- 1Graduate School, Bengbu Medical University, Bengbu, China
- 2Nursing Department, The Third People's Hospital of Bengbu, Bengbu, China
- 3School of Humanities and Health, Bengbu Medical University, Bengbu, China
Objectives: To investigate the stage-specific prevalence and progression mechanisms of sarcopenia in aging patients undergoing maintenance hemodialysis (MHD), and to identify modifiable risk and protective factors relevant to early public health interventions.
Methods: This multicenter cross-sectional study enrolled 448 eligible older adults (aged ≧ 60 years) undergoing maintenance hemodialysis (MHD) from three tertiary hospitals in Bengbu, China, between January and April 2025, using convenience sampling. Sarcopenia was classified according to the Asian Working Group for Sarcopenia (AWGS) 2019 criteria. Data on demographics, body composition, nutrition, self-efficacy, physical activity, and inflammation were collected. Logistic regression analyses identified factors associated with sarcopenia onset and progression.
Results: Sarcopenia prevalence was 54.0%, with 25.0% at the possible stage, 19.6% confirmed, and 9.4% severe. Key protective factors for sarcopenia onset included female sex, higher basal metabolic rate (BMR), higher Body Mass Index (BMI) and greater self-efficacy (SES6). Risk factors included low physical activity, diabetes, longer dialysis vintage, and malnutrition (MQSGA). Progression to confirmed/severe stages was independently associated with reduced BMI, protein mass, and self-efficacy, along with elevated BMR and physical inactivity. These findings highlight the importance of early screening and personalized preventive strategies in aging MHD populations.
Conclusion: Sarcopenia is highly prevalent among older adults receiving MHD, with distinct stage-specific progression patterns. This study identified key modifiable risk and protective factors related to both the onset and progression of sarcopenia. Early detection of possible sarcopenia and timely interventions targeting nutrition, physical activity, and self-efficacy may delay progression and promote healthy aging in this population.
1 Introduction
With global population aging accelerating, sarcopenia has emerged as a major public health concern among older adults. Characterized by progressive loss of skeletal muscle mass, strength, and physical performance, sarcopenia is strongly associated with functional decline, increased risk of falls and fractures, hospitalization, and mortality. It imposes a significant burden on healthcare systems and threatens the successful implementation of healthy aging strategies (1).
Among older populations with chronic diseases, patients receiving MHD are at particularly high risk for developing sarcopenia (2, 3). The pathogenesis in this population is multifactorial and includes protein-energy wasting (PEW), chronic inflammation, dialysis-related metabolic disturbances, reduced physical activity, and psychosocial stressors (4). These mechanisms lead to accelerated muscle loss and functional impairment, contributing to poor quality of life and adverse clinical outcomes. As such, MHD patients represent a key target group for sarcopenia prevention and early intervention strategies in aging-related public health planning (5).
To address the need for early detection, the Asian Working Group for Sarcopenia (AWGS) introduced the concept of “possible sarcopenia” in its 2019 consensus, referring to a stage where muscle strength and/or physical function are reduced, but muscle mass remains within normal limits (6). This stage is considered potentially reversible and critical for preventive interventions (7, 8). However, current evidence on the epidemiological characteristics, clinical significance, and progression mechanisms of “possible sarcopenia” in the MHD population remains limited.
To fill this gap, the present study employed the AWGS 2019 staging criteria to assess the prevalence and stage-specific features of sarcopenia in a multicenter sample of MHD patients. We aimed to identify modifiable risk and protective factors associated with both the onset and progression of sarcopenia, thereby providing a scientific basis for early screening, stratified intervention, and public health decision-making in older hemodialysis patients. This evidence is expected to support healthy aging goals and improve outcomes in this vulnerable population.
2 Materials and methods
2.1 Study design and participants
This study is a multi-center cross-sectional study conducted with convenience sampling. The participants were selected from patients receiving MHD treatment at blood purification centers in three Grade A tertiary hospitals in Bengbu city from January to April 2025. The inclusion criteria were: (1) aged 60 years or older; (2) receiving hemodialysis for at least 3 months; (3) undergoing dialysis 2–3 times per week, with each session lasting 4 h and a blood flow rate of no less than 200 mL/min; (4) able to communicate and complete questionnaires and physical examinations; (5) voluntarily participating in the study and providing informed consent. The exclusion criteria included: (1) severe cognitive impairment or mental illness that prevented cooperation; (2) acute severe diseases within the past 6 months, such as infections, malignant tumors, or decompensated heart failure; (3) physical disabilities or metal implants (e.g., pacemaker) that precluded bioelectrical impedance testing; (4) use of glucocorticoids or immunosuppressants within the last 6 months; (5) pregnancy or lactation. This study was approved by the Ethics Committee of Bengbu Medical University (Ethical Approval Number: [2025] No. 187), and conducted in accordance with the principles of the Declaration of Helsinki. All participants provided written informed consent.
2.2 Survey tools
2.2.1 General information questionnaire
This questionnaire, designed by the research team based on literature review and clinical expertise, was used to collect the basic demographic and disease-related information of the patients. It includes: (1) demographic data: gender, age, smoking and drinking habits, etc.; (2) disease characteristics: dialysis vintage, comorbid diabetes, etc.; (3) physical measurements: height, weight, grip strength, BMI, and pre-dialysis blood pressure; (4) body composition parameters: protein content, BMR measured by bioelectrical impedance analysis (BIA); and (5) laboratory indicators: C-reactive protein (CRP), serum albumin, hemoglobin, serum creatinine, calcium, phosphate, and Kt/V.
2.2.2 Modified quantitative subjective global assessment (MQSGA)
The MQSGA, developed by Kalantar-Zadeh et al. (9), was used to assess the nutritional status of patients. It includes 7 items: weight changes, dietary intake, gastrointestinal discomfort, daily functioning, complications, subcutaneous fat loss, and muscle wasting. Each item is rated on a scale from 1 to 5, with a total score ranging from 7 to 35. Higher scores indicate greater nutritional risk or malnutrition.
2.2.3 International physical activity questionnaire - short form (IPAQ-SF)
Physical activity was assessed using the Chinese version of the IPAQ-SF (10), which includes 7 items related to vigorous-intensity, moderate-intensity, walking, and sitting activities performed during the past week. Activity energy expenditure is calculated using metabolic equivalents (MET): vigorous activity = 8.0 MET, moderate activity = 4.0 MET, walking = 3.3 MET. Based on the IPAQ guidelines, physical activity is classified into low, moderate, and high levels according to the total MET value and activity type (11).
2.2.4 Self-efficacy for managing chronic disease 6-item scale (SES6)
The SES6, developed by Stanford University, assesses patients’ self-efficacy in managing chronic diseases (12). It includes 6 items related to confidence in controlling symptoms, emotional regulation, maintaining physical function, and performing daily activities. Each item is rated from 0 to 10, with higher scores indicating stronger health behavior execution and self-regulation. The average score of all items reflects the patient’s overall self-efficacy (13).
2.2.5 Sarcopenia diagnosis criteria
Sarcopenia screening and diagnosis in this study were based on the AWGS 2019 (14) guidelines, considering muscle mass, strength, and physical function. Muscle strength was measured using a handgrip dynamometer, with values <28 kg for men and <18 kg for women indicating muscle strength decline. Physical function was assessed using the 6-meter walking test, with speeds <1.0 m/s indicating functional decline. Muscle mass was measured by BIA to calculate the appendicular skeletal muscle index (ASM/height2), with <7.0 kg/m2 for men and <5.7 kg/m2 for women indicating low muscle mass. Sarcopenia is categorized into three stages: Possible Sarcopenia, where there is a decline in muscle strength and/or physical function but muscle mass is not yet reduced; Confirmed Sarcopenia, where low skeletal muscle mass is combined with either muscle strength decline or physical function reduction; and Severe Sarcopenia, which involves low skeletal muscle mass, muscle strength decline, and physical function reduction.
2.2.6 Body composition and basal metabolic rate assessment
Body composition was assessed using the BIA method. Measurements were performed using the InBody 770 multi-frequency bioelectrical impedance analyzer (manufactured by Biospace, Korea). All measurements were conducted with the patient in a standing position. To minimize the influence of fluid shifts related to dialysis therapy, all body composition measurements were scheduled and performed within 30 min to 2 h following the patient’s mid-week dialysis session. Before measurement, participants were instructed to empty their bladder and bowels and to remove socks and metal objects. The assessment was carried out by uniformly trained research personnel following the device’s standard operating procedures.
The InBody 770 reports protein mass and calculates BMR using proprietary algorithms that incorporate measured fat-free mass and other impedance derived parameters. In this study, protein mass (kg) was used as a proxy for whole body protein reserves and skeletal muscle protein stores.
2.3 Data collection and quality control
To ensure data consistency and comparability and to minimize the potential influence of the dialysis treatment itself on patients’ subjective feelings and physiological state, all questionnaire assessments (including MQSGA, IPAQ-SF, and SES6) as well as grip strength and gait speed tests were uniformly conducted before the initiation of their mid-week dialysis session, when their physical condition was relatively stable.
Data were collected concurrently across all participating dialysis centers by research personnel who received standardized training. Prior to study initiation, all investigators were instructed on questionnaire administration, measurement techniques, and device operation to ensure procedural consistency and data reliability. Eligible participants were informed in detail about the study’s purpose and procedures and provided written informed consent before enrollment. Demographic and clinical information, as well as subjective assessments (MQSGA, IPAQ-SF, SES6), were obtained through structured face-to-face interviews. All forms were reviewed by a second investigator to ensure completeness and logical consistency.
Handgrip strength was measured prior to dialysis using an electronic dynamometer. Participants stood upright and held the device with their non-fistula hand, arm naturally extended. The test was performed three times, and the highest value was used in analysis. Gait speed was evaluated using a 6-meter walking test, with the mean value of two trials at a comfortable walking pace recorded. Laboratory data, including CRP, hemoglobin, serum albumin, creatinine, calcium, and phosphate, were extracted from pre-dialysis venous blood and analyzed in the hospital’s certified laboratory. All data were anonymized using coded identifiers and managed by designated personnel, with regular audits performed to ensure data integrity and traceability.
2.4 Statistical analysis
All data analyses were performed using SPSS version 26.0 (IBM Corp., Armonk, NY, USA). The distribution of continuous variables was assessed for normality. Variables with a normal distribution were presented as mean ± standard deviation (SD) and compared using independent-sample t-tests. Non-normally distributed variables were reported as median with interquartile range (IQR) and compared using the Mann–Whitney U test. Categorical variables were summarized as frequencies and percentages, and intergroup comparisons were conducted using the chi-square (χ2) test. For ordinal variables such as physical activity level, the Kruskal–Wallis H test was applied to assess differences across groups.
To identify factors associated with both sarcopenia occurrence and progression, two separate logistic regression analyses were conducted. The dependent variables were defined as (1) presence of sarcopenia (yes vs. no) and (2) sarcopenia severity (confirmed/severe vs. possible). Variables with a p value < 0.05 in univariate analyses were included in multivariate logistic regression models. For each model, regression coefficients (β), standard errors, Wald χ2 values, p values, odds ratios (OR), and 95% confidence intervals (95% CI) were reported. Statistical significance was set at a two-sided p < 0.05.
3 Results
3.1 Prevalence and classification of sarcopenia in MHD patients
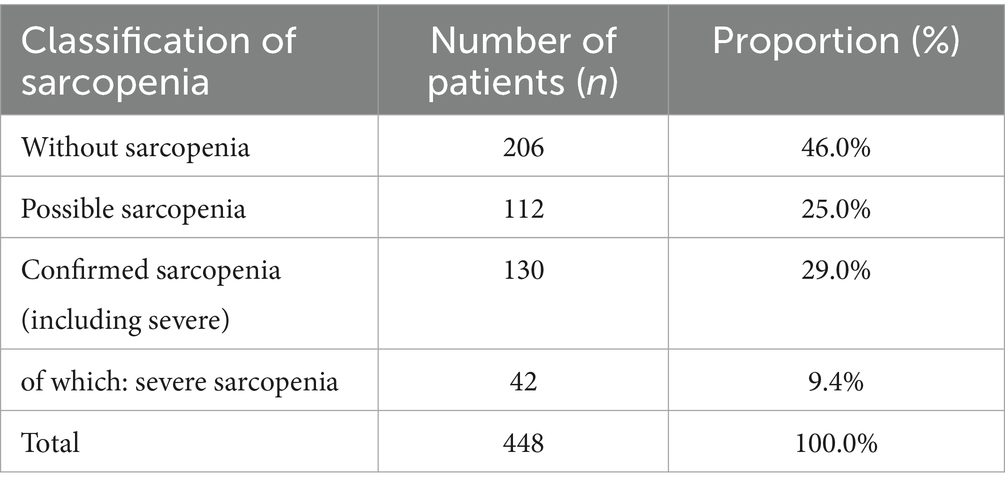
A total of 448 MHD patients were included in this study. Sarcopenia was classified according to the criteria established by the AWGS 2019. Among them, 206 patients (46.0%) were classified as without sarcopenia, 112 (25.0%) as possible sarcopenia, and 130 (29.0%) as confirmed sarcopenia. Of the confirmed cases, 42 (9.4% of all participants and 32.3% of those with confirmed sarcopenia) were further identified as having severe sarcopenia. The detailed distribution of sarcopenia classifications is shown in Table 1.
3.2 Clinical characteristics comparison between patients with and without sarcopenia
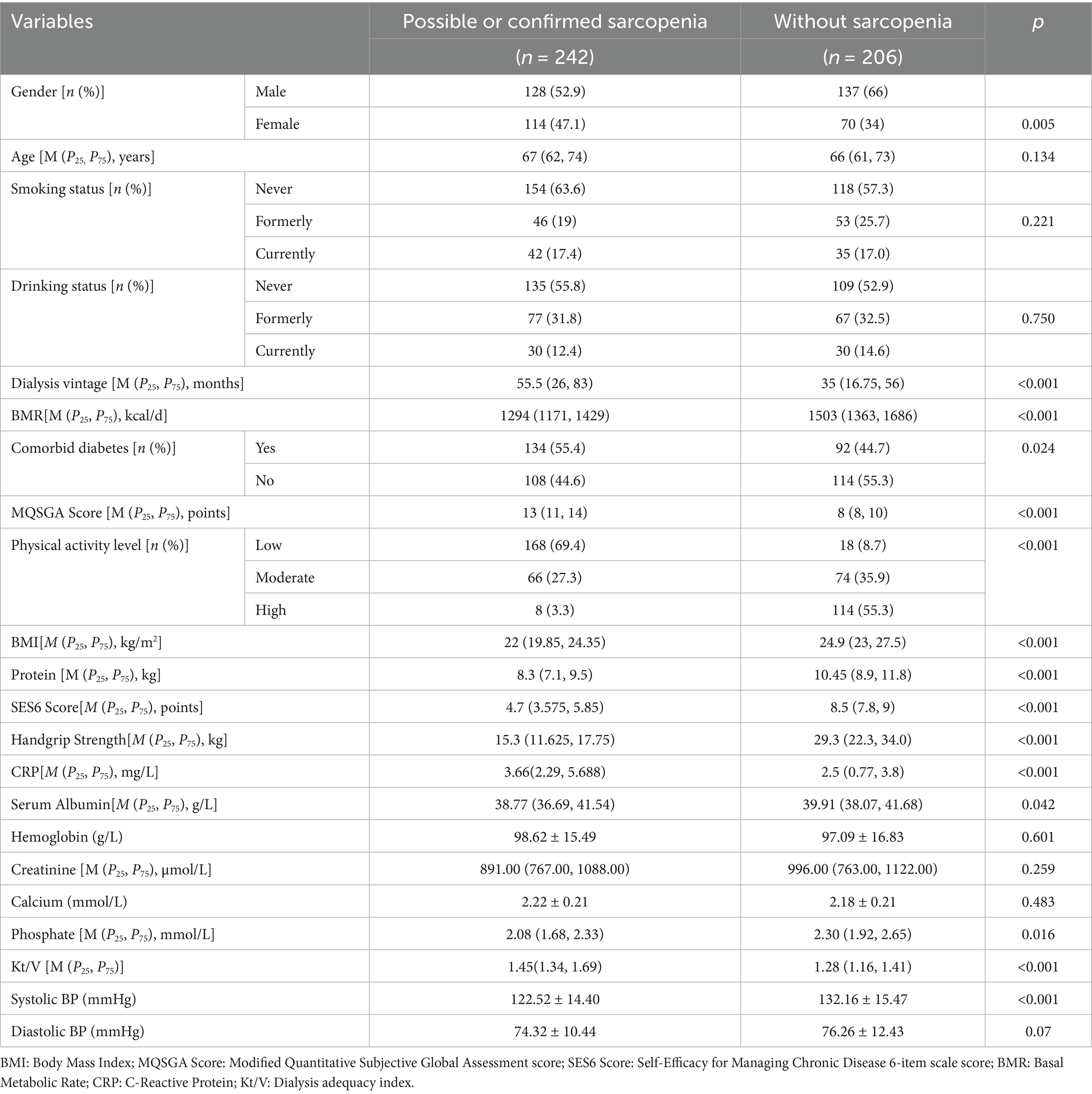
In this study, patients were classified into those with sarcopenia (n = 242), which included those with possible sarcopenia and confirmed sarcopenia (including severe cases), and those without sarcopenia (n = 206). The comparison of clinical characteristics between the two groups is shown in Table 2. Patients with sarcopenia had a significantly higher proportion of females (47.1% vs. 34.0%, p = 0.005), a longer median dialysis vintage [55.5 (26.0, 83.0) months vs. 35.0 (16.75, 56.0) months, p < 0.001], and a lower BMR [1,294 kcal/d vs. 1,503 kcal/d, p < 0.001]. The prevalence of comorbid diabetes was higher among those with sarcopenia (55.4% vs. 44.7%, p = 0.024). Regarding body composition and functional parameters, patients with sarcopenia had significantly lower BMI, protein mass, handgrip strength, and SES6 scores compared to those without sarcopenia (p < 0.001 for all). The MQSGA score was significantly higher in patients with sarcopenia (13 vs. 8, p < 0.001), indicating poorer nutritional status. Patients with sarcopenia also exhibited significantly lower physical activity levels, with 69.4% classified as having low physical activity, compared to only 8.7% in those without sarcopenia (p < 0.001). Additionally, CRP levels were significantly higher in patients with sarcopenia (p < 0.001), suggesting increased inflammation.
3.3 Analysis of factors influencing possible or confirmed sarcopenia in MHD patients
Logistic regression analysis was performed using patients without sarcopenia as the control and those with possible or confirmed sarcopenia as the case group. In the multivariable analysis, female sex (OR = 0.023, 95% CI: 0.001–0.402, p = 0.010), higher self-efficacy (SES6 score) (OR = 0.129, 95% CI: 0.062–0.269, p < 0.001), higher BMR (OR = 0.987, 95% CI: 0.976–0.998, p = 0.023), and higher BMI (OR = 0.738, 95% CI: 0.565–0.962, p = 0.025) were identified as protective factors against sarcopenia. Comorbid diabetes (OR = 4.639, 95% CI: 1.061–20.275, p = 0.041), longer dialysis vintage (OR = 1.025, 95% CI: 1.005–1.045, p = 0.013), poorer nutritional status (MQSGA score) (OR = 2.778, 95% CI: 1.710–5.450, p < 0.001), and low physical activity level (IPAQ: low vs. high: OR = 26.307, 95% CI: 2.802–247.004, p = 0.004) were identified as significant independent risk factors for sarcopenia (Table 3).
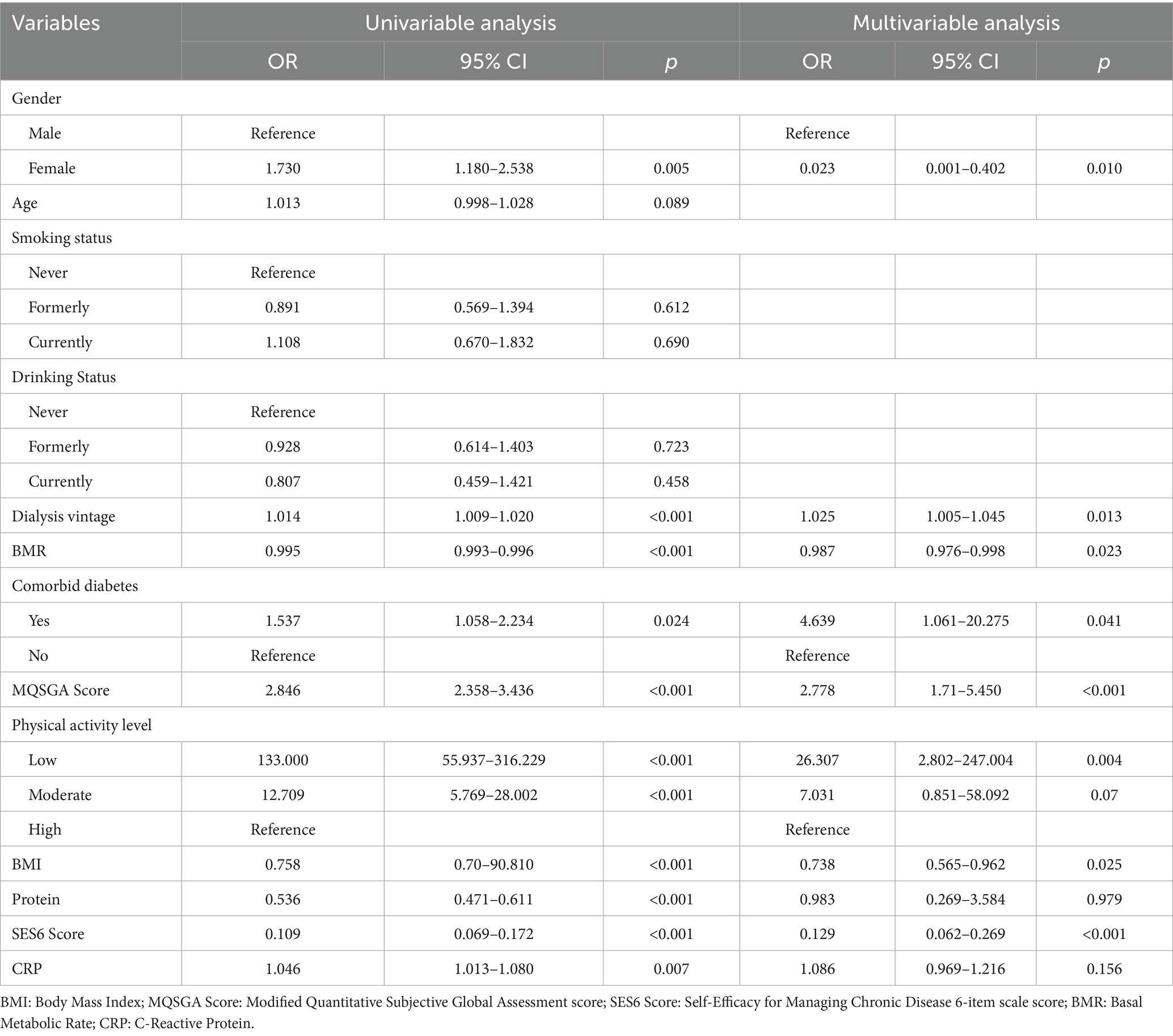
Table 3. Univariable and multivariable logistic regression analysis: factors affecting the possible or confirmed sarcopenia (n = 448).
3.4 Multivariate logistic regression analysis of factors influencing sarcopenia progression
To further identify the key factors influencing the progression of sarcopenia from the “possible” stage to the “confirmed or severe” stages, this study classified patients with confirmed or severe sarcopenia as the case group (n = 130), while possible sarcopenia patients were the control group (n = 112). Multivariate logistic regression analysis was conducted, as detailed in Table 4. Factors such as physical activity levels, body composition, metabolic indicators, and self-efficacy were all closely related to sarcopenia progression. Low physical activity levels significantly increased the risk of sarcopenia progression, with a risk increase of over 50 times compared to high physical activity levels (OR = 54.722, 95% CI: 2.224–962.841, p = 0.007). A decrease of 1 kg/m2 in BMI increased the risk of progression by approximately 51% (OR = 0.492, 95% CI: 0.378–0.646, p < 0.001). For every increase of 100 kcal/d in BMR, the risk increased by about 4.8% (OR = 1.048, 95% CI: 1.004–1.092, p = 0.042). A decrease of 1 kg in protein content significantly increased the risk of progression (OR = 0.004, 95% CI: 0.001–0.343, p = 0.020). For every 1-point decrease in SES6 score, the risk of sarcopenia progression increased by about 44% (OR = 0.558, 95% CI: 0.344–0.903, p = 0.018).
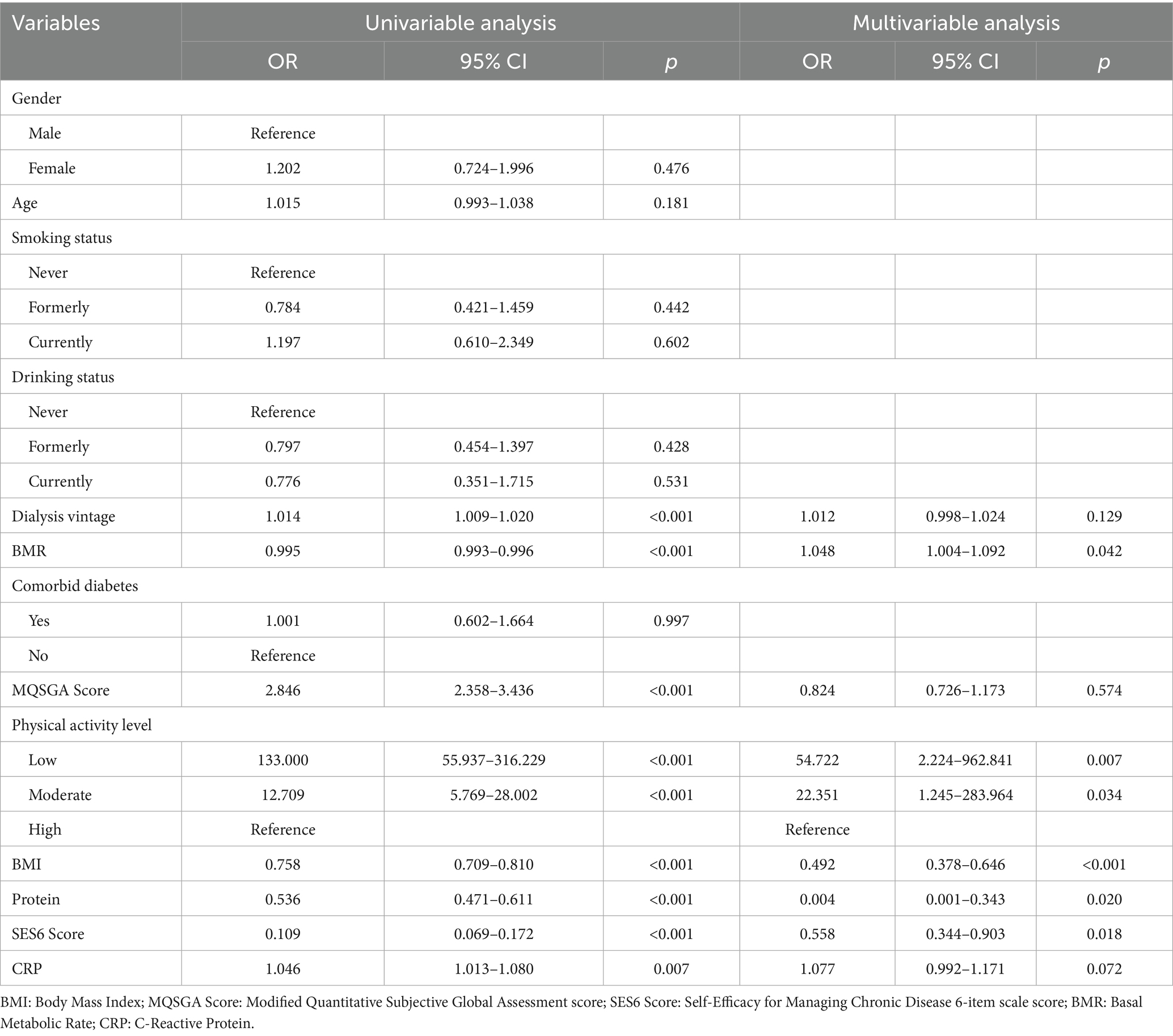
Table 4. Univariable and multivariable logistic regression analysis: confirmed sarcopenia + severe sarcopenia vs. possible sarcopenia (n = 242).
4 Discussion
In older MHD patients aged 60 years or above, the prevalence of possible or confirmed sarcopenia is relatively high (54%). Sarcopenia in MHD patients exhibits a graded progression from “possible” to “confirmed” and “severe,” with proportions of 25.0, 19.6, and 9.4%, respectively. This study revealed differences in body composition, physical activity levels, nutritional status, and self-management abilities across different stages of sarcopenia in MHD patients, highlighting the importance of early identification and intervention. In the “possible sarcopenia” stage, although muscle mass has not yet significantly declined, there is already noticeable deterioration in physical function or muscle strength, making it a critical period for delaying disease progression (15). Patients in this stage account for nearly one-quarter of the population, and early intervention at this point could potentially prevent progression to confirmed or severe sarcopenia (16). Severe sarcopenia accounts for more than one-third of the confirmed sarcopenia cases, indicating that some patients are already in a severe stage at the time of the first assessment, reflecting the current issue of “delayed recognition” in clinical practice.
This study identified several key, modifiable factors, specifically nutritional status, physical activity, and self-efficacy, that are independently associated with sarcopenia in aging MHD patients. Malnutrition was identified as a major determinant of sarcopenia in MHD patients (17). MQSGA comprehensively assesses dietary intake, gastrointestinal symptoms, and signs of muscle or fat loss (9), while serum albumin provides an objective biochemical marker of protein-energy status. In this study, patients with sarcopenia showed significantly higher MQSGA scores and lower serum albumin levels than those without sarcopenia. The findings suggest that deteriorating nutrition contributes to sarcopenia through insufficient amino acid availability, anabolic resistance, and persistent inflammation, which accelerate muscle protein breakdown (18). Although serum albumin may be influenced by hydration or inflammation, its combination with MQSGA offers a more comprehensive assessment of nutritional risk.
Inadequate physical activity represents another crucial factor. IPAQ results suggest that physical activity is seriously insufficient among dialysis patients. In this study, low levels of physical activity appear to be not only a potential contributor to the onset of sarcopenia but also a significant risk factor for its progression from the “possible” to the “confirmed/severe” stages. This aligns with the recognized decline in physical capacity and musculoskeletal function associated with sarcopenia, especially in its severe form (6). Low levels of physical activity mean that skeletal muscles are chronically deprived of effective loading, which may reduce the recruitment of type II muscle fibers and lead to decreased muscle tone and responsiveness (19–22). Among patients receiving MHD, common symptoms such as dialysis-related fatigue, myalgia, and joint stiffness can further exacerbate the vicious cycle of reduced mobility and progressive muscle atrophy (23–25). However, given the cross-sectional design of this study, it is not possible to establish a causal link between low physical activity and sarcopenia progression. Longitudinal research is warranted to explore this relationship further.
The SES6 score provides a validated measure of patients’ self-efficacy in managing their disease and engaging in health-promoting behaviors, including their confidence and capability in maintaining exercise routines, dietary regulation, and daily activity management (13). In this study, lower SES6 scores were significantly associated with an increased risk of both the onset and progression of sarcopenia. Evidence suggests that patients with lower levels of self-efficacy often lack the motivation and capacity to sustain healthy behaviors, such as regular exercise, adequate nutrition, and participation in rehabilitation, which may in turn accelerate the decline in muscle mass and function (26). Among MHD patients, dialysis-related fatigue, psychological distress, and diminished quality of life can further undermine self-management capacity, potentially weakening the engagement in muscle-preserving behaviors (27). Previous studies have demonstrated that enhancing self-efficacy can improve treatment adherence and overall quality of life (28).
During the progression phase of sarcopenia, reduced protein mass emerges as an independent risk factor. The underlying mechanism involves several key aspects: total body protein primarily reflects protein content in skeletal muscle, internal organs, and other tissues, with skeletal muscle protein constituting the predominant component. Therefore, a decline in total protein directly indicates reduced skeletal muscle mass and diminished muscle protein reserves (29). In patients undergoing MHD, this process is further exacerbated by chronic inflammation, metabolic disturbances, and sustained protein losses, which collectively suppress muscle protein synthesis while accelerating protein degradation, ultimately leading to gradual depletion of protein mass (30). Compared to serum markers such as albumin, protein mass measured by BIA devices offers distinct advantages in clinical practice, as it can more conveniently reflect dynamic changes in muscle tissue, particularly when body weight remains relatively stable, thereby providing valuable complementary information for monitoring alterations in muscle reserves.
The findings collectively demonstrate significant associations between sarcopenia in MHD patients and nutritional, behavioral, and psychosocial factors. MQSGA, IPAQ-SF, and SES6 reflect these distinct dimensions, together providing a multidimensional understanding of sarcopenia risk. Although the cross-sectional design precludes causal inferences, these findings offer valuable insights: incorporating these assessment tools into routine clinical monitoring may facilitate early identification and risk stratification of high-risk populations.
Building on the observed associations, we propose a staged management approach: at the preliminary stages, emphasis could be placed on nutritional support, physical activity promotion, and self-efficacy building; for patients already in the “possible” stage, particular attention should be paid to maintaining protein reserves and addressing physical inactivity. The effectiveness of these management approaches requires validation through prospective studies. Future research should include longitudinal studies to establish causal relationships between these factors and disease progression, and subsequently develop and test targeted intervention strategies based on these findings.
This study found that the BMR plays a complex role at different stages of sarcopenia in MHD patients. At the onset stage of sarcopenia, a higher BMR was associated with a lower risk of sarcopenia, suggesting that a favorable energy metabolism state may help maintain muscle mass, potentially by promoting protein synthesis and inhibiting protein degradation. This finding is consistent with previous studies (31). However, as sarcopenia progresses, an increased BMR becomes a risk factor. Existing literature suggests that dialysis patients often experience chronic low-grade inflammation (elevated IL-6 and TNF-α), which directly stimulates basal metabolism, leading to increased resting energy expenditure and an abnormally elevated BMR. Simultaneously, protein synthesis is inhibited, and muscle protein degradation is accelerated, resulting in a state of metabolic inefficiency (32–34). An elevated BMR at this stage likely reflects a pathological process of intensified energy consumption and muscle loss, rather than a simple increase in metabolic activity (35). The observed relationship between BMR and sarcopenia may also be confounded by inflammation, body composition, and sex-specific hormonal differences (36). Inflammation can blur this association because it not only elevates metabolic rate but also coexists with malnutrition and muscle wasting, making it difficult to distinguish whether high BMR represents adaptive metabolism or disease burden. Body composition is another major confounder, as BMR is predominantly determined by fat-free mass, particularly skeletal muscle, which has a much higher metabolic rate than adipose tissue. Therefore, individuals with a greater proportion of adipose tissue may exhibit a similar absolute BMR but lower metabolic efficiency per unit of lean mass. In addition, fluid retention and intramuscular fat infiltration in MHD patients may distort the estimation of lean tissue, leading to potential bias in BMR interpretation. Sex-specific hormonal differences may further modify this relationship: lower testosterone levels in men and estrogen-driven metabolic regulation in women can differentially influence substrate utilization and energy efficiency (37, 38). These overlapping influences suggest that BMR should not be interpreted in isolation but rather within an integrated physiological framework that accounts for inflammation, body composition, and sex-related characteristics. Future longitudinal studies using indirect calorimetry, precise body composition measurements, and inflammatory markers are warranted to validate and elucidate the causal and temporal relationships between BMR alterations and sarcopenia progression in MHD populations.
This study has several limitations. First, its cross-sectional design precludes causal inference, and longitudinal studies are needed to clarify temporal relationships and verify predictive value. Second, the study sample was drawn from three hospitals in Bengbu, China, which may restrict the external validity and generalizability of the findings. Third, some measures (such as BMR) were derived from bioimpedance-based estimates that can be influenced by hydration status and device-specific algorithms (39). Fourth, both IPAQ-SF and SES6 were self-reported instruments and may be subject to recall and social desirability bias. Future multicenter, prospective studies with more diverse populations and standardized multidimensional assessments are warranted to validate these findings and provide stronger evidence for clinical guidelines.
5 Conclusion
This study systematically investigated the prevalence and stage-specific risk factors of sarcopenia in older adults receiving MHD using the AWGS 2019 criteria. A high prevalence of sarcopenia was observed, with a clear pattern of stage-wise progression. During the onset phase, protective factors included female sex, higher self-efficacy, and elevated BMR, whereas low physical activity, diabetes, extended dialysis duration, and malnutrition were major risk contributors. Independent predictors for progression from possible to confirmed sarcopenia included reduced physical activity, declining BMI, decreased protein mass, and diminished self-efficacy. Interestingly, BMR demonstrated bidirectional changes across different stages, implying a complex regulatory role in sarcopenia development. These findings underscore the importance of early recognition of possible sarcopenia, regular monitoring of body composition and metabolic parameters, and implementing integrated interventions focusing on nutrition, physical activity, and self-management. Such strategies may help delay sarcopenia onset and progression, ultimately improving long-term outcomes. Future large-scale, multicenter, longitudinal studies are warranted to further elucidate the underlying mechanisms and validate intervention strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Bengbu Medical University (Ethical Approval Number: [2025] No. 187). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JL: Software, Funding acquisition, Resources, Writing – original draft, Data curation, Conceptualization, Investigation. FW: Writing – review & editing, Methodology, Software, Investigation. LS: Investigation, Resources, Project administration, Writing – review & editing, Formal analysis, Supervision. FF: Validation, Visualization, Investigation, Writing – review & editing. MS: Investigation, Data curation, Software, Writing – review & editing, Resources. HW: Conceptualization, Supervision, Writing – review & editing, Project administration, Funding acquisition, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was financially supported by the Anhui Provincial Scientific Research Program (Grant No. 2024AH052831) and the Bengbu Medical University Postgraduate Research and Innovation Project research funding (Grant No. Byycx24120).
Acknowledgments
We are grateful to all the participants and researchers in this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AWGS, Asian Working Group for Sarcopenia; BIA, Bioelectrical Impedance Analysis; BMI, Body Mass Index; BMR, Basal Metabolic Rate; CKD, Chronic Kidney Disease; CRP, C-Reactive Protein; MHD, Maintenance Hemodialysis; PEW, Protein-Energy Wasting; MQSGA, Modified Quantitative Subjective Global Assessment; IPAQ-SF, International Physical Activity Questionnaire - Short Form; SES6, Self-Efficacy for Managing Chronic Disease 6-item Scale.
References
1. Chen, Y, and Wu, J. Aging-related sarcopenia: metabolic characteristics and therapeutic strategies. Aging Dis. (2024) 16:1003–22. doi: 10.14336/AD.2024.0407
2. Zhao, Q, Zhu, Y, Zhao, X, Shi, R, Lu, T, Yu, R, et al. Prevalence and risk factors of sarcopenia in patients on maintenance hemodialysis: a retrospective cohort study. BMC Musculoskelet Disord. (2024) 25:424. doi: 10.1186/s12891-024-07546-3
3. Wu, YY, Li, JY, Xia, QJ, Gao, YY, Zhang, C, Xu, PJ, et al. Analysis of risk factors of sarcopenia in patients with maintenance hemodialysis and its correlation with emotional status and quality of life. J Multidiscip Healthc. (2024) 17:3743–51. doi: 10.2147/JMDH.S469900
4. Wang, Q, Wang, XY, Zhang, X, and Tao, MF. Research progress on sarcopenia in maintenance hemodialysis patients. J Qiqihar Med Coll. (2024) 45:886–91. doi: 10.3969/j.issn.1002-1256.2024.09.18
5. Zhang, JL, Lai, XT, Ke, Y, Xu, TF, and Ju, M. Investigation and analysis of symptom distress and quality of life in middle-aged and elderly maintenance hemodialysis patients. Chin J Blood Purif. (2022) 21:296–9. doi: 10.3969/j.issn.1671-4091.2022.04.017
6. Chen, LK, Woo, J, Assantachai, P, Auyeung, TW, Chou, MY, Iijima, K, et al. Asian working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012
7. Shao, HY, Hao, BT, Jia, LY, and Gao, FX. Association between TyG-WHtR index and possible sarcopenia in Chinese elderly population based on CHARLS database. Chin J Osteoporos Bone Miner Res. (2025) 18:28–35. doi: 10.3969/j.issn.1674-2591.2025.01.004
8. Lin, CM, Wei, WB, Lü, KJ, Wu, XW, and Ye, WB. Research progress on possible sarcopenia. J Pract Tradit Chin Intern Med. (2023) 37:33–6. doi: 10.13729/j.issn.1671-7813.Z20222391
9. Kalantar-Zadeh, K, Kleiner, M, Dunne, E, Lee, GH, and Luft, FC. A modified quantitative subjective global assessment of nutrition for dialysis patients. Nephrol Dial Transplant. (1999) 14:1732–8. doi: 10.1093/ndt/14.7.1732
10. Qu, NN, and Li, KJ. Study on the reliability and validity of the Chinese version of the international physical activity questionnaire. Chin J Epidemiol. (2004) 25:265–8. doi: 10.3760/j.issn:0254-6450.2004.03.021
11. Fan, MY, Lü, J, and He, PPI. Calculation method of physical activity level in the international physical activity questionnaire. Chin J Epidemiol. (2014) 35:961–4. doi: 10.3760/cma.j.issn.0254-6450.2014.08.019
12. Lorig, KR, Sobel, DS, Ritter, PL, Laurent, D, and Hobbs, M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. (2001) 4:256–62.
13. Zhang, MX, Pang, H, and Zhao, GM. Research progress on self-efficacy assessment tools for chronic disease management. Chin J Mult Organ Dis Elderly. (2023) 22:633–6. doi: 10.11915/j.issn.1671-5403.2023.08.133
14. Jiang, S, Kang, L, and Liu, XH. Interpretation of the 2019 Asian consensus on the diagnosis and treatment of sarcopenia. Chin J Geriatr. (2020) 39:373–6. doi: 10.3760/cma.j.issn.0254-9026.2020.04.002
15. Jiang, AQ, Liang, B, Wei, Y, and Pei, LJ. Study on potential risk factors for comorbidity of frailty and possible sarcopenia in middle-aged and elderly Chinese. Chin J Osteoporos Bone Miner Res. (2024) 17:564–73. doi: 10.3969/j.issn.1674-2591.2024.06.004
16. Pan, WM, Huang, G, Wang, B, Han, YB, He, CM, and Li, AP. Effects of comprehensive intervention in community hospitals on muscle mass and physical function in elderly patients with possible sarcopenia. Chin J Mult Organ Dis Elderly. (2022) 21:95–9. doi: 10.11915/j.issn.1671-5403.2022.02.021
17. Graterol Torres, F, Molina, M, Soler-Majoral, J, Romero-González, G, Rodríguez Chitiva, N, Troya-Saborido, M, et al. Evolving concepts on inflammatory biomarkers and malnutrition in chronic kidney disease. Nutrients. (2022) 14:4297. doi: 10.3390/nu14204297
18. Gao, MZ, Guo, Q, and Chen, XY. Research progress on pathogenesis, nutrition and exercise interventions in patients with protein-energy wasting. Acta Acad Med Sin. (2024) 46:281–6. doi: 10.3881/j.issn.1000-503X.15657
19. Nascimento, CM, Ingles, M, Salvador-Pascual, A, Cominetti, MR, Gomez-Cabrera, MC, and Viña, J. Sarcopenia, frailty and their prevention by exercise. Free Radic Biol Med. (2019) 132:42–9. doi: 10.1016/j.freeradbiomed.2018.08.035
20. Steffl, M, Bohannon, RW, Sontakova, L, Tufano, JJ, Shiells, K, and Holmerova, I. Relationship between sarcopenia and physical activity in older people: a systematic review and meta-analysis. Clin Interv Aging. (2017) 12:835–45. doi: 10.2147/CIA.S132940
21. Yasuda, T. Selected methods of resistance training for prevention and treatment of sarcopenia. Cells. (2022) 11:1389. doi: 10.3390/cells11091389
22. Zhao, Z, Yan, K, Guan, Q, Guo, Q, and Zhao, C. Mechanism and physical activities in bone-skeletal muscle crosstalk. Front Endocrinol (Lausanne). (2024) 14:1287972. doi: 10.3389/fendo.2023.1287972
23. Li, L, Ma, X, Xie, C, and Li, Y. Resistance exercise interventions for sarcopenia and nutritional status of maintenance hemodialysis patients: a meta-analysis. PeerJ. (2024) 12:e16909. doi: 10.7717/peerj.16909
24. Du, XJ, Zhang, HL, and Guo, G. Research progress on sarcopenia in maintenance hemodialysis patients. Nurs Res. (2021) 35:1194–8. doi: 10.12102/j.issn.1009-6493.2021.07.013
25. Meng, FH, Yang, HB, Tao, YL, Wang, YY, and Li, LL. Research progress on sarcopenia in maintenance hemodialysis patients. Evid Based Nurs. (2023) 9:2361–5. doi: 10.12102/j.issn.2095-8668.2023.13.015
26. Wu, R, Feng, S, Quan, H, Zhang, Y, Fu, R, and Li, H. Effect of Self-Determination Theory on Knowledge, Treatment Adherence, and Self-Management of Patients with Maintenance Hemodialysis. Contrast Media Mol Imaging. (2022) 2022:1416404. doi: 10.1155/2022/1416404
27. Kazak, A, Özkaraman, A, Topalı, H, and Duran, S. Evaluation of the relationship between health literacy and self-efficacy: a sample of hemodialysis patients. Int J Artif Organs. (2022) 45:659–65. doi: 10.1177/03913988221108754
28. Kang, E, Kim, S, Rhee, YE, Lee, J, and Yun, YH. Self-management strategies and comorbidities in chronic disease patients: associations with quality of life and depression. Psychol Health Med. (2021) 26:1031–43. doi: 10.1080/13548506.2020.1838585
29. Xiang, Q, Hu, Y, and Liu, WL. Research progress on gut microbiota and skeletal muscle protein metabolism in patients with sarcopenia. Chin J Microecol. (2024) 36:742–4. doi: 10.13381/j.cnki.cjm.202406020
30. Zhang, Y, Wang, XX, Zhang, XL, and Wang, B. Research progress on protein-energy wasting, sarcopenia and frailty in patients with chronic kidney disease. Chin J Nephrol. (2024) 40:757–64. doi: 10.3760/cma.j.cn441217-20231020-01033
31. Zhao, XY, Wang, DG, Lu, TT, Zheng, JJ, Bao, MZ, and Zhang, M. Current status and influencing factors of sarcopenia in maintenance hemodialysis patients. Chin Med Sci. (2024) 14:130–4. doi: 10.20116/j.issn2095-0616.2024.14.31
32. Hortegal, EVF, Alves, JJDA, Santos, EJF, Nunes, LCR, Galvão, JC, Nunes, RF, et al. Sarcopenia and inflammation in patients undergoing hemodialysis. Sarcopenia e inflamación en pacientes sometidos a hemodiálisis. Nutr Hosp. (2020) 37:855–62. doi: 10.20960/nh.03068
33. Hara, H, Nakamura, Y, Hatano, M, Iwashita, T, Shimizu, T, Ogawa, T, et al. Protein energy wasting and sarcopenia in Dialysis patients. Contrib Nephrol. (2018) 196:243–9. doi: 10.1159/000485729
34. Hanna, RM, Ghobry, L, Wassef, O, Rhee, CM, and Kalantar-Zadeh, K. A practical approach to nutrition, protein-energy wasting, sarcopenia, and cachexia in patients with chronic kidney disease. Blood Purif. (2020) 49:202–11. doi: 10.1159/000504240
35. Tuttle, CSL, Thang, LAN, and Maier, AB. Markers of inflammation and their association with muscle strength and mass: a systematic review and meta-analysis. Ageing Res Rev. (2020) 64:101185. doi: 10.1016/j.arr.2020.101185
36. Guan, L, Li, T, Wang, X, Yu, K, Xiao, R, and Xi, Y. Predictive roles of basal metabolic rate and body water distribution in sarcopenia and Sarcopenic obesity: the link to carbohydrates. Nutrients. (2022) 14:3911. doi: 10.3390/nu14193911
37. Romejko, K, Rymarz, A, Sadownik, H, and Niemczyk, S. Testosterone deficiency as one of the major endocrine disorders in chronic kidney disease. Nutrients. (2022) 14:3438. doi: 10.3390/nu14163438
38. Mustafa, M, Khaznah, I, Hrezat, D, Obaida, LA, and Aghbar, A. Does the hemodialysis program affect the testosterone serum level in patients with end-stage renal disease? Int Urol Nephrol. (2025) 57:785–91. doi: 10.1007/s11255-024-04265-5
Keywords: aging, Sarcopenia, maintenance hemodialysis, stage-based assessment, physical activity, nutritional status, self-efficacy, public health intervention
Citation: Li J, Wang F, Song L, Fang F, Shen M and Wang H (2025) Stage-specific prevalence and progression of sarcopenia among aging hemodialysis patients: a multicenter cross-sectional study. Front. Public Health. 13:1676139. doi: 10.3389/fpubh.2025.1676139
Edited by:
Yoshiyuki Morishita, Jichi Medical University Saitama Medical Center, JapanReviewed by:
Kiyonori Ito, Jichi Medical University Saitama Medical Center, JapanNaoki Nakagawa, Asahikawa Medical University, Japan
Copyright © 2025 Li, Wang, Song, Fang, Shen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyu Wang, d2FuZ2hvbmd5dTgxOTIwQDE2My5jb20=
 Jinguo Li
Jinguo Li Fei Wang2
Fei Wang2 Fang Fang
Fang Fang