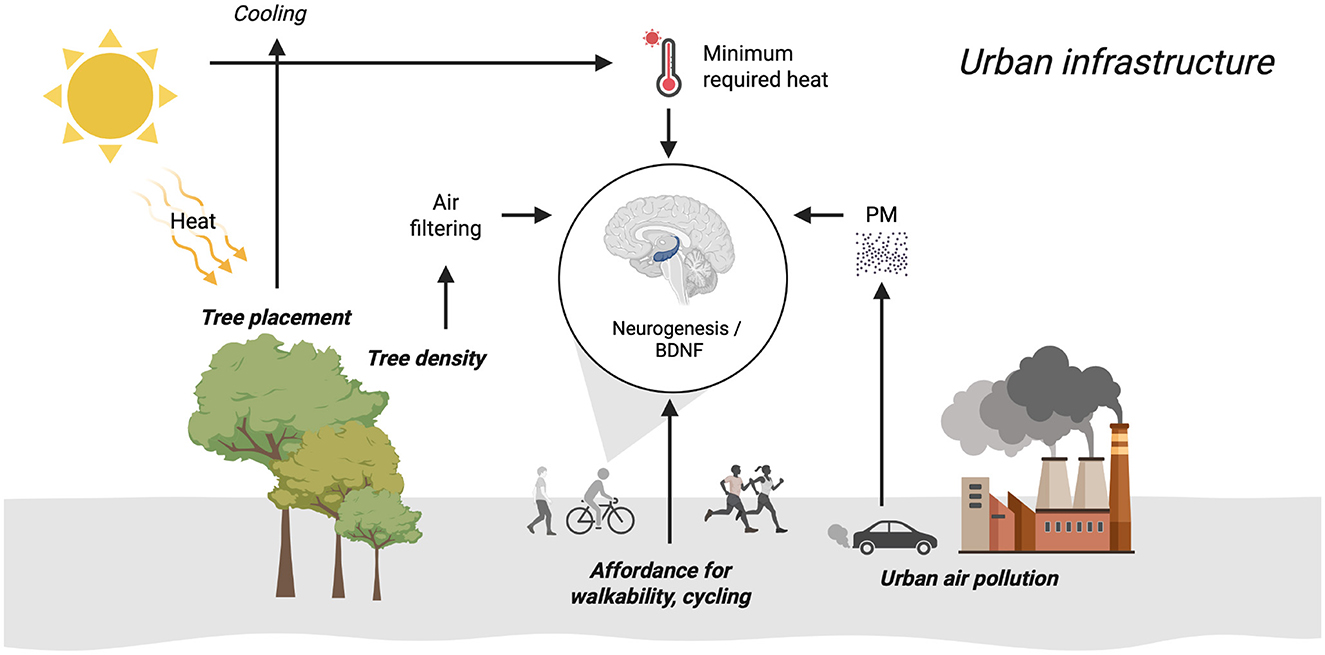
1 Introduction
Several urban physical activity infrastructure limitations can seriously impair the opportunity of sustaining the daily newborn neurons in the human brain.
Neurogenesis persists in the human brain's hippocampus until the tenth decade of life (1–4). Approximately 700 new neurons are born daily in each hemisphere's hippocampus (5). Nurturing newborn neurons heavily relies on physical activity that increases brain-derived neurotrophic factor (BDNF) (6), which is vital for cognition and mood improvements, and mood regulation (7–10).
Urban infrastructure can increase BDNF through the affordances for physical activity (e.g., walking, cycling) through planning and design (11). However, infrastructure solutions must be aware of the confounding temperature peaks in winter and summer, air pollution, and residential greenness and the tree placement strategy.
2 Urban infrastructure affordances for high-intensity physical activity to increase BDNF
The degree to which an environment affords higher metabolic equivalents (>3 METs) increases the likelihood of elevating BDNF in humans (11), where urban environments that support physical activity (e.g., walking and cycling) are more likely to help BDNF in their populations.
For cycling, Gibbons et al. (12) showed through their crossover study that 6 min of vigorous-intensity cycling elevated BDNF in both serum and plasma 4- to 5-fold more than 90 min of light-intensity physical activity. Cycling has been shown to increase serum BDNF and enhance the performance of a face-name-matching task that involves the hippocampus and associated medial temporal lobe structures (13). Similarly, the impact of walking on BDNF has been systematically reviewed (14), where 30 min of walking can be sufficient to increase BDNF (15).
If the environment has walkable pathways and cycling lanes, the affordances for METs increase, and if those lanes have steep slopes, METs can increase from 3 METs up to 16 METs (16), which increases the probability of elevating BDNF to a great extent.
To encourage urban walking and cycling to increase the likelihood of engaging in moderate-to-vigorous physical activity that can increase BDNF. This can be achieved through enhancing the urban infrastructure that encourages physical activity such as through understanding the environmental attributes that promote higher urban physical activity such as residential density, intersection density, public transport density, and number of parks (17), through improving accessibility, connectivity, safety and and the experience of walking and cycling (34).
However, the urban infrastructure affordance for physical activity depends on temperature peaks, pollution, residential greenness, and tree placement.
3 Seasonal BDNF differences and urban infrastructure responsivity to geographic temperature peak variances
Urban infrastructure does not face the limitation of affordances for METs alone, but solutions should be based on the geographic dynamics of temperature differences. For instance, Goulet et al. (18) showed that BDNF increased after 180 min of moderate-intensity treadmill walking in a 32°C environment, but not in a 16°C environment. This section discusses how BDNF rises in summer with longer-day periods due to heat increase, but extreme heat waves are likely to cause neuroinflammation that impairs the role of BDNF in nurturing neurogenesis. Those variances urge urban environments to be responsive to their seasonal dynamics.
Summertime is found to be significantly associated with higher BDNF changes. In humans, Molendijk et al. (19) examined seasonal fluctuations in serum concentrations of BDNF among 2,851 participants from the Netherlands Study of Depression and Anxiety (NESDA). The authors demonstrated pronounced elevations of serum BDNF concentrations during the spring and summer months, contrasting markedly with lower concentrations observed during the autumn and winter months. Monthly analyses highlighted substantial variations, with effect sizes (Cohen's d) ranging from moderate to large (0.27–0.66), reinforcing the biological relevance and magnitude of these seasonal differences. Further exploratory analyses illuminated the positive correlations between serum BDNF levels and sunlight exposure. Animal studies support those findings, where Hernandez et al. (20) revealed that hippocampal and hypothalamic BDNF protein peaks in the long-day, euthermic summer and troughs in the short-day, hypothermic winter. The seasonal effects on BDNF, however, could to a great extent be explained by some of the factors discussed subsequently, such as heat stress.
Daytime high temperatures, without severe heat stress, could explain the increase of BDNF in long days during summer. In humans, head-out immersion of males in hot water (42°C) for 20 min increased BDNF (21), and after daylong (9 h) exposure to hot ambient conditions (40°C) in younger and older adults, with slight variation in concentrations between both groups (22). Kirby et al. (23) studied the impact of 22°C (air-conditioned indoor environment), 26°C (recommended indoor temperature limit for health), 31°C, and 36°C (non-air-conditioned home). BDNF was increased by ~ 28% in the latter group compared to the first. They concluded that BDNF increases by 90 pg/mL per 1°C rise in ambient temperature. Animal models support this by explaining how neurogenesis increases in turn. Rats exposed to a 1-h heat treatment (36°C) for 7 days had a 1.4-fold increase in neurogenesis in the hippocampal dentate gyrus compared to controls in a normothermic environment (25°C) (24).
However, severe heat stress induces pathological alterations that encompass oxidative damage and apoptosis of hippocampal neurons and disrupts the BDNF-associated axis (25), which suggests that environments with high temperatures, not leading to severe heat stress, are more likely to increase BDNF.
Hence, the demands from urban infrastructure for physical activity vary accordingly, suggesting that urban planning and design should be geographically sensitive.
4 Urbanisation's air pollution inhibits the increase of BDNF via urban physical activity
Though the urban infrastructure can have the appropriate affordance for physical activity (e.g., walking, cycling) that corresponds to the context's temperature dynamics, neurotoxicity caused by air pollution can seriously impair BDNF increase via any form of urban physical activity.
Cycling near a major traffic route did not increase BDNF compared to a similar cycling activity in an air-filtered room due to particulate matter (PM) (26). PM is very common in urban environments (27), affecting brain structure (28–30). On the contrary, indoor physical activity can enhance BDNF levels since it reduces exposure to pollution (35), but household walking is unlikely to reach a moderate-intensity level (16).
Air pollution is a major challenge for most urban environments, making urban physical activity less effective than physical activity in natural environments due to the limited opportunities for walking or cycling, lack of slopes that enhance the effect of physical activity on BDNF, the the predominance of traffic-caused air pollution that is likely to inhibit any potential increase in BDNF via physical activity, which can seriously impair neurogenesis in humans.
5 Urban residential greenness, BDNF and tree placement strategies
Greening buildings can counteract the effect of pollution (31), and urban greenness generally overcomes neurotoxicity caused by air pollution as well (32).
Still, greening an urban environment is also geographically-sensitive. There are context-specific greening solutions to harness tree-based cooling in urban environments (33), where the authors found that trees generally cool cities in hot and dry climates, and less in hot and humid climates.
However, in light of the earlier discussion in this paper, reducing peak monthly temperatures to below 26°C can provide a cooling effect through which high-intensity urban physical activity may be needed and not just moderate-intensity walking.
6 Conclusion
Supporting neurogenesis in humans via BDNF through urban physical activity is very important. This paper brings the process of urban infrastructure decision making for physical activity to a full circle as illustrated in Figure 1. Several variables determine the effect of urban physical activity on BDNF such as affordance for physical activity, peak temperature is affected by climate change and geographical distributions, impact of temperature variances on the effect of physical activity, air pollution's inhibition of the impact of any potential physical activity even when temperature is taken into consideration, tree cover density counteracting neurotoxicity, and tree placement that can reduce temperatures beyond the effective threshold that increases BDNF.
Author contributions
MK: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Cambridge Commonwealth, European & International Trust and the Jameel Education Foundation for funding my doctoral thesis, which covers the open access charges of this article.
Acknowledgement
Thanks to Cambridge Commonwealth, European & International Trust and the Jameel Education Foundation for funding my doctoral thesis.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. (2018) 22:589–99. doi: 10.1016/j.stem.2018.03.015
2. Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat Med. (2019) 25:554–60. doi: 10.1038/s41591-019-0375-9
3. Moreno-Jiménez EP, Terreros-Roncal J, Flor-García M, Rábano A, Llorens-Martín M. Evidences for adult hippocampal neurogenesis in humans. J Neurosci. (2021) 41:2541–53. doi: 10.1523/JNEUROSCI.0675-20.2020
4. Tobin MK, Musaraca K, Disouky A, Shetti A, Bheri A, Honer WG, et al. Human hippocampal neurogenesis persists in aged adults and Alzheimer's disease patients. Cell Stem Cell. (2019) 24:974–82. doi: 10.1016/j.stem.2019.05.003
5. Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. (2013) 153:1219–27. doi: 10.1016/j.cell.2013.05.002
6. Liu PZ, Nusslock R. Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci. (2018) 12:52. doi: 10.3389/fnins.2018.00052
7. Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. (2008) 11:1169–80. doi: 10.1017/S1461145708009309
8. Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. (2012) 18:82–97. doi: 10.1177/1073858410397054
9. Piepmeier AT, Etnier JL. Brain-derived neurotrophic factor (BDNF) as a potential mechanism of the effects of acute exercise on cognitive performance. J Sport Health Sci. (2015) 4:14–23. doi: 10.1016/j.jshs.2014.11.001
10. Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. (2004) 20:2580–90. doi: 10.1111/j.1460-9568.2004.03720.x
11. Khalil MH. Environmental affordance for physical activity, neurosustainability, and brain health: quantifying the built environment's ability to sustain BDNF release by reaching metabolic equivalents (METs). Brain Sci. (2024) 14:1133. doi: 10.3390/brainsci14111133
12. Gibbons TD, Cotter JD, Ainslie PN, Abraham WC, Mockett BG, Campbell HA, et al. Fasting for 20 h does not affect exercise-induced increases in circulating BDNF in humans. J Physiol. (2023) 601:2121–37. doi: 10.1113/JP283582
13. Griffin ÉW, Mullally S, Foley C, Warmington SA, O'Mara SM, Kelly ÁM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. (2011) 104:934–41. doi: 10.1016/j.physbeh.2011.06.005
14. Khalil MH. The impact of walking on BDNF as a biomarker of neuroplasticity: a systematic review. Brain Sci. (2025) 15:254. doi: 10.3390/brainsci15030254
15. Hutchinson KA, Mohammad S, Garneau L, McInnis K, Aguer C, Adamo KB. Examination of the myokine response in pregnant and non-pregnant women following an acute bout of moderate-intensity walking. Front Physiol. (2019) 10:1188. doi: 10.3389/fphys.2019.01188
16. Herrmann SD, Willis EA, Ainsworth BE, Barreira TV, Hastert M, Kracht CL, et al. 2024 Adult compendium of physical activities: a third update of the energy costs of human activities. J Sport Health Sci. (2024) 13:6–12. doi: 10.1016/j.jshs.2023.10.010
17. Sallis JF, Cerin E, Conway TL, Adams MA, Frank LD, Pratt M, et al. Physical activity in relation to urban environments in 14 cities worldwide: a cross-sectional study. Lancet. (2016) 387:2207–17. doi: 10.1016/S0140-6736(15)01284-2
18. Goulet N, McCormick JJ, King KE, Notley SR, Goldfield GS, Fujii N, et al. Elevations in serum brain-derived neurotrophic factor following occupational heat stress are not influenced by age or common chronic disease. Temperature. (2023) 10:454–64. doi: 10.1080/23328940.2023.2176107
19. Molendijk ML, Haffmans JP, Bus BA, Spinhoven P, Penninx BW, Prickaerts J, et al. Serum BDNF concentrations show strong seasonal variation and correlations with the amount of ambient sunlight. PLoS ONE. (2012) 7:e48046. doi: 10.1371/journal.pone.0048046
20. Hernandez CM, Florant GL, Stranahan AM. Seasonal fluctuations in BDNF regulate hibernation and torpor in golden-mantled ground squirrels. Am J Physiol. (2024) 326:R311–8. doi: 10.1152/ajpregu.00186.2023
21. Kojima D, Nakamura T, Banno M, Umemoto Y, Kinoshita T, Ishida Y, et al. Head-out immersion in hot water increases serum BDNF in healthy males. Int J Hyperthermia. (2018) 34:834–9. doi: 10.1080/02656736.2017.1394502
22. Kirby NV, Meade RD, McCormick JJ, King KE, Kenny GP. Brain-derived neurotrophic factor response to daylong exposure to extreme heat in young and older adults: a secondary analysis. Applied Physiology, Nutrition, and Metabolism. (2025) 50:1–9. doi: 10.1139/apnm-2024-0289
23. Kirby NV, Meade RD, McCormick JJ, King KE, Notley SR, Kenny GP. Brain-derived neurotrophic factor in older adults exposed to simulated indoor overheating. Eur J Appl Physiol. (2025) 125:769–80. doi: 10.1007/s00421-024-05623-y
24. Koyama Y, Mukuda T, Hamasaki S, Nakane H, Kaidoh T. Short-term heat exposure promotes hippocampal neurogenesis via activation of angiotensin II type 1 receptor in adult rats. Neuroscience. (2018) 385:121–32. doi: 10.1016/j.neuroscience.2018.05.045
25. Chauhan NR, Kumar R, Gupta A, Meena RC, Nanda S, Mishra KP, et al. Heat stress induced oxidative damage and perturbation in BDNF/ERK1/2/CREB axis in hippocampus impairs spatial memory. Behav Brain Res. (2021) 396:112895. doi: 10.1016/j.bbr.2020.112895
26. Bos I, Jacobs L, Nawrot TS, De Geus B, Torfs R, Panis LI, et al. No exercise-induced increase in serum BDNF after cycling near a major traffic road. Neurosci Lett. (2011) 500:129–32. doi: 10.1016/j.neulet.2011.06.019
27. Alemayehu YA, Asfaw SL, Terfie TA. Exposure to urban particulate matter and its association with human health risks. Environ Sci Pollut Res. (2020) 27:27491–506. doi: 10.1007/s11356-020-09132-1
28. Ko J, Sohn J, Noh Y, Koh SB, Lee SK, Kim SY, et al. Effects of ambient air pollution on brain cortical thickness and subcortical volume: a longitudinal neuroimaging study. Neuroepidemiology. (2025) 59:120–30. doi: 10.1159/000539467
29. Pu F, Chen W, Li C, Fu J, Gao W, Ma C, et al. Heterogeneous associations of multiplexed environmental factors and multidimensional aging metrics. Nat Commun. (2024) 15:4921. doi: 10.1038/s41467-024-49283-0
30. Wilker EH, Preis SR, Beiser AS, Wolf PA, Au R, Kloog I, et al. Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke. (2015) 46:1161–6. doi: 10.1161/STROKEAHA.114.008348
31. Ysebaert T, Koch K, Samson R, Denys S. Green walls for mitigating urban particulate matter pollution—a review. Urban For Urban Green. (2021) 59:127014. doi: 10.1016/j.ufug.2021.127014
32. Khalil MH. Green environments for sustainable brains: parameters shaping adaptive neuroplasticity and lifespan neurosustainability—a systematic review and future directions. Int J Environ Res Public Health. (2025) 22:690. doi: 10.3390/ijerph22050690
33. Li H, Zhao Y, Wang C, Ürge-Vorsatz D, Carmeliet J, Bardhan R. Cooling efficacy of trees across cities is determined by background climate, urban morphology, and tree trait. Commun Earth Environ. (2024) 5:754. doi: 10.1038/s43247-024-01908-4
34. Panter J, Guell C, Humphreys D, Ogilvie D. Can changing the physical environment promote walking and cycling? A systematic review of what works and how. Health Place. (2019) 58:102161.
Keywords: outdoor exercise, urban physical activity, BDNF, hippocampal neurogenesis, brain health, heat stress, environmental health, walking and cycling
Citation: Khalil MH (2025) Urban physical activity for neurogenesis: infrastructure limitations. Front. Public Health 13:1638934. doi: 10.3389/fpubh.2025.1638934
Received: 31 May 2025; Accepted: 28 July 2025;
Published: 18 August 2025.
Edited by:
Yang Cao, Örebro University, SwedenReviewed by:
Tania Giraldo-Ospina, Universidad Nacional de Colombia sede Manizales, ColombiaCopyright © 2025 Khalil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed Hesham Khalil, bWhtaGsyQGNhbS5hYy51aw==
 Mohamed Hesham Khalil
Mohamed Hesham Khalil