- 1AIDS Clinical Research Center, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, China
- 2First Clinical Medical College, Henan University of Chinese Medicine, Zhengzhou, China
Introduction: The promotion and application of ART have significantly reduced the mortality rate of AIDS patients in China, but the problem of LLV that follows cannot be ignored. LLV is closely associated with viral failure and increased all-cause mortality. Conducting a systematic study on the prevalence of LLV among PLWH in China and its influencing factors has significant clinical implications for the comprehensive management, effective prevention, and intervention of LLV in the future.
Methods: Studies were included if research subjects were PLWH in China, with a duration of ART treatment of ≥6 months, the research types were cohort studies, case–control studies, and cross-sectional studies, the primary outcome indicator was LLV occurrence, as well as research findings were disseminated in Chinese or English. The systematic review was conducted using the R studio software.
Results: Our review included a total of 20 original studies, covering 225,687 HIV/AIDS patients, among which 45,265 were LLV. Our study showed that the overall prevalence rate of LLV among PLWH in China was 10.6% (95% CI: 6.8% ~ 16.1%). Further subgroup analysis revealed that Blip was higher than pLLV, the prevalence of LLV was the highest in the VL range of 50 ~ 199 cp/mL; there were no significant differences in the prevalence of LLV among different regions, type of study and ART regimens. The analysis of factors influencing LLV showed that poor ART adherence, baseline VL > 105 cp/mL, changing the treatment schemes, baseline CD4 < 350 cells/μL, PI-based regimen at initiation, age ≥50 years, and age of ART initiation ≥50 years were risk factors for LLV among PLWH, while homosexual transmission was a protective factor for LLV (p < 0.001).
Conclusion: The prevalence of LLV among PLWH was moderate to low in China. Special attention should be paid to older population with PLWH who had poor ART adherence, high baseline VL, had changed the treatment schemes, had a low baseline CD4 level, had an PI-based regimen at initiation, started ART relatively late, and needed enhanced compliance management and drug resistance testing, as well as increased VL testing frequency to avoid adverse outcomes.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251047912, identifier (CRD420251047912).
Background
By the end of 2023, an estimated 39.9 million individuals were living with HIV globally, of whom 30.7 million were receiving antiretroviral therapy (ART) (1). In China, 31 provinces reported 1,355,017 current people living with HIV(PLWH) by the end of 2024 (2). The ART coverage rate in China increased from 74.04% in 2016 to 92.9% in 2020, maintaining stability thereafter. ART effectively suppresses viral replication, reducing HIV viral load (VL) to undetectable levels and realizing the “U=U” principle (Undetectable = Uninfectious) (3). However, emerging evidence shows that persistent viral suppression is not achieved in all patients, leading to low level viraemia (LLV).
The US Department of Health and Human Services defines LLV as VL < 200 cp/mL (4); the Chinese AIDS Diagnosis and Treatment Guidelines (2024 Edition) specify VL of 50 ~ 200 cp/mL (5); while the World Health Organization and China’s National Free Antiviral Drug Treatment Manual (5th Edition) define it as VL between 50 and 1,000 cp/mL (6, 7). Evidence indicates that LLV among PLWH retains the potential for HIV transmission (8). LLV promotes drug resistance mutations, increasing the risk of virological failure (VF), and exacerbates immune activation and inflammatory responses, thereby elevating the risk of AIDS defining and non AIDS defining diseases (9–11). Compared to virologically suppressed PLWH, individuals with LLV exhibit a significantly elevated risk of all-cause mortality, warranting heightened attention to their disease management (12, 13).
China has made substantial progress in HIV/AIDS prevention and control, with both treatment coverage and viral suppression rates exceeding 90%, keeping the national epidemic at a low-prevalence level (14). However, the prevalence of LLV has risen gradually in recent years, surpassing 30% in certain provinces (15). This not only poses a significant threat to the survival of PLWH but also emerged as a critical gap hindering further improvements in the quality of China’s HIV/AIDS prevention and control efforts. Evidence serves as the cornerstone of clinical decision making, with high quality evidence providing invaluable references for researchers and clinicians (16). An integrated analysis of the prevalence and influencing factors of LLV among PLWH in China would facilitate the optimization of national HIV/AIDS prevention and control strategies and the standardized management of LLV. Accordingly, this study aims to synthesize existing clinical evidence via meta-analysis to characterize the current landscape of LLV among PLWH. The ultimate goal is to provide evidence-based support for the development of effective LLV intervention and prevention measures, as well as the formulation of personalized treatment regimens.
Materials and methods
Design and registration
This systematic review and meta-analysis were designed, conducted, and reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (17). This study was registered on PROSPERO (registration number: CRD420251047912).
Search strategy
A systematic review of literature was performed using eight databases: (1) PubMed; (2) EMBASE; (3) Cochrane Library; (4) Web of Science; (5) China National Knowledge Infrastructure (CNKI); (6) Wanfang; (7) Weipu database (VIP), and (8) China Biology Medicine disk (CBM). Search through various databases for papers published from their establishment until April 22, 2025. Keywords and MeSH terms synonymous with “HIV,” “AIDS,” “Human Immunodeficiency Viruses,” “Acquired Immunodeficiency Syndrome,” “low level viraemia,” “hypoviremia,” “low level viremia.” In addition, reference lists from previous related reviews were screened to ensure a comprehensive search. Details of the search strategies are provided in Supplementary Table S1.
Inclusion and exclusion criteria
Inclusion criteria: (1) The research subjects were PLWH in China, with a duration of ART treatment of ≥6 months; (2) The research types were cohort studies, case–control studies, and cross-sectional studies; (3) The primary outcome indicators were LLV occurrences, and the secondary outcome indicators were the influencing factors of LLV; (4) Research findings disseminated in either Chinese or English.
Exclusion criteria: (1) Review articles, scientific achievements, news reports, etc.; (2) Studies for which the full text cannot be obtained or the data cannot be extracted; (3) Republished literature; (4) Studies with a sample size of less than 300.
The specific definitions are as follows: (1) LLV, defined as the occurrence of at least one VL measurement of 50 ~ 1,000 cp/mL after virologic suppression is achieved or ART treatment of ≥6 months; (2) persistent LLV (pLLV), defined as two or more consecutive VL of 50 ~ 1,000 cp/mL after virologic suppression is achieved or ART treatment of ≥6 months, and Blip defined as only once or multiple times at intervals.
Data extraction
Two investigators independently extracted data using Excel spreadsheets, collecting information on first author, publication year, study design, geographic region, total sample size, LLV cases, LLV incidence, and multivariate analysis factors. In case of any disagreement, a third researcher would make the final decision.
Quality assessment
For cohort and case–control studies, literature quality was assessed using the Cochrane recommended Newcastle-Ottawa Scale (NOS), a 9-point tool where 0–3 points indicated low quality, 4–6 moderate quality, and 7–9 high quality. Cross-sectional studies were evaluated with the US Health Care and Research Institutions Quality Assessment Scale (11-point total), classifying 0–3 points as low, 4–7 as moderate, and 8–11 as high quality.
Statistical analysis
This meta analysis was performed using R Studio software. The prevalence of LLV was calculated for each included study using the total number of PLWH and the number of PLWH with LLV. The variation among the studies was evaluated through the application of Cochran’s Q statistic and the I-square statistics (I2). If I2 ≤ 50% and p ≥ 0.10, no significant heterogeneity was indicated among the included studies, and a common effect model was used for the meta analysis; if I2 > 50% and p < 0.10, substantial heterogeneity was suggested, and a random effects model was applied instead. Sensitivity and subgroup analyses were conducted to further investigate heterogeneity and validate the robustness of the study findings. The subgroup analyses examined the prevalence of LLV by frequency of LLV, VL level, geographic regions, type of study and treatment regimens. A funnel plot, Egger’s test and Begg’s test were used to analyze the publication bias in the studies included. The trim-and-fill method was used to identify and correct potential publication bias. For influencing factor analysis, odds ratios (OR) with 95% confidence intervals (CI) were used to pool effect sizes. All statistical tests were two-sided, with statistical significance set at p < 0.05.
Results
Literature screening process and results
Figure 1 shows the steps involved in searching for and screening literature. A total of 1,301 studies were retrieved by the database searches. After removing duplicates and screening titles, abstracts and full text, 20 studies (12 Chinese and 8 English studies) were included (15, 18–36) (Figure 1).
Basic characteristics of the included studies
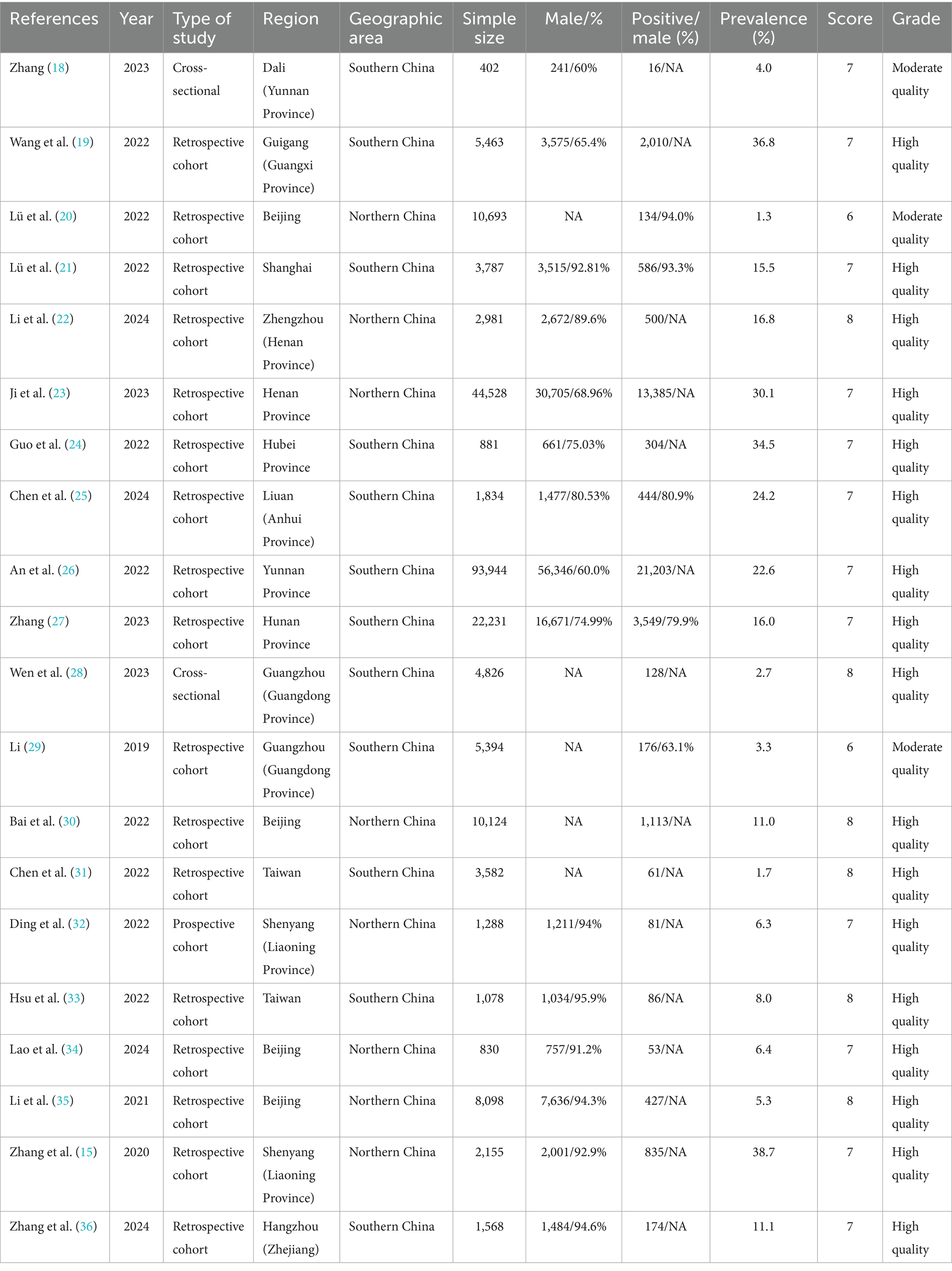
Table 1 summarizes the study’s baseline characteristics. A total of 20 literatures were finally included, involving 225,687 PLWH. The sample size ranged from 402 to 93,944 cases, predominantly male, among which 45,265 were LLV patients. The prevalence rate of LLV among PLWH ranges from 1.3 to 38.7%. The studies were conducted across 12 Chinese provinces, municipalities, and autonomous regions, with higher representation from Beijing (n = 4), Guangdong (n = 2), Henan (n = 2), Liaoning (n = 2), and Taiwan (n = 2). Geographic distribution showed a southern region predominance (n = 12). Publication years spanned 2019–2024, and the number of published literatures was the largest in 2022 (n = 9), followed by 2023 (n = 4) and 2024 (n = 4). The research types were dominated by retrospective cohort studies (n = 17).
Quality of included studies
Table 1 presents study quality and bias risk assessments. Findings indicated high overall quality among included studies, with 17 classified as high quality and 3 as moderate quality. The mean quality score for 18 cohort studies was 7.17. Major critical score deductions were attributed to inadequate comparability between exposed and non-exposed groups and insufficient cohort follow-up duration. Details of the quality evaluation are presented in Supplementary Table S2-S3.
The prevalence of LLV among PLWH in China
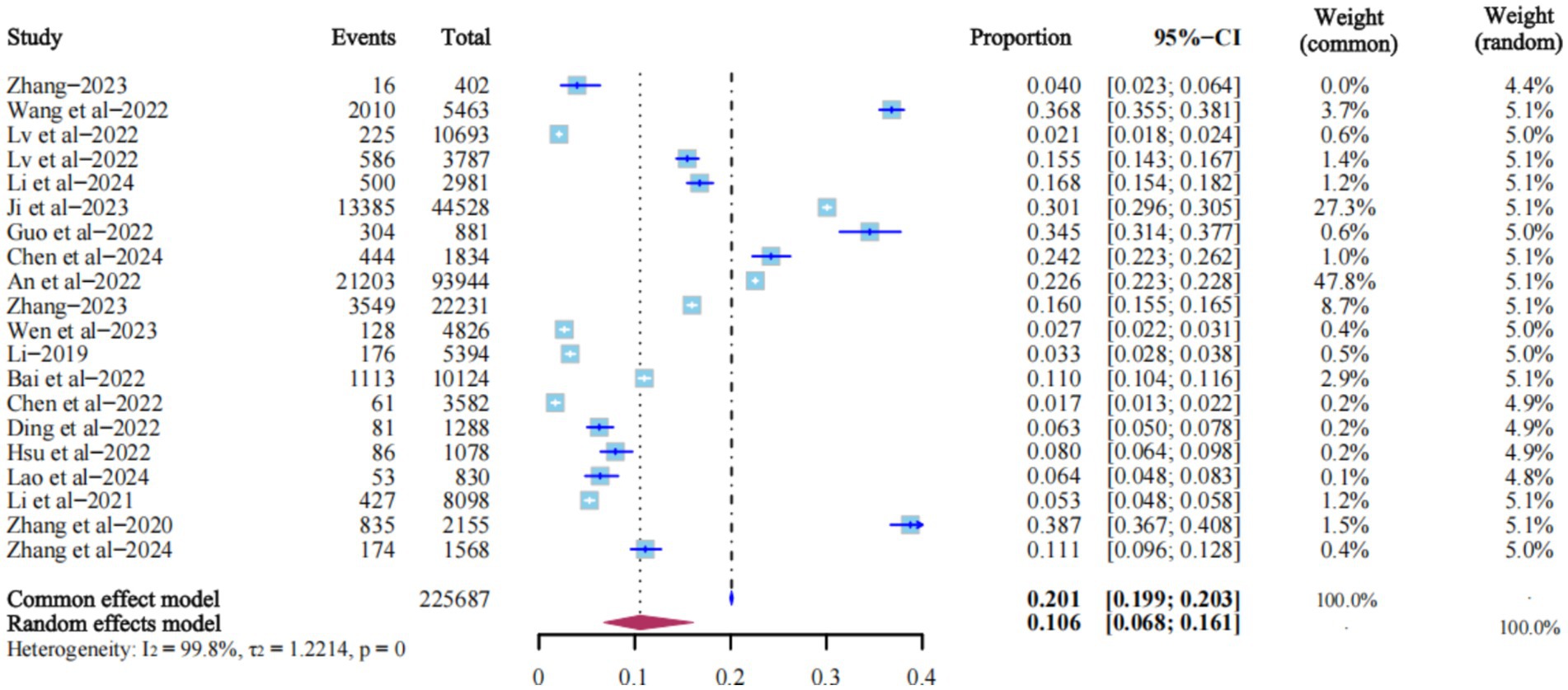
All 20 studies reported the prevalence of LLV. The heterogeneity test I2 = 99.8%, p = 0, so the random effects model was used. The results showed that the prevalence of LLV among PLWH in China was 10.6% (95% CI: 6.8% ~ 16.1%) (Figure 2).
Sensitivity analysis
Sensitivity analysis was performed by excluding studies individually. Despite the high heterogeneity, the change in the combined effect size was relatively small after each study was excluded, indicating that the results were relatively robust and not overly influenced by any single study. The combined prevalence of LLV among PLWH ranged from 10% (95% CI: 6% ~ 15%) to 12% (95% CI: 8% ~ 17%). We concluded that the prevalence of LLV was between these two values (Figure 3).
Subgroup analysis
To identify sources of heterogeneity, subgroup analyses were conducted for frequency of LLV, VL level, geographic regions, type of study and treatment regimens. Results showed Blip and pLLV prevalence rates of 14.2% (95% CI: 7.2% ~ 23.2%) and 2.6% (95% CI: 0.9% ~ 5.1%), cross-sectional study and cohort study prevalence rates of 3.0% (95% CI: 1.9% ~ 4.4%) and 14.0% (95% CI: 8.8% ~ 20.1%) (p < 0.05). VL level analysis revealed prevalence rates of 9.0%(5.5% ~ 13.3%) for 50 ~ 199 cp/mL, 3.3% (1.8% ~ 5.3%) for 200 ~ 399 cp/mL, and 3.0% (1.4% ~ 5.0%) for 400 ~ 1,000 cp/mL, with the 50 ~ 199 cp/mL range showing the highest prevalence (p < 0.05). Geographically, although the prevalence of LLV in southern regions was higher than that in northern regions, the difference was not statistically significant (p > 0.05). Among treatment regimens, second-line protease inhibitor (PI)-based regimens had the highest LLV prevalence, though differences compared to NNRTI- or INSTI-based regimens were not statistically significant (p > 0.05) (Table 2). Subgroup analysis forest plot was shown in Supplementary Figure S1.
Publication bias analysis
The funnel plot for bias assessment exhibited asymmetry. Egger’s test showed a significant result (t = −2.93, p = 0.0089), while Begg’s test was non-significant (z = −1.30, p = 0.1944), suggesting some publication bias in this meta-analysis. Following publication bias correction using the trim and fill method (8 missing studies imputed), the pooled prevalence of LLV increased from 10.6% (95% CI: 6.8% ~ 16.1%) to 22.2% (95% CI: 13.2% ~ 35.0%) indicating that our results might have been underestimated (Figure 4).
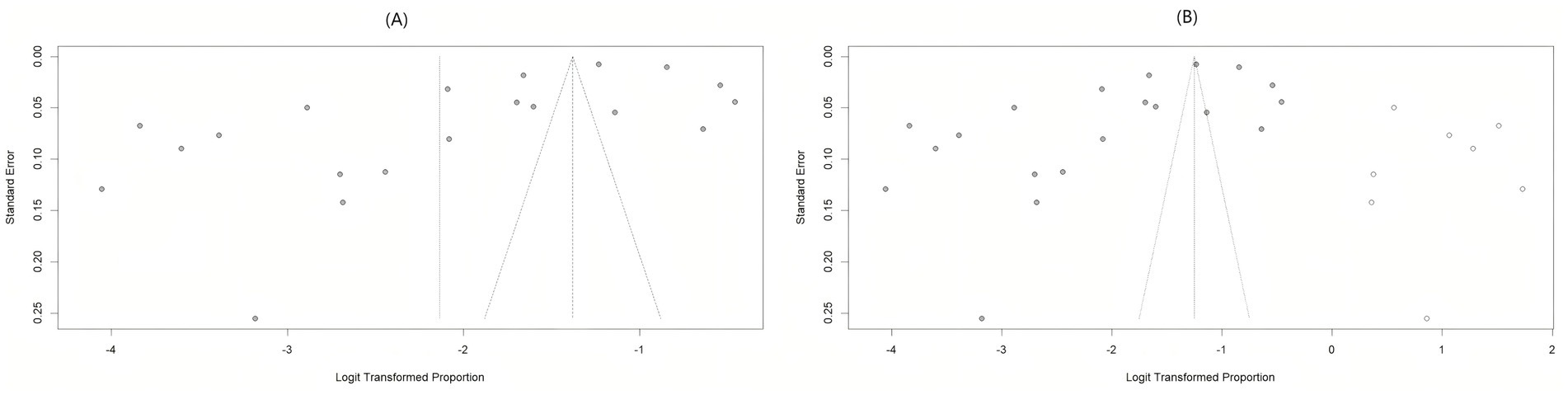
Figure 4. Funnel plot for risk of bias assessment. (A) Risk assessment funnel plot for original bias; (B) the funnel plot after correction by trim and fill method.
Influencing factors of LLV among PLWH
There were a total of 9 influencing factors for the effect size combination. Depending on the degree of heterogeneity, the common effect model and the random effect model were selected, respectively. Our study showed that baseline CD4 < 200 cells/μL (OR = 1.398, 95% CI: 1.318 ~ 1.484), baseline CD4 200 ~ 350 cells/μL (OR = 1.290, 95% CI: 1.207 ~ 1.379), PI-based regimen at initiation (OR = 1.377, 95% CI: 1.157 ~ 1.640), change the treatment schemes (OR = 1.883, 95% CI: 1.790 ~ 1.981), baseline VL > 105cp/mL (OR = 1.567, 95% CI: 1.340 ~ 1.833), poor ART adherence (OR = 3.019, 95% CI: 1.091 ~ 8.353), age ≥ 50 years (OR = 1.228, 95% CI: 1.135 ~ 1.474), and age of ART initiation ≥ 50 years (OR = 1.219, 95% CI: 1.161 ~ 1.280) were risk factors for the occurrence of LLV among PLWH. Homosexual transmission (OR = 0.523, 95% CI: 0.363 ~ 0.753) was a protective factor for LLV (p < 0.001) (Table 3). Analysis of influencing factors forest map was provided in Supplementary Figure S2.
Discussion
The “China AIDS Control and Prevention Plan (2024–2030)” aims to achieve the public health goal of ending AIDS by 2030 (37). While China has made substantial progress in AIDS prevention and control, persistent LLV poses risks of HIV transmission, VF, and drug resistance, challenging the realization of this goal. This systematic review synthesized data on LLV prevalence and associated factors among PLWH in China. Findings showed an overall LLV prevalence of 10.6%, with significant risk factors including baseline VL > 105 cp/mL, baseline CD4 < 350 cells/μL, initiation of protease inhibitor (PI)-based regimens, treatment regimen changes, poor ART adherence, age ≥ 50 years, and ART initiation at ≥ 50 years. Homosexual transmission was identified as a protective factor.
Our study revealed an overall LLV prevalence of 10.6% among PLWH in China, which is lower than the global rate (13.81%) reported by Zhao et al., and consistent with the domestic prevalence (10.12%) documented in this article (38). However, this study was subject to some publication bias, potentially attributed to limited sample size of included studies, heterogeneity in patient demographics (e.g., baseline CD4 counts, ART regimens, follow up durations), variations in VL detection sensitivity, and diverse data sources. Following bias correction via the trim and fill method, the pooled LLV prevalence increased from 10.6 to 22.2%, suggesting the true prevalence among PLWH may be even higher. These findings indicate prior studies might have underestimated LLV risk, prompting clinicians to remain alert for virologic “pseudosuppression” while advocating for standardized LLV definitions and enhanced reporting of negative outcomes.
Subgroup analysis of LLV prevalence revealed that the incidence of Blip viraemia was significantly higher than pLLV among PLWH. Previous studies have confirmed that viral blips are closely associated with abnormal immune activation (39). For such patients, increasing the frequency of VL testing and closely monitoring intra-patient viral dynamics are recommended. Timely intervention should be implemented to prevent disease progression and exacerbation. Our analysis further revealed a higher prevalence of LLV in the 50 ~ 200 cp/mL VL range compared to 201 ~ 400 cp/mL and 401 ~ 1,000 cp/mL tiers. Zhang et al. demonstrated that LLV confers an increased risk of VF only when VL reaches ≥ 400 cp/mL (15). However, emerging evidence indicated that patients with 50 ~ 200 cp/mL VL still exhibited significantly higher risks of virological non-suppression and treatment failure compared to those with persistently undetectable VL (40, 41). The southern region exhibited a slightly higher prevalence than the northern region, indicating that the prevalence of LLV varies by region in China. Differences in regional economic levels, ART regimens, VL detection technologies, and detection frequencies all lead to variations in the prevalence rates (42). However, the impact of these factors on the prevalence rate varies among different studies, and it is necessary to analyze them specifically according to different circumstances. Kanapathipillai et al. found that high-income regions perform VL testing more frequently than middle and low-income regions (43). This allows earlier detection of LLV at lower thresholds, enabling timely treatment intervention or regimen adjustment, which may explain regional prevalence disparities. Future research priorities should include upgrading detection technologies, reducing sequencing costs, enhancing assay efficiency, adopting highly sensitive VL testing, and implementing routine VL monitoring to enable prompt LLV detection and early intervention.
Baseline CD4 level is strongly associated with LLV. CD4 + T cell counts correlate positively with immune function, such that patients with low baseline CD4 levels exhibit more severe immune damage. This enhances persistent viral replication and drug resistance development, promoting LLV emergence (44). Additionally, LLV is linked to decay of viral reservoirs established prior to ART. PLWH with extremely low baseline CD4 counts typically harbor larger HIV reservoirs with prolonged decay kinetics, thereby increasing LLV risk (45).
Our analysis showed that while LLV prevalence did not differ significantly among NNRTI, PI, and INSTI-based treatment regimens, PI-based regimens exhibited numerically higher LLV rates. Notably, PI-based regimen at initiation was identified as an independent risk factor for LLV among PLWH. This could be attributed to two plausible mechanisms: (1) patients on PI-based regimens often have poorer baseline health, or switch to second-line PI regimens due to first-line ART resistance or VF, which may predispose to LLV (46); (2) prior research suggests that PI-based ART is associated with larger peripheral blood HIV reservoir sizes compared to NNRTI regimens (47).
This study identified poor ART Adherence as the most significant predictor (OR = 3.019), consistent with findings by Zaçe et al. (48). Unfortunately, among the included studies, three primary articles refer to this influencing factor (21, 22, 28), but their definitions of “poor ART adherence” are inconsistent, precluding the extraction and integration of a unified definition. Consequently, this paper only conducts a preliminary analysis of the literature mentioning this factor, without more in-depth and precise exploration. PLWH poor compliance are strongly linked to LLV and subsequent VF, likely due to missed doses leading to subtherapeutic drug concentrations and incomplete viral suppression (49, 50). Adherence management represents a key intervention target for LLV, as it significantly improves virological suppression rates in this population (51). Strategies should include comprehensive analysis of adherence determinants, patient-centered communication to address barriers, and ART regimen optimization to enhance compliance and prevent LLV.
In contrast to findings from other studies, our research identified ART treatment regimen changes as a critical risk factor for LLV among PLWH. Wen et al. demonstrated that frequent regimen changes may reduce plasma concentrations of antiretroviral drugs to subtherapeutic levels, thereby compromising viral suppression and predisposing patients to LLV (28). It has been proposed that treatment regimen changes may be associated with poor medication adherence and suboptimal disease control—factors that confer a higher risk of LLV among PLWH (25). Inter-drug variability in ADME (absorption, distribution, metabolism, excretion) properties may lead to subtherapeutic drug levels following regimen shifts. Additionally, drug–drug interactions (DDI) between ART or with co-administered medications, drug-food interactions, and absorption issues may reduce drug concentrations, increasing the risk of LLV. Recommendations include considering pharmacological factors, with ART regimen optimization guided by resistance test results, patient adherence, regimen resistance barriers, HIV RNA levels, and other treatment history. This also should account for DDI, drug-food interactions, adverse events, pill burden, and dietary requirements (52, 53).
Notably, age ≥ 50 years and ART initiation age ≥ 50 years were identified as independent risk factors for LLV, which is consistent with previous findings. Older adults typically exhibit lower educational attainment and risk awareness, accompanied by insufficient medication adherence. Additionally, they tend to have a suboptimal immune response to treatment, ultimately contributing to adverse clinical outcomes (54). Further studies have demonstrated that in older population receiving ART, age-related impairments in protein homeostasis, dysregulated stress responses, cellular senescence, and chronic inflammation are more likely to interfere with in vivo drug activity, diminish therapeutic efficacy, and thereby increase susceptibility to LLV (55). Mechanistically, LLV primarily arises from HIV reservoirs harbored in immature T cells of infected individuals (56). A larger HIV reservoir size and delayed viral clearance are associated with a higher propensity for LLV induction. Consequently, patients who initiate ART at a later stage face an elevated risk of developing LLV (57). In conclusion, to reduce the incidence of LLV, early initiation of ART should be prioritized for PLWH. Moreover, increased frequency of VL monitoring is recommended for older population, enabling timely detection and management of LLV.
This study identified a baseline VL > 105 cp/mL as a risk factor for LLV. VL is a key indicator for assessing the efficacy of ART, which functions by suppressing VL to undetectable levels through combination regimens—thereby delaying disease progression and extending survival. Baseline VL levels dictate the extent of immune reconstitution in AIDS patients post-ART; a high baseline VL (>105 cp/mL) reflects severe immune dysfunction, rendering such patients more susceptible to LLV (58). Single-cell sequencing data reveal that 23.5% of CD4 + T cells in patients with high baseline VL harbor proviral DNA, with integration sites predominantly near oncogenes—potentiating reactivation of latent viruses, a critical mechanism underlying LLV (59, 60). Additionally, high baseline VL correlates with a larger HIV reservoir, which exhibits enhanced capacity for releasing detectable virus, thereby increasing treatment difficulty and LLV risk (61). Thus, for confirmed PLWH with high baseline VL, early initiation of ART is imperative to facilitate immune reconstitution, prevent the establishment of a large viral reservoir, and mitigate LLV risk.
Significantly, homosexual transmission was identified as a protective factor for LLV. This may be ascribed to the relatively active social organizations among MSM. These organizations facilitate earlier detection of HIV infection, timely access to ART, and consistent follow-up interventions (25). Furthermore, following ART initiation, the viral suppression rate in this population rises rapidly—an outcome that ultimately lowers their risk of developing LLV (62).
Our systematic review and meta-analysis demonstrate several strengths regarding representativeness. First, the included studies encompass 12 cities across both southern and northern China, covering both economically developed and underdeveloped regions, which largely captures the major cities with HIV-infected populations in China. Second, prior studies have demonstrated that in medical research, studies with a sample size < 200 tend to systematically overestimate effect sizes in meta-analyses (63). Given the typical sample sizes of observational and cross-sectional studies in HIV/AIDS research, studies with a sample size of less than 300 were excluded to minimize potential effect size bias and overall risk of bias. Notably, most of the included studies (n = 15) reported age and sex distribution data, which largely reflect the epidemiological characteristics of PLWH with LLV. Furthermore, among the 20 included studies, 18 adopted the LLV diagnostic criterion of 50 ~ 1,000 cp/mL, and two studies simultaneously employed both the 50 ~ 1,000 cp/mL and 50 ~ 200 cp/mL criteria, which enhanced the robustness of the conclusions.
However, there are also several limitations: (1) The limited number of included studies, potentially influenced by study design and inclusion/exclusion criteria, warrants future re-analysis with expanded inclusion of high quality research. (2) Most included studies did not report data on VL detection methods or testing frequencies. The absence of such information may introduce uncertainty into our results. Future studies should prioritize evaluating outcomes associated with different detection methods and frequencies, rather than relying solely on pooled analyses of heterogeneous data. (3) The included studies had different definitions for certain influencing factors (such as “poor ART adherence”), which made it impossible to conduct more precise and in-depth analyses. In the future, this issue should be given due attention, and efforts should be made to promote the standardization and unification of standards and definitions in the field of AIDS, so as to carry out clinical research of higher quality and higher standards. (4) Evidence of publication bias was detected, and despite subgroup analysis of LLV prevalence, significant heterogeneity persisted across study results. Thus, these findings should be interpreted with caution.
Conclusion
This study demonstrates that the prevalence of LLV among PLWH is moderate to low following active ART in China. However, the results exhibit substantial heterogeneity. After adjusting for publication bias, the estimated LLV prevalence increased, indicating that the LLV burden in China has been underestimated. Given the adverse clinical consequences of LLV, screening for LLV-related risk factors prior to ART initiation is warranted. Future research should prioritize prospective studies to standardize LLV management and monitoring protocols, as well as implement early interventions tailored to identified risk factors.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XZ: Writing – review & editing, Writing – original draft. QX: Supervision, Project administration, Writing – review & editing. CL: Data curation, Writing – review & editing. YZ: Formal analysis, Methodology, Writing – review & editing. YJ: Formal analysis, Writing – review & editing, Methodology. PL: Visualization, Writing – review & editing. HG: Writing – review & editing, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Joint Fund Project of the Science and Technology Research Plan of Henan Province (No. 232301420089) and the Special Program for Henan Provincial TCM Science Study (No. 2022ZY1032).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1661253/full#supplementary-material
References
1. UNAlDS. Global HIV statistics fact sheet 2024. Available online at: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (2024) (Accessed June 18, 2025).
2. National Center for AIDS/STD Control and Prevention. National AIDS and STD epidemic situation in December 2024. Chin J AIDS STD. (2025) 31:225. doi: 10.13419/j.cnki.aids.2025.03.01
3. Crowell, TA, Ritz, J, Zheng, L, Naqvi, A, Cyktor, JC, Puleo, J, et al. Impact of antiretroviral therapy during acute or early HIV infection on virologic and immunologic outcomes: results from a multinational clinical trial. AIDS. (2024) 38:1141–52. doi: 10.1097/QAD.0000000000003881
4. The US Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Available online at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/archive/adult-adolescent-arv-2024-02-27.pdf (2024) (Accessed June 18, 2025).
5. Shen, YZ. Chinese guidelines for the diagnosis and treatment of AIDS (2024 edition). Chin J Viral Dis. (2025) 15:4–32. doi: 10.16505/j.2095-0136.2025.0001
6. World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring:recommendations for a public health approach. Available online at: https://www.who.int/publications/i/item/9789240031593 (2021) (Accessed August 22, 2025).
7. National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention. National Free Antiretroviral Therapy Manual for AIDS (5th edition). Beijing: People's Medical Publishing House (2023). 57 p.
8. Townsend, CL, Byrne, L, Cortina-Borja, M, Thorne, C, de Ruiter, A, Lyall, H, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000-2011. AIDS. (2014) 28:1049–57. doi: 10.1097/QAD.0000000000000212
9. Elvstam, O, Malmborn, K, Elén, S, Marrone, G, García, F, Zazzi, M, et al. Virologic failure following low-level viremia and viral blips during antiretroviral therapy: results from a European multicenter cohort. Clin Infect Dis. (2023) 76:25–31. doi: 10.1093/cid/ciac762
10. Lara-Aguilar, V, Llamas-Adán, M, Brochado-Kith, Ó, Crespo-Bermejo, C, Grande-García, S, Arca-Lafuente, S, et al. Low-level HIV-1 viremia affects T-cell activation and senescence in long-term treated adults in the INSTI era. J Biomed Sci. (2024) 31:80. doi: 10.1186/s12929-024-01064-z
11. Ganesan, A, Hsieh, HC, Chu, X, Colombo, RE, Berjohn, C, Lalani, T, et al. Low level viremia is associated with serious non-AIDS events in people with HIV. Open Forum Infect Dis. (2024) 11:ofae147. doi: 10.1093/ofid/ofae147
12. Yu, H, Yang, Y, Cao, D, Zhao, Y, Jin, C, Sun, H, et al. Association of low-level viremia with mortality among people living with HIV on antiretroviral therapy in Dehong, Southwest China: a retrospective cohort study. HIV Med. (2023) 24:37–45. doi: 10.1111/hiv.13320
13. Elvstam, O, Marrone, G, Medstrand, P, Treutiger, CJ, Sönnerborg, A, Gisslén, M, et al. All-cause mortality and serious non-AIDS events in adults with low-level human immunodeficiency virus viremia during combination antiretroviral therapy: results from a Swedish Nationwide observational study. Clin Infect Dis. (2021) 72:2079–86. doi: 10.1093/cid/ciaa413
14. Han, M. Analysis of the epidemic situation of AIDS in China and prospects for prevention and control. Chin J AIDS STD. (2023) 29:247–50. doi: 10.13419/j.cnki.aids.2023.03.01
15. Zhang, T, Ding, H, An, M, Wang, X, Tian, W, Zhao, B, et al. Factors associated with high-risk low-level viremia leading to virologic failure: 16-year retrospective study of a Chinese antiretroviral therapy cohort. BMC Infect Dis. (2020) 20:147. doi: 10.1186/s12879-020-4837-y
16. Liu, S, Guo, X, Wu, D, Anthony, LZ, Charlie, CX, and Wen, Z. Construction of a clinical evidence grading system for traditional Chinese medicine intervention based on the principles of evidence-based medicine. Chin J Integr Tradit West Med. (2023) 43:911–5. doi: 10.7661/cjim.20230628.272
17. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
18. Zhang, Y. Research on the current situation and influencing factors of low viral load in HIV/AIDS patients after HAART in Dali area. (master’s thesis). Yunnan: Dali University (2023).
19. Wang, X, Xu, Y, Liang, X, Qin, C, Chen, R, Qin, X, et al. Analysis of the incidence and influencing factors of low viral load in HIV-infected individuals in Guigang City, Guangxi. J Guangxi Med Univ. (2022) 39:677–81. doi: 10.16190/j.cnki.45-1211/r.2022.04.028
20. Lü, S, Bai, R, Dai, M, Wang, R, Hua, W, Lu, H, et al. Analysis of HIV-1 genotypic resistance characteristics in patients with low viral load after antiviral therapy. Chin J AIDS STD. (2022) 28:1187–90. doi: 10.13419/j.cnki.aids.2022.10.16
21. Lü, H, Liu, L, Chen, J, Hu, J, and Lu, H. Research on the influencing factors and clinical significance of HIV low viral load. J Dermatol Venereol. (2022) 44:353–8. doi: 10.3969/j.issn.1002-1310.2022.05.001
22. Li, C, Yang, X, Liu, J, Zhang, X, Chen, Y, Yang, X, et al. Analysis of the occurrence and influencing factors of low viral load in HIV/AIDS patients of different ages in Zhengzhou City. Chin Trop Med. (2024) 24:1513–8. doi: 10.13604/j.cnki.46-1064/r.2024.12.13
23. Ji, X, Li, N, Fan, P, Ma, Y, Zhang, G, Ne, Y, et al. Analysis of the occurrence and influencing factors of low viral load in HIV/AIDS patients in Henan Province. Dis Surveill. (2023) 38:1341–5. doi: 10.3784/jbjc.202303010079
24. Guo, M, Liu, C, Mei, F, Zheng, W, and Cai, K. Association and influencing factors of low viral load and failure of viral suppression in HIV-infected individuals in Hubei Province. Public Health Prev Med. (2022) 33:90–3. doi: 10.3969/j.issn.1006-2483.2022.06.021
25. Chen, H, Ma, G, and Chen, D. Analysis of the occurrence and influencing factors of low viral load in HIV/AIDS patients receiving antiviral treatment in Lu'an City. South China Prev Med. (2024) 50:1109–14. doi: 10.12183/j.scjpm.2024.1109
26. An, L, Lao, Y, and Tang, S. Analysis of the occurrence of HIV low viremia in antiviral treatment for AIDS in Yunnan Province. Chin J Public Health. (2022) 38:908–13. doi: 10.11847/zgggws1132975
27. Zhang, W. Research on the influencing factors of HIV-1 low viral load in Hunan Province and analysis of the risk of developing drug resistance. (master’s thesis). Guangdong: South China University of Technology (2023).
28. Wen, C, Li, H, Lan, Y, Guo, P, Zhong, H, Li, H, et al. Analysis of risk factors for low viral load in patients with HIV/AIDS undergoing antiviral therapy. Chin J Infect Dis. (2023) 41:122–7. doi: 10.3760/cma.j.cn311365-20220623-00267
29. Li, H. Clinical significance of low viral load in AIDS patients after antiviral treatment. (master’s thesis). Guangdong: Guangzhou Medical University (2019).
30. Bai, R, Lv, S, Hua, W, Su, B, Wang, S, Shao, Y, et al. Factors associated with human immunodeficiency virus-1 low-level viremia and its impact on virological and immunological outcomes: a retrospective cohort study in Beijing, China. HIV Med. (2022) 23:72–83. doi: 10.1111/hiv.13251
31. Chen, GJ, Sun, HY, Chen, LY, Hsieh, SM, Sheng, WH, Liu, WD, et al. Low-level viraemia and virologic failure among people living with HIV who received maintenance therapy with co-formulated bictegravir, emtricitabine and tenofovir alafenamide versus dolutegravir-based regimens. Int J Antimicrob Agents. (2022) 60:106631. doi: 10.1016/j.ijantimicag.2022.106631
32. Ding, H, Xu, J, Liu, J, Wang, Q, Kang, J, Li, X, et al. Outcomes of persistent low-level viremia among HIV patients on antiretroviral therapy: a prospective cohort study. HIV Med. (2022) 23:64–71. doi: 10.1111/hiv.13250
33. Hsu, JY, Sun, HY, Hsieh, TW, Chang, SY, Chuang, YC, Huang, YS, et al. Incidence of low-level viremia and its impact on virologic failure among people living with HIV-1 who switched to elvitegravir-based antiretroviral therapy. J Glob Antimicrob Resist. (2022) 29:7–16. doi: 10.1016/j.jgar.2022.02.007
34. Lao, X, Zhang, H, Deng, M, Li, Q, Xiao, Q, He, L, et al. Incidence of low-level viremia and its impact on virologic failure among people living with HIV who started an integrase strand transfer inhibitors: a longitudinal cohort study. BMC Infect Dis. (2024) 24:8. doi: 10.1186/s12879-023-08906-5
35. Li, Q, Chen, M, Zhao, H, Yu, F, Yan, L, Xiao, J, et al. Persistent low-level viremia is an independent risk factor for Virologic failure: a retrospective cohort study in China. Infect Drug Resist. (2021) 14:4529–37. doi: 10.2147/IDR.S332924
36. Zhang, W, Shi, J, Wang, Y, Li, E, Yan, D, Zhang, Z, et al. Risk factors and clinical prediction models for low-level viremia in people living with HIV receiving antiretroviral therapy: an 11-year retrospective study. Front Microbiol. (2024) 15:1451201. doi: 10.3389/fmicb.2024.1451201
37. General Office of the State Council of the People’s Republic of China. China's plan for controlling and preventing AIDS (2024 - 2030). Chin J Virol Dis. (2025) 15:1–3. doi: 10.16505/j.2095-0136.2024.0109
38. Zhao, S, Wang, W, Li, S, He, J, Duan, W, Fang, Z, et al. The prevalence of low-level viraemia and its association with virological failure in people living with HIV: a systematic review and meta-analysis. Emerg Microbes Infect. (2025) 14:2447613. doi: 10.1080/22221751.2024.2447613
39. Zoufaly, A, Kiepe, JG, Hertling, S, Hüfner, A, Degen, O, Feldt, T, et al. Immune activation despite suppressive highly active antiretroviral therapy is associated with higher risk of viral blips in HIV-1-infected individuals. HIV Med. (2014) 15:449–57. doi: 10.1111/hiv.12134
40. Laprise, C, de Pokomandy, A, Baril, JG, Dufresne, S, and Trottier, H. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis. (2013) 57:1489–96. doi: 10.1093/cid/cit529
41. Chun, HM, Abutu, A, Milligan, K, Ehoche, A, Shiraishi, RW, Odafe, S, et al. Low-level viraemia among people living with HIV in Nigeria: a retrospective longitudinal cohort study. Lancet Glob Health. (2022) 10:e1815-e1824. doi: 10.1016/S2214-109X(22)00413-2
42. Antiretroviral Therapy Cohort Collaboration (ART-CC)Vandenhende, MA, Ingle, S, May, M, Chene, G, Zangerle, R, et al. Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. AIDS. (2015) 29:373–83. doi: 10.1097/QAD.0000000000000544,
43. Kanapathipillai, R, McManus, H, Cuong, DD, Ng, OT, Kinh, NV, Giles, M, et al. The significance of low-level viraemia in diverse settings: analysis of the treat Asia HIV observational database (TAHOD) and the Australian HIV observational database (AHOD). HIV Med. (2014) 15:406–16. doi: 10.1111/hiv.12124
44. Liu, P, You, Y, Liao, L, Feng, Y, Shao, Y, Xing, H, et al. Impact of low-level viremia with drug resistance on CD4 cell counts among people living with HIV on antiretroviral treatment in China. BMC Infect Dis. (2022) 22:426. doi: 10.1186/s12879-022-07417-z
45. Bachmann, N, von Siebenthal, C, Vongrad, V, Turk, T, Neumann, K, Beerenwinkel, N, et al. Determinants of HIV-1 reservoir size and long-term dynamics during suppressive ART. Nat Commun. (2019) 10:3193. doi: 10.1038/s41467-019-10884-9
46. Esber, A, Polyak, C, Kiweewa, F, Maswai, J, Owuoth, J, Maganga, L, et al. Persistent low-level viremia predicts subsequent Virologic failure: is it time to change the third 90? Clin Infect Dis. (2019) 69:805–12. doi: 10.1093/cid/ciy989
47. Pasternak, AO, Vroom, J, Kootstra, NA, Wit, FW, de Bruin, M, De Francesco, D, et al. Non-nucleoside reverse transcriptase inhibitor-based combination antiretroviral therapy is associated with lower cell-associated HIV RNA and DNA levels compared to protease inhibitor-based therapy. eLife. (2021) 10:e68174. doi: 10.7554/eLife.68174
48. Zaçe, D, Rindi, LV, Compagno, M, Colagrossi, L, Santoro, MM, Andreoni, M, et al. Managing low-level HIV viraemia in antiretroviral therapy: a systematic review and meta-analysis. Sex Transm Infect. (2024) 100:460–8. doi: 10.1136/sextrans-2024-056198
49. Gaifer, Z, and Boulassel, MR. Low-level viremia predicts Virological failure in HIV-infected Omani patients receiving antiretroviral therapy. J Int Assoc Provid AIDS Care. (2020) 19:2325958220979817. doi: 10.1177/2325958220979817
50. Goupil de Bouillé, J, Collignon, M, Capsec, J, Guillon, L, Le Moal, G, Barin, F, et al. Low-level HIV viremia is associated with low antiretroviral prescription refill rates and social deprivation. AIDS Care. (2021) 33:1445–50. doi: 10.1080/09540121.2020.1806198
51. Nanyeenya, N, Nakanjako, D, Makumbi, F, Nakigozi, G, Nalugoda, F, Kigozi, G, et al. Effectiveness of intensive adherence counselling in achieving an undetectable viral load among people on antiretroviral therapy with low-level viraemia in Uganda. HIV Med. (2024) 25:245–53. doi: 10.1111/hiv.13568
52. European AIDS Clinical Society. European AIDS clinical society guideline, version 12. 0. Available online at: https://www.eacsociety.org/media/guidelines-12.0.pdf (2023) (Accessed June 18, 2025).
53. Ryscavage, P, Kelly, S, Li, JZ, Harrigan, PR, and Taiwo, B. Significance and clinical management of persistent low-level viremia and very-low-level viremia in HIV-1-infected patients. Antimicrob Agents Chemother. (2014) 58:3585–98. doi: 10.1128/AAC.00076-14
54. Wu, G, Zhou, C, Zhang, X, Zhang, W, Lu, R, Ouyang, L, et al. Higher risks of virologic failure and all-cause deaths among older people living with HIV in Chongqing, China. AIDS Res Hum Retrovir. (2019) 35:1095–102. doi: 10.1089/AID.2019.0096
55. Mackiewicz, MM, Overk, C, Achim, CL, and Masliah, E. Pathogenesis of age-related HIV neurodegeneration. J Neurovirol. (2019) 25:622–33. doi: 10.1007/s13365-019-00728-z
56. Bull, ME, Mitchell, C, Soria, J, Styrchak, S, Williams-Wietzikoski, C, Legard, J, et al. Monotypic low-level HIV viremias during antiretroviral therapy are associated with disproportionate production of X4 virions and systemic immune activation. AIDS. (2018) 32:1389–401. doi: 10.1097/QAD.0000000000001824
57. Chun, TW, Murray, D, Justement, JS, Hallahan, CW, Moir, S, Kovacs, C, et al. Relationship between residual plasma viremia and the size of HIV proviral DNA reservoirs in infected individuals receiving effective antiretroviral therapy. J Infect Dis. (2011) 204:135–8. doi: 10.1093/infdis/jir208
58. Jiang, TY, Hou, JH, Su, B, Zhang, T, Yang, Y, Liu, ZY, et al. Demographic and clinical factors associated with immune reconstitution in HIV/HBV co-infected and HIV mono-infected patients: a retrospective cohort study. HIV Med. (2020) 21:722–8. doi: 10.1111/hiv.13023
59. Crespo-Bermejo, C, de Arellano, ER, Lara-Aguilar, V, Valle-Millares, D, Gómez-Lus, ML, Madrid, R, et al. Persistent low-level viremia in persons living with HIV undertreatment: An unresolved status. Virulence. (2021) 12:2919–31. doi: 10.1080/21505594.2021.2004743
60. Brattgård, H, Björkman, P, Nowak, P, Treutiger, CJ, Gisslén, M, and Elvstam, O. Factors associated with low-level viraemia in people with HIV starting antiretroviral therapy: a Swedish observational study. PLoS One. (2022) 17:e0268540. doi: 10.1371/journal.pone.0268540
61. Leierer, G, Grabmeier-Pfistershammer, K, Steuer, A, Geit, M, Sarcletti, M, Haas, B, et al. Factors associated with low-level viraemia and virological failure: results from the Austrian HIV cohort study. PLoS One. (2015) 10:e0142923. doi: 10.1371/journal.pone.0142923
62. Zhou, C, Chen, Z, Zhou, Q, Wu, G, and Zhou, Y. Analysis of antiviral treatment effects of AIDS patients with male-male sexual behavior in Chongqing. Chongqing Med. (2015) 44:2205–7. doi: 10.3969/j.issn.1671-8348.2015.16.016
Keywords: HIV, AIDS, low-level viremia, prevalence, meta-analysis, China
Citation: Zhang X, Xu Q, Li C, Zhang Y, Jin Y, Li P and Guo H (2025) Prevalence of low-level viremia and related influencing factors among people living with HIV in China: a systematic review and meta-analysis. Front. Public Health. 13:1661253. doi: 10.3389/fpubh.2025.1661253
Edited by:
Wondwossen Amogne Degu, Addis Ababa University, EthiopiaReviewed by:
Samson Malwa Haumba, Georgetown University Medical Center, United StatesFu-Xiang Wang, Harbin Medical University, China
Copyright © 2025 Zhang, Xu, Li, Zhang, Jin, Li and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qianlei Xu, eHVxaWFubGVpNjY2QDEyNi5jb20=
 Xinxin Zhang
Xinxin Zhang Qianlei Xu
Qianlei Xu Chen Li
Chen Li Yue Zhang
Yue Zhang Yantao Jin
Yantao Jin Pengyu Li
Pengyu Li Huijun Guo
Huijun Guo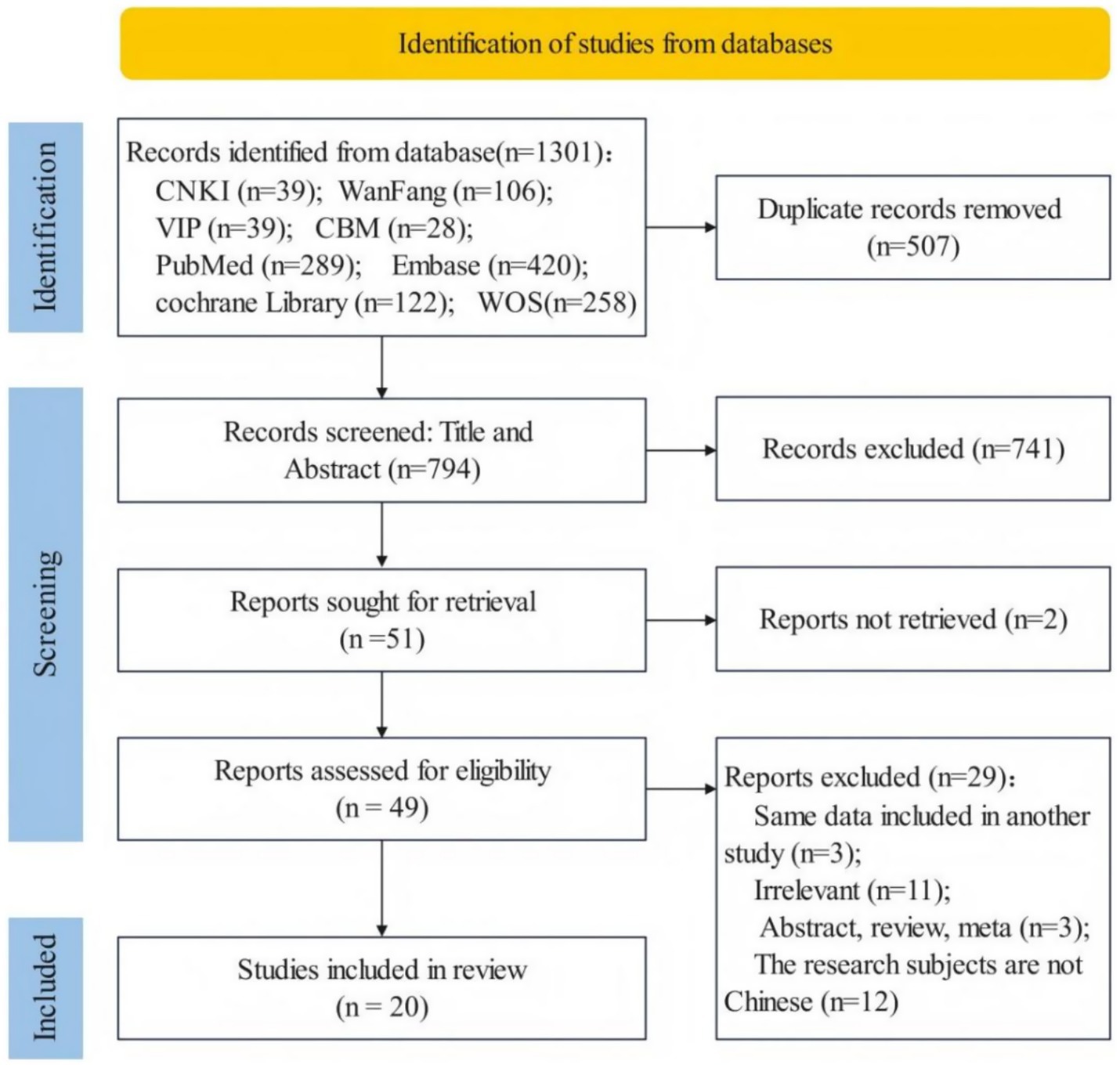
![Forest plot showing the proportions and confidence intervals of several studies, showing data across various omitted studies from 2019 to 2024. Each study has a corresponding proportion, 95% confidence interval, Tau2, Tau, and I-squared values. The random effects model is summarized at the bottom, with a proportion of 0.11 and confidence interval of [0.07, 0.16].](https://www.frontiersin.org/files/Articles/1661253/fpubh-13-1661253-HTML/image_m/fpubh-13-1661253-g003.jpg)