- 1School of Global Health, Chinese Center for Tropical Diseases Research, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2The First Hospital of Lanzhou University, Lanzhou, China
Objective: Rabies remains a preventable yet fatal zoonotic disease, disproportionately affecting low- and middle-income settings. Despite global progress in prevention and control, the extent of geographic, socioeconomic, and age-related disparities in rabies burden has not been fully quantified.
Methods: Using data from the Global Burden of Disease (GBD) Study 2021, we evaluated global, regional, national, and sociodemographic index (SDI)-specific patterns in rabies burden, focusing on age-standardized rate (ASR), including age-standardized incidence (ASIR), prevalence (ASPR), mortality (ASMR), and disability-adjusted life-year rates (ASDR). Temporal trends were quantified using the average annual percentage change (AAPC). Health inequalities were examined using the slope index of inequality (SII) and concentration index (CCI), while frontier analysis was conducted to estimate potential gains relative to socioeconomic development.
Results: In 2021, the global ASIR of rabies was 0.129 per 100,000 population [95% uncertainty interval (UI): 0.076, 0.182 per 100,000 population], the ASMR was 0.128 per 100,000 population (95% UI: 0.075, 0.181 per 100,000 population). All global ASRs declined from 1990 to 2021, yet the absolute burden remained concentrated in low-SDI countries. Marked inequalities persisted, with ASIR, ASMR, and ASDR highest in low-SDI regions and lowest in high-SDI regions, South Asia carried the greatest absolute number of deaths and disability-adjusted life-year (DALYs) cases, whereas Eastern Sub-Saharan Africa recorded the highest ASR. At the country level, Nepal showed the highest ASR, while India contributed the largest number of cases. Health inequality analysis demonstrated that the absolute gap in ASDR between the highest- and lowest-SDI countries narrowed substantially from 1990 to 2021, but relative inequalities remained stable. Frontier analysis revealed wide substantial room for improvement even in some low-resource and high-income settings.
Conclusion: Global rabies elimination requires comprehensive efforts to reduce disparities both between and within countries, primarily by expanding access to post-exposure prophylaxis, increasing dog vaccination coverage and addressing socioeconomic inequalities.
1 Introduction
Rabies is an acute and fatal zoonotic disease caused by the rabies virus (1–3). It is primarily transmitted through contact with the saliva of infected animals via scratches, bites, or direct exposure to mucous membranes (4, 5). Dogs are responsible for up to 99% of human rabies cases. Early symptoms include fever, pain, and neuropathic manifestations. Without timely post-exposure prophylaxis, rabies is nearly universally fatal. The disease has a prolonged incubation period, typically ranging from 1 to 3 months, during which prophylactic vaccination is nearly 100% effective at preventing the onset of clinical disease (1, 2, 6).
According to estimates from the World Health Organization (WHO), rabies causes approximately USD8.6 billion in global losses annually, encompassing the loss of life, livelihoods, healthcare costs, and the immeasurable psychological burden. Rabies is present on all continents except Antarctica, with an estimated 59,000 deaths worldwide each year (7). However, due to underreporting, the documented cases often differ from these estimates. Rabies is classified as a neglected tropical disease, disproportionately affecting marginalized populations. Despite the availability of highly effective human rabies vaccines and immunoglobulins, access remains limited for those in need, often due to affordability (7, 8). The average cost of post-exposure prophylaxis (PEP) is estimated at USD108, including travel expenses and lost income, posing a significant economic burden for individuals earning only USD1–2 per day. Globally, over 29 million people receive human rabies vaccines annually (7).
Studying the global burden of rabies is essential for understanding its epidemiology, health impact, and economic burden. As a nearly 100% fatal yet entirely preventable infectious disease, rabies remains a significant public health challenge in low- and middle-income countries, particularly in Asia and Africa (9, 10). Although previous studies have examined the global burden of rabies (11), they are often limited to specific time periods and regions, lacking comprehensive analyses of major rabies trends and associated health inequalities. In particular, studies utilizing the most recent data from the 2021 Global Burden of Diseases (GBD), Injuries, and Risk Factors Study remain scarce. GBD 2021 systematically analyzes and integrates global health and disease data, serving as a critical tool for assessing the global, regional, and national burden of diseases and injuries (12–14).
This study provides a comprehensive analysis of the burden of rabies. Health inequalities between countries and regions are evaluated using the slope index of inequality (SII) and concentration index (CCI) (15, 16). Frontier analysis estimates optimal health outcomes based on the sociodemographic index (SDI), while decomposition analysis identifies the main contributors to changes in disease burden (15, 16). Joinpoint regression analysis detects key inflection points in temporal trends (15, 16). Additionally, projections based on the Bayesian age-period-cohort (BAPC) model aim to identify potential public health challenges by 2035, offering policymakers a framework to optimize global health resource allocation and develop proactive intervention strategies (15, 16). Such analyses provide critical evidence for developing evidence-based rabies control strategies and resource allocation. Furthermore, they support the implementation of integrated policies for vaccination, dog population management, and post-exposure interventions, aligning with the WHO’s 2030 target of achieving zero human deaths from rabies.
2 Materials and methods
2.1 Date source
The GBD 2021 study assessed the burden of 371 diseases and injuries, along with 88 risk factors, across 204 countries and territories. Rates and counts of incidence, prevalence, mortality, and Disability-Adjusted Life Years (DALYs) were estimated using stratified models based on various classifications. The analysis systematically adjusted epidemiological data to account for biases arising from variations in data sources, definitions, and measurement methods (13, 14, 17). These adjustments were implemented through sophisticated statistical models, including Bayesian priors, regularization, and trimming using the MR-BRT (stands for meta-regression with Bayesian priors, regularization, and trimming) framework, as well as DisMod-MR 2.1 (a Bayesian mixed-effects meta-regression modeling tool developed for GBD analyses), ensuring internal consistency across estimates for global, five SDI regions, 7 super regions, 21 geographic regions, and 204 countries and territories, age groups, sexes, and time periods (13, 14, 17). The process employed standardization and calibration steps to minimize the impact of heterogeneity on study outcomes. Mortality data in GBD 2021 primarily originated from national vital registration systems, maternal and child health surveillance networks, and census data. Incidence and prevalence data were derived from disease surveillance systems, national health surveys, and published studies. Disability burden estimates were based on data from case notifications, hospital discharge records, household surveys, and cohort studies (13, 14, 17). Detailed information on study design, data collection, and estimation methodologies is provided in the GBD 2021 documentation (13, 14, 17).
Data on rabies were retrieved from the GBD 2021database using the GBD results tool on the Institute for Health Metrics and Evaluation (IHME) website (http://ghdx.healthdata.org), including rabies-related incidence, prevalence, mortality, and DALYs cases, alongside age-standardized incidence rate (ASIR), prevalence (ASPR), mortality (ASMR), and DALYs rate (ASDR) at global, super-regional, regional (21 geographic regions), and national (204 countries and territories) levels. For rabies, the relevant classification code in the WHO’s International Classification of Diseases (ICD-10) is B82 (13).
The SDI is a composite measure of social and demographic development widely used to compare health across settings. Countries and territories were grouped into five levels (low: 0–0.4658, low-middle: 0.4658–0.6188, middle: 0.6188–0.7120, high-middle: 0.7120–0.8103, high: 0.8103–1.0000), facilitating systematic assessment of socioeconomic influences on health outcomes (12, 13).
2.2 Statistical analysis
The disease burden of rabies was assessed using rates and total case counts for incidence, prevalence, mortality, and DALYs. Rate was expressed as estimates per 100,000 population, reflect the relative burden, while case counts represent the absolute burden. Both metrics are reported with 95% uncertainty intervals (UI) (16, 18, 19).
All statistical analyses were conducted using R software (version 4.4.1, R Foundation for Statistical Computing, Vienna, Austria; available at https://cran.r-project.org). Detailed descriptions of specific analytical methods, including BAPC models, the SII and CCI of inequality analysis, frontier analysis, and decomposition analysis, will be provided in subsequent sections (15, 16, 18).
2.2.1 Percentage change
The percentage change (PC) quantifies the changes in rabies incidence, prevalence, mortality, and DALYs, as well as in ASIR, ASPR, ASMR, and ASDR, by comparing 2021 values to those of 1990. The calculation for PC is as follows (18, 20):
PC = (value2021−value1990)/value2021 × 100%. When the lower bound of the 95% UI for the PC is greater than 0, it indicates an increasing trend. Conversely, if the upper bound of the 95% UI is less than 0, it indicates a decreasing trend (20).
2.2.2 Joinpoint regression analysis
The average annual percent change (AAPC) was used to evaluate the overall temporal trends in rabies burden indicators from 1990 to 2021. The formula for calculating AAPC is as follows (21, 22):
The number and location of joinpoints were identified using the Grid Search Method. In the model, represents the number of segments, while denotes the regression coefficients for each linear segment. The length of each segment is represented by . If the lower bound of the 95% Confidence Interval (CI) for the AAPC is greater than 0 and p < 0.05, this indicates an increasing trend. Conversely, if the upper bound of the 95% CI is less than 0 and p < 0.05, this indicates a decreasing trend (21, 22).
2.2.3 BAPC
BAPC model was employed to project global rabies rates for the period 2022–2035. The calculation formula is as follows (19, 21):
In this model, (1≤≤) represents time points, (1≤≤) denotes age groups, represents the intercept, represents the age effect, represents the period effect, represents the cohort effect.
2.2.4 Estimated annual percent change
The Estimated Annual Percent Change (EAPC) was utilized to quantify the annual trend of the age-standardized rate (ASR, including ASIR, ASPR, ASMR, ASDR). The formula for calculating EAPC is as follows (23, 24):
where represents the natural logarithm of the ASR, signifies the calendar year. If the lower bound of the 95% CI for the EAPC is greater than 0 with and p < 0.05, it indicates an increasing trend. Conversely, if the upper bound of the 95% CI is less than 0 with and p < 0.05, it indicates a decreasing trend (23, 25).
2.2.5 Decomposition analysis
The Das Gupta decomposition method was employed to analyze changes in rabies DALYs cases from 1990 to 2021, with these changes decomposed into three key contributing factors: aging, population growth, and epidemiological changes. Unlike traditional approaches (e.g., linear regression), which primarily explore relationships between variables, decomposition analysis was applied to enable a detailed assessment of the independent contribution of each factor to changes in DALYs cases. Through this deconstruction, a deeper understanding of the core drivers behind changes in rabies DALYs cases was achieved, thereby providing valuable insights for targeted interventions and policy development (15).
2.2.6 Cross-country inequality analysis
The study quantified the absolute and relative inequalities in rabies ASDR using the SII and CCI, as defined by the WHO. The SII was calculated by regressing ASDR against SDI, using the midpoint of the cumulative population distribution ranked by SDI (15). In addition, the CCI is calculated by matching the cumulative proportion of ASDR with the cumulative population distribution ranked by SDI and numerically integrating the area under the Lorenz curve (15).
2.2.7 Frontier analysis
To evaluate the association between rabies disease burden and socioeconomic development, frontier analysis was conducted to construct an ASDR-based model using SDI. A locally weighted regression combined with local polynomial regression was applied, with smoothing spans of 0.3, 0.4, and 0.5, to generate a smooth frontier line that accurately represented the nonlinear relationship between SDI and rabies ASDR. To ensure robustness, 1,000 bootstrap resampling iterations were performed, and the mean ASDR for each SDI value was calculated. The potential for improvement in reducing the rabies burden was assessed by measuring the absolute distance (effective difference) between ASDR and the estimated frontier line (15).
3 Results
3.1 Global
In 2021, the global ASIR of rabies was 0.129 per 100,000 population, the ASPR was 0.005 per 100,000 population, the ASMR was 0.128 per 100,000 population, and the ASDR was 7.496 per 100,000 population. From 1990 to 2021, all these rates showed significant declines, with the ASIR, ASPR, ASMR, and ASDR decreasing at annual average percentage changes (AAPC) of −0.010, −0.002%, −0.009%, and −0.552%, respectively (Tables 1–4). In absolute terms, there were an estimated 10,181 incident cases of rabies globally in 2021 and 10,084 deaths. From 1990 to 2021, global incident cases, prevalent cases, deaths, and DALYs attributable to rabies all showed a consistent decline (Supplementary Tables S1–S4).
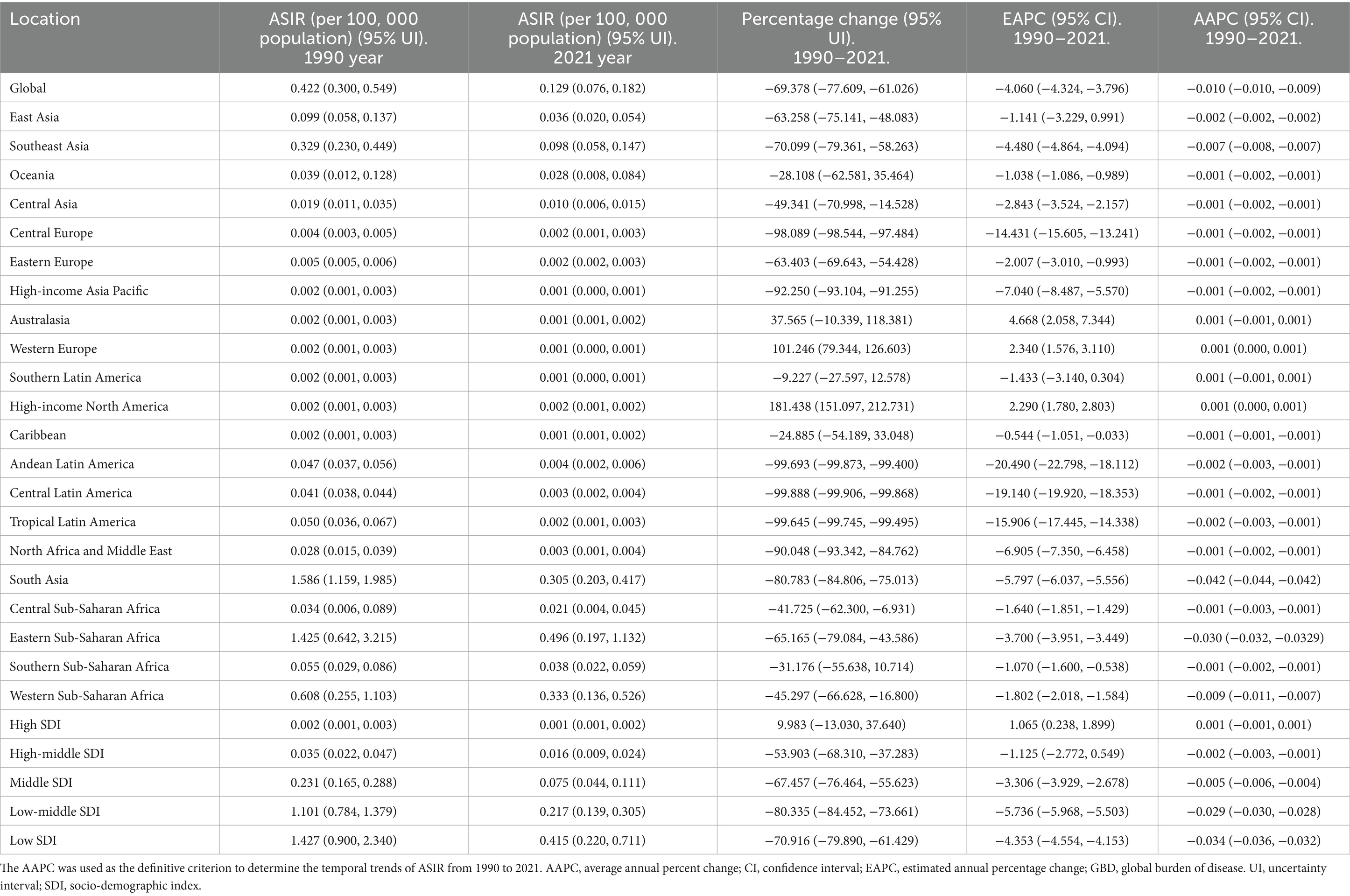
Table 1. The ASIR of rabies in 1990 year and 2021 year, and change trend of ASIR were analyzed across GBD regions.
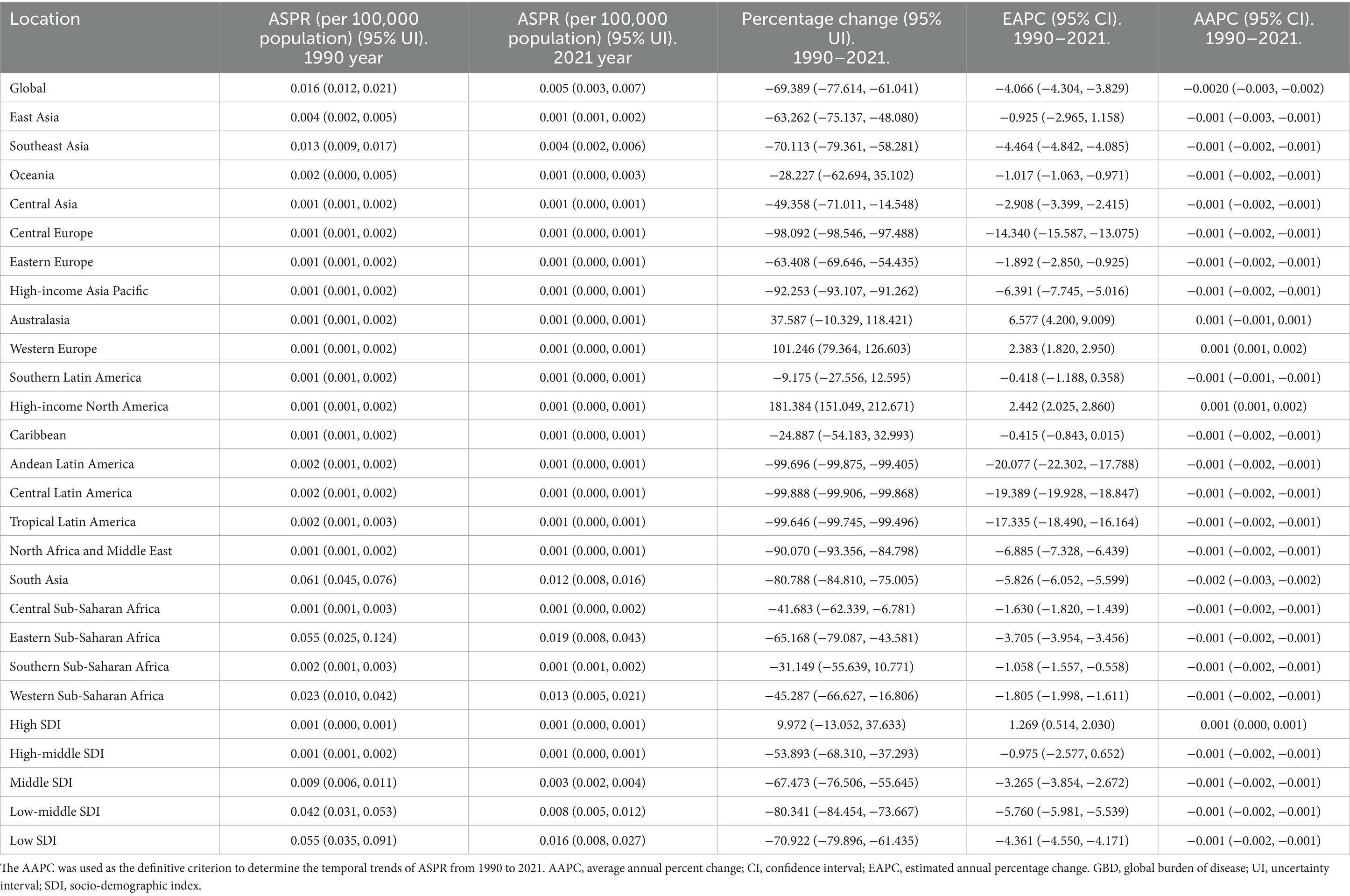
Table 2. The ASPR of rabies in 1990 year and 2021 year, and change trend of ASPR were analyzed across GBD regions.
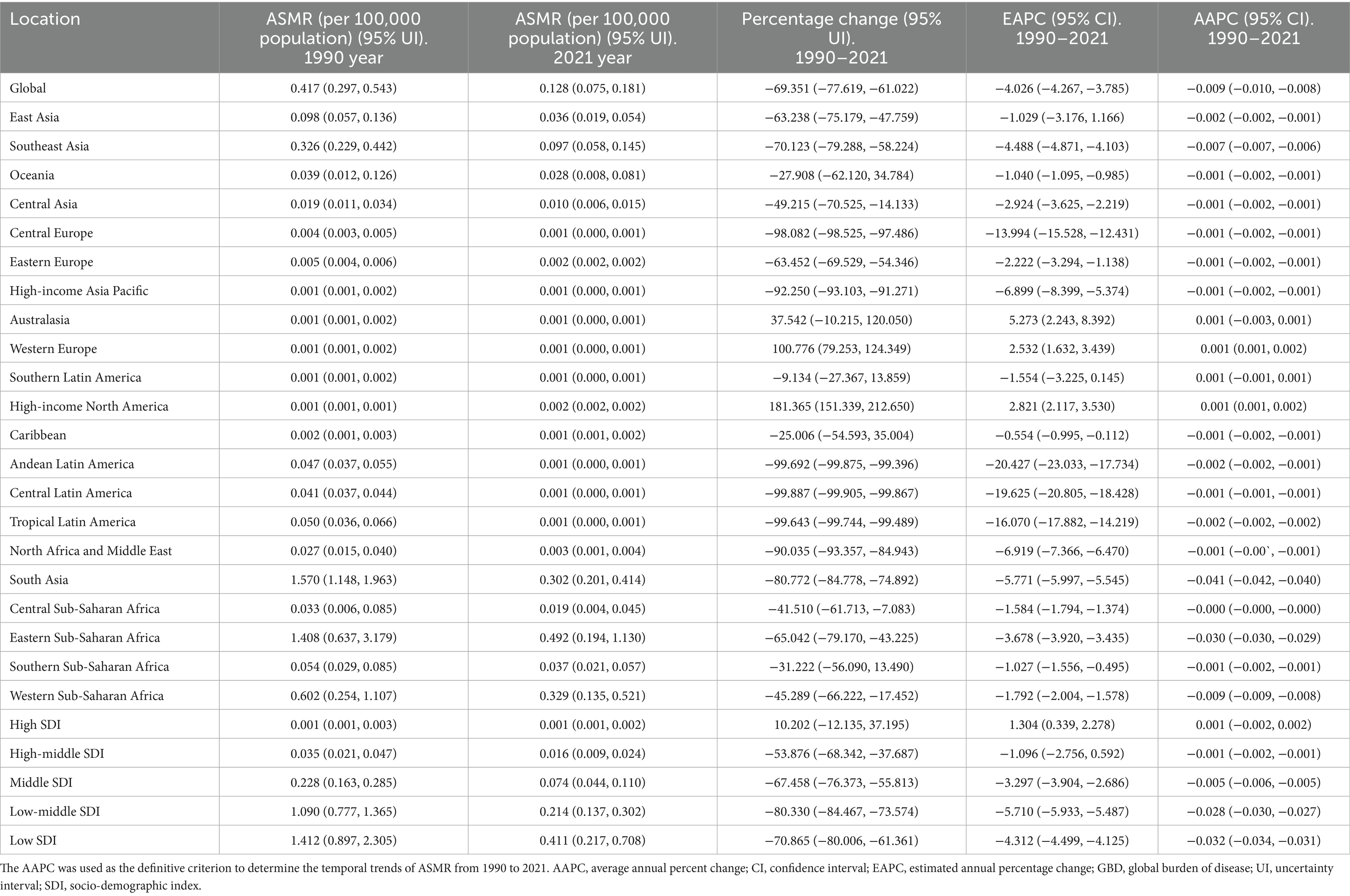
Table 3. The ASMR of rabies in 1990 year and 2021 year, and change trend of ASMR were analyzed across GBD regions.
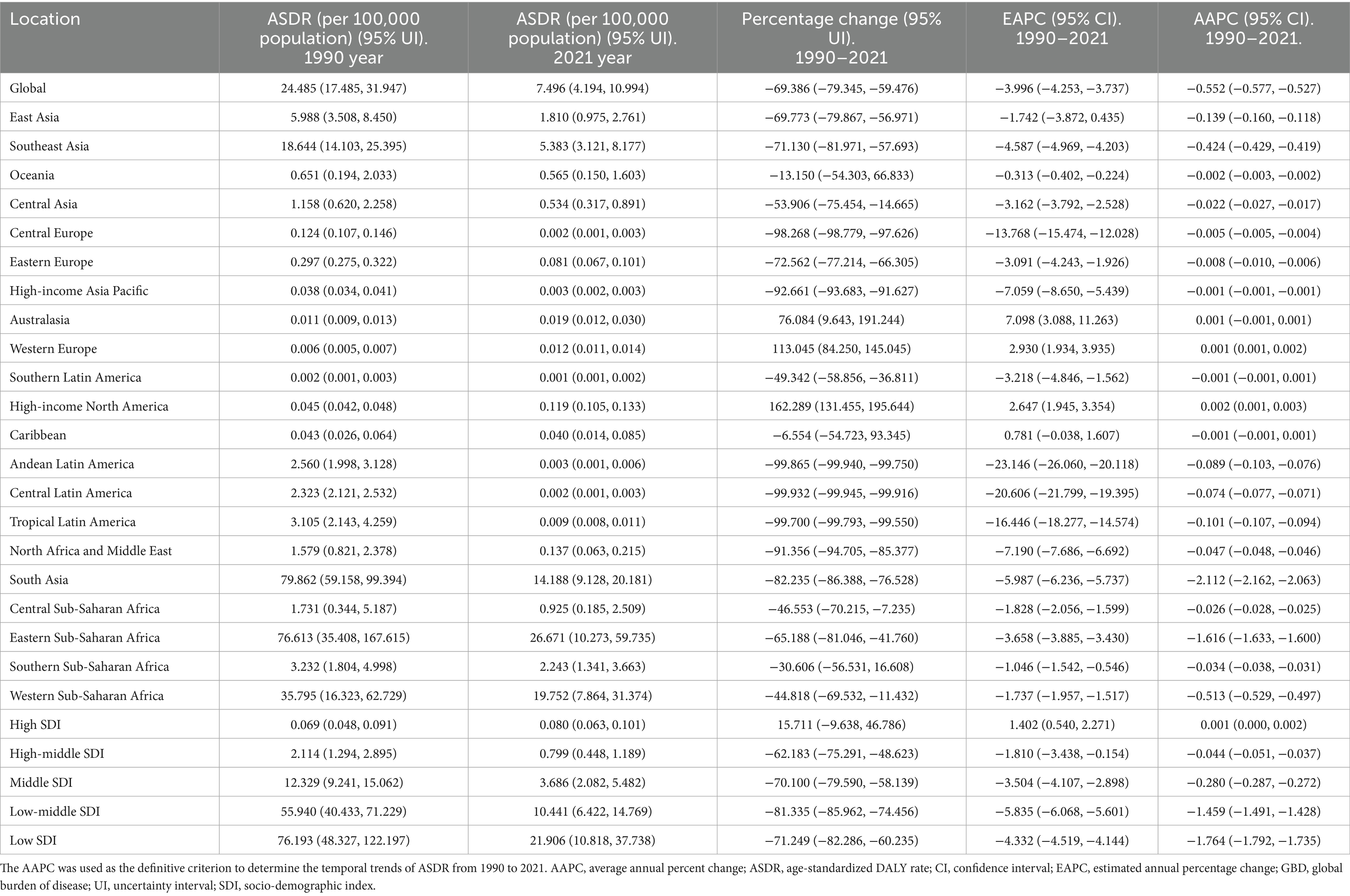
Table 4. The ASDR of rabies in 1990 year and 2021 year, and change trend of ASDR were analyzed across GBD regions.
3.2 Five SDI
In 2021, the burden of rabies, as measured by ASIR, ASPR, ASMR, and ASDR, was highest in low-SDI regions and lowest in high-SDI regions. Between 1990 and 2021, these indicators consistently declined across low, low-middle, middle, and high-middle SDI regions, while showing an increase in high-SDI regions (Tables 1–4). Similarly, the absolute burden of incident cases, prevalent cases, deaths, and DALYs was greatest in low-SDI regions and lowest in high-SDI regions, with a decrease observed in the former and an increase in the latter over the study period (Supplementary Tables S1–S4).
3.3 Geographical regions
In 2021, the highest ASIR of rabies was observed in Eastern Sub-Saharan Africa, while the greatest absolute number of incident cases was reported in South Asia. Between 1990 and 2021, the ASIR increased in Western Europe and High-income North America, but declined in 17 regions, with the most significant decrease in Eastern Sub-Saharan Africa (Table 1). In terms of absolute incident cases, the number increased in eight regions, particularly in Western Sub-Saharan Africa, but decreased in 13 regions, with the largest reduction observed in South Asia (Supplementary Table 1).
In 2021, the highest ASPR of rabies was recorded in Eastern Sub-Saharan Africa, while the greatest absolute number of prevalent cases occurred in South Asia. Between 1990 and 2021, the ASPR of rabies increased in Western Europe and High-income North America, but declined in 19 regions, with the steepest reduction observed in Eastern Sub-Saharan Africa (Table 2). Additionally, the absolute number of prevalent cases rose in eight regions, notably in Western Sub-Saharan Africa, while it decreased in 12 regions, with the most significant decline in South Asia (Supplementary Table 2).
In 2021, the highest ASMR of rabies was observed in Eastern Sub-Saharan Africa, while the greatest absolute number of deaths occurred in South Asia. From 1990 to 2021, the ASMR of rabies increased in Western Europe and High-income North America, but declined in 17 regions, with the most significant reduction seen in South Asia (Table 3). In addition, the absolute number of deaths rose in seven regions, particularly in Western Sub-Saharan Africa, while it decreased in 12 regions, with the largest decline in South Asia. (Supplementary Table 3).
In 2021, the highest ASDR of rabies was recorded in Eastern Sub-Saharan Africa, while the greatest absolute number of DALYs occurred in South Asia. Between 1990 and 2021, the ASDR of rabies increased in Western Europe and High-income North America, but declined in 17 regions, with the steepest reduction observed in South Asia (Table 4). Additionally, the absolute number of DALYs rose in four regions, particularly in High-income North America, but decreased in 15 regions, with the most significant decline in South Asia (Supplementary Table 4).
3.4 Countries and territories
In 2021, Nepal recorded the highest ASIR of rabies, while the largest absolute number of incident cases was observed in India. Between 1990 and 2021, the ASIR increased in 13 countries, with the greatest rise in Somalia, but declined in 165 locations, with the steepest reduction in Nepal. Additionally, the absolute number of incident cases rose in 80 countries, with the largest increase in Nigeria, while it decreased in 102 locations, with the most significant decline in India (Supplementary Tables 5–6).
In 2021, Nepal reported the highest ASPR of rabies, while the largest absolute number of prevalent cases was observed in India. Between 1990 and 2021, the ASPR increased in 31 countries, with the most pronounced rise in Jamaica, but declined in 160 locations, with the steepest reduction in Mexico. Additionally, the absolute number of prevalent cases increased in 90 countries, with the sharpest rise in Jamaica, while it decreased in 91 locations, with the largest decline in Mexico (Supplementary Tables 7, 8).
In 2021, Nepal reported the highest ASMR of rabies, while the largest absolute number of deaths occurred in India. Between 1990 and 2021, the ASMR increased in 20 countries, with the steepest rise in Canada, but declined in 153 locations, with the largest reduction in Guyana. Additionally, the absolute number of deaths rose in 74 countries, with the most pronounced increase in Turkmenistan, while it decreased in 76 locations, with the steepest decline in Saint Lucia (Supplementary Tables 9, 10).
In 2021, Nepal reported the highest ASDR of rabies, while the largest absolute number of DALYs occurred in India. Between 1990 and 2021, the ASDR increased in 15 countries, with the steepest rise in Turkmenistan, but declined in 150 locations, with the largest reduction in Jamaica. Additionally, the absolute number of DALYs rose in 52 countries, with the sharpest increase in Tunisia, while it decreased in 91 locations, with the steepest decline in Saint Vincent and the Grenadines (Supplementary Tables 11, 12).
3.5 Age-gender group analysis
In 2021, there were no significant differences in the incidence rate, prevalence rate, mortality rate, and DALYs rate of rabies between males and females across all age groups globally. Similarly, the absolute numbers of cases—incidence, prevalence, mortality, and DALYs—showed no significant gender-based differences across the various age groups (Figures 1A–D).
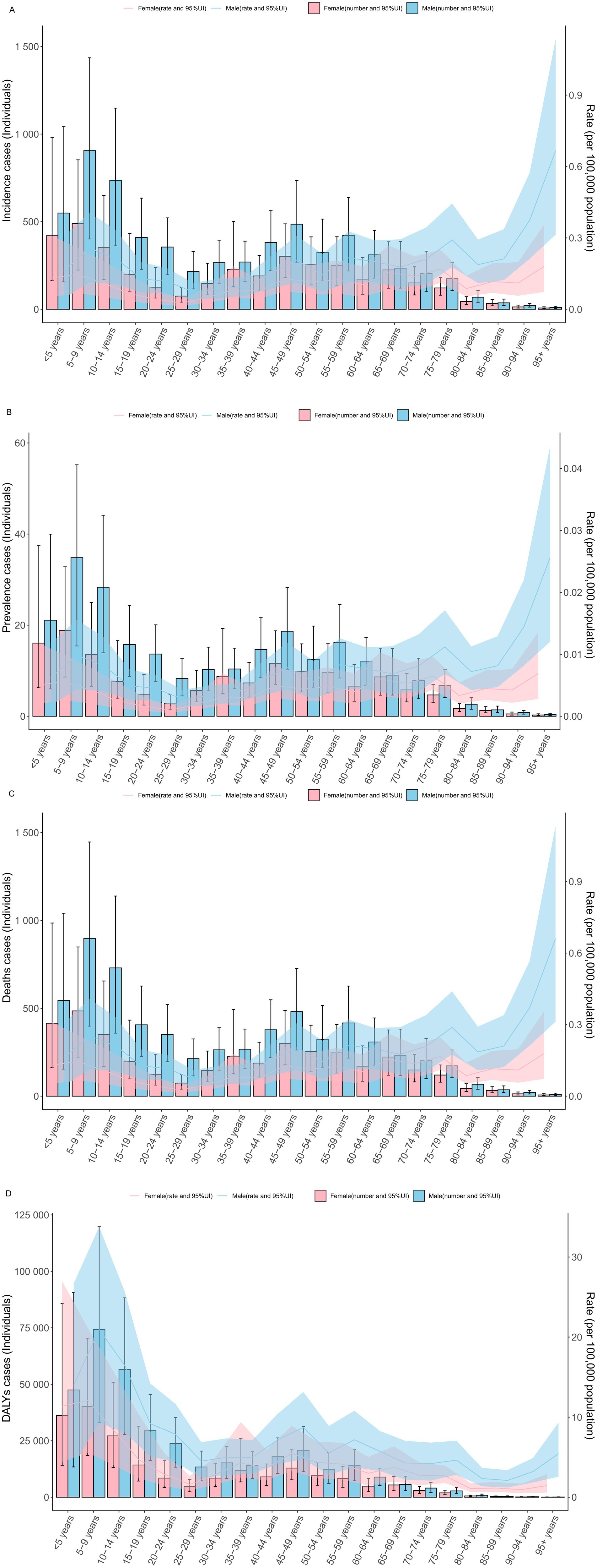
Figure 1. The specific rate of rabies showed notable differences across age and gender distributions in 2021 year (A) incidence rate. (B) prevalence rate. (C) mortality rate. (D) DALYs rate. DALYs, disability-adjusted life years; UI, uncertainty interval.
Notably, the specific DALYs rate and DALY cases were highest in the age groups under 5 years, 5–9 years, and 10–14 years, while these values were comparatively lower in all age groups over 60 years (Figures 1A–D).
3.6 Association between burden indicators and SDI
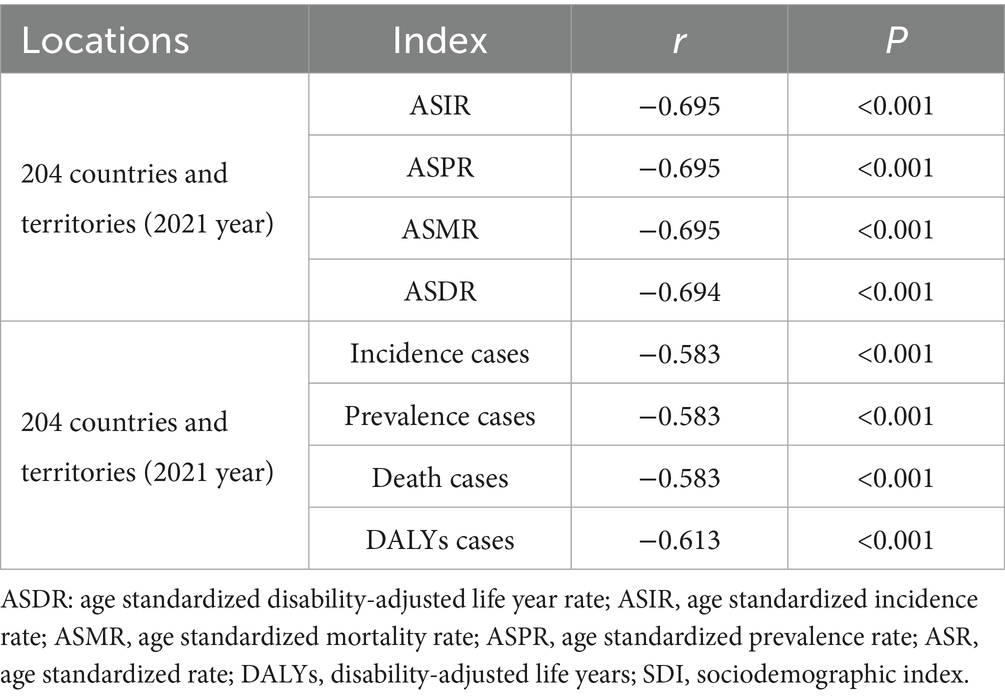
In 2021, the ASIR, ASPR, ASMR, and ASDR of rabies, together with the absolute numbers of incident cases, prevalent cases, deaths cases, and DALYs cases, were negatively correlated with the SDI across 204 countries and territories (Table 5).
3.7 Projections
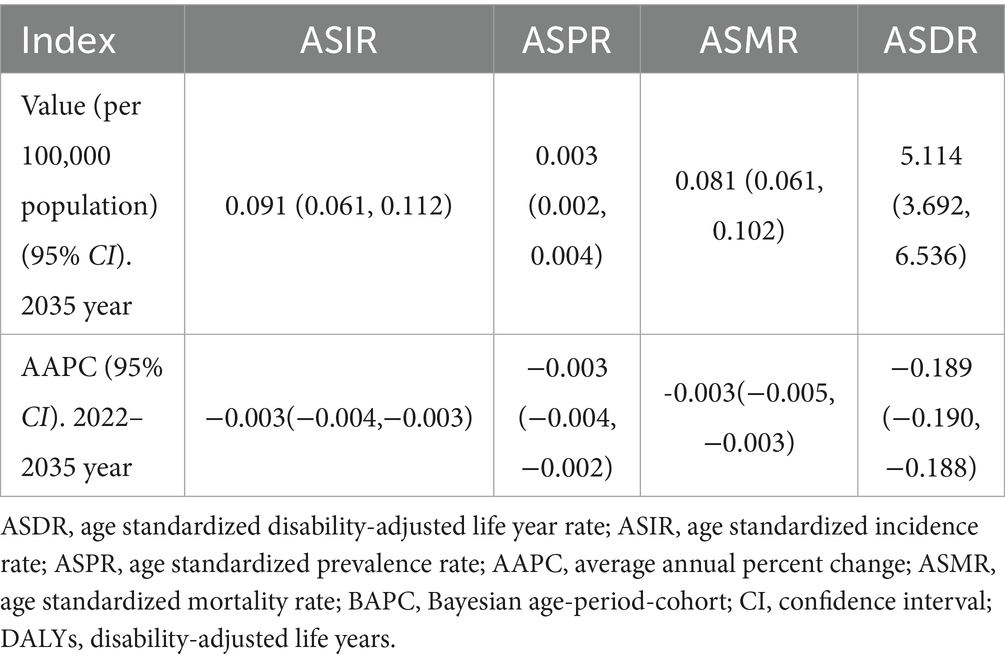
By 2035, globally, the ASIR of rabies is projected to decrease to 0.091 per 100,000 population (95% CI: 0.061, 0.112 per 100,000 population), while the ASMR is expected to decline to 0.081 per 100,000 population (95% CI: 0.061, 0.102 per 100,000 population). The AAPC values further highlight the gradual and steady decline in the global burden of rabies (Table 6).
3.8 Decomposition analysis
Globally, aging, population growth, and epidemiological changes contributed to changes in DALYs cases by 11.1615, −51.885%, and −1,124,615.561%, respectively. However, there were regional variations: DALYs cases increased in high-SDI regions, while it decreased in high-middle, middle, low-middle, and low-SDI regions (Figure 2; Supplementary Table 13).
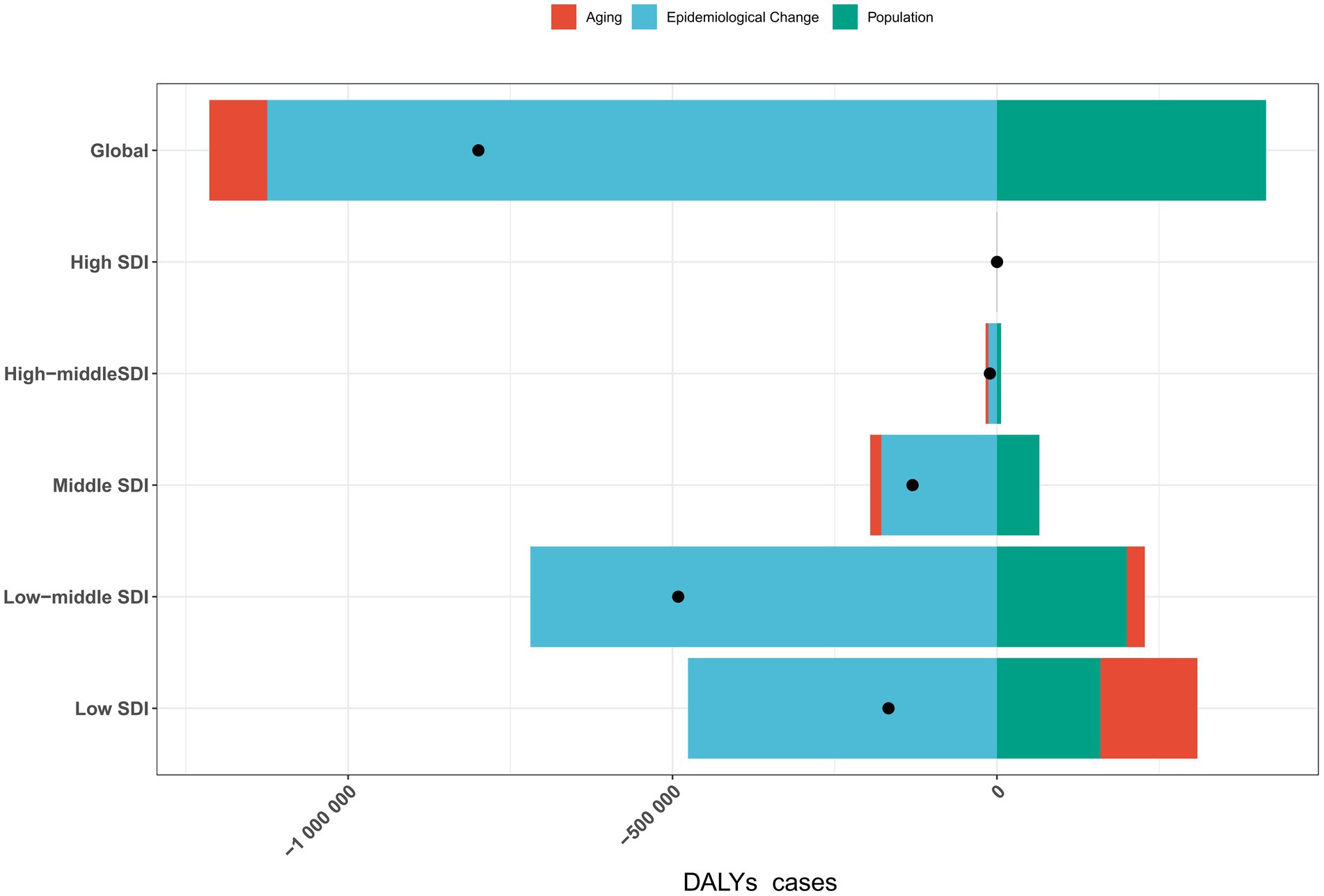
Figure 2. Impact of aging, population growth, and epidemiological changes on rabies DALYs cases globally and across five SDI regions in 2021. Black dots represent the total change contributed by all three factors. A positive value for each component indicates a corresponding increase in DALYs cases, while a negative value reflects a decrease in DALYs cases. DALYs, disability-adjusted life years; SDI, sociodemographic index.
3.9 Health inequality analysis
Significant absolute and relative health inequalities in rabies ASDR were observed in association with SDI (Figures 3A,B). The SSI reveals a decrease in the gap in ASDR between countries and territories with the highest and lowest SDI, from −22.331 (95% CI: −24.722, −19.952) in 1990 to −9.931 (95% CI: −11.352, −8.521) in 2021. In contrast, the CCI showed a slight change, moving from −0.712 (95% CI: −0.783, −0.616) in 1990 to −0.721 (95% CI: −0.770, −0.664) in 2021. These results suggest a reduction in absolute health inequalities in rabies ASDR from 1990 to 2021, while relative inequalities remained relatively stable.
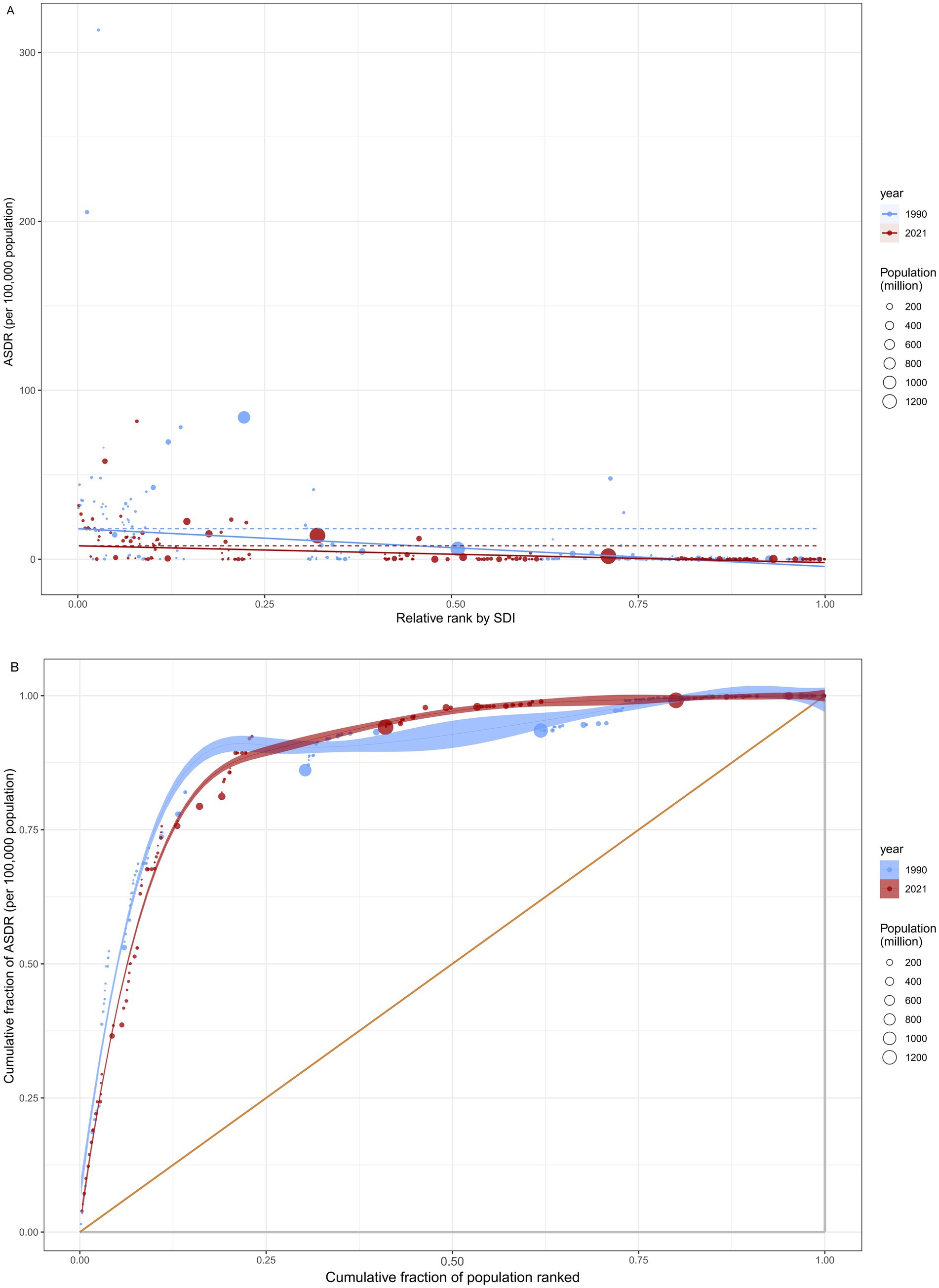
Figure 3. Health inequality regression analysis for rabies ASDR across 204 countries and territories. (A) illustrated the inequality slope index, depicting the relationship between rabies ASDR and SDI. Each point represents an individual country and territory, with the size of the point weighted by population. (B) presented the concentration index, which quantifies relative inequality by calculating the area under the Lorenz curve. This index aligns the distribution of rabies ASDR with the population distribution categorized by SDI. Data from 1990 is shown in blue, while data from 2021 is represented in red. ASDR, age standardized disability-adjusted life year rate; SDI, socio-demographic index.
3.10 Frontier analysis
A frontier analysis was performed to assess the potential for improvement in rabies ASDR across 204 countries and territories, relative to their respective levels of development as measured by the SDI (Figure 4; Supplementary Table 14). The 15 countries and territories exhibiting the largest disparities in potential improvement (effective difference range: 16.640–81.661) include Nepal, Ethiopia, Malawi, Mozambique, Myanmar, Chad, Nigeria, Ghana, Burkina Faso, Mali, Guinea, Burundi, South Sudan, Guinea-Bissau, and Eritrea. In contrast, low-SDI countries such as Haiti, Yemen, Afghanistan, Niger, and the Solomon Islands are close to the frontier line, indicating that although their development levels are low, their rabies ASDR is relatively close to the optimal level. In addition, high-SDI countries, including the United Arab Emirates, Puerto Rico, the United States, the United Kingdom, and Poland, despite having relatively ample resources, still exhibit significant potential for improvement in rabies ASDR (effective difference range: 302.981–371.140).
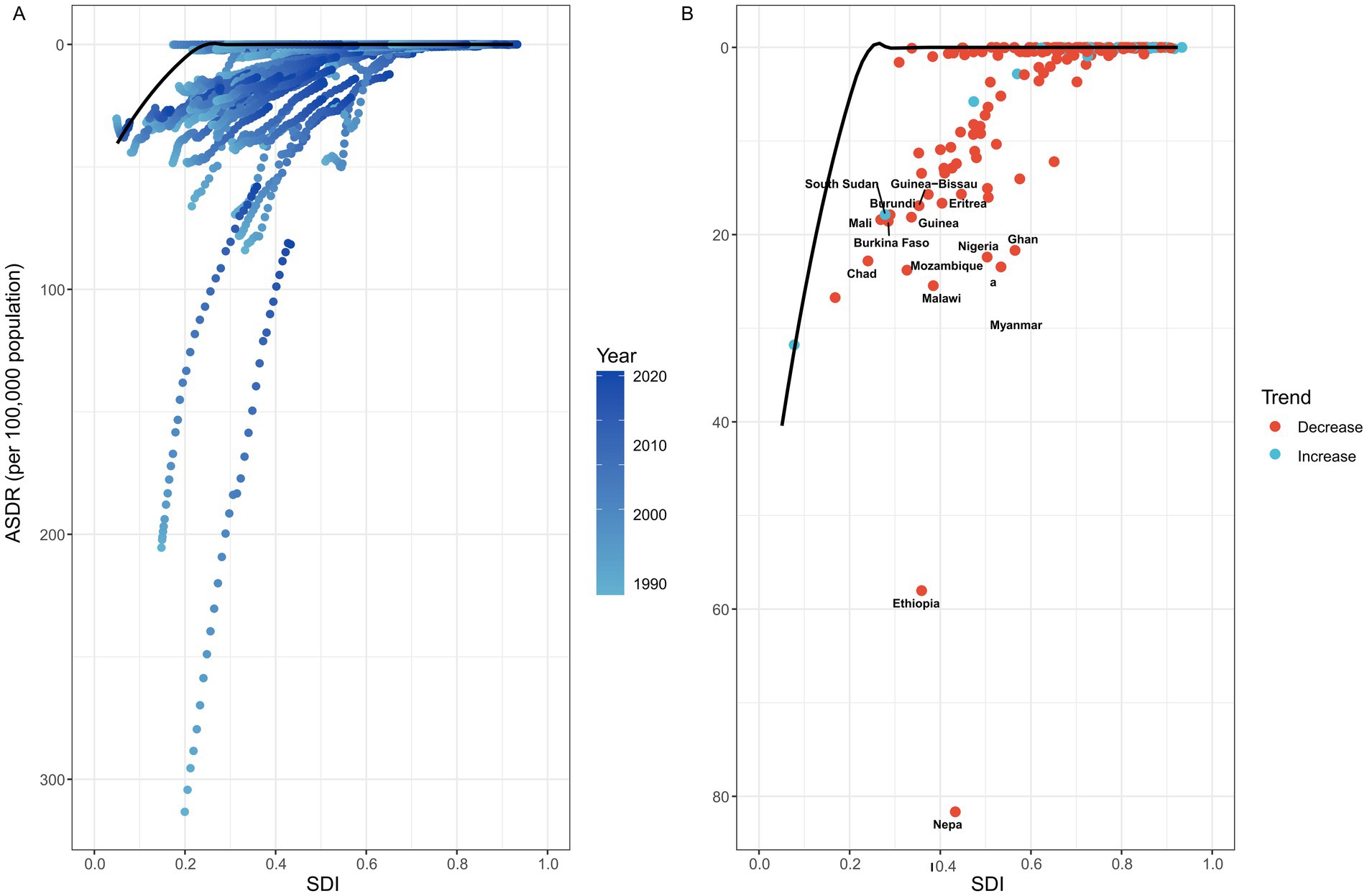
Figure 4. Frontier analysis was conducted to explore the association between ASDR and SDI for rabies in 204 countries and territories. (A) This panel showed the temporal trend of rabies ASDR changes, with a color gradient ranging from light blue (representing 1990) to dark blue (representing 2021). This gradient highlights how the ASDR has evolved globally over time. (B) Each point represented a specific country or territory in 2021, with the black frontier line indicating the optimal ASDR relative to the country’s SDI. Light blue points represent low-SDI countries that are close to the frontier line, signifying that these countries are approaching the optimal ASDR for their development level. Red points represent high-SDI countries exhibiting the largest gaps from the frontier, indicating significant room for improvement in rabies control despite relatively high development levels. In addition, blue points indicate a decrease in ASDR, while red points indicate an increase. ASDR, age standardized disability-adjusted life year rate; SDI, Socio-demographic index.
4 Discussion
This study systematically analyzed global and regional trends in the burden of rabies from 1990 to 2021 and projected future trajectories. Despite marked global declines in ASIR, ASPR, ASMR, and ASDR, the burden of rabies remains unequally distributed. Low SDI regions, particularly Sub-Saharan Africa and South Asia, continue to experience a disproportionately high burden. By contrast, in some high-income regions such as North America and Western Europe, overall levels remain low but incidence has shown an upward trend.
The global burden of rabies is characterized by striking inequalities. Rabies poses a particularly heavy burden in Nepal, South Asia, and many parts of Africa, especially in countries with low socioeconomic development (26). In these regions, where health systems are often under-resourced and structural inequities persist, weak dog population management, widespread stray dogs, and insufficient vaccination coverage have allowed sustained transmission of the virus between animals and humans. Limited health-care resources and the high cost or poor availability of PEP further increase population vulnerability (9, 27, 28). By contrast, in high-income countries, overall incidence is low, yet imported cases and wildlife reservoirs such as bats and raccoons continue to pose ongoing threats to public health (9, 27, 28).
Reducing this unequal burden requires coordinated, multilevel action. In low-income settings, priority should be given to expanding human and canine vaccine coverage, strengthening dog management and stray control, and ensuring affordable and accessible PEP (29, 30); public education campaigns are also essential to improve awareness of wound care and prevention after exposure. In high-income regions, enhanced cross-border surveillance, long-term wildlife reservoir control, and improved case-tracking and data-sharing systems are needed (31). At the global level, while financial, technical, and vaccine supply support from high-income countries and international organizations is crucial, it is essential to emphasize the foundational role of political will, national investment, and sustainable financing. A collaborative framework built on strong national leadership and sustainable financing strategies is critical for achieving lasting progress in the elimination of rabies (31–33).
In the past, successful and sustained interventions, such as dog vaccination campaigns in Latin America and the expansion of access to PEP in Asia, have significantly contributed to the reduction of the rabies burden in these regions. Building on this, to achieve the elimination of rabies in the future, priority should be given to strengthening canine vaccination coverage and standardizing and expanding human post-exposure prophylaxis networks. Additionally, enhancing public education and advocacy, raising awareness of prevention, and reducing exposure events will provide critical support for achieving the goal of rabies elimination. The implementation of these comprehensive measures will significantly reduce the global rabies burden and accelerate progress toward rabies elimination (34, 35).
This study has several limitations. First, the analysis is based on data from the GBD 2021 study, which faces notable challenges in quality and coverage, particularly in low-income and developing countries, where incomplete or inaccurate records may compromise reliability (13, 17). Second, the GBD estimates are model-derived rather than directly observed, it may introduce risks of overestimation of rabies burden (13, 36, 37). Third, the predictive models applied, including the BAPC model, rely heavily on the quality of available data and historical trends. Yet, key determinants such as human–dog interactions, canine infection rates, dog vaccination rate, and fluctuations in dog populations—critical drivers of rabies transmission—are not fully incorporated into the GBD modeling framework. Consequently, the projections may not adequately capture the complexity of future rabies dynamics.
5 Conclusion
Rabies remains disproportionately concentrated in low-SDI countries and among children, reflecting inequalities in human and dog vaccination, dog virus surveillance, post-exposure prophylaxis, and the distribution of health resources. Achieving the global elimination target for rabies will require not only increased financial investment, technical assistance, and vaccine support from high-income countries to strengthen North–South collaboration, but also the integration of dog population management, expanded vaccine coverage, cross-sectoral cooperation, and robust public health surveillance systems. Only through such comprehensive and equity-focused strategies can sustainable rabies elimination be accelerated.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
For Global Burden of Disease 2021 Study, a waiver of informed consent was reviewed and approved by the Institutional Review Board of the University of Washington (No. STUDY00009060). All the information about ethical standards is available through the official website (http://www.healthdata.org/gbd/2021).
Author contributions
LH: Investigation, Validation, Software, Conceptualization, Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Visualization, Data curation. E-LT: Formal analysis, Validation, Methodology, Data curation, Conceptualization, Software, Writing – original draft, Investigation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the Project of Shanghai Jiao Tong University School of Medicine (No. WK2405), Shanghai Natural Science Foundation (No. 23ZR1464000), Science and technology development project of Shanghai University of traditional Chinese medicine (No.24BZH07), Traditional Chinese Medicine Innovation Team of Shanghai Municipal Health Commission (No. 2022CX010), the Three-Year Action Plan for Strengthening the Construction of the Public Health System in Shanghai (2023—2025, No. GWVI-11.1-08), the Excellent Academic Leaders Program of Shanghai Science and Technology Commission (22XD1423500), the Multidisciplinary Innovation Team of Traditional Chinese Medicine of China (ZYYCXTD-D-202208), Talent Fund of Longhua Hospital affiliated to Shanghai University of Traditional Chinese Medicine (No. LH001.007).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1683863/full#supplementary-material
References
1. Shen, T, Welburn, SC, Sun, L, and Yang, GJ. Progress towards dog-mediated rabies elimination in PR China: a scoping review. Infect Dis Poverty. (2023) 12:30. doi: 10.1186/s40249-023-01082-3
2. Whitehouse, ER, Mandra, A, Bonwitt, J, Beasley, EA, Taliano, J, and Rao, AK. Human rabies despite post-exposure prophylaxis: a systematic review of fatal breakthrough infections after zoonotic exposures. Lancet Infect Dis. (2023) 23:e167–74. doi: 10.1016/S1473-3099(22)00641-7
3. Li, XC, Zhang, YY, Zhang, QY, Liu, JS, Ran, JJ, Han, LF, et al. Global burden of viral infectious diseases of poverty based on global burden of diseases study 2021. Infect Dis Poverty. (2024) 13:71. doi: 10.1186/s40249-024-01234-z
4. Nahata, KD, Bollen, N, Gill, MS, Layan, M, Bourhy, H, Dellicour, S, et al. On the use of Phylogeographic inference to infer the dispersal history of rabies virus: a review study. Viruses. (2021) 13:1628. doi: 10.3390/v13081628
5. Jaswant, G, Campbell, K, Czupryna, A, Mwatondo, A, Ogoti, B, Embregts,, et al. Molecular characterisation of human rabies in Tanzania and Kenya: a case series report and phylogenetic investigation. Infect Dis Poverty. (2024) 13:79. doi: 10.1186/s40249-024-01245-w
6. Tolentino Júnior, DS, Marques, MSV, Krummenauer, A, Duarte, MMS, Rocha, SM, de Santana, LF, et al. Rabies outbreak in Brazil: first case series in children from an indigenous village. Infect Dis Poverty. (2024) 12:78. doi: 10.1186/s40249-023-01130-y
7. World Health Organization. (2024). RabiesJ/OL. Available online at:https://www.who.int/news-room/fact-sheets/detail/rabies (Accessed 5 June 2024).
8. Lodha, L, Manoor Ananda, A, and Mani, RS. Rabies control in high-burden countries: role of universal pre-exposure immunization. Lancet Reg Health Southeast Asia. (2023) 19:100258. doi: 10.1016/j.lansea.2023.100258
9. Thangaraj, JWV, Krishna, NS, Devika, S, Egambaram, S, Dhanapal, SR, Khan, SA, et al. Estimates of the burden of human rabies deaths and animal bites in India, 2022-23: a community-based cross-sectional survey and probability decision-tree modelling study. Lancet Infect Dis. (2025) 25:126–34. doi: 10.1016/S1473-3099(24)00490-0
10. Gan, H, Hou, X, Wang, Y, Xu, G, Huang, Z, Zhang, T, et al. Global burden of rabies in 204 countries and territories, from 1990 to 2019: results from the global burden of disease study 2019. Int J Infect Dis. (2023) 126:136–44. doi: 10.1016/j.ijid.2022.10.046
11. Gibson, AD, Yale, G, Corfmat, J, Appupillai, M, Gigante, CM, Lopes, M, et al. Elimination of human rabies in Goa, India through an integrated one health approach. Nat Commun. (2022) 13:2788. doi: 10.1038/s41467-022-30371-y
12. GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2162–203. doi: 10.1016/S0140-6736(24)00933-4
13. GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2133–61. doi: 10.1016/S0140-6736(24)00757-8
14. GBD 2021 Forecasting Collaborators. Burden of disease scenarios for 204 countries and territories, 2022-2050: a forecasting analysis for the global burden of disease study 2021. Lancet. (2024) 403:2204–56. doi: 10.1016/S0140-6736(24)00685-8
15. Bai, Z, Han, J, An, J, Wang, H, Du, X, Yang, Z, et al. The global, regional, and national patterns of change in the burden of congenital birth defects, 1990-2021: an analysis of the global burden of disease study 2021 and forecast to 2040. EClinical Medicine. (2024) 77:102873. doi: 10.1016/j.eclinm.2024.102873
16. Zhang, J, Ou, D, Xie, A, Chen, D, and Li, X. Global burden and cross-country health inequalities of early-onset colorectal cancer and its risk factors from 1990 to 2021 and its projection until 2036. BMC Public Health. (2024) 24:3124. doi: 10.1186/s12889-024-20624-4
17. GBD 2021 Demographics Collaborators. Global age-sex-specific mortality, life expectancy, and population estimates in 204 countries and territories and 811 subnational locations, 1950-2021, and the impact of the COVID-19 pandemic: a comprehensive demographic analysis for the global burden of disease study 2021. Lancet. (2024) 403:1989–2056. doi: 10.1016/S0140-6736(24)00476-8
18. Li, K, Wu, J, Zhang, R, Xie, Y, Zhou, Z, Yin, Q, et al. Global, regional, and national burdens of pertussis among adults: a systematic analysis of age-specific trends using global burden of diseases 2021 data. Infect Dis Poverty. (2025) 14:85. doi: 10.1186/s40249-025-01355-z
19. Lv, H, Wang, L, Zhang, X, Dang, C, Liu, F, Zhang, X, et al. Further analysis of tuberculosis in eight high-burden countries based on the global burden of disease study 2021 data. Infect Dis Poverty. (2024) 13:70. doi: 10.1186/s40249-024-01247-8
20. Chen, Y, Chen, W, Cheng, Z, Chen, Y, Li, M, Ma, L, et al. Global burden of HIV-negative multidrug- and extensively drug-resistant tuberculosis based on global burden of disease study 2021. Sci One Health. (2024) 3:100072. doi: 10.1016/j.soh.2024.100072
21. Chu, C, Yang, G, Yang, J, Liang, D, Liu, R, Chen, G, et al. Trends in epidemiological characteristics and etiologies of diarrheal disease in children under five: an ecological study based on global burden of disease study 2021. Sci One Health. (2024) 3:100086. doi: 10.1016/j.soh.2024.100086
22. Xia, X, Tian, X, Xu, Q, Zhang, Y, Zhang, X, Li, J, et al. Global trends and regional differences in mortality of cardiovascular disease and its impact on longevity, 1980-2021: age-period-cohort analyses and life expectancy decomposition based on the global burden of disease study 2021. Ageing Res Rev. (2025) 103:102597. doi: 10.1016/j.arr.2024.102597
23. Lv, C, Chen, Y, Cheng, Z, Zhu, Y, Chen, W, Zhou, N, et al. Global burden of zoonotic infectious diseases of poverty, 1990-2021. Infect Dis Poverty. (2024) 13:82. doi: 10.1186/s40249-024-01252-x
24. Chen, J, Gong, Y, Chen, Q, Li, S, and Zhou, Y. Global burden of soil-transmitted helminth infections, 1990-2021. Infect Dis Poverty. (2024) 13:77. doi: 10.1186/s40249-024-01238-9
25. Li, T, Qiang, N, Bao, Y, Li, Y, Zhao, S, Chong, KC, et al. Global burden of enteric infections related foodborne diseases, 1990-2021: findings from the global burden of disease study 2021. Sci One Health. (2024) 3:100075. doi: 10.1016/j.soh.2024.100075
26. Cleaveland, S, Thumbi, SM, Sambo, M, Lugelo, A, Lushasi, K, Hampson, K, et al. Proof of concept of mass dog vaccination for thecontrol and elimination of canine rabies. Rev Sci Tech. (2018) 37:559–68. doi: 10.20506/rst.37.2.2824
27. Lu, WG, Ai, D, Song, H, Xie, Y, Liu, S, Zhu, W, et al. Epidemiological and numerical simulation of rabies spreading from canines to various human populations in mainland China. PLoS Negl Trop Dis. (2021) 15:e0009527. doi: 10.1371/journal.pntd.0009527
28. Ghosh, S, Hasan, MN, Nath, ND, Haider, N, Jones, DH, Islam, MK, et al. Rabies control in Bangladesh and prediction of human rabies cases by 2030: a one health approach. Lancet Reg Health Southeast Asia. (2024) 27:100452. doi: 10.1016/j.lansea.2024.100452
29. Rupprecht, CE, Mani, RS, Mshelbwala, PP, Recuenco, SE, and Ward, MP. Rabies in the tropics. Curr Trop Med Rep. (2022) 9:28–39. doi: 10.1007/s40475-022-00257-6
30. Chowdhury, S, Biswas, SK, Paul, S, Kaiser, SMG, Chowdhury, MGA, Ghosh, S, et al. One health investigation of the first human rabies death linked to a clinically suspected rabid bull calf in Bangladesh. One Health Outlook. (2025) 7:38. doi: 10.1186/s42522-025-00166-4
31. Shams, F, Jokar, M, Djalali, E, Abdous, A, Rahnama, M, Rahmanian, V, et al. Incidence and prevalence of rabies virus infections in tested humans and animals in Asia: a systematic review and meta-analysis study. One Health. (2025) 20:101102. doi: 10.1016/j.onehlt.2025.101102
32. Muhanga, M. Human-wildlife conflict and its consequences in Tanzania: advocating the use of one health approach as a mitigation measure. Sci One Health. (2025) 4:100109. doi: 10.1016/j.soh.2025.100109
33. Zhang, L, Guo, W, and Lv, C. Modern technologies and solutions to enhance surveillance and response systems for emerging zoonotic diseases. Sci One Health. (2024) 3:100061. doi: 10.1016/j.soh.2023.100061
34. Tidman, R, Thumbi, SM, Wallace, R, de Balogh, K, Iwar, V, Dieuzy-Labaye, I, et al. United against rabies forum: the one health concept at work. Front Public Health. (2022) 10:854419. doi: 10.3389/fpubh.2022.854419
35. Zhou, C, Wang, S, Wang, C, Qiang, N, Xiu, L, Hu, Q, et al. Integrated surveillance and early warning system of emerging infectious diseases in China at community level: current status, gaps and perspectives. Sci One Health. (2024) 4:100102. doi: 10.1016/j.soh.2024.100102
36. Zhang, YF, Li, SZ, Wang, SW, Mu, D, Chen, X, Zhou, S, et al. Zoonotic diseases in China: epidemiological trends, incidence forecasting, and comparative analysis between real-world surveillance data and global burden of disease 2021 estimates. Infect Dis Poverty. (2025) 14:60. doi: 10.1186/s40249-025-01335-3
37. Zhao, ZY, Li, JJ, Ouyang, HQ, Li, WH, Huang, SK, Ohore, OE, et al. Enhancing regional disease burden estimates: insights from the comparison of global burden of disease and China’s notifiable infectious diseases data with policy implications (2010-2020). Infect Dis Poverty. (2025) 14:81. doi: 10.1186/s40249-025-01351-3
Keywords: rabies, global burden of disease, health inequality, Bayesian age-period-cohort, disability-adjusted life-years
Citation: He L and Tan E-L (2025) From data to action: addressing geographic inequalities in rabies burden through targeted policies. Front. Public Health. 13:1683863. doi: 10.3389/fpubh.2025.1683863
Edited by:
Jinxin Zheng, Shandong Jiaotong University, ChinaReviewed by:
Kennedy Lushasi, Ifakara Health Institute, TanzaniaWanfu Xu, Guangzhou Medical University, China
Copyright © 2025 He and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: En-Li Tan, MTM2NTk0MjE1NTdAMTYzLmNvbQ==
 Lu He1
Lu He1 En-Li Tan
En-Li Tan