairment (16). Death rates quantify the number of deaths in a population over a designated time or area, illustrating the ratio of deaths to the total population. The GBD is processed using standardized algorithms (e.g., CODEm, ST-GPR, and DisMod-MR) to address issues such as incompleteness, misclassification, and stochastic variability. To further enhance data reliability, the framework not only generates point estimates but also calculates the 95% uncertainty interval. The 95% UI was calculated from 1,000 simulated samples, using the 2.5th and 97.5th percentiles to determine its bounds. Using the World Health Organization World Standard Population Distribution, age-standardized rates (ASR) were produced to allow for comparisons across groups with diverse age demographic compositions, which were calculated according to the following formula:
(n represents the number of age groups; Si: represents the standard population size of the i-th age group; Ri: represents the actual age-specific rate of the i-th age group). To estimate ASR trends over time, we used Joinpoint regression software (version 4.8.0.1, National Cancer Institute) to describe the change trends of KP infection from 1990 to 2021. Since data are time series and may exhibit autocorrelation, we applied an autocorrelation correction in the Joinpoint regression analysis (19). The annual percentage change (APC) with its 95% confidence interval (CI) indicates each trend segment (20). Based on the weighted average of the segmented annual percentage change over a specified interval, the average annual percentage change (AAPC) with its 95% CI meaning annual was calculated to explore the average change rate of the ASR-DALYs and ASDRs of KP infection during 1990–2021. An upward trend is indicated when both the AAPC and the lower boundary of the 95% CI are positive, whereas a downward trend is suggested when both the AAPC and the upper boundary of the 95% CI are negative. All statistical analyses and data visualizations were conducted using R software (version 4.4.1). For the trend analysis, a p-value of less than 0.05 was considered statistically significant.
Results
Global burden trends
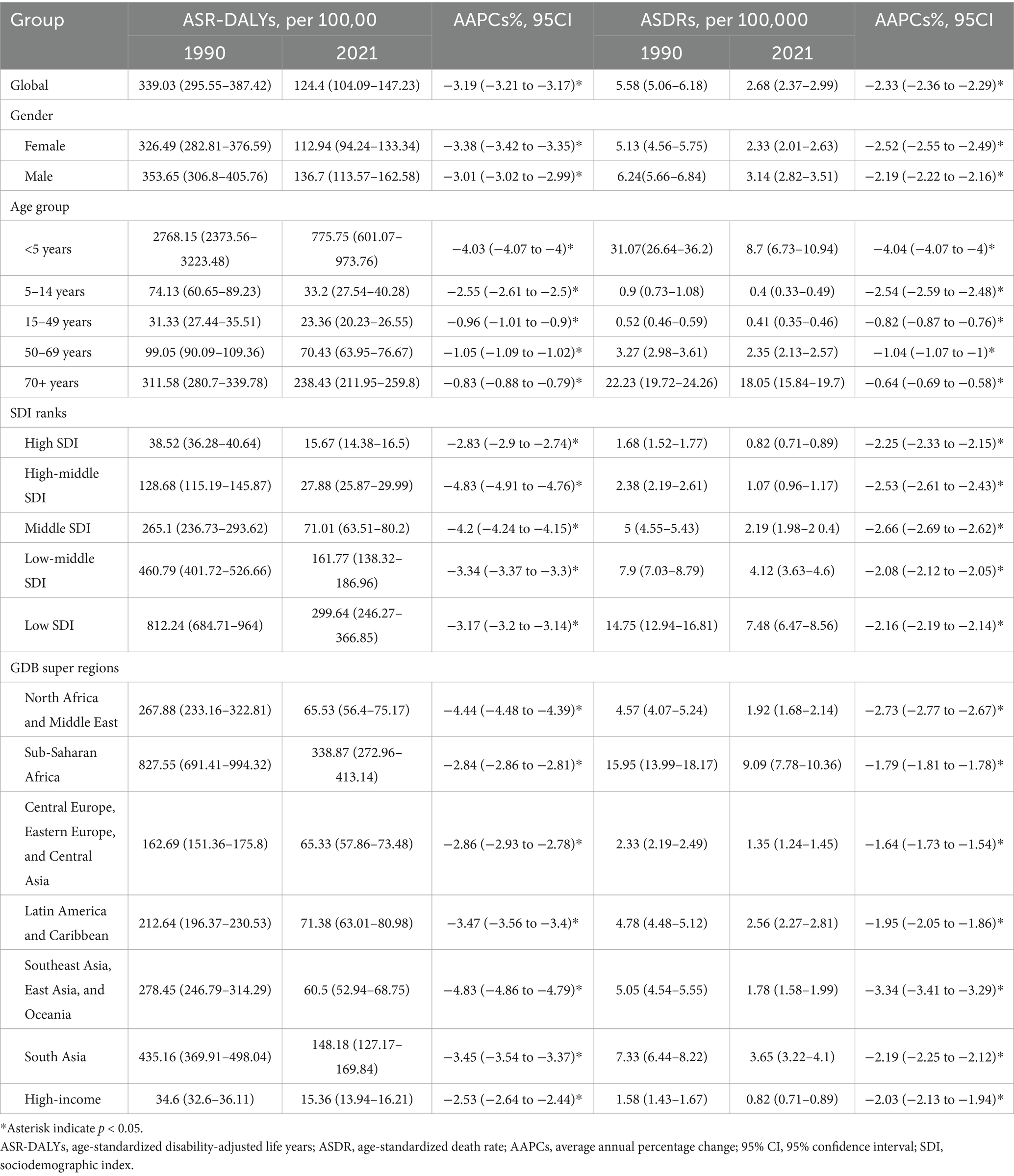
As shown in Table 1 and Figure 1, from 1990 to 2021, the global ASR-DALYs per 100,000 of KP infection decreased from 339.03 (95%UI: 295.55–387.42) to 124.4 (95%UI:104.09–147.23), with the AAPCs of −3.19 (95% CI: −3.21 to −3.17). ASDRs per 100,000 population also exhibited a downward trend during this period, declining from a rate of KP infection-related deaths at a rate of approximately from 5.58 (95% UI: 5.06–6.18) in the year 1990 to around 2.68 (95% UI: 2.37–2.99) in 2021, with the AAPCs of −2.33 (95% CI: −2.36 to −2.29). In 1990 and 2021, men experienced marginally higher ASR-DALYs and ASDRs burdens compared to women. Despite a significant decrease of ASR-DALYs in the younger than 5-year age group (AAPC = −4.03, 95% CI: −4.07 to −4.00), the burden remained the highest in 2021 (775.75 per 100,000, 95% UI: 601.07–973.76). In addition, for those over 70, ASDRs were on the decline, yet they experienced the greatest burden in 2021 compared to other age groups (18.05 per 100,000, 95% UI: 15.84–19.7).
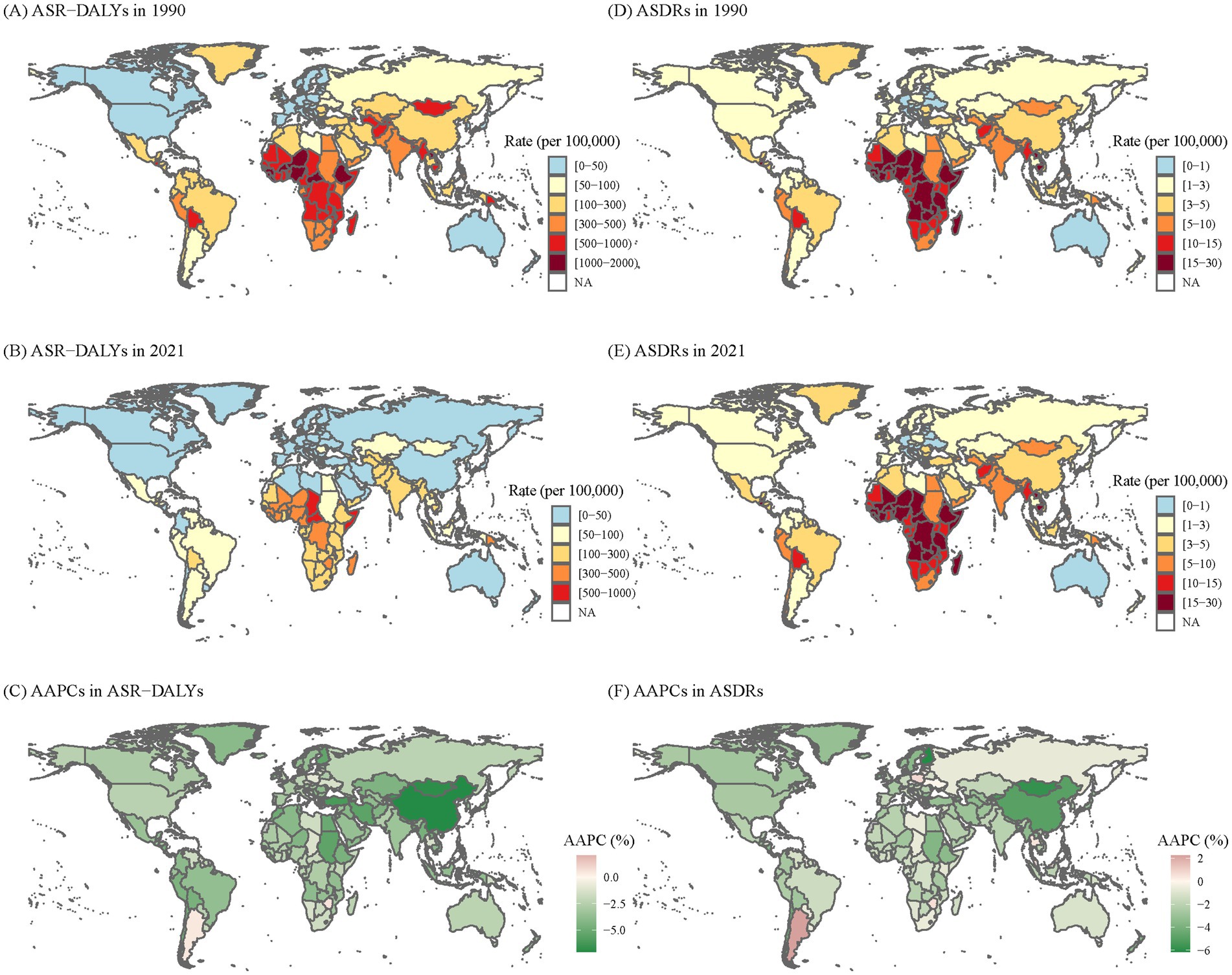
Figure 1. The global disease burden of KP infection in 204 countries and territories. (A) ASR-DALYs of KP infection in 1990. (B) ASR-DALYs of KP infection in 2021. (C) AAPCs of ASIR. (D) ASDRs of KP infection in 1990. (E) ASDRs of KP infection in 2021. (F) AAPCs of ASDRs. KP, Klebsiella pneumoniae; ASR-DALYs, age-standardized disability-adjusted life years; ASDR, age-standardized death rate; AAPCs, average annual percentage changes.
Trends of burdens among SDI quintiles
As shown in Table 1, according to SDI quintiles, the ASR-DALYs and ASDRs of KP infection exhibited a downward trend during the study period, but the burden varied considerably among different SDI regions. In 2021, the highest burden of ASR-DALYs (299.64 per 100,000, 95%UI: 246.27–366.85) and ASDRs (7.48 per 100,000, 95%UI: 6.47–8.56) both in low SDI regions; the lowest burden of ASR-DALYs (15.67 per 100,000, 95%UI: 14.38–16.5) and ASDRs (0.82 per 100,000, 95%UI: 0.71–0.89) attributable to KP infection both in high SDI regions. Among five SDI regions, the region where the ASR-DALYs decreased most significantly were in the high-middle SDI regions, with AAPCs of −4.83% (95% CI: −4.91 to −4.76).
As shown in Supplementary Table 1 and Supplementary Figure 1, the DALYs and death rates of KP infection in all age groups among SDI quintiles experienced a notable decline, with a more pronounced decline in younger than 5-year age group (AAPCs of DALYs among SDI quintiles were −5.72, −7.93%, −5.91, −4.49%, and −4.19%, respectively; AAPCs of death rates among SDI quintiles were −5.72, −7.94%, −5.91, −4.49%, and −4.20%, respectively).
Trends of burdens among super regions
As shown in Table 1 and Supplementary Figure 2, among the seven super regions defined by GBD, in 2021, the regions with the highest ASR-DALYs and ASDRs of KP infection were both in sub-Saharan Africa (338.87 per 100,000, 95% UI: 272.96–413.14; 9.09 per 100,000, 95%UI: 7.78–10.36, respectively); and the regions with the lowest ASR-DALYs and ASDRs were both in high-income regions (15.36 per 100,000, 95% UI: 13.94–16.21; 0.82 per 100,000, 95% UI: 0.71–0.89). Furthermore, the most decrease for ASR-DALYs and ASDRs of KP infection during the study period had been among the Southeast Asia, East Asia, and Oceania, with AAPCs of −4.83% (95% CI: −4.86 to −4.79) and −3.34% (95% CI: −3.41 to −3.29).
As shown in Figure 2 and Supplementary Table 2, from 1990 to 2021, the DALYs and death rates of KP infection exhibited declining trends across all super regions, with the exception of Central Europe, Eastern Europe, and Central Asia. In Central Europe, Eastern Europe, and Central Asia, the DALYs and death rates in all age groups older than 15 years had a significant increase, with AAPCs of DALYs were 0.35% (95% CI: 0.15–0.55), 0.82% (95% CI: 0.62–1.04) and 0.85% (95% CI: 0.64–1.05), while AAPCs of death rates were 0.63% (95% CI: 0.41–0.87), 0.92% (95% CI: 0.7–1.18) and 1.00% (95% CI: 0.85–1.17), respectively.
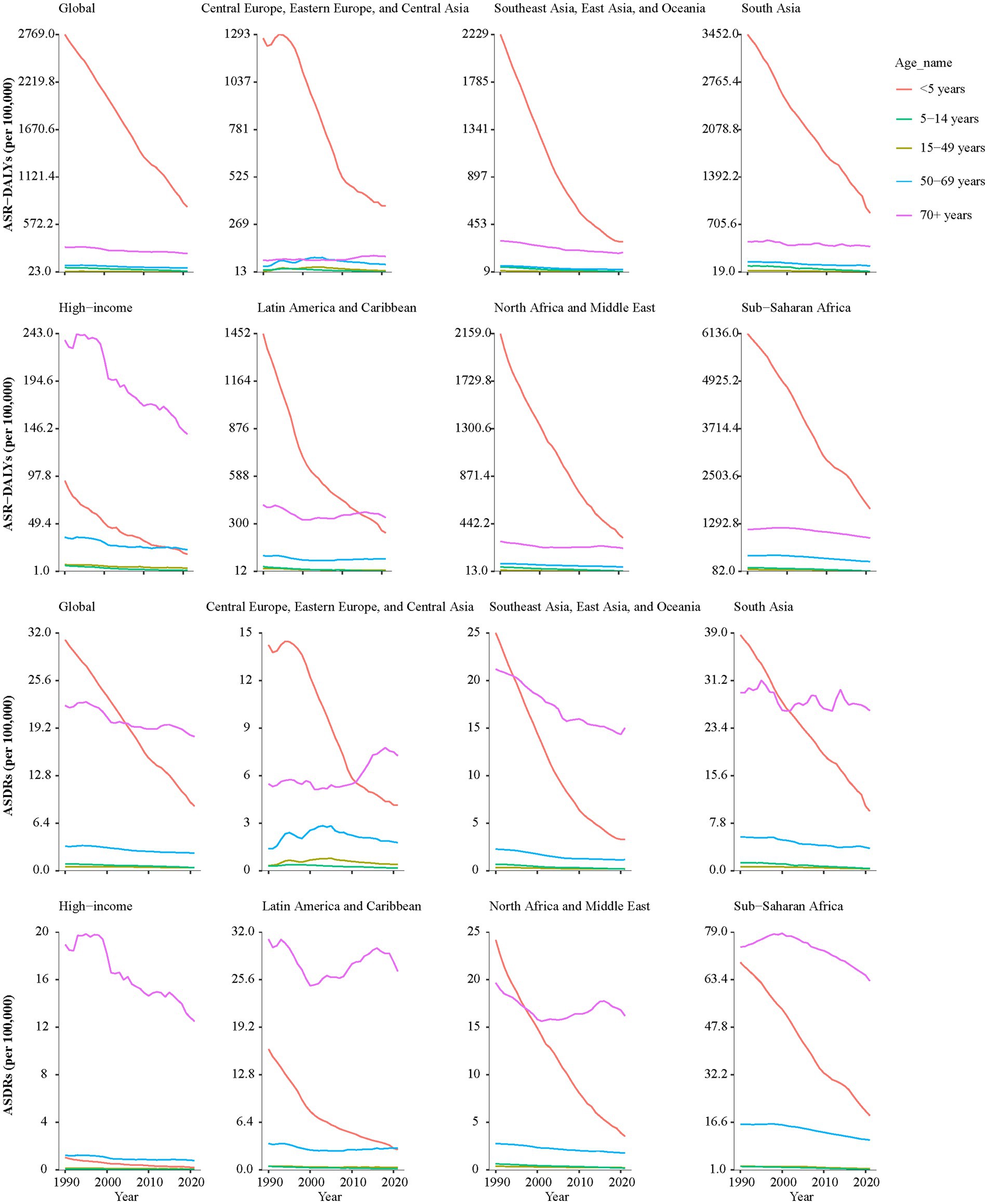
Figure 2. The AAPCs of ASR-DALYs and ASDRs of KP infection across all super regions in different age groups from 1990 to 2021. KP, Klebsiella pneumoniae; ASR-DALYs, age-standardized disability-adjusted life years; ASDR, age-standardized death rate; AAPCs, average annual percentage changes.
Relationship between the AAPCs of bureden with SDI scores
Figure 3 shows the relationships between AAPC of DALYs (Figure 3A) and death rates (Figure 3B) with SDI scores. There was no significant correlation between the AAPC of DALYs and SDI scores (r = 0.043, p = 0.53), nor between AAPC of death rates and SDI scores (r = −0.054, p = 0.44).
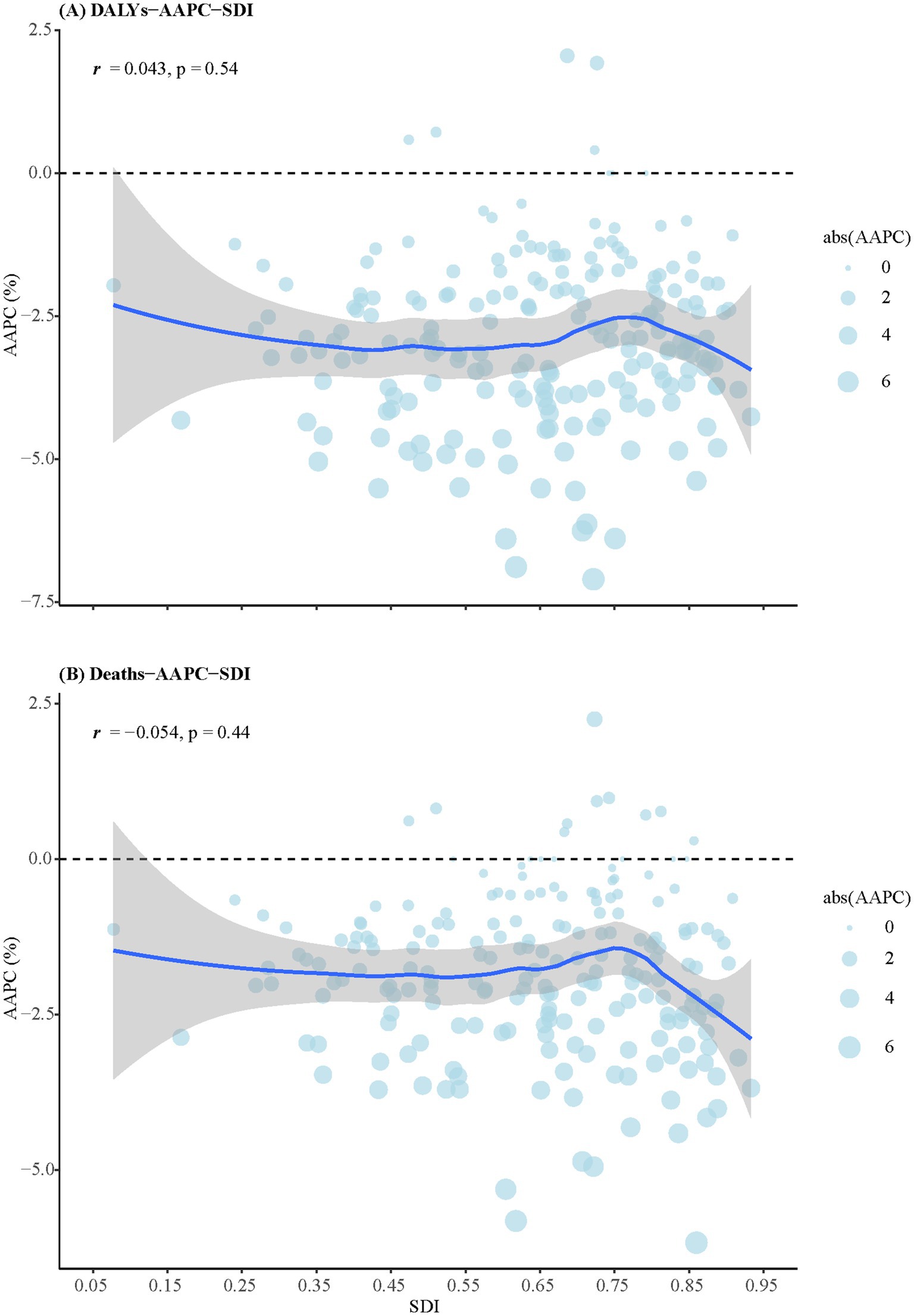
Figure 3. The relationships between AAPCs of DALYs and death rates with SDI scores. (A) DALYs. (B) Death rates. DALYs, disability-adjusted life years; SDI, sociodemographic index; AAPCs, average annual percentage changes.
Discussion
In this study, we systematically delineated the comprehensive epidemiological patterns and temporal dynamics of KP infection burden in the lower respiratory tract—including ASR-DALYs and ASDRs—across sexes, age groups SDI categories and geographic regions. Our study suggested that the worldwide decrease in ASR-DALYs and ASDRs of KP infection from 1990 to 2021. The decline in the global disease burden of KP infection is associated with multiple factors, including significantly strengthened hospital infection control measures (21), optimized antimicrobial stewardship and resistance management (22, 23), advances in medical technology and diagnostic technology (24, 25), enhanced public health interventions (26), and the synergistic effects of regional differences and global prevention and control strategies (25). However, compared to GBD level 3 underlying causes of death, five leading pathogens-related deaths, with KP being among them, have been the second leading cause of death globally in 2019 (13), suggesting that prevention and control of the disease burden of KP infection will be necessary and significant.
The global burden of KP infection varied significantly across different SDI levels. Our findings showed that the burden of KP was lowest and declining in high SDI regions, similar to trends in other infectious diseases like tuberculosis and malaria. This can be attributed to several factors, including stringent antibiotic usage protocols (encompassing precise drug selection and the restriction of broad-spectrum antibiotics) (27–29), advanced healthcare systems, and robust infection control measures (such as hand hygiene, contact isolation, and environmental disinfection) (27). Additionally, effective surveillance mechanisms (including accurate source tracing, outbreak prediction, and elucidation of transmission dynamics) (30), and significant investment in research and resources (for instance, the development of novel therapeutics and diagnostic technologies) play a crucial role (25). Although the burden of KP infection was also showing a downward trend in low SDI regions, it was still significantly heavier, particularly in sub-Saharan Africa. The results were similar to those found in previous studies (13, 31). Previous researches have suggested that the disproportionately high infection rates in low- and middle-income countries can largely be attributed to limited access to effective antimicrobials, fragile health systems, and insufficient prevention programs (32, 33). Furthermore, the frequent wars and conflicts in recent years have caused catastrophic damage to the public health system, directly leading to a significant increase in the burden of KP infections (25). Therefore, the United Nations Secretary-General has identified healthcare facilities as a critical area requiring urgent attention to achieve the Sustainable Development Goals (SDGs) by 2030 (34). In addition, targeted interventions should be designed to strengthen antimicrobial stewardship, enhance healthcare infrastructure, and resistance gene prevalence characteristics in high-burden areas (e.g., sub-Saharan Africa). However, there was no notable correlation between AAPC of DALYs or death rates and SDI scores. Disparities may arise from regional differences in infection control, healthcare access, antimicrobial resistance (AMR) issues, local antibiotic practices, diagnostic capacity, and healthcare reporting, as well as regional risk factors like demographics, comorbidities, and environmental conditions, affecting detection and reporting of KP infections independently of SDI (35, 36).
Our study found the burden of ASDRs in the age group older than 70 remained higher. KP is known for its high virulence and antimicrobial resistance (37), leading to a broad spectrum of clinical infections, particularly in older patients who are often more susceptible due to weakened immune systems and comorbidities (such as chronic lung disorders, diabetes, and malignant tumors) (38, 39). Highly virulent KP (especially those that produce ESBL and those with multi-drug resistance) is gradually increasing in the older population. The epidemiology of KP infections in geriatric care settings reveals that older patients are at increased risk for ESBL-producing infections (40). A network-based analysis study suggested that under-monitored settings such as long-term care facilities may serve as critical nodes for KP transmission (41). Meanwhile, research on antibiotic resistance in older patients with UTIs showed that KP resistance in nursing homes was similar to hospitals, indicating a comparable resistance burden (42). Another study reported that older nursing home residents have a 40% higher risk of antibiotic-resistant Enterobacteriaceae than those in the community (43). As the global population continues to age, the number of long-term care facilities is expected to rise, which will inevitably exacerbate the burden of KP infections. Notably, the DALYs and death rates associated with KP infections in Central Europe, Eastern Europe, and Central Asia had exhibited significant upward trends in all age groups older than 15 years, especially in individuals aged over 70 years. Besides antibiotic resistance and population aging, the improper use of antibiotics and the evolution of bacterial strains (particularly the high-risk clones ST258 and ST11) also contribute significantly to the burden of diseases in these areas (44, 45). And global migration accelerates KP transmission, with increased cases in countries receiving immigrants. In some European nations, crowded and unhygienic conditions for refugees exacerbate KP spread (46). These findings emphasize the importance of improving antibiotic management and monitoring drug resistance as key strategies. And preventive measures should be taken for high-risk groups, such as identifying high-risk groups, strengthening monitor the evolution of KP genome, and developing vaccines prevent KP infection.
This study is subject to certain limitations. Firstly, the GBD data may depend heavily on comprehensive statistical models due to the inconsistent quality of data, particularly in countries with limited raw data availability. These models have inherent limitations that have been discussed in other studies (47). Secondly, the GBD framework incorporates country-level data where available, utilizing advanced modeling techniques to provide accurate global burden estimates. But validating these estimates with country-level surveillance data remains challenging. To improve future estimates, we recommend strengthening the validation process with more robust country-level surveillance data. These efforts will help address current limitations and enhance the reliability of global health assessments. Finally, the underdeveloped state of economic and medical infrastructure in less developed regions poses significant challenges in diagnosing infections caused by KP, leading to an underestimated disease burden. Yadav et al. (48) reported that fewer than half of the hospitals in 10 low- and middle-income countries (LMICs) possessed the capability to conduct Gram staining. The GBD 2019 Antimicrobial Resistance Collaborators speculated that even fewer hospitals in these contexts could perform cultures and susceptibility testing (13). However, many LMICs lack systematic surveillance, hindering effective monitoring of these strains. Establishing regional AMR surveillance networks and databases is essential for reducing KP infections. To better prevent and control outbreaks, epidemiological surveillance must become more intelligent and precise, moving from passive to proactive monitoring. This involves using artificial intelligence and machine learning to predict infection risks and understand transmission, allowing for precise resource allocation and preemptive interventions (49).
Conclusion
This analysis found the persistent yet declining global burden of KP infection in the lower respiratory tract over the past three decades, evidenced by decreasing trends in ASR-DALYs and ASDRs. Nevertheless, significant disparities remain across regions, with low SDI regions, particularly sub-Saharan Africa, still experiencing the highest burden. Concurrently, the notable increase in DALYs and mortality rates among the older population in Central Europe, Eastern Europe, and Central Asia highlights emerging challenges related to inappropriate antibiotic use, widespread antimicrobial resistance, emerging virulent and multidrug-resistant strains, and an aging population. These findings emphasize the necessity for enhanced surveillance, targeted prevention strategies, and resource allocation tailored to high-risk populations and regions to mitigate the global impact of KP infections.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JJ: Formal analysis, Data curation, Writing – original draft, Funding acquisition. XL: Writing – original draft, Data curation, Formal analysis. QC: Writing – review & editing, Data curation. JW: Funding acquisition, Writing – review & editing, Data curation. LS: Formal analysis, Supervision, Data curation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by County-level Special Project of Zhejiang Province Traditional Chinese Medicine Science and Technology Plan (No. 2025ZX137), Public Welfare Scientific Research Guidance Project in the Field of Agriculture and Social Development in Hangzhou (No. 20241029Y141) and the Zhejiang Medical Health Science and Technology Program (No. 2023XY010).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1630262/full#supplementary-material
Footnotes
References
1. Pranavathiyani, G, Prava, J, Rajeev, AC, and Pan, A. Novel target exploration from hypothetical proteins of Klebsiella pneumoniae MGH 78578 reveals a protein involved in host-pathogen interaction. Front Cell Infect Microbiol. (2020) 10:109. doi: 10.3389/fcimb.2020.00109
2. Morozova, V, Babkin, I, Kozlova, Y, Baykov, I, Bokovaya, O, Tikunov, A, et al. Isolation and characterization of a novel Klebsiella pneumoniae N4-like bacteriophage KP8. Viruses. (2019) 11:1115. doi: 10.3390/v11121115
3. Navon-Venezia, S, Kondratyeva, K, and Carattoli, A. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev. (2017) 41:252–75. doi: 10.1093/femsre/fux013
4. Pitout, JD, Nordmann, P, and Poirel, L. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother. (2015) 59:5873–84. doi: 10.1128/AAC.01019-15
5. Zhang, Y, Yao, Z, Zhan, S, Yang, Z, Wei, D, Zhang, J, et al. Disease burden of intensive care unit-acquired pneumonia in China: a systematic review and meta-analysis. Int J Infect Dis. (2014) 29:84–90. doi: 10.1016/j.ijid.2014.05.030
6. Gajul, SV, Mohite, ST, Mangalgi, SS, Wavare, SM, and Kakade, SV. Klebsiella Pneumoniae in septicemic neonates with special reference to extended Spectrum β-lactamase, AmpC, Metallo β-lactamase production and multiple drug resistance in tertiary care hospital. J Lab Physicians. (2015) 7:32–7. doi: 10.4103/0974-2727.151689
7. Iredell, J, Brown, J, and Tagg, K. Antibiotic resistance in Enterobacteriaceae: mechanisms and clinical implications. BMJ. (2016) 352:h6420. doi: 10.1136/bmj.h6420
8. Lin, XC, Li, CL, Zhang, SY, Yang, XF, and Jiang, M. The global and regional prevalence of hospital-acquired carbapenem-resistant Klebsiella pneumoniae infection: a systematic review and meta-analysis. Open Forum Infect Dis. (2023) 11:ofad649. doi: 10.1093/ofid/ofad649
9. Zhang, C, Fu, X, Liu, Y, Zhao, H, and Wang, G. Burden of infectious diseases and bacterial antimicrobial resistance in China: a systematic analysis for the global burden of disease study 2019. Lancet Reg Health West Pac. (2023) 43:100972. doi: 10.1016/j.lanwpc.2023.100972
10. Ramatla, T, Mafokwane, T, Lekota, K, Monyama, M, Khasapane, G, Serage, N, et al. "One health" perspective on prevalence of co-existing extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae: a comprehensive systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. (2023) 22:88. doi: 10.1186/s12941-023-00638-3
11. Yan, Z, Li, Y, Ju, X, Wang, H, Zhang, J, Zhang, Y, et al. Dissemination of antimicrobial resistance in Klebsiella spp. from urban aquatic environments: a multi-country genomic perspective. J Adv Res. (2025):S2090-1232(25)00703-9). doi: 10.1016/j.jare.2025.09.020
12. Chuang, C, Su, CF, Lin, JC, Lu, PL, Huang, CT, Wang, JT, et al. Does antimicrobial therapy affect mortality of patients with Carbapenem-resistant Klebsiella pneumoniae bacteriuria? A Nationwide multicenter study in Taiwan. Microorganisms. (2020) 8:2035. doi: 10.3390/microorganisms8122035
13. GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2022) 400:2221–48. doi: 10.1016/S0140-6736(22)02185-7
14. GBD 2019 Tuberculosis Collaborators. Global, regional, and national sex differences in the global burden of tuberculosis by HIV status, 1990-2019: results from the global burden of disease study 2019. Lancet Infect Dis. (2022) 22:222–41. doi: 10.1016/S1473-3099(21)00449-7
15. GBD 2021 Lower Respiratory Infections and Antimicrobial Resistance Collaborators. Global, regional, and national incidence and mortality burden of non-COVID-19 lower respiratory infections and aetiologies, 1990-2021: a systematic analysis from the global burden of disease study 2021. Lancet Infect Dis. (2024) 24:974–1002. doi: 10.1016/S1473-3099(24)00176-2
16. 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2133–61. doi: 10.1016/S0140-6736(24)00757-8
17. GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 404:244. doi: 10.1016/S0140-6736(24)01458-2
18. Institute for Health Metrics and Evaluation. Global burden of disease study 2019 (GBD 2019) socio-demographic index (SDI) 1950–2019. (2023). Available online at: https://ghdx.healthdata.org/record/ihme-data/gbd-2019-socio-demographic-index-sdi-1950-2019 (Accessed February 20, 2025).
19. National Institutes of Health. (2025) Heteroscedastic/correlated errors option. Available online at: https://surveillance.cancer.gov/help/joinpoint/setting-parameters/input-file-tab/heteroscedastic-errors-option#Joinpoint_Regression_For_Correlated_Data (Accssed September 15, 2025).
20. Liu, X, Jiang, J, Yu, C, Wang, Y, Sun, Y, Tang, J, et al. Secular trends in incidence and mortality of bladder cancer in China, 1990-2017: a joinpoint and age-period-cohort analysis. Cancer Epidemiol. (2019) 61:95–103. doi: 10.1016/j.canep.2019.05.011
21. Changruenngam, S, Modchang, C, and Bicout, DJ. Modelling of the transmission dynamics of carbapenem-resistant Klebsiella pneumoniae in hospitals and design of control strategies. Sci Rep. (2022) 12:3805. doi: 10.1038/s41598-022-07728-w
22. López-Viñau, T, Muñoz-Rosa, M, Ruiz-Lara, LM, García-Martínez, L, Machuca, I, Gracia-Ahufinger, I, et al. Long-term clinical and ecological impact of an antimicrobial stewardship program on the incidence of Carbapenem-resistant Klebsiella pneumoniae infections in a high-endemic hospital. Antibiotics. (2024) 13:792. doi: 10.3390/antibiotics13090792
23. World Health Organization. Global antimicrobial resistance surveillance system (GLASS). Molecular methods for antimicrobial resistance (AMR) diagnostics to enhance the global antimicrobial resistance surveillance system. (2024). Available online at: https://www.who.int/publications/i/item/WHO-WSI-AMR-2019.1 (Accessed September 13, 2025)
24. Eskenazi, A, Lood, C, Wubbolts, J, Hites, M, Balarjishvili, N, Leshkasheli, L, et al. Combination of pre-adapted bacteriophage therapy and antibiotics for treatment of fracture-related infection due to pandrug-resistant Klebsiella pneumoniae. Nat Commun. (2022) 13:302. doi: 10.1038/s41467-021-27656-z
25. World Health Organization. Antimicrobial Resistance, Hypervirulent Klebsiella pneumoniae - Global situation. (2024). Available online at: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON527 (Accessed September 13, 2025).
26. Tsioutis, C, and Eichel, VM, Mutters, Nt,. Transmission of Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae: the role of infection control. J Antimicrob Chemother (2021); 76:i4–i11. doi: 10.1093/jac/dkaa492
27. Stefaniak, K, Kiedrzyński, M, Korzeniewska, E, Kiedrzyńska, E, and Harnisz, M. Preliminary insights on carbapenem resistance in Enterobacteriaceae in high-income and low−/middle-income countries. Sci Total Environ. (2024) 957:177593. doi: 10.1016/j.scitotenv.2024.177593
28. Akbari, M, Giske, CG, Alenaseri, M, Zarei, A, Karimi, N, and Solgi, H. Infection control interventions against carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae in an Iranian referral university hospital: a quasi-experimental study. Antimicrob Resist Infect Control. (2025) 14:48. doi: 10.1186/s13756-025-01569-8
29. Xu, Y, Liu, D, Han, P, Wang, H, Wang, S, Gao, J, et al. Rapid inference of antibiotic resistance and susceptibility for Klebsiella pneumoniae by clinical shotgun metagenomic sequencing. Int J Antimicrob Agents. (2024) 64:107252. doi: 10.1016/j.ijantimicag.2024.107252
30. Edwards, JR, Pollock, DA, Kupronis, BA, Li, W, Tolson, JS, Peterson, KD, et al. Making use of electronic data: the National Healthcare Safety Network eSurveillance initiative. Am J Infect Control. (2008) 36:S21–6. doi: 10.1016/j.ajic.2007.07.007
31. Buys, H, Muloiwa, R, Bamford, C, and Eley, B. Klebsiella pneumoniae bloodstream infections at a south African children's hospital 2006-2011, a cross-sectional study. BMC Infect Dis. (2016) 16:570. doi: 10.1186/s12879-016-1919-y
32. World Bank Group. (2025) World Bank world development indicators. Available online at: https://data.worldbank.org.cn/indicator (Accessed February 26, 2025).
33. Araya, Pablo, Hug, Julia, Joy, Genevieve, Oschmann, Felicia, and Rubinstein, Susana. The impact of water and sanitation on diarrhoeal disease burden and over-consumption of antibiotics. (2016). Available online at: https://amr-review.org/sites/default/files/LSE%20AMR%20Capstone.pdf (Accessed February 26, 2025).
34. IHME Pathogen Core Group. Global burden associated with 85 pathogens in 2019: a systematic analysis for the global burden of disease study 2019. Lancet Infect Dis. (2024) 24:868–95. doi: 10.1016/S1473-3099(24)00158-0
35. Li, W, Huang, T, Liu, C, Wushouer, H, Yang, X, Wang, R, et al. Changing climate and socioeconomic factors contribute to global antimicrobial resistance. Nat Med. (2025) 31:1798–808. doi: 10.1038/s41591-025-03629-3
36. World Health Organization. July issue of WHO bulletin focuses on health system performance assessment. (2024). Available online at: https://extranet.who.int/uhcpartnership/news/july-issue-who-bulletin-focuses-health-system-performance-assessment (Accessed September 14, 2025).
37. Boucher, HW, Talbot, GH, Bradley, JS, Edwards, JE, Gilbert, D, Rice, LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. (2009) 48:1–12. doi: 10.1086/595011
38. Chang, D, Sharma, L, Dela Cruz, CS, and Zhang, D. Clinical epidemiology, risk factors, and control strategies of Klebsiella pneumoniae infection. Front Microbiol. (2021) 12:750662. doi: 10.3389/fmicb.2021.750662
39. Seman, A, Mihret, A, Sebre, S, Awoke, T, Yeshitela, B, Yitayew, B, et al. Prevalence and molecular characterization of extended spectrum β-lactamase and carbapenemase-producing enterobacteriaceae isolates from bloodstream infection suspected patients in Addis Ababa, Ethiopia. Infect Drug Resist. (2022) 15:1367–82. doi: 10.2147/IDR.S349566
40. Gorrie, CL, Mirceta, M, Wick, RR, Judd, LM, Wyres, KL, Thomson, NR, et al. Antimicrobial-resistant Klebsiella pneumoniae carriage and infection in specialized geriatric care wards linked to acquisition in the referring Hospital. Clin Infect Dis. (2018) 67:161–70. doi: 10.1093/cid/ciy027
41. Luterbach, CL, Pasquale, DK, Henderson, HI, Cober, E, Richter, SS, Salata, RA, et al. A network analysis of carbapenem-resistant Klebsiella pneumoniae among healthcare facilities. Sci Rep. (2025) 15:27565. doi: 10.1038/s41598-025-04918-0
42. Biguenet, A, Bouxom, H, Bertrand, X, and Slekovec, C. Antibiotic resistance in elderly patients: comparison of Enterobacterales causing urinary tract infections between community, nursing homes and hospital settings. Infect Dis Now. (2023) 53:104640. doi: 10.1016/j.idnow.2022.12.005
43. Pulcini, C, Clerc-Urmes, I, Attinsounon, CA, Fougnot, S, and Thilly, N. Antibiotic resistance of Enterobacteriaceae causing urinary tract infections in elderly patients living in the community and in the nursing home: a retrospective observational study. J Antimicrob Chemother. (2019) 74:775–81. doi: 10.1093/jac/dky488
44. David, S, Reuter, S, Harris, SR, Glasner, C, Feltwell, T, Argimon, S, et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat Microbiol. (2019) 4:1919–29. doi: 10.1038/s41564-019-0492-8
45. Semenova, YA, and Mohamad,. (2025) Antimicrobial resistance study in Kazakhstan: community antibiotic consumption and resistance rates. Available online at: https://research.nu.edu.kz/en/projects/antimicrobial-resistance-study-in-kazakhstan-community-antibiotic (Accessed September 14, 2025).
46. Nellums, LB, Thompson, H, Holmes, A, Castro-Sánchez, E, Otter, JA, Norredam, M, et al. Antimicrobial resistance among migrants in Europe: a systematic review and meta-analysis. Lancet Infect Dis. (2018) 18:796–811. doi: 10.1016/S1473-3099(18)30219-6
47. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1562. doi: 10.1016/S0140-6736(20)32226-1
48. Yadav, H, Shah, D, Sayed, S, Horton, S, and Schroeder, LF. Availability of essential diagnostics in ten low-income and middle-income countries: results from national health facility surveys. Lancet Glob Health. (2021) 9:e1553–60. doi: 10.1016/S2214-109X(21)00442-3
49. Cheah, BCJ, Vicente, CR, and Chan, KR. Machine learning and artificial intelligence for infectious disease surveillance, diagnosis, and prognosis. Viruses. (2025) 17:882. doi: 10.3390/v17070882
Glossary
KP - Klebsiella pneumoniae
ASR-DALYs - age-standardized disability-adjusted life years
ASDRs - age-standardized death rates
DALYs - disability-adjusted life years
YLDs - years lived with disability
YLLs - years of life lost
AAPC - average annual percentage change
GBD - Global Burden of Disease
SDI - sociodemographic index
95% UI - 95% uncertainty intervals
95% CI - 95% confidence interval
LMICs - low- and middle-income countries
HAP - hospital-acquired pneumonia
CAP - community-acquired pneumonia
UTIs - urinary tract infection
ESBL - extended-spectrum β-lactamase
ESBL-KP - extended-spectrum β-lactamase-producing Klebsiella pneumoniae
CRKP - carbapenem-resistant Klebsiella pneumoniae
CODEm - cause of death ensemble model
SDGs - sustainable development goals
IHME - Institute for Health Metrics and Evaluation
AMR - antimicrobial resistance
Keywords: Klebsiella pneumoniae, global burden of disease, epidemiology, trend, average annual percentage changes
Citation: Ju J, Liu X, Chen Q, Wang J and Shen L (2025) Global burden and trends of Klebsiella pneumoniae infection, 1990–2021: insights from the global burden of disease study. Front. Public Health. 13:1630262. doi: 10.3389/fpubh.2025.1630262
Edited by:
Addisu Melese, Bahir Dar University, EthiopiaReviewed by:
Dongyu Wang, University of Oklahoma, United StatesPriyavardhan Mishra, Padmashree D. Y. Patil University, India
Copyright © 2025 Ju, Liu, Chen, Wang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linfeng Shen, c2xmMDkyMEBhbGl5dW4uY29t
†These authors have contributed equally to this work
 Jiangang Ju1†
Jiangang Ju1† Linfeng Shen
Linfeng Shen