- Key Specialty of Clinical Pharmacy, The First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou, China
Objective: The combination of second-generation androgen receptor (AR) antagonists with androgen deprivation therapy (ADT) has shown good efficacy and safety in advanced prostate cancer. This study aims to evaluate the cost-effectiveness of three second-generation AR antagonists in the treatment of metastatic hormone-sensitive prostate cancer (mHSPC) in China, providing pharmacoeconomic evidence for clinical drug selection.
Methods: A Markov model was constructed based on data from the ARCHES, TITAN, and ARANOTE phase III clinical trials, with a 28-day cycle period. Direct medical costs and quality-adjusted life years (QALYs) were simulated over a 15-year horizon. The incremental cost-effectiveness ratio (ICER) was used as the primary outcome, and a willingness-to-pay (WTP) threshold of three times the 2024 per capita GDP of China was set for cost-utility analysis. Sensitivity analysis was conducted to validate the model’s influencing factors and the robustness of the results.
Results: The cumulative cost of the apalutamide regimen was ¥776,807, resulting in 4.95 QALYs. Compared to apalutamide, the ICER for enzalutamide was ¥643,309/QALY, while for darolutamide, the ICER was -¥40,625/QALY.
Conclusion: For Chinese mHSPC patients, darolutamide is the most cost-effective treatment at a WTP threshold of ¥287,391/QALY, followed by apalutamide, with enzalutamide being less favorable.
1 Introduction
The GLOBOCAN 2022 data released by the International Agency for Research on Cancer in 2024 shows that prostate cancer is the second most common malignancy among men worldwide and ranks eighth in mortality among 36 types of cancer. In China, the incidence of prostate cancer in men aged 60 and above has shown a significant upward trend (1), and its diagnosis and treatment costs have placed a substantial economic burden on both patients and the healthcare system (2). Metastatic hormone-sensitive prostate cancer (mHSPC) refers to prostate cancer that has metastasized at diagnosis and has not yet received endocrine treatment. It can be divided into low-volume and high-volume diseases. In China, approximately 54% of patients are diagnosed with distant metastasis, indicating advanced disease (3). Although most patients respond well initially to androgen deprivation therapy (ADT), the majority will still progress to metastatic castration-resistant prostate cancer (mCRPC) within 1–3 years (4).
In recent years, with continuous advancements in medical technology, novel endocrine therapies—particularly second-generation androgen receptor (AR) antagonists—have provided new treatment options for patients with advanced prostate cancer. Second-generation AR antagonists demonstrate a more comprehensive mechanism of action compared to first-generation AR antagonists, which significantly improve patients’ prognosis and delay disease progression, and have been widely recognized and applied globally. Currently, four second-generation AR antagonists are available in China. Enzalutamide, apalutamide, and darolutamide have all received approval from the U.S. Food and Drug Administration (FDA). The fourth drug, rezvilutamide, a domestically developed medication, has been approved by China’s National Medical Products Administration (NMPA) but has not yet obtained FDA clearance. It is noteworthy that rezvilutamide is currently approved only for patients with high-volume mHSPC.
In the treatment of mHSPC, the CSCO Guidelines 2024 (5) and the NCCN Guidelines Version 2.2025 (6) have parallel recommendations for three second-generation AR antagonists approved by the FDA. Unlike the treatment regimen of enzalutamide or apalutamide combined with ADT, the standard regimen for darolutamide is a triple combination of ADT and docetaxel, though the toxicity of docetaxel limits its clinical use. The latest ARANOTE study (7) confirms that the darolutamide + ADT combination regimen can improve radiographic progression-free survival (rPFS) in mHSPC patients. A network meta-analysis (8, 9) shows that its efficacy is not significantly different from that of the triple combination regimen, while offering better safety. Real-world studies in China (10) also support its good efficacy and safety. The indication application for darolutamide + ADT in the treatment of mHSPC has been submitted globally. As treatment options increase, conducting pharmacoeconomic evaluations of second-generation AR antagonists is of great significance for assessing the value of the drugs, optimizing treatment options, and alleviating the financial burden on patients and healthcare security systems.
As an important method for improving clinical drug management and optimizing healthcare resource allocation, pharmacoeconomic evaluation methods include cost-effectiveness analysis, cost-utility analysis, cost-benefit analysis, and minimum cost analysis (11). Cost-utility analysis, as a subset of cost-effectiveness analysis (12), uses quality-adjusted life years (QALYs) as the health output indicator, and both methods use incremental cost-effectiveness ratio (ICER) as the evaluation metric, which refers to the additional cost required to gain one additional unit of health output (13). Cost-utility analysis is widely applied in the pharmacoeconomic evaluation of oncology drugs.
Given the differences in efficacy, safety, and cost of the second-generation AR antagonist combined with ADT treatment for mHSPC patients, and the fact that previous studies have only evaluated the economic viability of darolutamide + ADT + docetaxel (14, 15), there has been no economic study on the treatment of mHSPC with darolutamide + ADT. This study conducts a cost-utility analysis of second-generation AR antagonists for the treatment of mHSPC, based on the Chinese Pharmacoeconomic Evaluation Guidelines 2020 (16), from the perspective of the Chinese healthcare system, incorporating domestic drug pricing and residents’ income levels. The aim is to provide a basis for clinical, rational drug use and healthcare cost control.
2 Methods
2.1 Patient characteristics
Since there are no head-to-head clinical trials between second-generation AR antagonists, a network meta-analysis is required to further compare survival data. To this end, this study systematically searched the English databases PubMed, Embase, and self-built databases for literature published up to January 2025, as well as relevant conference reports from both domestic and international sources. In clinical trials for mHSPC, the ENZAMET, China AECHES, and ARASENS trials were excluded from the analysis because they did not fully publish rPFS and overall survival (OS) curves (17–21). The clinical trial of rezvilutamide (CHART) only included patients with high tumor burden (22), and the baseline characteristics differed from those of other second-generation AR antagonists’ clinical trials (which included both high and low tumor burdens), so rezvilutamide was also excluded from the cost-utility analysis. Finally, three Phase III randomized controlled trials—ARCHES (23, 24), TITAN (25, 26), and ARANOTE (7)—were included. The Markov model constructed for this study simulated a population whose characteristics were consistent with those of the populations in the aforementioned trials: ① histologically diagnosed as hormone-sensitive prostate cancer with confirmed metastasis through imaging (bone scan/CT/MRI); ② Eastern Cooperative Oncology Group performance status score of 0–1.
2.2 Clinical trial treatment regimen
2.2.1 Initial treatment for mHSPC patients entering the model
All patients receive ADT as the base treatment, combined with enzalutamide, apalutamide, or darolutamide.
2.2.2 The treatment after disease progression
Based on the design of each trial, subsequent treatment options include: docetaxel + ADT, abiraterone acetate + ADT, enzalutamide + ADT, apalutamide + ADT.
2.3 Model structure
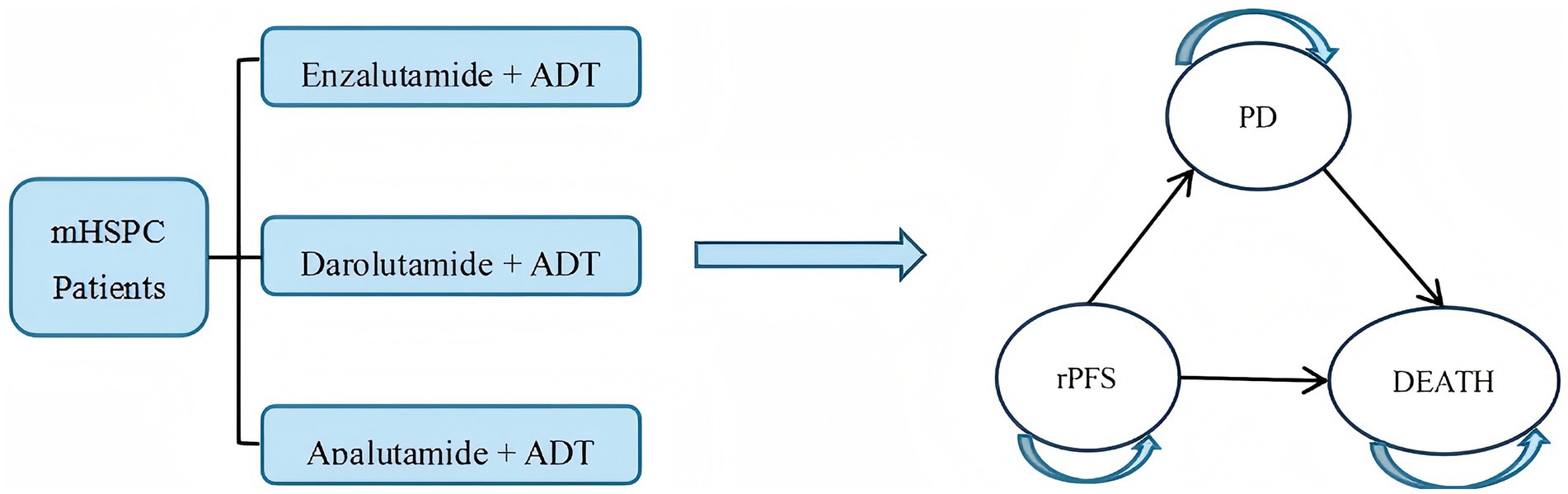
A Markov model can simulate the long-term, complex progression of diseases flexibly by defining mutually exclusive health states (e.g., disease stability, progression, death) and specifying the transition probabilities between these states. It enables extrapolation of lifetime treatment costs and health utility through cyclical periodic structures. It was widely applied in pharmacoeconomic evaluations of prostate cancer globally (27, 28). Given the multi-state and typically irreversible nature of mHSPC disease progression, this study employed Tree Age Pro 2022 to construct a Markov model incorporating three health states: rPFS, progressive disease (PD), and death. PD was defined as radiological progression: soft tissue lesion progression on CT/MR [RECIST 1.1 (29)] or ≥2 new bone lesions on bone scan [PCWG3 (30)]. To align with clinical reality, disease progression within the model was set as irreversible. The specific state transition rules are as follows: all patients start in the rPFS state; only unidirectional transitions are allowed: rPFS → PD → death or rPFS → death; after transitioning to the PD state, the initial treatment regimen is discontinued, and a preset subsequent treatment regimen is initiated; death is an irreversible endpoint state (see Figure 1).
Based on the ADT administration design used in the included clinical trials, the cycle length of the model was set to 28 days. This study assumes a patient body surface area of 1.72 m2 (14), with all patients receiving ADT treatment using leuprolide acetate microspheres for injection. Considering factors such as the median age of clinical trial participants and the average life expectancy in China, the simulation time was limited to 15 years.
2.4 Extraction of survival data and calculation of transition probabilities
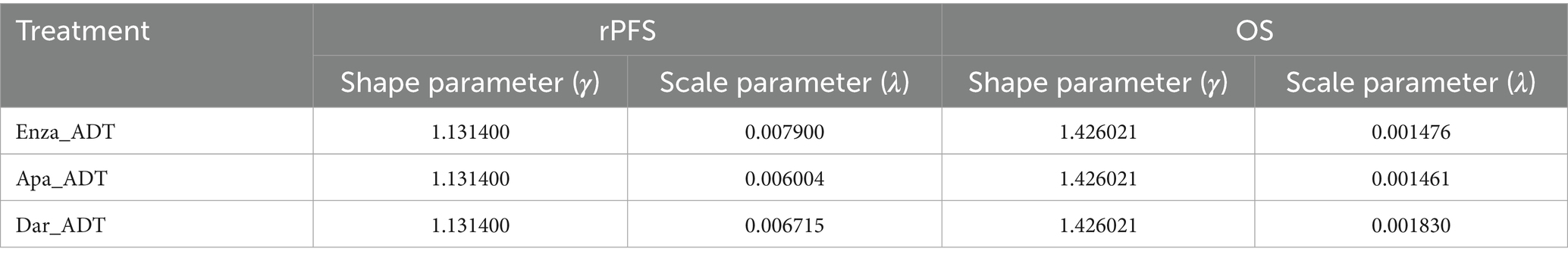
Data from survival curves published in mHSPC clinical trials were extracted using WebPlotDigitizer software. The rPFS and OS curves for three groups were digitized to obtain the time points and corresponding survival rates for each data point (31), and the data were organized accordingly. After organizing the mHSPC data into a format readable by the “IPDfromKM” package, we used the “IPDfromKM” package in R (v4.4.1) to reconstruct individual patient data, generating Kaplan–Meier survival curves. The survival curves were then fitted and extrapolated using the Weibull distribution to obtain the shape parameter (γ) and scale parameter (λ) (31, 32).
Due to the lack of head-to-head trials between second-generation AR antagonists, this study adjusted the survival curves for apalutamide and darolutamide based on the enzalutamide group survival curve, using the hazard ratio (HR) values from a network meta-analysis comparing apalutamide and darolutamide (7, 23–26). The survival curves for apalutamide and darolutamide were adjusted by assuming that the γ of these curves is equal to that of enzalutamide, and λ is equal to the λ of enzalutamide multiplied by the HR (other treatment regimens/enzalutamide) (32, 33). The parameter results for the adjusted mHSPC rPFS and OS curves are shown in Table 1.
Transition probability refers to the probability of a patient moving from one state to another during each cycle. Based on whether the transition probability changes over time, it can be classified as static or dynamic. Due to the time-dependent nature of disease progression in cancer patients, the Markov model in this study applied dynamic transition probabilities for analysis. The adjusted γ and λ parameters were incorporated into the transition probability formula (31), yielding time-dependent transition probabilities for different states during each cycle, where t represents survival time and u represents the cycle period.
2.5 Cost and utility value parameters
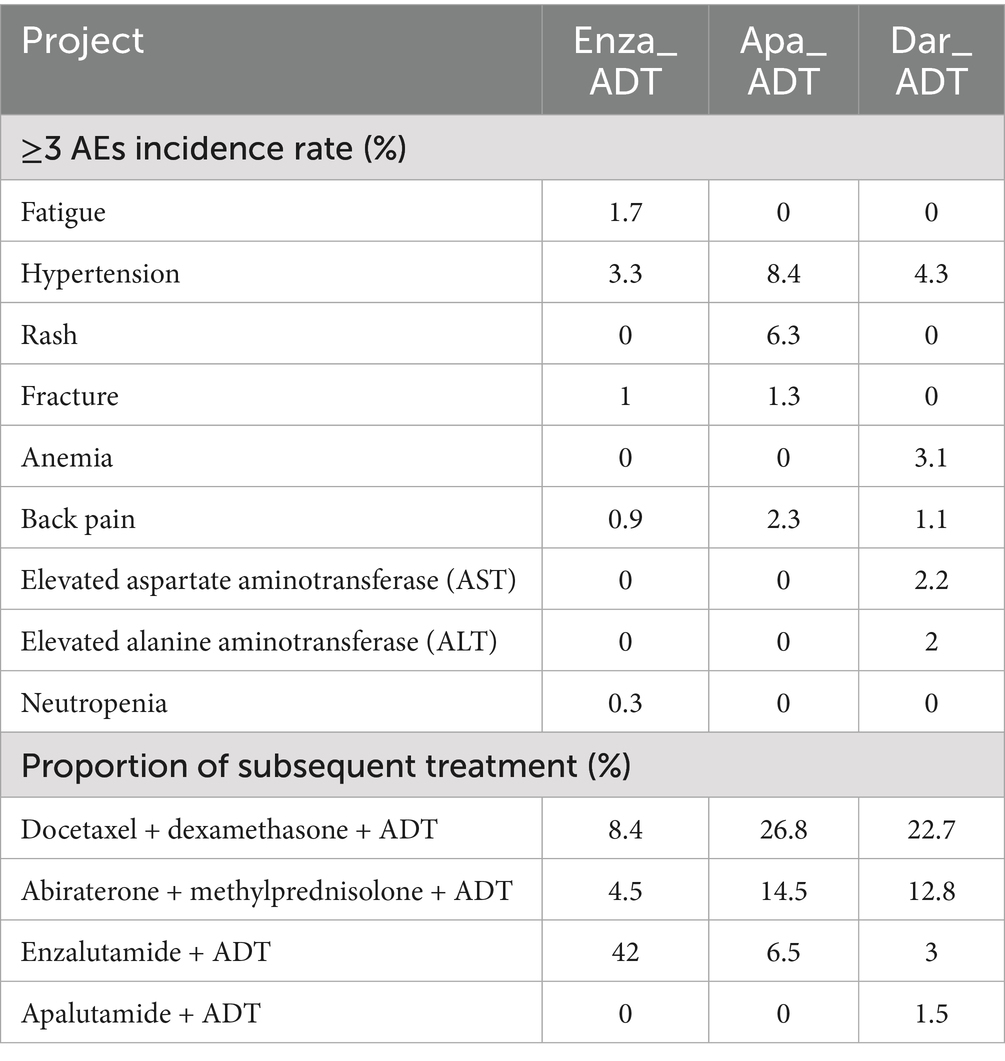
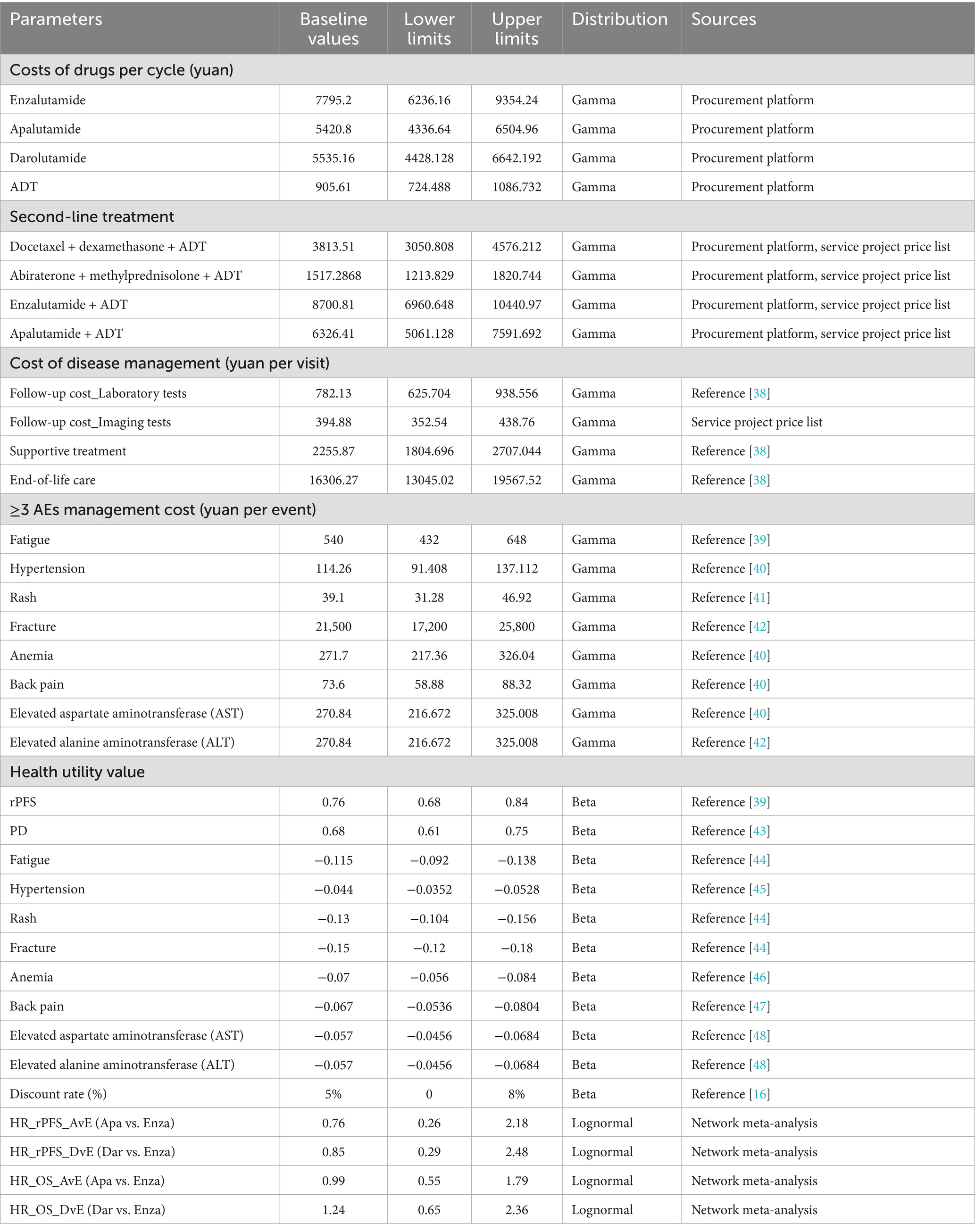
This study is based on the perspective of the Chinese healthcare system. Due to the difficulty in obtaining direct non-medical costs, only direct medical costs are included, which encompass drug costs, follow-up costs (including laboratory tests and imaging examinations), supportive treatment costs, end-of-life care costs, ≥3 grade adverse events (≥3 AEs) management costs, and subsequent treatment costs (34). Drug costs are sourced from the Guangzhou Drug and Medical Consumables Procurement Platform (https://gpo.gzggzy.cn/ referred to as the “Procurement Platform”). Injection and imaging examination costs are sourced from the Guangzhou Municipal Public Medical Institutions Basic Medical Service Project Price Summary Table (https://m12333.cn/qa/pidzc.html, December 2024, referred to as the “Service Project Price Table”). The price data was retrieved until December 2024, and other cost data come from published literature. The drug dosages, ≥3 AEs incidence rates, and subsequent treatment proportions for the mHSPC treatment regimens are cited from original clinical trial data, as detailed in Table 2.
Health utility values are mostly derived from standardized health-related quality of life measures, such as the EuroQol-5D and SF-6D scales (35, 36). Health utility values are typically represented by numbers ranging from 0 to 1, where 0 represents death and 1 represents perfect health. In addition to the disease itself affecting a patient’s health utility, AEs occurring during treatment can also lower quality of life, thus impacting the health utility value (14). Considering the varying incidence rates of ≥3 AEs among different treatment regimens, the health utility values in this study are adjusted based on the negative utility values caused by ≥3 AEs. Health utility values for different disease states are obtained from published literature related to mHSPC. The parameters for costs, health utility values, and their distributions in the model are provided in Table 3.
2.6 Cost-utility analysis
The transition probabilities, costs, health utility values, and other parameters mentioned above were input into the Markov model to conduct a cost-utility analysis for three second-generation AR antagonists. The simulation time horizon is 15 years, and to reduce bias caused by the discrete-time assumption, both costs and QALYs were adjusted with half-cycle correction. Based on the China Drug Economic Evaluation Guidelines 2020 (16), a 5% discount rate was applied to both treatment costs and QALYs. The model output evaluation indicators include the total cost, QALYs, and ICER for each treatment regimen. In this study, three times the 2024 per capita GDP of China (287,391 yuan/QALY) was used as the willingness-to-pay (WTP) threshold. When the ICER is less than the WTP, the treatment is considered cost-effective.
2.7 Sensitivity analyses
To assess the robustness of the model results and the impact of parameter uncertainty on the conclusions, this study performed both univariate sensitivity analysis and probabilistic sensitivity analysis.
2.7.1 One-way sensitivity analysis
For parameters derived from the literature, the 95% confidence interval (95% CI) was used as the upper and lower limits. If the 95% CI was unavailable, a fluctuation range of ±20% around the baseline value was applied. For healthcare service prices (e.g., examination and hospitalization costs), a sensitivity analysis was conducted using the fee standards of primary medical institutions (lower limit) and tertiary medical institutions (upper limit). The results were presented using a tornado diagram, showing the impact of each parameter on the outcome and ranking them.
2.7.2 Probabilistic sensitivity analysis
Probability distributions were assigned to the parameters for second-order Monte Carlo simulations. The model was randomly sampled 10,000 times, and the results were presented using cost-effectiveness scatter plots and cost-effectiveness acceptability curves (34).
3 Results
3.1 Cost-utility analysis
Under the 15-year simulation time horizon, the results of the cost-utility analysis based on the Markov model showed that darolutamide had the lowest total cost (751,369 yuan) and the highest QALYs (5.58 QALYs); enzalutamide had the highest total cost (872,033 yuan) and relatively high QALYs (5.10 QALYs); apalutamide had a mid-range total cost (776,807 yuan) and the lowest QALYs (4.95 QALYs), as detailed in Table 4.
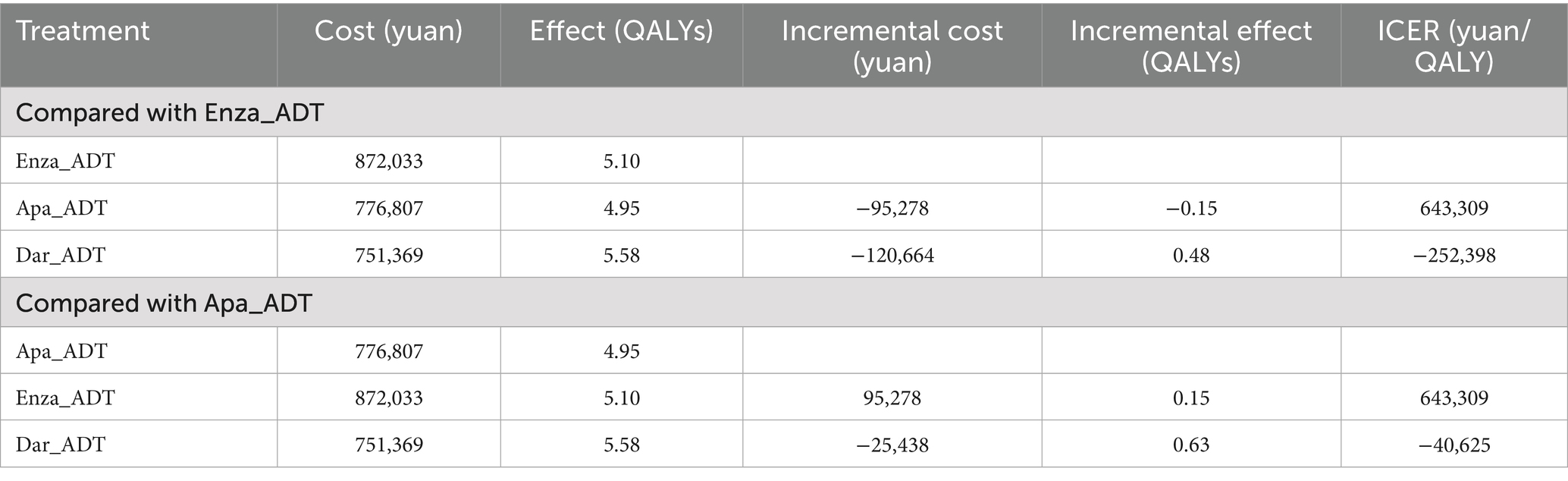
Table 4. Basic results of cost-utility analysis of second-generation AR antagonists in the treatment of mHSPC.
When using 287,391 yuan/QALY (three times the 2024 per capita GDP of China) as the WTP threshold, the results showed that the ICER of darolutamide was −252,398 yuan/QALY compared with enzalutamide and −40,625 yuan/QALY compared with apalutamide. Both ICER values were negative, indicating that the darolutamide regimen can reduce costs while improving health outcomes, making it an absolute dominant strategy in the treatment of mHSPC. On the other hand, compared with apalutamide, the ICER of enzalutamide was as high as 643,309 yuan/QALY. With both the incremental cost and incremental effect being positive, it meant that every additional QALY obtained with enzalutamide required an additional cost of 643,309 yuan, which significantly exceeded the WTP threshold. Therefore, apalutamide exhibited better cost-effectiveness than enzalutamide.
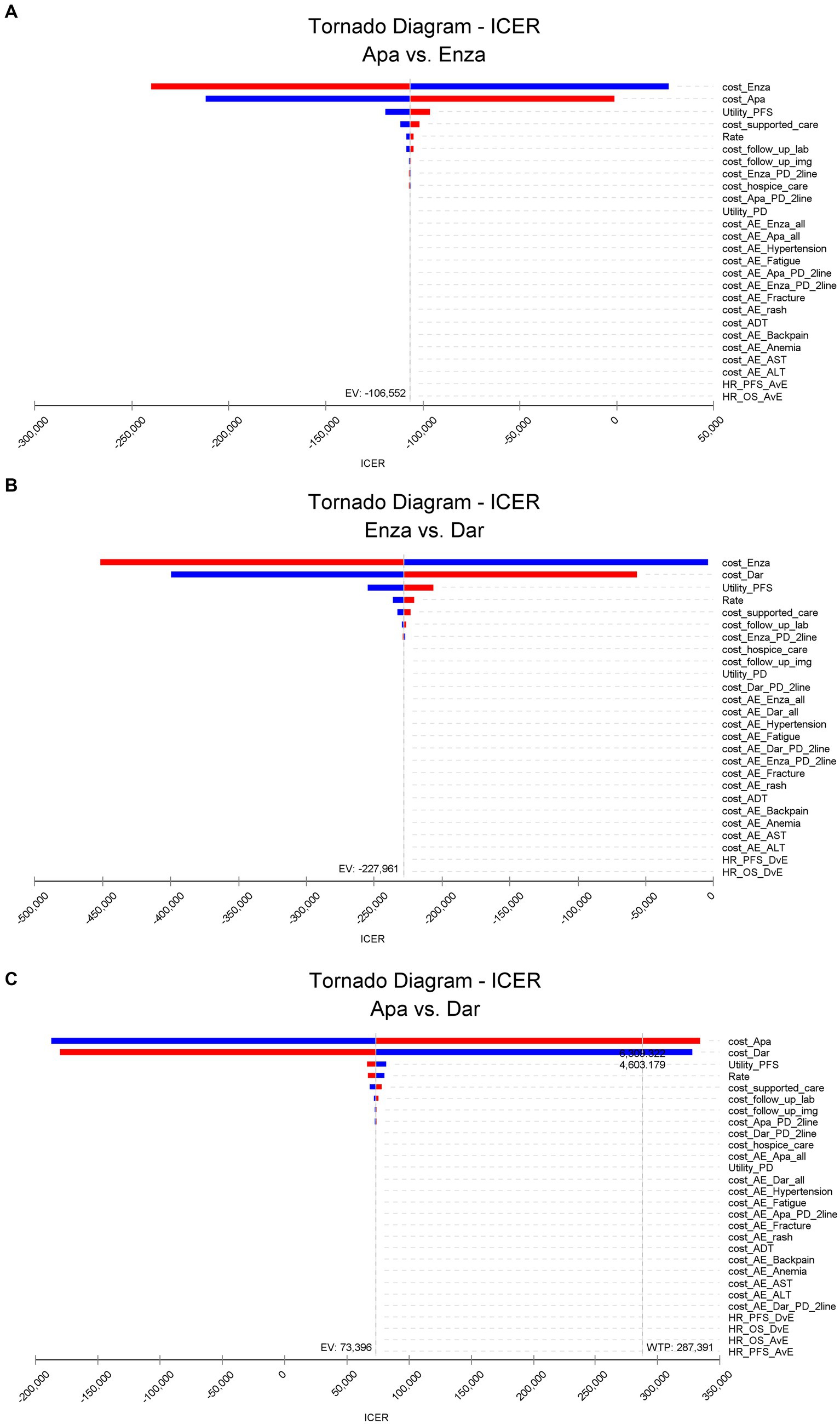
3.2 Single-factor sensitivity analysis
The results of the univariate sensitivity analysis for pairwise comparisons of apalutamide, darolutamide, and enzalutamide are shown in Figure 2 (include Figures 2A–C). The drug costs for first-line treatment and the health utility value for imaging progression-free survival have a significant impact on the ICER results, followed by the costs of supportive therapies, discount rates, and laboratory biochemical tests during follow-up. The cost of adverse event management and the HR between different treatment regimens have a minor effect on the ICER.
Apalutamide vs. enzalutamide: Apalutamide consistently demonstrates cost-effectiveness superiority across all parameter ranges; darolutamide vs. enzalutamide: Darolutamide’s ICER is consistently below the WTP, showing a greater cost-effectiveness advantage; darolutamide vs. apalutamide: If the drug cost of apalutamide exceeds 6300.322 yuan per cycle, its ICER will surpass the WTP and no longer demonstrate a cost-effectiveness advantage. The univariate sensitivity analysis confirmed the robustness of the base case analysis results.
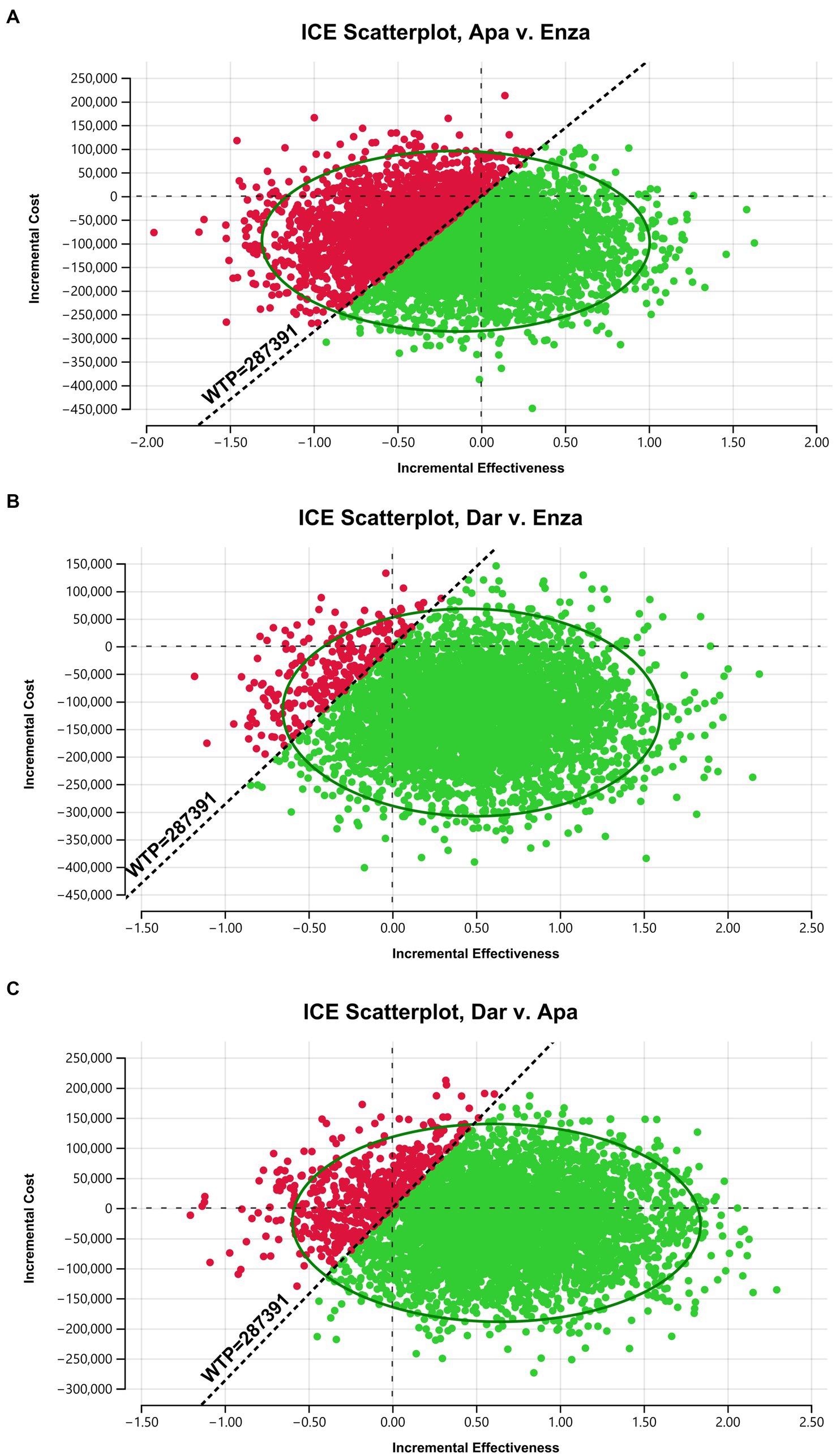
3.3 Probabilistic sensitivity analysis
The results of the probabilistic sensitivity analysis for pairwise comparisons of apalutamide, darolutamide, and enzalutamide are shown in the scatter plots in Figure 3 (include Figures 3A–C). The x-axis represents incremental effectiveness, and the y-axis represents incremental cost. The scatter points in each plot represent the ICER values for each sampling of model parameter combinations, which are mostly concentrated within an ellipse, with a small degree of data dispersion. This indicates that the model parameters are robust, and the probabilistic sensitivity analysis results are reliable. When the WTP is 287,391 yuan, compared to enzalutamide, more scatter points for apalutamide or darolutamide fall in the lower-right side below the WTP line, meaning that the probability of apalutamide and darolutamide having a cost-effectiveness advantage in treating mHSPC is higher. In contrast, compared to darolutamide, more scatter points for apalutamide fall above the WTP line, indicating a very low probability of apalutamide having a cost-effectiveness advantage.
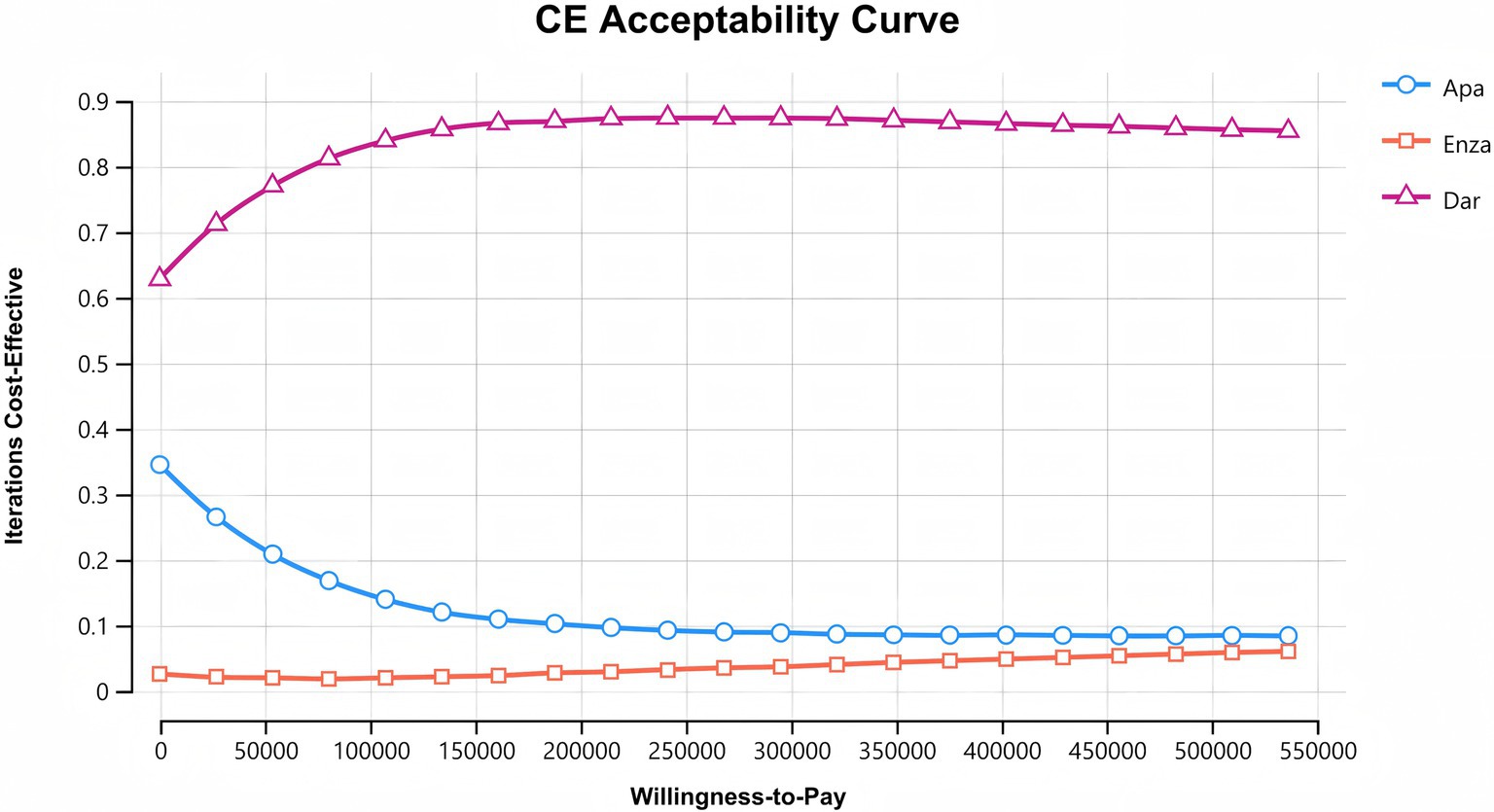
After 10,000 random samplings in the Monte Carlo simulation, the cost-effectiveness acceptability curves for the three second-generation AR antagonists are shown in Figure 4. The x-axis represents WTP, and the y-axis represents the probability of cost-effectiveness. Among the three drugs, darolutamide has the highest likelihood of cost-effectiveness advantage, which increases with WTP and then levels off. The probability of apalutamide having a cost-effectiveness advantage gradually decreases as WTP increases. The likelihood of enzalutamide having a cost-effectiveness advantage increases slowly with WTP, but it is still the lowest of being cost-effective, consistent with the base case analysis results.
4 Discussion
The growth of prostate cancer is highly dependent on the androgen signaling pathway, and most patients will eventually relapse and progress to mCRPC (4, 37). Due to the poor prognosis of mCRPC, the quality of life of patients is significantly reduced, and the mortality rate is high. Therefore, delaying the progression of mHSPC to mCRPC is of great importance for improving long-term prognosis and reducing the socio-economic burden.
Second-generation AR antagonists have demonstrated significant efficacy in patients with mHSPC, while their treatment costs remain a critical factor in clinical decision-making and reimbursement considerations. In this study, we evaluated the cost-effectiveness of enzalutamide, apalutamide, and darolutamide in the treatment of mHSPC by constructing a Markov model, providing an evidence-based basis for rational clinical drug use and health policy formulation.
The cost-effectiveness analysis results show that when the WTP threshold is set to three times the per capita GDP of China (287,391 yuan/QALY), the darolutamide regimen has the best cost-effectiveness advantage, and apalutamide offers a better cost-effectiveness advantage compared to enzalutamide. Sensitivity analysis results confirm the robustness of these results. The cost-acceptability curves for all three treatments indicate that when the WTP exceeds 550,000 yuan, the probability of enzalutamide becoming the cost-effective option may be higher than that of apalutamide. Given the outstanding economic advantages of darolutamide combination therapy, it is recommended that, once its indications are approved, darolutamide + ADT be prioritized for inclusion in the national medical insurance catalog and clinical pathway optimization, providing patients with a more cost-effective treatment option. For budget-constrained regions, price negotiations for apalutamide could further reduce costs and enhance accessibility, thereby maximizing public health benefits.
This study also has certain limitations. First, the clinical trial participants included in this study are from multiple regions, with Chinese patients accounting for only a small proportion, and most prostate cancer patients in China are diagnosed at later clinical stages, which introduces some variability. Therefore, it remains unclear whether the efficacy and safety results from these clinical trials can be directly extrapolated to the Chinese population. Second, due to the lack of head-to-head clinical trial data for second-generation AR antagonists in the treatment of mHSPC, this study extrapolated survival curves for the apalutamide and darolutamide groups based on HR obtained from network meta-analysis, which may introduce some uncertainty. Furthermore, in order to simplify the model calculations, the cost of ADT was represented by the cost of leuprolide acetate, and the cost for managing ≥3 AEs was sourced from published literature, which may lead to some bias between the model outputs and real-world data.
In the future, more clinical or real-world studies are still needed, especially localized studies targeting Chinese prostate cancer patients, to further clarify the direct efficacy differences of the second-generation AR antagonists in the treatment of mHSPC and improve the accuracy and applicability of economic assessment.
5 Conclusion
When the WTP is ¥287,391, darolutamide combined with ADT is the treatment plan with the most cost-effectiveness advantage for the treatment of mHSPC. It not only delays disease progression and improves the quality of life of patients but also significantly saves medical resources.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Visualization. Y-QC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. L-ZH: Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. YC: Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Key Clinical Specialty Construction Project (Clinical Pharmacy) and High-Level Clinical Key Specialty (Clinical Pharmacy) in Guangdong Province, with the funder being the subsidy fund for medical service and security capacity improvement of the Central Department of Finance, No. Z155080000004.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
mHSPC, Metastatic hormone-sensitive prostate cancer; ADT, Androgen deprivation therapy; mCRPC, Metastatic castration-resistant prostate cancer; AR, Androgen receptor; FDA, U.S. Food and Drug Administration; NMPA, National Medical Products Administration; rPFS, Radiographic progression-free survival; QALYs, Quality-adjusted life years; ICER, Incremental cost-effectiveness ratio; OS, Overall survival; PD, Disease progression; HR, Hazard ratio; AEs, Adverse events; ≥3 AEs, ≥3 grade adverse events; WTP, Willingness-to-pay; 95% CI, 95% confidence interval; Apa, Apalutamide; Enza, Enzalutamide; Dar, Darolutamide.
References
1. Qu, M, and Gao, X. Prostate cancer in the world and China in 2022: an epidemiological analysis. Acad J Nav Med Univ. (2025) 46:229–33. doi: 10.16781/j.CN31-2187/R.20240685
2. Yang, XY, Yang, YZ, Lv, C, Lyu, C, Yuan, Q, and Wang, SF. Advancements in health economic evaluation research of drug treatment regimens for prostate cancer. Chin J Cancer Prev Treat. (2023) 30:1452–8. doi: 10.16073/j.cnki.cjcpt.2023.23.09
3. Chinese Anticancer Association Genitourinary Oncology Committee. Chinese experts consensus on the treatment of metastatic prostate cancer 2018 edition. Chin J Surg. (2018) 56:646–52. doi: 10.3760/cma.j.issn.0529-5815.2018.09.002
4. Fizazi, K, Jenkins, C, and Tannock, IF. Should docetaxel be standard of care for patients with metastatic hormone-sensitive prostate cancer? Pro and contra. Ann Oncol. (2015) 26:1660–7. doi: 10.1093/annonc/mdv245
5. The Chinese Society of Clinical Oncology Guideline Working Committee. The Chinese Society of Clinical Oncology (CSCO): prostate cancer diagnosis and treatment guidelines. Beijing: People’s Medical Publishing House (2024).
6. NCCN. (2025). Clinical practice guidelines in oncology: prostate cancer (version 2.2025). Available online at: https://www.nccn.org. (Accessed April 16, 2025)
7. Saad, F, Vjaters, E, Shore, N, Olmos, D, Xing, N, Pereira de Santana Gomes, AJ, et al. Darolutamide in combination with androgen-deprivation therapy in patients with metastatic hormone-sensitive prostate cancer from the phase III ARANOTE trial. J Clin Oncol. (2024) 42:4271–81. doi: 10.1200/JCO-24-01798
8. Hoeh, B, Wenzel, M, Tian, Z, Karakiewicz, PI, Saad, F, Steuber, T, et al. Triplet or doublet therapy in metastatic hormone-sensitive prostate cancer patients: an updated network meta-analysis including ARANOTE data. Eur Urol Focus. (2024) 24:S2405–4569. doi: 10.1016/j.euf.2024.11.004
9. Matsukawa, A, Litterio, G, Cormio, A, Miszczyk, M, Kardoust Parizi, M, Fazekas, T, et al. An updated systematic review and network meta-analysis of first-line triplet vs. doublet therapies for metastatic hormone-sensitive prostate cancer. Cancers. (2025) 17:205. doi: 10.3390/cancers17020205
10. Zhi, B, Yu, SB, Cui, KK, Fan, YF, Tao, J, and Zhang, XP. Real world clinical observation on the efficacy and safety of darolutamide in the treatment of metastatic hormone-sensitive prostate cancer. J Mod Oncol. (2025) 33:81–4. doi: 10.3969/j.issn.1672-4992.2025.01.013
11. Liu, CY, and Liu, GX. Pharmacoeconomics: evaluation and application. Chin J Pharm Econ. (2019) 14:122–8.
12. Weinstein, MC, and Stason, WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med. (1977) 296:716–21. doi: 10.1056/NEJM197703312961304
13. Wu, J, Gan, L, and Bai, XF. The application and prospect of pharmacoeconomics in promoting rational use of medicines. Chin J Hosp Pharm. (2025) 45:485–8. doi: 10.13286/j.1001-5213.2025.05.01
14. Liu, S, Li, S, Dou, L, Wang, K, Shi, Z, Wang, R, et al. Cost-utility analysis of darolutamide in the treatment of metastatic hormone-sensitive prostate cancer. Herald Med. (2023) 42:1400–6. doi: 10.3870/j.issn.1004-0781.2023.09.022
15. Yoo, M, Nelson, RE, Haaland, B, Dougherty, M, Cutshall, ZA, Kohli, R, et al. Cost-effectiveness analysis of 7 treatments in metastatic hormone-sensitive prostate cancer: a public-payer perspective. J Natl Cancer Inst. (2023) 115:1374–82. doi: 10.1093/jnci/djad135
16. Chinese Pharmacoeconomics Evaluation Guideline Writing Group. Pharmacoeconomics evaluation guidelines of China 2020. Beijing: China Medical Science and Technology Press (2020).
17. Smith, MR, Hussain, M, Saad, F, Fizazi, K, Sternberg, CN, Crawford, ED, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. (2022) 386:1132–42. doi: 10.1056/NEJMoa2119115
18. Chang, Y, Li, Y, Zeng, G, Guo, H, Hu, Z, Zhang, X, et al. China ARCHES: a phase 3 trial of enzalutamide vs placebo in metastatic hormone-sensitive prostate cancer. JU Open Plus. (2024) 2:e00104. doi: 10.1097/ju9.0000000000000198
19. Sweeney, CJ, Martin, AJ, Stockler, MR, Begbie, S, Cheung, L, Chi, KN, et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): an international, open-label, randomised, phase 3 trial. Lancet Oncol. (2023) 24:323–34. doi: 10.1016/S1470-2045(23)00063-3
20. Davis, ID, Martin, AJ, Stockler, MR, Begbie, S, Chi, KN, Chowdhury, S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. (2019) 381:121–31. doi: 10.1056/NEJMoa1903835
21. Hussain, M, Tombal, B, Saad, F, Fizazi, K, Sternberg, CN, Crawford, ED, et al. Darolutamide plus androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer by disease volume and risk subgroups in the phase III ARASENS trial. J Clin Oncol. (2023) 41:3595–607. doi: 10.1200/JCO.23.00041
22. Gu, W, Han, W, Luo, H, Zhou, F, He, D, Ma, L, et al. Rezvilutamide versus bicalutamide in combination with androgen-deprivation therapy in patients with high-volume, metastatic, hormone-sensitive prostate cancer (CHART): a randomised, open-label, phase 3 trial. Lancet Oncol. (2022) 23:1249–60. doi: 10.1016/S1470-2045(22)00507-1
23. Armstrong, AJ, Szmulewitz, RZ, Petrylak, DP, Holzbeierlein, J, Villers, A, Azad, A, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. (2019) 37:2974–86. doi: 10.1200/JCO.19.00799
24. Armstrong, AJ, Azad, AA, Iguchi, T, Szmulewitz, RZ, Petrylak, DP, Holzbeierlein, J, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. (2022) 40:1616–22. doi: 10.1200/JCO.22.00193
25. Chi, KN, Agarwal, N, Bjartell, A, Chung, BH, Pereira de Santana Gomes, AJ, Given, R, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. (2019) 381:13–24. doi: 10.1056/NEJMoa1903307
26. Chi, KN, Chowdhury, S, Bjartell, A, Chung, BH, Pereira de Santana Gomes, AJ, Given, R, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. (2021) 39:2294–303. doi: 10.1200/JCO.20.03488
27. Pelloux-Prayer, R, Bataillard, T, Thiery-Vuillemin, A, Vincent, A, Fagnoni, P, and Nerich, V. Treatment of patients with metastatic hormone-sensitive prostate cancer: a systematic review of economic evaluations. Clin Genitourin Cancer. (2022) 20:594–602. doi: 10.1016/j.clgc.2022.04.014
28. Liu, SX, Li, SP, Dou, L, Wang, KX, Shi, Z, and Wang, RX. Current situation of economic evaluation on prostate cancer in China. Med Soc. (2023) 36:75–9. doi: 10.13723/j.yxysh.2023.01.014
29. Eisenhauer, EA, Therasse, P, Bogaerts, J, Schwartz, LH, Sargent, D, Ford, R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
30. Scher, HI, Morris, MJ, Stadler, WM, Higano, C, Basch, E, Fizazi, K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. (2016) 34:1402–18. doi: 10.1200/JCO.2015.64.2702
31. Zhou, T, and Ma, AX. The survival analysis applied in calculation of Markov model transition probability in pharmaceutical evaluation. Chin J Evid Based Med. (2018) 18:1129–33. doi: 10.7507/1672-2531.201801088
32. Liu, SX. Evaluation of efficacy, safety and economy of treatment regimens for metastatic hormone-sensitive prostate cancer. Shandong: Shandong University (2023).
33. Hoyle, M, Green, C, Thompson-Coon, J, Liu, Z, Welch, K, Moxham, T, et al. Cost-effectiveness of temsirolimus for first line treatment of advanced renal cell carcinoma. Value Health. (2010) 13:61–8. doi: 10.1111/j.1524-4733.2009.00617.x
34. Yang, L, Wang, FL, Huang, L, Li, Y, Zheng, H, Zheng, L, et al. Cost-effectiveness analysis of enzalutamide in the treatment of metastatic prostate cancer. Chin J Pharmacoepidemiol. (2024) 33:269–76. doi: 10.12173/j.issn.1005-0698.202304008
35. Rabin, R, and de Charro, F. EQ-5D: a measure of health status from the EuroQol group. Ann Med. (2001) 33:337–43. doi: 10.3109/07853890109002087
36. Wei, MY, Wang, JJ, and Shi, YM. Research progress on the application of SF-6D in measuring the health utility value of patients with chronic obstructive pulmonary disease. Chin Nurs Res. (2024) 38:1789–92. doi: 10.12102/j.issn.1009-6493.2024.10.016
37. Wang, XY. Time to entry into CRPC and survival time of patients with advanced prostate cancer after endocrine therapy. Nanchang: Nanchang University (2024).
38. Shen, YK, Gong, RX, Xi, YL, Li, L, and Han, Z. Cost-utility analysis on abiraterone in first-line treatment of metastatic castration-resistant prostate cancer. Eval Anal Drug-Use Hosp China. (2023) 23:1363–8. doi: 10.14009/j.issn.1672-2124.2023.11.018
39. Liu, M, Qu, S, Liu, Y, Yao, X, and Jiang, W. Comparative clinical effects and cost-effectiveness of maximum androgen blockade, docetaxel with androgen deprivation therapy and ADT alone for the treatment of mHSPC in China. J Comp Eff Res. (2019) 8:865–77. doi: 10.2217/cer-2018-0133
40. Hu, X, Qu, S, Yao, X, Li, C, Liu, Y, and Wang, J. Abiraterone acetate and docetaxel with androgen deprivation therapy in high-volume metastatic hormone-sensitive prostate cancer in China: an indirect treatment comparison and cost analysis. Cost Eff Resour Alloc. (2019) 17:27. doi: 10.1186/s12962-019-0193-4
41. Shu, Y, Ding, Y, He, X, Liu, Y, Wu, P, and Zhang, Q. Cost-effectiveness of osimertinib versus standard EGFR-TKI as first-line treatment for EGFR-mutated advanced non-small-cell lung cancer in China. Front Pharmacol. (2022) 13:920479. doi: 10.3389/fphar.2022.920479
42. Yan, J, Li, C, Zhang, X, Cheng, L, Ding, R, and Zhang, L. Degarelix vs. leuprorelin for the treatment of prostate cancer in China: a cost-utility analysis. Front Public Health. (2022) 10:942800. doi: 10.3389/fpubh.2022.942800
43. Castro, E, Figliuzzi, R, Walsh, S, Craigie, S, Nazari, J, Niyazov, A, et al. Systematic literature review and meta-analysis of health state utility values in metastatic castration-resistant prostate cancer. Oncologist. (2024) 30:oyae321. doi: 10.1093/oncolo/oyae321
44. Sung, WWY, Choi, HCW, Luk, PHY, and So, TH. A cost-effectiveness analysis of systemic therapy for metastatic hormone-sensitive prostate cancer. Front Oncol. (2021) 11:627083. doi: 10.3389/fonc.2021.627083
45. Nafees, B, Lloyd, AJ, Dewilde, S, Rajan, N, and Lorenzo, M. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. (2017) 13:e195–203. doi: 10.1111/ajco.12477
46. Liu, S, Dou, L, Wang, K, Shi, Z, Wang, R, Zhu, X, et al. Cost-effectiveness analysis of nivolumab combination therapy in the first-line treatment for advanced esophageal squamous-cell carcinoma. Front Oncol. (2022) 12:899966. doi: 10.3389/fonc.2022.899966
47. Barqawi, YK, Borrego, ME, Roberts, MH, and Abraham, I. Cost-effectiveness model of abiraterone plus prednisone, cabazitaxel plus prednisone and enzalutamide for visceral metastatic castration resistant prostate cancer therapy after docetaxel therapy resistance. J Med Econ. (2019) 22:1202–9. doi: 10.1080/13696998.2019.1661581
Keywords: second-generation AR antagonists, enzalutamide, apalutamide, darolutamide, metastatic prostate cancer, Markov model, cost-utility analysis
Citation: Yang Y, Chen Y-Q, Huang L-Z and Chen Y (2025) Cost-effectiveness analysis of second-generation androgen receptor antagonists for the treatment of metastatic hormone-sensitive prostate cancer. Front. Public Health. 13:1680002. doi: 10.3389/fpubh.2025.1680002
Edited by:
Roberto Ippoliti, University of Eastern Piedmont, ItalyReviewed by:
Zelalem G. Dessie, Bahir Dar University, EthiopiaMehdi Rezaee, Shiraz University of Medical Sciences, Iran
Copyright © 2025 Yang, Chen, Huang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Chen, cHVwbGUyMDAwQDE2My5jb20=
†These authors have contributed equally to this work
 Yang Yang
Yang Yang Ya-Qing Chen
Ya-Qing Chen Long-Zhuan Huang
Long-Zhuan Huang Yong Chen*
Yong Chen*