- 1Brain Injury Research Center, TIRR Memorial Hermann, Houston, TX, United States
- 2Department of Physical Medicine & Rehabilitation, University of Texas Southwestern Medical Center, Dallas, TX, United States
- 3Center for Community Health and Aging, Texas A&M University, College Station, TX, United States
- 4Center for Health Behavior, School of Public Health, Texas A&M University, College Station, TX, United States
- 5Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas, TX, United States
- 6Department of Epidemiology and Biostatistics, School of Public Health, Texas A&M University, College Station, TX, United States
- 7Department of Neurology, School of Medicine, Washington University in St. Louis, St. Louis, MO, United States
- 8School of Medicine, One West University Boulevard, University of Texas Rio Grande Valley, Brownsville, TX, United States
Introduction: Metacognitive strategy training interventions, like Problem-Solving Training/Descubriendo Soluciones Juntos (PST/DSJ), have efficacy for improving caregiver burden and depressive symptoms. We previously demonstrated that PST/DSJ improved caregiver burden and depressive symptoms among caregivers of adults with Alzheimer’s Disease and related dementias (ADRD), regardless of the number of sessions or boosters received. However, these results did not examine factors characterizing those who responded (improvement in caregiver burden or depressive symptoms) or did not respond to the intervention.
Objective: To identify key personal and clinical factors associated with response to PST/DSJ. Personal factors included age, gender, race, Hispanic ethnicity, education, and employment status. Clinical factors included care recipient diagnosis and dementia severity, caregiver problem-solving skills at baseline, caregiving experiences (caregiver life social support, satisfaction and resentment with the caregiving role, anger toward the care recipient, and care recipient aggressive, depressive, and forgetful behaviors), and social disconnection, caregiver burden, and depressive symptoms.
Method: We conducted a 2 × 2 randomized controlled optimization trial to test remotely delivered PST/DSJ to ADRD caregivers (NCT04748666). Primary outcomes were caregiver burden, measured by the Zarit Burden Interview (ZBI), and depressive symptoms, measured by the Patient Health Questionnaire-8 (PHQ-8). Response to PST/DSJ was defined for each primary outcome as a clinically important change (defined as ≥1 point on ZBI and ≥3 on PHQ) from baseline to 6-month follow-up.
Results: Ninety-one caregivers were included in responder analysis, with 55 (60.4%) demonstrating a clinically meaningful improvement in caregiver burden and/or depressive symptoms. No personal factors were associated with being a Responder (vs. Non-Responder). Clinical factors associated with being a Responder were greater care recipient dementia severity (FAST score, p < 0.01), lower baseline caregiver life satisfaction (p = 0.05), higher baseline caregiver overload (p = 0.05), higher baseline caregiver burden (p = 0.01), and more baseline depressive symptoms (p < 0.01).
Conclusion: Most caregivers demonstrated a clinically meaningful improvement in caregiver burden and/or depressive symptoms after receiving PST/DSJ. Notably, those who responded had higher symptoms of distress, including caregiver burden and overload and depressive symptoms and lower life satisfaction, and had care recipients with more severe dementia, indicating that those benefiting from the intervention were those most in need of this support.
Clinical trial registration: ClinicalTrials.gov, identifier is NCT04748666.
Introduction
Alzheimer’s disease or related dementias (ADRD), including vascular dementia, Lewy Body dementia, and other dementias, represent a substantial public and personal health burden. Informal caregivers—typically spouses, adult children, or other family members – provide day-to-day support to individuals with ADRD, totaling approximately 18.4 billion hours of unpaid care per year (based on 2023 data) with an estimated value of $346.6 billion (1–4). Over 11 million individuals are currently informal caregivers of a person with ADRD (4). Being an informal caregiver to someone with ADRD can lead to depression, health problems, increased alcohol use, caregiver burden, and poorer quality of life (5–10). Caregiver distress also affects the health and well-being of care recipients (11), with caregiver burden emerging as a direct predictor of institutionalization and of care recipient behavioral and psychological symptoms (11). Moreover, caregiver burden is substantial in the underserved US Hispanic/Latino population, with few available linguistically and culturally appropriate resources (12) despite the higher likelihood of developing dementia for older Hispanic/Latino adults (4, 13).
To date, most interventions for dementia caregivers are primarily focused on providing them with education about ADRD and providing support to manage the needs and behaviors of their care-recipient (14–16). There is growing evidence that interventions focused on emotional support and stress management for caregivers may help in reducing or managing caregiver burden (17, 18). This highlights the importance of evidence-based interventions to support caregivers in managing their own needs and stressors, including ones focused on enhancing problem-solving skills, rather than just the needs of their care-recipients (16).
Even when interventions are shown to improve caregiver outcomes, knowing who is most likely to benefit from these interventions and who may need additional support or intervention adaptations to benefits remains unclear. Very few studies identify factors that contribute to response (i.e., meaningful improvement in outcomes) vs. non-response after caregiver support interventions. One study evaluating factors affecting change in depressive symptoms after a stroke caregiver intervention found that responders generally had a more active coping style and were less reliant on the counseling relationship (19), suggesting the importance of intervention components that promote self-efficacy and self-management. Further, non-responders more frequently endorsed a history of psychologic disorder and had higher levels of anger compared to responders (19), so those with more psychological distress (and arguably in most need of support) may be less likely to benefit. A qualitative study that surveyed non-responders and interventionists about a caregiver support intervention identified specific supports to meet the needs of non-responders: providing more support specific to caregiving, spending more time processing the caregiver’s emotions, providing skills and psychoeducation materials based on the caregiver’s needs, and working with caregivers to identify ways they can ask for help or strengthen interpersonal relationships (20).
Problem-Solving Training (PST)/Descubriendo Soluciones Juntos (DSJ) is an evidence-based, bilingual strategy training intervention that promotes proactive coping skills and self-efficacy by teaching a simple, systematic approach to problem-solving, including thorough problem assessment, generating and selecting solutions for specific self-identified goals, developing detailed plans of action, and evaluating and adapting plans as needed to support goal achievement (21–33). PST has been translated and culturally adapted for Spanish-speaking caregivers (DSJ) (34) and caregivers can receive the intervention via telephone or videoconference (23, 35, 36), circumventing many known barriers to caregiver support. PST has demonstrated efficacy in improving caregiver burden and reducing mood symptoms (22, 24, 27–30, 37) and negative problem-solving orientation (38, 39). We previously demonstrated that PST/DSJ led to improvements in caregiver burden and depressive symptoms among caregivers of adults with ADRD, regardless of the number of sessions or boosters received (39). However, these results examined participants in aggregate and did not examine factors characterizing those who responded or did not respond to the intervention, which is important to understand for personalizing intervention approaches and providing the best support to all who need it.
The objective of this study was to identify key personal and clinical factors associated with response to PST/DSJ, defined as improvement in caregiver burden and/or improvement in depressive symptoms, among ADRD caregivers. Personal factors included age, gender, race, Hispanic ethnicity, education, and employment status. Clinical factors included care recipient diagnosis and dementia severity, caregiver social problem-solving skills at baseline (pre-intervention), caregiving experiences, and baseline caregiver burden and depressive symptoms.
Materials and methods
Design
The CaDeS study was a 2×2 factorial design randomized controlled trial to test differential effects of number of sessions and booster sessions of PST/DSJ on caregiver burden and depressive symptoms among English- and Spanish-speaking caregivers of adults with ADRD (NCT04748666). Details about the study design and methods are provided in the published study protocol (40) and in the primary outcomes paper (39). Participants were randomized to receive 3 or 6 sessions with or without booster sessions. As reported in the published results for the primary trial aim, we found a main effect of time (improvement in both caregiver burden and depressive symptoms from baseline to 6-months post-baseline) with no significant difference for number of sessions or presence of booster sessions (39). Therefore, for the aim of this study to examine differences in those who did and did not respond to PST/DJS, we pooled all participants across study arms and categorized them based on improvement in the two primary outcomes, regardless of group assignment.
Participants
Participants (n = 91) were informal caregivers of persons with ADRD. Inclusion criteria were that the participant identified as a caregiver (i.e., a family member, spouse/partner, or friend) with more than a 1-year relationship with the care recipient, spoke English or Spanish, was over 18 years old and able to self-consent, and endorsed some depressive symptoms and/or caregiver burden symptoms (scoring ≥2 on the PHQ-2 and/or ZBI-4). The PHQ-2 assesses the two hallmark symptoms of depression (41), with scores ranging from 0 to 6 and a score ≥2 validated as a cut-off for potential depression. The ZBI-4 is a short screener for caregiver burden with a score of ≥2 validated as a cut-off for notable caregiver burden (42).
We determined that a sample size of 26 per arm (n = 104 total) would achieve 80% power at a significance level of 0.05 to detect the improvements between any two arms of 30% vs. 65, 40% vs. 75, 50% vs. 83, and 60% vs. 90%, accounting for 10% attrition (40). We consented n = 106 participants, but randomized n = 104 (2 withdrawn prior to randomization), and 7 (6.7%) were lost prior to the intervention beginning. Of the n = 97 who started the intervention, 6 were lost to follow-up by the 6-month assessment (6.2%), leaving us with n = 91 participants to include in responder analysis. The percentage of participants who completed 100% of sessions ranged from 82.1 to 95.2% across study arms.
Intervention
PST/DSJ teaches individuals a simple step-by-step process, to solve problems and achieve goals. A trained coach teaches participants the PST/DSJ strategy and then guides them through iterative practice applying it to goals of their choosing (34, 39, 40, 43). The strategy employs an easy to remember mnemonic: A = Assess the problem/ A = Analice el problema; B = Brainstorm solutions/ B = Buscar soluciones; C = Consider solutions and Choose one/ C = Considere y escoja; D = Develop a plan and Do it/ D=Desarrollar un plan y ¡Desempeñelo!; E = Evaluate/E = Evaluar y Evolucionar; F = Flex. Sessions were conducted by telephone or Microsoft Teams by Coaches with master’s level training. Detailed description of the 3- and 6-session versions of PST/DSJ are provided in the published protocol. Briefly, both versions included training participants how to use the ABCDEF strategy, with the six session version allowing more sessions for coach-supported iterative practice applying the strategy (26). During booster sessions, which occurred monthly for 6 months if assigned, participants followed up with their coach about progress they had made using the strategy, received extra supported practice, and discussed opportunities for using PST/DSJ in the future. Intervention fidelity, assessed using our established fidelity protocol (27), was excellent at 95%.
Outcome measures
We collected:
1. Demographic data: age, gender, race, Hispanic ethnicity, education (≤ High School vs. > High school), and employment status (full-time or part-time vs. retired or unemployed).
2. Caregiver personal factors: social problem-solving skills [Social Problem-Solving Skills Inventory (44)] and social disconnectedness [Upstream Social Interaction Risk Scale (45, 46)].
3. Caregiving-related information: care recipient diagnosis (Alzheimer’s disease vs. Other), care recipient dementia severity [Functional Assessment Staging Tool (47) for dementia score], and family caregiving experiences (48), comprising caregiver life satisfaction, social support, overload, satisfaction and resentment with the caregiving role, anger toward the care recipient, help needed by and provided to the care recipient, and care recipient aggressive, depressive, and forgetful behaviors.
4. Clinical outcome measures: caregiver burden [Zarit Burden Interview (49, 50)] and depressive symptoms [Patient Health Questionnaire (41, 51)].
Primary outcomes were caregiver burden, measured by the Zarit Burden Interview (ZBI), and depressive symptoms, measured by the Patient Health Questionnaire-8 item version (PHQ-8). The ZBI (49, 50) consists of 22 items and measures self-reported caregiver burden with included items covering overall well-being, social and family life, finances, perceived control, and emotional health. ZBI scores range from 0 to 88. The PHQ (41, 51) is a depression screening tool based on the DSIM-IV-TR symptoms of a major depressive episode. Scores range from 0 to 24 for the 8-item version.
Response to the intervention was defined for each primary outcome as a clinically important change from baseline to 6-month follow-up (final follow-up, 3 months post-end of intervention). For ZBI, we used a distribution-based method (52), defining Responders as those who improved by ≥1 point, equivalent to 1 standard error of the mean (SEM) of the sample (53) vs. Non-responders who did not. For PHQ, we based our definition of response on consensus-methods (52), which ranged from 2 to 3 points. We defined Responders as those who improved by ≥3 points (54) vs. Non-responders who did not.
Statistical analysis plan
We calculated the percentage of responders for each outcome and overall (Responder for Caregiver Burden AND/OR Depressive symptoms vs. Non-responder for both). We first descriptively present differences between Responders and Non-Responders on all covariates. Next, we conducted bivariate analyses, including Mann–Whitney U tests and Chi-squared tests, to determine statistically significant differences between Responders and Non-Responders for all covariates. We used the overall Responder variable (improvement in either outcome) as our primary indicator of response to intervention, as inclusion criteria for the study were a positive screen for caregiver burden OR depressive symptoms (ZBI-4 or PHQ-2 scores) rather than both. A p-value of ≤0.05 was deemed statistically significant, and all tests were two-sided.
Ethics statement
All research procedures were in accordance with the Declaration of Helsinki, and all participants provided informed consent. The UT Southwestern Medical Center’s Institutional Review Board (IRB) served as the single IRB for the study, with other sites as reliance sites. Protocols were established for managing any crises that arose in the context of intervention delivery, and there were no serious adverse events. This trial is registered to ClinicalTrials.gov Identifier: NCT04748666.
Results
Ninety-one participants completed the 6-month follow-up assessment (88% retention of those who consented) and were included in these analyses (39). Based on initial screening, all participants (100%) met the criterion cut-off on the ZBI-4, and 27 participants (29.7%) met the criterion cut-off on the PHQ-2. The mean change in ZBI for all participants was −3.0 (SD = 9.2, Cohen’s d = 0.33) and for PHQ was −1.1 (SD = 3.6, Cohen’s d = 0.31).
For caregiver burden, there were 51 (56.0%) participants who improved by 6-month follow-up and 40 (44.0%) who did not improve. For depressive symptoms, there were 27 (29.7%) who improved and 64 (70.3%) who did not improve. Of these 64 who did not improve on the PHQ-8, almost all had stable depressive symptoms, with many not meeting the initial screening criteria for depressive symptoms (i.e., they did not have meaningful depressive symptoms to improve). Close to two-thirds of the sample (60.4%, n = 55) showed meaningful improvement on at least one of the outcomes (Responders), with a little over one-third of the sample (39.6%) not showing meaningful improvement for either caregiver burden or depressive symptoms (Non-Responders). Table 1 shows the cross tabulation of Responders and Non-Responders across both outcomes. Notably, among those whose depressive symptom scores improved, only 4 (14.8%) did not also improve for caregiver burden. Figure 1 shows the distribution of change scores for the ZBI and PHQ for Responders and Non-Responders.
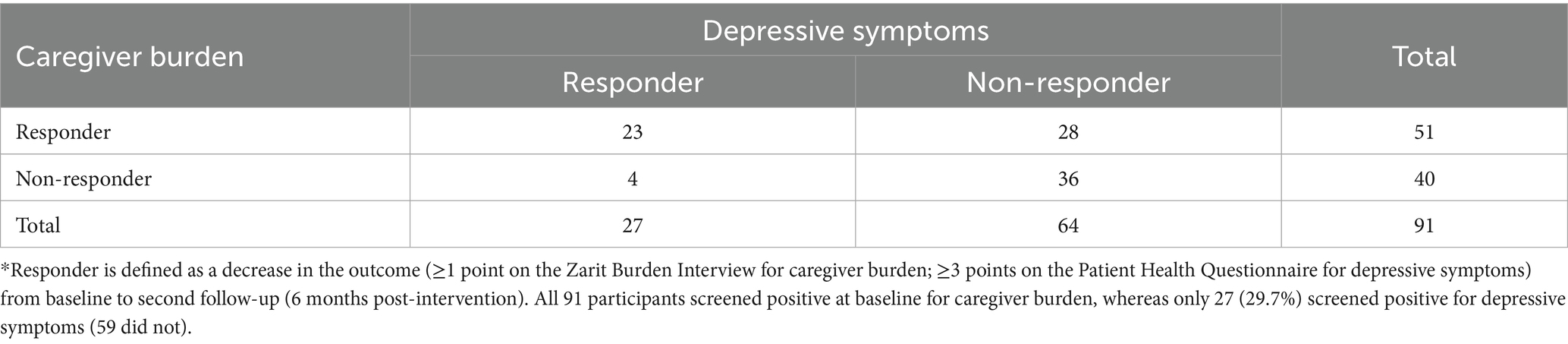
Table 1. Cross tabulation of responders and non-responders for caregiver burden and depressive symptoms.
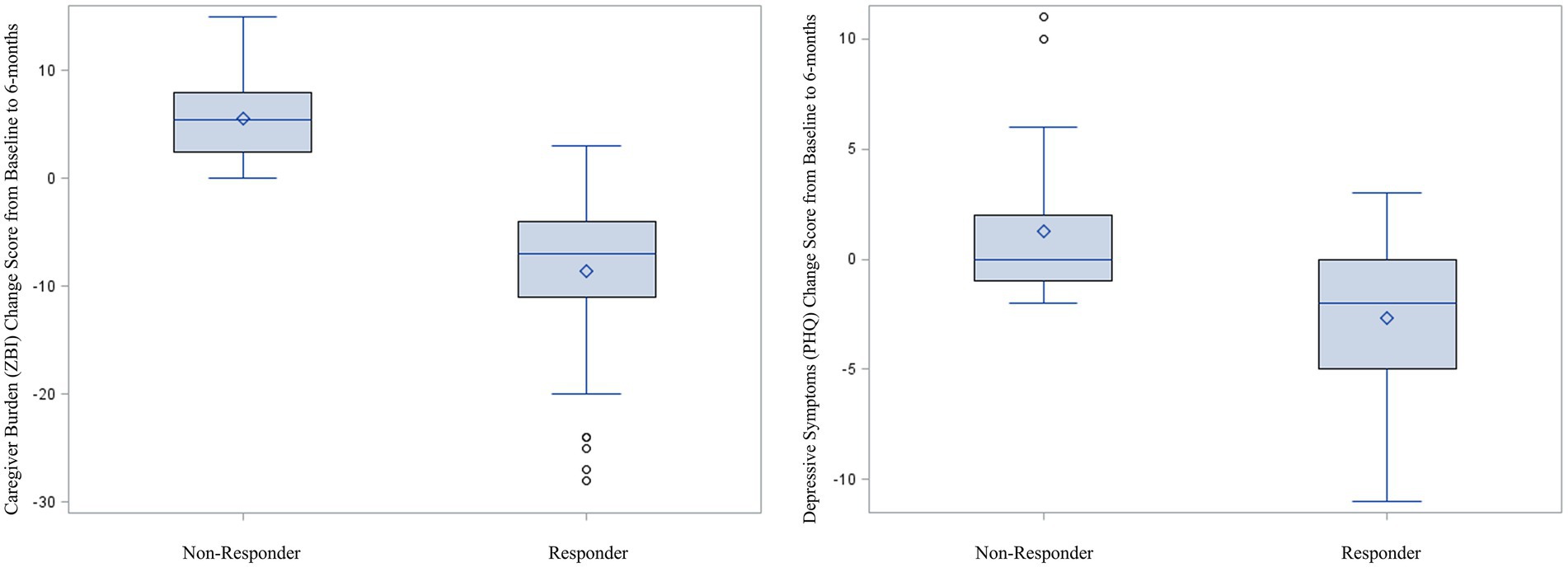
Figure 1. Boxplots comparing change in caregiver burden and depressive symptoms between responders and non-responders; positive scores indicate worsening symptoms; negative scores indicate improving symptoms.
Table 2 presents personal and clinical characteristics for all participants (n = 91) and descriptive and bivariate analyses between the combined Responder vs. Non-Responder groups. Statistically significant differences between groups for bivariate analyses were observed, with Responders reporting lower Caregiver Life Satisfaction (p = 0.05) and more feelings of Caregiver Overload (p = 0.05), more Caregiver Burden (p = 0.01), and greater Depressive Symptoms (p < 0.001) at baseline compared to Non-Responders. Responders had care recipients with higher FAST scores (dementia severity, p < 0.01) compared to their Non-Responder counterparts, with respective median scores of 6 (IQR = 5.7) indicating moderately severe dementia, vs. 5 (IQR = 4.6), indicating midstage dementia (see Table 2).
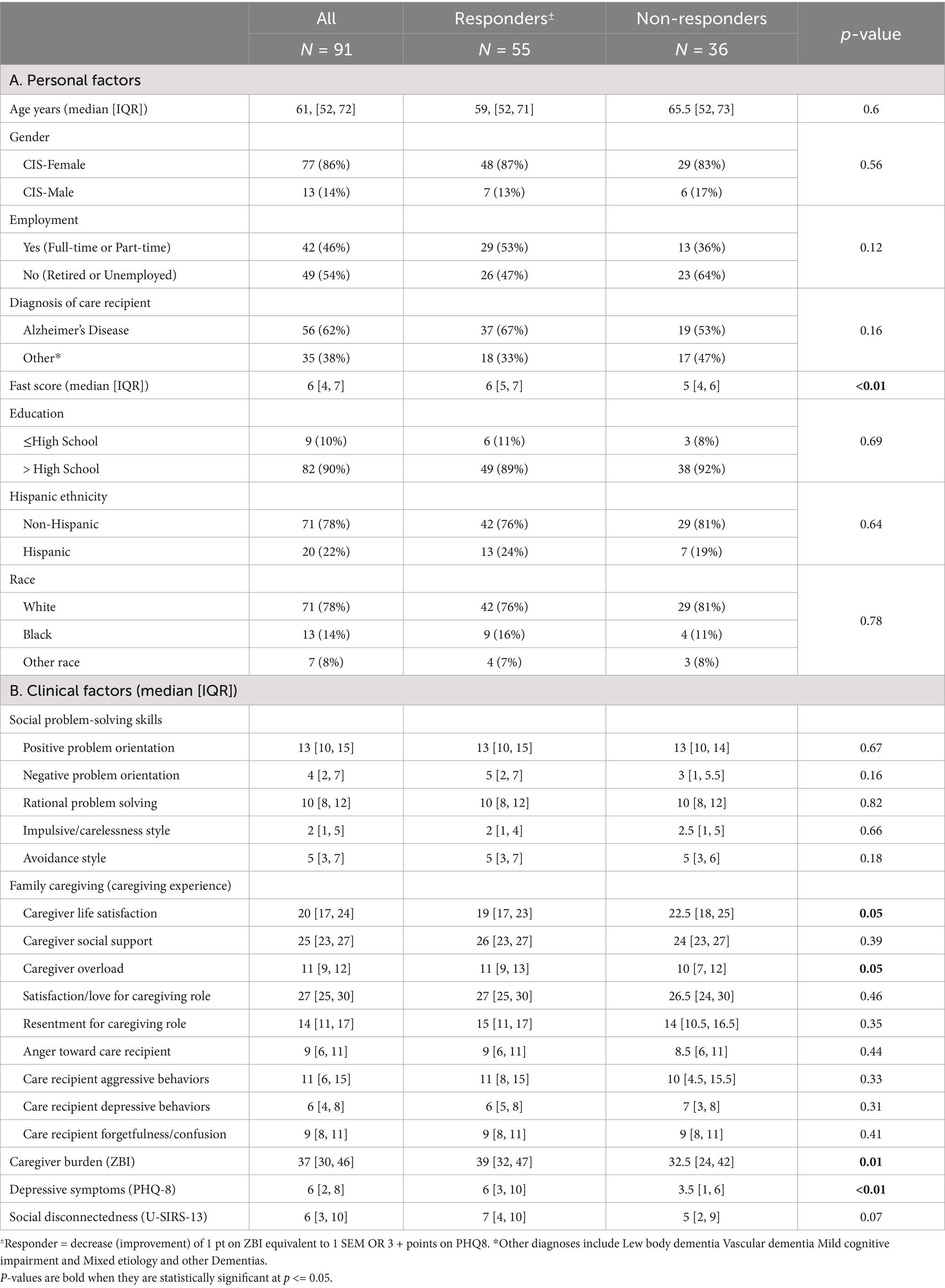
Table 2. Differences in personal and clinical characteristics between responders and non-responders to problem-solving training for either caregiver burden or depressive symptoms.
We also assessed differences in Responders vs. Non-Responders for caregiver burden and for depressive symptoms separately. Participant characteristics and group comparisons for each outcome are presented in Supplementary Table 1 (Caregiver Burden Responders) and Supplementary Table 2 (Depressive Symptom Responders).
Discussion
This study aimed to transcend outcome evaluation by examining the factors associated with treatment response among caregivers enrolled in a problem-solving intervention. We compared those achieving clinically significant improvement (i.e., Responders) with those who did not improve (i.e., Non-Responders) after PST/DSJ among 91 ADRD caregivers. Almost two-thirds of the sample were Responders (n = 55, 60.4%), supporting the benefits of PST/DSJ for dementia caregivers. Research on similar interventions has not always found statistically significant changes in burden/depression (55–57), and there is a paucity of dementia caregiver studies evaluating clinically, as opposed to just statistically, meaningful response to treatment (58).
In the current study, no personal characteristics of caregivers were associated with being a Responder (vs. Non-Responder). This suggests that the content of PST/DSJ was appropriate for caregivers in different situations and from different backgrounds and cultures, indicating success in our efforts to culturally translate the intervention while maintaining all evidence-based effective PST principles. Clinical factors associated with being a Responder (vs. Non-Responder) included providing care for individuals with greater dementia severity (moderately severe vs. midstage dementia) and, at baseline, reporting lower caregiver life satisfaction, higher caregiver overload, higher caregiver burden, and more depressive symptoms. This emphasizes that caregivers with greater need for intervention and support were also more likely to benefit from PST/DSJ.
This is contrary to a similar study in stroke caregivers who completed a cognitive-behavioral problem-solving and coping skills intervention that found that non-responders more often had a history of psychologic disorder and reported higher levels of anger than responders (19). Two notable differences between these studies may explain these somewhat discrepant findings. First, stroke and ADRD are notably different in their onset and progression, and participants in the stroke caregiver study were in their first year of caregiving. It may be that during this time of adjustment to a new, unexpected, and sudden-onset role, stroke caregivers experiencing the most emotional distress were not ready to engage in this kind of intervention. Second, related to the nature of the intervention, though both taught adaptive problem-solving based coping skills, cognitive behavioral approaches focus more on self-reflection and changing internal thoughts about a situation to improve emotional well-being, whereas PST/DSJ focuses only on the step-by-step applied process for goal setting and goal achievement known to have downstream effects on emotional health. Direct comparative studies of these different approaches may be warranted to best target interventions to caregivers’ needs and individual circumstances.
While the demand for caregiving interventions is growing alongside the rates of ADRD diagnoses and unpaid caregivers in the US (59, 60), the nature of caregiving demands, as well as feelings of burden and depression, may hinder caregivers from seeking out or participating in available and accessible interventions (61, 62). Additionally, although caregivers of care recipients with more severe dementia may be more likely to respond to intervention, the complexities of their caregiving situations and circumstances (and feelings of being overwhelmed and overburdened) may prevent them from engaging in multi-session interventions (63). The virtual delivery modality of PST/DSJ may overcome some of these barriers, as it is conducive to remotely reaching and serving caregivers with limited time and the inability to leave their care recipients alone while they travel to in-person interventions (39). Notably, though not statistically significant, a higher proportion of participants in the Responder group were employed (50% vs. 36%), lending support to the idea that those with the most demanding schedules may benefit the most from flexible and remotely delivered interventions.
This study is an important step to understanding differences between those who do and do not respond to interventions, which can provide insight into targeted recruitment strategies and adaptations for greater effectiveness. In the clinical trial from which this study was drawn (40), we employed brief screeners for caregiver burden and depression to ensure caregivers could potentially benefit from the intervention. As such, large proportions of caregivers engaged in the intervention had modifiable risk factors addressed by PST/DSJ, which may indicate why the intervention was generally successful across intervention doses and personal characteristics. While many studies have used this approach to recruit and engage appropriate participants (64–66), this strategy is recommended for future research and practice to avoid ‘floor effects’ (i.e., participants do not have risk at baseline and may not benefit from the intervention) and/or engaging participants with too high of risk at baseline, which may signal the need for advanced intervention with clinical professions (i.e., beyond the anticipated and feasible benefits participants can receive from the offered intervention) (67).
Given the smaller, yet considerable, proportion of caregivers categorized as Non-Responders to the intervention in the current study, there are clear opportunities to adapt or complement PST/DSJ to better meet the diverse and complex needs of caregivers. In these analyses, all participants across intervention arms were combined into a single group, thus not accounting for dose–response in the analyses. This is justified by the non-superiority effect of session number and booster sessions in a previous PST/DSJ publication (39); however, if all caregivers received the same dose, or if dose was more individually targeted to individual need, a larger proportion may have been Responders to the intervention. This may support future pragmatic trials that uniformly serve caregivers to assess clinical benefits in care burden and depression. While not all caregivers responded to the intervention in terms of caregiver burden and depression, other subjective benefits may have been obtained by these caregivers (and those who were responders to the intervention). Therefore, future research should assess other measures to document other benefits of PST/DSJ, such as relationship quality, perceived care quality, shared decision making, resilience, social connection, loneliness, and participants’ perceptions of benefit (68).
Finally, some among the Non-Responders may require additional or different intervention. PST/DSJ teaches a problem-solving strategy that provides a concrete adaptive coping skill for proactive problem solving and goal attainment. The downstream benefits of PST/DSJ on emotional outcomes like depressive symptoms likely result from enhance self-efficacy, goal attainment, and behavioral activation (55, 69–71). However, this may not be sufficient to address more severe depression that may require psychotherapy or pharmacological intervention. For caregiver burden, the skills learned in PST/DSJ help caregivers better manage their daily tasks and achieve goals that are important to them (55, 71), but this does not necessarily lessen the overall burden they still experience by the demands of caregiving and the potential lack of support available. Community-level interventions that provide tangible and instrumental support to caregivers are still needed to lessen these demands (14, 68).
Limitations and future directions
Though baseline assessment of caregivers in this study was robust, multiple factors that could contribute to treatment response were not measured, including access to resources, socioeconomic factors, and social support. Our sample was somewhat homogenous with regard to demographics (predominantly White care partners) and geography, which may introduce bias and limit generalizability. Additionally, though PST/DSJ is offered in both English and Spanish, there were not enough Spanish-speakers to examine whether response differed by language of delivery, which is an important direction for future research. Though care recipients had several different dementia diagnoses, most had AD, and the amount and nature of caregiving help provided was not captured in detail. Lastly, as previously noted, we only measured two common outcomes for caregivers: depressive symptoms and caregiver burden. However, PST/DSJ does not target any specific outcome and may have conferred unmeasured benefits for both caregivers and their care recipients.
Future work is needed to identify other potential benefits and to further determine for whom PST/DSJ would be most beneficial. Participants in the Non-responder group reported less severe symptoms of caregiver burden and depression compared to Responders, suggesting perhaps these were not outcomes for which they needed intervention. However, dementia caregivers experience a range of other challenges, from social participation restrictions to loneliness to anger and resentment, to name a few. Follow-up qualitative studies could reveal outcomes that are most meaningful and could benefit from PST/DSJ. Some in the Non-responder group actually reported worsening symptoms, indicating a need for more targeted and/or intensive intervention to address these potentially more serious emotional symptoms. Additionally, there are other approaches to operationalizing “response” to intervention, such as the participant’s perspective of whether they improved or not, which may yield different results.
Conclusion
This study is an important step in identifying the drivers of intervention response among caregivers of people living with ADRD. As seen in our study, most caregivers demonstrated a clinically meaningful improvement in caregiver burden and/or depressive symptoms after receiving PST/DSJ. Notably, those who responded to the intervention had higher symptoms of distress, caregiver burden, overload, and depressive symptoms; had lower life satisfaction; and had care recipients with more severe dementia. These results indicate that those benefiting may also be those most likely in need of this support. Additional studies are needed to drive adaptations and complementary support services to effective caregiver interventions to improve recruitment and increase impact.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Texas Southwestern Medical Center University of Texas Rio Grande Valley Texas A&M University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SJ: Investigation, Funding acquisition, Conceptualization, Resources, Writing – review & editing, Project administration, Supervision, Formal analysis, Methodology, Writing – original draft. MS: Conceptualization, Funding acquisition, Investigation, Writing – original draft, Resources, Writing – review & editing, Methodology. KW: Writing – review & editing, Writing – original draft, Project administration. BW: Writing – review & editing, Writing – original draft. GH: Validation, Writing – review & editing, Methodology, Formal analysis, Writing – original draft. CS: Writing – original draft, Conceptualization, Funding acquisition, Project administration, Writing – review & editing. GM: Writing – review & editing, Project administration, Funding acquisition, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the Texas Alzheimer’s Research and Care Consortium [TARCC 2020-06-25-CR]. The funder had no role in the published article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1682373/full#supplementary-material
References
1. Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement J Alzheimers Assoc. (2020) 16:77.
2. Arno, PS, Levine, C, and Memmott, MM. The economic value of informal caregiving. Health Aff (Millwood). (1999) 18:182–8.
3. U.S. Department of Labor, Bureau of Labor Statistics. Employment, hours, and earnings from the current employment statistics survey (10-CEU 6562160008, home health care services (NAICS code 6216), average hourly earnings, July 2018.). (2018). Available online at: www.bls.gov/ces/data.htm (Accessed February 16, 2021).
4. Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement J Alzheimers Assoc. (2024) 20:3708–821.
5. Wolff, JL, Spillman, BC, Freedman, VA, and Kasper, JD. A National Profile of family and unpaid caregivers who assist older adults with health care activities. JAMA Intern Med. (2016) 176:372–9. doi: 10.1001/jamainternmed.2015.7664
6. Rospenda, KM, Minich, LM, Milner, LA, and Richman, JA. Caregiver burden and alcohol use in a community sample. J Addict Dis. (2010) 29:314–24. doi: 10.1080/10550887.2010.489450
7. Sörensen, S, Duberstein, P, Gill, D, and Pinquart, M. Dementia care: mental health effects, intervention strategies, and clinical implications. Lancet Neurol. (2006) 5:961–73. doi: 10.1016/S1474-4422(06)70599-3
8. Vitaliano, PP, Zhang, J, and Scanlan, JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull. (2003) 129:946–72. doi: 10.1037/0033-2909.129.6.946
9. Napoles, AM, Chadiha, L, Eversley, R, and Moreno-John, G. Reviews: developing culturally sensitive dementia caregiver interventions: are we there yet? Am J Alzheimers Dis Other Dement. (2010) 25:389–406. doi: 10.1177/1533317510370957
10. Arango Lasprilla, JC, Moreno, A, Rogers, H, and Francis, K. The effect of dementia patient’s physical, cognitive, and emotional/ behavioral problems on caregiver well-being: findings from a Spanish-speaking sample from Colombia, South America. Am J Alzheimers Dis Other Dement. (2009) 24:384–95. doi: 10.1177/1533317509341465
11. Stall, NM, Kim, SJ, Hardacre, KA, Shah, PS, Straus, SE, Bronskill, SE, et al. Association of informal caregiver distress with health outcomes of community-dwelling dementia care recipients: a systematic review. J Am Geriatr Soc. (2019) 67:609–17. doi: 10.1111/jgs.15690
12. The Office of Minority Health. Hispanic/Latino - The Office of Minority Health. (2025). Available online at: https://minorityhealth.hhs.gov/hispaniclatino-health (Accessed March 24, 2021).
13. Mehta, KM, and Yeo, GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement. (2017) 13:72–83. doi: 10.1016/j.jalz.2016.06.2360
14. Parker, D, Mills, S, and Abbey, J. Effectiveness of interventions that assist caregivers to support people with dementia living in the community: a systematic review. Int J Evid Based Healthc. (2008) 6:137–72. doi: 10.1097/01258363-200806000-00002
15. Schulz, R, Burgio, L, Burns, R, Eisdorfer, C, Gallagher-Thompson, D, Gitlin, LN, et al. Resources for enhancing Alzheimer’s caregiver health (REACH): overview, site-specific outcomes, and future directions. The Gerontologist. (2003) 43:514–20. doi: 10.1093/geront/43.4.514
16. Prince, M, Guerchet, M, and Prina, M The global impact of dementia: 2013–2050. London, England.(2013)
17. Ko, E, Wongvibul, T, Rose, KM, and Jun, J. The effects of self-guided interventions on stress, burden, and mental health in caregivers of people living with dementia: a systematic review. Int J Nurs Stud Adv. (2023) 5:100141. doi: 10.1016/j.ijnsa.2023.100141
18. Cheng, ST, and Zhang, F. A comprehensive meta-review of systematic reviews and meta-analyses on nonpharmacological interventions for informal dementia caregivers. BMC Geriatr. (2020) 20:137. doi: 10.1186/s12877-020-01547-2
19. King, RB, Raad, JH, Flaherty, J, and Hartke, RJ. Stroke caregiver depression: qualitative comparison of treatment responders and nonresponders at 1 year. J Cardiovasc Nurs. (2022) 37:581–8. doi: 10.1097/JCN.0000000000000852
20. Nehrig, N, and Chen, CK. How to address the needs of non-responders to REACH VA: a qualitative analysis. Aging Ment Health. (2019) 23:1203–8. doi: 10.1080/13607863.2018.1484885
21. Rivera, PA, Elliott, TR, Berry, JW, and Grant, JS. Problem-solving training for family caregivers of persons with traumatic brain injuries: a randomized controlled trial. Arch Phys Med Rehabil. (2008) 89:931–41. doi: 10.1016/j.apmr.2007.12.032
22. Powell, JM, Fraser, R, Brockway, JA, Temkin, N, and Bell, KR. A telehealth approach to caregiver self-management following traumatic brain injury: a randomized controlled trial. J Head Trauma Rehabil. (2016) 31:180–90. doi: 10.1097/HTR.0000000000000167
23. Pfeiffer, K, Beische, D, Hautzinger, M, Berry, JW, Wengert, J, Hoffrichter, R, et al. Telephone-based problem-solving intervention for family caregivers of stroke survivors: a randomized controlled trial. J Consult Clin Psychol. (2014) 82:628–43. doi: 10.1037/a0036987
24. Elliott, TR, Berry, JW, and Grant, JS. Problem-solving training for family caregivers of women with disabilities: a randomized clinical trial. Behav Res Ther. (2009) 47:548–58. doi: 10.1016/j.brat.2009.03.006
25. Elliott, TR, and Berry, JW. Brief problem-solving training for family caregivers of persons with recent-onset spinal cord injuries: a randomized controlled trial. J Clin Psychol. (2009) 65:406–22. doi: 10.1002/jclp.20527
26. Elliott, TR, Shewchuk, RM, and Richards, JS. Family caregiver social problem-solving abilities and adjustment during the inital year of the caregiving role. J Couns Psychol. (2001) 48:223–32. doi: 10.1037/0022-0167.48.2.223
27. Juengst, SB, Silva, V, Goldin, Y, Cicerone, K, Lengenfelder, J, Chiaravalloti, N, et al. Care partner problem solving training (CP-PST) for care partners of adults with traumatic brain injury during inpatient rehabilitation: study protocol for a multisite, randomized, single-blind clinical feasibility trial. Contemp Clin Trials. (2019) 80:9–15. doi: 10.1016/j.cct.2019.03.004
28. Juengst, SB, Osborne, CL, Holavanahalli, R, Silva, V, Kew, CL, Nabasny, A, et al. Feasibility study of problem-solving training for care partners of adults with traumatic brain injury, spinal cord injury, burn injury, or stroke during the inpatient hospital stay. Arch Rehabil Res Clin Transl. (2019) 1:100009. doi: 10.1016/j.arrct.2019.100009
29. Garand, L, Morse, JQ, Barnes, J, Dadebo, V, and Lopez, OL. Problem solving therapy reduces subjective burden levels in caregivers of family members with mild cognitive impairment or early-stage dementia: secondary analysis of a randomized clinical trial. Int J Geriatr Psychiatry. (2019) 34:957–65.
30. Chiu, M, Pauley, T, Wesson, V, Pushpakumar, D, and Sadavoy, J. Evaluation of a problem-solving (PS) techniques-based intervention for informal carers of patients with dementia receiving in-home care. Int Psychogeriatr. (2015) 27:937–48. doi: 10.1017/S1041610214002798
31. Domingues, NS, Verreault, P, and Hudon, C. Reducing burden for caregivers of older adults with mild cognitive impairment: a systematic review. Am J Alzheimers Dis Other Dement. (2018) 33:401–14. doi: 10.1177/1533317518788151
32. Beinart, N, Weinman, J, Wade, D, and Brady, R. Caregiver burden and psychoeducational interventions in Alzheimer’s disease: a review. Dement Geriatr Cogn Disord Extra. (2012) 2:638–48. doi: 10.1159/000345777
33. McPherson, KM, Kayes, N, and Weatherall, MMembers of the Goals-SR Research Group. A pilot study of self-regulation informed goal setting in people with traumatic brain injury. Clin Rehabil. (2009) 23:296–309. doi: 10.1177/0269215509102980
34. Vega, M, Nabasny, A, and Juengst, SB. “Descubriendo Soluciones Juntos”—an argument for adapting problem-solving training for Latinx care partners after traumatic brain injury (TBI). Rehabil Psychol. (2020) 65:337–46. doi: 10.1037/rep0000310
35. Brockway, JA, St De Lore, J, Fann, JR, Hart, T, Hurst, S, Fey-Hinckley, S, et al. Telephone-delivered problem-solving training after mild traumatic brain injury: qualitative analysis of service members’ perceptions. Rehabil Psychol. (2016) 61:221–30. doi: 10.1037/rep0000077
36. Bell, KR, Fann, JR, Brockway, JA, Cole, WR, Bush, NE, Dikmen, S, et al. Telephone problem solving for service members with mild traumatic brain injury: a randomized, clinical trial. J Neurotrauma. (2017) 34:313–21. doi: 10.1089/neu.2016.4444
37. Baker, A, Barker, S, Sampson, A, and Martin, C. Caregiver outcomes and interventions: a systematic scoping review of the traumatic brain injury and spinal cord injury literature. Clin Rehabil. (2017) 31:45–60. doi: 10.1177/0269215516639357
38. Otero, MC, Walker, JA, Kumpula, MJ, Hernandez, B, Funderburk, JS, Wetherell, JL, et al. Negative problem orientation is associated with mental health outcomes for veterans enrolled in problem-solving training. Cogn Behav Pract. (2024) 31:203–14. doi: 10.1016/j.cbpra.2022.11.001
39. Juengst, SB, Holland, A, Wilmoth, K, Lee Smith, M, Han, G, Supnet-Bell, C, et al. Three versus six sessions of problem-solving training with or without boosters for care partners of adults with dementia (CaDeS): a randomised controlled optimization trial. Lancet Reg Health. (2025) 50:101222. doi: 10.1016/j.lana.2025.101222
40. Juengst, S, Supnet, C, Kew, CLN, Silva, V, Vega, M, Han, G, et al. Bilingual problem-solving training for caregivers of adults with dementia: a randomized, factorial-design protocol for the CaDeS trial. Contemp Clin Trials. (2021) 108:106506. doi: 10.1016/j.cct.2021.106506
41. Kroenke, K, Spitzer, RL, and Williams, JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
42. Bedard, M, Molloy, DW, Squire, L, Dubois, S, Lever, JA, and O’Donnell, M. The zarit burden interview: a new short version and screening version. Gerontologist. (2001) 41:652–7.
43. Juengst, SB, Wright, B, Driver, S, Calhoun, S, Muir, A, Dart, G, et al. Multisite randomized feasibility study of problem-solving training for care partners of adults with traumatic brain injury during inpatient rehabilitation. NeuroRehabilitation. (2023) 52:109–22. doi: 10.3233/NRE-220129
44. D’Zurilla, TJ, and Nezu, AM. Development and preliminary evaluation of the social problem-solving inventory. Psychol Assess J Consult Clin Psychol. (1990) 2:156–63.
45. Smith, ML, Steinman, LE, and Casey, EA. Combatting social isolation among older adults in a time of physical distancing: the COVID-19 social connectivity paradox. Front Public Health. (2020) 8:403. doi: 10.3389/fpubh.2020.00403
46. Smith, ML, and Barrett, ME. Development and validation of the upstream social interaction risk scale (U-SIRS-13): a scale to assess threats to social connectedness among older adults. Front Public Health. (2024) 12:1454847. doi: 10.3389/fpubh.2024.1454847
47. Sclan, SG, and Reisberg, B. Functional assessment staging (FAST) in Alzheimer’s disease: reliability, validity, and ordinality. Int Psychogeriatr. (1992) 4:55–69.
48. Schofield, HL, Murphy, B, Herrman, HE, Bloch, S, and Singh, B. Family caregiving: measurement of emotional well-being and various aspects of the caregiving role. Psychol Med. (1997) 27:647–57.
49. Hébert, R, Bravo, G, and Préville, M. Reliability, validity and reference values of the Zarit burden interview for assessing informal caregivers of community-dwelling older persons with dementia. Can J Aging Rev Can Vieil. (2000) 19:494–507.
50. Martín Carrasco, M, Salvadó, I, Nadal Álava, S, Miji, LC, Rico, JM, Lanz, P, et al. Adaptación para nuestro medio de la escala de sobrecarga del cuidador (Caregiver Burden Interview) de Zarit. Rev Gerontol. (1996) 6:338–45.
51. Pagán- Torres, OM, González- Rivera, JA, and Rosario-Hernández, E. Psychometric analysis and factor structure of the Spanish version of the eight-item patient health questionnaire in a general sample of Puerto Rican adults. Hisp J Behav Sci. (2020) 42:401–15. doi: 10.1177/0739986320926524
52. Dekker, J, de Boer, M, and Ostelo, R. Minimal important change and difference in health outcome: an overview of approaches, concepts, and methods. Osteoarthr Cartil. (2024) 32:8–17. doi: 10.1016/j.joca.2023.09.002
53. Liew, TM, and Yap, P. A 3-item screening scale for caregiver burden in dementia caregiving: scale development and score mapping to the 22-item Zarit burden interview. J Am Med Dir Assoc. (2019) 20:629–633.e12. doi: 10.1016/j.jamda.2018.11.005
54. Dy, SM, Waldfogel, JM, Sloan, DH, Cotter, V, Hannum, S, Heughan, JA, et al. Table A-11, Minimal clinically important differences and clinical cutoff scores for outcome assessment tools included in review. Agency for Healthcare Research and Quality (US); (2021). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK567826/table/appa.tab12/ (Accessed on 2025 May 7)
55. Tao, Q, and Zhang, J. Problem-solving based intervention for informal caregivers: a scoping review. Open J Nurs. (2019) 9:951–71.
56. Lopez-Hartmann, M, Wens, J, Verhoeven, V, and Remmen, R. The effect of caregiver support interventions for informal caregivers of community-dwelling frail elderly: a systematic review. Int J Integr Care. (2012) 12:e133. doi: 10.5334/ijic.845
57. Hopwood, J, Walker, N, McDonagh, L, Rait, G, Walters, K, Iliffe, S, et al. Internet-based interventions aimed at supporting family caregivers of people with dementia: systematic review. J Med Internet Res. (2018) 20:e216. doi: 10.2196/jmir.9548
58. McCurry, SM, Logsdon, RG, Vitiello, MV, and Teri, L. Successful behavioral treatment for reported sleep problems in elderly caregivers of dementia patients: a controlled study. J Gerontol B Psychol Sci Soc Sci. (1998) 53:P122–9.
59. AARP & National Alliance for Caregiving En español. Caregiving in the United States (2020). Available online at: https://www.aarp.org/pri/topics/ltss/family-caregiving/caregiving-in-the-united-states/ (Accessed on 2025 July 28)
60. ASPE (2014). Informal Caregiving for Older Americans: An Analysis of the 2011 National Study of Caregiving. Available online at: http://aspe.hhs.gov/reports/informal-caregiving-older-americans-analysis-2011-national-study-caregiving (Accessed on 2025 July 28)
61. Bieber, A, Nguyen, N, Meyer, G, and Stephan, A. Influences on the access to and use of formal community care by people with dementia and their informal caregivers: a scoping review. BMC Health Serv Res. (2019) 19:88. doi: 10.1186/s12913-018-3825-z
62. Teles, S, Ferreira, A, and Paúl, C. Access and retention of informal dementia caregivers in psychosocial interventions: a cross-sectional study. Arch Gerontol Geriatr. (2020) 93:104289
63. Deb, A, Thornton, JD, Sambamoorthi, U, and Innes, K. Direct and indirect cost of managing alzheimer’s disease and related dementias in the United States. Expert Rev Pharmacoecon Outcomes Res. (2017) 17:189–202. doi: 10.1080/14737167.2017.1313118
64. Offord, DR. Selection of levels of prevention. Addict Behav. (2000) 25:833–42. doi: 10.1016/S0306-4603(00)00132-5
65. Blom, MM, Zarit, SH, Groot Zwaaftink, RBM, Cuijpers, P, and Pot, AM. Effectiveness of an internet intervention for family caregivers of people with dementia: results of a randomized controlled trial. PLoS One. (2015) 10:e0116622. doi: 10.1371/journal.pone.0116622
66. Cheng, ST, Mak, EPM, Fung, HH, Kwok, T, Lee, DTF, and Lam, LCW. Benefit-finding and effect on caregiver depression: a double-blind randomized controlled trial. J Consult Clin Psychol. (2017) 85:521–9. doi: 10.1037/ccp0000176
67. Zarit, SH. Past is prologue: how to advance caregiver interventions. Aging Ment Health. (2018) 22:717–22. doi: 10.1080/13607863.2017.1328482
68. Gitlin, LN, Jutkowitz, E, and Gaugler, JE. Dementia caregiver intervention research now and into the future: Review and recommendations. Washington, DC: Commissioned paper for the National Academies of Science, Engineering and Medicine NIA Decadal Study (2020).
69. Bell, AC, and D’Zurilla, TJ. Problem-solving therapy for depression: a meta-analysis. Clin Psychol Rev. (2009) 29:348–53.
70. Artistico, D, Pinto, AM, Douek, J, Black, J, and Pezzuti, L. The value of removing daily obstacles via everyday problem-solving theory: developing an applied novel procedure to increase self-efficacy for exercise. Front Psychol. (2013) 4:20. doi: 10.3389/fpsyg.2013.00020
Keywords: caregiver, dementia, Alzheimer’s disease and related dementia, problem-solving, Spanish language, dementia care, psychosocial intervention, metacognitive strategies
Citation: Juengst SB, Smith ML, Wilmoth K, Wright B, Han G, Supnet C and Maestre G (2025) Problem-solving training to improve caregiver burden and depressive symptoms among dementia caregivers: personal and clinical factors of responders vs. non-responders. Front. Public Health. 13:1682373. doi: 10.3389/fpubh.2025.1682373
Edited by:
Vahid Rashedi, University of Social Welfare and Rehabilitation Sciences, IranReviewed by:
Thomas Wosch, University of Applied Sciences, Würzburg-Schweinfurt, GermanyMohammadjavad Hosseinabadi-farahani, University of Social Welfare and Rehabilitation Sciences, Iran
Mahmood Bahramizadeh, University of Social Welfare and Rehabilitation Sciences, Iran
Copyright © 2025 Juengst, Smith, Wilmoth, Wright, Han, Supnet and Maestre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shannon B. Juengst, c2hhbm5vbi5qdWVuZ3N0QG1lbW9yaWFsaGVybWFubi5vcmc=
 Shannon B. Juengst
Shannon B. Juengst Matthew Lee Smith
Matthew Lee Smith Kristin Wilmoth
Kristin Wilmoth Brittany Wright2
Brittany Wright2 Gang Han
Gang Han Charlene Supnet
Charlene Supnet Gladys Maestre
Gladys Maestre