- School of Stomatology and Optometry, Hubei University of Science and Technology, Xianning, Hubei, China
Background: Myopia is a highly prevalent eye disorder among adolescents, and an increasing body of research indicates that nutritional factors may have a significant impact on its development. However, the nature and extent of these relationships remain unclear. This systematic review and meta-analysis aimed to comprehensively evaluate the associations between various nutritional factors, including carbohydrates, proteins, cholesterol, and sodium, and myopia in adolescents.
Methods: Multiple databases, such as PubMed, Web of Science, Scopus, and Embase, were systematically searched up to February 15, 2025. The inclusion criteria encompassed observational studies published in English, involving adolescents (aged 6–18 years), and reporting data on the intake of the selected nutritional factors and myopia status. Two reviewers independently screened studies, extracted data, and assessed study quality using the Newcastle-Ottawa Scale. Standardized mean differences (SMDs) with 95% confidence intervals (CIs) were calculated for continuous outcomes. Random-effects models were applied to account for potential heterogeneity.
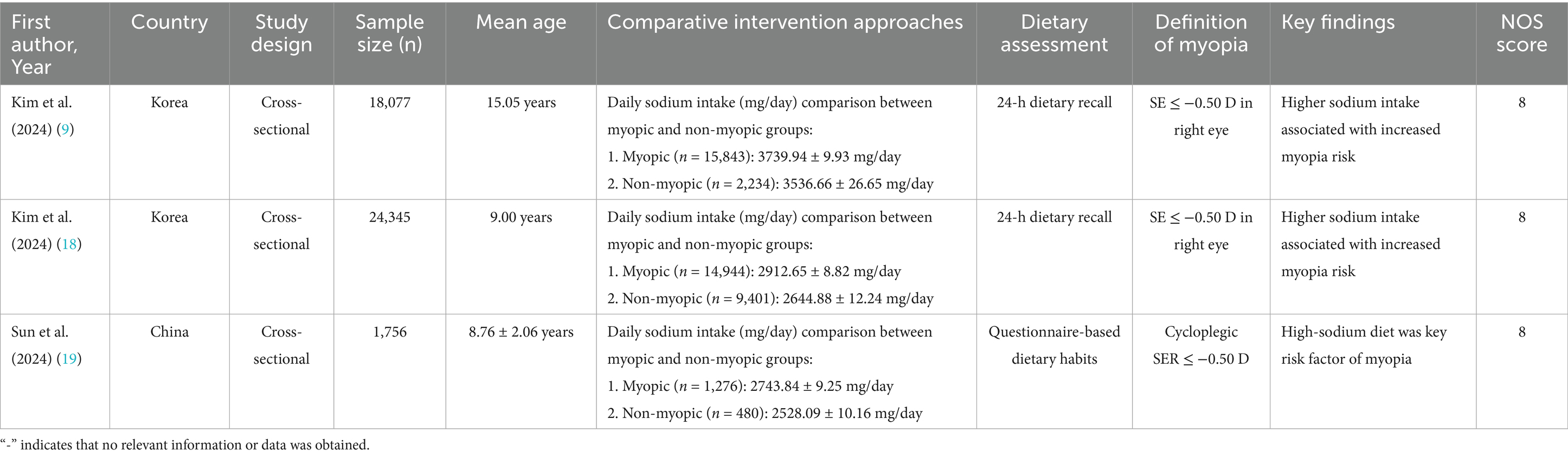
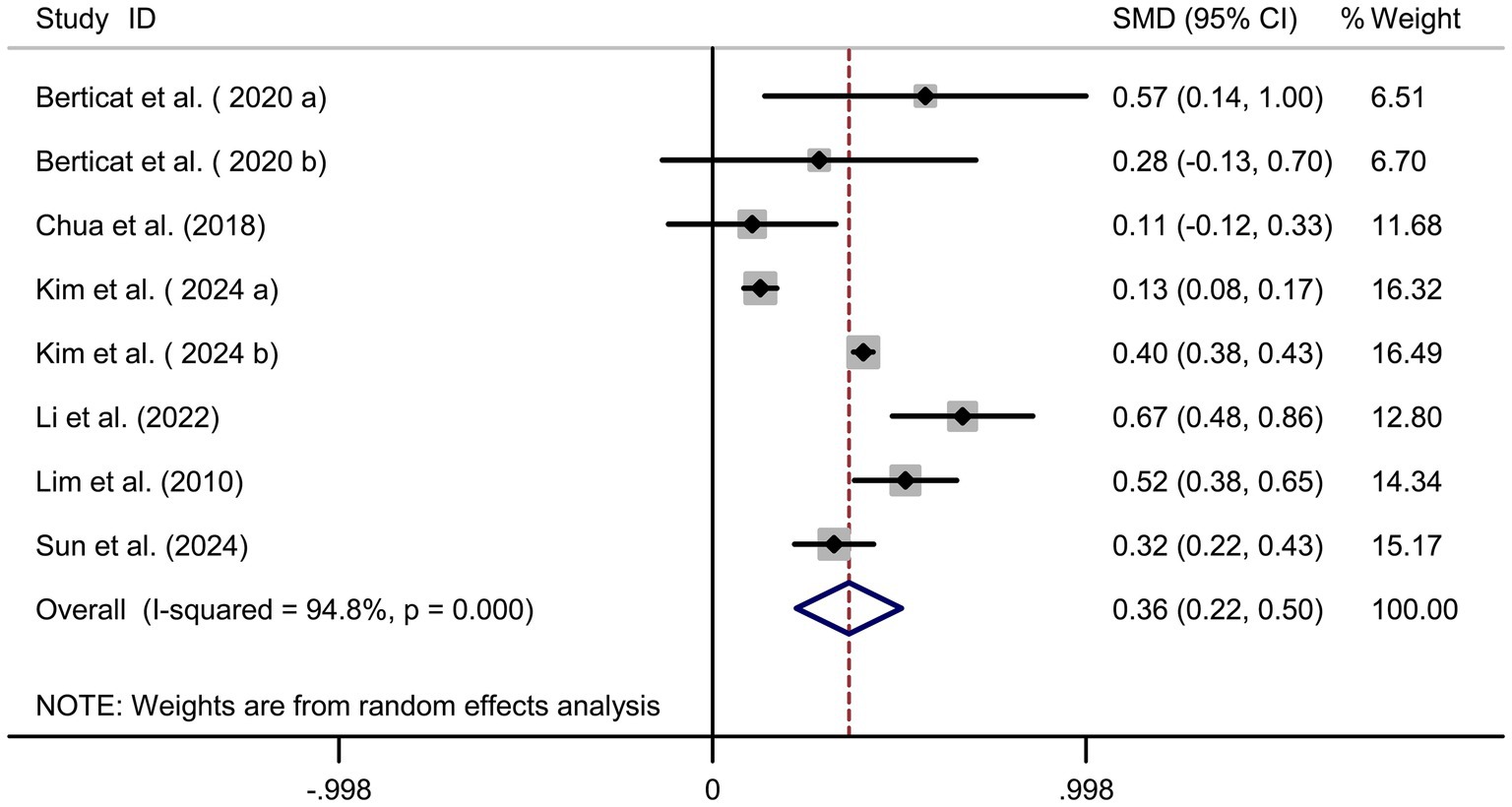
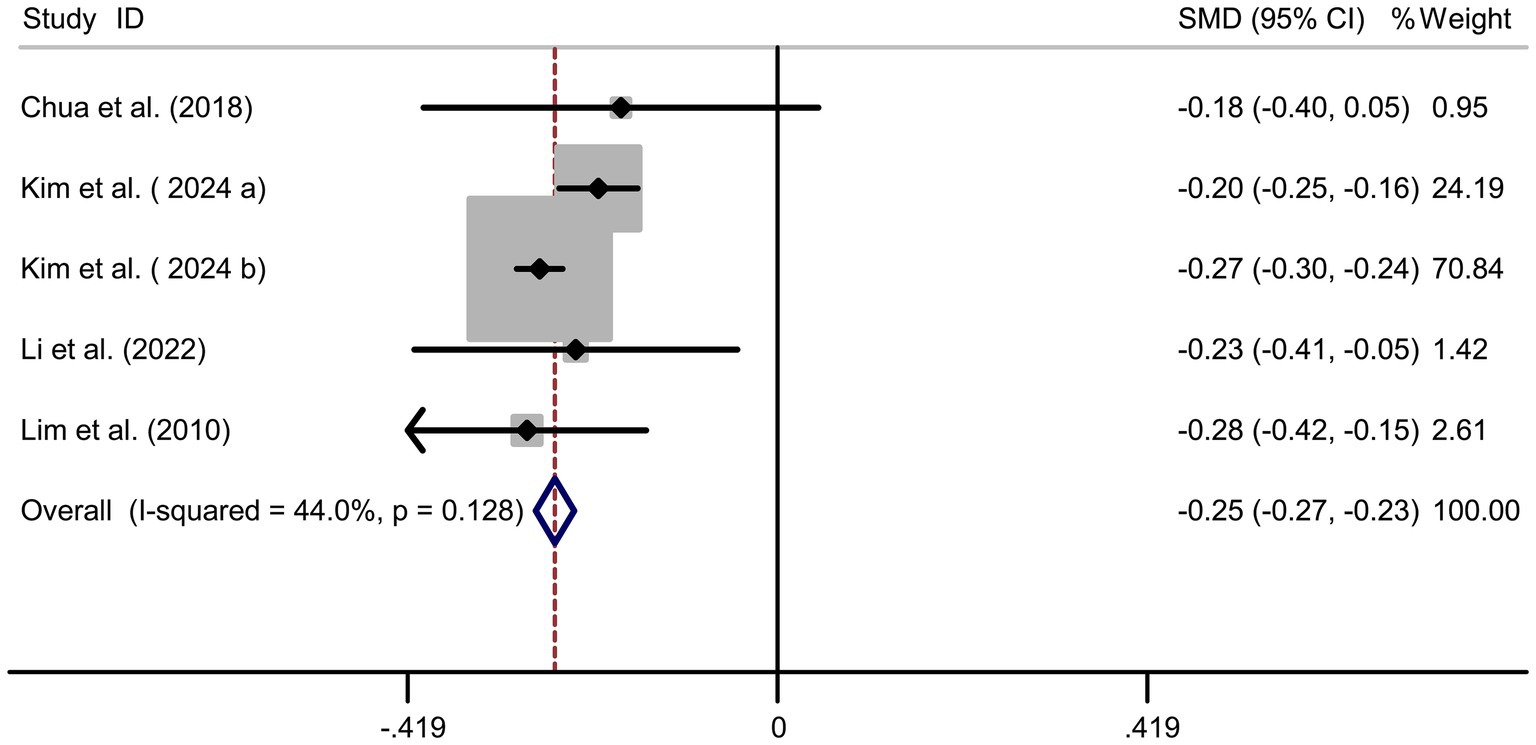
Results: A total of 7 articles (8 studies) involving 45,993 adolescents were included. Pooled analysis revealed significant associations between nutritional factors and myopia risk. Higher carbohydrate intake was positively linked to myopia (SMD = 0.36, 95% CI: 0.22–0.50, I2 = 94.8%, p < 0.001), while protein intake showed a protective effect (SMD = −0.25, 95% CI: −0.27 to −0.23, I2 = 44.0%, p < 0.001). Cholesterol intake was associated with increased myopia risk (SMD = 0.20, 95% CI: 0.10–0.31, I2 = 91.7%, p < 0.001), and sodium intake demonstrated a strong positive association (SMD = 1.07, 95% CI: 0.93–1.22, I2 = 96.0%, p < 0.001). Sensitivity analyses confirmed the robustness of results, and no publication bias was detected.
Conclusion: This study suggests potential associations between nutritional factors and myopia in adolescents. Carbohydrates, cholesterol, and sodium were positively associated with myopia, whereas proteins showed a possible protective effect. However, given the small number of available studies, the predominance of cross-sectional designs, and substantial heterogeneity, these findings should be considered preliminary. Future well-designed, longitudinal or interventional studies are required to confirm these associations before any firm dietary recommendations can be made for myopia prevention.
Introduction
Myopia, one of the most prevalent refractive errors, is characterized by impaired distance vision and has reached epidemic proportions worldwide, particularly among adolescents (1). Recent estimates indicate that approximately 30–50% of children and adolescents in East Asia are affected, with projections suggesting that by 2050, nearly half of the global population may be myopic (2–4). Beyond its immediate impact on visual acuity, myopia is associated with an increased risk of severe ocular complications, including retinal detachment, glaucoma, and cataracts, underscoring the urgent need for effective prevention strategies during childhood and adolescence (5, 6).
Well-established determinants of myopia progression include genetic predisposition, reduced outdoor activity, and prolonged near work or screen time, which together explain a large proportion of the current epidemic, particularly in East Asia (2). Nevertheless, these factors alone do not fully account for the rapid rise in prevalence, suggesting that additional modifiable influences may contribute.
Within this broader etiological framework, dietary factors have recently attracted increasing attention. Nutrition may interact with genetic and behavioral determinants to influence eye growth and refractive development through pathways involving insulin-like growth factor 1 (IGF-1) signaling, oxidative stress, and inflammation (4, 6). Situating nutrition alongside established factors such as outdoor exposure and digital device use provides a more comprehensive perspective on the multifactorial origins of myopia and underscores the potential relevance of investigating dietary contributions.
Within this broader etiological framework, nutrition has recently gained attention as a potentially modifiable factor. Emerging evidence suggests that dietary components, including macronutrients (carbohydrates, proteins, fats) and micronutrients (vitamins, minerals), may influence eye growth and refractive development via multiple mechanisms, such as modulation of IGF-1 signaling, oxidative stress, and inflammatory pathways (7–9). Nevertheless, studies investigating the association between specific nutrients and adolescent myopia have yielded conflicting results. For instance, some cross-sectional studies suggest that a high carbohydrate intake may elevate myopia risk by inducing hyperglycemic responses and accelerating axial elongation (10, 11), whereas others report no significant associations (12). Similarly, protein-rich diets have been postulated to confer protective effects by supporting collagen synthesis and maintaining scleral integrity (13, 14), yet the evidence remains inconsistent.
To address these knowledge gaps, this systematic review and meta-analysis aim to comprehensively synthesize current evidence on the associations between key nutritional factors—including carbohydrates, proteins, cholesterol, and sodium—and myopia in adolescents. By integrating data from observational studies, we seek to quantify the magnitude of these associations, identify potential sources of heterogeneity, and establish a more robust foundation for future research and public health interventions.
Materials and methods
Data sources and search strategy
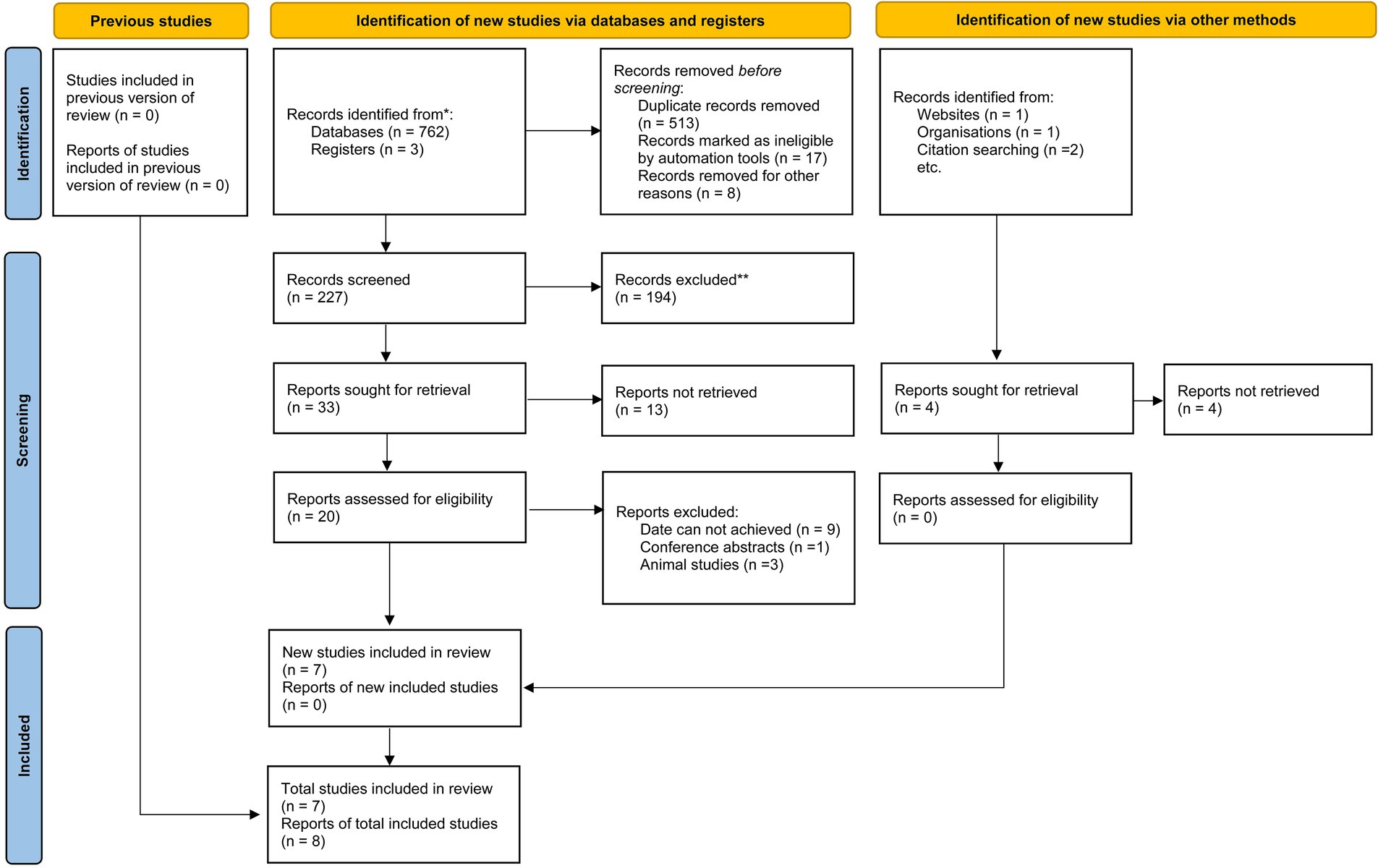
A systematic search was conducted across four databases: PubMed, Web of Science, Scopus, and Embase, up to February 15, 2025. The search strategy combined keywords related to nutritional factors (carbohydrates, proteins, cholesterol, sodium, dietary fats, vitamins, minerals), myopia (myopia, nearsightedness, refractive error), and adolescents (adolescents, children, juveniles, young adults). Detailed search terms for each database are listed in Table 1 and Figure 1.
Inclusion and exclusion criteria
Inclusion criteria: (1) Observational studies (cross-sectional, cohort) evaluating the association between dietary intake (carbohydrates, proteins, cholesterol, sodium) and myopia in adolescents aged 6–18 years. (2) Studies reporting quantitative data (e.g., mean intake, effect estimates) for at least one nutritional factor. (3) Myopia defined as spherical equivalent (SE) ≤ −0.50 D or cycloplegic refraction ≤ − 0.50 D. Exclusion criteria: (1) Interventional studies, case reports, reviews, or studies without original data. (2) Studies focusing on other refractive errors (e.g., hyperopia) or non-adolescent populations.
Although our search strategy was intentionally broad (including fats, vitamins, and minerals), only carbohydrates, proteins, cholesterol, and sodium had sufficient and comparable data across multiple studies to allow quantitative synthesis. Other nutrients were excluded from the pooled analysis due to the paucity of eligible studies, but they remain important topics for future research.
Study selection and data extraction
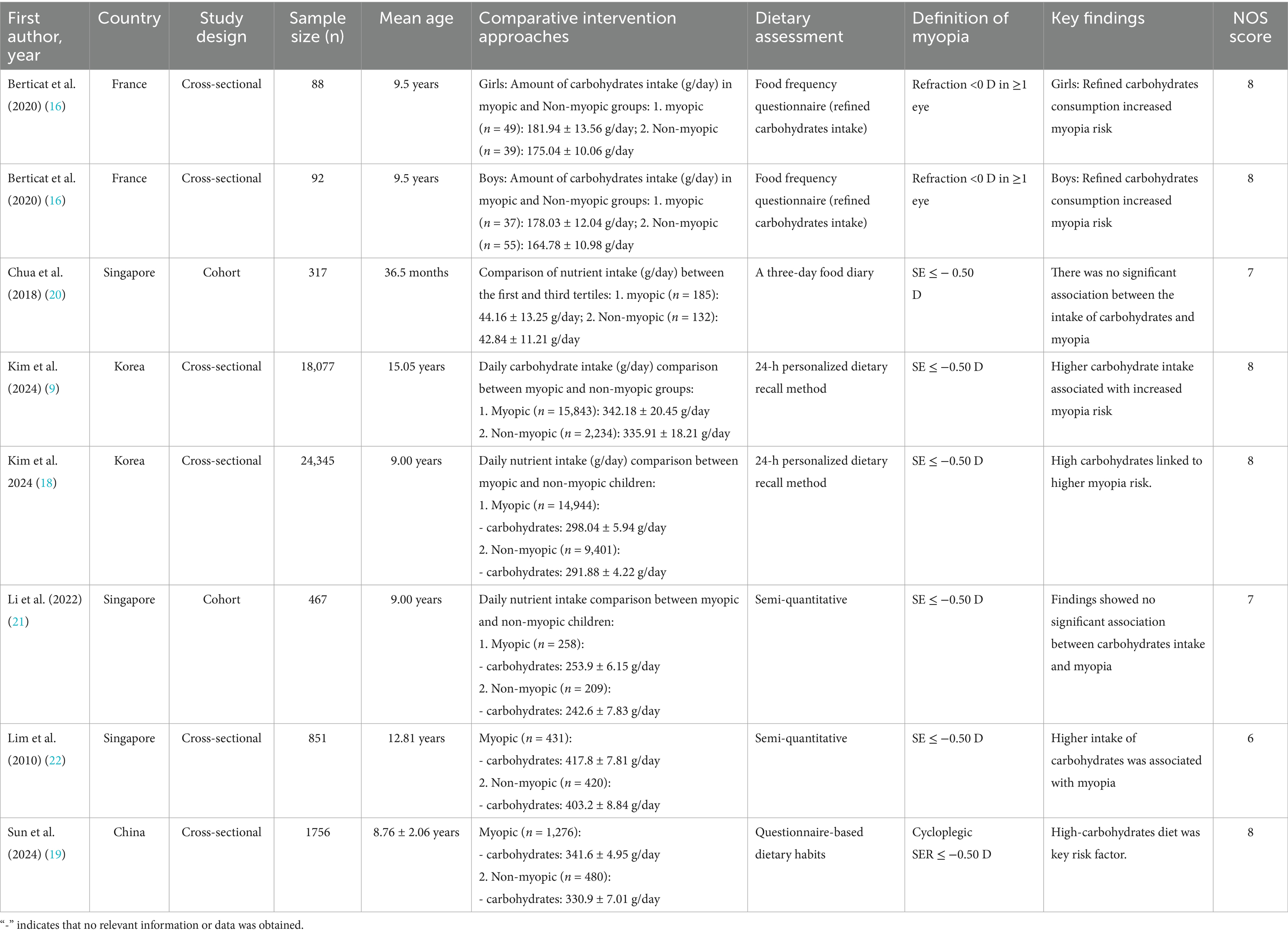
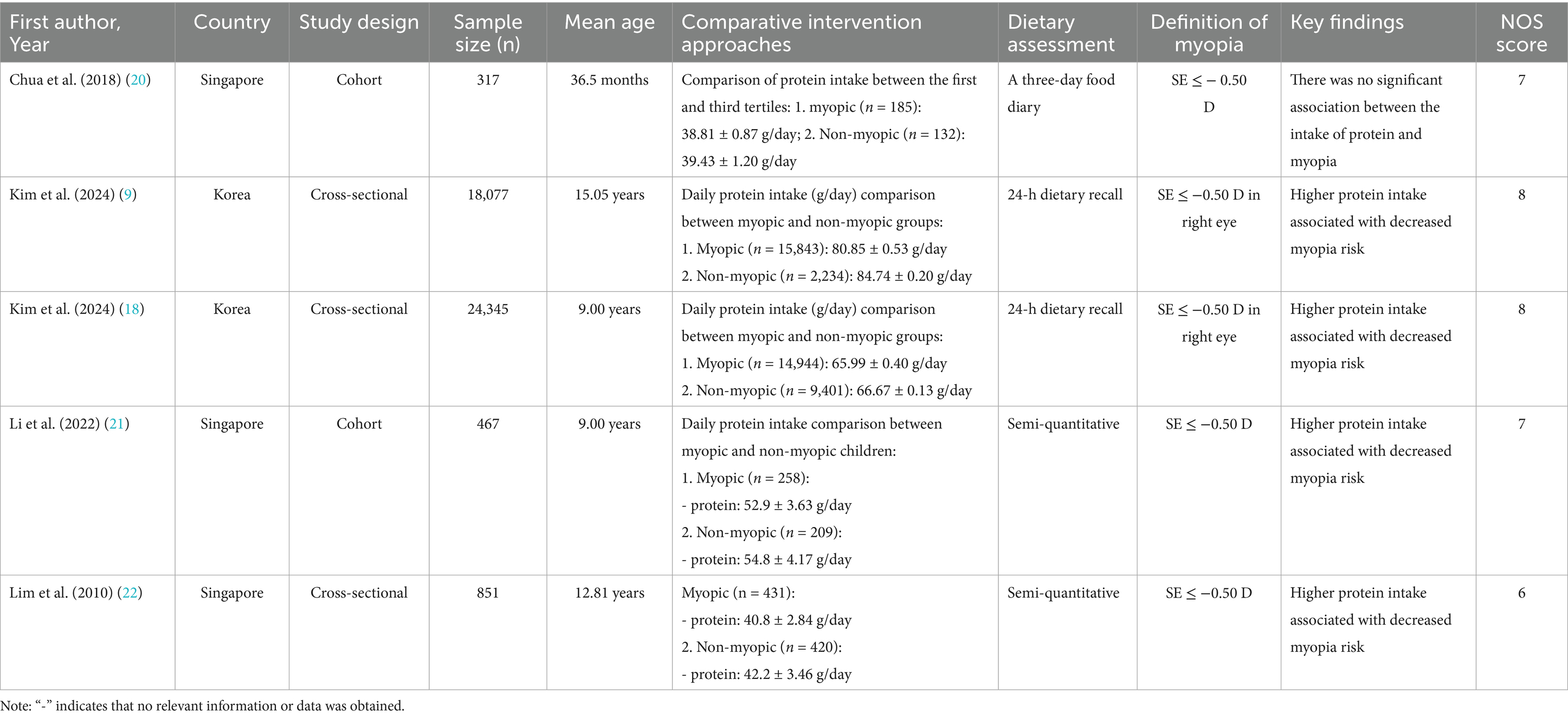
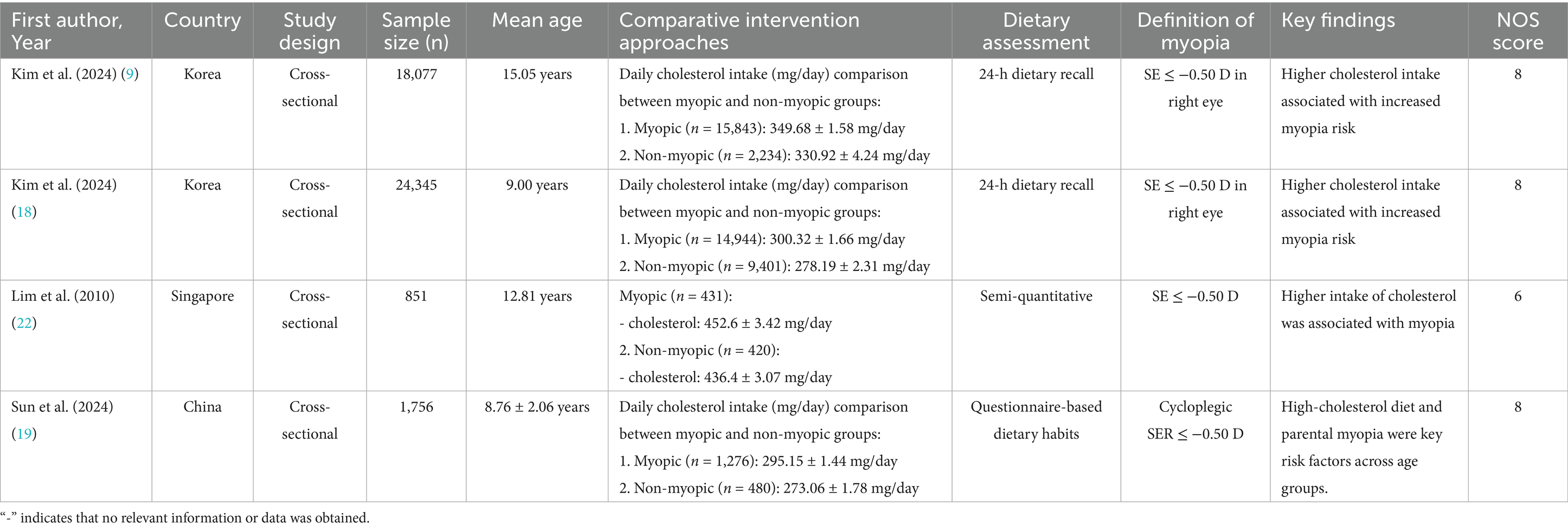
Two authors independently screened titles/abstracts and full texts for eligibility. Discrepancies were resolved through consensus. Data extracted included: (1) Study characteristics: Country, design, sample size, mean age, and NOS score. (2) Dietary assessment methods: Food frequency questionnaires (FFQ), 24-h dietary recall, or semi-quantitative diaries. (3) Myopia definition: SE thresholds and cycloplegic requirements. (4) Key findings: Mean nutrient intake (g/day or mg/day) in myopic vs. non-myopic groups. Full details of included studies are summarized in Tables 2–5.
Quality assessment
The quality of each included study was assessed using the Newcastle-Ottawa Scale (NOS) (15), which evaluates three key methodological domains in observational studies: selection of the study population, comparability of study groups, and outcome assessment. For cohort studies, the assessment criteria included the representativeness of the exposed cohort, the selection of an appropriate non-exposed cohort, and the adequacy of follow-up. In case–control studies, key factors such as the definition of cases and controls, the selection of control groups, and the assessment of exposure were considered. For cross-sectional studies, the evaluation focused on sampling methods, population definition, and the assessment of both exposure and outcome measures. Each study was assigned a score ranging from 0 to 9, with higher scores indicating better methodological rigor. Studies scoring ≥7 stars were classified as high-quality.
Statistical analysis
Standardized mean differences (SMDs) with 95% confidence intervals (CIs) were calculated for continuous outcomes (nutrient intake) using the inverse variance method, ensuring comparability across studies with different measurement units (e.g., grams/day vs. milligrams/day). Heterogeneity among studies was assessed using the Cochrane Q statistic and the I2 statistic, where a significant Q test (p < 0.05) indicated the presence of heterogeneity, and an I2 value greater than 50% suggested substantial heterogeneity. Given the substantial heterogeneity observed in preliminary analyses, we conducted predefined subgroup analyses according to study design (cross-sectional vs. cohort), dietary assessment method (24-h recall vs. FFQ vs. semi-quantitative diary), and study region (East Asia vs. Western countries). Meta-regression analyses were also performed where possible to further explore potential sources of heterogeneity. Sensitivity analyses were performed by sequentially excluding each study from the meta-analysis to evaluate the robustness of the pooled effect estimates and identify studies with disproportionate influence. Publication bias was assessed through visual inspection of funnel plot asymmetry and formally tested using Begg’s regression test, with p < 0.05 indicating potential bias. All statistical analyses were performed using Stata 17.0 (StataCorp, College Station, TX, USA) and Review Manager 5.4 (Cochrane Collaboration, Copenhagen, Denmark).
Results
Characteristics of included studies
A total of 7 articles (comprising 8 studies) (16–22) were included in the meta-analysis, involving 45,993 adolescents aged 6–18 years. The studies evaluated associations between carbohydrates (16–22) (8 studies, 45,993 participants), proteins (17, 18, 20–22) (5 studies, 44,057 participants), cholesterol (17–19, 22) (4 studies, 45,029 participants), and sodium (17–19) (3 studies, 44,178 participants) intake and myopia risk. Study designs included cross-sectional (6 studies) and cohort (2 studies) approaches. Dietary assessments varied, with 24-h dietary recalls (4 studies), food frequency questionnaires (3 studies), and semi-quantitative diaries (1 study) used. Study quality scores ranged from 6 to 8 stars on the NOS (Tables 2–5).
Carbohydrate intake and myopia risk
Pooled analysis of 7 articles (comprising 8 studies) (16–22) revealed a significant positive association between carbohydrate intake and myopia risk (SMD = 0.36, 95% CI: 0.22–0.50, I2 = 94.8%, p < 0.001; Figure 2). Subgroup analyses indicated that the very high heterogeneity (I2 = 94.8%) was partly attributable to study region and dietary assessment method. East Asian studies (Korea, China, Singapore) demonstrated a stronger positive association between carbohydrate intake and myopia (SMD = 0.38, 95% CI: 0.24–0.52; I2 = 88.2%), whereas the French cohorts showed weaker associations (SMD = 0.12, 95% CI: −0.05–0.29; I2 = 42.1%). Heterogeneity was also markedly reduced in studies using semi-quantitative dietary diaries or 24-h recalls (I2 = 70.4%) compared with those relying solely on food frequency questionnaires (I2 = 95.3%). Sensitivity analysis excluding individual studies did not alter the overall effect estimate (Figure 3A), indicating robustness. Funnel plot symmetry and Begg’s test (p = 0.12) suggested no publication bias (Figure 3B).
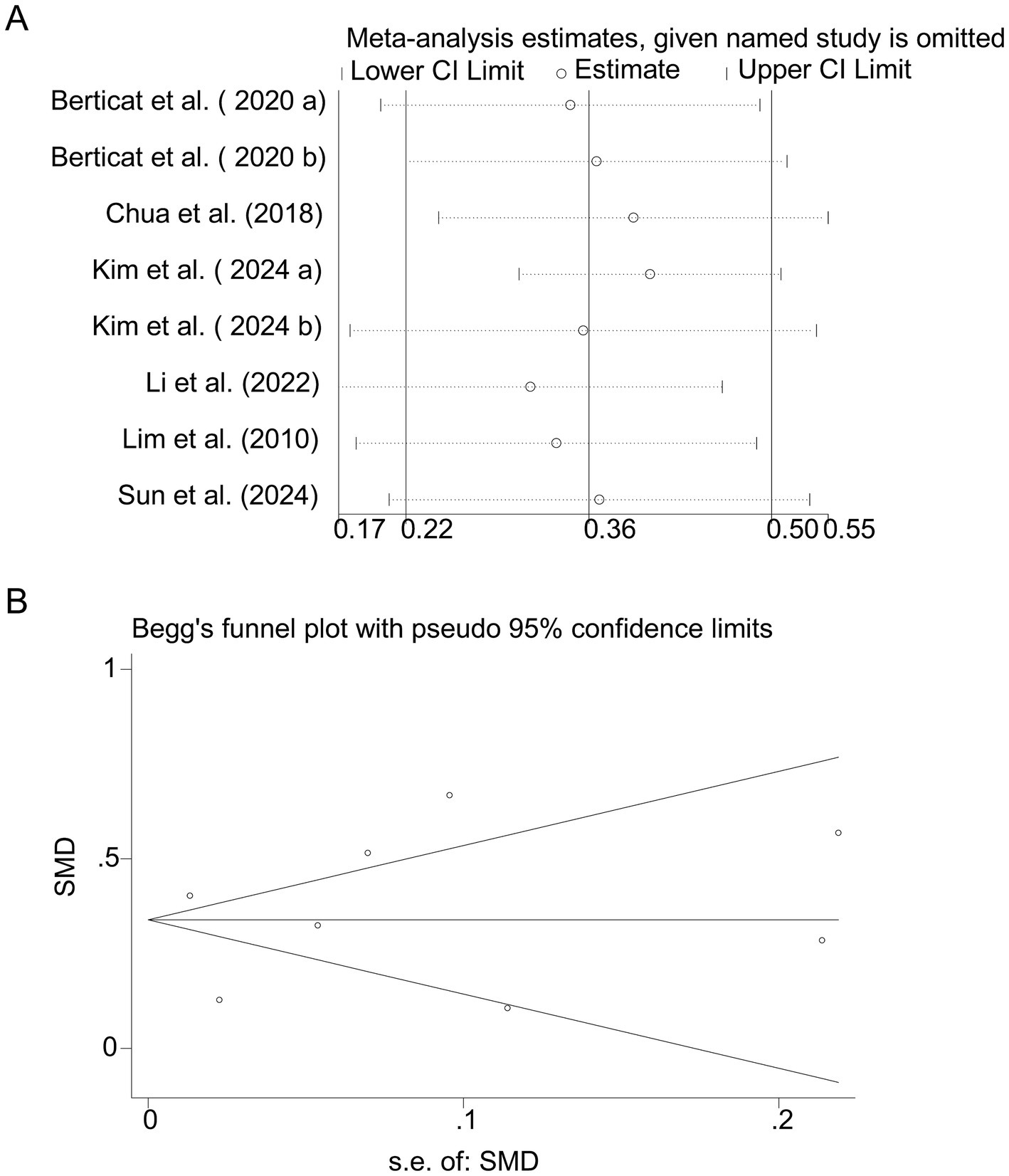
Figure 3. Sensitivity analysis and funnel plot for carbohydrate intake. (A) Sensitivity analysis for carbohydrate intake. (B) Funnel plot for carbohydrate intake publication bias.
Protein intake and myopia risk
Pooled analysis of 5 studies (17, 18, 20–22) demonstrated a protective effect of protein intake against myopia (SMD = −0.25, 95% CI: −0.27−−0.23, I2 = 44.0%, p < 0.001; Figure 4). Although heterogeneity was moderate (I2 = 44.0%), subgroup analyses showed that the protective association of protein intake remained stable across different study designs and regions. Animal-based protein–dominant studies demonstrated slightly stronger protective effects (SMD = −0.28, 95% CI: −0.33 to −0.23; I2 = 39.2%) compared with mixed or plant-based protein studies (SMD = −0.22, 95% CI: −0.27 to −0.18; I2 = 41.6%). Sensitivity analysis confirmed consistency across studies (Figure 5A), and no publication bias was detected (Begg’s test, p = 0.87; Figure 5B).
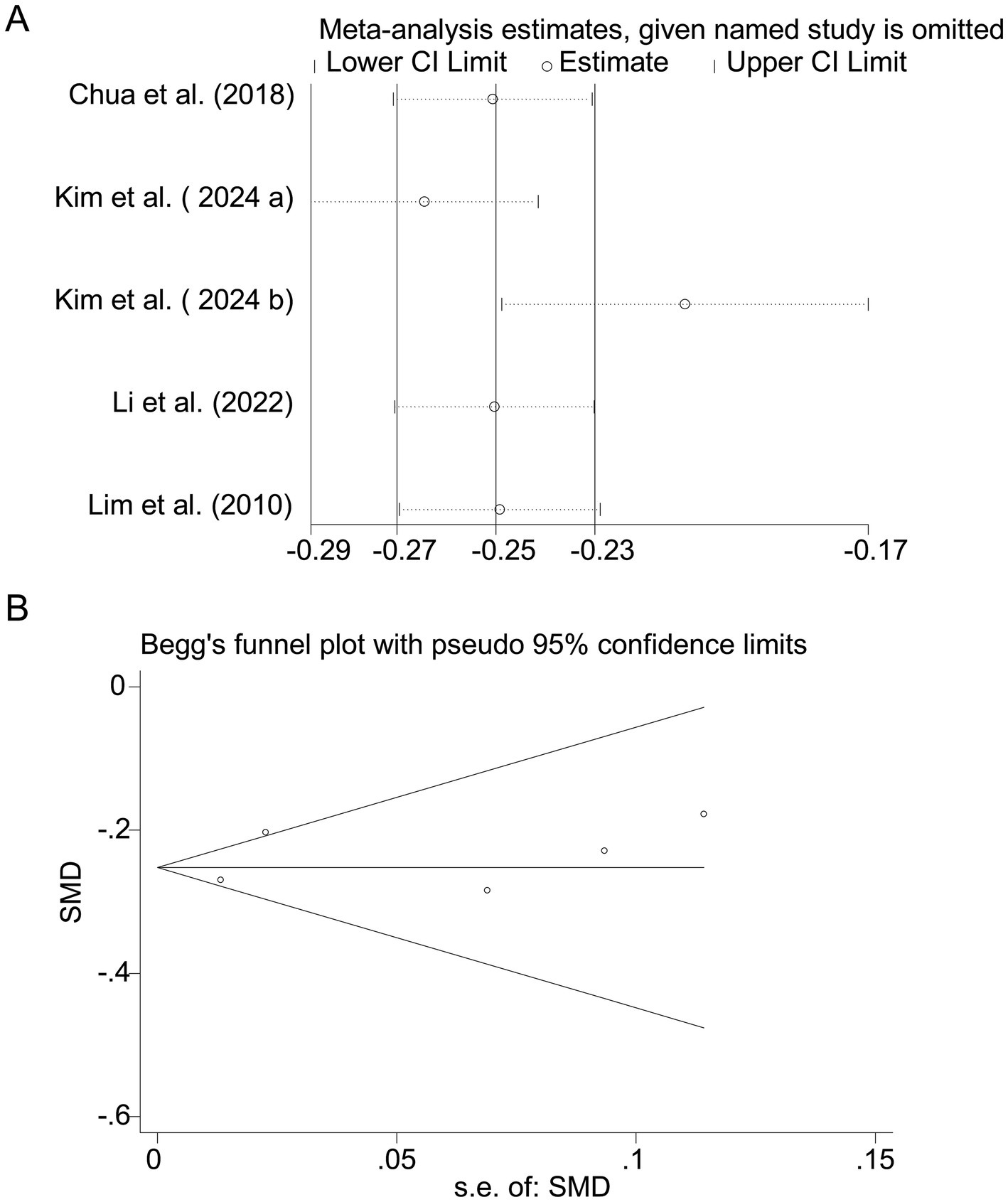
Figure 5. Sensitivity analysis and funnel plot for protein intake. (A) Sensitivity analysis for protein intake. (B) Funnel plot for protein intake publication bias.
Cholesterol intake and myopia risk
Four studies (17–19, 22) showed a significant positive association between cholesterol intake and myopia risk (SMD = 0.20, 95% CI: 0.10–0.31, I2 = 91.7%, p < 0.001; Figure 6). Sensitivity analysis supported the robustness of the effect (Figure 7A), and no publication bias was evident (Begg’s test, p = 0.53; Figure 7B). The high heterogeneity (I2 = 91.7%) was substantially influenced by dietary assessment methods. In studies using 24-h dietary recalls, the association between cholesterol intake and myopia was more consistent (SMD = 0.22, 95% CI: 0.15–0.30; I2 = 72.4%), whereas questionnaire-based studies exhibited extreme variability (SMD = 0.19, 95% CI: −0.05–0.43; I2 = 93.5%). Regional differences also contributed, with East Asian populations showing stronger associations than Western cohorts.
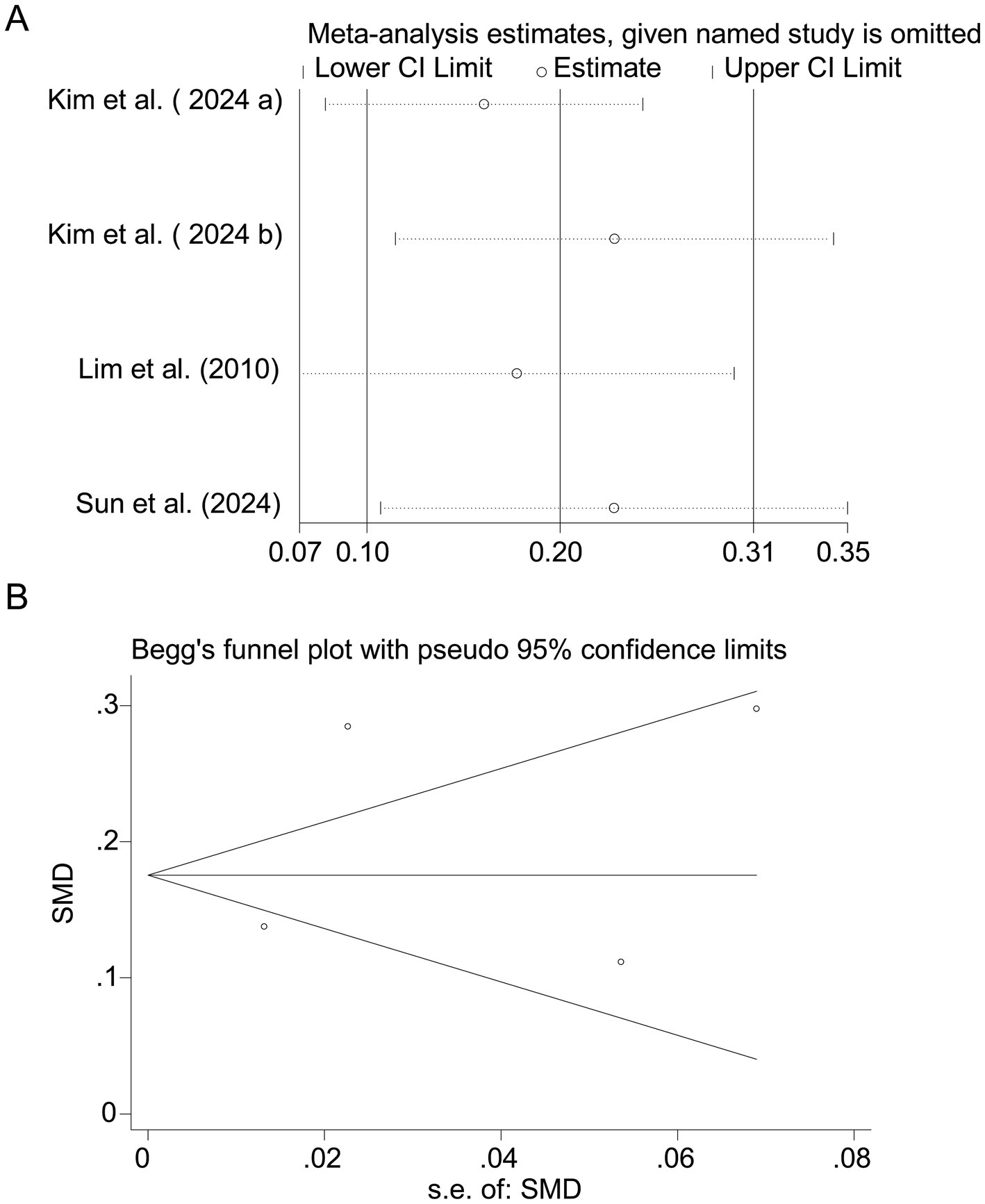
Figure 7. Sensitivity analysis and funnel plot for cholesterol intake. (A) Sensitivity analysis for cholesterol intake. (B) Funnel plot for cholesterol intake publication bias.
Sodium intake and myopia risk
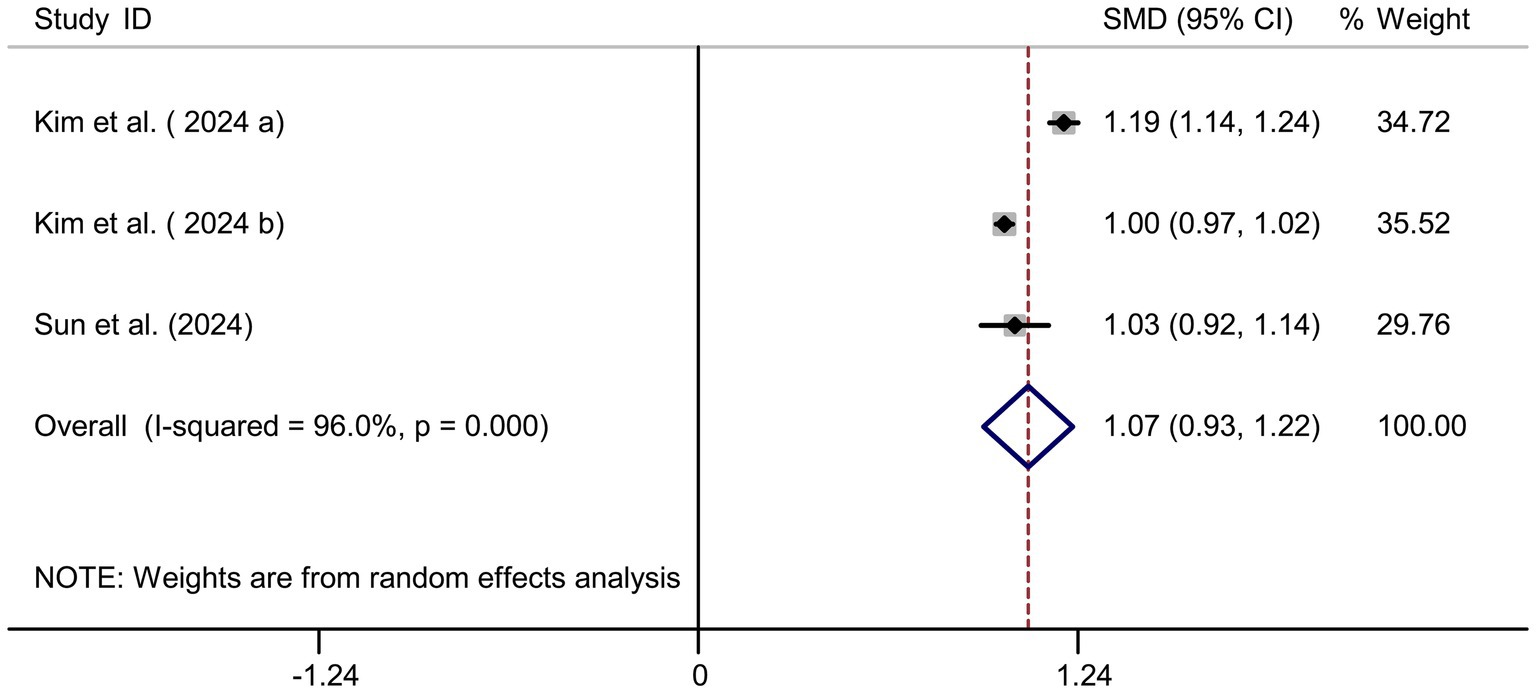
Pooled results from 3 studies (17–19) indicated a strong positive association between sodium intake and myopia (SMD = 1.07, 95% CI: 0.93–1.22, I2 = 96.0%, p < 0.001; Figure 8). Extreme heterogeneity likely stemmed from regional dietary habits (e.g., Korean vs. Chinese populations). Sensitivity analysis confirmed stable results (Figure 9A), and publication bias was absent (Begg’s test, p = 0.65; Figure 9B).
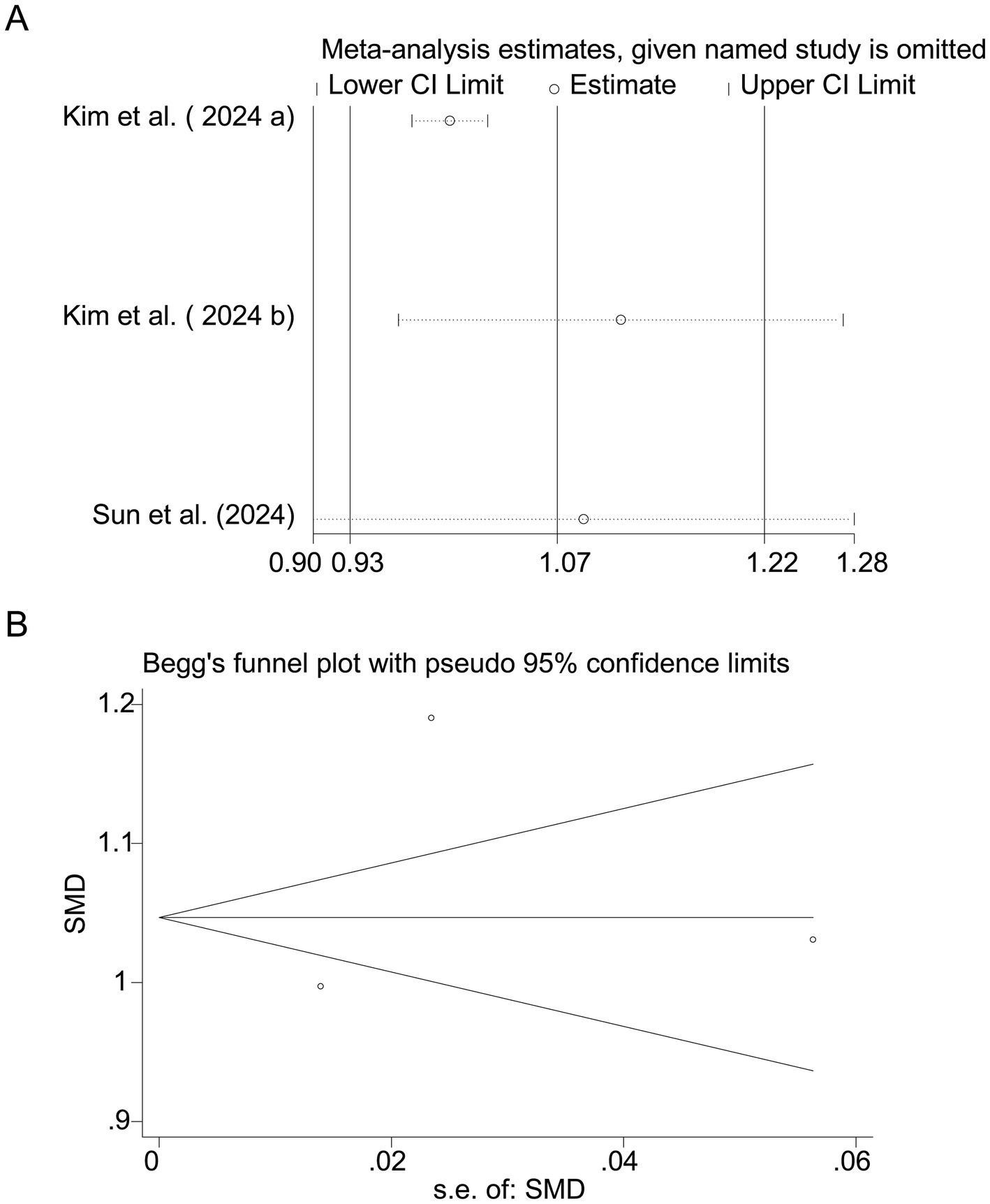
Figure 9. Sensitivity analysis and funnel plot for sodium intake. (A) Sensitivity analysis for sodium intake. (B) Funnel plot for sodium intake publication bias.
Discussion
Adolescent myopia has reached epidemic levels, particularly in East Asia, where prevalence rates exceed 30–50% (4, 11, 13). This rising trend is concerning due to the increased risk of sight-threatening complications such as retinal detachment and glaucoma (23). While genetic predisposition and environmental factors—including prolonged near work and reduced outdoor activity—are well-established contributors, our findings highlight the role of nutrition as a modifiable risk factor. Dietary modifications during adolescence could therefore serve as a complementary strategy to existing myopia control interventions, such as orthokeratology and atropine therapy.
Our meta-analysis identified dietary carbohydrates, cholesterol, and sodium as potential risk factors for myopia, whereas protein intake appeared to confer a protective effect. These associations align with proposed biological mechanisms. For instance, excessive carbohydrate intake may elevate insulin-like growth factor 1 (IGF-1) levels, thereby promoting scleral remodeling and axial elongation (24). Another hypothesized pathway is that high sodium intake may cause osmotic stress and disturb intraocular fluid balance, potentially contributing to axial elongation. Nutritional epidemiology provides some supportive human evidence: high dietary sodium has been linked to approximately a twofold increase in myopia risk in adolescents, and populations with traditionally low-salt diets (e.g., Amazonian natives) exhibit lower myopia prevalence (23). However, the causal role of sodium remains speculative, as longitudinal or interventional studies in youth are scarce. Conversely, proteins rich in essential amino acids (e.g., lysine, proline) may support collagen synthesis, enhancing scleral rigidity and resisting axial elongation (7). Importantly, subgroup analyses provided additional insights into the substantial heterogeneity observed in the main analyses. For carbohydrate intake, regional differences and dietary assessment methods explained a large proportion of variability: East Asian studies, characterized by higher refined carbohydrate consumption, demonstrated stronger associations than Western cohorts, while studies employing semi-quantitative diaries or 24-h recalls showed more consistent findings than those using food frequency questionnaires. For protein intake, the protective effect was stable across subgroups, but slightly stronger in studies emphasizing animal-based sources. Similarly, the heterogeneity in cholesterol and sodium analyses was substantially reduced when stratified by dietary assessment method, with 24-h recalls yielding more homogeneous results compared with questionnaire-based assessments. Regional dietary patterns, particularly the traditionally high sodium intake in Korea and China, also contributed to the heterogeneity. These subgroup findings strengthen the robustness of our results and highlight the influence of methodological and cultural factors on observed associations.
Quantitative synthesis indicated that higher carbohydrate intake was associated with increased myopia risk (SMD = 0.36, 95% CI: 0.22–0.50), particularly in East Asian cohorts. Subgroup analyses suggested that both study region and dietary assessment methods contributed to the observed heterogeneity. One possible mechanism is that excessive carbohydrate intake may influence insulin-like growth factor-1 (IGF-1) signaling, thereby promoting scleral remodeling and axial elongation. Some human data provide indirect support: IGF-1 gene polymorphisms have been associated with myopia susceptibility, and IGF-1/STAT3 pathways have been implicated in scleral remodeling in experimental studies (25, 26). Nevertheless, these mechanisms remain provisional, as direct confirmation in adolescent cohorts is limited.
Similarly, our pooled analysis demonstrated a protective association of protein intake with myopia (SMD = −0.25, 95% CI: −0.27 to −0.23), with relatively low heterogeneity (I2 = 44.0%). This protective effect was observed consistently across study designs and regions. A possible explanation is that protein intake, particularly rich in amino acids like lysine and proline, may support collagen synthesis and scleral rigidity, thereby reducing susceptibility to axial elongation. While this rationale is biologically plausible, current evidence is mostly experimental or animal-based, with limited direct validation in adolescent populations (27, 28). Beyond supporting collagen synthesis and scleral rigidity, dietary proteins could exert broader protective effects against myopia through their influence on skeletal growth, body composition, and energy metabolism. Adequate protein intake is essential for normal skeletal development during adolescence, a period of rapid axial elongation; balanced skeletal growth may help maintain proportional ocular development and reduce the tendency toward excessive axial lengthening (29). Proteins also modulate body composition by promoting lean muscle mass over adiposity, which is relevant because higher adiposity has been linked to metabolic disturbances and myopia risk (30). In addition, proteins contribute to efficient energy metabolism and glycemic regulation, potentially counteracting hyperinsulinemia and IGF-1 dysregulation that drive scleral remodeling (31). Together, these pathways suggest that sufficient protein intake may create a systemic environment less conducive to pathological eye growth, thereby offering a plausible biological basis for its protective association with myopia. Nevertheless, these pathways are hypothetical and require validation in experimental or longitudinal studies.
In addition to the four nutritional factors evaluated in our meta-analysis, other dietary components have also been investigated in relation to myopia (5, 32). For example, several large cross-sectional studies and meta-analyses have examined vitamin D, while others have focused on antioxidants such as anthocyanins and polyunsaturated fatty acids (5, 32). These nutrients may exert protective effects through anti-inflammatory and antioxidative mechanisms, as well as maintenance of scleral extracellular matrix integrity. However, despite the relatively larger body of work on vitamin D, the evidence across these nutrients remains inconsistent, with heterogeneity in study design, exposure measurement, and confounder adjustment. Our study therefore focused on carbohydrates, proteins, cholesterol, and sodium, which had sufficient comparable data for quantitative synthesis, but future work should incorporate these additional nutrients to build a more comprehensive understanding of the dietary determinants of myopia.
The associations between myopia and both cholesterol (SMD = 0.20, 95% CI: 0.10–0.31) and sodium (SMD = 1.07, 95% CI: 0.93–1.22) warrant cautious interpretation. High cholesterol intake has been speculated to promote ocular inflammation and oxidative stress, thereby contributing to myopia development. However, evidence in adolescents remains indirect, primarily from cross-sectional associations, and mechanistic data at the human level are scarce. These findings should therefore be interpreted cautiously until confirmed in prospective studies. However, the substantial heterogeneity in sodium-related studies (I2 = 96.0%) highlights the need for culturally specific dietary guidelines, as sodium intake varies significantly across populations (e.g., Korean diets typically contain higher sodium levels than Western diets). Although subgroup analyses helped to identify potential sources of heterogeneity—such as study design, dietary assessment method, and regional dietary patterns—residual heterogeneity remained high in some comparisons. This suggests that additional unmeasured factors, such as differences in carbohydrate quality, protein sources, cooking practices, or unadjusted confounders (e.g., parental myopia, physical activity), may further contribute to the variability. Future studies should incorporate standardized dietary assessment tools and harmonized outcome definitions to minimize these sources of heterogeneity.
This meta-analysis systematically synthesized evidence from eight studies, providing robust effect estimates for key nutrients. Our rigorous inclusion criteria, quality assessment (NOS scores ≥6), and sensitivity analyses strengthened the reliability of our conclusions. However, substantial heterogeneity across studies—arising from differences in dietary assessment methods, myopia definitions, and study designs—may limit the generalizability of our findings. Most included studies were cross-sectional, precluding causal inferences. Another important limitation is that key confounders such as obesity, socioeconomic status, parental myopia, and physical activity were not consistently adjusted for across the included studies. These factors are well-established contributors to myopia risk and may interact with dietary intake, potentially leading to residual confounding in our pooled estimates. For instance, obesity is associated with both dietary patterns and refractive development, while parental myopia strongly influences genetic susceptibility. Therefore, the observed associations between nutrition and myopia should be interpreted with caution. In addition, residual confounding by other unmeasured factors (e.g., differences in carbohydrate quality, protein sources, cooking practices) and variations in nutrient bioavailability may also have influenced the results. To establish causality, future research should prioritize longitudinal studies with validated dietary assessments and comprehensive adjustment for these confounders, as well as investigations into gene-diet interactions and clinical trials testing dietary interventions.
To establish causality, future research should prioritize longitudinal studies with validated dietary assessments, such as 24-h recalls combined with biomarkers. Additionally, investigations into gene-diet interactions (e.g., polymorphisms in IGF-1 signaling pathways) could provide insights into individual susceptibility to diet-induced myopia progression. Clinical trials evaluating dietary interventions, such as low-carbohydrate, high-protein diets, may further clarify the role of nutrition in myopia management. Nevertheless, the evidence base is limited, with only eight observational studies included—most of which are cross-sectional—severely restricting causal inference. Therefore, our results should not be interpreted as direct evidence to support policy-level dietary recommendations. At this stage, nutritional factors can be viewed as potential modifiable correlates rather than established causal determinants of myopia. Future large-scale, prospective cohort studies and randomized controlled trials are warranted to provide higher-level evidence.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: the data that support the findings of this study are available from the corresponding author upon reasonable request. Requests to access these datasets should be directed to ZX, Mjc0Mjc4OTYxQGhidXN0LmVkdS5jbg==.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
ZX: Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by The Special Scientific Research Fund of Medical College of Facial Features, Hubei University of Science and Technology (No. 2020XZ43); Science and Technology Plan Project of Xianning Municipal in 2022 (No. 2022SFYF014); Hubei Provincial-Level Teaching Reform Project (No. 2022419); Special Program on Ideological and Political Education in Medical Curricula, Hubei University of Science and Technology (No. 2023YSZ14); Teaching Research Project of Hubei University of Science and Technology (No. 2023XY052).
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Conflict of interest
The author(s) declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen, N, Sheng, Y, Wang, G, and Liu, J. Association between physical indicators and myopia in American adolescents: National Health and nutrition examination survey 1999-2008. Am J Ophthalmol. (2024) 260:132–9. doi: 10.1016/j.ajo.2023.12.014
2. Harb, EN, and Wildsoet, CF. Nutritional factors and myopia: An analysis of National Health and nutrition examination survey data. Optom Vis Sci. (2021) 98:458–68. doi: 10.1097/opx.0000000000001694
3. Lee, S, Lee, HJ, Lee, KG, and Kim, J. Obesity and high myopia in children and adolescents: Korea National Health and nutrition examination survey. PLoS One. (2022) 17:e0265317. doi: 10.1371/journal.pone.0265317
4. Zhang, RH, Yang, Q, Dong, L, Li, YF, Zhou, WD, Wu, HT, et al. Association between vitamin D and myopia in adolescents and young adults: evidence of national cross-sectional study. Eur J Ophthalmol. (2023) 33:1883–91. doi: 10.1177/11206721231161498
5. Tao, Q, Chang, Y, Day, AS, Wu, J, and Wang, X. Association between serum 25-hydroxyvitamin D level and myopia in children and adolescents: a cross-sectional study. Transl Pediatr. (2024) 13:310–7. doi: 10.21037/tp-23-617
6. Machluf, Y, Mezer, E, and Chaiter, Y. Comment on: association between physical indicators and myopia in American adolescents: NHANES 1999-2008. Am J Ophthalmol. (2024) 264:243–4. doi: 10.1016/j.ajo.2024.02.039
7. Chou, YL, Hsu, YA, Lin, CF, Chen, CS, Tien, PT, Wang, YC, et al. Complement decay-accelerating factor inhibits inflammation-induced myopia development. Mol Immunol. (2024) 171:47–55. doi: 10.1016/j.molimm.2024.05.005
8. Li, T, Deng, C, Li, J, Chen, Y, Chen, X, Zhang, N, et al. Mediation effect of sleep time on the association between outdoor activity and myopia in Chinese children and adolescents: a cross-sectional study. J Public Health (Oxf). (2024) 46:376–82. doi: 10.1093/pubmed/fdae104
9. Kim, H, Shahraki, K, and Suh, DW. Myopia trends among children and adolescents: a nationwide study in South Korea. J AAPOS. (2024) 28:103969. doi: 10.1016/j.jaapos.2024.103969
10. Xiao, K, Chen, R, Lin, R, Hu, W, Liu, J, Yusufu, M, et al. Association between micronutrients and myopia in American adolescents: evidence from the 2003-2006 National Health and nutrition examination survey. Front Nutr. (2024) 11:1477403. doi: 10.3389/fnut.2024.1477403
11. Zhao, L, Jiang, X, Zhang, W, Hao, L, Zhang, Y, Wu, S, et al. Prevalence and risk factors of myopia among children and adolescents in Hangzhou. Sci Rep. (2024) 14:24615. doi: 10.1038/s41598-024-73388-7
12. Ye, S, Hou, X, Song, K, Wang, L, Shi, Y, and Kang, Z. Association between dietary inflammatory index and adolescent myopia based on the National Health and nutrition examination survey. Sci Rep. (2024) 14:28048. doi: 10.1038/s41598-024-78629-3
13. Chen, Y, Xu, Q, Lv, L, Liu, Y, Zhang, Z, and Yang, Z. The association between anthocyanin intake and myopia in adolescents: a cross-sectional study of NHANES. Front Pediatr. (2024) 12:1503926. doi: 10.3389/fped.2024.1503926
14. Jin, Z, Liu, Y, and Luo, Y. Linear relationship between triglyceride-glucose-body mass index and myopia in United States adolescents. Eur J Ophthalmol. (2024) 35:11206721241306155. doi: 10.1177/11206721241306155
15. Wen, X, Xiong, Y, Qu, X, Jin, L, Zhou, C, Zhang, M, et al. The risk of endometriosis after exposure to endocrine-disrupting chemicals: a meta-analysis of 30 epidemiology studies. Gynecol Endocrinol. (2019) 35:645–50. doi: 10.1080/09513590.2019.1590546
16. Berticat, C, Mamouni, S, Ciais, A, Villain, M, Raymond, M, and Daien, V. Probability of myopia in children with high refined carbohydrates consumption in France. BMC Ophthalmol. (2020) 20:337. doi: 10.1186/s12886-020-01602-x
17. Kim, JM, and Choi, YJ. Association between dietary nutrient intake and prevalence of myopia in Korean adolescents: evidence from the 7th Korea National Health and nutrition examination survey. Front Pediatr. (2023) 11:1285465. doi: 10.3389/fped.2023.1285465
18. Kim, JM, and Choi, YJ. Nutritional intake, environmental factors, and their impact on myopia prevalence in Korean children aged 5-12 years. J Health Popul Nutr. (2024) 43:14. doi: 10.1186/s41043-024-00506-6
19. Sun, Y, Li, Z, Wang, Z, He, X, Yu, S, Hu, L, et al. Association of 10 genetic variations and 10 environmental factors with myopia of different severities in different age groups of people in Northeast China. Front Biosci. (2024) 29:9. doi: 10.31083/j.fbl2901009
20. Chua, SY, Sabanayagam, C, Tan, CS, Lim, LS, Toh, JY, Chong, YS, et al. Diet and risk of myopia in three-year-old Singapore children: the GUSTO cohort. Clin Exp Optom. (2018) 101:692–9. doi: 10.1111/cxo.12677
21. Li, M, Tan, CS, Foo, LL, Sugianto, R, Toh, JY, Sun, CH, et al. Dietary intake and associations with myopia in Singapore children. Ophthalmic Physiol Opt. (2022) 42:319–26. doi: 10.1111/opo.12929
22. Lim, LS, Gazzard, G, Low, YL, Choo, R, Tan, DTH, Tong, L, et al. Dietary factors, myopia, and axial dimensions in children. Ophthalmology. (2010) 117:993–997.e4. doi: 10.1016/j.ophtha.2009.10.003
23. Yin, C, Gan, Q, Xu, P, Yang, T, Xu, J, Cao, W, et al. Weight status and myopia in children and adolescents: a Nationwide cross-sectional study of China. Nutrients. (2025) 17:260. doi: 10.3390/nu17020260
24. Cheng, T, Wang, J, Xiong, S, Zhang, B, Li, Q, Xu, X, et al. Association of IGF1 single-nucleotide polymorphisms with myopia in Chinese children. PeerJ. (2020) 8:e8436. doi: 10.7717/peerj.8436
25. Zhang, X, Zhou, X, and Qu, X. The association between IGF-1 polymorphisms and high myopia. Int J Clin Exp Med. (2015) 8:10158–67.
26. Zidan, HE, Rezk, NA, Fouda, SM, and Mattout, HK. Association of Insulin-like Growth Factor-1 gene polymorphisms with different types of myopia in Egyptian patients. Genet Test Mol Biomarkers. (2016) 20:291–6. doi: 10.1089/gtmb.2015.0280
27. Wu, LJ, You, QS, Duan, JL, Luo, YX, Liu, LJ, Li, X, et al. Prevalence and associated factors of myopia in high-school students in Beijing. PLoS One. (2015) 10:e0120764. doi: 10.1371/journal.pone.0120764
28. Tao, Y, Pan, M, Liu, S, Fang, F, Lu, R, Lu, C, et al. cAMP level modulates scleral collagen remodeling, a critical step in the development of myopia. PLoS One. (2013) 8:e71441. doi: 10.1371/journal.pone.0071441
29. Darling, AL, Manders, RJF, Sahni, S, Zhu, K, Hewitt, CE, Prince, RL, et al. Dietary protein and bone health across the life-course: an updated systematic review and meta-analysis over 40 years. Osteoporos Int. (2019) 30:741–61. doi: 10.1007/s00198-019-04933-8
30. Phillips, SM. Nutritional supplements in support of resistance exercise to counter age-related sarcopenia. Adv Nutr. (2015) 6:452–60. doi: 10.3945/an.115.008367
31. Stubbs, J, R,, Horgan, G, Robinson, E, Hopkins, M, Dakin, C, et al. Diet composition and energy intake in humans. Philos Trans R Soc Lond Ser B Biol Sci. (2023) 378:20220449. doi: 10.1098/rstb.2022.0449
Keywords: myopia, adolescents, nutritional factors, dietary intake, systematic review
Citation: Xu Z (2025) Association between nutritional factors and myopia in adolescents: a systematic review and meta-analysis. Front. Public Health. 13:1670103. doi: 10.3389/fpubh.2025.1670103
Edited by:
Jennifer K. Frediani, Emory University, United StatesReviewed by:
Thiago Gonçalves dos Santos Martins, Federal University of São Paulo, BrazilFarheen Tariq, Xi'an Jiaotong University, China
Copyright © 2025 Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaoxia Xu, Mjc0Mjc4OTYxQGhidXN0LmVkdS5jbg==
 Zhaoxia Xu
Zhaoxia Xu