- 1Department of Psychology, University of Maryland, College Park, College Park, MD, United States
- 2Center for Substance Use, Addiction & Health Research (CESAR), University of Maryland-College Park, College Park, MD, United States
- 3Henry Ford Health, Detroit, MI, United States
- 4Department of Psychiatry, University of Maryland School of Medicine, Baltimore, MD, United States
Background: Although medications exist to effectively treat opioid use disorder (OUD), treatment retention is a pressing challenge. Peer recovery specialists (PRSs) may play an important role in OUD treatment retention, yet few evidence-based interventions to support OUD retention have been developed specifically for PRS delivery. Behavioral activation is a brief, reinforcement-based intervention with empirical support for improving depression and substance use outcomes, delivered typically by specialist mental health providers. Informed by key stakeholder feedback, our team adapted a behavioral activation and problem-solving intervention for PRS delivery (“Peer Activate”) to improve methadone treatment retention. Building on a successful open-label pilot trial demonstrating initial feasibility, acceptability, and preliminary effectiveness of Peer Activate, the current type 1 hybrid randomized controlled trial evaluates the effectiveness of Peer Activate compared to treatment as usual on six-month methadone retention (primary) and longer-term implementation outcomes.
Methods: The trial is being conducted at a large methadone treatment program in Baltimore City, Maryland. We are enrolling 200 patients who recently initiated methadone treatment or are experiencing challenges with methadone adherence in a randomized 1:1 ratio to receive Peer Activate plus treatment as usual (PA + TAU) or TAU only. Additionally, we are recruiting 12 stakeholders to provide feedback on implementation and sustainability. Peer Activate consists of four core intervention sessions delivered by a PRS with relevant lived experience and training in the intervention. Sessions focus on problem-solving barriers to retention and behavioral activation—increasing value-driven, substance-free activities—and continued skill practice and relapse prevention. Assessments are administered at baseline, post-treatment (approximately 3 months), and 6 months. The primary effectiveness outcome is methadone retention over 6 months, measured using chart review. Implementation outcomes are defined based on Proctor’s model, including feasibility, acceptability, and fidelity of the intervention.
Discussion: This trial will provide insight as to whether a PRS-delivered intervention may be effective and feasible for improving methadone treatment retention and other behavioral health outcomes. If findings are promising, Peer Activate may provide a platform on which to incorporate an evidence-based behavioral activation approach into PRS training nationally.
Clinical trial registration: NCT05299515.
1 Introduction
Opioid use disorder (OUD) and opioid overdose deaths have increased profoundly in the past decade, exacerbated further by the COVID pandemic (1, 2). Between October 2023 and September 2024, approximately 87,000 people died of drug overdoses in the United States (3), and opioids are a factor in seven out of every ten drug overdose deaths (4). While there has been a recent decrease (3), overdose deaths still disproportionately affect certain groups, including low-income racial and ethnic minoritized groups, particularly Black/African-American individuals living with OUD (5). In Maryland, the setting for the current trial, Black men have experienced an almost fivefold increase in mortality due to drug overdoses between 2010 and 2020 (6). Baltimore City, in particular, experiences a staggering number of drug overdose deaths with a fatal overdose rate of 15 per 100,000 residents between August 2023 and July 2024—nearly five times that of the national rate (7).
Medication for OUD (MOUD) is the gold standard evidence-based treatment for OUD (8, 9) to reduce cravings, drug use, and prevent overdoses (10, 11). MOUD includes the medication options of methadone and buprenorphine, which are prescribed and administered depending on patient and provider needs and access. Methadone treatment is the oldest and currently most common treatment option, especially among low-income and racially minoritized patients (12). This trial focused on methadone retention for a few important reasons. First, methadone treatment typically requires daily dosing that is highly regulated and includes frequent, often daily, in-person observed dosing; yet, retention in methadone treatment is often less than 60% within six-months of treatment initiation (13, 14), and even lower among low-income, marginalized individuals (14–16). Retention is associated with many other indirect benefits, including a reduction in criminal convictions and improved physical health and quality of life (17, 18). It is imperative to develop and test new strategies to improve methadone treatment retention rates and focus on the barriers faced by groups that are most affected by OUD.
There is growing evidence that the inclusion of peer recovery specialists (PRSs) in substance use treatment programs can improve MOUD retention (19, 20). PRSs are trained and often certified individuals with their own lived experience of substance use and recovery. PRS-delivered interventions can offer a more informal and personalized interaction for patients to receive support navigating the healthcare system, build healthy relationships, and strengthen their support system (21). There is evidence that PRS-delivered interventions are high in feasibility and acceptability (22–24), as well as evidence of positive patient interactions and support of PRSs (23). Moreover, research has established that PRSs can deliver brief, behavioral evidence-based interventions (EBIs), including behavioral activation and contingency management, with high fidelity while incorporating their lived experience into the interventions (22, 25–27). This may especially be true when PRSs are involved in the intervention adaptation process and view the intervention components as in-line with the PRS role (26). Yet, few trials have evaluated more structured EBI delivered by PRSs to support MOUD retention. By incorporating their lived experience, a PRS may increase trust and thereby foster greater patient engagement in EBIs while also maintaining fidelity to the PRS role and the intervention.
Grounded in reinforcement theory, behavioral activation (BA) is a promising EBI with empirical support to improve treatment retention. BA targets positive reinforcement to enhance retention in substance use treatment (28) and prevent relapse (29–32) by promoting engagement in value-driven, substance-free activities that increase enjoyment and mastery. Additionally, when combined with problem-solving interventions, BA improves medication adherence (e.g., for HIV) among low-income, minoritized populations with substance use disorder (29, 33–35). From an implementation perspective, we and others have shown that BA is feasibly delivered by PRSs and other lay health workers and represents an EBI that is in line with the PRS role (34, 36, 37). Additionally, it is able to be implemented by non-specialists (38, 39), particularly in low- and middle-income countries (22). However, limited prior work has evaluated PRS delivery of BA in the US to improve OUD treatment outcomes.
Our team conducted extensive prior work, including several rounds of iterative qualitative research with patients, PRSs and stakeholders, to adapt BA for PRS delivery, specifically focusing on improving methadone treatment retention (23, 40). We then conducted an open-label pilot trial (N = 37) of this adapted Peer Activate PRS-delivered BA approach. This pilot demonstrated preliminary effectiveness in improving methadone treatment retention, as well as acceptability, feasibility, and fidelity (24). Based on our pilot study and prior research, BA delivered by a PRS appears to be an ideal EBI for improving methadone treatment retention. The present trial builds upon this formative work and successful pilot (24).
1.1 Trial objectives
This hybrid type 1 effectiveness-implementation randomized trial aims to evaluate the effectiveness and implementation of Peer Activate compared to treatment as usual (TAU) at a large methadone treatment center in Baltimore. The primary effectiveness outcome is methadone treatment retention over six months. Guided by Proctor’s model (41) of implementation, we are evaluating feasibility, acceptability, and fidelity using mixed methods.
2 Materials and methods
2.1 Setting and recruitment
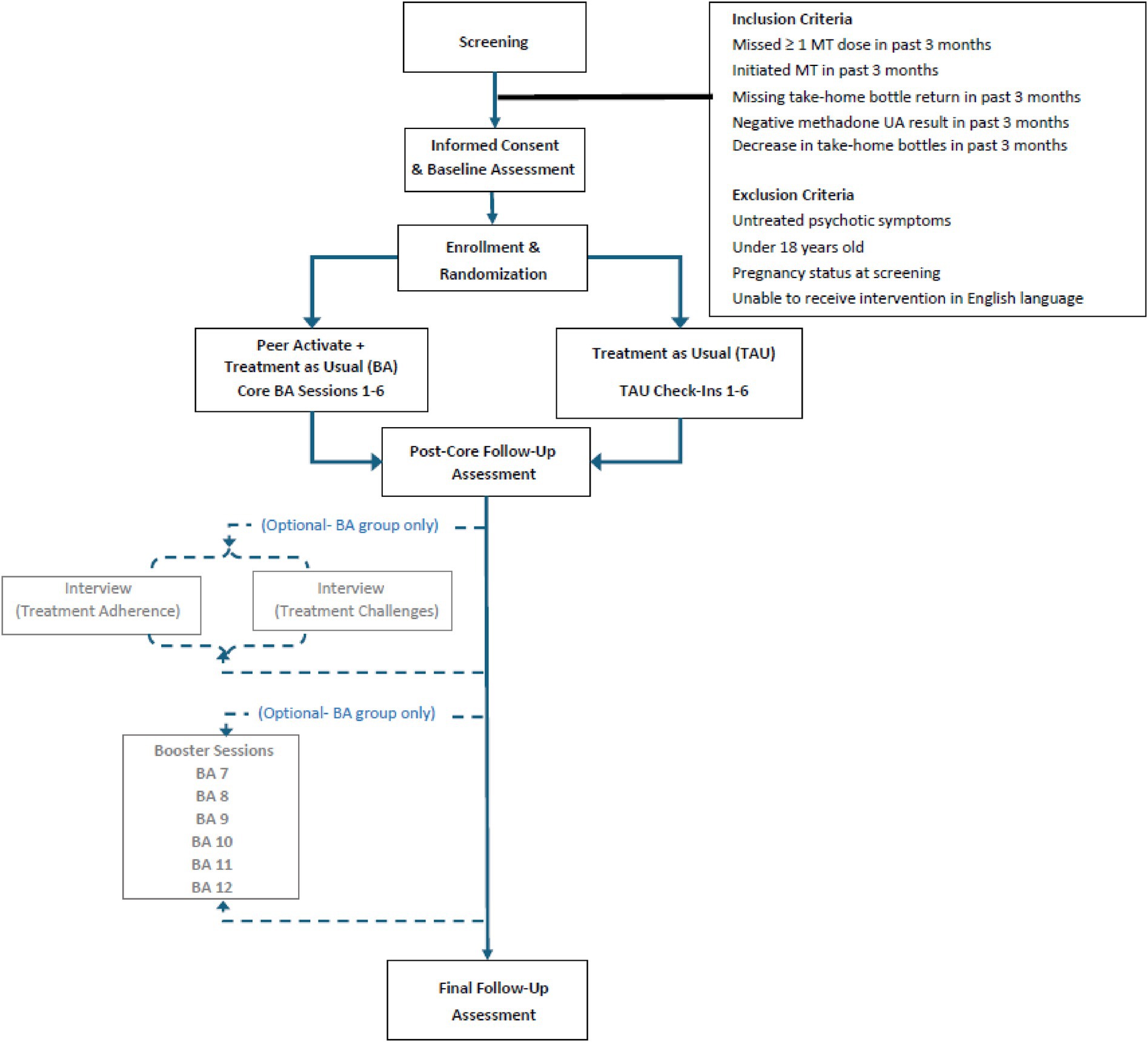
The study is conducted at a large substance use treatment center in Baltimore City that is affiliated with a local academic medical center, based in a community setting. The program currently serves over 425 active patients. Participants for the trial (N = 200) are recruited from the methadone treatment program through various screening methods: reviewing medical records of recently enrolled patients who have consented to being contacted for research at intake, provider/staff referrals, and through setting up informational tables to engage with participants in the clinic waiting room.
2.2 Eligibility criteria
Inclusion criteria for study eligibility are: (1) ≥18 years of age; and (2) initiating treatment in the methadone treatment program in the last three months (and having been enrolled in methadone treatment for at least two weeks) or demonstrating challenges with methadone adherence within the last three months, defined as: (a) at least one missed methadone dose in the past three months; (b) at least one missing methadone take-home bottle at the time of bottle return; (c) negative urine toxicology results for methadone in the past three months; and/or (d) transition from extended methadone take-homes to daily dosing due to concerns regarding adherence. These criteria were developed based on physician and stakeholder feedback and piloted previously (24). Exclusion criteria include: (1) pregnant at study enrollment; (2) untreated or undertreated psychosis or mania that would interfere with study participation; and/or (3) inability to provide informed consent in English.
2.3 Informed consent and ethical considerations
Trained members of the research team conduct informed consent with participants. After reading the consent form or having it read to them, participants are asked three structured questions to verify their understanding of the study, and any questions are answered. With participant permission during informed consent, all intervention sessions are audio recorded for supervision purposes and PRS intervention fidelity monitoring. This is noted in the consent process, and participants can refuse audio recording at any time (refusal of audio recording does not preclude participants from continuing to be part of the study). As part of the informed consent process, participants are advised that they may decline to answer any questions. This provides participants with the assurance of confidentiality around sensitive personal information relating to substance use and mental health. All personnel working on the project receive training on participants’ rights to confidentiality, and all study personnel are appropriately trained in the ethical conduct of human subjects’ research.
As this work includes collection of data relating to illegal behavior and substance use, the National Institutes of Health has provided the study with a Certificate of Confidentiality. As part of the consent process, participants are informed that, under the Certificate of Confidentiality, researchers cannot release or use information, documents, or samples that may identify participants in any action or suit unless participants provide permission, including in federal, state, or local legal action.
The University of Maryland, College Park Institutional Review Board (IRB) reviewed and approved all study procedures, which were approved through an Interagency Agreement with the University of Maryland, Baltimore. All study procedures are overseen by the PI.
This project is monitored by a Data and Safety Monitoring Board (DSMB) that meets annually to oversee the trial. The DSMB members function free of the career and financial interests of its members and consists of four members with experience in conducting clinical intervention research for psychiatric disorders, expertise in clinical trial ethics, human subject protection issues working with underserved populations, and peer recovery and policy expertise, including lived experience. The DSMB is updated annually. Annual meetings take place in the form of either a remote review of the annual DSMB report or a conference call to review project progress, barriers and challenges to study progress, as well as adverse events. The DSMB is presented with major amendments to the study protocol, study drop-outs, and determines whether study procedures should be changed or the study should be halted for reasons related to the safety of study subjects.
2.4 Assessment schedule
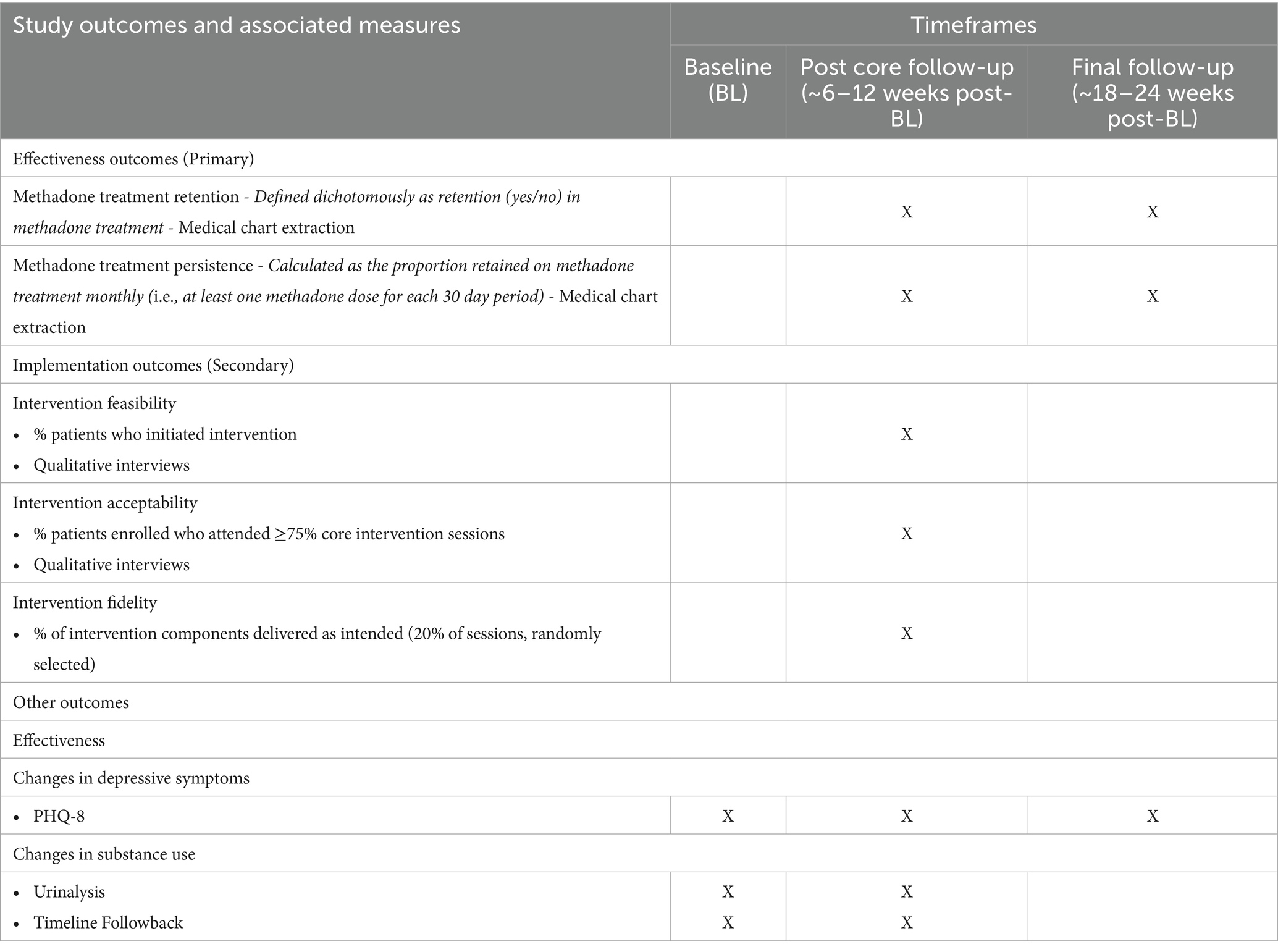
Participants first complete a baseline assessment, followed by assessments at approximately three- and six-months post baseline (see assessment measures in Figure 1). Both conditions (Peer Activate and TAU) have the same study assessment schedule. Participants are compensated $25 for each assessment. The study follows an intent-to-treat approach; participants can choose to continue study assessments without engaging in intervention sessions and/or withdraw at any time.
2.5 Baseline assessment
The baseline assessment includes a mix of self-report assessments of primary outcome variables at baseline (see Table 1) and other demographic and clinical characteristics. The baseline self-report assessments are administered by a research assistant using REDCap. Final eligibility is confirmed after conclusion of the baseline assessment. Patients who present with active psychotic or manic symptoms are further assessed using the Mini-International Neuropsychiatric Interview (MINI) (42); if symptoms are deemed to be active, untreated, and likely to interfere with study procedures, these participants are deemed ineligible and provided additional internal and external resources for treatment and other psychosocial support.
2.6 Randomization
Eligible participants are then randomized to either treatment as usual (TAU) or Peer Activate (PA + TAU), described below.
2.7 Treatment as usual (TAU)
TAU includes standard of care at the methadone treatment program, including a referral resource sheet, inclusive of general health centers, mental health providers, substance use support services, urgent care, domestic violence support, crisis hotlines, and other community services. Methadone treatment at the study site typically includes daily, observed methadone dosing 5 days per week with weekend take-home doses. Based on medical team determination, Saturday in-person dosing is also available and provided to patients who demonstrate the need for additional support (i.e., concern about the safety of medication storage in unstable housing settings, etc.). Depending on medication adherence (missed doses) and routine (approximately monthly) urine toxicology results, patients can receive up to 28 days of take-home doses of medication, provided at increasing intervals based on discussion across the treatment team (addiction medicine providers and addiction counselors). Patients who receive take-home bottles are required to return empty bottles at their next visit. Note, all data for this trial are being collected following COVID-19 modifications that eased federal regulations governing methadone dosing practices. Prior to these changes (issued in March 2020), patients were required to fulfill stringent criteria before considerations were given to decrease their observed dosing schedules and/or take-home eligibility (43, 44). Participants enrolled for this trial represent patients who have had variable experiences with pre- and post-COVID-19 pandemic methadone treatment dosing modifications. Clinic services also include wrap-around services such as individual and group counseling (which do not include BA or problem-solving therapy), primary care and infectious disease treatment, health home services, and access to peer recovery support at the site (i.e., general peer psychosocial support and service navigation). Thus, all participants in the trial have access to work with a peer in the traditional PRS role.
2.8 Peer-delivered BA intervention (“Peer Activate”)
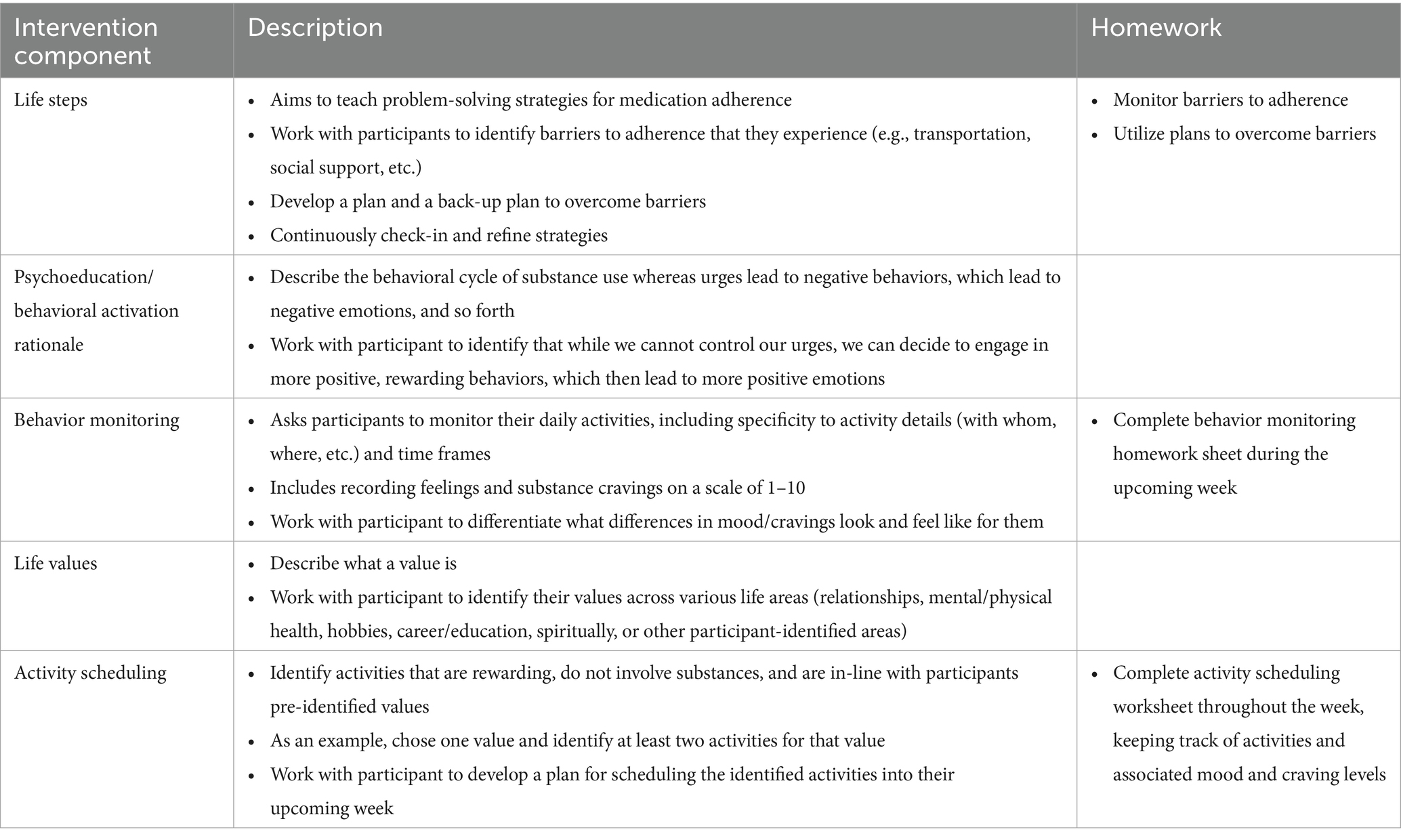
The core components of the PRS-delivered intervention (“Peer Activate”) include: (1) BA adapted for substance use (30), iteratively adapted by our team to be feasible for PRS delivery and focused on methadone treatment retention (23, 40); and (2) problem-solving strategies to support retention in methadone treatment adapted from Life Steps for medication adherence (45). Sessions begin with a check-in on substance use and methadone treatment adherence, followed by problem-solving to address barriers to adherence, for instance transportation and housing, and scheduling substance-free, value-driven rewarding activities.
Peer Activate consists of six weekly sessions (approximately 30–45 min each). The first four sessions introduce key content, followed by two core review sessions, and six optional booster sessions to further reinforce skill practice. Booster sessions address relapse prevention, balancing activities, building social support, and planning for challenges (24). This format and approach were adapted based on stakeholder and participant feedback from the pilot study (24). PRSs share their lived experiences to reduce stigma, increase engagement, and motivate participants (46). A flipbook format is used to promote peer fidelity to the behavioral intervention components, with specific prompts to incorporate one’s own lived experiences into intervention delivery as we have done in other trials evaluating PRS-delivered behavioral interventions in international settings (31). Table 2 outlines the core components of the intervention (see also (46) for greater detail on the intervention and flipbook delivery in a case series demonstration).
Sessions are held in-person at the methadone treatment center, with the option to complete some virtual study procedures (i.e., via phone, Zoom, or Google voice) when not feasible to meet in person for health/safety concerns, scheduling challenges, or other pertinent reasons. While all intervention sessions are scheduled, the PRS schedule is also flexible to meet patients on a walk-in basis to best support participants demonstrating ongoing challenges with treatment attendance and consistency in care. The PRSs document how and when sessions take place (method of communication, physical locations of PRS and participants, time of day) to further our understanding of how the PRS role can optimize participant engagement in treatment. PA + TAU participants receive $5 at each intervention session to support any additional travel costs.
2.9 PRS interventionists and training
Two study PRSs are trained and supervised in core peer recovery skills, knowledge, and abilities, are certified or working toward certification as PRSs in the State of Maryland and receive training in the study protocol and intervention procedures. Training is led by a PRS supervisor who was the primary PRS interventionist during the prior open-label pilot phase of this project (24). As an Internationally Certified Peer Recovery Specialist, a Maryland State Certified Peer Recovery Specialist, and a Registered Peer Supervisor through the Maryland Addictions and Behavioral Health Certification Board, the PRS supervisor has extensive experience in the training and certification of PRSs. The other PRS was recruited through hiring announcements and word of mouth, was a novice to the field of peer recovery who has lived experience in substance use and recovery. Training was conducted over approximately 1 month and includes a mix of didactic teaching, demonstrations, and role play of the peer role and Peer Activate intervention, with ongoing booster training and practice included in weekly supervision.
Peer Activate intervention training was guided by the intervention flipbook and reinforced through role-plays and shadowing. The PRS trainee shadowed the PRS supervisor in intervention sessions for up to 2 weeks before leading Peer Activate intervention sessions with the PRS supervisor present to observe for an additional 2 weeks. Once the PRS supervisor determined the PRS demonstrated competence, the PRS adopted an independent caseload under the supervision of the PRS supervisor. The PRS supervisor reviewed recorded practice sessions and guided the PRS through ongoing weekly clinical and PRS supervision as outlined below.
We did not restrict hiring to certified PRSs; rather, we provided support toward PRS certification as part of study procedures. Maryland Certified Peer Recovery Specialist (CPRS) certification is managed by Maryland Addictions and Behavioral Health Professional Certification Board and requires 46 hours of training, 500 work hours in peer recovery support, and 25 hours of documented supervision under a registered PRS supervisor (47). Maryland CPRS certification requires a core training that aligns knowledge, skills, and abilities in four subject domains: Advocacy, Mentoring & Education, Recovery & Wellness, and Ethical Responsibility (47). PRS training includes instruction on peer recovery skills, motivational interviewing, self-care and personal wellness planning, PRS role division and referral processes for clinical care (such as suicidal risk response and psychological support), ethics and boundary management, as well as disclosure as a recovery support tool. Once hired, the PRS also received supervised hours from a Registered Peer Supervisor toward PRS certification. The PRS supervisor provided support throughout the peer certification process, including sharing resources to acquire requisite trainings, preparing the peer for the examination, and supporting the PRS interventionist through the overall process. This support for general PRS training and certification was provided in addition to the study specific Peer Activate training.
2.10 PRS supervision
The approach to supervision includes both PRS supervision led by a PRS supervisor and clinical supervision led by a clinical psychologist. Weekly supervision sessions with a PRS supervisor include real-time case reviews of participant sessions where the PRS interventionist can raise questions and seek guidance, and the PRS supervisor can assess PRS performance in the intervention administration after listening to session recordings for fidelity monitoring. Based on these discussions, the PRS supervisor reviews relevant content and provides ongoing training to the PRS interventionist and supports resource brokering to ensure the PRS interventionist can refer participants to various community resources, as needed. PRS supervision sessions equally focus on knowledge-building and skills development with the goal of enhancing the understanding of systems of care, pathways for MOUD, the goals and agenda of MOUD care, person-centered support, and reducing barriers to receiving care. Additionally, PRS interventionists engage in twice weekly check-ins, ranging from 30- to 60 min in length, as required, to address real-time developments in training and delivery of the intervention, including regular monitoring of session fidelity as well as one’s own recovery and self-care. The PRS supervisor and PRS interventionists also consult on a weekly basis with clinical psychologists on the team to refer for higher level clinical needs and referrals, as well as to preserve role boundaries for PRSs and have sufficient support from licensed mental health clinicians. Clinical psychologists are also available to support ongoing booster training needs for BA and problem solving skills.
2.11 Follow-up assessments
Participants from both study groups (PA + TAU and TAU) complete follow-up assessments: post-core intervention sessions follow-up (PCFU) approximately six to 12 weeks post baseline and final follow-up (FFU) approximately 18–24 weeks post baseline. Follow up assessments are conducted by an independent blinded assessor who is trained on all study procedures but outside the core study team and blinded on randomization status. Measures administered are similar to the baseline assessment (see Table 1). Intervention implementation measures are completed during the PCFU assessment by an unblinded research team member and assess feasibility, acceptability, and fidelity among participants who received the Peer Activate intervention.
2.12 Effectiveness outcomes
2.12.1 Primary outcomes
2.12.1.1 Methadone treatment retention
The primary effectiveness outcome for this trial is methadone treatment retention, which is assessed over six months post baseline. Methadone treatment retention is examined through clinical records of appointment attendance and determined based on one or more methadone treatment-related visit(s) attended for each 30-day period during the six months following baseline. The primary outcome is retention assessed dichotomously (i.e., yes/no) at approximately six months post baseline. Participants who are referred to another internal treatment program or transferred to an external methadone treatment program are considered retained at six months based on clinic-verified records. To supplement the dichotomous measure of retention, we will also assess methadone treatment persistence, defined as the proportion retained on methadone treatment monthly over six months (i.e., at least one methadone dose for each 30-day period) post baseline, and days retained in treatment.
2.12.2 Secondary outcomes
We will also assess methadone treatment retention and persistence at three months post baseline, as well as changes in substance use over six months using both urinalysis and timeline follow back methods and depressive symptoms using the PHQ-8. For implementation outcomes, we will assess adoption of the intervention qualitatively through focus groups with providers and other key stakeholders (n = 12) about intentions to implement the intervention following the trial and to inform future adaptations to Peer Activate and plans for adoption and sustainability. Focus groups will be led with a semi-structured guide using Proctor’s model to define adoption (41).
2.12.3 Implementation outcomes
Implementation outcomes were defined based on Proctor’s model (41), including feasibility, acceptability, and fidelity, using mixed methods as detailed below.
2.12.3.1 Feasibility and acceptability
Feasibility and acceptability are assessed by session attendance (both initiation of the intervention and attendance at core sessions, respectively, See Table 1) and using a validated quantitative measure of feasibility and acceptability, the Applied Mental Health Research Group implementation outcome assessment (48). This assessment has been used to evaluate the implementation of interventions delivered by peer and lay health workers internationally and was designed to be adaptable for use in different resource-constrained settings globally (34, 49). In addition to quantitative measures, we are collecting qualitative feedback from patients (interviews offered to Peer Activate participants demonstrating either high treatment adherence or challenges with treatment during study engagement; n = 40) about their experiences with the intervention with specific probes related to feasibility and acceptability based on Proctor’s definitions (41). Qualitative and quantitative measures of feasibility are assessed at the posttreatment follow-up assessment (approximately three months post-baseline) or in the event of early participant discontinuation of the study.
2.12.3.2 Intervention fidelity
All Peer Activate sessions are audiotaped with patient permission as part of the consent process, and a randomly selected 20% are reviewed by a trained, independent rater, assessing both adherence and competence (50). The PRSs also self-report intervention adherence following each session. An adapted rating checklist for PRS adherence is utilized to determine whether the specific treatment components were delivered (both PRS report and independent ratings). Following recommendations for implementation science research (51), a “fidelity score” is calculated based on the proportion of key intervention components delivered as intended across sessions. Competence in the domain of general clinical skills (e.g., empathic listening, non-judgment) is rated using the ENhancing Assessment of Common Therapeutic Factors (ENACT) rating scale (52), a cross-cultural competency measure of skills of lay counselors, including PRSs specifically, in delivering a behavioral intervention. Prior research has seldom captured how to balance delivery of an evidence-based intervention, such as BA, with sharing one’s own identity as a PRS or adhering to the PRS role. The combination of these fidelity measures allows us to capture these unique elements of a PRS-delivered intervention (25).
2.13 Retention of study participants
As a strategy to support retention in a traditionally hard-to-reach population, both groups engage in approximately weekly contact (in-person or by phone) with the research team to continue to verify contact information to promote study retention. At these contact verifications, the research team asks brief questions about self-reported methadone treatment adherence and any changes to treatment enrollment (whether they are still enrolled in methadone treatment as well as any substance use treatment programs outside the study site clinic setting). Participants receive $5 compensation for completing each contact verification visit.
2.14 Power considerations and sample size calculations
Sample size estimates were calculated using PASS 16.0. Estimates for primary outcomes (retention and number of days in treatment) were calculated assuming: (1) 20% anticipated attrition in patient participation over six months, based on our preliminary work (24); (2) 2-tailed alpha = 0.05; and (3) a 1:1 enrollment ratio. Given prior findings suggesting the proportion of retention in the control group is 0.5 at 180 days, a sample size of 200 (100 patients per group) with an expected retention of 80% at six-month follow-up gives us 80% power to detect a 0.18 proportion difference in retention across conditions. While a binary outcome (retention/attrition) is used in our primary analyses, we can also examine a continuous measure of number of days in treatment. For these models, we would estimate 80% power to detect a small-to-medium effect size (d = 0.44).
As recommended in qualitative analysis, the proposed sample sizes for interviews and focus groups are estimates of the number of individuals needed to reach theoretical saturation of responses. The estimates are based on a sample that includes a range of patient, provider/staff and PRSs perspectives. If theoretical saturation is not met with the proposed sample size, we will seek additional approval to increase our sample size.
2.15 Data management and analysis
The research team developed a study-specific data management protocol and standard operating procedures for data collection, quality control, and data extraction. The PI and research coordinators provide ongoing oversight of data management throughout the study and are responsible for checking the accuracy of collected data on an ongoing basis and again prior to data analysis.
All protected health information (PHI) is stored on secure data storage servers or as paper records in a double-locked location at the University of Maryland campus, to which only authorized research personnel have access. Confidentiality is assured as participants are identified on all study materials by only a participant number and date of visit. All data management activities utilize REDCap (Research Electronic Data Capture), a software toolset and workflow methodology for electronic collection and management of research and clinical trial data (53). REDCap provides secure, web-based applications with an intuitive interface for users to enter data and have real time data checking such as validation rules (with automated data type and range checks) at the time of entry. All data input in REDCap are reviewed by a different research assistant to ensure completion and accuracy of data collection. Data files are downloaded from REDCap database on a weekly basis and saved on the study-designated, secure drive to maintain backup copies of the data. De-identified audio recordings and transcripts are stored on secure, password-protected drives at UMCP.
2.15.1 Data analysis
2.15.1.1 Quantitative
Utilizing a conservative intent-to-treat framework, all participants randomized will be included in analyses. We will examine the baseline equivalence of the groups by comparing participants receiving Peer Activate and TAU on sociodemographic characteristics, including sex, age, socioeconomic variables, severity of substance use, and days in treatment. Additional preliminary analyses will be conducted, including evaluating distributional assumptions of variables of interest. We will also examine patterns of missingness across treatment groups to detect deviations from missing at random (MAR) assumptions.
Effectiveness outcomes are evaluated primarily at the six-month follow up as well as at the three-month follow up post baseline. Dichotomous outcomes (including retention defined as retained/not retained at each data point) will be examined using generalized linear mixed modeling (GLMM) which allows for the inclusion of both categorical and continuous predictors as well as correlated observations across time. Two-level, mixed-effects models will be used to examine primary hypotheses. Time points (baseline, post-core follow-up, and final follow-up assessments) are nested within participants. Results will be expressed as either odds ratios (for binary, primary outcomes) or mean differences (for continuous, secondary and exploratory outcomes).
Using standard model-building procedures, treatment condition will be entered as the primary predictor in each model. We will then add additional covariates as indicated by our preliminary analyses, including days in treatment and substance use severity. We will also examine a time by group interaction which, if significant, would indicate that differences between treatment conditions become more or less pronounced across assessment points. Post hoc differences between treatment condition at three-month and six-month time points will also be examined using a logit link. To model days in treatment, we will conduct survival analysis using a frailty model to predict lapse in treatment and compare survival curves between treatment conditions, including random effect for PRS. We will assess levels of skew in the days in treatment outcome variable and accommodate our approach accordingly.
2.15.1.2 Qualitative and mixed methods
Qualitative interviews and focus groups will be audio-recorded and transcribed for analyses. Analysis of qualitative data will be guided by thematic analysis (54). A team of trained coders will develop detailed codebooks for the patient and staff data (separate codebooks) including definitions and examples for each code. The codebook will be developed iteratively, as done in our prior work. We will use a hybrid deductive-inductive approach for codebook development and analysis. A minimum of two members of the coding team will independently review a set of randomly selected transcripts and identify initial codes using an inductive approach. Coding team members will then discuss their codes and come to a consensus over a final code list of all identified themes grouped based on definitions of implementation outcomes as defined by Proctor’s model (41). Using the shared codebook, coders will code each transcript separately using NVivo and meet to discuss code discrepancies until consensus is reached. The coding team will also inductively identify themes that emerge in subsequent transcriptions and add to the codebook accordingly. We will also conduct a mixed methods analysis, integrating the qualitative feedback on implementation outcomes with quantitative results for each Proctor-defined implementation outcome assessed (41).
2.16 Dissemination plan
To disseminate our findings, we will prioritize dissemination to the academic community, PRSs, as well as to the local community. The study site has established two patient research advisory boards, and we will meet regularly with the patient advisory boards to share results from our study and receive input. We will also get their input on how best to disseminate study results to the larger patient population and community.
2.16.1 Dissemination of PRS training
At the end of the trial, if the approach is effective, feasible, and acceptable, we will offer capacity building workshops to train PRSs in the adapted manual, and we also will identify PRS supervisors for training. Feedback from these training sessions will be used to further refine the training and intervention materials and plans for future evaluation and dissemination will be completed. Once completed, this project has the potential to provide immediate benefit to the Maryland community and beyond by making modified training manuals and procedures available for dissemination to other community-based agencies engaging PRSs.
2.16.2 Data sharing plan
We will provide de-identified data from this project following achievement of the aims of the project (i.e., publication of the main outcome paper) through an approved data repository. These data will be provided in digital format (i.e., digital file or access to online repository) with clear labels for all variables. Our team will be available to address queries. All informed consent documents in this study include specific language relating to data sharing and confidentiality and data will also be uploaded to an NIH- and IRB-approved repository per HEAL data sharing requirements.
3 Discussion
Evidence-based behavioral interventions to support MOUD outcomes, including methadone, remain limited, including interventions specifically tailored for PRS delivery. Improving methadone retention is essential, and PRSs with their own lived experience with substance use recovery may be uniquely suited to support retention in methadone treatment and address common barriers to methadone retention, particularly among underserved populations. The current hybrid type 1 effectiveness-implementation RCT builds upon our team’s formative work (23, 34, 40, 46) and prior open-label trial to develop and pilot the PRS-delivered BA approach (24). We aim to establish the feasibility, acceptability, and fidelity of Peer Activate and effectiveness on improving methadone treatment retention over approximately six months.
3.1 Design considerations
We considered recruiting patients initiating methadone treatment or buprenorphine; however, we were concerned that the established differences in retention in methadone treatment vs. buprenorphine would confound our results. We intentionally selected a treatment site that provides both methadone treatment and buprenorphine to expedite the future application of this approach to buprenorphine. However, we also acknowledge that the program given its affiliation with an academic medical center may have greater resources compared to community-based sites without an academic affiliation, including greater support for the peer recovery specialist workforce and other retention supports. Other key considerations relate to the study being a hybrid effectiveness-implementation design (vs. an efficacy trial). We selected TAU as our comparison condition, following recommendations from implementation science to test the incremental clinical benefit of the intervention to existing services, as well as added costs to inform subsequent decisions to adopt the intervention after the trial. In our preliminary work, we have found the greatest challenges with retention in the first month after methadone treatment initiation, and other research also points to the importance of six-month retention for predicting long-term outcomes (55–58). Thus, we elected to focus on six-month methadone treatment retention in this study and designed the trial to lead to a subsequent cluster RCT that includes longer-term outcomes for effectiveness (including one-year retention and opioid abstinence), and implementation outcomes typically assessed well into or after implementation (sustainability, cost effectiveness).
4 Conclusion
This trial will test whether Peer Activate may be a feasible, scalable, reinforcement-based approach for improving methadone retention. Further, the trial will provide insight as to whether a PRS-delivered BA intervention may be effective and feasible for improving other behavioral health outcomes, such as depression. Positive findings of Peer Activate effectiveness may suggest an opportunity to incorporate an evidence-based approach into PRS training nationally.
Ethics statement
The studies involving humans were approved by University of Maryland College Park. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JM: Writing – original draft, Methodology, Conceptualization, Writing – review & editing, Funding acquisition, Supervision, Investigation. VB: Writing – original draft, Investigation, Project administration, Supervision, Writing – review & editing, Methodology. JA: Data curation, Project administration, Investigation, Writing – review & editing, Writing – original draft. MK: Writing – review & editing, Project administration, Methodology, Data curation, Writing – original draft, Supervision, Investigation, Conceptualization, Funding acquisition. JF: Methodology, Conceptualization, Funding acquisition, Writing – review & editing, Formal Analysis. AH: Data curation, Methodology, Project administration, Investigation, Supervision, Writing – review & editing. RB: Data curation, Writing – review & editing, Project administration. AG: Conceptualization, Project administration, Methodology, Writing – review & editing, Supervision, Investigation. DD: Supervision, Data curation, Writing – review & editing, Conceptualization, Resources, Funding acquisition, Project administration. MA: Writing – review & editing, Conceptualization, Methodology, Project administration. HF: Project administration, Supervision, Resources, Writing – review & editing. MB: Conceptualization, Writing – review & editing, Methodology, Funding acquisition, Supervision. AB: Funding acquisition, Project administration, Methodology, Investigation, Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the National Institutes of Health HEAL Initiative (R33DA057747; PI: Magidson and R61AT010799-01S1).
Acknowledgments
We extend our acknowledgement and gratitude to the patients, providers, and staff at the addiction treatment center, without whom this work would not be possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
BA, Behavioral activation; HEAL, Helping to End Addiction Long-term; IRB, Institutional Review Board; MAR, Missing at random; MOUD, Medication for opioid use disorder; TAU, Treatment as usual; NIH, National Institutes of Health; OUD, Opioid use disorder; PA, Peer Activate; PA + TAU, Peer-delivered behavioral activation intervention plus treatment as usual; PI, Principal Investigator; PRS, Peer recovery specialist; RCT, Randomized controlled trial; UMCP, University of Maryland-College Park.
References
1. Friedman, JR, and Hansen, H. Evaluation of increases in drug overdose mortality rates in the US by race and ethnicity before and during the COVID-19 pandemic. JAMA Psychiatry. (2022) 79:379–81. doi: 10.1001/jamapsychiatry.2022.0004
2. Patel, EU, Grieb, SM, Winiker, AK, Ching, J, Schluth, CG, Mehta, SH, et al. Structural and social changes due to the COVID-19 pandemic and their impact on engagement in substance use disorder treatment services: a qualitative study among people with a recent history of injection drug use in Baltimore, Maryland. Harm Reduct J. (2024) 21:91. doi: 10.1186/s12954-024-01008-8
3. Centers for Disease Control and Prevention. Provisional drug overdose death counts [internet]. Centers for Disease Control and Prevention (U.S.). National Center for Health Statistics; (2025) Available online at: https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
4. NCDAS. (2024). Drug Overdose Death Statistics [2023]: Opioids, Fentanyl & More. Available online at: https://drugabusestatistics.org/drug-overdose-deaths/
5. Gondré-Lewis, MC, Abijo, T, and Gondré-Lewis, TA. The opioid epidemic: a crisis disproportionately impacting black Americans and urban communities. J Racial Ethn Health Disparities. (2023) 10:2039–53. doi: 10.1007/s40615-022-01384-6
6. Cadet, K, Smith, BD, and Martins, SS. Intersectional racial and sex disparities in unintentional overdose mortality. JAMA Netw Open. (2025) 8:e252728–8. doi: 10.1001/jamanetworkopen.2025.2728
7. Maryland Department of Health. (2024). Fatal overdose: current overview. Available online at: https://health.maryland.gov/dataoffice/Pages/default.aspx
8. Mattick, RP, Breen, C, Kimber, J, and Davoli, M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. (2022). doi: 10.1002/14651858.CD002209.pub2
9. Mattick, RP, Breen, C, Kimber, J, and Davoli, M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. (2014) 2014:CD002207. doi: 10.1002/14651858
10. Bell, J, and Strang, J. Medication treatment of opioid use disorder. Biol Psychiatry. (2020) 87:82–8. doi: 10.1016/j.biopsych.2019.06.020
11. National Academies of Sciences E, Division H and M, Policy B on HS, Disorder C on MAT for OUMancher, M, and Leshner, AI. The effectiveness of medication-based treatment for opioid use disorder. Medications for opioid use disorder save lives National Academies Press (US) (2019). doi: 10.17226/25310
12. Nedjat, S, Wang, Y, Eshtiaghi, K, and Fleming, M. Is there a disparity in medications for opioid use disorder based on race/ethnicity and gender? A systematic review and meta-analysis. Res Social Adm Pharm. (2024) 20:236–45. doi: 10.1016/j.sapharm.2023.12.001
13. Anvari, MS, Kleinman, MB, Massey, EC, Bradley, VD, Felton, JW, Belcher, AM, et al. “In their mind, they always felt less than”: the role of peers in shifting stigma as a barrier to opioid use disorder treatment retention. J Subst Abus Treat. (2022) 138:108721. doi: 10.1016/j.jsat.2022.108721
14. O’Connor, AM, Cousins, G, Durand, L, Barry, J, and Boland, F. Retention of patients in opioid substitution treatment: a systematic review. PLoS One. (2020) 15:e0232086. doi: 10.1371/journal.pone.0232086
15. Biondi, BE, Vander Wyk, B, Schlossberg, EF, Shaw, A, and Springer, SA. Factors associated with retention on medications for opioid use disorder among a cohort of adults seeking treatment in the community. Addict Sci Clin Pract. (2022) 17:15. doi: 10.1186/s13722-022-00299-1
16. Samples, H, Williams, AR, Olfson, M, and Crystal, S. Risk factors for discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. J Subst Abus Treat. (2018) 95:9–17. doi: 10.1016/j.jsat.2018.09.001
17. Gossop, M, Trakada, K, Stewart, D, and Witton, J. Reductions in criminal convictions after addiction treatment: 5-year follow-up. Drug Alcohol Depend. (2005) 79:295–302. doi: 10.1016/j.drugalcdep.2005.01.023
18. Hser, YI, Longshore, D, and Anglin, MD. The life course perspective on drug use: a conceptual framework for understanding drug use trajectories. Eval Rev. (2007) 31:515–47. doi: 10.1177/0193841X07307316
19. Cos, TA, LaPollo, AB, Aussendorf, M, Williams, JM, Malayter, K, and Festinger, DS. Do peer recovery specialists improve outcomes for individuals with substance use disorder in an integrative primary care setting? A program evaluation. J Clin Psychol Med Settings. (2020) 27:704–15. doi: 10.1007/s10880-019-09661-z
20. Gormley, MA, Pericot-Valverde, I, Diaz, L, Coleman, A, Lancaster, J, Ortiz, E, et al. Effectiveness of peer recovery support services on stages of the opioid use disorder treatment cascade: a systematic review. Drug Alcohol Depend. (2021) 229:109123. doi: 10.1016/j.drugalcdep.2021.109123
21. Jack, HE, Oller, D, Kelly, J, Magidson, JF, and Wakeman, SE. Addressing substance use disorder in primary care: the role, integration, and impact of recovery coaches. Subst Abuse. (2018) 39:307–14. doi: 10.1080/08897077.2017.1389802
22. Anvari, MS, Hampton, T, Tong, MP, Kahn, G, Triemstra, JD, Magidson, JF, et al. Behavioral activation disseminated by non-mental health professionals, paraprofessionals, and peers: a systematic review. Behav Ther. (2023) 54:524–38. doi: 10.1016/j.beth.2022.12.007
23. Kleinman, MB, Anvari, MS, Bradley, VD, Felton, JW, Belcher, AM, Seitz-Brown, CJ, et al. “Sometimes you have to take the person and show them how”: adapting behavioral activation for peer recovery specialist-delivery to improve methadone treatment retention. Subst Abuse Treat Prev Policy. (2023) 18:15. doi: 10.1186/s13011-023-00524-3
24. Magidson, JF, Kleinman, MB, Bradley, V, Anvari, MS, Abidogun, TM, Belcher, AM, et al. Peer recovery specialist-delivered, behavioral activation intervention to improve retention in methadone treatment: results from an open-label, type 1 hybrid effectiveness-implementation pilot trial. Int J Drug Policy. (2022) 108:103813. doi: 10.1016/j.drugpo.2022.103813
25. Anvari, MS, Belus, JM, Kleinman, MB, Seitz-Brown, CJ, Felton, JW, Dean, D, et al. How to incorporate lived experience into evidence-based interventions: assessing fidelity for peer-delivered substance use interventions in local and global resource-limited settings. Transl Issues Psychol Sci. (2022) 8:153–63. doi: 10.1037/tps0000305
26. Peng, L, Stack, E, Cooke, A, Hartzler, B, Cook, R, Leichtling, G, et al. Centering peers in design and training for a peer-delivered contingency management program for self-identified harm reduction and treatment goals. Harm Reduct J. (2025) 22:72. doi: 10.1186/s12954-025-01213-z
27. Jaguga, F, Kwobah, EK, Giusto, A, Apondi, E, Barasa, J, Korir, M, et al. Feasibility and acceptability of a peer provider delivered substance use screening and brief intervention program for youth in Kenya. BMC Public Health. (2023) 23:2254. doi: 10.1186/s12889-023-17146-w
28. Magidson, JF, Gorka, SM, MacPherson, L, Hopko, DR, Blanco, C, Lejuez, CW, et al. Examining the effect of the life enhancement treatment for substance use (LETS ACT) on residential substance abuse treatment retention. Addict Behav. (2011) 36:615–23. doi: 10.1016/j.addbeh.2011.01.016
29. Daughters, SB, Magidson, JF, Schuster, RM, and Safren, SA. ACT HEALTHY: a combined cognitive-behavioral depression and medication adherence treatment for HIV-infected substance users. Cogn Behav Pract. (2010) 17:309–21. doi: 10.1016/j.cbpra.2009.12.003
30. Daughters, SB, Magidson, JF, Anand, D, Seitz-Brown, CJ, Chen, Y, and Baker, S. The effect of a behavioral activation treatment for substance use on post-treatment abstinence: a randomized controlled trial. Addiction. (2018) 113:535–44. doi: 10.1111/add.14049
31. Mimiaga, MJ, Reisner, SL, Pantalone, DW, O’Cleirigh, C, Mayer, KH, and Safren, SA. A pilot trial of integrated behavioral activation and sexual risk reduction counseling for HIV-uninfected men who have sex with men abusing crystal methamphetamine. AIDS Patient Care STDs. (2012) 26:681–93. doi: 10.1089/apc.2012.0216
32. Mimiaga, MJ, Pantalone, DW, Biello, KB, Hughto, JMW, Frank, J, O’Cleirigh, C, et al. An initial randomized controlled trial of behavioral activation for treatment of concurrent crystal methamphetamine dependence and sexual risk for HIV acquisition among men who have sex with men. AIDS Care. (2019) 31:1083–95. doi: 10.1080/09540121.2019.1595518
33. Magidson, JF, Seitz-Brown, CJ, Safren, SA, and Daughters, SB. Implementing behavioral activation and life-steps for depression and HIV medication adherence in a community health center. Cogn Behav Pract. (2014) 21:386–403. doi: 10.1016/j.cbpra.2013.10.002
34. Magidson, JF, Joska, JA, Belus, JM, Andersen, LS, Regenauer, KS, Rose, AL, et al. Project Khanya: results from a pilot randomized type 1 hybrid effectiveness-implementation trial of a peer-delivered behavioural intervention for ART adherence and substance use in HIV care in South Africa. J Int AIDS Soc. (2021) 24:e25720. doi: 10.1002/jia2.25720
35. Tull, MT, Berghoff, CR, Bardeen, JR, Schoenleber, M, and Konkle-Parker, DJ. An initial open trial of a brief behavioral activation treatment for depression and medication adherence in HIV-infected patients. Behav Modif. (2018) 42:196–209. doi: 10.1177/0145445517723901
36. Magidson, JF, Lejuez, CW, Kamal, T, Blevins, EJ, Murray, LK, Bass, JK, et al. Adaptation of community health worker-delivered behavioral activation for torture survivors in Kurdistan, Iraq. Glob Ment Health. (2015) 2:e24. doi: 10.1017/gmh.2015.22
37. Pott, SL, Kellett, S, Green, S, Daughters, S, and Delgadillo, J. Behavioral activation for depression delivered by drug and alcohol treatment workers: a pilot randomized controlled trial. J Subst Abus Treat. (2022) 139:108769. doi: 10.1016/j.jsat.2022.108769
38. Nadkarni, A, Weiss, HA, Weobong, B, McDaid, D, Singla, DR, Park, AL, et al. Sustained effectiveness and cost-effectiveness of counselling for alcohol problems, a brief psychological treatment for harmful drinking in men, delivered by lay counsellors in primary care: 12-month follow-up of a randomised controlled trial. PLoS Med. (2017) 14:e1002386. doi: 10.1371/journal.pmed.1002386
39. Richards, DA, Ekers, D, McMillan, D, Taylor, RS, Byford, S, Warren, FC, et al. Cost and Outcome of BehaviouRal Activation (COBRA): a randomised controlled trial of behavioural activation versus cognitive-behavioural therapy for depression. Health Technol Assess. (2017) 21:1–366. doi: 10.3310/hta21460
40. Satinsky, EN, Doran, K, Felton, JW, Kleinman, M, Dean, D, and Magidson, JF. Adapting a peer recovery coach-delivered behavioral activation intervention for problematic substance use in a medically underserved community in Baltimore City. PLoS One. (2020) 15:e0228084. doi: 10.1371/journal.pone.0228084. P
41. Proctor, E, Silmere, H, Raghavan, R, Hovmand, P, Aarons, G, Bunger, A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health Ment Health Serv Res. (2011) 38:65–76. doi: 10.1007/s10488-010-0319-7
42. Sheehan, DV, Lecrubier, Y, Sheehan, KH, Amorim, P, Janavs, J, Weiller, E, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 59:22–33.
43. Abidogun, TM, Cole, TO, Massey, E, Kleinman, M, Greenblatt, AD, Seitz-Brown, CJ, et al. Patient experiences of COVID-19-induced changes to methadone treatment in a large community-based opioid treatment program in Baltimore. J Subst Use Addict Treat. (2023) 145:208946. doi: 10.1016/j.josat.2022.208946
44. Greenblatt, AD, Magidson, JF, Belcher, AM, Gandhi, D, and Weintraub, E. Overdue for an Overhaul. Mayo Clin Proc. (2020) 95:2076–8. doi: 10.1016/j.mayocp.2020.08.011
45. Safren, SA, Otto, MW, and Worth, JL. Life-steps: applying cognitive behavioral therapy to HIV medication adherence. Cogn Behav Pract. (1999) 6:332–41. doi: 10.1016/S1077-7229(99)80052-2
46. Anvari, MS, Kleinman, MB, Dean, D, Bradley, VD, Abidogun, TM, Hines, AC, et al. Adapting a behavioral activation intervention for opioid use disorder and methadone treatment retention for peer delivery in a low-resource setting: a case series. Cogn Behav Pract. (2024) 31:437–50. doi: 10.1016/j.cbpra.2023.01.003
47. The Maryland Addiction & Behavioral-Health Professionals Certification Board (MABPCB). Certified peer recovery specialist (CPRS) | Maryland addiction and behavioral-health professionals certification board. (2025). Available online at: https://www.mabpcb.com/certified-peer-recovery-specialist-cprs
48. Haroz, EE, Bolton, P, Nguyen, AJ, Lee, C, Bogdanov, S, Bass, J, et al. Measuring implementation in global mental health: validation of a pragmatic implementation science measure in eastern Ukraine using an experimental vignette design. BMC Health Serv Res. (2019) 19:262. doi: 10.1186/s12913-019-4097-y
49. Moore, G, Campbell, M, Copeland, L, Craig, P, Movsisyan, A, Hoddinott, P, et al. Adapting interventions to new contexts—the ADAPT guidance. BMJ. (2021) 3:n1679. doi: 10.1136/bmj.n1679
50. Carroll, KM, Nich, C, Sifry, RL, Nuro, KF, Frankforter, TL, Ball, SA, et al. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug Alcohol Depend. (2000) 57:225–38. doi: 10.1016/S0376-8716(99)00049-6
51. Shelton, RC. and others, ‘Fidelity and Its Relationship to Effectiveness, Adaptation, and Implementation’, in Ross C. Brownson, Graham A. Colditz, and Enola K. Proctor (eds), Dissemination and Implementation Research in Health: Translating Science to Practice, 3rd edn (New York, 2023; online edn, Oxford Academic, 21 Mar. 2024), doi: 10.1093/oso/9780197660690.003.0007
52. Kohrt, BA, Ramaiya, MK, Rai, S, Bhardwaj, A, and Jordans, MJD. Development of a scoring system for non-specialist ratings of clinical competence in global mental health: a qualitative process evaluation of the enhancing assessment of common therapeutic factors (ENACT) scale. Glob Ment Health. (2015) 2:e23. doi: 10.1017/gmh.2015.21
53. Harris, PA, Taylor, R, Minor, BL, Elliott, V, Fernandez, M, O’Neal, L, et al. Duda SN; REDCap Consortium. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208
54. Braun, V., and Clarke, V. (2020). One size fits all? What counts as quality practice in (reflexive) thematic analysis? Qualitative Research in Psychology, 18, 328–352. doi: 10.1080/14780887.2020.1769238
55. Timko, C, Schultz, NR, Cucciare, MA, Vittorio, L, and Garrison-Diehn, C. Retention in medication-assisted treatment for opiate dependence: a systematic review. J Addict Dis. (2016) 35:22–35. doi: 10.1080/10550887.2016.1100960
56. Brorson, HH, Ajo Arnevik, E, Rand-Hendriksen, K, and Duckert, F. Drop-out from addiction treatment: a systematic review of risk factors. Clin Psychol Rev. (2013) 33:1010–24. doi: 10.1016/j.cpr.2013.07.007
57. Zhang, P, Tossone, K, Ashmead, R, Bickert, T, Bailey, E, Doogan, NJ, et al. Examining differences in retention on medication for opioid use disorder: an analysis of Ohio Medicaid data. J Subst Abus Treat. (2022) 136:108686. doi: 10.1016/j.jsat.2021.108686
Keywords: peer recovery specialists, behavioral activation, medication for opioid use disorder, retention, methadone, opioid use disorder
Citation: Magidson JF, Bradley VD, Anane JS, Kleinman MB, Felton JW, Hines AC, Baskar R, Greenblatt AD, Dean D, Anvari MS, Fitzsimons H, Bennett ME and Belcher AM (2025) “HEAL together”: a randomized, hybrid type 1 effectiveness-implementation trial protocol of a peer-delivered behavioral activation intervention to improve methadone treatment retention. Front. Public Health. 13:1637846. doi: 10.3389/fpubh.2025.1637846
Edited by:
Hannah Knudsen, University of Kentucky, United StatesReviewed by:
Jamey Lister, Rutgers University School of Social Work, United StatesBryan Hartzler, University of Washington, United States
Copyright © 2025 Magidson, Bradley, Anane, Kleinman, Felton, Hines, Baskar, Greenblatt, Dean, Anvari, Fitzsimons, Bennett and Belcher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica F. Magidson, am1hZ2lkc29AdW1kLmVkdQ==
†These authors share first authorship
 Jessica F. Magidson
Jessica F. Magidson Valerie D. Bradley1†
Valerie D. Bradley1† Jessica S. Anane
Jessica S. Anane Abigail C. Hines
Abigail C. Hines Rithika Baskar
Rithika Baskar Morgan S. Anvari
Morgan S. Anvari Annabelle M. Belcher
Annabelle M. Belcher