- 1School of Basic Medical Sciences & School of Nursing, Chengdu University, Chengdu, China
- 2Sichuan Provincial Orthopedic Hospital, Chengdu, China
- 3School of Nursing, Sun Yat-sen University, Guangzhou, China
- 4Chengdu University Affiliated Hospital, Chengdu, China
- 5Sichuan Nursing Vocational College, Chengdu, China
Background: Shift work, particularly night shift work, has become increasingly prevalent on a global scale and is associated with multiple health issues including type 2 diabetes and cardiovascular diseases (CVD). This study aimed to assess the relationship between night shift work and the incidence and mortality of CVD.
Method: Six electronic databases including PubMed, Embase, Cochrane Library, Web of Science, CINAHL, and Scopus were searched from inception until August 10, 2025. Cohort studies eligible for inclusion addressed the association between night shift work and outcomes of CVD. STATA 18.0 software was used for meta-analysis. The dose-response relationship was estimated using generalized least squares regression, and restricted cubic splines were used to analyze potential linear or nonlinear associations. The Newcastle-Ottawa Scale (NOS) was used to assess the quality of the studies. Quality assessment and data extraction were performed independently by two researchers.
Results: Twenty-three cohort studies were included. Overall, this meta-analysis revealed that night shift work significantly increased the risk of total CVD events (RR = 1.13, 95% CI = 1.10–1.16) and total CVD mortality (RR = 1.27, 95%CI = 1.18–1.36). Dose-response analysis indicated that each 5-year increment in shift work duration was associated with a 7% higher risk of CVD incidence (RR = 1.07, 95% CI: 1.04–1.09) and a 4% increased risk of CVD mortality (RR = 1.05, 95% CI: 1.03–1.06). Subgroup analyses identified elevated risks for incident coronary heart disease (CHD) (RR = 1.22, 95% CI = 1.16–1.28) and ischemic heart disease (IHD) (RR = 1.09, 95% CI = 1.05–1.14), but not stroke (RR = 1.06, 95% CI = 0.95–1.18), and night shift work was associated with an increased risk of mortality due to CHD (RR = 1.22, 95% CI = 1.10–1.36), IHD (RR = 1.39, 95% CI = 1.06–1.84), and stroke (RR = 1.49, 95% CI = 1.04–2.12).
Conclusion: These findings indicate that night shift work is significantly associated with increased CVD incidence and mortality risk, highlighting the need for targeted prevention strategies.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251060086. CRD: 420251060086.
Introduction
Contemporary socio-economic demands have led to the increasing prevalence of night shift work systems, particularly in the healthcare sector, service industries, and manufacturing industries (1). Studies indicate that approximately 18–20% of workers in Europe and the United States engage in shift work, encompassing various forms such as day shifts, night shifts, rotating shifts, and irregular schedules (2, 3). Shift work, commonly defined as work schedules not aligned with daytime hours, represents a widespread occupational arrangement globally (4). The shift toward such work patterns (especially the rise in night shifts) constitutes an independent risk factor for multisystem diseases by disrupting the body’s normal circadian rhythm.
Substantial evidence indicates that long-term night shift work is significantly associated with adverse health outcomes, including breast cancer (5), type 2 diabetes (6), mood disorders (7), and metabolic syndrome (8), and also contributes to the development and progression of cardiovascular diseases (CVD). CVD remains the leading cause of global mortality and a major contributor to the global disease burden (9). According to the World Heart Federation 2023 report, CVD caused approximately 20.5 million deaths globally, accounting for one-third of all global deaths (10). Among these CVD fatalities, 85% were attributable to heart attacks and strokes (11).
Accumulating evidence has linked prolonged night shift work to increased risks of CVD incidence and mortality (12–14). Although previous meta-analyses have reported associations between shift work and adverse CVD outcomes (15–18), one of these studies included only five cohort studies with limited sample sizes (15). More importantly, several recent cohort studies have since been published, providing substantial new evidence, however, some reported no significant association between night shift work and adverse CVD outcomes (14, 19–22). Given the current evidence remains inconclusive, an updated meta-analysis incorporating recent high-quality studies is imperative to clarify the potential impact of night shift exposure on cardiovascular disease morbidity and mortality.
Understanding the relationship between night shift work and CVD incidence and mortality is essential for establishing evidence-based occupational health standards. This study aims to systematically assess this relationship and synthesize evidence to provide clearer conclusions, ultimately informing interventions to mitigate night shift-associated CVD.
Methods
Protocol
The protocol for this systematic review and meta-analysis was registered to PROSPERO (CRD420251060086). This article followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (23).
Search strategy
We comprehensively searched six electronic databases, including PubMed, Embase, Cochrane Library, Web of Science, CINAHL, and Scopus, conducted from inception to August 10, 2025, without language restrictions. The search strategy entailed a combination of exposure-related factors (“shift work,” “night work,” “Shift Work Schedule,” “night shift work,” “rotating shift”) and a mixture of Medical Subject Headings (MESH) terms and keywords pertinent to outcomes (“Cardiovascular Diseases,” “coronary heart disease,” “Myocardial Ischemia,” “ischemic heart disease,” “stroke,” “cardiovascular events,” “cardiovascular mortality”) (Detailed search strategy can be found in the Supplementary Table S1). Additionally, a manual search of the reference lists from the retrieved articles was conducted to identify any additional relevant studies.
Eligibility
Inclusion criteria (based on PICO framework): P (Population): The studies examined outcomes in populations engaged in night shift work (including shift work schedules involving night shifts); I (Intervention): Any type of night shift work as the exposure variable (rotating shift work, fixed night shift work); C (comparisons): Daytime work exclusively, with no history of night shift work; O (Outcome): The outcomes focused on cardiovascular events (including both incidence and mortality) and encompassed major CVD subtypes such as coronary heart disease (CHD), ischemic heart disease (IHD), and stroke, and reported the provision of the 95% confidence intervals (CI), relative risk (RR), odds ratios (OR), hazard ratios (HR). When multiple publications utilized the same cohort, we prioritized the study with the most comprehensive follow-up, complete outcome data, or superior methodological quality to avoid double-counting of participants.
Exclusion criteria included: (1) studies with self-reported cardiovascular outcomes or those reporting only cardiovascular markers; (2) non-human studies; (3) reviews, case reports, letters, or conference abstracts; (4) incomplete data or inability to access full-text articles; (5) duplicate publications; and (6) case reports, systematic reviews, or meta-analyses.
Study selection
Literature management and screening were conducted using EndNote 20. The literature screening procedure was carried out in two phases. Initially, two authors (XJY and MWL) assessed the eligibility of studies by evaluating the titles and abstracts according to the predefined inclusion and exclusion criteria. The second phase entailed a thorough examination of the full texts to ascertain which studies qualified for inclusion in the meta-analysis. In cases of disagreement during the literature screening, the issues were deliberated with a third author (TYM) until a consensus was achieved.
Data extraction
Data regarding results and study characteristics were extracted to tables by the first author (XJY) and checked for accuracy by the second author (MWL). Microsoft Excel was used to extract the required data, including lead author, year of publication, location by country, sample source, study design, age, sex, sample size, follow-up duration, definition of night shift work, type of night shift work, occupation, Outcome, outcome assessment method, adjusted effect estimates (OR, RR, and HR) with 95% CI and a list of adjusted factors.
Assessment of study quality
Two authors (XJY and MWL) independently assessed the quality of the studies using the nine-star Newcastle–Ottawa Scale (NOS) (24), which evaluates three domains: selection (0–4 points), comparability (0–2 points), and outcomes (0–3 points). Total scores were categorized into three groups: 0–3 (fair), 4–6 (moderate), and 7–9 (good). Inter-rater agreement in study quality assessment was quantified using Cohen’s kappa coefficient. According to Cohen’s kappa coefficient, inter-rater agreement was categorized into three levels: coefficients ≤0.60 indicated poor agreement, values between 0.61 and 0.80 represented moderate agreement, and coefficients ≥0.81 signified good agreement (25). Any disagreements were resolved through consensus discussion with the third author (TYM).
Statistical analyses
Analysis of statistics performed with STATA 18.0. The RR was considered as the common measure of the association between night shift work and CVD. The HRs were deemed equivalent to RRs because HRs were considered to approximate RRs (26, 27). The ORs were transformed into RRs using the formula , where P0 indicates the incidence of the outcome of interest in the non-exposed group, and then synthesized with the RRs and the HRs into pooled RRs (28). For each included study, we extracted covariate-adjusted outcome measures. When multiple effect estimates were reported within a single study, these were first pooled at the study level prior to the overall meta-analysis. Heterogeneity across studies was evaluated using Cochran’s Q statistic test and I2 statistic (29, 30). A random-effects model was applied if significant heterogeneity was detected (p-value <0.05 or I2 > 50%); otherwise, a fixed-effects model was utilized (31). Sensitivity analysis was conducted by sequentially excluding individual studies to assess the robustness of the pooled results. At least three quantitative categories (involving the duration of night shift work) were reported in the included studies and were incorporated into the dose-response meta-analysis. The dose-response relationship model proposed by Greenland and Longnecker (32) was adopted to calculate the trend based on the estimated values of logRR in different shift work categories. According to this method, the distribution of cases and participants, as well as RR and 95% confidence intervals, were extracted for each shift work category. Additionally, we used restricted cubic splines with three knots set at the 10th, 50th, and 90th percentiles of the distribution to assess the potential curvilinear association between years of night shift work and the risk of CVD incidence and mortality. In addition, subgroup analyses were conducted to stratify the results on cardiovascular outcome type, sex, type of night shift work, and geographic region. Publication bias was examined through visual inspection of funnel plots and quantified using Egger’s test, if there was a large publication bias, correction is performed using trim-and-fill method (33, 34).
Quality of evidence
We assessed the strength of evidence for each outcome using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework. The evaluation included the following domains: risk of bias, inconsistency, indirectness, imprecision, and other considerations. Evidence quality was categorized into four levels: Very Low, Low, Moderate, and High.
Results
Search results
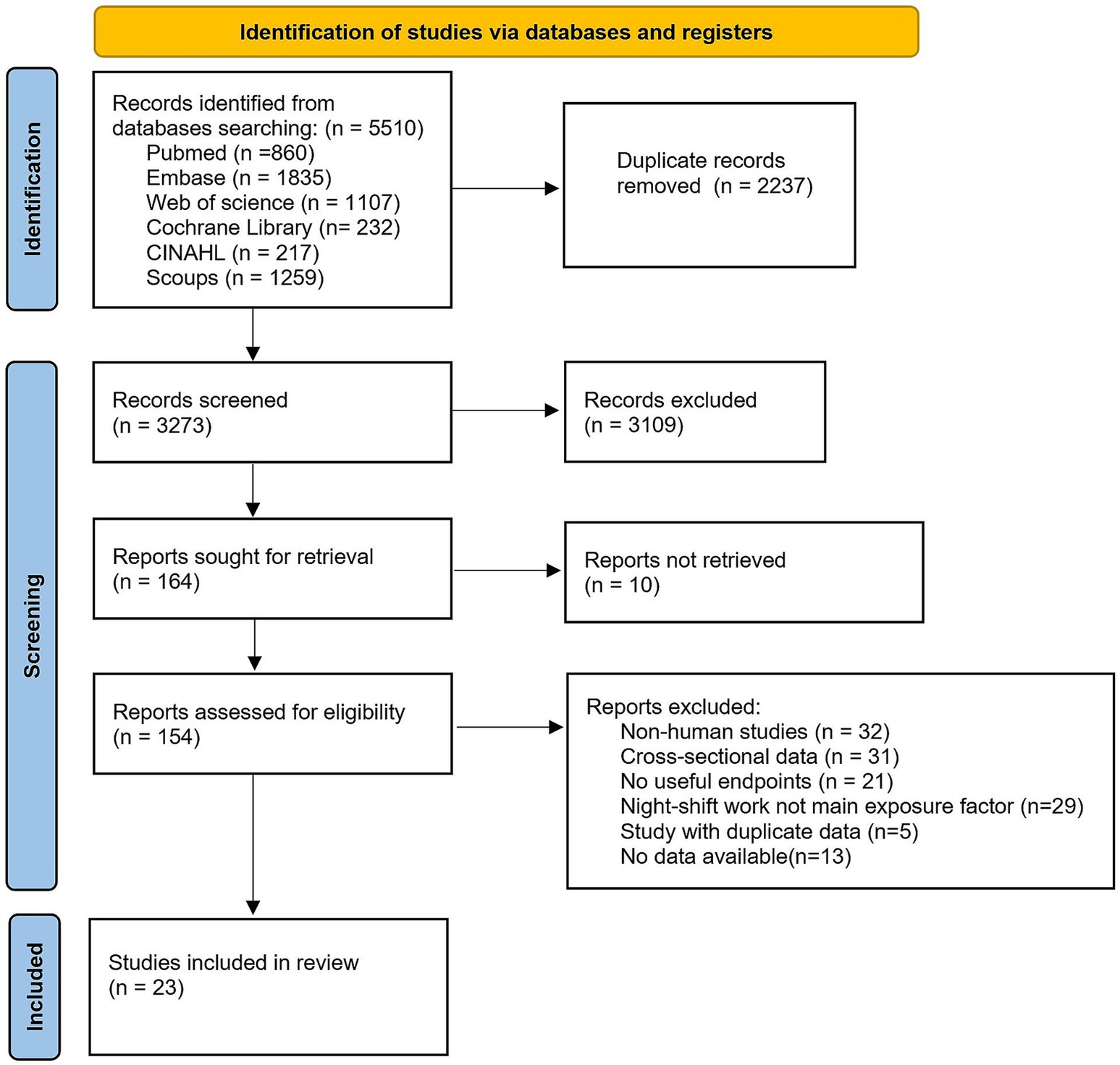
The initial database search identified 5,510 articles: 860 from PubMed, 1,107 from Web of Science, 1835 from Embase, 232 from Cochrane Library, 217 from CINAHL, and 1,259 from Scopus. After removing 2,237 duplicates, 3,273 unique records underwent title and abstract screening. Of these, 154 articles were deemed eligible for full-text review. Following detailed evaluation, 131 studies were excluded based on predefined criteria: (1) non-human studies; (2) cross-sectional design; (3) irrelevant outcomes; (4) duplicate data from overlapping cohorts; (6) insufficient data availability. Ultimately, 23 articles met all inclusion criteria and were included in the meta-analysis (selection process detailed in Figure 1).
Study characteristics
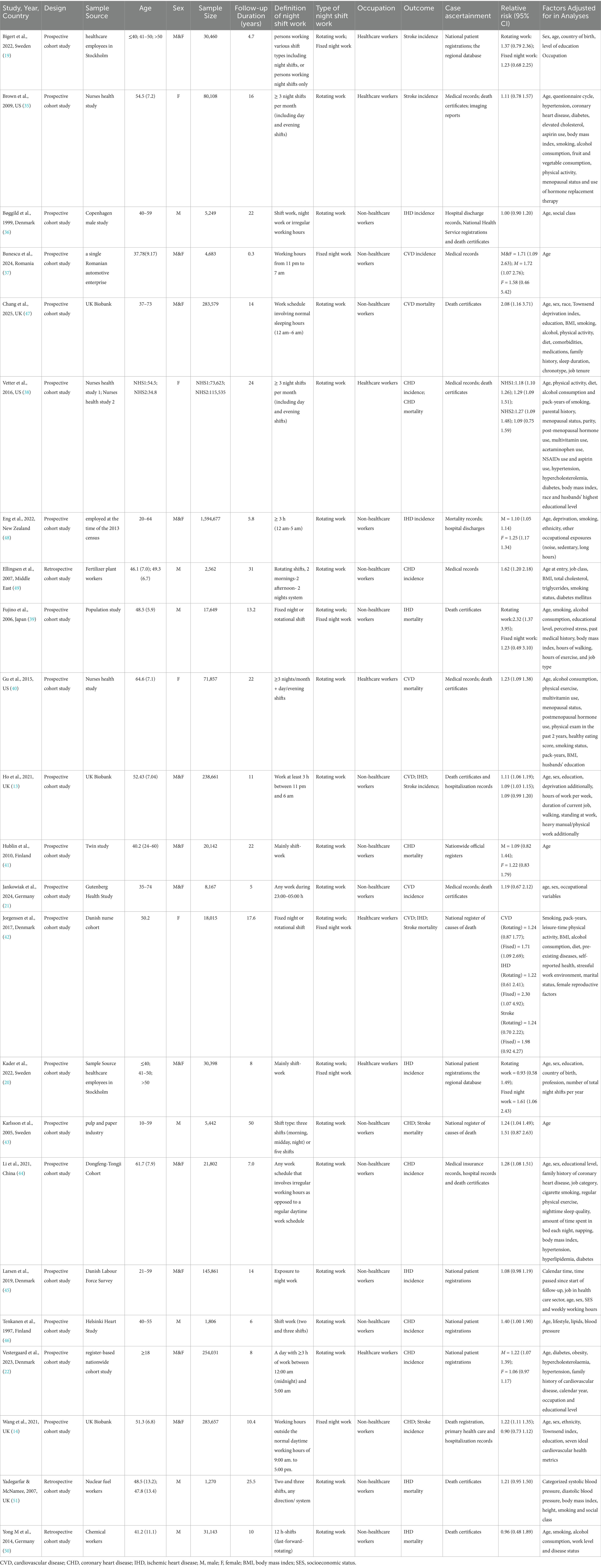
This meta-analysis incorporated 23 observational studies comprising 20 prospective cohort studies (13, 14, 19–22, 35–48), 3 retrospective cohort studies (49–51), with a total population of 3,340,377 participants. Geographically, 16 studies were conducted in Europe (13, 14, 19–22, 36, 37, 41–43, 45–47, 50, 51), 3 in North America (35, 38, 40), 1 in Oceania (48), and 3 in Asia (39, 44, 49). Regarding occupational distribution, 7 studies specifically focused on healthcare workers (19, 20, 22, 35, 38, 40, 42), while the remaining studies encompassed diverse occupations, predominantly involving industrial workers.
The included studies investigated associations between night shift work and CVD incidence/mortality risks, primarily targeting the following outcomes: CVD, CHD, IHD, and stroke. Case ascertainment was primarily based on local hospital admission records and death certificates. Definitions of night shift work varied across studies: most involved rotating night shifts, whereas others assessed fixed night shifts. Study characteristics are summarized in Table 1.
Quality assessment
Supplementary Table S2 shows the quality assessment of the included studies. All 23 studies were rated as high-quality based on the NOS, with scores ≥7. High methodological quality was primarily manifested in cohort selection, ascertainment of night shift work exposure, and assessment of CVD outcomes. Some potential biases were noted, primarily arising from inadequate follow-up and comparability limitations. Inter-rater reliability was assessed using Cohen’s kappa coefficient. The analysis yielded a kappa value of 0.857 (p < 0.001), indicating good agreement between the two assessors in quality evaluation.
Association between night shift work and total CVD incidence
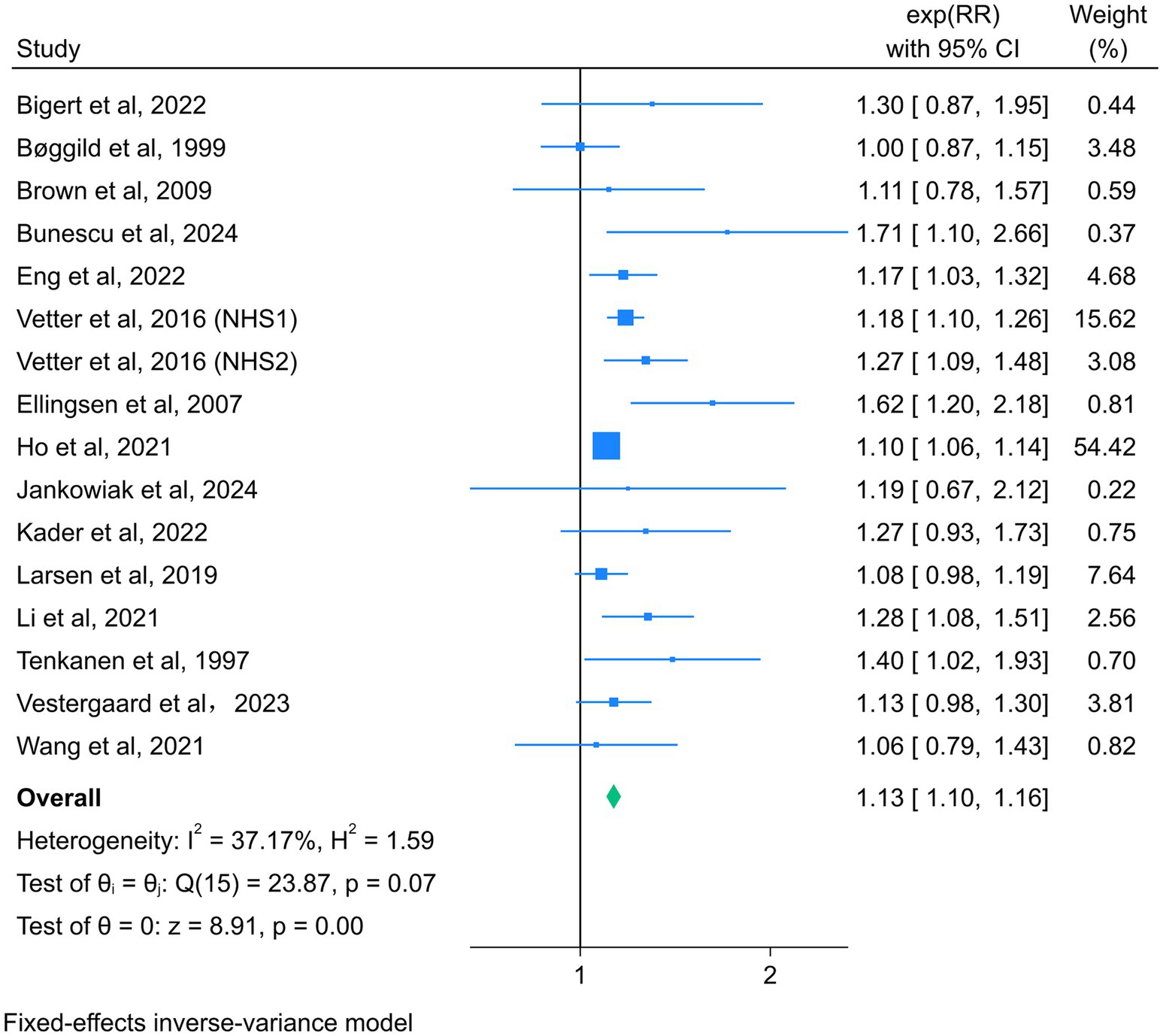
Fifteen studies reported the association between night shift work and the risk of total CVD events, with a total of 2,891,280 participants (13, 14, 19–22, 35–38, 44–46, 48, 49). Due to the absence of significant heterogeneity among the studies (I2 = 37.17%, p = 0.07), a fixed-effect model was selected for analysis. The summary RR (95% CI) was 1.13 (1.10–1.16) (Figure 2), suggesting that night shift work was associated with a higher risk of adverse cardiovascular events compared to daytime work.
Association between night shift work and total CVD mortality
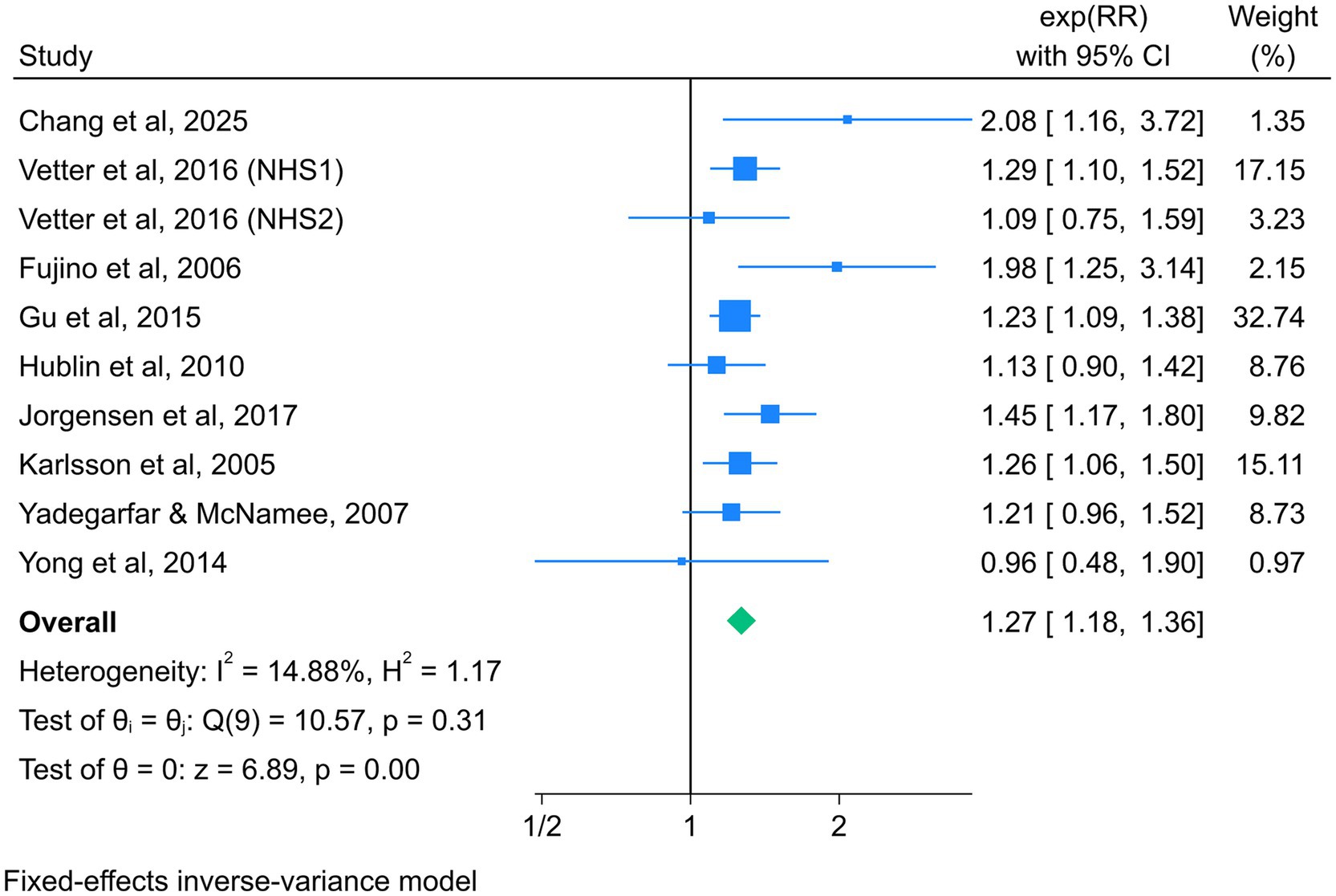
Nine studies reported the association between night shift work and the risk of total CVD mortality, with a total of 638,255 participants (38–43, 47, 50, 51). Given the absence of significant heterogeneity across studies (I2 = 14.88%, p = 0.31), a fixed-effect model was selected for analysis. The summary RR (95% CI) was 1.27 (1.18–1.36) (Figure 3), suggesting that night shift work was associated with a higher risk of adverse cardiovascular mortality outcomes compared to daytime work.
Dose response meta-analysis
Studies with ≥3 categories of shift work duration were included in the dose-response analysis. Results demonstrated a linear dose-response relationship between night shift work and the risk of CVD incidence and mortality. For each 5-year increase in shift work duration, the risk of CVD incidence increased by 7% (RR = 1.07, 95% CI: 1.04–1.09), and the risk of CVD mortality increased by 4% (RR = 1.05, 95% CI: 1.03–1.06) (Figure 4a). Using restricted cubic splines, we did not detect a significant nonlinear dose-response association between shift work duration and the risk of CVD incidence (p = 0.068) or mortality (p = 0.096) (Figure 4b).
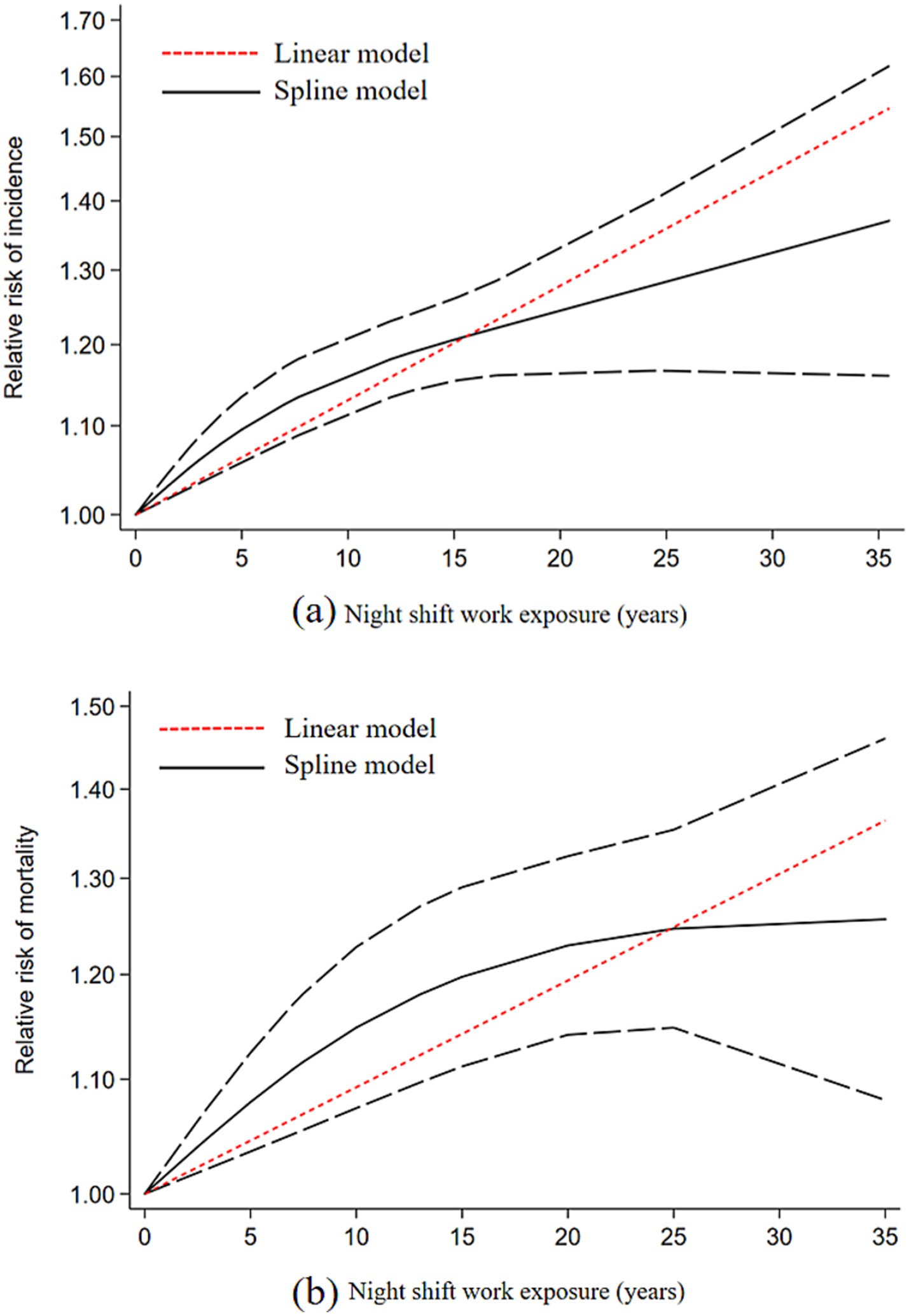
Figure 4. Dose-response relationship plot between duration of night shift work and CVD; (a) CVD incidence; (b) CVD mortality.
Subgroup analysis
In subgroup analyses examining the association between night shift work and CVD events, night shift workers exhibited significantly higher risks of CHD (RR = 1.22, 95% CI = 1.16–1.28) and IHD (RR = 1.09, 95% CI = 1.05–1.14) compared to daytime workers. However, no statistically significant associations were observed for CVD (RR = 1.24, 95% CI = 0.95–1.60) or stroke (RR = 1.06, 95% CI = 0.95–1.18). In contrast, subgroup analyses focusing on CVD mortality revealed elevated risks among night shift workers for CHD (RR = 1.22, 95% CI = 1.10–1.36), CVD (RR = 1.35, 95% CI = 1.10–1.66), IHD (RR = 1.39, 95% CI = 1.06–1.84), and stroke (RR = 1.49, 95% CI = 1.04–2.12) (Figures 5, 6).
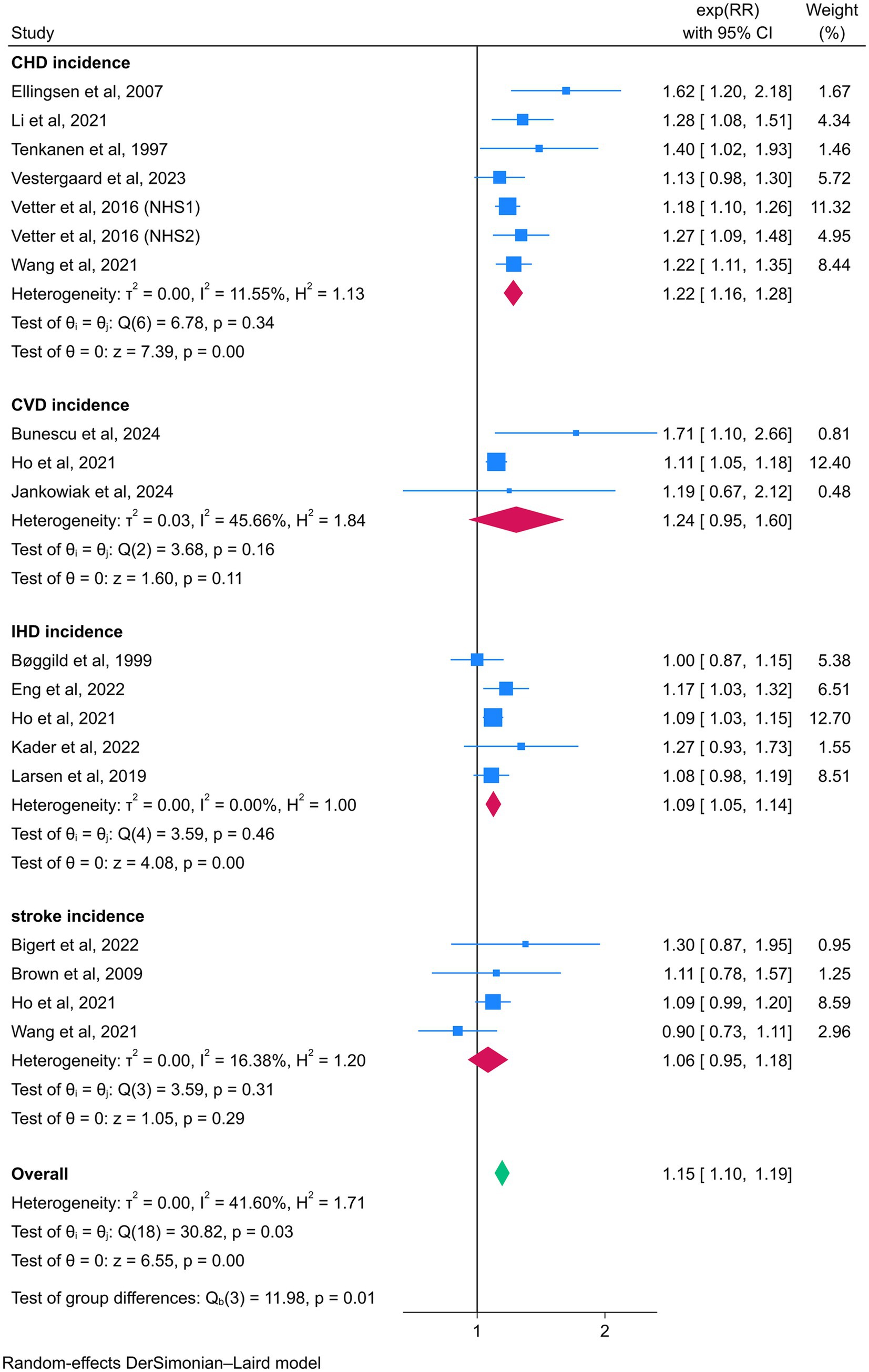
Figure 5. Forest plot of the associations between night shift work and CVD incidence by subgroup analysis.
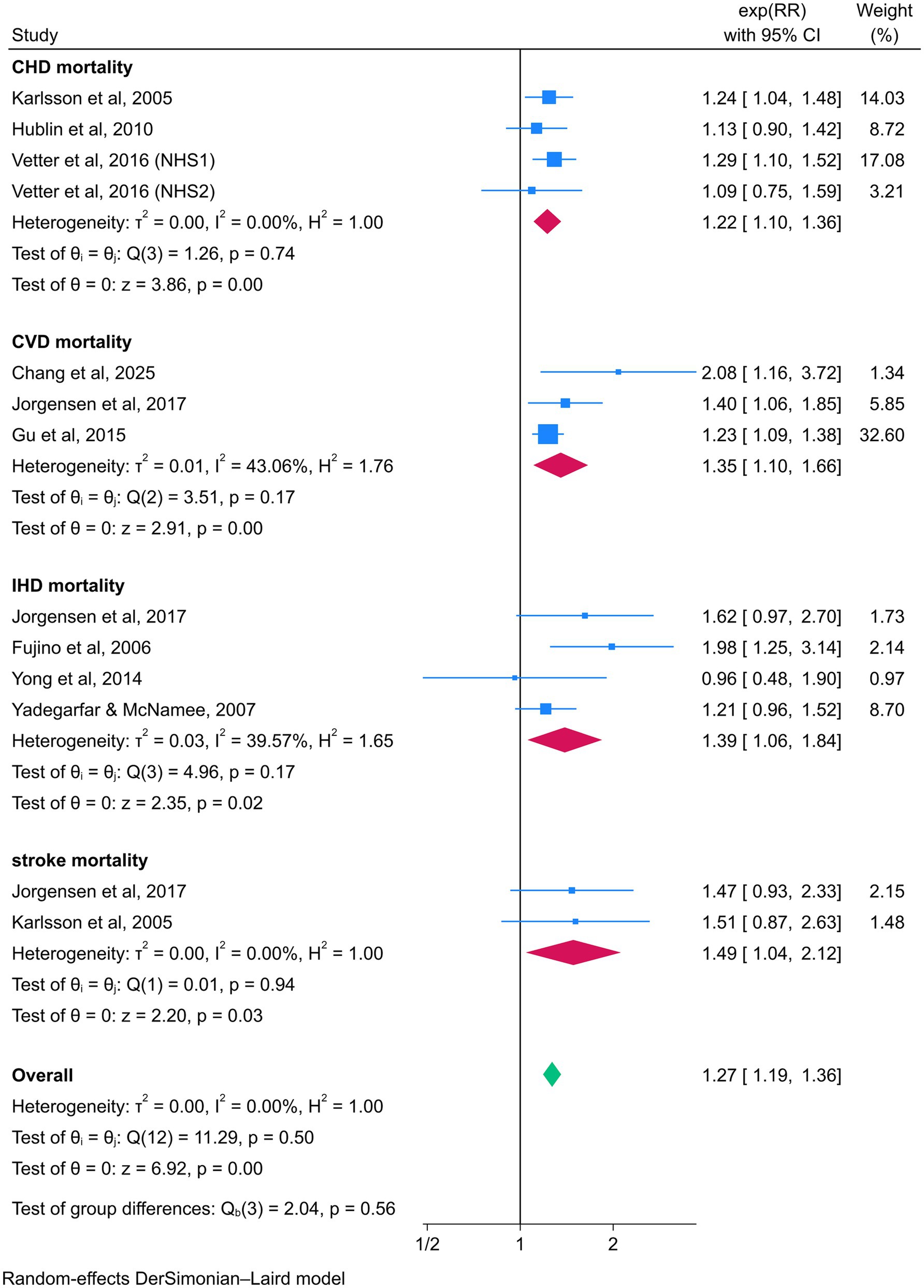
Figure 6. Forest plot of the associations between night shift work and CVD mortality by subgroup analysis.
Additionally, night shift work patterns were significantly associated with both cardiovascular event incidence and mortality: fixed night work (RR = 1.34, 95% CI = 1.04–1.72) and rotating work (RR = 1.15, 95% CI = 1.10–1.21) were positively correlated with CVD incidence, while fixed night work (RR = 1.77, 95% CI = 1.28–2.46) and rotating work (RR = 1.25, 95% CI = 1.16–1.35) were associated with increased CVD mortality. Sex-specific analyses indicated a 18% higher risk of CVD events in female (RR = 1.18, 95% CI = 1.11–1.26) and a 20% higher risk in male (RR = 1.20 95% CI = 1.07–1.36) among night shift workers. For CVD mortality, both male (RR = 1.27, 95% CI = 1.07–1.45) and female (RR = 1.27, 95% CI = 1.17–1.38) showed statistically significant increases in risk. Geographic region subgroup analyses further demonstrated that night shift workers from Asia, Europe, North America, and Oceania are all significantly associated with the incidence and mortality risk of CVD (Tables 2, 3).
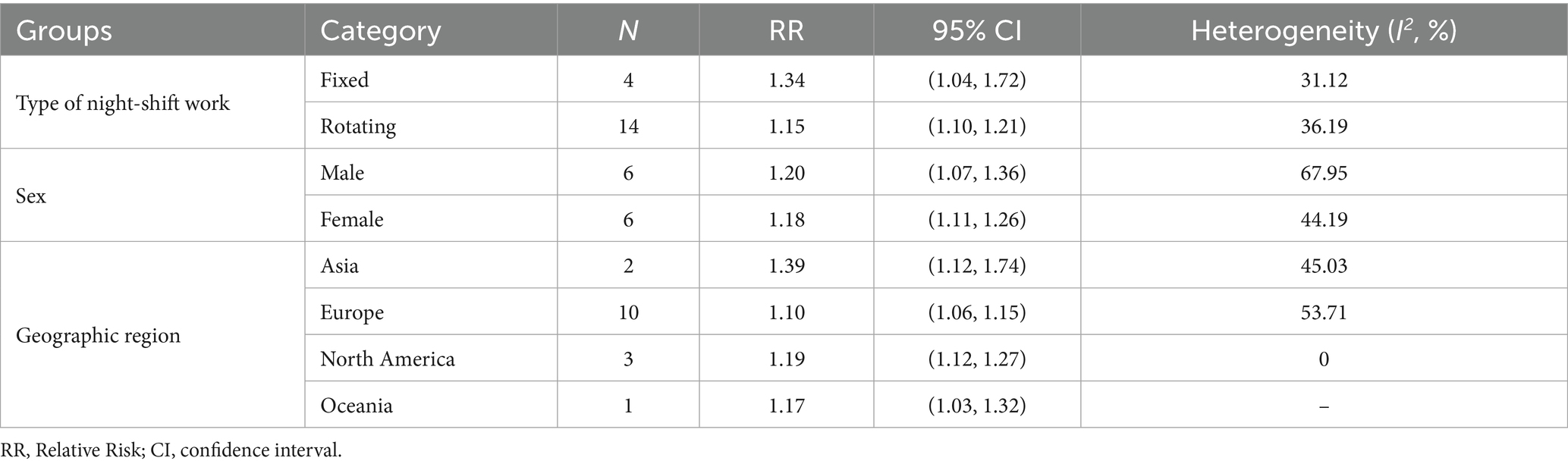
Table 2. Subgroup analyses of the risk of total CVD incidence (type of night shift work, sex, geographic region).
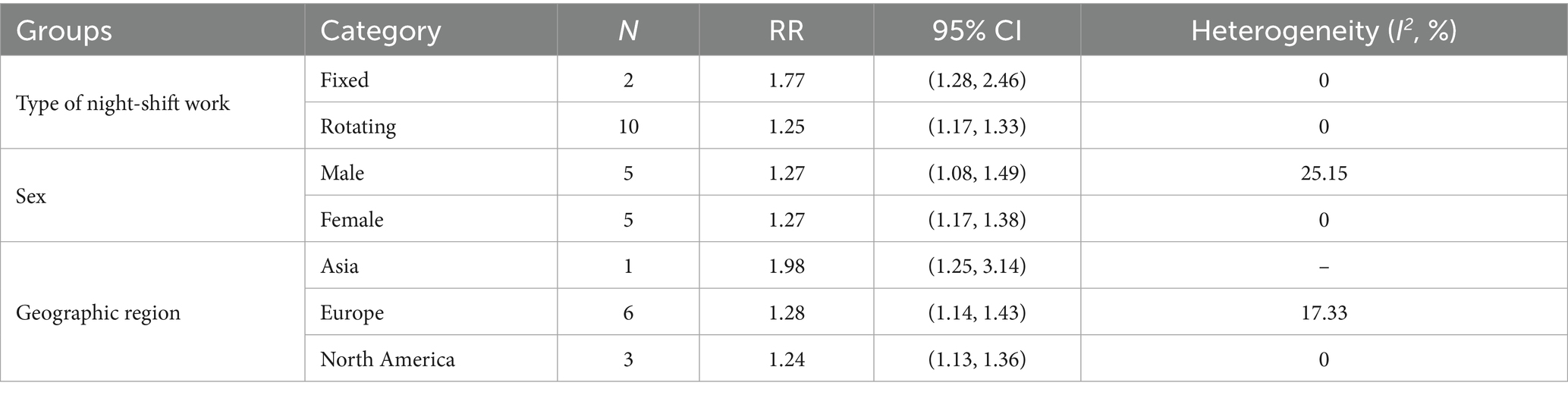
Table 3. Subgroup analyses of the risk of total CVD mortality (type of night shift work, sex, geographic region).
Sensitivity analysis
In this study, a sensitivity analysis was conducted on the association between night shift work and the risk of CVD incidence (Supplementary Figure S1) and mortality (Supplementary Figure S3). The application of sensitivity analysis confirmed the reliability of our meta-analysis results.
Publication bias
In this study, we used the Egger test and funnel plot to assess publication bias in the included studies. The analysis showed that the data distribution was slightly skewed in the association analysis between night shift work and overall CVD incidence risk (Supplementary Figure S2). The Egger test result (p = 0.025 < 0.05) indicated potential publication bias. However, the trim and fill method indicated that the impact of publication bias on the results was minimal. Imputation of six hypothetical studies showed no substantial alteration in the effect estimate (Supplementary Figure S2). This confirms the robustness of the primary conclusions. In the analysis of the association between night shift work and overall CVD mortality risk, the data distribution was generally symmetrical and relatively concentrated (Supplementary Figure S4). The Egger test results (p = 0.375 > 0.05) indicated no significant bias.
Grading of the evidence
GRADE evaluation indicated predominantly low to very low certainty evidence (Supplementary Table S3). Given the observational cohort designs, initial certainty was low. Evidence for CVD incidence was downgraded to very low due to publication bias, while CVD mortality maintained low certainty absent downgrading factors. All other outcomes were rated very low, primarily because of imprecision and publication bias risks from limited studies.
Discussion
This systematic review and meta-analysis of 23 cohort studies, encompassing data from over 3.3 million participants, provides a comprehensive evaluation of the association between night shift work and the risk of CVD incidence and mortality. Our results demonstrate that night shift work is significantly associated with an increased risk of both CVD incidence (RR = 1.13, 95% CI: 1.10–1.16) and CVD mortality (RR = 1.27, 95% CI: 1.18–1.36). Furthermore, dose-response analysis revealed a linear relationship between the duration of night shift work and CVD risk, with each additional 5-year period of exposure associated with a 7% increase in the risk of CVD incidence and a 4% increase in the risk of CVD mortality. These findings underscore a clear time-dependent effect of night shift work on cardiovascular health, providing crucial quantitative evidence for occupational health risk assessment.
Although our research findings are similar to some previous meta-analyses (15–18), there are still some differences. Regarding the risk of CVD incidence, our results are consistent with the conclusions of previous meta-analyses in this field. However, in terms of mortality risk, our study found a significant association, which is inconsistent with the results reported by Vyas et al. (16) and Torquati (2018) (16, 17). This difference may be related to the inclusion of more recent high-quality cohort studies in our research, with a larger sample size and higher statistical power, thereby identifying risk effects that may have been underestimated in previous studies. In terms of the dose-response relationship, our study supports the linear cumulative effect model reported by Wang et al. (15), indicating that risk accumulation begins from the start of night shift work (15). This is different from the non-linear model proposed by Torquati et al. (17), which suggests no significant risk in the first 5 years, and that meta-analysis only conducted dose-response analysis for CVD incidence. However, given the conflicting results from existing studies, there is a need for further longitudinal research to precisely quantify the exposure duration of night shift work in relation to cardiovascular health. In summary, in public health strategies and occupational health management, it is necessary to develop optimized shift cycle plans, avoid long-term consecutive night shifts, and reduce the risk of CVD.
Our findings indicate that the association between night shift work and CVD risk holds consistently across all subgroups, with the exception of stroke incidence. Notably, although stroke did not reach statistical significance in the risk of incidence, previous studies have suggested that male night shift workers may be more prone to stroke (52, 53). This difference may be related to gender differences in thrombosis tendency, hypertension management, or health behavior patterns. Additionally, in the subgroup analysis of stroke mortality risk, only two studies were included due to the limited number of studies, and the results should be interpreted with caution. Regarding shift types, we found that both rotating shift work and fixed night shift work were associated with the incidence and mortality risk of CVD, with fixed night shift work presenting a greater risk. This may be due to the fact that rotating shift work may include some non-night shifts as exposures, and the intensity of rotating shift work is lower than that of fixed night shift work (18). The gender subgroup analysis indicated that both men and women were associated with the incidence and mortality of CVD, with a more significant risk association in men. This gender difference may be attributed to men’s higher susceptibility to dyslipidemia, greater likelihood of engaging in physically demanding occupations, and higher prevalence of adverse lifestyle factors such as smoking and alcohol consumption (54). Notably, Torquati et al. previously did not find a significant association in men for the incidence of CVD (16). Geographically, the risk association was higher in Asian populations compared to Europe, North America, and Oceania. This regional difference may be related to variations in shift organization patterns and healthcare access among regions, but due to the limited data from Asia, this result should be interpreted with caution. Future studies could incorporate more detailed stratification based on sociodemographic factors, which is crucial for a comprehensive assessment of risk differences among populations.
Multiple mechanisms can account for the association between night shift work and the risks of CVD incidence and mortality. Night shift work is considered to be associated with circadian rhythm disruption, hormonal dysregulation, and stress responses, all of which are biological pathways that contribute to an increased incidence of CVD and mortality. Firstly, night shift work disrupts the synchronization between the endogenous circadian clock and the environmental light - dark cycle (55). This disruption impairs autonomic regulation and hormonal homeostasis, rendering individuals more susceptible to hypertension, insulin resistance, and metabolic dysfunctions (56). Furthermore, research has shown that night shift workers exhibit elevated levels of inflammatory biomarkers (57, 58). These include C-reactive protein, tumor necrosis factor-α (TNF-α), and white blood cell count. Such inflammatory responses accelerate the progression of atherosclerosis and elevate the risk of cardiovascular events. In addition, the high work stress associated with night shift work may trigger stress responses. This promotes the adrenal cortex to secrete glucocorticoids under the influence of adrenocorticotropic hormone, leading to obesity and insulin resistance (59, 60). With the accumulation of long-term exposure, this can further result in hypertension, hyperlipidemia, and other risk factors for cardiovascular disease (61, 62). Overall, the potential harm of night shift work to cardiovascular health is not single but multi-targeted.
A key strength of this study lies in the inclusion of a greater number of eligible studies than previous systematic reviews and meta-analyses on this topic, encompassing ten investigations not included in prior meta-analyses, which may support robust effect size estimates and increase the accuracy of meta-analysis. Furthermore, beyond examining overall CVD incidence and mortality risks, we specifically analyzed major CVD subtypes. Additionally, given the critical role of exposure duration in occupational health, we assessed both linear and nonlinear dose-response relationships between shift work duration and CVD incidence/mortality risks.
Limitations and prospects
Several limitations should be acknowledged in this meta-analysis. First, heterogeneity in the definition of night shift work across studies may introduce measurement bias. Second, residual confounding from unmeasured factors (dietary habits, physical activity) cannot be fully excluded. Third, most included studies were conducted in European and North American populations, limiting generalizability to low-income regions. Fourth, reliance on self-reported questionnaires or interviews to assess night shift work in some studies, coupled with potential changes in shift patterns during follow-up, may result in misclassification or measurement errors. Additionally, the search strategy was limited to studies related to cardiovascular events, and the strict inclusion and exclusion criteria may have led to the exclusion of potentially relevant studies. Finally, some subgroups included only 1–2 studies, which may affect the generalizability of the results, and the analysis results should be interpreted with caution. Future studies should actively include prospective cohort designs from low- and middle-income regions to evaluate the generalizability of findings across diverse geographic settings. Furthermore, primary studies are encouraged to incorporate objective exposure assessment (electronic shift records) with repeated measurements to reduce confounding bias, and to more deeply explore the underlying biological mechanisms.
Conclusion
In summary, this meta-analysis found an association between night shift workers and an increased risk of adverse cardiovascular outcomes. Further research into the underlying mechanisms and potential interactions between these factors is necessary in the future in order to develop targeted interventions and prevention strategies for individuals at risk of adverse cardiovascular outcomes due to shift work, while work organizations should try to scientifically and rationally design shift schedules to enable employees to be in a favorable occupational environment, in order to mitigate the risk of adverse cardiovascular outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JX: Writing – review & editing, Writing – original draft, Methodology, Software, Data curation, Investigation. WM: Investigation, Methodology, Data curation, Writing – review & editing. YT: Investigation, Methodology, Data curation, Writing – review & editing. XZ: Writing – review & editing, Formal analysis, Supervision, Methodology. LL: Data curation, Investigation, Writing – review & editing, Supervision. HW: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to express our sincere gratitude to our professor for his invaluable guidance and constructive suggestions throughout this research. We would also like to thank all members of the research team for their help and support during the preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1668848/full#supplementary-material
References
1. Costa, G. Shift work and health: current problems and preventive actions. Saf Health Work. (2010) 1:112–23. doi: 10.5491/shaw.2010.1.2.112
2. Parent-Thirion, A, Biletta, I, Cabrita, J, Vargas, O, Vermeylen, G, and Wilczynska, A Sixth European working conditions survey: Overview report. Luxembourg: Publications Office of the European Union. (2016)
3. Alterman, T, Luckhaupt, SE, Dahlhamer, JM, Ward, BW, and Calvert, GM. Prevalence rates of work organization characteristics among workers in the US: data from the 2010 National Health Interview Survey. Am J Ind Med. (2013) 56:647–59. doi: 10.1002/ajim.22108
4. Wright, KP Jr, Bogan, RK, and Wyatt, JK. Shift work and the assessment and management of shift work disorder (SWD). Sleep Med Rev. (2013) 17:41–54. doi: 10.1016/j.smrv.2012.02.002
5. Manouchehri, E, Taghipour, A, Ghavami, V, Ebadi, A, Homaei, F, and Latifnejad Roudsari, R. Night-shift work duration and breast cancer risk: an updated systematic review and meta-analysis. BMC Womens Health. (2021) 21:89. doi: 10.1186/s12905-021-01233-4
6. Gao, Y, Gan, T, Jiang, L, Yu, L, Tang, D, Wang, Y, et al. Association between shift work and risk of type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of observational studies. Chronobiol Int. (2020) 37:29–46. doi: 10.1080/07420528.2019.1683570
7. Torquati, L, Mielke, GI, Brown, WJ, Burton, NW, and Kolbe-Alexander, TL. Shift work and poor mental health: a Meta-analysis of longitudinal studies. Am J Public Health. (2019) 109:e13–20. doi: 10.2105/ajph.2019.305278
8. Sooriyaarachchi, P, Jayawardena, R, Pavey, T, and King, NA. Shift work and the risk for metabolic syndrome among healthcare workers: a systematic review and meta-analysis. Obes Rev. (2022) 23:e13489. doi: 10.1111/obr.13489
9. Roth, GA, Mensah, GA, Johnson, CO, Addolorato, G, Ammirati, E, Baddour, LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
10. Website World Heart Federation. (2023). World heart report 2023: navigating the heart of cardiovascular health. Available online at: https://heartreport23.world-heart-federation.org/ [Accessed July 18, 2025].
11. World Health Organization. Cardiovascular diseases (CVDs). (2021). Available online at: https://www.who.int/zh/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) [Accessed June 18, 2025].
12. Lunde, LK, Skare, Ø, Mamen, A, Sirnes, PA, Aass, HCD, Øvstebø, R, et al. Cardiovascular health effects of shift work with long working hours and night shifts: study protocol for a three-year prospective follow-up study on industrial workers. Int J Environ Res Public Health. (2020) 17:589. doi: 10.3390/ijerph17020589
13. Ho, FK, Celis-Morales, C, Gray, SR, Demou, E, Mackay, D, Welsh, P, et al. Association and pathways between shift work and cardiovascular disease: a prospective cohort study of 238 661 participants from UK biobank. Int J Epidemiol. (2022) 51:579–90. doi: 10.1093/ije/dyab144
14. Wang, N, Sun, Y, Zhang, H, Wang, B, Chen, C, Wang, Y, et al. Long-term night shift work is associated with the risk of atrial fibrillation and coronary heart disease. Eur Heart J. (2021) 42:4180–8. doi: 10.1093/eurheartj/ehab505
15. Wang, D, Ruan, W, Chen, Z, Peng, Y, and Li, W. Shift work and risk of cardiovascular disease morbidity and mortality: a dose-response meta-analysis of cohort studies. Eur J Prev Cardiol. (2018) 25:1293–302. doi: 10.1177/2047487318783892
16. Vyas, MV, Garg, AX, Iansavichus, AV, Costella, J, Donner, A, Laugsand, LE, et al. Shift work and vascular events: systematic review and meta-analysis. BMJ. (2012) 345:e4800. doi: 10.1136/bmj.e4800
17. Torquati, L, Mielke, GI, Brown, WJ, and Kolbe-Alexander, T. Shift work and the risk of cardiovascular disease. A systematic review and meta-analysis including dose-response relationship. Scand J Work Environ Health. (2018) 44:229–38. doi: 10.5271/sjweh.3700
18. Su, F, Huang, D, Wang, H, and Yang, Z. Associations of shift work and night work with risk of all-cause, cardiovascular and cancer mortality: a meta-analysis of cohort studies. Sleep Med. (2021) 86:90–8. doi: 10.1016/j.sleep.2021.08.017
19. Bigert, C, Kader, M, Andersson, T, Selander, J, Bodin, T, Gustavsson, P, et al. Night and shift work and incidence of cerebrovascular disease - a prospective cohort study of healthcare employees in Stockholm. Scand J Work Environ Health. (2022) 48:31–40. doi: 10.5271/sjweh.3986
20. Kader, M, Selander, J, Andersson, T, Albin, M, Bodin, T, Härmä, M, et al. Night and shift work characteristics and incident ischemic heart disease and atrial fibrillation among healthcare employees - a prospective cohort study. Scand J Work Environ Health. (2022) 48:520–9. doi: 10.5271/sjweh.4045
21. Jankowiak, S, Rossnagel, K, Bauer, J, Schulz, A, Liebers, F, Latza, U, et al. Night shift work and cardiovascular diseases among employees in Germany: five-year follow-up of the Gutenberg health study. Scand J Work Environ Health. (2024) 50:142–51. doi: 10.5271/sjweh.4139
22. Vestergaard, JM, Dalbøge, A, Bonde, JPE, Garde, AH, Hansen, J, Hansen, ÅM, et al. Night shift work characteristics and risk of incident coronary heart disease among health care workers: national cohort study. Int J Epidemiol. (2023) 52:1853–61. doi: 10.1093/ije/dyad126
23. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
24. Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
25. McHugh, ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). (2012) 22:276–82. doi: 10.11613/BM.2012.031
26. Li, L, Wu, C, Gan, Y, Qu, X, and Lu, Z. Insomnia and the risk of depression: a meta-analysis of prospective cohort studies. BMC Psychiatry. (2016) 16:375. doi: 10.1186/s12888-016-1075-3
27. Sun, J, Ma, C, Zhao, M, Magnussen, CG, and Xi, B. Daytime napping and cardiovascular risk factors, cardiovascular disease, and mortality: a systematic review. Sleep Med Rev. (2022) 65:101682. doi: 10.1016/j.smrv.2022.101682
28. Zhang, J, and Yu, KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. (1998) 280:1690–1. doi: 10.1001/jama.280.19.1690
29. Li, SJ, Jiang, H, Yang, H, Chen, W, Peng, J, Sun, MW, et al. The dilemma of heterogeneity tests in meta-analysis: a challenge from a simulation study. PLoS One. (2015) 10:e0127538. doi: 10.1371/journal.pone.0127538
30. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
31. Moola, S, Munn, Z, Tufanaru, C, Aromataris, E, Sears, K, and Sfetcu, R. Chapter 7: Systematic reviews of etiology and risk In: E Aromataris and Z Munn, editors. Joanna Briggs Institute reviewer’s manual : The Joanna Briggs Institute. (2017).
32. Greenland, S, and Longnecker, MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
33. Higgins, JP, Thomas, J, Chandler, J, Cumpston, M, Li, T, Page, MJ, et al. Cochrane handbook for systematic reviews of interventions. Hoboken: John Wiley & Sons (2019). doi: 10.1002/9781119536604
34. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
35. Brown, DL, Feskanich, D, Sánchez, BN, Rexrode, KM, Schernhammer, ES, and Lisabeth, LD. Rotating night shift work and the risk of ischemic stroke. Am J Epidemiol. (2009) 169:1370–7. doi: 10.1093/aje/kwp056
36. Bøggild, H, Suadicani, P, Hein, HO, and Gyntelberg, F. Shift work, social class, and ischaemic heart disease in middle aged and elderly men; a 22 year follow up in the Copenhagen male study. Occup Environ Med. (1999) 56:640–5. doi: 10.1136/oem.56.9.640
37. Bunescu, MG, Gheorman, V, Marcu, IR, Lungulescu, CV, and Dinescu, VC. Tackling shift work: cardiovascular health in the auto industry. Healthcare (Basel). (2024) 12.:1097. doi: 10.3390/healthcare12111097
38. Vetter, C, Devore, EE, Wegrzyn, LR, Massa, J, Speizer, FE, Kawachi, I, et al. Association between rotating night shift work and risk of coronary heart disease among women. JAMA. (2016) 315:1726–34. doi: 10.1001/jama.2016.4454
39. Fujino, Y, Iso, H, Tamakoshi, A, Inaba, Y, Koizumi, A, Kubo, T, et al. A prospective cohort study of shift work and risk of ischemic heart disease in Japanese male workers. Am J Epidemiol. (2006) 164:128–35. doi: 10.1093/aje/kwj185
40. Gu, F, Han, J, Laden, F, Pan, A, Caporaso, NE, Stampfer, MJ, et al. Total and cause-specific mortality of U.S. nurses working rotating night shifts. Am J Prev Med. (2015) 48:241–52. doi: 10.1016/j.amepre.2014.10.018
41. Hublin, C, Partinen, M, Koskenvuo, K, Silventoinen, K, Koskenvuo, M, and Kaprio, J. Shift-work and cardiovascular disease: a population-based 22-year follow-up study. Eur J Epidemiol. (2010) 25:315–23. doi: 10.1007/s10654-010-9439-3
42. Jørgensen, JT, Karlsen, S, Stayner, L, Hansen, J, and Andersen, ZJ. Shift work and overall and cause-specific mortality in the Danish nurse cohort. Scand J Work Environ Health. (2017) 43:117–26. doi: 10.5271/sjweh.3612
43. Karlsson, B, Alfredsson, L, Knutsson, A, Andersson, E, and Torén, K. Total mortality and cause-specific mortality of Swedish shift- and dayworkers in the pulp and paper industry in 1952-2001. Scand J Work Environ Health. (2005) 31:30–5. doi: 10.5271/sjweh.845
44. Li, W, Yu, K, Jia, N, Xu, X, Yuan, Y, Peng, R, et al. Past shift work and incident coronary heart disease in retired workers: a prospective cohort study. Am J Epidemiol. (2021) 190:1821–9. doi: 10.1093/aje/kwab074
45. Larsen, AD, Rugulies, R, Hansen, J, Kolstad, HA, Hansen, ÅM, Hannerz, H, et al. Night work and risk of ischaemic heart disease and anti-hypertensive drug use: a cohort study of 145 861 Danish employees. Eur J Pub Health. (2020) 30:259–64. doi: 10.1093/eurpub/ckz189
46. Tenkanen, L, Sjöblom, T, Kalimo, R, Alikoski, T, and Härmä, M. Shift work, occupation and coronary heart disease over 6 years of follow-up in the Helsinki heart study. Scand J Work Environ Health. (1997) 23:257–65. doi: 10.5271/sjweh.218
47. Chang, Q, Zhu, Y, Liang, H, Cheng, J, Li, D, Lin, F, et al. Night shift work associates with all-cause and cause-specific mortality: a large prospective cohort study. J Gen Intern Med. (2025) 40:1635–45. doi: 10.1007/s11606-024-08946-w
48. Eng, A, Denison, HJ, Corbin, M, Barnes, L, Mannetje, AT, Mc Lean, D, et al. Long working hours, sedentary work, noise, night shifts and risk of ischaemic heart disease. Heart. (2023) 109:heartjnl-2022-320999–9. doi: 10.1136/heartjnl-2022-320999
49. Ellingsen, T, Bener, A, and Gehani, AA. Study of shift work and risk of coronary events. J R Soc Promot Heal. (2007) 127:265–7. doi: 10.1177/1466424007083702
50. Yong, M, Nasterlack, M, Germann, C, Lang, S, and Oberlinner, C. Shift work and risk of non-cancer mortality in a cohort of German male chemical workers. Int Arch Occup Environ Health. (2014) 87:763–73. doi: 10.1007/s00420-013-0922-5
51. Yadegarfar, G, and McNamee, R. Shift work, confounding and death from ischaemic heart disease. Occup Environ Med. (2008) 65:158–63. doi: 10.1136/oem.2006.030627
52. Global, regional, and national burden of stroke and its risk factors, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet Neurol. (2024) 23:973–1003. doi: 10.1016/s1474-4422(24)00369-7
53. Li, X, He, Y, Wang, D, and Momeni, MR. Chronobiological disruptions: unravelling the interplay of shift work, circadian rhythms, and vascular health in the context of stroke risk. Clin Exp Med. (2024) 25:6. doi: 10.1007/s10238-024-01514-w
54. Townsend, N, Kazakiewicz, D, Lucy Wright, F, Timmis, A, Huculeci, R, Torbica, A, et al. Epidemiology of cardiovascular disease in Europe. Nat Rev Cardiol. (2022) 19:133–43. doi: 10.1038/s41569-021-00607-3
55. Golding, H, Ritonja, JA, Day, AG, Aronson, KJ, and Tranmer, J. Modeling the relationship between shift work and cardiometabolic risk through circadian disruption, sleep and stress pathways. Chronobiol Int. (2022) 39:704–13. doi: 10.1080/07420528.2022.2032124
56. Vasey, C, McBride, J, and Penta, K. Circadian rhythm dysregulation and restoration: the role of melatonin. Nutrients. (2021) 13:3480. doi: 10.3390/nu13103480
57. Erdem, JS, Das, MK, De Ryck, E, Skare, Ø, Lie, JS, Bugge, M, et al. Night shift work and indicators of cardiovascular risk: a systematic review and meta-analysis. Environ Res. (2025) 276:121503. doi: 10.1016/j.envres.2025.121503
58. Sooriyaarachchi, P, Jayawardena, R, Pavey, T, and King, NA. Shift work is associated with an elevated white blood cell count: a systematic review and Meta-analysis. Indian J Occup Environ Med. (2023) 27:278–85. doi: 10.4103/ijoem.ijoem_326_22
59. Rosa, D, Terzoni, S, Dellafiore, F, and Destrebecq, A. Systematic review of shift work and nurses' health. Occup Med (Lond). (2019) 69:237–43. doi: 10.1093/occmed/kqz063
60. Noushad, S, Ahmed, S, Ansari, B, Mustafa, UH, Saleem, Y, and Hazrat, H. Physiological biomarkers of chronic stress: a systematic review. Int J Health Sci. (2021) 15:46–59.
61. Kanki, M, Nath, AP, Xiang, R, Yiallourou, S, Fuller, PJ, Cole, TJ, et al. Poor sleep and shift work associate with increased blood pressure and inflammation in UK biobank participants. Nat Commun. (2023) 14:7096. doi: 10.1038/s41467-023-42758-6
Keywords: night shift work, cardiovascular disease, mortality, systematic review, meta-analysis
Citation: Xi J, Ma W, Tao Y, Zhang X, Liu L and Wang H (2025) Association between night shift work and cardiovascular disease: a systematic review and dose-response meta-analysis. Front. Public Health. 13:1668848. doi: 10.3389/fpubh.2025.1668848
Edited by:
Mohammad Hossein Ebrahimi, Shahroud University of Medical Sciences, IranReviewed by:
Johanna Samulin Erdem, National Institute of Occupational Health, NorwayMasoumeh Atefi, Shahroud University of Medical Sciences, Iran
Copyright © 2025 Xi, Ma, Tao, Zhang, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyan Wang, MTMyODEyMTgxOThAMTYzLmNvbQ==
 Jiayu Xi1
Jiayu Xi1 Hongyan Wang
Hongyan Wang