- 1School of Nursing, Nanjing University of Chinese Medicine, Nanjing, China
- 2Department of Cardiology, The Second Hospital of Nanjing, Affiliated to Nanjing University of Chinese Medicine, Nanjing, China
Objectives: This review aims to provide deeper insights into the patient experience by synthesizing qualitative findings from studies on DHTs used in CVD care. Specifically, it seeks to answer the question: What are the key barriers, facilitators, and trends in the use of digital interventions for CVD rehabilitation and self-management? Unlike traditional quantitative reviews, this study focuses on understanding the human factors, personal narratives, and contextual influences that shape the uptake and impact of DHTs in this area.
Methods: A qualitative meta-synthesis was conducted on articles from the following major electronic databases: PubMed, Cochrane Library, CINAHL, Web of Science, Embase, PsycINFO, and Chinese databases, including Chinese National Knowledge Infrastructure (CNKI), Wanfang Database (CECDB), VIP Database, and China Biomedical Database (CBM). Studies published between 2020 and 2025 were included, and a systematic search was conducted using predefined keywords. Papers were selected based on predefined inclusion and exclusion criteria. The synthesis employed the aggregative integration approach from the meta-synthesis method proposed by the Joanna Briggs Institute (JBI) Evidence-based Healthcare Center in Australia. The extracted qualitative data were compared and analyzed in-depth.
Results: A total of 21 studies were included; 92 findings were extracted, organized into 13 categories, and consolidated into five synthesized findings: positive effects of DHTs; barriers to adoption and sustained use of DHTs; optimization needs and design priorities for DHTs; and culture- and disease-specific needs.
Conclusion: Cardiovascular DHTs should be grounded in patients’ individualized needs and operationalized through age-friendly technological innovations, integration of professional support, and culturally sensitive design. Leveraging multiple pathways and modalities to strengthen the family–hospital–community support system will improve the acceptability of DHTs among people with cardiovascular disease. What this review uniquely adds is the patient-reported affective/relational value (e.g., feeling “continuously accompanied,” increased reassurance and trust through human-in-the-loop supervision) and the contextual value of digital health (age-friendly, culturally and disease-specific tailoring integrated with family/clinician support).
1 Introduction
Cardiovascular diseases (CVD) remain the leading cause of death and disability worldwide, and the global burden continues to grow. According to the World Heart Federation’s World Heart Report 2023 (1), CVD caused 20.5 million deaths in 2021—approximately one-third of all global deaths—with 80% occurring in low- and middle-income countries (LMICs). If current trends persist, CVD deaths could rise to 35.6 million by 2050 (2). In China, CVD likewise ranks first among causes of death in both urban and rural populations. The China Cardiovascular Health and Disease Report 2023 (3, 4) estimates that approximately 330 million people are living with CVD—nearly one quarter of adults. In 2021, CVD accounted for more than 40% of all deaths, with higher prevalence in Northeast and North China than in South China (5), underscoring the severity of the burden and regional inequities in prevention and control.
Against this backdrop, digital health tools (DHTs) are a pivotal pathway for optimizing patient management and reducing inequities in resource distribution and access to care. Representative tools—telerehabilitation, mobile health (mHealth) applications, and wearable devices—offer several advantages: continuous remote monitoring, enhanced self-management, strengthened clinician–patient collaboration, and potential gains in cost and efficiency. They show particular promise for improving access and continuity of care in primary care and resource-limited settings (6). Growing evidence (7–9) shows that DHTs improve process indicators such as medication adherence, self-monitoring frequency, and appointment attendance in secondary prevention. These tools have also been shown to improve clinical outcomes like blood pressure control and lipid management. For example, mHealth strategies using interactive voice response (IVR) and text messaging strengthen risk-factor management, facilitate blood-pressure and lipid control, and reduce heart-failure readmissions (10, 11). Wearable devices (e.g., smartwatches, ECG patches) support continuous rhythm surveillance and report positive predictive values of 84–98% for detecting atrial fibrillation, enabling earlier detection and intervention (12). Relevant qualitative study (13) further indicate that patients’ experiences include a sense of being “continuously accompanied” and emotionally supported, as well as identity affirmation, relational trust, informational transparency, perceived privacy, and burden. However, while several studies have explored the impact of DHTs, there is a lack of cross-study synthesis that clarifies how patients experience these tools, why they adopt or resist them, and under what conditions they are effective. Existing qualitative syntheses (14) have examined barriers and facilitators in cardiac rehabilitation, but there is still a gap in understanding the holistic experience of cardiovascular patients with DHTs, particularly in varied settings and across diverse patient populations.
Moreover, individuals with cardiovascular disease are typically older, have multiple comorbidities, and vary widely in functional status (15). These features heighten usability requirements, increase perceived burden, and demand higher levels of health literacy and digital health literacy (16). Differences across the digital health ecosystem—platforms (apps, mini-programs, wearables, telehealth portals), use contexts (post-discharge rehabilitation, community follow-up, home self-management), and health-system levels (primary care, specialty care, disease-specific programs)—produce substantial heterogeneity in patient experience and intervention effects (17, 18). This meta-synthesis aims to fill this gap by providing an integrative and comprehensive understanding of the barriers, facilitators, and optimization needs in DHTs for CVD patients. Unlike prior reviews that primarily emphasized technical efficacy, our meta-synthesis foregrounds patient-reported affective/relational value and contextualized design needs, showing how professional support and culture−/disease-specific tailoring convert data into safety, trust, and sustained action.
2 Methods
2.1 Inclusion and exclusion criteria
2.1.1 Study design (S)
The qualitative research studies from which qualitative data could be extracted, the primary qualitative research studies were included but were not limited to methodologies, such as phenomenology, grounded theory, action research, ethnography, and feminist research.
2.1.2 Participant (P)
Patients with CVD as defined by the World Health Organization (WHO).
2.1.3 Interest of phenomena (I)
Patients’ experiences with DHTs (e.g., mobile health applications, remote monitoring, and wearables), encompassing acceptance, barriers, and facilitators.
2.1.4 Context (Co)
We will exclude studies without accessible full text, duplicate publications, articles not published in Chinese or English, and mixed-methods studies in which the qualitative component cannot be extracted for synthesis.
2.2 Design
This study adopts an exploratory qualitative meta-synthesis design to identify, appraise, and synthesize primary qualitative evidence on CVD patients’ experiences of using DHTs (e.g., mHealth apps, telerehabilitation platforms, remote monitoring, and wearables). This review aims to generate higher-order, transferable insights to inform the patient-centered redesign and implementation of DHTs in CVD care. Reporting will follow PRISMA guidance for qualitative evidence syntheses. A meta-synthesis approach was used to combine and present the qualitative findings (19). Inspired by Sandelowski et al. (20), meta-synthesis of qualitative research is based on the premise of understanding its philosophical thoughts and methodology, repeatedly reading the included literature and extracting the themes and hidden meanings so as to conduct inductive analysis, form new categories, and finally integrate new results. By synthesizing new results, a more profound and substantial explanation can be given to specific phenomena, creating new perspectives and so-called “third-level” findings, providing a more influential and persuasive final conclusion. Relevant articles were searched, and data were extracted and critically evaluated using a thematic synthesis based on the three steps outlined by Thomas and Harden (21), i.e., text coding line by line, developing descriptive themes, and generating analytical themes. This study met the requirements of the Helsinki Declaration.
2.3 Search methods
To ensure adequate performance in searches (i.e., recall, precision, and number needed to read), we selected a combination of 11 databases for the literature search of systematic reviews, including both Chinese and English databases that are widely used in the field of health care. Qualitative studies published in PubMed, Cochrane Library, CINAHL, Web of Science, Embase, CINAHL, PsycINFO and Chinese databases, including Chinese National Knowledge Infrastructure (CNKI), Wanfang Database (CECDB), VIP Database, and China Biomedical Database (CBM) from January 2020 to June 2025 were searched by two researchers (YS and XH) in July 2025. The search terms were developed, and subject headings were used where possible and adjusted for different databases. Four groups of keywords or MeSH terms were included and combined using Boolean operators: (1) heart*, heart disease*, heart disorder*, cardiac*, cardiovascular, coronary heart disease*, myocardial infarction, acute coronary syndrome, angina, stable, heart failure, arrhythmias, cardiac, cardiac insufficiency, valvular heart disease*, aortic dissection, congenital heart disease*, hypertension; (2) Telemedicine, Mobile Applications, Wearable Electronic Devices, Digital Health, mHealth, eHealth, health app*, smartwatch, remote monitor*; (3) Patient Acceptance of Health Care, “Attitude to Health OR Health Knowledge, Attitudes, Practice,” acceptab*, barrier*, facilitator*, adherence, user experience, perception*; (4) Qualitative Research, Focus Groups, Interviews as Topic, Narratives, Ground Theory, phenomenolog*, ethnograph*, thematic analysis, semi-structured interview*, lived experience. To determine the eligibility of the potentially relevant studies, all titles and abstracts were reviewed by researchers. Box 1 showed the search strategy with PubMed as an example.
BOX 1. Search strategy in PubMed.
#1 (("Myocardial Infarction"[Mesh]) OR "Acute Coronary Syndrome"[Mesh] OR "Angina, Stable"[Mesh] OR "Heart Failure"[Mesh] OR "Arrhythmias, Cardiac"[Mesh] OR "Aortic Dissection"[Mesh] OR (heart*[Title/Abstract] OR heart disease*[Title/Abstract] OR heart disorder*[Title/Abstract] OR cardiac*[Title/Abstract] OR cardiovascular[Title/Abstract] OR coronary heart disease*[Title/Abstract] OR cardiac insufficiency[Title/Abstract] OR valvular heart disease*[Title/Abstract] OR congenital heart disease*[Title/Abstract] OR hypertension[Title/Abstract]))
#2 ((Telemedicine[MeSH Terms] OR Mobile Applications[MeSH Terms] OR Wearable Electronic Devices[MeSH Terms] OR Digital Health[Title/Abstract] OR mHealth[Title/Abstract] OR eHealth[Title/Abstract] OR health app*[Title/Abstract] OR smartwatch[Title/Abstract] OR remote monitor*[Title/Abstract]))
#3 (("Patient Acceptance of Health Care"[Mesh] OR "Attitude to Health"[Mesh] OR "Health Knowledge, Attitudes, Practice"[Mesh] OR acceptab*[Title/Abstract] OR barrier*[Title/Abstract] OR facilitator*[Title/Abstract] OR adherence[Title/Abstract] OR user experience[Title/Abstract] OR perception*[Title/Abstract]))
#4 (("Qualitative Research"[Mesh] OR "Focus Groups"[Mesh] OR "Interviews as Topic"[Mesh] OR "Narration"[Mesh] OR Ground Theory[Title/Abstract] OR phenomenolog*[Title/Abstract] OR ethnograph*[Title/Abstract] OR thematic analysis[Title/Abstract] OR semi-structured interview*[Title/Abstract] OR lived experience*[Title/Abstract]))
#5 #1 AND #2 AND #3 AND #4
Filters: 2020–2025
Search results:165
2.4 Search outcomes
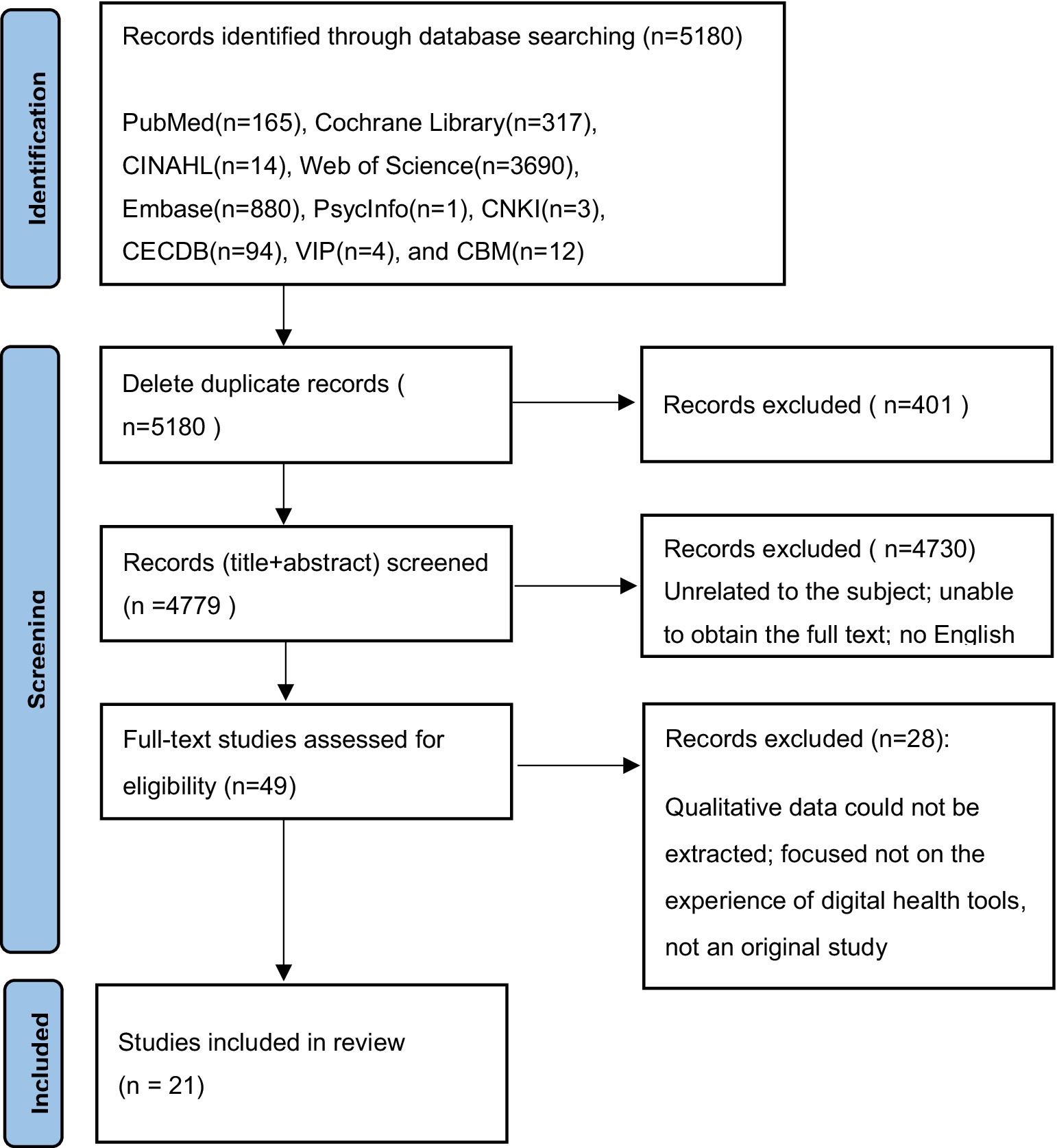
Two researchers independently screened and extracted the literature according to the inclusion and exclusion criteria. An initial search using the above strategy yielded a total of 5,180 articles. First, the titles and abstracts of the articles were read to exclude those unrelated to the subject, were repetitive, and full text could not be obtained. Subsequently, 5,131 articles were excluded. After reading the full texts, 28 articles were excluded, and finally, 21 articles were identified as relevant. Also, no articles were traced from references. This search process is illustrated in Figure 1.
2.5 Quality appraisal
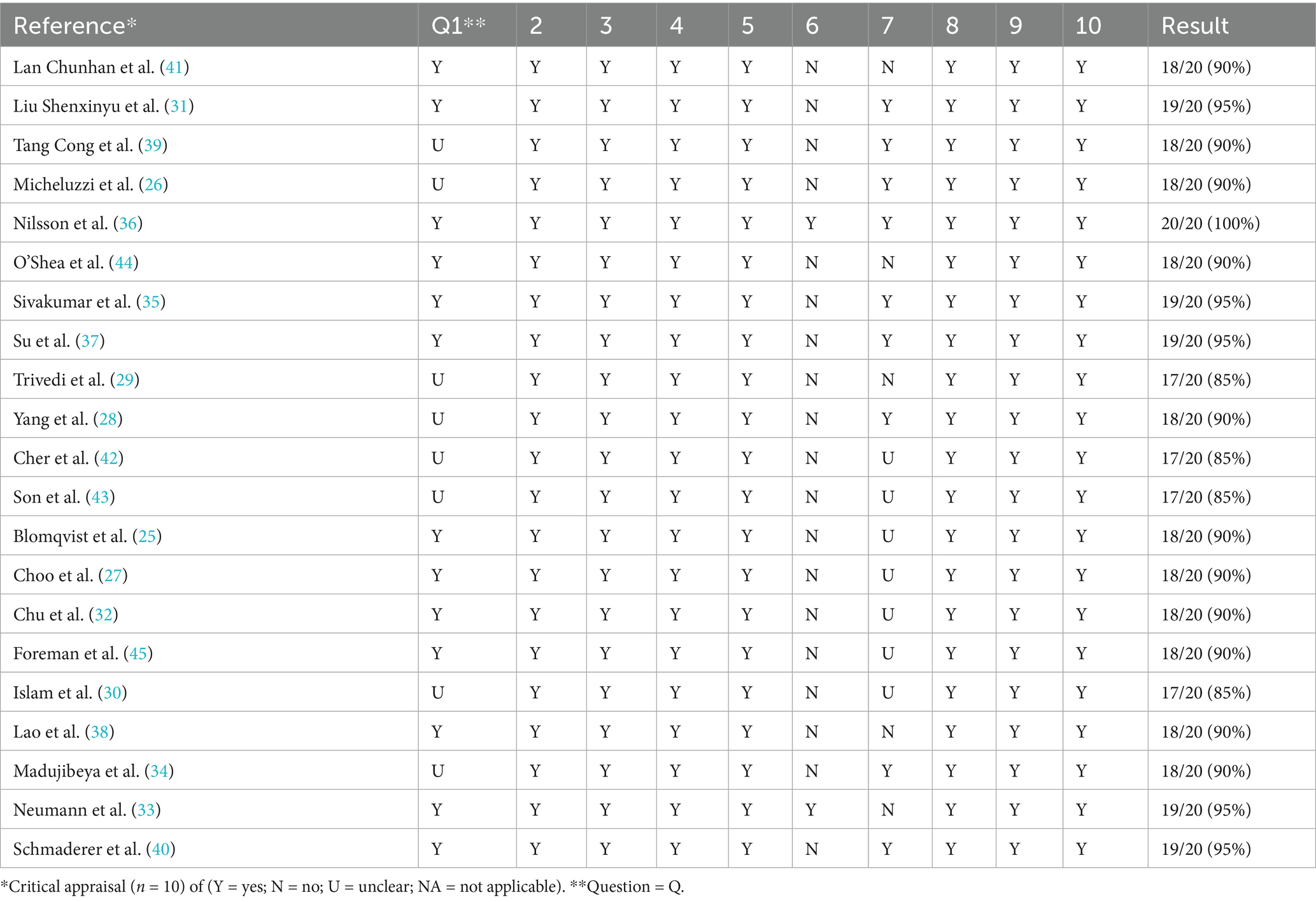
Two researchers independently assessed the methodological quality of the 21 included studies. Initially, the authors worked independently using the Joanna Briggs Critical Assessment Tool for Methodological Quality Assessment (22). This evaluation tool is widely applicable in the appraisal of qualitative research. The quality appraisal of research mainly focuses on the internal authenticity of the research, that is, the degree to which the research results are close to the true value, in order to determine whether there is bias. The evaluation tool consists of 10 questions designed to quickly and efficiently evaluate the studies with a simple yes, no, or unclear to each question. Each criterion was allocated a score (Yes = 2, No = 0, Unclear = l), giving a total score of 20 for each study. These scores were then converted to a percentage. Subsequently, the results were discussed to reach a consensus, studies with a score of more than 70% were considered acceptable after quality appraisal, otherwise they were excluded.
2.6 Data extraction
A comprehensive study was conducted to characterize the quality of the content and assess the methodological development in the collected studies (23). The extracted data included the author, the year of publication, country or region, research method, research subjects, interesting phenomena, and main research results. The results were cross-reviewed by two investigators, and any disagreement was resolved by discussion with a third investigator.
2.7 Data analysis and synthesis
We used meta-aggregation to synthesize the findings of the qualitative studies (24). This method of systematic review involves categorizing and re-categorizing the synthesized findings of two or more studies. First, each selected article was read several times to increase the understanding of research objectives, methods, and conclusions. Next, the results of each study are extracted, along with the research results showing that the results of data and text. The consistency between the research results and supporting data was assessed by two researchers independently, each finding provided its own level of credibility (23). For qualitative data, there are three levels of credibility: (1) Unequivocal (U): relates to evidence beyond a reasonable doubt, which may include findings that are matter of fact, directly reported/observed and not open to challenge; (2) Credible (C): those that are, albeit interpretations, plausible in light of data and the theoretical framework, They can be logically inferred from the data; (3) Not Supported (NS): when 1 nor 2 apply and when most notable findings are not supported by the data. Results were then encoded according to their meaning and content. Researchers looked for similarities and differences between the findings and the textual data, and the meanings of the original data set were classified. For each theme, when needed, sub-themes were also developed following the same process. Finally, these categories were repeatedly assessed to identify the similarities and obtain synthesized results.
2.8 Ethical considerations
This meta-analysis was carried out in compliance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines and referred only to published data. Therefore, no ethical approval was required. However, ethical approval in the included empirical qualitative studies were assessed.
3 Results
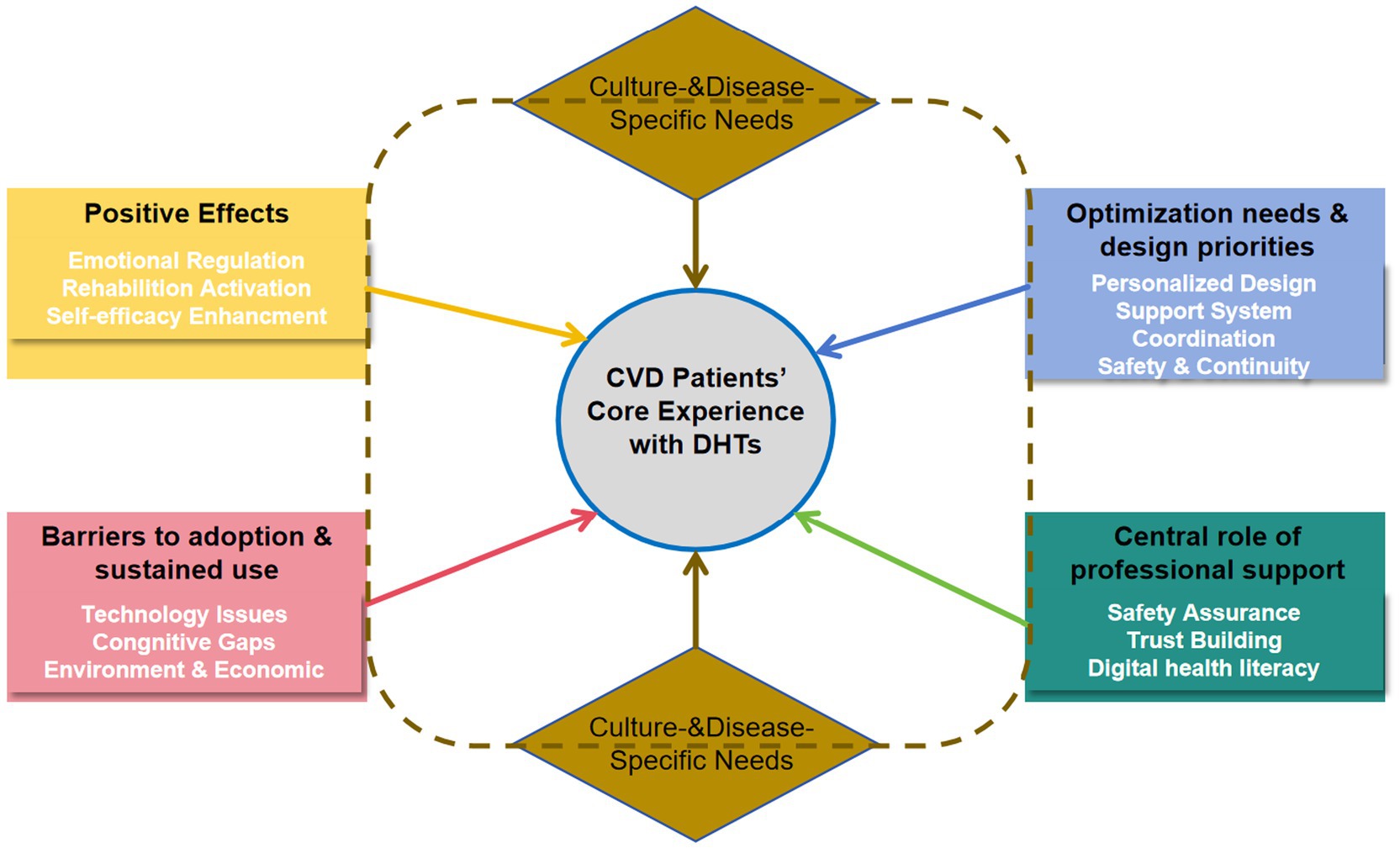
The 21 studies (25–45) were conducted in the following countries: China (n = 4), USA (n = 4), Italy (n = 1), Sweden (n = 2), Ireland (n = 1), Canada (n = 1), Australia (n = 2), Singapore (n = 1), Korea (n = 2), Chinese Hong Kong (n = 1), Chinese Macao (n = 1) and Germany (n = 1). These studies involved 442 CVD patients. Research methods included: phenomenological approaches (n = 12), descriptive qualitative analyses (n = 8) and grounded theory (n = 1). All studies were published after 2020 and were original articles. The results of the literature quality appraisal were shown in Table 1, the extraction results were presented in Table 2. The PRISMA was used as a basis for the results of syntheses. Five major themes emerged from the selected studies, reflecting the real experience of CVD Patients’ experiences of using DHTs. These themes were: Positive effects of DHTs, Barriers to adoption and sustained use of DHTs, Optimization needs and design priorities for DHTs, Central role of professional support in DHTs implementation, Culture- and disease-specific needs. The themes were divided into several sub-themes of meaningful units, as demonstrated in Table 3. The dynamic relationships between these five synthesized findings are illustrated in a conceptual model (Figure 2), which positions patient-centered DHI implementation as the ultimate goal influenced by the other four themes.
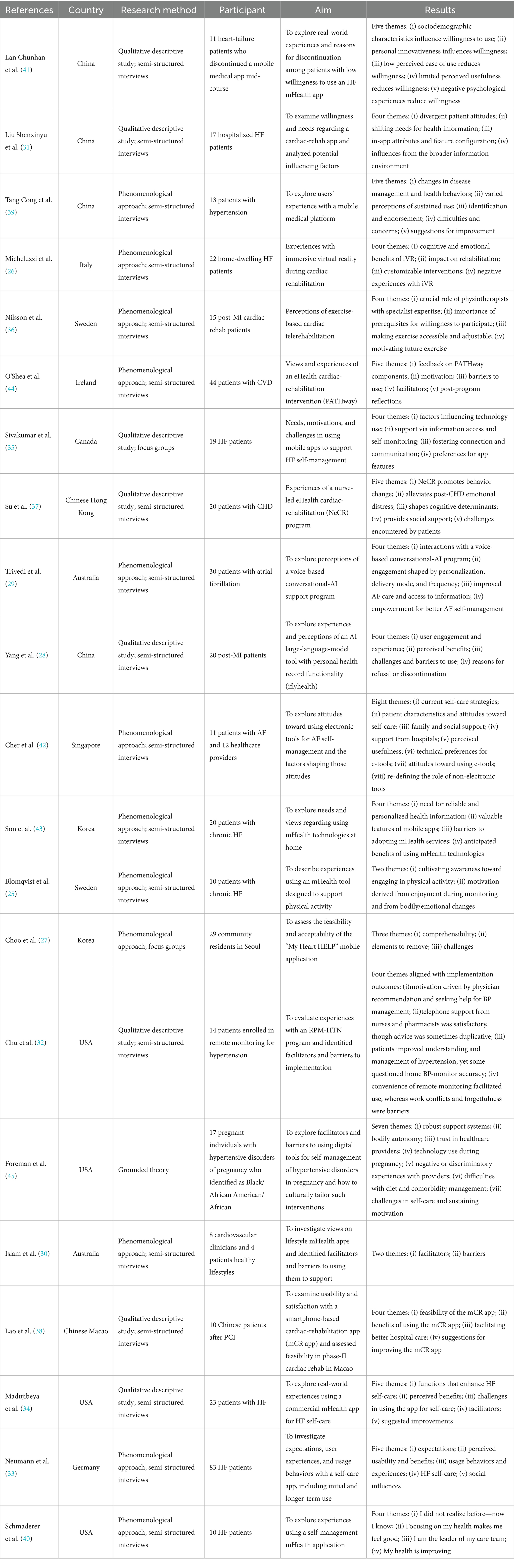
Table 2. Characteristics of included studies (n = 21): country/region, method, participants, aims, and key findings.
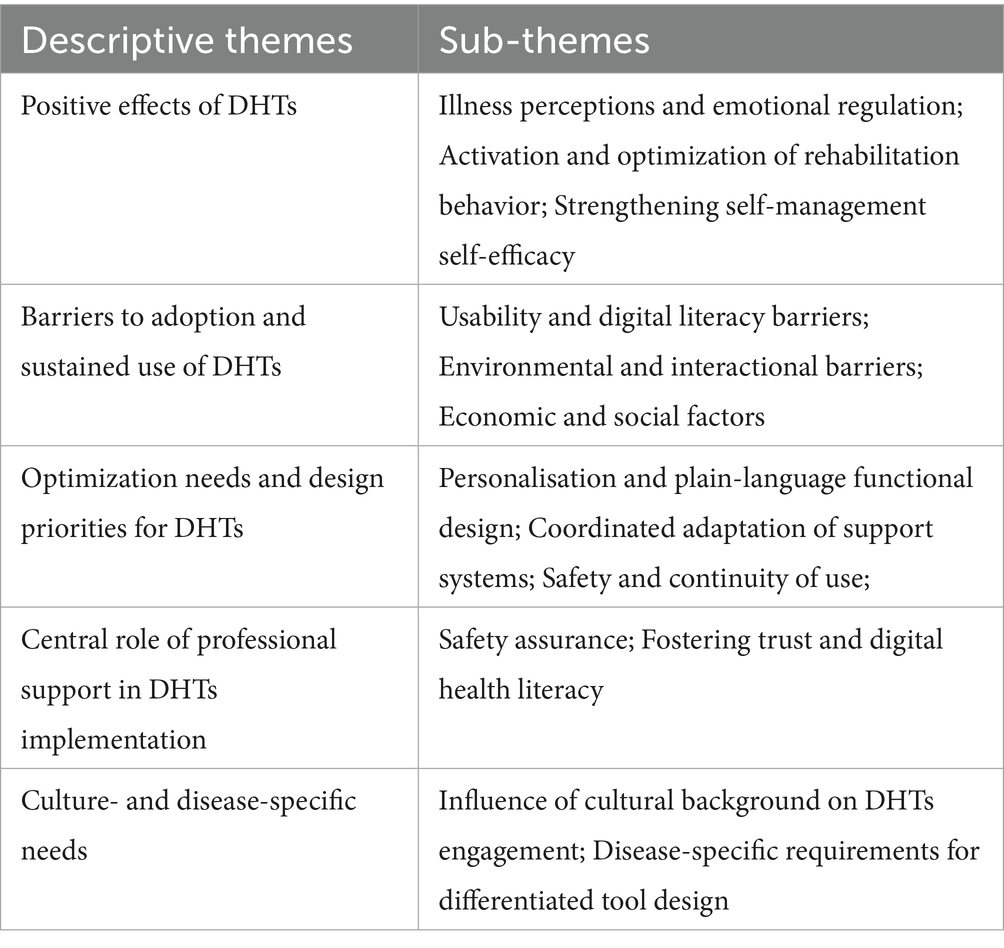
Table 3. Descriptive themes and sub-themes derived via meta-aggregation, mapped to the five synthesized findings.
3.1 Theme 1: positive effects of DHTs
3.1.1 Illness perceptions and emotional regulation
Some patients reported highly positive experiences with immersive virtual reality (VR). By presenting diverse scenes (e.g., forests, beaches) for people with heart failure, VR reframed rehabilitation from “monotonous repetition” to “travel-like exploration,” increasing acceptance and reducing anxiety “The experience exceeded my expectations; … It was interesting to see places I did not know… making the session feel more like an exploration…” (26). Smartphone applications provided education and peer support, alleviating loneliness and fear “…helps to talk to people who are going through the same stuff because family they do not always understand” (35). Some patients described a dampened sense of time that reduced aversion to prolonged exposure. Interactive guidance during telerehabilitation helped them focus on the task “… Time flies without you realizing it…; …Virtual reality makes you not think about the passing of time…; When you follow the video, you disconnect…” (26). Mobile apps and conversational agents delivered plain-language explanations that addressed knowledge gaps and, in turn, reduced anxiety. For example, patients with heart failure used app animations to understand reduced ejection fraction “maybe… reminder about a particular symptom that might occur that would require attention… I’ve got a pacemaker… I’ve got a couple of stents…; …would rather use an app, which would provide them ‘assurance’ that they are taking their medication” (35). After viewing educational videos distinguishing paroxysmal from permanent atrial fibrillation, patients reported less anxiety “having the ability to monitor their heart rhythm … can be reassuring … and to better manage their symptoms and anxiety” (29). This sub-theme highlights that DHTs can significantly improve the subjective illness experience by reducing anxiety and fostering positive engagement. Therefore, effective interventions should integrate emotional and cognitive support mechanisms alongside clinical monitoring to enhance holistic patient care.
3.1.2 Activation and optimization of rehabilitation behavior
Some patients reported that DHTs reduced constraints of time and place, enabling home-based training and supporting maintenance of rehabilitation routines. “It easily takes two, two and a half hours if you do not do the exercise at home and therefore, I think this is great, because it cuts the timeframe” (36). In-app prompts and progress feedback helped sustain long-term motivation. “I think it helped me stay motivated and made the sessions feel less like a chore; it feels almost as though you are pedaling with less effort because you are psychologically encouraged to follow the music and everything around you” (26), “Yes, I would like to continue, but my exercise period is over, I understand. But then it is good that I have already established a routine to continue on my own” (36). These findings emphasize the DHTs should provide positive feedback, facilitating sustained adherence to rehabilitation protocols, a key component of personalized and effective patient-centered care.
3.1.3 Strengthening self-management self-efficacy
Some patients reported that DHTs consolidate disease-related information (e.g., symptoms, diet) in one place, reducing the fragmentation and noise of general online sources and enhancing credibility. “I have everything in one particular place, I know because the app is geared specifically to heart issues, I know that I can trust the information on the app” (35). These applications also support precise self-monitoring by enabling recording of blood pressure, weight, and other indicators, thereby helping patients and clinicians track trends. “I measure my blood pressure twice a week; the platform prompts me to upload data at least once weekly, and I now have a clearer sense of my blood-pressure pattern” (39). Some patients added that goal setting and feedback incentives facilitated lifestyle change. “With that medication reminder and then weighing myself every day to see if I was retaining water, it helped me a lot…” (40). By centralizing information and facilitating self-monitoring, DHTs empower patients, increasing their confidence in managing their condition and provide actionable insights.
3.2 Theme 2: barriers to adoption and sustained use of DHTs
3.2.1 Usability and digital literacy barriers
Patients reported physical discomfort and performance issues with hardware-dependent tools (e.g., virtual reality), which impaired usability. “The headset sometimes bothered me a bit;… it’s often out of focus, never completely sharp…” (26), “My phone was too old, and it does not support apps like this” (28). These systems had software glitches, connectivity problems, and cumbersome workflows that hindered use. “Screening… was a bit cumbersome, it was another element to the whole start-up, so it was just another layer of… hassle” (44). Multi-device configurations (e.g., the PATHway system requiring both a computer and an Xbox) demand composite skills, including device configuration, troubleshooting, and procedural memory. Yet most CVD patients are older adults who lack experience with complex technologies “I found that there was too many bits… you had a computer, you had the X-Box… and everything had to be plugged in a certain way and you did not have enough power sockets. Then you kept running out of power and there were cables trailing. So, it was a bit cumbersome” (44). These technical challenges were often exacerbated by limited digital health literacy. Some older adults struggled to operate devices and applications. “I’m not used to using apps, not even using a cell phone, so figuring out the AI was really difficult at first. It took me two visits to the hospital to really learn how to use it… My son helped me too” (28). In addition, lower educational attainment may foster misconceptions about technology that hinder use. “I only attended school through third grade. With this high-tech app, I do not know how to keep using it” (31). Fundamentally, these challenges reflect an inadequate translation of professional information into accessible, plain language within digital tools. To overcome these barriers, DHTs should be designed with simplicity in mind, ensuring that they are intuitive, easy to use, and compatible with a range of devices.
3.2.2 Environmental and interactional barriers
Some middle-aged and younger adults reported less frequent data entry due to competing work demands. “When I first enrolled, I uploaded my blood pressure daily; now that work is busy, I log it every 3 days” (39). Comorbid conditions and age-related visual decline also impeded use. “The starting level was too high for me; I am 75 and I have never used a computer; Sometimes my body was very painful… I did not feel up to it at the time” (44). “My eyesight is poor. Even with large fonts, the platform has too many sections and not all display on my screen; I sometimes need my children’s help” (39). The design of DHTs must consider patients’ daily environments and health conditions, providing flexibility to accommodate varying schedules and physical capabilities. Features such as voice-assisted data entry or automated reminders can help overcome these interactional barriers, ensuring that DHTs remain useful and accessible for all patients, regardless of their circumstances.
3.2.3 Economic and social factors
Some patients expressed cost-related concerns about using DHTs. “But before that I will ask, all these, right, will you charge to us? Because my husband is not working, I’m not working myself …” (42). “It is free now, but will it remain free? If not, can health insurance reimburse the fees?” (41). Patients in remote areas, or those with limited hardware support, often face practical constraints. “Discontinuation of Internet access at home, loss of program information after changing the phone, and device failure” (37). Therefore, policymakers and healthcare providers should prioritize affordable, low-cost DHTs to ensure equitable access. Cost should not be a barrier to care, and health insurance reimbursement policies should cover DHTs to avoid exacerbating health inequities.
3.3 Theme 3: optimization needs and design priorities for DHTs
3.3.1 Personalisation and plain-language functional design
Patients emphasized the need for tailoring by disease type, stage, baseline knowledge, and personal preferences. Generic push notifications provided little disease-specific value. “…they wanted targeted information that was relevant to their disease status … and type of AF” (29). “Vague ‘how are you’ messages are not useful; I need professional, condition-specific information” (31). Older adults called for age-friendly design. Complex data entry, multiple steps, and non-essential features reduced willingness to use. “There are too many fields and screens; the selector is small, and if I forget to save I must re-enter everything” (41). Others reported excessive jargon and abbreviations in push content, limiting comprehension. “It is full of abbreviations and obscure terms” (31). A subset lacked intrinsic motivation and perceived low utility. “I am not convinced the management app helps beyond my medications” (41). The strong demand for personalized and plain-language design highlights a gap between current DHTs offerings and patient needs. This points to the necessity of user-centered design processes that actively involve patients, especially older adults, in co-creating tools that are both relevant and accessible.
3.3.2 Coordinated adaptation of support systems
Patients emphasized that problems are inevitable during use and called for stronger professional and social support to reduce barriers. “I messaged the nurse about stomach discomfort after taking my medication. She suggested ways to relieve it, reminded me of possible side effects, and advised seeing a gastroenterologist if symptoms persisted” (38). Participants wanted clinicians to access longitudinal app data to inform decisions, optimize treatment plans, and streamline consultations. “If the cardiologist could review 6 months of my blood-pressure and activity data in the app, that would be ideal” (35). Integrating social support helped family members understand the illness and enabled peer-to-peer communication. “Having a community means I can ask others, ‘Do you have this symptom? What happened?’ when symptoms start” (35). Several patients reported insufficient onboarding and troubleshooting support, leading to over-reliance on family members. “At the start, a 15 min onboarding session to demonstrate set-up and audio would help” (36). “My daughter is good at IT; I ask her, and she helps me” (44). So, DHTs should be integrated into a broader ecosystem of care that includes both clinical professionals and informal support systems like family and peer networks. This calls for policies that facilitate seamless communication and data sharing between patients, healthcare providers, and caregivers, ensuring that DHTs are not isolated but part of a holistic, patient-centered care model.
3.3.3 Safety and continuity of use
Some patients expressed concerns about potential disclosure of personal health information when using DHTs. “I recognize that some data sharing is unavoidable, but the possibility of online leakage and misuse makes me uncomfortable” (43). Others recommended extending the service period to support the internalization of health behaviors as daily habits. “If all functions of mCR app can be offered … for 1 year after hospital discharge, it’ll be much better for patient’s self-care as some medications (dual antiplatelet therapy) should be adhered for 1 year” (38). Therefore, two fundamental nursing strategies are crucial for DHTs success: fostering trust through proactive education on data security to empower patients, and securing long-term engagement by advocating for DHTs designs that correspond to clinical pathways and support behavior internalization.
3.4 Theme 4: central role of professional support in DHTs implementation
3.4.1 Safety assurance
Patients reported that clinicians’ real-time supervision and professional judgment mitigated risks associated with using DHTs. For example, during telerehabilitation, physiotherapists’ real-time monitoring (e.g., heart rate, movement quality) provided reassurance. “It’s a sense of security, that someone who actually has in-depth expertise and knows what I need to exercise in terms of fitness and strength, both how much and what is best for me, because I’m not fully aware of that” (36). Nurses also monitored app data and intervened proactively to reduce deterioration risk. “I used to just keep papers on the counter with my weight and I like this (OnTrack) better and if I see my weight go up, I send a note to a nurse and there is help” (34). This highlights the importance of integrating professional oversight into the use of DHTs. Therefore, DHTs should include features that facilitate healthcare providers access to real-time data, fostering collaboration between patients and their healthcare teams, a critical aspect of patient-centered care.
3.4.2 Fostering trust and digital health literacy
Clinicians’ endorsement of DHTs enhances patients’ trust and willingness to engage with the program. “If my treating physician emphasizes the importance of rehabilitation and recommends downloading an app for self-monitoring because it helps, I will use it” (31). Nurses deliver structured onboarding and hands-on training to support patients with limited digital health literacy; when needed, they also train relatives or younger family members. “This mCR app suits me, but older adults with limited literacy or unfamiliar with smartphones may have difficulties; nurses can teach relatives or younger family members how to use it” (38). Professional support is not merely an adjunct but a foundational component for successful DHT implementation. Healthcare professionals act as gatekeepers of trust, safety, and knowledge. This highlights the critical need to equip clinical staff with the skills to effectively support patients’ digital health journeys, integrating these new tools into standard care pathways.
3.5 Theme 5: culture- and disease-specific needs
3.5.1 Influence of cultural background on DHTs engagement
East Asian (China, Hong Kong, China, and Macao, China): Family involvement was a significant factor. Patients from these regions frequently requested that their caregivers, particularly family members, have access to app data. This reflects the culturally ingrained value placed on family support in healthcare. For example, one participant from China noted, “Could my daughter view my blood pressure on her phone? I am often home alone, and my daughter works in another city; real-time access would ease her concerns” (38). Such requests were more common among Chinese participants compared to other regions.
Western Countries (USA, Ireland): While family involvement was also noted, the engagement was more individualistic. Patients in these regions expressed a preference for clinicians to have access to digital health data to provide ongoing support. The desire for clinician involvement reflects a more clinical-centered approach to care, as exemplified by a participant in Ireland who stated, “If my treating physician emphasizes the importance of rehabilitation and recommends downloading an app for self-monitoring because it helps, I will use it” (31).
LMICS (Africa and African-American Communities): In specific populations such as Black pregnant women in the USA, cultural beliefs surrounding race and ethnicity influenced trust in healthcare providers. Participants in these studies expressed a preference for race-concordant clinicians, believing they would understand their lived experiences better. One participant shared, “A Black female physician would understand me; I would be more willing to confide in her” (44). This highlights how cultural identity can influence trust and the willingness to engage with DHTs. So culturally competent nursing strategies for DHT implementation include: (1) promote family-integrated care in East Asian contexts to leverage collective support, (2) strengthen the clinician-patient alliance in Western settings by highlighting professional monitoring, and (3) ensure cultural safety and address representation for marginalized groups to build foundational trust.
3.5.2 Disease-specific requirements for differentiated tool design
Patients with heart failure emphasized the importance of fluid management and daily weight monitoring. However, patients in Western countries also requested real-time feedback from healthcare professionals, such as “If the cardiologist could review 6 months of my blood-pressure and activity data in the app, that would be ideal” (35). Conversely, Chinese participants focused more on practical, day-to-day tracking without as much emphasis on professional monitoring.
Patients with AF in the USA and Europe prioritized heart-rate monitoring and stroke-risk alerts in their DHTs. The distinction between paroxysmal and permanent AF was often requested, with one patient noting, “When my AF symptoms occur, the program is more useful; I need information tailored to my status and AF type” (28). While these concerns were universal, USA patients tended to focus more on educational content regarding medication adherence and stroke prevention, whereas European patients expressed a need for emotional and psychological support during episodes.
In LMICs, where hypertension is a major health concern, patients preferred simpler, more accessible DHTs that could be used by individuals with lower health literacy. For instance, one participant from China emphasized, “I knew I should eat low salt and low fat, but I did not know what those terms actually meant” (38). These problems reflect effective DHTs must be disease-specific, offering tools tailored to the unique management needs of each condition. Additionally, they should be adaptable to local healthcare systems, patient literacy levels, and cultural practices.
4 Discussion
This qualitative meta-synthesis integrates related evidence to distill five core insights on DHTs for patients with cardiovascular disease. DHTs show substantial potential to strengthen emotional self-regulation, support rehabilitative behaviors, and enhance self-efficacy; however, uptake is constrained by multilevel barriers spanning usability, digital literacy, contextual demands of everyday life, and socioeconomic constraints. Together, these findings argue for multidimensional personalization, embedded professional support, and culturally adapted design to realize patient-centered implementation and align with the broader digital transformation of cardiovascular care. Our review offers a more comprehensive perspective than prior research that has primarily focused on clinical effectiveness.
A significant regional difference was observed in how cultural values influence patient engagement. In China, Hong Kong, China and Macao, China, patients demonstrated a strong preference for family involvement in managing their health via digital tools, reflecting the cultural value placed on family-centered healthcare. Research (46) shows that in these regions, DHTs should prioritize user-centered designs that integrate traditional values and address differences between patients and caregivers. This contrasts with Western countries (USA, Europe), where patients expressed a stronger preference for clinician engagement with digital tools. Patients in these regions valued healthcare professionals’ involvement in interpreting health data and providing continuous support. For instance, a national survey (47) of 352 mental health professionals in the USA found that 74.2% of respondents preferred to integrate DHTs into clinical practice. However, disconfirming evidence emerged in some populations, particularly among African-American patients in the USA, where a lack of trust in technology was reported. Some patients were skeptical about the accuracy and reliability of DHTs, especially when these tools were not directly linked to trusted healthcare providers. This finding highlights that cultural identity and healthcare system trust significantly impact technology acceptance. Related studies (48–50) indicate that African-American communities historically face mistrust in healthcare systems due to past discriminatory practices, which further compounds their reluctance toward adopting new technologies. This suggests that DHTs should not only account for digital literacy but also the trust patients have in the tools they use. By understanding these cultural and systemic factors, DHTs can be better designed to ensure broader acceptance and engagement.
Disease-specific needs also reveal important contextual nuances in HF. Across Western and Asian settings, patients consistently emphasize tools for fluid management (e.g., tailored fluid limits), daily weight monitoring, and tracking worsening symptoms—features reflected in HF self-care guidance and in reviews of HF self-management apps (51–53). However, preferences for clinician involvement vary by region. In Western health systems, qualitative studies of HF telemonitoring report that patients value real-time feedback and clinical staff oversight when sharing weight and symptom data (35, 54). By contrast, studies (55, 56) from China frequently highlight app-enabled, WeChat-based self-management models that emphasize independent daily tracking with intermittent professional input—an approach many patients find acceptable and effective for routine management.
Beyond cultural and disease-specific factors, regional disparities in economic conditions and technological infrastructure also presented significant barriers to the adoption of DHTs. In LMICs, patients faced significant barriers to engaging with these digital tools due to poor infrastructure, such as unreliable internet access and limited access to the required hardware (57). These barriers highlight the systemic limitations that hinder the success of DHTs, despite their potential to improve disease management. Disconfirming evidence from these regions suggested that remote monitoring and self-reported data were often insufficient for effectively managing acute conditions. For acute fluid retention in HF, self-report–dominant remote programs showed neutral effects (e.g., TELE-HF) (58), whereas approaches coupling continuous physiologic data with structured clinician oversight (e.g., TIM-HF2; PA-pressure monitoring) reduced events—highlighting the need for real-time clinical review in high-risk contexts (59, 60).
The internal design of DHTs—particularly their degree of personalization—profoundly influences their adoption and impact. In China, where family involvement is crucial, tools should incorporate features that allow caregivers to access health data. Meanwhile, in Western countries, the focus should be on clinician-centered features that enable healthcare providers to monitor progress in real-time. Moreover, as older adults are prevalent in many CVD patient populations, age-friendly design remains crucial for improving usability, particularly in Western populations where digital health literacy can be a barrier. However, disconfirming evidence arose from both European and North American studies, where patients voiced frustrations about receiving irrelevant or overly complex information (61). These findings suggest that while personalization is important, it must be executed carefully to avoid overwhelming users. Overly generic content and complex user interfaces can lead to disengagement, indicating that DHTs must strike a balance between personalization and simplicity.
Professional support is universally recognized as critical to the success of DHTs. In Western countries, patients typically prefer continuous engagement with healthcare providers to ensure effective use of these tools (62–64). In contrast, patients in Asian countries, such as China and Korea, demonstrate higher self-reliance, favoring tools that require minimal clinician involvement. Despite these cultural differences, clinician training emerges as a common systemic barrier (65). Even in high-income countries, many clinicians are unfamiliar with the digital tools used by their patients, limiting their ability to effectively interpret the digital health data (66, 67). This knowledge gap impedes the full potential of DHTs. Additionally, the lack of integration between DHTs and clinical workflows further hinders their successful implementation (68). These challenges underscore the need for comprehensive education programs for healthcare professionals to ensure effective data sharing, improve clinician familiarity with digital tools, and promote personalized care.
5 Limitations
This review focuses on qualitative studies, which provide in-depth insights but may limit generalisability due to the predominance of studies conducted in China, the USA, and Europe. Consequently, populations in regions such as Sub-Saharan Africa and Latin America are underrepresented. Cultural and healthcare system differences in these regions may influence patients’ engagement with and experiences of DHTs.
Although the analysis was conducted rigorously using Joanna Briggs Critical Assessment Tool and master tutor consultation to minimize bias, the inherent subjectivity of qualitative research remains a consideration.
Furthermore, the included studies varied in sample sizes and the duration of DHTs usage, which may influence the findings. The range of digital tools and platforms used across studies adds another layer of variability, though this diversity reflects real-world applications of DHTs in different contexts.
Author contributions
YS: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. XH: Conceptualization, Methodology, Writing – review & editing. QP: Conceptualization, Data curation, Formal analysis, Writing – review & editing. MH: Conceptualization, Formal analysis, Methodology, Writing – review & editing. CL: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Talent Support Project of The Second Hospital of Nanjing (Grant No. RCMS24010).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Murray, CJL. The global burden of disease study at 30 years. Nat Med. (2022) 28:2019–26. doi: 10.1038/s41591-022-01990-1
2. Chong, B, Jayabaskaran, J, Jauhari, SM, Chan, SP, Goh, R, Kueh, MTW, et al. Global burden of cardiovascular diseases: projections from 2025 to 2050. Eur J Prev Cardiol. (2025) 32:1001–15. doi: 10.1093/eurjpc/zwae281
3. Hu, S-S. Report on cardiovascular health and diseases in China 2021: an updated summary. J Geriatr Cardiol. (2023) 20:399–430. doi: 10.26599/1671-5411.2023.06.001
4. Center For Cardiovascular Diseases The Writing Committee Of The Report On Cardiovascular Health And Diseases In China N. Report on cardiovascular health and diseases in China 2023: an updated summary. Biomed Environ Sci. (2024) 37:949–92. doi: 10.3967/bes2024.162
5. Yichong, LI, Shiwei, LIU, Xinying, Z, and Maigeng, Z. Report on burden of cardiovascular diseases from 1990 to 2016 in China [in Chinese]. Chin Circ J. (2019) 34:729–40. doi: 10.3969/j.issn.1000-3614.2019.08.001
6. McDonagh, ST, Dalal, H, Moore, S, Clark, CE, Dean, SG, Jolly, K, et al. Home-based versus Centre-based cardiac rehabilitation - McDonagh, STJ (2023) | cochrane library. Available online at: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD007130.pub5/full?utm_source=chatgpt.com (accessed September 20, 2025).
7. Tam, HL, Wong, EML, Cheung, K, and Chung, SF. Effectiveness of text messaging interventions on blood pressure control among patients with hypertension: systematic review of randomized controlled trials. JMIR Mhealth Uhealth. (2021) 9:e24527. doi: 10.2196/24527
8. Umeh, CA, Torbela, A, Saigal, S, Kaur, H, Kazourra, S, Gupta, R, et al. Telemonitoring in heart failure patients: systematic review and meta-analysis of randomized controlled trials. World J Cardiol. (2022) 14:640–56. doi: 10.4330/wjc.v14.i12.640
9. Perez, MV, Mahaffey, KW, Hedlin, H, Rumsfeld, JS, Garcia, A, Ferris, T, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. (2019) 381:1909–17. doi: 10.1056/NEJMoa1901183
10. Zhu, Y, Gu, X, and Xu, C. Effectiveness of telemedicine systems for adults with heart failure: a meta-analysis of randomized controlled trials. Heart Fail Rev. (2020) 25:231–43. doi: 10.1007/s10741-019-09801-5
11. Thakkar, J, Kurup, R, Laba, T-L, Santo, K, Thiagalingam, A, Rodgers, A, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Intern Med. (2016) 176:340–9. doi: 10.1001/jamainternmed.2015.7667
12. Lubitz, SA, Faranesh, AZ, Selvaggi, C, Atlas, SJ, McManus, DD, Singer, DE, et al. Detection of atrial fibrillation in a large population using wearable devices: the Fitbit heart study. Circulation. (2022) 146:1415–24. doi: 10.1161/CIRCULATIONAHA.122.060291
13. Hamborg, T. G., Tang, L. H., Andersen, R. M., Skou, S. T., and Simonÿ, C. It is like someone holding your hand when you need it – lived experiences of patients with cardiovascular disease participating in a digital health intervention focusing on the maintenance of physical activity after cardiac rehabilitation. Disabil Rehabil Assist Technol (2024). Available online at: https://www.tandfonline.com/doi/abs/10.1080/17483107.2023.2228839 (accessed July 30, 2025).
14. Tadas, S, and Coyle, D. Barriers to and facilitators of technology in cardiac rehabilitation and self-management: systematic qualitative grounded theory review. J Med Internet Res. (2020) 22:e18025. doi: 10.2196/18025
15. James, K, Jamil, Y, Kumar, M, Kwak, MJ, Nanna, MG, Qazi, S, et al. Frailty and cardiovascular health. J Am Heart Assoc. (2024) 13:e031736. doi: 10.1161/JAHA.123.031736
16. Schorr, EN, Gepner, AD, Dolansky, MA, Forman, DE, Park, LG, Petersen, KS, et al. Harnessing mobile health technology for secondary cardiovascular disease prevention in older adults: a scientific statement from the American Heart Association. Circ Cardiovasc Qual Outcomes. (2021) 14:e000103. doi: 10.1161/HCQ.0000000000000103
17. Agarwal, S, LeFevre, AE, Lee, J, L’Engle, K, Mehl, G, Sinha, C, et al. Guidelines for reporting of health interventions using mobile phones: mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ. (2016) 352:i1174. doi: 10.1136/bmj.i1174
18. World Health Organization. (2019). WHO guideline recommendations on digital interventions for health system strengthening. Geneva: World Health Organization. Available online at: http://www.ncbi.nlm.nih.gov/books/NBK541902/ (accessed September 18, 2025).
19. Walsh, D, and Downe, S. Meta-synthesis method for qualitative research: a literature review. J Adv Nurs. (2005) 50:204–11. doi: 10.1111/j.1365-2648.2005.03380.x
20. Sandelowski, M, Barroso, J, and Voils, CI. Using qualitative metasummary to synthesize qualitative and quantitative descriptive findings. Res Nurs Health. (2007) 30:99–111. doi: 10.1002/nur.20176
21. Thomas, J, and Harden, A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol. (2008) 8:45. doi: 10.1186/1471-2288-8-45
22. Zachary, M, Sandeep, M, Karolina, L, and Dagmara, R. (PDF) the systematic review of prevalence and incidence data. ResearchGate. (2014). Available online at: https://www.researchgate.net/publication/301223587_The_Systematic_Review_of_Prevalence_and_Incidence_Data (accessed September 19, 2025).
23. Kastner, M, Antony, J, Soobiah, C, Straus, SE, and Tricco, AC. Conceptual recommendations for selecting the most appropriate knowledge synthesis method to answer research questions related to complex evidence. J Clin Epidemiol. (2016) 73:43–9. doi: 10.1016/j.jclinepi.2015.11.022
24. Pearson, A. Meta-aggregation: emergence of the “missing” piece in qualitative synthesis. JBI Database System Rev Implement Rep. (2016) 14:2–3. doi: 10.11124/JBISRIR-2016-003257
25. Blomqvist, A, Strömberg, A, Lundberg, M, Bäck, M, Jaarsma, T, and Klompstra, L. Exploring user experience: a qualitative analysis of the use of a physical activity support app for people with heart failure. PLoS One. (2025) 20:e0309577. doi: 10.1371/journal.pone.0309577
26. Micheluzzi, V, Casu, G, Burrai, F, Canu, A, Sircana, A, Merella, P, et al. The experience of immersive virtual reality in patients with heart failure during cardiac rehabilitation: a qualitative study. Front Med. (2025) 12:1578399. doi: 10.3389/fmed.2025.1578399
27. Choo, J, Noh, S, and Shin, Y. Evaluating feasibility and acceptability of the “my HeartHELP” mobile app for promoting heart-healthy lifestyle behaviors: mixed methods study. JMIR Form Res. (2025) 9:e66108–8. doi: 10.2196/66108
28. Yang, T, Zheng, H, Cao, S, Jing, M, Hu, J, Zuo, Y, et al. Harnessing an artificial intelligence–based large language model with personal health record capability for personalized information support in postsurgery myocardial infarction: descriptive qualitative study. J Med Internet Res. (2025) 27:e68762. doi: 10.2196/68762
29. Trivedi, R, Shaw, T, Sheahen, B, Chow, CK, and Laranjo, L. Patient perspectives on conversational artificial intelligence for atrial fibrillation self-management: qualitative analysis. J Med Internet Res. (2025) 27:e64325. doi: 10.2196/64325
30. Islam, SMS, Singh, A, Moreno, SV, Akhter, S, and Chandir Moses, J. Perceptions of healthcare professionals and patients with cardiovascular diseases on mHealth lifestyle apps: a qualitative study. Int J Med Inform. (2025) 194:105706. doi: 10.1016/j.ijmedinf.2024.105706
31. Liu, S, Sun, G, Gao, M, Wang, J, Yu, T, Tang, Z, et al. Strategies for the promotion and quality improvement of cardiac rehabilitation apps: a qualitative study based on the perspective of heart failure patients. Chin J Soc Med. (2025) 42:242–5. doi: 10.3969/j.issn.1673-5625.2025.02.025
32. Chu, F, Stark, A, Telzak, A, and Rikin, S. Patient experience in a remote patient monitoring program for hypertension: A qualitative study. Am J Hypertens. (2024) 37:861–7. doi: 10.1093/ajh/hpae086
33. Neumann, A, Steiner, B, Verket, M, Kanna, NDD, Hill, L, McNulty, A, et al. Patients’ expectations and experiences with the usage of a self-care application for heart failure: a qualitative interview study. Digit Health. (2024) 10:20552076241299649. doi: 10.1177/20552076241299649
34. Madujibeya, I, Lennie, TA, Pelzel, J, and Moser, DK. Patients’ experiences using a mobile health app for self-care of heart failure in a real-world setting: qualitative analysis. JMIR Form Res. (2023) 7:e39525. doi: 10.2196/39525
35. Sivakumar, B, Lemonde, M, Stein, M, Mak, S, Al-Hesayen, A, and Arcand, J. Patient perspectives on the use of mobile apps to support heart failure management: a qualitative descriptive study. PLoS One. (2023) 18:e0285659. doi: 10.1371/journal.pone.0285659
36. Nilsson, U, Öberg, B, and Bäck, M. Patients’ perceptions of exercise-based cardiac telerehabilitation after a myocardial infarction—a qualitative study. Int J Environ Res Public Health. (2023) 20:5420. doi: 10.3390/ijerph20075420
37. Su, JJ, Paguio, J, Baratedi, WM, Abu-Odah, H, and Batalik, L. Experience of coronary heart disease patients with a nurse-led eHealth cardiac rehabilitation: qualitative process evaluation of a randomized controlled trial. Heart Lung. (2023) 57:214–21. doi: 10.1016/j.hrtlng.2022.10.005
38. Lao, SSW, and Chair, SY. The feasibility of smartphone-based application on cardiac rehabilitation for Chinese patients with percutaneous coronary intervention in Macau: a qualitative evaluation. Int J Qual Stud Health Well-being. (2022) 17:2023940. doi: 10.1080/17482631.2021.2023940
39. Tang, C, Zhang, X, Li, B, and Zhang, A. Qualitative study on the application experience of Mobile health platforms for hypertensive patients [in Chinese]. Contemp Nurse. (2022) 29:61–6. doi: 10.19791/j.cnki.1006-6411.2022.10.018
40. Schmaderer, M, Miller, JN, and Mollard, E. Experiences of using a self-management mobile app among individuals with heart failure: qualitative study. JMIR Nurs. (2021) 4:e28139. doi: 10.2196/28139
41. Lan, C, Qiu, X, Shen, M, Xiao, X, and Li, J. A qualitative study on the real experiences of heart failure patients with low acceptance towards mobile health apps. Chin J New Clin Med. (2021) 14:1238–41. doi: 10.3969/j.issn.1674-3806.2021.12.18
42. Cher, BP, Kembhavi, G, Toh, KY, Audimulam, J, Chia, W-YA, Vrijhoef, HJ, et al. Understanding the attitudes of clinicians and patients toward a self-management eHealth tool for atrial fibrillation: qualitative study. JMIR Hum Factors. (2020) 7:e15492. doi: 10.2196/15492
43. Son, Y, Oh, S, and Kim, EY. Patients’ needs and perspectives for using mobile phone interventions to improve heart failure self-care: a qualitative study. J Adv Nurs. (2020) 76:2380–90. doi: 10.1111/jan.14455
44. O’Shea, O, Woods, C, McDermott, L, Buys, R, Cornelis, N, Claes, J, et al. A qualitative exploration of cardiovascular disease patients’ views and experiences with an eHealth cardiac rehabilitation intervention: the PATHway project. PLoS One. (2020) 15:e0235274. doi: 10.1371/journal.pone.0235274
45. Foreman, MA, Ross, A, Burgess, APH, Myneni, S, and Franklin, A. Barriers and facilitators of digital health use for self-management of hypertensive disorders by black pregnant women. AMIA Symposium (2025).
46. Zhou, S, Shen, M, Tao, X, and Han, S. Cultural adaptation of digital healthcare tools: a cross-sectional survey of caregivers and patients. Glob Health Res Policy. (2025) 10:36. doi: 10.1186/s41256-025-00439-5
47. Oskrochi, Y. Mind the gap: aligning clinician and patient priorities in digital mental health. BMJ Digit Health AI. (2025) 1:1–2. doi: 10.1136/bmjdhai-2025-000097
48. Yee, V, Bajaj, SS, and Stanford, FC. Paradox of telemedicine: building or neglecting trust and equity. Lancet Digit Health. (2022) 4:e480–1. doi: 10.1016/S2589-7500(22)00100-5
49. Gagnon, KW, Quinn, K, Walsh, JL, Amirkhanian, YA, and Kelly, JA. Characteristics of healthcare providers, healthcare systems, and patient strategies related to medical mistrust among black and african Americans. BMC Prim Care. (2025) 26:203. doi: 10.1186/s12875-025-02900-3
50. Marcondes, FO, Normand, S-LT, Le Cook, B, Huskamp, HA, Rodriguez, JA, Barnett, ML, et al. Racial and ethnic differences in telemedicine use. JAMA Health Forum. (2024) 5:e240131. doi: 10.1001/jamahealthforum.2024.0131
51. Giordan, LB, Tong, HL, Atherton, JJ, Ronto, R, Chau, J, Kaye, D, et al. The use of mobile apps for heart failure self-management: systematic review of experimental and qualitative studies. JMIR Cardio. (2022) 6:e33839. doi: 10.2196/33839
52. White, MF, Kirschner, J, and Hamilton, MA. Self-care guide for the heart failure patient. Circulation. (2014) 129:e293–4. doi: 10.1161/CIRCULATIONAHA.113.003991
53. CKS is only available in the UK. NICE website: the National Institute for health and care excellence. Available online at: https://www.nice.org.uk/cks-is-only-available-in-the-uk (accessed October 12, 2025).
54. P, F, J, U, J, H, L, M, M, D, A, S, et al. Telemonitoring for chronic heart failure: the views of patients and healthcare professionals - a qualitative study. J Clin Nurs. (2014) 23:132–44. doi: 10.1111/jocn.12137
55. Chen, K, and Xu, Y. A WeChat platform program (WCPP) for full-process management of patients with cardiac valve interventional surgery based on psycho-cardiology: protocol of a mixed-method study. Trials. (2024) 25:694. doi: 10.1186/s13063-024-08553-4
56. Lippke, S, Korte, L, Kumar, VA, Fach, A, Ratz, T, Lippke, S, et al. Health behavior and disease self-management indicators in patients with cardiovascular diseases using a health app: findings from an RCT. AIMS Public Health. (2025) 12:233–58. doi: 10.3934/publichealth.2025015
57. Lestari, HM, Miranda, AV, and Fuady, A. Barriers to telemedicine adoption among rural communities in developing countries: a systematic review and proposed framework. Clin Epidemiol Glob Health. (2024) 28:101684. doi: 10.1016/j.cegh.2024.101684
58. Chaudhry, SI, Mattera, JA, Curtis, JP, Spertus, JA, Herrin, J, Lin, Z, et al. Telemonitoring in patients with heart failure. N Engl J Med. (2010) 363:2301–9. doi: 10.1056/NEJMoa1010029
59. Clephas, PRD, Radhoe, SP, Boersma, E, Gregson, J, Jhund, PS, Abraham, WT, et al. Efficacy of pulmonary artery pressure monitoring in patients with chronic heart failure: a meta-analysis of three randomized controlled trials. Eur Heart J. (2023) 44:3658–68. doi: 10.1093/eurheartj/ehad346
60. Koehler, F, Koehler, K, Deckwart, O, Prescher, S, Wegscheider, K, Kirwan, B-A, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. (2018) 392:1047–57. doi: 10.1016/S0140-6736(18)31880-4
61. Hepburn, J, Williams, L, and McCann, L. Barriers to and facilitators of digital health technology adoption among older adults with chronic diseases: updated systematic review. JMIR Aging. (2025) 8:e80000. doi: 10.2196/80000
62. Klaiman, T, Iannotte, LG, Josephs, M, Russell, LB, Norton, L, Mehta, S, et al. Qualitative analysis of a remote monitoring intervention for managing heart failure. BMC Cardiovasc Disord. (2023) 23:440. doi: 10.1186/s12872-023-03456-9
63. Carter, J, Donelan, K, and Thorndike, AN. Patient perspectives on home-based care and remote monitoring in heart failure: A qualitative study. J Prim Care Community Health. (2022) 13:21501319221133672. doi: 10.1177/21501319221133672
64. Björck, CG, Bergqvist, D, Esquivel, C, Nilsson, B, and Rudsvik, Y. Effect of heparin, low molecular weight (LMW) heparin, and a heparin analogue on experimental venous thrombosis in the rabbit. Acta Chir Scand. (1984) 150:629–33.
65. Alotaibi, N, Wilson, CB, and Traynor, M. Enhancing digital readiness and capability in healthcare: a systematic review of interventions, barriers, and facilitators. BMC Health Serv Res. (2025) 25:500. doi: 10.1186/s12913-025-12663-3
66. Marra, C, Chico, T, Alexandrow, A, Dixon, WG, Briffa, N, Rainaldi, E, et al. Addressing the challenges of integrating digital health technologies to measure patient-centred outcomes in clinical registries. Lancet Digit Health. (2025) 7:e225–31. doi: 10.1016/S2589-7500(24)00223-1
67. World Health Organization. Digital health literacy key to overcoming barriers for health workers, WHO study says. (2023). Available online at: https://www.who.int/europe/news/item/18-09-2023-digital-health-literacy-key-to-overcoming-barriers-for-health-workers--who-study-says (accessed October 13, 2025).
Keywords: cardiovascular diseases, telehealth, self-management, qualitative study, meta-synthesis, evidence-based nursing science
Citation: Shao Y, Hou X, Peng Q, Hu M and Li C (2025) A meta-synthesis of qualitative studies on cardiovascular disease patients’ experiences using digital health tools. Front. Public Health. 13:1709562. doi: 10.3389/fpubh.2025.1709562
Edited by:
Han Feng, Tulane University, United StatesReviewed by:
Hadi Younes, Tulane University, United StatesHeping Wang, University of Texas MD Anderson Cancer Center, United States
Michel Abou Khalil, Tulane University, United States
Copyright © 2025 Shao, Hou, Peng, Hu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaofeng Li, ZnN5eTAxNTg1QG5qdWNtLmVkdS5jbg==
 Yuxuan Shao
Yuxuan Shao Xinyi Hou1
Xinyi Hou1 Qin Peng
Qin Peng Mengdie Hu
Mengdie Hu Chaofeng Li
Chaofeng Li