- 1Eye Center, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, China
- 2Department of Toxicology of School of Public Health and Department of Gynecologic Oncology of Women's Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, China
Background: Primary acquired lacrimal duct obstruction (PALDO) is the most common lacrimal drainage disease in clinics, which can be caused by multiple factors. However, few studies have investigated environmental risk factors contributing to PALDO exacerbation. This study aimed to investigate the potential association between short-term exposure to major ambient air pollutants and outpatient visits for PALDO.
Methods: Data of outpatients with PALDO who visited the Eye Center of the Second Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, Zhejiang Province, China) from January 1, 2014 to December 31, 2022 were collected. The concentrations of particulate matter (PM10 and PM2.5), nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide (CO) and ozone (O3), as well as the meteorological factors during the same period were obtained from Resource and Environment Science and Data Center, Chinese Academy of Science. A conditional logistic regression with a time-stratified case-crossover design was conducted to analyze the association between air pollutants and outpatient visits for PALDO.
Results: In the single-pollutant model, significant associations were observed between PM10 (Odds ratio (OR) = 1.0022; 95% confidence interval (CI):1.0008, 1.004), PM2.5 (OR = 1.0025; 95% CI: 1.0004, 1.005), NO2 (OR = 1.006; 95% CI: 1.0025, 1.010), SO2 (OR = 1.0124; 95% CI: 1.0027, 1.022) and CO (OR = 1.3273; 95% CI: 1.0183, 1.73) and outpatient visits for PALDO. These associations remained significant after adjusting for the certain pollutant in the multi-pollutant model except NO2. Moreover, variations occurred between sexes, among different age groups and different seasons.
Conclusions: Our study provided new and robust evidence that short-term exposure to air pollution may increase the risk of PALDO. Further studies are needed to decipher the underlying mechanisms.
Introduction
Air pollution is a leading environmental health risk worldwide and has been associated with numerous human diseases, such as ischemic heart disease, stroke, lung cancer and chronic obstructive pulmonary disease (COPD) (1–3). According to the report of the Lancet Commission on pollution and health, air pollution can cause an estimated 9 million premature deaths each year (4). In addition, nearly 99% of the global population still exposed to unhealthy air that had failed to meet the World Health Organization (WHO) air quality guidelines (5). As the largest developing country in the world, China is experiencing remarkable social and economic growth, which also has come at a significant cost to the environment (6–9). Although the government has implemented various measures such as stricter environmental policies, technological innovation, and public health initiatives, air pollution remains a prominent public health issue right now (10–15). In recent years, increasing studies have focused on the adverse impacts of air pollution on ocular health. The morbidities of ocular diseases, such as dry eye disease, conjunctivitis, pterygium and age-related macular degeneration, have been attributed to the exposure of air pollution (16–19). However, whether air pollution could affect the lacrimal drainage diseases remains unclear.
Lacrimal drainage system (LDS) is the excretory component of the lacrimal system, providing a route for tears from the ocular surface to the nasal cavity. Besides, LDS regulates tear dynamics and contributes to tear film homeostasis, which is crucial for ocular surface health (20, 21). Anatomically, LDS consists of punctum, canaliculus, common canaliculus, lacrimal sac and nasolacrimal duct, lined by non-keratinized stratified squamous or columnar epithelium. Stenosis or blockage within any of these components would induce symptoms of epiphora and secretions, thereby resulting in lacrimal duct obstruction diseases. The patients with lacrimal duct obstruction may also suffer from one or more complications, such as conjunctivitis, keratitis, dacryocystitis and orbital cellulitis (22). Amongst the overall lacrimal drainage diseases, primary acquired lacrimal duct obstruction (PALDO) was the most common (23). In the United States, the prevalence of symptomatic acquired lacrimal duct obstruction increased and was estimated to be 30.5 cases per 100,000 persons during the period 1976–2000 (24). In fact, the etiopathogenesis of PALDO is believed to be multifactorial and has not yet been fully elucidated. To date, several studies have suggested risk factors that are implicated in PALDO, including narrowed bony nasolacrimal duct, local hormonal imbalance, nasal abnormalities, autonomic dysregulation and gastroesophageal reflux (25). However, few studies have concerned environmental risk factors.
The formation of PALDO is a complicated process, involving multiple pathological changes and both genetic and environmental risk factors. Due to the position of LDS, the luminal mucosa is constantly exposed to various environmental agents like environmental allergens, chemical contaminants and pathogenic organisms, which could induce acute or chronic inflammation within ocular surface and airways (26, 27). Although the precise pathogenesis of PALDO is unclear, evidence has shown that active inflammatory infiltrate is the common pathological character in every stage of PALDO (28). The similar pathological changes were also found in the adjacent nasal mucosa of patients with PALDO (29). Besides, the pro-inflammatory cytokines of tears were significantly elevated in PALDO patients compared to healthy controls (30). These studies have indicated that the lacrimal mucosa could also be involved in a similar inflammatory process caused by the environmental harmful factors, consequently contributing to the development of PALDO. As the well-established environmental hazards, air pollutants especially fine particulate matter < 2.5 μm in diameter (PM2.5) could deposit and elicit inflammatory response both in human corneal and bronchial epithelial cells (31, 32). Moreover, epidemiological studies have shown that increases in air pollutants are associated with outpatient visits for several ocular surface and respiratory diseases (33, 34). Therefore, it is valid to speculate that air pollutants might be detrimental to LDS and play an important role in the pathological mechanism of PALDO. However, to the best of our knowledge, no epidemiological study has been conducted to examine the relationship between short-term exposure to air pollution and risk of PALDO outpatient visits.
In the present study, we performed a time-stratified case-crossover study to investigate the effects of major ambient air pollutants, including particulate matter < 10 μm in diameter (PM10), PM2.5, nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide (CO) and ozone (O3), as well as the meteorological factors, on outpatient visits for PALDO in Hangzhou, China.
Materials and methods
Outpatient information
Daily individual cases of PALDO during the period between January 1, 2014 and December 31, 2022 were obtained from the Eye Center of the Second Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, Zhejiang Province, China), which is the biggest ophthalmology clinic in Zhejiang Province, providing eye care service for patients from all districts of Hangzhou and the surrounding area. The definition for PALDO included H04.502-H04.506 according to the International Classification of Diseases, 10th Revision (ICD-10). H04.501 was not included because it represents congenital nasolacrimal duct obstruction. The data was obtained and comprised each patient's unique number, date of first confirmation of diagnosis, age, sex and residential address. This study adhered to the tenets of the Declaration of Helsinki and approved by the ethics committee of the Second Affiliated Hospital, Medical College of Zhejiang University.
Air pollutants and meteorological data
The data of six ambient air pollutants (PM10, PM2.5, NO2, SO2, CO, and O3) from January 1, 2014 to December 31, 2022 were obtained from the Department of Ecology and Environment of Zhejiang Province. Briefly, the 24-h mean values for PM10, PM2.5, SO2, NO2, CO and the maximum 8-h value for O3 were collected from 12 fixed air-quality monitoring stations in 10 districts of Hangzhou: Yunxi, Shifudalou, Zhenerzhong, Xixi, Zhejiangnongda, Wolongqiao, Xiaofangdadui, Hemuxiaoxue, Binjiang, Xiasha, Linpingzhen and Chengxiangzhen. The meteorological data (daily mean temperature, relative humidity and atmospheric pressure) during the study period were obtained from Resource and Environment Science and Data Center, Chinese Academy of Science.
Statistical analysis
A time-stratified case-crossover design was used in this study to explore the association between air pollutants and outpatient visits for PALDO. The case-crossover design, a modified version of the traditional case-control design, has been widely used to investigate transient effects of environmental exposures on health. By design, each patient serves as his/her own control, thereby reducing potential bias from time-invariant characteristics such as sex and genotype. Moreover, slowly changing confounders like smoking and drinking can be controlled by time-stratified method, since other days of the week, month and year matched to the day of the hospital visit for PALDO are selected as control days. As controls are chosen both before and after the case, each case matches to 3–4 controls depend on the number of days of the week in a particular month.
The descriptive statistics included the description of PALDO cases, air pollutants and meteorological factors, and correlation analyses were also carried out. The correlations between air pollutants and meteorological factors were analyzed by Spearman's correlation.
The association between PALDO cases and air pollution was estimated with the odds ratios (ORs) and their 95% confidence intervals (CI), which were calculated using conditional logistic regression by comparing the concentrations of air pollutants on the case and the matched control days. The conditional logistic regression analysis was performed by using a Cox proportional hazards regression model, which is a valid and efficient approach for matched case-control studies (including case-crossover designs), leveraging the mathematical equivalence between the two methods under specific conditions (35). The odds ratios were calculated with weights equal to the number of daily PALDO outpatient visits after case controls were selected and matched. As meteorological factors may be associated with PALDO, the daily mean values of each meteorological factor and air pollutant were viewed as covariates in the following formula to adjust for possible confounding by these variables:
where t refers to the day; X refers to the outpatient visit; h(t, X) refers to risk function; h0i(t) refers to the baseline risk function; T, RH, P, C(PM10), C(PM2.5), C(NO2), C(SO2), C(CO) and C(O3) refer to the daily mean temperature, relative humidity, atmospheric pressure and corresponding concentrations of air pollutants, respectively; and β refers to the coefficient for each covariate. In the single-pollutant model, the acute effects of each air pollutant were evaluated with both a single lag model (lag 0 to lag 7) and a moving average lag model (lag 0–1 to lag 0–7) up to 7 days before the outpatient visits. In lag models, the best lag time was selected according to the maximum OR and the minimum p-values. Moreover, considering the possible interactions between air pollutants, the multi-pollutant model was applied, wherein the concentrations of copollutants in the best lags were sequentially used as variables.
The subgroup analyses were performed by sex, age and season. Specifically, patients were divided into two gender groups: male and female, and three age groups: < 40, 40–60, and >60. The whole year was divided into four seasons: spring (March to May), summer (June to August), fall (September to November) and winter (December to February), according to the seasonal characteristics of Hangzhou.
All the data management and descriptive statistics were conducted using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA). The acute effects of air pollution were performed by survival package of R 3.5.2 software. All reported p-values were based on two-sided tests and a value of P < 0.05 was considered statistically significant.
Results
A total of 2,523 outpatients from 10 districts of Hangzhou visited our eye center with the diagnosis of PALDO from January 1, 2014 to December 31, 2022. Among these patients, females (74%) and those between the ages of 40 to 60 years (48.4%) were predominant. More PALDO cases were diagnosed in the spring (30.6%) (Table 1).
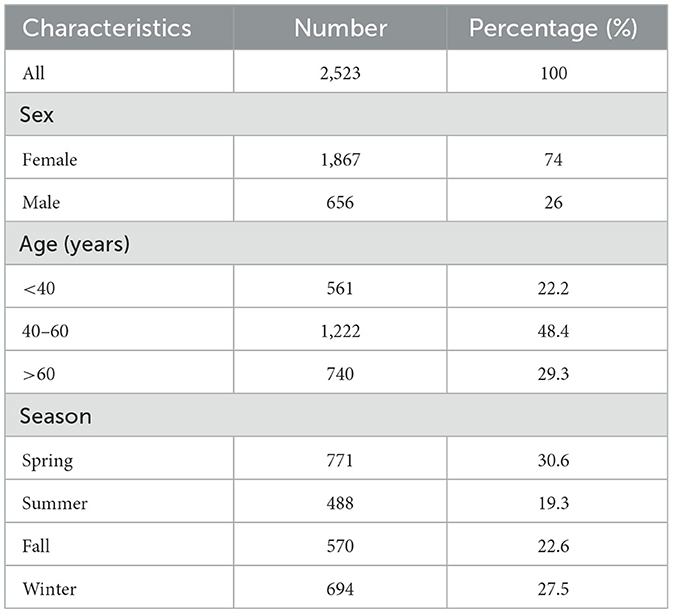
Table 1. Characteristics of outpatient for PALDO in Eye Center of the Second Affiliated Hospital, Zhejiang University School of Medicine (2014–2022).
Summary statistics of the ambient air pollutants (PM10, PM2.5, NO2, SO2, CO, and O3) and meteorological factors (temperature, relative humidity and atmospheric pressure) are shown in Table 2. The mean difference between concentrations of air pollutants measured on the case days and the average over control days was 2.93 ± 33.45 μg/m3 for PM10, 1.30 ± 22.45 μg/m3 for PM2.5, −0.16 ± 12.96 μg/m3 for NO2, 0.18 ± 5.44 μg/m3 for SO2, 0.01 ± 0.18 μg/m3 for CO, and 0.36 ± 37.72 μg/m3 for O3. Then the correlations between air pollutants and meteorological factors were analyzed. As shown in Supplementary Table S1, the daily concentrations of air pollutants were strongly correlated with each other (r = 0.49–0.94) except O3 (r = −0.27 to −0.02), and weakly or moderately correlated with meteorological factors (r = −0.46 to 0.61).
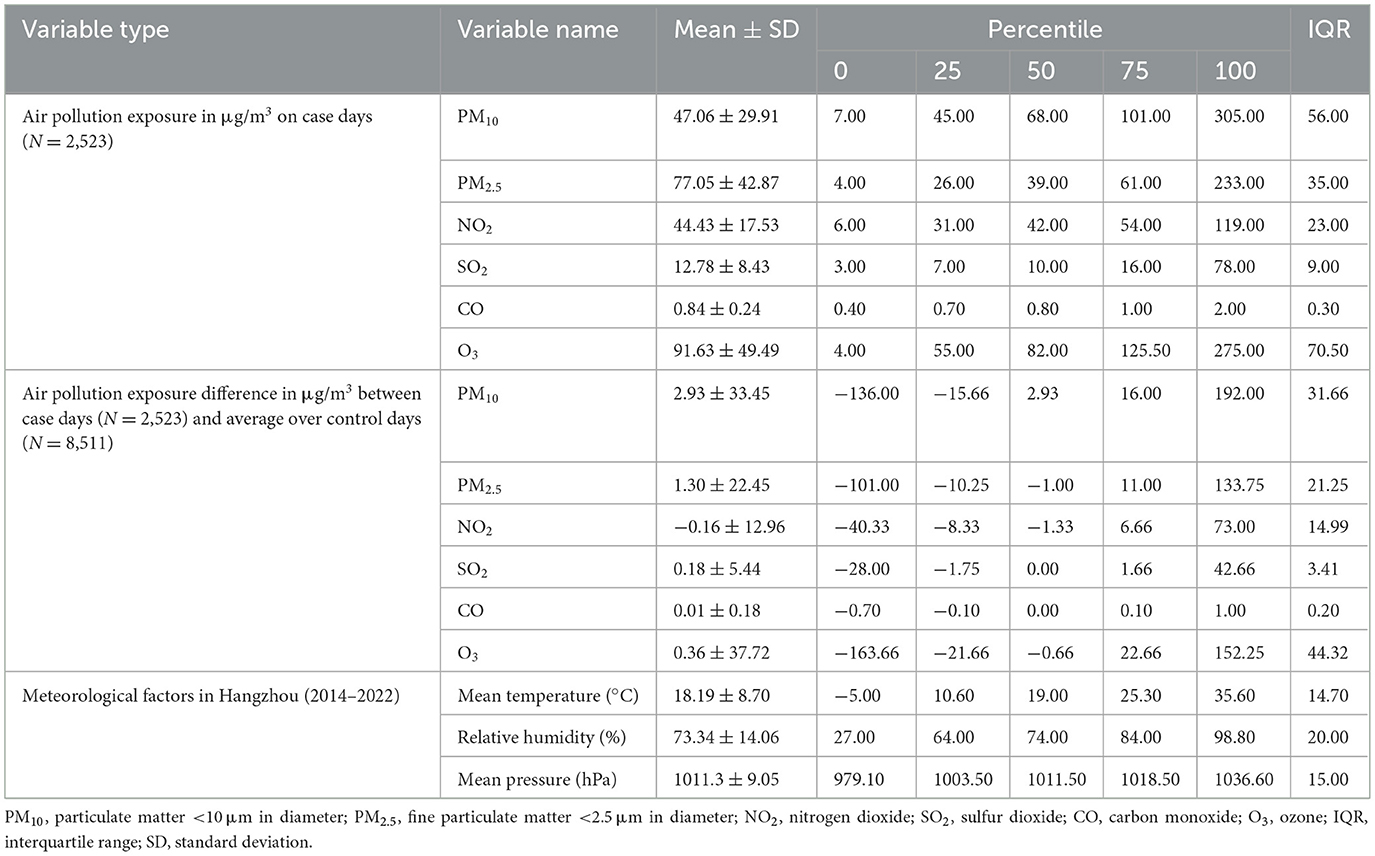
Table 2. Descriptive statistics of air pollutants, the absolute difference of air pollutants between case days and the average levels over control days, and meteorological factors in the case-crossover analysis in Hangzhou, 2014–2022.
To investigate the association between exposure to single air pollutant and risk of outpatient visits for PALDO, the single-pollutant model was applied. As shown in Figure 1 and Supplementary Table S2, the best lag times were lag 6 days for PM10, PM2.5 and CO, lag 0 days for NO2 and SO2, and lag 3 days for O3. In the single lag model, significant positive associations between air pollutants and outpatient visits for PALDO were observed with PM10 lags of 0, 2, 3, 5, 6, 7 days, with PM2.5 lag of 6 days, with NO2 lags of 0, 1, 3, 4, 5, 6, 7 days, with SO2 lag of 0 days, and with CO lag of 6 days. Positive associations were also observed in the moving average lag model from lag 0–3 to lag 0–7 for PM10, and from lag 0–1 to lag 0–7 for NO2. In our single-pollutant model, no evidence indicated the significant association between PALDO outpatient visits and O3.
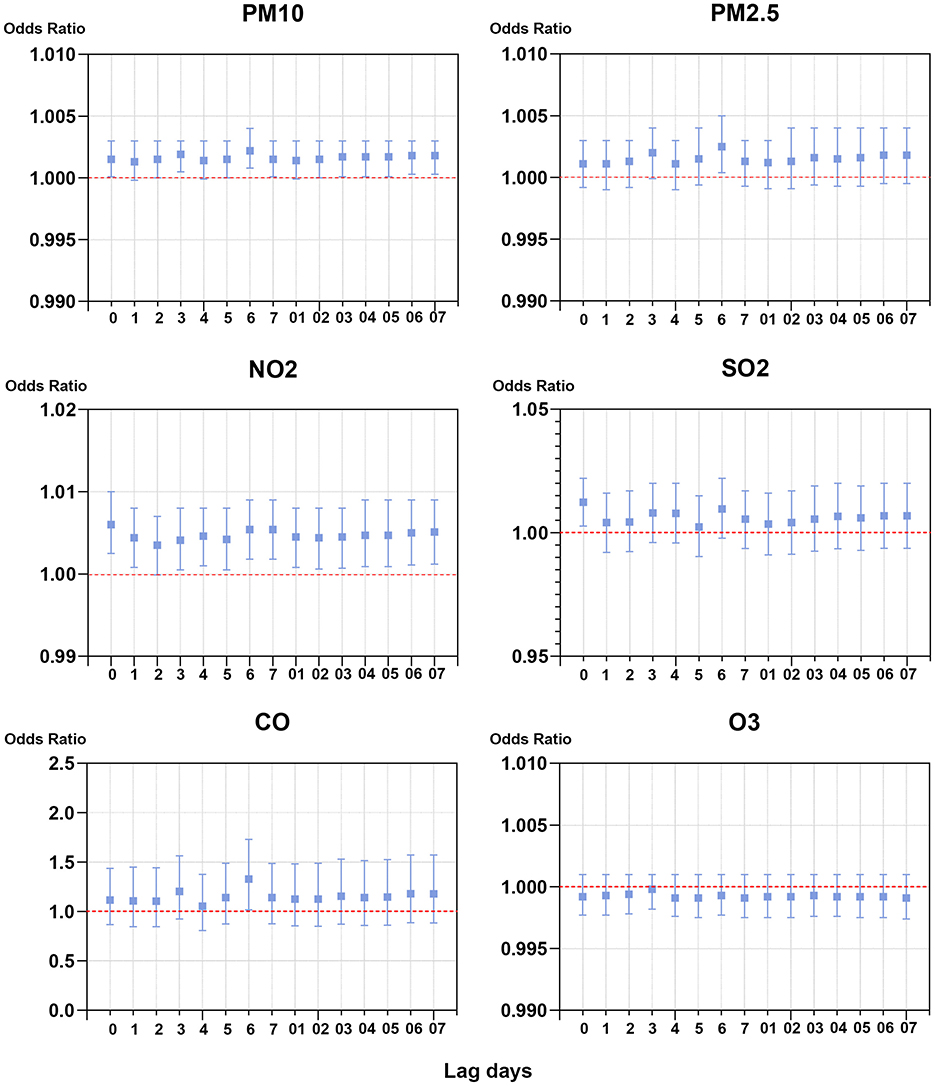
Figure 1. Associations of short-term exposure to air pollutants with outpatient visits for PALDO: single-pollutant model. Conditional logistic regression was performed using a Cox proportional hazards regression model to calculate the odds ratios (OR) and 95% confidence interval (CI). Each panel shows the association between PM10, PM2.5, NO2, SO2, CO, and O3 and PALDO cases with both a single lag model (lag 0 to lag 5) and a moving average lag model (lag 0–1 to lag 0–5). PM10, particulate matter <10 μm in diameter; PM2.5, fine particulate matter <2.5 μm in diameter; SO2, sulfur dioxide; NO2, nitrogen dioxide; CO, carbon monoxide; O3, ozone.
As the interactions between air pollutants were unavoidable, the multi-pollutant model was also used for analysis. In this model, the concentrations of air pollutants in the best lags and meteorological factors were chosen to be used as variables and controls, respectively. As previously mentioned, PM2.5 was strongly correlated with PM10 (r = 0.94) and its OR for PALDO outpatient visits was higher than that of PM10, so only PM2.5 was selected in the multi-pollutant model. Both PM10 and SO2 were significantly associated with PALDO outpatient visits, after adjusting for the other pollutants. Positive associations were also observed between PALDO outpatient visits and the pollutants PM2.5 and CO, after adjusting for a certain pollutant (Figure 2 and Supplementary Table S3).
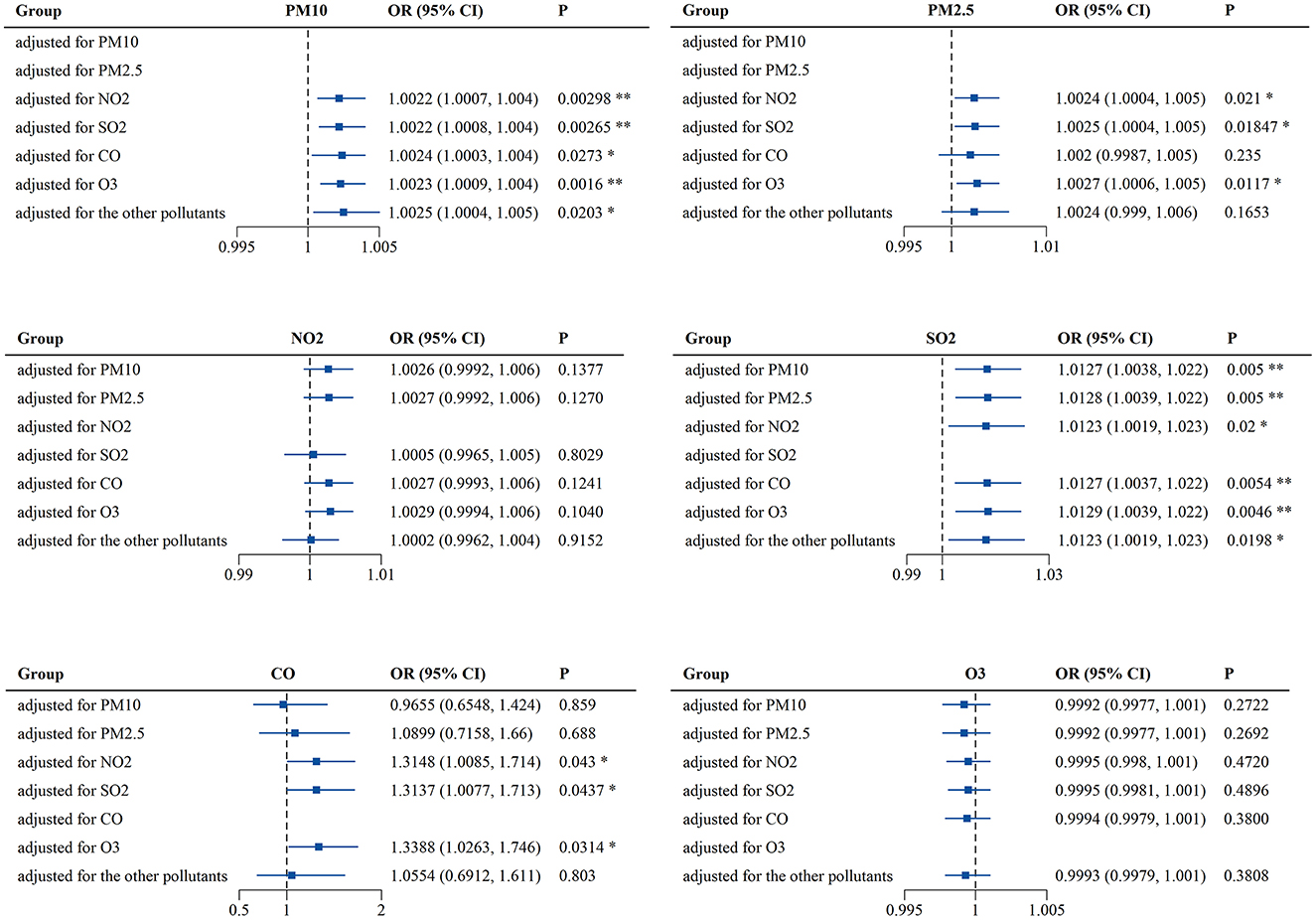
Figure 2. Associations of short-term exposure to air pollutants with outpatient visits for PALDO: multi-pollutant model. Conditional logistic regression was performed using a Cox proportional hazards regression model to calculate the odds ratios (OR) and 95% confidence interval (CI). Each panel shows the association between PM10, PM2.5, NO2, SO2, CO, and O3 and PALDO cases after adjustment for other pollutants. *P < 0.05, **P < 0.01. PM10, particulate matter <10 μm in diameter; PM2.5, fine particulate matter <2.5 μm in diameter; SO2, sulfur dioxide; NO2, nitrogen dioxide; CO, carbon monoxide; O3, ozone.
Finally, sex-, age- and season-specific associations between air pollutants and outpatient visits for PALDO were analyzed. As shown in Figure 3 and Supplementary Table S4, PM10 was significantly associated with PALDO outpatient visits for both genders, while the associations between the pollutants (PM2.5 and SO2) and PALDO outpatient visits were only significant among females. In addition, the associations were significant for PM10, PM2.5, and CO in patients with age under 40, for PM10 and SO2 in patients with age between 40 and 60, and for SO2 in patients with age over 60. The season-specific results showed significant associations for PM10 and PM2.5 in spring, as well as NO2 and SO2 in winter. Besides, PM2.5 was significantly associated with outpatient visits for PALDO in summer. The p-values for the interaction between air pollutants and sex, age and season were shown in Supplementary Table S5. There was evidence of effect modification by age groups for PM10 (p = 0.045) and by season groups for NO2 (p = 0.001).
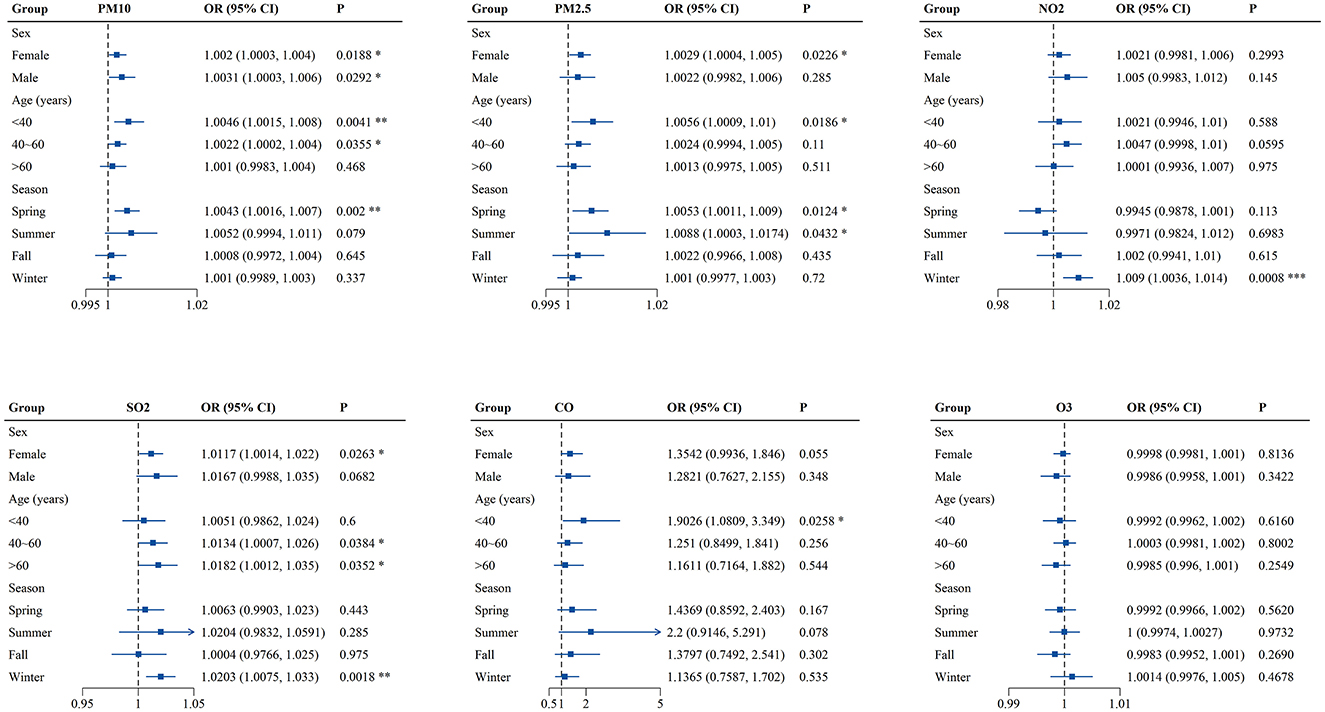
Figure 3. Associations of short-term exposure to air pollutants with outpatient visits for PALDO stratified by sex, age and season. Single-pollutant model was applied to calculate the odds ratios (OR) and 95% confidence interval (CI) in conditional logistic regression. Each panel shows sex-, age- and season-specific associations between PM10, PM2.5, NO2, SO2, CO, and O3 and PALDO cases. *P < 0.05, **P < 0.01, ***P < 0.001. PM10, particulate matter <10 μm in diameter; PM2.5, fine particulate matter <2.5 μm in diameter; SO2, sulfur dioxide; NO2, nitrogen dioxide; CO, carbon monoxide; O3, ozone.
Discussion
In the present study, we found that short-term exposure to higher levels of PM10, PM2.5, NO2, SO2, and CO was associated with increased risk of outpatient visits for PALDO in Hangzhou, China. Moreover, associations of PALDO outpatient visits with the pollutants (PM10, PM2.5, SO2, and CO) remained significant after adjusting for the certain pollutant in the multi-pollutant model. The subgroup differences associated with air pollutants varied by sex, age and season. To our knowledge, this is the first epidemiological study with a time-stratified case-crossover design to evaluate the risk of air pollution on outpatient visits for PALDO.
To date, several environmental risk factors have been reported to influence or contribute to the pathogenesis of PALDO. Tobacco smoke, one of the most common indoor pollutants, has been suspected as an etiological factor. Firstly, smoking has long been associated with dacryoliths, which are concretions formed in LDS. Early in 1965, Jones reported that the majority cases of dacryoliths were moderate or heavy smokers (36). Recent studies also have found a higher prevalence of smoking in patients with dacryoliths (37). The dacryoliths are believed to induce partial or complete obstruction of LDS, resulting in symptoms including epiphora, and acute dacryocystitis (38, 39). However, a large community based case-control study has refuted this claim by showing less prevalence of smoking among PALDO patients (40). Secondly, tobacco smoke can stimulate ocular surface and airways inflammation through an increase in inflammatory cells and the release of pro-inflammatory cytokines (41, 42). Pathological studies revealed that descending inflammation from the eye or ascending inflammation from the nose would contribute to the pathological changes of PALDO, yet severity of inflammation was not significantly different between smokers and nonsmokers with PALDO (43). Swimming pool exposure has been considered as another risk factor, as it was found to be independently associated with the development of PALDO (44). The possible explanation was chlorine, widely used to disinfect pool water, could react with ammonia in the water to form chloramines. The chloramines can cause significant eye and airways irritant symptoms, through the proposed mechanisms including inflammation, oxidative stress and hyperpermeability (45, 46). Since LDS is adjacent to the eye and nose, it would not be surprising to speculate the chloramines could also have similar effects on the luminal mucosa, which may contribute to PALDO pathogenesis. However, the pathogenic mechanism of these environmental factors on PALDO has not been clarified yet.
In this study, we evaluated the effects of six criteria air pollutants (PM10, PM2.5, NO2, SO2 CO, and O3) on outpatient visits for PALDO and found that short-term exposure to these air pollutants except O3 increased the possibility of outpatient visits. As a primary irritant, O3 can be hazardous to the eye, airways and skin owing to its oxidizing property (47). However, our results showed no significant association between O3 and PALDO outpatient visits in either single-pollutant or multi-pollutant model. The possible explanation is that the deleterious effects of O3 could take longer to present, and the relatively short analysis period may conceal the actual effect of O3 on the development of PALDO (48). In the subgroup analyses, female and young and middle-aged (< 40 and 40–60 years old) patients with PALDO were sensitive to more air pollutants. This gender difference may be related to the longer and narrowed bony nasolacrimal duct and lower levels of hormonal receptor expression in females, making them more susceptible to environmental risk factors such as air pollution (49, 50). Moreover, there are several plausible explanations for the age predilection observed in the estimates of pollutants effect by age. It is well known that young and middle-aged people are main labor force in the society, meaning that they spend more time outdoors for work and activity, so they may have greater exposure to air pollutants. On the other hand, air pollution may trigger a greater immune inflammatory response in these people due to their relatively active immune system than old people (51). Another interesting finding was that most air pollutants (PM10, PM2.5, NO2, and SO2) were significantly associated with PALDO outpatient visits in spring or winter. The differences are likely induced by seasonal differences in concentrations of air pollutants, as well as compromise of the mucosal barrier function in cold season (52). Further studies are needed to determine the dose-dependent effects of air pollutants on the pathogenesis of PALDO.
Multiple biological mechanisms could be involved in the association between air pollution exposure and the exacerbation of PALDO. First, air pollutants may damage the luminal mucosa of LDS. Several experimental studies found that PM2.5 led to cytotoxicity in human corneal epithelial cells, as well as goblet-cell hyperplasia in human conjunctiva (53, 54). In the nasolacrimal ducts from PALDO samples, ultrastructural changes were seen and characterized by focal areas of mucosal epithelial loss and basal layer hyperplasia (55). Second, inflammatory processes induced by air pollutants could be another explanation for this association. Previous studies have shown that air pollutants could stimulate both innate and adaptive immune responses, resulting in pro-inflammatory cytokines release, innate immune cells activation and B and T lymphocytes infiltration, which were also observed in the lacrimal sacs obtained from PALDO patients (56, 57). Third, air pollutants could indirectly influence the outpatient visits for PALDO with systemic comorbidities, such as hormonal imbalance, autonomic dysregulation and host defenses derangement (58–60).
It is noted that this study also has several limitations. Firstly, outpatient data from other clinics or hospitals in the city were not included, although our eye center attracted a large number of patients with eye diseases (about 1 million outpatient visits per year). Secondly, the city-level air pollution estimates may not represent the true exposures of patients, as the spatial-temporal variations of individual activities could not be measured. Thirdly, possible effect modifications by some risk factors like smoking and occupations were not evaluated due to limited patient information. Fourthly, we could not adjust for other potential confounders including holidays and the prevalence of Novel Coronavirus Disease 2019 (COVID-19). These confounding factors may affect the occurrence of PALDO to some extent.
Conclusions
Our results showed positive associations between short-term exposure to air pollutants (PM10, PM2.5, NO2, SO2, and CO) and outpatient visits for PALDO in Hangzhou, China. Additionally, differences were also found in sex-, age- and season-specific associations. This study provides an insight into the role of air pollution in the pathogenesis of PALDO, and indicates the urgency to accelerate environmental protection and governance through policies and laws. Moreover, our findings can help the public to establish prevention and control measures for PALDO, including reducing air pollution exposure and maintaining a suitable environment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Second Affiliated Hospital, Medical College of Zhejiang University. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/Institutional Review Board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because this study was a retrospective study.
Author contributions
QM: Conceptualization, Investigation, Methodology, Writing – original draft. YWa: Methodology, Software, Formal analysis, Writing – original draft. PX: Data curation, Visualization, Writing – review & editing. XS: Data curation, Resources, Writing – review & editing. YWu: Methodology, Formal analysis, Writing – review & editing. JY: Conceptualization, Resources, Supervision, Writing – review & editing. HW: Conceptualization, Supervision, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Zhejiang Provincial Natural Science Foundation of China (Grant No. ZCLY24H1201) and the National Natural Science Foundation of China (Grant No. 82101129).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1632109/full#supplementary-material
References
1. Al-Kindi SG, Brook RD, Biswal S, Rajagopalan S. Environmental determinants of cardiovascular disease: lessons learned from air pollution. Nat Rev Cardiol. (2020) 17:656–72. doi: 10.1038/s41569-020-0371-2
2. Kulick ER, Kaufman JD, Sack C. Ambient air pollution and stroke: an updated review. Stroke. (2023) 54:882–93. doi: 10.1161/STROKEAHA.122.035498
3. North CM, Rice MB, Ferkol T, Gozal D, Hui C, Jung SH, et al. Air pollution in the Asia-Pacific region a joint Asian Pacific Society of Respirology/American Thoracic Society perspective. Am J Resp Crit Care. (2019) 199:693–700. doi: 10.1164/rccm.201804-0673PP
4. Fuller R, Landrigan PJ, Balakrishnan K, Bathan G., Bose-O'Reilly S, Brauer M, et al. Pollution and health: a progress update. Lancet Planet Health. (2022) 6:E535–47. doi: 10.1016/S2542-5196(22)00090-0
5. WHO. Ambient (Outdoor) Air Pollution (2019). Available from: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed July 24, 2024).
6. Zhang Z, Hua C, Chen XL, Song M. The spatial impact of population semi-urbanization on sulfur dioxide emissions: empirical evidence from China. J Environ Manage. (2025) 375:124257. doi: 10.1016/j.jenvman.2025.124257
7. Zhang Z, Shang Y, Zhang G, Shao S, Fang J, Li P, et al. The pollution control effect of the atmospheric environmental policy in autumn and winter: evidence from the daily data of Chinese cities. J Environ Manage. (2023) 343:118164. doi: 10.1016/j.jenvman.2023.118164
8. Zhou C, Richardson-Barlow C, Fan L, Cai H, Zhang W, Zhang Z. Towards organic collaborative governance for a more sustainable environment: Evolutionary game analysis within the policy implementation of China's net-zero emissions goals. J Environ Manage. (2025) 373:123765. doi: 10.1016/j.jenvman.2024.123765
9. Zhang Z, Zhang G, Li L. The spatial impact of atmospheric environmental policy on public health based on the mediation effect of air pollution in China. Environ Sci Pollut Res Int. (2023) 30:116584–600. doi: 10.1007/s11356-022-21501-6
10. Yang J, Wang Y, Tang C, Zhang Z. Can digitalization reduce industrial pollution? Roles of environmental investment and green innovation. Environ Res. (2024) 240:117442. doi: 10.1016/j.envres.2023.117442
11. Zhang G, Wang Y, Zhang Z, Su B. Can green finance policy promote green innovation in cities? Evidence from pilot zones for green finance reform and innovation in China. J Environ Manage. (2024) 370:122816. doi: 10.1016/j.jenvman.2024.122816
12. Fan M, Zhang Z, Wei Y, Sun S. Does innovative city pilot policy improve carbon reduction? Quasi-experimental evidence from China. Environ Res. (2024) 262:119748. doi: 10.1016/j.envres.2024.119748
13. Weng Q, Zhu S, Luo L, Liu B, Zhang Z. Environmental and health benefits of clean winter heating policies in northern China. J Environ Manage. (2025) 382:125388. doi: 10.1016/j.jenvman.2025.125388
14. Zhang Z, Ling D, Tian W, Zhou C, Song M, Fang S. Public participation and outgoing audit of natural resources: evidence from tripartite evolutionary game in China. Environ Res. (2023) 236:116734. doi: 10.1016/j.envres.2023.116734
15. Gao X, Zhang G, Zhang Z, Wei Y, Liu D, Chen Y. How does new energy demonstration city pilot policy affect carbon dioxide emissions? Evidence from a quasi-natural experiment in China. Environ Res. (2024) 244:117912. doi: 10.1016/j.envres.2023.117912
16. Song F, Hao S, Gu Y, Yao K, Fu Q. Research advances in pathogenic mechanisms underlying air pollution-induced ocular surface diseases. Adv Ophthalmol Pract Res. (2021) 1:100001. doi: 10.1016/j.aopr.2021.100001
17. Liu L, Li C, Yu HH, Yang XH. A critical review on air pollutant exposure and age-related macular degeneration. Sci Total Environ. (2022) 840:156717. doi: 10.1016/j.scitotenv.2022.156717
18. Lu CW, Fu J, Liu XF, Chen WW, Hao JL Li XL, Pant OP. Air pollution and meteorological conditions significantly contribute to the worsening of allergic conjunctivitis: a regional 20-city, 5-year study in Northeast China. Light Sci Appl. (2021) 10:190. doi: 10.1038/s41377-021-00630-6
19. Lu CW, Fu J, Liu XF, Cui ZH, Chen WW, Guo L, et al. Impacts of air pollution and meteorological conditions on dry eye disease among residents in a northeastern Chinese metropolis: a six-year crossover study in a cold region. Light Sci Appl. (2023) 12:186. doi: 10.1038/s41377-023-01207-1
20. Thale A, Paulsen F, Rochels R, Tillmann B. Functional anatomy of the human efferent tear ducts: a new theory of tear outflow mechanism. Graef Arch Clin Exp. (1998) 236:674–8. doi: 10.1007/s004170050140
21. Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. pathophysiology report. Ocul Surf. (2017) 15:438–510. doi: 10.1016/j.jtos.2017.05.011
22. Vinciguerra A, Nonis A, Resti AG, Bussi M, Trimarchi M. Best treatments available for distal acquired lacrimal obstruction: a systematic review and meta-analysis. Clin Otolaryngol. (2020) 45:545–57. doi: 10.1111/coa.13551
23. Das AV, Rath S, Naik MN, Ali MJ. The incidence of lacrimal drainage disorders across a tertiary eye care network: customization of an indigenously developed electronic medical record system-eyesmart. Ophthal Plast Recons. (2019) 35:354–356. doi: 10.1097/IOP.0000000000001257
24. Woog JJ. The incidence of symptomatic acquired lacrimal outflow obstruction among residents of Olmsted County, Minnesota, 1976-2000 (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. (2007) 105:649–66. doi: 10.1016/j.ophtha.2007.05.024
25. Ali MJ. Etiopathogenesis of primary acquired nasolacrimal duct obstruction (PANDO). Prog Retin Eye Res. (2023) 96:101193. doi: 10.1016/j.preteyeres.2023.101193
26. Chen YH, Wang SD, Alemi H, Dohlman T, Dana R. Immune regulation of the ocular surface. Exp Eye Res. (2022) 218:109007. doi: 10.1016/j.exer.2022.109007
27. Raby KL, Michaeloudes C, Tonkin J, Chung KF, Bhavsar PK. Mechanisms of airway epithelial injury and abnormal repair in asthma and COPD. Front Immunol. (2023) 14:1201658. doi: 10.3389/fimmu.2023.1201658
28. Linberg JV, McCormick SA. Primary acquired nasolacrimal duct obstruction. A clinicopathologic report and biopsy technique. Ophthalmology. (1986) 93:1055–63. doi: 10.1016/S0161-6420(86)33620-0
29. Mauriello JA Palydowycz S DeLuca DeLuca J Clinicopathologic study of lacrimal sac and nasal mucosa in 44 patients with complete acquired nasolacrimal duct obstruction. Ophthalmic Plast Reconstr Surg. (1992) 8:13–21. doi: 10.1097/00002341-199203000-00002
30. Ali MJ, Patnaik S, Kelkar N, Ali MH, Kaur I. Alteration of tear cytokine expressions in primary acquired nasolacrimal duct obstruction—potential insights into the etiopathogenesis. Curr Eye Res. (2020) 45:435–9. doi: 10.1080/02713683.2019.1665186
31. Somayajulu M, Ekanayaka S, McClellan SA, Bessert D, Pitchaikannu A, Zhang KZ, et al. Airborne particulates affect corneal homeostasis and immunity. Invest Ophth Vis Sci. (2020) 61:23. doi: 10.1167/iovs.61.4.23
32. Onishi T, Honda A, Tanaka M, Chowdhury PH, Okano H, Okuda T, et al. Ambient fine and coarse particles in Japan affect nasal and bronchial epithelial cells differently and elicit varying immune response. Environ Pollut. (2018) 242:1693–701. doi: 10.1016/j.envpol.2018.07.103
33. Upaphong P, Thonusin C, Wanichthanaolan O, Chattipakorn N, Chattipakorn SC. Consequences of exposure to particulate matter on the ocular surface: Mechanistic insights from cellular mechanisms to epidemiological findings. Environ Pollut. (2024) 345:123488. doi: 10.1016/j.envpol.2024.123488
34. Tran HM, Tsai FJ, Lee YL, Chang JH, Chang LT, Chang TY, et al. The impact of air pollution on respiratory diseases in an era of climate change: a review of the current evidence. Sci Total Environ. (2023) 898:166340. doi: 10.1016/j.scitotenv.2023.166340
35. Ni WL, Stafoggia M, Zhang SQ, Ljungman P, Breitner S, de Bont J, et al. Short-Term Effects of lower air temperature and cold spells on myocardial infarction hospitalizations in Sweden. J Am Coll Cardiol. (2024) 84:1149–59. doi: 10.1016/j.jacc.2024.07.006
36. Jones LT. Tear-Sac foreign bodies. Am J Ophthalmol. (1965) 60:111. doi: 10.1016/0002-9394(65)92403-7
37. Yazici B, Hammad AM, Meyer DR. Lacrimal sac dacryoliths—predictive factors and clinical characteristics. Ophthalmology. (2001) 108:1308–12. doi: 10.1016/S0161-6420(01)00596-6
38. Repp DJ, Burkat CN, Lucarelli MJ. Lacrimal excretory system concretions: canalicular and lacrimal sac. Ophthalmology. (2009) 116:2230–5. doi: 10.1016/j.ophtha.2009.04.029
39. Andreou P, Rose GE. Clinical presentation of patients with dacryolithiasis. Ophthalmology. (2002) 109:1573–4. doi: 10.1016/S0161-6420(02)01107-7
40. Nemet AY, Vinker S. Associated morbidity of nasolacrimal duct obstruction-a large community based case-control study. Graef Arch Clin Exp. (2014) 252:125–30. doi: 10.1007/s00417-013-2484-3
41. Li J, Zhang GW, Nian S, Lv Y, Shao Y, Qiao NN, et al. Dry eye induced by exposure to cigarette smoke pollution: an in vivo and in vitro study. Free Radical Bio Med. (2020) 153:187–201. doi: 10.1016/j.freeradbiomed.2020.04.007
42. Lugg ST, Scott A, Parekh D, Naidu B, Thickett DR. Cigarette smoke exposure and alveolar macrophages: mechanisms for lung disease. Thorax. (2022) 77:94–101. doi: 10.1136/thoraxjnl-2020-216296
43. Kashkouli MB, Sadeghipour A, Kaghazkanani R, Bayat A, Pakdel F, Aghai GH. Pathogenesis of primary acquired nasolacrimal duct obstruction. Orbit. (2010) 29:11–5. doi: 10.3109/01676830903207828
44. Ohtomo K, Ueta T, Toyama T, Nagahara M. Predisposing factors for primary acquired nasolacrimal duct obstruction. Graef Arch Clin Exp. (2013) 251:1835–9. doi: 10.1007/s00417-013-2288-5
45. Jacobs JH, Spaan S, van Rooy GB, Meliefste C, Zaat VA, Rooyackers JM, et al. Exposure to trichloramine and respiratory symptoms in indoor swimming pool workers. Eur Respir J. (2007) 29:690–8. doi: 10.1183/09031936.00024706
46. Bonetto G, Corradi M, Carraro S, Zanconato S, Alinovi R, Folesani G, et al. Longitudinal monitoring of lung injury in children after acute chlorine exposure in a swimming pool. Am J Resp Crit Care. (2006) 174:545–9. doi: 10.1164/rccm.200509-1392OC
47. Salonen H, Salthammer T, Morawska L. Human exposure to ozone in school and office indoor environments. Environ Int. (2018) 119:503–14. doi: 10.1016/j.envint.2018.07.012
48. Fan K, Dong N, Fang M, Xiang Z, Zheng L, Wang M, et al. Ozone exposure affects corneal epithelial fate by promoting mtDNA leakage and cGAS/STING activation. J Hazard Mater. (2024) 465:133219. doi: 10.1016/j.jhazmat.2023.133219
49. Takahashi Y, Kakizaki H, Nakano T. Bony nasolacrimal duct entrance diameter: gender difference in cadaveric study. Ophthal Plast Recons. (2011) 27:204–5. doi: 10.1097/IOP.0b013e3182078e47
50. Ali MJ, Schicht M, Paulsen F. Qualitative hormonal profiling of the lacrimal drainage system: potential insights into the etiopathogenesis of primary acquired nasolacrimal duct obstruction. Ophthal Plast Recons. (2017) 33:381–8. doi: 10.1097/IOP.0000000000000962
51. Nikolich-Zugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol. (2018) 19:10–9. doi: 10.1038/s41590-017-0006-x
52. Zhou TY, Liao WJ, Wang XF, Wang YY, Yang PC, Zuo L, et al. Low temperature reduces occludin expression in bronchial epithelial cells: Implications in cold-induced asthma. Mol Immunol. (2023) 157:176–85. doi: 10.1016/j.molimm.2023.03.018
53. Kashiwagi K, Iizuka Y. Effect and underlying mechanisms of airborne particulate matter 2.5 (PM2.5) on cultured human corneal epithelial cells. Sci Rep. (2020) 10:19516. doi: 10.1038/s41598-020-76651-9
54. Torricelli AAM, Matsuda M, Novaes P, Braga ALF, Saldiva PHN, Alves MR, et al. Effects of ambient levels of traffic-derived air pollution on the ocular surface: Analysis of symptoms, conjunctival goblet cell count and mucin 5AC gene expression. Environ Res. (2014) 131:59–63. doi: 10.1016/j.envres.2014.02.014
55. Paulsen FP, Thale AB, Maune S, Tillmann BN. New insights into the pathophysiology of primary acquired dacryostenosis. Ophthalmology. (2001) 108:2329–36. doi: 10.1016/S0161-6420(01)00946-0
56. Glencross DA, Ho TR, Camilla N, Hawrylowicz CM, Pfeffer PE. Air pollution and its effects on the immune system. Free Radical Bio Med. (2020) 151:56–68. doi: 10.1016/j.freeradbiomed.2020.01.179
57. Ali MJ, Mulay K, Pujari A, Naik MN. Derangements of lacrimal drainage-associated lymphoid tissue (LDALT) in human chronic dacryocystitis. Ocul Immunol Inflamm. (2013) 21:417–23. doi: 10.3109/09273948.2013.797473
58. Saleem A, Awan T, Akhtar MF. A comprehensive review on endocrine toxicity of gaseous components and particulate matter in smog. Front Endocrinol. (2024) 15:1294205. doi: 10.3389/fendo.2024.1294205
59. Taylor-Clark TE. Air pollution-induced autonomic modulation. Physiology. (2020) 35:363–74. doi: 10.1152/physiol.00017.2020
Keywords: air pollution, air pollutant, primary acquired lacrimal duct obstruction, case-crossover study, public health
Citation: Miao Q, Wang Y, Xu P, Shi X, Wu Y, Ye J and Wu H (2025) Association between ambient air pollution and outpatient visits for primary acquired lacrimal duct obstruction in Hangzhou, China. Front. Public Health 13:1632109. doi: 10.3389/fpubh.2025.1632109
Received: 20 May 2025; Accepted: 12 September 2025;
Published: 29 September 2025.
Edited by:
Cheng-wei Lu, First Affiliated Hospital of Jilin University, ChinaReviewed by:
Zhenhua Zhang, Lanzhou University, ChinaXiu-fen Liu, First Affiliated Hospital of Jilin University, China
Copyright © 2025 Miao, Wang, Xu, Shi, Wu, Ye and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Ye, eWVqdWFuQHpqdS5lZHUuY24=; Han Wu, ZG9jdG9yd3VoYW5Aemp1LmVkdS5jbg==
†These authors have contributed equally to this work
 Qi Miao1†
Qi Miao1† Yihua Wu
Yihua Wu Han Wu
Han Wu