- 1School of Nursing, Southern Medical University, Guangzhou, China
- 2Nanfang Hospital, Southern Medical University, Guangzhou, China
- 3Shenzhen Hospital of Southern Medical University, Shenzhen, China
Background: The third trimester of pregnancy is the most frequent period of prenatal depression. Its prevalence is associated with differences in weight status. Pre-pregnancy overweight and obesity (OWOB) pregnant women have a high risk of third-trimester pregnancy depression. The aim of this study was to investigate the depression status of pre-pregnancy OWOB women in the third trimester of pregnancy, and to explore the influence of modifiable lifestyle behaviors during pregnancy on their depression status, so as to provide scientific basis for the prevention and intervention of third-trimester pregnancy depression in OWOB pregnant women.
Methods: A cross-sectional survey was conducted among 441 pregnant women with pre-pregnancy OWOB recruited from two tertiary hospitals in Guangdong Province. Participants completed a basic information form, the Self-Rating Depression Scale, the Food Frequency Questionnaire, the Perinatal Pregnant Women’s Health Literacy Scale, the Physical Activity Rating Scale, and the Pittsburgh Sleep Quality Index. Binary logistic step regression was used to identify the associated factors of pregnancy depression in the third trimester.
Results: Among the 441 participants, 411 (93.20%) were overweight and 30 (6.80%) were obesity prior to pregnancy. The mean score of depression was 48.50 ± 9.48. A total of 289 (65.53%) women had no depression, while 107 (24.26%) had mild, 44 (9.98%) had moderate, and 1 (0.23%) had severe depression. Binary logistic regression analysis showed that educational level, career, family income, and first pregnancy or not were the influencing factors for third-trimester pregnancy depression among pre-pregnancy OWOB women. The balanced dietary pattern, higher physical activity level and health literacy were protective factors, while the highly processed dietary pattern and sleep disorders were risk factors (p < 0.05).
Conclusion: About one-third of pre-pregnancy OWOB pregnant women experienced depression in the third trimester of pregnancy. The depression was most likely associated with modifiable lifestyle behaviors. Although the cross-sectional design may not necessarily indicate a cause-effect relationship, the findings suggest that early lifestyle interventions may still be an effective strategy for improving mental health outcomes in this high-risk population.
1 Introduction
Overweight and obesity (OWOB) have become increasingly serious public health concerns worldwide. According to the World Health Organization (WHO), the global prevalence of obesity rose from 4.7% in 1975 to 16% in 2022, with 44% of adult women overweight (1). China has the highest number of individuals with obesity in the world (2). By 2030, China’s medical expenses caused by OWOB will reach 417.8 billion CNY, accounting for 21.5% of total national healthcare expenditures (3–6), and the economic burden will be huge. This trend is also evident among women of childbearing age. A national study conducted across 31 provinces in mainland China reported 24.5% overweight rate and 9.0% obesity rate among women aged 18–44 years (7), with 25.1% pregnant women overweight (8). In this regard, the National Health Commission, together with 15 other ministries, launched the “Weight Management Year” campaign (9). Additionally, the Chinese Nutrition Society published the Dietary Guidelines for Pregnant and Lactating Women (2022), with the core of achieving a normal pre-pregnancy weight (10). These actions reflect growing national attention to weight management in pregnant women.
In 2024, the National Health Commission and other three departments jointly issued the Guidelines on Promoting the Development of Fertility-Friendly Hospitals, which explicitly proposed to include prenatal depression screening into routine maternal healthcare services (11). Maternal mental health issues are also included in national health policy priority areas. The third trimester of pregnancy, defined as the period from 28 weeks of gestation to delivery, is considered the peak period for prenatal depression (12). In China, the prevalence of depression among the third-trimester pregnant women ranged from 12.01 to 44.4% (13, 14), posing significant risks to maternal well-being, neonatal outcomes and even family functioning (15, 16). Previous studies have shown that OWOB pregnant women are more susceptible to negative emotion such as depression and anxiety (17). On the one hand, excessive weight is associated with metabolic abnormalities and increased risk of pregnancy complications; on the other hand, these women may experience heightened body image concerns, social stigma, and health-related anxiety. The combined burden of physical and psychological stressors contributes to a higher risk of depression (18). Given the rising proportion of OWOB women among the pregnant population, investigating the prevalence and influencing factors of third-trimester depression in this group is of great importance, so as to inform the early identification of high-risk individuals and support the development of targeted personalized interventions.
Previous studies also identified that lower education level and family income, advanced maternal age, unemployment and other demographic factors are predictors of depression in third trimester of pregnancy (19–21). Gravidity, gestational diabetes mellitus, gestational hypertension, and other pregnancy complications and other obstetric variables are also closely related to the occurrence of depression (22). However, most existing studies have not stratified participants according to the body mass index (BMI), and pregnant women with underweight, normal weight, overweight, and obesity are often analyzed as a single group, without accounting for the potential differential impact of BMI categories on mental health. Considering that OWOB may elevate the risk of prenatal depression through multiple pathways (including metabolic abnormalities, chronic inflammation, psychological stress, etc.), such undifferentiated approaches may obscure the specific mechanism and risk profiles unique to OWOB pregnant women. Therefore, it is necessary to investigate third-trimester depression specifically in pre-pregnancy OWOB women, and further clarify the high-risk features and intervention priorities of this population.
Meanwhile, as the research on “lifestyle medicine” continues to deepen, researchers have increasingly recognized the important role of lifestyle in mental health and the prevention and treatment of depression (23–25). In terms of diet, Silva’s review of 10 studies found a negative association between adherence to a “healthy dietary pattern” and perinatal depression (26). Regarding sleep, Poeira noted that poor sleep quality during pregnancy predicts prenatal depression, with sleep quality progressively deteriorating as gestational age increases (27). Reduced physical activity during pregnancy has also elevates depression risk (28). Furthermore, a study of 1,478 pregnant women in Beijing found that passive smoking increased the risk of prenatal depression by 1.85 times, with husband-related exposure playing an important role (29). Health literacy refers to the ability of individuals to obtain, understand and apply health information to maintain and promote health. It is the basis of health-related behaviors and affects lifestyle choices. Studies have shown that lacking health literacy can negatively predict the occurrence of depression during pregnancy (30). These unhealthy lifestyle behaviors may contribute to depression through common biological pathways, including disrupting the hypothalamic–pituitary–adrenal (HPA) axis, increasing cortisol, low-grade systemic inflammation, and oxidative stress, and resulting in neurodegeneration and reduced neurogenesis (31, 32). OWOB, as inflammatory disease of adipose tissue, further enhance the production of pro-inflammatory cytokines, which in turn affect neurotransmitter expression and promote the development of depression (33). Notably, pre-pregnancy OWOB women often exhibit one or more unhealthy lifestyle behaviors, such as excessive intake of high-calorie and high-fat foods, physical inactivity, etc., which substantially heightens their risk of prenatal depression. Thus, examining the relationship between lifestyle behaviors and depression during third-trimester pregnancy among this specific population may help identify feasible avenues to improve their physical health and thus alleviate adverse psychological outcomes.
Therefore, the aim of this study was to assess the prevalence and influencing factors of depression during the third trimester of pregnancy among pre-pregnancy OWOB women, in order to provide evidence for the development and implementation of targeted prevention and intervention strategies for depression in pre-pregnancy OWOB pregnant women.
2 Methods
2.1 Study design
The study adopted a cross-sectional design and a convenience sampling method. Data were collected from February 2023 to September 2024. Based on geographical location and convenience of implementation, Guangzhou city and Shenzhen city were selected as representatives of Guangdong Province. Participants were recruited from the obstetric outpatient departments of Nanfang hospital Southern Medical University and Shenzhen hospital of Southern Medical University, both of which are the tertiary general teaching hospitals. Two trained researchers screened the electronic medical records of pregnant women attending routine prenatal examinations in the waiting areas. Eligible individuals were consecutively invited to participate until the required sample size was reached. Two trained researchers explained the study’s objective, content, and cooperation to the participants. Then participants signed the informed consent and completed several electronic questionnaires. This study has been registered in the clinical trial registry, with the registration number: ChiCTR2300077450.
2.2 Study population
Calculate the sample size required for the study. As no prior studies have reported the prevalence of third-trimester depression specifically among pre-pregnancy OWOB pregnant women in China, given the fact that pre-pregnancy OWOB pregnant women may face a higher risk of depression in the third trimester of pregnancy than general pregnant women, we referred to the findings of Shen (34), which indicated that the prevalence of depression in high-risk pregnant women was 29.3%. According to the cross-sectional survey sample size calculation formula (35), set the two-sided significance level (α) of 0.05 and a margin of error (δ) of 0.05, the required sample size was: n = 1.962 × 0.293 × (1–0.293) / 0.052 ≈ 319. Considering a potential 20% dropout or error rate, 319/(1–0.20) ≈ 399, the sample size required for this study is at least 399. A total of 441 participants were ultimately recruited.
Pre-pregnancy weight status was classified based on BMI according to WHO criteria. Following the recommended thresholds for the Chinese population proposed by the Chinese Obesity Working Group, pre-pregnancy overweight was defined as 24 kg/m2 ≤ BMI < 28 kg/m2, and pre-pregnancy obesity as BMI ≥ 28 kg/m2, and this overweight or obesity status would continue throughout pregnancy (36). Pre-pregnancy BMI was calculated as the self-reported pre-pregnancy weight divided by the square of the height measured at the first prenatal examination. For participants who were unsure of their pre-pregnancy weight or whose reported weight was deemed unreliable, BMI was calculated using the hospital-recorded weight measured at the first prenatal examination, provided that it was measured before 12 weeks of gestation (37). Participants need to wear light clothes and no shoes when weighing.
The target population was determined to be pregnant women over 18 years old with pre-pregnancy BMI ≥ 24 kg/m2, diagnosed as singleton intrauterine pregnancy, no signs of labor or abortion, and had adequate reading and communication abilities. According to the definition of the third trimester of pregnancy, only women with gestational age of ≥ 28 weeks were considered. However, since this study used the Pittsburgh Sleep Quality Index and the Physical Activity Rating Scale to assess sleep quality and physical activity over the past month (i.e., 4 weeks), we restricted the inclusion to women between 32 weeks of gestation and before delivery to ensure that these behaviors reflected the third-trimester pregnancy. Women with a history of severe hypertension, heart disease, diabetes, pre-pregnancy depression or other diagnosed mental illness were excluded.
2.3 Data collection
In the Basic information form, participants provided detailed demographic information such as age, ethnicity, education level, career, marital status, monthly per capita family income (CNY), and residence. Medical status was collected through medical records, including whether it was the first pregnancy, and whether there was gestational diabetes mellitus or gestational hypertension.
The Self-Rating Depression Scale (SDS) by Zung was used to assess depression over the past week (38). It has been demonstrated as a valuable and effective tool for detecting depression in pregnancy (39). The scale includes four dimensions with 20 items. A 4-point scale was used for each entry. The higher the score, the more severe the depression tendency. A total score ≤ 52 is no depression, 53 ~ 62 is mild depression; 63 ~ 72 is moderate depression; and ≥ 73 is severe depression. The Cronbach’ s α coefficient of this scale was 0.86 (40). The Cronbach’s α coefficient for the scale in this study was 0.74.
The Food Frequency Questionnaire (FFQ) represents a shortened version of the validated semi-quantitative FFQ for Chinese pregnant women and was revised to incorporate the dietary habits of the Guangdong region (41, 42). The questionnaire consisted of 23 food categories. According to previous researches, for most foods, the frequency division is more applicable with a finer division scale at high frequency levels, and if the intake frequency of a food is less than once a month, the contribution of this food to nutrient intake will also be negligible (43), and to reduce recall bias, pregnant women’s dietary habits over the past week were investigated. Therefore, we determined the frequency as: ① no eating/drinking; ② 1 ~ 2 times a week; ③ 3 ~ 4 times a week; ④ 5 ~ 6 times a week; ⑤ once a day; and ⑥ ≥ 2 times a day. Food intake: not eaten in the last week, ≤ 50 g, 100 g, 150 g, 200 g, and ≥ 250 g. Daily intake of each food (g/d) = average daily intake × total weekly intake frequency / 7. Each individual has a factor score on each dietary pattern (DP). The higher the score, the more inclined to this DP. The Cronbach ‘s α coefficient of the questionnaire in this study was 0.86, the content validity was 0.80.
The Perinatal Pregnant Women’s Health Literacy Scale was compiled by Wang (44) in 2017, including three dimensions of functional health literacy, communicative health literacy, and critical health literacy, 51 items. The scale uses the Likert 5-level scoring method, from “very disagree” to “very agree,” 1 to 5 points in turn. The higher the score, the higher the health literacy, and ≥ 204 points indicate having health literacy. The Cronbach’s α coefficient of the scale was 0.885, and the Cronbach’s α coefficients of each dimension were 0.802, 0.773 and 0.823. The Cronbach’s α coefficient of the scale in this study was 0.943.
The Physical Activity Rating Scale (PARS-3) was compiled by the Japanese scholar, Takao Hashimoto, and revised by Liang (45). The activity level was examined from 3 aspects: exercise intensity, exercise time and exercise frequency. Activity level = intensity × time × frequency. Each aspect was divided into 5 grades. Intensity and frequency were scored 1 ~ 5, and time was scored 0 ~ 4, so the highest amount of exercise is 100 points, the lowest is 0 points. Activity level was rated on a scale of low (≤ 19 points), moderate (20 ~ 42 points) and high (≥ 43 points). The retest reliability of the scale was 0.82, and Javalle’s research proved that the scale has high reliability (46). The Cronbach’s α coefficient for the scale in this study was 0.65.
The Pittsburgh sleep quality index (PSQI) is a subjective sleep quality assessment scale developed by Buysse (47) of the Pittsburgh Medical Center in 1989. It was translated into Chinese by Liu et al. in 1996 and applied in the Chinese population (48), with a Cronbach’s α coefficient of 0.84. The scale contains 7 dimensions, each dimension is scored from 0 to 3, and the cumulative score of each dimension is the total score of PSQI (0–21), and a higher score indicates poorer sleep quality. The Cronbach’s α coefficient of the PSQI was 0.83 in this study.
2.4 Data analysis
Epi Data 3.1 was used to establish database, with logic checks applied and double data entry conducted to ensure accuracy. SPSS 27.0 was used for data analysis. When analyzing DP, in order to reduce the number of variables and the variation degree of food intake among individuals, improving interpretability, according to the Chinese Residents Balanced Diet Pagoda and the relationships between food attributes and nutritional composition (49), 23 items were divided into 18 non-overlapping food groups (Supplementary Table S1). Factor analysis was used to analyze DP to reduce the dimensionality of food frequency questionnaire data and to identify underlying dietary patterns. KMO and Bartlett’s test of sphericity were used to determine whether the data was suitable for factor analysis. Main DPs were determined according to scree plot, the proportion of variance explained and the eigenvalue. According to nutritional knowledge and clinical plausibility of the retained factors number, this study selected the eigenvalue beyond the statistical cut-off value as the criteria to determine the number of retained DPs (λ > 1.4) (25, 50). To achieve a relatively simple and more interpretable factor structure, the maximum variance rotation of the initial factor load matrix is performed. Food groups with absolute factor loadings > 0.25 are generally considered to contribute meaningfully to a given factor (51); in this study, the retention standard of food group items in DP was set as 0.3 (52). DPs were then named after the combination of food groups with high factor loadings and relevant nutritional knowledge. After the DP was extracted, each participant had a corresponding DP score (factor score) for each pattern. The higher the score, the more consistent the individual’s dietary situation with the DP, and the score was then included in the logistic regression model.
Measurement data with a normal distribution were expressed in , while those with a non-normal distribution were expressed as M (P25, P75), and count data was expressed in rate (%). Independent samples t-test and one-way ANOVA were used to compare the data with normal distribution in different characteristic groups. Mann–Whitney U and Kruskal-Wallis H tests were used to compare the data with skewed distribution. Chi-square tests were used to compare categorical variables. Binary logistic regression analysis was performed to identify predictors of depression in third-trimester pregnancy, with the presence or absence of depression as the dependent variable. Independent variables included demographic and lifestyle-related factors that were statistically significant (p < 0.05) in univariate analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. p < 0.05 was considered statistically significant.
2.5 Rigour
Before the study commenced, all personnel involved in data collection received standardized training to ensure a consistent guidance explanation was used during the collection process. All variables were collected through self-administered online questionnaires completed by the participants. The questionnaire was developed using the online survey platform (Wenjuanxing platform), with restrictions to allow only one submission per device and account. All questions were set as mandatory to prevent missing data. Upon completion, the researchers checked each questionnaire for completeness and obvious errors. If any missing responses or inconsistencies were identified, participants were asked to provide corrections or complete the items immediately. After each questionnaire, there are participants’ contact information. When questionable data were identified, participants were contacted by phone for verification. After collection, two independent researchers checked and eliminated the questionnaires with the answer time < 15 min or more than 50% of the same responses in a row.
2.6 Ethics approval and consent to participate
All procedures in the study were conducted in accordance with the principles outlined in the Declaration of Helsinki. Ethical approval was obtained from both the Ethics Committee of Southern Medical University (Approval No. NFYKDX003-2023–64) and the Medical Ethics Committee of Nanfang Hospital, Southern Medical University (Approval No. NFEC-2024-493). Participants were informed of the study’s purpose, scope, ethical sensitivity, and potential benefits, assuring them that participation is voluntary. Before data analysis, participants were explicitly informed of their right to refuse participation or withdraw from the study. All participants provided written informed consent. According to the scale score, the participants who were judged for depression will be further provided with professional mental health resources by the hospital.
3 Results
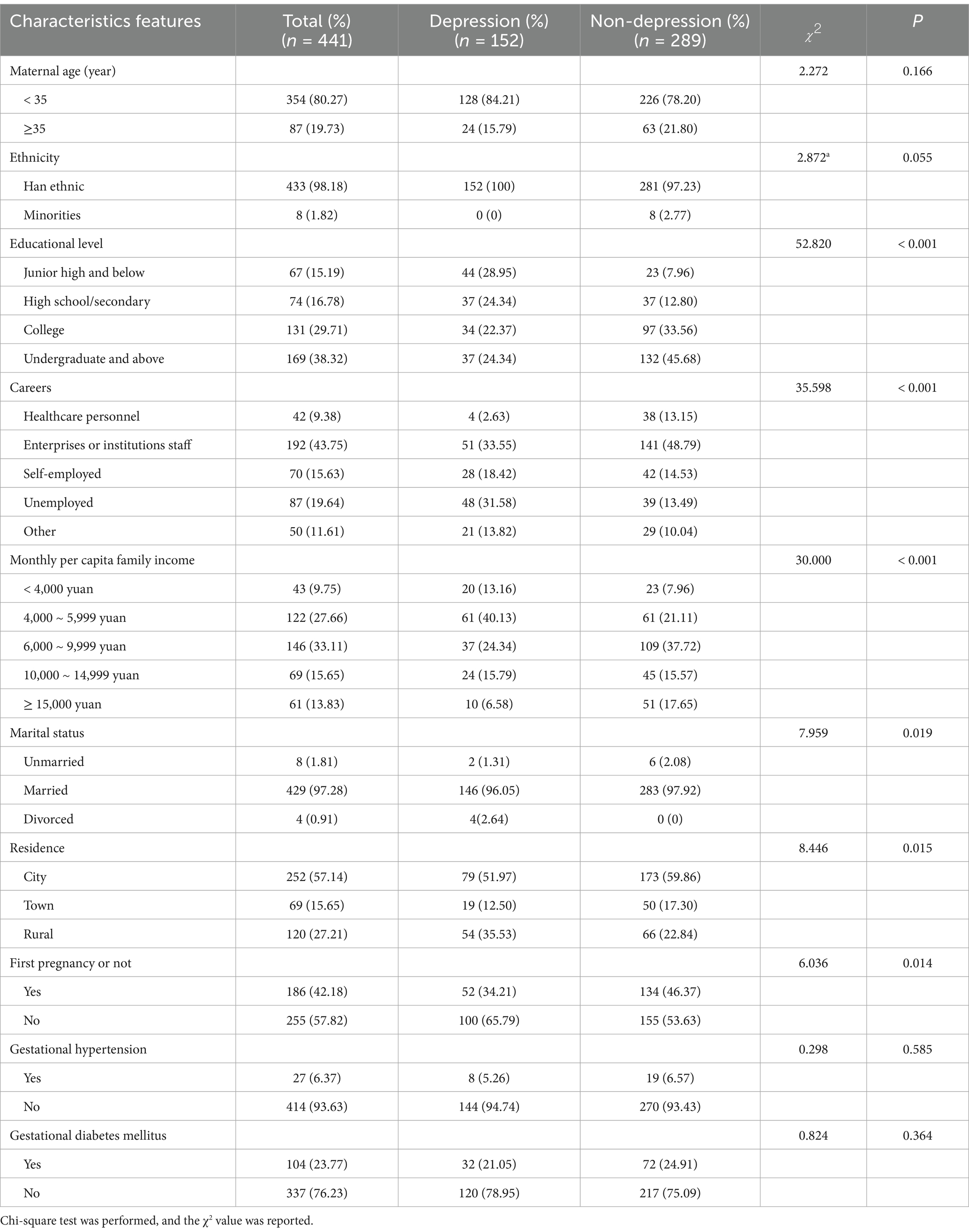
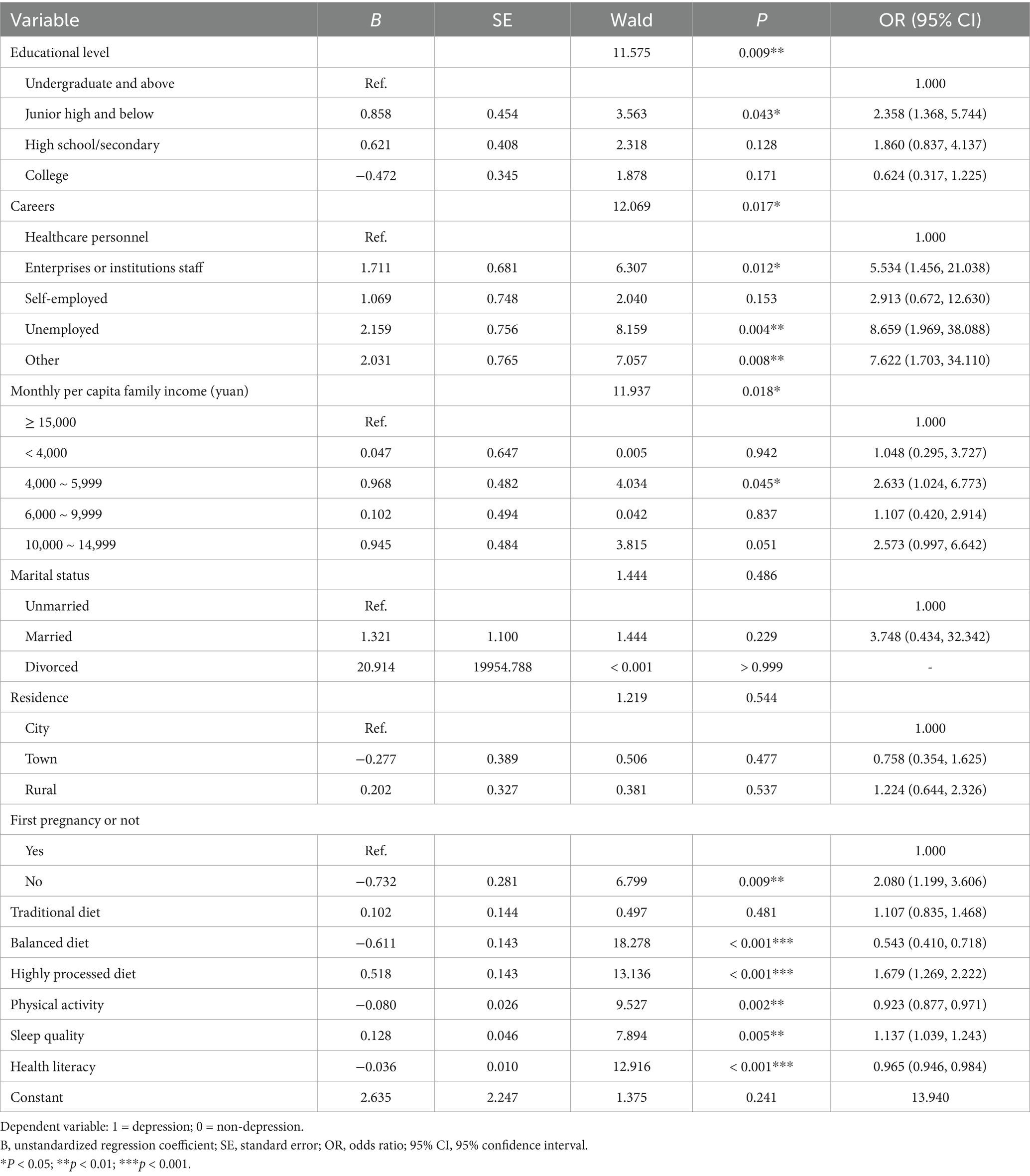
A total of 461 questionnaires were distributed, and 441 questionnaires were left after deleting missing key variables or invalid questionnaires. The effective response rate was 95.66%. Finally, 441 pregnant women were included, with an average age of 31.15 ± 4.03 years. The average pre-pregnancy BMI was 25.44 ± 1.79 kg/m2. There were 411 (93.20%) women classified as overweight and 30 (6.80%) as obesity before pregnancy. Most participants had an undergraduate educational level or above (n = 169, 38.32%), and the majority were enterprises or institutions staff (n = 192, 43.75%). The mean SDS score of pre-pregnancy OWOB pregnant women was 48.50 ± 9.48. Of the participants, 289 (65.53%) had no depression, 107 (24.26%) had mild depression, 44 (9.98%) had moderate depression, and 1 (0.23%) had severe depression. Additional demographic characteristics of the participants are presented in Table 1. Univariate analysis showed that significant differences were observed between women with and without third-trimester depression in terms of educational level, career, family income, marital status, residence, and whether it was the first pregnancy (p < 0.05).
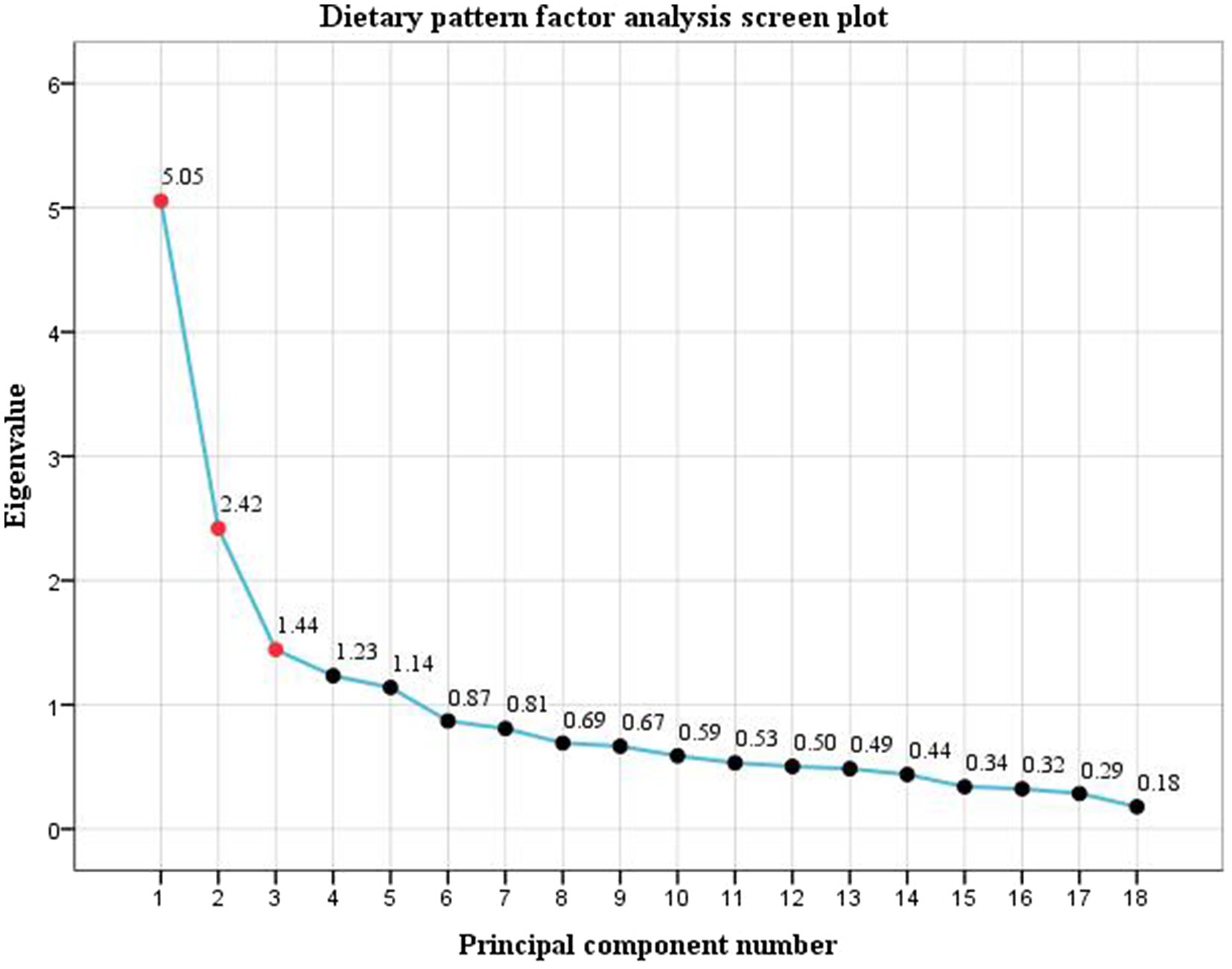
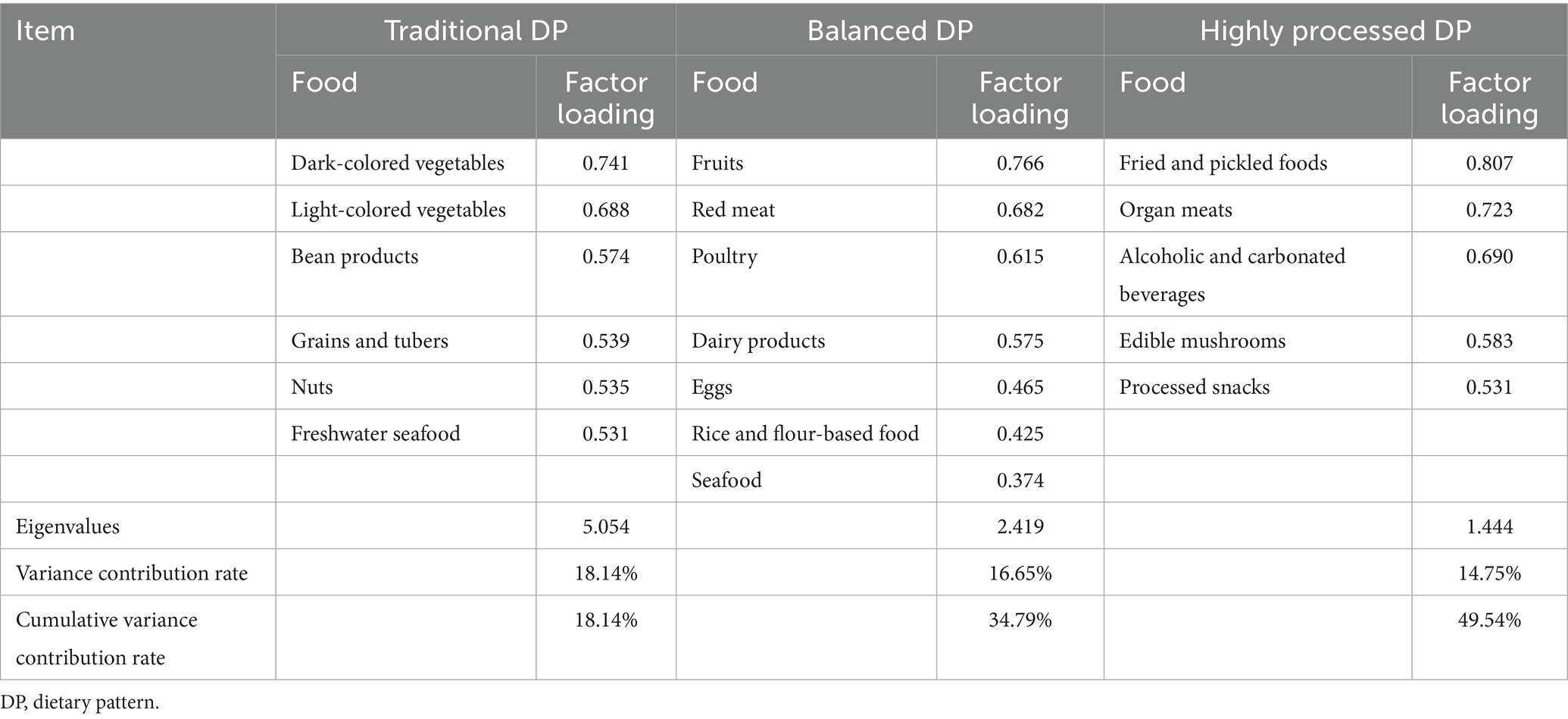
The factor analysis showed a KMO statistic of 0.793 and Bartlett’s test of sphericity χ2 = 2812.973 (p < 0.001). Three dietary patterns were extracted according to the eigenvalues, scree plot (Figure 1) and the proportion of variance explained. The cumulative variance contribution rate reached 49.54%, accounting for 18.14, 16.65, and 14.75%, respectively. The mean factor scores for each pattern among the participants were 0. The first DP is mainly composed of vegetables, beans, freshwater seafood, nuts, and grains and tubers, reflecting traditional eating habits in southern China, and was named the Traditional DP. The second DP included fruits, red meat, poultry, dairy products, eggs, rice/flour products, and seafood, representing a more nutritionally balanced composition suitable for maternal and fetal needs during pregnancy, which was named the Balanced DP. In the third pattern, the factor loads of fried and pickled foods, organ meats, and alcoholic and carbonated beverages were high, and most of them were highly processed foods, which were named highly processed DP. The food groups and factor loadings for each pattern are presented in Table 2. Spearman correlation analysis showed that pre-pregnancy BMI was negatively correlated with balanced DP (r = −0.263, p < 0.001), and positively correlated with traditional DP (r = 0.097, p = 0.043) and highly processed DP (r = 0.224, p < 0.001).
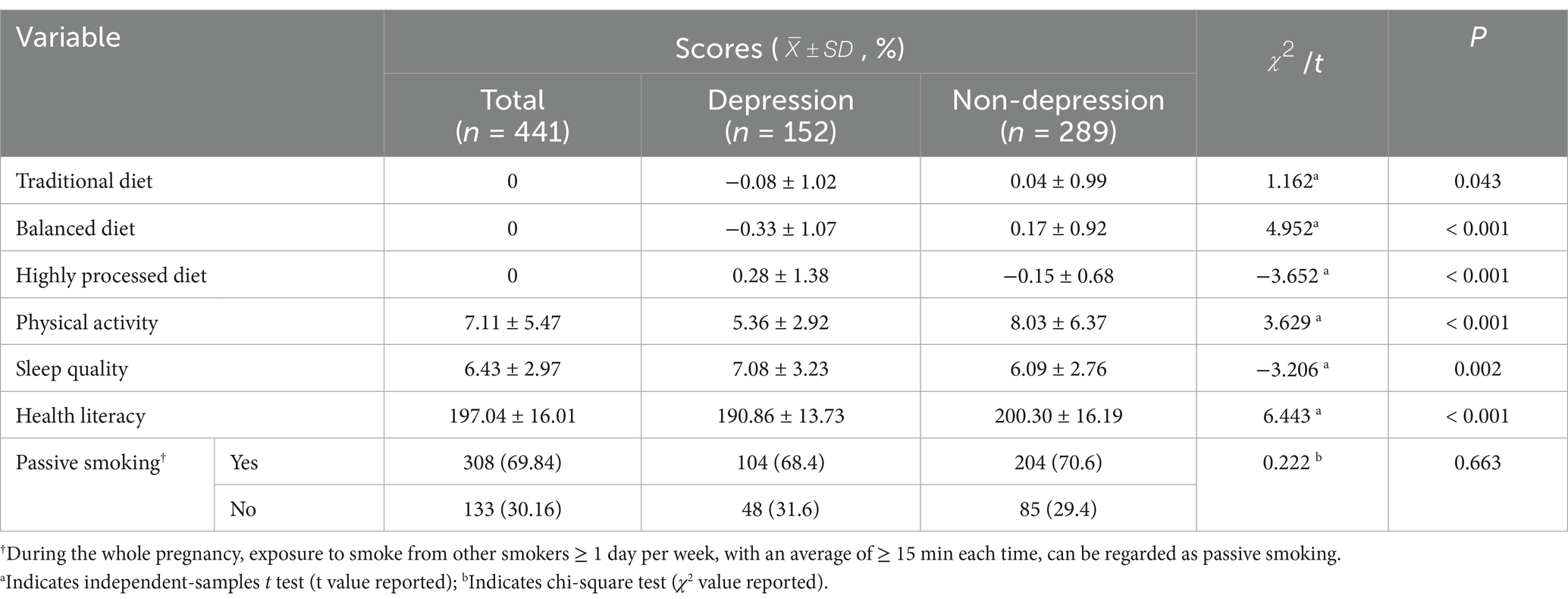
Among pre-pregnancy OWOB pregnant women, the mean PSQI score for sleep quality was 6.43 ± 2.97. The mean PARS-3 score for physical activity was 7.11 ± 5.47, and the mean score for perinatal health literacy was 197.04 ± 16.01. During the whole pregnancy, exposure to smoke from other smokers ≥ 1 day per week, with an average of ≥ 15 min each time, can be regarded as passive smoking (53). A total of 308 (69.84%) participants reported exposure to secondhand smoke during pregnancy, while 133 (30.16%) did not. Analysis showed that women with third-trimester pregnancy depression had significantly different scores in DPs, physical activity, sleep quality, and health literacy compared to those without depression, with all differences reaching statistical significance (p < 0.05). However, the presence of depression was not associated with passive smoking during pregnancy (Detailed results are presented in Table 3).
Binary logistic regression analysis was conducted using the third-trimester pregnancy depression as the dependent variable. Independent variables included demographic and lifestyle factors that were statistically significant (p < 0.05) in the univariate analysis. A forward stepwise selection method was applied. The results showed that among demographic factors, education level, career, monthly per capita family income, and whether it was the first pregnancy were influencing factors of third-trimester pregnancy depression in pre-pregnancy OWOB women. Among them, the risk of third-trimester depression for pre-pregnancy OWOB women with an educational level of junior high and below was 2.358 times that of undergraduate and above (95% CI: 1.368 ~ 5.744, p = 0.043), and the risk of depression for unemployed women was 7.622 times that of healthcare personnel women (95% CI: 1.703 ~ 34.110, p = 0.008). Regarding lifestyle behaviors, adherence to a balanced DP, higher levels of physical activity, and better health literacy were identified as protective factors, while adherence to a highly processed DP and poor sleep quality were identified as risk factors (p < 0.05). The binary logistic regression Hosmer and Lemeshow test showed p = 0.267 > 0.05 and the model was valid (Table 4).
4 Discussion
There is growing research in the field of prenatal mental health. However, pregnant women with pre-pregnancy OWOB as the vulnerable group to the mental disorder are still overlooked. This study investigated the association between demographic factors and depression in the third trimester of pregnancy among this high-risk group and comprehensively explored associations with various modifiable lifestyle factors, including dietary patterns, sleep quality, physical activity, passive smoking, and health literacy. In this study, the prevalence of third-trimester pregnancy depression among pre-pregnancy OWOB women was 34.47%, which is similar to the 30% prevalence reported by Salehi (54) for the second and third trimesters of pregnancy in the same population. Given China’s large population base and the rising proportion of women entering pregnancy with OWOB [according to the 1.13 million live births reported in Guangdong Province in 2024, the number of pre-pregnancy OWOB women suffering from depression in third-trimester pregnancy in Guangdong alone is as high as 98,000 (8)], the mental health of pre-pregnancy OWOB pregnant women should be highly valued by maternal and child health care personnel.
In this study, 93.20% of participants were overweight before pregnancy, while 6.80% were obesity. The findings predominantly reflect the situation of pre-pregnancy overweight women. In this study, educational level was one of the influencing factors of third-trimester pregnancy depression among pre-pregnancy OWOB women, consistent with findings from a retrospective study by Liu et al. on 14,329 pregnant women in southwestern China (55). Previous research has also shown that prenatal depression is more prevalent among women with lower educational (19). Our results indicated that women with an education level of junior high school and below were 2.358 times more likely to experience depression in third-trimester pregnancy compared to those with an undergraduate degree and above (95% CI: 1.368 ~ 5.744, p = 0.043). This may be explained by the link between lower educational and lower socioeconomic status, which is an important risk factor for depression (56). Furthermore, higher education levels are associated with better understanding of pregnancy- and childbirth-related health knowledge, enabling OWOB women to better access and adhere to health guidance. This may help reduce their anxiety about adverse pregnancy outcomes and, in turn, lower the risk of prenatal depression.
Binary logistic regression results revealed the association between career and third-trimester pregnancy depression among pre-pregnancy OWOB women. Compared with healthcare personnel, the risk of third-trimester pregnancy depression increased by 5.534 times (95% CI: 1.456 ~ 21.038, p = 0.012), 8.659 times (95% CI: 1.969 ~ 38.088, p = 0.004), and 7.622 times (95% CI: 1.703 ~ 34.110, p = 0.008) for pregnant women in enterprises or institutions, unemployed, and other careers, respectively, which was consistent with the results of He in 4,564 pregnant women in Beijing (57). Healthcare personnels may be able to manage emotional changes more effectively due to their strong professional knowledge and depression recognition ability. Among all career categories, unemployed women had the highest risk of depression, which aligns with previous findings (20). This may be attributed to individuals with lower socioeconomic status who often lack adequate social support, experience reduced social interactions that can lead to loneliness and low self-esteem, and face financial stress due to limited income (58). In addition, our study found that family income was also an influencing factor of third-trimester pregnancy depression. Women with income of 4,000–5,999 CNY had a 2.633 times higher risk of depression compared to those with income exceeding 15,000 CNY (95% CI: 1.024 ~ 6.773 p = 0.045). This result further supports the link between economic conditions and maternal mental health and also supported the finding that unemployed women were more vulnerable to prenatal depression.
We also found that having had a previous pregnancy was a risk factor for depression in third-trimester pregnancy among pre-pregnancy OWOB women, and the risk was 2.080 times higher than that of first-pregnancy women (95% CI: 1.199 ~ 3.606 p = 0.009). Current evidence on the relationship between the number of pregnancies and prenatal depression remains inconsistent. Studies have suggested that first pregnancy may be a risk factor for third-trimester depression (59), while Ngocho reported no significant association between the number of pregnancies and prenatal depression (60). On the other hand, Qi found that multiparous women were at greater risk for anxiety and depression than primiparous women (61). Therefore, the prediction of the first pregnancy may be affected by the characteristics of pregnant women. In this study, non-first-time pregnant women have a higher risk of depression in third-trimester pregnancy, which may be related to their more family and childcare responsibilities. Additionally, previous adverse pregnancy or birth experiences may negatively influence women’s emotional state during subsequent pregnancies (62). OWOB women are more likely to experience adverse outcomes in previous pregnancy, which may aggravate their depression level in subsequent pregnancy. This suggests that maternal healthcare providers should provide more attention and intervention measures in line with the characteristics of pre-pregnancy OWOB pregnant women according to the specific conditions of women, so as to reduce the risk of depression and ensure maternal and child health.
Although an increasing number of studies focused on how mothers cope with depression at the emotional and psychological levels (63, 64), examining the association between lifestyle behaviors and mental health during pregnancy is particular significance for pre-pregnancy OWOB pregnant women. On one hand, pregnant women are generally more motivated and receptive to adopting healthy behaviors compared to non-pregnant periods (65). On the other hand, OWOB pregnant women are often accompanied by one or more unhealthy lifestyles (66). It is important to note that the relationship between lifestyle and depression may be bidirectional: unhealthy lifestyle behaviors may increase the risk of depression, whereas depression itself may impair the ability to maintain healthy behaviors. Within this context, as a non-drug intervention, lifestyle modification is relatively easy to implement. Investigating its relationship with third-trimester depression can help maternal healthcare providers achieve earlier prevention and intervention among OWOB pregnant women in a more cost-effective, safer, and feasible manner.
This study identified DPs as significant influencing factors of depression in third-trimester pregnancy among pre-pregnancy OWOB women. This may be explained by the fact that dietary habits are key lifestyle factors contributing to excessive weight gain (67, 68), which in turn is closely associated with the risk of depression. Specifically, balanced DP was a protective factor for depression, while highly processed DP increased the risk of depression; correspondingly, we found that the balanced DP was negatively correlated with pre-pregnancy BMI, while the highly processed DP was positively correlated with pre-pregnancy BMI (p < 0.05). The balanced DP in our study was rich in calcium, vitamin B complex, and protein, which are known to support nervous system stability, reduce anxiety, and alleviate depression. Both the quality and quantity of dietary protein are important. In this study, red meat was the primary protein source in the balanced DP, supporting prior evidence linking iron status to depression (69). In contrast, the highly processed DP was rich in high-sugar, high-fat, and low-fiber foods, which can easily lead to physiological reactions closely related to depression, such as blood glucose fluctuations, insulin resistance, and chronic inflammation (70). Alcohol intake, also included in this DP, may further exacerbate depression (71). Moreover, women who favor highly processed DP may experience internal conflict—motivated to improve health but simultaneously feeling frustration or guilt over their food choices—which may intensify psychological stress and contribute to depression. With the growing adoption of mobile health technologies, maternal healthcare providers can utilize smartphone applications and digital platforms to monitor the daily diet of OWOB pregnant women (72). In addition, encouraging the involvement of family members, especially partners, in healthy eating has been shown to improve dietary adherence and enhance intervention effectiveness (73). Such strategies may help shift dietary preferences toward more nutritional balanced DP and reduce dependence on highly processed foods in this high-risk population.
Physical activity level was also found to be associated with depression in third-trimester pregnancy. Consistent with previous research, our findings support the inverse association between physical activity and prenatal depression (23). Although moderate physical activity was considered as a promising non-drug therapy to alleviate depression (74), the mean PARS-3 score among participants in this study was 7.11 ± 5.47, indicating an overall low level of physical activity. This may be attributed to the physical limitations of OWOB pregnant women (such as joint burden, poor activity endurance), which can hinder their ability to engage in high-intensity or regular exercise (75). These barriers may confine the direct mental health benefits of physical activity. Therefore, maternal healthcare providers should offer individualized activity recommendations that consider both the physical risks and the specific capacities of OWOB pregnant women (76). For example, OWOB pregnant women are less likely to engage in physical activity during pregnancy than normal-weight women (77). Low-intensity leisure activities, such as walking, may be more feasible and sustainable to increase their activity levels (78). At the same time, well-designed and adequately powered randomized trials are needed to further investigate the effects of various intensities of physical activity on the mental status of OWOB pregnant women.
Sleep disorder was identified as a risk factor for depression among pre-pregnancy OWOB pregnant women. Physiological, psychological, and lifestyle changes during pregnancy may interfere with sleep quality (79). Additionally, OWOB may also lead to obstructive sleep apnea (OSA), affecting sleep quality, and triggering or exacerbating depression through multiple pathways (24). Dominguez have noted that existing OSA screening tools may lack sensitivity in extremely obese pregnant women, and further research is needed to adjust the evaluation criteria and thresholds (80). Wearable sleep-monitoring technologies represent a promising tool for identifying sleep disorder in OWOB pregnant women, offering maternal healthcare providers more objective data to inform diagnosis and management strategies (81). In our study, health literacy was found to be negatively associated with depression, consistent with findings in other populations (82). Pregnant women with low health literacy may perceive depressive symptoms as a normal emotional response and ignore seeking help, whereas those with higher health literacy are usually better able to recognize emotional changes and respond proactively (83). Health literacy is the foundation of health-promoting behaviors. When providing lifestyle intervention guidance for the mental status of pre-pregnancy OWOB pregnant women, maternal healthcare providers should also focus on enhancing their health literacy. One effective approach is the Teach-back method, which involves encouraging pregnant women to repeat key information in their own words. This approach helps confirm understanding and strengthens their ability to follow health recommendations (84).
In our study, passive smoking among pre-pregnancy OWOB women was not statistically associated with third-trimester depression. Although many studies reported second-hand smoke exposure as a risk factor for depression, several well-conducted investigations have not found a significant link (85–87). A large-scale German study involving 28,000 never-smokers reported no significant association between second-hand smoke exposure and depression among women, and even observed an inverse relationship among men (88). Such inconsistent findings may reflect differences in exposure assessment, population or contextual factors, or residual confounding. For instance, individuals exposed to second-hand smoke may be more frequently involved in social interactions, which may be protective for the development of depression (89). These considerations may help explain why no significant association was observed in our sample. However, this does not imply that passive smoking has no effect on third-trimester depression; more precise exposure measurement or higher thresholds of exposure may be needed to identify or elicit such an effect.
This study is associated with certain limitations. First, due to the cross-sectional design, depression and demographic or lifestyle factors may not necessarily indicate a cause-effect relationship. Second, the prevalence of clinically diagnosed prenatal depression in our OWOB pregnancy population was not determined as mental status examinations and biomarker measurements were not performed. Third, the sample was unbalanced between overweight and obesity subgroups; therefore, the findings primarily reflect overweight women and should be generalized to obese women with caution. Finally, potential unmeasured confounders, such as social support, psychiatric history, and obstetric complications, may also have influenced the results and should be addressed in future research.
5 Conclusion
This study revealed that about one-third of pre-pregnancy OWOB women experienced depression in third-trimester pregnancy. It is important to pay attention to associated factors of depression in these women. In addition to demographic factors, several modifiable lifestyle behaviors—including DP, physical activity, sleep quality, and health literacy—were found to be associated with depression. These findings not only contribute to identifying high-risk individuals but also provide practical guidance for the development of subsequent interventions. Given the modifiable and cost-effective nature of lifestyle factors, maternal healthcare providers are encouraged to consider them as key entry points for addressing depression among pre-pregnancy OWOB women. By combining mental health management with lifestyle intervention, maternal and child health can be guaranteed in a more economical, safe and accessible way.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Southern Medical University (Approval No. NFYKDX003-2023–252 64) and the Medical Ethics Committee of Nanfang Hospital, Southern Medical University (Approval No. NFEC-2024-493). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZY: Methodology, Conceptualization, Software, Validation, Writing – review & editing, Writing – original draft, Visualization, Formal analysis. YZ: Supervision, Project administration, Data curation, Resources, Writing – review & editing. JC: Resources, Project administration, Supervision, Data curation, Writing – review & editing. XL: Writing – original draft, Formal analysis, Conceptualization, Software, Methodology. DL: Investigation, Supervision, Project administration, Resources, Writing – review & editing. JZ: Methodology, Supervision, Funding acquisition, Writing – original draft, Resources, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the 2025 Higher Education Reform Project of Guangdong Province (202430553) and 2025 Natural Science Foundation of Guangdong Province, Guangdong (2025A1515011703). The funding source was not involved in study design, data collection, interpretation, or writing of this study.
Acknowledgments
We appreciate Southern Medical University and Southern Medical University Nanfang Hospital for their support for this study. Thank the nurse and midwife team of the obstetric outpatient department of the hospital for their cooperation in this data collection process. We also thank to all the pregnant women who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1687185/full#supplementary-material
References
1. World Health Organization. (2025). Obesity and overweight. Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (Accessed Jul 10, 2025).
2. Xin, Z, and Huixia, Y. Influence of maternal obesity and excessive weight gain during pregnancy on perinatal outcome and long-term health in offspring: a review. Chinese J Perinat Med. (2020) 239:640–4. doi: 10.3760/cma.j.cn113903-20200114-00022
3. Obesity prevention and control branch of the Chinese nutrition society, clinical nutrition branch of Chinese nutrition society, behavioral health branch of Chinese preventive medicine association, sports and health branch of Chinese preventive medicine association. Expert consensus on obesity prevention and treatment in China. Chinese J Epidemiol. (2022) 435:609–26. Avaiable online at: https://rs.yiigle.com/cmaid/1377701
4. Pan, XF, Wang, L, and Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. (2021) 9:373–92. doi: 10.1016/s2213-8587(21)00045-0
5. Wang, Y, Zhao, L, Gao, L, Pan, A, and Xue, H. Health policy and public health implications of obesity in China. Lancet Diabetes Endocrinol. (2021) 9:446–61. doi: 10.1016/s2213-8587(21)00118-2
6. Zeng, Q, Li, N, Pan, XF, Chen, L, and Pan, A. Clinical management and treatment of obesity in China. Lancet Diabetes Endocrinol. (2021) 9:393–405. doi: 10.1016/s2213-8587(21)00047-4
7. Xiaoyan, L, Jiang Yong, H, Nan, LY, Mei, Z, and Zhengjing, H. Prevalence and characteristic of overweight and obesity among adults in China, 2010. Chinese J Preventive Medicine. (2012) 4608:683–6. doi: 10.3760/cma.j.issn.0253-9624.2012.08.003
8. Li, N, Liu, E, Guo, J, Pan, L, Li, B, Wang, P, et al. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS One. (2013) 8:e82310. doi: 10.1371/journal.pone.0082310
9. China Daily. (2025). 6th national campaign for fitness underway. Available online at: https://english.www.gov.cn/news/202504/22/content_WS68072ac6c6d0868f4e8f1f60.html (Accessed April 22, 2025).
10. Chinese Nutrition Society. (2022). Dietary guidelines for pregnant and lactating women (2022). Available online at: http://dg.cnsoc.org/article/04/hjgfxca3Ra69sKbvqDETbg.html (Accessed July 10, 2025).
11. Xinhua. (2025). China to make mental health screening part of routine hospital care. Available online at: https://english.www.gov.cn/news/202504/26/content_WS680c15c4c6d0868f4e8f214b.html (Accessed July 10, 2025).
12. Okagbue, HI, Adamu, PI, Bishop, SA, Oguntunde, PE, Opanuga, AA, and Akhmetshin, EM. Systematic review of prevalence of antepartum depression during the trimesters of pregnancy. Open Access Maced J Med Sci. (2019) 7:1555–60. doi: 10.3889/oamjms.2019.270
13. Fan, F, Chen, Y, Jin, W, Julan, X, and Wenting, L. Relationship between prenatal depressive emotion and rumination and research on attentional Bias among pregnant women in the middle or late pregnancy. Military Nursing. (2021) 381:24. doi: 10.3969/j.issn.1008-9993.2021.01.004
14. Zhang, S, Ma, X, Wei, Q, Zhang, Y, Wang, L, and Shi, H. Maternal pre-pregnancy BMI and gestational weight gain modified the association between prenatal depressive symptoms and toddler’s emotional and behavioral problems: a prospective cohort study. Nutrients. (2022) 15:151. doi: 10.3390/nu15010181
15. McEwen, BS. Hormones and behavior and the integration of brain-body science. Horm Behav. (2020) 119:104619. doi: 10.1016/j.yhbeh.2019.104619
16. Admas, WT, Teoh, AN, and Chonu, K. The effects of prenatal psychosocial work stress on adverse pregnancy outcomes: a comprehensive systematic review and meta-analysis. Scand J Work Environ Health. (2025) 51:355–69. doi: 10.5271/sjweh.4236
17. Schuette, SA, Kominiarek, MA, Wisner, KL, and Massey, SH. Pre-pregnancy body mass index and third-trimester depressive symptoms in a healthy privately insured sample. AJP reports. (2018) 8:e13–7. doi: 10.1055/s-0038-1625974
18. Ertop, F, and Cetisli, NE. Postpartum depression and breastfeeding in overweight/obese and non-obese mothers. JPMA. (2020) 702:219–24. doi: 10.5455/jpma.302642854
19. Akçalı Aslan, P, Aydın, N, Yazıcı, E, Aksoy, AN, Kirkan, TS, and Daloglu, GA. Prevalence of depressive disorders and related factors in women in the first trimester of their pregnancies in Erzurum, Turkey. Int J Soc Psychiatry. (2014) 60:809–17. doi: 10.1177/0020764014524738
20. González-Mesa, E, Kabukcuoglu, K, Körükcü, O, Blasco, M, Ibrahim, N, and Kavas, T. Cultural factors influencing antenatal depression: a cross-sectional study in a cohort of Turkish and Spanish women at the beginning of the pregnancy. J Affect Disord. (2018) 238:256–60. doi: 10.1016/j.jad.2018.06.003
21. Gomà, M, Martínez, M, Blancafort, X, Muniente, G, Antón, S, Lara, S, et al. Detection of depressive-anxiety symptomatology and associated risk factors among pregnant women in a low-income neighborhood. J Psychosom Obstet Gynaecol. (2021) 42:293–9. doi: 10.1080/0167482x.2020.1761319
22. Al-Abri, K, Edge, D, and Armitage, CJ. Prevalence and correlates of perinatal depression. Soc Psychiatry Psychiatr Epidemiol. (2023) 58:1581–90. doi: 10.1007/s00127-022-02386-9
23. Dipietro, L, Evenson, KR, Bloodgood, B, Sprow, K, Troiano, RP, Piercy, KL, et al. Benefits of physical activity during pregnancy and postpartum: an umbrella review. Med Sci Sports Exerc. (2019) 51:1292–302. doi: 10.1249/mss.0000000000001941
24. Pandi-Perumal, SR, Monti, JM, Burman, D, Karthikeyan, R, BaHammam, AS, Spence, DW, et al. Clarifying the role of sleep in depression: a narrative review. Psychiatry Res. (2020) 291:113239. doi: 10.1016/j.psychres.2020.113239
25. Chan, LC, Wang, HH, Wahlqvist, ML, Liu, CC, Liu, JY, and Lee, MS. Perinatal dietary patterns and symptomatic depression: a prospective cohort study. Matern Child Nutr. (2024) 20:e13561. doi: 10.1111/mcn.13561
26. Silva, DFO, Cobucci, RN, Gonçalves, AK, and Lima, S. Systematic review of the association between dietary patterns and perinatal anxiety and depression. BMC Pregnancy Childbirth. (2019) 19:212. doi: 10.1186/s12884-019-2367-7
27. Poeira, AF, and Zangão, MO. Construct of the association between sleep quality and perinatal depression: a literature review. Healthcare. (2022):107. doi: 10.3390/healthcare10071156
28. Ribeiro, MM, Andrade, A, and Nunes, I. Physical exercise in pregnancy: benefits, risks and prescription. J Perinat Med. (2022) 50:4–17. doi: 10.1515/jpm-2021-0315
29. Junxi, C, Jiamei, W, Qinfeng, S, Jie, Z, Le, Z, and Zhiwen, L. Association between passive smoking and depressive emotion in the first trimester among pregnant women. Chinese J Reproductive Health. (2021) 324:23. doi: 10.3969/j.issn.1671-878X.2021.04.001
30. Li, J, Zhu, Y, Zhen, D, Luo, Y, Jia, J, and Yang, Y. Relationship between maternal and infant health literacy and prenatal depression in pregnant women:the mediating role of family function. Modern Preventive Medicine. (2023) 5010:1807–12. doi: 10.20043/j.cnki.MPM.202210478
31. Anderson, T, Berry, NT, and Wideman, L. Exercise and the hypothalamic–pituitary–adrenal axis: a special focus on acute cortisol and growth hormone responses. Curr Opin Endocr Metab Res. (2019) 9:74–7. doi: 10.1016/j.coemr.2019.08.002
32. Wang, T, Han, W, Wang, C, Kang, Y, Wang, Y, Lei, S, et al. Interaction effects of sleep duration and activities of daily living on depressive symptoms among Chinese middle-aged and older adult individuals: evidence from the CHARLS. Front Public Health. (2025) 13:1547329. doi: 10.3389/fpubh.2025.1547329
33. Dębski, J, Przybyłowski, J, Skibiak, K, Czerwińska, M, Walędziak, M, and Różańska-Walędziak, A. Depression and obesity-do we know everything about it? A narrative review. Nutrients. (2024) 16:1619. doi: 10.3390/nu16193383
34. Beibei, S, Qiping, L, Liping, M, and Jing, W. Combined use of Edinburgh postpartum depression scale and postpartum depression screening scale to screen and study the depression status of high-risk pregnant women in late pregnancy. Chinese J Practical Nursing. (2018) 3426:2031–4. doi: 10.3760/cma.j.issn.1672-7088.2018.26.007
35. Starzec-Proserpio, M, Węgrzynowska, M, Sys, D, Kajdy, A, Rongies, W, and Baranowska, B. Prevalence and factors associated with postpartum pelvic girdle pain among women in Poland: a prospective, observational study. BMC Musculoskelet Disord. (2022) 23:928. doi: 10.1186/s12891-022-05864-y
36. Wang, M, Xu, PS, Liu, W, Zhang, C, Zhang, X, Wang, L, et al. Prevalence and changes of BMI categories in China and related chronic diseases: cross-sectional National Health Service Surveys (NHSSs) from 2013 to 2018. EClinicalMedicine. (2020) 26:100521. doi: 10.1016/j.eclinm.2020.100521
37. Roberto, A, Parenti, ABH, de Barros Gomes, C, Carvalhaes, M, and Parada, C. Association between sleep quality and weight gain in pregnancy: a cross-sectional study. BMC Pregnancy Childbirth. (2024):241:779. doi: 10.1186/s12884-024-06965-3
38. Zung, WW. A self-rating depression scale. Arch Gen Psychiatry. (1965) 12:63–70. doi: 10.1001/archpsyc.1965.01720310065008
39. Ren, F, Zhu, X, Liu, J, Zhai, Q, Wang, J, Gao, Y, et al. Associations of multiple risk factors with prenatal depression and anxiety: evidence from the Tianjin birth cohort (TJBC) study. J Affect Disord. (2024) 366:411–22. doi: 10.1016/j.jad.2024.08.122
40. Dunstan, DA, Scott, N, and Todd, AK. Screening for anxiety and depression: reassessing the utility of the Zung scales. BMC Psychiatry. (2017) 17:329. doi: 10.1186/s12888-017-1489-6
41. Zhang, H, Qiu, X, Zhong, C, Zhang, K, Xiao, M, Yi, N, et al. Reproducibility and relative validity of a semi-quantitative food frequency questionnaire for Chinese pregnant women. Nutr J. (2015) 14:56. doi: 10.1186/s12937-015-0044-x
42. Dan, L, Li, H, Xin, Z, Qian, Z, Jian, Z, Xiaoguang, Y, et al. Establishment and application of food frequency questionnaire method among Chinese. J Hygiene Research. (2018) 475:55. Available online at: https://pubmed.ncbi.nlm.nih.gov/30593302/
43. Qin, L. Associations of dietary habits, physical activity, and cognitive views and gestational diabetes mellitus among Cantonese women [Doctoral thesis]. Guangzhou, China: Southern Medicial University (2013).
44. Ya, W. The development and application of health literacy scale for perinatal pregnant women [Master thesis]. Chongqing, China: Chongqing Medical University (2017).
45. Deqin, L. Stress level of college students and its relationship with physical exercise. Chin Ment Health J. (1994) 1:5–6.
46. Javelle, F, Vogel, A, Laborde, S, Oberste, M, Watson, M, and Zimmer, P. Physical exercise is tied to emotion-related impulsivity: insights from correlational analyses in healthy humans. Eur J Sport Sci. (2023) 23:1010–7. doi: 10.1080/17461391.2022.2065927
47. Buysse, DJ, Reynolds, CF, Monk, TH, Berman, SR, and Kupfer, DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
48. Xianchen, L, Maoqin, T, Lei, H, Aizhen, W, Hongxin, W, Guifang, Z, et al. Reliability and validity of the Pittsburgh sleep quality index. Chinese J Psychiatry. (1996) 2:103–7.
49. Chinese Nutrition Society. (2022). Revision and analysis of Chinese residents’ balanced diet pagoda (2022). Available online at: http://dg.cnsoc.org/article/04/RMAbPdrjQ6CGWTwmo62hQg.html. (Accessed July 11, 2025).
50. Hu, FB, Rimm, EB, Stampfer, MJ, Ascherio, A, Spiegelman, D, and Willett, WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. (2000) 72:912–21. doi: 10.1093/ajcn/72.4.912
51. Zhang, J, Wang, Z, Du, W, Huang, F, Jiang, H, Bai, J, et al. Twenty-Five-Year Trends in dietary patterns among Chinese adults from 1991 to 2015. Nutrients. (2021) 13:134. doi: 10.3390/nu13041327
52. Chen, Y, Chen, G, Liang, Y, Huang, H, Cai, Y, and Ni, X. Associations of nutrient intake, dietary behaviours, and patterns with metabolic profiles and obesity measures: findings from a cross-sectional study in a southern Chinese population. Front Nutr. (2025) 12:1586106. doi: 10.3389/fnut.2025.1586106
53. World Health Organization. Guidelines for controlling and monitoring the tobacco epidemic. Geneva: World Health Organization (1998).
54. Salehi-Pourmehr, H, Dolatkhah, N, Gassab-Abdollahi, N, Farrin, N, Mojtahedi, M, and Farshbaf-Khalili, A. Screening of depression in overweight and obese pregnant women and its predictors. J Obstet Gynaecol Res. (2019) 4511:2169–77. doi: 10.1111/jog.14100
55. Liu, Y, Zhang, R, Zhang, Z, Zhou, L, Cheng, B, Liu, X, et al. Risk factors and predictive model for prenatal depression: a large retrospective study in China. J Affect Disord. (2024) 354:1–10. doi: 10.1016/j.jad.2024.02.090
56. Khan, R, Waqas, A, Mustehsan, ZH, Khan, AS, Sikander, S, Ahmad, I, et al. Predictors of prenatal depression: a cross-sectional study in rural Pakistan. Front Psych. (2021) 12:584287. doi: 10.3389/fpsyt.2021.584287
57. He, X, Wang, X, Li, G, Zhu, S, Wu, Y, Sun, X, et al. Influencing factors of depressive symptoms during pregnancy in Beijing, China. Front Psych. (2025) 16:1500034. doi: 10.3389/fpsyt.2025.1500034
58. Redinger, S, Norris, SA, Pearson, RM, Richter, L, and Rochat, T. First trimester antenatal depression and anxiety: prevalence and associated factors in an urban population in Soweto, South Africa. J Dev Orig Health Dis. (2018) 9:30–40. doi: 10.1017/s204017441700071x
59. Shujian, Q, Meifen, W, and Chaoling, W. Analysis of anxiety status and its influencing factors in pregnant women in late pregnancy. Maternal Child Health Care China. (2019) 34:4067–9. doi: 10.7620/zgfybj.j.issn.1001-4411.2019.17.60
60. Ngocho, JS, Minja, LM, Mwamba, RN, Knettel, BA, Kisigo, GA, Mmbaga, BT, et al. Prevalence and predictors of depression among women attending antenatal care in Moshi, Tanzania: a cross-sectional study. BMC Pregnancy Childbirth. (2022) 22:594. doi: 10.1186/s12884-022-04917-3
61. Hong, Q, Yun, S, and Chunyu, H. Investigation of prenatal anxiety and depression in second pregnant women and analysis of influencing factors. Medical J National Defending Forces Southwest China. (2016) 26:957–9. doi: 10.3969/j.issn.1004-0188.2016.08.051
62. Nahaee, J, Rezaie, M, Abdoli, E, Mirghafourvand, M, Ghanbari-Homaie, S, and Jafarzadeh, M. Association of childbirth experience with long-term psychological outcomes: a prospective cohort study. Reprod Health. (2024) 21:71. doi: 10.1186/s12978-024-01819-9
63. Zhang, L, Wang, L, Cui, S, Yuan, Q, Huang, C, and Zhou, X. Prenatal depression in women in the third trimester: prevalence, predictive factors, and relationship with maternal-fetal attachment. Front Public Health. (2020) 8:602005. doi: 10.3389/fpubh.2020.602005
64. Tokumitsu, K, Sugawara, N, Maruo, K, Suzuki, T, Shimoda, K, and Yasui-Furukori, N. Prevalence of perinatal depression among Japanese women: a meta-analysis. Ann General Psychiatry. (2020) 19:41. doi: 10.1186/s12991-020-00290-7
65. Kurti, AN, Redner, R, Bunn, JY, Tang, K, Nighbor, T, Lopez, AA, et al. Examining the relationship between pregnancy and quitting use of tobacco products in a U.S. national sample of women of reproductive age. Prev Med. (2018) 117:52–60. doi: 10.1016/j.ypmed.2018.08.019
66. Jia, C, Chunyan, G, Huaimei, C, and Yadong, W. Investigation of mental health status of pregnant women in late pregnancy and analysis of the influencing factors. Chinese J Woman Child Health Research. (2021) 328:1162–5. doi: 10.3969/j.issn.1673-5293.2021.08.016
67. Li, Y, Lv, MR, Wei, YJ, Sun, L, Zhang, JX, Zhang, HG, et al. Dietary patterns and depression risk: a meta-analysis. Psychiatry Res. (2017) 253:373–82. doi: 10.1016/j.psychres.2017.04.020
68. Xinhua. (2024). China unveils obesity diagnosis, treatment guidelines. Available online at: https://english.www.gov.cn/news/202410/21/content_WS67158eadc6d0868f4e8ec220.html. (Accessed Jul 11, 2025).
69. Lee, HS, Chao, HH, Huang, WT, Chen, SC, and Yang, HY. Psychiatric disorders risk in patients with iron deficiency anemia and association with iron supplementation medications: a nationwide database analysis. BMC Psychiatry. (2020) 20:216. doi: 10.1186/s12888-020-02621-0
70. Patil, R, Aswar, U, and Vyas, N. Pterostilbene alleviates cafeteria diet-induced obesity and underlying depression in adolescent male Swiss albino mice and affects insulin resistance, inflammation, HPA axis dysfunction and SIRT1 mediated leptin-ghrelin signaling. Horm Behav. (2024) 161:105504. doi: 10.1016/j.yhbeh.2024.105504
71. Qi, P, Huang, M, and Zhu, H. Association between alcohol drinking frequency and depression among adults in the United States: a cross-sectional study. BMC Psychiatry. (2024) 24:836. doi: 10.1186/s12888-024-06296-9
72. Zhou, M, Wang, L, Deng, Y, Ge, J, Zhao, S, and You, H. Effects of a Mobile health intervention based on behavioral integrated model on cognitive and behavioral changes in gestational weight management: randomized controlled trial. J Med Internet Res. (2025) 27:e55844. doi: 10.2196/55844
73. Nicklas, JM, Zera, CA, Seely, EW, Abdul-Rahim, ZS, Rudloff, ND, and Levkoff, SE. Identifying postpartum intervention approaches to prevent type 2 diabetes in women with a history of gestational diabetes. BMC Pregnancy Childbirth. (2011) 11:23. doi: 10.1186/1471-2393-11-23
74. Vargas-Terrones, M, Barakat, R, Santacruz, B, Fernandez-Buhigas, I, and Mottola, MF. Physical exercise programme during pregnancy decreases perinatal depression risk: a randomised controlled trial. Br J Sports Med. (2019) 53:348–53. doi: 10.1136/bjsports-2017-098926
75. Niemiro, GM, Rewane, A, and Algotar, AM. Exercise and fitness effect on obesity. Treasure Island: StatPearls Publishing (2025).
76. Hunter, E, Avenell, A, Maheshwari, A, Stadler, G, and Best, D. The effectiveness of weight-loss lifestyle interventions for improving fertility in women and men with overweight or obesity and infertility: a systematic review update of evidence from randomized controlled trials. Obes Rev. (2021) 22:e13325. doi: 10.1111/obr.13325
77. Bacchi, E, Bonin, C, Zanolin, ME, Zambotti, F, Livornese, D, Donà, S, et al. Physical activity patterns in Normal-weight and overweight/obese pregnant women. PLoS One. (2016) 11:e0166254. doi: 10.1371/journal.pone.0166254
78. Donofry, SD, Germeroth, LJ, Kolko Conlon, RP, Venditti, EM, and Levine, MD. Correlates of physical activity engagement among pregnant women with overweight and obesity. Women’s Health Iss. (2020) 30:393–400. doi: 10.1016/j.whi.2020.06.001
79. Ertmann, RK, Nicolaisdottir, DR, Kragstrup, J, Siersma, V, and Lutterodt, MC. Sleep complaints in early pregnancy. A cross-sectional study among women attending prenatal care in general practice. BMC Pregnancy Childbirth. (2020) 20:123. doi: 10.1186/s12884-020-2813-6
80. Dominguez, JE, Grotegut, CA, Cooter, M, Krystal, AD, and Habib, AS. Screening extremely obese pregnant women for obstructive sleep apnea. Am J Obstet Gynecol. (2018) 2196:613.e1–e10. doi: 10.1016/j.ajog.2018.09.001
81. Wirth, MD, Liu, J, Wallace, MK, McLain, AC, Turner-McGrievy, GM, Davis, JE, et al. Dietary inflammatory index and sleep quality and duration among pregnant women with overweight or obesity. Sleep. (2022):4512. doi: 10.1093/sleep/zsac241
82. Hsu, YL, Su, DH, and Kuo, SC. Health literacy and depression in women with type 2 diabetes mellitus. Clinics. (2020) 75:e1436. doi: 10.6061/clinics/2020/e1436
83. König, L, Schröder, R, Hamer, T, and Suhr, R. Depression and health literacy among adolescents and adults in Germany: findings from two representative samples. Front Psychol. (2024) 15:1494333. doi: 10.3389/fpsyg.2024.1494333
84. Cheng, GZ, Chen, A, Xin, Y, and Ni, QQ. Using the teach-back method to improve postpartum maternal-infant health among women with limited maternal health literacy: a randomized controlled study. BMC Pregnancy Childbirth. (2023) 23:13. doi: 10.1186/s12884-022-05302-w
85. Bot, M, Vink, JM, Willemsen, G, Smit, JH, Neuteboom, J, Kluft, C, et al. Exposure to secondhand smoke and depression and anxiety: a report from two studies in the Netherlands. J Psychosom Res. (2013) 75:431–6. doi: 10.1016/j.jpsychores.2013.08.016
86. Lam, E, Kvaavik, E, Hamer, M, and Batty, GD. Association of secondhand smoke exposure with mental health in men and women: cross-sectional and prospective analyses using the U.K. health and lifestyle survey. European Psychiatry. (2013) 28:276–81. doi: 10.1016/j.eurpsy.2012.04.001
87. Gim, W, Yoo, JH, Shin, JY, and Goo, AJ. Relationship between secondhand smoking with depressive symptom and suicidal ideation in Korean non-smoker adults: the Korean National Health and nutrition examination survey 2010-2012. Korean J Family Medicine. (2016) 37:97–104. doi: 10.4082/kjfm.2016.37.2.97
88. Erdsiek, F, and Brzoska, P. Association between second-hand smoke exposure and depression and its moderation by sex: findings from a nation-wide population survey in Germany. J Affect Disord. (2019) 253:102–6. doi: 10.1016/j.jad.2019.04.081
89. Allem, JP, Ayers, JW, Unger, JB, Vollinger, RE Jr, Latkin, C, Juon, HS, et al. The environment modifies the relationship between social networks and secondhand smoke exposure among Korean nonsmokers in Seoul and California. Asia Pac J Public Health. (2015) 27:Np437-47. doi: 10.1177/1010539512459750
Keywords: overweight, obesity, depression, the third trimester, lifestyle
Citation: Yao Z, Zhou Y, Chen J, Liu X, Li D and Zhai J (2025) The prevalence and associated factors of third-trimester pregnancy depression in pre-pregnancy overweight and obesity women: a cross-sectional study in Guangdong, China. Front. Public Health. 13:1687185. doi: 10.3389/fpubh.2025.1687185
Edited by:
Emanuela Bianciardi, University of Rome Tor Vergata, ItalyReviewed by:
Rossella Mattea Quinto, European University of Rome, ItalyIlaria Adulti, University of Rome Tor Vergata, Italy
Copyright © 2025 Yao, Zhou, Chen, Liu, Li and Zhai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinguo Zhai, aGVsZW5qeHpoYWlAZ21haWwuY29t
 Zheng Yao
Zheng Yao Yanli Zhou2
Yanli Zhou2 Xuantian Liu
Xuantian Liu Jinguo Zhai
Jinguo Zhai