- Laboratory of Bio-Analytical Chemistry, Faculty of Pharmacy, Research Institute of Pharmaceutical Sciences, Musashino University, Nishitokyo, Tokyo, Japan
Although Sustainable Development Goal 3.9 states the global goal of reducing air pollution-related death and disease, innovative ways to reduce air pollution-related deaths and diseases have not yet been established. To address this issue, we have established animal models using urban air dust containing the major air pollutants, suspended particulate matter (PM10) and fine particulate matter (PM2.5), and are promoting research to discover protective factors in the respiratory tract where air pollutants first reach the human body. Therefore, in this Perspective manuscript, we provide an overview of our research on the preventive effects of the endogenous protein metallothionein on air pollutant-dependent lung injury and prospects for the clinical application of metallothionein inducers. We hope that this manuscript will help to solve health hazards caused by air pollution.
1 Health hazards caused by air pollution
The increase in the number of deaths and diseases associated with air pollution worldwide is a major problem. Alarmingly, the annual global death toll from air pollution has risen to more than 8.8 million, exceeding the annual global death toll from smoking (1). The global goal of reducing air pollution-related deaths and diseases is clearly stated in Sustainable Development Goals 3.9 (2). Unfortunately, innovative ways to reduce deaths and diseases associated with air pollution have not yet been established. Suspended particulate matter (PM10) and fine particulate matter (PM2.5) are the main health hazards caused by air pollution. Because these particles are very small (less than 10 micrometers in diameter), they remain in the air for long periods of time, easily pass through the human airways, and reach the alveoli at the periphery of the lungs, causing various respiratory and cardiovascular diseases (1, 3). Previous studies have suggested that the induction of oxidative stress, such as the production of reactive oxygen species (ROS), is associated with exposure to these air pollutants (e.g., PM2.5 and PM10) and is an important contributing factor in respiratory diseases such as chronic obstructive pulmonary disease (COPD), asthma, pulmonary fibrosis, and lung cancer, as well as cardiovascular diseases such as heart failure, myocardial infarction, and stroke (4, 5). Therefore, we are conducting research to analyze the molecular mechanism of injury in bronchioles and alveoli, the first sites in the human body where air pollutants reach, and to discover substances that can prevent such injury. We believe that this will lead to the establishment of preventive methods against the health hazards caused by air pollution.
2 Overview of our previous studies
In our research to date, we have created a mouse model of acute lung injury and a cell experiment system using urban aerosol particulate matter, 99% of which is composed of particles with a diameter of 10 μm or less [purchased from the National Institute for Environmental Studies (Tsukuba, Japan)] (6), and are conducting research using these models. We also used these models to analyze the function of protective factors that may prevent respiratory diseases caused by air pollutants. In our first study on air pollution, we analyzed the effectiveness of human serum albumin-fused thioredoxin (HSA-Trx), a drug delivery system (DDS) formulation that improves the in vivo stability of the endogenous antioxidant protein, thioredoxin (7). Using an in vivo imaging system, we found that HSA-Trx significantly suppressed neutrophil-associated inflammatory responses, such as neutrophil extracellular traps and acute lung injury, by suppressing ROS production in mouse lungs caused by urban aerosols. This first study showed that suppressing oxidative stress such as ROS production caused by air pollutants is important for preventing respiratory diseases caused by air pollutants (8). In fact, other clinical studies and analyses using animal and cell models have also pointed out that oxidative stress, including ROS production, may be the main cause of health hazards caused by air pollutants (9–12).
In contrast, we are conducting research to analyze the protective function of metallothionein, an endogenous metal-binding protein, against various respiratory diseases and to elucidate its molecular mechanisms. Metallothionein is present in all animal cells and can bind up to 7–12 metal ions; thus, it plays a role in maintaining the homeostasis of essential trace elements and detoxifying heavy metal elements such as cadmium (13). In addition, metallothionein has approximately 20 thiol groups per molecule, which not only bind to excess heavy metals for detoxification but also react with ROS to exert an antioxidant effect (14–16). Four isoforms of metallothionein have been identified, of which metallothionein-1 and -2 are expressed in most tissues and play a role in heavy metal detoxification and antioxidant functions (13). Because of these physiological activities of metallothionein, there have been many efficacy analyses of metallothionein for liver and kidney injuries (17–19), but there have not been many analyses of metallothionein for respiratory diseases such as COPD and pulmonary fibrosis. Based on this background, we have conducted functional analysis of metallothionein in various respiratory diseases, and have found that porcine pancreatic elastase-induced lung injury and cigarette smoke-induced lung injury, which are animal models of COPD, are more pronounced in metallothionein-knockout (MT-KO) mice (129/Sv-MT1MT2 tm1Bri ) than in wild-type mice (129/SvCPJ) (20, 21). In another study, we found that treatment of alveolar epithelial cells with polaprezinc or zinc acetate induced metallothioneins in the cells, and that this induction prevented cell injury in the alveolar epithelium caused by cadmium, a heavy metal found in tobacco smoke (22). These results indicate a new function of metallothionein that has a preventive effect against the onset and exacerbation of COPD and suggest that compounds such as zinc preparations that have the ability to induce metallothionein may be a preventive method for COPD and other respiratory diseases.
Because air pollutants such as PM2.5 contain metal elements such as aluminum, iron, magnesium, and titanium (6), we thought it was important to clarify the relationship between metallothionein, a metal-binding protein, and lung injury caused by air pollutants. Thus, we recently analyzed the efficacy of metallothionein against air pollutant-dependent acute lung injury in MT-KO mice to identify novel functions of metallothionein (23). As described below, in part in the results published in Biomedicine and Pharmacotherapy, we found that acute lung injury caused by urban aerosols was significantly exacerbated in MT-KO mice compared to that in wild-type mice due to enhanced lung ROS production. ROS are known to activate NF-kB, a transcription factor that promotes inflammatory responses induced by various stimuli (24). Thus, we speculate that excessive ROS production in MT-KO mice may have promoted the NF-kB-mediated inflammatory responses in the lungs. Furthermore, we found that the administration of zinc acetate (20 mg/kg) to wild-type mice markedly enhanced metallothionein induction (Mt1 and Mt2 mRNA) in the lungs, and that this induction significantly suppressed acute lung injury caused by urban aerosols. In contrast, as shown in Figure 1, pre-administration of zinc acetate (20 mg/kg) to MT-KO mice hardly suppressed the increase in total inflammatory cell counts, neutrophil counts, and protein levels in the bronchoalveolar lavage fluid caused by urban aerosols. These results strongly support our hypothesis in Biomedicine and Pharmacotherapy that the preventive effect of zinc acetate on air pollutant-dependent acute lung injury is mediated by metallothionein induction. In other words, we suggest that compounds with metallothionein-inducing properties, such as zinc acetate, are promising candidates for the prevention of air pollution-induced health hazards (Scheme 1).
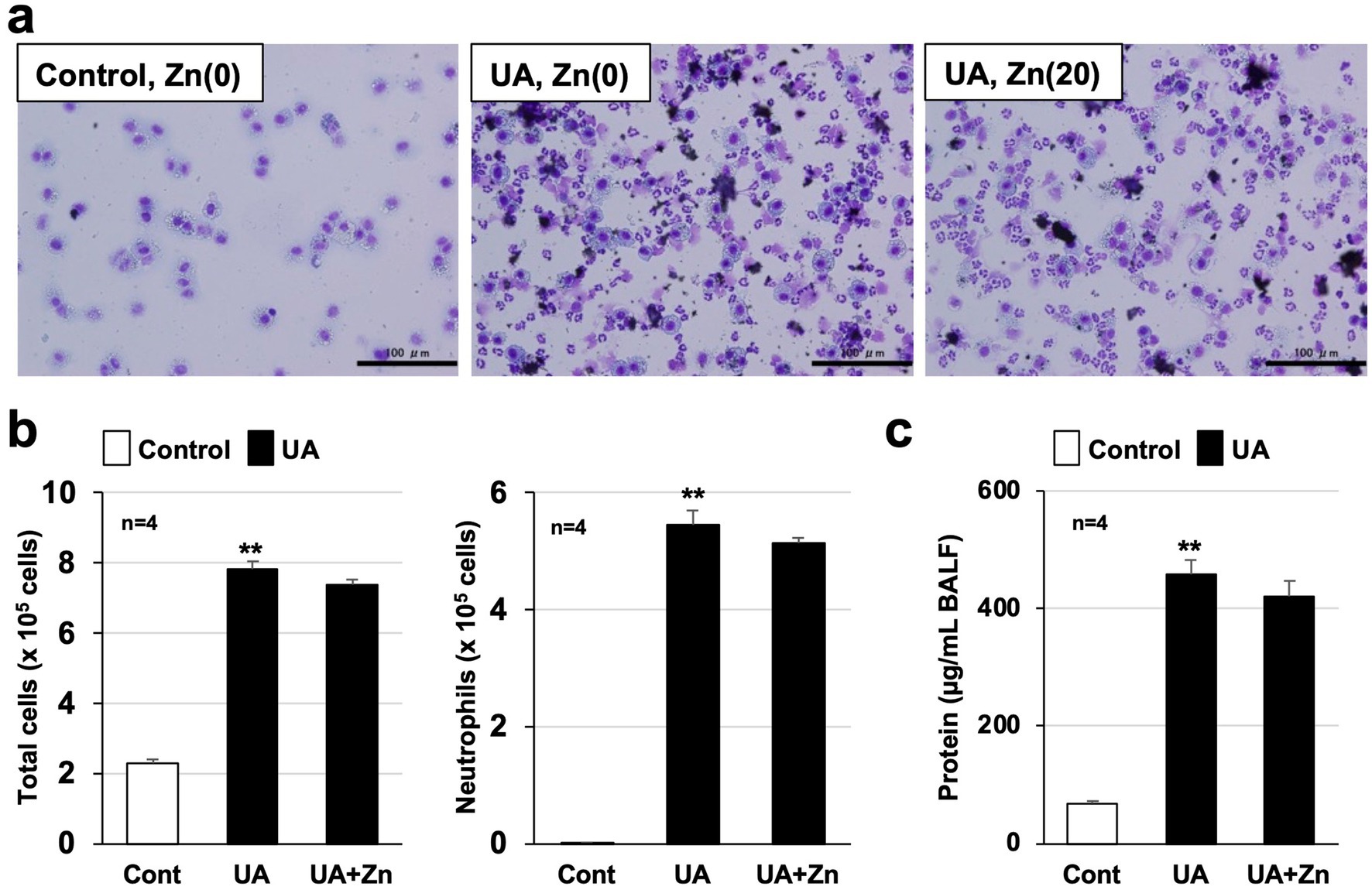
Figure 1. Zinc acetate had little effect on metallothionein-knockout (MT-KO) mice. Metallothionein-knockout (MT-KO) mice received a single intraperitoneal dose of sterile saline (control or urban aerosols alone group) or zinc acetate (20 mg/kg) at 0 h. Twenty-four hours after intraperitoneal administration, MT-KO mice received a single intratracheal dose of sterile saline (Cont) or an urban aerosol particle suspension (UA or UA + Zn, 1.0 mg/60 μL sterile saline per mouse). Twenty-four hours after intratracheal administration, bronchoalveolar lavage fluid (BALF) was collected from mice; cells present in the BALF were applied to slides using a Cytospin 4 cell centrifuge and stained with Diff-Quik reagent, and visualized under a light microscope (scale bar, 100 μm) (a). Total cell counts were determined using a hemocytometer and neutrophil counts were calculated based on Diff-Quik staining (n = 4) (b). Protein levels in BALF were measured using Bradford reagent (n = 4) (c). Values are the mean ± SEM; **p < 0.01. (**, vs. Cont, one-way analysis of variance followed by Dunnett’s test). The results showing that pre-administration of zinc acetate suppresses air pollutant-dependent acute lung injury in wild-type mice have already been published in Biomedicine and Pharmacotherapy (23). In contrast, pre-administration of zinc acetate showed little efficacy in MT-KO mice (in this figure). These results strongly support the notion that metallothionein inducers prevent air pollutant-dependent acute lung injury.
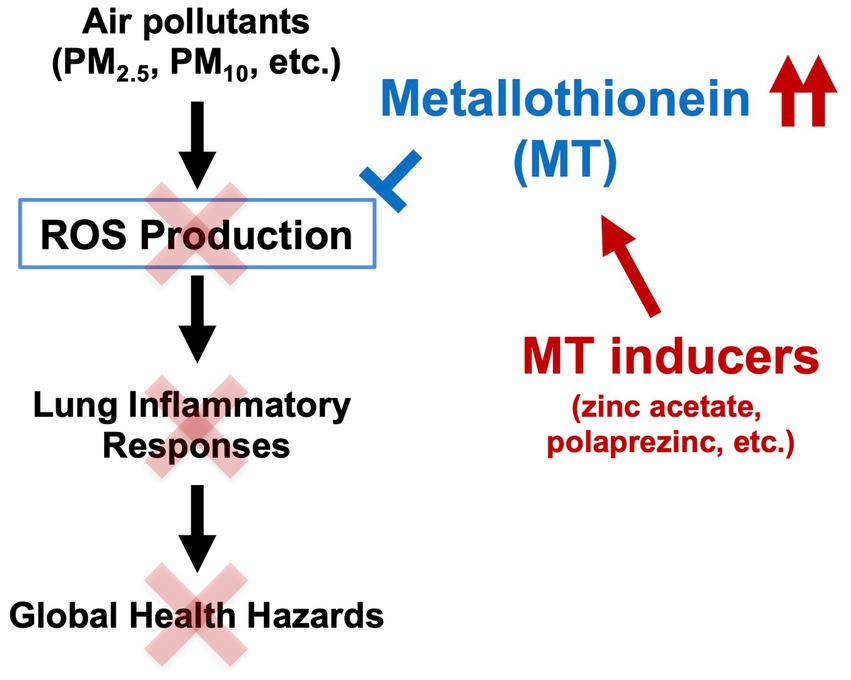
SCHEME 1. Mechanisms of acute lung injury induced by air pollutants and preventive effects of metallothionein. Metallothionein inducers, such as zinc acetate or polaprezinc, exert their antioxidant effects via induction of metallothionein in the mouse lung, ultimately preventing acute lung injury caused by air pollutants.
3 Future perspective of our study
To establish metallothionein inducers as a method for preventing health hazards caused by air pollution, several issues remain to be resolved, and we are currently attempting to resolve them. First, if zinc acetate is to be applied as a method of preventing health hazards caused by air pollution, it is necessary to discover the optimal method of administration. In our recent study, we conducted an efficacy analysis of intraperitoneal administration of zinc acetate (23); however, its clinical application in the intraperitoneal setting is not very realistic. The results of clinical trials investigating the pharmacokinetics of oral zinc acetate have already been reported. Following the administration of 50 mg of zinc acetate to healthy adult males, the AUC0–24 h was 672.44 ± 165.38 μg•h/dL and the Cmax was 144.67 ± 31.11 μg/dL (https://pins.japic.or.jp/pdf/newPINS/00071288.pdf). Although inhaled zinc acetate has not yet been clinically applied, it is simple and can deliver zinc acetate directly to the respiratory tract damaged by air pollutants. Therefore, we believe that these administration methods are clinically feasible and that the efficacy of zinc acetate should be analyzed using these methods. In addition, we also need to clarify in pre-clinical studies the side effects that may occur with prolonged clinical use of zinc acetate in these dosing regimens. It is well known that insufficient zinc intake in humans can cause health problems, but excessive zinc intake can cause acute health problems, such as nausea, vomiting, loss of appetite, abdominal cramps, diarrhea, and headaches (25). Moreover, chronic zinc intake is associated with adverse effects on urinary physiology (26). We believe that the effectiveness of oral or inhalation administration of zinc acetate in the treatment of air pollutant-dependent lung injury, while also conducting safety studies on zinc acetate, is likely to lead to practical applications. In addition, as previous studies have only examined zinc acetate administration before treatment, it is important to analyze the efficacy of zinc acetate administration after exposure to urban aerosols in future studies.
Furthermore, our model of air pollutant-dependent acute lung injury has limitations. In our previous studies, acute lung injury was assessed 24–48 h after a single intratracheal administration of urban aerosols (8, 23, 27). People living in areas with severe air pollution are repeatedly exposed to air pollutants over long periods, which can lead to devastating health consequences. In particular, long-term exposure to air pollutants has been reported to induce respiratory symptoms similar to the clinical manifestations of COPD, pneumonia, and asthma (28–32). Our model of acute lung injury due to a single exposure to air pollutants is very useful for people who travel to areas with severe air pollution in the short term. However, to solve global health problems, it is important to establish animal and cellular models for long-term repeated exposure to air pollutants and to analyze the efficacy of metallothionein inducers, such as zinc acetate, based on indicators such as the degree of inflammatory response, tissue damage, and respiratory dysfunction. We are convinced that such efforts will lead to a solution for global health problems.
4 Potential clinical application of MT inducers other than zinc acetate
It has been reported that various metals other than zinc, such as cadmium and iron, induce metallothionein through pathways mediated by metal regulatory transcription factor 1 (MTF-1) and metal responsive element (MRE). In addition, it has been reported that ROS, glucocorticoids, and various cytokines induce metallothionein through antioxidant responsive element (ARE), glucocorticoid responsive element (GRE), and activator protein 1 (AP-1) sites (14–16). Our research group is focusing on selenium as a metal other than zinc. Selenium is an essential trace element present in the active centers of antioxidant enzymes, such as glutathione peroxidase (GPX) and thioredoxin reductase (TrxR) (33, 34). Selenium has been reported to alleviate cadmium-induced cerebellar injury by activating metal responses, including metallothionein induction via MTF-1, in both in vivo and in vitro models (35). For these reasons, we speculate that selenium, like zinc, may be effective in preventing air pollutant-dependent lung injury via metallothionein induction, and we look forward to further research developments. We would argue that establishing metallothionein inducers, such as zinc and selenium, as a method of preventing health problems caused by air pollution would be one of the major contributions to public health and would also help reduce healthcare costs in an aging society.
5 Limitations of our previous study
The animal model used in previous studies was a model of acute lung injury caused by a single dose of urban aerosol. In future studies, it is extremely important to establish a chronic lung injury model resulting from multiple doses of urban aerosols and analyze the preventive effects of metallothionein.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Animal Care Committee of Musashino University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
K-iT: Conceptualization, Funding acquisition, Investigation, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Kakenhi Kiban (C) 22K06588 and 25K09977), Takeda Science Foundation, and DAIGAKUTOKUBETSU KENKYUHI Grant (Musashino University).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author declares that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lelieveld, J, Klingmuller, K, Pozzer, A, Poschl, U, Fnais, M, Daiber, A, et al. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J. (2019) 40:1590–6. doi: 10.1093/eurheartj/ehz135
2. Beloconi, A, and Vounatsou, P. Substantial reduction in particulate matter air pollution across Europe during 2006-2019: a spatiotemporal modeling analysis. Environ Sci Technol. (2021) 55:15505–18. doi: 10.1021/acs.est.1c03748
3. Losacco, C, and Perillo, A. Particulate matter air pollution and respiratory impact on humans and animals. Environ Sci Pollut Res Int. (2018) 25:33901–10. doi: 10.1007/s11356-018-3344-9
4. Hou, T, Zhu, L, Wang, Y, and Peng, L. Oxidative stress is the pivot for PM2.5-induced lung injury. Food Chem Toxicol. (2024) 184:114362. doi: 10.1016/j.fct.2023.114362
5. Mudway, IS, Kelly, FJ, and Holgate, ST. Oxidative stress in air pollution research. Free Radic Biol Med. (2020) 151:2–6. doi: 10.1016/j.freeradbiomed.2020.04.031
6. Mori, I, Sun, Z, Ukachi, M, Nagano, K, Mcleod, CW, Cox, AG, et al. Development and certification of the new NIES CRM 28: urban aerosols for the determination of multielements. Anal Bioanal Chem. (2008) 391:1997–2003. doi: 10.1007/s00216-008-2076-y
7. Ikuta, S, Chuang, VT, Ishima, Y, Nakajou, K, Furukawa, M, Watanabe, H, et al. Albumin fusion of thioredoxin--the production and evaluation of its biological activity for potential therapeutic applications. J Control Release. (2010) 147:17–23. doi: 10.1016/j.jconrel.2010.05.020
8. Tanaka, KI, Kubota, M, Shimoda, M, Hayase, T, Miyaguchi, M, Kobayashi, N, et al. Thioredoxin-albumin fusion protein prevents urban aerosol-induced lung injury via suppressing oxidative stress-related neutrophil extracellular trap formation. Environ Pollut. (2021) 268:115787. doi: 10.1016/j.envpol.2020.115787
9. Li, J, Li, H, Li, H, Guo, W, An, Z, Zeng, X, et al. Amelioration of PM2.5-induced lung toxicity in rats by nutritional supplementation with fish oil and vitamin E. Respir Res. (2019) 20:76. doi: 10.1186/s12931-019-1045-7
10. Li, W, Wilker, EH, Dorans, KS, Rice, MB, Schwartz, J, Coull, BA, et al. Short-term exposure to air pollution and biomarkers of oxidative stress: the Framingham heart study. J Am Heart Assoc. (2016) 5:e002742. doi: 10.1161/JAHA.115.002742
11. Lin, Y, Ramanathan, G, Zhu, Y, Yin, F, Rea, ND, Lu, X, et al. Pro-oxidative and proinflammatory effects after traveling from Los Angeles to Beijing: a biomarker-based natural experiment. Circulation. (2019) 140:1995–2004. doi: 10.1161/CIRCULATIONAHA.119.042054
12. Piao, CH, Fan, Y, Nguyen, TV, Shin, HS, Kim, HT, Song, CH, et al. PM2.5 exacerbates oxidative stress and inflammatory response through the Nrf2/NF-kappaB signaling pathway in OVA-induced allergic rhinitis mouse model. Int J Mol Sci. (2021) 22:8173. doi: 10.3390/ijms22158173
13. Davis, SR, and Cousins, RJ. Metallothionein expression in animals: a physiological perspective on function. J Nutr. (2000) 130:1085–8. doi: 10.1093/jn/130.5.1085
14. Ruttkay-Nedecky, B, Nejdl, L, Gumulec, J, Zitka, O, Masarik, M, Eckschlager, T, et al. The role of metallothionein in oxidative stress. Int J Mol Sci. (2013) 14:6044–66. doi: 10.3390/ijms14036044
15. Schanz, M, Schaaf, L, Dippon, J, Biegger, D, Fritz, P, Alscher, MD, et al. Renal effects of metallothionein induction by zinc in vitro and in vivo. BMC Nephrol. (2017) 18:91. doi: 10.1186/s12882-017-0503-z
16. Yang, R, Roshani, D, Gao, B, Li, P, and Shang, N. Metallothionein: a comprehensive review of its classification, structure, biological functions, and applications. Antioxidants (Basel). (2024) 13:825. doi: 10.3390/antiox13070825
17. Lian, Y, Zhao, J, Wang, YM, Zhao, J, and Peng, SQ. Metallothionein protects against isoniazid-induced liver injury through the inhibition of CYP2E1-dependent oxidative and nitrosative impairment in mice. Food Chem Toxicol. (2017) 102:32–8. doi: 10.1016/j.fct.2017.01.016
18. Lv, Y, Zhang, B, Xing, G, Wang, F, and Hu, Z. Protective effect of naringenin against acetaminophen-induced acute liver injury in metallothionein (MT)-null mice. Food Funct. (2013) 4:297–302. doi: 10.1039/c2fo30213f
19. Wu, H, Zhou, S, Kong, L, Chen, J, Feng, W, Cai, J, et al. Metallothionein deletion exacerbates intermittent hypoxia-induced renal injury in mice. Toxicol Lett. (2015) 232:340–8. doi: 10.1016/j.toxlet.2014.11.015
20. Masters, BA, Kelly, EJ, Quaife, CJ, Brinster, RL, and Palmiter, RD. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci USA. (1994) 91:584–8. doi: 10.1073/pnas.91.2.584
21. Tanaka, KI, Shiota, S, Sakakibara, O, Shimoda, M, Takafuji, A, Takabatake, M, et al. Exacerbation of elastase-induced emphysema via increased oxidative stress in Metallothionein-knockout mice. Biomolecules. (2022) 12:583. doi: 10.3390/biom12040583
22. Kimura, K, Nakano, Y, Sugizaki, T, Shimoda, M, Kobayashi, N, Kawahara, M, et al. Protective effect of polaprezinc on cadmium-induced injury of lung epithelium. Metallomics. (2019) 11:1310–20. doi: 10.1039/c9mt00060g
23. Tanaka, KI, Uehara, Y, Shimoda, M, Funayama, R, Shiota, S, Yamaguchi, A, et al. Metallothionein, an endogenous antioxidant protein, protects against acute lung injury caused by air pollutants. Biomed Pharmacother. (2025) 185:117965. doi: 10.1016/j.biopha.2025.117965
24. Xu, G, Dong, F, Su, L, Tan, ZX, Lei, M, Li, L, et al. The role and therapeutic potential of nuclear factor kappaB (NF-kappaB) in ischemic stroke. Biomed Pharmacother. (2024) 171:116140. doi: 10.1016/j.biopha.2024.116140
25. Lewis, MR, and Kokan, L. Zinc gluconate: acute ingestion. J Toxicol Clin Toxicol. (1998) 36:99–101. doi: 10.3109/15563659809162595
26. Johnson, AR, Munoz, A, Gottlieb, JL, and Jarrard, DF. High dose zinc increases hospital admissions due to genitourinary complications. J Urol. (2007) 177:639–43. doi: 10.1016/j.juro.2006.09.047
27. Tanaka, KI, Nakaguchi, S, Shiota, S, Nakada, Y, Oyama, K, Sakakibara, O, et al. Preventive effect of epigallocatechin gallate, the main component of green tea, on acute lung injury caused by air pollutants. Biomolecules. (2022) 12:1196. doi: 10.3390/biom12091196
28. Katanoda, K, Sobue, T, Satoh, H, Tajima, K, Suzuki, T, Nakatsuka, H, et al. An association between long-term exposure to ambient air pollution and mortality from lung cancer and respiratory diseases in Japan. J Epidemiol. (2011) 21:132–43. doi: 10.2188/jea.je20100098
29. Li, J, Sun, S, Tang, R, Qiu, H, Huang, Q, Mason, TG, et al. Major air pollutants and risk of COPD exacerbations: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. (2016) 11:3079–91. doi: 10.2147/COPD.S122282
30. Liu, S, Jorgensen, JT, Ljungman, P, Pershagen, G, Bellander, T, Leander, K, et al. Long-term exposure to low-level air pollution and incidence of asthma: the ELAPSE project. Eur Respir J. (2021) 57:2003099. doi: 10.1183/13993003.030992020
31. Scibor, M, Balcerzak, B, Galbarczyk, A, and Jasienska, G. Associations between daily ambient air pollution and pulmonary function, asthma symptom occurrence, and quick-relief inhaler use among asthma patients. Int J Environ Res Public Health. (2022) 19:4852. doi: 10.3390/ijerph19084852
32. Wang, L, Xie, J, Hu, Y, and Tian, Y. Air pollution and risk of chronic obstructed pulmonary disease: the modifying effect of genetic susceptibility and lifestyle. EBioMedicine. (2022) 79:103994. doi: 10.1016/j.ebiom.2022.103994
33. Cai, Z, Zhang, J, and Li, H. Selenium, aging and aging-related diseases. Aging Clin Exp Res. (2019) 31:1035–47. doi: 10.1007/s40520-018-1086-7
34. Lee, KH, and Jeong, D. Bimodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: the selenium paradox (review). Mol Med Rep. (2012) 5:299–304. doi: 10.3892/mmr.2011.651
Keywords: metallothionein, antioxidant, endogenous protein, oxidative stress, PM2.5, lung injury
Citation: Tanaka K-i (2025) Toward the application of metallothionein inducers as a method of prevention of air pollutant-dependent lung injury. Front. Public Health. 13:1652263. doi: 10.3389/fpubh.2025.1652263
Edited by:
Hector Gonzalez-Iglesias, Spanish National Research Council (IPLA-CSIC), SpainReviewed by:
Yazmin Debray, Instituto Nacional de Enfermedades Respiratorias, MexicoCopyright © 2025 Tanaka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ken-ichiro Tanaka, ay10YW5hQG11c2FzaGluby11LmFjLmpw
 Ken-ichiro Tanaka
Ken-ichiro Tanaka