- 1Department of Biomedical and Health Informatics, University of Missouri-Kansas City, Kansas City, MO, United States
- 2Department of Biomedical Informatics, University of Arkansas for Medical Sciences, Little Rock, AR, United States
- 3Department of Civil and Environmental Engineering, University of Missouri, Columbia, MO, United States
Microplastics (MPs) and nanoplastics (NPs) have become pervasive contaminants in food, water, and air, leading to widespread human exposure, primarily through ingestion. Although MPs are increasingly detected in human tissues, including the placenta, blood, and brain, their long-term health implications are poorly understood. This review compiles emerging evidence on the systemic distribution and biological effects of ingested MPs, particularly on neurological risks. MPs can disrupt gut microbiota, breach intestinal and blood–brain barriers, and accumulate in neural tissues. Mechanistic studies reveal that MPs induce oxidative stress, neuroinflammation, protein aggregation, and neurotransmitter alterations, which may contribute to the development of cognitive dysfunction and neurodegenerative disease pathways. Recent work using brain organoids, single-cell and multi-omics technologies provides deeper mechanistic insights, linking MP/NP exposure to mitochondrial injury, inflammatory signaling, and impaired protein homeostasis. We also identify important gaps in exposure assessment, NPs detection, and epidemiological evidence. Human studies remain scarce but initial reports associating elevated MP/NP burdens in brain tissue with dementia highlight the urgency of this research. To address these gaps, we suggest critical next steps in the research agenda, integrating omics technologies, real-world exposure models, and human-relevant in vitro systems. As MP contamination grows, it is critical to understand its neurotoxic potential for informing public health policy and protecting vulnerable populations.
Introduction
Microplastics (MPs), defined as plastic particles <5 mm (with nanoplastics (NPs) generally <1 μm) (1), arise either from the fragmentation of larger plastics (secondary MPs) or are manufactured as small particles (primary MPs, e.g., microbeads, resin pellets) (2). These particles are now pervasive across ecosystems, detected in marine and freshwater environments, soil, and air (3–5). Primary sources include single-use plastics, synthetic fibers, personal care products, and tire wear particles (4, 6, 7). Human exposure occurs through ingestion of contaminated food and water, inhalation and dermal contact (8–10).
MPs are ingested through diverse dietary items. They have been detected in seafood (particularly shellfish consumed whole), sea salt, tap and bottled water, and even fruits and vegetables (11–15). Cox et al. (16) estimated annual human ingestion at tens to hundreds of thousands of particles, equating to several grams per week. A 2019 study by Schwabl et al., found MPs in all analyzed stool samples, with a median of 20 particles per 10 g of feces (17), confirming routine dietary exposure. MPs have also been identified in human tissues such as the placenta and breast milk (18, 19) raising significant concerns about potential health impacts on infants and children. This review focuses on how ingested MPs enter and distribute in the body, their general health effects (on gastrointestinal, cardiovascular, immune, and reproductive systems), and, most critically, their emerging neurological implications.
To identify relevant literature, we conducted a structured search of PubMed, Web of Science, and Scopus for publications from 2015 through January 2025. Search terms included combinations of “microplastics,” “nanoplastics,” “neurotoxicity,” “ingestion,” “blood–brain barrier,” and “gut–brain axis.” We included peer-reviewed primary research and review articles that addressed exposure, toxicokinetics, systemic or neurological health effects, and human biomonitoring. Studies not published in English or lacking relevance to human or mammalian systems were excluded. References summarized in Tables 1–4 as well as those discussed in the main text, were identified through this process.
Mechanisms of microplastic ingestion and absorption
Sources of ingestion
Humans continuously ingest micro- and nanoplastics (MNPs) through food and beverages. Both tap and bottled water contain MPs, with plastic bottles showing particularly high loads (1). Heating liquids in plastic teabags or baby bottles can also release large quantities of MPs (20). Seafood, especially species consumed whole, are known vectors due to marine contamination (11, 21). Other foods, including salt, sugar, honey, beer, and produce, may contain MPs due to environmental or processing contamination (1). Additionally, indoor dust and synthetic fibers shed from textiles contribute to unintentional ingestion (22). Fang et al. (23) estimated that atmospheric deposition alone can contribute up to 1 million MPs per year to the human diet.
Fate post-ingestion
Most MPs are excreted via feces; infants show significantly higher levels than adults (20). However, smaller particles, especially NPs, can cross the intestinal barrier (24–27). MPs < 150 μm may penetrate the gut lining, particularly via M-cells in Peyer’s patches and mucosal immune tissues (28, 29). While larger particles remain in the GI tract, smaller ones may enter circulation, depending on their size, charge, and surface chemistry. Continuous dietary exposure ensures a steady internal presence of MPs, underscoring the need to understand their bioavailability and health implications.
Toxicokinetics of microplastics in the human body
Once ingested, MPs’ absorption and distribution are primarily governed by particle size and physicochemical properties (30, 31). Larger particles (>150 μm) are typically confined to the gastrointestinal tract and excreted, acting locally within the gut. In contrast, smaller MPs (<150 μm), especially NPs (<1 μm), can cross the intestinal barrier to some extent (31). Toxicological data estimate that ≤0.3% of small MPs may be absorbed, while NPs may achieve higher uptake, potentially several percent (31). Experimental studies confirm that polystyrene NPs between 20 and 100 nm can penetrate the intestinal lining and enter the bloodstream in rodents (32), likely through endocytosis or paracellular transport (33).
Once in the systemic circulation, MPs can travel to various organs. A landmark biomonitoring study detected particles ≥700 nm in human whole blood, including polyethylene and polyethylene terephthalate (PET), in 77% of donors (34). This confirms the systemic bioavailability of MPs in humans. Subsequent studies have found MPs in human lungs (35, 36), liver (37, 38), spleen (37), kidney (39), and placenta (40, 41). Notably, MPs were detected on both maternal and fetal sides of the placenta, demonstrating their ability to cross placental barriers.
Of critical concern is the brain. Animal studies have shown that NPs can cross the blood–brain barrier (BBB). For example, mice fed 30–50 nm polystyrene NPs showed brain accumulation and cognitive impairment (32). Two main routes are proposed: (1) via the bloodstream, where particles may breach the BBB by forming a protein corona or exploiting endothelial pathways (33), and (2) via the olfactory nerve, where inhaled particles migrate directly from the nasal cavity to the olfactory bulb (42). One autopsy case series detected polypropylene fragments in the olfactory bulbs of 8 of 15 human cadavers (42), suggesting direct nose-to-brain translocation.
Once in tissues, MPs may persist due to limited clearance. A 2023 autopsy study found higher concentrations of MPs in brain tissue than in the liver or kidney of the same individuals (43). Many particles were nanoscale, shard-like fragments consistent with environmental degradation products. Alarmingly, the total plastic burden in brains appeared to increase over the past decade (43). While clearance may occur via immune cells or the glymphatic system, recent findings suggest NPs may impair glymphatic clearance mechanisms (1).
Thus, the neurotoxic potential of MPs is strongly influenced by their physicochemical properties, particularly particle size and shape. Smaller NPs are more likely to cross biological barriers such as the intestinal epithelium and blood–brain barrier, while shape characteristics (e.g., rod-like or spiked forms) may enhance tissue penetration and cellular interactions. Table 4 summarizes how these properties affect brain accumulation and neurotoxicity based on current evidence.
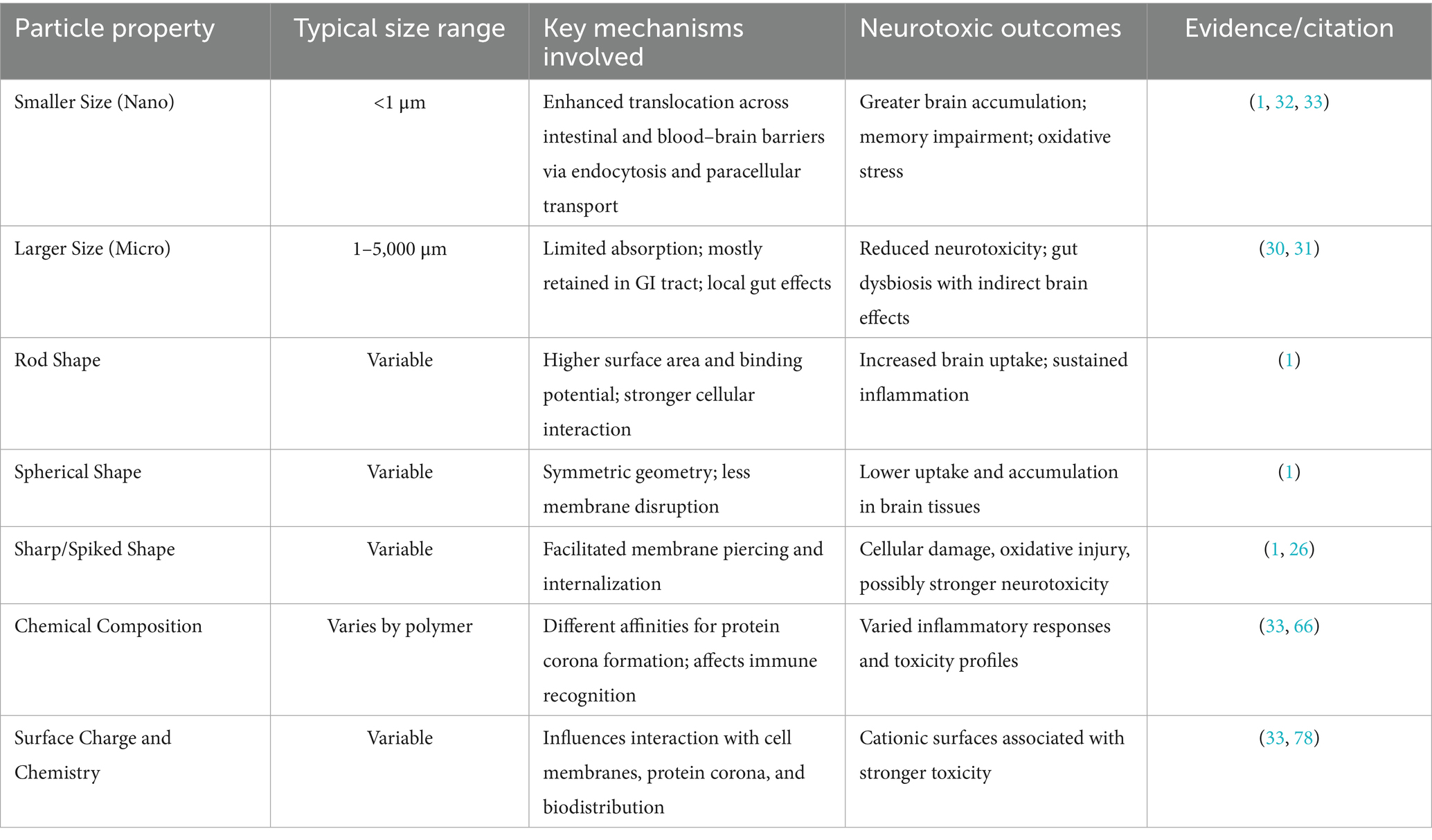
Table 4. Influence of microplastic physicochemical properties on neurotoxicity and brain penetration.
Taken together, MPs show minimal absorption when large, but measurable systemic uptake when small, with the ability to cross biological barriers—including the placenta and BBB—and accumulate particularly in the brain. This underscores their potential for chronic internal exposure and associated neurological risks.
General health effects of microplastic exposure
Although human data remains limited, growing evidence suggests that ingested MPs may pose risks to gastrointestinal, immune, cardiovascular, and reproductive health.
Gastrointestinal (GI) tract
The GI tract is the primary site of contact with ingested MPs and is particularly vulnerable (44–46). Physical interactions between MPs and intestinal linings can cause irritation, inflammation, and even microlesions (45–47). Polystyrene MPs have been shown to disrupt intestinal integrity in animals and induce inflammatory responses (36, 47). A significant concern is the impact on the gut microbiome: MPs can lead to dysbiosis, shifting microbial balance toward pro-inflammatory organisms (44–48). These shifts are observed across species, from fish to rodents to humans, and beyond disrupting the microbiome, MPs also increase gut permeability, commonly referred to as ‘leaky gut. The weakening of tight junctions between intestinal cells (44–46) allows microbes and particles to translocate into circulation, potentially triggering systemic inflammation. These changes are associated with chronic disorders like inflammatory bowel disease and metabolic syndrome (45–47). Human data are still emerging, but the GI tract remains a critical site of concern for MP exposure with potential consequences extending along the gut-liver and gut-brain axes.
Immune and inflammatory responses
MPs can elicit immune activation as foreign particles, especially when they cross mucosal barriers (49). Human immune cells internalize MPs in vitro. The result is the release of pro-inflammatory cytokines and Reactive Oxygen Species (ROS), a typical cellular response to MPs (49, 50). Persistent exposure may cause low-grade systemic inflammation. Chronic immune activation raises concern for links to autoimmune conditions, although direct evidence remains limited (51, 52).
Additionally, MPs can act as carriers for bacteria and toxins. Environmental MPs have been shown to adsorb pathogens and microbial metabolites, which may exacerbate immune responses (53, 54). They also bind heavy metals like lead and cadmium, but the health risks of such co-exposures remain underexplored (55, 56). Overall, MP-induced oxidative stress and immune activation likely underlie many health disturbances. These immune responses may not only affect peripheral systems but may also influence brain health. Chronic inflammation and cytokine signaling can disrupt the blood–brain barrier and contribute to neuroinflammation and neurodegenerative risks.
Cardiovascular and metabolic effects
Though research is still in the early stages, there is growing concern about cardiovascular toxicity. MPs entering the bloodstream may damage the vascular endothelium and promote inflammation, a driver of atherosclerosis (57–59). MNPs have been detected in human atherosclerotic plaques, and higher burdens correlate with myocardial infarction, stroke, and mortality (58, 60). MPs also transport endocrine-disrupting additives (e.g., bisphenol A, phthalates) that are linked to obesity, insulin resistance, and cardiovascular disease (61). Disruption of gut microbiota by MPs may further exacerbate these effects through metabolic inflammation (61). Notably, MPs have been found in the cardiac tissues of patients undergoing surgery, though their pathological significance remains uncertain (59). Collectively, cardiovascular and metabolic disturbances provide plausible indirect routes to neurological harm via vascular injury, impaired cerebral perfusion, and systemic inflammation.
Reproductive health
MPs have been detected in reproductive tissues, raising concerns about their impact on fertility and fetal development. The presence of MPs in the human placenta (“plasticenta”) suggests possible interference with placental function (62). Though the pregnancies in those studies were clinically normal, MPs may cause localized inflammation or oxidative stress that impairs nutrient exchange. Recent studies also found MPs in testicular tissue, correlating with reduced sperm quality (63). Endocrine-disrupting additives (e.g., phthalates, bisphenols) could further disrupt spermatogenesis and hormone signaling. Animal studies corroborate these risks: female or maternal exposure reduces fertility and offspring size (64). Maternal MP exposure has also been linked to lower birth weights and metabolic disturbances in offspring. While human data remain limited, reproductive and developmental effects are relevant to neurodevelopmental vulnerability, particularly via placental inflammation, endocrine disruption, and early-life metabolic programming.
Other health considerations
Respiratory exposure to airborne MPs may contribute to lung inflammation or fibrosis (65). There is also concern about carcinogenesis. MPs can carry carcinogenic compounds like polycyclic aromatic hydrocarbons (PAHs), and chronic inflammation from particle exposure is a recognized risk factor for cancer (65, 66). However, human evidence for MP-induced carcinogenicity remains inconclusive. Most insights currently derive from in vitro systems or high-dose animal studies. Although MPs clearly have biological activity, their long-term effects in humans, especially at real-world exposure levels, require further investigation (66).
In summary, MP ingestion has been associated with multi-system inflammation and dysfunction. These gastrointestinal, immune, cardiovascular, and reproductive perturbations create conditions like systemic inflammation, endothelial/vascular injury, endocrine disruption, and microbiome-mediated signaling that potentially heighten vulnerability of the nervous system. We next examine mechanistic links to neurotoxicity.
Neurological effects of microplastic exposure
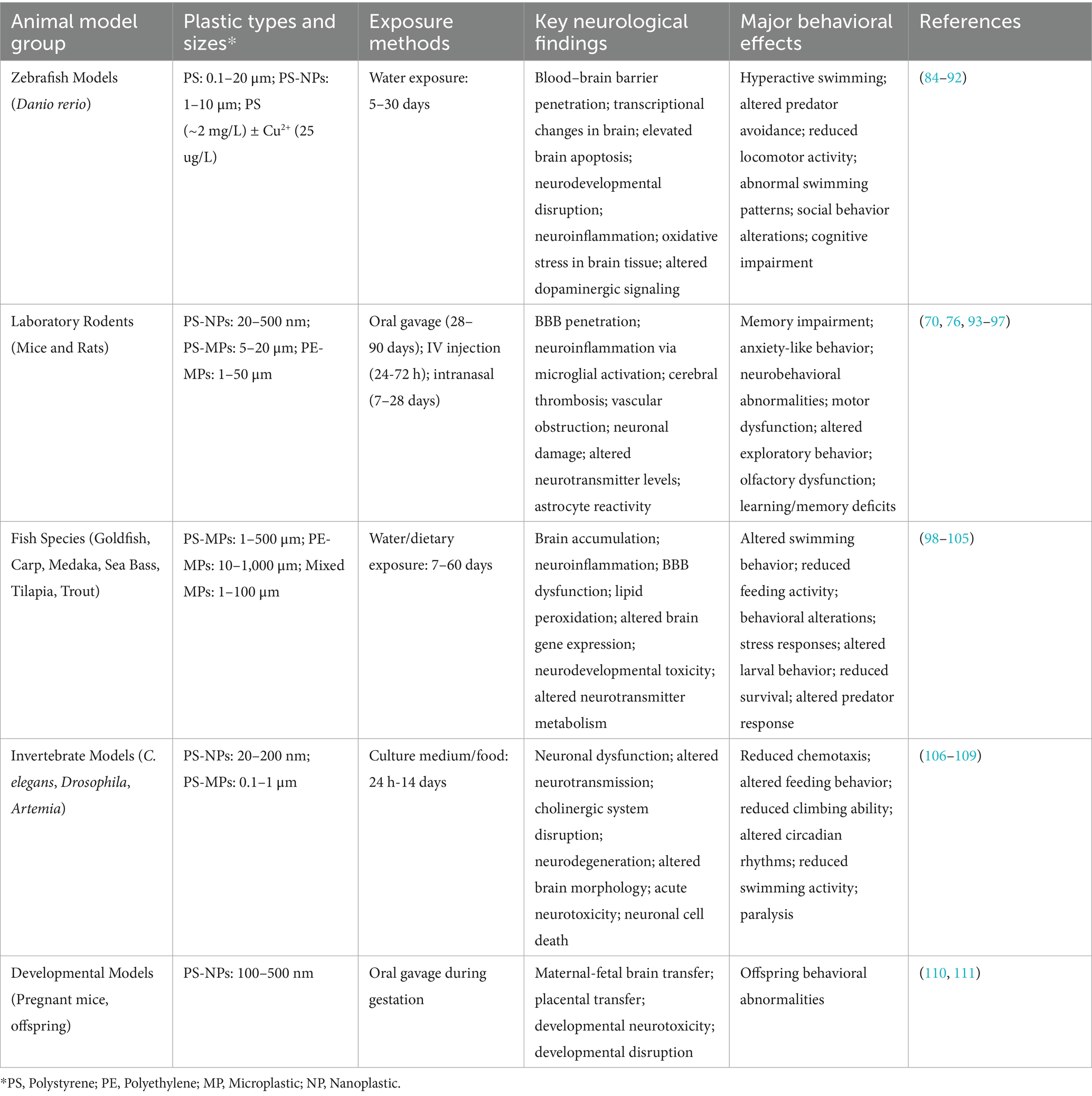
Emerging evidence from experimental studies suggests that exposure to MNPs can lead to neurological impairments, including cognitive and behavioral dysfunctions. There are ethical and practical barriers to direct human studies, but animal models provide compelling insights. Numerous animal studies have provided mechanistic insights into how MNPs impair brain function. These studies span diverse species and exposure regimens, consistently reporting neurobehavioral changes, oxidative stress, and protein aggregation. Table 1 provides a summary of key experimental findings across various animal models.
In rodent models, oral exposure to polystyrene NPs (10–20 mg/kg/day) over several weeks has resulted in significant memory and learning deficits without affecting general health or motor function (32). These findings suggest subtle but specific neurobehavioral toxicity. Similarly, aquatic models such as zebrafish and nematodes have exhibited behavioral abnormalities, ranging from reduced exploration and impaired prey capture to locomotor disruption and convulsive activity at high MP concentrations (67). Collectively, these studies indicate that MPs may impair core neurological functions.
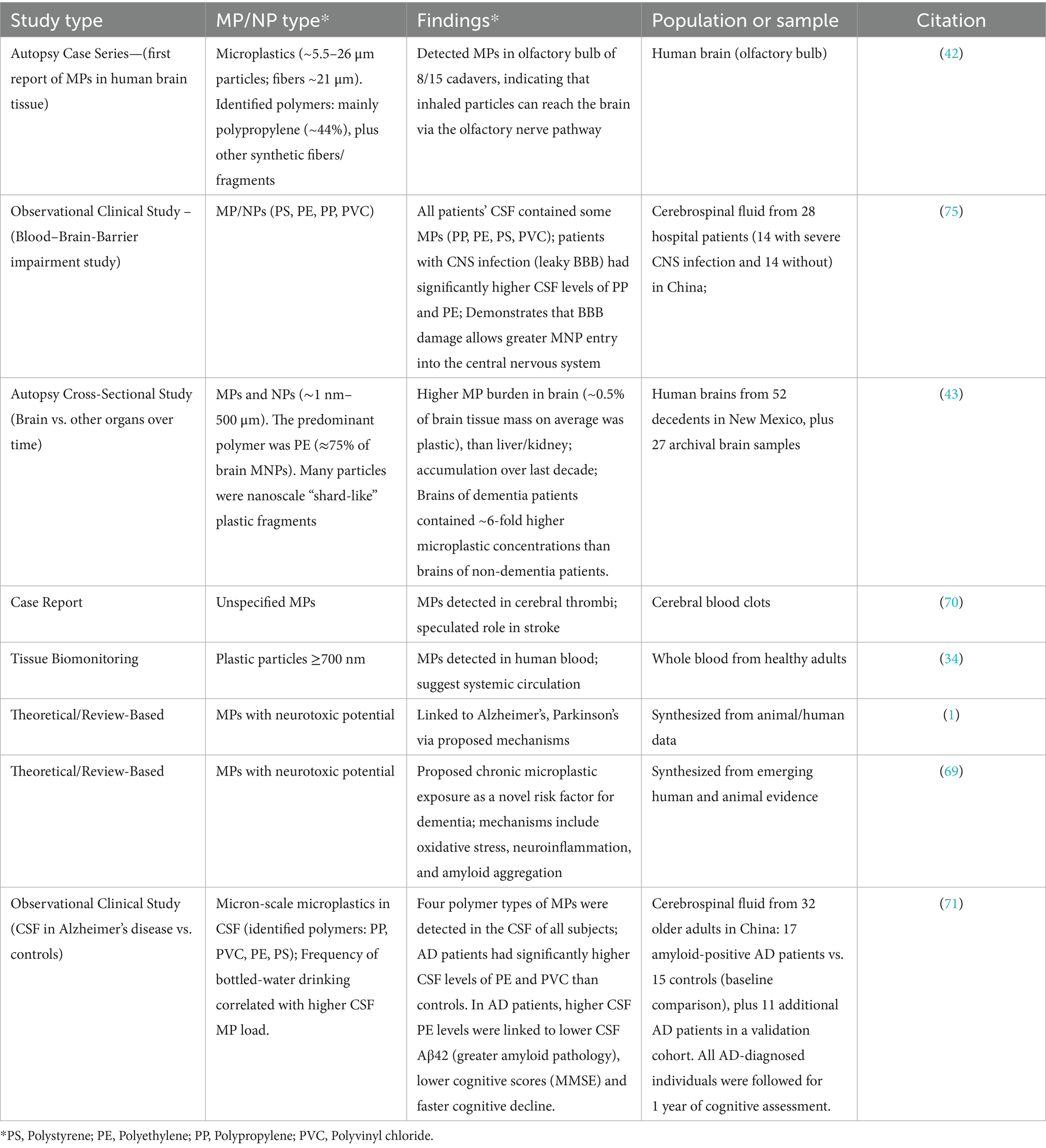
Human epidemiological data directly linking MP exposure to neurological outcomes are still lacking, but recent autopsy and case reports raise important concerns. Microplastics have been found in human brain tissue, including in individuals with dementia (43, 68). A recent autopsy study by Nihart et al. (43) reported that microplastic concentrations were significantly higher in human brain tissue compared to liver or kidney, and that dementia patients had markedly higher brain plastic burdens than non-dementia patients. Similarly, Gecegelen et al. (69) proposed chronic microplastic exposure as a novel risk factor for dementia. These findings provide compelling human evidence linking chronic microplastic accumulation to neurodegenerative risk. Although causality cannot be inferred from most cross-sectional findings, the accumulation of MPs in brain regions like the cortex and olfactory bulb (42) certainly raises the possibility of neurotoxic effects. Additionally, MPs have been detected in cerebral thrombi, prompting speculation that they may contribute to stroke risk by inducing microvascular obstruction (70). Detection of these particles in the human brain/CNS, although preliminary, raises important questions about chronic exposure and neurotoxicity. As summarized in Table 2, multiple recent human studies, including cerebrospinal fluid analyses (71) and brain autopsy series (43) provide direct clinical evidence of microplastics in the central nervous system. In their study He et al. reported microplastics in CSF along the AD continuum and linked higher CSF polyethylene and PVC to worse cognitive trajectories, reinforcing the clinical relevance of CSF plastic burden. These studies not only underscore the clinical relevance of microplastic neurotoxicity but also strengthen the rationale for investigating links with dementia and other neurodegenerative outcomes.
A particularly provocative area of investigation is the potential link between chronic MP exposure and neurodegenerative diseases. Preclinical studies suggest MPs may accelerate pathological processes underlying Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and Multiple Sclerosis (MS). For instance, when in sufficient quantities, polystyrene NPs have been shown to promote alpha-synuclein aggregation, a hallmark of PD. (72) Other studies report that MPs facilitate amyloid-β aggregation in vitro, enhancing neurotoxicity in AD models (1). There is also evidence from fetal rat studies that MPs disrupt myelin formation, which could have relevance for MS. (1) While these disease-focused studies remain preclinical, they raise important hypotheses about MPs as environmental risk factors for neurodegeneration.
Cumulative evidence from animal studies suggests that MPs can impair memory, learning, and behavior and may promote the aggregation of neurotoxic proteins. These outcomes mirror features of neurodevelopmental and neurodegenerative disorders. Although most findings are from high-dose animal models, they raise critical questions about whether chronic, low-level human exposures could cause similar, albeit subtler, effects (67). Addressing this gap is essential, especially as MP contamination becomes increasingly pervasive. Overall, experimental data strongly support the neurotoxicity potential of MPs and underscore the need for more research.
Molecular mechanisms of microplastic-induced neurotoxicity
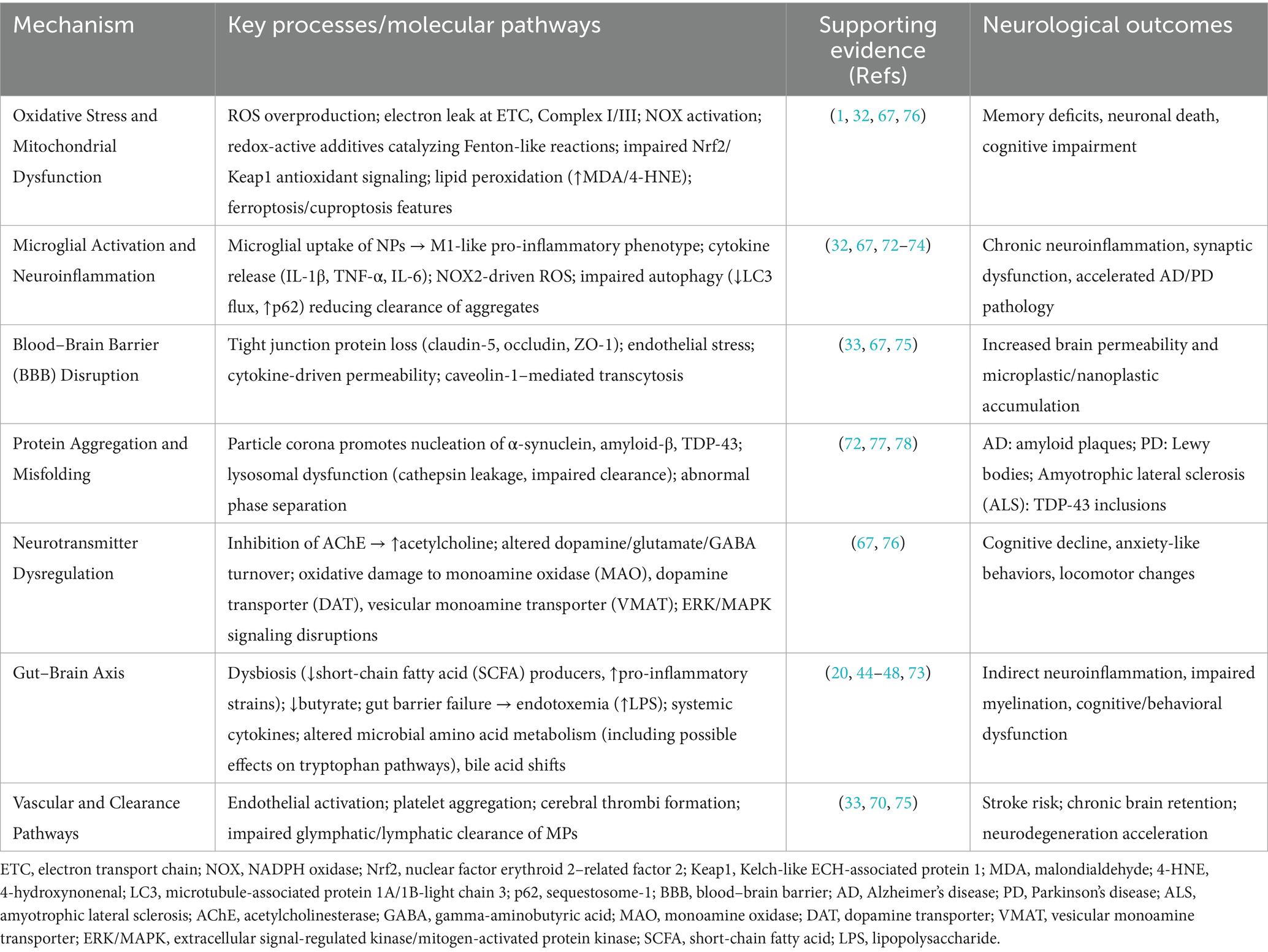
The neurotoxicity of MNPs is based on interconnected biological processes. The mechanistic pathways can include oxidative stress, neuroinflammation, disruption of the blood–brain barrier (BBB), neurotransmitter dysregulation, protein aggregation, and/or modulation of the gut-brain axis (Figure 1). These mechanisms are discussed in detail below and an integrated summary of the key molecular mechanisms is presented in Table 3.
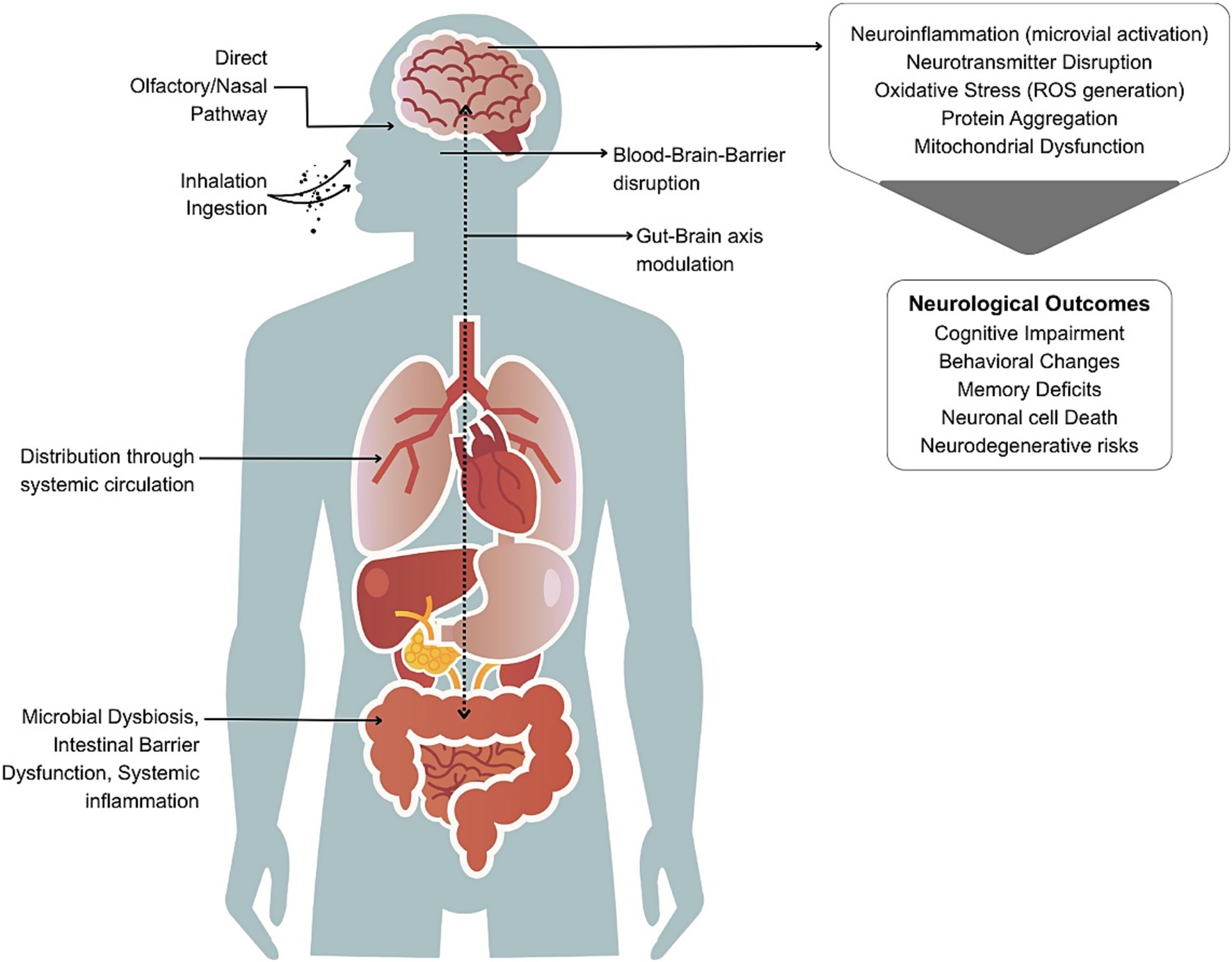
Figure 1. Pathways through which micro- and nanoplastics (MNPs) may cause neurological effects. MNPs from food, water, and air enter the body via ingestion or inhalation. Inhaled particles may bypass the blood–brain barrier (BBB) via the nasal/olfactory route. Ingested particles can disrupt gut microbiota and intestinal barriers, leading to systemic inflammation and translocation into circulation, ultimately affecting the brain through BBB disruption and gut-brain axis modulation. Once in the brain, MNPs may trigger neuroinflammation, oxidative stress, neurotransmitter imbalance, and protein aggregation contributing to cognitive, behavioral, and neurodegenerative outcomes.
Oxidative stress
Oxidative stress is a consistent and early response to MP exposure. Both animal models and in vitro studies show that MPs induce ROS generation in neuronal tissues (1, 67). Excessive ROS damages cellular biomolecules, leading to impaired neural function and cell death. Mechanistic studies indicate mitochondrial dysfunction as a central source of ROS, particularly electron leakage at complexes I and III of the electron transport chain, leading to loss of mitochondrial membrane potential and reduced ATP synthesis (1, 32). In rodents, oxidative injury in the hippocampus has been linked to memory deficits (32). ROS also activates signaling pathways like Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB), contributing to neuroinflammation and apoptosis. In addition, Nrf2/Keap1 antioxidant defenses appear downregulated in MNP-exposed neurons, suggesting impaired adaptive responses (67). Given its central role in neurodegenerative diseases MP-induced oxidative stress is considered a major mechanistic trigger of neural dysfunction (1).
Neuroinflammation and microglial activation
MPs can provoke inflammatory responses once they enter the brain. Microglia, the brain’s resident immune cells, preferentially internalize NPs (32). They undergo morphological changes upon uptake and release pro-inflammatory cytokines and ROS, creating a neurotoxic environment. This is accompanied by activation of NADPH oxidase (NOX2), which amplifies oxidative and inflammatory signaling (67). Conditioned media from MP-exposed microglia has been shown to reduce neuronal firing activity, an effect reversible with anti-inflammatory inhibitors (32). Chronic microglial activation can damage neurostructures, driving disease progression. Additionally, impaired microglial autophagy, reflected in reduced LC3-II flux and p62 accumulation, further limits clearance of amyloid and α-synuclein aggregates, compounding proteostatic stress and thus exacerbating AD and PD pathology (72–74).
Blood–brain barrier disruption
MPs can not only cross the BBB but also compromise its structural integrity. In vitro models reveal that polystyrene nanoparticles disrupt tight junction proteins in endothelial cells (33). Key targets include claudin-5, occludin, and ZO-1, whose downregulation increases paracellular permeability (33). Inflammatory cytokines released in response to MP exposure further degrade BBB tightness, potentially increasing brain exposure to other neurotoxicants (67). Sustained oxidative stress is another factor that weakens barrier function. Endothelial activation markers such as caveolin-1, VCAM-1, and ICAM-1 are also upregulated, suggesting active transcytosis and immune cell recruitment as additional routes of barrier compromise (67, 75). Thus, MPs may act both as direct neurotoxicants and facilitators of broader CNS vulnerability by impairing the brain’s primary defense.
Neurotransmitter and synaptic effects
MNPs disrupt neurotransmitter systems. Studies have reported that inhibition of a Acetylcholinesterase (AChE) results in elevated acetylcholine levels at synapses, disrupting cholinergic signaling (67). This hypercholinergic state may disrupt normal long-term potentiation (LTP) and synaptic plasticity, processes essential for learning and memory. MPs also alter brain levels of dopamine, glutamate, and Gamma-Aminobutyric Acid (GABA) (1, 67). These neurochemical imbalances correspond with behavioral changes observed in exposed animals. In zebrafish and rodents, MP exposure has been associated with altered serotonin and dopamine signaling (67). Evidence also points to oxidative modification of dopamine transporter (DAT) and vesicular monoamine transporter (VMAT), which impair dopamine reuptake and storage (76). Enzymatic changes affecting neurotransmitter metabolism (e.g., monoamine oxidase inhibition) have also been reported. Together these suggest widespread disruption of synaptic communication.
Protein aggregation and misfolding
Nanoplastics may serve as nucleation sites for the aggregation of neurodegeneration-related proteins. Experimental studies demonstrate that polystyrene NPs bind α-synuclein, accelerating its conversion to insoluble fibrils associated with Parkinson’s disease (77). Similarly, MPs promote amyloid-β aggregation, enhancing neurotoxicity in AD models (1). MPs also interfere with the normal degradation of proteins. Once internalized, they accumulate in lysosomes and impair their function, hindering the clearance of misfolded proteins (77). Lysosomal destabilization causes cathepsin leakage into the cytoplasm, further promoting neuronal apoptosis and inflammation (77). Promoting aggregation and inhibiting degradation contributes to toxic protein buildup, a hallmark of many neurodegenerative conditions. Additionally, NPs have been shown to induce TDP-43 aggregation, linked to amyotrophic lateral sclerosis (ALS) (78).
Gut-brain axis and indirect effects
Ingested MPs may influence brain function indirectly via the gut-brain axis. MPs disturb the intestinal microbiome, reducing beneficial bacteria and increasing pro-inflammatory strains (20). Notably, depletion of butyrate-producing taxa reduces availability of short-chain fatty acids that are critical for maintaining gut barrier and microglial homeostasis (44–48). These microbiota shifts can affect brain health through altered production of microbial metabolites, e.g., short-chain fatty acids, amino acids and neurotransmitter precursors with potential downstream effects on neuroactive compounds (71) MPs also compromise gut barrier integrity. They promote systemic inflammation, a known contributor to neuroinflammatory and neurodegenerative processes. Behavioral and neural changes in MP-exposed rodents have been associated with these gut-level alterations (20, 73). Therefore, neurological consequences may result not only from MPs reaching the brain but also from cascading systemic effects originating in the gut.
Integrated mechanisms
These mechanisms are not isolated. Oxidative stress can initiate microglial activation; neuroinflammation can impair BBB integrity, and disrupted autophagy can intensify protein aggregation. These synergistic interactions create positive feedback loops, for example, BBB disruption increases brain MNP accumulation, which further exacerbates oxidative and inflammatory stress. MPs can also alter membrane fluidity and intracellular signaling, which amplifies stress responses. Experimental studies using single-nucleus RNA sequencing in MP-exposed mice have revealed widespread transcriptional changes in neuronal pathways, particularly those regulating energy metabolism. This implicates mitochondrial dysfunction in MP-related neurotoxicity (79). Together, these findings indicate that MNPs are biologically active and capable of perturbing multiple molecular systems within the Central Nervous System (CNS). The cumulative effect of these disruptions may increase susceptibility to cognitive impairments, behavioral alterations, and progressive neurodegenerative diseases.
Knowledge gaps
Despite rapid progress in understanding microplastic-induced neurotoxicity, several critical knowledge gaps remain. These are concerning human exposure levels, NPs detection, mechanistic specificity, and the effects of combined exposures and individual vulnerability.
Human exposure levels and risk thresholds
We still lack precise data on typical brain exposures to MPs. MP intake has been quantified at tens of thousands of particles annually through food and water. They have been detected in blood and tissue (22, 34), but the internal dose required to cause neurological harm remains unclear. Moreover, the relationship between MPs’ physicochemical characteristics and their health impacts is poorly understood. Most toxicological studies use doses that exceed environmental exposure levels by orders of magnitude (67). Whether chronic, low-level exposures contribute to subtle neurofunctional changes has not been explored in humans. The absence of epidemiological studies linking MP exposure to neurodegenerative outcomes is a key barrier. This is partly due to the lack of validated exposure biomarkers. Future work should prioritize the development of sensitive, non-invasive biomarkers for MP burden.
Detection of nanoplastics
A major technical challenge is the detection and characterization of NPs (<1 μm) in human tissues because most conventional analytical methods, such as micro-FTIR or Raman microscopy, have lower detection limits in the micrometer range (43). NPs, due to their small size and surface reactivity, are the most likely to cross biological barriers like the blood–brain barrier and accumulate in the brain (50, 51, 66). Their actual concentration in human tissues may be significantly underestimated. High-resolution pyrolysis gas chromatography mass spectrometry (py-GC/MS) or field-flow fractionation coupled with light scattering are needed to detect, quantify, and characterize NPs in biological matrices. Without such tools, risk assessments are likely to overlook the most neurotoxic fraction of plastic particles.
Mechanistic specificity
While general mechanisms such as oxidative stress, neuroinflammation, and protein misfolding have been identified, our understanding of how specific MP characteristics drive these effects remains limited. Particle size, shape, charge, and polymer composition likely influence toxicity, but systematic comparisons are rare. For instance, whether spherical MPs are more neurotoxic than fibers or whether polystyrene elicits stronger microglial activation than polyethylene is not well established (67). Moreover, most mechanistic studies have been short-term. The potential for cumulative effects, such as protein aggregation, synaptic remodeling, or epigenetic changes, from chronic exposure has not been explored. Longitudinal studies and multi-omics approaches (transcriptomics, proteomics, metabolomics) could elucidate molecular pathways and identify markers of early neurotoxicity.
Combined exposures and real-world conditions
Environmental MPs do not act in isolation. They often adsorb and transport other pollutants such as heavy metals, persistent organic pollutants (POPs), and microbial toxins (51, 66). Yet most laboratory studies use pristine, single-polymer spheres, which do not reflect the heterogeneous, weathered particles encountered in the environment (66). Surface oxidation, changes in hydrophobicity, and chemical loading can significantly alter toxicity profiles (80, 81). Studies comparing new vs. aged MPs and those incorporating adsorbed contaminants are urgently needed. For example, co-exposure models could test whether MPs carrying lead or per- and poly-fluoroalkyl substances (PFAS) have synergistic neurotoxic effects. Likewise, MPs may facilitate microbial translocation or endotoxin delivery across the intestinal or nasal mucosa, heightening immune responses (53, 54). Experimental designs must better mirror environmental conditions to ensure relevance to human health.
Individual vulnerability and life stages
Susceptibility to MP neurotoxicity likely varies. Infants and children who ingest more MPs per body weight and have developing nervous systems may be particularly vulnerable (20). However, data on developmental neurotoxicity are virtually nonexistent. Do prenatal or early-life exposures affect long-term cognition? Maternal exposure studies suggest MPs can cross the placenta, but whether they impair fetal brain development remains unknown. Similarly, the role of MPs in accelerating age-related neurodegeneration is unexplored. Could the accumulation of NPs in aging brains worsen outcomes in AD or PD models? Genetic factors such as polymorphisms in oxidative stress pathways may also mediate susceptibility. These questions require targeted studies across life stages and in genetically diverse models.
Thresholds, reversibility, and chronicity
It is unclear whether neurotoxicity from MPs exhibits a dose threshold or is reversible. Some rodent studies show effects at very low doses, while others require much higher exposure to elicit changes (67). This inconsistency suggests potential nonlinear or threshold-dependent effects. Longitudinal studies are needed to determine whether neural changes (e.g., inflammation or synaptic loss) resolve after exposure ends or persist, potentially leading to lasting dysfunction. Identifying whether damage accumulates over time or reaches a plateau will help refine risk assessments. Further, it is not known whether intermittent vs. continuous exposure has differential effects on brain accumulation and damage.
Future directions
To advance the field of microplastic (MP) neurotoxicity and bridge critical knowledge gaps, a coordinated, interdisciplinary research agenda is essential. Below, we outline streamlined priorities that integrate epidemiology, exposure science, mechanistic toxicology, and public health policy.
Advancing human exposure assessment and epidemiology
Robust epidemiological studies are urgently needed to evaluate the potential contribution of MP exposure to neurodevelopmental, neurobehavioral, and neurodegenerative outcomes. Currently, no population-level data link MP burden to diseases such as AD, PD, or cognitive decline, largely due to the lack of validated biomarkers of MP exposure. Research should focus on developing high-throughput, cost-effective methods to detect MNPs in biological matrices such as blood, urine, cerebrospinal fluid, and feces. These biomarkers must consider particle size, polymer type, surface properties, and adsorbed chemicals. Integrating such tools into existing cohorts (e.g., birth registries and aging studies) offers a scalable approach to human data generation.
Improving nanoplastic detection technologies
The biological detection of nanoplastics remains technically challenging, particularly due to their small size and complex interactions with biological matrices. Spectroscopic techniques μFTIR and μRaman fail to detect the smallest, potentially most toxic particles. To move the field forward, efforts should focus on refining and standardizing these techniques for biological samples. Integrative strategies that combine imaging, spectrometry, and machine learning may enhance sensitivity and specificity. Establishing validated protocols and inter-laboratory benchmarks will be critical for generating reproducible, comparable data across studies.
Mechanistic insights from organoid and in vitro systems
Advanced human-relevant in vitro systems, including neural organoids, microfluidic BBB models, and gut-brain-on-chip platforms enable detailed study of MP-induced neurotoxicity. Recent brain organoid studies show NPs reduce neural progenitors/neurons and perturb neurodevelopmental programs, underscoring translational relevance for human brain biology (82). These models support high-resolution investigations of particle size, polymer type, surface chemistry, and co-contaminant effects. Transcriptomic and proteomic profiling can identify early molecular changes preceding neurological damage. Studies using organoids from genetically susceptible donors (e.g., APOE4 for AD) can help uncover gene–environment interactions influencing vulnerability.
Systems toxicology and multi-omics integration
A systems-level understanding of MP effects is needed. Multi-omics approaches; transcriptomics, metabolomics, epigenomics, and proteomics can help build integrated toxicity networks. For example, single-cell RNA-seq in MP-exposed brain tissue has highlighted disruptions in mitochondrial metabolism and synaptic signaling pathways (79). Emerging 2024 multi-omics work integrating brain transcriptomics with metabolomics similarly highlights synaptic and mitochondrial pathway disruption after MNP exposure, extending single-cell findings (83). Coupling omics data with functional assessments (e.g., behavior, electrophysiology) and applying machine learning can elucidate causal pathways and inform biomarker discovery.
Transdisciplinary collaboration and stakeholder integration
Addressing MP neurotoxicity requires collaborative efforts across neuroscience, environmental health, materials science, microbiology, and computational biology. Equally important is engagement with policymakers, risk communication experts, and communities. Transdisciplinary centers and consortia can facilitate data sharing, method harmonization, and consensus-building on exposure thresholds. Including citizen science and open-access databases can increase transparency, trust, and relevance of findings.
Enhancing real-world relevance of exposure models
Toxicological studies often use pristine MPs, which differ from environmentally aged particles present in food, air, and water. These aged MPs exhibit surface oxidation, biofilm accumulation, and chemical adsorption that alter biological interactions (66). Future models must simulate realistic exposure conditions, including mixed MPs, co-contaminants, and chronic low-dose regimens. Studies should also assess the bio-corona that forms in vivo and its role in MP uptake and immune interactions.
Identifying vulnerable populations and windows of susceptibility
The developing brain is especially vulnerable to environmental insults. Prenatal and early-life exposure to MPs may disrupt neurodevelopment, as evidenced in animal studies showing impaired myelination and neuronal differentiation (1). Aging populations may also be at risk due to cumulative MP burden and comorbidities. Research should evaluate sex-based, genetic, and life-stage differences in MP absorption, distribution, and toxicity. Stratification by risk profiles will enhance the precision of epidemiological insights and interventions.
Exploring combined effects with environmental co-stressors
MPs often act synergistically with other pollutants, enhancing the bioavailability and toxicity of co-adsorbed chemicals such as heavy metals, PFAS, or microbial toxins (51, 54, 66). Additionally, MPs may compromise host defenses, including the gut microbiome, immune system, and blood–brain barrier. Future research must adopt multi-stressor models that mirror real-world exposures and uncover interactive effects on neurological health.
Bridging science and policy for risk reduction
Scientific findings must inform actionable regulations. Despite recognizing MP contamination, bodies like the World Health Organization (WHO) and the European Food Safety Authority (EFSA) have not yet issued enforceable health-based guidelines due to limited toxicological data. Research should help establish evidence-based exposure limits, prioritize high-risk plastic sources, and guide interventions (e.g., safer food contact materials, improved water filtration, waste reduction policies). Scientists must engage early with regulators to ensure timely translation of findings. Public outreach and educational campaigns can empower consumers to adopt exposure-reducing behaviors, especially among high-risk groups like pregnant women and children.
Conclusion
Long-term health impacts are a pressing concern, particularly in the brain, because microplastics are inescapable. They are pervasive in the environment and have been detected in human tissues. Experimental studies provide compelling evidence of microplastic-induced neurotoxicity but direct evidence in humans remains limited. Addressing this problem will require research integrating human exposure assessment coupled with advanced in vitro and omics-based tools and real-world toxicological models. Moving beyond laboratory findings toward translational science that informs public health and regulatory action is essential. Ultimately, understanding and mitigating the neurological risks of microplastics is not only a scientific imperative but a public health priority.
Author contributions
SB: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing. MG: Supervision, Visualization, Writing – review & editing. MS: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding for this work was provided by the United States National Science Foundation grant #CBET-2305189.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. Generative AI was used to correct some of the grammatical errors or typos, not the scientific content.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Salehi, M, Pincus, LN, Deng, B, and Peters, CA. Microplastics: from intrinsic properties to environmental fate. Environ Eng Sci. (2024) 41:425–35. doi: 10.1089/ees.2024.0232
2. Bhattacharjee, L, Jazaei, F, and Salehi, M. Insights into the mechanism of plastics’ fragmentation under abrasive mechanical forces: An implication for agricultural soil health. Clean – soil, air. Water. (2023) 51:2200395. doi: 10.1002/clen.202200395
3. Center for International Environmental Law (CIEL). (2023). Breathing Plastic: The Health Impacts of Invisible Plastics in the Air. Available online at: https://www.ciel.org/breathing-plastic-the-health-impacts-of-invisible-plastics-in-the-air/ (Accessed March 14, 2025)
4. Osman, AI, Hosny, M, and Eltaweil, AS. Microplastic sources, formation, toxicity and remediation: a review. Environ Chem Lett. (2023) 21:2129–69. doi: 10.1007/s10311-023-01593-3
5. Ziani, K, Ioniță-Mîndrican, CB, and Mititelu, M. Microplastics: a real global threat for environment and food safety: a state of the art review. Nutrients. (2023) 15:617. doi: 10.3390/nu15030617
6. European Environment Agency (EEA). (2022). Microplastics from Textiles: Towards a Circular Economy for Textiles in Europe. Available online at: https://www.eea.europa.eu/publications/microplastics-from-textiles-towards-a (Accessed March 14, 2025)
7. HORIBA Scientific. (2024). Where Do Microplastics Come From? Available online at: https://www.horiba.com/usa/scientific/resources/science-in-action/where-do-microplastics-come-from/ (Accessed July 02, 2025).
8. Zuri, G, Karanasiou, A, and Lacorte, S. Microplastics: human exposure assessment through air, water, and food. Environ Int. (2023) 179:108150. doi: 10.1016/j.envint.2023.108150
9. Down to Earth (2024). What are exposure pathways and health risks of microplastics in our body? Available online at: https://www.downtoearth.org.in/waste/what-are-exposure-pathways-and-health-risks-of-microplastics-in-our-body--95840 (Accessed May 26, 2025).
10. Sun, A, and Wang, W-X. Human exposure to microplastics and its associated health risks. Environ Health. (2023) 1:139–49. doi: 10.1021/envhealth.3c00053
11. Smith, M, Love, DC, Rochman, CM, and Neff, RA. Microplastics in seafood and the implications for human health. Curr Environ Health Rep. (2018) 5:375–86. doi: 10.1007/s40572-018-0206-z
12. Traylor, SD, Granek, EF, Duncan, M, and Brander, SM. From the ocean to our kitchen Table: anthropogenic particles in the edible tissue of U.S. West coast seafood species. Environ Pollut. (2023) 316:120553. doi: 10.1016/j.envpol.2022.120553
13. Di Fiore, C, Sammartino, MP, Giannattasio, C, Avino, P, and Visco, G. Microplastic contamination in commercial salt: an issue for their sampling and quantification. Food Chem. (2023) 404 Part B:134682. doi: 10.1016/j.foodchem.2022.134682
14. Singh, T. Generation of microplastics from the opening and closing of disposable plastic water bottles. J Water Health. (2021) 19:488–98. doi: 10.2166/wh.2021.025
15. Oliveri Conti, G, Ferrante, M, Banni, M, Favara, C, Nicolosi, I, Cristaldi, A, et al. Micro- and Nano-plastics in edible fruit and vegetables: the first diet risks assessment for the general population. Environ Res. (2020) 187:109677. doi: 10.1016/j.envres.2020.109677
16. Cox, KD, Covernton, GA, Davies, HL, Dower, JF, Juanes, F, and Dudas, SE. Human consumption of microplastics. Environ Sci Technol. (2019) 53:7068–74. doi: 10.1021/acs.est.9b01517
17. Schwabl, P, Köppel, S, Königshofer, P, Bucsics, T, Trauner, M, Reiberger, T, et al. Detection of various microplastics in human stool: a prospective case series. Ann Intern Med. (2019) 171:453–7. doi: 10.7326/M19-0618
18. Saraluck, A, Techarang, T, Bunyapipat, P, Boonchuwong, K, Pullaput, Y, and Mordmuang, A. Detection of microplastics in human breast Milk and its association with changes in human Milk bacterial microbiota. J Clin Med. (2024) 13:4029. doi: 10.3390/jcm13144029
19. Liu, S, Guo, J, Liu, X, Yang, R, Wang, H, Sun, Y, et al. Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: a pilot prospective study. Sci Total Environ. (2023) 854:158699. doi: 10.1016/j.scitotenv.2022.158699
20. Ke, D, Zheng, J, Liu, X, Xu, X, Zhao, L, Gu, Y, et al. Occurrence of microplastics and disturbance of gut microbiota: a pilot study of preschool children in Xiamen, China. EBioMedicine. (2023) 97:104828. doi: 10.1016/j.ebiom.2023.104828
21. De-la-Torre, GE. Microplastics: An emerging threat to food security and human health. J Food Sci Technol. (2020) 57:1601–8. doi: 10.1007/s13197-019-04138-1
22. Harvard Medicine Magazine. (2024). Microplastics Everywhere. Available online at: https://magazine.hms.harvard.edu/articles/microplastics-everywhere (Accessed May 20, 2025).
23. Fang, M, Liao, Z, Ji, X, Zhu, X, Wang, Z, Lu, C, et al. Microplastic ingestion from atmospheric deposition during dining/drinking activities. J Hazard Mater. (2022) 432:128674. doi: 10.1016/j.jhazmat.2022.128674
24. Kannan, K, and Vimalkumar, K. A review of human exposure to microplastics and insights into microplastics as Obesogens. Front Endocrinol. (2021) 12:724989. doi: 10.3389/fendo.2021.724989
25. Schwarzfischer, M, and Rogler, G. The intestinal barrier—shielding the Body from Nano- and Microparticles in our diet. Meta. (2022) 12:223. doi: 10.3390/metabo12030223
26. Dzierżyński, E, Gawlik, PJ, Puźniak, D, Flieger, W, Jóźwik, K, Teresiński, G, et al. Microplastics in the human Body: exposure, detection, and risk of carcinogenesis: a state-of-the-art review. Cancer. (2024) 16:3703. doi: 10.3390/cancers16213703
27. EFSA Panel on Contaminants in the Food Chain (CONTAM). Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. (2016) 14:4501. doi: 10.2903/j.efsa.2016.4501
28. Corr, SC, Gahan, CCGM, and Hill, C. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol Med Microbiol. (2008) 52:2–12. doi: 10.1111/j.1574-695X.2007.00359.x
29. Kobayashi, N, Takahashi, D, Takano, S, Kimura, S, and Hase, K. The roles of Peyer's patches and microfold cells in the gut immune system: relevance to autoimmune diseases. Front Immunol. (2019) 10:2345. doi: 10.3389/fimmu.2019.02345
30. Prata, JC. Microplastics and human health: integrating pharmacokinetics. Crit Rev Environ Sci Technol. (2023) 53:1489–511. doi: 10.1080/10643389.2023.2195798
31. Campanale, C, Massarelli, C, Savino, I, Locaputo, V, and Uricchio, VF. A detailed review study on potential effects of microplastics and additives of concern on human health. Int J Environ Res Public Health. (2020) 17:1212. doi: 10.3390/ijerph17041212
32. Paing, YMM, Eom, Y, Song, GB, Kim, B, Choi, MG, Hong, S, et al. Neurotoxic effects of polystyrene Nanoplastics on memory and microglial activation: insights from In vivo and In vitro studies. Sci Total Environ. (2024) 924:171681. doi: 10.1016/j.scitotenv.2024.171681
33. Kopatz, V, Wen, K, Kovács, T, Keimowitz, AS, Pichler, V, Widder, J, et al. Micro- and nanoplastics breach the blood–brain barrier (BBB): biomolecular corona’s role revealed. Nano. (2023) 13:1404. doi: 10.3390/nano13081404
34. Leslie, HA, van Velzen, MJM, Brandsma, SH, Vethaak, AD, Garcia-Vallejo, JJ, and Lamoree, MH. Discovery and quantification of plastic particle pollution in human blood. Environ Int. (2022) 163:107199. doi: 10.1016/j.envint.2022.107199
35. Baeza-Martínez, C, Garcia-Pachon, E, and Bayo, J. Environmental microplastics and the lung. Arch Bronconeumol. (2023) 59:352–3. doi: 10.1016/j.arbres.2022.09.019
36. Zhu, L, Kang, Y, Ma, M, Wu, Z, Zhang, L, Hu, R, et al. Tissue accumulation of microplastics and potential health risks in human. Sci Total Environ. (2024) 915:170004. doi: 10.1016/j.scitotenv.2024.170004
37. Garcia, MM, Romero, AS, Merkley, SD, Meyer-Hagen, JL, Forbes, C, Hayek, EE, et al. In vivo tissue distribution of microplastics and systemic Metabolomic alterations after gastrointestinal exposure. bioRxiv. (2023):2023.06.02.542598. doi: 10.1101/2023.06.02.542598
38. PTGLAB (2024). Exposure to microplastics increases risk of liver fibrosis. Available online at: https://www.ptglab.com/news/blog/exposure-to-microplastics-increases-risk-of-liver-fibrosis/ (Accessed May 27, 2025).
39. Massardo, S, Verzola, D, Alberti, S, Caboni, C, Santostefano, M, Verrina, EE, et al. MicroRaman spectroscopy detects the presence of microplastics in human urine and kidney tissue. Environ Int. (2024) 184:108444. doi: 10.1016/j.envint.2024.108444
40. Anifowoshe, AT, Akhtar, MN, Majeed, A, Singh, AS, Ismail, TF, and Nongthomba, U. Microplastics: a threat to Fetoplacental unit and reproductive systems. Toxicol Rep. (2025) 14:101938. doi: 10.1016/j.toxrep.2025.101938
41. Zurub, RE, Cariaco, Y, Wade, MG, and Bainbridge, SA. Microplastics exposure: implications for human fertility, pregnancy and child health. Front Endocrinol. (2023) 14:1330396. doi: 10.3389/fendo.2023.1330396
42. Amato-Lourenço, LF, Dantas, KC, Júnior, GR, Paes, VR, Ando, RA, de Oliveira Freitas, R, et al. Microplastics in the olfactory bulb of the human brain. JAMA Netw Open. (2024) 7:e2440018. doi: 10.1001/jamanetworkopen.2024.40018
43. Nihart, AJ, Garcia, MA, El Hayek, E, Liu, R, Olewine, M, Kingston, JD, et al. Bioaccumulation of microplastics in decedent human brains. Nat Med. (2025) 31:1114–9. doi: 10.1038/s41591-024-03453-1
44. Wang, Y-F, Wang, X-Y, Chen, B-J, Yang, Y-P, Li, H, and Wang, F. Impact of microplastics on the human digestive system: from basic to clinical. World J Gastroenterol. (2025) 31:100470. doi: 10.3748/wjg.v31.i4.100470
45. Bora, SS, Gogoi, R, Sharma, MR, Anshu,, Borah, MP, Deka, P, et al. Microplastics and human health: unveiling the gut microbiome disruption and chronic disease risks. Front Cell Infect Microbiol. (2024) 14:1492759. doi: 10.3389/fcimb.2024.1492759
46. Sofield, CE, Anderton, RS, and Gorecki, AM. Mind over microplastics: exploring microplastic-induced gut disruption and gut-brain-Axis consequences. Curr Issues Mol Biol. (2024) 46:4186–202. doi: 10.3390/cimb46050256
47. Lee, AG, Kang, S, Yoon, HJ, Im, S, Oh, SJ, and Pak, YK. Polystyrene microplastics exacerbate systemic inflammation in high-fat diet-induced obesity. Int J Mol Sci. (2023) 24:12421. doi: 10.3390/ijms241512421
48. Su, Q-L, Wu, J, Tan, S-W, Guo, X-Y, Zou, D-Z, and Kang, K. The impact of microplastics polystyrene on the microscopic structure of mouse intestine, tight junction genes and gut microbiota. PLoS One. (2024) 19:e0304686. doi: 10.1371/journal.pone.0304686
49. Hwang, J, Choi, D, Han, S, Choi, J, and Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci Total Environ. (2019) 684:657–69. doi: 10.1016/j.scitotenv.2019.05.071
50. Schirinzi, GF, Pérez-Pomeda, I, Sanchís, J, Rossini, C, Farré, M, and Barceló, D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ Res. (2017) 159:579–87. doi: 10.1016/j.envres.2017.08.043
51. Prata, JC, da Costa, JP, Lopes, I, Duarte, AC, and Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci Total Environ. (2020) 702:134455. doi: 10.1016/j.scitotenv.2019.134455
52. Yong, CQY, Valiyaveettil, S, and Tang, BL. Toxicity of microplastics and nanoplastics in mammalian systems. Int J Environ Res Public Health. (2020) 17:1509. doi: 10.3390/ijerph17051509
53. Bowley, J, Baker-Austin, C, Porter, A, Hartnell, R, and Lewis, C. Oceanic hitchhikers – assessing pathogen risks from marine microplastic. Trends Microbiol. (2021) 29:107–16. doi: 10.1016/j.tim.2020.06.011
54. Hirt, N, and Body-Malapel, M. Immunotoxicity and intestinal effects of Nano- and Microplastics: a review of the literature. Part Fibre Toxicol. (2020) 17:57. doi: 10.1186/s12989-020-00387-7
55. Li, J, Zhang, K, and Zhang, H. Adsorption of antibiotics on microplastics. Environ Pollut. (2018) 237:460–7. doi: 10.1016/j.envpol.2018.02.050
56. Herath, A, Datta, DK, Bonyadinejad, G, and Salehi, M. Partitioning of heavy metals in sediments and microplastics from Stormwater runoff. Chemosphere. (2023) 332:138844. doi: 10.1016/j.chemosphere.2023.138844
57. Prattichizzo, F, Ceriello, A, Pellegrini, V, La Grotta, R, Graciotti, L, Olivieri, F, et al. Micro-Nanoplastics and cardiovascular diseases: evidence and perspectives. Eur Heart J. (2024) 45:4099–110. doi: 10.1093/eurheartj/ehae552
58. Goldsworthy, A, O’Callaghan, LA, Blum, C, Horobin, J, Tajouri, L, Olsen, M, et al. Micro-nanoplastic induced cardiovascular disease and dysfunction: a scoping review. J Expo Sci Environ Epidemiol. (2025) 35:746–69. doi: 10.1038/s41370-025-00766-2
59. Zhang, T, Liao, Y, Ling, J, Zhang, J, Zhang, D, Yin, X, et al. Tiny trouble: microplastics, Nanoplastics, and their heartfelt impact on cardiovascular health. Cardiovasc Res. (2025) 121:992–1010. doi: 10.1093/cvr/cvaf068
60. Marfella, R, Prattichizzo, F, Sardu, C, Fulgenzi, G, Graciotti, L, Spadoni, T, et al. Microplastics and nanoplastics in atheromas and cardiovascular events. N Engl J Med. (2024) 390:900–10. doi: 10.1056/NEJMoa2309822
61. Martínez-Pinna, J, Sempere-Navarro, R, Medina-Gali, RM, Fuentes, E, Quesada, I, Sargis, RM, et al. Endocrine disruptors in plastics Alter β-cell physiology and increase the risk of diabetes mellitus. Am J Physiol Endocrinol Metab. (2023) 324:E488–505. doi: 10.1152/ajpendo.00068.2023
62. Ragusa, A, Svelato, A, Santacroce, C, Catalano, P, Notarstefano, V, Carnevali, O, et al. Plasticenta: first evidence of microplastics in human placenta. Environ Int. (2021) 146:106274. doi: 10.1016/j.envint.2020.106274
63. Zhao, Q, Zhu, L, Weng, J, Jin, Z, Cao, Y, Jiang, H, et al. Detection and characterization of microplastics in the human testis and semen. Sci Total Environ. (2023) 877:162713. doi: 10.1016/j.scitotenv.2023.162713
64. Wei, Z, Wang, Y, Wang, S, Xie, J, Han, Q, and Chen, M. Comparing the effects of polystyrene microplastics exposure on reproduction and fertility in male and female mice. Toxicology. (2022) 465:153059. doi: 10.1016/j.tox.2021.153059
65. Prata, JC. Airborne microplastics: consequences to human health? Environ Pollut. (2018) 234:115–26. doi: 10.1016/j.envpol.2017.11.043
66. Wright, SL, and Kelly, FJ. Plastic and human health: a Micro issue? Environ Sci Technol. (2017) 51:6634–47. doi: 10.1021/acs.est.7b00423
67. Prüst, M, Meijer, J, and Westerink, RHS. The plastic brain: neurotoxicity of micro- and nanoplastics. Part Fibre Toxicol. (2020) 17:24. doi: 10.1186/s12989-020-00358-y
68. Campen, M, Nihart, A, Garcia, M, Liu, R, Olewine, M, Castillo, E, et al. Bioaccumulation of microplastics in decedent human brains assessed by pyrolysis gas chromatography-mass spectrometry. Res Sq. (2024):rs.3.rs-4345687. doi: 10.21203/rs.3.rs-4345687/v1
69. Gecegelen, E, Ucdal, M, and Dogu, BB. A novel risk factor for dementia: chronic microplastic exposure. Front Neurol. (2025) 16:1581109. doi: 10.3389/fneur.2025.1581109
70. Huang, H, Hou, J, Li, M, Wei, F, Liao, Y, and Xi, B. Microplastics in the bloodstream can induce cerebral thrombosis by causing cell obstruction and Lead to neurobehavioral abnormalities. Sci Adv. (2025) 11:eadr8243. doi: 10.1126/sciadv.adr8243
71. He, P, Wang, F, Xi, G, Li, Y, Wang, F, Wang, H, et al. Association of microplastics in human cerebrospinal fluid with Alzheimer’s disease-related changes. J Hazard Mater. (2025) 494:138748. doi: 10.1016/j.jhazmat.2025.138748
72. Liu, Z, Sokratian, A, Duda, AM, Xu, E, Stanhope, C, Fu, A, et al. Anionic Nanoplastic contaminants promote Parkinson's disease-associated α-Synuclein aggregation. Sci Adv. (2023) 9:eadi8716. doi: 10.1126/sciadv.adi8716
73. Zheng, Y, Xu, S, Liu, J, and Liu, Z. The effects of micro- and nanoplastics on the central nervous system: a new threat to humanity? Toxicology. (2024) 504:153799. doi: 10.1016/j.tox.2024.153799
74. Wang, Z, Wang, Q, Li, S, Li, XJ, Yang, W, and He, D. Microglial autophagy in Alzheimer's disease and Parkinson's disease. Front Aging Neurosci. (2023) 14:1065183. doi: 10.3389/fnagi.2022.1065183
75. Xie, J, ji, J, Sun, Y, Ma, Y, Wu, D, and Zhang, Z. Blood-brain barrier damage accelerates the accumulation of micro- and nanoplastics in the human central nervous system. J Hazard Mater. (2024) 480:136028. doi: 10.1016/j.jhazmat.2024.136028
76. Chen, Y, Nan, Y, Xu, L, Dai, A, Orteg, RMM, Ma, M, et al. Polystyrene Nanoplastics exposure induces cognitive impairment in mice via induction of oxidative stress and ERK/MAPK-mediated neuronal Cuproptosis. Part Fibre Toxicol. (2025) 22:13. doi: 10.1186/s12989-025-00633-w
77. NIH (2024). Nanoplastics may help set the stage for Parkinson’s risk. National Institutes of Health (NIH). Available online at: https://www.nih.gov/news-events/nih-research-matters/nanoplastics-may-help-set-stage-parkinson-s-risk (Accessed May 30, 2025).
78. Sun, H, Yang, B, Li, Q, Zhu, X, Song, E, Liu, C, et al. Polystyrene nanoparticles trigger aberrant condensation of TDP-43 and amyotrophic lateral sclerosis-like symptoms. Nat Nanotechnol. (2024) 19:1354–65. doi: 10.1038/s41565-024-01683-5
79. Liang, B, Huang, Y, Zhong, Y, Li, Z, Ye, R, Wang, B, et al. Brain single-nucleus transcriptomics highlights that polystyrene Nanoplastics potentially induce Parkinson's disease-like neurodegeneration by causing energy metabolism disorders in mice. J Hazard Mater. (2022) 430:128459. doi: 10.1016/j.jhazmat.2022.128459
80. Aghilinasrollahabadi, K, Salehi, M, and Fujiwara, T. Investigate the influence of microplastics weathering on their heavy metals uptake in stormwater. J Hazard Mater. (2021) 408:124439. doi: 10.1016/j.jhazmat.2020.124439
81. Hadiuzzaman, M, Salehi, M, and Fujiwara, T. Plastic litter fate and contaminant transport within the urban environment, photodegradation, fragmentation, and heavy metal uptake from storm runoff. Environ Res. (2022) 212:113183. doi: 10.1016/j.envres.2022.113183
82. Chen, S, Chen, Y, Gao, Y, Han, B, Wang, T, Dong, H, et al. Toxic effects and mechanisms of nanoplastics on embryonic brain development using brain organoids model. Sci Total Environ. (2023) 904:166913. doi: 10.1016/j.scitotenv.2023.166913
83. Shi, J, Yu, X, Zhao, J, Wang, T, Li, N, Yu, J, et al. Integrated transcriptomics and metabolomics reveal the mechanism of polystyrene nanoplastics toxicity to mice. Ecotoxicol Environ Saf. (2024) 284:116925. doi: 10.1016/j.ecoenv.2024.116925
84. Limonta, G, Mancia, A, Benkhalqui, A, Bertolucci, C, Abelli, L, Fossi, MC, et al. Microplastics induce transcriptional changes, immune response and behavioral alterations in adult zebrafish. Sci Rep. (2019) 9:15775. doi: 10.1038/s41598-019-52292-5
85. Chen, Q, Lackmann, C, Wang, W, Seiler, T-B, Hollert, H, and Shi, H. Microplastics Lead to hyperactive swimming behaviour in adult zebrafish. Aquat Toxicol. (2020) 224:105521. doi: 10.1016/j.aquatox.2020.105521
86. Lu, Y, Zhang, Y, Deng, Y, Jiang, W, Zhao, Y, Geng, J, et al. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ Sci Technol. (2016) 50:4054–60. doi: 10.1021/acs.est.6b00183
87. Pitt, JA, Kozal, JS, Jayasundara, N, Massarsky, A, Trevisan, R, Geitner, N, et al. Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquat Toxicol. (2018) 194:185–94. doi: 10.1016/j.aquatox.2017.11.017
88. Savuca, A, Curpan, A-S, Hritcu, LD, Buzenchi Proca, TM, Balmus, I-M, Lungu, PF, et al. Do microplastics have neurological implications in relation to schizophrenia zebrafish models? A brain immunohistochemistry, neurotoxicity assessment, and oxidative stress analysis. Int J Mol Sci. (2024) 25:8331. doi: 10.3390/ijms25158331
89. Santos, D, Luzio, A, Félix, L, Cabecinha, E, Bellas, J, and Monteiro, SM. Microplastics and copper induce apoptosis, Alter Neurocircuits, and cause behavioral changes in zebrafish (Danio rerio) brain. Ecotoxicol Environ Saf. (2022) 242:113926. doi: 10.1016/j.ecoenv.2022.113926
90. Yang, B, Han, Y, Hu, S, Xie, X, Zhu, X, and Yuan, L. Polystyrene microplastics induce depression-like behavior in zebrafish via neuroinflammation and circadian rhythm disruption. Sci Total Environ. (2025) 959:178085. doi: 10.1016/j.scitotenv.2024.178085
91. Sarasamma, S, Audira, G, Siregar, P, Malhotra, N, Lai, Y-H, Liang, S-T, et al. Nanoplastics cause neurobehavioral impairments, reproductive and oxidative damages, and biomarker responses in zebrafish: throwing up alarms of wide spread health risk of exposure. Int J Mol Sci. (2020) 21:1410. doi: 10.3390/ijms21041410
92. Brun, NR, van Hage, P, Hunting, ER, Haramis, A-PG, Vink, SC, Vijver, MG, et al. Polystyrene nanoplastics disrupt glucose metabolism and cortisol levels with a possible link to behavioural changes in larval zebrafish. Commun Biol. (2019) 2:382. doi: 10.1038/s42003-019-0629-6
93. Dong, C-D, Chen, C-W, Chen, Y-C, Chen, H-H, Lee, J-S, and Lin, C-H. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J Hazard Mater. (2020) 385:121575. doi: 10.1016/j.jhazmat.2019.121575
94. Luo, T, Zhang, Y, Wang, C, Wang, X, Zhou, J, Shen, M, et al. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ Pollut Barking Essex. (2019) 255:113122. doi: 10.1016/j.envpol.2019.113122
95. Gan, AJW, Chia, KF, Lim, CL, Tan, BK, Wong, SF, Chye, SM, et al. Neurotoxicity of Nanoplastics: a review. F1000Res. (2024) 13:793. doi: 10.12688/f1000research.149068.1
96. An, R, Wang, X, Yang, L, Zhang, J, Wang, N, Xu, F, et al. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology. (2021) 449:152665. doi: 10.1016/j.tox.2020.152665
97. Shan, S, Zhang, Y, Zhao, H, Zeng, T, and Zhao, X. Polystyrene Nanoplastics penetrate across the blood-brain barrier and induce activation of microglia in the brain of mice. Chemosphere. (2022) 298:134261. doi: 10.1016/j.chemosphere.2022.134261
98. Ding, J, Zhang, S, Razanajatovo, RM, Zou, H, and Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red Tilapia (Oreochromis Niloticus). Environ Pollut Barking Essex. (1987) 238:1–9. doi: 10.1016/j.envpol.2018.03.001
99. Yang, H, Xiong, H, Mi, K, Xue, W, Wei, W, and Zhang, Y. Toxicity comparison of Nano-sized and Micron-sized microplastics to goldfish Carassius Auratus larvae. J Hazard Mater. (2020) 388:122058. doi: 10.1016/j.jhazmat.2020.122058
100. Rochman, CM, Hoh, E, Kurobe, T, and Teh, SJ. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci Rep. (2013) 3:3263. doi: 10.1038/srep03263
101. Barboza, LGA, Vieira, LR, Branco, V, Figueiredo, N, Carvalho, F, Carvalho, C, et al. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat Toxicol. (2018) 195:49–57. doi: 10.1016/j.aquatox.2017.12.008
102. Khalil, AM. Toxicological effects and oxidative stress responses in freshwater snail, Lanistes Carinatus, following exposure to Chlorpyrifos. Ecotoxicol Environ Saf. (2015) 116:137–42. doi: 10.1016/j.ecoenv.2015.03.010
103. Pannetier, P, Morin, B, Le Bihanic, F, Dubreil, L, Clérandeau, C, Chouvellon, F, et al. Environmental samples of microplastics induce significant toxic effects in fish larvae. Environ Int. (2020) 134:105047. doi: 10.1016/j.envint.2019.105047
104. Ehsanifar, M, and Yavari, Z. Neurotoxicity following exposure to micro and nanoplastics. OBM Neurobiol. (2025) 9:1–20. doi: 10.21926/obm.neurobiol.2501277
105. Bhuyan, MS. Effects of microplastics on fish and in human health. Front Environ Sci. (2022) 10:10. doi: 10.3389/fenvs.2022.827289
106. Lei, L, Wu, S, Lu, S, Liu, M, Song, Y, Fu, Z, et al. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio Rerio and nematode Caenorhabditis Elegans. Sci Total Environ. (2018) 619-620:1–8. doi: 10.1016/j.scitotenv.2017.11.103
107. Nasser, F, and Lynch, I. Secreted protein eco-Corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia Magna. J Proteome. (2016) 137:45–51. doi: 10.1016/j.jprot.2015.09.005
108. Yan, W, Li, Z-J, Lin, Z-Y, Ji, S-Q, Tse, WKF, Meng, Z-Q, et al. Microplastic exposure disturbs sleep structure, reduces lifespan, and decreases ovary size in Drosophila melanogaster. Zool Res. (2024) 45:805–20. doi: 10.24272/j.issn.2095-8137.2024.038
109. Xiong, F, Liu, J, Xu, K, Huang, J, Wang, D, Li, F, et al. Microplastics induce neurotoxicity in aquatic animals at environmentally realistic concentrations: a Meta-analysis. Environ Pollut. (2023) 318:120939. doi: 10.1016/j.envpol.2022.120939
110. Luo, T, Wang, C, Pan, Z, Jin, C, Fu, Z, and Jin, Y. Maternal polystyrene microplastic exposure during gestation and lactation altered metabolic homeostasis in the dams and their F1 and F2 offspring. Environ Sci Technol. (2019) 53:10978–92. doi: 10.1021/acs.est.9b03191
Keywords: microplastics, nanoplastics, environmental health, neurological effects, public health, plastic pollution
Citation: Bhattacharyya S, Greer ML and Salehi M (2025) Impact of micro- and nanoplastics exposure on human health: focus on neurological effects from ingestion. Front. Public Health. 13:1681776. doi: 10.3389/fpubh.2025.1681776
Edited by:
Tong Wang, University of Connecticut, United StatesReviewed by:
Watcharin Joemsittiprasert, New York Institution for Continuing Education, United StatesYuanyin Teng, Institute of Hematology, Zhejiang University, China
Mengting Li, University of Florida, United States
Dongjie Zhu, University of Massachusetts Medical School, United States
Copyright © 2025 Bhattacharyya, Greer and Salehi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sudeepa Bhattacharyya, cy5iaGF0dGFjaGFyeXlhQHVta2MuZWR1
 Sudeepa Bhattacharyya
Sudeepa Bhattacharyya Melody L. Greer
Melody L. Greer Maryam Salehi
Maryam Salehi