- 1Department of Oncology, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, China
- 2Department of Oncology, Xiangya Hospital, Central South University, Changsha, China
- 3Department of Pulmonary and Critical Care Medicine, Affiliated Hainan Hospital of Hainan Medical University, Haikou, China
- 4Key Laboratory of Emergency and Trauma of Ministry of Education, Hainan Medical University, Haikou, China
- 5NHC Key Laboratory of Tropical Disease Control, Hainan Medical University, Haikou, China
Background: The international Phase 3 LAURA trial (NCT03521154) demonstrated that the use of osimertinib following chemoradiotherapy markedly improved survival outcomes in unresectable stage III NSCLC with epidermal growth factor receptor (EGFR) mutations. Considering the high cost of targeted therapy, the popularization of osimertinib in clinical practice should be considered comprehensively in terms of cost and efficacy. This study was to investigate the cost-effectiveness of osimertinib for unresectable stage III EGFR-mutated NSCLC without disease progression after chemoradiotherapy from the perspective of payers in the USA and China.
Methods: The main health outcomes were evaluated by measuring life-years (LYs), quality-adjusted life-years (QALYs), incremental cost-effectiveness ratio (ICER), and incremental net health benefit (INHB). An integrated Markov model with three separate health states over a 15-year horizon was established. The sensitivity of the model was assessed, and subgroup analyses were conducted.
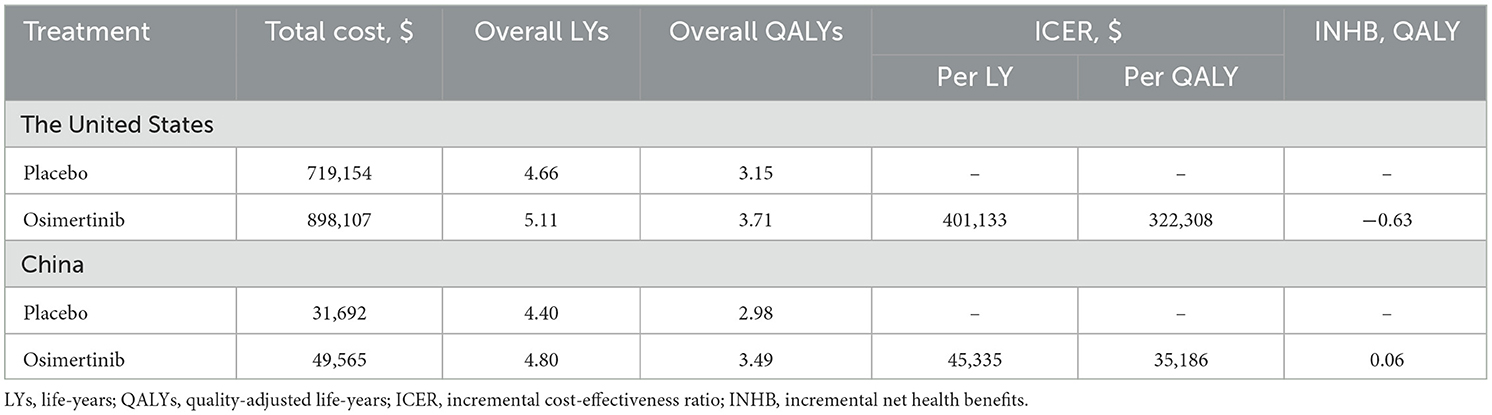
Results: Compared with placebo in stage III EGFR-mutated NSCLC after chemoradiation, osimertinib [$898,107 (3.70 QALYs) and $49,565 (3.49 QALYs)] increased costs (efficacy) by $178,953 (0.56 QALYs) in the USA and $17,872 (0.51 QALYs) in China. The corresponding ICERs were $322,308/QALY and $35,186/QALY, respectively, with an INHB of −0.63 and 0.06 QALYs. The sensitivity analysis showed that the results were influenced significantly by progression-free survival.
Conclusions: In China, treatment with osimertinib rather than placebo appears to be an effective and economically accessible option for patients with stage III EGFR-mutated NSCLC with no disease progression after chemoradiotherapy. This applied especially to the eastern and central economic regions of China but not the USA currently.
1 Background
Lung cancer remains the most common malignancy in the world with approximately 2.5 million new diagnoses and over 1.8 million deaths in 2022, with a 5-year survival of below 20% (1). Non-small cell lung cancer (NSCLC) accounts for about 85% of these diagnoses, with adenocarcinoma forming 40% of these cases and representing the most prevalent subtype since 2020 (2, 3). Stage III NSCLC shows a high degree of heterogeneity, and treatments are thus divided into three categories, namely, resectable, potentially resectable, and unresectable, depending on appropriateness of radical surgery (4, 5). Over 20% of patients have unresectable stage III locally advanced disease when diagnosed, for which the recommended treatment is concurrent chemoradiotherapy (CCRT) (6–9). The introduction of immunotherapy in 2017 has provided a new treatment model for these patients, where it is used as consolidation therapy in patients without progression following chemoradiotherapy, markedly improving patient survival (10–12). Approximately one-third of patients with unresectable stage III NSCLC treated with chemoradiotherapy have mutations in the epidermal growth factor receptor (EGFR) worldwide (13, 14). In China and other East Asian countries, EGFR mutations were detected in ~30% to 50% of patients with lung adenocarcinoma, whereas in the USA, the mutation rates were observed in only 8% to 21% (15).These patients have been found to have shorter or similar progression-free survival (PFS) compared with those without mutations as although there is a lower risk of treatment failure, the incidence of distant metastasis is higher, a finding subsequently confirmed in several real-world studies (16–18). Therefore, the control of systemic metastasis remains crucial for improving the long-term prognosis of these patients.
Osimertinib, a tyrosine kinase inhibitor (TKI) that inhibits EGFR, can effectively block the effects of various EGFR mutations, including deletions of exon 19 and the L858R substitution in exon 21 (19). It is approved for use as a postoperative adjuvant therapy for early-stage resectable lung cancers as well as a first-line therapy for advanced NSCLC with mutated EGFR or in combination with platinum-based chemotherapy (20). Due to its remarkable efficacy and minimal side effects in lung adenocarcinoma, osimertinib has become one of the most prescribed targeted drugs in recent years (21).
Addressing the huge unmet treatment need for patients with EGFR-mutated stage III unresectable NSCLC, the LAURA trial represents the first international Phase 3 clinical trial to directly compare the safety and efficacy of osimertinib with placebo in these patients after chemoradiotherapy, potentially establishing osimertinib as a new standard of care (20). The trial showed that osimertinib significantly improved PFS in cases without progression during or after chemoradiotherapy, with an estimated median PFS of 38.9 months vs. 7.3 months [hazard ratio (HR), 0.19; 95% confidence interval (CI), 0.12 to 0.29], resulting in an 81% reduction in disease progression and death (20). Although overall survival (OS) data have not yet reached maturity, interim analyses a trend towards OS benefit in the osimertinib group (HR, 0.81; 95% CI, 0.42 to 1.56), with a decrease of 19% in the risk of death. The PFS benefit of osimertinib over placebo was consistent across all key subgroups. Radiation pneumonitis was the most frequently reported treatment-related adverse event (TRAE) in both groups, the majority of which were low-grade, non-serious, and manageable, with no grade 4 or 5 events observed.
The LAURA trial has transformed the treatment landscape for these patients, suggesting the potential of osimertinib therapy.
Over the past few decades, despite a global decline in the overall incidence and mortality of lung cancer in most countries, the burden of lung cancer has been largely driven by changes in the morphological subtype patterns, with a continuous increase in the incidence of adenocarcinoma (22). This necessitates further accurate identification and in-depth analysis of differences among subtypes by clinical researchers, as well as increased attention from healthcare policymakers to the clinical accessibility of personalized treatment strategies. However, the high cost of targeted therapies and the large patient population render it unaffordable for both individuals and healthcare systems. It is thus necessary and urgent to conduct an economic analysis of the clinical benefits associated with specific targeted therapies. Here, the relative cost-effectiveness of osimertinib vs. placebo was assessed as consolidation treatment for unresectable stage III NSCLC post-chemoradiotherapy, from the perspectives of healthcare systems in high- and middle-income countries, represented by the USA and China, respectively.
2 Materials and methods
This study was entirely based on previous research and publicly available disease progression and therapy data, and it did not include any new research involving human participants or animals by any of the authors and therefore does not require approval from an independent ethics committee. The analysis followed the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement, as shown in Supplementary Table S1.
2.1. Patient population and intervention
Using a simulated patient cohort with identical characteristics to that used in the LAURA clinical trial, patients with sensitizing EGFR mutations and stage III unresectable NSCLC without progressive disease post-chemoradiotherapy were hypothetically randomized in a 2:1 ratio to be treated with either osimertinib or placebo, with a daily dose of 80 mg until disease progression, death, or discontinuation for other reasons (20). Imaging assessments were conducted every 8 weeks. For consistency, any patient in the osimertinib or placebo group who showed progressive disease (PD) received subsequent systemic anticancer therapy [42 cases (29.4%) and 57 cases (78.1%)], respectively. The majority of patients in both groups received subsequent osimertinib treatment after disease progression, as reported in the corresponding trial (20). Patients who did not receive further treatment were considered to have undergone the best supportive care (BSC) before death, and none of those who died of treatment-related causes were included. Supplementary Table S2 details the drug dosages and unit prices.
2.2. Model design
An integrated Markov model was utilized to simulate the disease course of the patients in both cohorts using TreeAge Pro (version 2022). The model included three states of health, specifically, PFS, PD, and death. At the start of treatment, all patients fell into the PFS category, while after receiving osimertinib or placebo, they either remained in the PFS category or moved to the next categories, namely, PD or death (Supplementary Figure S1).
The Markov model had a cycle length of 4 weeks and a horizon of 15 years, during which it was anticipated that over 99% of patients would die within this time frame. Key outcomes predicted included life years (LYs), quality-adjusted life years (QALYs), the incremental cost-effectiveness ratio (ICER), and the incremental net health benefit (INHB) for the two interventions. Based on previous literature with an international perspective, the medical benefits and costs were discounted by 5% and 3%, respectively, each year (23, 24). The willingness-to-pay (WTP) thresholds were set at $150,000 for the USA and $39,632 [three times the per capita gross domestic product (GDP) in 2024] for China, with all costs expressed in US dollars (25, 26).
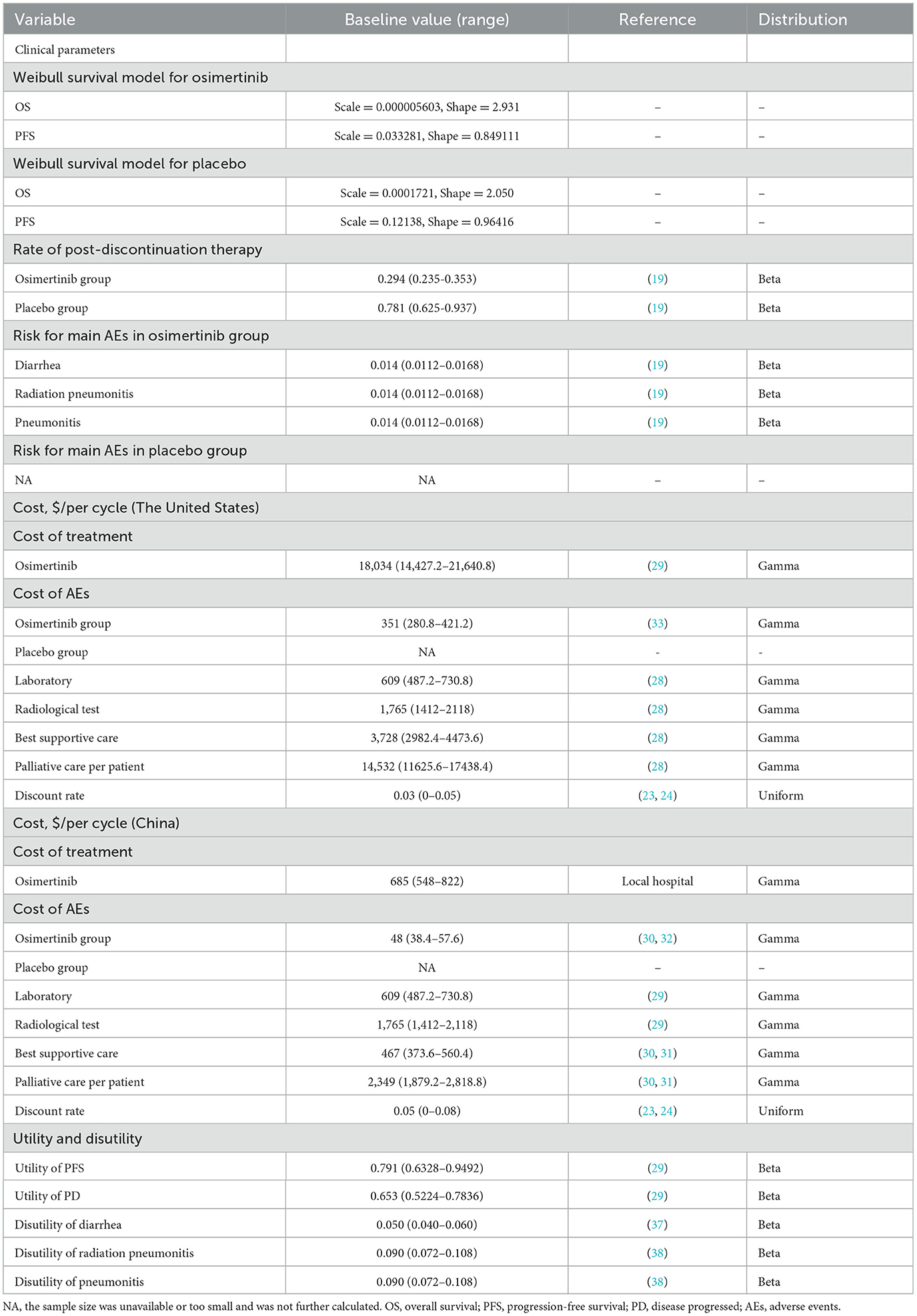
Patient survival curves were utilized to calculate the likelihood of change between the different states of health. Due to the lack of precise individual patient information in the study, survival information was extracted from the OS and PFS Kaplan-Meier (KM) curves reported by Hoyle et al., using the Get Data Graph Digitizer (version 2.26) (27). After the integration of this patient survival information, a parametric survival model was established (Supplementary Figure S1). According to the Akaike and Bayesian information criteria, visual analysis was used to select the Weibull distribution as the most suitable parameter distribution among the Exponential, Log-normal, Log-logistic, Weibull, and Gompertz distributions for reconstructing the model parameters (Supplementary Figure S2, Supplementary Table S3) (28). Subsequently, R Studio (version 1.2.5042) was used to obtain the specific distributions of the γ (scale) and λ (shape) parameters (Table 1).
2.3. Costs and utility
The investigation included only direct medical costs, and indirect and hidden medical costs were excluded. The direct medical costs covered major interventional drugs, necessary laboratory and radiological tests, BSC, palliative care, and management of TRAEs (Table 1). The drug prices aligned with real hospital data and online queries of drug prices (29). Other direct medical costs were derived from the existing literatures (30–35). The analysis included TRAEs of grade 3 or above with a frequency of ≥1%. Notably, no grade 4/5 TRAEs were reported. All costs were corrected to the 2024 prices in terms of the US Consumer Price Index (CPI); in contrast, due to government regulation, drug prices in China did not require inflation adjustment to ensure cost stability (36). The costs were all calculated in US dollars based on the exchange rate of $1 = ¥7.2478 (June 2024).
Health utility values reflect patients' health-related quality of life (HRQOL) during the natural course of the disease, ranging from 0 (worst) to 1 (perfect) health status. As the LAURA trial did not provide similar information, health utility values of 0.791 and 0.653 were assigned for PFS and PD, respectively, based on previous studies with adjustment for the disutility values of adverse events (AEs) (30). These AE-associated disutility values were derived from previous reports (37, 38). It was found that the cost data conformed to a gamma distribution, while the utility values and AE incidence confirmed to a beta distribution (39).
2.4. Statistical analysis
Various methods for sensitivity assessments were used to elucidate the performance of the integrated model and the effects of variables on the outcomes. One-way sensitivity analysis covered extreme changes in each model parameter. The parameters were randomly altered by 20% within the range of their baseline values to identify parameters having a significant impact on the ICER value (40). A tornado diagram was used for displaying the importance of each parameter on the model outcomes. To simulate the impact of simultaneously changing several uncertain parameters that varied randomly within their distribution ranges on the ICER, probabilistic sensitivity analysis (PSA) was performing using 10,000 Monte Carlo simulations, and the results were displayed in scatter plots and cost-effectiveness acceptability curves to assess the cost-effectiveness of osimertinib relative to placebo (40). Additionally, the potential effects of different patient subgroup characteristics on the results were evaluated using targeted subgroup analyses of stratified patients, following the method of Hoyle et al. (41).
3 Results
3.1. Base-case analyses
The mature model with a 15-year projection showed that the life expectancy for patients with sensitising EGFR mutations stage III unresectable NSCLC in the USA and China, post-chemoradiotherapy and without disease progression increased by 0.45 LYs (5.40 months) and 0.39 LYs (4.68 months) respectively with osimertinib consolidation therapy relative to placebo. In terms of improvements in quality of life, in the USA and China, osimertinib incurred additional costs (efficacy) of $178,953 (0.56 QALYs) and $17,872 (0.51 QALYs) compared to placebo, respectively. Using a WTP threshold of $150,000/QALY (USA) and 39,632/QALY (China), the respective ICERs were 322,308/QALY and 35,186/QALY, with an INHB of−0.63 and 0.06 QALYs. The detailed results are presented in Table 2.
3.2. Sensitivity and subgroup analyses
Tornado diagrams were utilized to display parameters that the one-way sensitivity analyses had shown to significantly influence the ICER values (Figure 1) The results indicated that in both the USA and China, the utility value of the patient's health status was the primary factor affecting the ICER, surpassing the cost of osimertinib and other follow-up expenses. The PFS utility value had the most significant impact on the ICER. In the USA, when the PFS utility value ranged from 0.6328 to 0.9492, the ICER per QALY was between $208,346 and $711,474. Regardless of parameter changes, the ICER values were consistently greater than the WTP threshold of $150,000/QALY. However, in China, when the PFS utility value rose to its upper limit, the ICER per QALY decreased to $22,465, indicating that osimertinib provided greater clinical benefits at a lower cost. When the PFS utility value dropped to its lower limit, the ICER increased to $81,368/QALY, exceeding the threshold of $39,632/QALY. Then we conducted a scenario analysis based on plausible ranges of health-utility values reported in NSCLC populations (42). Across utility scenarios A–C, all ICERs exceeded the WTP threshold of $150,000/QALY across all parameter settings In the United States. In China, only under the most conservative utility set (Scenario C) did the ICER ($43,942/QALY) exceed the prespecified threshold of $39,632/QALY (Supplementary Table S4). Other parameters, such as the cost of osimertinib, laboratory and radiological tests, and BSC costs, had a relatively limited impact on the model inputs.
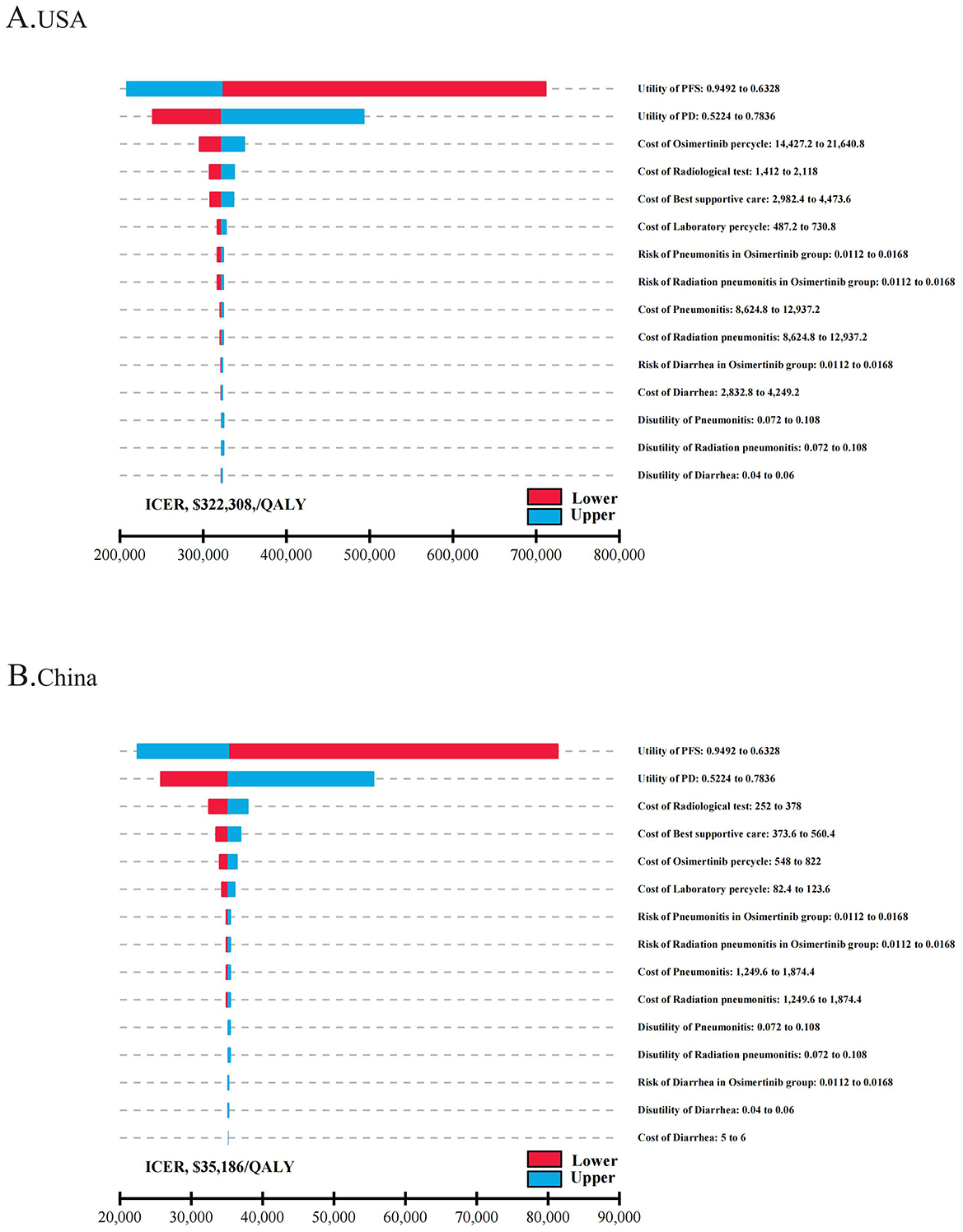
Figure 1. The one-way sensitivity analyses for osimertinib versus placebo strategy in the USA (A) and China (B). PFS, progression-free survival; PD, progressive disease; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
The PSA results indicated that patients treated with osimertinib consolidation therapy had a 21.1% likelihood of it being a more cost-effective option, compared with 50.0% for the placebo, at the WTP thresholds of $150,000/QALY and $39,632/QALY, respectively (Figure 2 and Supplementary Figure S3). The cost-effectiveness acceptability curves indicated a strong correlation between economic advantage and increasing WTP thresholds. When the WTP thresholds increased to ~$400,000 in the USA and $45,000 in China, respectively, the probability of cost-effectiveness approached 60% in both settings, suggesting that the WTP threshold significantly influenced the chance of a specific treatment strategy becoming the primary option.
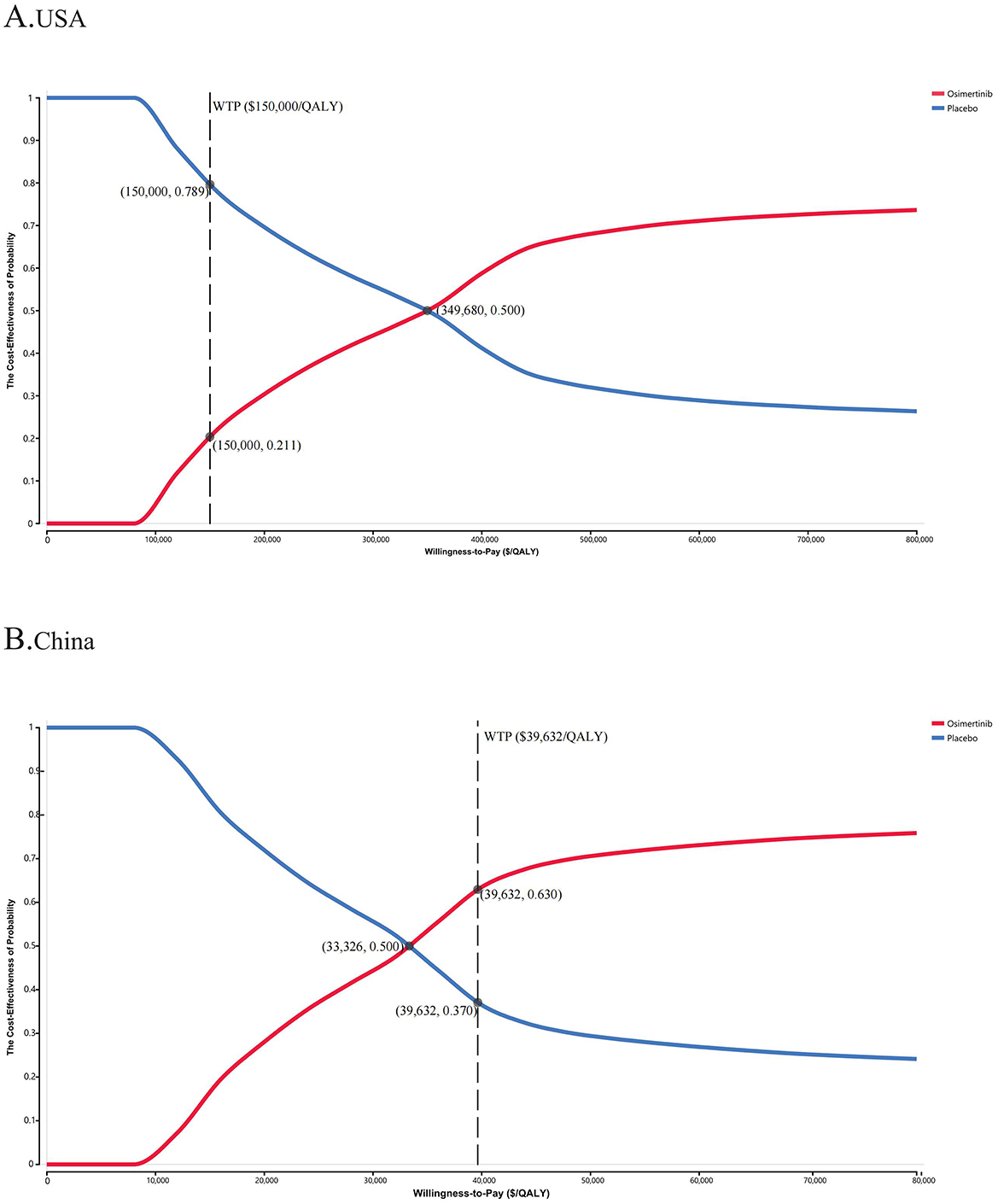
Figure 2. The cost-effectiveness acceptability curves for osimertinib versus placebo strategy in the USA (A) and China (B). WTP, willingness-to-pay; QALY, quality-adjusted life-year.
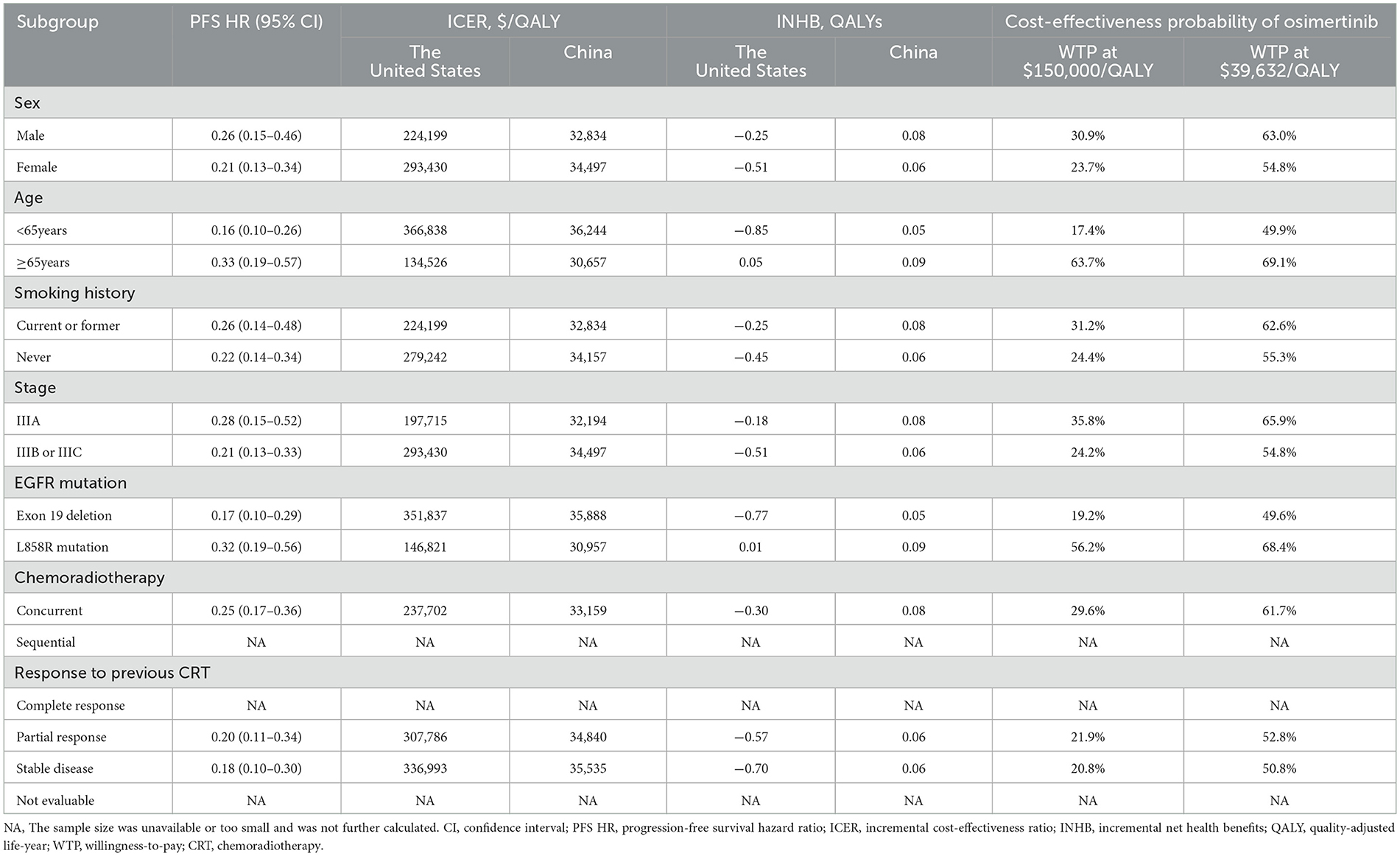
Subgroup analyses in the United States indicated that, aside from patients with the L858R EGFR mutation and those aged 65 years or older, sensitivity analyses revealed ICER values exceeding the WTP thresholds in nearly all subgroups, correlating with negative INHBs (Table 3). In the subgroup aged 65 years and older, osimertinib offered an additional 0.45 QALYs (0.21 LYs) compared to placebo, resulting in an ICER of $134,526 and an INHB of 0.05 QALYs. For the L858R EGFR mutation subgroup, the additional cost (efficacy) of osimertinib compared to placebo was $66,875 (0.46 QALYs), with corresponding ICERs of $146,821/QALY and an INHB of 0.01 QALYs. Conversely, in China, ICER values below WTP thresholds were observed across all patient subgroups, with the probability of osimertinib being cost-effective exceeding 50% in most analyzed subgroups. Osimertinib demonstrated superior cost-effectiveness and emerged as the optimal treatment for patients with unresectable stage III EGFR-mutated NSCLC without disease progression after chemoradiotherapy.
4 Discussion
EGFR mutations are active in all stages of NSCLC and represent the most common oncogenic mutations associated with stage III unresectable locally advanced NSCLC (43). To date, CCRT followed by immunotherapy consolidation remains the standard treatment. However, the effectiveness of immunotherapy in these patients remains unclear (44, 45). Despite better response rates to radical CCRT and lower local recurrence rates compared to patients with wild-type EGFR, they exhibit higher rates of distant metastasis, particularly to the central nervous system (16, 18). Over the past decade or so, the identification of specific driver mutation genes and the development and upgrading of targeted therapies has profoundly affected the treatment paradigm for NSCLC patients.
Osimertinib is one of the most effective new EGFR inhibitors and can inhibit both EGFR mutations and T790M resistance mutations, selectively targeting the EGFR tyrosine kinase and leading to significant and sustained tumor regression(19). According to the latest research data, osimertinib consolidation therapy can markedly extend PFS in these patients relative to placebo without significant toxicity, demonstrating better efficacy and safety, and greatly improving the quality of life of the patients. However, while providing clinical benefits and improving quality of life, these treatments inevitably impose a significant social burden and macroeconomic cost on healthcare systems worldwide. Additionally, in recent years, global guidelines have rarely addressed the issue of the costs associated with new anticancer drugs or treatments. Even for approved anti-cancer drugs, affordability is a key factor in determining their accessibility in clinical practice. Therefore, it is necessary to conduct economic evaluations of new treatments.
Given that the clinical evidence for the use of osimertinib consolidation therapy in these patients is relatively new, there is an absence of evidence on cost-effectiveness. The present study evaluated the cost-effectiveness of osimertinib consolidation therapy relative to placebo in these patients based on the most recent evidence and the healthcare systems of the USA and China. The economic analyses show that the efficacy of osimertinib treatment relative to placebo exceeded 0.5 QALYs. In the USA, the total treatment cost for the osimertinib group was $898,107, with an ICER of $322,308/QALY and a corresponding INHB of −0.63 QALYs. For Chinese patients, the total treatment cost for the osimertinib group was $49,565, with an ICER of $35,186/QALY and a corresponding INHB of 0.06 QALYs. From the perspective of healthcare services in both countries, the use of osimertinib relative to placebo for these patients is a cost-effective option in China but not in the USA, suggesting that patients in China will be more inclined to choose osimertinib as their first-line treatment. It also highlights the importance of local cost-effectiveness analysis that can be tailored to a country or specific region.
Although China and the United States bear the largest shares of global cancer-related economic costs, their per-capita health expenditure diverges substantially, with China at $672.5 and the United States at $12,434.4 in 2022 (46, 47). The US healthcare system is predominantly driven by private insurance, emphasizing market orientation and efficiency. Drug prices are largely determined by market competition, with pricing and reimbursement policies shaped by rigorous pharmacoeconomic evaluations. Reimbursement negotiations mainly focus on cost containment, often favoring established therapies with proven effectiveness and lower budget impact. Centers for medicare services may prompt pharmaceutical companies in the USA to re-evaluate their pricing strategies, with tiered pricing emerging as a potential approach to enhance both cost-effectiveness and market competitiveness. In contrast, China's healthcare system is primarily government-led, with drug prices set through state regulation and national policy. Within the constraints of the national health insurance budget, reimbursement negotiations aim to balance the sustainability of the fund with population access, thereby promoting more efficient resource allocation. Consequently, regulatory approval and inclusion in the national reimbursement drug list will be an essential step to enhance access to innovative therapies in China. Therefore, differences in cost-effectiveness outcomes between China and the United States arise not only from drug pricing disparities but also from fundamental differences in their healthcare systems.
In health-related cost-effectiveness analyses, the WTP threshold represents the estimated amount that consumers are willing to pay for health benefits and is often used as a non-monetary measure of the cost-effectiveness of health interventions(48, 49), inherently reflecting each society's capacity and preference for financing health-care resource allocation. Given systematic differences in national conditions and social preferences, these thresholds are not strictly fair or comparable. In economic decision-making, when the ICER is below the relevant WTP threshold, the intervention is generally considered cost-effective. In this study, we used the conventional US threshold of $150,000/QALY, as commonly applied by the US Institute for Clinical and Economic Review, although the World Health Organization has suggested that values of up to 3 times the GDP per capita may be justified(49, 50). It is worth mentioning that the use of a higher WTP threshold ($257,430/QALY, three times the US per capita GDP in 2024) did not result in osimertinib becoming the most cost-effective strategy in the USA, although it did improve the cost-effectiveness. In China, due to the uneven and insufficient economic development of different parts of the country, there are significant differences in the per capita GDP among different economic regions. In 2024, the WTP thresholds in Beijing (eastern region), Hubei (central), Liaoning (northeastern), and Gansu (western) were $94,443, $42,564, $32,383, and $21,865/QALY, respectively (51). At a WTP threshold of $39,632/QALY, osimertinib thus remains the most cost-effective treatment option in most provinces in eastern and central China, while placebo may be a more suitable choice in the less developed northeastern and western regions. Nevertheless, this does not mean that these patients should be treated with less effective therapeutic options. The healthcare systems and drug pricing policymakers should establish assistance strategies and insurance policies based on differences in the economic development of different regions.
This study has several significant strengths. Firstly, it used the latest clinical evidence to evaluate the cost-effectiveness of osimertinib vs. placebo in patients with stage III unresectable EGFR-mutated NSCLC following chemoradiotherapy. Secondly, the use of treatment strategies targeting disease-specific molecular targets has become indispensable in lung cancer, greatly advancing precision medicine and personalized treatment (52). This study analyzed the economic outcomes of different patient subgroups, providing strong guidance for personalized management and treatment decisions based on the characteristics of specific patient subgroups. In addition, considering the differences between healthcare systems in different countries, to enhance the applicability of the results, the study evaluated cost-effectiveness from the perspectives of a high-income country (the USA) and a middle-income country (China), providing scientific references to guide health-related decisions in countries with varying income levels.
The study also has several limitations. Due to the relatively short follow-up time of the Phase 3 LAURA clinical trial, survival data may change with the prolongation of the follow-up, particularly in the median OS data, and the extrapolated survival estimates are likely to be uncertain. Thus, despite conducting sensitivity and subgroup analyses to assess this uncertainty, it is an inevitable limitation of our model that requires updated data from the LAURA trial for validation. Second, since the LAURA trial did not include information on HRQOL, health utility data for PFS and PD were obtained from the literature, which may have introduced bias into the results of the model, although the results of subsequent scenario analysis based on health utilities showed osimertinib vs. placebo remained the relatively most cost-effective option in the eastern economic regions of China. Third, consistent with most cost-effectiveness analyses, the use of the same virtual patient cohort with different national healthcare systems due to the absence of data is an inescapable issue in clinical trial-based cost-effectiveness analyses, even if the trial showed similar trends in terms of safety and efficacy in subgroups and the population as a whole. Fourth, placebo was selected as the control group, which may not be representative of the real-world practice for typical international patients with unresectable stage III EGFR-mutated NSCLC. However, it is particularly relevant for populations who are PD-L1 negative, have contraindications to immunotherapy, or are unable to afford costly treatments—especially given the current limited evidence indicating that the benefits of consolidation therapy with immunotherapy for patients with unresectable stage III EGFR-mutated NSCLC remain uncertain (16). Finally, without the availability of separate survival curves based on patient subgroups, economic estimates may show differences from real-life survival outcomes by using specific HRs, requiring additional follow-up studies on these subgroups.
5 Conclusion
In China, treatment with osimertinib rather than placebo appears to be an effective and economically accessible option for patients with stage III EGFR-mutated NSCLC with no disease progression during or after chemoradiotherapy, especially in the eastern and central economic regions of China. However, this does not apply to the USA currently.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
DT: Visualization, Data curation, Software, Writing – original draft, Conceptualization, Validation, Formal analysis, Writing – review & editing, Methodology. XZ: Writing – review & editing, Software, Data curation, Writing – original draft, Formal analysis. CW: Project administration, Visualization, Validation, Writing – original draft, Supervision, Funding acquisition, Writing – review & editing, Resources, Investigation. XZ: Funding acquisition, Writing – review & editing, Investigation, Validation, Project administration, Writing – original draft, Resources, Visualization, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by grants from Hainan Provincial Natural Science Foundation of China (No. 824QN374), NHC Key Laboratory of Tropical Disease Control, Hainan Medical University (No. 2023NHCTDCKFKT11002), and Key Laboratory of Emergency and Trauma of Ministry of Education (Hainan Medical University) (Grant. KLET-202213).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1698562/full#supplementary-material
Supplementary Figure S1 | Model Structure.
Supplementary Figure S2 | AIC and BIC statistics for alternate parametric survival distributions.
Supplementary Figure S3 | Probability Sensitivity Analysis Scatter Plot.
Supplementary Table S1 | The CHEERS 2022 checklist.
Supplementary Table S2 | Details of Treatment Strategy and Unit Costs.
Supplementary Table S3 | Summary of Statistical Goodness-of-fit of K-M Curve.
Supplementary Table S4 | Scenario analysis for health utilities.
Abbreviations
AEs, adverse events; BSC, best supportive care; BSA, body surface area; CCRT, concurrent chemoradiotherapy; CPI, consumer price index; EGFR, epidermal growth factor receptor; GDP, gross domestic product; HRQOL, health-related quality of life; HR, hazard ratio; ICER, incremental cost-effectiveness ratio; INHB, incremental net health benefit; KM, Kaplan-Meier; LYs, life-years; NSCLC, non-small cell lung cancer; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PSA, probabilistic sensitivity analysis; QALYs, quality-adjusted life-years; TKI, tyrosine kinase inhibitor; TRAE, treatment-related adverse event; WTP, willingness-to-pay threshold.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med. (2017) 377:849–61. doi: 10.1056/NEJMra1703413
3. Zhang Y, Vaccarella S, Morgan E, Li M, Etxeberria J, Chokunonga E, et al. Global variations in lung cancer incidence by histological subtype in 2020: a population-based study. Lancet Oncol. (2023) 24:1206–18. doi: 10.1016/S1470-2045(23)00444-8
4. Wu Y-L, Lu S, Zhou Q, Li P, Cheng Y, Wang J, et al. Expert consensus on treatment for stage III non-small cell lung cancer. Med Adv. (2023) 1:3–13. doi: 10.1002/med4.7
5. Casal-Mouriño A, Ruano-Ravina A, Lorenzo-González M, Rodríguez-Martínez Á, Giraldo-Osorio A, Varela-Lema L, et al. Epidemiology of stage III lung cancer: frequency, diagnostic characteristics, and survival. Transl Lung Cancer Res. (2021) 10:506–18. doi: 10.21037/tlcr.2020.03.40
6. Walters S, Maringe C, Coleman MP, Peake MD, Butler J, Young N, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–2007. Thorax. (2013) 68:551–64. doi: 10.1136/thoraxjnl-2012-202297
7. Agbarya A, Shalata W, Addeo A, Charpidou A, Cuppens K, Brustugun OT, et al. Real-world journey of unresectable stage III NSCLC patients: current dilemmas for disease staging and treatment. J Clin Med. (2022) 11:1738. doi: 10.3390/jcm11061738
8. NCCN. NCCN Guidelines, Non-Small Cell Lung Cancer (2024). Available online at: https://www.nccn.org/guidelines/category_1 (Accessed July 8, 2024).
9. CSCO. Chinese Society of Clinical Oncology (2024). Available online at: http://www.csco.org.cn/cn/index.aspx (Accessed July 8, 2024).
10. Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-Year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. (2022) 40:1301–11. doi: 10.1200/JCO.21.01308
11. Daly ME, Singh N, Ismaila N, Antonoff MB, Arenberg DA, Bradley J, et al. Management of stage III non-small-cell lung cancer: ASCO guideline. J Clin Oncol. (2022) 40:1356–84. doi: 10.1200/JCO.21.02528
12. Zhou Q, Chen M, Jiang O, Pan Y, Hu D, Lin Q, et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. (2022) 23:209–19. doi: 10.1016/S1470-2045(21)00630-6
13. Park SE, Noh JM, Kim YJ, Lee HS, Cho JH, Lim SW, et al. EGFR mutation is associated with short progression-free survival in patients with stage III non-squamous cell lung cancer treated with concurrent chemoradiotherapy. Cancer Res Treat. (2019) 51:493–501. doi: 10.4143/crt.2018.125
14. Ochiai S, Nomoto Y, Watanabe Y, Yamashita Y, Toyomasu Y, Kawamura T, et al. The impact of epidermal growth factor receptor mutations on patterns of disease recurrence after chemoradiotherapy for locally advanced non-small cell lung cancer: a literature review and pooled analysis. J Radiat Res. (2016) 57:449–59. doi: 10.1093/jrr/rrw075
15. Ma F, Laster K, Dong Z. The comparison of cancer gene mutation frequencies in Chinese and U.S. patient populations. Nat Commun. (2022) 13:5651. doi: 10.1038/s41467-022-33351-4
16. Naidoo J, Antonia S, Wu YL, Cho BC, Thiyagarajah P, Mann H, et al. Brief report: durvalumab after chemoradiotherapy in unresectable stage III EGFR-mutant NSCLC: a post hoc subgroup analysis from PACIFIC. J Thorac Oncol. (2023) 18:657–63. doi: 10.1016/j.jtho.2023.02.009
17. Bi N, Xu K, Ge H, Chen MEM, Zhang L, Cao J, et al. Real-world treatment patterns and clinical outcomes in EGFR-mutant locally advanced lung adenocarcinoma: a multi-center cohort study. J Nat Cancer Center. (2023) 3:65–71. doi: 10.1016/j.jncc.2022.11.003
18. Nassar AH, Kim SY, Aredo JV, Feng J, Shepherd F, Xu C, et al. Consolidation osimertinib versus durvalumab versus observation after concurrent chemoradiation in unresectable EGFR-mutant NSCLC: a multicenter retrospective cohort study. J Thorac Oncol. (2024) 19:928–40. doi: 10.1016/j.jtho.2024.01.012
19. Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. (2014) 4:1046–61. doi: 10.1158/2159-8290.CD-14-0337
20. Lu S, Kato T, Dong X, Ahn MJ, Quang LV, Soparattanapaisarn N, et al. Osimertinib after chemoradiotherapy in stage III EGFR-mutated NSCLC. N Engl J Med. (2024) 391:587–97. doi: 10.1056/NEJMoa2402614
21. Nawaz K, Webster RM. The non-small-cell lung cancer drug market. Nat Rev Drug Discov. (2023) 22:264–5. doi: 10.1038/d41573-023-00017-9
22. George JE, George PS, Krishna JKM, Mathew A. Global trends in lung cancer incidence, mortality by age, gender, gender and morphology and forecast: a bootstrap-based analysis. Lung Cancer. (2025) 205:108626. doi: 10.1016/j.lungcan.2025.108626
23. Zhu Y, Liu K, Zhu H. Immune checkpoint inhibitor for patients with advanced biliary tract cancer: a cost-effectiveness analysis. Liver Int. (2023) 43:2292–301. doi: 10.1111/liv.15699
24. Goldstein DA, Chen Q, Ayer T, Howard DH, Lipscomb J, El-Rayes BF, et al. Cost-effectiveness of atezolizumab plus bevacizumab vs sorafenib as first-line treatment of unresectable hepatocellular carcinoma. J Clin Oncol. (2015) 33:1112–8. doi: 10.1200/JCO.2014.58.4904
25. Su D, Wu B, Shi L. Cost-effectiveness of Atezolizumab plus Bevacizumab vs Sorafenib as first-line treatment of unresectable hepatocellular carcinoma. JAMA Netw Open. (2021) 4:e210037. doi: 10.1001/jamanetworkopen.2021.0037
26. Murray CJ, Evans DB, Acharya A, Baltussen RM. Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ. (2000) 9:235–251.3.0.CO;2-O" doi: 10.1002/(SICI)1099-1050(200004)9:3<235::AID-HEC502>3.0.CO;2-O
27. Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol. (2011) 11:139. doi: 10.1186/1471-2288-11-139
28. Kohn CG, Zeichner SB, Chen Q, Montero AJ, Goldstein DA, Flowers CR. Cost-effectiveness of immune checkpoint inhibition in BRAF wild-type advanced melanoma. J Clin Oncol. (2017) 35:1194–202. doi: 10.1200/JCO.2016.69.6336
29. Drugs.com. Tagrisso Prices, Coupons and Patient Assistance Programs. Available online at: https://www.drugs.com/price-guide/tagrisso (Accessed July 2, 2024).
30. Han J, Tian K, Yang J, Gong Y. Durvalumab vs placebo consolidation therapy after chemoradiotherapy in stage III non-small-cell lung cancer: an updated PACIFIC trial-based cost-effectiveness analysis. Lung Cancer. (2020) 146:42–9. doi: 10.1016/j.lungcan.2020.05.011
31. Zhu Y, Liu K, Qin Q, Zhu H. Serplulimab plus chemotherapy as first-line treatment for extensive-stage small-cell lung cancer: a cost-effectiveness analysis. Front Immunol. (2022) 13:1044678. doi: 10.3389/fimmu.2022.1044678
32. Chen X, Yang Z, Xiang G, Gu L, Qi Z, Wan B, et al. Durvalumab consolidation therapy in patients with stage III non-small cell lung cancer after concurrent chemoradiation: a China-based cost-effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res. (2022) 22:647–54. doi: 10.1080/14737167.2022.1993062
33. Pei Z, Xiao N, Yang P. Cost-effectiveness analysis of tumor treating fields treatment in Chinese patients with metastatic non-small cell lung cancer. Front Public Health. (2024) 12:1276049. doi: 10.3389/fpubh.2024.1276049
34. Kareff SA, Han S, Haaland B, Jani CJ, Kohli R, Aguiar PN Jr, et al. International cost-effectiveness analysis of durvalumab in stage III non-small cell lung cancer. JAMA Netw Open. (2024) 7:e2413938. doi: 10.1001/jamanetworkopen.2024.13938
35. Guan H, Liu G, Xie F, Sheng Y, Shi L. Cost-effectiveness of Osimertinib as a second-line treatment in patients with EGFR-mutated advanced non-small cell lung cancer in China. Clin Ther. (2019) 41:2308–20.e2311. doi: 10.1016/j.clinthera.2019.09.008
36. US Bureau of Labor Statistics. CPI Inflation Calculator. Available online at: https://www.bls.gov/data/inflation_calculator.htm (Accessed July 2, 2024).
37. Ding D, Hu H, Li S, Zhu Y, Shi Y, Liao M, et al. Cost-effectiveness analysis of durvalumab plus chemotherapy in the first-line treatment of extensive-stage small cell lung cancer. J Natl Compr Canc Netw. (2021) 19:1141–7. doi: 10.6004/jnccn.2020.7796
38. Aguiar PN Jr, Perry LA, Penny-Dimri J, Babiker H, Tadokoro H, de Mello RA, Lopes GL Jr. The effect of PD-L1 testing on the cost-effectiveness and economic impact of immune checkpoint inhibitors for the second-line treatment of NSCLC. Ann Oncol. (2017) 28:2256–63. doi: 10.1093/annonc/mdx305
39. Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM modeling good research practices task force working group-6. Med Decis Making. (2012) 32:722–32. doi: 10.1177/0272989X12458348
40. Wan X, Zhang Y, Tan C, Zeng X, Peng L. First-line nivolumab plus Ipilimumab vs Sunitinib for metastatic renal cell carcinoma: a cost-effectiveness analysis. JAMA Oncol. (2019) 5:491–6. doi: 10.1001/jamaoncol.2018.7086
41. Hoyle M, Green C, Thompson-Coon J, Liu Z, Welch K, Moxham T, et al. Cost-effectiveness of temsirolimus for first line treatment of advanced renal cell carcinoma. Value Health. (2010) 13:61–8. doi: 10.1111/j.1524-4733.2009.00617.x
42. Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. (2017) 13:e195–203. doi: 10.1111/ajco.12477
43. Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR, Threapleton D, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. (2016) 7:78985–93. doi: 10.18632/oncotarget.12587
44. Riudavets M, Auclin E, Mosteiro M, Dempsey N, Majem M, Lobefaro R, et al. Durvalumab consolidation in patients with unresectable stage III non-small cell lung cancer with driver genomic alterations. Eur J Cancer. (2022) 167:142–148. doi: 10.1016/j.ejca.2022.02.014
45. Aredo JV, Wakelee HA, Hui AB, Padda SK, Joshi ND, Guo HH, et al. Induction EGFR tyrosine kinase inhibitors prior to definitive chemoradiotherapy in unresectable stage III EGFR-mutated non-small cell lung cancer. Cancer Treat Res Commun. (2022) 33:100659. doi: 10.1016/j.ctarc.2022.100659
46. World Bank Group. World Bank Open Data. Available online at: https://data.worldbank.org.cn/ (Accessed September 25, 2025).
47. Chen S, Cao Z, Prettner K, Kuhn M, Yang J, Jiao L, et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. (2023) 9:465–72. doi: 10.1001/jamaoncol.2022.7826
48. Robinson LA, Hammitt JK, Chang AY, Resch S. Understanding and improving the one and three times GDP per capita cost-effectiveness thresholds. Health Policy Plan. (2017) 32:141–5. doi: 10.1093/heapol/czw096
49. Bertram MY, Lauer JA, De Joncheere K, Edejer T, Hutubessy R, Kieny MP, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. (2016) 94:925–30. doi: 10.2471/BLT.15.164418
50. Institute for Clinical and Economic Review: 2020–2023 Value Assessment Framework. Available online at: https://icer.org/wp-content/uploads/2020/10/ICER_2020_2023_VAF_102220.pdf (Accessed September 25, 2025).
51. National Bureau of Statistics of China. National Data. Available online at: https://data.stats.gov.cn/search.htm (Accessed September 25, 2025).
Keywords: EGFR-mutated, non-small cell lung cancer, osimertinib, chemoradiotherapy, cost-effectiveness
Citation: Tang D, Zou X, Wei C and Zhang X (2025) International cost-effectiveness analysis in osimertinib after chemoradiotherapy in stage III EGFR-mutated non-small cell lung cancer. Front. Public Health 13:1698562. doi: 10.3389/fpubh.2025.1698562
Received: 03 September 2025; Accepted: 09 October 2025;
Published: 29 October 2025.
Edited by:
Nan Zhang, Shandong Cancer Hospital, ChinaReviewed by:
Yuhang Liu, Guangdong Pharmaceutical University, ChinaHarsh Sahu, Jawaharlal Nehru Cancer Hospital and Research Center, India
Copyright © 2025 Tang, Zou, Wei and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaochao Wei, d2NjOTA1OEAxMjYuY29t; Xiaoyu Zhang, emhhbmd4aWFveXUyMDA0QDE2My5jb20=
 Diya Tang1
Diya Tang1 Chaochao Wei
Chaochao Wei