- 1The Eighth Clinical Medical College of Guangzhou University of Chinese Medicine, Foshan, China
- 2Foshan Hospital of Traditional Chinese Medicine, Foshan, China
Objective: This study reports a familial Gelsemium elegans poisoning case and systematically evaluates the efficacy of gastric lavage in G. elegans intoxication through a meta-analysis.
Methods: We systematically searched PubMed, Web of Science, CNKI, and Wanfang Data (2000–2024). Eligible randomized controlled trials and case reports comparing gastric lavage versus non-lavage approaches were included. Data were extracted from published studies, and pooled odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using Mantel–Haenszel random-effects models.
Results: Thirteen studies involving 160 patients demonstrated an overall in-hospital mortality of 18.75%. Gastric lavage suggested potential survival benefit compared to controls (OR 0.39; 95% CI 0.15–0.99; p = 0.62).
Conclusion: G. elegans poisoning is life-threatening, with severe cases rapidly progressing to respiratory/circulatory failure requiring urgent support. Gastric lavage may offer survival advantage in hemodynamically stable patients when performed with airway protection. Prompt respiratory support should be prioritized in the therapeutic management.
Introduction
Gelsemium elegans (G. elegans) is a highly toxic plant that contains various poisonous alkaloids (Figure 1) (1). It was found in a broad range of geographic regions, with reports of its presence in Southeast Asian countries and Chinese southern provinces (2). History reports that Shen Nong was fatally poisoned b G. elegans, which led to the plant being referred to as “Duan Changcao”in Chinese (1). Due to its resemblance to several traditional Chinese medicines with health benefits, such as Lonicera japonica (Figure 2) and Radix Millettiae Speciosae (Figure 3), accidental ingestion can occur, leading to acute poisoning caused by mistaken ingestion (3). This report describes three successfully treated cases of G. elegans poisoning. And through a systematic review we evaluated the clinical impact of gastric lavage on outcomes in G. elegans poisoning while synthesizing reported clinical presentations, treatment modalities, and outcome determinants.

Figure 2. Flowers and leaves of Lonicera japonica. Pictures courtesy of Mr. Li Xibeiyang and Plant Photo Bank of China. Lonicera japonica is a semi-evergreen climbing shrub characterized by tubular flowers (3–4.5 cm in length) that transition from white to yellow coloration. The flowering period primarily occurs from April to June (with frequent autumn flowering periods observed), featuring ovate to oblong-ovate leaf morphology. Lonicera japonica predominantly inhabits montane shrublands at elevations below 1,500 meters, demonstrating notable adaptability to various soil conditions. This plant serves as a fundamental herb in traditional Chinese medicine, where the medicinal components are derived from either dried flower buds or partially opened flowers at early developmental stages.

Figure 3. Roots from radix Millettiae Speciosae. Millettia speciosa (commonly called Cattle Strength Vine) is a climbing shrub with thick, fleshy roots that look like twisted intestines or strings of irregular beads. These roots are juicy but contain tough fibers. Primarily found in southern China, it grows in provinces including Guangdong, Guangxi, Yunnan, Fujian, Hunan, Guizhou, and Hainan. This plant thrives in various environments such as valleys, roadsides, shrublands, and open forests. The dried roots are used in traditional medicine and can be collected any time of year. Source from https://www.douyin.com/note/7384251535108148492
Case history
Three family members ingested a home-prepared herbal broth at approximately 7:00 PM. All individuals manifested symptoms of dizziness, visual disturbance, generalized numbness with weakness, and dyspnea following ingestion of herbal soup. Emergency medical services (EMS) were contacted at 19:20, with personnel arriving promptly to collect samples (Figure 4) within 30 min. All patients were transferred back to emergency department of Foshan Hospital of Traditional Chinese Medicine for continued resuscitation and therapeutic management.
Case 1
A 55-year-old male, who was healthy all along. He presented with symptoms of dizziness, weakness, blurred vision, and shortness of breath about 20 min after ingestion. Upon admission, his Glasgow Coma scale was 13/15, blood pressure 144/96 mmHg, respiratory rate of 30 breaths/min, pulse rate of 100 bpm, and blood oxygen saturation level of 100%. His dyspnea worsened progressively, accompanied by unconsciousness and cyanosis of the lips and fingernails at 20:10. Due to acute respiratory failure, the patient was intubated and transferred to the emergency intensive care unit (EICU). Arterial blood gas analysis revealed pH 7.395, PaO2 182.3 mmHg, PaCO2:36.5 mmHg, CO2 22.3 mmol/L, BE:-2.5 mmol/L, K+3.7 mmol/L, Glu:7.8 mmol/L, SO2 55%, lactate 4.2 mmol/L.
Case 2
A 49-year-old previous heathy female, was the wife of case 1. She developed symptoms of blurred vision, peripheral numbness with weakness, and nausea by accompanied emetic tendency about 20 min post-ingestion. Her Glasgow Coma scale was 14/15, blood pressure 133/76 mmHg, respiratory rate of 20 breaths/min, pulse rate of 98 bpm, and temperature 36.8°C. Both her pupils were 3 mm in size and were reactive to light. The limb muscle power was 3/5 and deep tendon reflexes were normal. At 20:42, the patient developed somnolence with intermittent limb convulsions accompanied by a decline in oxygen saturation (SpO2) to 68%. Endotracheal intubation with mechanical ventilation was subsequently initiated, followed by ICU admission for continuous intensive monitoring and therapeutic management.
Case 3
A 33-year-old woman with history of Sjögren’s syndrome, was the daughter in law of case 1. Her clinical manifestations resembled those observed in Case 2, with concomitant development of generalized erythema. Her Glasgow Coma scale was 13/15, blood pressure 119/86 mmHg, respiratory rate of 24 breaths/min, pulse rate of 115 bpm, and temperature 36.5°C. Both her pupils were 3 mm in size and were reactive to light. At 20:40, the patient developed a comatose state accompanied by intermittent limb convulsions and frothy oral secretions. Endotracheal intubation was emergently performed with subsequent initiation of mechanical ventilation. The patient was transferred to the intensive care unit (ICU) for advanced life support and ongoing critical care management.
Given the short poisoning interval (30 min), the medical team performed emergent gastric lavage (4) and hemoperfusion was subsequently initiated to enhance toxin elimination following admission to the EICU (5). Laboratory findings revealed leukocytosis in all patients (Case 1: 11.15 × 109/L; Case 2: 11.73 × 109/L; Case 3: 13.76 × 109/L), accompanied by elevated serum amyloid A (SAA) levels. There were no notable abnormalities in the patients’ coagulation function, cardiac enzymes, chest X-ray, and brain CT scan. Adjunctive therapies including anti-infection, gastrointestinal protection and correction of electrolyte imbalances. All patients regained spontaneous respiration by hospital day 2 and were discharged after a five-day treatment period without any neurological complications.
On April 7, the Foshan Center for Disease Control and Prevention (CDC) confirmed the cause of poisoning as G. elegans through liquid chromatography analysis of food samples collected on-site (Figure 5).
Figure 5. The report of liquid chromatography from the FoShan Center for Disease Control and Prevention (FoShan CDC).
Materials and methods
Database search strategy
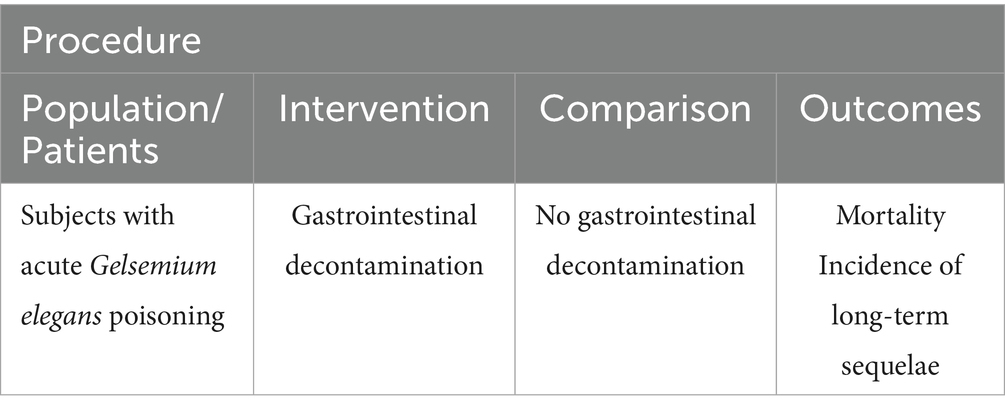
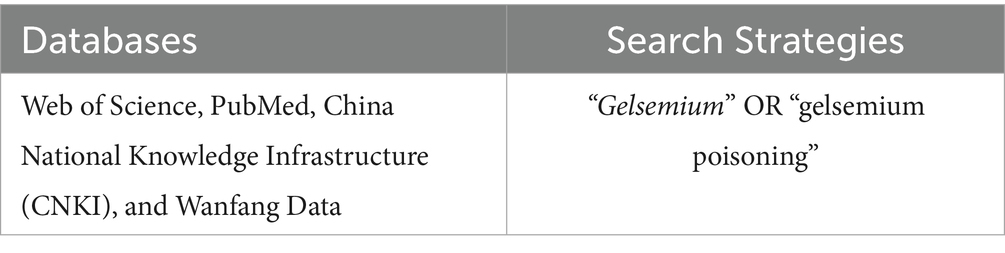
Literature screening and data analysis were conducted in accordance with PRISMA guidelines (6). The study protocol has been resisted in the PROSPERO database (Registration number: 2025 CRD420251027380). The scope of the review was to answer the following PICO question: “In patients with acute Gelsemium elegans poisoning, does gastric lavage compared with supportive care without gastric lavage reduce mortality and incidence of long-term sequelae?.” The primary research question was formulated according to the PICO framework (P: population, I: intervention, C: comparator, O: outcome; Table 1). A comprehensive literature search, encompassing databases Web of Science, PubMed, China National Knowledge Infrastructure (CNKI), and Wanfang Data has been performed with the following Boolean Indicators: (“Gelsemium” OR “gelsemium poisoning” Table 2).
Inclusion/exclusion criteria
The inclusion criteria specified clinical trials, case reports or case series on patients with no follow up limitations. The exclusion criteria were limited to animal studies, systematic reviews, short communications and subjects who died prior to hospital admission were excluded from the analysis. All databases were queried for entries indexed from 2000 onward.
Screening process
Following the search strategy across the designated databases, all retrieved records were exported into NoteExpress software for duplication remove. Two independent reviewers (Y.Z, B.S.) conducted an initial screening based on the titles and abstracts of all unique records. Full texts were obtained for all eligible articles. Excluded publications were categorized, specifying the reasons for exclusion (Figure 6).
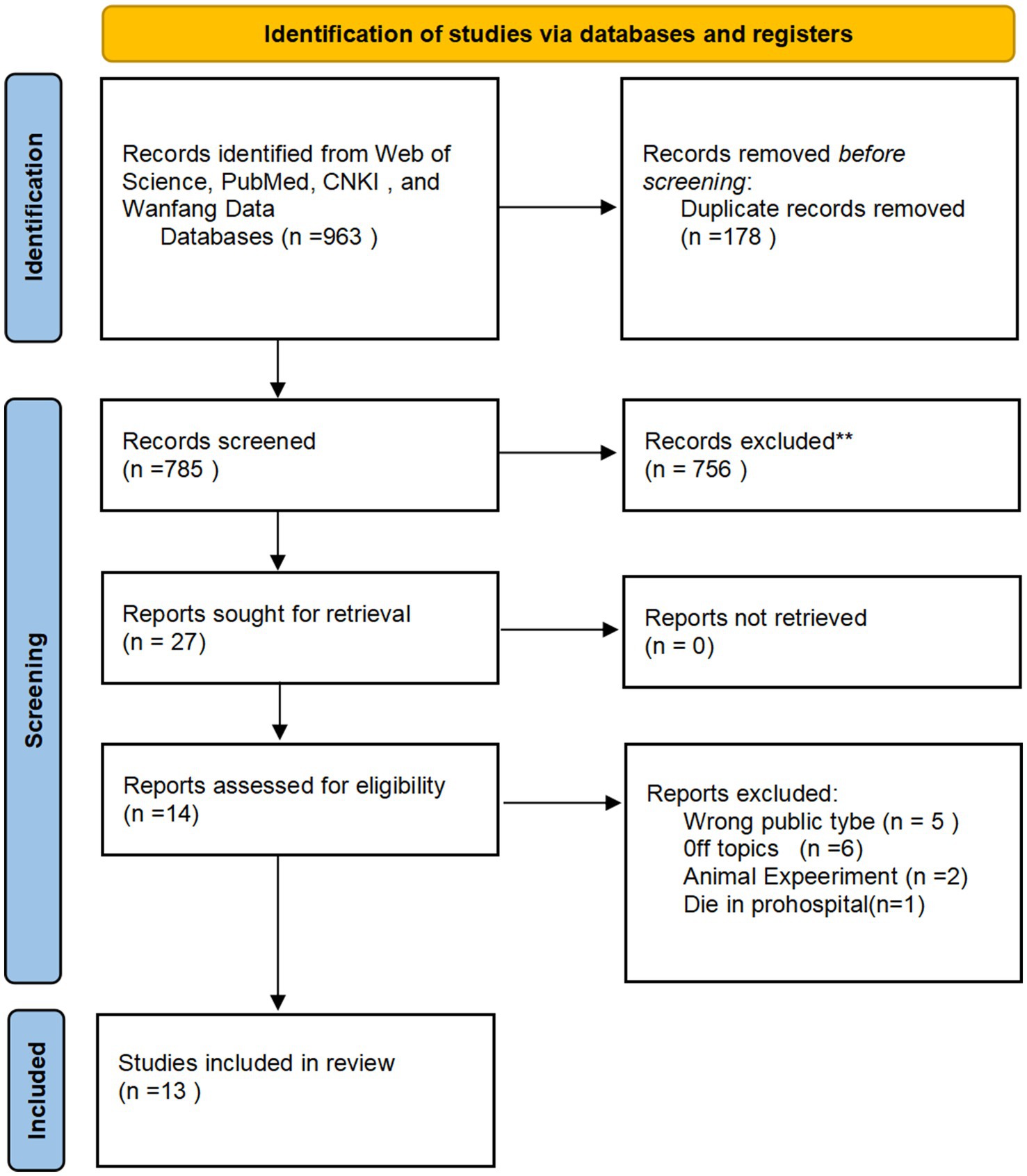
Figure 6. Work-flow of the screening and eligibility process of the systematic review according the PRISMA guidelines.
The following data were recorded and collected: publication year, analytical methodology, patient cohort size, geographic provenance (province), age and sex distribution, therapeutic interventions administered, time to first medical contact, implicated food types, and outcome.
Risk of bias assessment
Bias risk was assessed using the software package Review Manager (RevMan) software, Version 5.4 (The Cochrane Collaboration).and the OHAT guidelines. The final evaluation considered the following parameters: randomization sequence, allocation concealment, blinding of outcome assessment, completeness of procedure description, clarity of inclusion criteria, attrition bias, reporting bias, follow-up duration, and other biases. Bias risk was categorized as low, unclear, or high.
Statistical analysis
Odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were calculated for all reported outcomes using RevMan 5.4 (The Cochrane Collaboration). The Mantel–Haenszel method was applied under a random-effects model to account for anticipated cross-trial variations in study designs and intervention characteristics. Between-study heterogeneity was quantified using the I2 statistic in accordance with Cochrane Handbook guidelines.
Result
Publication screen
Database screening initially identified 963 publications. During the initial screening phase, 178 duplicate records were removed for duplication. Subsequently, 756 articles were excluded based on title and abstract evaluation. 27 articles proceeded to the eligibility assessment stage. Following full-text review, 14 publications were excluded from the final synthesis for the following reasons:5 articles were excluded due to off-topic,5 were review articles,2 involved animal or non-human subjects, One study was excluded because the subjects experienced prehospital mortality.
Risk of bias assessment
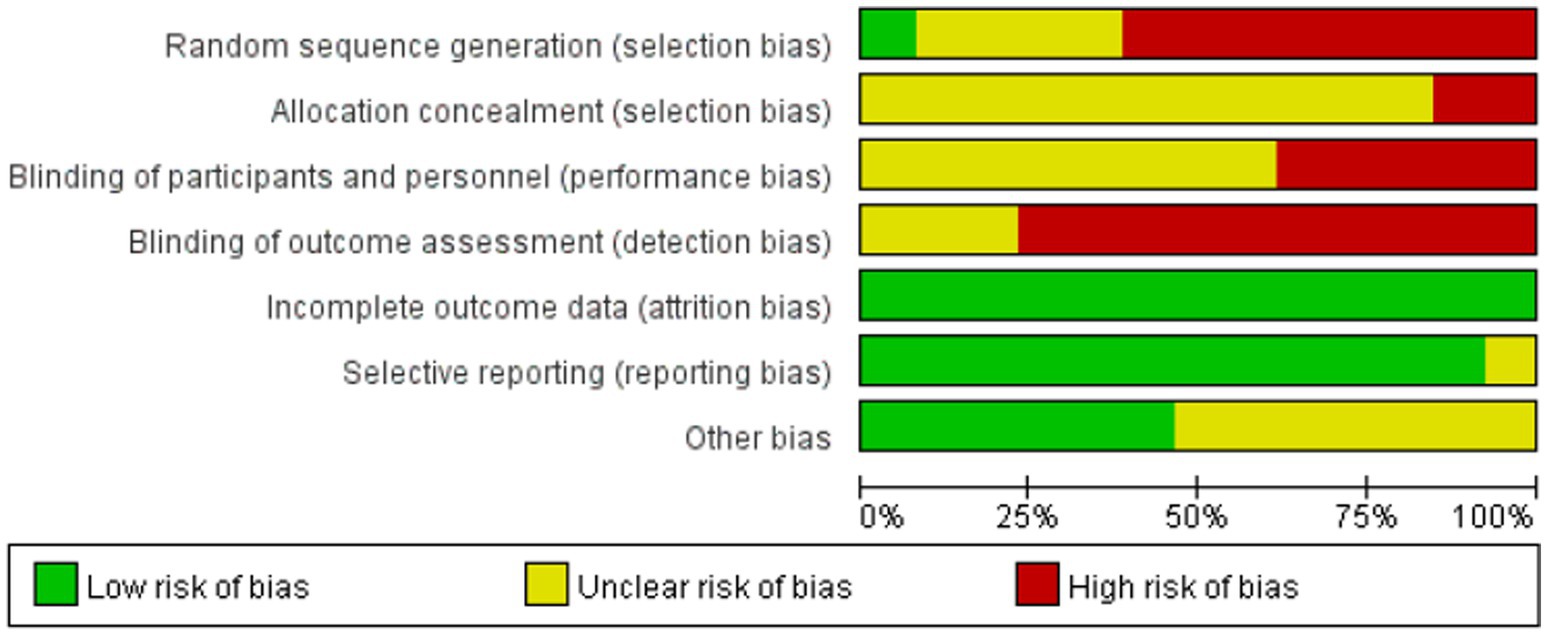
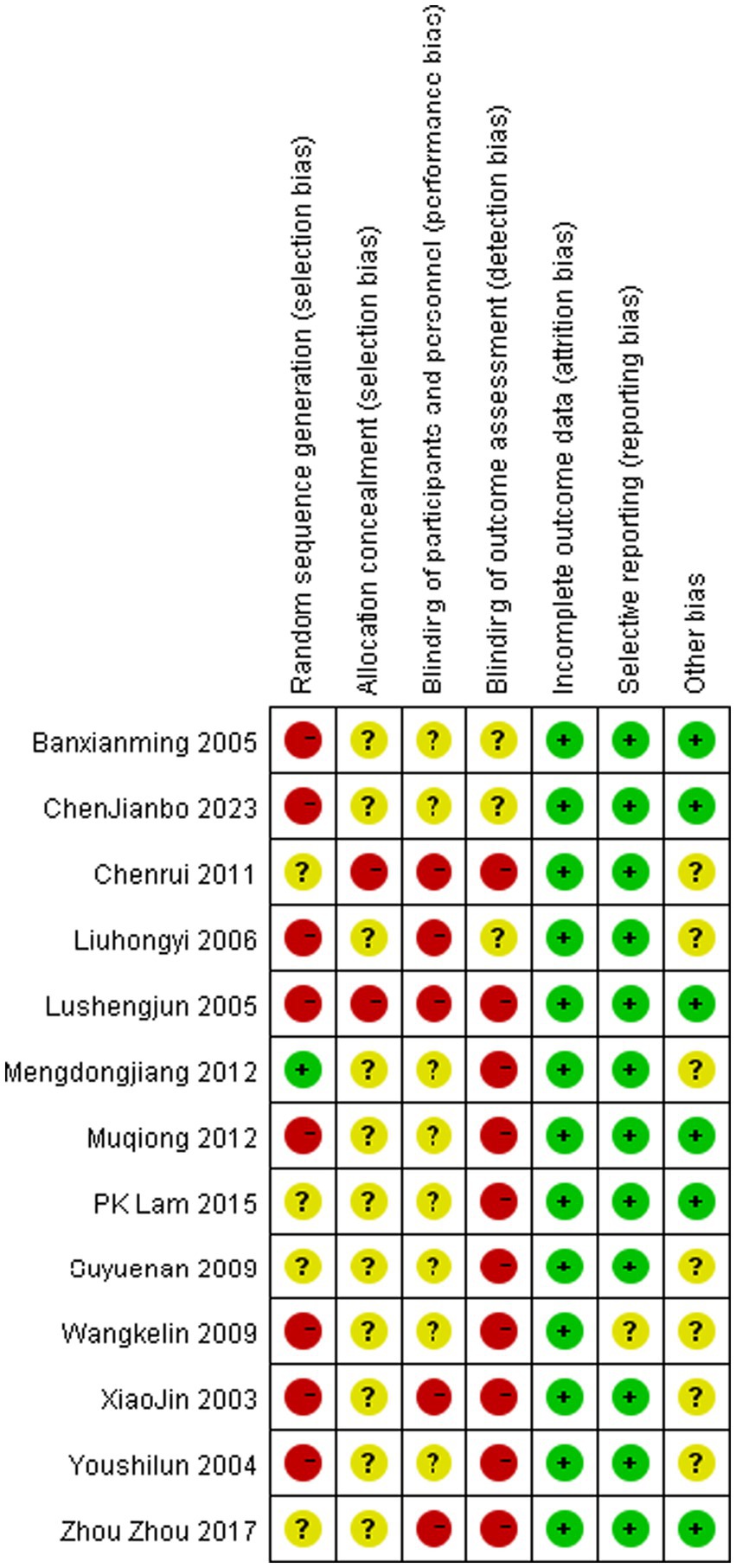
Risk of bias assessment for all included studies is presented in Figures 7, 8. All publications exhibited elevated bias risk (Figures 7, 8) attributable to extensive study design divergence, constrained observational periods, and abundant non-comparative clinical data.
Study characteristics
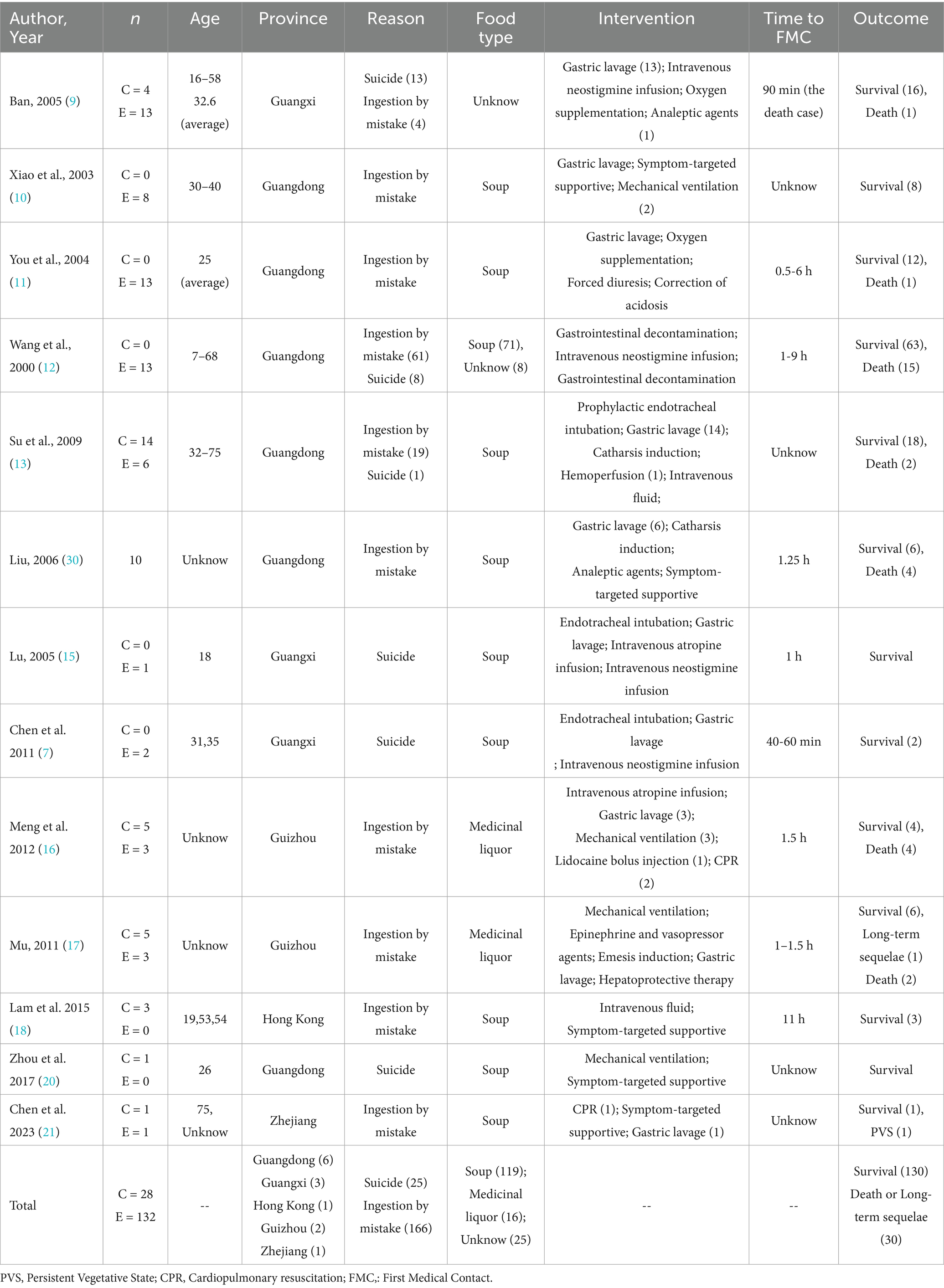
A summary is depicted in the Table 3. Thirty fatalities occurred among 160 patients, yielding an in-hospital mortality rate of 18.75%. Among the thirteen case reports, six cases occurred in Guangdong, three in Guangxi, and one case each in Guizhou, Zhejiang, and Hong Kong SAR. The age distribution of cases is relatively wide, covering age groups including adolescents, middle-aged people, and the older adult. Due to the limited number of patients with clearly-defined gender data, a comprehensive gender-based analysis was not feasible. In all previous reports, no gender differences among deceased patients have been mentioned. According to summary of food type, the primary cause is accidental ingestion of soups, medicinal liquors containing liquids. Other causes include suicide and homicide (7, 8).
Data statistic
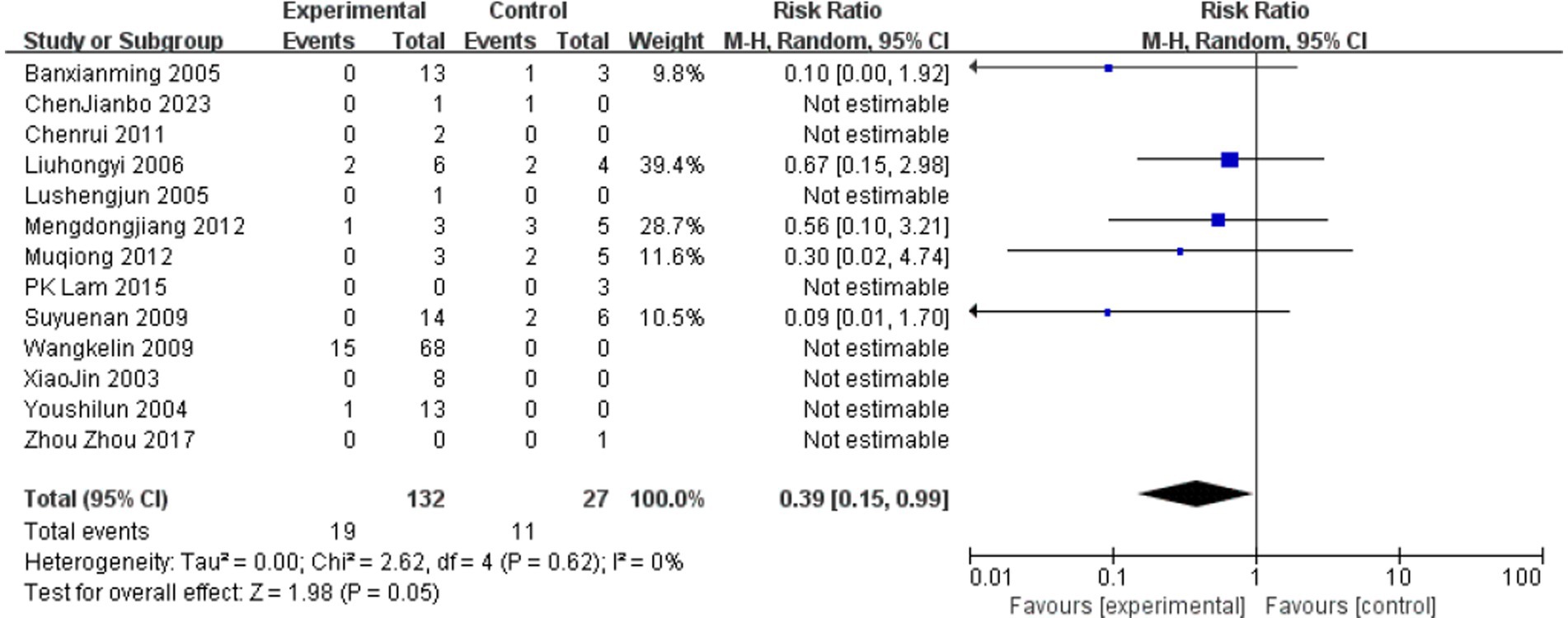
We used Mantel–Haenszel methods and forest plots to test if gastric lavage helps patients. Because age, toxin types, and other factors affect outcomes, we chose a random-effects model. The results surprised us: Lavage cut death rates by 61% versus controls (OR 0.39, 95% CI 0.15–0.99, p = 0.05). Studies agreed closely (I2 = 0%, Figure 9). We conducted a subgroup analysis of the clinical characteristics of 27 deceased patients for whom relatively detailed data were available (3, 7, 9–21).
Discussion
According to phytochemical analyses, G. elegans plants contain 121 alkaloids and 25 iridoid compounds, which are primarily concentrated in the roots though also distributed in the entire plant (22). Pharmacological studies have shown that gelsemine, which is abundant in G. elegans, acts as a modulator of glycine receptors and type A GABA receptors in the central nervous system (CNS), causing CNS depression and respiratory and circulatory failure in poisoned individuals (23, 24). The main components of G. elegans alkaloids include gelsemicine, koumicine, kouminicine, and koumine with the most abundant alkaloid being koumine (25). Gelsenicine has the highest toxicity (LD 50 ~ 0.26 mg/kg rat, i.p.; and 0.15 mg/kg rat, i.v.), while gelsemine and koumine are also present in significant amounts (gelsemine, LD50 ~ 56 mg/kg mice, i.p.; koumine, LD50 ~ 100 mg/kg mice, i.p.) (22, 26).
This report describes three cases of acute poisoning resulting from the accidental ingestion of soup containing G. elegans. The patients presented with symptoms such as dizziness and shortness of breath, which rapidly deteriorated into respiratory failure and CNS depression within half an hour. The medical team administered treatments including endotracheal intubation, gastric lavage, hemoperfusion, and anti-microbial medications. They recovered due to timely respiratory support, and the pharmacologic treatment administered effectively reduced the risk of severe sequelae.
The conventional treatment approach includes respiratory support, gastric lavage, anti-emetics, blood perfusion, intravenous fluid therapy, and correction of acid–base imbalance and electrolyte disorders (20). Gastric lavage is a method for gastrointestinal decontamination following toxin ingestion, remains widely practiced in China (4). However, the clinical benefits derived by patients from this intervention are currently contentious. Another reason to avoid routine use involves toxins like gelsemine alkaloids absorb quickly through mucous membranes. Still, we have yet to clinically confirm this rapid-absorption theory. The animal studies showed that if gastric lavage was undertaken within 60 min, the mean recovery of marker was 13 and 8.6% (27). Among the included studies, two patients experienced cardiac arrest following gastric lavage (with one having recurrent episodes), though causality remains indeterminate between the procedure and underlying gelsemine alkaloid poisoning. Although data analysis showed an slight benefit, clinical significance remains uncertain due to uncontrolled variables like lavage fluid selection, severity variability, and treatment timing. Consequently, forest plot results failed to demonstrate significant clinical benefit. Recent studies suggest that endotracheal intubation should take precedence over gastric lavage due to the risk of respiratory failure from gelsemium poisoning and the need to ensure airway protection during gastric lavage (to reduce complications like aspiration pneumonia and cardiopulmonary arrest) (28). This approach may hold clinical value in treating gelsemium poisoning.
Excluding the 10 patients who died before reaching the hospital and the 8 cases without pre-hospital time-table information. Among those who died after consuming medicinal liquor, 81.25% were male. This phenomenon might be attributed to the traditional practice among middle-aged and older adult men in China of using medicinal liquor, they believed the herbal plant could tonify kidney from TCM theory.
There is currently no specific antidote for G. elegans poisoning. Some cases reports have noted that naloxone is particularly effective in rapidly alleviating respiratory depression and shortening the duration of patient unconsciousness, a mechanism believed to be related to its antagonism of enkephalin release induced by G. elegans poisoning (11). A 2022 study in mice found that the combination of flumazenil and epinephrine showed promising therapeutic effects in treating G. elegans poisoning (29). However, both therapies rely solely on limited preclinical data and isolated human cases, lacking robust evidence from large-scale clinical trials.
The main limitation of this study stems from the heterogeneous reporting format across the included clinical cases. Furthermore, nearly all poisoning reports lacked Poisoning Severity Score (PSS) data. This precluded meaningful subgroup analysis based on patient stratification and compromised the interpretation of the final results.
Conclusion
G. elegans poisoning is a life-threatening condition that requires prompt clinical attention. It manifests as dizziness, respiratory depression, neuromuscular paralysis, and multi-organ failure, with a mortality rate of ~18.75%. Common symptoms of G. elegans poisoning include dizziness, chest tightness, respiratory depression, vertigo, nausea and vomiting (7, 8, 15). G. elegans alkaloids also inhibit spinal motor neurons, causing respiratory muscle paralysis. Gastric lavage may benefit patients presenting with acute poisoning, but its performance requires prior airway protection. Therefore, management should prioritize prompt respiratory support and airway protection. Although agents like naloxone and the combination of flumazenil with epinephrine show potential in alleviating symptoms (e.g., respiratory depression) based on limited preclinical and case report data, their efficacy lacks robust validation through large-scale clinical trials.
Author contributions
YZ: Formal analysis, Project administration, Writing – review & editing, Investigation, Writing – original draft, Data curation. BS: Data curation, Writing – original draft, Writing – review & editing. QX: Writing – original draft, Data curation, Investigation, Formal analysis. YW: Data curation, Formal analysis, Writing – original draft. YF: Formal analysis, Data curation, Writing – review & editing. QT: Investigation, Writing – review & editing. ZL: Supervision, Investigation, Writing – review & editing, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding for this study was provided by the Self-funded Science and Technology Innovation Projects of Foshan Municipal Science and Technology Bureau (no. 2420001004373).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fpubh.2025.1717881.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Peng, YL, Liang, JJ, Xue, Y, Khan, A, Zhang, PP, Feng, TT, et al. Genus Gelsemium and its Endophytic Fungi - comprehensive review of their traditional uses, phytochemistry, pharmacology, and toxicology. Curr Top Med Chem. (2023) 23:2452–87. doi: 10.2174/1568026623666230825105233
2. Liu, YS, Tang, Q, Cheng, P, Zhu, MF, Zhang, H, Liu, JZ, et al. Whole-genome sequencing and analysis of the Chinese herbal plant Gelsemium elegans. Acta Pharm Sin B. (2020) 10:374–82. doi: 10.1016/j.apsb.2019.08.004
3. Qu, D, Qiao, D-F, Chen, X-C, Feng, C-Q, Luo, Q-Z, and Tan, X-H. Fatal poisoning by accidental ingestion of the “heartbreak grass” (Gelsemium elegans) verified by toxicological and medico-legal analyses. Forensic Sci Int. (2021) 321:110745. doi: 10.1016/j.forsciint.2021.110745
4. Chinese Medical Doctor Association EMB, Chinese Society of Toxicology Poisoning and Treatment of Specialized Committee. Chinese expert consensus on diagnosis and treatment of acute poisoning. Chin J Emerg Med. (2016) 25:15. doi: 10.3760/cma.j.issn.2095-4352.2016.11.003
5. Lavergne, V, Nolin, TD, Hoffman, RS, Roberts, D, Gosselin, S, Goldfarb, DS, et al. The EXTRIP (EXtracorporeal TReatments in poisoning) workgroup: guideline methodology. Clin Toxicol. (2012) 50:403–13. doi: 10.3109/15563650.2012.683436
6. Hutton, B, Salanti, G, Caldwell, DM, Chaimani, A, Schmid, CH, Cameron, C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
7. Chen, R. The experience of rescuing respiratory and cardiac arrest caused by Gelsemium elegans poisoning treatment. China Pract Med. (2011) 6:195–6. doi: 10.3969/j.issn.1673-7555.2011.19.149
8. Chen, C, He, j, Wei, J, Chen, Y, Tan, N, He, J, et al. Literature analysis of 1034 cases of Gelsemium elegans poisoning. J Wuzhou Univ. (2020) 30:11–9. doi: 10.3969/j.issn.1673-8535.2020.03.003
9. Ban, X. Clinical analysis of 17 cases of Gelsemium elegans poisoning. J Min Invasive Med. (2005) 24:225–6. doi: 10.3969/j.issn.1673-6575.2005.02.058
10. Xiao, J, Wang, L, Liu, YM, and Ma, WE. Analysis of diagnosis and treatment in 8 cases of gelsemiun elegans poisoning. J Front Med. (2016) 3:357.
11. You, SL, and Lian, ZF. 13 cases of Gelsemium elegans poisoning resuscitation. Chin J Gen Pract. (2004) 3:280. doi: 10.3760/cma.j.issn.1671-7368.2004.04.043
12. Wang, KL, Tang, XX, and Su, ZY. Clinical analysis of 78 cases of acute poisoning of Duanchangcao. Modem Hospit. (2009) 9:69. doi: 10.3969/j.issn.1671-332X.2009.05.033
13. Su, YN, Zeng, W, and Huang, W. Preventive tracheal intubation in the emergeney treatment of an important role in gelsemism. Int Med Health Guid News. (2009) 15:22–4. doi: 10.3760/cma.j.issn.1007-1245.2009.23.008
14. Marileo, AM, Gavilán, J, San Martín, VP, Lara, CO, Sazo, A, Muñoz-Montesino, C, et al. Modulation of GABAA receptors and of GABAergic synapses by the natural alkaloid gelsemine. Front Mol Neurosci. (2023) 15:1083189. doi: 10.3389/fnmol.2022.1083189
15. Lu, SJ, Su, X, and Huang, ZB. A case report of rescuing respiratory arrest due to Gelsemium elegans poisoning. Acta Med Sin. (2006) 19:184. doi: 10.3969/j.issn.1008-2409.2006.02.121
16. Meng, DJ, and Luo, YJ. Experience and lessons from the resuscitation of 8 cases of Gelsemiun elegans poisoning. Chinese Commun Doctors. (2012) 14:315. doi: 10.3969/j.issn.1007-614x.2012.16.309
17. Mu, Q, Yu, HJ, Wei, WQ, and Cai, YH. Clinical experience in the treatment of Gelsemium elegans poisoning. J Guizhou Med Univ. (2012) 37:10:206. doi: 10.3969/j.issn.1000-2707.2012.02.033
18. Lam, PK, Au, TTS, Leung, JKS, and Wong, TW. Gelsemium poisoning in a family after consumption of Cassytha filiformis Linn. Collected in the countryside. Hong Kong J Emerg Med. (2015) 22:60–3. doi: 10.1177/102490791502200110
19. Zhong, YX, Xie, YH, Jiang, YY, Liu, YP, Shi, MM, and Yang, WM. Analysis of gelsemine poisoning events in the Guangxi Zhuang autonomous region during 2015-2017. Chinese J Food Hyg. (2019) 1:81–3. doi: 10.13590/j.cjfh.2019.01.017
20. Zhou, Z, Wu, L, Zhong, Y, Fang, X, Liu, Y, Chen, H, et al. Gelsemium elegans poisoning: a case with 8 months of follow-up and review of the literature. Front Neurol. (2017) 8:204. doi: 10.3389/fneur.2017.00204
21. Chen, J, Guo, Y, Chen, Z, Bao, R, and Kong, F. Dead hair detected from Gelsemium elegans poisoning. J Forensic Med. (2023) 39:509–11. doi: 10.12116/j.issn.1004-5619.2022.420903
22. Jin, GL, Su, YP, Liu, M, Xu, Y, Yang, J, Liao, KJ, et al. Medicinal plants of the genus Gelsemium (Gelsemiaceae, Gentianales)--a review of their phytochemistry, pharmacology, toxicology and traditional use. Ethnopharmacol. (2014) 152:33–52. doi: 10.1016/j.jep.2014.01.003
23. Rujjanawate, C, Kanjanapothi, D, and Panthong, A. Pharmacological effect and toxicity of alkaloids from Gelsemium elegans Benth. J Ethnopharmacol. (2003) 89:91–5. doi: 10.1016/S0378-8741(03)00267-8
24. Marileo, AM, Lara, CO, Sazo, A, Contreras, OV, González, G, Castro, PA, et al. Molecular pharmacology of Gelsemium alkaloids on inhibitory receptors. Int J Mol Sci. (2024) 25:3390. doi: 10.3390/ijms25063390
25. Sun, M-X, Cui, Y, Li, Y, Meng, W-Q, Xu, Q-Q, Zhao, J, et al. Indole alkaloids from Gelsemium elegans. Phytochemistry. (2019) 162:232–40. doi: 10.1016/j.phytochem.2019.03.016
26. Lai, C-K, and Chan, Y-W. Confirmation of Gelsemium poisoning by targeted analysis of toxic Gelsemium alkaloids in urine. J Anal Toxicol. (2009) 33:56–61. doi: 10.1093/jat/33.1.56
27. Vale, JA. Position statement: gastric lavage American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol-Clin Toxic. (2004) 35:711–9. doi: 10.1081/clt-200045006
28. Pan, G. Resuscitation of 8 cases of gelsemiun elegans poisoning. China Pharmaceut. (2008) 17:56–7. doi: 10.3969/j.issn.1006-4931.2008.21.039
29. Li, Y, Xiao, N, Jia, W, Li, X, Zheng, X, and Sun, Z. The effectiveness of flumazenil-epinephrine combination on treatment for acute Gelsemium elegans poisoning. Acta Vet Zootech Sin. (2022) 53:938–46. doi: 10.11843/j.issn.0366-6964.2022.03.026
Keywords: Gelsemium elegans, poisoning, case report, review-systematic, gastric lavage
Citation: Zhao Y, Shen B, Xiao Q, Wang Y, Fu Y, Tang Q and Liang Z (2025) Caution on the severe damage of Gelsemium elegans poisoning: a case report on family poisoning and systematic review. Front. Public Health. 13:1633727. doi: 10.3389/fpubh.2025.1633727
Edited by:
Mansoor C. Abdulla, Sultan Qaboos Hospital, OmanReviewed by:
Lorenzo Franceschetti, University of Milan, ItalyCopyright © 2025 Zhao, Shen, Xiao, Wang, Fu, Tang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhangrong Liang, MjAyMTA5ODE3MUBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
 Yawen Zhao
Yawen Zhao Bisheng Shen1,2†
Bisheng Shen1,2†