- 1Luoyang Key Laboratory of Transplantation and Immunological Studies for Haematological Diseases, Department of Clinical Laboratory, The First Affiliated Hospital, and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China
- 2Department of Laboratory Medicine, First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 3Department of Medical Equipment, The First Affiliated Hospital, and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China
- 4Department of Rehabilitation, The Third People's Provincial Hospital of Henan Province, Zhengzhou, China
- 5Henan Key Laboratory of Rare Diseases, Endocrinology and Metabolism Center, The First Affiliated Hospital, and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China
- 6Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital, and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China
- 7Department of Intensive Care Unit (Internal Medicine), The First Affiliated Hospital, and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China
Background: Neonatal sepsis remains a major disease threatening the lives of newborns. With the escalating global air pollution, substantial evidence indicates that air pollution is among the primary environmental threats to children's health. However, its contribution to the global burden of neonatal sepsis and other neonatal infections remains unclear. Although existing studies have established associations between air pollution and adverse neonatal outcomes, a comprehensive evaluation differentiating pollution types and accounting for socio-economic disparities across geographic regions remains lacking. This study fills this critical evidence gap.
Methods: Based on data from the Global Burden of Disease (GBD) Study 2021, we analyzed associations between air pollution, particulate matter pollution, household air pollution from solid fuels, and ambient particulate matter pollution and neonatal sepsis and other neonatal infections, calculating deaths, disability-adjusted life years (DALYs), and their corresponding age-standardized rates (ASRs). Subsequently, cluster analysis and decomposition analysis were conducted to identify regional patterns and quantify contributing factors. Finally, an autoregressive integrated moving average (ARIMA) model was employed to forecast the disease burden from 2022 to 2050.
Result: In 2021, global deaths from neonatal sepsis and related infections attributable to air pollution numbered 54,026 (95% UI: 45,371–64,084), a 23.48% decrease from 1990, with age-standardized death rates dropping 1.49% annually (EAPC = −1.49). Deaths from household solid fuel pollution fell by 30.65%, while ambient particulate matter pollution caused a 13.05% increase to 13,080 deaths. Low-SDI regions bore the highest death burden with 31,063 cases, and Western Africa showed the highest age-standardized mortality rate of 2.21. African countries like Sierra Leone ranked top globally. Male deaths and DALYs consistently exceeded female figures. Population growth was the primary driver of global burden increase, contributing 621.99% to deaths, mitigated by epidemiological improvements. Projections indicate continuous declines in air/household pollution-related deaths 2022–2050, steeper in females, while ambient particulate matter deaths may peak in 2027 before easing.
Conclusion: Overall, air pollution remains a significant public health challenge threatening neonatal health. Implementing targeted, geographically tailored interventions is essential to reduce disease burden resulting from air pollution.
1 Introduction
Neonatal and under-five mortality rates are among the core indicators reflecting child health status, healthcare quality, and social development within countries and regions (1). Despite significant global progress in reducing child mortality over recent decades, the mortality rate among children under five remains alarmingly high. According to 2022 data, ~4.9 million children under the age of five died worldwide (2), with neonatal deaths (occurring within the first 28 days of life) accounting for 47% of this total (1), and this proportion continues to rise.
Neonatal infections, preterm births, and birth complications (including birth asphyxia and trauma) collectively contribute to nearly 90% of neonatal deaths (3, 4). Neonatal sepsis, defined as a systemic inflammatory response syndrome caused by infections (bacterial, viral, or fungal) during the neonatal period (typically within the first 28 days postpartum), is classified as either early-onset or late-onset based on a 72-h threshold post-birth (5). Neonatal sepsis affects ~3 million newborns annually, with a mortality rate as high as 17% (6), making it a significant cause of neonatal death and a considerable global health challenge. Neonatal infections account for ~26% of deaths among children under five (7). Additionally, neonatal sepsis incidence is notably higher in low- and middle-income countries compared to high-income nation (8), with studies identifying Sub-Saharan Africa as having one of the highest neonatal sepsis mortality rates globally (9, 10). Existing research data indicate that sub-Saharan Africa experiences an estimated 380,000 to 2,000,000 cases of neonatal sepsis and 270,000 associated deaths annually (11). Despite this, current prevention strategies exhibit substantial limitations.
Air pollution represents one of the most significant environmental risks to child health, encompassing particulate matter pollution, which can be further categorized into ambient particulate matter pollution and household air pollution from solid fuels (12). According to the Global Air Quality Report 2021, air pollution was associated with more than 700,000 deaths among children under 5 years old globally, making it the second leading global risk factor for mortality within this age group (13). Numerous studies have also demonstrated that exposure to PM2.5 during pregnancy is associated with adverse effects on neonatal health, leading to negative outcomes (14–16). Recently, a time stratified, case crossover analyses conducted in the United States found that short-term exposure to PM2.5 is associated with increased risk of hospital admissions due to septicaemia (17). Alarmingly, household air pollution due to cooking with polluting fuels accounted for ~500,000 of these deaths (18). Furthermore, research has linked exposure to ambient air pollution, household air pollution, and particulate matter with adverse birth outcomes such as low birth weight, preterm birth, and respiratory infection (19–21). Prenatal air pollution exposure has also been associated with an increased risk of neonatal sepsis (22). Recent studies further indicate a positive correlation between long-term air pollution exposure and sepsis-related mortality in older adults populations (23). However, the direct and indirect roles of these pollutants in neonatal sepsis and other neonatal infections have not been comprehensively examined on a global scale. As climate change and urbanization continue to exacerbate pollution levels, understanding the long-term trends in pollution-associated neonatal mortality is critical for developing effective public health interventions (24).
Therefore, we utilized the Global Burden of Disease (GBD) database to evaluate trends and burdens of neonatal sepsis and other neonatal infections attributable to air pollution, particulate matter pollution, household air pollution from solid fuels, and ambient particulate matter pollution from 1990 to 2021, and forecasted disease burdens from 2022 to 2050. This study aims to inform healthcare professionals and policymakers, facilitating the development of targeted public health strategies to reduce the substantial disease burden caused by air pollution.
2 Methods
2.1 Overview
This study utilized the latest data from the GBD Study 2021 database (25), analyzing the burden of neonatal sepsis and other neonatal infections (age < 5 years) attributable to air pollution, particulate matter pollution, household air pollution from solid fuels, and ambient particulate matter pollution at global, regional, and national levels. Developed by the Institute for Health Metrics and Evaluation (IHME) in the United States, the GBD database integrates data from 204 countries and territories, covering 371 diseases and injuries with indicators such as prevalence, incidence, deaths, disability-adjusted life years (DALYs), and age-standardized rates (ASRs), stratified by age, gender, geographic region, and socio-demographic index (SDI).
GBD classifies countries and regions into 21 regions and seven super-regions based on geographic location. SDI is a composite measure used to assess socio-economic development, typically encompassing factors such as income, education, and fertility rates. The SDI facilitates the analysis of regional health burdens and helps identify underlying causes of health inequalities (26). Based on SDI, countries and regions are categorized into five groups: high SDI, high-middle SDI, middle SDI, low-middle SDI, and low SDI.
2.2 Data acquisition and preliminary analysis
We extracted deaths, DALYs, and their corresponding ASRs for neonatal sepsis and other neonatal infections attributable to air pollution, particulate matter pollution, household air pollution from solid fuels, and ambient particulate matter pollution from the GBD database for the period 1990 to 2021. Disease burden was modeled and estimated by gender, region, and year using the Disease Modeling Meta-Regression (DisMod-MR) tool to ensure data consistency and accuracy. DisMod-MR is a Bayesian statistical modeling and meta-regression tool specifically used for estimating epidemiological parameters in burden-of-disease studies. Subsequently, bias adjustment was performed using MR-BRT.
2.3 Statistics
Our study reported global and subgroup-specific data (SDI, region, and country) on deaths, DALYs, and ASRs attributable to neonatal sepsis and other neonatal infections caused by air pollution, particulate matter pollution, household air pollution from solid fuels, and ambient particulate matter pollution in 2021. The estimated annual percentage change (EAPC) was utilized to evaluate temporal trends in disease burden globally and across various subgroups from 1990 to 2021. Furthermore, cluster analyses based on EAPC values were conducted to characterize patterns in disease burden changes across GBD regions and to identify regions exhibiting similar trends. To further investigate the underlying drivers of changes in DALYs and deaths related to neonatal sepsis and other neonatal infections between 1990 and 2021, decomposition analysis was performed, quantifying the contributions of population dynamics and epidemiological shifts (27). Additionally, autoregressive integrated moving average (ARIMA) models, a class of statistical models used for time series analysis and forecasting, were applied to predict gender-specific deaths and DALYs attributable to neonatal sepsis and other neonatal infections associated with air pollution, particulate matter pollution, household air pollution from solid fuels, and ambient particulate matter pollution from 2022 to 2050, thereby providing a scientific basis for future public health interventions. Finally, we conducted a stability analysis, selected the model combination with the smallest AIC/BIC values, and verified its applicability. The white noise test (α = 0.05) was used to evaluate the model residuals. The prediction performance was assessed using root mean square error (RMSE), mean absolute error (MAE), and mean absolute percentage error (MAPE).
3 Results
3.1 Global epidemiological patterns of air pollution-attributable neonatal sepsis (1990–2021)
In 2021, at the national level, deaths attributable to neonatal sepsis and other neonatal infections caused by air pollution totaled 54,026 (95% UI: 45,371–64,084), representing a decrease of 23.48% from 1990 to 2021. Correspondingly, the age-standardized deaths rate exhibited a declining trend, with an average annual reduction of 1.49% (EAPC = −1.49; 95% CI: −1.65to −1.34) during this period (Table 1, Supplementary Figure S1). Deaths due to neonatal sepsis and other neonatal infections associated with particulate matter pollution numbered 54,026 (95% UI: 45,371–64,084), also declining by 23.48% from 1990 to 2021, and the age-standardized deaths rate decreased annually by 1.49% (EAPC = −1.49; 95% CI: −1.65 to −1.34; Table 1, Supplementary Figure S4B). Deaths attributed to household air pollution from solid fuels accounted for 40,937 cases (95% UI: 32,377–51,168), reflecting a 30.65% reduction between 1990 and 2021, with an annual decline in the age-standardized deaths rate of 1.92% (EAPC = −1.92; 95% CI: −2.09 to −1.74; Table 1, Supplementary Figure S4C). Conversely, deaths due to neonatal sepsis and other neonatal infections associated with ambient particulate matter pollution increased to 13,080 (95% UI: 7,635–20,048), representing a 13.05% rise from 1990 to 2021, accompanied by an upward trend in the age-standardized deaths rate (EAPC = 0.15; 95% CI: −0.05 to 0.36; Table 1, Supplementary Figure S4D).

Table 1. Deaths of neonatal sepsis and other neonatal infections in 1990 and 2021 by different characteristics.
In 2021, DALYs attributable to neonatal sepsis and other neonatal infections caused by air pollution were 4,861,948 (95% UI: 4,083,310–5,767,176), with an age-standardized DALYs rate of 78.57 per 100,000 people (95% UI: 65.98–93.19). From 1990 to 2021, the number of DALYs decreased by 23.49%, while the age-standardized DALYs rate declined by 20.96%, with an EAPC of −1.50 (95% CI: −1.65 to −1.34; Table 2, Supplementary Figure S4A). DALYs due to particulate matter pollution were also 4,861,948 (95% UI: 4,083,310–5,767,176), with the same corresponding age-standardized DALYs rate and EAPC values as air pollution (Table 2, Supplementary Figure S4B). For household air pollution from solid fuels, DALYs totaled 3,683,938 (95% UI: 2,913,626–4,604,472), and the age-standardized DALYs rate was 59.53 per 100,000 people (95% UI: 47.08–74.41), decreasing by 30.66% for DALY numbers and 28.36% for the age-standardized DALYs rate between 1990 and 2021 (EAPC = −1.92; 95% CI: −2.09 to −1.74; Table 2, Supplementary Figure S4C). Conversely, DALYs attributable to ambient particulate matter pollution rose to 1,177,162 (95% UI: 687,185–1,804,264), representing an increase of 13.04%, and the age-standardized DALYs rate increased by 15.76% to 19.02 per 100,000 people (95% UI: 11.1–29.16; EAPC = 0.15; 95% CI: −0.05 to 0.36; Table 2, Supplementary Figure S4D).

Table 2. DALYs of neonatal sepsis and other neonatal infections in 1990 and 2021 by different characteristics.
3.2 Sex and socioeconomic disparities
In 2021, both deaths and DALYs attributable to neonatal sepsis and other neonatal infections caused by air pollution, particulate matter pollution, household air pollution from solid fuels, and ambient particulate matter pollution were consistently higher in males than females at the global level, with corresponding ASRs also higher in males (Tables 1, 2, Figure 1). Trends in deaths and DALYs from 1990 to 2021 were consistent across genders and aligned with the overall population trend (Supplementary Figure S2).
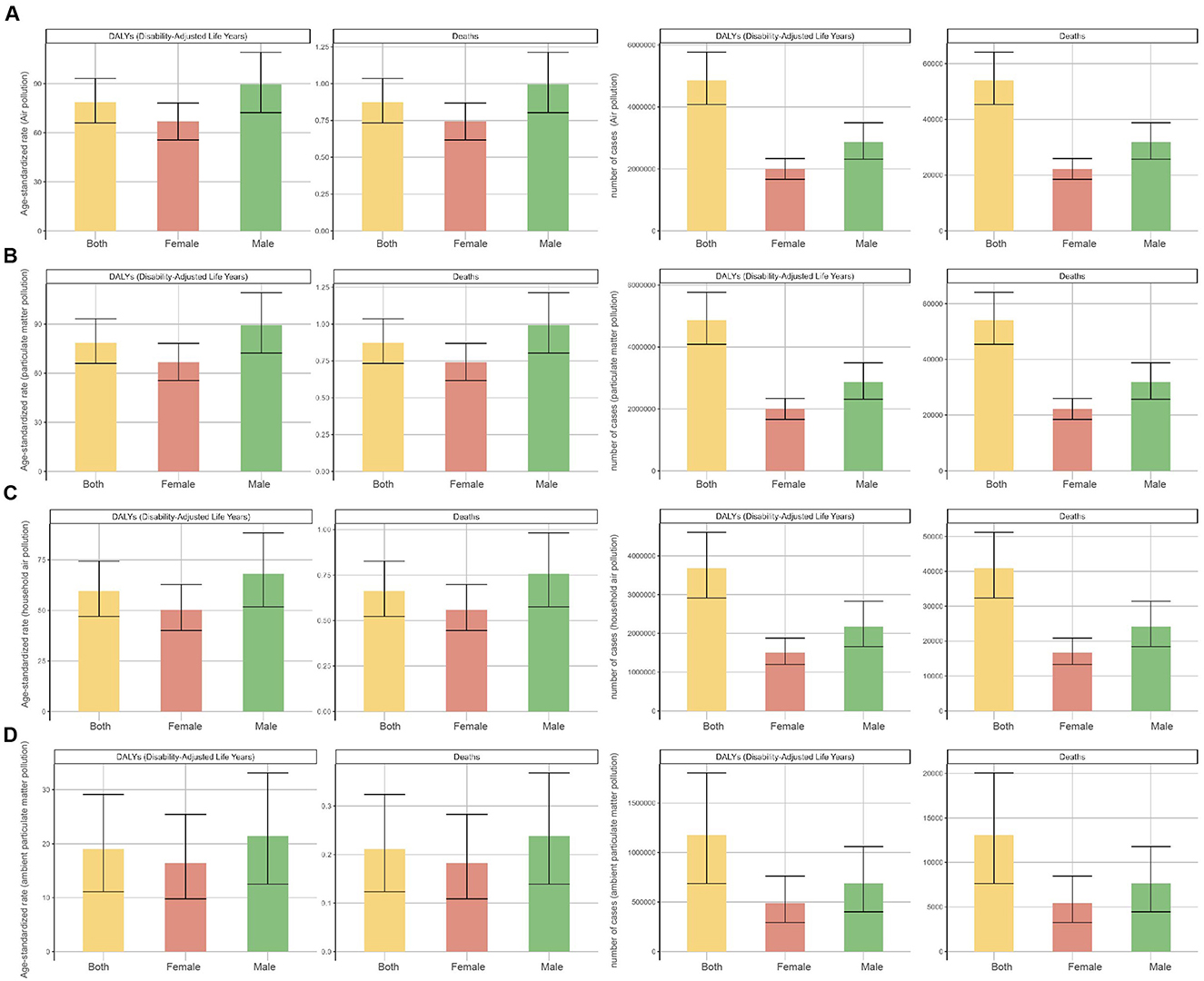
Figure 1. Numbers and age-standardized rates (ASR) of deaths and DALYs attributable to neonatal sepsis and other neonatal infections, by sex, in 2021. (A) Air pollution, (B) particulate matter pollution, (C) household air pollution, and (D) ambient particulate matter pollution.
The global burden of neonatal sepsis and other neonatal infections from various air pollution sources showed notable regional disparities closely associated with SDI levels. In 2021, the low-SDI region had the highest number of deaths from air pollution [31,063 (95% UI: 25,026–38,875)], particulate matter pollution [31,063 (95% UI: 25,026–38,875)], and household air pollution from solid fuels [26,378 (95% UI: 20,833–32,927)] (Tables 1, 2, Figures 2A–C). Ambient particulate matter pollution resulted in the highest number of deaths [4,970 (95% UI: 2,557–8,211)] and age-standardized deaths rate in the low-middle SDI region (Tables 1, 2, Figure 2D). However, high-SDI regions exhibited significant reductions in EAPC for all four types of pollution: air pollution [−4.12 (95% CI: −4.4 to −3.85)], particulate matter pollution [−4.12 (95% CI: −4.4 to −3.85)], household air pollution from solid fuels [−16.91 (95% CI: −17.47 to −16.36)], and ambient particulate matter pollution [−3.86 (95% CI: −4.17 to −3.55)], reflecting effective prevention and management strategies. In contrast, the burden in low-middle SDI regions from ambient particulate matter pollution continued to rise significantly, with the highest EAPC of 0.82 (95% CI: 0.45–1.19; Tables 1, 2). Regional differences were further emphasized by age-standardized DALYs rate, consistent with death trends described above (Tables 1, 2). Analyzing trends from 1990 to 2021 revealed continuous declines in deaths and DALYs attributable to air pollution, particulate matter pollution, and household air pollution from solid fuels in middle SDI and low-middle SDI regions. High SDI and high-middle SDI regions showed stable trends, whereas low SDI regions initially increased and subsequently decreased (Supplementary Figure S3). Notably, significant intersection points occurred in 1998 between low SDI and low-middle SDI regions for air pollution and particulate matter pollution, and in 1997 for household air pollution from solid fuels. For ambient particulate matter pollution, deaths and DALYs gradually declined to stability in high SDI and high-middle SDI regions, whereas low SDI, low-middle SDI, and middle SDI regions experienced minor fluctuations between 2009 and 2010, followed by rapid increases reaching a peak in 2015 before decreasing. Trends between low SDI and low-middle SDI regions closely overlapped during this rising phase (Supplementary Figure S2D).

Figure 2. Numbers and age-standardized rates (ASR) of deaths and DALYs attributable to neonatal sepsis and other neonatal infections, by SDI, in 2021. (A) Air pollution, (B) particulate matter pollution, (C) household air pollution, and (D) ambient particulate matter pollution.
3.3 Regional hotspots and cluster analysis
Our results indicated that Western Africa experienced the highest global burden of neonatal sepsis and other neonatal infections attributable to air pollution and particulate matter pollution, with an age-standardized deaths rate (air pollution/particulate matter pollution: 2.21, 95% UI: 1.82–2.69) and an age-standardized DALYs rate (air pollution/particulate matter pollution: 199.15, 95% UI: 163.93–241.80). Western Sub-Saharan Africa ranked second (deaths: 2.18, 95% UI: 1.79–2.64; DALYs: 196.06, 95% UI: 160.90–237.16), followed by Eastern Sub-Saharan Africa (deaths: 2.06, 95% UI: 1.55–2.60; DALYs: 184.99, 95% UI: 139.63–233.57; Supplementary Tables S1, S2). Regarding neonatal sepsis and other neonatal infections associated with household air pollution from solid fuels, Eastern Sub-Saharan Africa exhibited the highest age-standardized deaths rate (1.90, 95% UI: 1.42–2.41) and DALYs rate (170.86, 95% UI: 127.88–216.68). Eastern Africa ranked second (deaths: 1.71, 95% UI: 1.27–2.18; DALYs: 153.57, 95% UI: 114.22–196.62), followed by the Commonwealth Low-Income region (deaths: 1.67, 95% UI: 1.33–2.15; DALYs: 150.33, 95% UI: 119.40–193.59; Supplementary Tables S1, S2). For ambient particulate matter pollution, the regions with the highest age-standardized deaths and DALYs rate due to neonatal sepsis and other neonatal infections were Western Africa (deaths: 0.55, 95% UI: 0.28–0.95; DALYs: 49.76, 95% UI: 24.98–85.52), Western Sub-Saharan Africa (deaths: 0.53, 95% UI: 0.27–0.90; DALYs: 47.74, 95% UI: 24.30–80.97), and Commonwealth Middle-Income regions (deaths: 0.39, 95% UI: 0.21–0.63; DALYs: 34.93, 95% UI: 18.91–56.30; Supplementary Tables S1, S2).
Subsequently, hierarchical cluster analyses were performed to identify regions with similar changes in disease burden. For neonatal sepsis and other neonatal infections due to air pollution and particulate matter pollution, age-standardized deaths and DALYs rate significantly increased in 19 regions including Southern Sub-Saharan Africa and Central Africa, whereas Tropical Latin America, East Asia, and Central Europe exhibited significant decreases (Figures 3A, B). In relation to household air pollution from solid fuels, 18 regions, including Eastern Sub-Saharan Africa and Eastern Africa, showed significant increases, while 21 regions, including the Middle East and Northern Africa, showed significant decreases (Figure 3C). For ambient particulate matter pollution, Central Asia, the Caribbean, Central Sub-Saharan Africa, and Commonwealth middle-income regions demonstrated significant increases in deaths and DALY rates, whereas 25 regions, including Southern Africa and Africa, showed significant declines (Figure 3D).
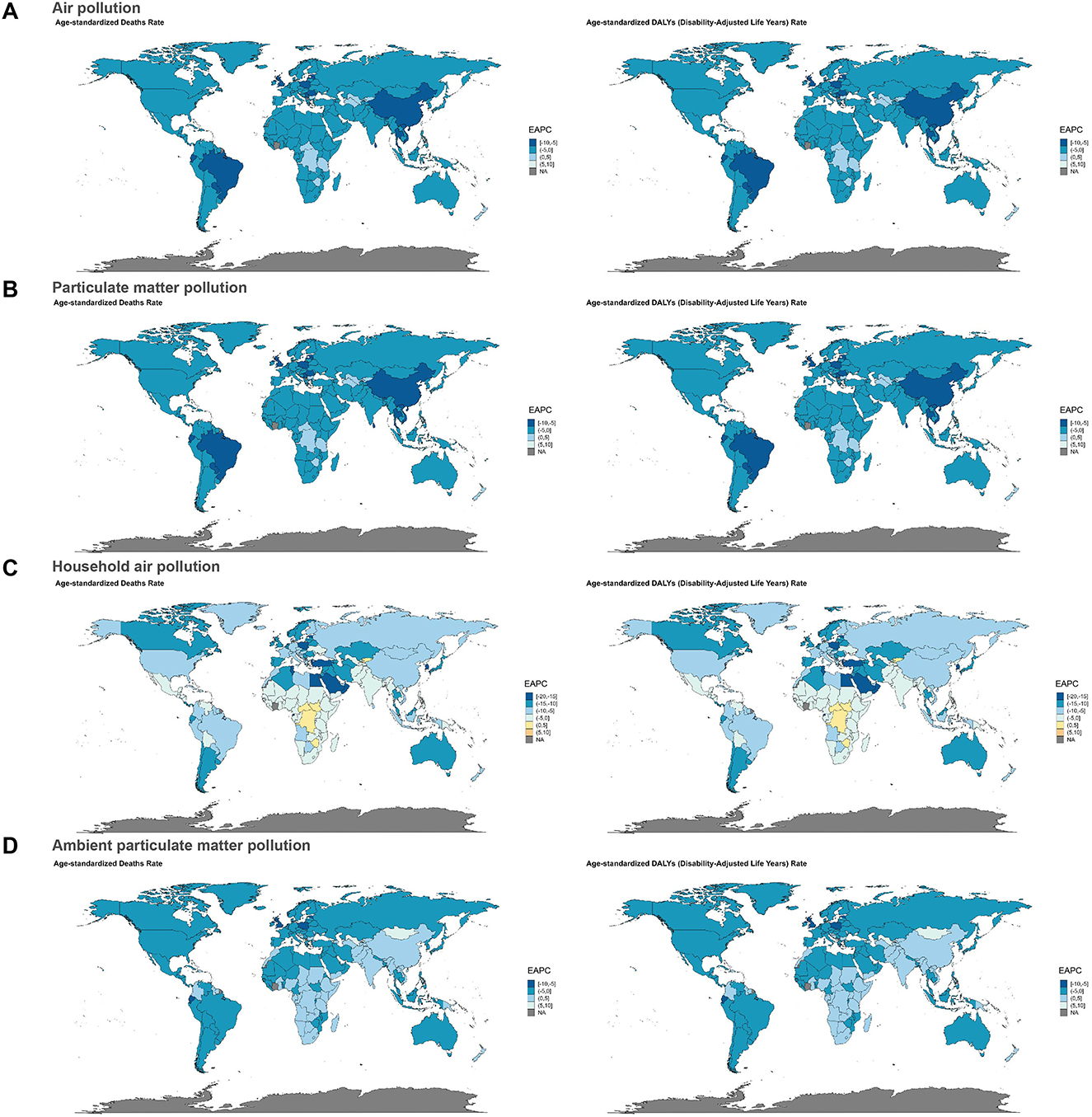
Figure 3. Results of cluster analysis based on the EAPC values from 1990 to 2021. (A) Air pollution, (B) particulate matter pollution, (C) household air pollution, and (D) ambient particulate matter pollution.
3.4 National heterogeneity in attributable burden
Among 204 countries, Sierra Leone, Mozambique, and Somalia ranked as the top three nations in terms of age-standardized deaths and DALYs rate due to neonatal sepsis and other neonatal infections attributed to air pollution, particulate matter pollution, and household air pollution from solid fuels (Supplementary Tables S1, S2, Supplementary Figure S4). Notably, all three countries with the highest ASRs are located in Africa-specifically, Sierra Leone in Western Sub-Saharan Africa, Mozambique in southeastern Africa, and Somalia in Eastern Sub-Saharan Africa. Additionally, two of the top three countries with the highest age-standardized deaths and DALYs rate attributable to ambient particulate matter pollution-Ghana and Nigeria-are also in Africa, specifically Western Sub-Saharan Africa (Supplementary Tables S1, S2, Supplementary Figure S3). These findings are consistent with our regional analysis, which identifies Africa as the region experiencing the highest global burden in terms of deaths and DALYs from neonatal sepsis and other neonatal infections attributable to air pollution, particulate matter pollution, household air pollution from solid fuels, and ambient particulate matter pollution. In contrast, high-income countries (such as those in North America, Europe, and the high-income Asia-Pacific region) showed significantly lower deaths and DALYs rate compared to low-income countries.
3.5 Decomposition analysis
The decomposition analysis indicated that globally, population was the primary driver of the increase in disease burden associated with air pollution (deaths: 621.99%; DALYs: 622.57%), whereas epidemiological changes significantly reduced this burden (deaths: −521.99%; DALYs: −522.57%). These two factors had opposite effects, but the influence of population was stronger, resulting in a net increase in burden (deaths: 40,294.84; DALYs: 3,623,017.97). Among the different SDI regions, the low-SDI region was most profoundly affected by population (deaths: 234.46%; DALYs: 234.49%), although epidemiological changes partly mitigated this adverse impact (deaths: −134.46%; DALYs: −134.49%). Conversely, the high-SDI region experienced the smallest impact from population (deaths: −23.84%; DALYs: −23.86%), achieving a net reduction in burden due to favorable epidemiological changes (deaths: 123.84%; DALYs: 123.86%; Supplementary Table S3, Figure 4A). For particulate matter pollution, global and regional trends were consistent with those observed for air pollution, with the low-SDI region again identified as a critical target for intervention (Supplementary Table S3, Figure 4B). Regarding household air pollution from solid fuels, globally, population substantially increased the disease burden (deaths: −1,181.79%; DALYs: −1,179.57%), yet epidemiological changes exerted an even stronger counter-effect (deaths: 1,281.79%; DALYs: 1,279.57%), resulting in a net global reduction in burden (deaths: −17,034.57; DALYs: −1,535,914.84). Among SDI regions, the low-SDI region experienced the greatest increase due to population (deaths: 263.70%; DALYs: 263.74%), with epidemiological changes having a smaller mitigating effect (deaths: −163.70%; DALYs: −163.74%), leading to a net increase in burden. The high-SDI region saw minimal impact from population (deaths/DALYs: −12.34%), with epidemiological changes contributing significantly to reductions in burden (deaths/DALYs: 112.34%). The low-middle SDI region showed the greatest benefit from epidemiological changes (deaths: 259.65%; DALYs: 259.63%; Supplementary Table S3, Figure 4C). In terms of ambient particulate matter pollution, globally, population remained the dominant factor driving the increase in disease burden (deaths: 86.03%; DALYs: 86.05%), while epidemiological changes had a smaller impact (deaths: 13.97%; DALYs: 13.95%). The low-SDI region was disproportionately impacted by population (deaths/DALYs: 135.03%), though this was partially alleviated by epidemiological changes (deaths/DALYs: −35.03%). In the high-SDI region, epidemiological changes significantly reduced the disease burden (deaths: 125.63%; DALYs: 125.64%), more than compensating for the adverse effect of population (deaths: −25.63%; DALYs: −25.64%; Supplementary Table S3, Figure 4D).
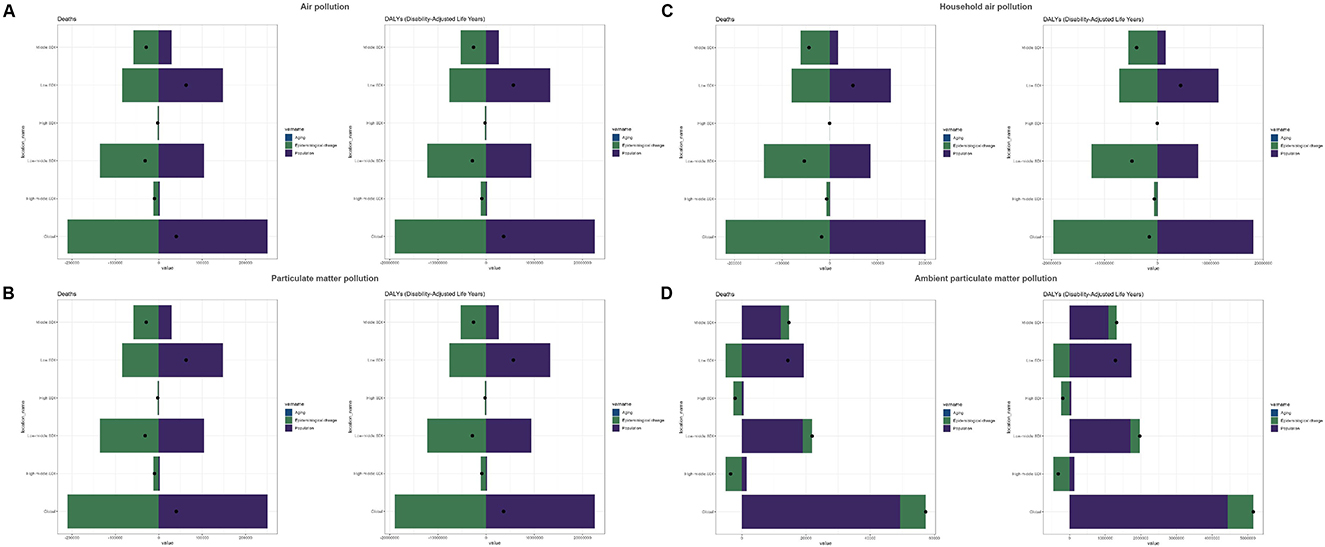
Figure 4. Decomposition analysis results for the global population and five SDI regions. (A) Air pollution, (B) particulate matter pollution, (C) household air pollution, and (D) ambient particulate matter pollution.
3.6 The predicted results from 2022 to 2050
Forecast results from the ARIMA model indicated that from 2022 to 2050, deaths and DALYs attributable to neonatal sepsis and other neonatal infections associated with air pollution, particulate matter pollution, and household air pollution from solid fuels are predicted to decrease annually in both males and females, with females experiencing a more rapid decline. However, age-standardized deaths and DALYs rate in males are projected to slightly increase, while females are expected to show a continuous decreasing trend (Supplementary Tables S4, S6, Figures 5A–C). Conversely, deaths and DALYs associated with ambient particulate matter pollution in males are anticipated to initially increase, reaching a peak in 2027, before subsequently declining, with stable trends observed for corresponding age-standardized deaths and DALYs rate. Females are predicted to exhibit similar trends, with deaths, DALYs, and corresponding ASRs first increasing, peaking in 2027, and then declining thereafter (Supplementary Table S7, Figure 5D).
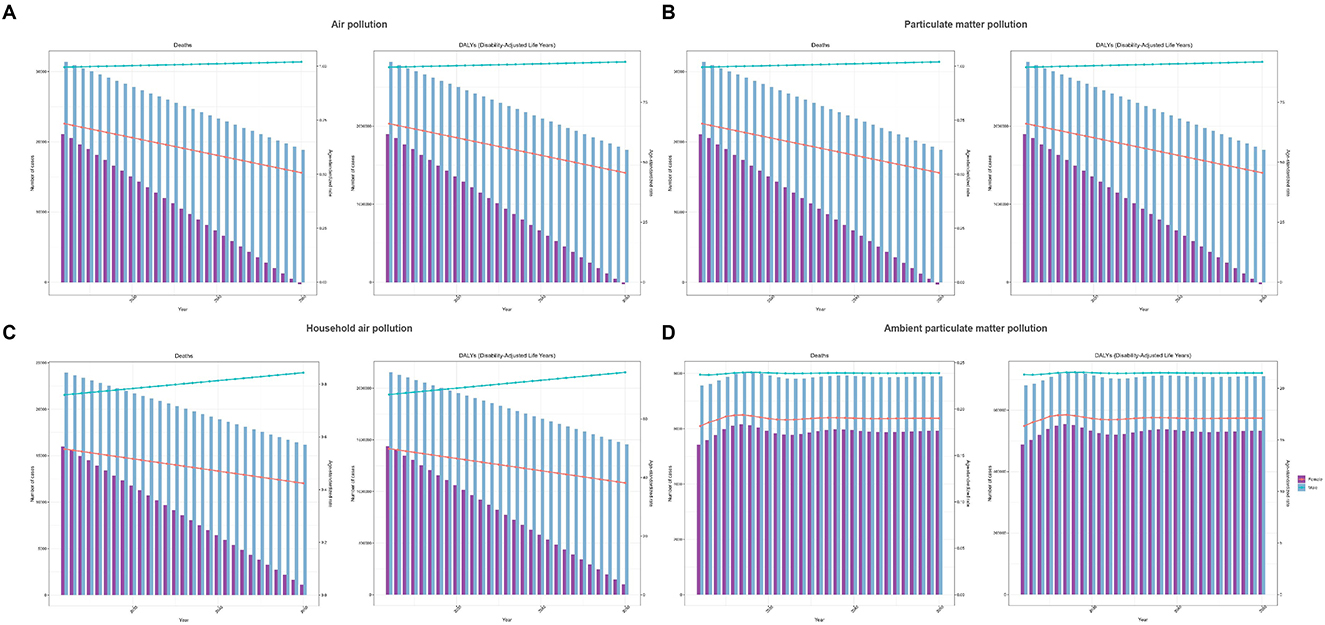
Figure 5. Predicted trends for neonatal sepsis and other neonatal infections deaths and DALYs. (A) Air pollution, (B) particulate matter pollution, (C) household air pollution, and (D) ambient particulate matter pollution.
4 Discussion
Air pollution has emerged as a critical global public health issue. With rapid industrialization and ongoing urbanization, emissions of air pollutants continue to rise, posing serious threats to human health and ecosystems (28, 29). Neonates are particularly vulnerable due to their rapid growth stage and immature immune defense systems, making them more susceptible to health damage from exposure to air pollutants (30, 31). Previous studies indicate that inhaled air pollutants may induce the accumulation of free radicals in the lungs, exacerbating oxidative stress and subsequently triggering inflammatory cascades, resulting in substantial cytokine release and eventual damage to overall health (32). Furthermore, prenatal exposure to air pollutants can cross the placental barrier, enter fetal circulation, impair immune system development, and potentially lead to neonatal mortality (33). Prior research also suggests that air pollutants may increase the risk of neonatal sepsis and other neonatal infections, with a gender disparity, as males experience higher DALYs compared to females (34).
Multiple studies have revealed significant associations between prenatal exposure to air pollution and the risk of neonatal sepsis. A large-scale European study encompassing 10 birth cohorts (n = 16,059) found that exposure to air pollution at birth increased the risk of pneumonia in neonates. During the 0–1 year period, exposure to pollutants such as PM2.5 and NO2 exhibited a stronger association with neonatal pneumonia compared to the entire early childhood phase (e.g., 0–5 years). Specifically, each 5 μg/m3 increase in PM2.5 was associated with a four-fold increased risk of pneumonia (OR = 4.06; 95% CI: 1.93–8.57) (35), which may further progress to sepsis. Another retrospective cohort study focusing on singleton pregnancies further confirmed that elevated prenatal exposure to nitrogen dioxide (NO2) significantly increased the risk of neonatal sepsis evaluation, particularly in cases of early preterm birth (22). Additionally, gestational exposure to fine PM2.5 may lead to alveolar developmental abnormalities and immune dysregulation (12, 15), thereby impairing the neonate's defense against infections. Mechanistic studies suggest that air pollution promotes the onset and progression of sepsis through multiple pathways. On one hand, pollutants such as diesel exhaust particles can impair pulmonary antibacterial function, reduce macrophage-mediated bacterial clearance efficiency, and enhance the colonization capacity of Streptococcus pneumoniae, increasing its risk of hematogenous dissemination (36–38). Notably, S. pneumoniae is a key pathogen implicated in early-onset neonatal sepsis (39). Importantly, compared to sepsis originating from other sources, pulmonary sepsis is associated with significantly higher mortality. For instance, one study reported a 76% higher mortality rate in sepsis secondary to pneumonia compared to non-pulmonary sepsis (95% CI: 11%−178%) (40). This suggests that the impact of air pollutants on respiratory infections may directly contribute to the increased risk of sepsis-related mortality. Furthermore, air pollution may indirectly exacerbate sepsis mortality through its well-documented systemic inflammatory and oxidative stress effects-both of which disrupt immune responses and impair the body's ability to clear potential infections.
Our study is the first to systematically assess and quantify the global burden of neonatal sepsis and other neonatal infections attributable to air pollution (including particulate matter pollution, household air pollution from solid fuels, and ambient particulate matter pollution), and further forecast future trends. The results highlight air pollution as a major environmental risk factor contributing to neonatal sepsis and other neonatal infections, with significant disparities observed across socio-economic levels and geographic regions. Globally, deaths, DALYs, and corresponding ASRs due to neonatal sepsis and other neonatal infections associated with air pollution (including particulate matter pollution and household air pollution from solid fuels) have generally exhibited a decreasing trend, whereas the burden associated with ambient particulate matter pollution has continued to increase. This declining burden is largely attributed to the widespread implementation of control measures for household air pollution from solid fuels and improvements in healthcare conditions (41). Conversely, the rising burden from ambient particulate matter pollution highlights the inadequacy of environmental management strategies in certain regions (42, 43).
Substantial research evidence indicates that the exposure risks and health impacts of air pollution exhibit significant inequalities, with disproportionately higher burdens experienced by low-income and marginalized communities (44, 45). Existing evidence explicitly demonstrates that air pollution has emerged as a leading cause of mortality in low- and middle-income countries (46). These countries commonly face challenges including lenient air-quality regulations, widespread use of outdated and highly polluting machinery and vehicles, persistent fossil fuel subsidy policies, congested urban transportation systems, rapid industrial sector expansion, and slash-and-burn agricultural practices-collectively driving elevated concentrations of air pollutants (47). Our data similarly indicate that the burden of neonatal sepsis and other neonatal infections attributable to air pollution (including particulate matter pollution, household air pollution from solid fuels, and ambient particulate matter pollution) is significantly greater in low SDI regions compared to high SDI regions. This aligns with previous findings reporting high prevalence rates of neonatal sepsis in Sub-Saharan Africa (48), and also matches the spatial distribution of PM2.5 exposure (25). Furthermore, earlier studies combining satellite-derived atmospheric PM2.5 data with household survey data found a strong linear association between infant mortality rates and PM2.5 levels in Sub-Saharan Africa, with infant mortality under 1 year increasing by 9.2% for every 10 μg/m3 rise in PM2.5 concentration (49). These studies collectively suggest that socioeconomic factors play a crucial role in mediating pollution-related health outcomes. The global air quality report highlighted that African countries rank among the highest globally in terms of deaths attributed to PM2.5 pollution-a phenomenon compounded by limited access to medical resources, socioeconomic development disparities, and demographic shifts (50). Notably, even in regions experiencing modest declines in PM2.5 concentrations, continued population growth may increase the overall burden of air pollution-related diseases, potentially explaining the persistently high child mortality rates observed in Africa (51).
Additionally, gender disparities were evident in our results, revealing higher deaths, DALYs, and corresponding ASRs for neonatal sepsis and other neonatal infections attributable to air pollution among males compared to females, suggesting males may face greater risk of neonatal sepsis and related deaths associated with air pollution. Prior research has shown clear variations in air pollution-related public health impacts across different age groups and genders, noting heightened vulnerability among older adults individuals and children under five, with males experiencing more severe disease burdens from air pollution than females (52). Furthermore, forecasting results using the ARIMA model for the next 29 years warn that without stronger intervention measures, the burden of neonatal infections caused by ambient particulate matter pollution will continue to rise, peaking notably around 2027. These findings underscore the necessity of prioritizing air pollution-targeted interventions-particularly in low-income countries-as essential to advancing progress toward achieving the United Nations Sustainable Development Goal (SDG 3.2) of reducing childhood mortality (53). Results from decomposition analyses revealed a complex interaction between population dynamics and epidemiological changes across different environmental risk factors (air pollution, particulate matter pollution, household air pollution from solid fuels, and ambient particulate matter pollution). Globally, population was identified as the main driving force behind the rising disease burden attributable to air pollution (54). Conversely, epidemiological changes partially counteracted this increase, indicating the effectiveness of public health interventions such as clean air policies and improved healthcare (43, 55). Low SDI regions, notably Sub-Saharan Africa, experienced the most pronounced negative impact from population, although epidemiological improvements provided some mitigation (56). High SDI regions, such as countries in Europe and North America, experienced relatively minor impacts, reflecting their advanced pollution control measures and mature healthcare systems (55). For particulate matter pollution, global trends mirrored those observed for overall air pollution, with low SDI regions again bearing the heaviest burden, underscoring particulate matter pollution as a significant health threat in developing countries, possibly due to rapid urbanization, industrialization, and increasing vehicular emissions (47). In terms of household air pollution from solid fuels, population contributed substantially to the global disease burden, likely related to continued reliance on solid fuels (such as wood and coal) in certain low-income countries (57, 58). Regarding ambient particulate matter pollution, population remained the dominant driver, especially in low SDI nations (e.g., India and parts of Africa), suggesting that urbanization and industrial expansion continue to exacerbate pollution (59, 60). Although global air pollution control efforts have made some progress, ambient particulate matter pollution continues to pose a significant challenge. For example, almost all African cities with available PM2.5 monitoring data exceed World Health Organization Air Quality Guidelines (WHO AQG) (61). Additionally, research specifically addressing neonatal vulnerability to PM2.5 exposure remains limited, leading to insufficient evidence to guide targeted policy interventions.
Our projections suggest that the disease burden from ambient particulate matter pollution will reach its maximum level in 2027. Therefore, countries and regions should establish air pollution control frameworks tailored to their respective developmental stages. Strengthening emissions regulation in manufacturing and transportation sectors, promoting electrified public transportation infrastructure, advancing clean energy transitions, and progressively replacing high-carbon energy sources with cleaner alternatives are critical strategies. Furthermore, implementing clean household fuel programs, reducing solid fuel and kerosene usage, and expanding access to clean domestic energy could significantly decrease exposure to indoor and outdoor PM2.5 and CO. Additionally, governments must develop differentiated air-quality-based management policies and strengthen public education to raise awareness of the health hazards associated with environmental pollution, systematically reducing air pollution levels (62, 63).
Based on the identified association between air pollution exposure and neonatal sepsis burden and its regional disparities, the following integrated intervention strategies are proposed: in low-SDI regions (e.g., Sub-Saharan Africa), priority should be given to promoting clean cooking stoves to replace solid fuels, while concurrently strengthening early diagnostic capability for neonatal sepsis. In middle- and high-SDI countries, industrial and transportation emission regulations must be enhanced, particularly targeting particulate matter restrictions for diesel vehicles. All regions should establish pollution-health co-monitoring systems, integrating real-time PM2.5 data into maternal and child health platforms to enable risk warnings for pregnant women and newborns. The international community should support clean energy transitions in low-SDI countries through the Green Climate Fund and incorporate neonatal PM2.5 exposure metrics into revised WHO Air Quality Guidelines. Additionally, sex-differentiated research is needed to elucidate the mechanisms underlying higher risks in male neonates, while emergency response plans should be developed for the projected peak pollution burden in 2027.These measures must be adapted to local socioeconomic conditions and implemented through cross-sector collaboration to achieve SDG 3.2's child health targets.
This study has certain limitations. First, our analyses were conducted at the national level and did not incorporate provincial or state-level variations. Given substantial heterogeneity in air pollution levels, demographic structures, and healthcare resource distributions within countries, findings might have limited applicability at subnational scales. Additionally, our analyses relied exclusively on the GBD database without integrating other internationally recognized datasets. Differences in data collection methodologies, coverage, and quality control across databases could introduce systematic biases or underestimations of region-specific impacts. Finally, the selection of the ARIMA model may have certain limitations. For instance, when making long-term predictions, the ARIMA model may exhibit certain deviations. Subsequently, the prediction model needs to be optimized and further research should be conducted.
5 Conclusion
In summary, at a global level, deaths and DALYs attributable to neonatal sepsis and other neonatal infections caused by air pollution (including particulate matter pollution and household air pollution from solid fuels) have declined. However, the disease burden from ambient particulate matter pollution is projected to rise continuously, particularly in low-income countries. This underscores ambient particulate matter pollution as an ongoing critical public health issue requiring targeted institutional strategies and interventions to further alleviate the burden in low SDI regions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. YY: Conceptualization, Data curation, Visualization, Writing – original draft, Writing – review & editing. ZQ: Investigation, Resources, Writing – review & editing. HF: Project administration, Writing – review & editing. TJ: Data curation, Project administration, Writing – original draft. CL: Conceptualization, Visualization, Writing – original draft. XB: Visualization, Writing – original draft. MW: Investigation, Writing – review & editing. HH: Visualization, Writing – review & editing. RC: Investigation, Writing – original draft. DL: Visualization, Writing – review & editing. HC: Investigation, Writing – review & editing. QL: Funding acquisition, Investigation, Supervision, Writing – review & editing. QF: Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by youth talent support project of Henan association for science and technology (2025HYTP084).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1644191/full#supplementary-material
References
1. Sharrow D, Hug L, You D, Alkema L, Black R, Cousens S, et al. Global, regional, and national trends in under-5 mortality between 1990 and 2019 with scenario-based projections until 2030: a systematic analysis by the UN inter-agency group for child mortality estimation. Lancet Glob Health. (2022) 10:e195–206. doi: 10.1016/S2214-109X(21)00515-5
2. Azevedo JP, Banerjee A, Wilmoth J, Fu H, You D. Hard truths about under-5 mortality: call for urgent global action. Lancet. (2024) 404:506–8. doi: 10.1016/S0140-6736(24)00501-4
3. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
4. Lawn JE, Kerber K, Enweronu-Laryea C, Massee Bateman O. Newborn survival in low resource settings–are we delivering? Bjog. (2009) 116 Suppl 1:49–59. doi: 10.1111/j.1471-0528.2009.02328.x
5. Strunk T, Molloy EJ, Mishra A, Bhutta ZA. Neonatal bacterial sepsis. Lancet. (2024) 404:277–93. doi: 10.1016/S0140-6736(24)00495-1
6. Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. (2018) 6:223–30. doi: 10.1016/S2213-2600(18)30063-8
7. Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet. (2016) 388:3027–35. doi: 10.1016/S0140-6736(16)31593-8
9. Seale AC, Mwaniki M, Newton CR, Berkley JA. Maternal and early onset neonatal bacterial sepsis: burden and strategies for prevention in sub-Saharan Africa. Lancet Infect Dis. (2009) 9:428–38. doi: 10.1016/S1473-3099(09)70172-0
10. Bech CM, Stensgaard CN, Lund S, Holm-Hansen C, Brok JS, Nygaard U, et al. Risk factors for neonatal sepsis in Sub-Saharan Africa: a systematic review with meta-analysis. BMJ Open. (2022) 12:e054491. doi: 10.1136/bmjopen-2021-054491
11. Ranjeva SL, Warf BC, Schiff SJ. Economic burden of neonatal sepsis in sub-Saharan Africa. BMJ Glob Health. (2018) 3:e000347. doi: 10.1136/bmjgh-2017-000347
12. Johnson NM, Hoffmann AR, Behlen JC, Lau C, Pendleton D, Harvey N, et al. Air pollution and children's health-a review of adverse effects associated with prenatal exposure from fine to ultrafine particulate matter. Environ Health Prev Med. (2021) 26:72. doi: 10.1186/s12199-021-00995-5
13. Collaborative CsEH. Air Pollution 2024 (2024). Available online at: https://ceh.unicef.org/spotlight-risk/air-pollution (Accessed April 16, 2025).
14. Macchi C, Iodice S, Persico N, Ferrari L, Cantone L, Greco MF, et al. Maternal exposure to air pollutants, PCSK9 levels, fetal growth and gestational age - an Italian cohort. Environ Int. (2021) 149:106163. doi: 10.1016/j.envint.2020.106163
15. Korten I, Ramsey K, Latzin P. Air pollution during pregnancy and lung development in the child. Paediatr Respir Rev. (2017) 21:38–46. doi: 10.1016/j.prrv.2016.08.008
16. Proietti E, Röösli M, Frey U, Latzin P. Air pollution during pregnancy and neonatal outcome: a review. J Aerosol Med Pulm Drug Deliv. (2013) 26:9–23. doi: 10.1089/jamp.2011.0932
17. Wei Y, Wang Y, Di Q, Choirat C, Wang Y, Koutrakis P, et al. Short term exposure to fine particulate matter and hospital admission risks and costs in the medicare population: time stratified, case crossover study. BMJ. (2019) 367:l6258. doi: 10.1136/bmj.l6258
18. AIR SOG. State of Global Air Report 2024 (2024). Available online at: https://www.stateofglobalair.org/resources/report/state-global-air-report-2024 (Accessed April 16, 2025).
19. Márquez-Lázaro J, Madera M, Bernabe E. Particulate matter 25 exposure during pregnancy and birth outcomes: evidence from Colombia. Sci Total Environ. (2024) 927:172369. doi: 10.1016/j.scitotenv.2024.172369
20. Milner J, Hughes R, Chowdhury S, Picetti R, Ghosh R, Yeung S, et al. Air pollution and child health impacts of decarbonization in 16 global cities: modelling study. Environ Int. (2023) 175:107972. doi: 10.1016/j.envint.2023.107972
21. Soesanti F, Hoek G, Brunekreef B, Meliefste K, Chen J, Idris NS, et al. Perinatal exposure to traffic related air pollutants and the risk of infection in the first six months of life: a cohort study from a low-middle income country. Int Arch Occup Environ Health. (2024) 97:575–86. doi: 10.1007/s00420-024-02064-0
22. Jones SI, Pruszynski JE, Spong CY, Nelson DB. Traffic-related air pollution is associated with spontaneous extremely preterm birth and other adverse perinatal outcomes. Am J Obstet Gynecol. (2023) 229:455.e1–55.e7. doi: 10.1016/j.ajog.2023.07.040
23. Honda TJ, Kazemiparkouhi F, Henry TD, Suh HH. Long-term PM(25) exposure and sepsis mortality in a US medicare cohort. BMC Public Health. (2022) 22:1214. doi: 10.1186/s12889-022-13628-5
24. D'Amato G, Pawankar R, Vitale C, Lanza M, Molino A, Stanziola A, et al. Climate change and air pollution: effects on respiratory allergy. Allergy Asthma Immunol Res. (2016) 8:391–5. doi: 10.4168/aair.2016.8.5.391
25. Global Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations 1990-2021: 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2162–203. doi: 10.1016/S0140-6736(24)00933-4
26. Global regional and and national burden of chronic kidney disease 1990-2017: 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2020) 395:709–33. doi: 10.1016/S0140-6736(19)32977-0
27. Global Global Burden of Disease Cancer Collaboration; Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. (2017) 3:524−48. doi: 10.1001/jamaoncol.2016.5688
28. Li G, Fang C, Wang S, Sun S. The effect of economic growth, urbanization, and industrialization on fine particulate matter (PM(25)) concentrations in china. Environ Sci Technol. (2016) 50:11452–59. doi: 10.1021/acs.est.6b02562
29. Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front Public Health. (2020) 8:14. doi: 10.3389/fpubh.2020.00014
30. Perera F. Pollution from fossil-fuel combustion is the leading environmental threat to global pediatric health and equity: solutions exist. Int J Environ Res Public Health. (2017) 15:16 doi: 10.3390/ijerph15010016
31. Carvajal V, Jorques Molla JV, Luo Y, Zhao Y, Moncunill G, Gascon M. Air pollution and systemic immune biomarkers in early life: a systematic review. Environ Res. (2025) 269:120838. doi: 10.1016/j.envres.2025.120838
32. Russo RC, Togbe D, Couillin I, Segueni N, Han L, Quesniaux VFJ, et al. Ozone-induced lung injury and inflammation: pathways and therapeutic targets for pulmonary diseases caused by air pollutants. Environ Int. (2025) 198:109391. doi: 10.1016/j.envint.2025.109391
33. Bongaerts E, Lecante LL, Bové H, Roeffaers MBJ, Ameloot M, Fowler PA, et al. Maternal exposure to ambient black carbon particles and their presence in maternal and fetal circulation and organs: an analysis of two independent population-based observational studies. Lancet Planet Health. (2022) 6:e804–11. doi: 10.1016/S2542-5196(22)00200-5
34. Julaiti M, Wubuli D, Cui T, Nijiati N, Huang P, Hu B. Analysis of the relationship between environmental particulate matter exposure and congenital diseases, as well as the epidemiological trends and burden of impact on newborns. Ecotoxicol Environ Saf. (2025) 289:117465. doi: 10.1016/j.ecoenv.2024.117465
35. MacIntyre EA, Gehring U, Mölter A, Fuertes E, Klümper C, Krämer U, et al. Air pollution and respiratory infections during early childhood: an analysis of 10 European birth cohorts within the ESCAPE Project. Environ Health Perspect. (2014) 122:107–13. doi: 10.1289/ehp.1306755-erratum
36. Yang HM, Antonini JM, Barger MW, Butterworth L, Roberts BR, Ma JK, et al. Diesel exhaust particles suppress macrophage function and slow the pulmonary clearance of listeria monocytogenes in rats. Environ Health Perspect. (2001) 109:515–21. doi: 10.1289/ehp.01109515
37. Zhao H, Li W, Gao Y, Li J, Wang H. Exposure to particular matter increases susceptibility to respiratory Staphylococcus aureus infection in rats via reducing pulmonary natural killer cells. Toxicology. (2014) 325:180–8. doi: 10.1016/j.tox.2014.09.006
38. Sigaud S, Goldsmith CA, Zhou H, Yang Z, Fedulov A, Imrich A, et al. Air pollution particles diminish bacterial clearance in the primed lungs of mice. Toxicol Appl Pharmacol. (2007) 223:1–9. doi: 10.1016/j.taap.2007.04.014
39. Ortiz de Zárate M, Sáenz C, Cimbaro Canella R, Díaz M, Mucci J, Dinerstein A, et al. Prevalence of microbiologically confirmed neonatal sepsis at a maternity center in the city of Buenos Aires. Arch Argent Pediatr. (2023) 121:e202202779. doi: 10.5546/aap.2022-02779.eng
40. Kim WY, Lee YJ, Yeon Lim S, Ok Koh S, Choi WI, Chan Kim S, et al. Clinical characteristics and prognosis of pneumonia and sepsis: multicenter study. Minerva Anestesiol. (2013) 79:1356–65.
41. Ward TJ, Semmens EO, Weiler E, Harrar S, Noonan CW. Efficacy of interventions targeting household air pollution from residential wood stoves. J Expo Sci Environ Epidemiol. (2017) 27:64–71. doi: 10.1038/jes.2015.73
42. Wu Y, Song P, Lin S, Peng L, Li Y, Deng Y, et al. Global burden of respiratory diseases attributable to ambient particulate matter pollution: findings from the global burden of disease study 2019. Front Public Health. (2021) 9:740800. doi: 10.3389/fpubh.2021.740800
43. Kelly FJ, Fussell JC. Air pollution and public health: emerging hazards and improved understanding of risk. Environ Geochem Health. (2015) 37:631–49. doi: 10.1007/s10653-015-9720-1
44. Jbaily A, Zhou X, Liu J, Lee TH, Kamareddine L, Verguet S, et al. Air pollution exposure disparities across US population and income groups. Nature. (2022) 601:228–33. doi: 10.1038/s41586-021-04190-y
45. Bell ML, Zanobetti A, Dominici F. Evidence on vulnerability and susceptibility to health risks associated with short-term exposure to particulate matter: a systematic review and meta-analysis. Am J Epidemiol. (2013) 178:865–76. doi: 10.1093/aje/kwt090
46. Lelieveld J, Pozzer A, Pöschl U, Fnais M, Haines A, Münzel T. Loss of life expectancy from air pollution compared to other risk factors: a worldwide perspective. Cardiovasc Res. (2020) 116:1910–17. doi: 10.1093/cvr/cvaa025
47. McDuffie EE, Martin RV, Spadaro JV, Burnett R, Smith SJ, O'Rourke P, et al. Source sector and fuel contributions to ambient PM(25) and attributable mortality across multiple spatial scales. Nat Commun. (2021) 12:3594. doi: 10.1038/s41467-021-23853-y
48. Traoré FB, Sidibé CS, Diallo EHM, Camara BS, Sidibé S, Diallo A, et al. Prevalence and factors associated with maternal and neonatal sepsis in sub-Saharan Africa: a systematic review and meta-analysis. Front Public Health. (2024) 12:1272193. doi: 10.3389/fpubh.2024.1272193
49. Heft-Neal S, Burney J, Bendavid E, Burke M. Robust relationship between air quality and infant mortality in Africa. Nature. (2018) 559:254–58. doi: 10.1038/s41586-018-0263-3
50. Kousar S, Shabbir A, Shafqat R. Investigation of socioeconomic determinants on child death in south Asian countries: a panel cointegration analysis. Omega. (2022) 84:811–36. doi: 10.1177/0030222820915023
51. AIR SOG. Health Impacts of PM2.5 (2024). Available online at: https://www.stateofglobalair.org/health/pm (Accessed April 16, 2025).
52. Behera DK, Viswanathan PK, Mishra S. Effects of air pollution on global health: evidence from the global burden of disease study in the BRICS countries. Int Arch Occup Environ Health. (2024) 97:813–32. doi: 10.1007/s00420-024-02087-7
53. Lai PS, Lam NL, Gallery B, Lee AG, Adair-Rohani H, Alexander D, et al. Household air pollution interventions to improve health in low- and middle-income countries: an official American thoracic society research statement. Am J Respir Crit Care Med. (2024) 209:909–27. doi: 10.1164/rccm.202402-0398ST
54. Babatola SS. Global burden of diseases attributable to air pollution. J Public Health Afr. (2018) 9:813. doi: 10.4081/jphia.2018.813
55. Mao Q, Zhu X, Zhang X, Kong Y. Effect of air pollution on the global burden of cardiovascular diseases and forecasting future trends of the related metrics: a systematic analysis from the global burden of disease study 2021. Front Med. (2024) 11:1472996. doi: 10.3389/fmed.2024.1472996
56. Juginović A, Vuković M, Aranza I, Biloš V. Health impacts of air pollution exposure from 1990 to 2019 in 43 European countries. Sci Rep. (2021) 11:22516. doi: 10.1038/s41598-021-01802-5
57. Adjei-Mantey K, Takeuchi K. The effect of in utero exposure to household air pollution on child health: evidence from Ghana. Health Policy Open. (2021) 2:100029. doi: 10.1016/j.hpopen.2020.100029
58. Health and economic impact of air pollution in the states of India: the global burden of disease study 2019. Lancet Planet Health. (2021) 5:e25–38. doi: 10.1016/S2542-5196(20)30298-9
59. Sharma GK, Ghuge VV. How urban growth dynamics impact the air quality? A case of eight Indian metropolitan cities. Sci Total Environ. (2024) 930:172399. doi: 10.1016/j.scitotenv.2024.172399
60. S SK, Bagepally BS, Rakesh B. Air pollution attributed disease burden and economic growth in India: estimating trends and inequality between states. Lancet Reg Health Southeast Asia. (2022) 7:100069. doi: 10.1016/j.lansea.2022.100069
61. Agbo KE, Walgraeve C, Eze JI, Ugwoke PE, Ukoha PO, Langenhove HV. A review on ambient and indoor air pollution status in Africa. Atmos Pollut Res. (2021) 12:243–60. doi: 10.1016/j.apr.2020.11.006
62. Zheng B, Chen J, Zhang Q. Air pollution control and health economic burdens: evidence from a megacity in China from 2014 through 2022. Environ Res. (2025) 264(Pt 2):120392. doi: 10.1016/j.envres.2024.120392
63. Puzzolo E, Fleeman N, Lorenzetti F, Rubinstein F, Li Y, Xing R, et al. Estimated health effects from domestic use of gaseous fuels for cooking and heating in high-income, middle-income, and low-income countries: a systematic review and meta-analyses. Lancet Respir Med. (2024) 12:281–93. doi: 10.1016/S2213-2600(23)00427-7
Keywords: neonatal sepsis, neonatal infections, air pollution, particulate matter pollution, GBD
Citation: Duan J, Yu Y, Qu Z, Fu H, Jiang T, Liu C, Bai X, Wang M, Hu H, Chen R, Liu D, Chen H, Liu Q and Fu Q (2025) The global burden of neonatal sepsis attributable to air pollution from 1990 to 2021: findings from the global burden of disease study 2021. Front. Public Health 13:1644191. doi: 10.3389/fpubh.2025.1644191
Received: 10 June 2025; Accepted: 02 September 2025;
Published: 24 September 2025.
Edited by:
James Milner, University of London, United KingdomReviewed by:
Xiaochun Zhao, Anhui University, ChinaPinelopi Petropoulou, University of West Attica, Greece
Copyright © 2025 Duan, Yu, Qu, Fu, Jiang, Liu, Bai, Wang, Hu, Chen, Liu, Chen, Liu and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qizhi Fu, cWl6aGlmdV9reUAxMjYuY29t; Qiang Liu, MTMyNTM0MzgyOTBAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Jiajia Duan
Jiajia Duan Ying Yu2†
Ying Yu2† Tao Jiang
Tao Jiang Chuanxin Liu
Chuanxin Liu Xiaoyang Bai
Xiaoyang Bai Min Wang
Min Wang Ruyan Chen
Ruyan Chen Hetao Chen
Hetao Chen Qizhi Fu
Qizhi Fu