- Department of Public Health Policy, School of Public Health, University of West Attica, Athens, Greece
Background: Obesity is a major public health issue associated with significant humanistic and economic burden. In Greece, liraglutide 3.0 mg is currently the only reimbursed pharmacotherapy for obesity, restricted to patients with morbid obesity and selected comorbidities. Semaglutide 2.4 mg has demonstrated superior clinical efficacy in the STEP-8 clinical trial; however, its cost-effectiveness relative to liraglutide requires further investigation to ensure informed reimbursement decision-making.
Methods: A state-transition model was developed in Microsoft Excel to evaluate the long-term cost-effectiveness of semaglutide 2.4 mg compared with liraglutide 3.0 mg in adults with obesity (BMI ≥ 35 kg/m2 and ≥ one weight-related comorbidity). Clinical efficacy and safety inputs were derived from the STEP 8 trial, while cost inputs (expressed in 2025 euros) and utility values were obtained from the literature and published local sources. The analysis was conducted over a 40-year time horizon, with both costs and outcomes discounted at an annual rate of 3.5%. Health outcomes were reported as life-years (LYs) and quality-adjusted life-years (QALYs). The evaluation was conducted from the perspective of the Greek third-party payer, and deterministic, scenario, and probabilistic sensitivity analyses were performed.
Results: Semaglutide 2.4 mg was associated with an incremental mean increase in quality-adjusted life expectancy of 0.09 at modestly incremental higher costs of 1,083 compared with liraglutide 3.0 mg, yielding an incremental cost-effectiveness ratio (ICER) of €12,724 per QALY gained, below the willingness-to-pay threshold of €27,117. Probabilistic sensitivity analysis showed semaglutide dominated liraglutide in 80.8% of simulations (greater QALYs and lower costs) and reached 100% probability of cost-effectiveness at a willingness-to-pay threshold of €9,000 per QALY. Deterministic and scenario analysis identified treatment duration, time horizon, discount rates, and diabetes-related complication costs as key drivers of ICER variability.
Conclusions: Semaglutide 2.4 mg is likely to be a cost-effective treatment option compared to liraglutide 3 mg for patients with a BMI ≥ 35 and at least one weight-related comorbidity in Greece.
1 Introduction
Obesity is a major global public health issue, defined as excessive adiposity that adversely affects health and is typically evaluated using body mass index (BMI ≥30 kg/m2) (1). Its prevalence has increased sharply since 1990, with an estimated 890 million adults affected worldwide in 2022 and projections indicating that more than 1.2 billion will be living with obesity by 2030 (2). In Greece, 27.98% of adults—approximately 2.5 million individuals—were classified as obese in 2022, placing the country among the highest in Europe (3).
Obesity increases the risk of chronic conditions and is associated with reduced life expectancy, increased all-cause mortality, and diminished health-related quality of life (HRQoL) (4–6). Globally, high BMI accounts for more than 5 million premature deaths and approximately 9% of all disability-adjusted life years (DALYs) annually (7, 8). Beyond its health implications, obesity imposes a considerable economic burden, with its global cost projected to reach USD 2.47 trillion by 2025 (9). In Greece, the total cost of adult obesity was estimated at EUR 4.92 billion in 2024, equivalent to 2.07% of GDP (10).
Lifestyle modification, including diet, physical activity, and behavioral therapy, is the cornerstone of obesity management (11). However, lifestyle interventions alone generally result in modest and challenging-to-maintain weight loss due to physiological mechanisms that favor weight regain (12, 13). Pharmacotherapy, therefore, represents an important adjunct for individuals unable to achieve or maintain sufficient weight reduction. Among currently available agents, glucagon-like peptide-1 (GLP-1) receptor agonists—liraglutide and semaglutide—have shown significant efficacy in reducing weight, improving glycemic control, and lowering cardiometabolic risk factors in the SCALE and STEP clinical programs (14–19).
In Greece, liraglutide 3.0 mg is currently the only reimbursed pharmacotherapy for obesity. Its reimbursement is restricted to adults aged 18–74 years with BMI >40 kg/m2 and established CVD or obstructive sleep apnea and requires prior authorization from the National Organization for the Provision of Health Services (EOPYY) (20). Semaglutide 2.4 mg, given its clinical efficacy profile and broad eligible population, is anticipated to generate substantial uptake if reimbursed. Therefore, assessing its cost-effectiveness relative to liraglutide is crucial to inform reimbursement decisions, support HTA evaluations, and optimize the allocation of scarce healthcare resources.
International cost-effectiveness studies consistently show semaglutide 2.4 mg to be cost-effective for chronic weight management. In the UK, a NICE-aligned analysis projected an ICER of £14,827/QALY vs. diet and exercise, with a 90% probability of cost-effectiveness at a £20,000/QALY threshold (21). In Portugal, semaglutide yielded an ICER of €13,459/QALY, with all subgroup ICERs below the conventional threshold of €20,000 (22). In Canada, semaglutide dominated orlistat, naltrexone–bupropion, and liraglutide, with ICERs of CAD 29,014–31,243/QALY vs. standard care from a societal perspective (23). In the US, semaglutide 2.4 mg was cost-effective compared with lifestyle intervention and other branded anti-obesity medications, including liraglutide, with QALY gains of 0.138–0.925 over 30 years and ICERs well below the USD 150,000 threshold (24). Systematic reviews reinforce these findings, whereas more recently, evaluations in patients with obesity and established CVD also found semaglutide cost-effective compared with standard care in the US, Australia, and Canada (25–30).
However, the transferability of existing international findings to the Greek setting remains limited. Most published analyses rely on foreign unit costs and health system structures, while differing in assumptions regarding treatment duration, adherence, discontinuation, and post-treatment weight regain. In Greece, economic evidence is currently limited to a short-term cost-effectiveness analysis, which demonstrates favorable results for semaglutide vs. liraglutide over a 68-week horizon (31), without capturing long-term outcomes. Similarly, a recent evaluation from Egypt confirmed the short-term cost-effectiveness of semaglutide compared with liraglutide, further reinforcing its economic value across diverse healthcare contexts (32).
The objective of this study is therefore to evaluate the long-term cost-effectiveness of semaglutide 2.4 mg compared with liraglutide 3.0 mg for adults with obesity (BMI ≥ 35 kg/m2 and ≥ one weight-related comorbidity) in Greece, from the perspective of the third-party payer (EOPYY).
2 Materials and methods
2.1 Model structure and description
A Markov state-transition model was developed in Microsoft Excel (Microsoft Corp, Redmond, WA, USA) to estimate the long-term cost-effectiveness of semaglutide 2.4 mg compared with liraglutide 3.0 mg for the treatment of adults in Greece with a BMI greater than 35 kg/m2 and at least one weight-related comorbidity. Liraglutide 3.0 mg was selected as the comparator because it is the only reimbursed obesity pharmacotherapy in Greece, while other anti-obesity agents, such as naltrexone–bupropion and tirzepatide, were excluded as they are paid for exclusively out-of-pocket.
Unlike more complex multi-biomarker frameworks such as the Core Obesity Model (COM) (21–24), the present model adopted a parsimonious structure in which the BMI served as the sole surrogate risk factor. Changes in BMI under treatment or natural progression were dynamically translated into the incidence of obesity-related complications using published risk equations or transition probabilities. This simplified approach was chosen to maximize transparency, reproducibility, and adaptability to Greek decision-making while still capturing the principal health and economic consequences of obesity. It is critical to note that while multi-biomarker models, such as the COM, simultaneously capture changes in additional surrogate endpoints, including blood pressure, lipids, and glycemic control, this analysis employed BMI as the sole surrogate risk factor for three reasons. First, it is a well-established and consistently the strongest and most validated predictor of obesity-related outcomes, including type 2 diabetes, cardiovascular disease, and mortality (33, 34). Second, BMI is systematically collected in both clinical trials and population-based epidemiological studies, and is the only measure for which robust, representative, and longitudinal data are consistently available in the Greek setting (35, 36). This availability allows the model to be parameterised and externally validated with local data, thereby enhancing its credibility and relevance for Greek decision-makers. Third, several previously published cost-effectiveness studies of obesity pharmacotherapies have successfully applied BMI-only frameworks, producing reliable results that are broadly consistent with multi-biomarker models and policy-relevant in high-income settings (37–40). These precedents demonstrate that a BMI-driven structure is a methodologically accepted and pragmatic approach for long-term decision modeling. Nonetheless, as discussed later, future research should validate the findings of the present single-biomarker model against multi-biomarker frameworks to confirm robustness under alternative structural assumptions.
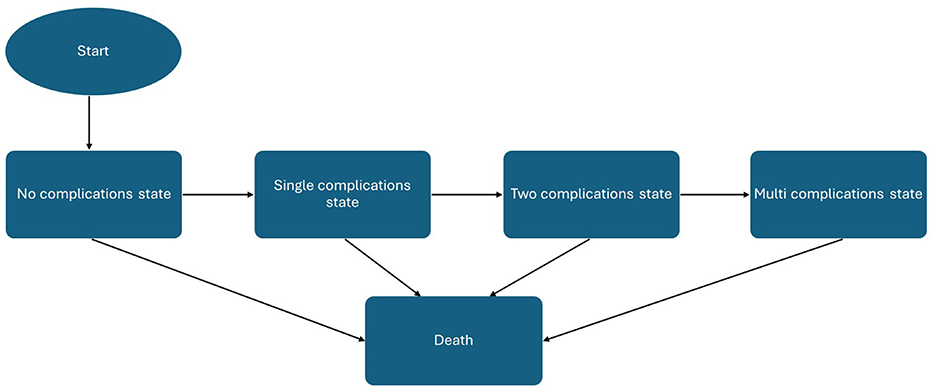
The model followed a hypothetical cohort of 1,000 adults whose baseline characteristics were aligned with those of the STEP 8 clinical trial population (41), supplemented with clinical expert opinion where local adjustment was necessary. Health states included no complication, single complications (acute coronary syndrome, type 2 diabetes, hypertension, dyslipidemia, asthma, chronic kidney disease, and sleep apnea), combined complications, and death. All patients enter the model in the “no complications” state, characterized by their baseline BMI, demographic characteristics, and utility value. During each cycle, individuals could transition from the non-complication state to one of the single-complication states, representing the first onset of type 2 diabetes, acute coronary syndrome (myocardial infarction or unstable angina), hypertension, dyslipidemia, asthma, sleep apnea, or chronic kidney disease. From there, patients could progress into multi-morbidity states, defined as the coexistence of two or three of these comorbidities, thereby reflecting the clustering of obesity-related diseases observed in real-world populations. All health states were linked to an absorbing death state, allowing transitions from any condition to mortality according to age-, sex-, and risk-adjusted probabilities.
The model was run over a 40-year time horizon to approximate a lifetime perspective, with 3-month cycles in the first year (to capture treatment response and discontinuation) and annual cycles thereafter. A half-cycle correction was applied to reflect the mid-cycle timing of events. Both interventions were assumed to be administered for a maximum of 2 years, with a non-responder stopping rule at 12 weeks (failure to achieve ≥5% weight loss from baseline) applied in line with STEP 8 (41). Following discontinuation, patients reverted to the diet-and-exercise trajectory, and treatment benefits diminished progressively until values returned to baseline (21–24).
Outcomes were expressed in terms of costs, life-years (LYs), quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs). Costs and outcomes were discounted at an annual rate of 3.5%, consistent with prior published Greek cost-effectiveness studies (42–46), as no official national HTA guideline currently specifies a discounting rate. Cost-effectiveness was assessed against a willingness-to-pay (WTP) threshold of €27,117 per QALY gained, with an additional threshold of €34,000 per QALY gained also examined, reflecting the only two published estimates available for Greece (47, 48). A schematic of the model structure is presented in Figure 1. The analysis was conducted in line with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 guidelines (49).
2.2 Cohort of patients
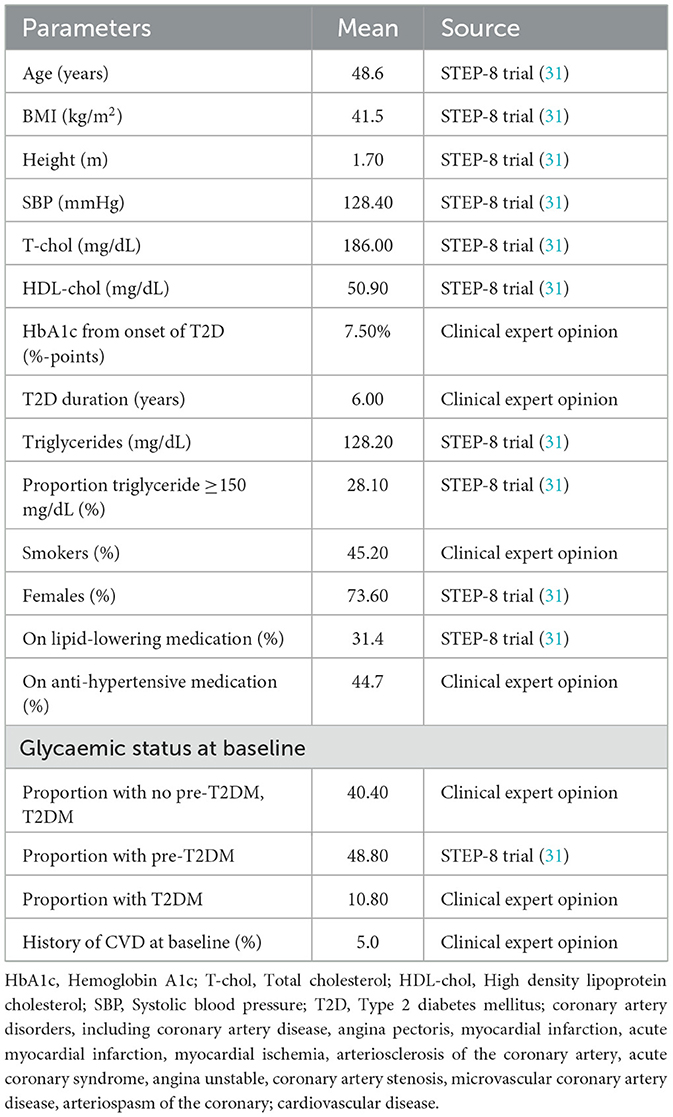
The modeled population reflects individuals eligible for obesity pharmacotherapy under the criteria of the Greek National Organization for the Provision of Health Services (EOPYY) (20), specifically adults with a BMI greater than 40 kg/m2 and at least one weight-related comorbidity. Baseline characteristics were sourced from the STEP-8 trial, the only randomized controlled trial directly comparing semaglutide 2.4 mg with liraglutide 3.0 mg in obesity, thereby providing robust and internally consistent data for this head-to-head evaluation. Recognizing that the STEP-8 population may not fully represent the Greek obesity population, trial-based characteristics were reviewed and adapted using local epidemiological data and expert clinical opinion (e.g., smoking prevalence, hypertension, and diabetes distribution) to enhance relevance for the Greek setting. This approach ensured internal validity while improving external applicability, though it is acknowledged that comprehensive local baseline data remain limited.
The simulated cohort had a mean age of 48.6 years, a mean BMI of 41.5 kg/m2, and was predominantly female (73.6%). Cardiometabolic risk factors included a mean systolic blood pressure of 128.4 mmHg, a mean total cholesterol level of 186.0 mg/dL, a mean HDL-cholesterol level of 50.9 mg/dL, and a mean triglyceride level of 128.2 mg/dL. Smoking prevalence was assumed at 45.2%, while 31.4% and 44.7% of patients were on lipid-lowering and anti-hypertensive therapy, respectively. A complete list of baseline cohort characteristics is provided in Table 1.
2.3 Clinical efficacy and safety
Treatment efficacy for semaglutide 2.4 mg and liraglutide 3.0 mg was represented by their effect on BMI, with clinical inputs derived from the STEP-8 trial (full analysis set) (41) (Supplementary Table 1). A maximum treatment duration of 2 years was assumed, with efficacy estimates based on an intention-to-treat analysis using the treatment-policy estimand, thereby capturing population-level effects irrespective of adherence or treatment modifications (41). A non-responder stopping rule was applied at 12 weeks, defined as failure to achieve ≥5% weight loss from baseline. Non-responder rates were 24.6% for semaglutide and 38.6% for liraglutide. These patients were assumed to continue with diet and exercise alone, with efficacy values informed by the corresponding arm of STEP-8 (41).
Following treatment discontinuation (after 2 years, or earlier due to non-response or adverse events), treatment effects were assumed to decline according to a catch-up rate until BMI returned to baseline. Thereafter, BMI was assumed to increase at a natural rate of 0.47 kg/year, based on longitudinal data from the UK General Practice Research Database reported by Ara et al. (50). This approach has been widely adopted in prior obesity modeling studies and in economic evaluations of semaglutide and other pharmacotherapies (21–24). To address uncertainty around post-treatment weight trajectories, additional scenarios were tested, including accelerated weight regain to baseline within 1 year and immediate reversion to natural progression without residual diet-and-exercise benefit.
Safety inputs were also derived from STEP-8 (41). Treatment-related adverse events (AEs) captured in the model included gastrointestinal events (e.g., nausea, vomiting, diarrhea) and non-severe hypoglycaemia. These were applied during active treatment, with discontinuations due to AEs transitioning patients onto the diet-and- exercise pathway.
2.4 Transition probabilities
Consistent with previous obesity modeling studies (21–24), transition probabilities between health states and the incidence of obesity-related complications were derived from published risk equations that link changes in BMI to the risk of developing comorbidities. Specifically, the incidence of T2D was estimated using the QDiabetes risk prediction algorithm, a validated tool based on large UK primary care cohorts (51). The risk of ACS was estimated using the QRisk3 equation, which integrates demographic and clinical risk factors (52). For individuals with T2D, the probability of recurrent coronary events was derived from the Framingham Recurrent CHD equations (53). The incidence of dyslipidemia was estimated from the PERSINA Guilan Cohort study (54), while the risk of hypertension was predicted using longitudinal data from the Johns Hopkins Precursors Study (55). The probability of asthma onset was informed by analyses from the National Health and Nutrition Examination Survey (56). The incidence of sleep apnea was estimated using pooled estimates from a systematic review and meta-analysis (57). Finally, the risk of chronic kidney disease (CKD) was informed by prospective analyses of a primary care cohort of 1.4 million adults in England (58).
2.5 Mortality
Obesity is associated with increased risk of premature mortality, particularly through complications such as T2D and CVD (59). Age- and sex-specific all-cause mortality rates for the general population were obtained from the WHO life tables for Greece (60). We followed the approach adopted by Miguel et al. (22) to avoid double counting of mortality attributable to obesity-related complications. Consequently, all-cause mortality was adjusted by subtracting deaths due to specific modeled diseases. The resulting disease-specific mortality was then modified using hazard ratios for BMI, derived from an extensive cohort study based on the UK Clinical Practice Research Datalink (CPRD) (61). These HRs capture the residual mortality risk associated with higher BMI levels that are not otherwise explained by the explicitly modeled comorbidities (22). Full parameter values for BMI-related hazard ratios and mortality adjustments are reported in Supplementary Table 2.
2.6 Utilities
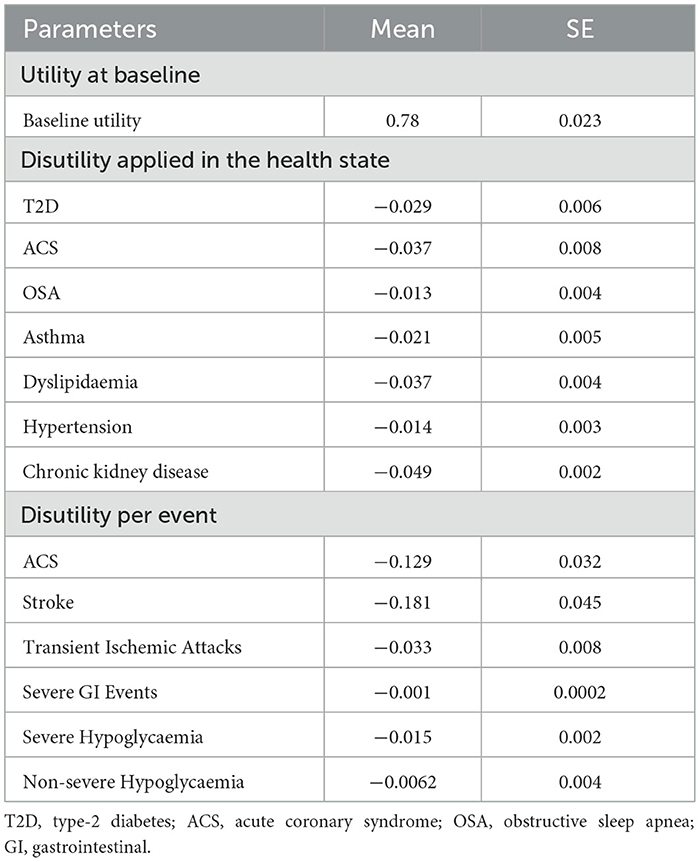
Baseline HRQoL was estimated using a regression-based function of BMI, derived from baseline data in the STEP 8 trial, an approach also adopted in previous cost-effectiveness studies (21–24). Utility scores were regressed against baseline BMI, controlling for age, sex, coronary artery disease, prediabetes, hypertension, and smoking status. This generated a baseline, complication-free utility value that varied with BMI across cycles, consistent with prior obesity economic models (21–24). The explicit regression equation applied in the model was: “Baseline Utility = 0.942975 – 0.0005414 *Age – 0.0742818 *HeartCirc – 0.0097531 *Hypertension + 0.0039044 *Smoke_Current – 0.0081972 * Smoke_Previous + 0.0065954 *BMI – 0.0002476 * BMI2 + 0.00000175 * BMI3 – 0.0031133 * Prediabetes” (Supplementary Table 3).
Utilities were dynamically updated in each cycle to reflect both BMI trajectories and the onset of comorbidities. As individuals transitioned into health states corresponding to obesity-related complications, condition-specific disutilities were applied additively to the baseline utility. Disutilities for chronic comorbidities were obtained from published literature (62–66). For acute events such as stroke or transient ischemic attack, temporary disutilities were applied in the cycle of occurrence. Treatment-related adverse events, primarily gastrointestinal symptoms and non-severe hypoglycaemia, were also incorporated as short-duration disutilities during active therapy, consistent with prior studies (21–24). The baseline utilities and disutilities applied in the model are reported in Table 2.
2.7 Costs
The analysis was conducted from the perspective of the Greek third-party payer (EOPYY) and therefore included only direct medical costs. Indirect costs, such as productivity losses, were excluded from the analysis. Confidential, mandatory or voluntary discounts were not applied as such data are confidential and not readily accessible.
Drug acquisition costs for semaglutide 2.4 mg and liraglutide 3.0 mg were obtained from the most recent official price bulletin (67). Payer costs were derived by applying the statutory 25% co-payment to retail prices, consistent with EOPYY reimbursement rules. The annual cost of obesity monitoring comprised three general practitioner visits, four visits to an obesity or surgical specialist, two visits to a dietitian/nutritionist, and three complete blood and urine panels per patient per year. Unit costs for visits and diagnostic tests were extracted from the Government Gazette and the EOPYY reimbursement schedule (68, 69). Costs associated with the treatment of obesity-related complications and with adverse events were sourced from published Greek literature and national unit cost databases. Acute event costs—including hospitalizations for acute coronary syndrome, stroke, or hypoglycemia—were retrieved from publicly available Diagnosis-Related Groups (DRGs) (70). All costs were expressed in 2025 euros. Where necessary, earlier cost estimates were adjusted to 2025 values using the Consumer Price Index (CPI) published by the Hellenic Statistical Authority (ELSTAT) (71). Table 3 illustrates the cost inputs used in the present analysis.
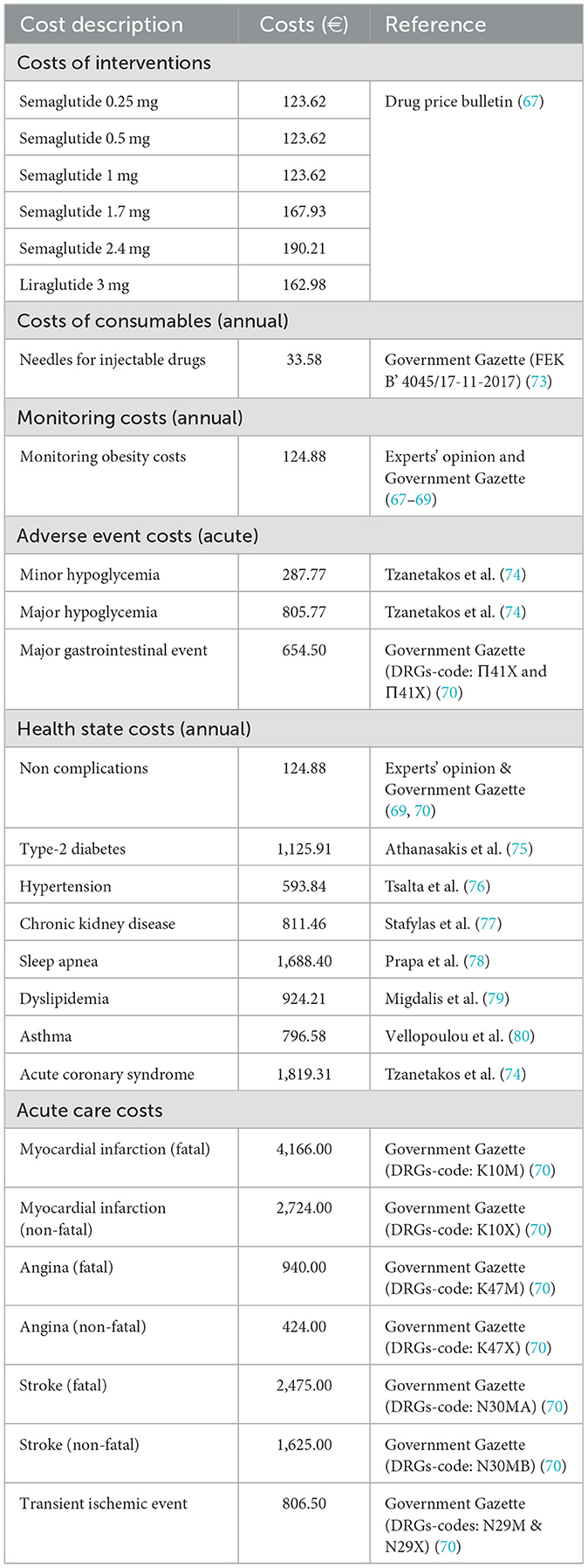
Table 3. Health state, event, drug acquisition and consumable costs used in the analysis, expressed in 2025 Euros (€).
2.8 Sensitivity analyses
Deterministic sensitivity analyses (DSA) were conducted to assess the impact of parameter uncertainty on model outcomes by varying one parameter at a time while holding all others constant. Key clinical and economic inputs—including drug acquisition costs, complication costs, utility values, discount rate, and assumptions regarding post-treatment weight regain—were varied. When empirical measures of parameter uncertainty (e.g., standard errors or confidence intervals) were not available from published sources, a conservative range of ±25% was applied around the base-case value. This approach follows established practice in cost-effectiveness modeling and aligns with previous economic evaluations in obesity (21–24). The eleven most influential parameters were identified and ranked according to their effect on the incremental cost-effectiveness ratio (ICER), with results presented in a tornado diagram.
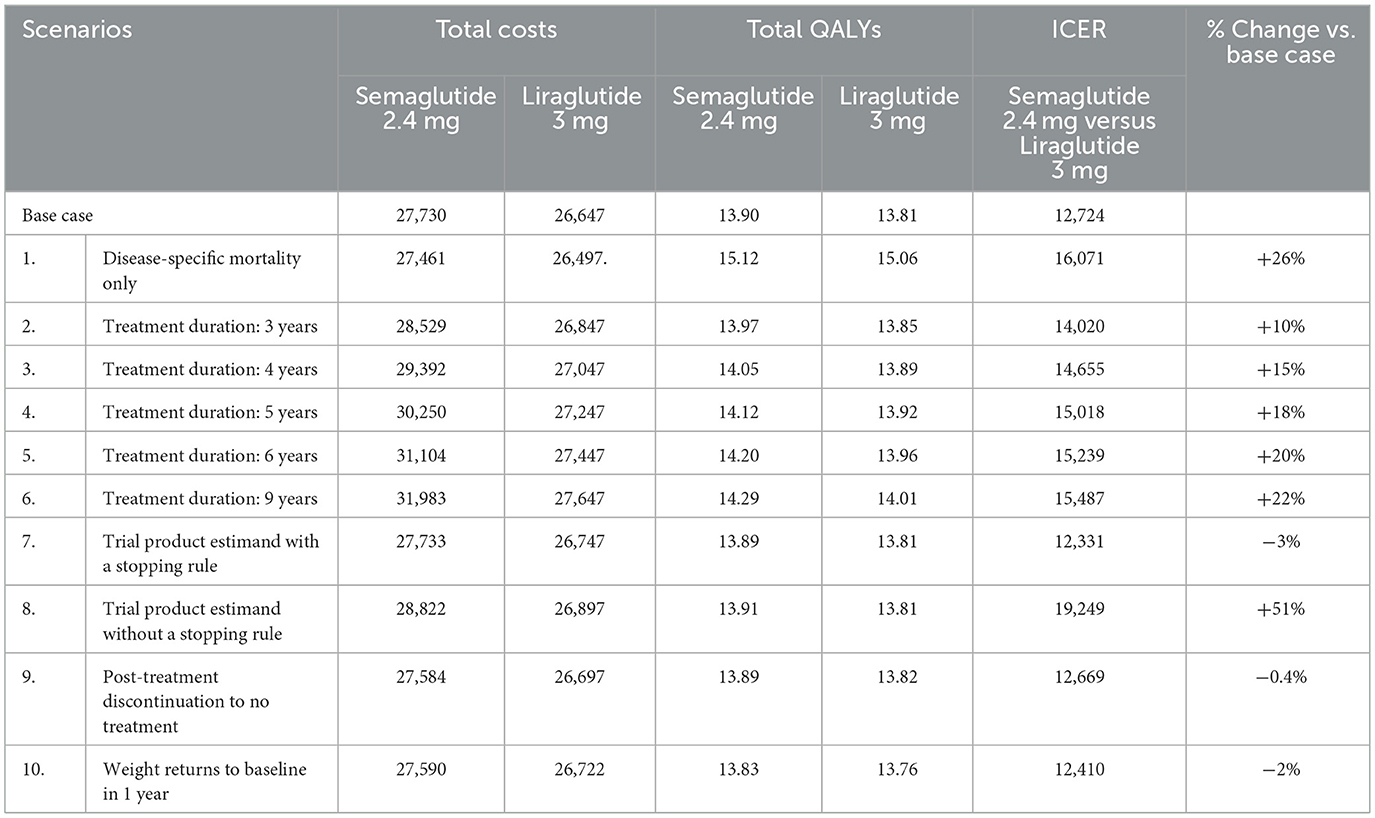
A series of scenario analyses was conducted to explore the impact of alternative input parameters and structural assumptions on cost-effectiveness outcomes. These included: restricting to disease-specific mortality only, extending treatment duration beyond the base case to 3, 4, 5, 6, and 9 years; applying alternative efficacy estimands, namely the trial product estimand (with and without treatment discontinuation for semaglutide 2.4 mg); and modifying post-treatment trajectories, with scenarios assuming reversion to natural progression without the benefit of diet and exercise, as well as accelerated catch-up with treatment effects fading completely within 1 year.
Probabilistic sensitivity analysis (PSA) was undertaken to explore joint parameter uncertainty and estimate the probability that semaglutide 2.4 mg is cost-effective compared with liraglutide 3.0 mg. A Monte Carlo simulation with 1,000 iterations was implemented, drawing inputs from predefined probability distributions consistent with recommended practice, namely Gamma for costs, Beta for probabilities and utilities, and Normal or Lognormal for relative risks and treatment effects (72). Standard errors were taken from original data sources when reported; in their absence, a standard error of 20% of the point estimate was assumed.
PSA outcomes were summarized on a cost-effectiveness plane and through a cost-effectiveness acceptability curve (CEAC). Results were reported as mean costs, QALYs, and ICERs across simulations, with 95% confidence intervals derived using the percentile method.
3 Results
3.1 Base case analysis
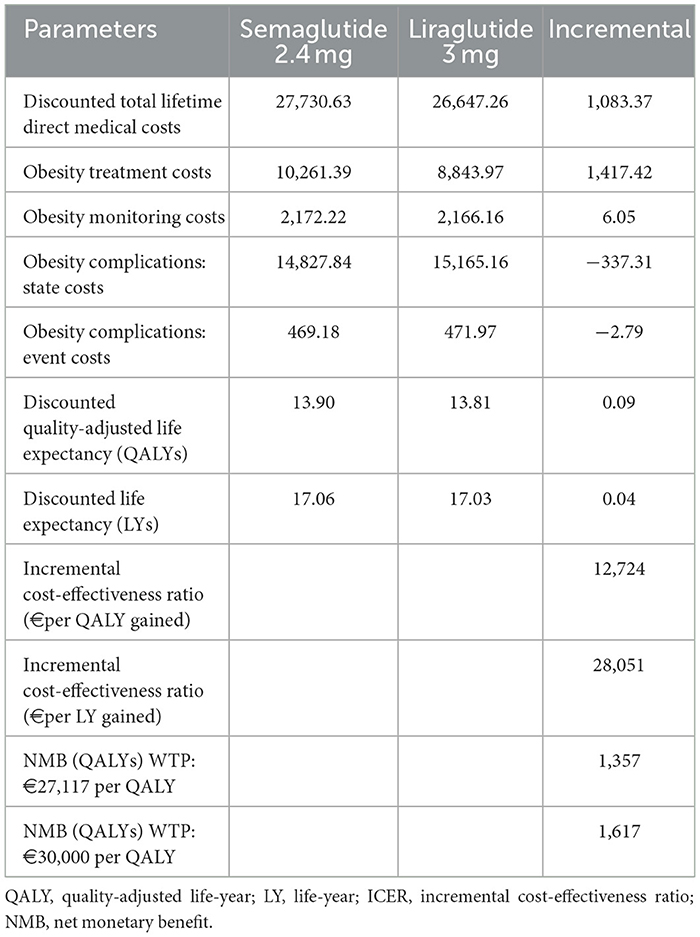
Total discounted lifetime costs, life-years (LYs), quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs) are presented in Table 4. Over a 40-year horizon, semaglutide 2.4 mg was associated with higher total direct medical costs than liraglutide 3.0 mg (€27,731 vs. €26,647; incremental €1,083). This difference was primarily attributable to higher drug acquisition costs in the semaglutide arm (€10,261 vs. €8,844; +€1,417). Monitoring costs were nearly identical between groups (€2,172 vs. €2,166). Significantly, these additional treatment costs were partially offset by reductions in downstream complications. Compared with liraglutide, semaglutide reduced the costs of obesity-related disease states (€14,828 vs. €15,165; –€337) and event-related complications (€469 vs. €472; –€3).
Health outcomes favored semaglutide, which provided an additional 0.09 QALYs (13.90 vs. 13.81) and 0.04 LYs (17.06 vs. 17.03). The resulting ICERs were €12,724 per QALY gained and €28,051 per LY gained. At the WTP threshold of €27,117 per QALY, semaglutide yielded a positive net monetary benefit (NMB) of €1,357. When applying a threshold of €34,000 per QALY, as proposed by recent research for non-oncology interventions in Greece (47), the NMB increased to €1,616.
3.2 Scenario analysis
Scenario analyses (Table 5) confirmed the robustness of the base case findings. Extending treatment duration from 3 to 9 years resulted in ICERs ranging from €14,020 to €15,487 per QALY. The application of the trial product estimand with a 12-week stopping rule resulted in a slight improvement in cost-effectiveness (ICER: €12,331/QALY), whereas removing the non-responder stopping rule increased the ICER to €19,249/QALY. Post-treatment assumptions had a limited impact on the cost-effectiveness results, with reversion to no-treatment progression resulting in an ICER of €12,669/QALY, while accelerated weight regain to baseline within 1 year yielded an ICER of €12,410/QALY. Finally, restricting the model to disease-specific mortality (excluding BMI-dependent excess mortality) increased the ICER to €16,071/QALY. Overall, semaglutide remained cost-effective vs. liraglutide across all structural and clinical assumptions, reinforcing the robustness of the base case findings.
3.3 Sensitivity analysis
The results of the DSA are presented in Figure 2 in the form of a tornado graph illustrating the 11 most influential parameters impacting the base-case ICER. It is worth noting that in all DSA scenarios, the resulting ICER remained below the WTP threshold of €27,117/QALY.
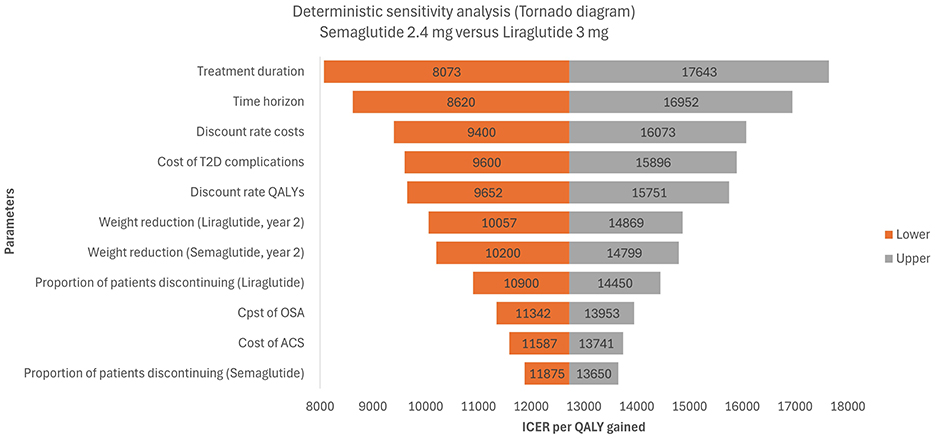
Figure 2. Tornado diagram based on a deterministic-sensitivity analysis for semaglutide 2.4 mg vs. liraglutide 3 mg. T2D, type-2 diabetes; QALY, quality-adjusted life year; OSA, obstructive sleep apnea; ACS, acute coronary syndrome; ICER, incremental cost-effectiveness ratio.
The parameters with the most significant influence on cost-effectiveness were treatment duration, time horizon, discount rates, and the cost of T2D complications. Extending treatment duration or shortening the time horizon increased the ICER (up to €17,643). Conversely, reducing treatment duration or extending the time horizon improved cost-effectiveness (ICERs as low as €8,073–€8,620). Discounting assumptions were also important: varying the discount rates for costs and QALYs shifted the ICER between €9,400 and €16,073 for costs and €9,652 to €15,751 for QALYs. Similarly, varying the cost of T2D complications yielded ICERs ranging from €9,600 to €15,896.
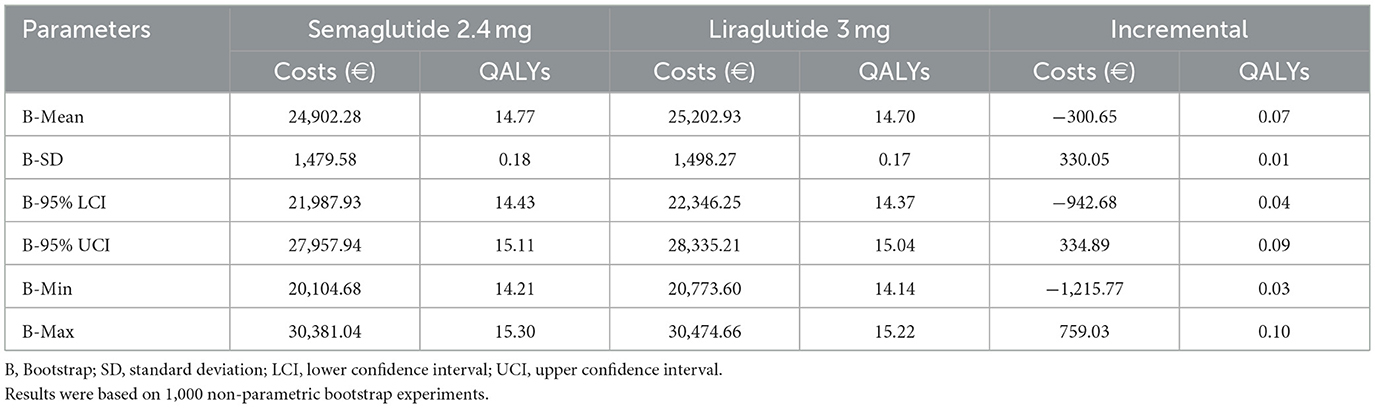
The results of the probabilistic sensitivity analysis (PSA) are summarized in Table 6. Across 1,000 Monte Carlo simulations, semaglutide 2.4 mg was associated with lower mean lifetime costs (€24,902 vs. €25,203 for liraglutide 3.0 mg) and higher mean QALYs (14.77 vs. 14.70). This translated into mean incremental cost savings of €301 and incremental health gains of 0.07 QALYs in favor of semaglutide. A decomposition of incremental costs is presented in Supplementary Table 4. In the deterministic base case, semaglutide incurred higher total lifetime costs (€1,083) compared with liraglutide, primarily due to higher drug acquisition expenses (€1,417), which were only partially offset by lower costs associated with obesity complications (€340 in total). In contrast, the probabilistic sensitivity analysis (PSA) produced a mean incremental cost saving (–€301). This difference reflects the incorporation of parameter uncertainty in the PSA, where joint sampling across treatment effects, complication risks, and cost inputs allowed scenarios in which semaglutide achieved larger reductions in obesity-related complications. On average, savings in complication-related “state” costs (–€1,287.99) and event costs (–€14.14) outweighed the higher drug and monitoring costs (+€997.23 and +€4.25, respectively). This resulted in a net mean saving of €300.65 for semaglutide, demonstrating that the cost-effectiveness of semaglutide remains robust when uncertainty and inter-parameter variability are considered.
Uncertainty around these estimates was modest. The standard deviation of incremental costs was €330, with a 95% confidence interval (CI) ranging from €−943 to €335. For incremental QALYs, the standard deviation was 0.01, with a 95% CI of +0.04 to +0.09. Across all simulations, semaglutide consistently generated positive incremental QALYs (minimum +0.03, maximum +0.10).
The cost-effectiveness (CE) plane (Figure 3) illustrates that 80.8% of simulations fell in the south-east quadrant, where semaglutide dominated liraglutide (more QALYs at lower costs). The remaining simulations were in the north-east quadrant, where semaglutide provided more QALYs but at higher costs. No simulations indicated fewer QALYs with semaglutide.
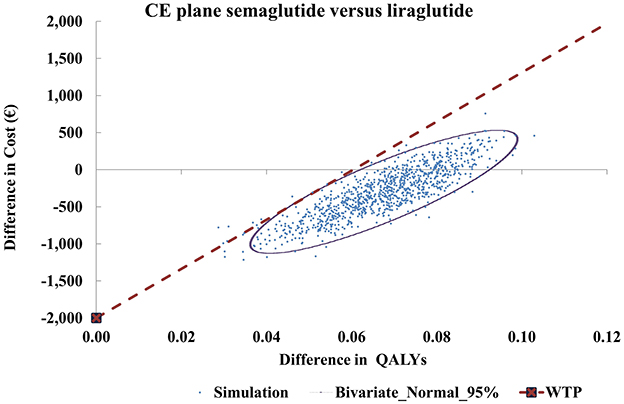
Figure 3. Cost-effectiveness plane for semaglutide 2.4 mg vs. liraglutide 3 mg. CE, cost-effectiveness; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; WTP, willingness-to-pay.
Consistent with these results, the cost-effectiveness acceptability curve (CEAC) (Figure 4) showed that semaglutide achieved a positive net monetary benefit (NMB) in 80.8% of simulations. The probability of semaglutide being cost-effective reached 100% at a WTP threshold of approximately €9,000 per QALY gained. At the examined Greek thresholds of €27,117 or €34,000 per QALY, semaglutide was cost-effective vs. liraglutide in all simulations.
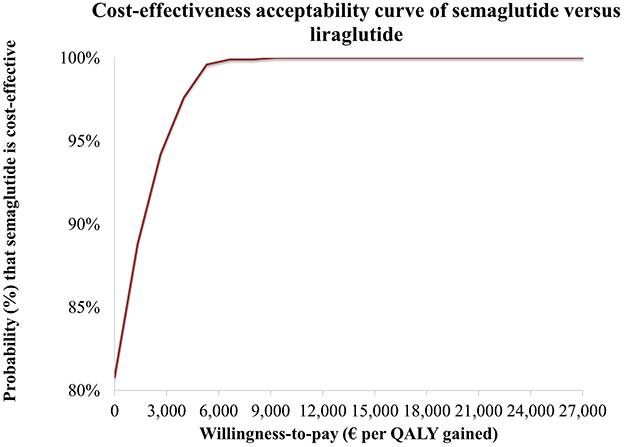
Figure 4. Cost-effectiveness acceptability curve (CEAC) for semaglutide 2.4 mg vs. liraglutide 3 mg.
4 Discussion
This study presents the first long-term cost-effectiveness analysis of semaglutide 2.4 mg compared with liraglutide 3.0 mg for the treatment of adults with obesity (BMI ≥ 35 kg/m2 and at least one comorbidity) from the perspective of the Greek third-party payer. Using a state-transition model over a 40-year horizon, semaglutide was shown to be cost-effective relative to liraglutide, with an ICER of €12,724 per QALY gained, well below the commonly used Greek WTP thresholds. The probabilistic sensitivity analysis confirmed the robustness of these findings, with semaglutide dominating liraglutide in most simulations, generating greater QALYs and lower costs in 80.8% of cases, and achieved a 100% probability of cost-effectiveness at a WTP threshold of approximately €9,000 per QALY. The findings remained robust when evaluated against various cost-effectiveness thresholds (€27,117 and €34,000 per QALY which reinforced semaglutide's cost-effectiveness profile and strengthened the policy relevance of the analysis for HTA and reimbursement decision-making. Deterministic and scenario analyses further demonstrated that the results were most sensitive to assumptions regarding treatment duration, time horizon, discounting, and the costs of diabetes-related complications; however, semaglutide remained cost-effective in all scenarios.
Our results align with a growing international evidence base. Cost-effectiveness studies conducted in the UK, Portugal, Canada, and the United States consistently concluded that semaglutide is cost-effective relative to lifestyle management and other pharmacotherapies, including liraglutide (21–27). In Portugal, semaglutide yielded an ICER of €13,459 per QALY compared with diet and exercise alone in adults with BMI >30 and comorbidities, with a 100% probability of cost-effectiveness at a €20,000 threshold (22). In the UK, Sandhu et al. (21) reported that semaglutide was cost-effective in 90% of simulations at a willingness-to-pay threshold of £20,000 per QALY, whereas Olivieri et al. (23) demonstrated semaglutide to be cost-effective vs. liraglutide and other agents in Canada, with extended dominance over naltrexone–bupropion. Our results are consistent with the findings of Alshahawey et al. (32), who conducted a decision analysis in the Egyptian setting and reported semaglutide 2.4 mg as a cost-effective and clinically superior alternative to liraglutide 3.0 mg for achieving significant weight loss. It is noteworthy that the probabilistic analysis yielded mean cost savings despite the deterministic base case showing marginally higher costs for semaglutide. This discrepancy reflects the probabilistic sampling of interdependent parameters—treatment effects, complication risks, and healthcare costs—enabling scenarios where greater reductions in obesity-related complications result in substantial downstream savings. The resulting net cost savings observed in the PSA further reinforce the robustness of semaglutide's cost-effectiveness under uncertainty.
The present analysis builds on this literature by providing country-specific evidence for Greece, where liraglutide remains the only reimbursed obesity pharmacotherapy and is restricted to adults with morbid obesity and established cardiovascular disease or sleep apnoea. Methodologically, this study advances previous work by broadening the scope of obesity-related complications considered. While earlier models often focused on type 2 diabetes, cancer and cardiovascular disease (20–23), we also incorporated chronic kidney disease, sleep apnoea, hypertension, asthma, and dyslipidaemia, thereby capturing a broader spectrum of obesity-related morbidity. This increases face validity and decision-making relevance. Nevertheless, important comorbidities such as osteoarthritis, several cancers (e.g., colorectal, endometrial), and mental health disorders were not included, potentially leading to underestimation of the full clinical and economic benefits of treatment.
Several limitations should be acknowledged. First, although semaglutide's efficacy estimates were derived from the robust STEP 8 trial, long-term extrapolation of weight trajectories and complication risks required assumptions and the application of published risk equations. These may not fully reflect real-world outcomes in Greece and, given the BMI-only surrogate approach, may underestimate pathways not directly mediated through BMI. Second, tirzepatide, a dual GIP/GLP-1 receptor agonist with superior weight-loss efficacy, was not considered. Although it is currently available in Greece only on an out-of-pocket basis, its potential future reimbursement makes it a key comparator for subsequent analyses. Third, this analysis was conducted from the perspective of the Greek third-party payer (EOPYY) and therefore excluded indirect costs, such as productivity losses, absenteeism, presenteeism, and premature mortality, associated with obesity. As these costs represent a substantial component of the overall economic burden of obesity, excluding a societal perspective may underestimate the broader economic benefits of effective pharmacotherapy. Fourth, although STEP-8 provides the only head-to-head randomized evidence directly comparing semaglutide and liraglutide, its trial population may not fully reflect the demographic and clinical profile of Greek patients with obesity. Given the limited availability of comprehensive local data, some differences between the modeled cohort and the real-world Greek population may remain unaccounted for, which should be considered when interpreting the findings. Fifth, while the STEP-8 trial primarily informed baseline characteristics, specific parameters (such as smoking prevalence, hypertension, and diabetes distribution) were supplemented with expert clinical input to reflect the Greek population more accurately. Although this approach enhances local relevance, it may introduce a degree of subjectivity and should be interpreted as a potential source of uncertainty. Finally, although this analysis incorporated more comorbidities than many prior studies, several obesity-related conditions (e.g., certain cancers, osteoarthritis, and mental health disorders) were not included. Similarly, bariatric surgery was not modeled as a comparator or as an acute event manifesting across health states. These omissions suggest that our results may be conservative estimates of semaglutide's cost-effectiveness profile.
Future research should expand on these findings in several directions. First, the application of alternative frameworks, such as the Core Obesity Model (20–23), would allow for the validation of results across different structural assumptions and the incorporation of additional risk factors beyond BMI, thereby enabling more accurate long-term projections. Second, societal-perspective analyses in Greece are warranted to capture the broader economic implications of obesity management, including productivity losses and informal care costs. Third, head-to-head evaluations of semaglutide and emerging therapies, particularly tirzepatide, will be crucial in informing future reimbursement and treatment decisions. Fourth, the integration of real-world data on adherence, weight trajectories, safety, and long-term outcomes in Greek patients will strengthen the external validity of future models. Finally, subgroup analyses in patients with obesity and coexisting conditions such as type 2 diabetes or non-alcoholic steatohepatitis could provide deeper insights into the dual benefits of obesity management for both metabolic and hepatic outcomes.
In summary, this study provides robust evidence that semaglutide 2.4 mg is a cost-effective treatment for obesity in Greece compared with liraglutide 3.0 mg. These results have important implications for reimbursement and resource allocation in Greece, where obesity imposes a substantial health and economic burden, and support the expansion of access to clinical and cost-effective pharmacotherapies such as semaglutide.
5 Conclusion
The present cost-effectiveness analysis demonstrates that semaglutide 2.4 mg is a cost-effective option compared with liraglutide 3.0 mg for adults with obesity (BMI ≥ 35 kg/m2 and at least one weight-related comorbidity) in Greece, at a willingness-to-pay threshold of €27,117 per QALY gained. Deterministic and probabilistic sensitivity analyses confirmed the robustness of these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
PP: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NM: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Part of the article processing charge (APC) fees were covered by the Special Account for Research Grants of the University of West Attica, Athens, Greece.
Acknowledgments
The authors are grateful to the local clinical expert for his critical input and to the reviewers and the editors for their valuable comments and suggestions for improvement during the review process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1690211/full#supplementary-material
References
1. World Health Organization. Obesity and Overweight. Geneva: WHO (2024). Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (Accessed May 8, 2025).
2. World Health Organization. European regional obesity report 2022. Copenhagen: WHO Regional Office for Europe (2022).
3. World Health Organization. The Global Health Observatory. Prevalence of obesity among adults, BMI ≥30 (age-standardised estimate) (%). Geneva: WHO (2024). Available online at: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-obesity-among-adults-bmi-=-30-(age-standardized-estimate)-(-) (Accessed May 15, 2025).
4. Hruby A, Manson JE Qi L, Malik VS, Rimm EB, Sun Q, et al. Determinants and consequences of obesity. Am J Public Health. (2016) 106:1656–62. doi: 10.2105/AJPH.2016.303326
5. Fruh SM. Obesity: risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract. (2017) 29:S3–14. doi: 10.1002/2327-6924.12510
6. Sarma S, Sockalingam S, Dash S. Obesity as a multisystem disease: trends in obesity rates and obesity-related complications. Diabetes Obes Metab. (2021) 23:3–16. doi: 10.1111/dom.14290
7. World Health Organization. Disability-Adjusted Life Years (DALYs). Geneva: WHO (2025). Available online at: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/156 (Accessed June 12, 2025).
8. Zhou XD, Chen QF, Yang W, Zuluaga M, Targher G, Byrne CD, et al. Burden of disease attributable to high body mass index: an analysis of data from the Global Burden of Disease Study 2021. EClinicalMedicine. (2024) 76:102848. doi: 10.1016/j.eclinm.2024.102848
9. World Obesity Federation. World Obesity Atlas 2024. London: World Obesity Federation (2024). Available online at: https://data.worldobesity.org/publications/?cat=22 (Accessed June 10, 2025).
10. Papantoniou P, Maniadakis N. The Economic Cost of Obesity: A Cost-of-Illness Study in Greece. Appl Health Econ Health Policy. (2025) 27:1–21. doi: 10.1007/s40258-025-01002-6
11. Ryan DH, Kahan S. Guideline recommendations for obesity management. Med Clin North Am. (2018) 102:49–63. doi: 10.1016/j.mcna.2017.08.006
12. Ochner CN, Barrios DM, Lee CD, Pi-Sunyer FX. Biological mechanisms that promote weight regain following weight loss in obese humans. Physiol Behav. (2013) 120:106–13. doi: 10.1016/j.physbeh.2013.07.009
13. Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. (2001) 74:579–84. doi: 10.1093/ajcn/74.5.579
14. Xie Z, Yang S, Deng W, Li J, Chen J. Efficacy and safety of liraglutide and semaglutide on weight loss in people with obesity or overweight: a systematic review. Clin Epidemiol. (2022) 14:1463–76. doi: 10.2147/CLEP.S391819
15. Deng Y, Park A, Zhu L, Xie W, Pan CQ. Effect of semaglutide and liraglutide in individuals with obesity or overweight without diabetes: a systematic review. Ther Adv Chronic Dis. (2022) 13:20406223221108064. doi: 10.1177/20406223221108064
16. Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomised, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. (2015) 373:11–22. doi: 10.1056/NEJMoa1411892
17. Wilding JP, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. (2021) 384:989–1002. doi: 10.1056/NEJMoa2032183
18. European Medicines Agency. Saxenda (2025). Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/saxenda (Accessed July 17, 2025).
19. European Medicines Agency. Wegovy (2025). Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/wegovy (Accessed July 17, 2025).
20. Government Gazette. Reimbursement of Liraglutide 3 mg for obesity (2023). Available online at: https://eopyyfiles.blob.core.windows.net/eopyysite/ServiceCategories/d5769ccc-59c5-48ac-a55c-ad7f1d69f586.pdf (Accessed January 17, 2025).
21. Sandhu H, Xu W, Olivieri AV, Lübker C, Smith I, Antavalis V. Once-weekly subcutaneous semaglutide 2.4 mg injection is cost-effective for weight management in the United Kingdom. Adv Ther. (2023) 40:1282–91. doi: 10.1007/s12325-022-02423-8
22. Miguel LS, Soares M, Olivieri A, Sampaio F, Lamotte M, Shukla S, et al. Cost-effectiveness of semaglutide 2.4 mg in chronic weight management in Portugal. Diabetol Metab Syndr. (2024) 16:97. doi: 10.1186/s13098-024-01338-4
23. Olivieri AV, Muratov S, Larsen S, Luckevich M, Chan K, Lamotte M, et al. Cost-effectiveness of weight-management pharmacotherapies in Canada: a societal perspective. Int J Obes. (2024) 48:683–93. doi: 10.1038/s41366-024-01467-w
24. Kim N, Wang J, Burudpakdee C, Song Y, Ramasamy A, Xie Y, et al. Cost-effectiveness analysis of semaglutide 2.4 mg for the treatment of adult patients with overweight and obesity in the United States. J Manag Care Spec Pharm. (2022) 28:740–52. doi: 10.18553/jmcp.2022.28.7.740
25. Xue Y, Zou H, Ruan Z, Chen X, Lai Y, Yao D, et al. Pharmacoeconomic evaluation of anti-obesity drugs for chronic weight management: a systematic review of literature. Front Endocrinol. (2023) 14:1254398. doi: 10.3389/fendo.2023.1254398
26. Asiabar AS, Rezaei MA, Jafarzadeh D, Rajaei S, Atefimanesh P, Soleimanpour S, et al. The cost-effectiveness analysis of semaglutide for the treatment of adult and adolescent patients with overweight and obesity: a systematic review. Eur J Clin Pharmacol. (2024) 80:1857–70. doi: 10.1007/s00228-024-03755-w
27. Neovius M, Narbro K. Cost-effectiveness of pharmacological anti-obesity treatments: a systematic review. Int J Obes. (2008) 32:1752–63. doi: 10.1038/ijo.2008.189
28. Zomer E, Zhou J, Nelson A, Nicholls S, Stub D, Zoungas S. Is semaglutide cost-effective for the secondary prevention of cardiovascular disease in people with overweight or obesity in Australia? Heart Lung Circ. (2024) 33:S523. doi: 10.1016/j.hlc.2024.06.870
29. McEwan P, Bøg M, Faurby M, Foos V, Lingvay I, Lübker C, et al. Cost-effectiveness of semaglutide in people with obesity and cardiovascular disease without diabetes. J Med Econ. (2025) 28:268–78. doi: 10.1080/13696998.2025.2459529
30. Rennert-May E, Manns B, Clement F, Spackman E, Collister D, Sumner G, et al. Cost-effectiveness of semaglutide in patients with obesity and cardiovascular disease. Can J Cardiol. (2025) 41:128–36. doi: 10.1016/j.cjca.2024.09.025
31. Papantoniou P, Maniadakis N. Semaglutide 2.4 mg vs liraglutide 3 mg for the treatment of obesity in Greece: a short-term cost-effectiveness analysis. Pharmacoecon Open. (2025) 9:487–97. doi: 10.1007/s41669-025-00561-7
32. Alshahawey M, Ghazy M, El Morshedy M, El Said NO. Cost-effectiveness analysis of once-weekly semaglutide vs. once-daily liraglutide administered subcutaneously in patients with overweight and obesity: a decision analysis. Eur Rev Med Pharmacol Sci. (2024) 28:3365–3374. doi: 10.26355/eurrev_202405_36181
33. Taieb AB, Roberts E, Luckevich M, Larsen S, le Roux CW, de Freitas PG, et al. Understanding the risk of developing weight-related complications associated with different body mass index categories: a systematic review. Diabetol Metab Syndr. (2022) 14:186. doi: 10.1186/s13098-022-00952-4
34. Bioletto F, Ponzo V, Goitre I, Stella B, Rahimi F, Parasiliti-Caprino M, et al. Complementary Role of BMI and EOSS in Predicting All-Cause and Cause-Specific Mortality in People with Overweight and Obesity. Nutrients (2024) 16:3433. doi: 10.3390/nu16203433
35. Touloumi G, Karakosta A, Kalpourtzi N, Gavana M, Vantarakis A, Kantzanou M, et al. High prevalence of cardiovascular risk factors in adults living in Greece: the EMENO National Health Examination Survey. BMC Public Health (2020) 20:1665. doi: 10.1186/s12889-020-09757-4
36. Panagiotakos D, Sigala EG, Damigou E, Loukina A, Dalmyras D, Mentzantonakis G, et al. The burden of cardiovascular disease and related risk factors in Greece: The ATTICA epidemiological study (2002–2022). Hellenic J Cardiol. (2024). doi: 10.1016/j.hjc.2024.05.009
37. Cawley J, Meyerhoefer C, Biener A, Hammer M, Wintfeld N. Savings in medical expenditures associated with reductions in body mass index among US adults with obesity, by diabetes status. Pharmacoeconomics. (2015) 33:707–22. doi: 10.1007/s40273-014-0230-2
38. Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS ONE. (2021) 16:e0247307. doi: 10.1371/journal.pone.0247307
39. Muscogiuri G, Verde L, Colao A. Body mass index (BMI): still be used? Eur J Intern Med. (2023) 117:50–1. doi: 10.1016/j.ejim.2023.09.002
40. Byker Shanks C, Bruening M, Yaroch AL, BMI. or not to BMI? Debating the value of body mass index as a measure of health in adults. Int J Behav Nutr Phys Activity. (2025) 22:23. doi: 10.1186/s12966-025-01719-6
41. Rubino DM, Greenway FL, Khalid U, O'Neil PM, Rosenstock J, Sørrig R, et al. STEP 8 Investigators. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomised clinical trial. JAMA. (2022) 327:138–50. doi: 10.1001/jama.2021.23619
42. Gourzoulidis G, Tzanetakos C, Ioannidis I, Tsapas A, Kourlaba G, Papageorgiou G, et al. Cost-effectiveness of empagliflozin for the treatment of patients with type 2 diabetes mellitus at increased cardiovascular risk in Greece. Clin Drug Investig. (2018) 38:417–26. doi: 10.1007/s40261-018-0620-x
43. Gourzoulidis G, Solakidi A, Psarra M, Nikitopoulou E, Tzanetakos C. Cost-effectiveness of tofacitinib for the treatment of active ankylosing spondylitis in Greece. Clin Drug Investig. (2024) 44:59–69. doi: 10.1007/s40261-023-01333-z
44. Gourzoulidis G, Kourlaba G, Kakisis J, Matsagkas M, Giannakoulas G, Gourgoulianis KI, et al. Cost-effectiveness analysis of rivaroxaban for treatment of deep vein thrombosis and pulmonary embolism in Greece. Clin Drug Investig. (2017) 37:833–44. doi: 10.1007/s40261-017-0540-1
45. Tzanetakos C, Melidonis A, Verras C, Kourlaba G, Maniadakis N. Cost-effectiveness analysis of liraglutide vs. sitagliptin or exenatide in patients with inadequately controlled type 2 diabetes on oral antidiabetic drugs in Greece. BMC Health Serv Res. (2014) 14:419. doi: 10.1186/1472-6963-14-419
46. Tzanetakos C, Psarra M, Kotsis I, Gourzoulidis G. Cost-effectiveness analysis of upadacitinib in patients with moderately to severely active ulcerative colitis in Greece. Value Health Reg Issues. (2025) 46:101091. doi: 10.1016/j.vhri.2025.101091
47. Tzanetakos C, Gourzoulidis G. Does a standard cost-effectiveness threshold exist? The case of Greece. Value Health Reg Issues (2023) 36:18–26. doi: 10.1016/j.vhri.2023.02.006
48. Athanasakis K, Agorastos G, Kyriopoulos I. Estimating a cost-effectiveness threshold for healthcare decision-making in the Greek NHS. Health Policy Technol. (2024) 13:100882. doi: 10.1016/j.hlpt.2024.100882
49. Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. MDM Policy Pract. (2022) 7:23814683211061097. doi: 10.1177/23814683211061097
50. Ara R, Blake L, Gray L, Hernández M, Crowther M, Dunkley A, et al. What is the clinical effectiveness and cost-effectiveness of using drugs in treating obese patients in primary care? A systematic review. Health Technol Assess. (2012) 16:iii–xiv. doi: 10.3310/hta16050
51. Wilson PWF, Meigs JB, Sullivan L, Fox CS, Nathan DM, D'Agostino RB Sr. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. (2007) 167:1068–74. doi: 10.1001/archinte.167.10.1068
52. Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. (2017) 357:j2099. doi: 10.1136/bmj.j2099
53. D'Agostino RB, Russell MW, Huse DM, Ellison RC, Silbershatz H, Wilson PWF, et al. Primary and subsequent coronary risk appraisal: new results from the Framingham study. Am Heart J. (2000) 139:272–81. doi: 10.1016/S0002-8703(00)90236-9
54. Shahraz J, Joukar F, Sheida F, Yeganeh S, Maroufizadeh S, Baghaee M, et al. Associations between body mass index (BMI) and dyslipidemia: results from the PERSIAN Guilan Cohort Study (PGCS). Obes Sci Pract. (2025) 11:e70055. doi: 10.1002/osp4.70055
55. Shihab HM, Meoni LA, Chu AY, Wang NY, Ford DE, Liang KY, et al. Body mass index and risk of incident hypertension over the life course: the Johns Hopkins Precursors Study. Circulation. (2012) 126:2983–9. doi: 10.1161/CIRCULATIONAHA.112.117333
56. Kong W, Zhang X, Gu H, Chen M, Li M, Zhang X, et al. Association between BMI and asthma in adults over 45 years of age: analysis of Global Burden of Disease 2021, China Health and Retirement Longitudinal Study, and National Health and Nutrition Examination Survey data. EClinicalMedicine. (2025) 82:102836. doi: 10.1016/j.eclinm.2025.103163
57. Dong Z, Xu X, Wang C, Cartledge S, Maddison R, Islam SMS. Association of overweight and obesity with obstructive sleep apnoea: a systematic review and meta-analysis. Obes Med. (2020) 17:100185. doi: 10.1016/j.obmed.2020.100185
58. Herrington WG, Smith M, Bankhead C, Matsushita K, Stevens S, Holt T, et al. Body-mass index and risk of advanced chronic kidney disease: prospective analyses from a primary care cohort of 1.4 million adults in England. PLoS ONE. (2017) 12:e0173515. doi: 10.1371/journal.pone.0173515
59. Abdelaal M. le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med. (2017) 5:161. doi: 10.21037/atm.2017.03.107
60. World Health Organization. Life Tables by Country. Geneva: WHO (2025). Available online at: https://apps.who.int/gho/data/view.main.60640 (Accessed May 20, 2025).
61. Bhaskaran K, dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 36 million adults in the UK Lancet Diabetes Endocrinol. (2018) 6:944–53. doi: 10.1016/S2213-8587(18)30288-2
62. Gough SC, Kragh N, Ploug UJ, Hammer M. Impact of obesity and type 2 diabetes on health-related quality of life in the general population in England. Diabetes Metab Syndr Obes. (2009) 2:179–84. doi: 10.2147/DMSO.S7088
63. Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making. (2011) 31:800–4. doi: 10.1177/0272989X11401031
64. Campbell J, McGarry LA, Shikora SA, Hale BC, Lee JT, Weinstein MC. Cost-effectiveness of laparoscopic gastric banding and bypass for morbid obesity. Am J Manag Care. (2010) 16:e174–87. doi: 10.1016/j.bbrc.2023.01.014
65. Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making. (2002) 22:340–9. doi: 10.1177/027298902400448902
66. Foos V, McEwan P. Conversion of hypoglycemia utility decrements from categorical units reflecting event history into event-specific disutility scores applicable to diabetes decision models. Value Health. (2018) 21:S223. doi: 10.1016/j.jval.2018.04.1506
67. Greek Ministry of Health. Bulletin of revised prices of medicines for human use, December 2023. Athens: Ministry of Health (2024). Available online at: https://www.moh.gov.gr/articles/times-farmakwn/deltia-timwn/12148 (Accessed May 20, 2025).
68. Presidential Decree. FEK 262A'/16-12-2011. Government Gazette of the Hellenic Republic. Athens: National Printing Office
69. EOPYY. Reimbursement prices of tests. Athens: National Organization for the Provision of Health Services (EOPYY) (2025). Available online at: https://eservices.eopyy.gov.gr/eSearchAllMaterial/ (Accessed May 20, 2025).
70. Common Ministerial Decree. FEK 946B'/27-3-2012. DRG list: Government Gazette of the Hellenic Republic. Athens: National Printing Office
71. Hellenic Statistical Authority. Consumer Price Index. Piraeus: ELSTAT (2025). Available online at: https://www.statistics.gr/en/statistics/-/publication/DKT87/ (Accessed June 17, 2025).
72. Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press (2006). p. 238.
74. Tzanetakos C, Bargiota A, Kourlaba G, Gourzoulidis G, Maniadakis N. Cost effectiveness of exenatide once weekly vs. insulin glargine and liraglutide for the treatment of type 2 diabetes mellitus in Greece. Clin Drug Investig. (2018) 38:67–77. doi: 10.1007/s40261-017-0586-0
75. Athanasakis K, Ollandezos M, Angeli A, Gregoriou A, Geitona M, Kyriopoulos J. Estimating the direct cost of type 2 diabetes in Greece: the effects of blood glucose regulation on patient cost. Diabet Med. (2010) 27:679–84. doi: 10.1111/j.1464-5491.2010.03004.x
76. Tsalta D, Athanasakis K, Konstantopoulou A, Kyriopoulos J, Papadogiannis D. Estimating the cost of treatment in a specialised National Health System hypertension centre in Greece: J Hypertens. (2010) 28:e398–9. doi: 10.1097/01.hjh.0000379431.55876.b7
77. Stafylas P, Sarafidis P, Tychala C, Pella E, Karaiskou M, Valsami R, et al. The clinical and economic burden of chronic kidney disease in Greece. Nephrol Dial Transplant. (2023) 38:gfad063c_5632. doi: 10.1093/ndt/gfad063c_5632
78. Prapa P, Geitona M, Gourgoulianis K. PHS29 Cost of treating patients with obstructive sleep apnea/hypopnea syndrome in the Sotiria Chest Hospital in Greece. Value Health. (2012) 15:A523. doi: 10.1016/j.jval.2012.08.1802
79. Migdalis I, Rombopoulos G, Hatzikou M, Manes C, Kypraios N, Tentolouris N. The cost of managing type 2 diabetes mellitus in Greece: a retrospective analysis of 10-year patient-level data “The HERCULES Study”. Int J Endocrinol. (2015) 2015:520759. doi: 10.1155/2015/520759
Keywords: cost-effectiveness, obesity, semaglutide, liraglutide, Greece
Citation: Papantoniou P and Maniadakis N (2025) Cost-effectiveness of semaglutide 2.4 mg versus liraglutide 3 mg for the treatment of obesity in Greece. Front. Public Health 13:1690211. doi: 10.3389/fpubh.2025.1690211
Received: 21 August 2025; Accepted: 09 October 2025;
Published: 28 October 2025.
Edited by:
Charalampos Tzanetakos, Health Through Evidence Consulting G.P., GreeceReviewed by:
Nouran Omar El Said, Future University in Egypt, EgyptGeorge Mavridoglou, University of the Peloponnese, Greece
Copyright © 2025 Papantoniou and Maniadakis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Panagiotis Papantoniou, cHBhcGFudG9uaW91QHVuaXdhLmdy
 Panagiotis Papantoniou
Panagiotis Papantoniou Nikolaos Maniadakis
Nikolaos Maniadakis