- Hunan Province Maternal and Children Care Hospital, Changsha, China
Background: China’s evolving fertility policies (one-child to three-child) have shaped maternal and neonatal outcomes, but specific gaps in stillbirth epidemiology during policy transitions.
Methods: This retrospective cohort study analyzed 721,860 singleton pregnancies in 2011–2023, from 18 maternal near-miss surveillance hospitals in Hunan. Stillbirth rates were assessed across four policy periods: one-child (2011–2013), partial two-child (2013–2015), universal two-child (2016–2020), and three-child (2021–2023). Multivariable logistic regression identified risk factors, adjusting for fertility policy period, maternal demographics and maternal comorbidities. Trends over time were analyzed using segmented regression models.
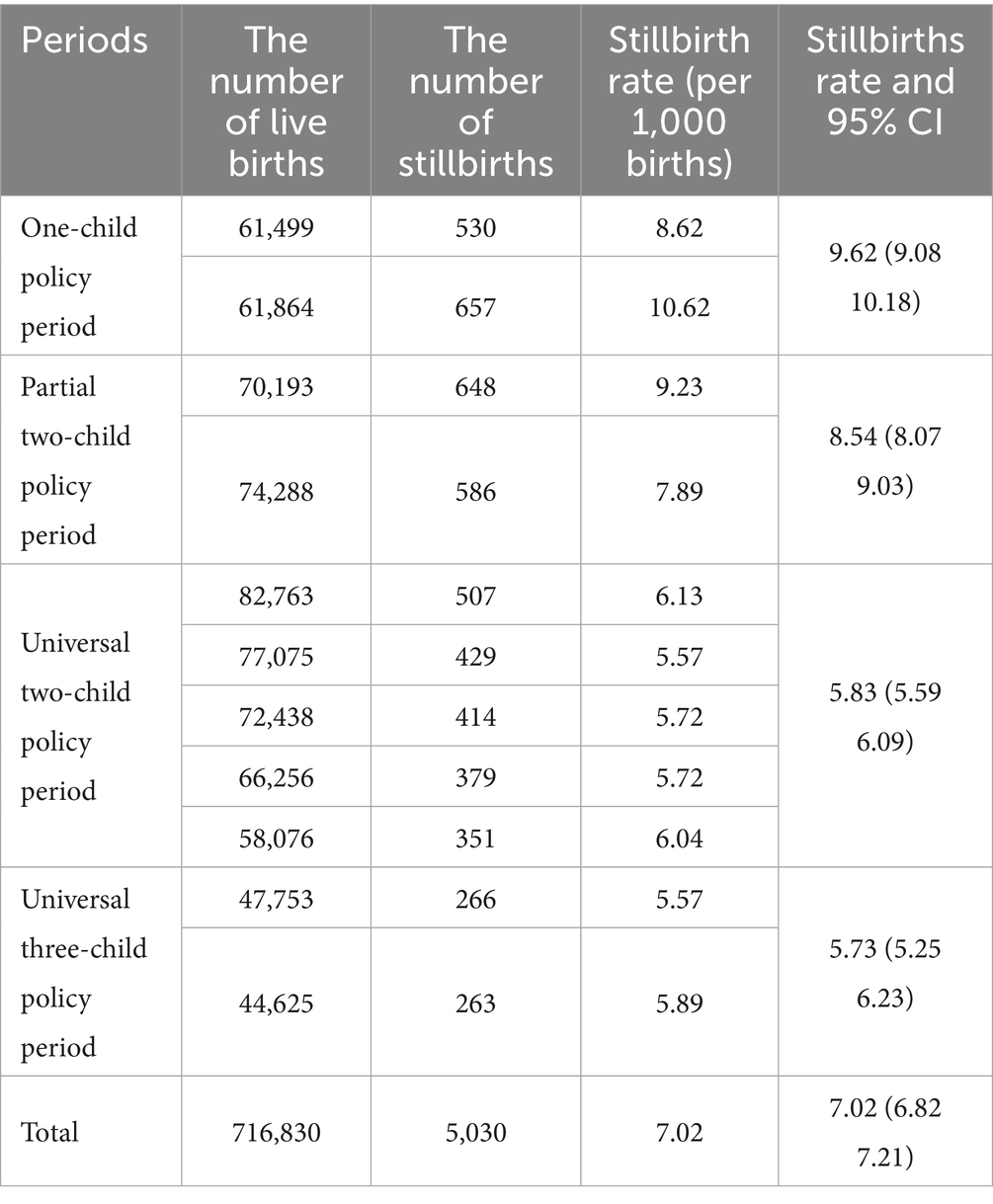
Results: The overall stillbirth rate was 7.02‰ (95% confidence interval [CI]: 6.82–7.21), declining significantly from 9.62‰ during the one-child policy to 5.73‰ (95%CI: 5.25–6.23) under the three-child policy (t = −4.22, p < 0.01). Key risk factors included maternal age < 24 years (adjusted odds ratio [aOR] = 1.77, 95%CI:1.63–1.92), multiparity (aOR = 1.27–2.82. p < 0.01), non-rural hospital delivery (aOR = 4.00–11.13, p < 0.01), education ≤9 years (aOR = 1.51–2.20, p < 0.01), not being married (aOR = 2.92–5.60, p < 0.01), and comorbidities: severe preeclampsia (aOR = 3.80, 95%CI: 3.36–4.29), chronic hypertension (aOR = 2.67, 95%CI: 2.09–3.37), placental abruption (aOR = 5.06, 95%CI: 4.11–6.16), and placenta previa (aOR = 1.55, 95%CI: 1.29–1.84). Paradoxically, prenatal diabetes was associated with reduced stillbirth risk (aOR = 0.86, 95%CI: 0.77–0.95). Temporal shifts revealed elevated stillbirth rates among advanced-age mothers pre-2016 versus rising rates in women <24 years post-policy liberalization. Only the partial two-child policy period (aOR = 1.15, 95%CI: 1.05–1.25) was associated with the risk of stillbirth.
Conclusion: China’s fertility policy transitions correlate with dynamic stillbirth epidemiology, emphasizing age- and parity-specific vulnerabilities. Targeted interventions for high-risk subgroups, especially younger, less well-educated, multiparous women, and those with hypertensive or placental disorders, are critical amid ongoing implementation of the three-child policy.
Background
The definition of stillbirth varies among countries and regions around the world. The World Health Organization (WHO) defines it as fetal death as occurring at ≥28 weeks’ gestation, with birth weight ≥1,000 g or body length ≥35 cm (1). Contrastingly, the American College of Obstetricians and Gynecologists (ACOG) uses a lower viability threshold, classifying stillbirths as fetal demise at ≥20 weeks’ gestation (or undetermined gestational age) with birth weight ≥350 g (2). Other major developed economies show further variation: Australia and European nations typically use either 20-week gestational criteria (24 weeks in certain jurisdictions) or 500 g weight thresholds (3). The definition of stillbirths in China is consistent with the WHO definition.
Stillbirth is a significant component of perinatal mortality and a primary cause of medical disputes in healthcare institutions. It is also a crucial indicator of the quality and accessibility of perinatal care, particularly antenatal and intrapartum care (4). Despite sharing 72% of modifiable risk factors with maternal and neonatal mortality (including hypertensive disorders, infections, and placental insufficiency) (5), stillbirth reduction lags markedly in global health agendas, evidenced by an annual decline of a mere 2.3% since 2000, compared with reduction of 2.9% in neonatal mortality and 4.3% in mortality of children under 5 years old (6). This stagnation translates to preventable losses of 2 million viable fetuses annually. The United Nations has therefore explicitly included stillbirth prevention in its Global Strategy (2016–2030) alongside maternal and child mortality, through the landmark commitment to “end preventable stillbirths” (7). However, China lacks standardized integration of stillbirth metrics into core maternal and neonatal quality indicators, such as maternal and neonatal mortality rates, reflecting insufficient focus on this problem. Current research on stillbirth rate there remains fragmented, limited to Zhu’s national report (8, 9), and Shenzhen’s regional reports (10). Hunan Province-a demographic epicenter with 66 million residents-exemplifies this data desert, with no provincial-level stillbirth epidemiology information.
Global stillbirth surveillance shows significant epidemiological disparities, with an estimated 2 million late-gestation (≥28 weeks) fetal deaths annually (13.9‰ of total births) (11). When applying the 20-week gestational threshold, this increases to over 4 million deaths worldwide (4). Notably, 98% of stillbirths occur in low- and middle-income countries, with China ranking fifth in absolute numbers globally (5, 12). China’s stillbirth epidemiology shows dynamic temporal patterns influenced by evolving fertility regulations. National research data shows a prevalence of 8.8‰(≥28 weeks) during 2012–2014 (9), 13.2‰ (≥24 weeks) in 2015–2016 (8), and 5.5‰ (≥28 weeks) by 2019 (13). The prevalence of stillbirths may be linked to the effects of child birth policy. China introduced the one-child policy in 1978 (14), the partial two-child policy in November 2013 (15), and the universal two-child policy in October 2015 (16). However, the fertility rate in China has been declining every year since 2016. Since 2021, the Chinese government and many regions have introduced policies to encourage people to have second and third children (17) and China implemented a full three-child policy on May 31, 2021 (18).
China has accumulated substantial research evidence on the relationship between fertility policy adjustments and maternal and neonatal health outcomes. Investigations have primarily focused on two dimensions: maternal health aspects, including fertility intentions (19), second-time mothers (20), cesarean section rates (21), obstetric infection (22), uterine rupture (23), the characteristics of pregnancy and delivery (24), and perinatal outcomes, including birth defects (25–27), gender equity (17), early-term birth (28) and so on. Internationally, few studies have examined temporal changes in stillbirth rates, especially specific gaps in stillbirth epidemiology during policy transitions.
It is therefore both feasible and important to study the incidence of stillbirths and the characteristics of pregnancies that ended in stillbirths under different fertility policies. This will provide a reference for the incidence of stillbirths in central provinces of China, examine the causes of stillbirths, and demonstrate changes in the characteristics of stillbirths in central regions.
Materials and methods
Study design and data sources
This retrospective cohort study was conducted across 18 maternal near miss (MNM) surveillance hospitals in 14 cities within Hunan province, which aimed to enumerate all maternal deaths and near misses(women who nearly died from a severe complication of pregnancy or delivery) using the same approach as that proposed by WHO (29). The study population was pregnant or post-partum women admitted to obstetrics departments, with data collection spanning from hospital admission through to discharge. Data were systematically collected using the standardized “Maternal Case Survey Form” designed by the China Office of Maternal and Child Health Monitoring. This includes maternal characteristics, maternal and perinatal health, and pregnancy-related comorbidities and complications. The surveillance protocol incorporated three-tier quality control measures at hospital, municipal, and provincial levels to ensure data integrity. Detailed methodological specifications for sampling strategies, data reporting procedures, and quality assurance mechanisms have previously been documented in established protocols (9).
This study was approved by the Ethics Review Committee of Hunan Province Maternal and Children Care Hospital and conducted in accordance with the principles of the Declaration of Helsinki. Because of the retrospective design of this study, the Ethics Review Committee of Hunan Province Maternal and Children Care Hospital has waived the requirement for informed consent for this study.
Definition of variables
Stillbirth was defined using WHO criteria as fetal deaths occurring at ≥ 28 weeks gestation or with birthweight ≥ 1,000 g, restricted to antepartum cases. To account for conception-to-delivery time lags in policy impact assessment, we operationalized policy exposure windows based on last menstrual period dates (23). The study timeline was stratified into four distinct policy phases: one-child policy (October 2011–September 2013), partial two-child policy (November 2013–November 2015), universal two-child policy (January 2016–December 2020), and universal three-child policy (June 2021–May 2023).
Covariates covering maternal characteristics included: demographics variables such as age (≤24, 25–29, 30–34, ≥35 years) and parity (0, 1, 2, ≥3 prior pregnancies), socioeconomic factors including education level (primary/none, junior high, senior high, college or more) and marital status (married, single/widowed, divorced/cohabiting), attendance at antenatal care visits categorized in line with guidelines (≤5, 6–9, ≥10) and hospital levels classified by geographic proximity to Changsha, the provincial capital(rural, peri-urban, metropolitan).
Obstetric comorbidities were defined as: hypertensive disorders including chronic hypertension (pre-existing or secondary hypertension ± proteinuria), severe preeclampsia (gestational hypertension + proteinuria >0.3 g/24 h or ≥30 mg/mmol) and eclampsia (seizures complicating preeclampsia), endocrine metabolism of preexisting diabetes mellitus and placental pathologies including placenta previa defined as the placenta abnormally covering the endocervical os (30), and placental abruptiondefined as the premature separation of the placenta from its uterine attachment before the delivery of a fetus (31).
Data selection and sample size
We extracted 917,855 maternal care records from China’s National Maternal Near Miss Surveillance System, spanning October 2011 to May 2023 in Hunan Province. We excluded 71,184 records falling outside the target study period were, giving an initial dataset of 846,671 documented pregnancies. Subsequent application of predefined exclusion criteria sequentially removed multiple gestations (n = 18,009), records with missing delivery outcomes (n = 83,677), records with incomplete maternal age documentation (n = 11,977), pregnancies lacking gestational week documentation (n = 3,602), preterm deliveries before 28 weeks’ gestation (n = 6,037), and statistical outliers (n = 236). Considering that intrapartum stillbirth is a sensitive marker of delay and low quality of care, reflecting scarcity of intrapartum monitoring and delays in the rapid delivery of a compromised fetus, and the 18 Maternal Near Miss Surveillance hospitals quality obstetric and neonatal care is different, the intrapartum stillbirths (n = 1,273) were deleted considering that it is a sensitive marker of delay and low quality of care, reflecting scarcity of intrapartum monitoring and delays in the rapid delivery of a compromised fetus but not the health condition of the fetus itself. The final cohort for analysis included 721,860 singleton pregnancies with complete perinatal records, including 5,030 cases of stillbirth. The facility-specific data extraction was listed in Supplementary Table 1.
Based on the following formula for estimating sample size for population incidence rates, the sample size was 6,743 based on the expected incidence rate p of 0.46%, allowable absolute error less than 0.05%, and width of the 95% confidence interval (CI) is within an acceptable range. The current 721,860 subjects was fully sufficient to meet the sample requirements.
Formula: N
Statistical analysis temporal and stratified analysis
We initially calculated annual stillbirth rates across fertility policy periods (2012–2015: one-child policy; 2016–2020: universal two-child policy; 2021–2023: three-child policy). We used stratified analyses to examine the demographic distribution of stillbirths and live births by maternal characteristics (age, parity, education level, marital status, hospital level, antenatal care frequency) and pregnancy complications across policy epochs. Temporal trends were quantified using segmented regression models.
Policy period effect estimation
We constructed binary logistic regression to assess unadjusted associations between maternal characteristics and stillbirth risk across policy periods, using the one-child policy era as reference. We also assessed both crude and adjusted associations for pregnancy complications during each policy period, with adjustment for policy exposure duration, maternal age, parity, education attainment, marital status, hospital level and antenatal visit frequency.
Comprehensive risk profiling
We used integrated models to evaluate population-level stillbirth risks associated with maternal characteristics, adjusting for policy period, demographic factors, and complication status. We also examined complication-specific stillbirth risks, controlling for policy period and demographic covariates.
Statistical operations
Descriptive statistics were used to characterize baseline maternal profiles. Temporal trends were assessed through segmented regression models with year as the continuous predictor. Multivariable logistic regression generated odds ratios (ORs) with 95% confidence intervals, comparing stillbirths (cases) against live births (controls). All analyses were conducted in R 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria) using two-tailed significance testing (α = 0.05).
Results
Stillbirth rates across different birth policy periods
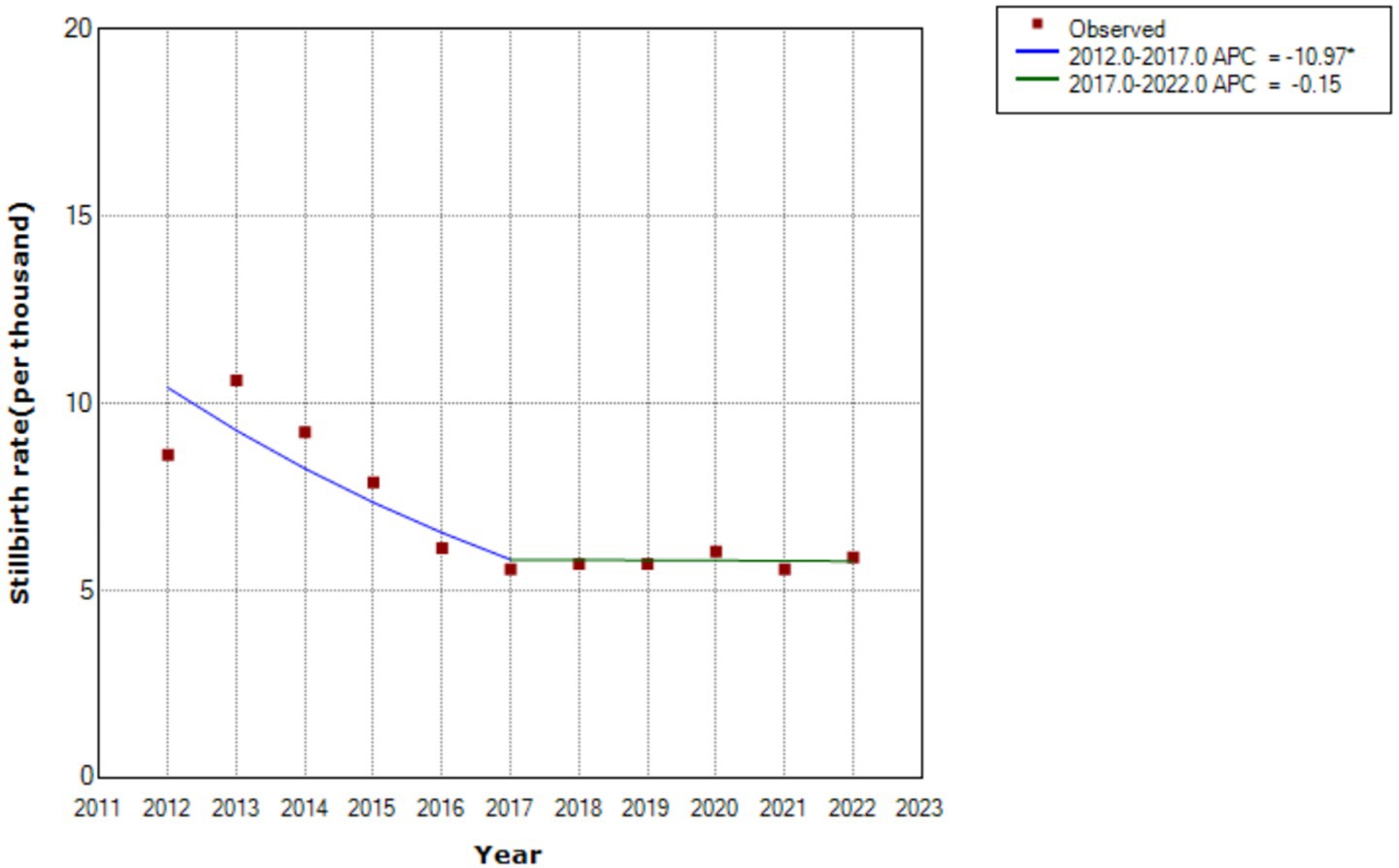
The overall stillbirth rate was 7.02 per 1,000 births (95% CI: 6.83–7.21). However, Table 1 shows, significant temporal variations across policy periods. There was a consistent downward trend from the one-child policy era (9.62‰, 95% CI: 9.08–10.18) to the universal three-child policy phase (5.73‰, 95% CI: 5.25–6.23), with statistically significant linear progression (t = −4.22, p < 0.01) (Figure 1).
The stillbirth rate by maternal characteristics under different birth policy periods
Figure 2 shows the annual stillbirth rate by different maternal characteristics under different birth policy periods. Table 2 shows the unadjusted odds ratio of stillbirth rate by different maternal characteristics in different childbirth periods, compared with that in the one-child policy period. The aggregate stillbirth rates were 10.12‰, 6.39‰, 6.03‰ and 7.62‰ for women aged <24, 25–29, 30–34, and ≥35 years. Only those aged < 24 years showed an increasing stillbirth rate during the universal three-child policy period. In contrast, women aged 25–29 years showed no significant temporal decline in stillbirth incidence (t = 0.32, p = 0.76). Parity analysis showed descending stillbirth rates for pregnancies with no more than two previous pregnancies, while multiparous women (at least one previous pregnancy) maintained consistently higher rates than primiparous women. Lower maternal education levels were correlated with higher stillbirth incidence, showing progressive declines across policy periods except for primary school-educated women. Pregnancies with non-normative marital status were associated with increased stillbirth risks during policy transition phases compared with those during the one-child policy period. The stillbirth rate of pregnancies in different types of hospital gradually decreased with the highest stillbirth rate in hospitals around the provincial capital. Having more antenatal was significantly associated with reduced stillbirth incidence across all policy periods. The frequencies of different maternal characteristics associated with stillbirth across different birth policy periods are shown in Supplementary Table 2.
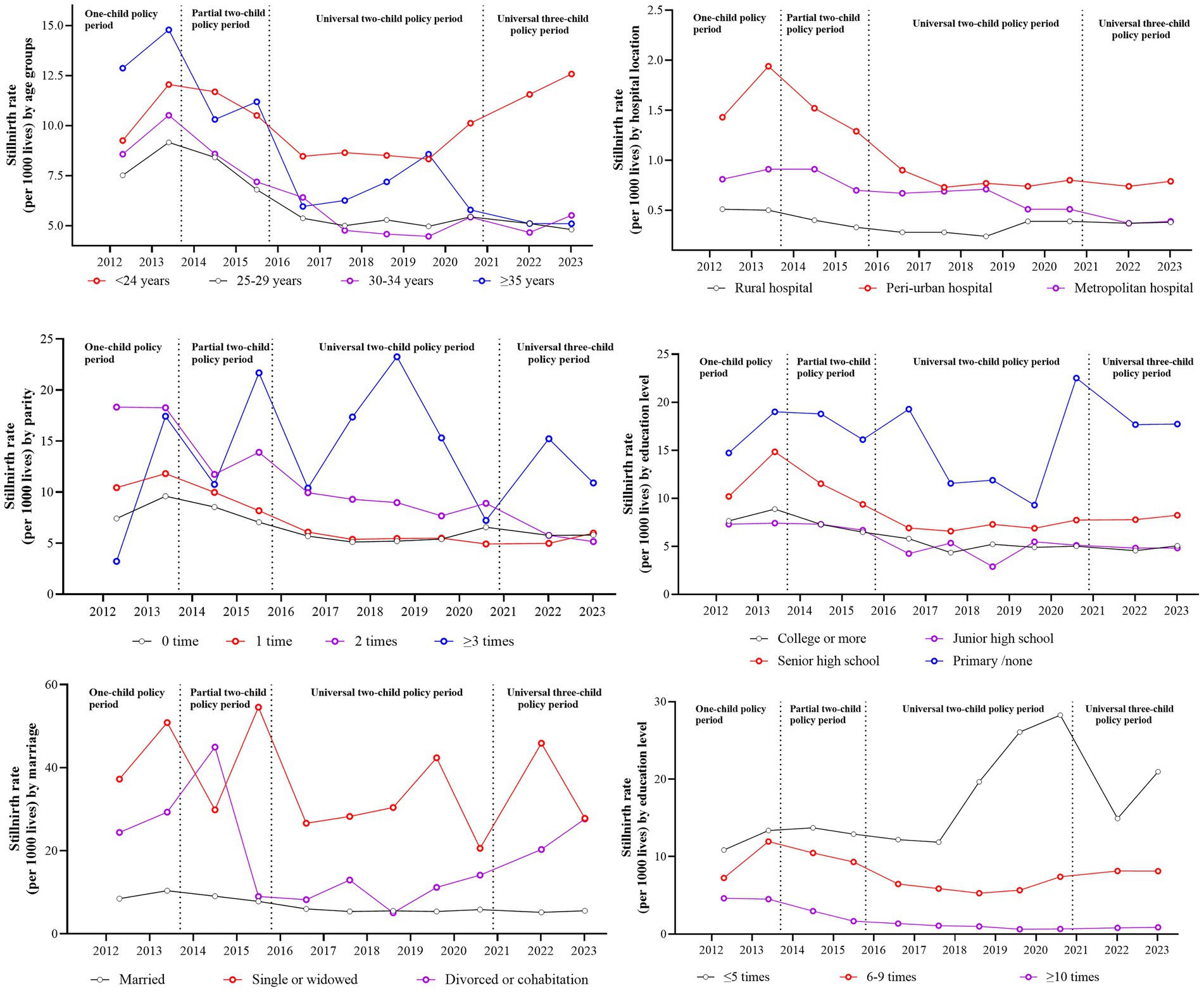
Figure 2. The annual stillbirth incidence by maternal characteristics under different birth policy periods.
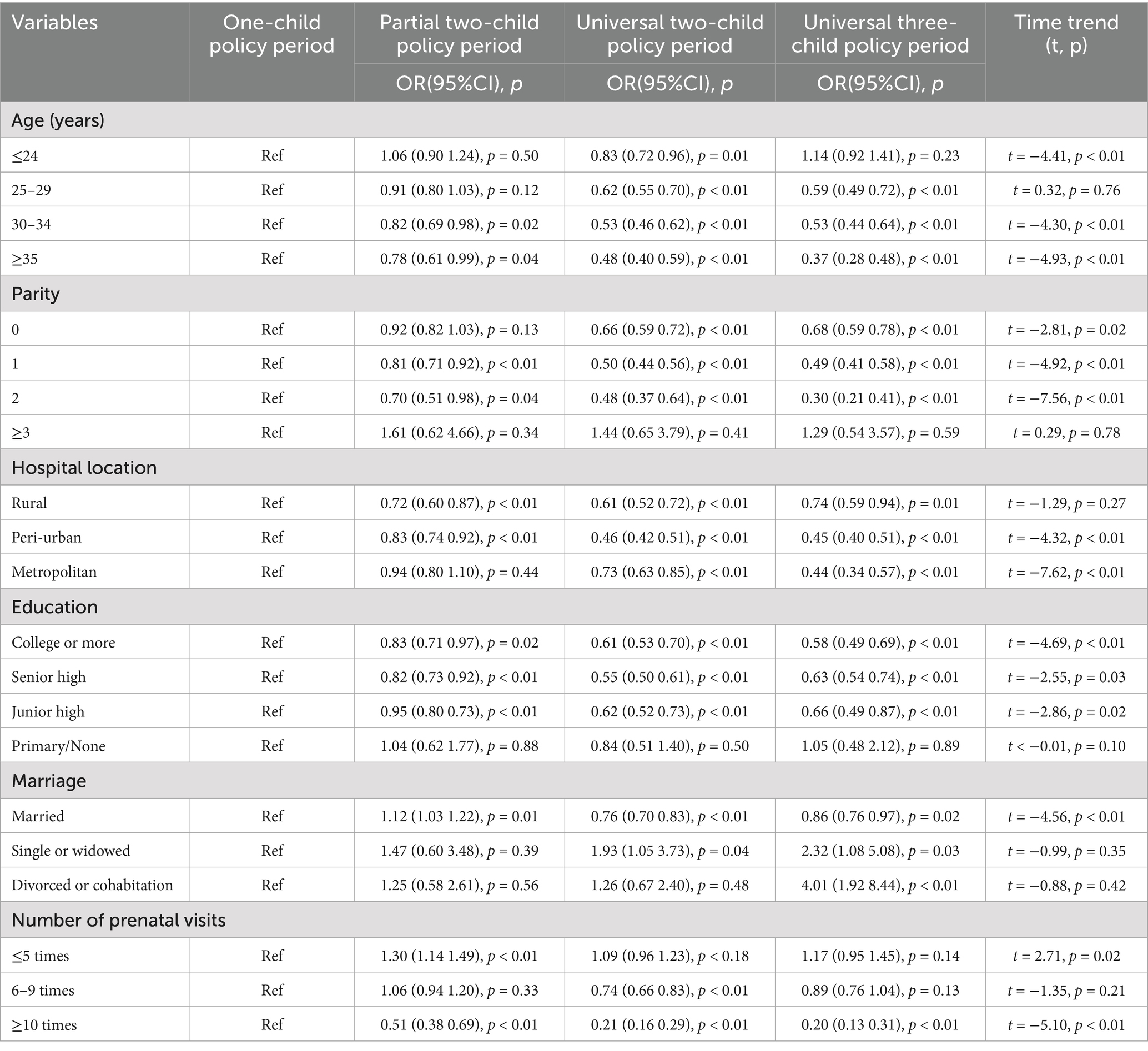
Table 2. The time trend and unadjusted odds ratio of maternal characteristic variables in different childbirth periods compared with one-child policy period.
The stillbirth rate by maternal complications across birth policy periods
Figure 3 shows annual stillbirth rates stratified by maternal complications under different birth policy periods. Table 3 shows unadjusted and adjusted odds ratios for stillbirth risk across these policy periods, and Supplementary Table 3 shows the frequency distribution of pregnancies with complications and stillbirths across policy phases. Within the study cohort of 458,636 pregnancies with complications (63.54%), the prevalence of specific complications was as follows: prenatal diabetes mellitus (15.85%, N = 72,705), severe preeclampsia (2.07%, N = 9,471), placenta previa (1.92%, N = 8,810), chronic hypertension (0.84%, N = 3,858), placental abruption (0.61%, N = 2,775), and eclampsia (0.03%, N = 153). Comparative analysis showed significantly higher stillbirth rates in pregnancies complicated by prenatal hypertension, severe preeclampsia, placenta previa, and placental abruption compared with the rates in uncomplicated pregnancies across all policy periods.
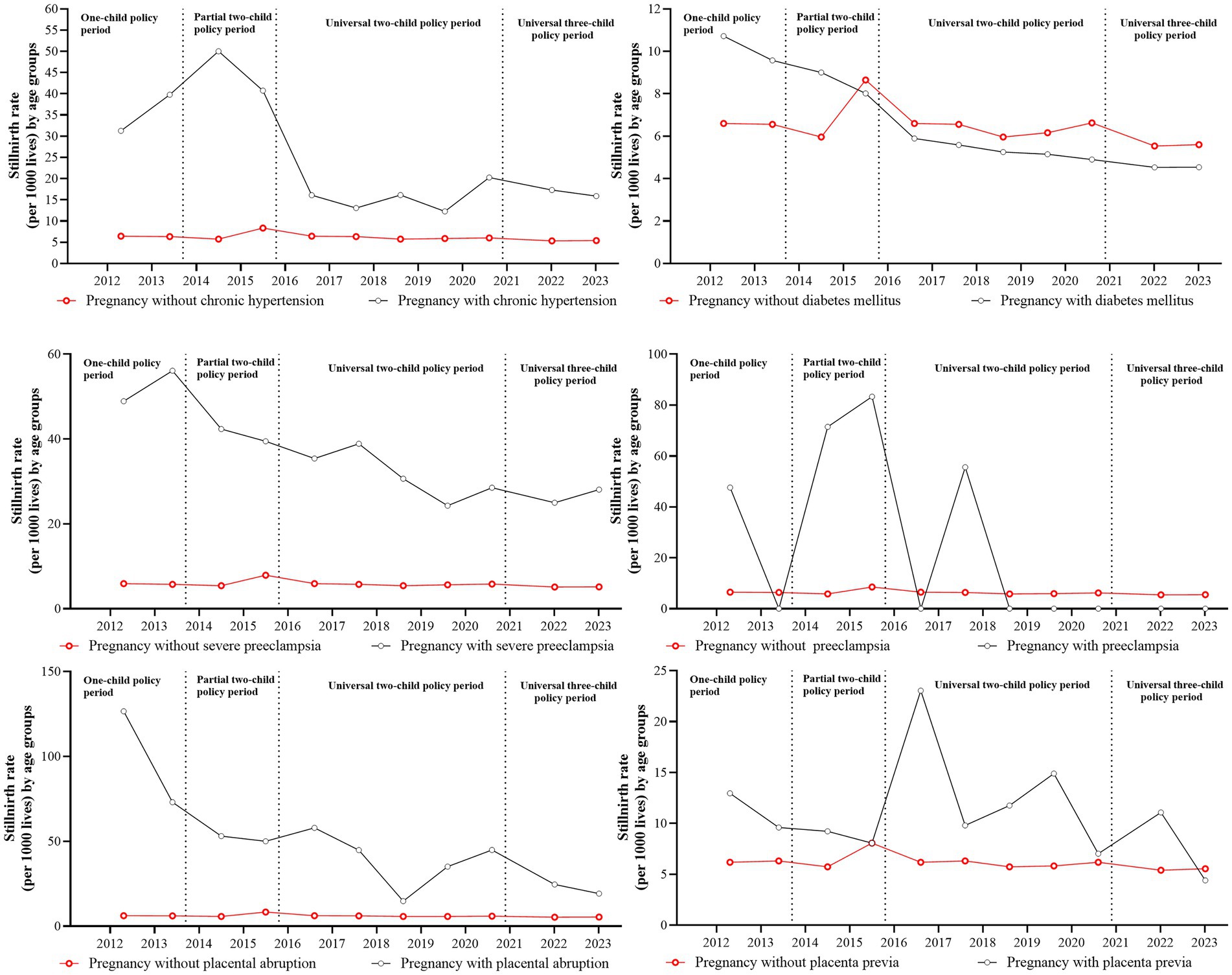
Figure 3. The incidence and related trends of stillbirths by complications during different birth policy periods.
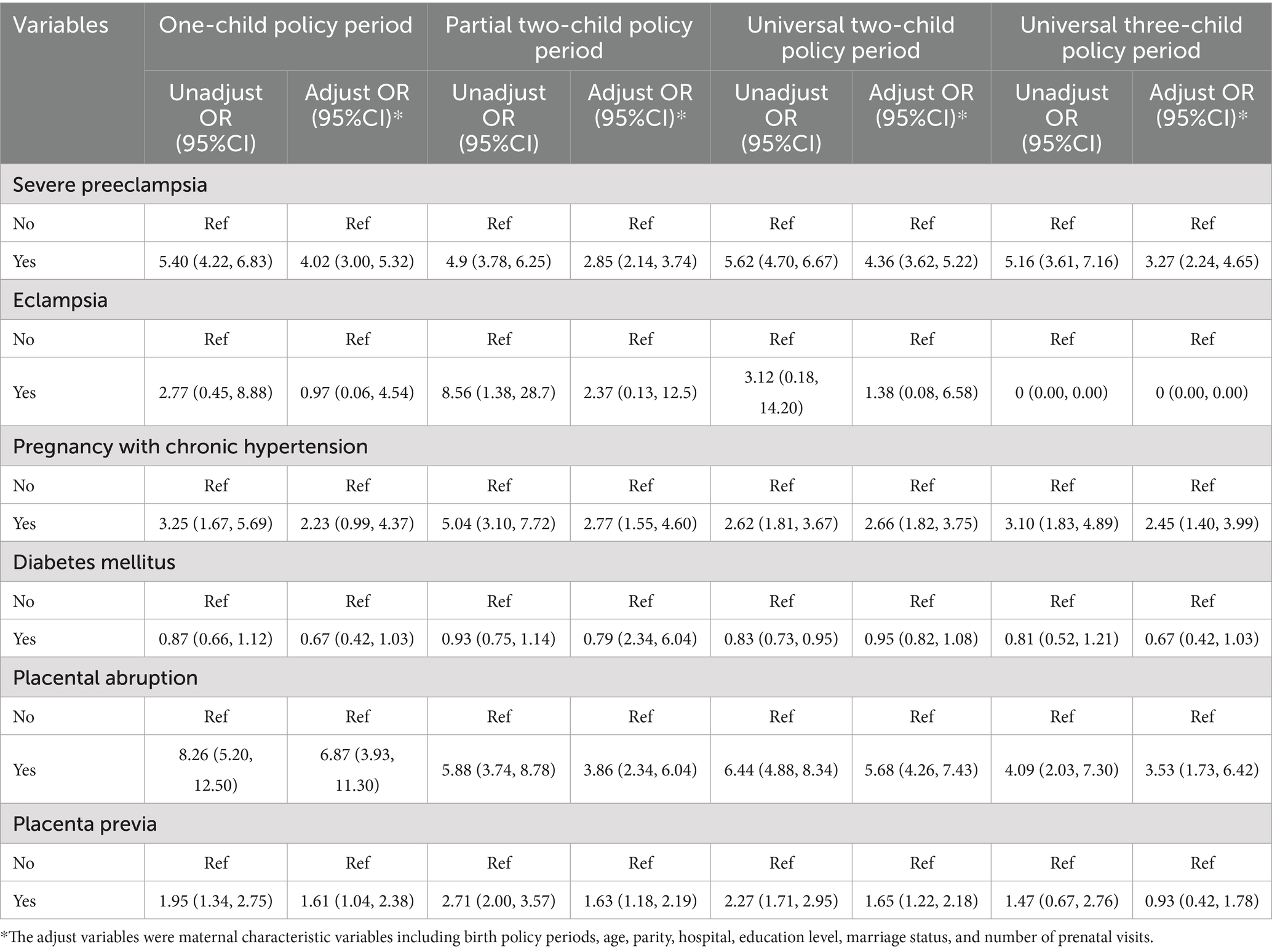
Table 3. The unadjusted and adjusted odds ratio of different maternal near misses in each child policy period.
Two complications showed consistent associations with elevated stillbirth risk across all policy periods: severe preeclampsia (adjusted OR range: 2.85–4.36) and placental abruption (adjusted OR range: 3.53–6.87). Hypertension-related complications and placenta previa both showed policy-dependent risk patterns, with non-significant associations observed specifically during the one-child policy and universal three-child policy periods.
Risk factors associated with stillbirth across birth policy periods
The unadjusted and adjusted odds ratio for stillbirth associated with maternal characteristics and six pregnancy complications across all the birth policy periods are shown in Table 4. Binary logistic regression analysis showed several significant risk factors for stillbirth after adjustment for confounding variables. These included exposure to the partial two-child policy period, maternal age ≤24 years (aOR = 1.77, 95%CI: 1.63–1.92), multiparity (aOR = 1.27–2.82), non-rural hospital location (aOR = 4.00–11.13), educational attainment below university level, being unmarried, and the presence of severe preeclampsia, prenatal hypertension, placental abruption, or placenta previa. There were also two protective factors: pregnancies complicated by diabetes mellitus (aOR = 0.86, 95%CI: 0.77–0.95) and receiving more frequent prenatal check-ups (aOR = 0.06–0.34) were associated with lower stillbirth risk.
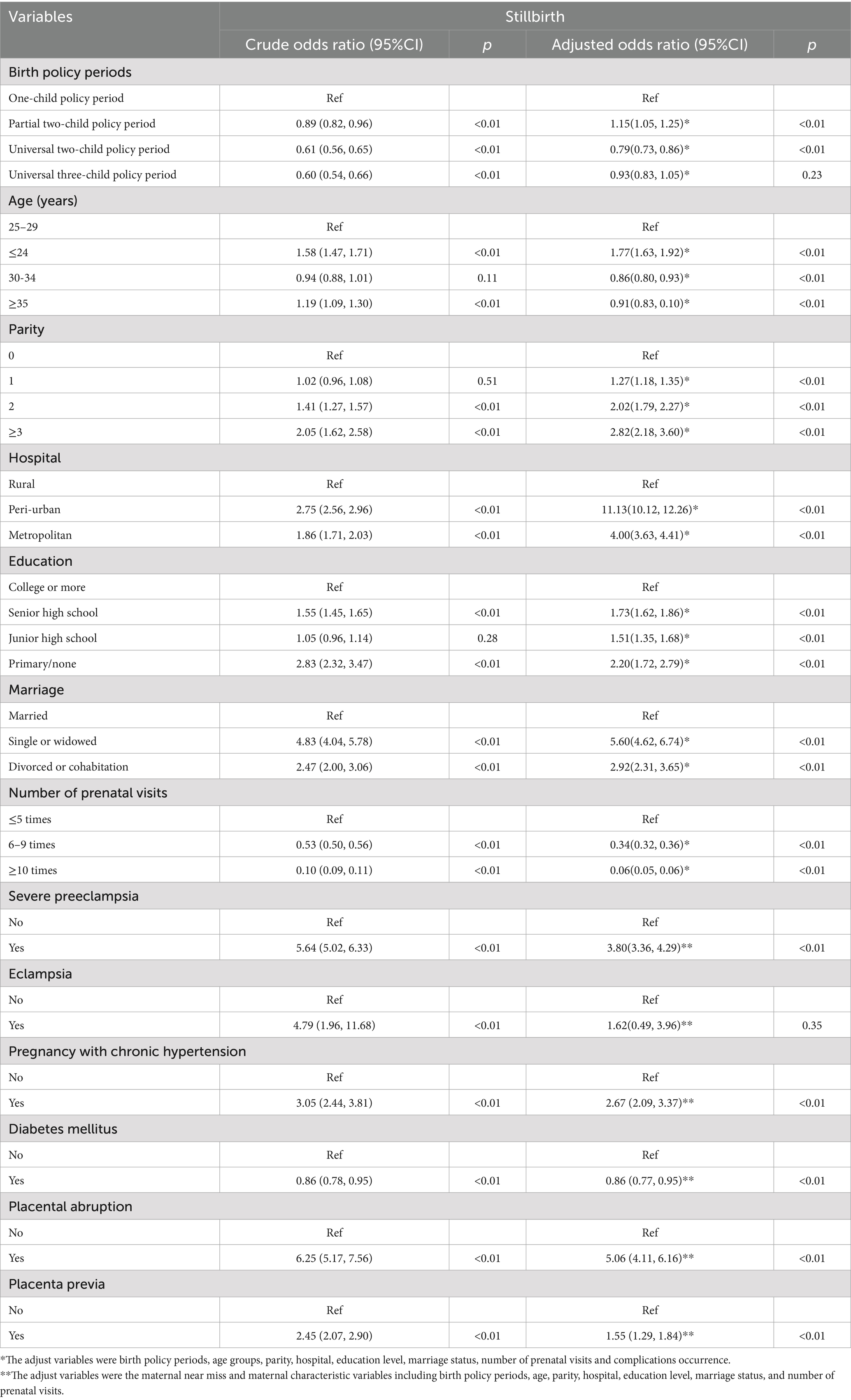
Table 4. The total unadjusted and adjusted odds ratio of stillbirth among maternal characteristics and different maternal near misses.
Discussion
This longitudinal policy-sensitive analysis shows critical patterns in the epidemiology of stillbirth across changes in China’s fertility policy form 2011 to 2024. We found an overall stillbirth rate of 7.02 per 1,000 live births (95%CI 6.82–7.21), with a significant annual decline of 4.82% and a J-shaped association with stillbirth risk, with the lowest rate among women aged 25–29 years. Those aged < 24 years showed a paradoxical increase in stillbirth rate after the COVID-19 pandemic. Multiparity, educational attainment of ≤9 years, being unmarried, delivery in a secondary hospital, attending less than five antenatal care appointments, preconception hypertension, severe preeclampsia, placenta accreta, and placenta previa were all risk factors of stillbirths. Pre-existing diabetes mellitus showed a paradoxical risk, with diabetic mothers showing lower stillbirth incidence than non-diabetic controls during the universal two−/three-child policy periods.
The stillbirth rate of Hunan province in the Central region of China, was higher than that in South, and lower than that in the Northwest, highlighting the uneven distribution of stillbirths across geographical regions in China (8). The stillbirth rate was also higher than that reported (4.5 per 1,000 births from January 2010 to December 2019 in Baoan, Shenzhen) (10), and lower than that the average of 8.8 per 1,000 births reported for China during 2012 to 2014 (9). Our stillbirth rate was also higher than the 5.8 per 1,000 total births at ≥24 weeks in the United states in 2020 (32), and 0.09 per 1,000 births of term intrapartum stillbirths in Norway from 1999 to 2018 (33). However, it was lower than the global stillbirth rate of 13.9 per 1,000 total births at or beyond 28 weeks of gestation (34), and the rate for most other low and middle-income countries (12), such as India (35). The average annual reduced rate was the same as the national average of 4–6% (36). The decline in rate of stillbirth was greatest (37%) from the partial two-child policy period to the universal two-child policy period. After that, it continued to decrease slightly. This phenomenon may be related to fertility intention and the total numbers of live births. After the enactment of China’s new universal two- child policy, the change in fertility intention among Chinese women (intention to have a second child) was short-term (19). The total number of births only increased during the partial two-child policy period (25). This study therefore found that only the partial two-child policy period was associated with the risk of stillbirth, which can be attributed to factors such as a higher proportion of pregnant women over 35 years, an increase in pregnancy complications and comorbidities, and potential impacts on obstetric service capabilities (37–39).
The prevention and reduction of stillbirths require targeted interventions for key risk groups, particularly pregnant women under 24 and over 35 years of age. Additional support is also needed for multiparous women, and those with limited education, or who are unmarried, delivering in rural hospitals, and have fewer than five antenatal care visits. Our analysis uncovered a paradoxical age-specific pattern in stillbirth rates following China’s evolving fertility policy framework. Women aged under 24 years had the highest stillbirth incidence across all age groups following the implementation of the 2016 universal two-child policy. This trend intensified post-2021, when the three-child policy started, establishing this group as the sole demographic exhibiting with rising stillbirth rates during policy transitions. This epidemiological pattern aligns with established risk profiles for stillbirth, such as being young, unmarried, and with low educational attainment (9). These risk factors predominantly cluster in those under 24 years, particularly given the diminished fertility intentions among women of optimal childbearing age since the implementation of the three-child policy (40). These findings underscore the need for focused stillbirth prevention strategies targeting women under 24, including enhanced contraceptive education and access (9, 41). Contrastingly, the period before 2016 showed higher stillbirth risk among older women, attributable to both inherent biological risks (5, 8) and fertility demand release during the partial two-child policy phase. We also found parity-dependent risk escalation, consistent with perinatal outcome studies (28, 42). This association may reflect the dual mechanisms of older women being more likely to have other children and early pregnancies occurring in socioeconomically disadvantaged contexts where there is limited health literacy.
This study identified placenta accreta and placenta previa among placental factors, and severe preeclampsia and prenatal hypertension among hypertensive disorders, as significant risk factors for stillbirth. These findings are consistent with other evidence showing elevated stillbirth rates in pregnancies with maternal comorbidities. For instance, singleton births in Gambian hospitals from January to June 2006 showed an eight-fold increase in risk of stillbirth among women with severe obstetric complications compared with the risk among those without complications (43, 44). Hypertensive disorders in pregnancy are categorized into four major subtypes: pregnancy-induced hypertension (PIH, mild to moderate hypertension without proteinuria), preeclampsia (hypertension with proteinuria), severe preeclampsia, and eclampsia (45). Hypertension during pregnancy is widely recognized as a risk factor for stillbirth, but our analysis showed no significant association between eclampsia and stillbirth, potentially attributable to pharmacological interventions during eclamptic episodes. Emerging evidence suggests that calcium supplementation during pregnancy may reduce stillbirths by 19%, though this finding was not statistically significant, while aspirin use showed no protective association (46). Future investigations should explore mediating factors between hypertensive disorders and stillbirth pathogenesis.
As China’s maternal medical care system has improved, general gestational health has also improved and there have been better outcomes among infants, as well as lower incidence of hypertensive disorders in pregnancy and placenta previa after the introduction of the universal two-child policy in one of our monitoring hospitals (20). Our results, together with others, suggest that placental factors may contribute to stillbirth (47–49). Nationwide studies also showed placenta accreta was a critical risk factor for antepartum stillbirth in central China (8). However, contrary to conventional risk paradigms, prenatal diabetes was not associated with increased stillbirth risk in our study. Notably, since the implementation of the universal two-child policy in China, diabetic pregnancies have resulted in a lower incidence of stillbirth than non-diabetic pregnancies. This observation is consistent with studies identifying maternal blood glucose levels and body mass index as modifiable risk factors for stillbirth in diabetic pregnancies (50). Future investigations should explore mediating factors between prenatal diabetes and stillbirth.
It is still unclear whether maternal comorbidities precipitate stillbirths or vice versa. We had no data on prior history of stillbirth, but cohort data from Western Australian from 2000 to 2015 show that mothers who have stillbirths, regardless of any comorbidities have a great risk of severe maternal morbiditythan mothers who have live births (48). Future research should prioritize understanding the temporal relationship between comorbidities and fetal death (51). Healthcare quality improvements, socioeconomic changes during China’s rapid development, Body Mass Index, smoking status, previous obstetric history, COVID-19 pandemic impact overlaps with policy influence should be taken account for analysis of factors influencing stillbirths.
Our study is important for several reasons. First, it is the first study of which we are aware to characterize stillbirth patterns and identify influencing factors across different policy periods. Second, our research framework incorporated a critical assessment of policy lag effects, a temporal dimension frequently overlooked in epidemiological studies examining the impact of fertility policies. Third, the investigation covered 18 surveillance hospitals across the province, ensuring a large sample size that enhances the statistical power of our findings.
However, the study also had several limitations. First, it was a cross-sectional retrospective investigation, and the observed associations between risk factors and stillbirth therefore require validation through long-term longitudinal observations and prospective cohort studies. Second, hospital selection followed the urban–rural distribution patterns from the 2010 national census to ensure national representativeness, but this sampling strategy may not fully reflect the specific demographic characteristics of Hunan Province. What’s more, this hospital-based sampling data may not represent the whole province population-level estimates for the selection bias and generalizability limitations. Third, the dataset lacked detailed clinical parameters such as maternal anthropometrics (weight/height) and obstetrical history indicators (previous occurrence of stillbirth), which could potentially influence the analysis outcomes.
Conclusion
We found that the selective two-child policy, maternal age of <24 years, multiparity, non-rural hospital delivery, lower levels of education, inadequate antenatal care, and comorbidities including prenatal hypertension, severe preeclampsia, placenta accreta, and placenta previa were significantly associated with elevated risk of stillbirth. Temporal trend analysis showed distinct patterns: advanced maternal age was linked to higher stillbirth rates before the two-child policy liberalization. However, post-liberalization, younger mothers (age <24 years) had higher incidence of stillbirth. Pregnancies complicated by prenatal diabetes actually showed a lower risk of stillbirth than non-diabetic pregnancies. Future preventive strategies should prioritize high-risk subgroups, including younger mothers, those with lower educational attainment, women with other children, and pregnancies complicated by hypertensive disorders or placental pathologies. This should help to mitigate stillbirths. As national efforts move from reducing infant mortality to enhancing perinatal care, together with the promotion of fertity after the implementation of China’s three-child policy, addressing stillbirth remains a critical clinical and public health priority. Integrating stillbirth metrics into maternal and neonatal care quality surveillance and enhancing perinatal care accessibility remain urgent public health priorities.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Review Committee of Hunan Province Maternal and Children Care Hospital and conducted in accordance with the principles of the Declaration of Helsinki. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LX: Methodology, Writing – original draft, Software, Conceptualization, Writing – review & editing. DX: Writing – review & editing, Data curation, Project administration. QJ: Writing – review & editing. JF: Writing – review & editing, Project administration, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by the high-level talent plan project of Hunan province Maternal and Child Health Hospital and Hunan Provincial Department of Science and Technology General Project (2025JJ50655). The funders did not play any role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Acknowledgments
We would like to express our thanks to all the medical staff and participants who took part in the 18 maternal near miss surveillance hospitals in Hunan province.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1635120/full#supplementary-material
References
1. United Nations Inter-agency Group for Child Mortality Estimation. A Neglected Tragedy: the Global Burden of Stillbirths. New York: United Nations Children’s Fund (2020).
2. American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine in collaboration with, Metz TD, Berry RS, Fretts RC, Reddy UM, et al. Obstetric Care Consensus #10: Management of Stillbirth: (Replaces Practice Bulletin Number 102, March 2009). Am J Obstet Gynecol. (2020) 222:B2–B20. doi: 10.1016/j.ajog.2020.01.017
3. Winsloe, C, and Pasupathy, D. Understanding perinatal mortality. Obstet Gynaecol Reprod Med. (2024) 34:1–5. doi: 10.1016/j.ogrm.2023.10.001
4. Silver, RM, and Reddy, U. Stillbirth: we can do better. Am J Obstet Gynecol. (2024) 231:152–65. doi: 10.1016/j.ajog.2024.05.042
5. Lawn, JE, Blencowe, H, Waiswa, P, Amouzou, A, Mathers, C, Hogan, D, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. (2016) 387:587–603. doi: 10.1016/S0140-6736(15)00837-5
6. Alkema, L, New, JR, Pedersen, J, and You, DUN, Inter-agency Group for Child Mortality Estimation, Technical Advisory Group. Child mortality estimation 2013: an overview of updates in estimation methods by the United Nations Inter-agency Group for Child Mortality Estimation. PLoS One. (2014) 9:e101112. doi: 10.1371/journal.pone.0101112
7. Kuruvilla, S, Bustreo, F, Kuo, T, Mishra, CK, Taylor, K, Fogstad, H, et al. The Global strategy for women’s, children’s and adolescents’ health (2016-2030): a roadmap based on evidence and country experience. Bulletin World Health Org. (2016) 94:398–400. doi: 10.2471/BLT.16.170431
8. Zhu, J, Zhang, J, Xia, H, Ge, J, Ye, X, Guo, B, et al. Stillbirths in China: a nationwide survey. BJOG. (2021) 128:67–76. doi: 10.1111/1471-0528.16458
9. Zhu, J, Liang, J, Mu, Y, Li, X, Guo, S, Scherpbier, R, et al. Sociodemographic and obstetric characteristics of stillbirths in China: a census of nearly 4 million health facility births between 2012 and 2014. Lancet Glob Health. (2016) 4:e109–18. doi: 10.1016/S2214-109X(15)00271-5
10. Ma, R, and Zou, L. Stillbirth trends by maternal sociodemographic characteristics among a large internal migrant population in Shenzhen, China, over a 10-year period: a retrospective study. BMC Public Health. (2022) 22:325. doi: 10.1186/s12889-022-12734-8
11. Hug, L, You, D, Blencowe, H, Mishra, A, Wang, Z, Fix, MJ, et al. Global, regional, and national estimates and trends in stillbirths from 2000 to 2019: a systematic assessment. Lancet. (2021) 398:772–85. doi: 10.1016/S0140-6736(21)01112-0
12. Goldenberg, RL, Saleem, S, Aziz, A, and McClure, EM. International progress on stillbirth reduction: changes in stillbirth rates in selected low and middle-income countries from 2000 to 2021. Semin Perinatol. (2024) 48:151868. doi: 10.1016/j.semperi.2023.151868
13. Hug, L, You, D, Blencowe, H, Mishra, A, and Alkema, L. Global, regional, and national levels and trends in stillbirths from 2000 to 2019: a systematic assessment. Lancet. (2021) 398:772–85.
14. Hesketh, T, and Zhu, WX. The one child family policy: the good, the bad, and the ugly. BMJ. (1997) 314:1685–7. doi: 10.1136/bmj.314.7095.1685
15. Hesketh, T, Zhou, X, and Wang, Y. The end of the one-child policy: lasting implications for China. JAMA. (2015) 314:2619–20. doi: 10.1001/jama.2015.16279
16. Zeng, Y, and Hesketh, T. The effects of China's universal two-child policy. Lancet. (2016) 388:1930–8. doi: 10.1016/S0140-6736(16)31405-2
17. Wang, Y, Kong, F, and Qiao, J. Gender equity, caregiving, and the 1-2-3-child policy in China - authors' reply. Lancet. (2021) 398:953–4. doi: 10.1016/S0140-6736(21)01749-9
19. Liu, J, Liu, M, Zhang, S, Ma, Q, and Wang, Q. Intent to have a second child among Chinese women of childbearing age following China's new universal two-child policy: a cross-sectional study. BMJ Sex Reprod Health. (2019) 46:59–66. doi: 10.1136/bmjsrh-2018-200197
20. Liu, Y, Qin, Q, Xiao, Y, Li, H, Guang, S, Tao, S, et al. Changes of second-time mothers and their infants under the universal two-child policy in Changsha, China. Midwifery. (2019) 77:32–6. doi: 10.1016/j.midw.2019.06.005
21. Tang, W, Zeng, D, Wu, W, Wu, S, Ou, Y, Huang, Y, et al. How two-child policy affects cesarean section in women with advanced maternal age (AMA): using the Robson classification system. BMC Pregnancy Childbirth. (2024) 24:641. doi: 10.1186/s12884-024-06784-6
22. Yuan, H, Zhang, C, Maung, ENT, Fan, S, Shi, Z, Liao, F, et al. Epidemiological characteristics and risk factors of obstetric infection after the universal two-child policy in North China: a 5-year retrospective study based on 268,311 cases. BMC Infect Dis. (2022) 22:878. doi: 10.1186/s12879-022-07714-7
23. Zhou, Y, Mu, Y, Chen, P, Xie, Y, Zhu, J, and Liang, J. The incidence, risk factors and maternal and foetal outcomes of uterine rupture during different birth policy periods: an observational study in China. BMC Pregnancy Childbirth. (2021) 21:360. doi: 10.1186/s12884-021-03811-8
24. Zhang, HX, Zhao, YY, and Wang, YQ. Analysis of the characteristics of pregnancy and Delivery before and after implementation of the two-child policy. Chin Med J. (2018) 131:37–42. doi: 10.4103/0366-6999.221268
25. Xie, D, Wei, J, Wang, A, Xiong, L, Zou, K, Xie, Z, et al. The effect of China's many-child policy on the number of births and the prevalence of serious teratogenic and disabling defects in Hunan Province. BMC Public Health. (2023) 23:2226. doi: 10.1186/s12889-023-16583-x
26. Zhang, X, Chen, L, Wang, X, Wang, X, Jia, M, Ni, S, et al. Changes in maternal age and prevalence of congenital anomalies during the enactment of China's universal two-child policy (2013-2017) in Zhejiang Province, China: an observational study. PLoS Med. (2020) 17:e1003047. doi: 10.1371/journal.pmed.1003047
27. Chen, H, Wei, T, Wang, H, Zhou, Y, Chen, H, Sun, L, et al. Association of China's two-child policy with changes in number of births and birth defects rate, 2008-2017. BMC Public Health. (2022) 22:434. doi: 10.1186/s12889-022-12839-0
28. Zhang, J, Williams, GJ, Wang, G, Chen, J, Zhang, M, Du, W, et al. Early-term birth and its association with universal two-child policy: a national cross-sectional study in China. BMJ Open. (2021) 11:e054959. doi: 10.1136/bmjopen-2021-054959
29. Lumbiganon, P, Laopaiboon, M, Gülmezoglu, AM, Souza, JP, and Taneepanichskul, S. Method of delivery and pregnancy outcomes in Asia: the WHO global survey on maternal and perinatal health 2007–08. Lancet. (2010) 375:490–9. doi: 10.1016/S0140-6736(09)61870-5.
30. Svanvik, T, Jacobsson, AK, and Carlsson, YA-O. Prenatal detection of placenta previa and placenta accreta spectrum: evaluation of the routine mid-pregnancy obstetric ultrasound screening between 2013 and 2017. Int J Gynaecol Obstet. (2022) 157:647–53. doi: 10.1002/ijgo.13876
31. Brandt, JS, and Ananth, CV. Placental abruption at near-term and term gestations: pathophysiology, epidemiology, diagnosis, and management. Am J Obstet Gynecol. (2023) 228:S1313–29. doi: 10.1016/j.ajog.2022.06.059
32. Ananth, CV, Fields, JC, Brandt, JS, Graham, HL, Keyes, KM, and Zeitlin, J. Evolving stillbirth rates among black and White women in the United States, 1980-2020: a population-based study. Lancet Reg Health Am. (2022) 16:100380. doi: 10.1016/j.lana.2022.100380
33. Murzakanova, G, Raisanen, S, Jacobsen, AF, Yli, BM, Tingleff, T, and Laine, K. Trends in term intrapartum stillbirth in Norway. JAMA Netw Open. (2023) 6:e2334830. doi: 10.1001/jamanetworkopen.2023.34830
35. Siva, N, Nayak, BS, Roy, A, Edward, SLL, G, S, Noronha, JA, et al. Causes and risk factors for stillbirth in India: a systematic review protocol. Public Health. (2025) 239:32–6. doi: 10.1016/j.puhe.2024.12.029
36. Chen, D, Cui, S, Liu, C, Qi, H, and Zhong, N. Stillbirth in China. Lancet. (2016) 387:1995–6. doi: 10.1016/S0140-6736(16)30461-5
37. Zhou, Y, Yin, S, Sheng, Q, Yang, J, Liu, J, Li, H, et al. Association of maternal age with adverse pregnancy outcomes: a prospective multicenter cohort study in China. J Glob Health. (2023) 13:04161. doi: 10.7189/jogh.13.04161
38. Zhu, H, Zhao, Z, Xu, J, Chen, Y, Zhu, Q, Zhou, L, et al. The prevalence of gestational diabetes mellitus before and after the implementation of the universal two-child policy in China. Front Endocrinol. (2022) 13:960877. doi: 10.3389/fendo.2022.960877
39. Zhu, H, Cai, J, Liu, H, Zhao, Z, Chen, Y, Wang, P, et al. Trajectories tracking of maternal and neonatal health in eastern China from 2010 to 2021: a multicentre cross-sectional study. J Glob Health. (2024) 14:04069. doi: 10.7189/jogh.14.04069
40. Yang, H, Han, R, and Wang, Z. Third-child fertility intention and its socioeconomic factors among women aged 20-34 years in China. BMC Public Health. (2023) 23:821. doi: 10.1186/s12889-023-15719-3
41. Zeng, X, Cheng, L, Wu, S, Qian, X, Gu, B, and Zhang, J. Optimising contraceptive services as fertility rates fall in China. BMJ. (2024) 386:e078642. doi: 10.1136/bmj-2023-078642
42. Thiruvengadam, R, Ayushi,, Murugesan, DR, Desiraju, BK, Misra, S, Sharma, D, et al. Incidence of and risk factors for small vulnerable newborns in North India: a secondary analysis of a prospective pregnancy cohort. Lancet Glob Health. (2024) 12:e1261–77. doi: 10.1016/S2214-109X(24)00212-2
43. Cham, M, Sundby, J, and Vangen, S. Fetal outcome in severe maternal morbidity: too many stillbirths. Acta Obstet Gynecol Scand. (2009) 88:343–9. doi: 10.1080/00016340902730318
44. Lewkowitz, AK, Rosenbloom, JI, Lopez, JD, Keller, M, Macones, GA, Olsen, MA, et al. Association between stillbirth at 23 weeks of gestation or greater and severe maternal morbidity. Obstet Gynecol. (2019) 134:964–73. doi: 10.1097/AOG.0000000000003528
45. Abalos, E, Duley, L, Steyn, DW, and Gialdini, C. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. (2018) 10:CD002252. doi: 10.1002/14651858.CD002252
46. Jabeen, M, Yakoob, MY, Imdad, A, and Bhutta, ZA. Impact of interventions to prevent and manage preeclampsia and eclampsia on stillbirths. BMC Public Health. (2011) Suppl 3. doi: 10.1186/1471-2458-11-S3-S6
47. Wall-Wieler, E, Carmichael, SL, Gibbs, RS, Lyell, DJ, Girsen, AI, El-Sayed, YY, et al. Severe maternal morbidity among stillbirth and live birth deliveries in California. Obstet Gynecol. (2019) 134:310–7. doi: 10.1097/AOG.0000000000003370
48. Bailey, HD, Adane, AA, White, SW, Farrant, BM, and Shepherd, CCJ. Severe maternal morbidity following stillbirth in Western Australia 2000-2015: a population-based study. Arch Gynecol Obstet. (2023) 308:1175–87. doi: 10.1007/s00404-022-06782-z
49. Nyarko, SH, Greenberg, LT, Phibbs, CS, Buzas, JS, Lorch, SA, Rogowski, J, et al. Association between stillbirth and severe maternal morbidity. Am J Obstet Gynecol. (2024) 230: 364.e1–e14. doi: 10.1016/j.ajog.2023.08.029
50. Mackin, ST, Nelson, SM, Wild, SH, Colhoun, HM, Wood, R, Lindsay, RS, et al. Factors associated with stillbirth in women with diabetes. Diabetologia. (2019) 62:1938–47. doi: 10.1007/s00125-019-4943-9
Keywords: stillbirth, fertility policy, incidence, maternal comorbidities, risk factors
Citation: Xiong L, Xie D, Jiang Q and Fang J (2025) The incidence, characteristics, and complications of pregnant women who delivered stillbirths under different child policies in central China. Front. Public Health. 13:1635120. doi: 10.3389/fpubh.2025.1635120
Edited by:
Matilda Aberese-Ako, University of Health and Allied Sciences, GhanaReviewed by:
Ganchimeg Togoobaatar, University of Tsukuba, JapanBright Senyo Diapim, Ho Polytechnic, Ghana
Copyright © 2025 Xiong, Xie, Jiang and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donghua Xie, MjIxMDY4NTM1MEBxcS5jb20=; Qingyun Jiang, MzE1MDk2MTA0QHFxLmNvbQ==; Junqun Fang, NDAxMTIwNzlAcXEuY29t
 Lili Xiong
Lili Xiong Donghua Xie*
Donghua Xie*