- 1Antimicrobial Resistance Coordinating Committee, Zambia National Public Health Institute, Lusaka, Zambia
- 2Department of Pharmacy, School of Health Sciences, University of Zambia, Lusaka, Zambia
- 3Education and Continuous Professional Development Committee, Pharmaceutical Society of Zambia, Lusaka, Zambia
- 4Infection Prevention Network-Kenya (IPNET-Kenya), Mombasa, Kenya
- 5Department of Pathology and Microbiology, University Teaching Hospitals, Lusaka, Zambia
- 6Department of Medicine, Kanyama Level 1 Hospital, Lusaka, Zambia
- 7Strengthening Pandemic Preparedness, Eastern, Central, and Southern Africa Health Community, Arusha, Tanzania
- 8Education and Research, Clinical Research Education and Management Services (CREAMS), Lilongwe, Malawi
- 9Department of Clinical Services, Kamuzu Central Hospital (KCH), Lilongwe, Malawi
- 10Department of Medicine, Lusaka Apex Medical University, Lusaka, Zambia
- 11Resident Doctors Association of Zambia, Ministry of Health Zambia, Lusaka, Zambia
- 12Sinazeze Hills Mini Hospital, Ministry of Health Zambia, Sinazongwe, Zambia
- 13Department of Clinical Pharmacology and Therapeutics, Kairuki University, Dar Es Salaam, Tanzania
- 14Health Systems Strengthening Unit, World Health Organization, Harare, Zimbabwe
- 15The Global Fund, Geneva, Switzerland
- 16Action on Antibiotic Resistance (ReAct) Africa, Lusaka, Zambia
- 17Hokudai Center for Zoonosis Control in Zambia, Hokkaido University, Lusaka, Zambia
- 18Division of Bioresources, Hokkaido University International Institute for Zoonosis Control, Sapporo, Japan
- 19Department of Disease Control, School of Veterinary Medicine, University of Zambia, Lusaka, Zambia
- 20Department of Surgery, Macerata Hospital, Macerata, Italy
- 21Department of Public Health Pharmacy and Management, School of Pharmacy, Sefako Makgatho Health Sciences University, Ga-Rankuwa, South Africa
- 22Department of Pharmacoepidemiology, Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, United Kingdom
- 23Antibiotic Policy Group, Institute for Infection and Immunity, City St. George’s, University of London, London, United Kingdom
Background: Infection Prevention and Control (IPC) is key to preventing healthcare-associated infections (HAIs) and the spread of antimicrobial resistance (AMR). This study evaluated the implementation of IPC in Zambian hospitals.
Materials and methods: We conducted a multicentric cross-sectional study in nine hospitals across Zambia using the WHO IPCAF tool. Data were collected from September 1 to 30, 2024 and analyzed using the self-scoring Excel and IBM SPSS version 25.0.
Results: Out of the nine hospitals assessed, four were tertiary-level hospitals while the rest were secondary-level hospitals. Overall, the implementation of IPC core components was intermediate (IPCAF Score of 594 out of 800). Four hospitals had IPCAF scores between 401 and 600, indicating an intermediate level of IPC implementation. Five hospitals scored between 601 and 800, indicating an advanced implementation of IPC in these hospitals. Three tertiary hospitals scored between 601 and 800, demonstrating their advanced implementation of IPC core components.
Conclusion: This study found that the overall implementation of IPC in the surveyed hospitals was intermediate, indicating that further improvements were needed. There is a need to provide peer-learning support and strengthen IPC implementation to respond to new or re-emerging infections and AMR in the country and beyond.
Introduction
Infection prevention and control (IPC) is a systematic, data-driven practice aimed at curbing the transmission of preventable infections in the healthcare setting (1). It is a cornerstone of effective healthcare delivery, and essential for safeguarding patients and healthcare workers (2–4). In recent years, the growing threat of healthcare-associated infections (HAIs) and antimicrobial resistance (AMR) has underscored the urgent need for robust IPC programmes across health systems (5–9), particularly in low- and middle-income countries (LMICs) (10–14), including Zambia.
Healthcare-associated infections (HAIs) are a significant concern in the context of AMR, with virulent and high-risk microorganism strains such as “ESKAPE” pathogens – (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species) of global significance (15–17). Evidence has indicated that HAIs are acquired by patients while receiving healthcare treatment (18, 19). These include Central Line-Associated Bloodstream Infections (CLABSI), Surgical Site Infections (SSI), respiratory infections like Hospital-Acquired Pneumonia (HAP), Ventilator-Associated Pneumonia (VAP), Catheter-Associated Urinary Tract Infections (CAUTI), gastrointestinal tract infections such as Clostridioides difficile infections (antibiotic-associated diarrhoea), and wound infections (18, 20–24). These infections are prevalent in intensive care units (ICUs), where patients are particularly vulnerable due to invasive procedures, immunocompromised, and extensive antibiotic use (5, 8, 15, 25).
The occurrence of these HAIs contributes to the overuse of antibiotics, increases costs and eventually the development and spread of AMR (15, 26). The economic and clinical burden associated with HAIs is substantial, with patients experiencing longer hospital stays and higher medical costs (27, 28). In the USA, attributable costs to HAIs have been estimated at $3,384 ($885–$7,717) per patient for vancomycin-resistant enterococci, increasing to $39,787 ($20,813–$64,140) for MDR Acinetobacter and further rising to $74,306 ($20,377–$128,235) for carbapenem-resistant (CR) Acinetobacter (29–31). Subsequently, AMR also increases patient morbidity and mortality and harms the global economy (26, 32–36).
The prevalence and burden of HAIs in LMICs are significant public health and economic concerns (5, 37, 38). Fraser et al. (39) estimated that the prevalence of HAIs across Africa between 2009 and 2018 ranged from 3 to 15% of patients; however, this was likely to be an underestimate with considerable under-reporting of HAIs across the continent (39). Abubakar et al. in 2022 estimated the prevalence of HAIs in Africa at 12.76%, with surgical site infections being the most common type (5). More recently, Hutton et al. (40) estimated that the number of HAIs among 14 sub-Saharan African countries was 4.8 million in 2022, with the number of deaths resulting from these estimated at 502,000. The total economic costs across the 14 sub-Saharan African countries were estimated to be at least US$13 billion in 2022, with the costs per capita of the population higher among lower-middle-income African countries at US$23.9 per capita versus low-income African countries at US$7.2 per capita (40).
Rates of HAIs are typically elevated across sub-Saharan Africa as a result of poor IPC practices due to limited resources, as well as typically overcrowding in hospitals, coupled with understaffing and underfunding of healthcare facilities (41, 42). Overall, it is estimated that up to 50% or more of public healthcare facilities across sub-Saharan Africa, especially in rural areas, lack routine access to clean water, basic hygiene, and basic waste management, adding to the development of HAIs (43–45). It is against these aforementioned challenges that strategies need to be instigated across countries to prevent infections and address AMR and its consequences (40, 46–56).
To help address the challenge of HAIs, the WHO developed the Infection Prevention and Control Assessment Framework (IPCAF) to evaluate and monitor IPC programs and support the implementation of IPC guidelines in healthcare facilities, particularly among LMICs (57–59). This user-friendly tool aligns with WHO’s eight core IPC components, providing a baseline assessment and enabling ongoing monitoring to track progress and identify gaps (57, 60). By guiding targeted interventions, the IPCAF aims to improve healthcare quality, reduce the level of HAIs, and combat AMR (58). Its structured approach supports data-driven IPC strategies, fostering accountability and continuous improvement, making it valuable, especially among LMICs where there are more concerns (4, 61–64).
Effective IPC is a multifaceted endeavor requiring a synergistic approach across various critical components (65, 66). Further, robust IPC programs, underpinned by evidence-based national and facility-level guidelines, standardize practices and ensure a systematic approach to safety (1, 57). Furthermore, comprehensive education and training empower healthcare workers (56), while continuous HAI surveillance (47, 67), coupled with diligent monitoring and feedback (57, 68), enables data-driven adjustments and fosters a culture of improvement. Alongside this, ensuring appropriate workload, staffing, and a well-maintained built environment with functional equipment provides the necessary infrastructure for safe care (59, 69–72). Collectively, these interconnected components are foundational to minimizing infection transmission, safeguarding patient safety, and playing a pivotal role in the global fight against AMR.
In Zambia, multidrug-resistant pathogens have been reported in hospitals, demonstrating the current significant burden of AMR (73–81). Very few studies, however, have been conducted on the extent of IPC implementation in Zambian hospitals (82, 83), hence this study. Furthermore, the implementation of IPC programmes in Zambia faces significant challenges, including resource limitations, inadequate infrastructure, and varying levels of IPC knowledge among healthcare workers. Notwithstanding, the authorities in Zambia have made notable strides toward improving IPC practices at the national and facility levels as part of ongoing initiatives to tackle AMR in the country as part of the National Action Plan (NAP) for AMR (84).
The adoption of tools like the IPCAF by the Antimicrobial Resistance Coordinating Committee (AMRCC) of the Zambia National Public Health Institute (ZNPHI) has the potential to further strengthen IPC programmes by providing a standardized mechanism to assess and improve practice. This is welcomed, given ongoing concerns regarding the excessive prescribing of antibiotics across different hospitals in Zambia (85–93). This study assessed the level and extent of IPC implementation in nine hospitals across Zambia. The findings of this study will inform the status of objective number three of the NAP and future directions toward preventing the occurrence of infections and AMR in Zambia (84).
Materials and methods
Study design, setting and population
A self-reporting, descriptive multicentric cross-sectional survey was conducted to assess the IPC situation in hospitals in Zambia against the WHO minimum requirements for IPC programmes. The study was conducted from September 1 to 30, 2024 and included nine hospitals, out of which five were secondary-level public hospitals namely CGH, KGH, LGH, MGH, and SGH, respectively, and four were tertiary-level ADCH, CCH, LUTH, and NTH, respectively (Figure 1). In Zambia, Secondary-Level Hospitals, on the one hand, provide specialized referral services for areas of internal medicine, general surgery, paediatrics, obstetrics and gynecology, dentistry, psychiatry, and intensive care services. They are intended to serve a population range between 200,000 and 800,000, including referral patients from First-Level Hospitals. On the other hand, Tertiary-Level Hospitals provide medical services in internal medicine, surgery, paediatrics, obstetrics, gynecology, intensive care, and psychiatry, including health training and research. These hospitals are intended to serve a population of 800,000, including referrals from Secondary-Level Hospitals. The hospitals were selected using a purposive sampling method as they were implementing antimicrobial stewardship (AMS) programs to promote the rational use of antimicrobials, as reported in previous studies (94, 95). The nine hospitals included in this study were purposively selected to capture a range of facility types (secondary and tertiary) and geographical regions, with the aim of reflecting diverse IPC contexts within the country. However, these hospitals represent only a fraction of the total number of hospitals nationally, and therefore, the findings may not be fully representative of all healthcare facilities. To be eligible for inclusion, all hospitals were to be run and owned by the Government of the Republic of Zambia and with an established IPC Committee. Additionally, implementing effective IPC programmes in these hospitals is critical to prevent the occurrence and spread of infections and subsequently reduce the current overuse of antimicrobials. Furthermore, all targeted hospitals were secondary or tertiary-level. The targeted respondents were healthcare professionals or teams responsible for organizing and implementing IPC activities in the respective hospitals. Overall, 27 respondents were purposively selected from the respective IPC committees to respond to the questionnaire. This included the committee chairperson, secretary and IPC nurse from each of the nine hospitals.
Data collection
Data collection was done using the pre-validated WHO IPCAF tool (57). We adopted the WHO globally validated IPCAF tool organized into eight IPC core components and multiple choice questions (57). The IPCAF is a globally validated evaluation tool designed to benchmark IPC performance at national and facility levels while supporting the implementation of the eight IPC core components (Supplementary material). The WHO IPCAF instrument used in this study was applied in its original form without local modifications. This decision was based on the instrument’s status as a globally standardized and validated tool designed for cross-country comparability. While the IPCAF has not undergone formal psychometric validation in Zambia, it has been widely used in LMIC settings with similar healthcare contexts, including in the African region. The research team ensured contextual relevance by providing brief clarifications during administration where necessary, without altering the structure, wording, or scoring system of the tool. Data collection was executed by 15 data collectors who were specifically trained for this purpose and the team visited each hospital for a period of 5 days translating into a 15-day data collection and dissemination period. Each data collection team visited three hospitals and conducted face-to-face interviews with the IPC committee members. The data collectors (medical doctors, pharmacists, nurses, public health staff, and biomedical scientists) were members of the IPC Technical Working Group (TWG) under the AMRCC who were trained on the use of the tool and had previous training on IPC using the national curriculum on AMS and IPC. The trainers were part of the Antimicrobial Resistance Coordinating Committee (AMRCC) who are experts in implementing IPC and AMS activities in Zambia. The training was done for a period of 3 days to ensure that the data collectors understood the need to collect complete and good quality data. REDCap accounts were opened for all the data collectors and testing of data entry was done on day two and three of training. After completion of the study, a meeting was held with the hospital management and staff where the findings were disseminated and recommendations were provided.
The IPCAF tool generates a final score ranging from 0 to 800, categorizing IPC implementation as inadequate, basic, intermediate, and advanced (57). This approach identifies gaps in current practices and fosters quality improvement initiatives. The scoring in the IPCAF tool is done as follows: Scores of 0–200 (Inadequate): IPC core component implementation is deficient and thus requires significant improvement; Scores of 201–400 (Basic): Some aspects of the IPC core components are in place, but not sufficiently implemented, hence, requires further improvement; Scores of 401–600 (Intermediate): Most aspects of IPC core components are appropriately implemented but there is a need to continue to improve the scope and quality of implementation and focus on the development of long-term plans to sustain and further promote the existing IPC program; Scores of 601–800 (Advanced): Means that the IPC core components are fully implemented according to the WHO recommendations and appropriate to the needs of the facility (57). All survey questions addressing the core components were multiple-choice, with response options such as “yes,” “no,” or “choose one answer.” The findings of the study were discussed with the IPC committee and disseminated across the surveyed hospitals.
Data analysis
The collected data were checked for completeness before analysis. Data analysis was done using the self-scoring Excel sheet and IBM Statistical Package for Social Sciences (SPSS) version 25.0. Scoring for implementation of IPC core components was assigned as follows: 0–200 (Inadequate): 201–400 (Basic): 401–600 (Intermediate): and 601–800 (Advanced). Descriptive statistics were used to determine the overall implementation of IPC core components, and the status of IPC core components for each hospital was expressed as proportions. Additionally, descriptive statistics, including the mean and standard deviation, were used to summarize the overall IPCAF scores and the scores for each of the eight core components across the surveyed hospitals. Frequencies and percentages were used to describe the distribution of hospitals within the defined IPCAF implementation levels. To compare the mean IPC implementation scores across the nine hospitals, a One-Way Analysis of Variance (ANOVA) was performed. The statistical test (F-statistic) was conducted at a 95% confidence level and the level of statistical significance was set at p < 0.05.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Tropical Diseases Research Centre (TRC/C4/09/2023). Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the participants and facilities to publish this paper. Respondents and hospital management were informed that the findings of the study were to be published in peer-reviewed journals.
Results
Demographics of the surveyed hospitals
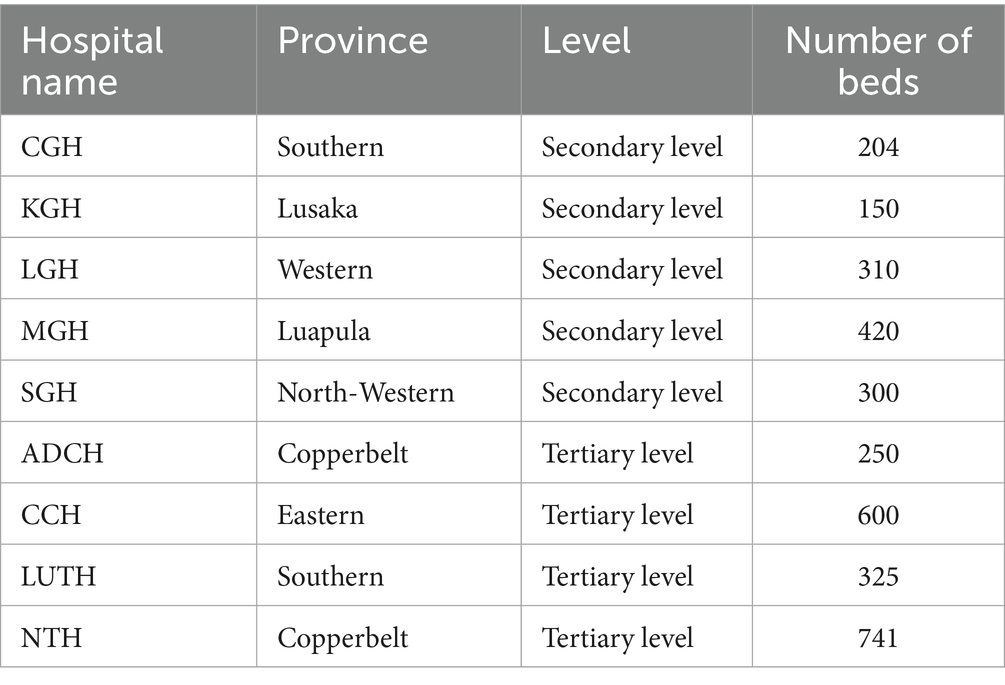
Overall, nine hospitals, including five (55.6%) secondary-level and four (44.4%) tertiary-level hospitals, were included in this study across seven provinces in Zambia, with an overall inpatient bed capacity of 3,300. Of the nine hospitals, two had bed capacities above 500, while the rest had between 150 and 420 beds (Table 1). The average bed capacity for secondary-level hospitals was 277 while that for tertiary-level hospitals was 479.
The average overall IPCAF score across the hospitals was 594, indicating a generally intermediate level of IPC implementation (Figure 2). The distribution of hospital IPCAF scores revealed that 55.6% (n = 5) of the hospitals demonstrated advanced IPC implementation (score range 601–800), while 44.4% (n = 4) exhibited intermediate implementation (score range 401–600) (Figure 2). No hospitals scored within the basic level (<400). The F-statistic was found to be 3.72 and a p = 0.001, indicating that there is a statistically significant difference in the mean IPC implementation scores across the nine hospitals.
Tertiary hospitals outperformed secondary hospitals across most IPCAF core components, with the greatest differences observed in IPC education and training and HAI surveillance, reflecting stronger capacity-building initiatives and monitoring systems in higher-level facilities (Table 2). Secondary hospitals scored higher only in workload, staffing, and bed occupancy, likely due to lower patient volumes and reduced overcrowding. Both hospital categories demonstrated moderate performance in built environment, materials, and equipment for IPC, suggesting shared infrastructural gaps. The overall IPCAF score for tertiary hospitals was 641 compared to secondary hospitals at 556 (Table 2). The F-statistic was found to be 4.65 and a p = 0.049, indicating that there is a statistically significant difference in mean scores between secondary and tertiary hospitals, suggesting that hospital level is associated with variations in IPC performance across the IPCAF components.
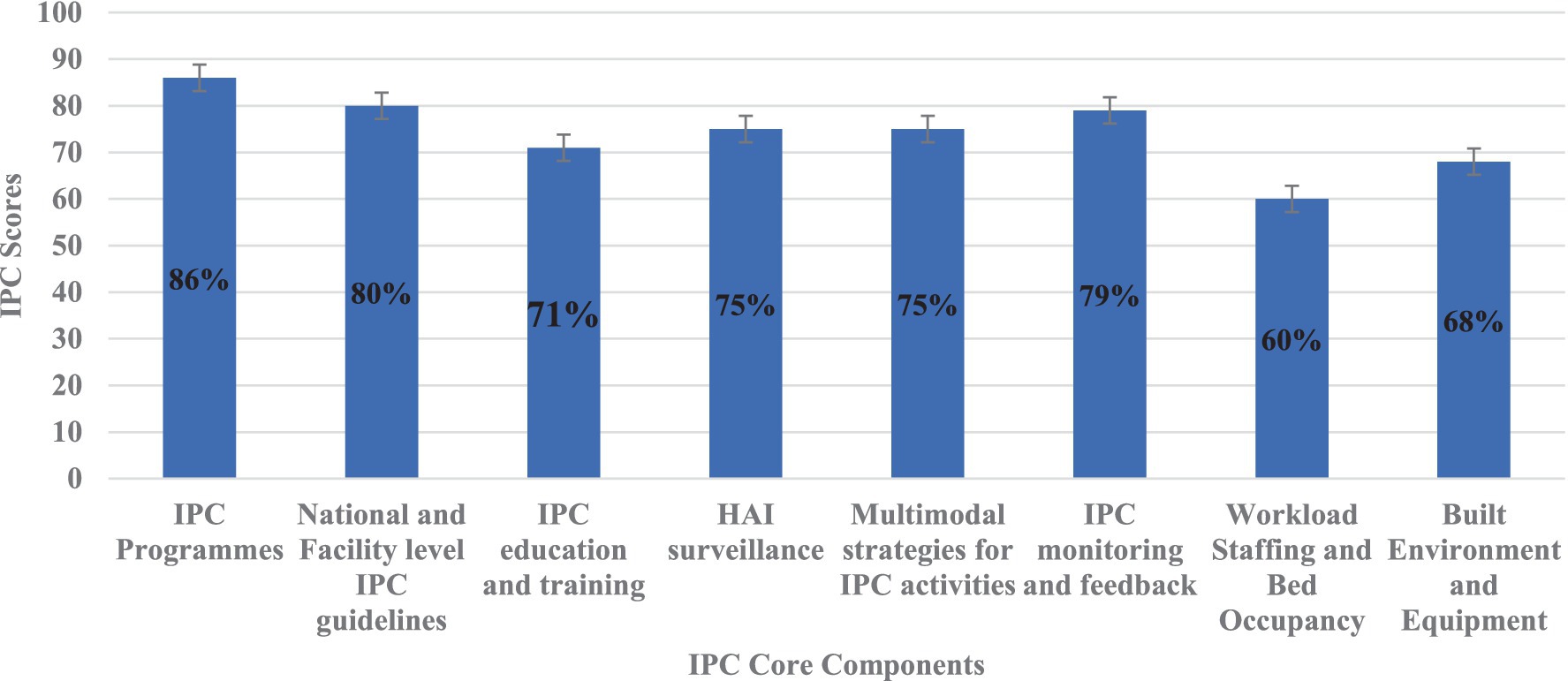
In this study, the IPC core components with the highest scores included IPC programmes (86%), National and Facility level IPC guidelines (80%), and IPC monitoring and feedback (79%) (Figure 3). Conversely, the IPC core components with the lowest scores included workload staffing and bed occupancy (60%), build environment and equipment (68%), IPC education and training (71%), HAI surveillance (75%), and multimodal strategies for IPC activities (75%) (Figure 3).
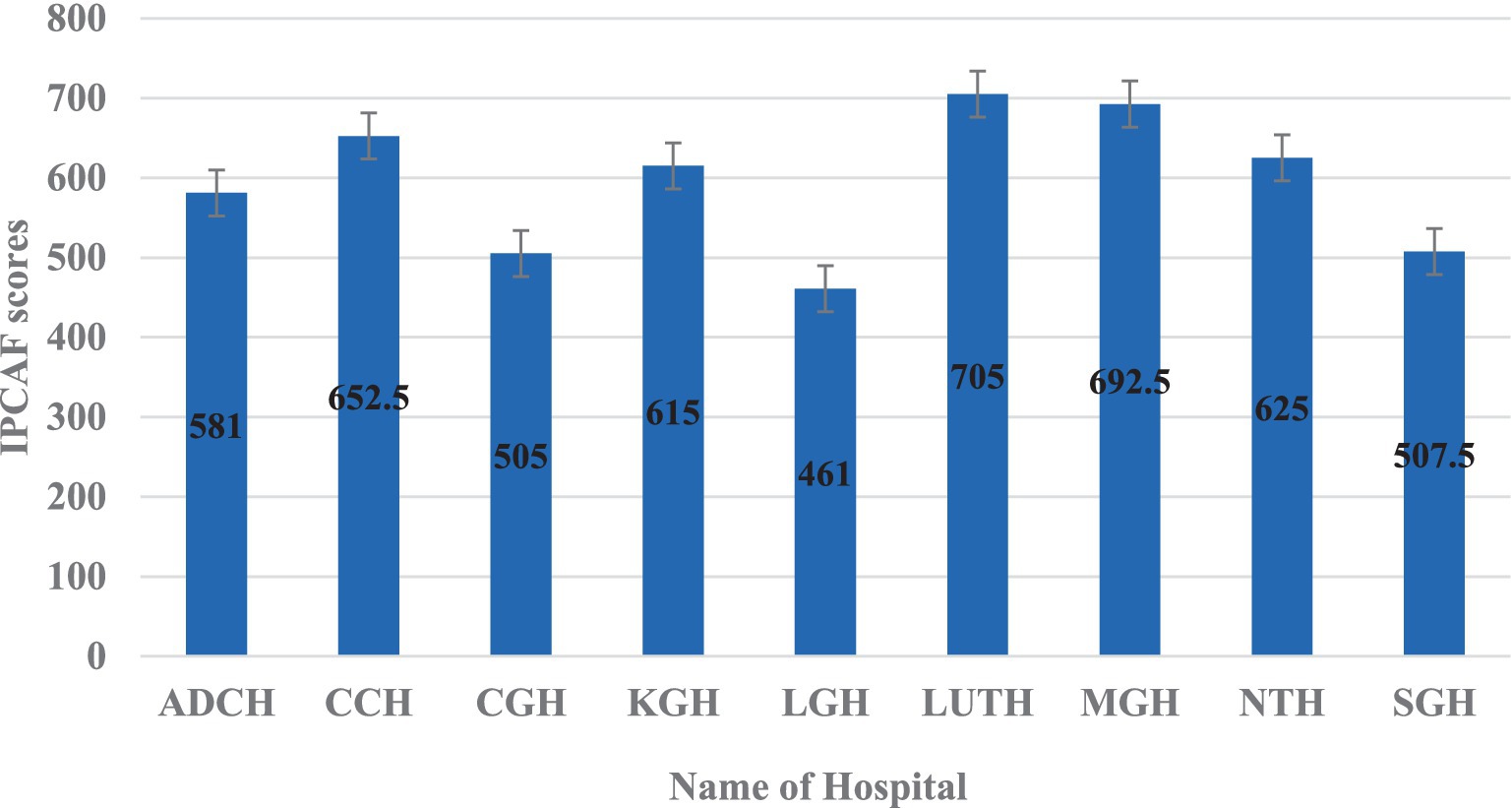
The hospital with the highest IPCAF score was LUTH (705) – a tertiary level hospital, followed by MGH (692.5), CCH (652.5), NTH (625), and KGH (615) all indicating an advanced level of IPC implementation (Figure 4). Consequently, the hospitals with the lowest IPCAF scores were LGH (461), CGH (505), SGH (507.5), and ADCH (581) which showed an intermediate implementation of IPC in these hospitals (Figure 4).
The IPCAF levels are shown in Figure 4 which shows that four hospitals (44.4%) had an intermediate IPCAF level while five hospitals (55.6%) had an advanced level (Figure 5).
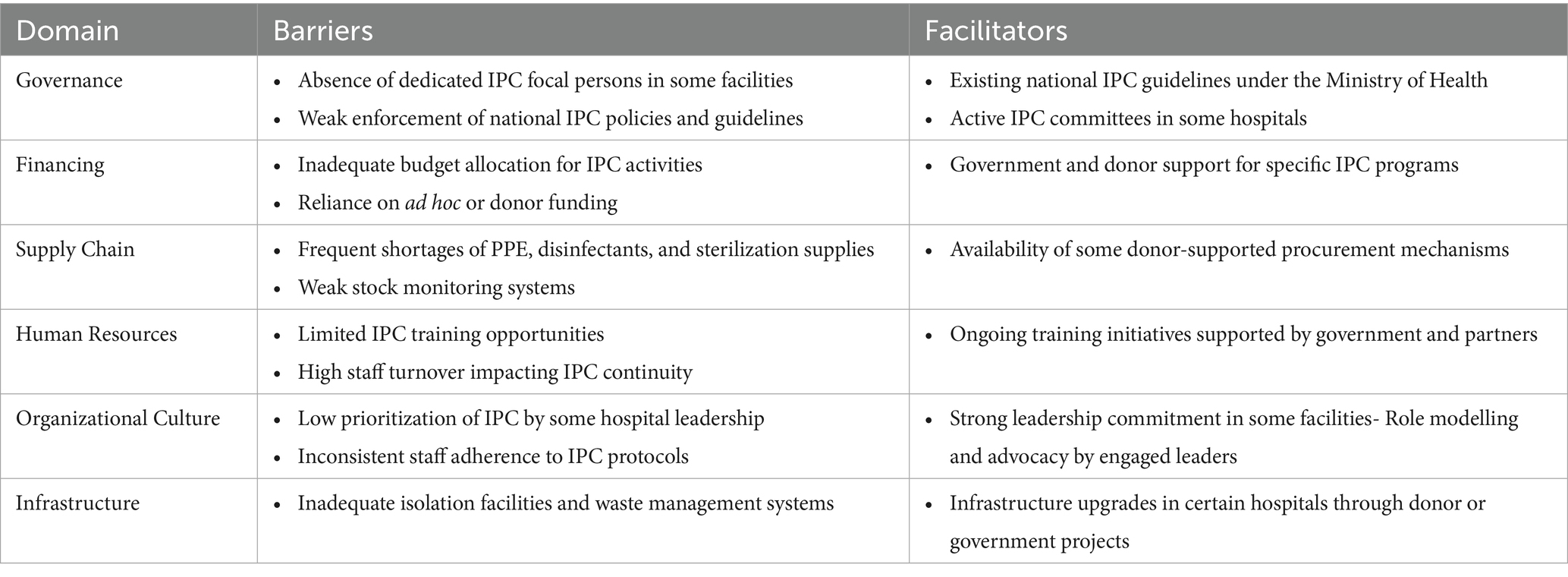
Implementation of IPC in Zambia’s hospitals faces challenges such as limited dedicated staff, weak policy enforcement, insufficient funding, supply shortages, and inadequate training and infrastructure (Table 3). However, support from national guidelines, active committees, donor-funded programs, leadership commitment, and some infrastructure improvements act as facilitators to IPC efforts (Table 3).
Discussion
To the best of our knowledge, this study represents the first nationwide assessment of the level and extent of IPC program implementation across different levels of public hospitals in the country. While previous reports (82, 83) may have touched upon aspects of IPC in specific settings, none have provided a comprehensive and validated nationwide assessment of IPC implementation. This study found an intermediate implementation of IPC core components in most secondary-level hospitals but an advanced implementation in most tertiary-level hospitals. Overall, the study found an intermediate implementation of IPC programs in the surveyed hospitals, which indicates a need to continue improving the scope and quality of IPC implementation. Alongside this, the focus should be on the development of long-term plans to sustain and further promote existing IPC programs in the country to reduce the prevalence of HAIs and the overuse of antibiotics among hospitals in Zambia.
The present study found an IPCAF score of 594, which indicates an intermediate level of IPC implementation in Zambian hospitals. These findings are similar to those reported across six hospitals in Uganda with an IPCAF score of 547 (96) and in Rwanda across 25 hospitals with an IPCAF score of 545 (97), potentially reflecting similar resource constraints and healthcare system structures within the East African region. Intermediate implementation of IPC, though lower than scores reported in Zambia, was also reported in Malawi across 33 hospitals with an IPCAF score of 445 (62) and in Burkina Faso with an IPCAF overall score of 415 (68), might indicate more significant challenges in basic IPC infrastructure and implementation in those contexts. Our findings are also better than those reported in the Democratic Republic of Congo (DRC) and Cote d’Ivoire. In the DRC, only a basic level implementation of IPC was reported, with an overall IPCAF score of 392.5 (68) while in Cote d’Ivoire, the score was only 242.5 (98), indicating the key barriers at play as reported in many LMICs, including inadequate allocation of budget for IPC, insufficient staffing of full-time IPC professionals, absence of clear IPC goals, challenges in staff training, no HAI surveillance, no periodic monitoring and inconsistent availability of IPC supplies (63, 99, 100). Conversely, the advanced implementation in Latin America (73) and high-income countries (HICs) (58, 60, 101–107) likely benefits from high-income level, greater investment in healthcare infrastructure, staffing, more attention given to IPC, and established IPC protocols (63). These findings indicate that most HICs have advanced implementation of IPC in their hospitals. Consequently, evidence has shown that significantly lower scores of IPC implementation were observed in LMICs and public healthcare facilities (58, 63).
Our study found that most tertiary-level hospitals had an advanced implementation of IPC core components compared to secondary-level hospitals. Arguably, this could be attributed to a higher density of specialized IPC-trained personnel serving in the facilities, more consistent access to funding for IPC supplies and programme support, or the presence of established IPC committees with more actions and implementing activities in the hospital. These findings underscores persistent disparities in resources and capacity that warrant targeted interventions to strengthen IPC performance nationwide. Our findings differ from those reported in Malawi, in which tertiary-level hospitals had an intermediate implementation of IPC programs (62). Our findings are also better than those reported in Bangladesh, where most tertiary-level hospitals had an inadequate implementation of IPC components (51). The findings in Malawi and Bangladesh, where tertiary hospitals showed intermediate implementation of IPC programs, suggest potentially different national-level resource allocation or policy implementation strategies.
Of the eight core components of IPC, the highest IPCAF scores were in IPC programmes and national and facility-level IPC guidelines. Our findings corroborate those reported in a study that was conducted in the DRC, in which the highest was the presence of IPC programmes, and national and facility IPC guidelines (68). This is in line with established global guidelines and the 17th International Congress on Infectious Diseases workshop on establishing IPC and developing IPC resources for LMICs (108, 109). However, our findings differ from those reported in Burkina Faso, where the highest IPCAF scores were in HAI surveillance, built environment, and equipment (68). The difference could be due to the fact that in Zambia, IPC program guidelines scored well because they were instigated but the Zambian program had not implemented HAI surveillance. However, our findings guide future activities in this area in Zambia.
In our study, the lowest IPCAF scores were in workload, staffing, bed occupancy, built environment and equipment, and IPC education and training, which may reflect systemic issues within the Zambian healthcare system and limitations in infrastructure planning. Low scores in workload staffing and bed occupancy have also been reported in other LMICs, including the DRC and Malawi (62, 68). In Zambia, staffing, workload, and bed occupancy scored 60% better than the 45% reported in Malawi (62). Studies in Latin America, Germany, and Turkey also found low scores in workload, staffing and bed occupancy, followed by IPC training and multimodal strategies (102, 107, 110). Low scores in IPC education and training were also recorded in Burkina Faso (68). The low scores in IPC education and training highlight a crucial gap that has been recognized in other settings and likely hinders the effective implementation of IPC practices on the ground (111, 112). Providing education and training for healthcare workers in IPC is an essential area for the improvement of IPC practices and must be implemented in all countries (70). A study in China reported that multimodal strategies had the lowest average scores among the core components of IPC, thereby demonstrating gaps in adopting and using evidence-based strategies to implement IPC in hospitals (61).
The present study reported a 75% score in HAI surveillance. The scoring on HAI surveillance in our study may be biased due to the lack of participant understanding related to what constitutes HAI surveillance, because of the lack of training on HAI surveillance standards and requirements. This highlights a critical need for targeted education and capacity-building initiatives to ensure accurate data collection and effective utilization of HAI surveillance for IPC program improvement, as recommended by WHO guidelines on HAI surveillance (56, 109, 113). However, our findings are better than those reported in Malawi, where the HAI surveillance score was 40% (62). Our findings and those reported in other studies indicate the need to strengthen all the IPC core components in hospitals to control HAIs and prevent the emergence and spread of AMR. Furthermore, there is a need to develop IPC resources, especially for LMICs, where the burden of infections is high (108). Leadership support at the facility, national, and global levels is also needed to achieve implementation of the core components across all countries (109).
The gaps identified in IPC performance across the nine hospitals can be better understood by examining the underlying systemic barriers and facilitators. The study found that governance challenges, such as the absence of dedicated IPC focal persons in some facilities and weak enforcement of national guidelines, undermine consistent implementation of IPC policies. These gaps affect the full implementation of IPC strategies in hospitals, similar to findings from other studies (109, 114). For a functional and effective IPC programme, there is a need of having a dedicated focal point person to implement IPC measures (115). In the present study, inadequate financing, characterized by limited or non-ring-fenced budget allocations, restricts investment in essential supplies, infrastructure, and training. These challenges have been reported in other studies and affect the implementation of IPC in hospitals (99, 116–118). Our study further found that weaknesses in supply chain systems further exacerbate these constraints, with frequent shortages of PPE, disinfectants, and sterilization materials disrupting adherence to IPC protocols. Similar findings were reported in Nigeria where inadequate IPC materials affected the implementation of IPC in hospitals (119). The present study also found that in some hospitals, low prioritization of IPC by leadership and inconsistent staff compliance reflect competing demands, limited training opportunities, and insufficient role modelling. Lack of leadership support to implement IPC in hospitals has been reported to affect the effective prevention of infections (64, 120). Addressing these gaps is critical to full implementation of IPC strategies in hospitals.
Our study revealed several facilitators of IPC implementation in some hospitals, including the presence of active IPC committees, supportive leadership in certain facilities, and existing national guidelines that provide a structured framework for implementation. Donor-supported programs offering training and procurement support also helped to strengthen IPC in specific settings. These facilitators of IPC implementation have been recommended for LMICs, such as Zambia (59, 64). Therefore, these findings underscore the need for a multi-pronged approach that addresses governance, financing, supply chain resilience, and cultural change to close IPC performance gaps and sustain improvements over time.
We are aware that cross-sectional studies have limitations. Our study relied on self-reported measurements which may be prone to recall bias, including social desirability bias, whereby participants may overstate compliance with IPC standards, and recall bias, particularly for activities or events occurring in the past. The study was limited to nine hospitals, which may restrict the generalizability of the findings to other healthcare facilities across the country, particularly those with differing levels of resources, staffing, or patient populations. The purposive sampling approach may overrepresent facilities with better-established IPC programs or stronger administrative support, and underrepresent those with fewer resources. Alongside this, the cross-sectional nature of this study captured IPC performance at a single point in time within a month, which limits the ability to assess temporal changes, monitor trends, or establish causal relationships between identified factors and IPC outcomes. For example, infectious disease episodes may be higher during the rainy season, which may have an impact on the implementation of IPC strategies. These factors may have led to overestimation or underestimation of IPC performance in some hospitals. As a result, while the findings provide valuable insights into the current IPC landscape, they should be interpreted with caution and complemented by future longitudinal and observational studies. Notwithstanding, this study provides baseline findings that can be used to develop strategies for improving IPC in hospitals across Zambia. In doing so, this can impact reductions in antimicrobial use, and the emergence and spread of AMR. Future research should employ nationally representative longitudinal study designs that include both public and private hospitals across all provinces. Such studies would enhance the generalizability of findings, allow for the monitoring of IPC performance trends over time, and enable a more robust assessment of causal relationships between governance, financing, supply chain resilience, and IPC outcomes over time. Alongside this, future IPC research in Zambia should adopt mixed-methods approaches, combining quantitative assessments with qualitative studies to explore healthcare workers’ perspectives, organizational culture, and practical barriers to IPC adherence. Such approaches would provide deeper contextual understanding, uncover underlying systemic challenges, and inform the design of interventions that are both feasible and culturally appropriate.
Policy recommendations and implications
The proposed policy recommendations address key systemic gaps identified in IPC implementation across Zambia’s hospitals (Table 4). Strengthening governance through the appointment of trained IPC focal persons and regular audit mechanisms would improve accountability and ensure adherence to national guidelines. Dedicated and ring-fenced funding at both national and facility levels is essential to sustain IPC programs and prioritize expenditure on critical supplies. Enhancing supply chain resilience via robust procurement systems and pooled purchasing arrangements would minimize shortages of essential IPC commodities. Building the skills and knowledge of healthcare workers through regular refresher training and integration of IPC into continuous professional development would reinforce best practices. Promoting a culture of safety through leadership advocacy, role modelling, and recognition systems would encourage staff adherence and institutional commitment. Finally, leveraging partnerships with development agencies for infrastructure upgrades, technical support, and collaborative research would expand the resource base and support innovation in IPC strategies. Together, these measures offer a practical and context-specific framework for improving IPC performance nationwide.

Table 4. Policy recommendations to strengthen infection prevention and control (IPC) in Zambia’s hospitals.
Conclusion
In conclusion, this study found an intermediate implementation of IPC core components in most secondary-level hospitals but an advanced implementation in most tertiary-level hospitals in Zambia. Overall, the average IPCAF level was found to be intermediate. The implementation of the WHO IPCAF tool in Zambia represents a significant opportunity to enhance IPC practices, reduce the burden of HAIs, and combat AMR. By leveraging this systematic and evidence-based approach, healthcare facilities can achieve measurable improvements in patient safety and healthcare quality. While this study provides valuable baseline data, future research should focus on conducting a larger, nationally representative survey, employing qualitative methods to explore barriers to IPC implementation at different hospital levels, and undertaking longitudinal studies to evaluate the impact of targeted IPC interventions on HAI rates and antibiotic use. Notably, our results contribute to ongoing efforts to optimize IPC programmes in Zambia, ensuring alignment with global best practices while addressing local healthcare challenges. Finally, there is a need for quality improvement in education and training of IPC measures among healthcare workers across all the hospitals in Zambia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Tropical Diseases Research Centre Committee (TRC/C4/09/2023). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SM: Project administration, Methodology, Validation, Conceptualization, Writing – original draft, Formal analysis, Writing – review & editing, Software, Investigation, Data curation, Resources, Visualization. JC: Writing – review & editing, Writing – original draft. LN: Writing – review & editing, Writing – original draft. MK: Writing – review & editing, Writing – original draft. IM: Writing – review & editing, Writing – original draft. AK: Writing – original draft, Writing – review & editing. EW: Writing – original draft, Writing – review & editing. AL: Writing – review & editing, Writing – original draft. JCLM: Writing – review & editing, Writing – original draft. MMw: Writing – original draft, Writing – review & editing. AM: Writing – review & editing, Writing – original draft. NS: Writing – review & editing, Writing – original draft. TM: Writing – review & editing, Writing – original draft. MMu: Writing – original draft, Writing – review & editing. PS: Writing – original draft, Writing – review & editing. DL: Writing – review & editing, Writing – original draft. KY: Writing – review & editing, Writing – original draft. MiS: Writing – review & editing, Writing – original draft. CN: Writing – original draft, Writing – review & editing. YS: Writing – review & editing, Writing – original draft. JBM: Writing – review & editing, Writing – original draft. MaS: Writing – review & editing, Writing – original draft. BG: Writing – review & editing, Writing – original draft. RC: Writing – original draft, Funding acquisition, Writing – review & editing, Conceptualization, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Pandemic Fund.
Acknowledgments
We are grateful to all the Management Staff of the public hospitals that were involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1642119/full#supplementary-material
References
1. World Health Organization. Guidelines on core components of infection prevention and control programmes at the national and acute health care facility level. Geneva: WHO (2016). Available at: https://www.who.int/publications/i/item/9789241549929 (Accessed October 9, 2024).
2. Tsioutis, C, Birgand, G, Bathoorn, E, Deptula, A, ten Horn, L, Castro-Sánchez, E, et al. Education and training programmes for infection prevention and control professionals: mapping the current opportunities and local needs in European countries. Antimicrob Resist Inf Control BioMed Central Ltd. (2020) 9:183. doi: 10.1186/s13756-020-00835-1
3. Hill, B, Lamichhane, G, and Wamburu, A. Infection prevention and control: critical strategies for nursing practice. Br J Nurs. (2024) 33:804–11. doi: 10.12968/bjon.2024.0286
4. Barrera-Cancedda, AE, Riman, KA, Shinnick, JE, and Buttenheim, AM. Implementation strategies for infection prevention and control promotion for nurses in sub-Saharan Africa: A systematic review. Impl Sci. BMC. (2019) 14:111. doi: 10.1186/s13012-019-0958-3
5. Abubakar, U, Amir, O, and Rodríguez-Baño, J. Healthcare-associated infections in Africa: a systematic review and meta-analysis of point prevalence studies. J Pharm Policy Pract. (2022) 15:500. doi: 10.1186/s40545-022-00500-5
6. Sievert, DM, Ricks, P, Edwards, JR, Schneider, A, Patel, J, Srinivasan, A, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. (2013) 34:1–14. doi: 10.1086/668770
7. Sartorius, B, Gray, AP, Davis Weaver, N, Robles Aguilar, G, Swetschinski, LR, Ikuta, KS, et al. The burden of bacterial antimicrobial resistance in the WHO African region in 2019: a cross-country systematic analysis. Lancet Glob Heal. (2024) 12:e201–16. doi: 10.1016/s2214-109x(23)00539-9
8. Alp, E, and Damani, N. Healthcare-associated infections in intensive care units: epidemiology and infection control in low-to-middle income countries. Journal of Infection in Developing Countries J Infect Dev Ctries. (2015) 9:1040–5. doi: 10.3855/jidc.6832
9. Sartelli, M, Barie, PS, Coccolini, F, Abbas, M, Abbo, LM, Abdukhalilova, GK, et al. Ten golden rules for optimal antibiotic use in hospital settings: the WARNING call to action. World J Emerg Surg. (2023) 18:50. doi: 10.1186/s13017-023-00518-3
10. Avortri, GS, and Nabyonga-Orem, J. The global call for action on infection prevention and control: implication for low income countries. Int J Health Care Qual Assur. (2019) 32:927–40. doi: 10.1108/IJHCQA-03-2018-0063
11. Kinyenje, E, Hokororo, J, Eliakimu, E, Yahya, T, Mbwele, B, Mohamed, M, et al. Status of infection prevention and control in Tanzanian primary health care facilities: learning from star rating assessment. Infect Prev Pract. (2020) 2:100071. doi: 10.1016/j.infpip.2020.100071
12. Oppong, TB, Amponsem-Boateng, C, Kyere, EKD, Wang, Y, Gheisari, Z, Oppong, EE, et al. Infection prevention and control preparedness level and associated determinants in 56 acute healthcare facilities in Ghana. Infect Drug Resist. (2020) 13:4263–71. doi: 10.2147/IDR.S273851
13. Nejad, SB, Allegranzi, B, Syed, SB, Ellisc, B, and Pittetd, D. Health-care-associated infection in Africa: a systematic review. Bull World Health Organ. (2011) 89:757–65. doi: 10.2471/BLT.11.088179
14. Bunduki, GK, Masoamphambe, E, Fox, T, Musaya, J, Musicha, P, and Feasey, N. Prevalence, risk factors, and antimicrobial resistance of endemic healthcare-associated infections in Africa: a systematic review and meta-analysis. BMC Infect Dis. (2024) 24. doi: 10.1186/s12879-024-09038-0
15. Abban, MK, Ayerakwa, EA, Mosi, L, and Isawumi, A. The burden of hospital acquired infections and antimicrobial resistance. Heliyon. (2023) 9:e20561. doi: 10.1016/j.heliyon.2023.e20561
16. Machado, E, Costa, P, and Carvalho, A. Occurrence of healthcare-associated infections (HAIs) by Escherichia coli and Klebsiella spp. producing extended-spectrum β-lactamases (ESBL) and/or carbapenemases in Portuguese long-term care facilities. Pathogens. (2022) 11:1019. doi: 10.3390/pathogens11091019
17. Mudenda, S, Hakayuwa, CM, Lubanga, AF, Kasanga, M, Daka, V, Salachi, KI, et al. Global antimicrobial stewardship, surveillance, and infection prevention and control programs: leveraging one health, nanotechnology, and artificial intelligence to combat antimicrobial resistance in a climate-impacted world. Pharmacol Pharm. (2025) 16:197–291. doi: 10.4236/PP.2025.167014
18. Haque, M, Sartelli, M, McKimm, J, and Bakar, MA. Health care-associated infections – An overview. Infect Drug Resist. (2018) 11:2321–33. doi: 10.2147/IDR.S177247
19. Centers for Disease Control. About healthcare-associated infections (HAIs). (2025). Available online at: https://www.cdc.gov/healthcare-associated-infections/about/index.html (Accessed April 15, 2025).
20. Teus, JK, Mithen, L, Green, H, Hutton, A, and Fernandez, R. Impact of infection prevention and control practices, including personal protective equipment, on the prevalence of hospital-acquired infections in acute care hospitals during COVID-19: a systematic review and meta-analysis. J Hosp Infect. (2024) 147:32–9. doi: 10.1016/j.jhin.2024.02.010
21. Hu, Y, Li, D, Xu, L, Hu, Y, Sang, Y, Zhang, G, et al. Epidemiology and outcomes of bloodstream infections in severe burn patients: a six-year retrospective study. Antimicrob Resist Infect Control. (2021) 10:969. doi: 10.1186/s13756-021-00969-w
22. Ketata, N, Ben Ayed, H, Ben Hmida, M, Trigui, M, Ben Jemaa, M, Yaich, S, et al. Point prevalence survey of health-care associated infections and their risk factors in the tertiary-care referral hospitals of southern Tunisia. Infect Dis Heal. (2021) 26:284–91. doi: 10.1016/j.idh.2021.06.004
23. Magill, SS, Edwards, JR, Bamberg, W, Beldavs, ZG, Dumyati, G, Kainer, MA, et al. Multistate point-prevalence survey of health care–associated infections. N Engl J Med. (2014) 370:1198–208. doi: 10.1056/NEJMoa1306801
24. Al-Tawfiq, JA, and Tambyah, PA. Healthcare associated infections (HAI) perspectives. J Infect Public Health. (2014) 7:339–44. doi: 10.1016/j.jiph.2014.04.003
25. Vincent, JL, Rello, J, Marshall, J, Silva, E, Anzueto, A, Martin, CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. (2009) 302:2323–9. doi: 10.1001/jama.2009.1754
26. Ahmed, SK, Hussein, S, Qurbani, K, Ibrahim, RH, Fareeq, A, Mahmood, KA, et al. Antimicrobial resistance: impacts, challenges, and future prospects. J Med Surgery, Public Heal. (2024) 2:100081. doi: 10.1016/j.glmedi.2024.100081
27. Gidey, K, Gidey, MT, Hailu, BY, Gebreamlak, ZB, and Niriayo, YL. Clinical and economic burden of healthcare-associated infections: A prospective cohort study. PLoS One. (2023) 18:e0282141. doi: 10.1371/journal.pone.0282141
28. Lv, Y, Huang, X, Wu, J, Xiao, X, Ma, C, Jiang, X, et al. Economic burden attributable to healthcare-associated infections at western China hospitals: 6 year, prospective cohort study. J Infect. (2024) 88:112–22. doi: 10.1016/j.jinf.2023.12.008
29. Nelson, RE, Schweizer, M, Jones, M, Stevens, VW, Khader, K, Perencevich, E, et al. The cost and mortality burden of hospital-onset antimicrobial-resistant healthcare-associated infections in the USA. Open Forum Infect Dis. (2017) 4:S177–8. doi: 10.1093/ofid/ofx163.323
30. Nelson, RE, Hatfield, KM, Wolford, H, Samore, MH, Scott, RD, Reddy, SC, et al. National estimates of healthcare costs associated with multidrug-resistant bacterial infections among hospitalized patients in the United States. Clin Infect Dis. (2021) 72:S17–26. doi: 10.1093/cid/ciaa1581
31. Magill, SS, O’Leary, E, Janelle, SJ, Thompson, DL, Dumyati, G, Nadle, J, et al. Changes in prevalence of health care–associated infections in U.S. hospitals. N Engl J Med. (2018) 379:1732–44. doi: 10.1056/NEJMoa1801550
32. Salam, MA, Al-Amin, MY, Salam, MT, Pawar, JS, Akhter, N, Rabaan, AA, et al. Antimicrobial resistance: A growing serious threat for global public health. Healthcare. (2023) 11:1946. doi: 10.3390/healthcare11131946
33. Ikuta, KS, Swetschinski, LR, Robles Aguilar, G, Sharara, F, Mestrovic, T, Gray, AP, et al. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2022) 400:2221–48. doi: 10.1016/S0140-6736(22)02185-7
34. Murray, CJ, Ikuta, KS, Sharara, F, Swetschinski, L, Robles Aguilar, G, Gray, A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. (2022) 399:629–55. doi: 10.1016/S0140-6736(21)02724-0
35. Naghavi, M, Vollset, ES, Ikuta, KS, Swetschinski, LR, Gray, AP, Wool, EE, et al. Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with forecasts to 2050. Lancet. (2024) 404:1199–226. doi: 10.1016/S0140-6736(24)01867-1
36. Prestinaci, F, Pezzotti, P, and Pantosti, A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. (2015) 109:309–18. doi: 10.1179/2047773215Y.0000000030
37. Shahida, SM, Islam, A, Dey, BR, Islam, F, Venkatesh, K, and Goodman, A. Hospital acquired infections in low and middle income countries: root cause analysis and the development of infection control practices in Bangladesh. Open J Obstet Gynecol. (2016) 6:28–39. doi: 10.4236/ojog.2016.61004
38. Raoofi, S, Kan, FP, Rafiei, S, Hosseinipalangi, Z, Mejareh, ZN, Khani, S, et al. Global prevalence of nosocomial infection: a systematic review and meta-analysis. PLoS One. (2023) 18:e0274248. doi: 10.1371/journal.pone.0274248
39. Fraser, JL, Mwatondo, A, Alimi, YH, Varma, JK, and Vilas, VJDR. Healthcare-associated outbreaks of bacterial infections in Africa, 2009–2018: A review. Int J Infect Dis. (2021) 103:469–77. doi: 10.1016/j.ijid.2020.12.030
40. Hutton, G, Chase, C, Kennedy-Walker, R, and Hamilton, H. Financial and economic costs of healthcare-associated infections in Africa. J Hosp Infect. (2024) 150:1–8. doi: 10.1016/j.jhin.2024.04.015
41. Irek, EO, Amupitan, AA, Obadare, TO, and Aboderin, AO. A systematic review of healthcare-associated infections in Africa: an antimicrobial resistance perspective. Afr J Lab Med. (2018) 7:796. doi: 10.4102/ajlm.v7i2.796
42. Ogunsola, FT, and Mehtar, S. Challenges regarding the control of environmental sources of contamination in healthcare settings in low-and middle-income countries - a narrative review. Antimicrob Resist Infect Control. (2020) 9:81. doi: 10.1186/s13756-020-00747-0
43. World Health Organization & United Nations Children’s Fund (UNICEF). Progress on WASH in health care facilities 2000–2021: special focus on WASH and infection prevention and control (IPC). Geneva: WHO (2022). Available at: https://iris.who.int/handle/10665/366657 (Accessed March 14, 2025).
44. World Health Organization. WASH in health care facilities. Geneva: WHO (2024). Available at: https://www.who.int/publications/m/item/wash-in-health-care-facilities-2023-data-update (Accessed April 15, 2025).
45. Tseole, NP, Mindu, T, Kalinda, C, and Chimbari, MJ. Barriers and facilitators to water, sanitation and hygiene (WaSH) practices in southern Africa: A scoping review. PLoS ONE. (2022) 17:e0271726. doi: 10.1371/journal.pone.0271726
46. Godman, B, Egwuenu, A, Wesangula, E, Schellack, N, Kalungia, AC, Tiroyakgosi, C, et al. Tackling antimicrobial resistance across sub-Saharan Africa: current challenges and implications for the future. Expert Opin Drug Saf. (2022) 21:1089–111. doi: 10.1080/14740338.2022.2106368
47. Haque, M, McKimm, J, Sartelli, M, Dhingra, S, Labricciosa, FM, Islam, S, et al. Strategies to prevent healthcare-associated infections: A narrative overview. Risk Manag Healthc Policy. (2020) 13:1765–80. doi: 10.2147/RMHP.S269315
48. Gamalathge, PU, Kularatna, S, Carter, HE, Senanayake, S, and Graves, N. Cost-effectiveness of interventions to reduce the risk of healthcare-acquired infections in middle-income countries: A systematic review. J Infect Prev. (2019) 20:266–73. doi: 10.1177/1757177419852662
49. Godman, B, Egwuenu, A, Haque, M, Malande, OO, Schellack, N, Kumar, S, et al. Strategies to improve antimicrobial utilization with a special focus on developing countries. Life. (2021) 11:528. doi: 10.3390/life11060528
50. Mudenda, S, Chabalenge, B, Daka, V, Mfune, RL, Salachi, KI, Mohamed, S, et al. Global strategies to combat antimicrobial resistance: a one health perspective. Pharmacol Pharm. (2023) 14:271–328. doi: 10.4236/PP.2023.148020
51. Harun, MGD, Anwar, MMU, Sumon, SA, Hassan, MZ, Mohona, TM, Rahman, A, et al. Rationale and guidance for strengthening infection prevention and control measures and antimicrobial stewardship programs in Bangladesh: a study protocol. BMC Health Serv Res. (2022) 22:8603. doi: 10.1186/s12913-022-08603-0
52. Gahamanyi, N, Umuhoza, T, Saeed, SI, Mayigane, LN, and Hakizimana, JN. A review of the important weapons against antimicrobial resistance in sub-Saharan Africa. Appl Biosci. (2023) 2:136–56. doi: 10.3390/applbiosci2020011
53. Oliveira, M, Antunes, W, Mota, S, Madureira-Carvalho, Á, Dinis-Oliveira, RJ, and Dias da Silva, D. An overview of the recent advances in antimicrobial resistance. Microorganisms. (2024) 12:1920. doi: 10.3390/microorganisms12091920
54. Woolhouse, MEJ. One health approaches to tackling antimicrobial resistance. Sci One Heal. (2024) 3:100082. doi: 10.1016/j.soh.2024.100082
55. Walsh, TR, Gales, AC, Laxminarayan, R, and Dodd, PC. Antimicrobial resistance: Addressing a global threat to humanity. PLoS Med. Public Library Sci. (2023) 20:e1004264. doi: 10.1371/journal.pmed.1004264
56. Storr, J, Twyman, A, Zingg, W, Damani, N, Kilpatrick, C, Reilly, J, et al. Core components for effective infection prevention and control programmes: new WHO evidence-based recommendations. Antimicrob resist. Infect Control. (2017) 6:6. doi: 10.1186/s13756-016-0149-9
57. World Health Organization. Infection prevention and control assessment framework at the facility level. Geneva: WHO (2018). Available at: https://www.who.int/publications/i/item/WHO-HIS-SDS-2018.9 (Accessed August 7, 2024).
58. Tomczyk, S, Twyman, A, de Kraker, MEA, Coutinho Rehse, AP, Tartari, E, Toledo, JP, et al. The first WHO global survey on infection prevention and control in health-care facilities. Lancet Infect Dis. (2022) 22:845–56. doi: 10.1016/S1473-3099(21)00809-4
59. Tomczyk, S, Storr, J, Kilpatrick, C, and Allegranzi, B. Infection prevention and control (IPC) implementation in low-resource settings: a qualitative analysis. Antimicrob Resist. Infect Control. (2021) 10:113. doi: 10.1186/s13756-021-00962-3
60. Aghdassi, SJS, Aghdassi, SJS, Grisold, A, Grisold, A, Wechsler-Fördös, A, Hansen, S, et al. Evaluating infection prevention and control programs in Austrian acute care hospitals using the WHO infection prevention and control assessment framework. Antimicrob Resist Infect Control. (2020) 9:92. doi: 10.1186/s13756-020-00761-2
61. Lu, Q, Sun, L, Wang, W, Li, Z, Wu, F, and Ni, K. Assessment of IPCAF scores and incidence of health care-associated infections: A cross-sectional study in eastern China. Am J Infect Control. (2025) 53:527–9. doi: 10.1016/j.ajic.2024.12.015
62. Ngambi, D, Jingini, E, Chadwala, H, Musopole, O, Kamchedzera, W, et al. An assessment of infection prevention and control implementation in Malawian hospitals using the WHO infection prevention and control assessment framework (IPCAF) tool. Infect Prev Pract. (2024) 6:100388. doi: 10.1016/j.infpip.2024.100388
63. Asgedom, AA. Status of infection prevention and control (IPC) as per the WHO standardised infection prevention and control assessment framework (IPCAF) tool: existing evidence and its implication. Infect Prev Pract. (2024) 6:100351. doi: 10.1016/j.infpip.2024.100351
64. Sengupta, S, Barman, P, and Lo, J. Opportunities to overcome implementation challenges of infection prevention and control in low-middle income countries. Curr Treat Options Infect Dis. (2019) 11:267–80. doi: 10.1007/s40506-019-00200-w
65. Lacotte, Y, Årdal, C, and Ploy, MC. Infection prevention and control research priorities: what do we need to combat healthcare-associated infections and antimicrobial resistance? Results of a narrative literature review and survey analysis. Antimicrob Resist Infect Control. (2020) 9:142. doi: 10.1186/s13756-020-00801-x
66. World Health Organization. Infection prevention and control. IPC and antimicrobial resistance (AMR). Geneva: WHO (2025). Available at: https://www.who.int/teams/integrated-health-services/infection-prevention-control/ipc-and-antimicrobial-resistance (Accessed April 13, 2025).
67. Hearn, P, Miliya, T, Seng, S, Ngoun, C, Day, NPJ, Lubell, Y, et al. Prospective surveillance of healthcare associated infections in a Cambodian pediatric hospital. Antimicrob Resist Infect Control. (2017) 6:16. doi: 10.1186/s13756-017-0172-5
68. Wood, R, Tembele, W, Hema, A, Somé, A, Kinganda-Lusamaki, E, Basilubo, C, et al. Implementation of the WHO core components of an infection prevention and control programme in two sub-Saharan African acute health-care facilities: a mixed methods study. Antimicrob Resist Infect Control. (2024) 13:1358. doi: 10.1186/s13756-023-01358-1
69. Allegranzi, B, Aiken, AM, Zeynep Kubilay, N, Nthumba, P, Barasa, J, Okumu, G, et al. A multimodal infection control and patient safety intervention to reduce surgical site infections in Africa: a multicentre, before–after, cohort study. Lancet Infect Dis. (2018) 18:507–15. doi: 10.1016/S1473-3099(18)30107-5
70. Sonpar, A, Hundal, CO, Totté, JEE, Wang, J, Klein, SD, Twyman, A, et al. Multimodal strategies for the implementation of infection prevention and control interventions—update of a systematic review for the WHO guidelines on core components of infection prevention and control programmes at the facility level. Clin Microbiol Infect. (2025) 31:948–57. doi: 10.1016/j.cmi.2025.01.011
71. Aiken, LH, Sloane, DM, Bruyneel, L, Van Den Heede, K, Griffiths, P, Busse, R, et al. Nurse staffing and education and hospital mortality in nine European countries: A retrospective observational study. Lancet. (2014) 383:1824–30. doi: 10.1016/S0140-6736(13)62631-8
72. Sehulster, L, and Chinn, RYW. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the healthcare infection control practices advisory committee (HICPAC). MMWR Recomm Reports. (2003) 52:1–42. Available at: https://pubmed.ncbi.nlm.nih.gov/12836624/ (Accessed July 31, 2025).
73. Kasanga, M, Gajdács, M, Muleya, W, Ikhimiukor, OO, Mudenda, S, Kasanga, M, et al. Genotypic characterisation and antimicrobial resistance of extended-spectrum β-lactamase-producing Escherichia coli in humans, animals, and the environment from Lusaka, Zambia: public health implications and one health surveillance. Antibiotics. (2024) 13:951. doi: 10.3390/ANTIBIOTICS13100951
74. Siame, A, Yamba, K, Samutela, M, Mukubesa, A, and Mulundu, G. Carriage and antimicrobial susceptibility patterns of rectal ESBL E. coli in surgical patients at the university teaching hospitals in Lusaka, Zambia. JAC-Antimicrobial Resist. (2024) 6:159. doi: 10.1093/jacamr/dlae159
75. Kasanga, M, Kwenda, G, Wu, J, Kasanga, M, Mwikisa, MJ, Chanda, R, et al. Antimicrobial resistance patterns and risk factors associated with ESBL-producing and MDR Escherichia coli in hospital and environmental settings in Lusaka, Zambia: implications for one health, antimicrobial stewardship and surveillance systems. Microorganisms. (2023) 11:1951. doi: 10.3390/MICROORGANISMS11081951
76. Kasanga, M, Shempela, DM, Daka, V, Mwikisa, MJ, Sikalima, J, Chanda, D, et al. Antimicrobial resistance profiles of Escherichia coli isolated from clinical and environmental samples: findings and implications. JAC-Antimicrobial Resist. (2024) 6:dlae061. doi: 10.1093/jacamr/dlae061
77. Bumbangi, FN, Llarena, A-K, Skjerve, E, Hang’ombe, BM, Mpundu, P, Mudenda, S, et al. Evidence of community-wide spread of multi-drug resistant Escherichia coli in young children in Lusaka and Ndola districts, Zambia. Microorganisms. (2022) 10:1684. doi: 10.3390/microorganisms10081684
78. Chanda, W, Manyepa, M, Chikwanda, E, Daka, V, Chileshe, J, Tembo, M, et al. Evaluation of antibiotic susceptibility patterns of pathogens isolated from routine laboratory specimens at Ndola teaching hospital: A retrospective study. PLoS One. (2019) 14:e0226676. doi: 10.1371/journal.pone.0226676
79. Yamba, K, Lukwesa-Musyani, C, Samutela, MT, Kapesa, C, Hang’ombe, MB, Mpabalwani, E, et al. Phenotypic and genotypic antibiotic susceptibility profiles of gram-negative bacteria isolated from bloodstream infections at a referral hospital, Lusaka, Zambia. PLoS Glob Public Health. (2023) 3:e0001414. doi: 10.1371/journal.pgph.0001414
80. Mudenda, S, Mufwambi, W, and Mohamed, S. The burden of antimicrobial resistance in Zambia, a sub-Saharan African country: a one health review of the current situation, risk factors, and solutions. Pharmacol Pharm. (2024) 15:403–65. doi: 10.4236/PP.2024.1512024
81. Yamba, K, Chizimu, JY, Chanda, R, Mpundu, M, Samutela, MT, Chanda, D, et al. Antibiotic resistance profiles in gram-negative Bacteria causing bloodstream and urinary tract infections in Paediatric and adult patients in Ndola District, Zambia, 2020-2021. Infect Prev Pract. (2025) 7:100462. doi: 10.1016/j.infpip.2025.100462
82. Mudenda, S, Chizimu, J, Chabalenge, B, Kasanga, M, Matafwali, SK, Daka, V, et al. Knowledge, attitude, and practices toward infection prevention and control among undergraduate pharmacy students in Zambia: findings and implications. Antimicrob Steward Healthc Epidemiol. (2023) 3:e154. doi: 10.1017/ash.2023.428
83. Mukwato, K, Ngoma, C, and Maimbolwa, M. Compliance with infection prevention guidelines by health Care Workers at Ronald Ross General Hospital Mufulira District. Med J Zambia. (2009) 35:110–6. doi: 10.4314/mjz.v35i3.46530
84. Zambia National Public Health Institute. Multi-sectoral National Action Plan on antimicrobial resistance. Lusaka: Zambia National Public Health Institute (2017). (Accessed 24 July, 2022).
85. Kalungia, AC, Mukosha, M, Mwila, C, Banda, D, Mwale, M, Kagulura, S, et al. Antibiotic use and stewardship indicators in the first- and second-level hospitals in Zambia: findings and implications for the future. Antibiotics. (2022) 11:11626. doi: 10.3390/antibiotics11111626
86. Mudenda, S, Chilimboyi, R, Matafwali, SK, Daka, V, Lindizyani Mfune, R, Arielle, L, et al. Hospital prescribing patterns of antibiotics in Zambia using the WHO prescribing indicators post-COVID-19 pandemic: findings and implications. JAC-Antimicrobial Resist. (2024) 6:dlae023. doi: 10.1093/jacamr/dlae023
87. Mudenda, S, Chomba, M, Chabalenge, B, Hikaambo, CN, Banda, M, Daka, V, et al. Antibiotic prescribing patterns in adult patients according to the WHO aware classification: a multi-facility cross-sectional study in primary healthcare hospitals in Lusaka, Zambia. Pharmacol Pharm. (2022) 13:379–92. doi: 10.4236/PP.2022.1310029
88. Mudenda, S, Lubanga, AF, Jamshed, S, Biemba, B, Sakala, R, Chiyabi, M, et al. Point prevalence survey of antibiotic use in level 1 hospitals in Zambia: future prospects for antimicrobial stewardship programs. Infect Drug Resist. (2025) 18:887–902. doi: 10.2147/IDR.S509522
89. Chizimu, JY, Mudenda, S, Yamba, K, Lukwesa, C, Chanda, R, Nakazwe, R, et al. Antibiotic use and adherence to the WHO AWaRe guidelines across 16 hospitals in Zambia: a point prevalence survey. JAC-Antimicrobial Resistance. (2024) 6:dlae170. doi: 10.1093/jacamr/dlae170
90. Kalonga, J, Hangoma, J, Banda, M, Munkombwe, D, and Mudenda, S. Antibiotic prescribing patterns in Paediatric patients at levy Mwanawasa university teaching Hospital in Lusaka. Zambia Int J Pharm Pharmacol. (2020) 4:1–9. doi: 10.31531/2581-3080.1000138
91. Masich, AM, Vega, AD, Callahan, P, Herbert, A, Fwoloshi, S, Zulu, PM, et al. Antimicrobial usage at a large teaching hospital in Lusaka, Zambia. PLoS One. (2020) 15:e0228555. doi: 10.1371/journal.pone.0228555
92. Shawa, M, Paudel, A, Chambaro, H, Kamboyi, H, Nakazwe, R, Alutuli, L, et al. Trends, patterns and relationship of antimicrobial use and resistance in bacterial isolates tested between 2015–2020 in a national referral hospital of Zambia. PLoS One. (2024) 19:e0302053. doi: 10.1371/journal.pone.0302053
93. Mudenda, S, Simbaya, R, Moonga, G, Mwaba, F, Zulu, M, Tembo, R, et al. Surveillance of antibiotic use and adherence to the WHO/INRUD core prescribing indicators at a primary healthcare hospital in southern Zambia: opportunities for antimicrobial stewardship programs. Pharmacol Pharm. (2025) 16:1–19. doi: 10.4236/PP.2025.161001
94. Kalungia, AC, Kampamba, M, Banda, D, Bambala, AM, Marshall, S, Newport, M, et al. Impact of a hub-and-spoke approach to hospital antimicrobial stewardship programmes on antibiotic use in Zambia. JAC-Antimicrobial Resistance. (2024) 6:dlae178. doi: 10.1093/jacamr/dlae178
95. Chizimu, JY, Mudenda, S, Yamba, K, Lukwesa, C, Chanda, R, Nakazwe, R, et al. Antimicrobial stewardship situation analysis in selected hospitals in Zambia: findings and implications from a national survey. Front Public Health. (2024) 12:1367703. doi: 10.3389/fpubh.2024.1367703/full
96. Kasujja, H, Waswa, JP, Kiggundu, R, Murungi, M, Kwikiriza, G, Bahatungire, R, et al. Enhancing infection prevention and control through hand hygiene compliance in six Ugandan hospitals using quality improvement approaches. Front Public Health. (2024) 12:1465439. doi: 10.3389/fpubh.2024.1465439/full
97. Irakiza, JJ, Mazimpaka, C, Ndatimana, D, Kalach, JB, Hatangimbabazi, V, Kamuhangire, E, et al. Status of infection prevention and control programs in 25 facilities of Rwanda: results from the WHO infection prevention and control assessment framework. Public heal. Challenges. (2024) 3:e183. doi: 10.1002/puh2.183
98. Cissé, DM, Laure, EEM, Blaise, KA, Jean Paul, NN, Gbonon, MV, Mayaka, CRA, et al. Evaluation of the implementation of hospital hygiene components in 30 health-care facilities in the autonomous district of Abidjan (cote d’Ivoire) with the WHO infection prevention and control assessment framework (IPCAF). BMC Health Serv Res. (2023) 23:870. doi: 10.1186/s12913-023-09853-2
99. Asghar, S, Atif, M, Masood, I, and Khan, M. A comprehensive review of current status of infection prevention and control program in low- and middle-income countries. Infect Dis Heal. (2025) 30:260–83. doi: 10.1016/J.IDH.2025.03.005
100. Opollo, MS, Otim, TC, Kizito, W, Thekkur, P, Kumar, AMV, Kitutu, FE, et al. Infection prevention and control at lira university hospital, Uganda: more needs to be done. Trop Med Infect Dis. (2021) 6:69. doi: 10.3390/tropicalmed6020069
101. Katoch, O, Katyal, S, Srivastav, S, Rodrigues, C, Rupali, P, Chakrabarti, A, et al. Self-reported survey on infection prevention and control structures in healthcare facilities part of a national level healthcare associated infection surveillance network in India, 2019. Am J Infect Control. (2022) 50:390–5. doi: 10.1016/j.ajic.2021.09.019
102. Azak, E, Sertcelik, A, Ersoz, G, Celebi, G, Eser, F, Batirel, A, et al. Evaluation of the implementation of WHO infection prevention and control core components in Turkish health care facilities: results from a WHO infection prevention and control assessment framework (IPCAF)—based survey. Antimicrob Resist Infect Control. (2023) 12:11. doi: 10.1186/s13756-023-01208-0
103. Nomoto, H, Saito, H, Ishikane, M, Gu, Y, Ohmagari, N, Pittet, D, et al. First nationwide survey of infection prevention and control among healthcare facilities in Japan: impact of the national regulatory system. Antimicrob Resist. Infect Control. (2022) 11:1175. doi: 10.1186/s13756-022-01175-y
104. Ni, K, Jin, D, Wu, Z, Sun, L, and Lu, Q. The status of infection prevention and control structures in eastern China based on the IPCAF tool of the World Health Organization. Antimicrob Resist Infect Control. (2022) 11:46. doi: 10.1186/s13756-022-01087-x
105. Aghdassi, SJS, Hansen, S, Bischoff, P, Behnke, M, and Gastmeier, P. A national survey on the implementation of key infection prevention and control structures in German hospitals: results from 736 hospitals conducting the WHO infection prevention and control assessment framework (IPCAF). Antimicrob Resist Infect Control. (2019) 8:73. doi: 10.1186/s13756-019-0532-4
106. Supriadi, IR, Haanappel, CP, Saptawati, L, Widodo, NH, Sitohang, G, Usman, Y, et al. Infection prevention and control in Indonesian hospitals: identification of strengths, gaps, and challenges. Antimicrob Resist. Infect Control. (2023) 12:1211. doi: 10.1186/s13756-023-01211-5
107. Fabre, V, Secaira, C, Herzig, C, Bancroft, E, Bernachea, MP, Galarza, LA, et al. Contextual barriers to infection prevention and control program implementation in hospitals in Latin America: a mixed methods evaluation. Antimicrob Resist Infect Control. (2024) 13:132. doi: 10.1186/s13756-024-01484-4
108. Sastry, S, Masroor, N, Bearman, G, Hajjeh, R, Holmes, A, Memish, Z, et al. The 17th international congress on infectious diseases workshop on developing infection prevention and control resources for low- and middle-income countries. Int. J. Inf. Dis. (2017) 57:138–43. doi: 10.1016/j.ijid.2017.01.040
109. Tartari, E, Tomczyk, S, Pires, D, Zayed, B, Coutinho Rehse, AP, Kariyo, P, et al. Implementation of the infection prevention and control core components at the national level: a global situational analysis. J Hosp Infect. (2021) 108:94–103. doi: 10.1016/j.jhin.2020.11.025
110. Rüther, FD, Gropmann, A, Hansen, S, Behnke, M, Geffers, C, and Aghdassi, SJS. Assessing infection prevention and control structures in German hospitals after the COVID-19 pandemic using the WHO infection prevention and control assessment framework (IPCAF): results from 660 hospitals and comparison with a pre-pandemic survey. Antimicrob Resist Infect Control. (2024) 13:103. doi: 10.1186/s13756-024-01465-7
111. Leong, M, Picton, R, Wratten, M, Mahe, A, and Zimmerman, PA. Baseline evaluation of the World Health Organization (WHO) infection prevention and control (IPC) core components in Pacific Island countries and territories (PICTs). Antimicrob Resist Infect Control. (2024) 13:108. doi: 10.1186/s13756-024-01447-9
112. Abbas, S. The challenges of implementing infection prevention and antimicrobial stewardship programs in resource-constrained settings. Antimicrob Steward Healthc Epidemiol. (2024) 4:e45. doi: 10.1017/ash.2024.35
113. Moghnieh, R, Al-Maani, AS, Berro, J, Ibrahim, N, Attieh, R, Abdallah, D, et al. Mapping of infection prevention and control education and training in some countries of the World Health Organization’s eastern Mediterranean region: current situation and future needs. Antimicrob Resist Infect Control. (2023) 12:1299. doi: 10.1186/s13756-023-01299-9
114. Tartari, E, Tomczyk, S, Twyman, A, Rehse, APC, Gomaa, M, Talaat, M, et al. Evaluating national infection prevention and control minimum requirements: evidence from global cross-sectional surveys, 2017–22. Lancet Glob Heal. (2024) 12:e1620–8. doi: 10.1016/S2214-109X(24)00277-8
115. Kabego, L, Balde, T, Barasa, D, Ndoye, B, Hilde, OB, Makamure, T, et al. Analysing the implementation of infection prevention and control measures in health care facilities during the COVID-19 pandemic in the African region. BMC Infect Dis. (2023) 23:824. doi: 10.1186/s12879-023-08830-8
116. Lowe, H, Woodd, S, Lange, IL, Janjanin, S, Barnett, J, and Graham, W. Challenges and opportunities for infection prevention and control in hospitals in conflict-affected settings: a qualitative study. Confl Heal. (2021) 15:94. doi: 10.1186/s13031-021-00428-8
117. Büchler, AC, Haddad Galas, M, Buetti, N, Alp, E, Apisarnthanarak, A, Dziekan, G, et al. Challenges and success stories of the implementation of infection control and antimicrobial stewardship strategies: proceedings of the 5th global ministerial summit on patient safety, 2023. Antimicrob Resist Infect Control. (2024) 13:16. doi: 10.1186/s13756-023-01344-7
118. Madran, B, Demir, ZI, Yalcin, B, Ayaz, OT, Iyikosker, K, Keskin, A, et al. Reasons for insufficient compliance with infection prevention and control measures in the intensive care unit: a qualitative study conducted in Türkiye in 2024. Antimicrob Resist Infect Control. (2025) 14:71. doi: 10.1186/s13756-025-01544-3
119. Falana, ROA, Ogidan, OC, and Fajemilehin, BR. Barriers to infection prevention and control implementation in selected healthcare facilities in Nigeria. Infect Dis Now. (2024) 54:104877. doi: 10.1016/j.idnow.2024.104877
Keywords: healthcare-associated infections, infection prevention and control, IPCAF, antimicrobial resistance, Zambia
Citation: Mudenda S, Chizimu JY, Ndegwa L, Kasanga M, Mutwale I, Kalungia AC, Wesangula E, Lubanga AF, Mwansa JCL, Mwaba M, Massele AY, Sinyange N, Mashe T, Mutila M, Simujayang’ombe P, Lowrance D, Yamba K, Shawa M, Nakajima C, Suzuki Y, Muma JB, Sartelli M, Godman B and Chilengi R (2025) Evaluating infection prevention and control programs in Zambian hospitals using the WHO infection prevention and control assessment framework tool. Front. Public Health. 13:1642119. doi: 10.3389/fpubh.2025.1642119
Edited by:
Mouloudj Kamel, University of Medea, AlgeriaReviewed by:
Dachel Martínez Asanza, National School of Public Health (ENSAP), CubaMariya Dimitrova, Plovdiv Medical University, Bulgaria
Latifa Merzougui, University of Sousse, Tunisia
Copyright © 2025 Mudenda, Chizimu, Ndegwa, Kasanga, Mutwale, Kalungia, Wesangula, Lubanga, Mwansa, Mwaba, Massele, Sinyange, Mashe, Mutila, Simujayang’ombe, Lowrance, Yamba, Shawa, Nakajima, Suzuki, Muma, Sartelli, Godman and Chilengi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steward Mudenda, c3Rld2FyZC5tdWRlbmRhQHVuemEuYWMuem0=
 Steward Mudenda
Steward Mudenda Joseph Yamweka Chizimu
Joseph Yamweka Chizimu Linus Ndegwa
Linus Ndegwa Maisa Kasanga
Maisa Kasanga Ilunga Mutwale1,6
Ilunga Mutwale1,6 Evelyn Wesangula
Evelyn Wesangula Adriano Focus Lubanga
Adriano Focus Lubanga James C. L. Mwansa
James C. L. Mwansa Nyambe Sinyange
Nyambe Sinyange Misheck Shawa
Misheck Shawa Yasuhiko Suzuki
Yasuhiko Suzuki Brian Godman
Brian Godman