- 1Huzhou Central Blood Station, Huzhou, China
- 2Huzhou College of Life and Health, Huzhou, China
Background: Blood is a critical yet scarce medical resource, and improving the efficiency with which it is utilised remains a major global challenge. In 2019, China introduced Quality Control Indicators for Clinical Blood Use in an attempt to standardise management. However, significant discrepancies remain between the intended policy and its practical implementation, resulting in inefficiencies and safety concerns.
Objective: This study aims to quantitatively evaluate the impact of administrative policies on clinical blood use, identify the main factors affecting the efficiency with which blood is utilised, and analyse how hospital level and type influence transfusion practices.
Methods: A retrospective, multicentre study was conducted using data from 24 secondary and tertiary hospitals in Huzhou between 2020 and 2024. Key quality control indicators and a 25-point transfusion record scoring system were employed. Trends were analysed using ANOVA and chi-square tests, and hospital stratification was analysed using MANOVA. Predictors of blood use per discharged patient were identified using multiple linear regression and linear mixed-effects models.
Results: Over 5 years, the number of transfusion technicians increased by 72%, transfusion record scores improved by 34.6%, and per capita blood use decreased by 46.9%. However, blood use in low-complexity surgeries increased by an abnormal 200%. Tertiary hospitals showed higher blood use but better documentation than secondary hospitals. Regression analysis revealed that technician density (β = −0.280) and transfusion record score (β = −0.202) were negatively associated with blood use, whereas surgical complexity was positively associated with it. Hospital grade and type also significantly influenced outcomes.
Conclusion: Efficiency in blood utilisation is more strongly influenced by process standardisation and human resources than by hospital level or type alone. Rather than rigid indicators, policy incentives should emphasise precision transfusion strategies and dynamic quality management to align resource use with clinical need and support sustainable blood management systems.
Introduction
As a non-renewable strategic reserve for saving lives, blood resources are increasingly becoming a serious challenge to the global public health system in terms of supply and demand tension and utilisation efficiency. To address this challenge, China promulgated the Indicators for Quality Control (QC) of Clinical Blood Use in 2019, aiming to improve the effectiveness of blood resource utilisation through standardised management. However, the policy has encountered significant resistance at the grassroots level—insufficient compliance and uneven dissemination of key technologies have led to a worrying “disconnect” between the policy vision and clinical practice. This disconnect is not only reflected at the level of specific operation (1–4), but also reflects systemic issues such as resource allocation, capacity building, and incentives at a deeper level. The serious consequences cannot be ignored: inefficient blood utilisation, increased potential safety risks of blood transfusion, and waste of valuable healthcare resources may ultimately affect patient outcomes and increase the burden on the system. As shown by Owusu-Ofori et al. (1) and studies in countries such as India (2), inconsistent guidelines, fragmented delivery systems, and lack of knowledge and training of healthcare professionals on current policies (3, 4) are common factors contributing to the policy-practice divide globally. At the same time, the rapid evolution of healthcare needs has placed greater demands on the timeliness and adaptability of policies (5, 6). Therefore, a central scientific question needs to be answered: how to effectively quantify and deconstruct the key drivers and mechanisms affecting the effectiveness of clinical blood use policy implementation, so as to bridge the gap between policy regulation and clinical practice?
To explore this issue in depth, this study focuses on the core QC indicator of ‘per capita blood use of discharged patients’. We systematically integrated the clinical blood use data of 24 secondary and above hospitals in the city from 2020 to 2024 with the QC centre’s supervisory and assessment records, constructing a “policy-implementation-impact” linkage assessment model through long-term, multi-centre retrospective analysis. This study aims to: (1) quantitatively monitor the actual impact of administrative regulatory measures on the implementation of clinical blood use specifications, and (2) provide an in-depth analysis of the underlying mechanisms that lead to changes in clinical blood use practices. The results of this study will provide solid quantitative evidence to inform the scientific evaluation of regulatory effectiveness, the precise identification of intervention targets and the optimisation of blood safety management strategies. Ultimately, this will serve the major goal of enhancing the efficiency of blood resource utilisation, ensuring patient safety and promoting the sustainable development of the national blood protection system.
Research methods
Data collection
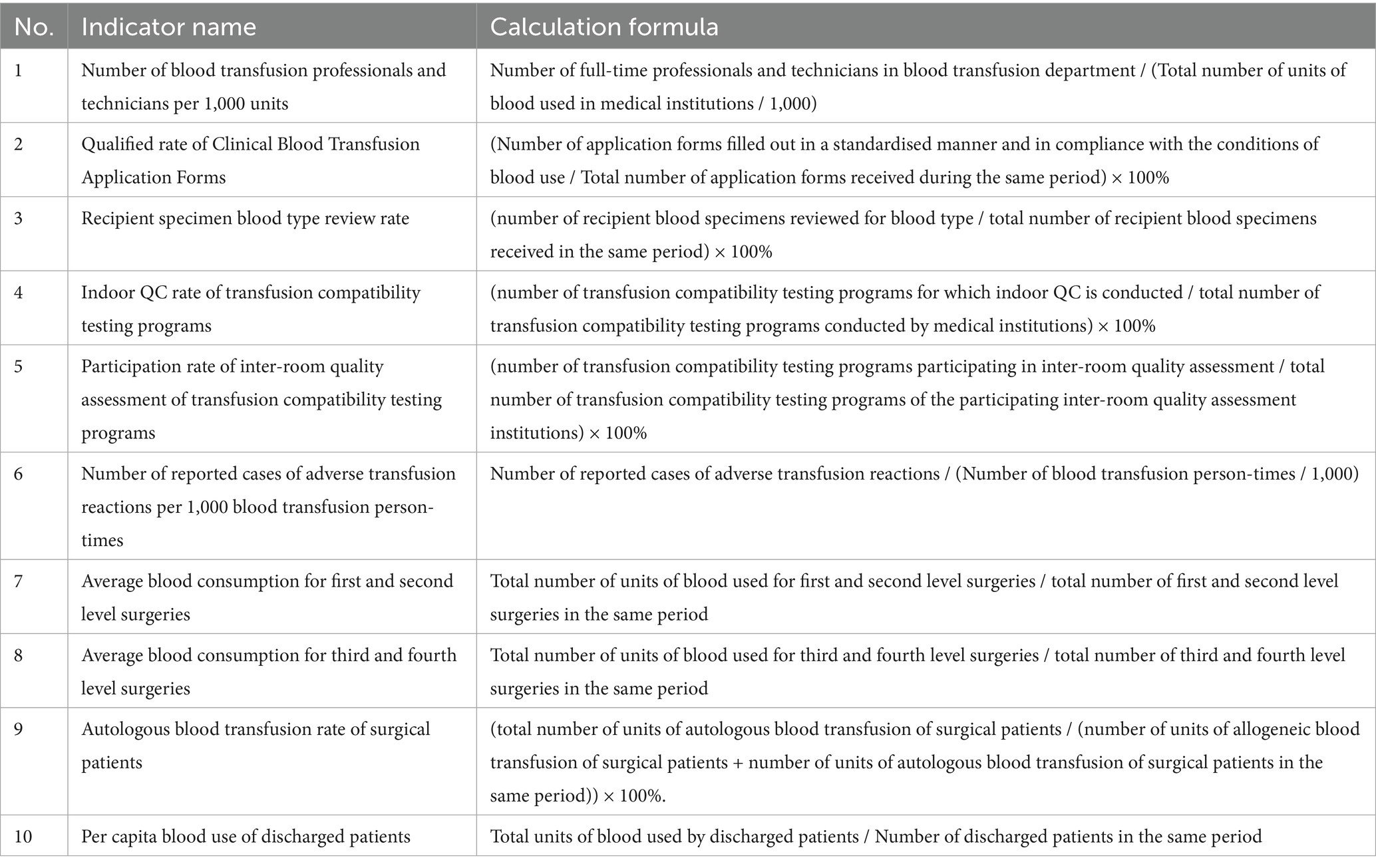
The annual QC reports of all secondary and above hospitals (n = 24) in Huzhou City from 2020 to 2024 were extracted. The QC indexes and calculation formulas are shown in Table 1. The statistical values of the three indexes, “blood type review rate of blood recipient specimens,” “indoor QC rate of blood transfusion compatibility testing program” and “participation rate of inter-room quality assessment program of blood transfusion compatibility testing,” are at or close to 100% for each clinical blood hospital. As these three indicators reached or were close to 100% in each clinical blood hospital, they were excluded from the statistical analysis.
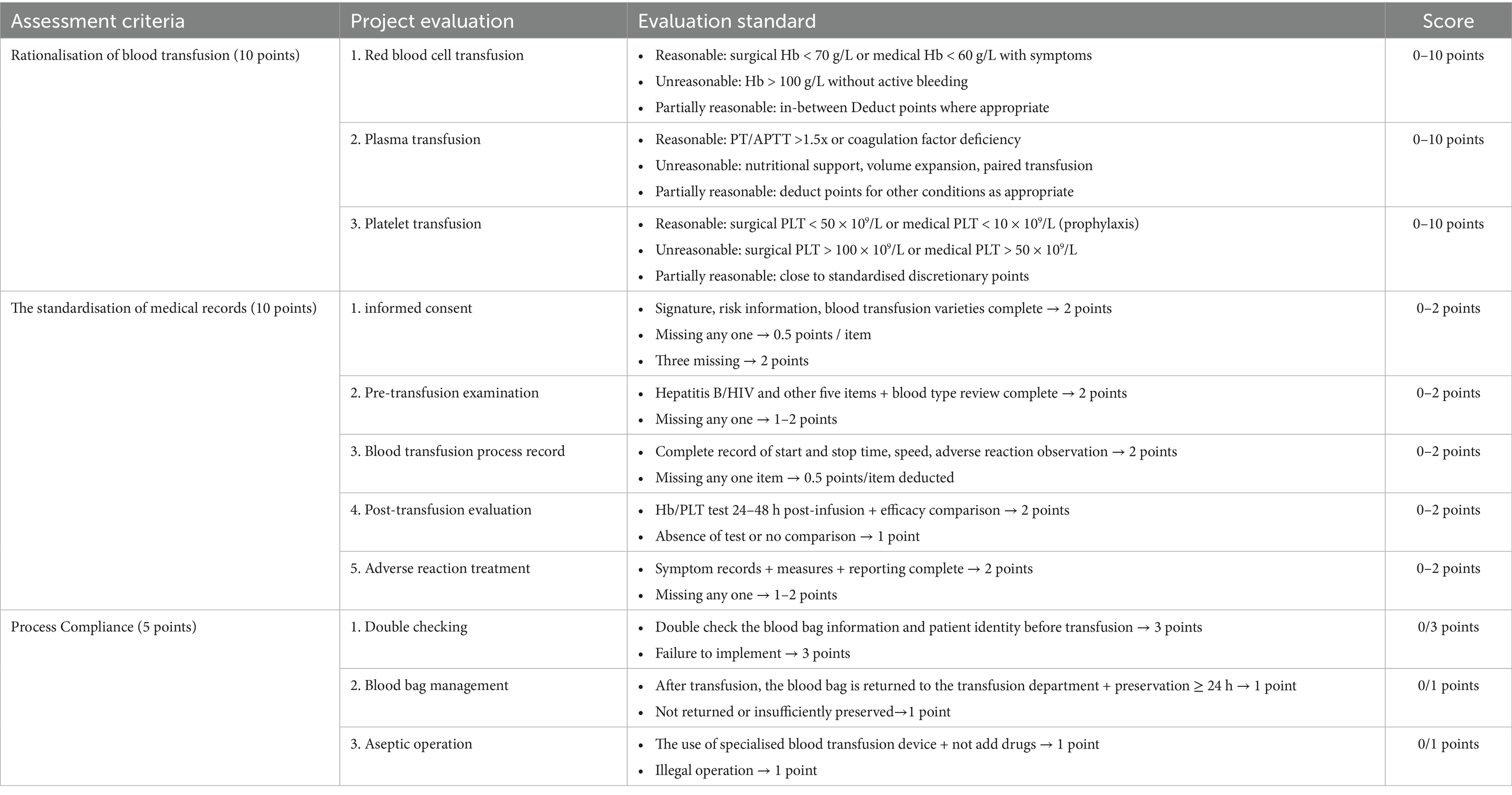
Scoring of blood transfusion medical records, 25-point scale (Table 2) was designed based on the Clinical Blood Transfusion Specifications and a double-blind evaluation by an expert group from the QC Centre (intra-group correlation coefficient ICC = 0.85). The annual score for each hospital was calculated as the mean value of six medical records.
Trend analysis
The statistics cover blood transfusion medical record scores and the remaining seven QC indicators of clinical blood use in hospitals above level two in Huzhou City from 2019 to 2024. These include the number of blood transfusion professionals and technicians per 1,000 units of blood used and the qualification rate of clinical blood transfusion application forms., the number of adverse transfusion reaction cases reported per 1,000 transfusions, the average amount of blood used per first- and second-degree surgery, the average amount of blood used per third- and fourth-degree surgery, the autologous transfusion rate for surgical patients, and the average amount of blood used per patient discharged from hospital. We analysed the trend of each data set over the past 5 years.
Stratified comparison
The 24 hospitals were classified according to hospital class and type as tertiary (11), secondary (13), general (15) and specialised (9) hospitals. The differences in QC index data and transfusion chart scores between the different classes and types of hospitals over a five-year period were analysed using multivariate analysis of variance (ANOVA).
Multiple linear regression modelling
The dependent variable was “blood use per discharged patient” (Box-Cox transformed to satisfy normality), and the remaining six QC indicators were the independent variables. The control variables were hospital grade (tertiary = “0,” secondary = “1”), hospital type (specialty hospital = “0,” general hospital = “1”), and transfusion medical record score, which were inflated by variance. Multiple linear regression equations were constructed using stepwise regression (entry criteria p < 0.05, exclusion p > 0.10) and the variance inflation factor (VIF) test was used to remove the covariance (threshold VIF < 5). Residual independence was tested using the Durbin-Watson test (1.5 < DW < 2.5).
Linear mixed effects model analysis
A linear mixed-effects model was used to account for clustering effects at hospital level, and a compound symmetric (CS) covariance structure was employed to model temporal correlations in data from different years within the same hospital.
Statistical analysis
Categorical variables were compared using the chi-squared test (with Yates’s correction for expected frequencies of less than 5). Rates were compared using the chi-squared test. Continuous variables were compared using the Shapiro–Wilk normality test, followed by the independent samples t-test, the variance test (chi-squared test) or the Mann–Whitney U-test (for non-normal distributions). Model diagnosis was performed using residual normality (Q–Q plots) and heteroscedasticity (Breusch–Pagan test). Statistical software: SPSS 27.0 (statistical analysis, linear regression and model diagnosis) and GraphPad Prism 10.4 (trend visualisation).
Results
Trends in QC indicators and “transfusion history score”
A one-way analysis of variance (ANOVA) and a Pearson’s chi-square test revealed a significant upward trend in the number of specialised blood transfusion technicians per 1,000 units of blood used from 2020 to 2024 (1.14 → 1.96, an increase of 72%), peaking in 2024 (M = 1.96, 95% CI [1.93, 2.00]). One-way ANOVA also revealed a significant year effect (F(4, 469,098) = 862.54, p < 0.001). Transfusion requisition pass rates continued to improve (2020: 92.0% → 2024: 98.4%), and a chi-square test confirmed a significant year effect (χ2 = 3333.06, p < 0.001). The reporting rate of adverse transfusion reactions increased continuously (2020: 0.72% → 2024: 1.15%), rising by 59.7%, and was significantly higher from 2023 onwards (2023: 1.01, 95% CI [1.00, 1.02] to 2024: 1.15, 95% CI [1.14, 1.16]). A chi-squared test confirmed that the reporting rates in different years were qualitatively different (χ2 = 83.378, p < 0.001). There was a substantial increase in perioperative autotransfusion rates (2020: 28.8% → 2024: 44.6%), peaking at 45.5% in 2023 (95% CI [45.27, 45.73]). There was a significant increase between 2022 and 2023 (38.1% → 45.5%), followed by a small decrease in 2024 which was significant according to the chi-square test (χ2 = 849.60, p < 0.001). Regarding the efficiency of surgical blood use, the average blood usage per procedure in first- and second-level surgeries fluctuated (2020: 0.017 U → 2022: 0.010 U → 2024: 0.030 U), with a sudden increase in usage in 2023–2024 (0.021 U → 0.030 U). This was contrary to the overall optimisation trend, as shown by the analysis of variance (F(4, 580,880) = 13,630; 0.20, p < 0.001). The average blood usage per case for tertiary and quaternary surgeries continued to decrease significantly (2020, 0.311 U → 2024: 0.133 U, a 57.2% decrease), as revealed by a significant ANOVA (F(4, 254,818) = 425.73, p < 0.001). A significant trend was also observed over time in the ‘blood use per patient discharged and transfusion history score’, with F-values of 76,244.46 and 15.312, respectively (p < 0.001). The mean value of blood use per patient discharged decreased gradually from 0.123 units (95% CI, 0.122–0.123) in 2020 to 0.065 units (95% CI, 0.065–0.065) in 2024, representing a 46.9% decrease. Conversely, the mean transfusion medical record score increased from 14.67 (95% CI, 13.30–16.03) in 2020 to 19.75 (95% CI, 18.81–20.69) in 2024, indicating an improvement in the standardisation of transfusion practice in clinical blood hospitals. See Figures 1A–H.

Figure 1. Trends in clinical blood QC indicators (A: Number of blood transfusion professionals and technicians per 1,000 units; B: Qualified rate of Clinical Blood Transfusion Application Forms; C: Number of reported cases of adverse transfusion reactions per 1,000 blood transfusion persontimes; D: Average blood consumption for first and second level surgeries; E:Average blood consumption for third and fourth level surgeries; F: Autologous blood transfusion rate of surgical patients; G: Per capita blood use of discharged patients) and transfusion history scores, 2020–2024.
Stratified comparison
A multivariate analysis of variance (MANOVA) was conducted to examine the effects of hospital grade (Level III vs. Level II) and hospital type (general vs. specialised) on eight transfusion-related performance indicators. The analysis incorporated 120 annual reports from 24 hospitals over a five-year period. The reports were stratified by hospital level (tertiary hospitals: n = 55; secondary hospitals: n = 65) and type (general hospitals: n = 75; specialty hospitals: n = 45). Key transfusion-related indicators are presented in Table 3 as means with 95% confidence intervals (CIs) and standard deviations (SDs; Figure 2).
1. Level III general hospitals.
2. Level III specialised hospitals.
3. Level II general hospitals.
4. Level II specialised hospitals.
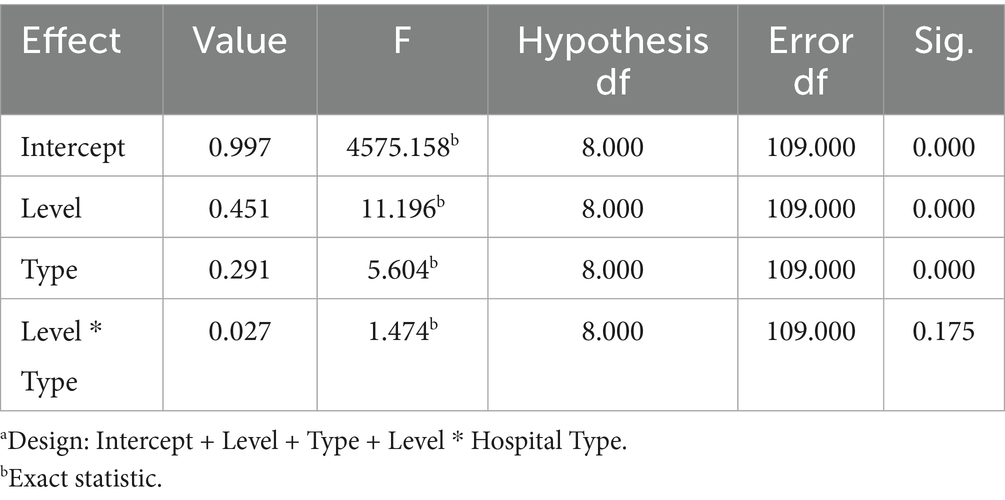
Table 3. Multivariate testsa.
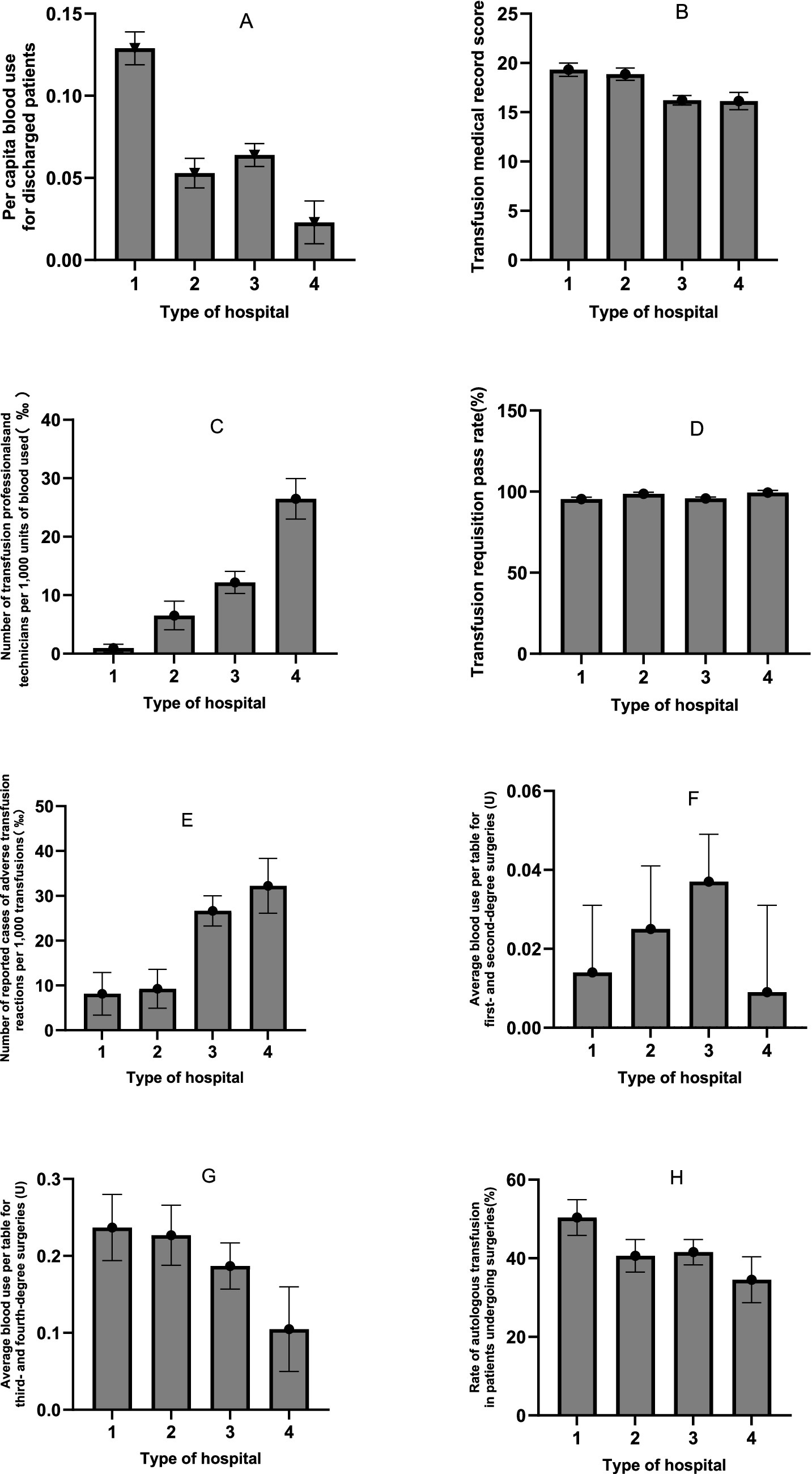
Figure 2. Means and standard deviations of indicators (A: Pre capita blood use for discharged patients; B: Transfusion medical record score; C: Number of transfusion professionals and technicians per 1,000 units of biood used(‰); D: Transfusion requisition pass rate; E: Number of reported cases of adverse transfusion reactions per 1,000 transfusions; F: Average blood use per table for first- and second-degree surgeries(U); G: Average blood use per table for third- and fourth-degree surgeries(U); H: Rate of autologous transfusion in patients undergoing surgeries) for different hospital grades and types.
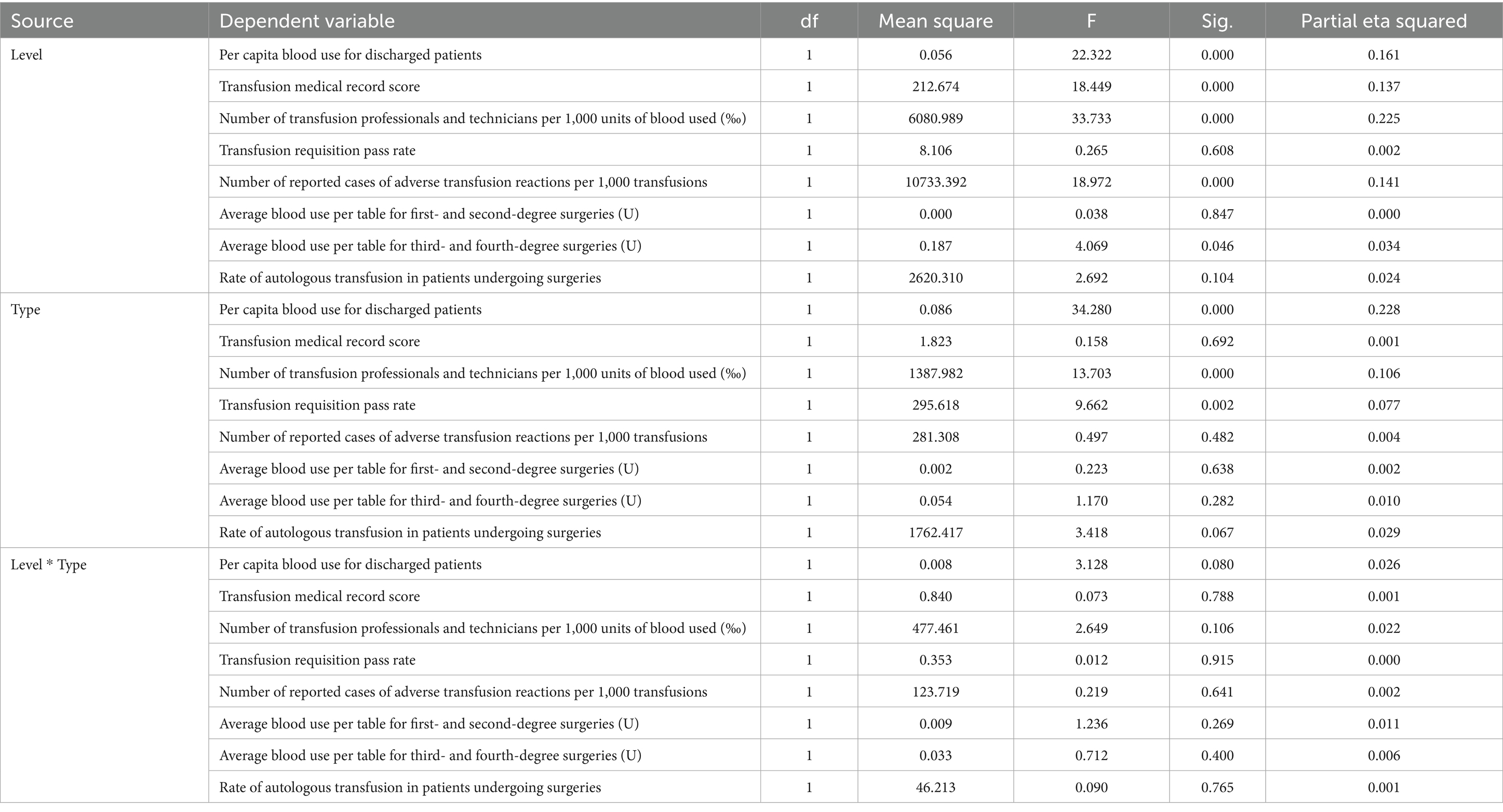
Box’s M test (M = 739.426, F = 5.759, p < 0.001) permitted subsequent analyses to proceed, given that MANOVA is robust to chi-squaredness of the covariance matrix (especially when sample sizes between groups are similar). Multiple effects tests (Pillai’s trace prevailed) were significant for the main effects of hospital class (Pillai’s trace = 0.451, F = 11.196, p < 0.001, partial η2 = 0.451) and hospital type (Pillai’s trace = 0.291, F = 4.86, p < 0.001). The hospital class × hospital type interaction effect was not significant (Pillai’s Trace = 0.098, F = 1.474, p = 0.175; see Table 3).
The between-subjects effect test for tertiary vs. secondary hospitals showed the following results:
Main effect of hospital grade
The results revealed a significant main effect of hospital grade on multiple indicators (Pillai’s trace; p < 0.05). Specifically:
• Per capita blood use for discharged patients was significantly higher in Level III hospitals than in Level II hospitals (F(1, 119) = 22.322, p < 0.001, partial η2 = 0.161).
• Transfusion medical record scores were significantly higher in Level III hospitals (F(1, 119) = 18.449, p < 0 0.001, partial η2 = 0.137).
• There was a significant difference in the number of transfusion professionals and technicians per 1,000 units of blood between grades (F(1, 119) = 33.733, p < 0 0.001, partial η2 = 0.225), with a higher mean count in Level II hospitals.
• The number of reported adverse transfusion reactions per 1,000 transfusions was also significantly higher in Level II hospitals (F(1, 119) = 18.972, p < 0 0.001, partial η2 = 0.141).
• The average amount of blood used per case for third- and fourth-degree surgeries was also significantly influenced by grade (F(1, 119) = 4.069, p = 0 0.046, partial η2 = 0.034), with level III hospitals using more blood.
• However, no significant main effect of grade was found for the transfusion requisition pass rate (p = 0 0.608), blood use in minor surgeries (p = 0 0.847) or the autologous transfusion rate (p = 0.104).
Main effect of hospital type
A significant main effect of hospital type was also observed.
• Per capita blood use for discharged patients was significantly higher in general hospitals than in specialised hospitals (F(1, 119) = 34.280, p < 0.001, partial η2 = 0.228).
• There was a significant difference in the number of transfusion professionals and technicians per 1,000 units of blood between types (F(1, 119) = 13.703, p < 0.001, partial η2 = 0.106), with specialised hospitals showing a higher mean count.
• The transfusion requisition pass rate was also significantly higher in specialised hospitals (F(1, 119) = 9.662, p = 0.002, partial η2 = 0.077).
• However, hospital type did not have a significant effect on transfusion record scores (p = 0 0.692), adverse reaction reports (p = 0.482), blood use for any surgical grade (p > 0.05) or the autologous transfusion rate (p = 0.067).
Interaction effect (grade × type)
The interaction effect between hospital grade and type was not statistically significant for any of the eight dependent variables (all p > 0.05). This indicates that the effect of hospital grade on these indicators is consistent across different hospital types, and vice versa.
Effect size
The effect sizes (partial eta squared) for the significant findings ranged from small to medium (0.034 to 0.228). The largest effect was observed for the impact of hospital type on per capita blood use (partial eta squared = 0.228), as shown in Table 4.
a. R Squared = 0.310 (Adjusted R Squared = 0.293)
b. R Squared = 0.157 (Adjusted R Squared = 0.135)
c. R Squared = 0.243 (Adjusted R Squared = 0.224)
d. R Squared = 0.074 (Adjusted R Squared = 0 0.050)
e. R Squared = 0.147 (Adjusted R Squared = 0.125)
f. R Squared = 0.015 (Adjusted R Squared = −0.010)
g. R Squared = 0.036 (Adjusted R Squared = 0.011)
h. R Squared = 0.050 (Adjusted R Squared = 0.026)
Multiple linear regression modelling
The model constructed using stepwise regression was statistically significant (F = 15.067, p < 0.001) and explained 39.2% of the variation in blood usage per patient discharged from hospital (adjusted R2 = 0.392). The model residuals were independent (Durbin-Watson statistic = 2.009) and there were no serious multicollinearity issues between the predictor variables (VIF < 2.23). Hospital type was the primary negative predictor (standardised β = −0.457), with a significant increase in per capita blood use of 0.056 units in general hospitals compared to specialty hospitals (95% CI: −0.077 to −0.035, t(114) = −5.261, p < 0.001). Technician staffing (standardised β = −0.295) and transfusion history score (standardised β = −0.212) synergistically suppressed blood use: an increase of 1 technician per 1,000 units of blood used decreased per capita blood use by 0.001 units (p = 0.001), and each 1-point improvement in the transfusion history score decreased blood use by 0.003 units (p = 0.021). Surgical complexity was also a significant factor, with per capita blood use rising by 0.018 units for every 1-unit increase in blood use for grades 3 and 4 surgeries (β = 0.226, p = 0.003), which supports the idea of a rigid demand for high-difficulty surgeries. Conversely, an increase in hospital grade (low grade to high grade) decreased blood use (β = −0.343, p = 0.002), suggesting that high-grade hospitals offset the effect of surgical complexity through effective management (Table 5). The standardised regression coefficients (β) and significance of each predictor variable are shown in Table 6. The final regression equation is as follows:
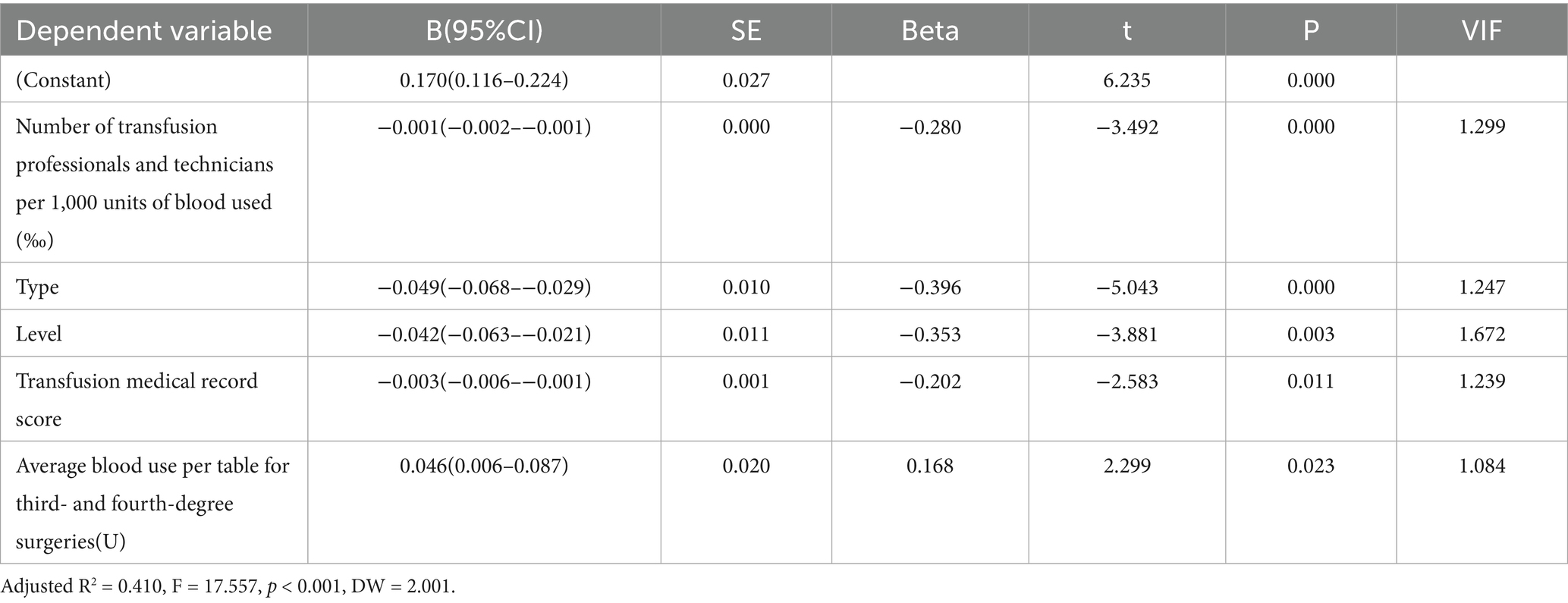
Table 5. Results of multiple linear regression analysis of blood use per patient discharged from the hospital (n = 120).
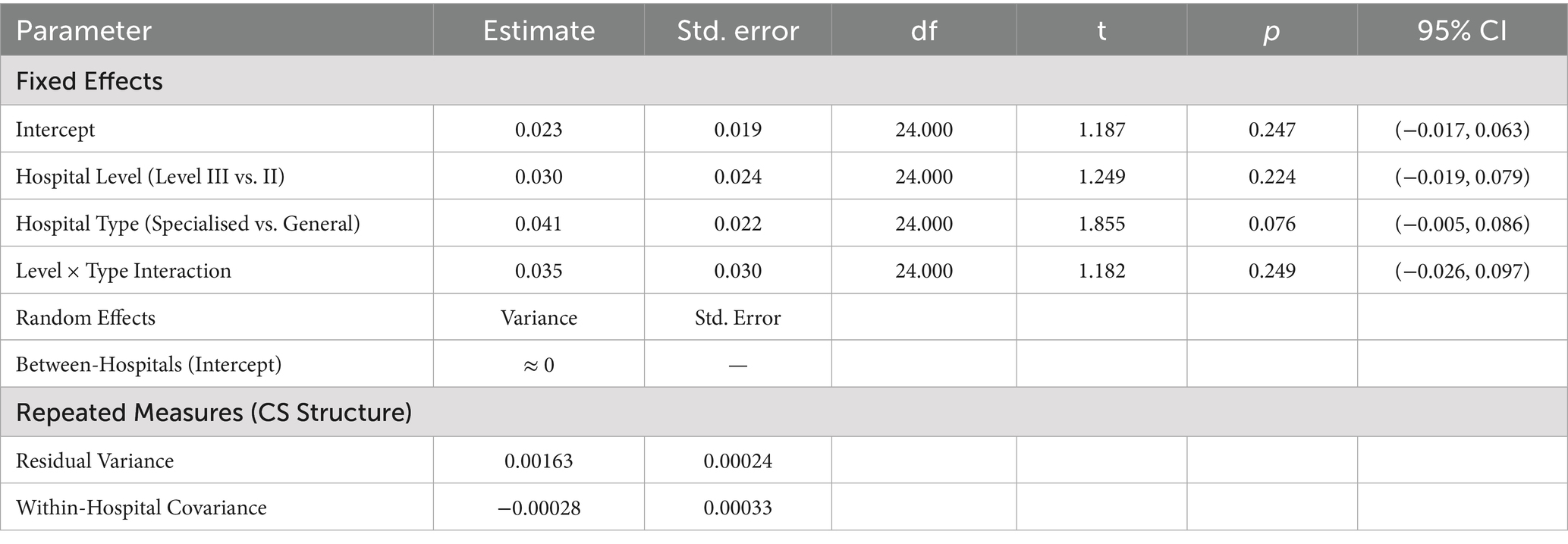
Table 6. Factors associated with per capita blood use for discharged patients: a linear mixed-effects model analysis.
Blood use per patient discharged from hospital = 0.170–0.001 × number of transfusion professionals and technicians per 1,000 units of blood used − 0.049 × hospital grade − 0.042 × hospital grade − 0.003 × transfusion chart score + 0.046 × average blood use per surgical table for three or four levels of surgery.
Linear mixed effects model analysis
The mixed-model results indicate that the variance estimate for the random intercept is close to zero and has been flagged as redundant (Variance ≈ 0), which suggests that there are no significant differences in baseline per capita blood usage among hospitals. The covariance parameters show that variability in the data is mainly due to fluctuations within hospitals over time (residual variance = 0.001625). Furthermore, none of the fixed effects (hospital grade, type, and their interaction) were significant (all p > 0.05). These findings align with those from the random effects analysis, confirming that macro-level hospital classification characteristics are ineffective predictors of per capita blood usage, as shown in Table 6.
Discussion
This study uses multi-indicator trend analysis to reveal the synergistic effects of implementing blood management policies within regional healthcare systems. The analysis indicates that, over a five-year period, the number of blood transfusion technicians increased significantly by 72.0%. This change exhibits a clear temporal correlation with improvements in transfusion record quality (as measured by transfusion history scores), which increased by 34.6%. This finding corroborates the view proposed by Naveen Bansal et al. that ‘professional staffing forms the foundation of transfusion safety’ (7–9). Furthermore, multivariate regression analysis indicates that technician density is a significant negative predictor of per capita blood consumption (β = −0.280, p < 0.001). This suggests that investments in human capital and process standardisation are more critical policy drivers than administrative oversight alone.
Concurrently, the volume of red blood cell transfusions per capita decreased by 46.9%, equivalent to approximately 123,000 unnecessary transfusions being avoided each year. The compliance rate for completed transfusion request forms increased to 98.4%, and the rate of adverse event reports rose by 59.7%, indicating strengthened pre-approval review and post-event feedback mechanisms.
However, an abnormal increase in blood usage was observed in Classes I and II surgeries (from 0.010 U in 2022 to 0.030 U in 2024, representing a 200% increase; F = 13,630.20, p < 0.001). However, multivariate analysis of variance revealed no significant differences in blood usage across hospital types/grades for these procedures (p > 0.05). This suggests a potential widespread misuse of transfusion indications in low-risk surgeries. Autologous transfusion rates notably peaked in 2023 at 45.5% (95% CI [45.27, 45.73]), which coincided with the rise in blood usage for Class I and II surgeries. However, this increase in autologous transfusion rates did not improve overall blood utilisation efficiency (linear regression p = 0.299). Further analysis revealed no significant differences in perioperative autologous transfusion rates among hospitals (F = 3.418 for level and F = 2.032 for type; p > 0.05), which contradicts the expectation that higher-level hospitals would have higher rates due to a greater proportion of complex surgeries.
The aforementioned anomalous results may be related to the design of the assessment mechanism. Although autologous blood transfusion can reduce the risks associated with allogeneic transfusion (26, 27), when the implementation rate is used as a rigid assessment indicator, it can encourage institutions to collect autologous blood for non-indicated purposes during low-risk surgeries. This results in the misallocation of resources (e.g., resources needed for high-risk surgeries being diverted) (10–13). In the regression model, medical record scores showed a negative correlation (B = −0.003), with tertiary hospitals scoring significantly higher than secondary hospitals. This suggests that lower-level hospitals could improve their blood management systems, reflecting the potential ‘double-edged sword’ effect of policy incentives on clinical practice (14–16).
Therefore, evidence-based autotransfusion guidelines referencing the AABB Perioperative Autologous Blood Collection and Transfusion Standard (17) are necessary. The assessment system should shift from evaluating implementation rates alone to evaluating both the implementation rate and the indication compliance rate, while promoting a ‘personalised threshold strategy’ (18). For example, autologous blood collection should be restricted to low-risk procedures (e.g., Grade I hernia repair) with a restrictive transfusion threshold (Hb < 7 g/dL). Conversely, for moderate-to-high-risk surgeries (e.g., radical tumour resection), multimodal blood management plans should be tailored based on factors such as preoperative anaemia status and surgical scope. This approach ensures precise alignment between resource allocation and clinical needs, thus optimising academic transfusion practices.
Tertiary general hospitals exhibited the typical coexistence of ‘high blood usage and high quality control’, demonstrating the highest per capita blood usage (M = 0.129 U) and optimal transfusion record scores (19.32 points). Multivariate linear regression analysis indicates that, although increased surgical complexity leads to higher blood demand, a higher hospital tier is significantly associated with reduced per capita blood usage (β = −0.353, p = 0.003). However, mixed-effects modelling revealed a more complex mechanism. As shown in Table 6, variations in blood usage were driven less by inherent attributes such as hospital grade or type and more by dynamic factors over time, such as the implementation of short-term clinical quality improvement initiatives. This suggests that future research on blood utilisation efficiency should focus on tracking and evaluating process-oriented management measures rather than merely comparing outcome metrics across different hospital categories.
Lasocki et al. (19) demonstrated that implementing standardised PBM significantly reduced red blood cell transfusion rates by 28% (p < 0.001). Mitchell’s team further validated the efficacy of PBM in high-complexity orthopaedic surgeries, demonstrating that adopting a restrictive transfusion strategy (haemoglobin threshold <8 g/dL) reduced blood usage by 32% in Level III and IV surgeries without increasing the risk of complications (p = 0.03) (20). Furthermore, multiple pieces of clinical evidence indicate that standardised practice models are essential for achieving precision transfusion. In specialised fields such as transplantation, oncology and sickle cell disease, compliance with standardised transfusion protocols is between 50 and 75% (21). In contrast, general hospitals (particularly orthopaedic and community hospitals) still exhibit significant heterogeneity between hospitals in transfusion indication management and process control (22).
This study also found that the overall per capita blood usage in specialist hospitals was significantly lower than in general hospitals (one-way ANOVA, F = 34.280, p < 0.001). This finding further supports the positive role of precision transfusion strategies in enhancing blood utilisation efficiency.
This study has several limitations. Firstly, the analysis did not adjust for patient-specific complexity or case mix variation [e.g., by using measures such as Diagnosis-Related Groups (DRGs) or Case Mix Index (CMI)] (23). This implies that the observed differences in blood usage between hospitals of different levels and types may be due to disparities in patient disease severity and surgical complexity rather than differences in management efficiency or a combination of both. Therefore, the results reported in this study should be interpreted as unadjusted observational differences. Although the proportion of high-complexity surgeries was included as a proxy for complexity, future studies should incorporate patient-level data to more accurately distinguish the independent contributions of case-mix characteristics and management practices to disparities in blood usage (24, 25).
Secondly, empirical evidence from China’s urban context suggests that policy interventions focused on strengthening technical staffing and standardising processes may significantly improve blood utilisation efficiency and safety culture levels. However, the generalisability of these findings may be influenced by patient demographics, hospital organisational structures, and prevailing governance models in target regions. Therefore, while core principles such as strengthening human resource development, standardising operational procedures and reforming incentive mechanisms are universally relevant, the design and implementation of specific policies require adaptation to local conditions.
In summary, this study further highlights that the future focus of blood management should shift from simple metrics to a patient-centred, data-driven, intelligent framework. This encompasses performance evaluation with data-based risk adjustment, widespread adoption of validated Patient Blood Management (PBM) protocols across all surgical specialties and incentive policies that emphasise clinical outcomes and transfusion rationality rather than quantitative indicators alone. By implementing these measures, healthcare systems can work together to improve the quality and efficiency of transfusion practices.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethical Review Committee of Huzhou Central Blood Station. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this study complied with the Declaration of Helsinki and domestic ethical norms. It was reviewed by the Ethics Committee of the Huzhou Central Blood Station (approval no. HZXZLL2025-06, 15 May 2025). The data originated from anonymised summary data from the provincial clinical blood use quality control centre for the years 2020–2023. This data was de-identified and encrypted for storage and contained only anonymous information such as age and gender, with no patient privacy information. As the data has been anonymised and analysed as a group, individual informed consent is exempt according to the relevant regulations. The researchers are committed to ensuring the authenticity of the data and the compliance of the process, and to using the results for academic purposes only to prevent any disclosure of privacy or misuse.
Author contributions
FW: Writing – original draft, Writing – review & editing, Formal analysis, Data curation, Project administration, Conceptualization. ZX: Validation, Conceptualization, Writing – review & editing, Writing – original draft. KL: Writing – review & editing, Writing – original draft, Investigation. JF: Writing – review & editing, Writing – original draft, Data curation, Formal analysis. HY: Writing – review & editing, Writing – original draft, Validation. ZO: Writing – original draft, Investigation, Software, Writing – review & editing. YW: Writing – review & editing, Formal analysis, Visualization, Writing – original draft. JS: Writing – original draft, Resources, Supervision, Writing – review & editing. YC: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration. LW: Conceptualization, Data curation, Project administration, Methodology, Writing – original draft, Writing – review & editing, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work was funded by the Public Welfare Application Research Project of Huzhou (Grant Number: 2024GYB29) Institute.
Acknowledgments
We would like to express our gratitude to the individuals who have provided support and assistance for this study. We apologize for not being able to individually acknowledge all the individuals or institutions that have supported this study but are not mentioned in this acknowledgment. We would like to thank Huzhou Central Blood Station and Huzhou University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Owusu-Ofori, AK, and Bates, I. Impact of inconsistent policies for transfusion-transmitted malaria on clinical practice in Ghana. PLoS One. (2012) 7:e34201. doi: 10.1371/journal.pone.0034201
2. Bray, TJ, and Prabhakar, K. Editorial: blood policy and transfusion practice - India. Trop Med Int Health. (2002) 7:477–8. doi: 10.1046/j.1365-3156.2002.00895.x
3. Isbister, JP. Frozen blood transfusion practices: time for change. Intern Med J. (2008) 38:683–5. doi: 10.1111/j.1445-5994.2008.01770.x
4. Mackeprang, P-H, Bryjova, K, Heusel, AE, Henzen, D, Scricciolo, M, and Elicin, O. Consideration of image guidance in patterns of failure analyses of intensity-modulated radiotherapy for head and neck cancer: a systematic review. Radiat Oncol. (2024) 19:1748-717X. doi: 10.1186/s13014-024-02421-w
5. Howard, SW, Counte, MA, Vriesman, LJ, and Brink, HD. Standing in the gap: the academic and professional divide between health administration and health policy. Front Public Health. (2023) 11:1147646. doi: 10.3389/fpubh.2023.1147646
6. Eiroa-Orosa, FJ, and MacIntyre, G. Editorial: from individual to collective: bridging the gap between clinical practice and public policies. Front Psychol. (2023) 14:1169159. doi: 10.3389/fpsyg.2023.1169159
7. Bansal, N, Saluja, GP, and Raturi, M. Need for standardization of technical manpower norms for blood centres: lessons from the Indian scenario. Transfus Clin Biol. (2023) 30:456–7. doi: 10.1016/j.tracli.2023.09.002
8. Yetmgeta Abdella,, Hajjeh, R, and Smit Sibinga, CT. Availability and safety of blood transfusion during humanitarian emergencies. East Mediterr Health J. (2018) 24:778–88. doi: 10.26719/2018.24.8.778
9. Robins, JV, Haran, RDL, Kumar, HA, I, S, and James, S. Evaluating the crossmatch-to-transfusion ratio as a tool for analyzing and optimizing blood bank resource utilization: a retrospective observational study. Cureus. (2024) 16:e69862. doi: 10.7759/cureus.69862
10. Davies, L, Brown, TJ, Haynes, S, Payne, K, Elliott, RA, and McCollum, C. Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model. Health Technol Assess. (2006) 10:1–210. doi: 10.3310/hta10440
11. Martinez, V, Monsaingeon-Lion, A, Cherif, K, Judet, T, Chauvin, M, and Fletcher, D. Transfusion strategy for primary knee and hip arthroplasty: impact of an algorithm to lower transfusion rates and hospital costs. Br J Anaesth. (2007) 99:794–800. doi: 10.1093/bja/aem266
12. Murphy, MF, Stanworth, SJ, and Yazer, M. Transfusion practice and safety: current status and possibilities for improvement. Vox Sang. (2011) 100:46–59. doi: 10.1111/j.1423-0410.2010.01366.x
13. Perez, A, Bakhtary, S, Nedelcu, E, and Manuel, S. Overtransfusion of autologous blood identifies opportunities for improving patient blood management. Cureus. (2019) 11:e4202. doi: 10.7759/cureus.4202
14. Chegondi, M, Hernandez Rivera, JF, Alkhoury, F, and Totapally, BR. The need for blood transfusion therapy is associated with increased mortality in children with traumatic brain injury. PLoS One. (2023) 18:e0279709. doi: 10.1371/journal.pone.0279709
15. Frank, SM, Oleyar, MJ, Ness, PM, and Tobian, AAR. Reducing unnecessary preoperative blood orders and costs by implementing an updated institution-specific maximum surgical blood order schedule and a remote electronic blood release system. Anesthesiology. (2014) 121:501–9. doi: 10.1097/ALN.0000000000000338
16. Masud, F, Larson-Pollock, K, Leveque, C, and Vykoukal, D. Establishing a culture of blood management through education a quality initiative study of postoperative blood use in CABG patients at Methodist DeBakey Heart & Vascular Center. Am J Med Qual. (2011) 26:349–56. doi: 10.1177/1062860611398532
17. Yazer, MH, and Triulzi, DJ. AABB red blood cell transfusion guidelines: something for almost everyone. JAMA. (2016) 316:1984–5. doi: 10.1001/jama.2016.10887
18. Couvret, C, Tricoche, S, Baud, A, Dabo, B, Buchet, S, Palud, M, et al. The reduction of preoperative autologous blood donation for primary total hip or knee arthroplasty: the effect on subsequent transfusion rates. Anesth Analg. (2002) 94:815–23. doi: 10.1097/00000539-200204000-00008
19. Lasocki, S, Belbachir, A, Mertes, P-M, Le Pelley, E, Bosch, L, Bezault, C, et al. Changes in practices after implementation of a patient blood management program in French surgical departments: the National Multicenter Observational PERIOPES study. Anesth Analg. (2025) 140:453–64. doi: 10.1213/ANE.0000000000006917
20. Mitchell, MD, Betesh, JS, Ahn, J, Hume, EL, Mehta, S, and Umscheid, CA. Transfusion thresholds for major orthopedic surgery: a systematic review and Meta-analysis. J Arthroplast. (2017) 32:3815–21. doi: 10.1016/j.arth.2017.06.054
21. Plantinga, LC, Bender, AA, Urbanski, M, Douglas-Ajayi, C, Morgan, JC, and Woo, K. Patient care technician staffing and outcomes among US patients receiving in-center hemodialysis. JAMA Netw Open. (2024) 7:e241722. doi: 10.1001/jamanetworkopen.2024.1722
22. Choi, S, Choi, SJ, Shin, JW, and Yoon, YA. Common data model-based analysis of selective leukoreduction protocol compliance at three hospitals. Ann Lab Med. (2023) 43:187–95. doi: 10.3343/alm.2023.43.2.187
23. Ferreira, DC, and Marques, RC. Should inpatients be adjusted by their complexity and severity for efficiency assessment? Evidence from Portugal. Health Care Manag Sci. (2016). 19:43–57. doi: 10.1007/s10729-014-9286-y
24. Ozawa, S, Ozawa-Morriello, J, Rock, R, Sromoski, MA, Walbolt, S, Hall, T, et al. Patient blood management as an emerging concept in quality: the role of nurses. J Nurs Care Qual. (2024) 39:129–35. doi: 10.1097/NCQ.0000000000000734
25. Goobie, SM, Gallagher, T, Gross, I, and Shander, A. Society for the advancement of blood management administrative and clinical standards for patient blood management programs. 4th edition (pediatric version). Paediatr Anaesth. (2019) 29:231–6. doi: 10.1111/pan.13574
26. Hirose, H, and Jaekel, A. Commentary: autologous blood transfusion effects. J Thorac Cardiovasc Surg. (2022) 164:1582–3. doi: 10.1016/j.jtcvs.2021.01.085
Keywords: discharged patients, per capita blood use, policy implementation linkage model, regional control of blood resources, patient blood management
Citation: Wang F, Xu Z-g, Lv K, Fei J, Yang H, Ou Z, Wang Y, Song J, Chen Y and Wang L (2025) Analysis of blood utilisation efficiency driven by clinical management and hospital heterogeneity. Front. Public Health. 13:1668449. doi: 10.3389/fpubh.2025.1668449
Edited by:
Bernadette Pfang, University Hospital Fundación Jiménez Díaz, SpainReviewed by:
Ricardo De Moraes E. Soares, Instituto Politecnico de Setubal (IPS), PortugalTrupti Lokhande, All India Institute of Medical Sciences Raebareli, India
Copyright © 2025 Wang, Xu, Lv, Fei, Yang, Ou, Wang, Song, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Le Wang, d2FuZ2xlNTIxZ29vZEAxNjMuY29t
 Feng Wang
Feng Wang Zhi-guo Xu
Zhi-guo Xu Ke Lv1
Ke Lv1