- 1Department of Neurosurgery, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, China
- 2Department of Computer Science and Information Technologies, University of A Coruña, A Coruña, Spain
- 3School of Life Science, South China Normal University, Guangzhou, China
Background: Subarachnoid hemorrhage (SAH) is increasingly recognized as a PM2.5-linked neurological emergency, yet global spatiotemporal burden evidence across socioeconomic, demographic, and geographic subgroups remains scarce, impeding tarsgeted prevention. This study quantifies current burden, trends, and future SAH projections attributable to PM2.5 using the latest data.
Methods: Using data from the Global Burden of Disease Study 2021, we analyzed deaths and disability-adjusted life years (DALYs) from SAH attributable to ambient PM2.5 pollution (1990–2021) across 204 countries/territories, stratified by age, sex, region, and Socio-demographic Index (SDI). Temporal trends were quantified using estimated annual percentage changes (EAPCs), and Bayesian age-period-cohort modeling projected disease burden through 2050.
Results: Between 1990 and 2021, global age-standardized mortality (ASMR) and DALY rates (ASDR) for PM2.5-related SAH declined by 36% (0.99 to 0.63 per 100,000) and 34% (27.42 to 17.96 per 100,000), respectively. However, absolute deaths surged 40% (38,130 to 53,562), driven by aging populations and demographic shifts. Burden disparities were stark: Middle SDI regions had the highest ASMR (1.07, 95% UI, 0.68–1.43) and ASDR (27.42, 95% UI: 17.96–35.65), while high SDI regions achieved the steepest declines (−67% ASMR). South Asia (+246% deaths) and Southeast Asia (+147% deaths) experienced the most rapid mortality growth, contrasting with East Asia’s high absolute burden (229,553 deaths in 2021). Males faced higher risks (ASMR: 0.72, 95% UI: 0.48–0.99) compared with females (0.55, 95% UI: 0.36–0.75). In South Asia, the female mortality share was rising from 31 to 41%. Mongolia had the highest national burden [2.49 (95% UI, 1.23–3.82) and ASDR of 61.92 (95% UI, 30.6–93.24)], while Central Asia and Southern Sub-Saharan Africa exhibited worsening trends. Projections indicate a resurgence in ASMR and ASDR by 2050, disproportionately impacting low-middle SDI regions.
Conclusion: Despite declining age-standardized rates, a 40% surge in absolute PM2.5-attributable SAH deaths over three decades, due to aging populations and regional inequalities (e.g., South Asia +246% deaths, Middle SDI highest ASMR), demands urgent air-quality and healthcare policies for high-growth Asian and African regions and vulnerable low-middle SDI populations to curb projected 2050 increases.
Introduction
Subarachnoid hemorrhage (SAH), a devastating form of hemorrhagic stroke caused by bleeding into the subarachnoid space, represents a critical global health challenge with substantial regional variations in disease burden (1, 2). SAH, a devastating form of hemorrhagic stroke caused by bleeding into the subarachnoid space, accounts for 5–10% of all stroke cases globally and is associated with high mortality rates, particularly in regions such as Central Asia and Eastern Europe (3). According to the Global Burden of Disease Study 2021, while the crude incidence of SAH increased by 37.09% from 1990 to 2021, the age-standardized incidence rates decreased significantly (EAPC: -1.52; 95% UI -1.66 to −1.37) (4). International comparisons reveal striking disparities, with the highest age-standardized incidence rates observed in the high-income Asia Pacific region at 14.09 per 100,000 population, while regions such as East Asia experienced substantial decreases with an estimated annual percentage change of −3.60 (1, 4). The global incidence declined from 10.2 per 100,000 person-years in 1980 to 6.1 in 2010, with notable variations according to region, blood pressure levels, and smoking prevalence (5). These epidemiological disparities between countries and regions highlight the necessity for comprehensive global research initiatives to understand underlying risk factors, optimize prevention strategies, and improve treatment protocols across diverse populations and healthcare systems.
The emerging evidence for environmental pollutants as modifiable risk factors for SAH has garnered increasing attention in recent epidemiological research, with multiple studies demonstrating significant associations between air pollution exposure and cerebrovascular events (6, 7). PM2.5, a pollutant capable of penetrating the bloodstream and inducing systemic inflammation and oxidative stress, has been linked to cerebrovascular damage and aneurysm rupture (8–10). Recent studies suggest that even short-term exposure to PM2.5 increments as low as 1 μg/m3 may elevate SAH risk by 1.7%, underscoring its public health significance (11). A landmark study in South Korea revealed gender-specific associations, showing that districts with higher interquartile range concentrations of NO₂ (12.2 ppb), SO₂ (1.41 ppb), and PM₁₀ (9.4 μg/m3) had 1.06, 1.06, and 1.05-fold higher mortality rates from SAH in females, respectively, while no significant associations were observed in male (12). This finding is corroborated by research indicating that air pollution effects on stroke mortality demonstrate stronger associations in women than men, potentially due to differential susceptibility mechanisms (13). Additional research from Seoul demonstrated that among meteorological and pollutant variables, ozone was independently associated with subarachnoid hemorrhage occurrence (14), while global burden studies indicate that air pollution-related stroke deaths, including SAH, reached 1,989,686 deaths globally in 2021 (15). These findings collectively emphasize the critical importance of environmental pollution as a modifiable risk factor for SAH and highlight the urgent need for public health interventions targeting air quality improvement as a strategy for cerebrovascular disease prevention.
Despite growing recognition of PM2.5’s role in SAH, critical knowledge gaps persist. First, the burden of PM2.5-attributable SAH across diverse geographic and demographic subgroups remains poorly quantified. For instance, sex-specific susceptibility (e.g., heightened male vulnerability potentially tied to vascular physiology) (16), and age-related disparities (e.g., increased risks in children and the older adults due to developmental or immunosenescence factors) (17, 18) warrant systematic investigation. Second, socioeconomic inequities, as reflected by the Socio-Demographic Index (SDI), may exacerbate PM2.5-related SAH burdens in low-resource settings with limited air quality regulations (19–21). Third, while prior ecological studies have examined regional PM2.5-SAH associations (22, 23), no global analysis has projected long-term trends to inform policy planning. Therefore, this study uses the latest GBD 2021 data to assess the global disease burden (mortality and DALYs) of SAH caused by PM2.5 (both APMP and HAP), analyze the trends from 1990 to 2021 with the EAPC to better understand the complex patterns, forecast the future burden of PM2.5-attributable SAH until 2050, and examine these burdens and trends across different countries, regions, genders, and age groups.
Methods
Study data
The GBD 2021 delivers through evaluation of disease, injury, and risk factor burden across 204 countries and territories, encompassing 88 risk factors (24, 25). This research offers findings on incidence, prevalence, mortality, DALYs, YLDs, and YLL for 371 diseases in 204 countries and regions. It uses data on 88 risk factors from 1990–2021, along with associated uncertainty intervals (UIs). Study data were sourced from the GBD 2021, accessible through https://ghdx.healthdata.org/gbd-2021. The risks are organized into a four-tier hierarchy: Level 1 encompasses environmental, occupational, behavioral, and metabolic risks; Level 2 details include 20 broader categories such as air pollution and high BMI; Level 3 encompasses more nuanced risks, including particulate matter pollution and child growth failure, representing some of the most detailed categorizations. Further refinement occurs at Level 4, which breaks down risks from Level 3 into even more specific classification, such as ambient particulate matter pollution and child stunning. The four-level risk hierarchy is based on the well-established comparative risk assessment (CRA) framework developed by the Global Burden of Disease (GBD) Study, which has been widely adopted as the international standard for risk factor quantification (26, 27). Data processing followed standardized GBD protocols (24, 25). Input data underwent: Quality grading (0–5 stars based on completeness, diagnostic specificity, and representativeness), Bias adjustment via spatial–temporal meta-regression, Ensemble modeling integrating 43 cause-of-death models. This framework replaces conventional systematic review tools by directly quantifying uncertainty from source heterogeneity.
The Socio-demographic Index (SDI) measures development by amalgamating income, education, and fertility data, classifying regions into five development stages from Low to High SDI, reflecting population wealth and education levels. Specifically, the SDI ranges are as follows: Low SDI from 0 to 0.4658, low-middle SDI from 0.4658 to 0.6188, Middle SDI from 0.6188 to 0.7120, High-middle SDI from 0.7120 to 0.8103, and High SDI from 0.8103 to 1.
In the 10th edition of the International Classification of Disease (ICD-10), subarachnoid hemorrhage is classified under codes I60-I60.9, I62.0, I67.0-I67.1, and I69.0. The ICD-10 assigns the code 430–430.9 to subarachnoid hemorrhage.
DALY (Disability-Adjusted Life Years): A summary measure of population health that quantifies the burden of disease by combining years of life lost due to premature mortality and years lived with disability, weighted by the severity of the disability (28). ASMR (Age-Standardized Mortality Rate): A mortality rate that has been adjusted to account for differences in age structure between populations, allowing for valid comparisons across different populations and time periods (29). ASDR (Age-Specific Death Rate): The number of deaths in a specific age group per 100,000 population in that same age group during a given time period (30).
Definition of ambient PM2.5
Ambient particulate matter pollution refers to the annual average concentration of PM2.5, particles smaller than 2.5 micrometers in diameter, in the air, weighted by population exposure. This estimation integrates data from various sources, such as satellite aerosol observations, ground-level air quality monitors, chemical transport models, demographic data, and land-use information. For the GBD 2021, the dataset was expanded with newer ground monitor readings from both pre-existing and newly added sites. Additional contributions to the database came from sources such as the European Environment Agency, the United States Environmental Protection Agency, and the OpenAQ initiative (31).
Statistical analysis
The Estimated Annual Percentage Change (EAPC) serves as a pivotal indicator for monitoring the progression of Age-Standardized Rate (ASR) across time. It is determined within a regression framework defined as y = α + βx + ε, where y corresponds to the annual rate change to per 100,000 individuals, α represents the intercept, β represents the slope, x is the calendar year, and ε is the error term. The EAPC calculation is based on the formula:
With the 95% confidence interval (CI) extracted directly from the linear regression model parameters. Statistical significance is established for two-sided p-values below 0.05.
In parallel, the Pearson correlation coefficient was applied to evaluate the relationship between ASR and the SDI, with significance indicated by p-values less than 0.001. These analyses were conducted using R software, version 4.4.1.
Projection analysis
The Bayesian Age-Period-Cohort (BAPC) R package is a statistical tool designed for projecting future disease burdens using a Bayesian framework (29). We employed age-specific population data spanning from 1990 to 2021, along with projections for 2022 to 2050, to assess trends in mortality and DALYs among populations. For our analysis, we adhered to the standard parameters provided with the BAPC packages, leveraging its default settings to effectively model.
Results
Global PM2.5-attributable SAH burden by regions from 1990 to 2021
The trends in age-standardized mortality rates (ASMR) and age-standardized DALY rates (ASDR) for SAH attributed to ambient PM2.5 have shown a downward trend from 1990 to 2021, except for regions with low-middle SDI (Figure 1 and Supplementary Figure S1). Despite a decline in ASMR from 0.99 (95% UI, 0.60–1.50) in 1990 to 0.63 (95% UI, 0.43–0.82) in 2021, the death toll rose from 38,129.84 (95% UI, 23,179.25–57695.62) in 1990 to 53,561.65 (95% UI, 36,516.80-69,717.63) in 2021. Within the spectrum of SDI regions, middle SDI exhibited the highest ASMR and ASDR at 1.07 and 27.42, respectively. In addition, high SDI regions experienced the most pronounced decline in ASMR and ASDR, from 0.66 and 20.9 in 1990 to 0.22 and 7.14 in 2021. Turning to gender differences, ASMR for SAH due to PM2.5 was higher in male at 0.72 compared to female at 0.55 in 2021 (Tables 1, 2).
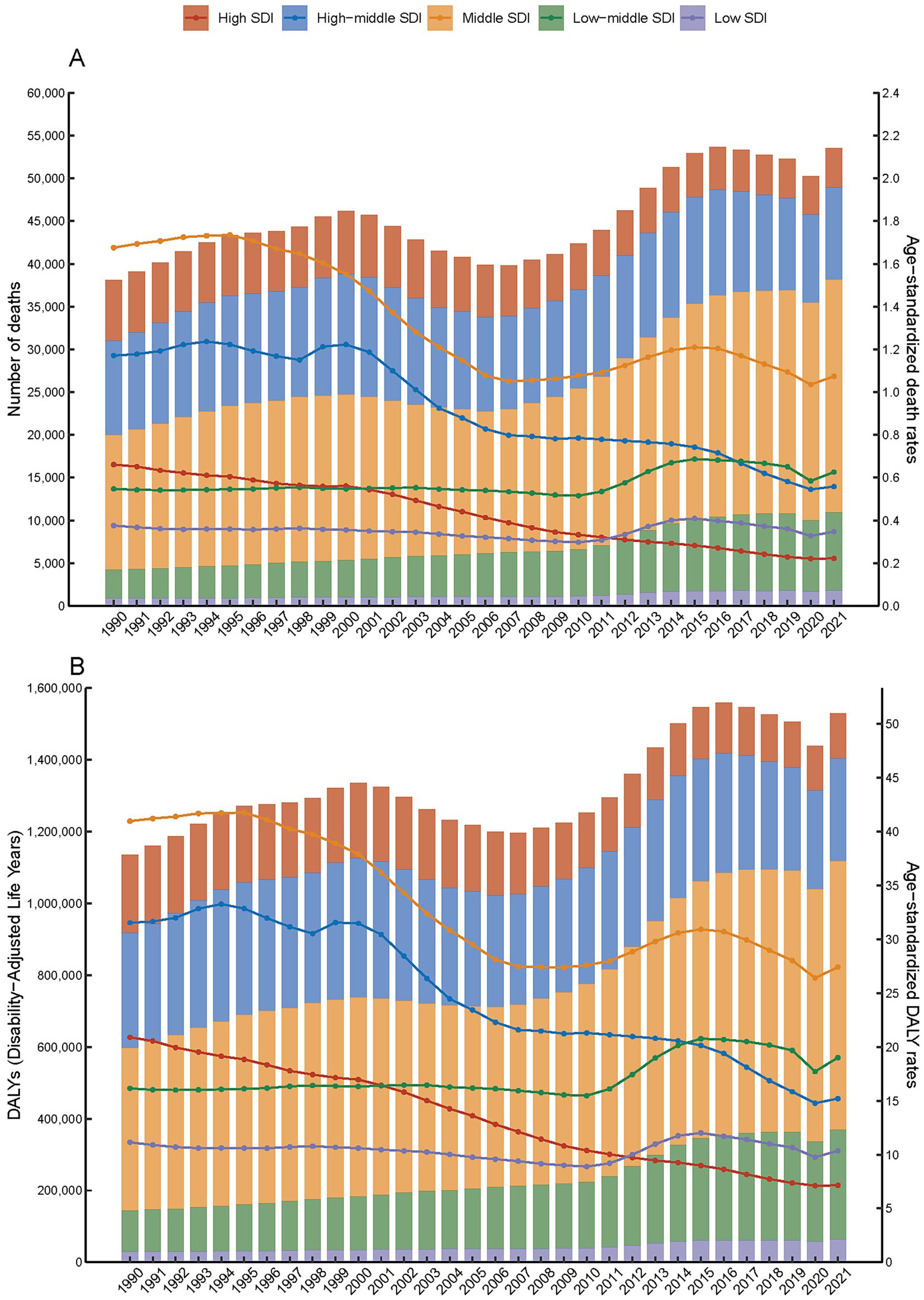
Figure 1. Number and age-specific rates of disease burden (A. number of death and age-standardized death rates; B. DALYs and age-standardized DALYs rates) for subarachnoid hemorrhage attributed to PM2.5 across five SDI quintiles, 1990–2021.
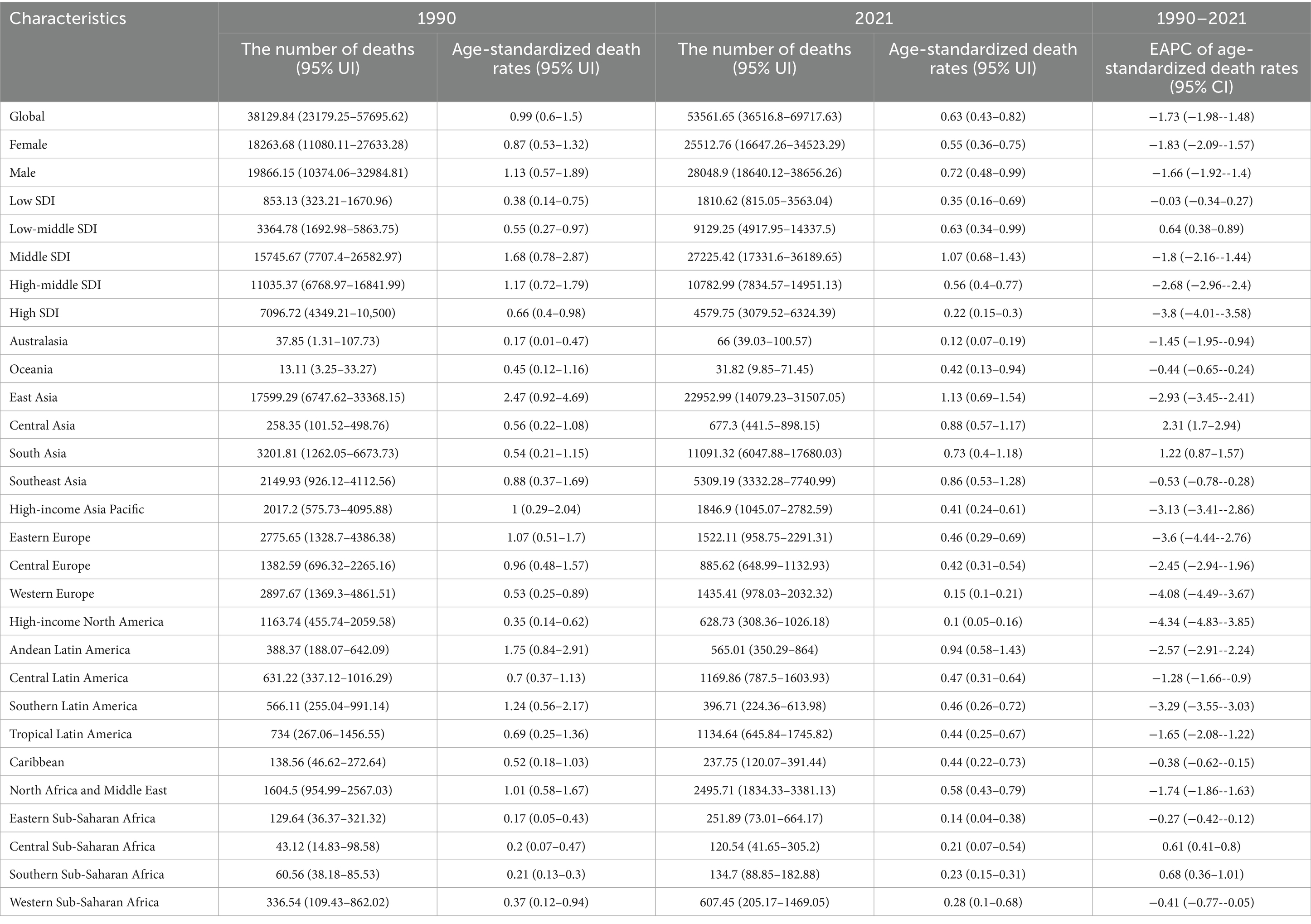
Table 1. Trends in PM2.5-Attributable subarachnoid hemorrhage mortality from 1990 to 2021 by geographic region.

Table 2. Trends in PM2.5-attributable subarachnoid hemorrhage DALYs from 1990 to 2021 by geographic region.
Among the 21 GBD regions, South Asia and Southeast Asia experienced the most notable increase in SAH deaths attributed to PM2.5 by 2021 (Figure 2). Death numbers in South and Southeast Asia increased from 3,201.81 and 2,149.93 in 1990 to 11,091.32 and 5,309.19 in 2021. Nevertheless, East Asia bore the greatest burden, with the highest death and DALY figures peaking at 229,552.99 and 563,441.41 (Figure 2, Supplementary Figure S1, and Tables 1, 2). In contrast, high SDI regions such as Australasia and Oceania exhibited lower PM2.5-attributable SAH burdens, with death numbers recorded at 66 (95% UI, 39.03–100.57) and 31.82 (95% UI, 9.85–71.45), and DALY numbers at 1,630.4 (95% UI, 964.32–2,422.15) and 1,204.59 (95% UI, 378.66–2,724.32) (Tables 1, 2). Furthermore, in South Asia, the proportion of female deaths increased significantly, from 31.0% in 1990 to 40.9% in 2021 (Figure 2).
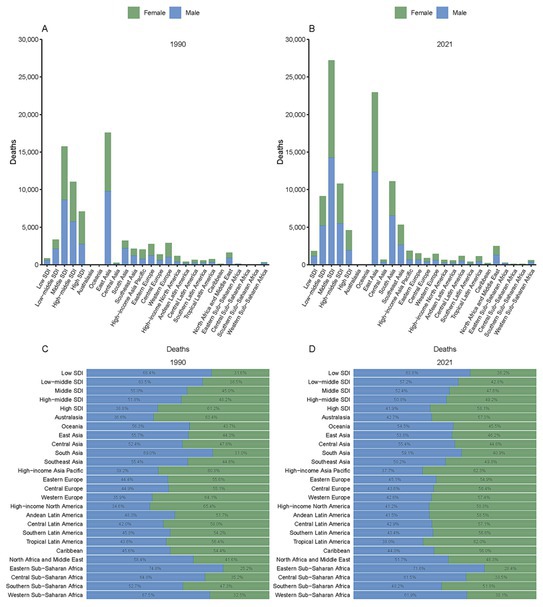
Figure 2. Deaths from subarachnoid hemorrhage attributed to PM2.5 by GBD and SDI regions in 1990 (A) and 2021 (B), with proportional distributions in 1990 (C) and 2021 (D).
Global trends of PM2.5-related SAH burden
Nationally, Mongolia had the highest burden of subarachnoid hemorrhage attributable to ambient PM2.5, with an ASMR of 2.49 (95% UI, 1.23–3.82) and ASDR of 61.92 (95% UI, 30.60–93.24) in 2021. Moreover, the EAPC in ASMR showed the most significant increasing trend at 5.26 (95% CI, 4.69–5.84) (Supplementary Tables S1, S2). Similarly, increasing trends in ASMR and ASDR were noted across South Asia, Central Asia and Southern Sub-Saharan Africa (Figure 3).
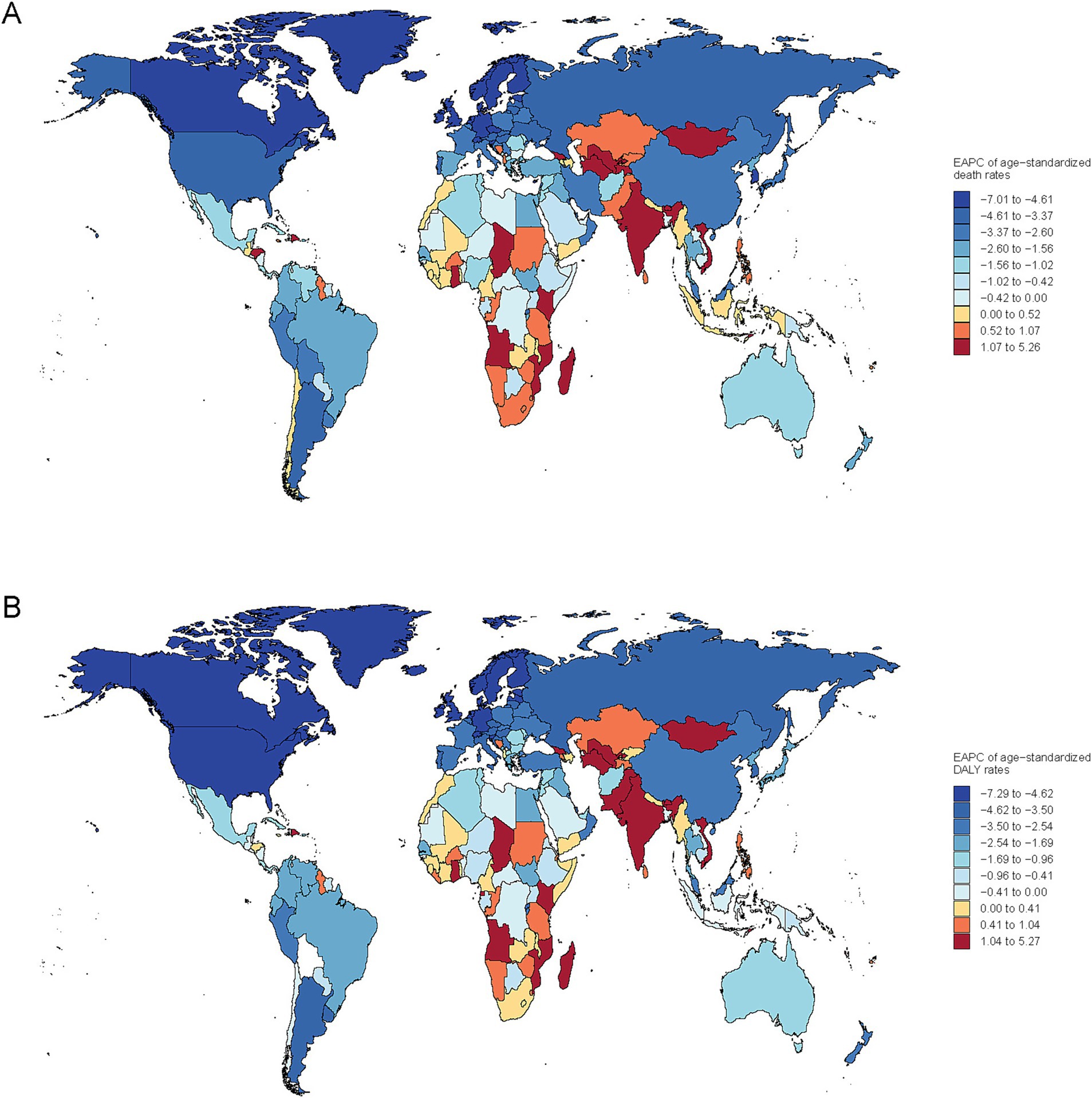
Figure 3. Global map of EAPC for age-standardized rates of subarachnoid hemorrhage due to PM2.5 for deaths (A) and DALYs (B).
Conversely, on a global scale, the EAPC for both ASMR and ASDR showed a decline in most countries, particularly in North America, Europe, Oceania, and South America. Notably, exhibited the most pronounced decreases in EAPC for ASMR and ASDR, at −7.01 (95% CI, −7.48 to −6.53) and −7.25 (95% CI, −7.66 to −6.84), respectively.
Age-specific global burden of SAH due to PM2.5: 1990–2021
Between 1990 and 2021, the PM2.5-attributable death and DALYs of SAH saw a consistent annual decline across all age groups. By 2021, the disease burden had lessened in comparison to 1990 for every age demographic (Figure 4 and Supplementary Figure S2). Notably, a downward trend in the burden for those over 50 years old was observed starting around 2000. For those under 50, although the death burden was comparatively minor, a decline has been noted since the early 2000s (Figure 4). The death among those aged 85 and above showed a brief increase between 1999 and 2003, followed by a decline. Between 1990 and 2021, there was a notable decrease in global age-stratified DALYs from SAH across all age groups. However, from 2012 to 2017, there was a slight increase in DALYs for individuals aged 70–74 (Supplementary Figure S2).
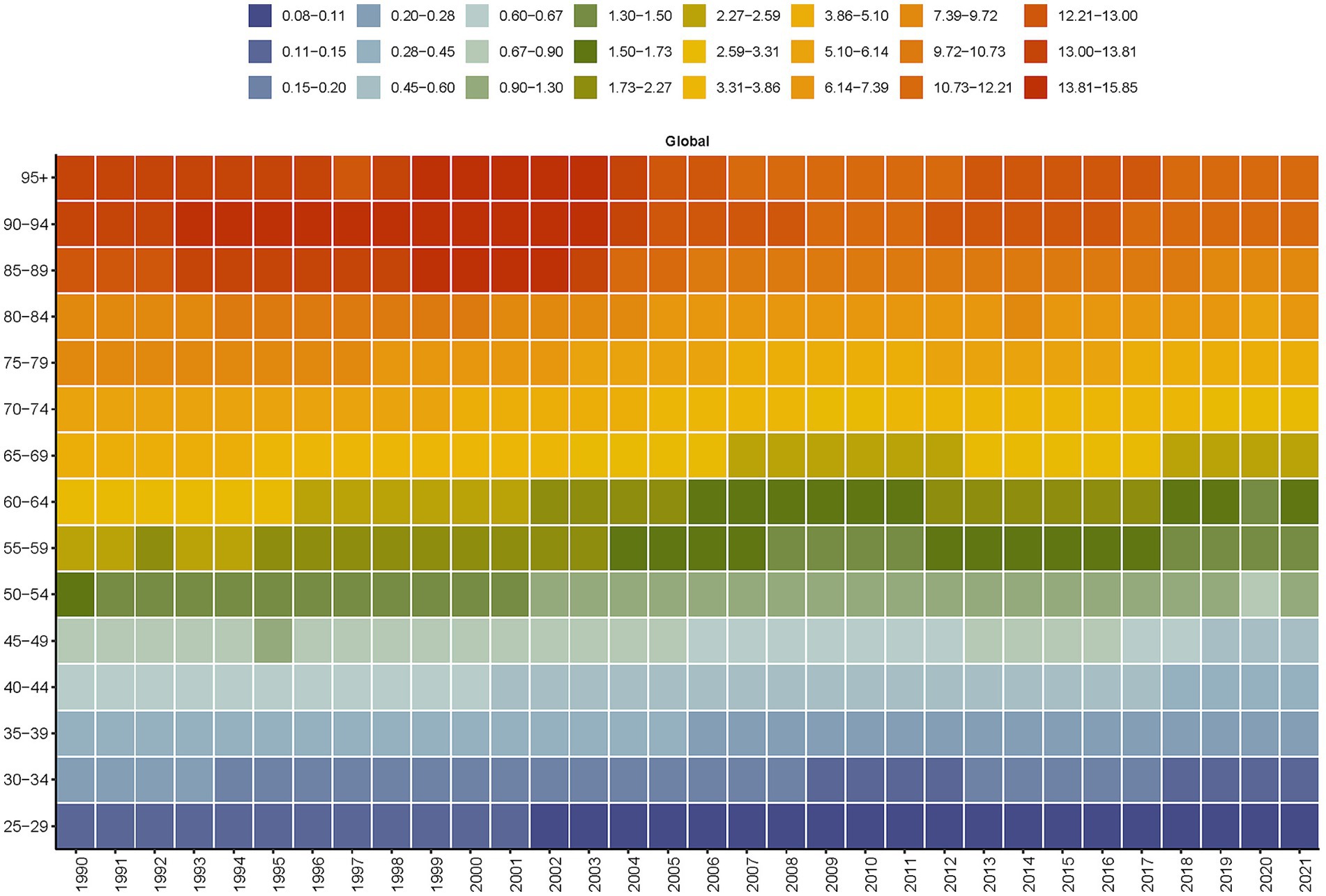
Figure 4. Global age-stratified deaths from subarachnoid hemorrhage attributable to PM2.5 from 1990 to 2021.
PM2.5-attributable SAH burden in 2021, SDI-stratified
In the comparative analysis of PM2.5-attributable SAH burden across countries and territories with varying SDI levels. It was found that the correlation between ASMR and SDI mirrored that between ASDR and SDI. However, a significant trend was not observed, with the p-value exceeding 0.05. The disease burden due to PM2.5 in SAH was predominantly greater in countries or regions with an SDI ranging from 0.6 to 0.7 and lower in those with an SDI below 0.4 or above 0.8 (Figure 5).
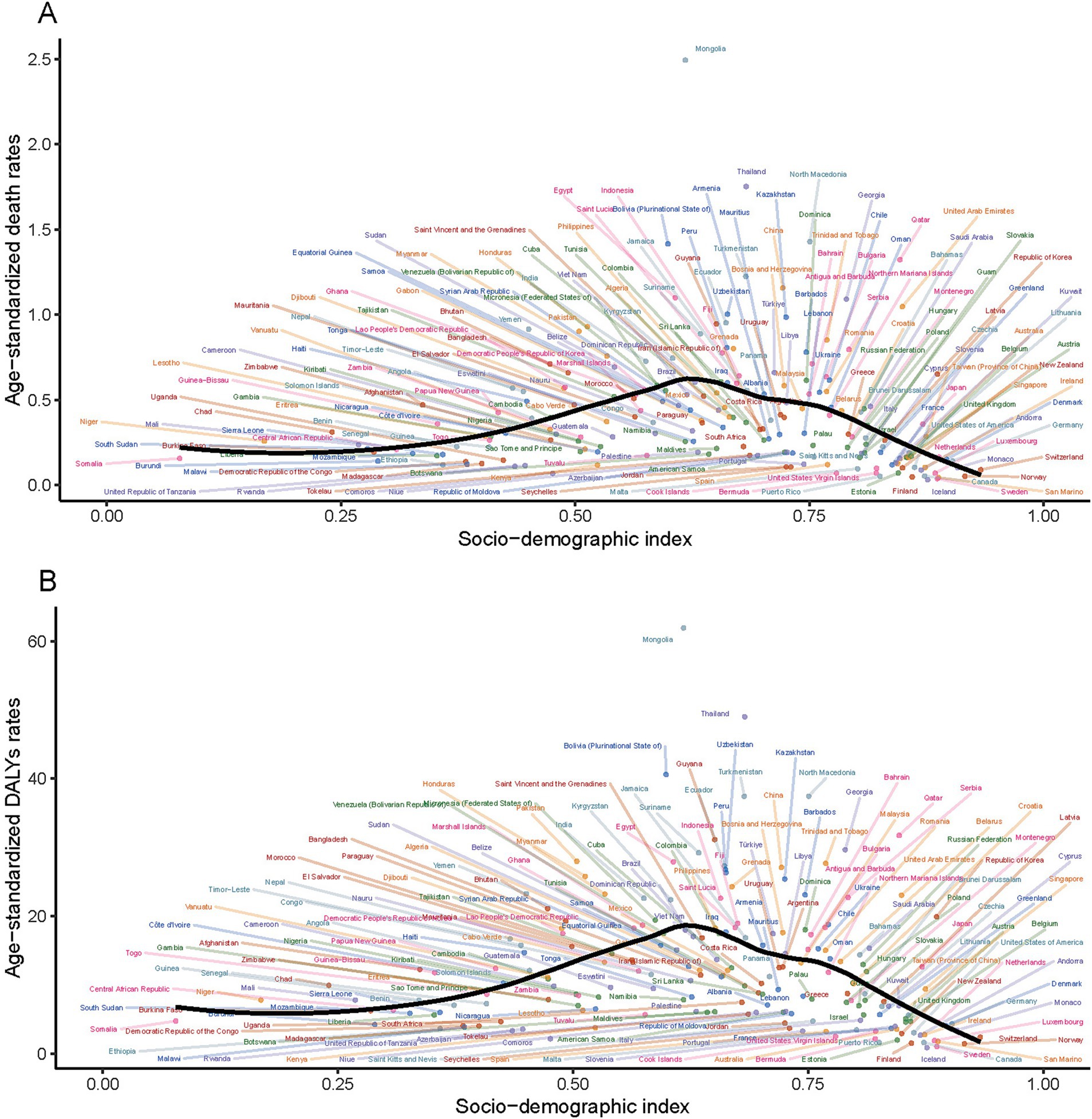
Figure 5. Association between age-standardized rates and the sociodemographic index for subarachnoid hemorrhage due to PM2.5 for deaths (A) and DALYs (B).
Future projection of global SAH burden
Between 1990 and 2021, the ASMR and ASDR for SAH attributable to ambient PM2.5 exhibited slightly fluctuations. This period witnessed a complex interplay of factors influencing the health outcomes related to ambient PM2.5 exposure. Looking ahead, from 2022 to 2050, there is a projected increase in both ASMR and ASDR for SAH due to ambient PM2.5, indicating a potential escalation in the disease burden (Figure 6 and Supplementary Figure S3).
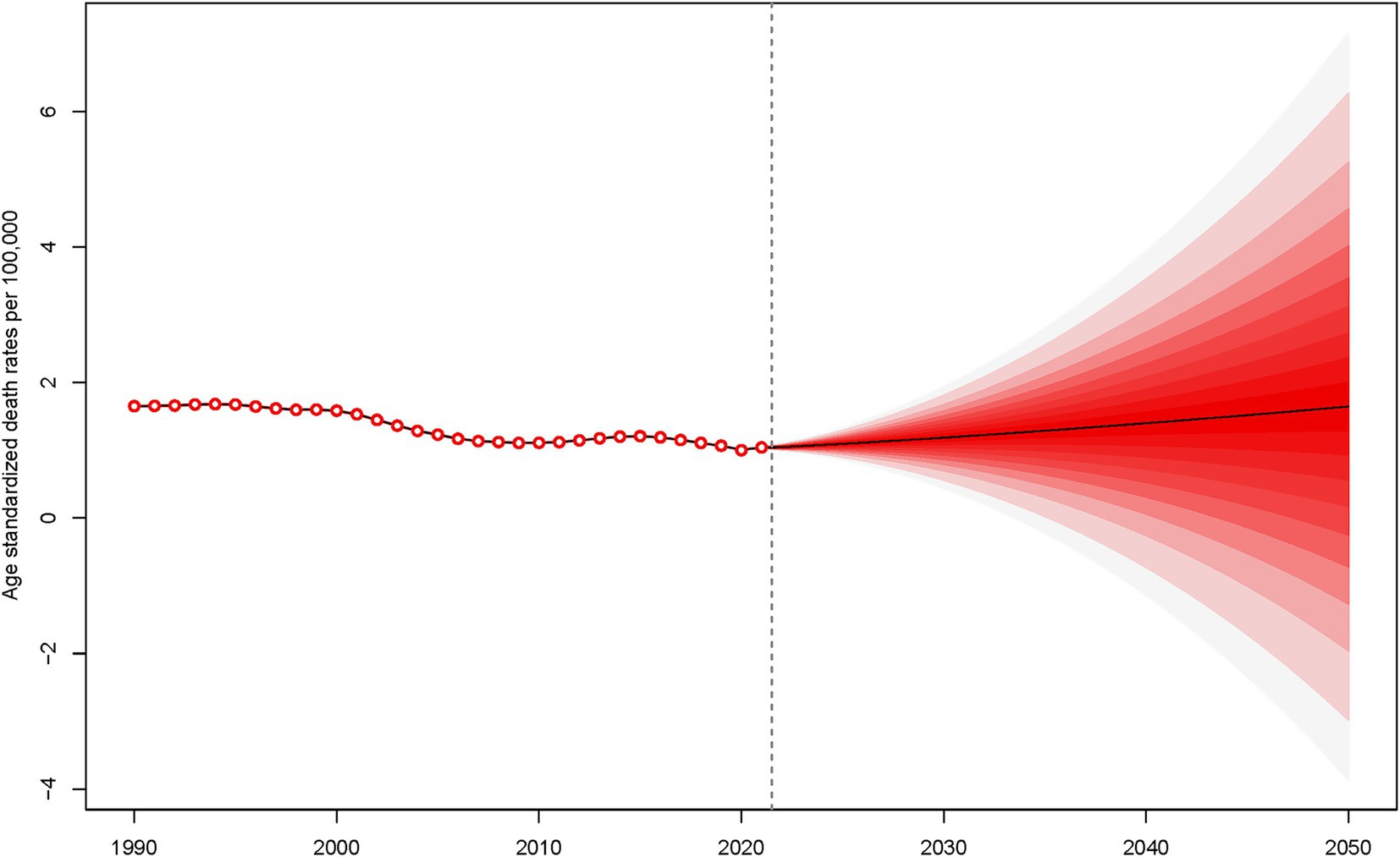
Figure 6. Projection of age-standardized death rates for subarachnoid hemorrhage due to PM2.5 from 2022 to 2050.
Discussion
Global trends and paradoxical findings
This study provides a comprehensive analysis of the global disease burden of SAH attributable to ambient PM2.5 pollution across regions, genders, age groups, and SDI levels from 1990 to 2021, with projections extending to 2050. While previous studies have investigated PM2.5-associated stroke burden, this work uniquely quantifies the sex-specific temporal trends and aging-related vulnerability patterns in SAH burden, while integrating future projections through robust modeling approaches (32–34).
The paradoxical 40.4% increase in absolute SAH deaths from 38,129.84 to 53,561.65 despite a 36.4% decline in age-standardized mortality rates (from 0.99 to 0.63 per 100,000) reflects the complex interplay of demographic and epidemiological factors over the 32-year study period. This apparent contradiction can be attributed to several key demographic transitions: (1) population aging dynamics-the global population aged ≥60 years increased by approximately 180% during this period, creating a substantially larger at-risk population despite improved per-capita risk profiles (35). (2) overall population growth - the world population expanded from 5.3 billion in 1990 to 7.9 billion in 2021, representing a 49% increase in the denominator for absolute case calculations (36). and (3) persistent environmental health disparities - while high-income regions achieved substantial PM2.5 reductions, rapid industrialization in South Asia and Sub-Saharan Africa maintained or increased exposure levels for large populations.
This demographic-epidemiologic paradox illustrates a critical limitation in public health assessment: age-standardized rates, while essential for comparing risk across populations and time periods, may underestimate the true societal burden when applied to aging populations with age-sensitive health outcomes. The divergence between these metrics emphasizes that successful risk reduction strategies at the individual level can be overwhelmed by demographic shifts, necessitating integrated approaches that address both exposure reduction and healthcare system capacity for aging populations. Furthermore, the absolute increase in deaths predominantly occurred in low-and middle-income countries (contributing 78% of the excess deaths), highlighting persistent global health inequities in environmental protection and healthcare access (37, 38).
Regional disparities and socioeconomic development index patterns
The pronounced regional disparities underscore differential progress in environmental health. High SDI regions achieved the most substantial improvements (ASMR: 0.66 to 0.22), likely reflecting stringent air quality standards and advanced healthcare systems (39). The 65.8% ASDR reduction in high SDI regions (20.9 to 7.14) demonstrates the effectiveness of integrated environmental-health interventions, providing a roadmap for middle SDI countries (40).
Conversely, middle SDI regions bear the highest SAH burden (ASMR 1.07), trapped in a developmental phase combining industrializing economies with insufficient pollution controls. These regions experience rapid industrialization, environmental pollution, and limited medical resources, leading to a higher disease burden of PM2.5-related SAH, particularly in South and East Asia (41). Low-middle SDI regions’ unfavorable trends may indicate persistent barriers in pollution control and healthcare access (42).
Geographical distribution and country-specific findings
The striking geographical disparities in subarachnoid hemorrhage burden attributable to ambient PM2.5 exposure reflect the complex interplay between environmental pollution levels, healthcare infrastructure, and socioeconomic development across different regions. Mongolia’s position as having the highest national burden, with an age-standardized mortality rate of 2.49 per 100,000 and a concerning annual increase of 5.26%, underscores the urgent need for targeted air quality interventions in this region.
South and Southeast Asia exhibited the steepest increases in PM2.5-attributable SAH deaths (South Asia: +246.5%, Southeast Asia: +147.0%), aligning with satellite-derived PM2.5 concentration trends showing population-weighted annual averages exceeding 75 μg/m3 in Bangladesh and India (43). These regions face compounded challenges including inadequate neurosurgical infrastructure (44) and limited implementation of WHO air quality guidelines (45, 46) creating a “double burden” of environmental and healthcare system deficiencies. The pronounced increasing trends observed in South Asia, Central Asia, and Southern Sub-Saharan Africa align with the rapid industrialization and urbanization occurring (Figure 3) (47, 48). Air pollution in South Asia results from a complex interplay of emission sources beyond industrial activities, including the combustion of solid fuels for cooking and heating, emissions from small industries such as brick kilns, the burning of municipal and agricultural waste, and cremation practices (49, 50). In contrast, East Asia’s substantial absolute burden (229,552.99 deaths in 2021) reflects legacy pollution effects from rapid industrialization, though recent policy interventions show promising declines in PM2.5 levels (51). The declining trends in North America, Europe, Oceania, and South America reflect successful implementation of air quality improvement policies and stricter environmental regulations over the past decades, demonstrating the potential for effective public health interventions to reduce PM2.5-related health burdens (36, 40). East Asia region includes China, North Korea, and Taiwan, with China contributing the vast majority of the 229,553 deaths in 2021 due to its large population size. Despite China’s middle SDI classification, this region showed significant improvements in age-standardized rates (ASMR decline), reflecting substantial investments in healthcare infrastructure and air quality management over the study period (52). The apparent contradiction between high absolute burden and improving rates reflects China’s demographic transition and successful implementation of pollution control policies since 2013 (53). High-income Asia-Pacific region encompasses Australia, Brunei, Japan, New Zealand, Singapore, and South Korea. These countries consistently demonstrate the lowest PM2.5-attributable SAH burden, with marked improvements in both absolute and age-standardized metrics, directly correlating with their high SDI status and advanced healthcare systems (48). The steep declining trends in this region exemplify how combined high socioeconomic development and stringent environmental regulations effectively reduce pollution-related health burdens.
Sex and age dimensions
The male predominance in PM2.5-related SAH mortality (ASMR 0.72 vs. 0.55 in females) likely reflects differential exposure patterns and biological susceptibility. Men experience higher occupational exposure through industrial work, while sex-linked differences in inflammatory responses and hormonal status may influence cardiovascular vulnerability to PM2.5 (54, 55). However, evidence on gender differences in air pollution health effects remains inconsistent across studies (56).
The global increase in female SAH mortality proportion (particularly +9.9% in South Asia) may be explained through two complementary mechanisms: biological susceptibility via enhanced oxidative stress responses (20, 21), and socioeconomic factors limiting women’s access to preventive healthcare in LMICs (57). The age-dependent burden escalation (Figure 4 and Supplementary Figure S2) demonstrates a 3.2-fold higher mortality risk in populations >70 years compared to <50 years, likely mediated through PM2.5-induced exacerbation of hypertension (58) and cumulative blood–brain barrier damage (16). This aging-related vulnerability is projected to intensify as the global population over 60 years grows by 56% by 2050 (23).
Future projections and public health implications
The increased burden of SAH projected by the model over the next 29 years (Figure 6 and Supplementary Figure S1), potentially due to a significant association between PM2.5 exposure and SAH, with a particularly sharp increase in risk over short periods. As the global population ages, the older adults, who are more sensitive to the health effects of PM2.5, become a larger proportion of society (59). Additionally, industrialization and urbanization have exacerbated PM2.5 pollution, and regions lacking effective health protection measures and resources experience more significant adverse health effects from PM2.5 (41, 60). Therefore, it is imperative that the world invests more time and effort into controlling PM2.5 pollution and reducing the medical burden on SAH patients (61).
Previous research has examined the global burden of PM2.5 in relation to SAH, yet this study extends these findings, revealing that between 1990 and 2021, the proportion of SAH attributed to PM2.5 increased among women across all GBD regions. This underscores a concerning trend, particularly as the disease burden of SAH due to PM2.5 escalating with advancing age. Our projections for future trends indicate a global increase in both SAH deaths and DALYs attributed to PM2.5.
Since the database used in this study covers different countries and regions, cultural, geographical, climatic, and even genetic differences have influenced the study’s results. Although these factors are unlikely to fundamentally alter the relationship between ambient air pollution and the risk of SAH (62, 63). Genetic polymorphisms in oxidative stress pathways and inflammatory responses may modulate individual susceptibility to PM2.5-induced cerebrovascular damage. This could potentially explain some of the striking regional differences, such as East Asia having a disproportionately high absolute burden despite relatively lower PM2.5 concentrations (64, 65). Climatic factors, including temperature extremes and seasonal variations, may interact with PM2.5 toxicity through altered particulate composition and enhanced inflammatory responses. This could partially account for Mongolia’s exceptionally high burden and the seasonal patterns observed in temperate regions (66). However, the consistent dose–response relationship between PM2.5 exposure and SAH risk documented across diverse populations in epidemiological studies suggests that ambient particulate matter remains an independent risk factor regardless of these population-specific modifiers (67).
While the GBD 2021 methodology employs comparative risk assessment that inherently adjusts for major confounders through integrated exposure-response functions derived from epidemiological meta-analyses, residual confounding from traditional SAH risk factors may influence our estimates (68, 69). Chronic diseases (hypertension, diabetes), lifestyle factors (smoking, alcohol consumption), and healthcare access disparities may cluster geographically with PM2.5 exposure patterns, potentially inflating the pollution-attributable burden in regions with limited diagnostic capacity and neurosurgical infrastructure (70). The stark regional disparities observed—particularly Mongolia’s exceptionally high burden and South Asia’s 246% mortality growth—likely reflect complex interactions between air pollution exposure and unmeasured socioeconomic, healthcare, and behavioral confounders that extend beyond PM2.5 effects alone (71). Despite these limitations, the consistent global patterns and projected 2050 increases suggest that PM2.5 reduction efforts would yield substantial health benefits, warranting urgent air quality interventions in high-burden regions regardless of residual confounding concerns.
Our study had some limitations including: (1) Data heterogeneity in cause-of-death certification and PM2.5 exposure modeling across regions may affect burden estimates; (2) Residual confounding (e.g., unmeasured comorbidities, socioeconomic factors) could bias risk associations; (3) Limited granularity in occupational/lifestyle exposure data hinders precise gender-risk stratification; (4) Model uncertainties (e.g., counterfactual exposure thresholds) warrant sensitivity analyses in future work. Future there are urgent actions remain imperative: Prioritize air quality interventions in high-burden regions (Mongolia/South/Southeast Asia); Strengthen stroke care systems in low-resource settings; Implement gender-responsive strategies addressing occupational (male) and household (female) exposures; Target hypertension control in older populations. Integrating SAH burden metrics into global health frameworks is essential to mitigate this preventable crisis, despite current methodological constraints.
Conclusion
This study uncovers a critical paradox in PM2.5-attributable subarachnoid hemorrhage (SAH): despite a 36.4% global decline in age-standardized mortality (1990–2021), absolute deaths surged by 40.4%-driven by population aging and growth. Stark inequities persist, with South Asia experiencing a 246.5% death increase and Mongolia bearing the highest burden (ASMR 2.49). Projections indicate rising rates by 2050, disproportionately affecting aging populations and regions with weak pollution controls. Urgent actions are warranted: (1) Prioritize air quality interventions in high-burden regions (Mongolia/South/Southeast Asia); (2) Strengthen stroke care systems in low-resource settings; (3) Implement gender-responsive strategies addressing occupational (male) and household (female) exposures; (4) Target hypertension control in older populations. Integrating SAH burden metrics into global health frameworks is essential to mitigate this preventable crisis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the GBD adheres to the Guidelines for Accurate and Transparent Health Estimates Reporting statement. The Institute for Health Metrics and Evaluation, which is responsible for administering the GBD, provides only deidentified and aggregated data. All research adhered to the tenets of the Declaration of Helsinki. There requirement for informed consent was waived because of the retrospective nature of the study. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
EW: Conceptualization, Writing – original draft, Data curation, Formal analysis. TT: Writing – original draft, Software, Methodology, Visualization. RS: Writing – original draft, Visualization. YL: Writing – original draft. MM: Writing – original draft, Software. GaZ: Writing – original draft, Formal analysis. ML: Writing – original draft, Software. YZ: Visualization, Writing – original draft. CD: Validation, Writing – review & editing. GuZ: Data curation, Writing – original draft, Formal analysis. DG: Conceptualization, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Science and Technology Program of Xinjiang Uyghur Autonomous Region (No. 2025E01032).
Acknowledgments
We highly appreciate the work by the GBD 2021 collaborators. We are truly grateful to Zayatta Zungar for providing the final linguistic revisions to our manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1652872/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | DALYs from subarachnoid hemorrhage attributed to PM2.5 by GBD and SDI regions in 1990 (A) and 2021 (B), with proportional distributions in 1990 (C) and 2021 (D).
SUPPLEMENTARY FIGURE S2 | Global age-stratified DALYs from subarachnoid hemorrhage attributable to PM2.5 from 1990 to 2021.
SUPPLEMENTARY FIGURE S3 | Projection of age-standardized DALY rates for subarachnoid hemorrhage due to PM2.5 from 2022 to 2050.
References
1. Gu, L, Zhou, J, Zhang, L, Li, C, Bao, K, Du, F, et al. Global, regional, and national burden of subarachnoid hemorrhage: trends from 1990 to 2021 and 20-year forecasts. Stroke. (2025) 56:887–97. doi: 10.1161/STROKEAHA.124.048950
2. Thilak, S, Brown, P, Whitehouse, T, Gautam, N, Lawrence, E, Ahmed, Z, et al. Diagnosis and management of subarachnoid haemorrhage. Nat Commun. (2024) 15:1850. doi: 10.1038/s41467-024-46015-2
3. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
4. Lv, B, Lan, J-X, Si, Y-F, Ren, Y-F, Li, M-Y, Guo, F-F, et al. Epidemiological trends of subarachnoid hemorrhage at global, regional, and national level: a trend analysis study from 1990 to 2021. Mil Med Res. (2024) 11:46. doi: 10.1186/s40779-024-00551-6
5. Etminan, N, Chang, H-S, Hackenberg, K, de Rooij, NK, Vergouwen, MDI, Rinkel, GJE, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. (2019) 76:588–97. doi: 10.1001/jamaneurol.2019.0006
6. Lin, H, Yin, Y, Li, J, Liu, S, Long, X, and Liao, Z. Exploring the causal links between cigarette smoking, alcohol consumption, and aneurysmal subarachnoid hemorrhage: a two-sample Mendelian randomization analysis. Front Nutr. (2024) 11:1397776. doi: 10.3389/fnut.2024.1397776
7. Zhang, M, Long, Z, Liu, P, Qin, Q, Yuan, H, Cao, Y, et al. Global burden and risk factors of stroke in young adults, 1990 to 2021: a systematic analysis of the global burden of disease study 2021. J Am Heart Assoc. (2025) 14:e039387. doi: 10.1161/JAHA.124.039387
8. Nikmanesh, Y, Mohammadi, MJ, Yousefi, H, Mansourimoghadam, S, and Taherian, M. The effect of long-term exposure to toxic air pollutants on the increased risk of malignant brain tumors. Rev Environ Health. (2023) 38:519–30. doi: 10.1515/reveh-2022-0033
9. Wu, F, Liu, Z, Li, G, Zhou, L, Huang, K, Wu, Z, et al. Inflammation and oxidative stress: potential targets for improving prognosis after subarachnoid hemorrhage. Front Cell Neurosci. (2021) 15:739506. doi: 10.3389/fncel.2021.739506
10. Taimuri, B, Lakhani, S, Javed, M, Garg, D, Aggarwal, V, Mehndiratta, MM, et al. Air pollution and cerebrovascular disorders with special reference to Asia: An overview. Ann Indian Acad Neurol. (2022) 25:S3–8. doi: 10.4103/aian.aian_491_22
11. Ustinaviciene, R, Venclovienė, J, Luksiene, D, Tamosiunas, A, Jasukaitiene, E, Augustis, S, et al. Impact of ambient air pollution with PM2.5 on stroke occurrence: data from Kaunas (Lithuania) stroke register (2010–2022). Atmos. (2024) 15:1327. doi: 10.3390/atmos15111327
12. Hwang, J, Yi, H, Jang, M, Kim, J-G, Kwon, SU, Kim, N, et al. Air pollution and subarachnoid hemorrhage mortality: a stronger association in women than in men. J Stroke. (2022) 24:429–32. doi: 10.5853/jos.2022.02180
13. Shah, ASV, Lee, KK, McAllister, DA, Hunter, A, Nair, H, Whiteley, W, et al. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ. (2015) 350:h1295. doi: 10.1136/bmj.h1295
14. Han, M-H, Yi, H-J, Ko, Y, Kim, Y-S, and Lee, Y-J. Association between hemorrhagic stroke occurrence and meteorological factors and pollutants. BMC Neurol. (2016) 16:59. doi: 10.1186/s12883-016-0579-2
15. Fan, Y-X, Zhang, W, Li, W, Ma, Y-J, and Zhang, H-Q. Global, regional, and national impact of air pollution on stroke burden: changing landscape from 1990 to 2021. BMC Public Health. (2024) 24:2786. doi: 10.1186/s12889-024-20230-4
16. Li, W, Lin, G, Xiao, Z, Zhang, Y, Li, B, Zhou, Y, et al. A review of respirable fine particulate matter (PM2.5)-induced brain damage. Front Mol Neurosci. (2022) 15:967174. doi: 10.3389/fnmol.2022.967174
17. Chen, S-J, Lee, M, Wu, B-C, Muo, C-H, Sung, F-C, and Chen, P-C. Meteorological factors and risk of ischemic stroke, intracranial hemorrhage, and subarachnoid hemorrhage: a time-stratified case-crossover study. Int J Stroke. (2024) 19:1172–81. doi: 10.1177/17474930241270483
18. Guo, Y, Luo, C, Cao, F, Liu, J, and Yan, J. Short-term environmental triggers of hemorrhagic stroke. Ecotoxicol Environ Saf. (2023) 265:115508. doi: 10.1016/j.ecoenv.2023.115508
19. Cheng, Z, Luo, L, Wang, S, Wang, Y, Sharma, S, Shimadera, H, et al. Status and characteristics of ambient PM2.5 pollution in global megacities. Environ Int. (2016) 89-90:212–21. doi: 10.1016/j.envint.2016.02.003
20. Véliz, KD, Alcantara-Zapata, DE, Chomalí, L, and Vargas, J. Gender-differentiated impact of PM2.5 exposure on respiratory and cardiovascular mortality: a review. Air Qual Atmos Health. (2024) 17:1565–86. doi: 10.1007/s11869-024-01525-2
21. Yue, H, Ji, X, Ku, T, Li, G, and Sang, N. Sex difference in bronchopulmonary dysplasia of offspring in response to maternal PM2.5 exposure. J Hazard Mater. (2020) 389:122033. doi: 10.1016/j.jhazmat.2020.122033
22. Amnuaylojaroen, T, and Parasin, N. Pathogenesis of PM2.5-related disorders in different age groups: children, adults, and the elderly. Epigenomes. (2024) 8. doi: 10.3390/epigenomes8020013
23. Yin, H, Brauer, M, Zhang, JJ, Cai, W, Navrud, S, Burnett, R, et al. Population ageing and deaths attributable to ambient PM2·5 pollution: a global analysis of economic cost. Lancet Planet Health. (2021) 5:e356–67. doi: 10.1016/S2542-5196(21)00131-5
24. GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2100–32. doi: 10.1016/S0140-6736(24)00367-2
25. GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2133–61. doi: 10.1016/S0140-6736(24)00757-8
26. GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. (2017) 390:1345–422. doi: 10.1016/S0140-6736(17)32366-8
27. Murray, CJ, and Lopez, AD. Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet. (1997) 349:1436–42. doi: 10.1016/S0140-6736(96)07495-8
28. Murray, CJ, and Acharya, AK. Understanding DALYs (disability-adjusted life years). J Health Econ. (1997) 16:703–30.
29. World Health Organization. Global health estimates: Methods and data sources. Geneva: World Health Organization (2014).
30. Boyle, P, and Parkin, DM. Cancer registration: principles and methods. Statistical methods for registries. IARC Sci Publ. (1991):126–58.
31. GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2162–203. doi: 10.1016/S0140-6736(24)00933-4
32. Chen, H, Zhou, Z, Li, Z, Liang, S, Zhou, J, Zou, G, et al. Time trends in the burden of stroke and subtypes attributable to PM2.5 in China from 1990 to 2019. Front Public Health. (2022) 10:1026870. doi: 10.3389/fpubh.2022.1026870
33. Kim, JH, Lee, S-H, Park, S-H, Lim, D-J, and Park, D-H. The relationship between air pollutant levels and aneurysmal subarachnoid hemorrhage. Medicine (Baltimore). (2022) 101:e30373. doi: 10.1097/MD.0000000000030373
34. Cao, H, Han, J, Hou, W, and Yuan, J. Associations of greenhouse gases, air pollutants and dynamics of scrub typhus incidence in China: a nationwide time-series study. BMC Public Health. (2025) 25:1977. doi: 10.1186/s12889-025-23156-7
35. Magpantay, FMG, King, AA, and Rohani, P. Age-structure and transient dynamics in epidemiological systems. J R Soc Interface. (2019) 16:20190151. doi: 10.1098/rsif.2019.0151
36. GBD 2021 HAP Collaborators. Global, regional, and national burden of household air pollution, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2025), 405:1167–1181. doi: 10.1016/S0140-6736(24)02840-X
37. Hou, W. Effect and prediction of long-term weather and pollutant exposure on hemorrhagic fever with renal syndrome: based on statistical models. Front Public Health. (2025) 13:1393763. doi: 10.3389/fpubh.2025.1393763
38. Hou, W, and Song, Z. Exploring the risk and predictive study of outdoor air pollutants on the incidence and mortality of HIV/AIDS. Ecotoxicol Environ Saf. (2024) 287:117292. doi: 10.1016/j.ecoenv.2024.117292
39. Wu, E, Su, R, Tang, T, Zhu, G, and Geng, D. Ambient versus household PM2.5 exposure and socioeconomic disparities in intracerebral hemorrhage burden: a 32-year global analysis (1990-2021) with projections to 2050. Front Public Health. (2025) 13:1615934. doi: 10.3389/fpubh.2025.1615934
40. Bo, Y, Zhu, Y, and Zhang, X. Spatiotemporal trends of stroke burden attributable to ambient PM (2.5) in 204 countries and territories, 1990-2019: a global analysis. Neurology. (2023) 101:e764-ee76. doi: 10.1212/WNL.0000000000207503
41. Yue, H, He, C, Huang, Q, Yin, D, and Bryan, BA. Stronger policy required to substantially reduce deaths from PM2.5 pollution in China. Nat Commun. (2020) 11:1462. doi: 10.1038/s41467-020-15319-4
42. Li, W, Ruan, X, Yang, H, Zhang, S, Rui, F, and Xiong, J. Global, regional and national trends in the burden of intracranial hemorrhage, 1990-2021: results from the global burden of disease study. Heliyon. (2025) 11:e42608. doi: 10.1016/j.heliyon.2025.e42608
43. Shi, Y, Matsunaga, T, Yamaguchi, Y, Li, Z, Gu, X, and Chen, X. Long-term trends and spatial patterns of satellite-retrieved PM2.5 concentrations in south and Southeast Asia from 1999 to 2014. Sci Total Environ. (2018) 615:177–86. doi: 10.1016/j.scitotenv.2017.09.241
44. Sodhi, HBS, Savardekar, AR, Mohindra, S, Chhabra, R, Gupta, V, and Gupta, SK. The clinical profile, management, and overall outcome of aneurysmal subarachnoid hemorrhage at the neurosurgical unit of a tertiary care center in India. J Neurosci Rural Pract. (2014) 5:118–26. doi: 10.4103/0976-3147.131650
45. Xu, R, Ye, T, Huang, W, Yue, X, Morawska, L, Abramson, MJ, et al. Global, regional, and national mortality burden attributable to air pollution from landscape fires: a health impact assessment study. Lancet. (2024) 404:2447–59. doi: 10.1016/S0140-6736(24)02251-7
46. WHO. Global air quality guidelines: Particulate matter (PM (2.5) and PM(10)), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. Geneva: World Health Organization (2021).
47. Cohen, AJ, Brauer, M, Burnett, R, Anderson, HR, Frostad, J, Estep, K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the global burden of diseases study 2015. Lancet. (2017) 389:1907–18. doi: 10.1016/S0140-6736(17)30505-6
48. Burnett, R, Chen, H, Szyszkowicz, M, Fann, N, Hubbell, B, Pope, CA 3rd, et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci USA. (2018) 115:9592–7. doi: 10.1073/pnas.1803222115
49. Abdul Jabbar, S, Tul Qadar, L, Ghafoor, S, Rasheed, L, Sarfraz, Z, Sarfraz, A, et al. Air quality, pollution and sustainability trends in South Asia: a population-based study. Int J Environ Res Public Health. (2022) 19:7534. doi: 10.3390/ijerph19127534
50. India State-Level Disease Burden Initiative Air Pollution Collaborators. Health and economic impact of air pollution in the states of India: the global burden of disease study 2019. Lancet Planet Health. (2021) 5:e25–38. doi: 10.1016/S2542-5196(20)30298-9
51. Jin, H, Chen, X, Zhong, R, and Liu, M. Influence and prediction of PM2.5 through multiple environmental variables in China. Sci Total Environ. (2022) 849:157910. doi: 10.1016/j.scitotenv.2022.157910
52. Zhang, Q, Zheng, Y, Tong, D, Shao, M, Wang, S, Zhang, Y, et al. Drivers of improved PM2.5 air quality in China from 2013 to 2017. Proc Natl Acad Sci USA. (2019) 116:24463–9. doi: 10.1073/pnas.1907956116
53. Huang, J, Pan, X, Guo, X, and Li, G. Health impact of China’s air pollution prevention and control action plan: an analysis of national air quality monitoring and mortality data. Lancet Planet Health. (2018) 2:e313–23. doi: 10.1016/S2542-5196(18)30141-4
54. Clougherty, JE. A growing role for gender analysis in air pollution epidemiology. Ciênc Saúde Colet. (2011) 16:2221–38. doi: 10.1590/s1413-81232011000400021
55. Shin, HH, Maquiling, A, Thomson, EM, Park, I-W, Stieb, DM, and Dehghani, P. Sex-difference in air pollution-related acute circulatory and respiratory mortality and hospitalization. Sci Total Environ. (2022) 806:150515. doi: 10.1016/j.scitotenv.2021.150515
56. Kraus, U, Horstmann, S, Dandolo, L, Bolte, G, Peters, A, Schneider, A, et al. Sex/gender in the association between ambient air pollution and cardiovascular mortality: systematic review and meta-analysis. Ecotoxicol Environ Saf. (2025) 300:118443. doi: 10.1016/j.ecoenv.2025.118443
57. Turner, RJ, and Avison, WR. Status variations in stress exposure: implications for the interpretation of research on race, socioeconomic status, and gender. J Health Soc Behav. (2003) 44:488–505. doi: 10.2307/1519795
58. Tada, Y, Wada, K, Shimada, K, Makino, H, Liang, EI, Murakami, S, et al. Roles of hypertension in the rupture of intracranial aneurysms. Stroke. (2014) 45:579–86. doi: 10.1161/STROKEAHA.113.003072
59. Son, J-Y, Sabath, MB, Lane, KJ, Miranda, ML, Dominici, F, Di, Q, et al. Long-term exposure to PM2.5 and mortality for the older population: effect modification by residential greenness. Epidemiology. (2021) 32:477–86. doi: 10.1097/EDE.0000000000001348
60. Maji, KJ, Dikshit, AK, Arora, M, and Deshpande, A. Estimating premature mortality attributable to PM2.5 exposure and benefit of air pollution control policies in China for 2020. Sci Total Environ. (2018) 612:683–93. doi: 10.1016/j.scitotenv.2017.08.254
61. Thompson, JC, Chalet, F-X, Manalastas, EJ, Hawkins, N, Sarri, G, and Talbot, DA. Economic and humanistic burden of cerebral vasospasm and its related complications after aneurysmal subarachnoid hemorrhage: a systematic literature review. Neurol Ther. (2022) 11:597–620. doi: 10.1007/s40120-022-00348-6
62. Brook, RD, Rajagopalan, S, Pope, CA 3rd, Brook, JR, Bhatnagar, A, Diez-Roux, AV, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. (2010) 121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1
63. Wang, M, Aaron, CP, Madrigano, J, Hoffman, EA, Angelini, E, Yang, J, et al. Association between long-term exposure to ambient air pollution and change in quantitatively assessed emphysema and lung function. JAMA. (2019) 322:546–56. doi: 10.1001/jama.2019.10255
64. Yang, B-Y, Qian, Z, Howard, SW, Vaughn, MG, Fan, S-J, Liu, K-K, et al. Global association between ambient air pollution and blood pressure: a systematic review and meta-analysis. Environ Pollut. (2018) 235:576–88. doi: 10.1016/j.envpol.2018.01.001
65. Li, M-H, Fan, L-C, Mao, B, Yang, J-W, Choi, AMK, Cao, W-J, et al. Short-term exposure to ambient fine particulate matter increases hospitalizations and mortality in COPD: a systematic review and meta-analysis. Chest. (2016) 149:447–58. doi: 10.1378/chest.15-0513
66. Schneider, A, Neas, L, Herbst, MC, Case, M, Williams, RW, Cascio, W, et al. Endothelial dysfunction: associations with exposure to ambient fine particles in diabetic individuals. Environ Health Perspect. (2008) 116:1666–74. doi: 10.1289/ehp.11666
67. Hou, Q, An, XQ, Wang, Y, and Guo, JP. An evaluation of resident exposure to respirable particulate matter and health economic loss in Beijing during Beijing 2008 Olympic games. Sci Total Environ. (2010) 408:4026–32. doi: 10.1016/j.scitotenv.2009.12.030
68. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1223–49. doi: 10.1016/S0140-6736(20)30752-2
69. GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1923–94. doi: 10.1016/S0140-6736(18)32225-6
70. Nieuwkamp, DJ, Setz, LE, Algra, A, Linn, FHH, de Rooij, NK, and Rinkel, GJE. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. (2009) 8:635–42. doi: 10.1016/S1474-4422(09)70126-7
Keywords: PM2.5, subarachnoid hemorrhage, disease burden, estimated annual percentage changes, Bayesian age-period-cohort modeling
Citation: Wu E, Tang T, Su R, Li Y, Mijiti M, Zhang G, Lian M, Zhang Y, Du C, Zhu G and Geng D (2025) Global burden of subarachnoid hemorrhage attributable to ambient PM2.5 in low-resource regions (1990–2050). Front. Public Health. 13:1652872. doi: 10.3389/fpubh.2025.1652872
Edited by:
Chris Fook Sheng Ng, The University of Tokyo, JapanReviewed by:
Masoume Taherian, Ahvaz Jundishapur University of Medical Sciences, IranWeiming Hou, Air Force General Hospital PLA, China
Copyright © 2025 Wu, Tang, Su, Li, Mijiti, Zhang, Lian, Zhang, Du, Zhu and Geng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guohua Zhu, emh1Z3VvaHVhNDI3QHNpbmEuY29t; Dangmurenjiafu Geng, ZGFtcmphYkAxNjMuY29t
 Erman Wu
Erman Wu Tong Tang
Tong Tang Riqing Su
Riqing Su Yandong Li1
Yandong Li1 Maimaitili Mijiti
Maimaitili Mijiti Chang Du
Chang Du Dangmurenjiafu Geng
Dangmurenjiafu Geng