- 1School of Management, Shandong Second Medical University, Weifang, China
- 2Weifang People’s Hospital, Shandong Second Medical University, Weifang, China
Introduction: Chronic pain (CP) is a prevalent comorbidity in patients with chronic diseases, yet the relationship between CP and health-related quality of life (HRQoL) remain unclear, particularly through the mediating roles of frailty and depression.
Methods: We conducted a cross-sectional survey in two provinces (eastern and central China) from October 2024 to January 2025, enrolling 3,094 patients with chronic diseases. HRQoL was assessed using the EQ-5D scale, while CP status was determined through structured logic-based questions. Frailty was evaluated using the FRAIL scale, and depressive symptoms were measured with the CES-D10. Spearman’s correlation analysis was performed to assess associations among CP, frailty, depression, and HRQoL. A chain mediation model (PROCESS 4.1, Model 6) was constructed, and mediation effects were tested using a bootstrap approach with 5,000 resamples.
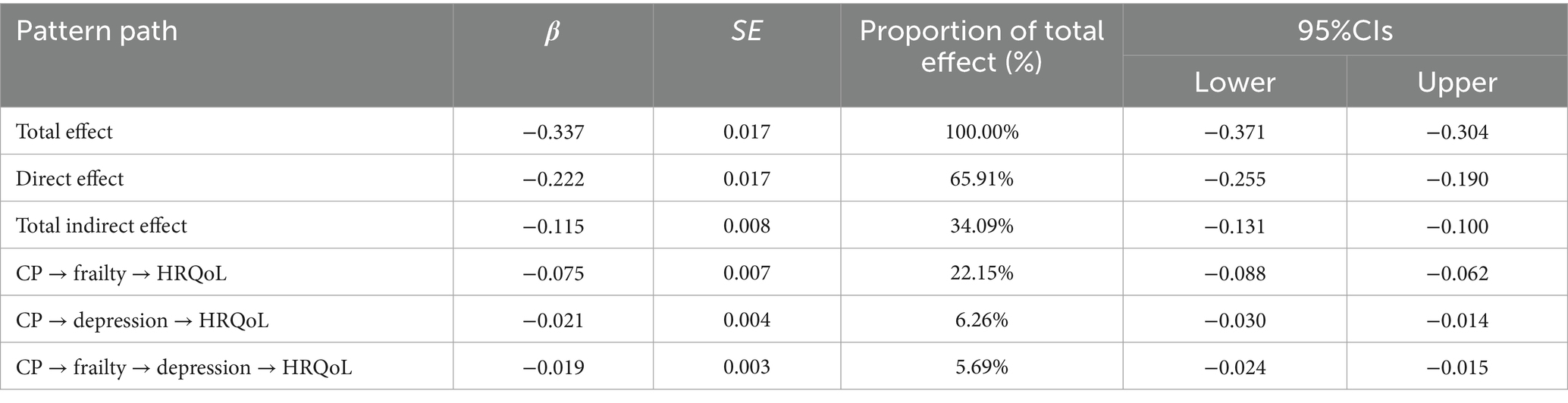
Results: Frailty and depression exhibited significant mediating effects in the relationship between CP and HRQoL. The indirect effects of frailty and depression on HRQoL were −0.0747 (95% CI: −0.0881, −0.0618) and −0.0211 (95% CI: −0.0297, −0.0135). Additionally, a significant chain mediation effect was observed (−0.0192, 95% CI: −0.0242, −0.0145). The indirect effect of frailty and depression accounted for 34.09% of the association between CP and HRQoL (total effect: −0.1150, 95% CI: −0.1305, −0.0999).
Conclusion: The study findings demonstrated that frailty and depression serve as significant chain mediators in the relationship between CP and diminished HRQoL. Measures should be taken to reduce the incidence and severity of CP in patients with chronic diseases, improve frailty and depression, and thus improve HRQoL.
1 Introduction
With sustained improvements in global population health and significant gains in life expectancy, chronic diseases have emerged as the leading disease burden worldwide. Recent data from the World Health Statistics 2024 revealed that chronic diseases accounted for 65.3% of global mortality in 2021 (1). National epidemiological studies indicated an even more pronounced burden in China, where chronic diseases contributed to over 80% of total deaths (2). Chronic disease patients experience heavier health burdens than healthy individuals, including more severe pain, fatigue, psychological distress, functional impairments, and reduced Health-related quality of life (HRQoL) (3).
HRQoL is a multidimensional concept reflecting an individual’s subjective perception of physical, psychological, and social well-being within their cultural and personal context (4, 5). For chronic disease patients (CDPs), who often face prolonged illness, poor HRQoL—driven by physical limitations and reduced daily functioning—is common (6–8). Understanding these determinants is key to designing targeted interventions that improve well-being, uphold dignity, and mitigate the societal impacts of aging.
Chronic pain (CP), defined as pain lasting over 3 months (9), is the world’s third most prevalent health condition after cardiovascular disease and cancer (10–12). Globally affecting about 33% of people (13), China has over 300 million cases, with 10–20 million new annual diagnoses (14). CP frequently coexists with chronic diseases, often causing more severe pain (15). In China, 63.4% of middle-aged/older adults report chronic disease-related pain (16), with prevalence rising alongside chronic conditions (17, 18). Persistent pain leads to mobility issues, higher fall risk, depression, psychological distress, and reduced HRQoL (19, 20). While the CP-HRQoL link is established, underlying mechanisms need further study.
Frailty, characterized by reduced physiological resilience and multisystem decline (21), is prevalent among CDPs and ranks as their third most common comorbidity (22, 23). Frailty prevalence varied substantially among chronic conditions: exceeding 30% in chronic pancreatitis patients (24), reaching 61.8% in type 2 diabetes populations (25), and tripling morbidity risk in older adults cardiovascular patients versus controls (26). Growing evidence showed frailty was a major consequence of CP (27–30). Research demonstrates CP patients show elevated frailty rates compared to pain-free individuals (31), with frailty substantially increasing risks for falls, hospitalization, disability, and mortality (32, 33). Frail older adults also exhibit higher susceptibility to physiological dysregulation, depression, and cognitive decline (34–36) key determinants of health span and quality of life in aging populations (37–40).
Depression, characterized by persistent low mood and anhedonia, affects 3.8% of the global population and worsens outcomes for chronic diseases (41–43). Prevalence is particularly high in CDPs—38.3% in rheumatoid arthritis (44) 19.97% in U. S. resettlement communities (45), and 41.3% in eastern China (46). Among CP patients, 30–50% experience depressive symptoms (47, 48). Depression accelerates physical decline through fatigue and sleep disturbances while causing cognitive dysfunction and social withdrawal (49). As the world’s most common mental disorder, it significantly impairs HRQoL (50–53).
While prior research has established connections between CP, frailty, depression and HRQoL, the specific mediating pathways remain unclear. This study investigated how frailty and depression transmit CP’s impact on HRQoL in chronic disease patients. We propose four hypotheses:
Hypothesis 1: CP will demonstrate a significant negative association with HRQoL in patients with chronic diseases.
Hypothesis 2: Frailty will significantly mediate the relationship between CP and reduced HRQoL.
Hypothesis 3: Depression will significantly mediate the association between CP and impaired HRQoL.
Hypothesis 4: Frailty and Depression will operate as sequential mediators in the pathway between CP and reduced HRQoL.
2 Methods
2.1 Sample selection
This study utilized data from a cross-sectional survey conducted between October 2024 and January 2025 across two Chinese provinces representing eastern and central regions. The survey employed a multi-stage stratified random sampling approach to ensure population representativeness. The first stage was to select sample cities according to the low, middle, and high levels of economic development. The second stage was to select 3 districts (counties) randomly in each city and exact 4 townships (streets) in each selected district (county), and then choose 6 villages (communities) from each township (street). The third stage was to choose 23 patients with chronic diseases randomly from each village (community) for the survey. Finally, samples were screened for inclusion in the analysis according to the needs of the study.
The inclusion criteria for this study were the completion of all frailty, depression scales, and HRQoL assessments during follow-up. Exclusion criteria for this study: (1) Patients with severe hearing, visual, and communication (i.e., language expression) disorders, mental illness, and serious physical illness; (2) Subjects who gave incomplete answers. Finally, 3,094 people were included in the analysis. Written informed consent was obtained from each participant before the survey.
2.2 Chronic pain
Chronic pain (CP) was defined as pain that persists or recurs for more than 3 months (9). CP status was ascertained through a validated algorithmic questionnaire employing sequential logic-based screening items. In the questionnaire, “Do you have CP?”? No/Yes “If the respondent answers” No, the respondent is classified as “painless”. If the respondent has CP, further ask “What do you think is the level of CP?.” Respondents were asked to rate the level of CP as “not severe at all,” not too severe, “fair,” moderately severe, “and” very severe, “on a numerical scale of 1 to 5. If the respondent did not have CP, a score of 0 was given. The higher the score, the more severe the CP.
2.3 Health-related quality of life
EQ-5D (Euro Qol Five Dimensions Questionnaire) was used to measure the HRQoL. HRQoL was quantified using the EQ-5D-5L instrument, with utility scores calculated according to the Chinese value set (coefficient-based scoring algorithm) (54).
2.4 Frailty
The FRAIL scale was used to assess the frailty status of the participants. The scale consists of 5 items with a full score of 5. The higher the score, the more severe the frailty status (55).
2.5 Depression
The CES-D10 scale measured depression. The scale contains 10 items. The overall score of the scale ranges from 0 to 30. The higher the score, the higher the degree of depression (56).
2.6 Covariates
Based on prior evidence (52–57), we adjusted for established HRQoL covariates including socio-demographics, health behaviors, and physical health status in our analyses. (1) Socio-demographic characteristics: a self-made demographic questionnaire was used, including age (years), gender (male, female), marital status [married, not married], living status (living alone, not living alone), education level (primary school and below, junior high school, senior high school and technical secondary school, junior college and above), working status (employed, not employed). (2) Health behaviors include smoking status (smoking, smoking before, not smoking now, never smoking), drinking status (drinking, drinking before, not drinking now, never drinking), physical exercise (never doing physical exercise, 1–2 times/week, 3–5 times/week, more than 5 times/week), social activities (no, yes). (3) Physical health status includes chronic disease status [(number of diseases: 1, 2, ≥3), course of disease (years)]. Chronic disease status was defined as the presence of a chronic disease that the subject was considered to have.
2.7 Statistical analysis
First, the mean (SD) of the continuous variable and the frequency (%) of the categorical variable were applied to report the sociodemographic characteristics of the sample. Normality of CP, HRQoL, debilitation, and depression was examined by the Kolmogorov–Smirnov normality test. The results showed that not all variables were normally distributed (p < 0.001). The Mann–Whitney test (U test) and the Kruskal-Wallis test (H test) were used to compare the EQ-5D utility values of patients with chronic diseases with different socio-demographic characteristics. Because of the large sample size, Dunn’s test was used for further multiple comparisons. Bivariate associations among CP, HRQoL, frailty, and depression were assessed using Spearman’s rank correlation coefficients. The items of CP, HRQoL, frailty, and depression scales were tested for common method bias using the Harman univariate test.
To demonstrate whether there is a series of multiple mediating effects, such as debilitation and depression, between CP and HRQoL, we used the SPSS macroprocess program (Model 6) designed by Hayes (58) to complete the data analysis. A p value of less than 0.05 was considered statistically significant. The statistical significance of the mediating effect was tested using the Bootstrap method, and estimates of the mediating effect were generated, and bias-corrected 95% confidence intervals (CIs) were calculated by a resampling procedure with 5,000 samples. Two-tailed p < 0.05 was considered statistically significant.
3 Result
3.1 Basic information of participants
Table 1 showed the demographic characteristics of the study participants. Of the 3,094 participants, 62.09% were female, 71.68% were patients with chronic diseases over 70 years old, their average age was (70.00 ± 8.32) years old, and most of the participants were currently married (73.92%). Overall, the education level of the participants was generally low, with 63.87% of them having an education level of primary school or below. Additionally, 35.42% of the participants had one chronic disease, while 64.58% were comorbid with multiple chronic diseases. 29.64% of the participants exhibited frailty. The prevalence of CP was 63.45%, with the majority (55.82%) having experienced it for 10 years or less. The health utility value of EQ-5D was higher in males, younger, married, more educated, employed, less chronic diseases, shorter disease course, smoking, drinking, more physical exercise, not living alone, less CP, and less debilitating participants (p < 0.001). See Table 1 for details.
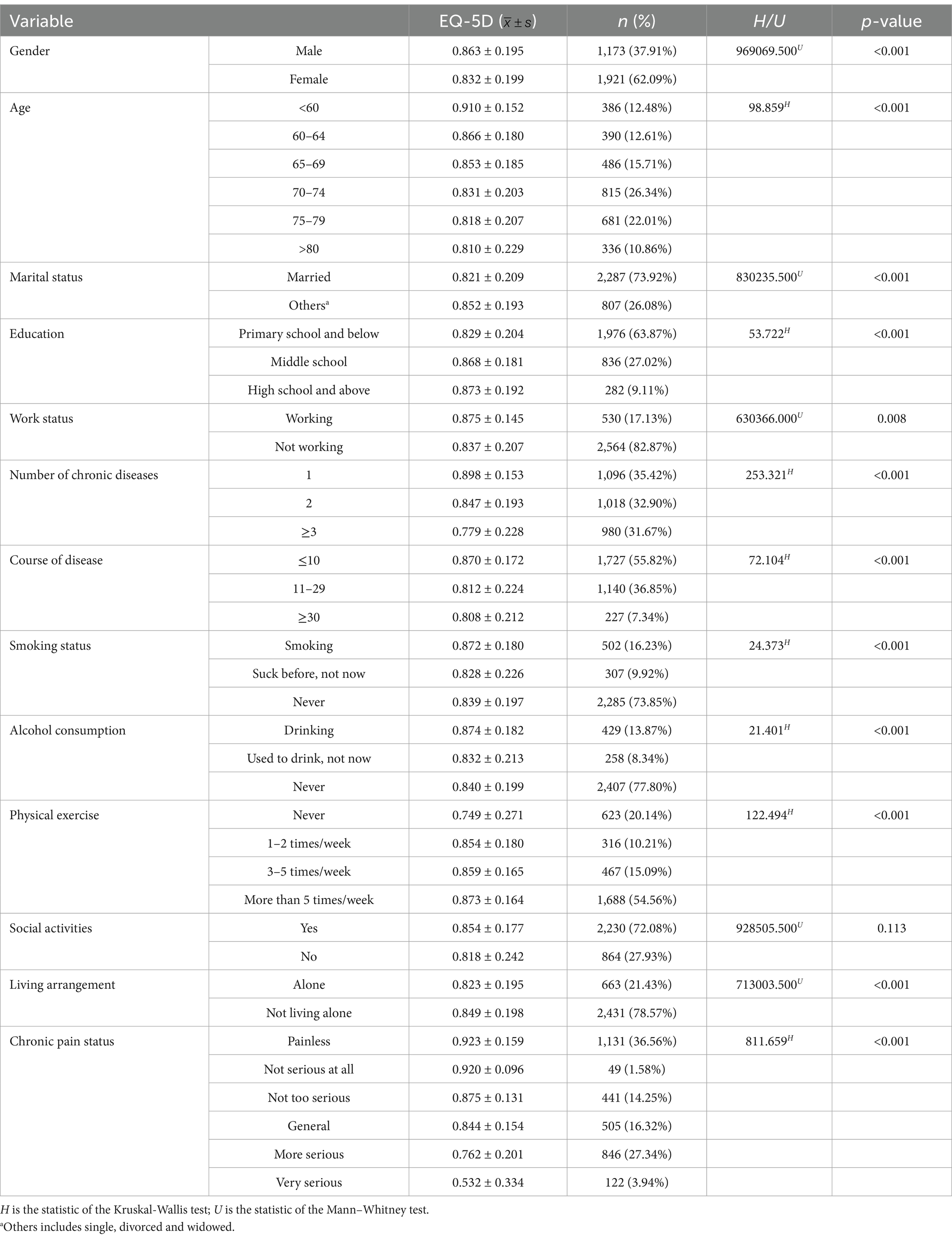
Table 1. Distribution of EQ-5D health utility values with different demographic characteristics (N = 3,094).
3.2 Common deviation test
Harman’s single-factor test method was used to test the input common deviation of variables in each scale (59). The results showed that there were 6 factors with characteristic roots greater than 1 extracted by the factors. The explanation rate of the first common factor was 29.40%, which was less than the critical value of 40.00%, indicating that the common deviation of this study was not significant and the research results are credible.
3.3 Variable description statistics and correlations
The scores of frailty, depression, and HRQoL were (1.502 ± 1.322), (18.344 ± 6. 393), and (0.844 ± 0. 198), respectively. The mean and difference of CP, frailty, depression, and HRQoL, and the Spearman correlation test results between the variables are shown in Table 2. Results showed that CP was negatively associated with HRQoL (r = −0.509, p < 0.001) and positively associated with frailty (r = 0.383, p < 0.001) and depression (r = 0.281, p < 0.001). Frailty was positively associated with depression (r = 0.434, p < 0.001) and negatively associated with HRQoL (r = −0.513, p < 0.001). Depression was negatively associated with health-related life (r = −0.412, p < 0.001).
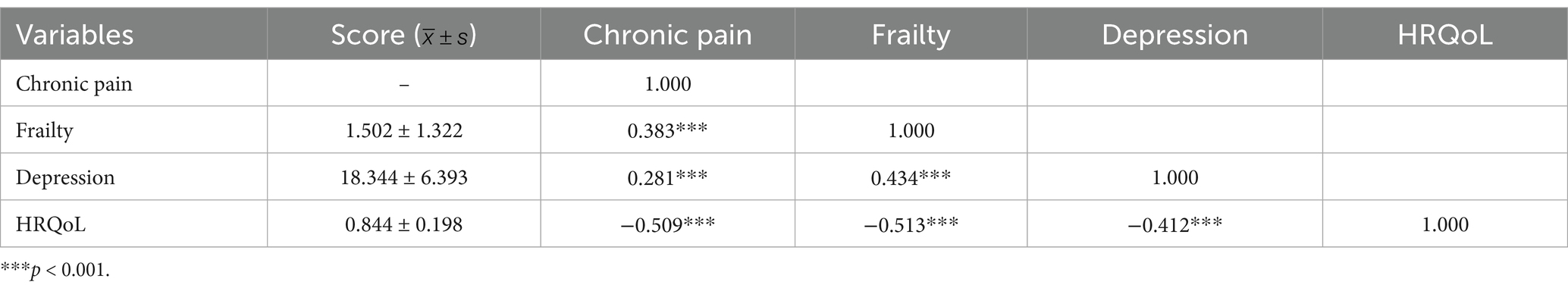
Table 2. Statistical description of pain, frailty, depression, and HRQoL and Spearman correlation analysis between them.
3.4 Chain mediating effect of frailty and depression between CP and HRQoL
Using model 6 of the SPSS PROCESS V4.1 macro program, after controlling for the covariates, the 95% confidence interval of each effect was estimated by using the Bootstrap method, sampling 5,000 times, and the mediating effects of frailty and depression on CP and HRQoL were tested.
Regression showed that R2 indicated that the model explained 22.46% of the variance in frailty, 23.92% of the variance in depression, and 33.74% of the variance in HRQoL. Specifically, the direct effect of CP situations on HRQoL was significant (β = −0.337, p < 0.001) before the inclusion of mediating variables. With the introduction of mediators of frailty and depression, the positive effect of CP situations on frailty g was significant (β = 0.300, p < 0.001), positive effect on depression was also significant (β = 0.105, p < 0.001), and the direct effect on HRQoL remained significant (β = −0.223, p < 0.001). This suggests that there is a partial mediating effect between frailty and depression. Frailty significantly positively affected depression (β = 0.318, p < 0.001) and negatively affected HRQoL (β = −0.250, p < 0.001). Meanwhile, depression also negatively affected HRQoL (β = −0.201, p < 0.001). This suggests that CP not only directly contributes to HRQoL but also indirectly exacerbates negative effects through chained mediating pathways of frailty and depression, as shown in Tables 3, 4.
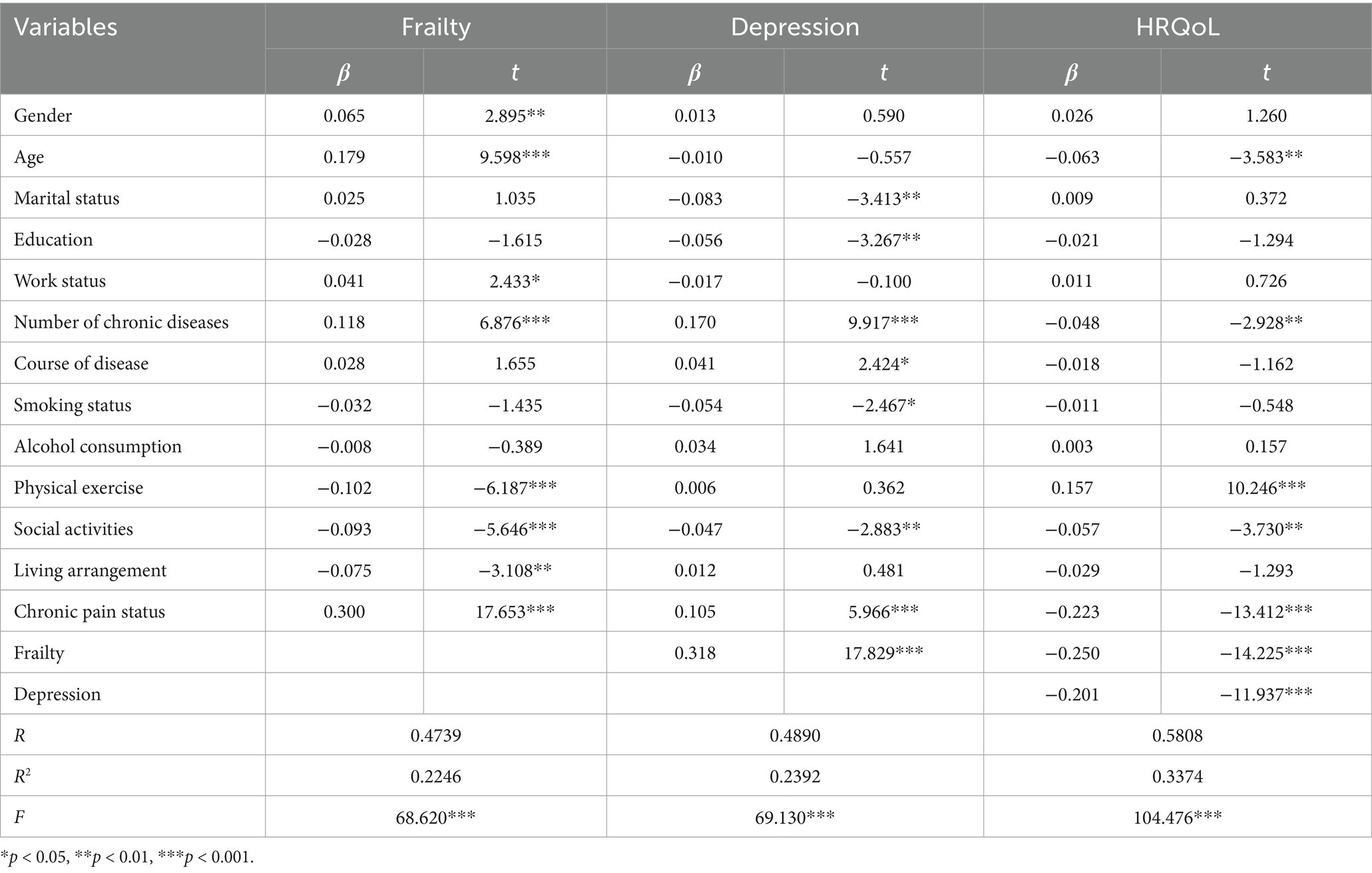
Table 3. Results of multiple mediating utility tests of frailty and depression between pain and HRQoL.
3.5 Estimation of the confidence interval of the mediation effect test
Table 4 presented the results of the mediating effects of frailty and depression on the relationship between CP and HRQoL. None of the 95% confidence intervals of the 3 paths contained 0, indicating that the indirect effects of the 3 paths reached a significant level, as shown in Figure 1. In the indirect effect model, the total indirect effect of CP caused by frailty and depression was −0.1150 (95%CI = −0.1305 ∼ −0.0999), with 34.09% of the total effect mediated. Specifically, frailty and depression explained 22.15 and 6.26% of the total effect, respectively, and 5.69% through the frailty-mediated depression pathway.
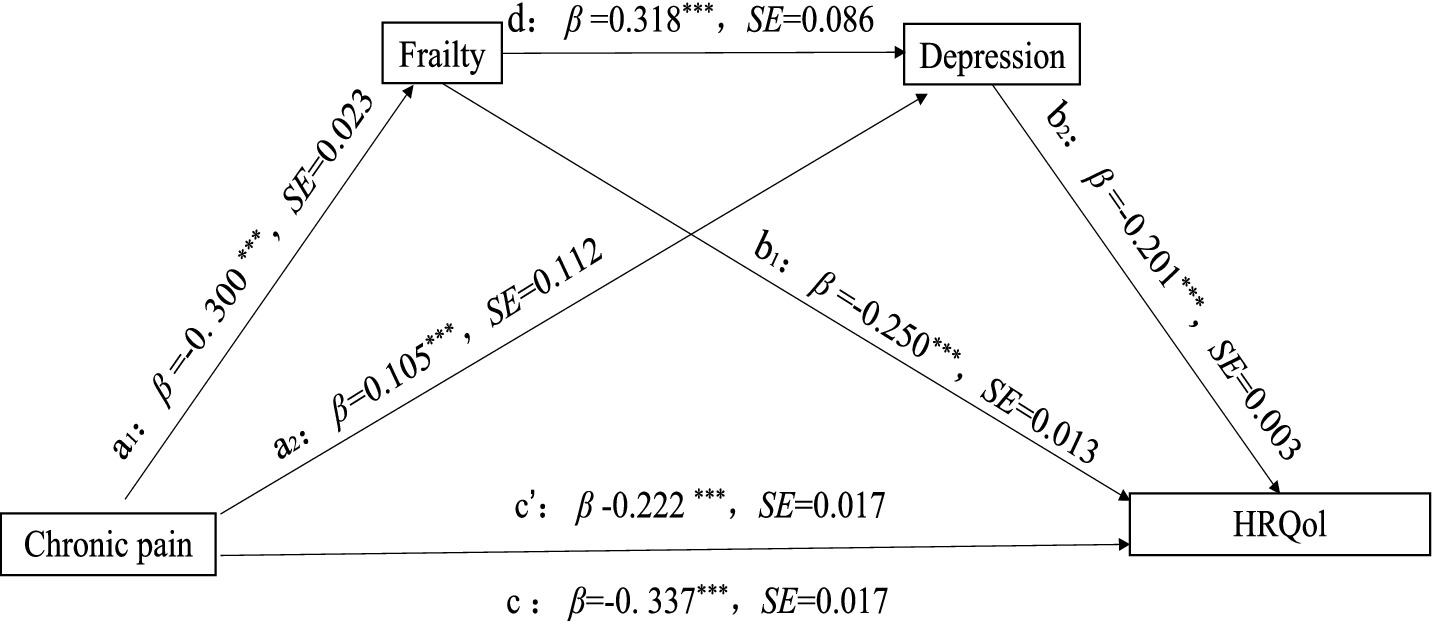
Figure 1. Correlation pathway diagram of frailty and depression as mediators of pain and HRQoL. ***p < 0.001, **p < 0.01, *p < 0.05. The model controls sex, age, sex, marital status, education level, work situation, number (species) of chronic diseases, course (years) of diseases, smoking and drinking, physical exercise, social activities, and living conditions.
4 Discussion
HRQoL serves as a crucial prognostic indicator for mortality and hospitalization risk (60), offering a comprehensive evaluation of physical, psychological, social, and life satisfaction domains (6, 7) that surpasses traditional health metrics. The WHO incorporates HRQoL in Healthy Life Years (HALE) calculations as a key health metric (61). This study pioneered the examination of frailty and depression as chain mediators in the CP-HRQoL relationship among CDPs. The results supported all hypotheses, establishing CP as a significant negative predictor of HRQoL-CDPs with greater CP severity showed markedly lower HRQoL scores, revealing a novel mechanistic framework.
These results corroborate previous findings on CP’s detrimental effect on HRQoL (62), mediated through both physiological and psychological pathways. CP commonly accompanies tissue inflammation (63), neuropathy (64), and disease progression (65), causing functional impairments like mobility limitations (66) and sleep disturbances (67) that reduce physical capacity and daily functioning. These chronic impairments foster learned helplessness and social withdrawal (68), exacerbating isolation and ultimately worsening HRQoL.
CP directly impairs HRQoL while also indirectly reducing it via frailty mediation, potentially through hypothalamic–pituitary–adrenal (HPA) axis activation. This neuroendocrine response leads to sustained hypercortisolemia, which subsequently triggers a cascade of pathophysiological consequences including immunosuppression, metabolic dysregulation, and enhanced fatigue perception. These changes accelerate frailty by impairing immunity, disrupting metabolism, and increasing disease susceptibility (69), worsening physical decline. CP-induced inflammation and catabolism (63) impair energy metabolism, promoting frailty and creating a debilitating cycle of muscle weakness and functional decline that worsens HRQoL. Alternatively, CP may exacerbate frailty through multiple interconnected pathways. First, pain-induced activity restriction can lead to musculoskeletal atrophy and progressive functional deterioration. Second, it negatively impacts treatment adherence and reduces participation in rehabilitation programs among CDPs (66). Third, CP induces systemic inflammation and metabolic dysfunction while disrupting sleep and appetite, leading to nutritional deficits. Finally, the psychological sequelae of CP, including depression and cognitive dysfunction, further compound physical decline. These multifactorial mechanisms collectively accelerated the frailty trajectory in affected individuals. The progressive loss of self-management capacity (28–30, 70) critically exacerbates frailty and impairs HRQoL, creating a vicious cycle where declining function further reduces health-preserving behaviors and accelerates physical deterioration.
CP negatively impacted HRQoL in CDPs through depression mediation, aligning with known CP-depression associations (71). As a chronic stressor, CP consistently correlates with psychological distress and depression risk (72, 73). Neurobiological mechanisms include CNS pathway dysregulation, particularly altered opioid/dopamine receptor function (73, 74), while pro-inflammatory cytokines may disrupt 5-HT and dopamine synthesis (75), impairing emotional regulation and increasing depression susceptibility. CP reduced patients’ daily activities and social participation, increasing loneliness and depression risk (76). Depression worsened treatment adherence and well-being while impairing functional capacity and social adaptation, creating perceived loss of control that further diminished self-efficacy and HRQoL.
This study demonstrated that frailty and depression sequentially mediate 34.09% of CP’s total negative effect on HRQoL in chronic disease patients, representing a substantial mediation pathway. CP-induced functional decline (21) establishes frailty and depression as key sequential mediators in reducing HRQoL. Physical decline fosters depression through psychological vulnerability (77, 78) and subsequent physiological dysregulation (79). These pathophysiological alterations subsequently impaired subjective well-being, thereby accelerating the progressive deterioration of HRQoL in affected patients.
5 Conclusion
The study revealed that CP not only directly reduces HRQoL in patients with chronic diseases, but also indirectly impacts it through frailty and depression. These factors act as both individual and sequential mediators, forming a significant mediation pathway. This indirect pathway explains 34.09% of CP’s total effect on HRQoL. While the findings provide important insights, the cross-sectional design limits causal inferences and cannot address potential bidirectional relationships between mediators, highlighting the need for future longitudinal studies to verify these pathways and further elucidate the complex interrelationships between CP, frailty, depression and HRQoL in this vulnerable population.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Weifang Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YS: Writing – original draft, Conceptualization. MX: Conceptualization, Writing – original draft. WY: Conceptualization, Writing – original draft. ZL: Data curation, Writing – review & editing. PD: Writing – review & editing, Data curation. XZha: Writing – original draft, Investigation, Data curation. HL: Writing – review & editing, Investigation. XZhu: Investigation, Writing – review & editing. XL: Formal analysis, Writing – review & editing. MG: Formal analysis, Writing – review & editing. DM: Formal analysis, Writing – review & editing. KS: Writing – review & editing, Supervision. HC: Supervision, Writing – review & editing. ZC: Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by a grant from by the National Natural Science Foundation of China (72274140), Natural Science Foundation of Shandong Province (ZR2024MG028), Talent Project of Shandong Province (tsqn202312250).
Acknowledgments
The authors thank all participants for their contributions to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HRQoL, Health-related quality of life; CP, Chronic pain; CIs, Confidence intervals; HALE, Healthy life years; CDPs, Chronic disease patients; HPA, Hypothalamic–pituitary–adrenal.
References
1. WHO. World health statistics 2024: monitoring health for the SDGs, sustainable development goals. Available online at: https://www.who.int/publications/i/item/9789240094703.
2. Publicity Department of the National Health Commission. Transcript of the National Health Commission's press conference on November 15, 2023. Available online at: http://www.nhc.gov.cn/xcs/s3574/202311/53b7a4cfc1804f0e9eb1f369cf4e21f7.shtml.
3. He, L, Biddle, SJH, Lee, JT, Duolikun, N, Zhang, L, Wang, Z, et al. The prevalence of multimorbidity and its association with physical activity and sleep duration in middle aged and elderly adults: a longitudinal analysis from China. Int J Behav Nutr Phys Act. (2021) 18:77. doi: 10.1186/s12966-021-01150-7
4. WHO. The World Health Organization Quality of Life (WHOQOL). Available online at: https://www.who.int/publications/i/item/WHO-HIS-HSI-Rev.2012.03
5. The WHOQOL Group. The World Health Organization quality of life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med. (1995) 41:1403–9. doi: 10.1016/0277-9536(95)00112-K
6. Alzarea, AI, Khan, YH, Alzarea, SI, Alanazi, AS, Alsaidan, OA, Alrowily, MJ, et al. Assessment of health-related quality of life among patients with chronic diseases and its relationship with multimorbidity: a cross-sectional study from Saudi Arabia. Patient Prefer Adherence. (2024) 18:1077–94. doi: 10.2147/PPA.S448915
7. Al-Jabi, SW, Seleit, DI, Badran, A, Koni, A, and Zyoud, SH. Impact of socio-demographic and clinical characteristics on functional disability and health-related quality of life in patients with rheumatoid arthritis: a cross-sectional study from Palestine. Health Qual Life Outcomes. (2021) 19:241. doi: 10.1186/s12955-021-01874-x
8. Wong, ELY, Xu, RH, and Cheung, AWL. Measurement of health-related quality of life in patients with diabetes mellitus using EQ-5D-5L in Hong Kong, China. Qual Life Res. (2020) 29:1913–21. doi: 10.1007/s11136-020-02462-0
9. Treede, RD, Rief, W, Barke, A, Aziz, Q, Bennett, MI, Benoliel, R, et al. A classification of chronic pain for ICD-11. Pain. (2015) 156:1003–7. doi: 10.1097/j.pain.0000000000000160
10. Yongjun, Z, Tingjie, Z, Xiaoqiu, Y, Zhiying, F, Feng, Q, Guangke, X, et al. A survey of chronic pain in China. Libyan J Med. (2020) 15:1730550. doi: 10.1080/19932820.2020.1730550
11. Lasalvia, P, Gil-Rojas, Y, and Rosselli, D. Burden of disease of chronic pain in Ecuador. Expert Rev Pharmacoecon Outcomes Res. (2023) 23:547–54. doi: 10.1080/14737167.2023.2193689
12. Cruz-Almeida, Y, Fillingim, RB, Riley, JL 3rd, Woods, AJ, Porges, E, Cohen, R, et al. Chronic pain is associated with a brain aging biomarker in community-dwelling older adults. Pain. (2019) 160:1119–30. doi: 10.1097/j.pain.0000000000001491
13. Fan, Bifa. Report on the Development of Chronic Pain Medicine in China (2020). Tsinghua University Press, Beijing (2020). Available online at: https://wqbook.wqxuetang.com/book/3219665.
14. Wang, S, Du, J, Xi, D, Shao, F, Qiu, M, Shao, X, et al. Role of GABAAR in the transition from acute to chronic pain and the analgesic effect of Electroacupuncture on Hyperalgesic priming model rats. Front Neurosci. (2021) 15:691455. doi: 10.3389/fnins.2021.691455
15. Heikkala, E, Oura, P, Paananen, M, Ho, E, Ferreira, P, Tanguay-Sabourin, C, et al. Chronic disease clusters are associated with prolonged, bothersome, and multisite musculoskeletal pain: a population-based study on northern Finns. Ann Med. (2023) 55:592–602. doi: 10.1080/07853890.2023.2177723
16. 21st Century New Health Research Institute. Community Prevention and Management Initiative for Chronic Disease Pain Risk (2024). 21st Century Business Herald (2024–10). Available online at: https://img.21jingji.com/uploadfile/cover/20241021/5bbc6d76adbc4215cf38dfc9087f9eb3.pdf
17. Corran, TM, Farrell, MJ, Helme, RD, and Gibson, SJ. The classification of patients with chronic pain: age as a contributing factor. Clin J Pain. (1997) 13:207–14. doi: 10.1097/00002508-199709000-00005
18. Baum, E, Abdi, S, Hattendorf, J, van Eeuwijk, P, Tschopp, R, Vosseler, B, et al. Burden of chronic pain among adult pastoralists in Ethiopia: a cross-sectional household survey. Pain. (2024) 165:2629–43. doi: 10.1097/j.pain.0000000000003282
19. De La Rosa, JS, Brady, BR, Herder, KE, Wallace, JS, Ibrahim, MM, Allen, AM, et al. The unmet mental health needs of U.S. adults living with chronic pain. Pain. (2024) 165:2877–87. doi: 10.1097/j.pain.0000000000003340
20. Ryan, E, Grol-Prokopczyk, H, Dennison, CR, Zajacova, A, and Zimmer, Z. Is the relationship between chronic pain and mortality causal? A propensity score analysis. Pain. (2025) 166:183–95. doi: 10.1097/j.pain.0000000000003336
21. Thillainadesan, J, Scott, IA, and Le Couteur, DG. Frailty, a multisystem ageing syndrome. Age Ageing. (2020) 49:758–63. doi: 10.1093/ageing/afaa112
22. Chen, X, Yang, S, Wang, Y, and Liu, S. Study on influencing factors and intervention strategies of frailty in elderly diabetic patients. Chin Gen Med. (2019) 22:1772–7. doi: 10.12114/j.issn.1007-9572.2019.00.14
23. Liu, F, Yang, Q, Yang, K, Sun, J, Li, Y, Ban, B, et al. Cortisol circadian rhythm and sarcopenia in patients with type 2 diabetes: a cross-sectional study. J Cachexia Sarcopenia Muscle. (2025) 16:e13727. doi: 10.1002/jcsm.13727
24. Bundred, J, Thakkar, RG, and Pandanaboyana, S. Systematic review of sarcopenia in chronic pancreatitis: prevalence, impact on surgical outcomes, and survival. Expert Rev Gastroenterol Hepatol. (2022) 16:665–72. doi: 10.1080/17474124.2022.2091544
25. GGolchha, S, Sondhi, S, Gupta, S, and Goel, A. Frailty in diabetic population: a study from northern India. Cureus. (2024) 16:e68494. doi: 10.7759/cureus.68494
26. Singh, M, Stewart, R, and White, H. Importance of frailty in patients with cardiovascular disease. Eur Heart J. (2014) 35:1726–31. doi: 10.1093/eurheartj/ehu197
27. Hruschak, V, and Cochran, G. Psychosocial predictors in the transition from acute to chronic pain: a systematic review. Psychol Health Med. (2018) 23:1151–67. doi: 10.1080/13548506.2018.1446097
28. Brown, L, Mossabir, R, Harrison, N, Lam, N, Grice, A, Clegg, A, et al. Developing the evidence and associated service models to support older adults living with frailty to manage their pain and to reduce its impact on their lives: protocol for a mixed-method, co-design study (the POPPY study). BMJ Open. (2023) 13:e074785. doi: 10.1136/bmjopen-2023-074785
29. Máximo, RO, Lopes, IC, Brigola, AG, Luchesi, BM, Gratão, ACM, Inouye, K, et al. Pre-frailty, frailty and associated factors in older caregivers of older adults. Rev Saude Publica. (2020) 54:17. doi: 10.11606/s1518-8787.2020054001655
30. Otones, P, García, E, Sanz, T, and Pedraz, A. A physical activity program versus usual care in the management of quality of life for pre-frail older adults with chronic pain: randomized controlled trial. BMC Geriatr. (2020) 20:396. doi: 10.1186/s12877-020-01805-3
31. Figueiredo, T, Midão, L, Sampaio, R, Carrilho, J, Coelho, C, Cerullo, G, et al. Managing non-Cancer chronic pain in frail older adults: a pilot study based on a multidisciplinary approach. Int J Environ Res Public Health. (2023) 20:7150. doi: 10.3390/ijerph20247150
32. Vermeiren, S, Vella-Azzopardi, R, Beckwée, D, Habbig, A-K, Scafoglieri, A, Jansen, B, et al. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. (2016) 17:1163.e1–1163.e17. doi: 10.1016/j.jamda.2016.09.010
33. Kapan, A, Ristic, M, Leser, A, Felsinger, R, and Waldhoer, T. Assessment of muscle fatigability using isometric repetitive handgrip strength in frail older adults. A cross-sectional study. J Transl Med. (2025) 23:215. doi: 10.1186/s12967-025-06239-2
34. Muntsant, A, and Giménez-Llort, L. Crosstalk of Alzheimer's disease-phenotype, HPA axis, splenic oxidative stress and frailty in late-stages of dementia, with special concerns on the effects of social isolation: a translational neuroscience approach. Front Aging Neurosci. (2022) 14:969381. doi: 10.3389/fnagi.2022.969381
35. Dugravot, A, Fayosse, A, Dumurgier, J, Bouillon, K, Rayana, TB, Schnitzler, A, et al. Social inequalities in multimorbidity, frailty, disability, and transitions to mortality: a 24-year follow-up of the Whitehall II cohort study. Lancet Public Health. (2020) 5:e42–50. doi: 10.1016/S2468-2667(19)30226-9
36. Liu, C, Zhou, R, Peng, X, Chen, X, Xia, Z, Wei, W, et al. The longitudinal study of the relationship between social participation pattern and depression symptoms in frail older adults. Front Psych. (2024) 15:1440641. doi: 10.3389/fpsyt.2024.1440641
37. Wang, H, Chen, Y, Ding, H, Li, Y, Li, M, and Wang, X. Current status and influencing factors of frailty among rural elderly in Changfeng County, Anhui Province. Med Soc. (2025) 38:67–73. doi: 10.13723/j.yxysh.2025.02.010
38. Yu, S, Che, Y, Zhang, N, Mehmeti, S, Feng, X, and Yan, P. The chain mediating effect of physical function and nutritional status of rural elderly on sleep quality and frailty. Mod Prevent Med. (2024) 51:4473–9. doi: 10.20043/j.cnki.MPM.202407426
39. Singh, A, Gamboa-Cárdenas, RV, Pimentel-Quiroz, V, Rodriguez-Bellido, Z, Pastor-Asurza, C, Perich-Campos, R, et al. Systemic lupus international collaborating clinics frailty index (SLICC-FI) predicts worsening health-related quality of life, data from the Almenara lupus cohort. Arthritis Care Res (Hoboken). (2025) 77:1135–40. doi: 10.1002/acr.25544
40. Choi, JY, Yoon, YS, Kim, KI, and Kim, CH. Multiple domain resilience components and frailty, postoperative complications, and one year quality of life deterioration after pancreatectomy in older patients. Sci Rep. (2025) 15:11047. doi: 10.1038/s41598-024-82627-w
41. Jing, Z, Li, J, Fu, PP, Wang, Y, Yuan, Y, Zhao, D, et al. Physical multimorbidity and lifetime suicidal ideation and plans among rural older adults: the mediating role of psychological distress. BMC Psychiatry. (2021) 21:78. doi: 10.1186/s12888-021-03087-4
42. Luo, J, Zhao, D, Gao, T, Wang, X, Wang, X, Chai, S, et al. The mediating effect of sleep quality on solid cooking fuel use and psychological distress among rural older adults: evidence from Shandong, China. BMC Geriatr. (2024) 24:750. doi: 10.1186/s12877-024-05327-0
43. WHO. Depressive disorder (depression). Available online at: https://www.who.int/zh/news-room/fact-sheets/detail/depression
44. Matcham, F, Rayner, L, Steer, S, and Hotopf, M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology (Oxford). (2013) 52:2136–48. doi: 10.1093/rheumatology/ket169
45. Karmacharya, I, Chapadia, B, Shrestha, A, Subedi, J, Yadav, UN, Mistry, SK, et al. Depressive symptoms among resettled Bhutanese older adults in Ohio: a cross-sectional study. BMC Psychol. (2025) 13:239. doi: 10.1186/s40359-024-02255-x
46. Shen, Y, Chen, Y, Huang, S, Yao, X, Kanwar, YS, and Zhan, M. The association between symptoms of depression and anxiety, quality of life, and diabetic kidney disease among Chinese adults: a cross-sectional study. Int J Environ Res Public Health. (2022) 20:475. doi: 10.3390/ijerph20010475
47. Liu, G, Liu, D, Shi, D, Wang, Z, and Fu, W. Electroacupuncture ameliorates chronic inflammatory pain and depression comorbidity by inhibiting Nrf 2-mediated ferroptosis in hippocampal neurons. Neurochem Res. (2025) 50:149. doi: 10.1007/s11064-025-04401-2
48. Gong, H, Leng, S, Wang, X, and Han, D. The impact of physical activity on depression in elderly Chinese people. Chin J Gerontol. (2024) 44:5343–7. doi: 10.3969/j.issn.1005-9202.2024.21.055
49. Makris, UE, Higashi, RT, Marks, EG, Fraenkel, L, Gill, TM, Friedly, JL, et al. Physical, emotional, and social impacts of restricting Back pain in older adults: a qualitative study. Pain Med. (2017) 18:1225–35. doi: 10.1093/pm/pnw196
50. Jiang, Q, Feng, M, Yuan, L, Liu, L, Hou, B, and Sun, J. Analysis of the current status and influencing factors of disability among elderly people with depressive symptoms in China—based on the 8th round of CLHLS data. J Naval Med Univ. (2023) 44:1268–75. doi: 10.16781/j.CN31-2187/R.20230234
51. Freire, ACC, Pondé, MP, Liu, A, and Caron, J. Anxiety and depression as longitudinal predictors of mild cognitive impairment in older adults. Can J Psychiatr. (2017) 62:343–50. doi: 10.1177/0706743717699175
52. Atkins, J, Naismith, SL, Luscombe, GM, and Hickie, IB. Psychological distress and quality of life in older persons: relative contributions of fixed and modifiable risk factors. BMC Psychiatry. (2013) 13:249. doi: 10.1186/1471-244X-13-249
53. Qaisar, R, Hussain, MA, Karim, A, Ahmad, F, Awad, A, Alsaeed, M, et al. Cystatin-c/total cholesterol ratio as a predictor of probable sarcopenia in geriatric population from 12 European countries. Aging Clin Exp Res. (2025) 37:94. doi: 10.1007/s40520-025-03007-6
54. Luo, N, Liu, G, Li, M, Guan, H, Jin, X, and Rand-Hendriksen, K. Estimating an EQ-5D-5L value set for China. Value Health. (2017) 20:662–9. doi: 10.1016/j.jval.2016.11.016
55. Abellan van Kan, G, Rolland, Y, Bergman, H, Morley, JE, Van Kan, GA, Kritchevsky, SB, et al. The I.A.N.A task force on frailty assessment of older people in clinical practice. J Nutr Health Aging. (2008) 12:29–37. doi: 10.1007/BF02982161
56. Hong, S, Lu, B, Wang, S, and Jiang, Y. Comparison of logistic regression and machine learning methods for predicting depression risks among disabled elderly individuals: results from the China health and retirement longitudinal study. BMC Psychiatry. (2025) 25:128. doi: 10.1186/s12888-025-06577-x
57. Xu, PR, Wei, R, Cheng, BJ, Wang, AJ, Li, XD, Li, HB, et al. The association of marital status with cognitive function and the role of gender in Chinese community-dwelling older adults: a cross-sectional study. Aging Clin Exp Res. (2021) 33:2273–81. doi: 10.1007/s40520-020-01743-5
58. Hayes, AF. Beyond baron and kenny: statistical mediation analysis in the new millennium. Commun Monogr. (2009) 76:408–20. doi: 10.1080/03637750903310360
59. Zhou, H, and Long, L. Statistical tests and control methods for common method bias. Adv Psychol Sci. (2004) 12:942–50. doi: 10.3969/j.issn.1671-3710.2004.06.018
60. Bakzaza, B, Lemmih, H, Errachidi, F, el Bouazzi, O, Rachiq, S, and Raoui, SM. The life quality of people living with chronic disease in Africa: a systematic narrative synthesis. Pan Afr Med J. (2024) 49:115. doi: 10.11604/pamj.2024.49.115.42393
61. Cai, Y, Wu, S, and Chen, Y. Overview of the international application of health expectancy indicators. Chin Med J. (2023) 103:229–34. doi: 10.3760/cma.j.cn112137-20221111-02372
62. Clement, ND, Duthie, RA, Mac Donald, DJ, Yapp, LZ, and Scott, CEH. Chronic knee pain while awaiting arthroplasty is associated with worsening joint-specific function, health-related quality of life and personal wellbeing, and increased use of opioid analgesia. Bone Jt Open. (2025) 6:237–45. doi: 10.1302/2633-1462.63.BJO-2024-0210.R1
63. Kurniasari, MD, Karwur, FF, Rayanti, RE, Shih, YW, Yuliana, S, Miao, NF, et al. Immersion in water between 20-30oC mediated inflammations marker to reduced pain among Indonesian with gout arthritis: a community-based randomized controlled trial. Biol Res Nurs. (2023) 25:267–81. doi: 10.1177/10998004221132843
64. Veronese, N, Koyanagi, A, Barbagallo, M, Dominguez, LJ, Maggi, S, Soysal, P, et al. Pain increases the risk for sarcopenia in community-dwelling adults: results from the English longitudinal study of ageing. J Gerontol A Biol Sci Med Sci. (2023) 78:1013–9. doi: 10.1093/gerona/glad062
65. Barron, DS, Saltoun, K, Kiesow, H, Fu, M, Cohen-Tanugi, J, Geha, P, et al. Pain can't be carved at the joints: defining function-based pain profiles and their relevance to chronic disease management in healthcare delivery design. BMC Med. (2024) 22:594. doi: 10.1186/s12916-024-03807-z
66. Kato, S, Demura, S, Shinmura, K, Yokogawa, N, Kabata, T, Matsubara, H, et al. Association of low back pain with muscle weakness, decreased mobility function, and malnutrition in older women: a cross-sectional study. PLoS One. (2021) 16:e0245879. doi: 10.1371/journal.pone.0245879
67. Valdes-Hernandez, PA, Montesino-Goicolea, S, Laffitte Nodarse, C, Johnson, AJ, Fillingim, RB, and Cruz-Almeida, Y. Widespread and prolonged pain may reduce brain clearance capacity only via sleep impairment: evidence from participants with knee pain. J Pain. (2025) 30:105356. doi: 10.1016/j.jpain.2025.105356
68. Ekholm, O, Herling, SF, Lykke, C, Skurtveit, S, Hamina, A, Sjøgren, P, et al. Monitoring chronic non-Cancer pain in Denmark over two decades: prevalence, mental health and loneliness. Eur J Pain. (2025) 29:e 4776. doi: 10.1002/ejp.4776
69. Fan, HB, Zhang, T, Sun, K, Song, SP, Cao, SB, Zhang, HL, et al. Corticotropin-releasing factor mediates bone cancer induced pain through neuronal activation in rat spinal cord. Tumour Biol. (2015) 36:9559–65. doi: 10.1007/s13277-015-3670-1
70. Shen, Y, and Lin, P. Association between frailty and postherpetic neuralgia in the older adult with herpes zoster. Front Public Health. (2025) 13:1511898. doi: 10.3389/fpubh.2025.1511898
71. Nissen, A, Hynek, KA, Scales, D, Hilden, PK, and Straiton, M. Chronic pain, mental health and functional impairment in adult refugees from Syria resettled in Norway: a cross-sectional study. BMC Psychiatry. (2022) 22:571. doi: 10.1186/s12888-022-04200-x
72. Simning, A, and Seplaki, CL. Association of the cumulative burden of late-life anxiety and depressive symptoms with functional impairment. Int J Geriatr Psychiatry. (2020) 35:80–90. doi: 10.1002/gps.5221
73. Harris, RE, Clauw, DJ, Scott, DJ, McLean, SA, Gracely, RH, and Zubieta, JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. (2007) 27:10000–6. doi: 10.1523/JNEUROSCI.2849-07.2007
74. Miu, DK. Pain and frailty among community-dwelling older people in Hong Kong. Asian J Gerontol Geriatr. (2022) 17:22–7. doi: 10.12809/ajgg-2022-529-oa
75. Arias, HR, Micheli, L, Jensen, AA, Galant, S, Vandermoere, F, Venturi, D, et al. Ibogalogs decrease neuropathic pain in mice through a mechanism involving crosstalk between 5-HT2A and mGlu2 receptors. Biomed Pharmacother. (2025) 184:117887. doi: 10.1016/j.biopha.2025.117887
76. Wippold, GM, Tucker, CM, Roncoroni, J, and Henry, MA. Impact of stress and loneliness on health-related quality of life among low income senior African Americans. J Racial Ethn Health Disparities. (2021) 8:1089–97. doi: 10.1007/s40615-020-00865-w
77. Li, Y, Chen, X, Hu, D, Peng, X, and Wang, J. The relationship between psychological distress and frailty in stroke patients: the mediating effect of depression. BMC Psychol. (2025) 13:159. doi: 10.1186/s40359-025-02454-0
78. Soysal, P, Veronese, N, Thompson, T, Kahl, KG, Fernandes, BS, Prina, AM, et al. Relationship between depression and frailty in older adults: a systematic review and meta-analysis. Ageing Res Rev. (2017) 36:78–87. doi: 10.1016/j.arr.2017.03.005
Keywords: frailty, depression, chronic disease management, chronic pain, chain mediation
Citation: Shi Y, Xu M, Yin W, Li Z, Dong P, Zhang X, Li H, Zhuge X, Li X, Gao M, Ma D, Sun K, Cao H and Chen Z (2025) The relationship between chronic pain and health-related quality of life: the mediating roles of frailty and depression in chronic disease patients. Front. Public Health. 13:1658008. doi: 10.3389/fpubh.2025.1658008
Edited by:
Alberto Sardella, University of Catania, ItalyReviewed by:
Rola Angga Lardika, Riau University, IndonesiaAndrés Ramírez, Salesian Polytechnic University, Ecuador
Copyright © 2025 Shi, Xu, Yin, Li, Dong, Zhang, Li, Zhuge, Li, Gao, Ma, Sun, Cao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kui Sun, d2VpeWlndWFubGlAMTYzLmNvbQ==; Haihong Cao, MTU4OTg5MzEyNzNAMTYzLmNvbQ==; Zhongming Chen, Y3ptMzMwNjE5NkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Yongli Shi
Yongli Shi Mengyuan Xu1†
Mengyuan Xu1† Ziyuan Li
Ziyuan Li Xiaona Li
Xiaona Li Min Gao
Min Gao Dongping Ma
Dongping Ma Zhongming Chen
Zhongming Chen