- 1School of Public Health and Management, Jiangsu Medical College, Yancheng, China
- 2Department of Epidemiology, School of Public Health, Nanjing Medical University, Nanjing, China
- 3Department of Thoracic Surgery, The First People’s Hospital of Yancheng City, The Yancheng Clinical College of Xuzhou Medical University, Yancheng, China
- 4Department of Chronic Communicable Disease, Nanjing Municipal Center for Disease Control and Prevention, Nanjing, China
- 5Department of Endocrinology, The First People’s Hospital of Yancheng City, The Yancheng Clinical College of Xuzhou Medical University, Yancheng, China
- 6Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States
- 7State Key Laboratory of Systems Medicine for Cancer, Center for Single-Cell Omics, School of Public Health, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 8Department of Epidemiology, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China
- 9Department of Chronic and Non-Communicable Disease, Yancheng Municipal Center for Disease Control and Prevention, Yancheng, China
- 10Department of Pharmacy, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, China
Background: Gastrointestinal (GI) cancers account for 43.1% of cancer-related deaths in China, with aging populations exacerbating this burden. While chronic air pollution exposure is linked to GI carcinogenesis, evidence on acute effects remains limited. This study investigates short-term ambient pollutant exposure and GI cancer mortality in a coastal Chinese city with moderate pollution levels.
Methods: Using death registry data from Yancheng, China (2013–2022; n = 104,216 GI cancer deaths), we employed a time-stratified case-crossover design combined with distributed lag nonlinear models (DLNM) to assess associations between daily PM2.5, PM10, SO2, NO2, and O3 concentrations (lag 0–7 days) and mortality. Stratified analyses by age, sex, and cancer subsite were conducted, with sensitivity analyses evaluating model robustness.
Results: A 10 μg/m3 increase in PM2.5, PM10, and O3 was associated with acute GI cancer mortality, peaking at lag 0–5 days (relative risk [RR] = 1.011, 95% CI: 1.000–1.022 for PM2.5; RR = 1.009, 95% CI: 1.001–1.017 for PM10; RR = 1.008, 95% CI: 1.001–1.016 for O3). The older males (≥65 years) exhibited heightened vulnerability, with maximal cumulative RRs of 1.018 (PM2.5), 1.010 (PM10), and 1.014 (O3). Esophageal cancer showed acute PM sensitivity (lag 0–4 days: RR = 1.021 for PM2.5), while colorectal cancer mortality correlated with delayed O3 effects (lag 0–7 days: RR = 1.031). No associations were observed for SO2 or NO2. Sensitivity analyses confirmed model stability across pollutant co-exposure adjustments and temporal confounders.
Conclusion: Short-term exposure to PM2.5, PM10, and O3 elevates GI cancer mortality risk, particularly among the older males and upper GI malignancies. These findings highlight the need for revised air quality standards addressing acute exposure thresholds and targeted protections for high-risk populations to mitigate pollution-related cancer mortality.
1 Introduction
Gastrointestinal (GI) cancer presents a significant global public health challenge. According to the 2022 cancer mortality estimates from the Global Cancer Observatory (GLOBOCAN), GI cancers at specific sites constitute five of the top 10 causes of cancer mortality. These include colorectal, liver, stomach, pancreatic, and esophageal cancers, accounting for 9.3%, 7.8%, 6.8%, 4.8% and 4.6% of total cancer deaths, respectively (1). China is expected to have 1.6 million new cases and 1.11 million deaths from digestive system cancers, representing 43.1% of all cancer-related deaths in 2022, with older people most affected (2). With the advent of China’s aging population, the burden of the expected future gastrointestinal cancer mortality and morbidity will increase.
Mounting epidemiological evidence positions ambient pollutants as critical modulators of carcinogenesis and tumor evolution, with their multifaceted biological mechanisms now constituting a priority research domain in environmental oncology. Environmental air pollution encompasses various contaminants including gaseous pollutants like sulfur dioxide (SO₂), nitrogen dioxide (NO₂), ozone (O3), and volatile organic compounds (VOCs) along with particulate matter (PM) (3). These pollutants often occur together, posing health risks to the population, particularly the harm of PM. The International Agency for Research on Cancer (IARC) has classified PM as a human carcinogen (4). Previous research has demonstrated a robust correlation between the short-term effects of air pollution on all-cause deaths and deaths from cardiovascular and respiratory diseases (5–8). Epidemiological literature demonstrates chronic particulate matter exposure exhibits dose–response relationships with both total and site-specific gastrointestinal cancer mortality (9, 10). A meta-analysis of 20 cohort studies quantified this association, revealing that 80% (16/20) of included research confirmed statistically significant associations between prolonged PM2.5/PM10 exposure and elevated GI cancer risk (11). Notably, current evidence exhibits three critical limitations: (1) paucity of investigations on acute (<7 days) pollution exposures’ impacts on digestive tract cancer outcomes; (2) absence of population-level studies in regions with ambient pollutant concentrations below WHO thresholds (e.g., Yancheng, China); (3) insufficient mechanistic exploration of particulate-induced gastrointestinal carcinogenesis. These knowledge gaps underscore the imperative for methodologically standardized investigations addressing geospatial heterogeneity in pollution exposure-response dynamics.
In this study, we analyzed death registry data from Yancheng City, China for the years 2013–2022. The dataset included over 100,000 deaths related to GI cancer. Our analytic framework combined a case-time-control design with distributed lag nonlinear modeling (DLNM) within a quasi-Poisson generalized additive model architecture to quantify concentration-response relationships between acute ambient pollutant exposures (lag 0–7 days) and gastrointestinal cancer mortality outcomes. Furthermore, we conducted stratified analyses by sex and age to explore the potential moderating effects and identify potentially susceptible populations. These findings not only helped identify high-risk susceptible populations but also provided a crucial epidemiological foundation for the development of effective preventive measures against GI cancer.
2 Materials and methods
2.1 Study area and population
Yancheng is a coastal city situated in the eastern part of China, within the transitional belt from the subtropical to warm temperate zones. It serves as a pivotal link between northern and southern China, encompassing an area of 17,718 square kilometers with a population of 6,689,700 in 2022. The average annual temperature is recorded at 16.1 °C. Despite a decreasing trend over the past decade, PM2.5 levels in Yancheng persistently exceed the World Health Organization (WHO) air quality guideline (annual mean of 5 μg/m3) by a substantial margin.
2.2 Daily mortality data
This study analyzed GI cancer mortality patterns using de-identified records from Yancheng, China, spanning 2013–2022. Mortality data were systematically collected through the municipal Death Registration System under the supervision of Yancheng Municipal Center for Disease Control and Prevention (CDC)., covering all residential areas within the jurisdiction. GI cancer cases were specifically identified using ICD-10 codes C15-C26. To ensure data reliability, the CDC implemented multilevel quality control protocols, including routine audits and validation processes for all reported deaths. The surveillance framework adheres to standardized national procedures for cause-of-death certification and coding practices.
2.3 Daily air quality and meteorological data
We extracted data on meteorological factors (temperature, relative humidity, wind speed, barometric pressure) in Yancheng between January 1, 2013 and December 31, 2022 from the China Meteorological Data Sharing Center1, as well as daily average concentrations of five ambient air pollutants, including PM10, PM2.5, SO2, NO2, and O3 (the concentration of O3 was the maximum 8-h moving average) in Yancheng during the same period from the National urban air quality real-time release platform2. Air-pollution data obtained from this monitoring system have been used extensively to evaluate the health effects of air pollution both regionally and nationally (12, 13). The 2013–2022 timeframe was selected because 2013 marked a pivotal year for air quality monitoring in China: the national ambient air monitoring network was officially operationalized on January 1, 2013. This network enabled 74 key cities (including Yancheng) to commence standardized monitoring and real-time public disclosure of the 6 pollutants (including PM2.5) and AQI indices, ensuring high-quality, consistent data from 2013 onward. The monitoring sites in Yancheng were deployed following the Technical Specifications for Ambient Air Quality Monitoring Network (HJ 664–2013), ensuring effective representation of spatial variations in pollutant concentrations.
2.4 Statistical analysis
This study adopted a time-stratified case-crossover design to control for both known (e.g., age, socioeconomic status, sex) and unknown confounding factors by matching each death case to its exposure status across different time periods (14, 15). Specifically, the stratification variable “year-month-day of the week” was used to select control periods (i.e., dates with the same year, month, and day of the week) for each case, thereby eliminating interference from long-term trends, seasonal variations, and weekday-related effects. Building on this, a quasi-Poisson regression model was employed to analyze the stratified data. This approach not only addresses overdispersion and autocorrelation in daily GI cancer death counts but also ensures robustness by conditionally adjusting for stratification variables through a fixed-effects framework (16, 17). To further capture delayed and nonlinear effects of environmental exposures, the study integrated a Distributed Lag Non-linear Model (DLNM) to quantify the dynamic impacts of exposure factors (e.g., air pollutants) across varying lag periods (e.g., 0–7 days post-exposure) and to dissect their nonlinear relationships with mortality risk. By combining these methods, the model leverages the self-matching advantages of the case-crossover design to control for confounders while utilizing the DLNM to flexibly model complex temporal exposure-response patterns. This integration enables a more precise assessment of the acute effects of environmental factors on gastrointestinal cancer mortality (14, 15).
Specifically, the model uses the expected number of deaths due to GI cancer on observation day t, denoted as E(Yt), as the dependent variable. The log-linear formulation is expressed as:
Yt~ quasi-Poisson[E(Yt)]
Log[E(Yt)] = α + βPt,l + γTt,l + ns(Relative Humidityt, df = 3) + ns(Wind Speedt, df = 3) + ns(time,7*10) + stratum + dow + as factor(holiday)
Pt,l and Tt,l represent cross-basis functions of daily average concentration for air pollutants and daily mean temperature, respectively, which quantify the exposure-response relationships of pollutants and temperature across varying lag days; The coefficients β and γ correspond to these functions, respectively; Natural cubic splines (ns) with 3 degrees of freedom (df) are employed to control for nonlinear confounding effects of relative humidity and wind speed (18). The stratification variable (stratum) inherently adjusts for seasonality and long-term trends by restricting comparisons to days within the same year, month, and day of the week. The time-stratified case-crossover approach inherently adjusts for temporal confounders including weekly variations, seasonal cycles, and longitudinal trends through its self-matching design framework. Furthermore, to refine control over short-term temporal variations, the model incorporates day-of-week effects (dow) to capture mortality fluctuations linked to differences between weekdays and weekends, and a categorical holiday variable to account for public holidays. In the analysis of lagged exposure effects, the study evaluates both single-day lags (lag0–lag7, corresponding to the exposure day up to 7 days prior) and cumulative lags (lag01–lag07, defined as moving averages of the current day and the preceding 1–7 days) to comprehensively assess the acute health impacts of pollutants (19).
Single-pollutant models were established for PM2.5, PM10, SO2, NO2, and O3, independently assessing the lag-specific and cumulative effects of each pollutant on GI cancer mortality per 10-unit increase in concentration. Subsequently, to address the collinearity between air pollutants, multi-pollutant models were constructed by excluding highly correlated variables (Pearson correlation coefficient r < 0.8) based on statistically significant pollutants identified in single-pollutant analyses. Relative risk (RR) estimates and corresponding 95% confidence intervals (CI) were employed to represent the lag-specific and cumulative exposure impacts. Further, stratified analyses by age (<65 years vs. ≥65 years), and sex (male vs. female) were conducted to reveal population heterogeneity in risk distribution and identify potential high-risk subpopulations.
We finally performed several sensitivity analyses to evaluate the robustness of the results. Firstly, to evaluate model parameter sensitivity, we systematically varied the df for the time variable in single-pollutant models, testing a range of 6–10 df per year as supported by prior methodological studies (20, 21). Secondly, to address multicollinearity concerns, dual-pollutant models were implemented using conditional inclusion criteria (Spearman’s ρ < 0.7) to quantify confounding interactions between co-varying atmospheric contaminants. Thirdly, recognizing the unprecedented environmental and healthcare disruptions caused by the SARS-CoV-2 pandemic, we introduced binary dummy variables (2020–2021 coded as 1 vs. other years as 0) into our primary models to isolate and control for pandemic-related confounding factors.
Analytical procedures were implemented in R version 4.2.2 (R Foundation for Statistical Computing) with specialized computational libraries: the ‘dlnm’ package for distributed lag nonlinear modeling, and ‘splines’ for nonparametric smoothing. A two-tailed alpha level of 0.05 served as the prespecified statistical significance threshold.
2.5 Ethics approval
Ethics approval was not required for secondary analysis of the anonymous data in this study.
3 Results
3.1 Descriptive statistics
As shown in Table 1, there were 104,216 GI cancer deaths identified in Yancheng from 2013 to 2022 averaging 28.62 deaths per day. The majority of deaths died from esophagus cancer (29.94%), stomach cancer (28.11%), and liver cancer (22.14%), while the number of colorectum cancer (10.41%) and pancreas cancer (7.89%) deaths was relatively small. A total of 68,247(65.49%) males and 35,969 (34.51%) females were among all GI cancer deaths. The number of deaths per day attributed to GI cancer was 18.74 for males and 9.88 for females. In all cases of death caused by GI cancer, 71.21% of patients were aged 65 or older, whereas 28.79% were younger than 65 years old.
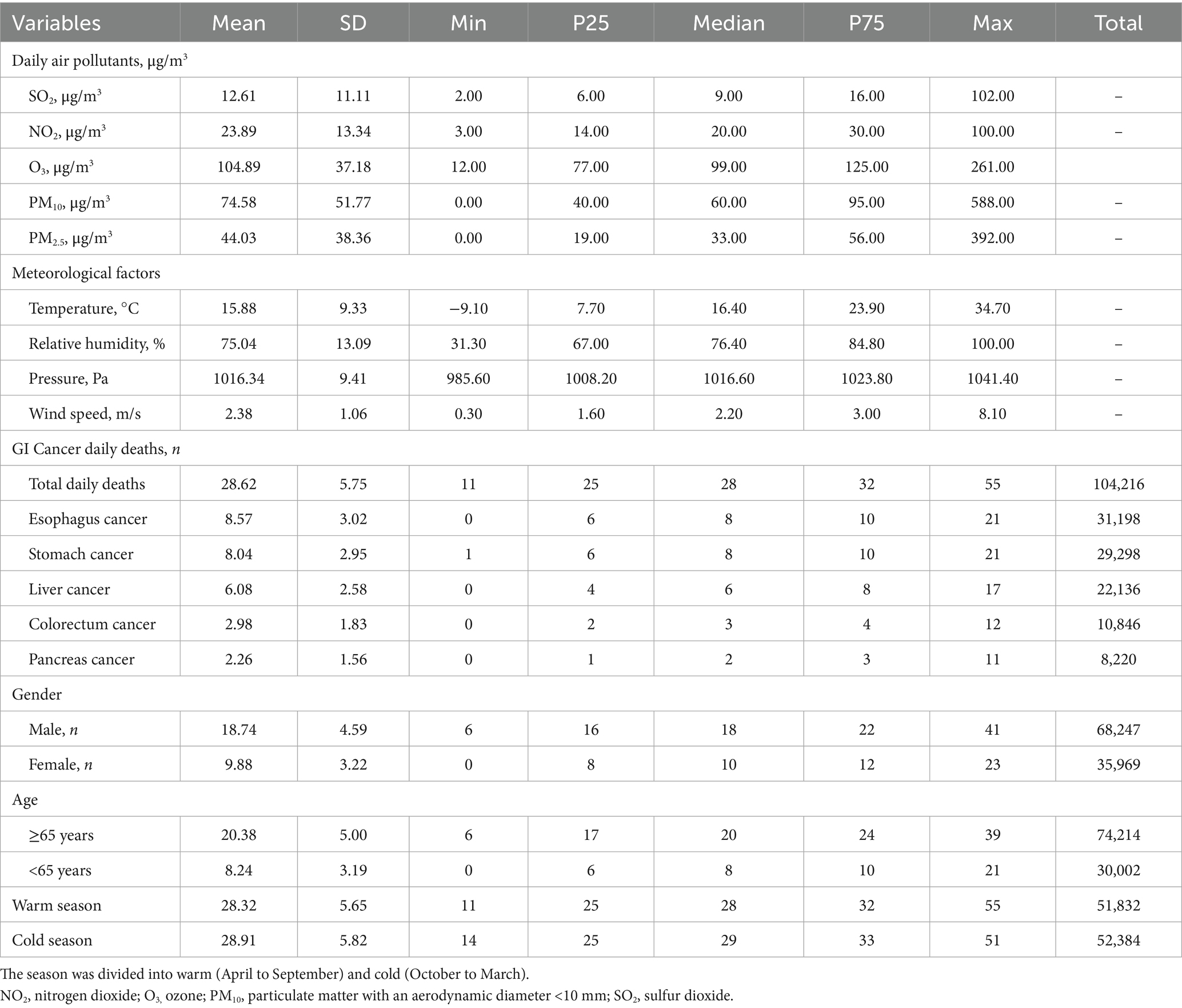
Table 1. Summary statistics for air pollutants, meteorological parameters and GI cancer daily deaths from January 2013 to December 2022 in Yancheng, China.
The daily average concentrations of SO2, NO2, O3, PM10 and PM2.5 in Yancheng were 12.61 μg/m3, 23.89 μg/m3, 104.89 μg/m3, 74.58 μg/m3 and 44.03 μg/m3, respectively. During the study period, the daily average temperature was 15.88 °C, the mean pressure was 1016.34 kPa, the mean wind speed was 2.38 m/s, and the relative humidity was 75.04%. The time-series patterns of ambient air pollution, daily GI cancer deaths, and meteorological factors between 2013 and 2022 are shown in Figure 1.
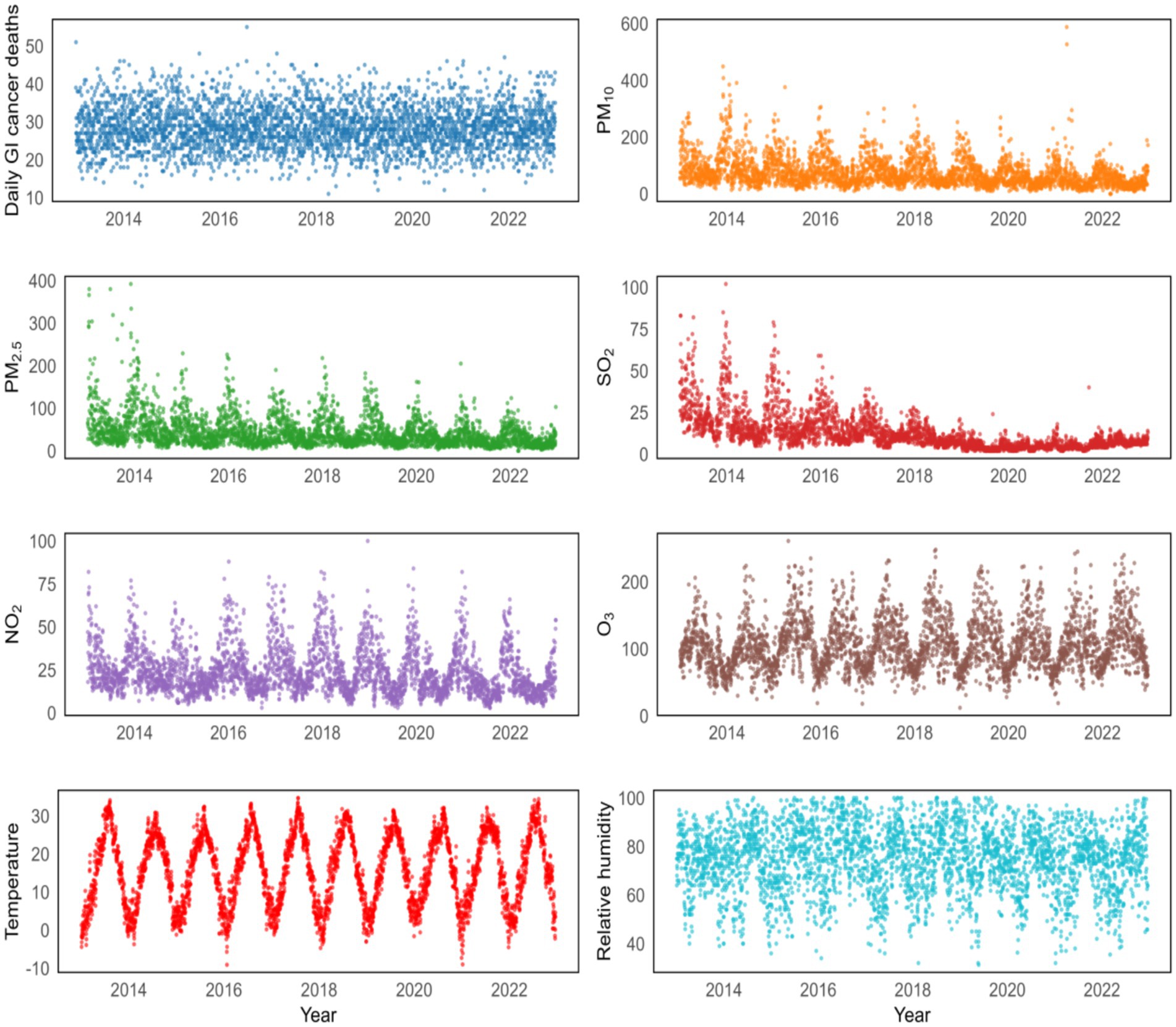
Figure 1. Temporal patterns of daily GI cancer deaths and various environmental factors from 2013 to 2022.
3.2 Spearman rank correlation analysis
Supplementary Table S1 presents Pearson correlation coefficients among air pollutant concentrations. Significant inter-correlations were observed (p < 0.001) among sulfur dioxide (SO₂), nitrogen dioxide (NO₂), and particulate matter (PM10 and PM2.5), while ozone (O3) demonstrated associations exclusively with PM10 and NO₂ in the analysis. Our research results indicated that temperature exhibits a positive correlation with O₃ concentration, yet a negative correlation with PM2.5, PM10, SO₂, and NO₂ concentrations (all p < 0.001). Relative humidity also demonstrated a negative correlation with these five pollutant concentrations (all p < 0.001). Furthermore, atmospheric pressure was positively correlated with PM2.5, PM10, SO₂, and NO₂ concentrations, and negatively correlated with O₃ concentration (all p < 0.001). Wind speed exhibited a negative correlation with PM2.5, PM10, O₃, and NO₂ concentrations, but a positive correlation with SO₂ concentration (all p < 0.01).
3.3 The short-term exposure to air pollution and GI cancer deaths
Figure 2 and Table 2 estimated lag-response and cumulative relative risk of GI cancer deaths associated with a 10 μg/m3 increase in air pollutant concentrations using a single pollutant model. For single–day lags, significant positive associations were found between these pollutants (PM2.5 and O3) and GI cancer deaths at lag 0, lag 1 and lag 2 (lag 0, lag 1, lag 2 and lag 3 days for PM10), with all these pollutants peaking on lag 0 day. For multi-day lags, significant positive associations were also found between these pollutants (PM2.5, PM10, and O3) and GI cancer deaths. The cumulative risk of GI cancer deaths was associated with PM2.5 exposure, ranging from lag 0 day (RR = 1.0031, 95%CI: 1.0001–1.0061) to lag 0–5 days (RR = 1.0112, 95%CI: 1.0001–1.0224). PM10 exposure, from lag 0 day (RR = 1.0029, 95%CI: 1.0007–1.0051) to lag 0–6 days (RR = 1.0089, 95%CI: 1.0005–1.0174), was linked to an increased cumulative risk of GI cancer deaths. O3 exposure, from lag 0 days (RR = 1.0024, 95%CI: 1.0002–1.0045) to lag 0–5 days (RR = 1.0084, 95%CI: 1.0005–1.0163), also increased the cumulative risk of GI cancer deaths. Moreover, the estimated cumulative relative risk of GI cancer deaths associated with a 10 μg/m3 increment in pollutant concentrations all reached a maximum at lag 0–5 days for PM2.5, PM10, and O3. There was no single-day or multi-day lag effect on SO2 and NO2.
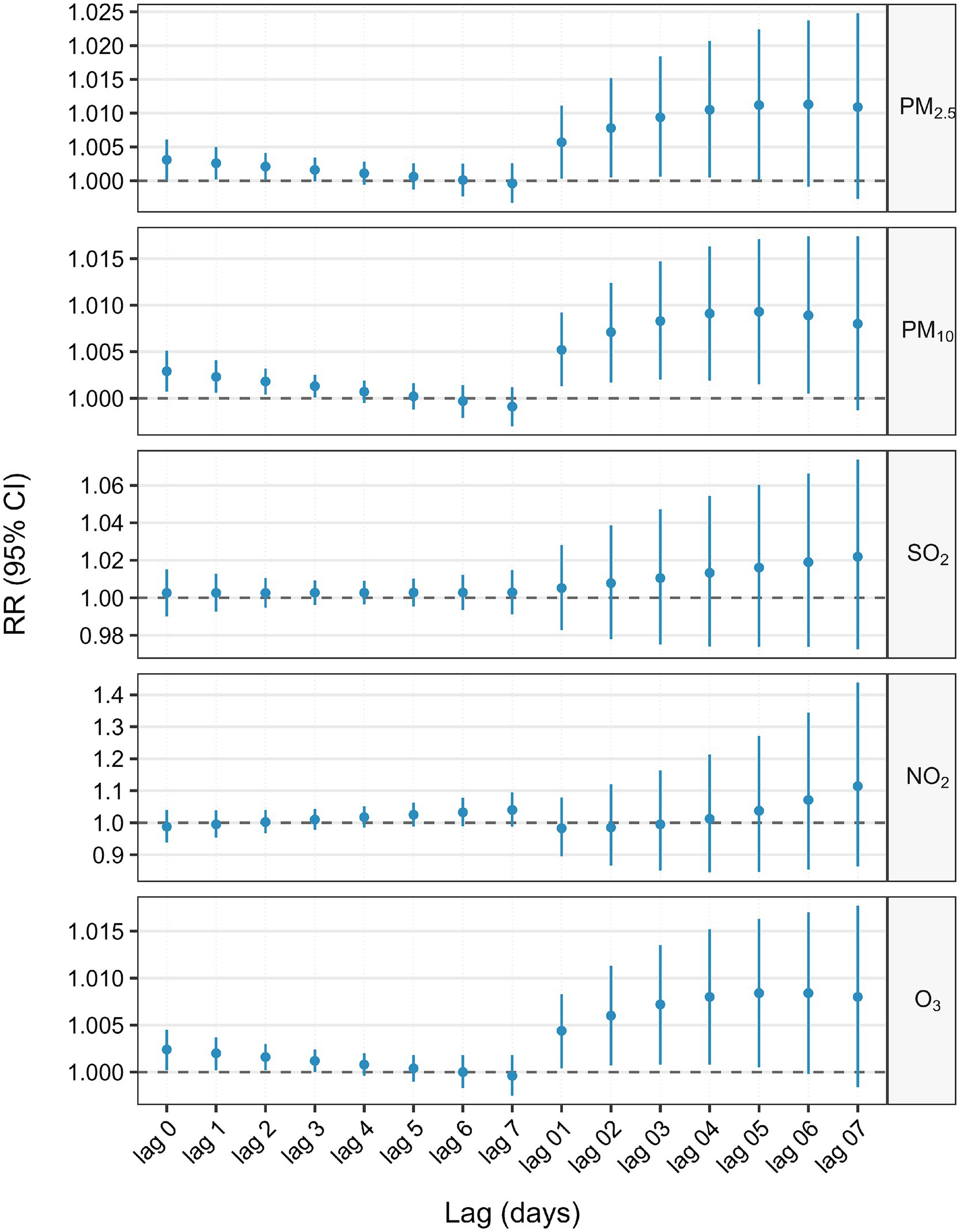
Figure 2. Association between 10 μg/m3 air pollution exposure and lagged GI cancer mortality risk: single-pollutant model.
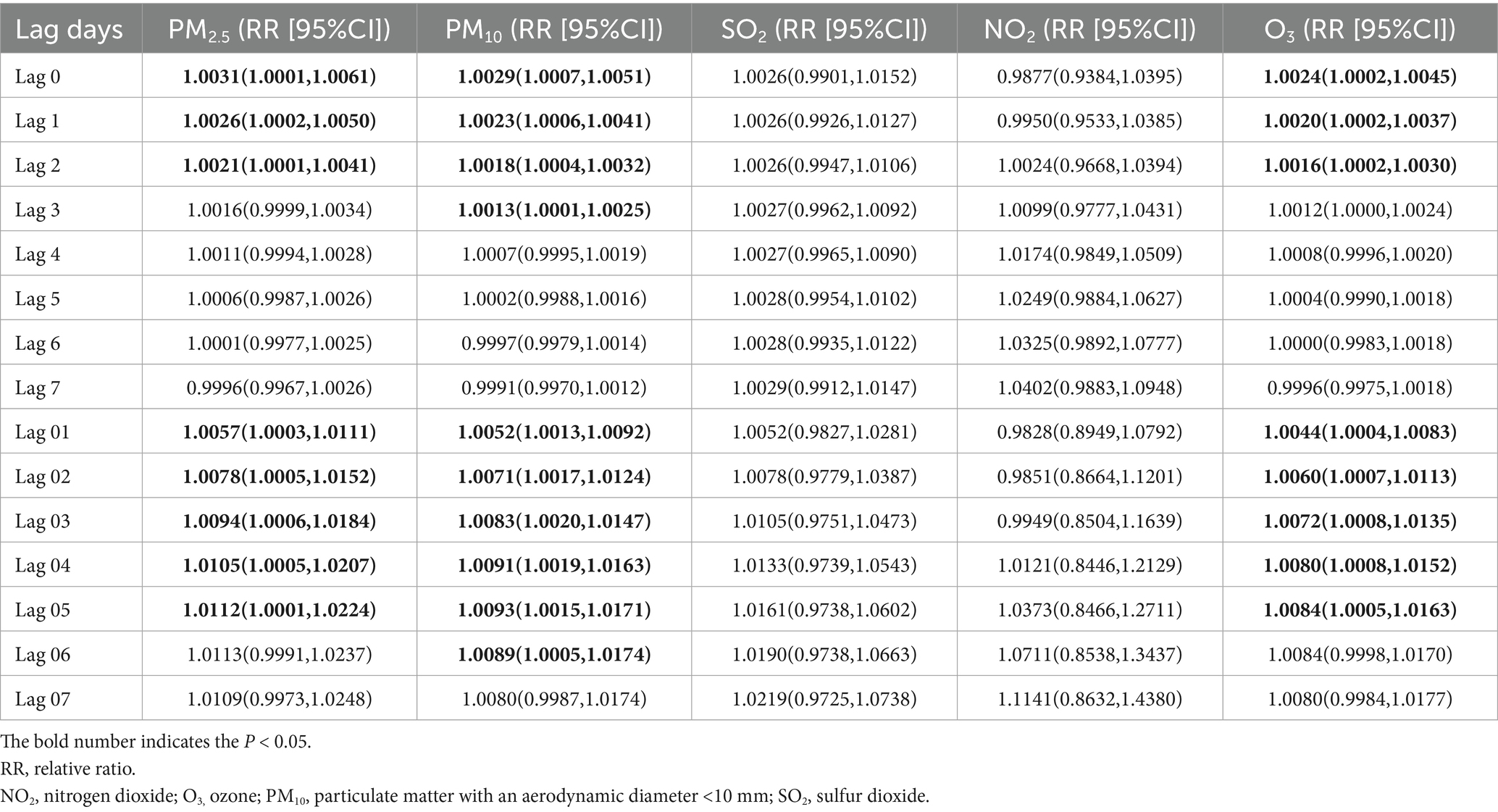
Table 2. Association between 10 μg/m3 air pollution exposure and lagged GI cancer mortality risk: single-pollutant model.
3.4 Subgroup analysis
Figure 3 and Supplementary Tables S2, S3 presented the results of the analysis stratified by various age groups within the single pollutant model. When categorized by age, the correlation between GI cancer deaths and exposure to PM2.5, PM10, and O3 was significant exclusively in the older population (age ≥65 years). The highest cumulative RR for PM2.5 was observed at a lag of 0–7 days (RR = 1.0175, 95%CI: 1.0011–1.0342), for PM10 at a lag of 0–5 days (RR = 1.0101, 95%CI: 1.0008–1.0195), and for O3 at a lag of 0–6 days (RR = 1.0143, 95%CI: 1.0040–1.0247). Additionally, we also performed a stratified analysis by gender (Figure 4 and Supplementary Tables S4, S5). We found that the statistically significant correlation between exposure to PM2.5, PM10, and O3 and GI cancer mortality was evident only in males. The association between PM2.5 exposure and GI cancer deaths was strongest at a lag of 0–7 days (RR = 1.0188, 95%CI: 1.0021–1.0359). For PM10, the peak cumulative risk was at a lag of 0–4 days (RR = 1.0116, 95%CI: 1.0029–1.0205), and the cumulative RR for O3 peaked at a lag of 0–5 days (RR = 1.0102, 95%CI: 1.0005–1.0199). No link with GI cancer mortality was identified for SO2 and NO2 in the subgroup analysis. Furthermore, our stratified analysis of five GI cancers revealed distinct anatomic and temporal susceptibility patterns to airborne pollutants (Supplementary Tables S6–S10). Esophageal cancer mortality exhibited multipollutant sensitivity, with PM2.5 demonstrating peak cumulative risk at lag 0–4 days (RR = 1.0216 per 10 μg/m3 increase; 95% CI: 1.0035–1.0401), concurrent with PM10’s maximal effect window during the same exposure period (RR = 1.0208, 95% CI: 1.0078–1.0340 per 10 μg/m3 increment). Notably, O3 displayed acute-phase toxicity in esophageal cancer, showing a significant mortality elevation at lag 0–2 days (RR = 1.0095, 95% CI: 1.0000–1.0192 per 10 μg/m3 increment). In contrast, colorectal cancer mortality was uniquely associated with ozone exposure, manifesting maximal risk at lag 0–7 days (RR = 1.0312, 95% CI: 1.0019–1.0614 per 10 μg/m3 increment). This divergence in temporal dynamics–early particulate matter dominance in upper gastrointestinal malignancies versus delayed ozone effects in lower gastrointestinal sites – was further reinforced by null associations observed in gastric, hepatic, and pancreatic cancers.
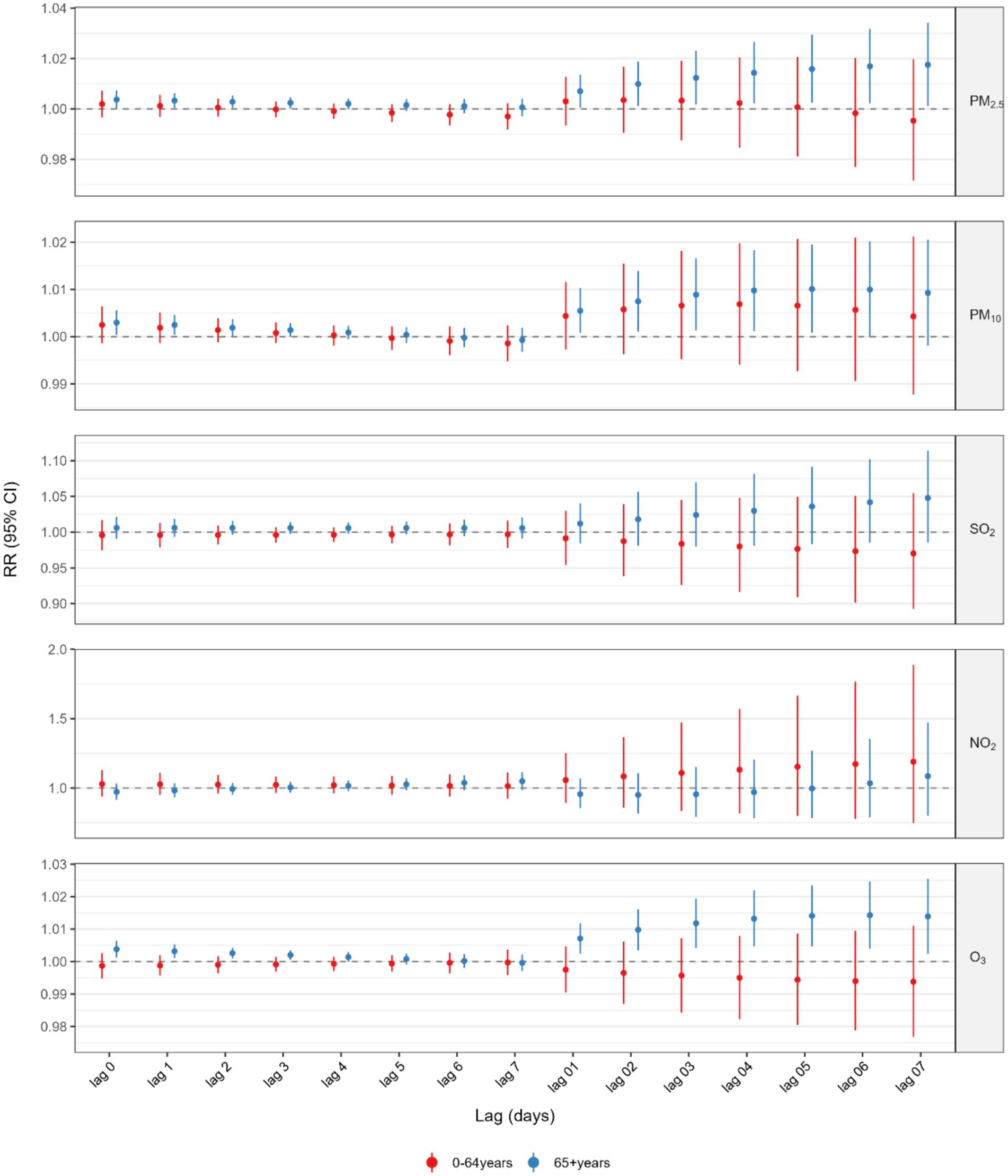
Figure 3. Association between 10 μg/m3 air pollution exposure and lagged GI cancer mortality risk: age-satisfied analysis.
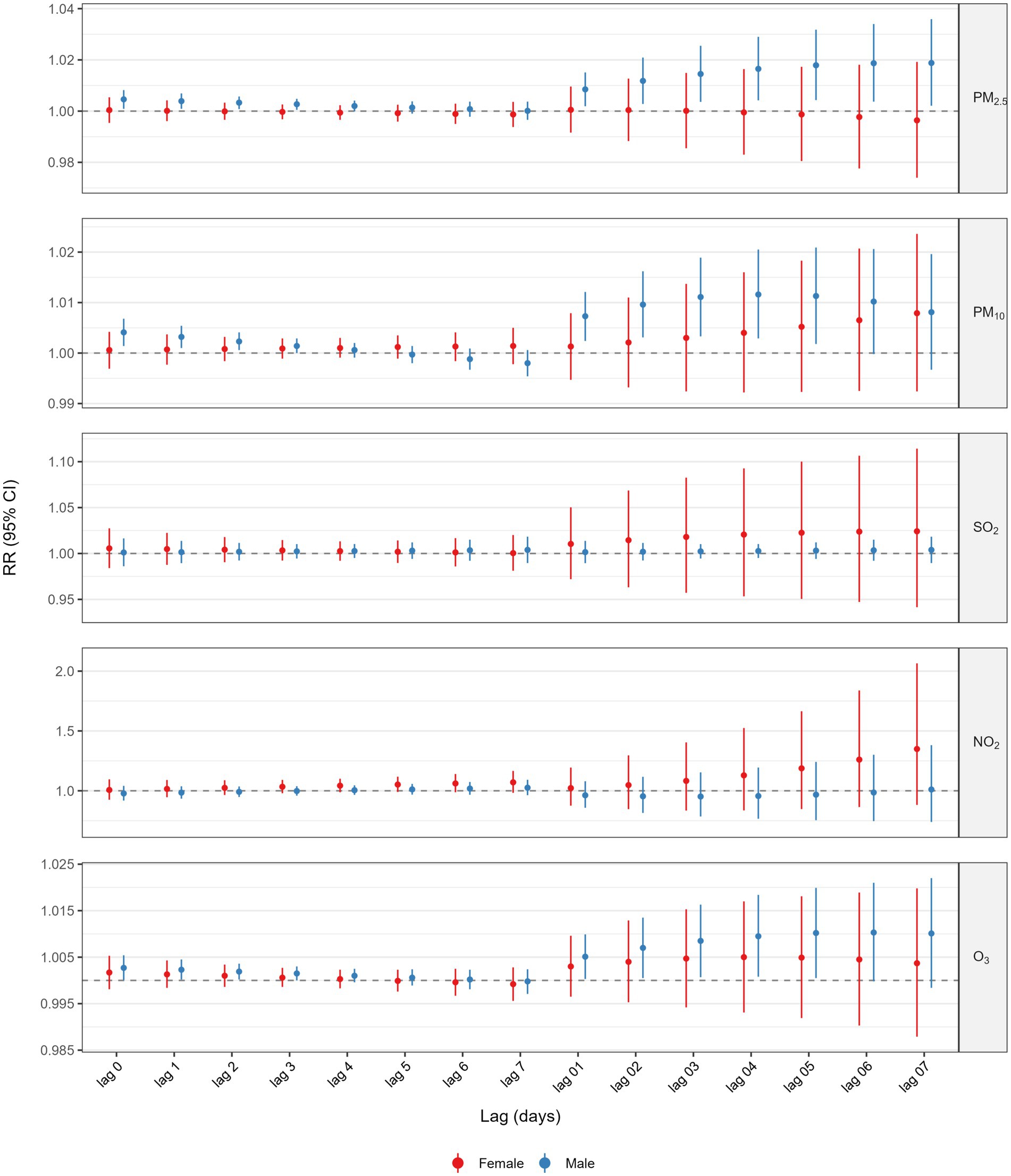
Figure 4. Association between 10 μg/m3 air pollution exposure and lagged GI cancer mortality risk: sex-stratified analysis.
3.5 Sensitivity analysis
The robustness of the primary findings was systematically evaluated through multiple approaches. First, varying degrees of freedom (6–10 df per year) to adjust for long-term temporal trends yielded notably stable results across all exposure-pollutant models (Supplementary Tables S11–S14), demonstrating the consistency of effect estimates under different smoothing parameterizations. Furthermore, in two-pollutant models adjusting for potential confounding by co-pollutants, the associations between a 10 μg/m3 increase in PM2.5, PM10, SO2, NO2, and O3 concentrations and GI cancer mortality revealed no substantial differences compared to single-pollutant models (Supplementary Table S15). Additionally, the inclusion of dummy variables to account for the SARS-CoV-2 pandemic period revealed no substantial alterations in exposure-response relationships compared to the main model (Supplementary Table S16). Finally, we conducted additional sensitivity analyses examining the cumulative lag effect of air pollutants on GI cancer deaths over 0-21 days and found that the cumulative relative risks (RRs) for PM2.5, PM10, SO2, NO2, and O3 (per 10 μg/m3 increase) showed no evidence of decline over the 0–21 day period; instead, they remained stable or slightly increased (Supplementary Table S17).
4 Discussion
Our study reveals novel associations between short-term exposure to ambient pollutants and elevated GI cancer mortality in a coastal city with moderate pollution. Acute-phase risks peaked at lag 0–5 days (PM2.5, PM10, and O3), aligning mechanistically with pollutant-triggered systemic inflammation and oxidative stress, despite concentrations below WHO interim targets. These findings also demonstrated that transient pollution spikes may accelerate GI cancer mortality in vulnerable populations (e.g., the older population and men). Anatomic-temporal divergence—acute upper GI effects versus delayed lower GI impacts—highlights subsite-specific vulnerabilities, addressing prior ecological studies’ lack of subsite stratification.
The association between air pollutants and GI cancer mortality exhibits significant spatiotemporal heterogeneity. While evidence on the short-term effects of air pollution on GI cancer mortality remains limited, accumulating studies highlight significant associations with long-term exposure to air pollution on GI cancer mortality (22–26). Regarding the effects of PM2.5, discrepancies exist between our findings and previous short-term exposure studies: A Brazilian cohort demonstrated that each 10 μg/m3 increase in PM2.5 (lag 0–2 days) elevated mortality risks for esophageal, gastric, and colorectal cancers by 4%, 5%, and 4%, respectively (27), while a time-series study in Xi’an confirmed PM2.5’s association with gastric cancer mortality (RR = 1.0003) (28). Although our results align directionally with these studies, the effect magnitude is notably lower. Given the heterogeneity in genetic profiles, socioeconomic conditions, climatic factors, pollutant composition, and methodological approaches across existing investigations, current evidence remains insufficient to confirm whether adaptive mechanisms contribute to attenuated cancer mortality trends. Differences from the umbrella review (29) stem from exposure time scales and methods: we focused on short-term dynamic exposure (0–7 days) and acute mortality via time-stratified case-crossover (controlling confounders), whereas it used long-term static exposure (annual averages) and cohort studies for chronic incidence effects.
In terms of O3, this study provides novel evidence: Each 10 μg/m3 increase in O3 concentration at lag 0–5 days corresponds to a 0.84% elevation in GI cancer mortality risk. This finding echoes a Brazilian nationwide case-crossover study linking 8-h O3 exposure to increased all-cancer mortality, including gastric cancer (30). However, Chinese studies present conflicting results—while a study conducted in Guangzhou revealed positive O3-all-cancer mortality associations (31), the other two studies focusing on lung cancer showed null associations (32, 33). These inconsistencies may arise from: (1) methodological variations (case-crossover vs. time-series designs); (2) cancer-type specificity (all-cancer vs. single-site analyses); and (3) sample size limitations. Notably, this study pioneers verification of O3’s short-term health effects in a moderately polluted city (Yancheng’s daily mean O3: 104.89 μg/m3), offering new evidence for regional air quality standard revisions.
Regarding the lack of significant associations for SO2 and NO2, we attribute this primarily to Yancheng’s unique coastal atmospheric conditions: (1) prevailing southeastern winds enhance pollutant dispersion, yielding daily mean concentrations of 12.61 μg/m3 (SO2) and 23.89 μg/m3 (NO2)—significantly lower than industrial clusters like the Beijing-Tianjin-Hebei region; (2) marine-derived secondary aerosols may alter pollutant chemical profiles, potentially mitigating carcinogenicity (34). This low-concentration, low-variability exposure profile likely reduced statistical power, necessitating future multicenter studies to validate these findings’ generalizability.
Although the potential mechanisms linking ambient air pollutants to GI cancer mortality remain unclear, current research proposes a multi-pathway hypothesis. Particulate matter harbors carcinogenic constituents such as heavy metals and persistent organic pollutants, with demonstrated potential to initiate oncogenic processes and promote tumorigenesis through chronic exposure pathways. First, pollutants such as PM2.5 can enter the GI tract through dual routes: On one hand, inhaled PM2.5 partially crosses alveolar membranes into the bloodstream and deposits in intestinal tissues via systemic circulation (35); on the other hand, particles retained in bronchioles and alveoli are phagocytosed by macrophages (36) and subsequently transported to the upper GI tract through the mucociliary clearance mechanism (37, 38), a process confirmed in human studies of nonsmokers (39). Second, pollutants may directly disrupt intestinal barrier function: PM2.5 synergizes with toxic gasses like SO₂ and NOx to induce tight junction protein rearrangement in gut epithelial cells, increasing intestinal permeability (40), while its heavy metals and carcinogens may trigger localized oxidative stress and DNA damage. Third, the synergistic effects of systemic inflammation and microbial dysbiosis: PM exposure induces systemic inflammatory responses (40), and animal studies show air pollution alters gut microbiota composition, exacerbating susceptibility to mucosal inflammation (36, 41). This dual impact is particularly critical in GI cancer patients, who already exhibit chronic inflammation and microbial imbalance, potentially accelerating cancer progression. Notably, upper GI cancers (e.g., esophageal and gastric cancers) may have unique exposure patterns due to direct contact with PM cleared via mucus, though experimental evidence validating this hypothesis remains lacking.
Emerging evidence suggests that short-term O₃ exposure may elevate all-cancer mortality risk through interrelated pathways involving hemostatic imbalance, neuro-inflammatory activation, and systemic dysregulation. Short-term O₃ exposure induces a hypercoagulable state by upregulating coagulation factor X while suppressing anticoagulant proteins Z and ZPI, thereby increasing thrombotic susceptibility (42). Additionally, inhaled O₃ triggers sensory nerve stimulation in the respiratory tract, initiating local reflex reactions and propagating signals to the central nervous system. This neural activation disrupts autonomic function regulation, potentially exacerbating cardiovascular stress and systemic inflammation, which can lead to potential mortality (43). Concurrently, O₃ exposure promotes respiratory and systemic inflammation, which synergizes with coagulation abnormalities to amplify tissue damage and organ dysfunction and ultimately contributes to mortality in cancer patients. Clinically significant immune compromise in oncology populations, stemming from underlying malignancy and iatrogenic factors, amplifies susceptibility to airborne toxicants’ pathobiological effects, thereby elevating mortality risks. Moreover, emerging evidence identifies dysregulation of oncogenesis-associated mRNA/miRNA signatures following brief (≤2 h) low-concentration ambient pollutant exposures, suggesting accelerated carcinogenic pathways (44).
Current evidence on the modifying factors that influence the association between short-term exposure to air pollution and GI cancers is limited. Therefore, we conducted subgroup analyses to explore the potential modifying effects of sex, age, and tumor type. Our analyses revealed that the relationship between PM2.5, PM10, and O3 and GI cancer mortality is more robust in the older population, males, and patients with esophageal cancer. The heightened vulnerability of older adults aligns with age-related declines in immune resilience and increased comorbidity burden, which may exacerbate pollutant-induced oxidative damage. Most studies have found that women were more sensitive to the acute effects of air pollution, but our results contrast with this consensus, revealing stronger associations in males. This discrepancy may arise from sex-specific exposure patterns: in China, males exhibit a substantially higher smoking prevalence (47.2% in males vs. 2.7% in females (45)), which likely synergizes with air pollution to amplify acute cardiopulmonary stress and cancer progression. Furthermore, the robust association observed in esophageal cancer—a malignancy with rapid progression and shorter survival—contrasts with weaker effects in gastric, hepatic, and colorectal cancers. This is consistent with large cohort studies in Europe (46, 47). For malignancies with prolonged survival periods, such as gastric, hepatic, and colorectal cancers, patients may survive for years post-diagnosis, with eventual mortality often attributed to competing causes (e.g., cardiovascular events or infectious complications). When mortality alone is utilized as an endpoint, this approach may fail to capture cases where cancer contributes indirectly to death, thereby leading to a potential underestimation of the association between air pollutant exposure and carcinogenesis (23, 48).
This study pioneers the investigation of short-term air pollution effects on GI cancer mortality in a coastal area characterized by moderate pollution. Utilizing a robust methodology (case-crossover design combined with lagged modeling), it effectively controlled for individual confounders while capturing acute exposure risks. Furthermore, stratified analyses revealed higher susceptibility in the the older males and identified site-specific responses—for example, esophageal cancer was strongly linked to particulate matter, whereas colorectal cancer exhibited sensitivity to ozone. Despite its innovation, exposure misclassification from fixed-site pollution data may lead to an underestimation of individual risks. Additionally, unmeasured confounders (e.g., smoking, diet) and unclear biological mechanisms limit causal interpretation. Moreover, the findings may lack generalizability to high-pollution regions or other cancer types, emphasizing the need for broader validation.
5 Conclusion
This study demonstrates that short-term exposure to PM2.5, PM10, and O3 significantly increases the mortality risk of GI cancer patients in a moderately polluted coastal area. We also identified a higher susceptibility in males and the vulnerability of the older population, consistent with sex-specific inflammatory responses and age-related detoxification decline. The differences in anatomical timing—acute effects of PM on upper gastrointestinal cancers and delayed effects of O3 on lower gastrointestinal malignancies—reflect the observed patterns of pollutant deposition. These results necessitate urgent updates to air quality standards, emphasizing short-term exposure controls and targeted protections for susceptible populations to improve the survival of GI cancer patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
This study was performed according to the Declaration of Helsinki, 1964 convention. The research protocol was approved by the ethics committee or review committee of Xuzhou Medical University.
Author contributions
ZW: Methodology, Writing – original draft, Software, Visualization, Formal analysis, Data curation. CW: Methodology, Writing – review & editing, Visualization, Formal analysis, Validation, Resources. JS: Formal analysis, Validation, Methodology, Resources, Conceptualization, Writing – review & editing. BQ: Writing – review & editing, Methodology, Formal analysis, Data curation, Validation. WK: Writing – review & editing, Resources, Validation, Methodology, Data curation, Formal analysis. YY: Validation, Methodology, Data curation, Formal analysis, Writing – review & editing. YH: Writing – review & editing, Formal analysis, Data curation, Validation, Methodology. CL: Methodology, Writing – review & editing, Validation, Data curation, Formal analysis. LW: Methodology, Validation, Data curation, Writing – review & editing, Formal analysis. FL: Writing – review & editing, Conceptualization, Resources. XW: Visualization, Formal analysis, Methodology, Investigation, Writing – review & editing, Writing – original draft, Funding acquisition, Supervision, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Science and Technology project of Xuzhou Health Commission [grant number: XWKYHT-20230059 (to XW)], Talent introduction research start-up funds [grant number: 2024203010 (to XW)], Project of the Affiliated Hospital of Xuzhou Medical University [grant number: 2023ZY10 (to XW)].
Acknowledgments
I am indebted to my tutors and team members for their efforts and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1666928/full#supplementary-material
Abbreviations
GI, Gastrointestinal; PM2.5, Particulate Matter ≤2.5 μm; PM10, Particulate Matter ≤10 μm; SO₂, Sulfur Dioxide; NO₂, Nitrogen Dioxide; O₃, Ozone; DLNM, Distributed Lag Nonlinear Model; RR, Relative Risk; CI, Confidence Interval; df, Degrees of Freedom.
Footnotes
References
1. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Han, B, Zheng, R, Zeng, H, Wang, S, Sun, K, Chen, R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Center. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
3. Manisalidis, I, Stavropoulou, E, Stavropoulos, A, and Bezirtzoglou, E. Environmental and health impacts of air pollution: a review. Front Public Health. (2020) 8:14. doi: 10.3389/fpubh.2020.00014
4. Loomis, D, Grosse, Y, Lauby-Secretan, B, El Ghissassi, F, Bouvard, V, Benbrahim-Tallaa, L, et al. The carcinogenicity of outdoor air pollution. Lancet Oncol. (2013) 14:1262–3. doi: 10.1016/s1470-2045(13)70487-x
5. Liu, S, Li, X, Wei, J, Shu, L, Jin, J, Fu, TM, et al. Short-term exposure to fine particulate matter and ozone: source impacts and attributable mortalities. Environ Sci Technol. (2024) 58:11256–67. doi: 10.1021/acs.est.4c00339
6. Khoshakhlagh, AH, Mohammadzadeh, M, Gruszecka-Kosowska, A, and Oikonomou, E. Burden of cardiovascular disease attributed to air pollution: a systematic review. Glob Health. (2024) 20:37. doi: 10.1186/s12992-024-01040-0
7. Ma, C, Jung, CR, Nakayama, SF, Tabuchi, T, Nishihama, Y, Kudo, H, et al. Short-term association of air pollution with lung cancer mortality in Osaka, Japan. Environ Res. (2023) 224:115503. doi: 10.1016/j.envres.2023.115503
8. Gouveia, N, Rodriguez-Hernandez, JL, Kephart, JL, Ortigoza, A, Betancourt, RM, Sangrador, JLT, et al. Short-term associations between fine particulate air pollution and cardiovascular and respiratory mortality in 337 cities in Latin America. Sci Total Environ. (2024) 920:171073. doi: 10.1016/j.scitotenv.2024.171073
9. Yu, P, Xu, R, Li, S, Coelho, M, Saldiva, PHN, Sim, MR, et al. Associations between long-term exposure to PM(2.5) and site-specific cancer mortality: a nationwide study in Brazil between 2010 and 2018. Environ Pollut. (2022) 302:119070. doi: 10.1016/j.envpol.2022.119070
10. Stafoggia, M, Oftedal, B, Chen, J, Rodopoulou, S, Renzi, M, Atkinson, RW, et al. Long-term exposure to low ambient air pollution concentrations and mortality among 28 million people: results from seven large European cohorts within the ELAPSE project. Lancet Planetary Health. (2022) 6:e9–e18. doi: 10.1016/S2542-5196(21)00277-1
11. Pritchett, N, Spangler, EC, Gray, GM, Livinski, AA, Sampson, JN, Dawsey, SM, et al. Exposure to outdoor particulate matter air pollution and risk of gastrointestinal cancers in adults: a systematic review and Meta-analysis of epidemiologic evidence. Environ Health Perspect. (2022) 130:36001. doi: 10.1289/EHP9620
12. Chen, R, Yin, P, Meng, X, Liu, C, Wang, L, Xu, X, et al. Fine particulate air pollution and daily mortality. A Nationwide analysis in 272 Chinese cities. Am J Respir Crit Care Med. (2017) 196:73–81. doi: 10.1164/rccm.201609-1862OC
13. Tian, Y, Liu, H, Liang, T, Xiang, X, Li, M, Juan, J, et al. Fine particulate air pollution and adult hospital admissions in 200 Chinese cities: a time-series analysis. Int J Epidemiol. (2019) 48:1142–51. doi: 10.1093/ije/dyz106
14. He, C, Liu, C, Chen, R, Meng, X, Wang, W, Ji, J, et al. Fine particulate matter air pollution and under-5 children mortality in China: a national time-stratified case-crossover study. Environ Int. (2022) 159:107022. doi: 10.1016/j.envint.2021.107022
15. Wang, Y, Wu, Y, Zheng, F, Zhang, T, Wang, M, Huang, L, et al. Health threat of PM(2.5)-bound trace elements exposure on asthma hospital admission: a time-stratified case-crossover study. Environ Int. (2022) 170:107604. doi: 10.1016/j.envint.2022.107604
16. Armstrong, BG, Gasparrini, A, and Tobias, A. Conditional Poisson models: a flexible alternative to conditional logistic case cross-over analysis. BMC Med Res Methodol. (2014) 14:122. doi: 10.1186/1471-2288-14-122
17. Tobias, A, Kim, Y, and Madaniyazi, L. Time-stratified case-crossover studies for aggregated data in environmental epidemiology: a tutorial. Int J Epidemiol. (2024) 53:20. doi: 10.1093/ije/dyae020
18. Liu, C, Chen, R, Sera, F, Vicedo-Cabrera, AM, Guo, Y, Tong, S, et al. Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med. (2019) 381:705–15. doi: 10.1056/NEJMoa1817364
19. Sun, S, Cao, W, Pun, VC, Qiu, H, Ge, Y, and Tian, L. Respirable particulate constituents and risk of cause-specific mortality in the Hong Kong population. Environ Sci Technol. (2019) 53:9810–7. doi: 10.1021/acs.est.9b01635
20. Dong, J, You, J, Wang, J, and Bao, H. Association between short-term ambient air pollution and outpatient visits for acute exacerbation of chronic obstructive pulmonary disease in Lanzhou, 2013-19. Environ Geochem Health. (2023) 45:2495–509. doi: 10.1007/s10653-022-01363-0
21. Yan, R, Ying, S, Jiang, Y, Duan, Y, Chen, R, Kan, H, et al. Associations between ultrafine particle pollution and daily outpatient visits for respiratory diseases in Shanghai, China: a time-series analysis. Environ Sci Pollut Res Int. (2024) 31:3004–13. doi: 10.1007/s11356-023-31248-3
22. Yang, CY. Association between long-term exposure to fine particulate air pollution and risk of death attributed to esophageal cancer in Taiwan. J Toxicol Environ Health A. (2025) 88:34–42. doi: 10.1080/15287394.2024.2415318
23. Guo, C, Chan, TC, Teng, YC, Lin, C, Bo, Y, Chang, LY, et al. Long-term exposure to ambient fine particles and gastrointestinal cancer mortality in Taiwan: a cohort study. Environ Int. (2020) 138:105640. doi: 10.1016/j.envint.2020.105640
24. Li, Y, He, Z, Wei, J, Xu, R, Liu, T, Zhong, Z, et al. Long-term exposure to ambient fine particulate matter constituents and mortality from total and site-specific gastrointestinal cancer. Environ Res. (2024) 244:117927. doi: 10.1016/j.envres.2023.117927
25. Wong, CM, Tsang, H, Lai, HK, Thomas, GN, Lam, KB, Chan, KP, et al. Cancer mortality risks from long-term exposure to ambient fine particle. Cancer Epidemiol. (2016) 25:839–45. doi: 10.1158/1055-9965.EPI-15-0626
26. Xu, X, Zhang, L, An, Y, Han, H, Chen, R, Zhang, M, et al. The association between ambient air pollution and colorectal cancer: a Mendelian randomization study. Int J Environ Health Res. (2025) 35:495–505. doi: 10.1080/09603123.2024.2361453
27. Yu, P, Xu, R, Wu, Y, Huang, W, Coelho, M, Saldiva, PHN, et al. Cancer mortality risk from short-term PM(2.5) exposure and temporal variations in Brazil. J Hazard Mater. (2024) 473:134606. doi: 10.1016/j.jhazmat.2024.134606
28. Ethan, CJ, Mokoena, KK, Yu, Y, Shale, K, Fan, Y, Rong, J, et al. Association between PM(2.5) and mortality of stomach and colorectal cancer in Xi'an: a time-series study. Environ Sci Pollut Res Int. (2020) 27:22353–63. doi: 10.1007/s11356-020-08628-0
29. Zhao, H, Zheng, X, Lin, G, Wang, X, Lu, H, Xie, P, et al. Effects of air pollution on the development and progression of digestive diseases: an umbrella review of systematic reviews and meta-analyses. BMC Public Health. (2025) 25:183. doi: 10.1186/s12889-024-21257-3
30. Yu, P, Xu, R, Huang, W, Yang, Z, Coelho, M, Saldiva, PHN, et al. Short-term ozone exposure and cancer mortality in Brazil: a nationwide case-crossover study. Int J Cancer. (2024) 155:1731–40. doi: 10.1002/ijc.35069
31. Li, M, Dong, H, Wang, B, Zhao, W, Zare Sakhvidi, MJ, Li, L, et al. Association between ambient ozone pollution and mortality from a spectrum of causes in Guangzhou, China. Sci Total Environ. (2021) 754:142110. doi: 10.1016/j.scitotenv.2020.142110
32. Xue, X, Chen, J, Sun, B, Zhou, B, and Li, X. Temporal trends in respiratory mortality and short-term effects of air pollutants in Shenyang, China. Environ Sci Pollut Res Int. (2018) 25:11468–79. doi: 10.1007/s11356-018-1270-5
33. Wang, N, Mengersen, K, Tong, S, Kimlin, M, Zhou, M, Wang, L, et al. Short-term association between ambient air pollution and lung cancer mortality. Environ Res. (2019) 179:108748. doi: 10.1016/j.envres.2019.108748
34. Chen, J, Dan, L, Sun, Y, Yuan, S, Liu, W, Chen, X, et al. Ambient air pollution and risk of Enterotomy, gastrointestinal Cancer, and all-cause mortality among 4,708 individuals with inflammatory bowel disease: a prospective cohort study. Environ Health Perspect. (2023) 131:77010. doi: 10.1289/EHP12215
35. Beamish, LA, Osornio-Vargas, AR, and Wine, E. Air pollution: An environmental factor contributing to intestinal disease. J Crohns Colitis. (2011) 5:279–86. doi: 10.1016/j.crohns.2011.02.017
36. Mutlu, EA, Comba, IY, Cho, T, Engen, PA, Yazıcı, C, Soberanes, S, et al. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ Pollut. (2018) 240:817–30. doi: 10.1016/j.envpol.2018.04.130
37. Bustamante-Marin, XM, and Ostrowski, LE. Cilia and Mucociliary clearance. Cold Spring Harb Perspect Biol. (2017) 9:a028241. doi: 10.1101/cshperspect.a028241
38. Munkholm, M, and Mortensen, J. Mucociliary clearance: pathophysiological aspects. Clin Physiol Funct Imaging. (2014) 34:171–7. doi: 10.1111/cpf.12085
39. Möller, W, Häussinger, K, Winkler-Heil, R, Stahlhofen, W, Meyer, T, Hofmann, W, et al. Mucociliary and long-term particle clearance in the airways of healthy nonsmoker subjects. J Appl Physi. (2004) 97:2200–6. doi: 10.1152/japplphysiol.00970.2003
40. Feng, S, Gao, D, Liao, F, Zhou, F, and Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol Environ Saf. (2016) 128:67–74. doi: 10.1016/j.ecoenv.2016.01.030
41. Bosch, AJT, Rohm, TV, AlAsfoor, S, Low, AJY, Keller, L, Baumann, Z, et al. Lung versus gut exposure to air pollution particles differentially affect metabolic health in mice. Part Fibre Toxicol. (2023) 20:7. doi: 10.1186/s12989-023-00518-w
42. Niu, Y, Li, H, Wang, W, Wang, C, Liu, C, Du, X, et al. Ozone exposure and prothrombosis: mechanistic insights from a randomized controlled exposure trial. J Hazard Mater. (2022) 429:128322. doi: 10.1016/j.jhazmat.2022.128322
43. Vinikoor-Imler, LC, Owens, EO, Nichols, JL, Ross, M, Brown, JS, and Sacks, JD. Evaluating potential response-modifying factors for associations between ozone and health outcomes: a weight-of-evidence approach. Environ Health Perspect. (2014) 122:1166–76. doi: 10.1289/ehp.1307541
44. Espín-Pérez, A, Krauskopf, J, Chadeau-Hyam, M, van Veldhoven, K, Chung, F, Cullinan, P, et al. Short-term transcriptome and microRNAs responses to exposure to different air pollutants in two population studies. Environ Pollut. (2018) 242:182–90. doi: 10.1016/j.envpol.2018.06.051
45. Wang, M, Luo, X, Xu, S, Liu, W, Ding, F, Zhang, X, et al. Trends in smoking prevalence and implication for chronic diseases in China: serial national cross-sectional surveys from 2003 to 2013. Lancet Respir Med. (2019) 7:35–45. doi: 10.1016/S2213-2600(18)30432-6
46. Nagel, G, Chen, J, Jaensch, A, Skodda, L, Rodopoulou, S, Strak, M, et al. Long-term exposure to air pollution and incidence of gastric and the upper aerodigestive tract cancers in a pooled European cohort: the ELAPSE project. Int J Cancer. (2024) 154:1900–10. doi: 10.1002/ijc.34864
47. Weinmayr, G, Chen, J, Jaensch, A, Skodda, L, Rodopoulou, S, Strak, M, et al. Long-term exposure to several constituents and sources of PM(2.5) is associated with incidence of upper aerodigestive tract cancers but not gastric cancer: results from the large pooled European cohort of the ELAPSE project. Sci Total Environ. (2024) 912:168789. doi: 10.1016/j.scitotenv.2023.168789
Keywords: ambient particulate matter, gastrointestinal cancer, mortality risk, distributed lag nonlinear model, case-crossover study
Citation: Wu Z, Wei C, Sun J, Qiu B, Kong W, Yang Y, Huang Y, Li C, Wu L, Liu F and Wang X (2025) Acute air pollution exposure and gastrointestinal cancer mortality: a case-crossover study in coastal China. Front. Public Health. 13:1666928. doi: 10.3389/fpubh.2025.1666928
Edited by:
Arthit Phosri, Mahidol University, ThailandReviewed by:
Watcharin Joemsittiprasert, New York Institution for Continuing Education, United StatesXingshun Qi, General Hospital of Northern Theater Command, China
Copyright © 2025 Wu, Wei, Sun, Qiu, Kong, Yang, Huang, Li, Wu, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fudong Liu, MjUwMDkwNzgyQHFxLmNvbQ==; Xiaojie Wang, eGlhb2ppZXdhbmc4ODZAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Zhuchao Wu
Zhuchao Wu Chaohua Wei2†
Chaohua Wei2† Beibei Qiu
Beibei Qiu Yanqiu Huang
Yanqiu Huang