- 1Anhui Polytechnic University, Wuhu, Anhui, China
- 2Hefei Preschool Education College, Hefei, Anhui, China
- 3Changzhou University, Changzhou, Jiangsu, China
Objective: Insulin-like growth factor-1 (IGF-1) is thought to play an important role in regulating skeletal muscle mass and function, with its decline potentially linked to age-related frailty and sarcopenia. Given the limitations of pharmacological and nutritional interventions, exercise may serve as a potential non-pharmacological strategy to modulate IGF-1 levels. The purpose of this study is to systematically evaluates the effects of exercise interventions on serum IGF-1 levels in older adults with frailty and/or sarcopenia using a meta-analysis approach.
Methods: A systematic search was conducted in PubMed, Web of Science, Cochrane Library, EMBASE and Scopus (from inception to July 2025) to identify randomized controlled trials (RCTs) investigating the impact of exercise interventions on serum IGF-1 levels in older adults with frailty and/or sarcopenia. Data were analyzed using RevMan 5.4 and Stata 15.1, with standardized mean differences (SMD) and 95% confidence intervals (95% CI) calculated via a random-effects model. The protocol was registered with PROSPERO (CRD420251085472).
Results: A total of 11 studies (comprising 16 RCTs) were included, involving 604 participants (intervention group: 314; control group: 290), age range: 63.6 to 85.8 years old. Meta-analysis revealed that exercise interventions significantly increased serum IGF-1 levels in older adults with frailty and/or sarcopenia (SMD = 0.42, 95% CI: 0.23–0.60, p < 0.0001, I2 = 15%). Subgroup analysis demonstrated that combined training (aerobic + resistance) yielded the most pronounced effect (SMD = 0.60, 95% CI: 0.36–0.84, p < 0.00001, I2 = 0%), followed by resistance training alone (SMD = 0.35, 95% CI: 0.05–0.66, p = 0.02, I2 = 28%), whereas aerobic training alone showed no significant effect [SMD = 0.01, 95%CI: (−0.46, 0.48), p = 0.96, I2 = 0%]. Similarly, subgroup analysis revealed that exercise intervention could effectively improve serum IGF-1 levels in older adult individuals with frailty (SMD = 0.53, 95%CI: 0.07–0.98, I2 = 0%) or sarcopenia (SMD = 0.40, 95%CI: 0.19–0.61, I2 = 25%), with no statistically significant difference in effect sizes between the two groups.
Conclusion: Exercise intervention can effectively increase serum IGF-1 concentrations in older adults with frailty and/or sarcopenia. The research results may provide key evidence-based basis for clinical non-pharmacological interventions.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251085472.
1 Introduction
Sarcopenia is a geriatric syndrome characterized by progressive and generalized decline in muscle strength and mass, along with impaired balance ability, which increases the risk of falls, physical disability, mortality, and other adverse outcomes (1). This condition exhibits clinical heterogeneity and frequently coexists with metabolic disorders such as osteoporosis and obesity, leading to complications including osteosarcopenia, sarcopenic obesity, and osteosarcopenic obesity (2–4). Frailty, on the other hand, is defined by a decline in physiological reserve, impaired stress response, and disrupted homeostasis in older adults, often presenting with multisystem dysfunction such as reduced mobility, weight loss, and cognitive impairment (5, 6). Sarcopenia is considered an early manifestation of frailty, with shared pathological mechanisms including chronic low-grade inflammation, hormonal dysregulation, malnutrition, and sedentary behavior (7). Both conditions are clinically characterized by functional decline, particularly in balance impairment, muscle function, and strength reduction (7). Against the backdrop of global population aging, the prevalence of sarcopenia and frailty continues to rise (2, 8–10), and both are strongly associated with adverse health outcomes such as falls, hospitalization, chronic disease progression, and all-cause mortality (11–13). Given their profound impact on geriatric health and healthcare burdens, effective prevention and management of frailty and sarcopenia have become critical medical priorities for improving healthy longevity and optimizing healthcare resource allocation.
Insulin-like growth factor-1 (IGF-1) is a pleiotropic polypeptide hormone structurally similar to insulin. It is mainly synthesized in the liver and secreted into the bloodstream (endocrine effect), regulated by growth hormone (GH). Meanwhile, peripheral tissues such as skeletal muscle can also locally synthesize and secrete IGF-1, exerting autocrine/paracrine effects (14). IGF-1 is a key factor regulating the anabolic and catabolic balance of skeletal muscle and determining muscle size and function (15). After the age of 21, serum IGF-1 concentrations in humans show a progressive downward trend with increasing age, and this decline is closely associated with the onset and progression of sarcopenia (16). The reason why maintaining IGF-1 levels is effective in preventing and delaying sarcopenia lies in its dual regulatory role in skeletal muscle protein metabolism: IGF-1 binds to IGF-1 receptors (IGF-1R) on muscle cell surfaces, activating multiple intracellular signaling cascades (such as the PI3K/Akt/mTOR pathway, PI3K/Akt/GSK3β pathway, and MAPK/ERK pathway), thereby promoting muscle protein synthesis (17, 18). At the same time, IGF-1 can also “turn off” the expression of muscle atrophy genes at the transcriptional level through signal transduction via its PI3K/Akt/FoxO pathway, thereby inhibiting protein degradation (19–21). A Mendelian randomization study based on genetic evidence further suggests a causal association between genetically predicted higher IGF-1 levels and lower sarcopenia risk (22). In addition, the relationship between IGF-1 and sarcopenia/frailty is not a one-way causal chain but a complex interaction network. Pathological states themselves can in turn profoundly affect the function of the IGF-1 system. Studies have shown that factors such as malnutrition, chronic low-grade inflammation, and reduced physical activity in the context of sarcopenia may further inhibit the synthesis and secretion of IGF-1 in the liver and muscles (23); in local tissues, hydrolytic cleavage of IGF-binding proteins (IGFBPs) is a key step in releasing free IGF-1 and regulating its bioavailability (24), but this regulatory process may be disrupted in the pathological microenvironment of sarcopenia, thereby inhibiting IGF-1 signaling (25).
While pharmacological interventions (e.g., recombinant human growth hormone [rhGH], somatostatin analogs) and nutritional strategies (e.g., high-protein diets, amino acid supplementation) can effectively elevate serum IGF-1 levels, they are limited by poor adherence, high costs, and potential side effects (26–28). Compared with drug and nutritional therapies, exercise training is characterized by greater safety and more cost-effective alternative. However, its controversy in the effect of increasing serum IGF-1 concentrations has attracted much attention, with the controversy focusing on whether exercise intervention can effectively increase serum IGF-1 concentrations. Some studies have shown that endurance and resistance training can promote the release of inflammatory cytokines and growth factors including IGF-1 by inducing remodeling of muscle ultrastructure, suggesting that exercise may have the potential to increase serum IGF-1 (29, 30). In contrast, other studies have found that serum IGF-1 concentrations do not increase significantly or even decrease following either acute or long-term exercise interventions, which is in clear disagreement with the aforementioned conclusions (31, 32).
Additionally, individual randomized controlled trials (RCT) often suffer from small sample sizes and heterogeneous intervention protocols. Existing studies have conducted comprehensive quantitative analyses of results from multiple randomized controlled trials through meta-analysis. For instance, Titus et al., Behrad et al., and Jiang et al. all used meta-analytic methods to, respectively, verify the improvement effect of exercise intervention on serum IGF-1 concentration in healthy middle-aged and older adult individuals (33–35). However, none have specifically focused on older adults with frailty and/or sarcopenia, limiting their clinical applicability. These inconsistencies highlight the need for more systematic investigations to clarify the effects of exercise on IGF-1 in this population.
To address these gaps, based on the proposed hypothesis (exercise interventions, particularly those combining resistance and aerobic training, will effectively elevate serum IGF-1 levels in older adults specifically diagnosed with frailty and/or sarcopenia), this study employs meta-analysis to quantitatively synthesize existing RCT evidence on the effects of exercise interventions on serum IGF-1 levels in older adults with frailty and/or sarcopenia. Through systematic review, we further elucidate the underlying mechanisms by which different exercise modalities may enhance IGF-1 production. Additionally, subgroup analyses are conducted to compare the efficacy of various exercise types. By integrating these approaches, this study aims to address existing research gaps by validating the effects of exercise interventions on serum IGF-1 levels in these patients, thereby providing comprehensive theoretical foundations and practical guidance for the prevention and treatment of related conditions, as well as the development of clinical trials.
2 Methods
This study was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for systematic reviews and meta-analyses (36). The protocol has been registered in the PROSPERO database of the Centre for Reviews and Dissemination at the University of York: registration number CRD420251085472.1
2.1 Search strategy
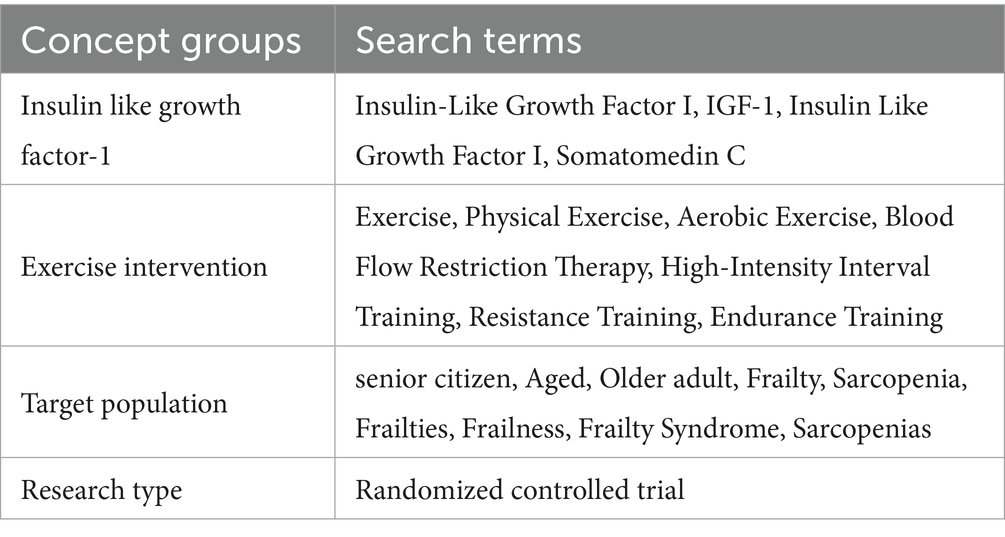
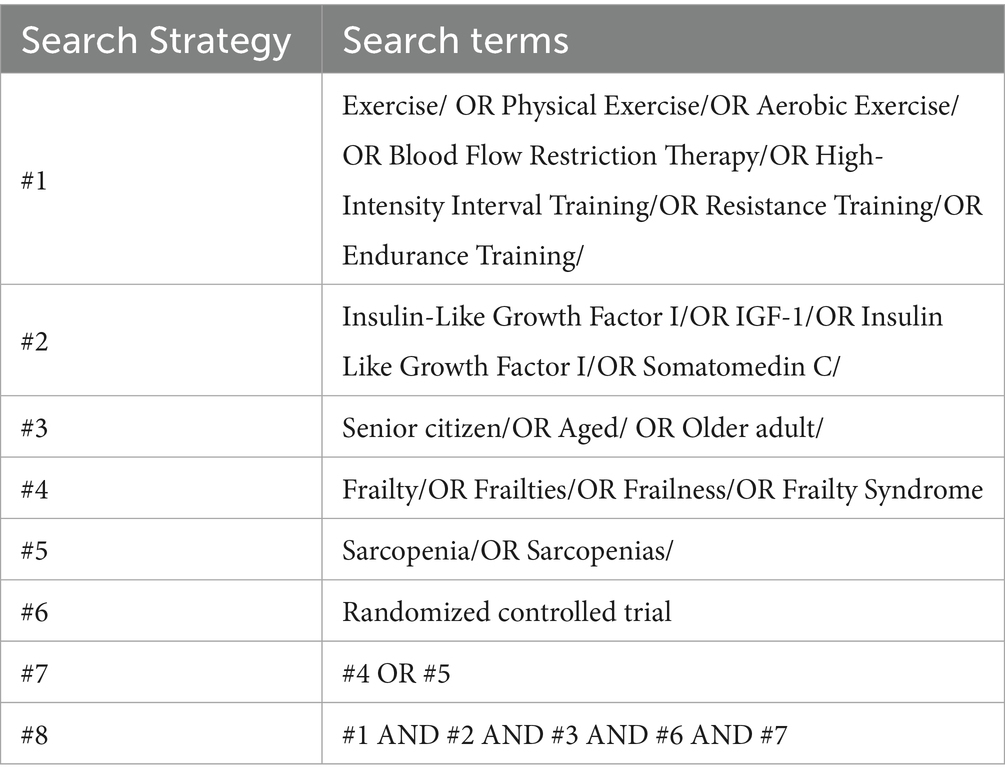
The articles search process involved independent screening by multiple reviewers (MML and YND). The retrieval time span covers from the establishment of each database to July 20, 2025, and the retrieval scope includes electronic databases such as Web of Science, Cochrane Library, PubMed, EMBASE, Scopus, and other relevant electronic databases. The search strategy adopted a combination of subject terms and free terms, covering the following concept groups: (1) Terms related to IGF-1; (2) Terms related to exercise intervention; (3) Terms related to the target population (older adults + frailty/sarcopenia). A detailed list of specific search terms and Search Strategy is provided in Tables 1, 2.
After the retrieval, duplicate articles were first removed by EndNote articles management software. Then, according to the pre-established inclusion and exclusion criteria, the titles and abstracts of the articles were initially screened to exclude obviously irrelevant studies. For the articles that passed the initial screening, the full texts were further obtained, and two independent researchers (MML and YND) conducted secondary screening, quality evaluation and data extraction, respectively. In case of disputes, the corresponding author organized a discussion meeting to negotiate and resolve them.
2.2 Inclusion and exclusion criteria
Based on the PICOS principle of Cochrane, the inclusion and exclusion criteria for articles formulated in this study are as follows:
2.2.1 Inclusion criteria
(1) Study subjects: older adult individuals aged ≥60 years (regardless of gender) from all settings (including communities, nursing homes, hospitals, etc.) who met the clinical diagnostic criteria for sarcopenia and/or frailty were included. Among them, the diagnostic criteria adopted for sarcopenia are as follows: EWGSOP2 (2010): grip strength: male < 27 kg, female < 16 kg; DXA: male < 7.0 kg/m2, female < 5.5 kg/m2; BIA: male < 7.0 kg/m2; female < 5.7 kg/m2, gait speed ≤ 0.8 m/s (37); AWGS (2019): grip strength: male < 28 kg, female < 18 kg; gait speed: < 1.0 m/s; muscle mass: DXA male < 7.0 kg/m2, female < 5.4 kg/m2 (38). The diagnostic indicators for older adult frailty are as follows: Frailty Phenotype (2001): among the five criteria of unintentional weight loss, self-reported fatigue, low grip strength, slow gait speed, and insufficient physical activity, ≥3 criteria indicate frailty; 1–2 criteria indicate pre-frailty; 0 criteria indicate health (39); Frailty Index: the proportion is calculated based on more than 30 health deficits (symptoms, diseases, dysfunctions), and a Frailty Index (FI) ≥ 0.25 is used to determine frailty (40); Clinical Frailty Scale, CFS (2005): the frailty degree of the older adult is quantitatively evaluated through scoring in terms of their self-perceived ability, walking ability, cognitive ability, emotional state, etc. The CFS scale is usually divided into 9 levels, ranging from very healthy to end-stage, among which levels 1–3 are defined as healthy, levels 4–5 as intermediate frailty, and levels 6 and above as frailty) (41). (2) The experimental group received interventions using single or multiple exercise modalities, including aerobic training (such as brisk walking, stationary cycling, running, balance training, calisthenics, etc.), resistance training (including resistance exercises performed with various equipment), and combined training (conducting both aerobic and resistance training in the same training session). Among these, the exercise interventions were required to be continuous periodic interventions with a duration of at least 4 weeks and a frequency of 2–5 times per week. (3) Control measures: The control group adopts measures such as health education, routine nursing, and routine exercise (Maintain one’s original daily living activities), which were different from the experimental group in terms of intervention methods or intervention objects. (4) Study type: Only randomized controlled trials (RCTs) are included. (5) Outcome indicators: The outcome indicators involved in the study should include Serum IGF-1 Levels(ng/ml). (6) Language restriction: To cover all existing relevant studies as comprehensively as possible, this study does not impose restrictions on the language of the articles included.
2.2.2 Exclusion criteria
(1) Non-randomized controlled trial studies. (2) The intervention measures in the experimental group are inconsistent, such as using other drug interventions or failing to clearly define the type of exercise. (3) The study subjects do not meet the requirements, including non-human subjects, the average age of the intervention objects is under 60 years old, or the diagnostic criteria or guidelines for frailty and sarcopenia are not mentioned. (4) The study data are incomplete and cannot be supplemented. (5) The study results do not report IGF-1 data or the data cannot be extracted. (6) Grey literature: including unpublished articles, conference papers, dissertations, theses, and other similar materials.
2.3 Data extraction
Two reviewers (MML and YND) independently extracted data. If there were disagreements during the extraction process, the corresponding author would organize a meeting to negotiate and resolve them. The specific contents of the extracted data are as follows:
1. Basic information of the articles: first author and year of publication.
2. Subject information: subject characteristics such as sample size, age, physical status (frailty and/or sarcopenia) and diagnostic criteria.
3. Intervention parameters: Specific implementation details of the intervention measures adopted in the experimental group were extracted, and information on intervention parameters such as intervention duration, frequency, and intensity was analyzed according to research needs.
4. Experimental result data: for the intervention results of IGF-1, the final values of the experimental group and the control group after intervention were extracted, and the data were presented in the form of mean ± standard deviation (mean ± SD).
2.4 Quality assessment
Risk of bias was assessed using the Cochrane Risk of Bias Tool (RoB2) (42), conducted independently by two reviewers (MML and YND). Disagreements were resolved through discussion with the corresponding author. The following domains were evaluated:
1. Selection bias (random sequence generation): It is necessary to judge whether the allocation of research subjects has truly achieved randomness, and whether the allocation scheme has been effectively concealed before the completion of allocation, so as to prevent researchers or subjects from predicting the grouping situation and thus avoiding selection bias.
2. Performance bias (deviations from intended interventions): Assess whether the trial successfully implemented blinding for participants and personnel delivering the intervention (such as doctors and nurses). If blinding was not achieved, determine whether there were systematic deviations in the intervention that might affect the outcomes.
3. Attrition bias (incomplete outcome data): Judge whether the outcome data are complete. If incomplete, determine whether the proportion and reasons for data missing may be related to the true outcome values, thereby leading to bias in the effect estimates between groups.
4. Detection bias (inappropriate outcome measurement): Judge whether the measurement methods of outcomes are objective and reliable, and whether outcome assessors remain blinded to participants’ grouping information to prevent subjective judgments from being influenced by grouping information.
5. Reporting bias (selective reporting of results): Judge whether the research results presented in the final report are derived from pre-specified research protocols and analysis plans, or are presented after “data mining” or “data cherry-picking.”
6. Other potential sources of bias: It logically integrates the assessment results of the previous five core domains rather than being an independent source of bias. The overall risk of bias is judged as “high risk” if at least one of the five core domains is rated “high risk”; with no domain rated “high risk,” it is “some concerns” if at least one domain is rated so; and it can only be “low risk” when all five core domains are rated “low risk.”
Each domain was rated as “low risk,” “high risk,” or “unclear risk.” Based on the overall quality, studies were categorized into three grades:
• Grade A: All domains rated as low risk.
• Grade B: Some domains rated as low risk.
• Grade C: No domains rated as low risk.
2.5 Statistical analysis
In this study, RevMan 5.4 and Stata 15.1 software were used to conduct a meta-analysis on the outcome indicators of the included articles, aiming to clarify the intervention effect of exercise intervention on IGF-1 in older adults with frailty and/or sarcopenia. Considering that all outcome indicators of the included articles are continuous variables with inconsistent measurement units, the standardized mean difference (SMD) and its 95% confidence interval (CI) were used for statistical analysis.
In terms of heterogeneity assessment, the I2 statistic and Q test were used for judgment: when I2 ≤ 50% and p ≥ 0.01, it was judged as low heterogeneity; when I2 > 50% and p < 0.01, it was judged as high heterogeneity. In cases of high heterogeneity, subgroup analysis and meta-regression analysis were employed to identify the sources of heterogeneity. Additionally, sensitivity analysis and publication bias detection were conducted to analyze the reliability of the research results. To evaluate potential publication bias, Egger’s test was used for statistical analysis in this study; if publication bias was detected, the trim-and-fill method was used to assess the stability of the overall effect of the study. In addition, sensitivity analysis was performed by excluding the included articles one by one to test the robustness of the meta-analysis results. Meanwhile, due to differences in the biological mechanisms of IGF-1 affected by different types of exercise interventions (such as aerobic training, resistance training, and comprehensive training, etc.), different intervention effects may occur. Therefore, subgroup analysis will be conducted to explore the differences in the impact of different exercise intervention types on outcome indicators.
3 Results
3.1 Studies search results
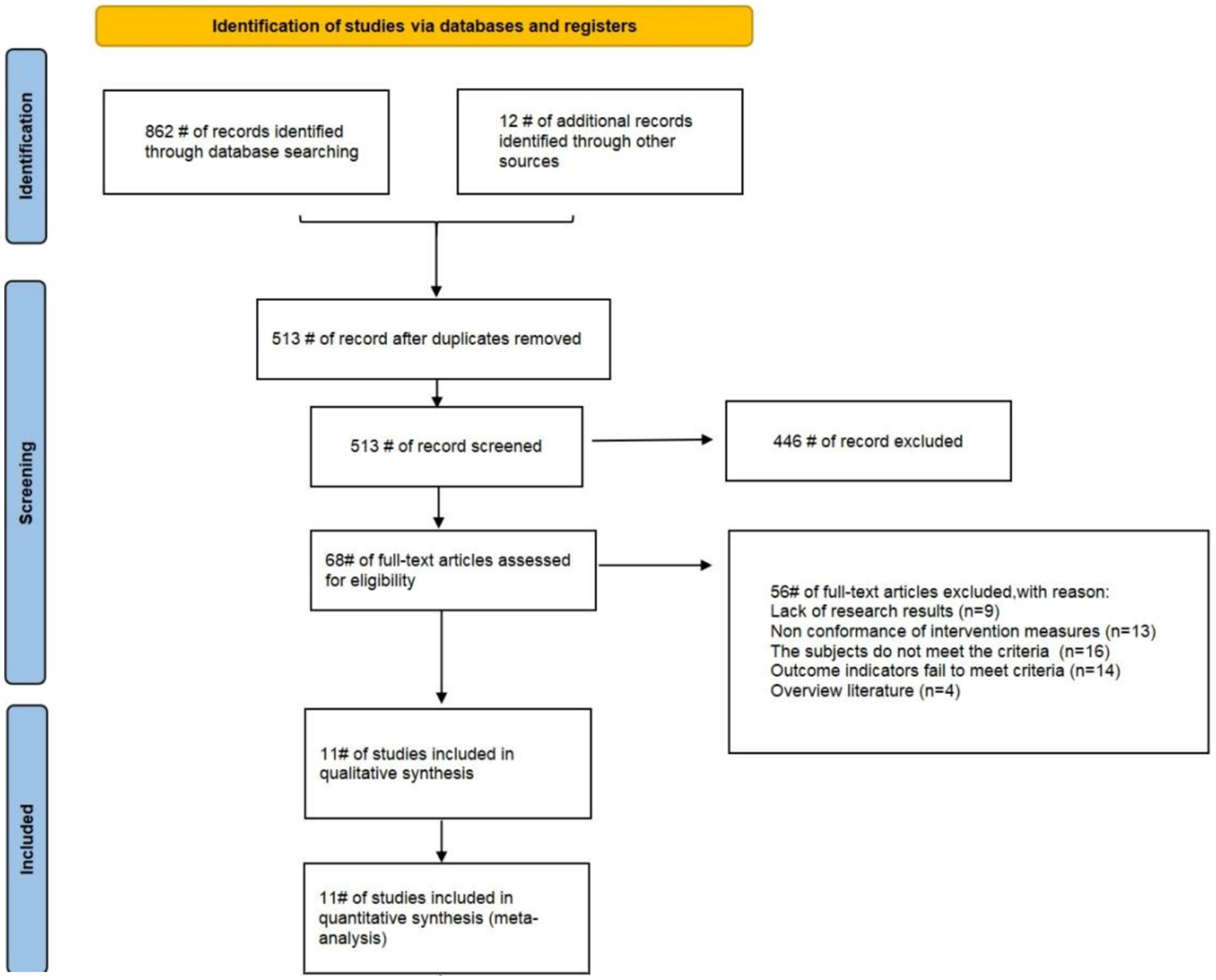
This study comprehensively searched multiple electronic databases and initially identified 874 relevant articles. First, EndNote was used for duplicate checking, 361 duplicate articles were excluded, leaving 513 valid articles. Then, preliminary screening was conducted by reading titles and abstracts, and 446 irrelevant articles were excluded based on research topics and keywords. Finally, the remaining 67 articles were evaluated through full-text intensive reading in accordance with the pre-established inclusion and exclusion criteria, and 11 eligible articles were ultimately included. Among them, since some articles adopted different exercise intervention measures or exercise intervention frequencies in multiple groups, 16 RCTs were included in these 11 articles. The studies screening process is shown in Figure 1.
3.2 Characteristics of the studies
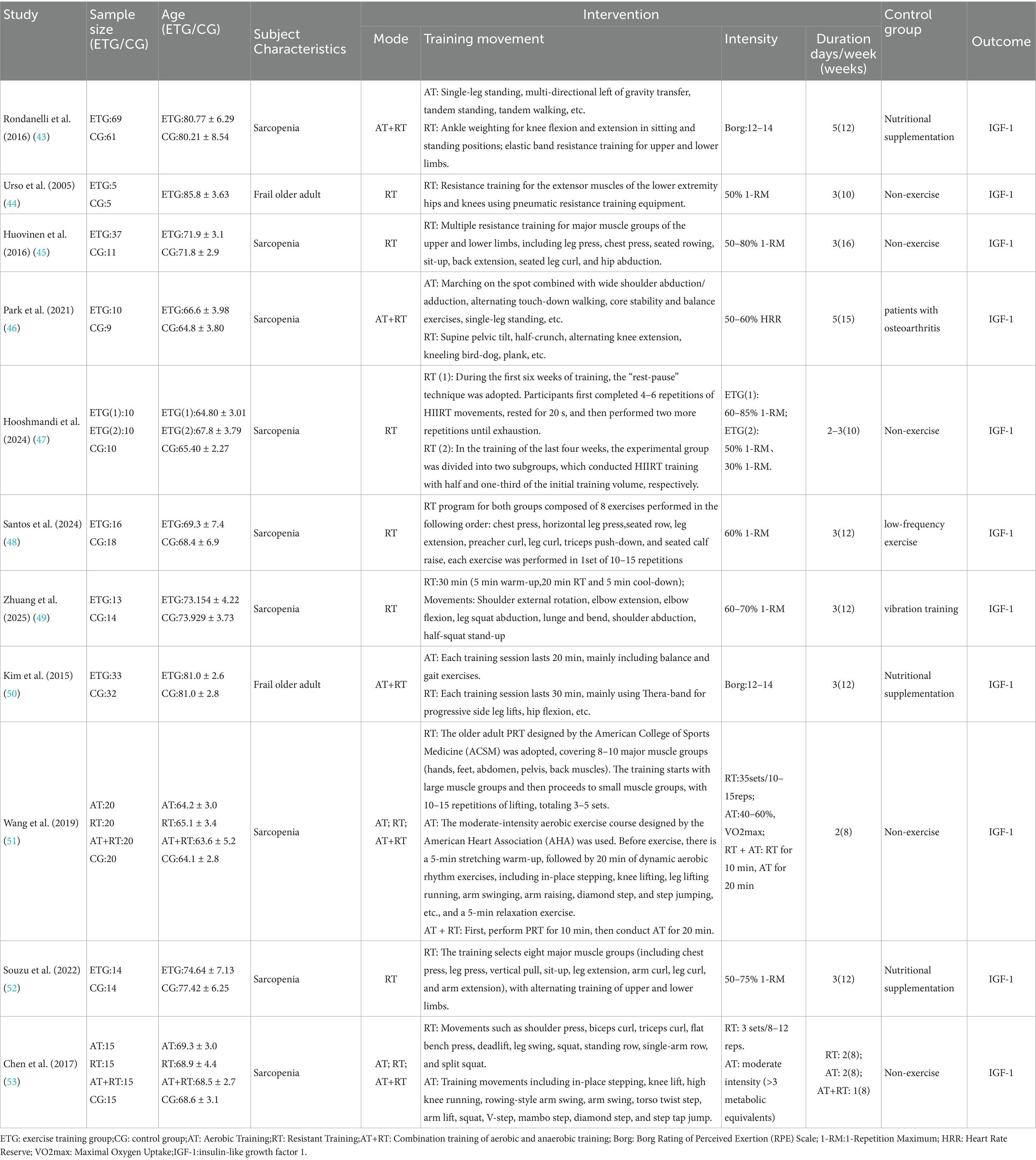
Table 3 systematically summarizes the subject characteristics, experimental grouping design, exercise intervention protocols, and related outcome indicators of the 11 included articles (including 16 RCTs) (43–53). Among them, the total sample size is 604 cases, including 314 cases in the experimental group and 290 cases in the control group. The average age of all participants in the experiment is approximately 71.8 years old.
Among the included articles, 9 articles (14 RCTs) involved older adult subjects with sarcopenia (43, 45–49, 51–53), and 2 articles (2 RCTs) involved frail older adult subjects (44, 50). 5 articles (5 RCTs) adopted the exercise intervention method of combined aerobic training and resistance training (43, 46, 50, 51, 53), 7 articles (9 RCTs) used resistance training as the exercise intervention method (44, 45, 47, 48, 51–53), and 2 articles (2 RCTs) used aerobic training as the exercise intervention method (51, 53). All studies took IGF-1 as the outcome indicator.
3.3 Risk of bias
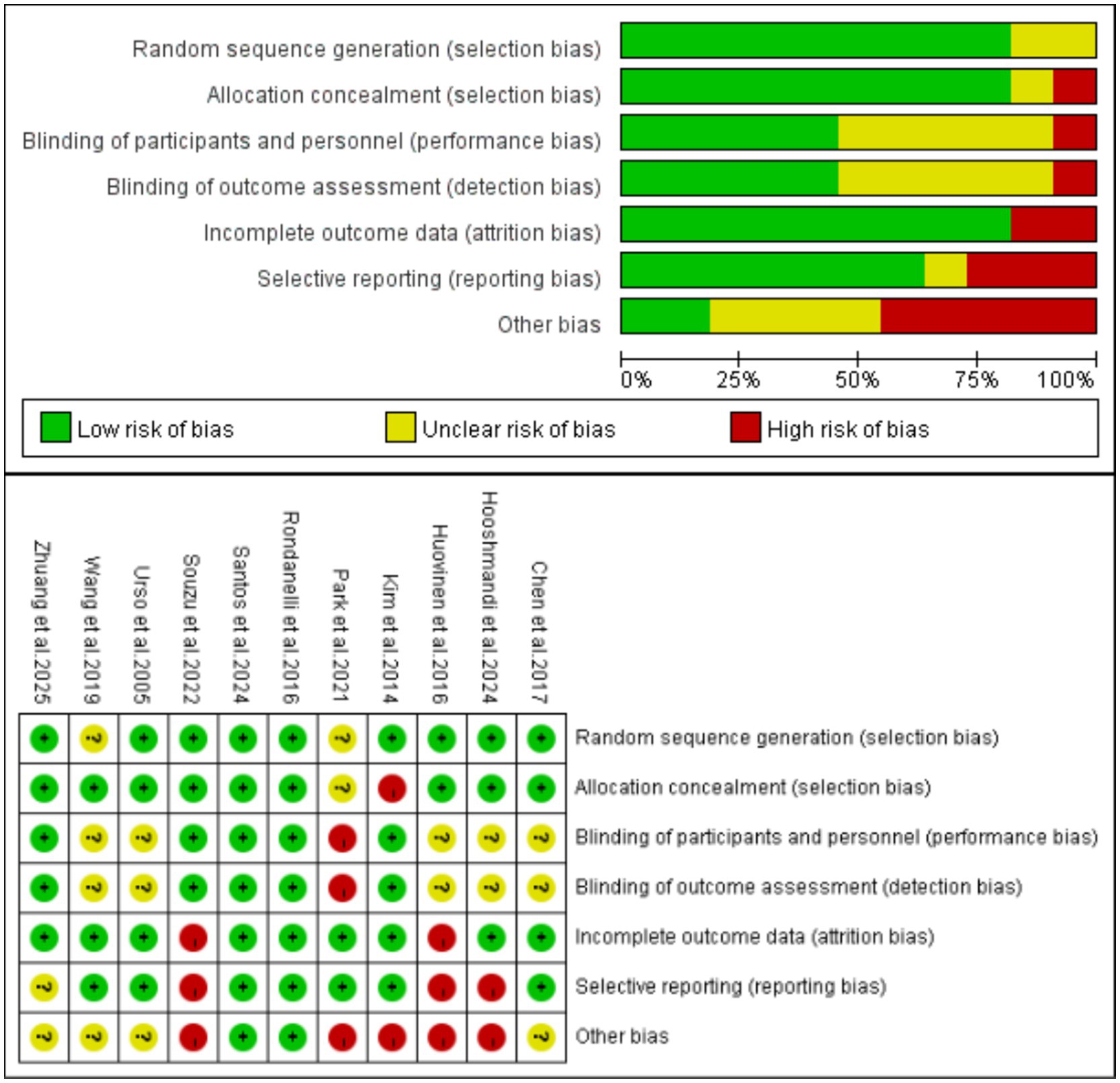
The risk of bias assessment results for the included studies are presented in Figure 2. Among them, 2 studies was rated as Grade A and 9 studies were rated as Grade B, indicating an overall good quality of the articles. Among the included studies, nine studies used random sequence generation (including but not limited to computer-generated random numbers, random number tables, etc.) (43–45, 47–50, 52, 53), while two studies did not clearly report this (46, 51). Nine studies provided detailed descriptions of allocation concealment methods (e.g., computer-generated random allocation sequences with concealment via opaque sealed envelopes) (43–45, 47–49, 51–53), with 1 failing to specify this information (50). Five studies reported their blinding methods (43, 48–50, 52), 5 did not clearly state this (44, 45, 47, 51, 53), and 1 indicated no blinding implementation, being judged to have a high risk of performance bias (46). Additionally, five studies reported that outcome assessors were blinded (43, 48–50, 52), 5 did not clearly state this (44, 45, 47, 51, 53), and 1 indicated no assessor blinding, being judged to have a high risk of detection bias (46). Eight studies described complete outcome data (including measurement results, dropout and attrition rates, etc.) (43, 44, 46, 48–52), whereas 3 lacked reports on dropout and attrition rates (45, 47, 52). Seven studies reported trial registration (low risk of selective reporting bias) (43, 44, 46, 48, 50, 51, 53), while 3 did not register trials and were judged to have a high risk of selective reporting bias (45, 47, 52). Based on analysis of the first five core indicators, other bias risks of the 12 studies were assessed using the “barrel shortcoming effect” principle: only 2 were low risk (43, 48), 4 unclear risk (44, 49, 51, 53), and 5 high risk (45–47, 50, 52).
3.4 Meta-analysis results
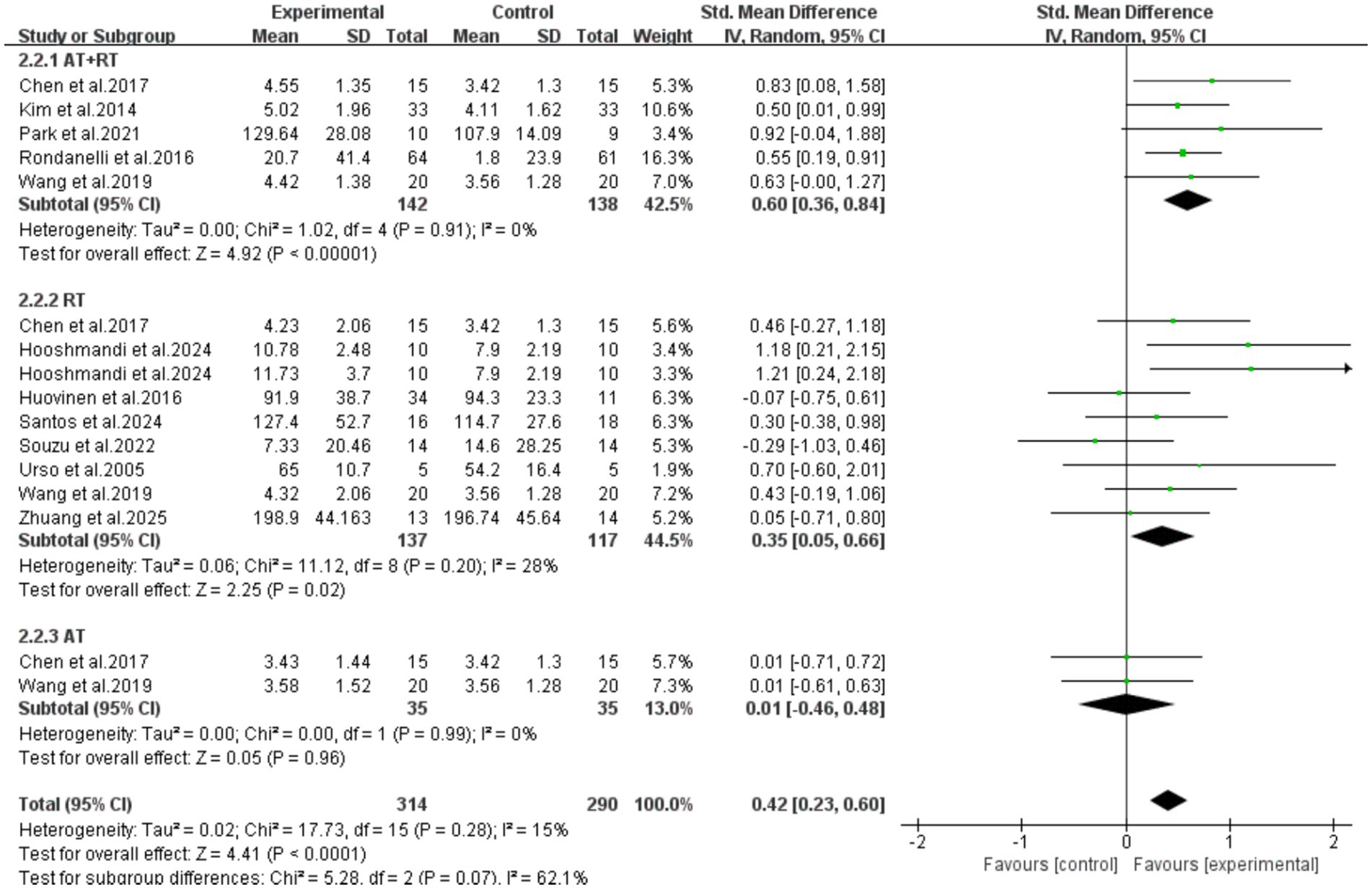
All 11 included articles (including 16 RCTs) adopted exercise as the intervention measure to explore its intervention effect on the serum IGF-1 content in older adults with frailty and/or sarcopenia. Through Meta-analysis (Figure 3), the effect size was obtained as follows: SMD = 0.42, 95% CI: 0.23–0.60, p < 0.0001. The difference was statistically significant, indicating that exercise intervention can significantly increase the serum IGF-1 concentration in older adults with frailty and/or sarcopenia.
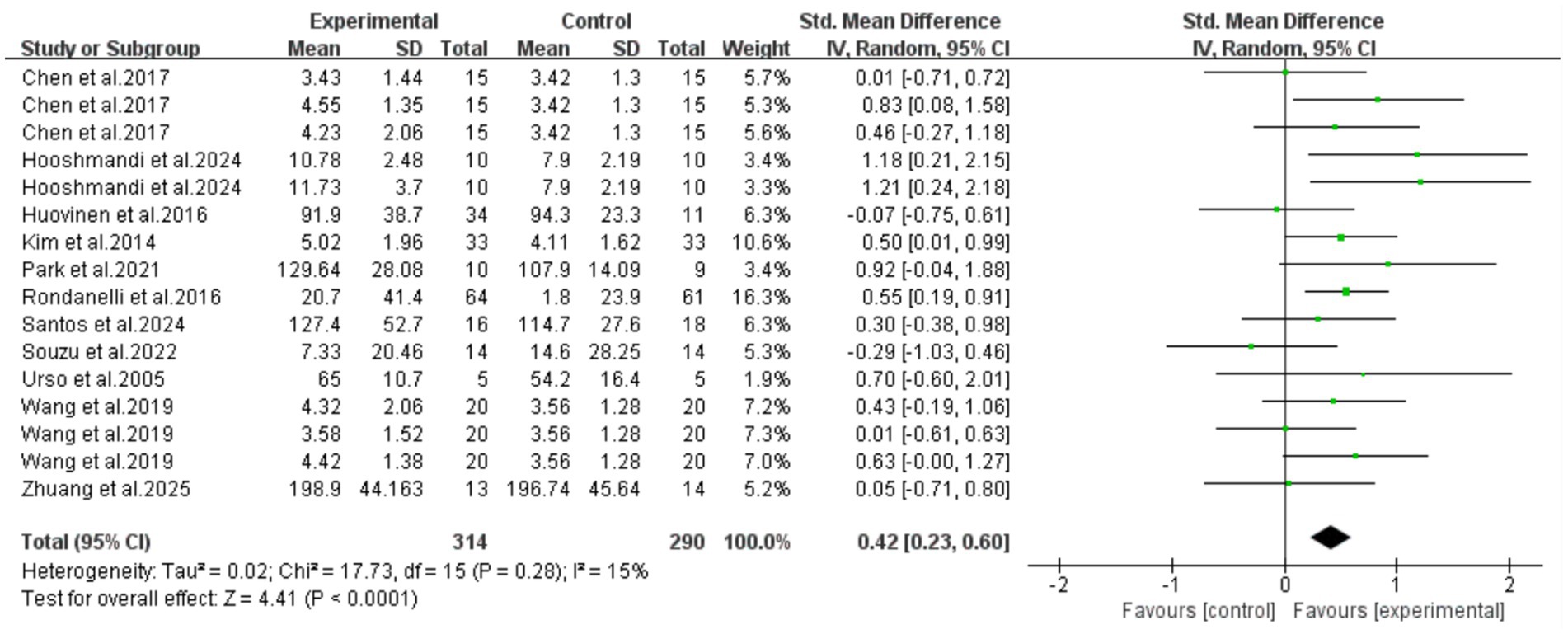
Figure 3. Analysis of the effect of exercise intervention on IGF-1 in older adults with frailty or/and sarcopenia.
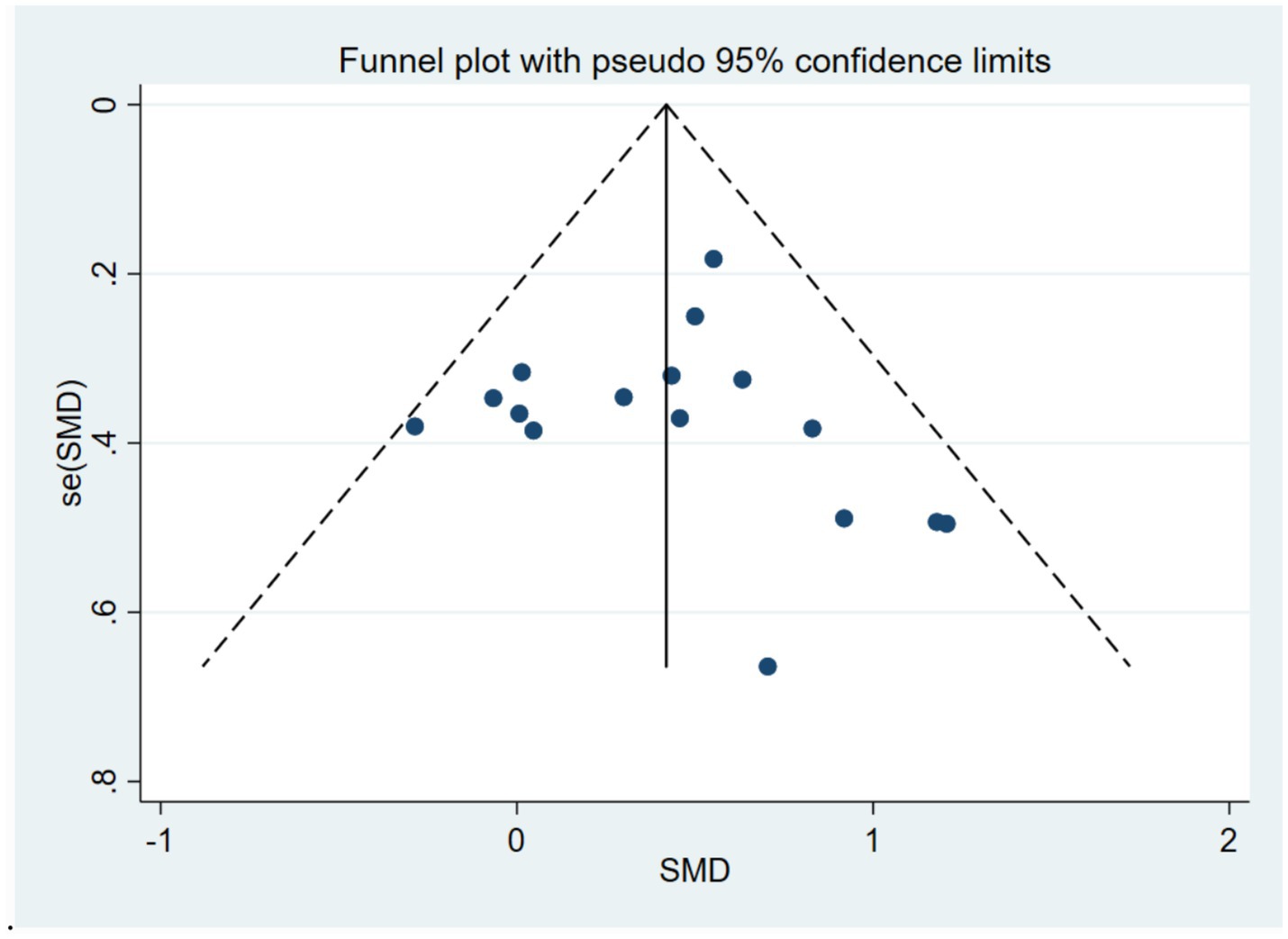
The results of the heterogeneity test showed that there was moderate heterogeneity among the studies (I2 = 15%, p = 0.28). The Egger’s test was used to evaluate the publication bias of the 16 included studies. The results showed that p = 0.700, the results indicated the absence of publication bias, suggesting that the conclusions of this study have good reliability. To further verify publication bias, a funnel plot was generated for visual assessment as Egger’s test showed no significant bias (p = 0.700) (Figure 4). Results indicated approximately symmetric data point distribution; however, the trim-and-fill method was still needed to examine potential small-sample study missing impacts on effect sizes.
After imputing two studies using the trim-and-fill method (Figure 5), the pooled effect size was slightly adjusted from SMD = 0.42 (95%CI: 0.23–0.60, p < 0.0001) to SMD = 0.355 (95%CI: 0.158–0.553, p < 0.0001). The confidence interval still did not include zero and maintained statistical significance, indicating that the core conclusions are robust.
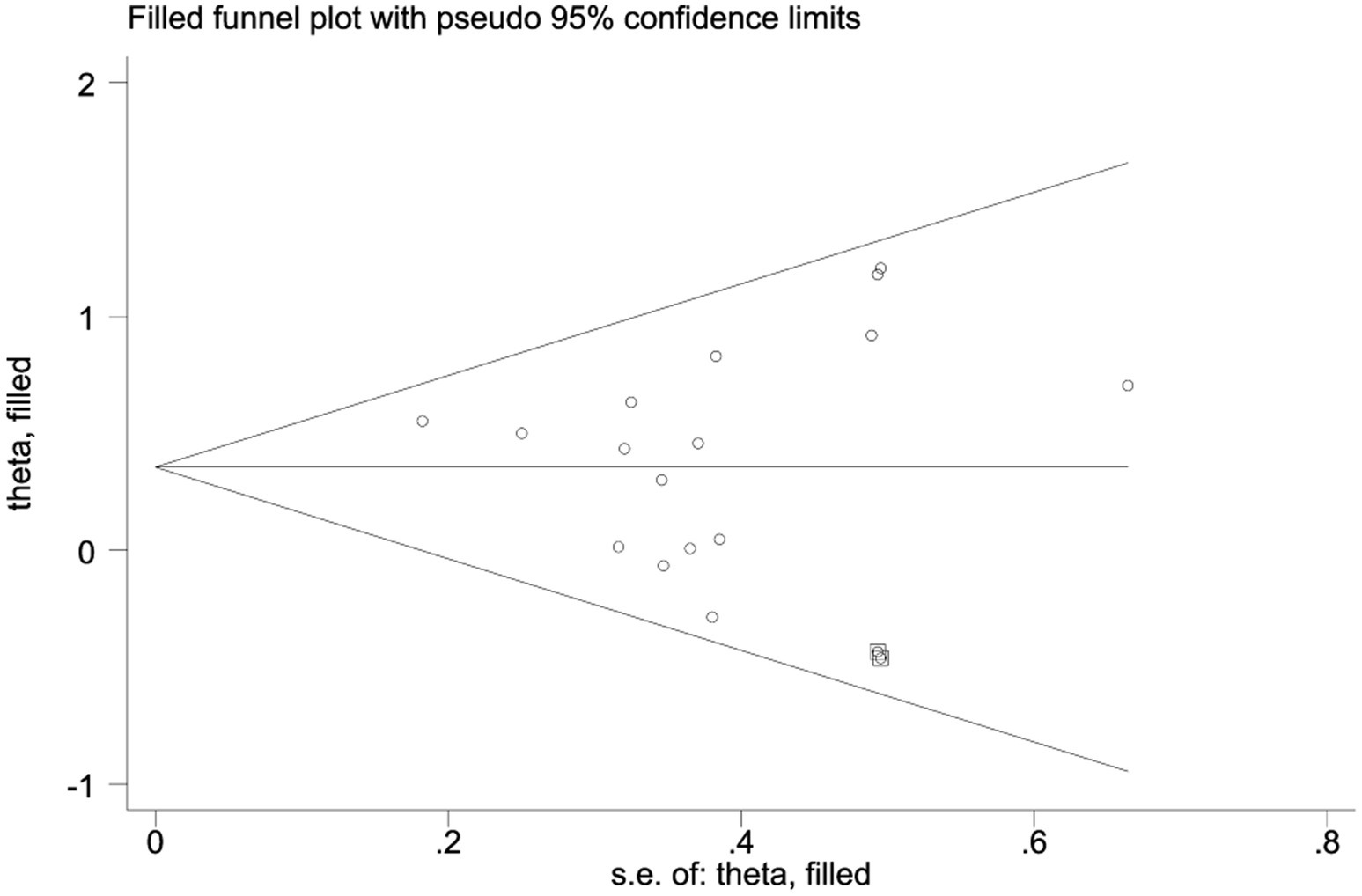
Figure 5. Application results of trim-and-fill method (including 11 included studies and 2 filled Studies).
Further subgroup analysis results showed that the intervention effect of combined aerobic training and resistance training was the best (SMD = 0.60, 95%CI: 0.36–0.84, p < 0.0001). Resistance training alone could also achieve an improvement with statistical significance (SMD = 0.35, 95%CI: 0.05–0.66, p = 0.02). However, aerobic training alone could not statistically significant increase the serum IGF-1 concentration in older adults with frailty and/or sarcopenia [SMD = 0.01, 95%CI: (−0.46, 0.48), p = 0.96] (Figure 6).
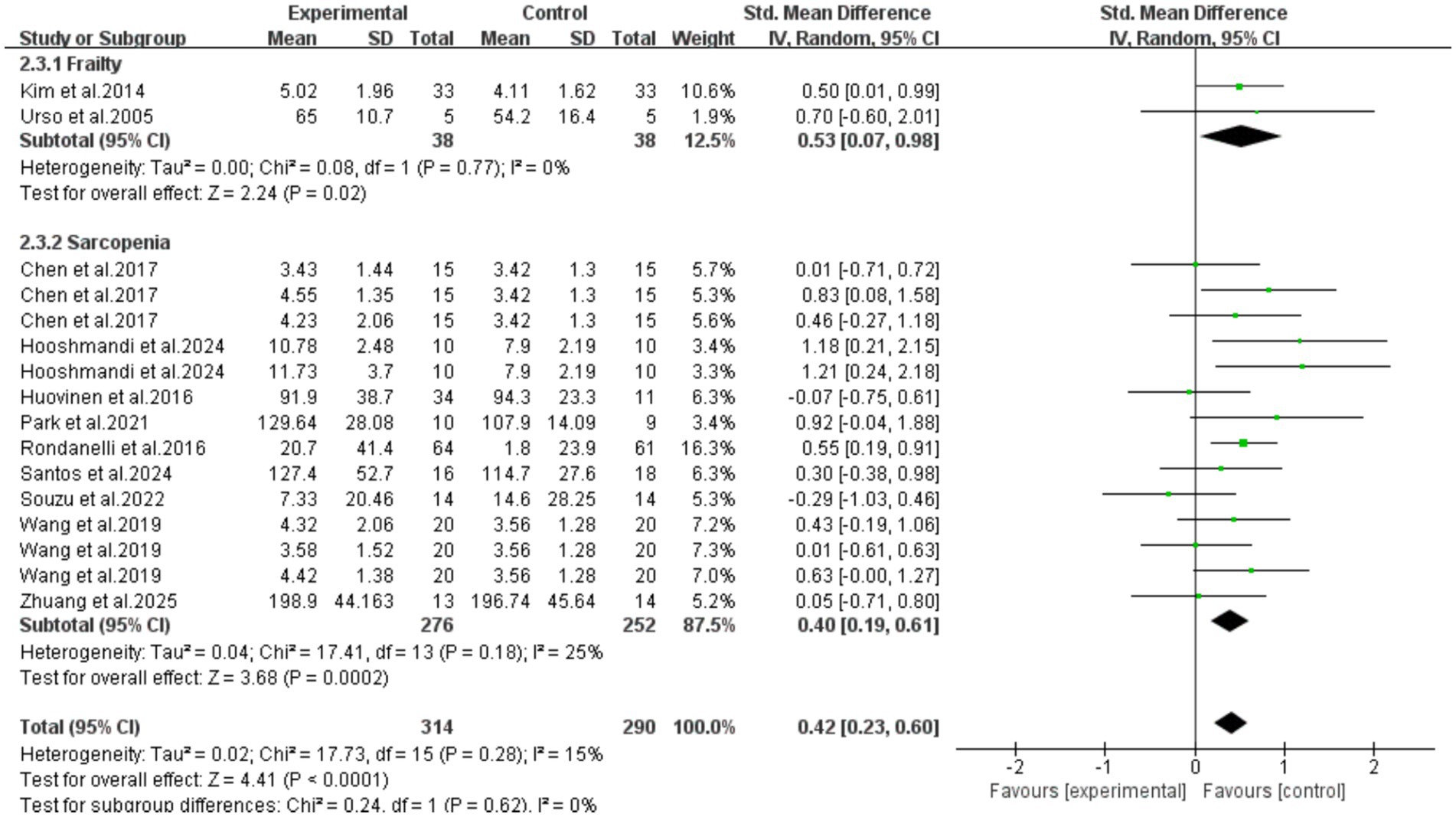
Subgroup analysis was performed based on participant characteristics (frailty/sarcopenia). The results showed that exercise intervention could effectively increase serum IGF-1 concentrations in older adult individuals with frailty (SMD = 0.53, 95%CI: 0.07–0.98, p = 0.02) and those with sarcopenia (SMD = 0.40, 95%CI: 0.19–0.61, p = 0.0002). Meanwhile, there was no significant difference between the two groups (p = 0.62), indicating that there was no statistical difference in the improvement effect of exercise intervention on these two conditions. However, it should be noted that the number of frail patients included in this subgroup analysis was small (2 RCTs), and the extrapolation of its conclusions may be limited in clinical application. More large-sample studies are needed in the future for further verification (Figure 7).
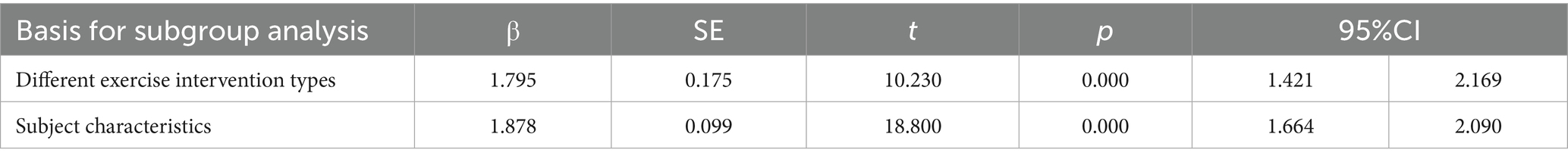
Results of the meta-regression analysis showed that both subgroup analyses produced significant effects, indicating that the results of the two subgroup analyses had high robustness (Table 4).
3.5 Sensitivity analysis
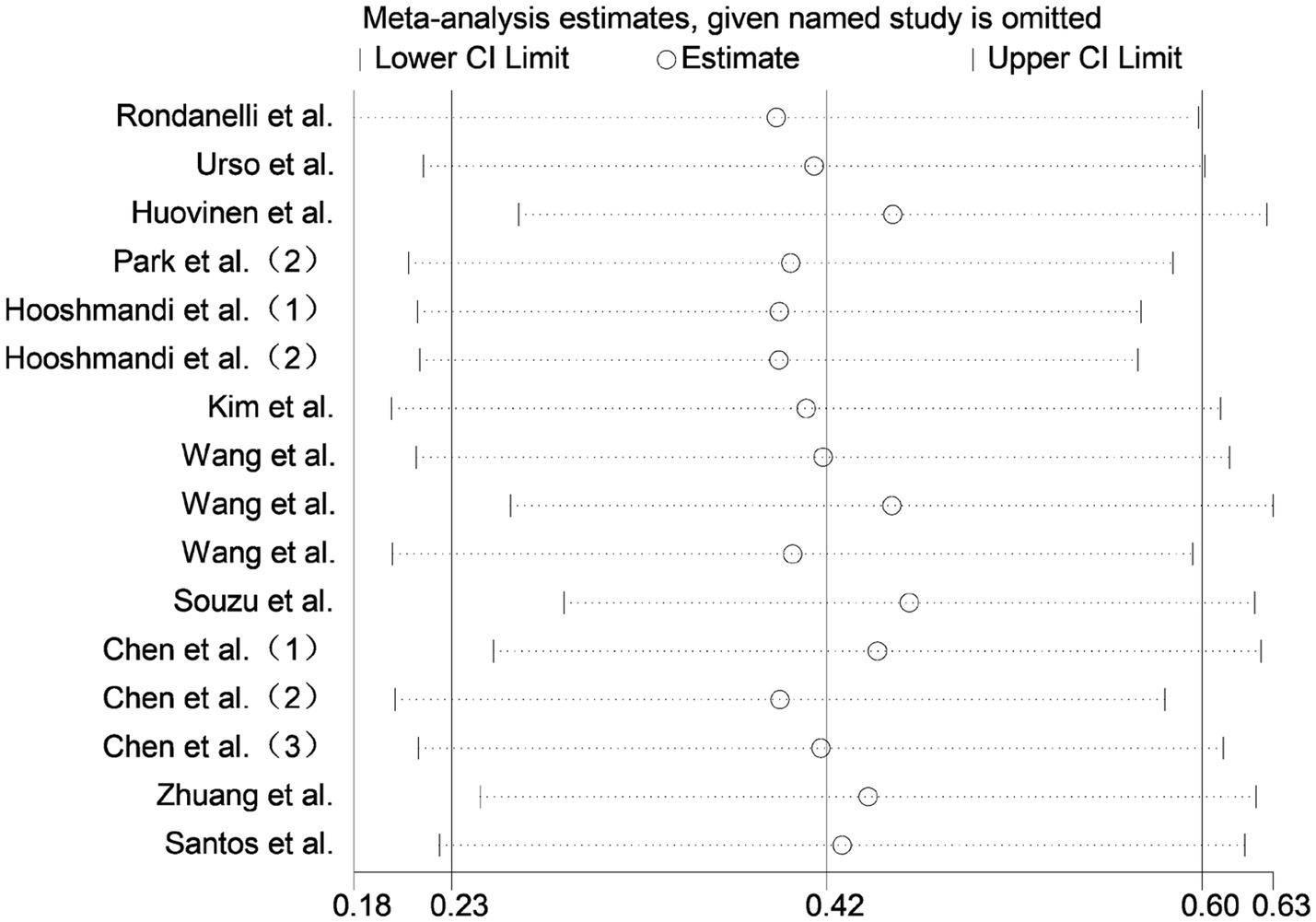
Sensitivity analysis was conducted by sequentially excluding each included study. The overall effect estimates remained stable, indicating that the meta-analysis results were robust and reliable (Figure 8).
4 Discussion
In this study, through the Meta-analysis method, we summarized multiple RCTs on the effect of exercise intervention on serum IGF-1 concentration in older adults with frailty and/or sarcopenia. The results showed that exercise, as an intervention method, can effectively increase the serum IGF-1 concentration in patients with the above-mentioned conditions, thereby providing support for the prevention and treatment of related diseases.
This study’s results consistent with Behrad et al.’s meta-analysis, which summarized 11 RCTs and concluded that physical exercise effectively increased serum IGF-1 levels in healthy older adults (SMD = 0.276, 95%CI: 0.065–0.487) (34). Praksch et al.’s RCT further supported the beneficial effects of exercise on serum IGF-1 in the healthy older adult population (54). Our subgroup analysis demonstrated that resistance training (either alone or combined with aerobic training) exerted significantly greater effects on serum IGF-1 levels in the target population compared to aerobic training alone, which is consistent with Jiang et al.’s meta-analysis. The latter study, which pooled data from 22 RCTs on resistance training, reported a significant increase in serum IGF-1 (WMD = 10.34, 95%CI: 4.94–15.75), though the high heterogeneity (I2 = 90.3%) may relate to the broad age range of included participants (22.7–82 years) (35).
Compared with previous studies that mostly focused on healthy middle-aged and older adult people and specific intervention methods, given that older adult populations with frailty and/or sarcopenia may have pathological bases such as chronic inflammation, hormonal imbalances, and abnormal muscle metabolism, with exercise’s IGF-1 regulatory mechanisms potentially differing from those in healthy populations, this study is the first to conduct a meta-analysis targeting older adult populations with frailty and/or sarcopenia. And the research results showed that exercise may not only improve serum IGF-1 concentration in adolescents and healthy older adult people, but also may have a promoting effect on older adult populations with frailty and/or sarcopenia. Meanwhile, most previous studies adopted a single type of exercise (such as aerobic training intervention, resistance training intervention, etc.) or used mixed exercises without subdividing subgroups. For this reason, this study categorized exercises into three types of movement patterns according to the characteristics of different exercises, and directly compared their improvement effects through subgroup analyses to clarify the differential effects of different exercise types on the target population, thereby providing a basis for precise prescriptions; in addition, subgroup analysis was conducted based on the characteristics of the subjects (frailty/sarcopenia), and the results suggested that the benefits of exercise intervention for these two groups of people may be universal.
4.1 IGF-1 and frailty, sarcopenia
IGF-1 is a single-chain polypeptide composed of 70 amino acids, mainly synthesized and secreted by the liver, and also present in other tissues such as muscle, lung, kidney, and cartilage (55). As an important mediator of growth hormone, it plays a significant role in the development of the skeleton, muscle, and nervous system (56). The potential mechanism by which IGF-1 promotes muscle hypertrophy and alleviates the development of frailty and sarcopenia may lie in: As noted in Studies by Pedersen et al. have pointed out that IGF-1 is a myokine produced and secreted by muscle fibers (57). It can activate the IGF-1 receptor (IGF-1R) on the cell membrane, trigger and activate the downstream PI3K/Akt/mTOR and Ras/MAPK signaling pathways, promote protein synthesis in skeletal muscle cells, thereby promoting skeletal muscle hypertrophy and reducing fibrosis in muscle tissue (58). Meanwhile, the activation of PI3K/AKT by IGF-1 can also inhibit ubiquitin-mediated degradation of proteins and apoptosis in skeletal muscle cells, reduce necrosis of muscle tissue, and thus alleviate the development of sarcopenia and its comorbidities (59, 60). Specifically, specific inactivation of the IGF-1 receptor leads to a reduction in the number and diameter of muscle fibers, thereby slowing down muscle growth (61); in contrast, muscle-specific overexpression of IGF-1 results in muscle hypertrophy (62). The signal transduction of IGF-1 is initially mediated by the binding of IGF-1 ligand to IGF-1R, which induces the phosphorylation of IGF-1R dimers. The resulting phosphorylated tyrosine residues generate docking sites to recruit insulin receptor substrate 1 (IRS-1) (63). Phosphorylation of IRS-1 is required for most downstream signal transduction and represents a key step in the regulation of IGF-1 signal transduction. After IGF-1R phosphorylates IRS-1, it further activates phosphatidylinositol 3-kinase (PI3K) and its downstream protein kinase B (Akt), forming the classic IGF1R/IRS1/PI3K/Akt pathway. This is the main pathway that drives protein synthesis, inhibits protein degradation, and thereby promotes muscle cell proliferation and hypertrophy (64, 65).
It is worth noting that the concentration of IGF-1 in the human body gradually decreases with age, which can lead to growth hormone release dysfunction, decreased immune capacity, and reduced activity of the growth hormone-insulin-like growth factor axis (66). This is also one of the reasons why the older adult population is a high-risk group for frailty, sarcopenia, and their comorbidities. A study on healthy and frail older adult people found that low IGF-1 levels are associated with decreased knee extensor strength and difficulty in mobility. Additionally, other studies have shown that there is a cross-sectional relationship between IGF-1 levels and thigh muscle area and density (67). It can thus be seen that low circulating IGF-1 levels are significantly associated with sarcopenia, decreased muscle strength, and impaired physical function in the older adult (68). Meanwhile, a Japanese study also showed that in men, older adult individuals with low IGF-1 levels (≤88 ng/mL) had a 1.58-fold and 3.42-fold higher probability of developing pre-frailty and frailty, respectively, compared to those with high IGF-1 levels (≥125 ng/mL). In women, low IGF-1 levels (≤78 ng/mL) were also positively correlated with an increased risk of frailty (69).
In summary, as an important factor regulating bone and muscle metabolism, the decline in IGF-1 levels associated with aging, reduced exercise, malnutrition, and other factors may be one of the pathological bases for the development of older adult frailty and sarcopenia. This further suggests that exercise intervention to increase serum IGF-1 concentrations in this population may have certain clinical significance in the present study.
4.2 IGF-1 and exercise
Compared with exogenous treatments such as nutritional supplementation and drug therapy, exercise is a safer and lower-cost preventive and therapeutic method by promoting the endogenous secretion of IGF-1 in the body (70–72). A study by Gomarasca et al. found that skeletal muscle, as an organ with endocrine function, can secrete bioactive protein IGF-1 after exercise stimulation, promoting the improvement of skeletal muscle mass, thereby preventing or treating the occurrence and development of sarcopenia (73). Meanwhile, IGF-1 in skeletal muscle has autocrine and paracrine functions, among which a muscle-specific form is called mechano growth factor (MGF), whose concentration is significantly upregulated during stretching and increased load (74). A long-term resistance training study on the older adult population found that the serum IGF-1 of the subjects increased significantly, and at the same time, cognitive function and lean body mass were also improved (75). Both resistance exercise and aerobic endurance exercise can effectively activate the IGF-1/PI3K/Akt/mTOR signaling pathway, thereby increasing the levels of IGF-1, IGF-1R and mTOR, as well as the activities of PI3K and Akt (76, 77). In terms of acute exercise effects, studies have found that 10 min of high-intensity resistance training or high-intensity aerobic training can effectively promote the increase of IGF-1 concentration in the human circulation and enhance the bioavailability of IGF-1 (78).
Despite extensive evidence that exercise intervention can effectively promote an increase in circulating IGF-1 concentration in the body, some studies have drawn opposite conclusions, suggesting that exercise intervention cannot increase or even reduce IGF-1 concentration in the body (79, 80). A study by Nindl et al. explained that such differences in results may stem from the lack of dietary control, heterogeneity of subject characteristics, differences in plasma volume, variations in exercise modes, and differences in sampling time and process after exercise (81). In addition, experimental results have shown that the decrease in IGF-1 levels during high-intensity exercise may promote the secretion of GH by weakening the negative feedback effect, thereby increasing circulating GH levels and promoting fatty acids as an energy source for exercising muscles. Meanwhile, long-term exercise may also affect the recovery of IGF-1 after exercise (82).
Through subgroup analysis of different exercise intervention types, this study found that the combined training of aerobic and resistance training had a relatively better effect on improving serum IGF-1 concentration in older adult patients with frailty and/or sarcopenia, followed by resistance training alone, while the improvement effect of aerobic training did not reach statistical significance. Based on the logical review of existing studies, the potential mechanism may be as follows: resistance training, by applying mechanical stimulation to skeletal muscles, may promote the acute secretion of growth hormone (GH) and activate proteases such as ADAM10 (A Disintegrin And Metalloproteinase 10) and ADAM17 (A Disintegrin And Metalloproteinase 17) on the cell membrane (83, 84). GH is the main upstream signal promoting hepatic synthesis of IGF-1, and the activation of proteases may also create conditions for the release of IGF-1 in local tissues. Subsequent aerobic training can promote vasodilation, reduce intravascular resistance, and increase blood flow, which may improve the delivery efficiency of GH, ADAM10, ADAM17 and other proteases generated or activated by resistance training to the liver and related target tissues (85). Therefore, a reasonable hypothesis is that resistance training can promote GH secretion and activate ADAMs activity through generating local mechanical stress, while aerobic training further accelerates the above process by improving blood circulation. The combination of the two training methods may result in a higher efficiency of IGF-1 secretion than a single training method.
Results of this subgroup analysis showed that combined aerobic and resistance training had a better effect on improving serum IGF-1 concentration in older adults with frailty and/or sarcopenia than single training methods, which provides a reference basis for clinically formulating targeted exercise prescriptions. Meanwhile, the hypotheses formed by deducing the potential mechanisms in the subgroup analysis results based on existing theories may provide certain research directions for future clinical trials targeting frailty and sarcopenia. In addition, the research results further suggest the potential of exercise intervention in increasing serum IGF-1 concentration in specific populations, providing support for non-pharmacological treatment of sarcopenia and frailty.
5 Strengths and limitations
The innovations and advantages of this study are mainly reflected in the following aspects: First, by precisely focusing on the research gaps in the field, it is the first to target the high-risk population of older adult patients with frailty and/or sarcopenia, and specifically explore the intervention effect of exercise intervention on their serum IGF-1 concentration, which helps to address the lack of population specificity in existing meta-analyses. Second, the study strictly followed the PRISMA guidelines and completed the pre-registration of the research protocol on the PROSPERO platform (registration number: CRD420251071083), which is conducive to ensuring the transparency and reproducibility of the research process. In addition, subgroup analysis showed that resistance training and combined training with resistance training had relatively better improvement effects on outcome indicators, providing a relatively intuitive basis for selecting exercise types when formulating targeted exercise prescriptions.
However, at the same time, this study still has the following limitations: (1) Limitations in the quality of original articles. The overall quality of the included studies is limited, with only 2 study being of Grade A and the rest being Grade B. Some studies did not implement blinding and allocation concealment, which may lead to a certain degree of selection bias and performance bias. (2) Constraints from sample size and population heterogeneity. Although systematic searches have been conducted in major databases, there are few articles that meet the inclusion criteria. This study only included 614 subjects (including 16 RCTs), and both the frailty subgroup and the aerobic training subgroup included only 2 studies. Meanwhile, the types of sarcopenia-related comorbidities (such as sarcopenic osteoporosis, sarcopenic obesity, etc.) were not subdivided, which may result in insufficient consideration of disease-specific manifestations. (3) Insufficient standardization of exercise intervention parameters. The included studies have large heterogeneity in intervention cycle, duration, intensity and frequency, making it difficult to extract the optimal dose-effect relationship. (4) Lack of analysis on long-term effects. The intervention cycles of the included RCTs are all ≤16 weeks, so the long-term intervention effect of exercise on IGF-1 cannot be evaluated.
In view of the existing limitations of this study, future research can be deepened and improved from the following dimensions: (1) Further strengthen the research on targeted exercise prescriptions, standardize the specific parameters of exercise intervention, further explore its dose-effect relationship, and clarify the optimal intervention parameter matrix; at the same time, combine new training modes such as blood flow restriction training and high-intensity interval training to enrich the range of exercise intervention options. (2) Expand the clinical trial cycle and carry out exercise intervention experiments with a duration of ≥48 weeks to evaluate the long-term effect of exercise intervention and its role in delaying disease progression. (3) Explore combined intervention strategies, carry out intervention studies on the combination of exercise with nutrition and drugs, and clarify the impact of various nutritional interventions on the effect of exercise intervention, as well as the role of exercise in reducing drug side effects. (4) Strengthen the evidence base for special populations, carry out multi-left, large-sample RCTs to improve the credibility of research evidence; at the same time, incorporate economic cost-effectiveness into the analysis to provide more references for the selection of disease prevention and treatment methods and the formulation of public health policies.
6 Conclusion
This study employed the Meta-analysis method to systematically integrate and quantitatively synthesize existing relevant studies. The results showed that exercise intervention can help increase the serum IGF-1 concentration in older adults with frailty and/or sarcopenia. Meanwhile, further subgroup analysis clarified that resistance training and combined training with resistance training are more effective than aerobic training alone in increasing serum IGF-1 in such patients. Therefore, to enhance clinical practicability, it is proposed that a combined resistance and aerobic training program could be adopted under safety supervision. The specific program settings are as follows: the training period is recommended to be ≥12 weeks, with 3–5 training sessions per week; the intensity of resistance training is suggested to be set at 60–80% 1-RM, and the intensity of aerobic training is moderate (i.e., Borg scale 12–14 or 40–60% of VO₂max). The total duration of each intervention session is 50–60 min, with approximately 25–30 min allocated to resistance training and 25–30 min to aerobic training, respectively. This evidence-based prescription may serve as a basic intervention module in community rehabilitation and geriatrics departments, helping delay muscle loss by regulating the IGF-1 pathway and laying a practical foundation for exercise intervention strategies. In the context where current exogenous treatment methods have certain limitations, this study provides preliminary evidence for the prevention and treatment of older adult frailty and sarcopenia, and may have certain guiding significance for clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
RC: Supervision, Investigation, Writing – original draft, Writing – review & editing, Methodology. ML: Writing – review & editing, Investigation, Conceptualization, Methodology, Writing – original draft. YX: Writing – review & editing, Methodology, Formal analysis, Investigation, Data curation, Project administration. YD: Data curation, Conceptualization, Writing – review & editing, Investigation, Funding acquisition, Resources, Formal analysis. TN: Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was funded by Anhui Philosophy and Social Sciences Planning Project (AHSKY2023D076).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1660694/full#supplementary-material
Footnotes
References
1. Voulgaridou, G, Tyrovolas, S, Detopoulou, P, Tsoumana, D, Drakaki, M, Apostolou, T, et al. Diagnostic criteria and measurement techniques of sarcopenia: a critical evaluation of the up-to-date evidence. Nutrients. (2024) 16:436. doi: 10.3390/nu16030436
2. Nielson, CM, Srikanth, P, and Orwoll, ES. Obesity and fracture in men and women: an epidemiologic perspective. J Bone Miner Res. (2012) 27:1–10. doi: 10.1002/jbmr.1486
3. Ormsbee, MJ, Prado, CM, Ilich, JZ, Purcell, S, Siervo, M, Folsom, A, et al. Osteosarcopenic obesity: the role of bone, muscle, and fat on health. J Cachexia Sarcopenia Muscle. (2014) 5:183–92. doi: 10.1007/s13539-014-0146-x
4. Sun, JH, Shi, JP, Zhu, TR, Quan, HL, and Xu, HQ. Effects of exercise on older adults with sarcopenia and its comorbidities: a meta-analysis. Chinese J Tissue Eng Res. (2026) 30:997–1007. doi: 10.12307/2026.019
5. Alonso-Bouzón, C, Carcaillon, L, García-García, FJ, Amor-Andrés, MS, El Assar, M, and Rodríguez-Mañas, L. Association between endothelial dysfunction and frailty: the Toledo study for healthy aging. Age (Dordr). (2014) 36:495–505. doi: 10.1007/s11357-013-9576-1
6. Barzilay, JI, Blaum, C, Moore, T, Xue, QL, Hirsch, CH, Walston, JD, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular health study. Arch Intern Med. (2007) 167:635–41. doi: 10.1001/archinte.167.7.635
7. Fried, LP, Tangen, CM, Walston, J, Newman, AB, Hirsch, C, Gottdiener, J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56:M146–56. doi: 10.1093/gerona/56.3.M146
8. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
9. Bianchi, L, Maietti, E, Abete, P, Bellelli, G, Bo, M, Cherubini, A, et al. Comparing EWGSOP2 and FNIH sarcopenia definitions: agreement and 3-year survival prognostic value in older hospitalized adults: the GLISTEN study. J Gerontol A Biol Sci Med Sci. (2020) 75:1331–7. doi: 10.1093/gerona/glz249
10. Reijnierse, EM, Trappenburg, MC, Blauw, GJ, Verlaan, S, de van der Schueren, MA, Meskers, CG, et al. Common ground? The concordance of sarcopenia and frailty definitions. J Am Med Dir Assoc. (2016) 17:371.e7–371.e12. doi: 10.1016/j.jamda.2016.01.013
11. Landi, F, Liperoti, R, Russo, A, Giovannini, S, Tosato, M, Capoluongo, E, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. (2012) 31:652–8. doi: 10.1016/j.clnu.2012.02.007
12. Ensrud, KE, Ewing, SK, Cawthon, PM, Fink, HA, Taylor, BC, Cauley, JA, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. (2009) 57:492–8. doi: 10.1111/j.1532-5415.2009.02137.x
13. Zhang, H, Wang, J, Xi, J, Xu, J, and Wang, L. Functional fitness and risk factors of older patients with diabetes combined with sarcopenia and/or frailty: a cross-sectional study. Nurs Open. (2024) 11:e2042. doi: 10.1002/nop2.2042
14. Yoshida, T, and Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells. (2020) 9:1970. doi: 10.3390/cells9091970
15. Musarò, A, and Scicchitano, BM. Counteracting sarcopenia: the role of IGF-1 isoforms. Aging (Albany NY). (2019) 11:3410–1. doi: 10.18632/aging.102027
16. Barbieri, M, Ferrucci, L, Ragno, E, Corsi, A, Bandinelli, S, Bonafè, M, et al. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. (2003) 284:E481–7. doi: 10.1152/ajpendo.00319.2002
17. Lu, W, Xiao, W, Xie, W, Fu, X, Pan, L, Jin, H, et al. The role of osteokines in sarcopenia: therapeutic directions and application prospects. Front Cell Dev Biol. (2021) 9:735374. doi: 10.3389/fcell.2021.735374
18. Gao, Y, Arfat, Y, Wang, H, and Goswami, N. Muscle atrophy induced by mechanical unloading: mechanisms and potential countermeasures. Front Physiol. (2018) 9:235. doi: 10.3389/fphys.2018.00235
19. Zhu, J, Peng, F, Yang, H, Luo, J, Zhang, L, Chen, X, et al. Probiotics and muscle health: the impact of Lactobacillus on sarcopenia through the gut-muscle axis. Front Microbiol. (2025) 16:1559119. doi: 10.3389/fmicb.2025.1559119
20. Kwak, JY, Hwang, H, Kim, SK, Choi, JY, Lee, SM, Bang, H, et al. Prediction of sarcopenia using a combination of multiple serum biomarkers. Sci Rep. (2018) 8:8574. doi: 10.1038/s41598-018-26617-9
21. Schiaffino, S, Dyar, KA, Ciciliot, S, Blaauw, B, and Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. (2013) 280:4294–314. doi: 10.1111/febs.12253
22. Liu, J, Chen, M, Xia, X, Wang, Z, Wang, Y, and Xi, L. Causal associations between the insulin-like growth factor family and sarcopenia: a bidirectional mendelian randomization study. Front Endocrinol (Lausanne). (2024) 15:1422472. doi: 10.3389/fendo.2024.1422472
23. Doi, T, Makizako, H, Tsutsumimoto, K, Hotta, R, Nakakubo, S, Makino, K, et al. Association between insulin-like growth Factor-1 and frailty among older adults. J Nutr Health Aging. (2018) 22:68–72. doi: 10.1007/s12603-017-0916-1
24. Yamada, PM, and Lee, KW. Perspectives in mammalian IGFBP-3 biology: local vs. systemic action. Am J Physiol Cell Physiol. (2009) 296:C954–76. doi: 10.1152/ajpcell.00598.2008
25. Huang, XY, Huang, ZL, Yang, JH, Xu, YH, Sun, JS, Zheng, Q, et al. Pancreatic cancer cell-derived IGFBP-3 contributes to muscle wasting. J Exp Clin Cancer Res. (2016) 35:46. doi: 10.1186/s13046-016-0317-z
26. Jellinger, PS, Handelsman, Y, Rosenblit, PD, Bloomgarden, ZT, Fonseca, VA, Garber, AJ, et al. American association of clinical endocrinologists and American College of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. (2017) 23:1–87. doi: 10.4158/EP171764.APPGL
27. Gaskin, FS, Farr, SA, Banks, WA, Kumar, VB, and Morley, JE. Ghrelin-induced feeding is dependent on nitric oxide. Peptides. (2003) 24:913–8. doi: 10.1016/S0196-9781(03)00160-8
28. Murad, MH, Elamin, KB, Abu Elnour, NO, Elamin, MB, Alkatib, AA, Fatourechi, MM, et al. Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab. (2011) 96:2997–3006. doi: 10.1210/jc.2011-1193
29. Landi, F, Marzetti, E, Martone, AM, Bernabei, R, and Onder, G. Exercise as a remedy for sarcopenia. Curr Opin Clin Nutr Metab Care. (2014) 17:25–31. doi: 10.1097/MCO.0000000000000018
30. Thornell, LE. Sarcopenic obesity: satellite cells in the aging muscle. Curr Opin Clin Nutr Metab Care. (2011) 14:22–7. doi: 10.1097/MCO.0b013e3283412260
31. Maass, A, Düzel, S, Brigadski, T, Goerke, M, Becke, A, Sobieray, U, et al. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. NeuroImage. (2016) 131:142–54. doi: 10.1016/j.neuroimage.2015.10.084
32. Maddalozzo, GF, and Snow, CM. High intensity resistance training: effects on bone in older men and women. Calcif Tissue Int. (2000) 66:399–404. doi: 10.1007/s002230010081
33. Titus, J, Bray, NW, Kamkar, N, Camicioli, R, Nagamatsu, LS, Speechley, M, et al. The role of physical exercise in modulating peripheral inflammatory and neurotrophic biomarkers in older adults: a systematic review and meta-analysis. Mech Ageing Dev. (2021) 194:111431. doi: 10.1016/j.mad.2021.111431
34. Behrad, S, Dezfuli, SAT, Yazdani, R, Hayati, S, and Shanjani, SM. The effect of physical exercise on circulating neurotrophic factors in healthy aged subjects: a meta-analysis and meta-regression. Exp Gerontol. (2024) 196:112579. doi: 10.1016/j.exger.2024.112579
35. Jiang, Q, Lou, K, Hou, L, Lu, Y, Sun, L, Tan, SC, et al. The effect of resistance training on serum insulin-like growth factor 1(IGF-1): a systematic review and meta-analysis. Complement Ther Med. (2020) 50:102360. doi: 10.1016/j.ctim.2020.102360
36. Liberati, A, Altman, DG, Tetzlaff, J, Mulrow, C, Gøtzsche, PC, Ioannidis, JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
37. Cruz-Jentoft, AJ, Baeyens, JP, Bauer, JM, Boirie, Y, Cederholm, T, Landi, F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
38. Chen, LK, Woo, J, Assantachai, P, Auyeung, TW, Chou, MY, Iijima, K, et al. Asian working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012
39. Huang, EYZ, Cheung, J, Liu, JYW, Kwan, RYC, and Lam, SC. Groningen frailty Indicator-Chinese (GFI-C) for pre-frailty and frailty assessment among older people living in communities: psychometric properties and diagnostic accuracy. BMC Geriatr. (2022) 22:788. doi: 10.1186/s12877-022-03437-1
40. Wong, L, Duque, G, and McMahon, LP. Sarcopenia and frailty: challenges in mainstream nephrology practice. Kidney Int Rep. (2021) 6:2554–64. doi: 10.1016/j.ekir.2021.05.039
41. Kelaiditi, E, Cesari, M, Canevelli, M, van Kan, GA, Ousset, PJ, Gillette-Guyonnet, S, et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. (2013) 17:726–34. doi: 10.1007/s12603-013-0367-2
42. Higgins, JP, Altman, DG, Gøtzsche, PC, Jüni, P, Moher, D, Oxman, AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
43. Rondanelli, M, Klersy, C, Terracol, G, Talluri, J, Maugeri, R, Guido, D, et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr. (2016) 103:830–40. doi: 10.3945/ajcn.115.113357
44. Urso, ML, Fiatarone Singh, MA, Ding, W, Evans, WJ, Cosmas, AC, and Manfredi, TG. Exercise training effects on skeletal muscle plasticity and IGF-1 receptors in frail elders. Age (Dordr). (2005) 27:117–25. doi: 10.1007/s11357-005-1629-7
45. Huovinen, V, Ivaska, KK, Kiviranta, R, Bucci, M, Lipponen, H, Sandboge, S, et al. Bone mineral density is increased after a 16-week resistance training intervention in elderly women with decreased muscle strength. Eur J Endocrinol. (2016) 175:571–82. doi: 10.1530/EJE-16-0521
46. Park, J, Bae, J, and Lee, J. Complex exercise improves anti-inflammatory and anabolic effects in osteoarthritis-induced sarcopenia in elderly women. Healthcare (Basel). (2021) 9:711. doi: 10.3390/healthcare9060711
47. Hooshmandi, Z, Daryanoosh, F, Ahmadi Hekmatikar, AH, and Awang Daud, DM. Highlighting the effect of reduced training volume on maintaining hormonal adaptations obtained from periodized resistance training in sarcopenic older women. Expert Rev Endocrinol Metab. (2024) 19:187–97. doi: 10.1080/17446651.2023.2294091
48. Dos Santos, VR, Antunes, M, Dos Santos, L, Nascimento, MA, Pina, FLC, Carneiro, NH, et al. Effects of different resistance training frequencies on body composition, muscular strength, muscle quality, and metabolic biomarkers in sarcopenic older women. J Strength Cond Res. (2024) 38:e521–8. doi: 10.1519/JSC.0000000000004827
49. Cha, HJ, Kim, KB, and Baek, SY. Square-stepping exercise program effects on fall-related fitness and BDNF levels in older adults in Korea: a randomized controlled trial. Int J Environ Res Public Health. (2022) 19:7033. doi: 10.3390/ijerph19127033
50. Kim, H, Suzuki, T, Kim, M, Kojima, N, Ota, N, Shimotoyodome, A, et al. Effects of exercise and milk fat globule membrane (MFGM) supplementation on body composition, physical function, and hematological parameters in community-dwelling frail Japanese women: a randomized double blind, placebo-controlled, follow-up trial. PLoS One. (2015) 10:e0116256. doi: 10.1371/journal.pone.0116256
51. Wang, LZ, Guo, YB, and Luo, JH. Effects of home-based exercise training on elderly patients with sarcopenic obesity. Chinese J Rehab Theory Prac. (2019) 25:90–6. doi: 10.3969/j.issn.1006-9771.2019.01.012
52. de Sá Souza, H, de Melo, CM, Piovezan, RD, Miranda, REEPC, Carneiro-Junior, MA, Silva, BM, et al. Resistance training improves sleep and anti-inflammatory parameters in sarcopenic older adults: a randomized controlled trial. Int J Environ Res Public Health. (2022) 19:16322. doi: 10.3390/ijerph192316322
53. Chen, HT, Chung, YC, Chen, YJ, Ho, SY, and Wu, HJ. Effects of different types of exercise on body composition, muscle strength, and IGF-1 in the elderly with sarcopenic obesity. J Am Geriatr Soc. (2017) 65:827–32. doi: 10.1111/jgs.14722
54. Praksch, D, Sandor, B, Kovacs, D, Petrovics, P, Kovacs, K, Toth, K, et al. Impact of home- and left- based physical training program on cardio-metabolic health and IGF-1 level in elderly women. Eur Rev Aging Phys Act. (2019) 16:13. doi: 10.1186/s11556-019-0220-7
55. Rinderknecht, E, and Humbel, RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. (1978) 253:2769–76. doi: 10.1016/S0021-9258(17)40889-1
56. Neirijnck, Y, Papaioannou, MD, and Nef, S. The insulin/IGF system in mammalian sexual development and reproduction. Int J Mol Sci. (2019) 20:4440. doi: 10.3390/ijms20184440
57. Pedersen, BK, and Febbraio, MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. (2012) 8:457–65. doi: 10.1038/nrendo.2012.49
58. Martín, AI, Priego, T, and López-Calderón, A. Hormones and muscle atrophy. Adv Exp Med Biol. (2018) 1088:207–33. doi: 10.1007/978-981-13-1435-3_9
59. Nakamura, S, Sato, Y, Kobayashi, T, Kaneko, Y, Ito, E, Soma, T, et al. Insulin-like growth factor-I is required to maintain muscle volume in adult mice. J Bone Miner Metab. (2019) 37:627–35. doi: 10.1007/s00774-018-0964-6
60. Barclay, RD, Burd, NA, Tyler, C, Tillin, NA, and Mackenzie, RW. The role of the IGF-1 signaling Cascade in muscle protein synthesis and anabolic resistance in aging skeletal muscle. Front Nutr. (2019) 6:146. doi: 10.3389/fnut.2019.00146
61. Hu, B, Zhao, C, Pan, X, Wei, H, Mo, G, Xian, M, et al. Local GHR roles in regulation of mitochondrial function through mitochondrial biogenesis during myoblast differentiation. Cell Commun Signal. (2023) 21:148. doi: 10.1186/s12964-023-01166-5
62. Chou, CH, and Barton, ER. Phosphorylation of AMPKα at Ser485/491 is dependent on muscle contraction and not muscle-specific IGF-I overexpression. Int J Mol Sci. (2023) 24:11950. doi: 10.3390/ijms241511950
63. Böhni, R, Riesgo-Escovar, J, Oldham, S, Brogiolo, W, Stocker, H, Andruss, BF, et al. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell. (1999) 97:865–575. doi: 10.1016/S0092-8674(00)80799-0
64. Fanzani, A, Conraads, VM, Penna, F, and Martinet, W. Molecular and cellular mechanisms of skeletal muscle atrophy: an update. J Cachexia Sarcopenia Muscle. (2012) 3:163–79. doi: 10.1007/s13539-012-0074-6
65. Lee, SW, Dai, G, Hu, Z, Wang, X, Du, J, and Mitch, WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol. (2004) 15:1537–45. doi: 10.1097/01.ASN.0000127211.86206.E1
66. Bando, H, Zhang, C, Takada, Y, Yamasaki, R, and Saito, S. Impaired secretion of growth hormone-releasing hormone, growth hormone and IGF-I in elderly men. Acta Endocrinol. (1991) 124:31–6. doi: 10.1530/acta.0.1240031
67. Sattler, FR. Growth hormone in the aging male. Best Pract Res Clin Endocrinol Metab. (2013) 27:541–55. doi: 10.1016/j.beem.2013.05.003
68. Maggio, M, De Vita, F, Lauretani, F, Buttò, V, Bondi, G, Cattabiani, C, et al. IGF-1, the cross road of the nutritional, inflammatory and hormonal pathways to frailty. Nutrients. (2013) 5:4184–205. doi: 10.3390/nu5104184
69. Lana, A, Valdés-Bécares, A, Buño, A, Rodríguez-Artalejo, F, and Lopez-Garcia, E. Serum leptin concentration is associated with incident frailty in older adults. Aging Dis. (2017) 8:240–9. doi: 10.14336/AD.2016.0819
70. Aguirre, GA, De Ita, JR, de la Garza, RG, and Castilla-Cortazar, I. Insulin-like growth factor-1 deficiency and metabolic syndrome. J Transl Med. (2016) 14:3. doi: 10.1186/s12967-015-0762-z
71. Puche, JE, and Castilla-Cortázar, I. Human conditions of insulin-like growth factor-I (IGF-I) deficiency. J Transl Med. (2012) 10:224. doi: 10.1186/1479-5876-10-224
72. Anderson, LJ, Tamayose, JM, and Garcia, JM. Use of growth hormone, IGF-I, and insulin for anabolic purpose: pharmacological basis, methods of detection, and adverse effects. Mol Cell Endocrinol. (2018) 464:65–74. doi: 10.1016/j.mce.2017.06.010
73. Gomarasca, M, Banfi, G, and Lombardi, G. Myokines: the endocrine coupling of skeletal muscle and bone. Adv Clin Chem. (2020) 94:155–218. doi: 10.1016/bs.acc.2019.07.010
74. Ratamess, N. ACSM'S foundations of strength training and conditioning. Lippincott Williams & Wilkins (2021).
75. Cassilhas, RC, Viana, VA, Grassmann, V, Santos, RT, Santos, RF, Tufik, S, et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc. (2007) 39:1401–7. doi: 10.1249/mss.0b013e318060111f
76. Bao, C, Yang, Z, Li, Q, Cai, Q, Li, H, and Shu, B. Aerobic endurance exercise ameliorates renal vascular sclerosis in aged mice by regulating PI3K/AKT/mTOR signaling pathway. DNA Cell Biol. (2020) 39:310–20. doi: 10.1089/dna.2019.4966
77. Yin, L, Lu, L, Lin, X, and Wang, X. Crucial role of androgen receptor in resistance and endurance trainings-induced muscle hypertrophy through IGF-1/IGF-1R-PI3K/Akt- mTOR pathway. Nutr Metab (Lond). (2020) 17:26. doi: 10.1186/s12986-020-00446-y
78. Schwarz, AJ, Brasel, JA, Hintz, RL, Mohan, S, and Cooper, DM. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J Clin Endocrinol Metab. (1996) 81:3492–7. doi: 10.1210/jcem.81.10.8855791
79. Spanoudaki, M, Giaginis, C, Karafyllaki, D, Papadopoulos, K, Solovos, E, Antasouras, G, et al. Exercise as a promising agent against Cancer: evaluating its anti-Cancer molecular mechanisms. Cancers (Basel). (2023) 15:5135. doi: 10.3390/cancers15215135
80. Campbell, KL, and McTiernan, A. Exercise and biomarkers for cancer prevention studies. J Nutr. (2007) 137:161s–9s. doi: 10.1093/jn/137.1.161S
81. Nindl, BC, and Pierce, JR. Insulin-like growth factor I as a biomarker of health, fitness, and training status. Med Sci Sports Exerc. (2010) 42:39–49. doi: 10.1249/MSS.0b013e3181b07c4d
82. Nindl, BC, Alemany, JA, Rarick, KR, Eagle, SR, Darnell, ME, Allison, KF, et al. Differential basal and exercise-induced IGF-I system responses to resistance vs. calisthenic-based military readiness training programs. Growth Hormon IGF Res. (2017) 32:33–40. doi: 10.1016/j.ghir.2016.12.001
83. Bamman, MM, Shipp, JR, Jiang, J, Gower, BA, Hunter, GR, Goodman, A, et al. Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab. (2001) 280:E383–90. doi: 10.1152/ajpendo.2001.280.3.E383
84. Sommer, A, Kordowski, F, Büch, J, Maretzky, T, Evers, A, Andrä, J, et al. Phosphatidylserine exposure is required for ADAM17 sheddase function. Nat Commun. (2016) 7:11523. doi: 10.1038/ncomms11523
Keywords: sarcopenia, frailty, insulin-like growth factor-1, exercise intervention, meta-analysis
Citation: Chu R, Li M, Xie Y, Du Y and Ni T (2025) Exercise interventions and serum IGF-1 levels in older adults with frailty and/or sarcopenia: a systematic review and meta analysis. Front. Public Health. 13:1660694. doi: 10.3389/fpubh.2025.1660694
Edited by:
Bojan Masanovic, University of Montenegro, MontenegroReviewed by:
Peerasak Lerttrakarnnon, Chiang Mai University, ThailandLeo Delaire, Hospices Civils de Lyon, France
Xinyu Yang, Huzhou University, China
Dimitrios Anagnostou, Simanogleio-Amalia Fleming General Hospital, Greece
Thinakaran Kandayah, Pejabat Kesihatan Daerah Hilir Perak, Malaysia
Copyright © 2025 Chu, Li, Xie, Du and Ni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yeshou Xie, MjIzMTMxMjEzNEBzdHUuYWhwdS5lZHUuY24=; Rui Chu, MTk5NjMyMjI2NzJAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Rui Chu
Rui Chu Mingming Li2†
Mingming Li2†