- 1ICMR Regional Medical Research Centre, Bhubaneswar, Odisha, India
- 2Model Rural Health Research Unit, Ranchi, Jharkhand, India
- 3ICMR-National Institute of Epidemiology, Chennai, Tamil Nadu, India
- 4Rajendra Institute of Medical Sciences, Ranchi, Jharkhand, India
Background: Despite widespread vaccination programs, mumps has resurged globally. This systematic review and meta-analysis assessed the epidemiological characteristics, attack rates (ARs), and complications of mumps outbreaks worldwide from 2004 to 2024.
Methods: We systematically searched MEDLINE, Embase, CINAHL and Google Scholar for studies reporting on mumps outbreaks. Confirmed mumps cases, defined by WHO criteria, were included across all age groups. Epidemiological characteristics were summarized using the Time-Place-Person format. Pooled ARs and complication rates were calculated using random-effects models. Subgroup analyses examined variations by age, region, vaccination status, and outbreak period. A random-effects meta-regression and leave one out sensitivity analysis was used to explore the influence of study-level characteristics heterogeneity in the attack rate among mumps outbreak studies. Heterogeneity was assessed using Cochran’s Q and I2, and publication bias was evaluated with funnel plots and Egger’s test.
Results: A total of 47 studies from 21 countries reporting 71,174 mumps cases, were included in the systematic review, with 30 studies in the meta-analysis. The pooled AR of mumps outbreaks was 14.5% (95% CI: 12.91–16.11), with adults having the highest AR (31.8%). The pooled complication rate was 10.3% (95% CI: 5.7–14.9), with orchitis being the most common complication (63.1%). Temporal trends showed peaks during 2004–2009 and 2016–2020, while regional analysis revealed higher ARs in the Americas (29.2%) and Eastern Mediterranean (28.8%) regions compared to Europe (7.6%) and South-East Asia (9.6%). Among vaccinated individuals, ARs were highest with a single dose (35.7%) and lowest with three doses (10.1%).
Conclusion: Mumps outbreaks remain a global concern due to waning vaccine-induced immunity. Incorporating a third MMR booster dose into vaccination schedules is recommended, particularly for high-risk groups, to reduce ARs and complications effectively.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/.
Introduction
Mumps, a highly contagious viral illness caused by a member of the Paramyxoviridae family, spreads primarily through respiratory droplets (1). Clinically, it is characterised by inflammation and swelling of the parotid glands. However, complications such as meningitis, orchitis, encephalitis, pancreatitis, nephritis, and, in rare cases, mortality significantly contribute to the disease’s public health burden, highlighting the critical importance of prevention and control strategies (2, 3). The advent of widespread vaccination programs, particularly with the Measles, Mumps, and Rubella (MMR) vaccine in the 1970s, has dramatically reduced the global mumps incidence worldwide (4). Mumps vaccination was first introduced in high-resource settings (e.g., the U. S., Canada, and Europe), but its global rollout has been uneven and World Health Organization (WHO) data indicates that as of 2023, numerous low- and middle-income countries (LMICs) such as India, Lao People Democratic Republic, and most African nations still lack routine mumps immunization, highlighting persistent inequities in vaccine access (5, 6). Despite this progress, mumps continues to pose significant global health challenges (7, 8). The attack rate (AR), defined as the proportion of individuals who develop the disease among those exposed during an outbreak, is a critical metric for understanding the dynamics of mumps outbreaks (9). The WHO estimates approximately 500,000 mumps cases are reported annually worldwide, with fluctuations in incidence reflecting its complex epidemiological landscape (7, 8). In 2023, WHO reported 384,785 mumps cases globally, a 1% increase from the previous year (10). Recent resurgences, such as those observed in Europe between 2021 and 2022, particularly among individuals aged 10 years and older, suggest shifting patterns of transmission and immunity (11). Moreover, mumps outbreaks impose a substantial economic burden; a U. S. study estimated a per-case cost of $9,459, including direct medical expenses and productivity losses (12). In LMICs, endemic mumps circulation remains a persistent challenge due to disparities in vaccination coverage and healthcare access, underscoring the urgent need for adaptive vaccination strategies and robust surveillance systems (13).
Several key factors influence mumps outbreaks, including vaccination coverage, timing of vaccination, waning immunity, viral strain variability, and demographic shifts (14). These determinants play pivotal roles in outbreak dynamics, affecting population vulnerabilities, predicting future outbreaks, and guiding the implementation of control measures (15, 16). Notably, recent outbreaks have disproportionately affected adolescents and young adults, marking a transition from the traditional burden among younger children (13, 17). For example, in Spain from 1998 to 2003 (Period 1, P1), children aged 1–4 years experienced the highest incidence rate (71.7 cases per 100,000 population). Over subsequent periods, this pattern shifted towards adolescents and young adults, with higher incidence rate ratios observed among those aged 15–24 years (P2 = 1.46; P3 = 2.68) and adults aged 25–34 years (P2 = 2.17; P3 = 4.05) during 2004–2009 (P2) and 2010–2014 (P3) (18). These trends are compounded by behavioural and societal challenges such as vaccine hesitancy, inconsistent immunization adherence, and strained public health resources (19, 20). Understanding these shifts is critical for developing targeted vaccination strategies to address the evolving dynamics of mumps transmission.
Vaccination remains the cornerstone of mumps prevention. Two-dose vaccine schedules offer higher protection (vaccine effectiveness, VE: 64.0–92.4%) compared to single-dose regimens (VE: 47.4–86.0%) (21, 22). Vaccination has reduced mumps incidence by 66–88% in high-risk areas (23, 24). However, waning immunity, particularly among individuals vaccinated over a decade ago, presents a significant challenge (25–27). Individuals vaccinated 13 years prior are nine times more likely to contract mumps than those recently vaccinated (19, 20). To address waning immunity, the Centres for Disease Control and Prevention (CDC) recommend administering a third dose of MMR vaccine during outbreaks for individuals previously vaccinated with two doses. This approach aims to bolster immunity among high-risk groups and mitigate outbreak severity (19, 28, 29).
Measuring AR globally and regionally provides invaluable insights into the effectiveness of vaccination programs, susceptibility patterns, and outbreak severity across populations (30). Such analyses help identify immunity gaps, emerging risk factors, and the role of waning immunity in propagating outbreaks. Additionally, AR quantifies the public health impact of outbreaks, aiding resource allocation and outbreak response planning (31). Recent estimates show ARs ranging from 5.6 to 13.2% even in vaccinated communities, highlighting the complex waning vaccine efficacy, shifting epidemiological trends, and evolving viral strains underscoring the pressing need for ongoing research (15, 32–35). Given these challenges, it is imperative to generate robust evidence regarding the shifting epidemiology of mumps. Despite these concerns, synthesized global evidence on ARs, booster dose efficacy, and complications remains limited. In this context, we aimed to determine epidemiological characteristics and determinants of mumps outbreaks globally by assessing global and regional ARs, identifying key outbreak determinants, and evaluating complication rates associated with mumps.
Methods
Protocol and registration
We conducted a systematic review and meta-analysis of cross-sectional, cohort and case–control studies reporting on the epidemiological characteristics of mumps outbreaks. This review was registered with PROPSPERO (CRD42024572629) (36). The study design, selection, screening, analysis, and reporting adhered to the latest PRISMA-2020 guidelines (37), with the detailed PRISMA checklist provided in Supplementary Table 1.
Information source and search strategy
A systematic literature search was initially conducted on July 30, 2024, and updated on August 19, 2024. We searched peer-reviewed databases, including MEDLINE (via PubMed), Embase (via Ovid), and CINAHL (via EBSCO), along with Google Scholar to capture relevant grey literature including the studies published between 2004 and 2024. A predefined search strategy combined text words and Medical Subject Headings (MeSH) terms. For example, in PubMed, we searched for Mumps (MeSH) combined with title and abstract searches for “Epidemic parotitis,” “Mumps viruses,” and “Myxovirus parotitis.” Then, we searched with the MeSH term Disease Outbreaks, combined with a search of title and abstract using “Disease hotspot,” “Outbreaks,” and “Epidemics.” The search was restricted to studies published in English. Reference lists of included studies were manually screened by two independent reviewers (RA, SM) to identify additional relevant articles. Systematic review experts (MS, TR) assisted in refining the search strategy. The detailed search methodology is outlined in Supplementary Table 2.
Eligibility criteria
We defined the inclusion criteria using the CoCoPop framework (38).
Condition: Confirmed mumps cases, as defined by WHO.
Context: Cases reported during an outbreak.
Population: Global populations of any age group.
We defined mumps cases according to WHO clinical and laboratory criteria (39). Suspected cases were identified by acute onset of unilateral or bilateral parotid gland swelling lasting >2 days without an alternative cause. Confirmed cases included laboratory-confirmed results (e.g., mumps-specific IgM antibodies, mumps virus RNA detected via RT-PCR, or virus isolation) or cases linked to a known outbreak.
Eligible studies met the following criteria:
• Reported a mumps outbreak in the last two decades (between 2004 and 2024).
• Included an epidemiological curve.
• Specified the outbreak duration (short or extended).
The exclusion criteria were as follows:
• Not original research (e.g., reviews, abstracts, editorials, commentaries).
• Lacked methodological details or diagnostic confirmation.
• Focused on diseases other than mumps or co-infections without specific mumps data. Potential confounders, such as misdiagnoses of parainfluenza or Epstein–Barr virus, were excluded (40–42).
Study selection
We identified, extracted, and compiled the eligible studies into CSV and RIS formats. Preliminary deduplication and screening were conducted in Rayyan software (43). The selection process included:
1. Primary screening: Two independent reviewers (DS, PC) screened titles, abstracts and keywords (inter-rater agreement: 85–94%). Disagreements were resolved through discussion or consultation with a third reviewer (RA). Full-text retrieval was based on eligibility criteria.
2. Secondary screening: Full-text articles were reviewed by the same reviewers (inter-rater agreement: 94–95%).
3. Final selection: Inclusion decisions were finalized through consensus among investigators (TR, MS).
Data collection and data items
We extracted data using a pre-designed Excel spreadsheet (by DS and PC) and cross-checked it (by RA, TR, and MS). Disagreements were resolved through discussion. Missing data were requested from corresponding authors until September 21, 2024. Extracted data included:
• Study Characteristics: Authors, year, and country.
• Methodological Details: Study setting, design, population demographics (age, gender).
• Outbreak Information: Duration, timeline, diagnostic methods, case definitions, confirmed cases, population at risk.
• Clinical Features: Symptoms (e.g., fever, gland swelling), complications (e.g., orchitis, meningitis, encephalitis).
• Vaccination Data: Status of unvaccinated individuals and those with one, two, or three doses.
Data analysis
We analyzed data using R (V.3.0.3, The R Foundation for Statistical Computing, Vienna, Austria) (44). Epidemiological characteristics were summarized using the Time-Place-Person (TPP) format (45, 46). Descriptive statistics included proportions, means with standard deviations (SD), and medians with interquartile ranges (IQR), depending on data distribution. Temporal trends were analysed by grouping studies into three intervals: 2004–2009, 2010–2015, and 2016–2020, ensuring even distribution. While our search extended to studies published up to 2024, we found no published evidence of outbreaks beyond 2020 in the available literature. Geographic distribution was analysed using the WHO’s regional classification system (47). A choropleth map was generated in QGIS software to visually depict regional AR trends, highlighting variations across different WHO regions (48). Subgroup analyses were conducted to examine patterns of mumps complications. We estimated the pooled ARs and complication rate of mumps globally. The AR was calculated for each study using the reported number of mumps cases (numerator) divided by the total at-risk population (denominator). The complication rate was computed as the proportion of mumps cases that developed any of the specific complications: orchitis, meningitis, encephalitis, pancreatitis, oophoritis, or hearing loss expressed as a percentage of total cases. We assessed statistical heterogeneity across studies using Cochran’s Q test (with p < 0.10 indicating significant heterogeneity) and the I2 statistic, interpreting I2 values as follows: 0–40% as negligible, 30–60% as moderate, 50–90% as substantial, and 75–100% as considerable heterogeneity (49–51). Based on the degree of heterogeneity, a random-effects model was applied when heterogeneity was significant, while a fixed-effects model was used otherwise. Forest plots were created to visually summarize pooled estimates of ARs and complication rates, along with their confidence intervals (CIs). Sensitivity analyses, including “leave-one-out” methods, were performed to assess the robustness of pooled estimates by sequentially removing individual studies and observing their impact (52). Additionally, subgroup analyses explored AR determinants by age groups, WHO regional classifications, vaccination statuses, and years of outbreak. This approach accounted for potential geopolitical and socioeconomic differences influencing outbreak dynamics. A random-effects meta-regression with restricted maximum likelihood (REML) method was used to explore the influence of study-level characteristics heterogeneity in the attack rate among mumps outbreak studies (53). Different packages were used in R for the data analysis such as the meta-analyses of proportions were performed using the meta package (metaprop), while meta-regression and random-effects models were conducted with the metafor package (rma, rma.glmm). Forest plots and funnel plots were generated using a combination of meta, metafor with diagnostic checks supported by dmetar. Publication bias was assessed through visual inspection of funnel plots, and Egger’s regression test was used for quantitative evaluation, with p ≤ 0.05 considered statistically significant (54).
Risk of bias assessment
Three reviewers (DS, PC, and RA) independently assessed bias using Joanna Briggs Institute (JBI) tools for cross-sectional, cohort, and case–control studies (55–57). Criteria included sample appropriateness, research aim clarity, and outcome accuracy. Scores (Yes = 1, No = 0, Unclear = 0.5) provided objective insights, helping us to determine the reliability of the findings and the potential for bias.
Results
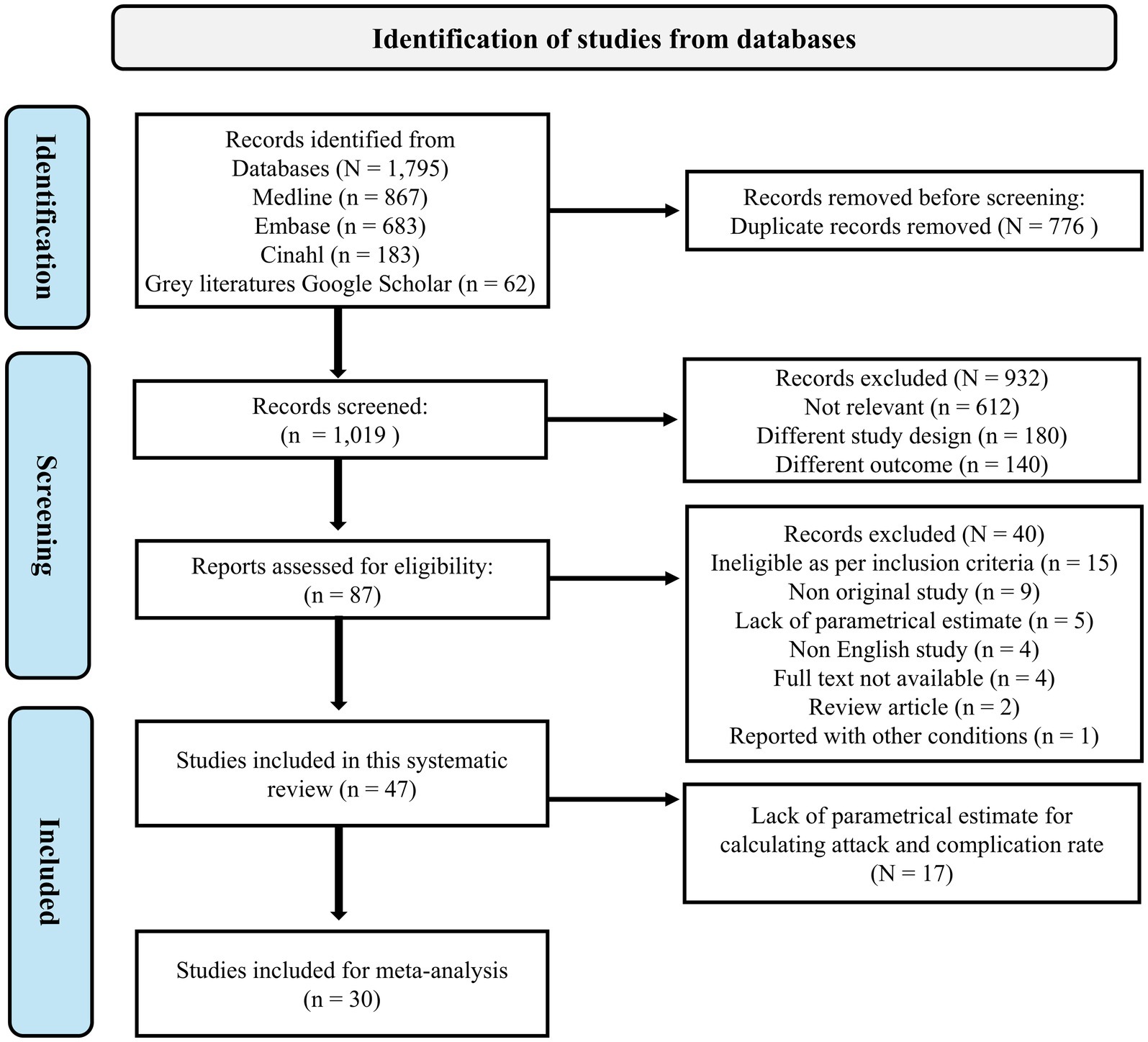
We identified a total of 1,795 articles published between 2004 and 2024 through a systematic search. After applying the eligibility criteria, 87 articles were deemed potentially relevant, and 47 studies were included in the systematic review. Of these, 30 studies met the criteria for inclusion in the meta-analysis, while 17 studies were excluded due to insufficient parametric estimates for calculating ARs and complication rates. The details of all the studies identified with their reason for exclusion are provided in the Supplementary Table 3. The study selection process is illustrated in the PRISMA flow diagram (Figure 1).
General characteristics of the included studies
The 47 studies represented data from 21 countries, documenting 71,174 cumulative mumps cases across 61 outbreaks between 2004 and 2024. The affected population ranged from 0 to 90 years of age (12, 16, 34, 58–101). Most studies reported a single outbreak whereas a few reported multiple outbreaks (62, 68, 74, 77, 83, 96). Among the cases, three fatalities were reported in a study from the Lao People’s Democratic Republic by Hubschen et al. (74). Study designs predominantly included cross-sectional studies, with two cohort studies (51, 70) and two case–control studies (49, 68). Diagnostic approaches varied, with 31 studies (65.9%) using a combination of laboratory criteria for mumps confirmation. The included studies are summarized in Table 1. The detailed template for data extracted from the included studies are provided in Supplementary Table 4.

Table 1. Summary characteristics of individual studies included in a systematic review of a mumps outbreak globally.
Mumps vaccination policies across countries
Table 2 summarizes the mumps vaccination policies of countries included in the systematic review and meta-analysis. It details the year of mumps vaccine introduction, the number of doses in the national immunization schedule, the recommended ages for the first and second doses, and notes on booster policies. This information provides context for understanding the variability in mumps outbreak dynamics and the potential impact of booster doses.

Table 2. Mumps vaccination policies across countries included in this systematic review and meta-analysis as per WHO immunization schedule (6).
Epidemiological characteristics of mumps outbreaks
Time
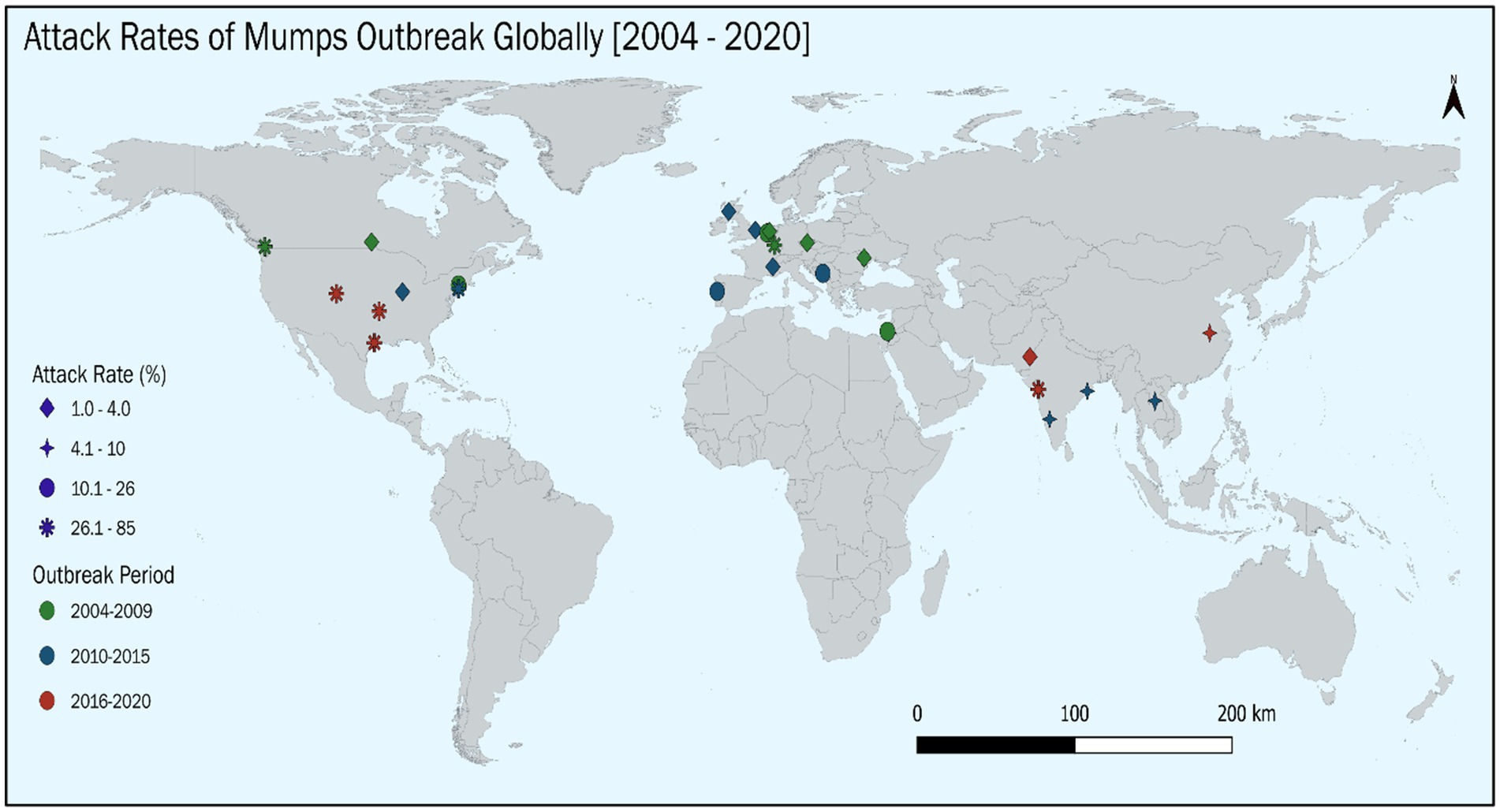
Outbreaks occurred consistently over the 20-year study period, with peaks during 2004–2009 (38.3%, n = 18) and 2016–2020 (34.0%, n = 16), while fewer outbreaks were documented between 2010 and 2015 (27.7%, n = 13). The mean (SD) outbreak duration was 10.95 (12.42) months. These figures represent the distribution of published studies reporting outbreaks, and not surveillance-based incidence data; hence, they should be interpreted descriptively rather than as statistically significant temporal trends.
Place
The geographical distribution of outbreaks revealed distinct patterns across WHO regions:
• European Region (EUR): Represented 44.7% (n = 21) of outbreaks, with the highest concentration in the Netherlands (5 studies, 10.6%) (34, 65, 68, 85, 94), and the United Kingdom (3 studies, 6.4%) (72, 80, 86).
• Region of the Americas (AMR): Accounted for 25.5% (n = 12) of outbreaks, primarily in the United States (8 studies, 17.0%) (62, 66, 71, 78, 93, 98, 100, 101), and Canada (4 studies, 8.5%) (61, 77, 95, 96).
• South-East Asia Region (SEAR): Represented 12.8% (n = 6), with outbreaks in India (5 studies, 10.6%) (64, 70, 79, 82, 91), and Lao People’s Democratic Republic (1 study, 2.1%) (72).
• Western Pacific Region (WPR): Comprised 8.5% (n = 4), with outbreaks in Australia (2 studies, 4.3%) (58, 81), China (76), and the Federated States of Micronesia (97).
• Eastern Mediterranean Region (EMR): Contributed 8.5% (n = 4), with all outbreaks reported in Israel (12, 16, 73, 88).
• Africa Region (AFR): No outbreaks were reported from this region.
Person
Demographic and clinical characteristics of outbreaks were as follows:
• Age Distribution: Children aged 0–10 years accounted for 66.7% (32 studies) of cases, followed by adolescents aged 11–18 years (25.0%, 12 studies), often linked to university or workplace outbreaks. Adults (≥19 years) accounted for 8.3% (3 studies) of cases.
• Gender: Most studies (91.5%) included mixed-gender populations. However, males represented 57% (n = 40,811) of cases, compared to females (38%, n = 27,254).
• Clinical Features: Parotitis was the most frequently reported symptom, accompanied by fever, cold, and cough in some cases. Rare symptoms included difficulty swallowing (64, 79, 82) and earache (64, 79, 88, 100). Complications such as orchitis, meningitis were reported in majority of the cases whereas, encephalitis and oophoritis were reported in a fewer subset of cases.
• Vaccination Status: A large proportion of mumps cases occurred in individuals who had received two or more doses of the MMR vaccine 57.4% had received two doses, and 21.3% had received three doses. In contrast, 8.5% of cases occurred among unvaccinated individuals and 6.4% among those who had received only one dose.
Further details are presented in Table 3.
Complication rates of mumps outbreaks
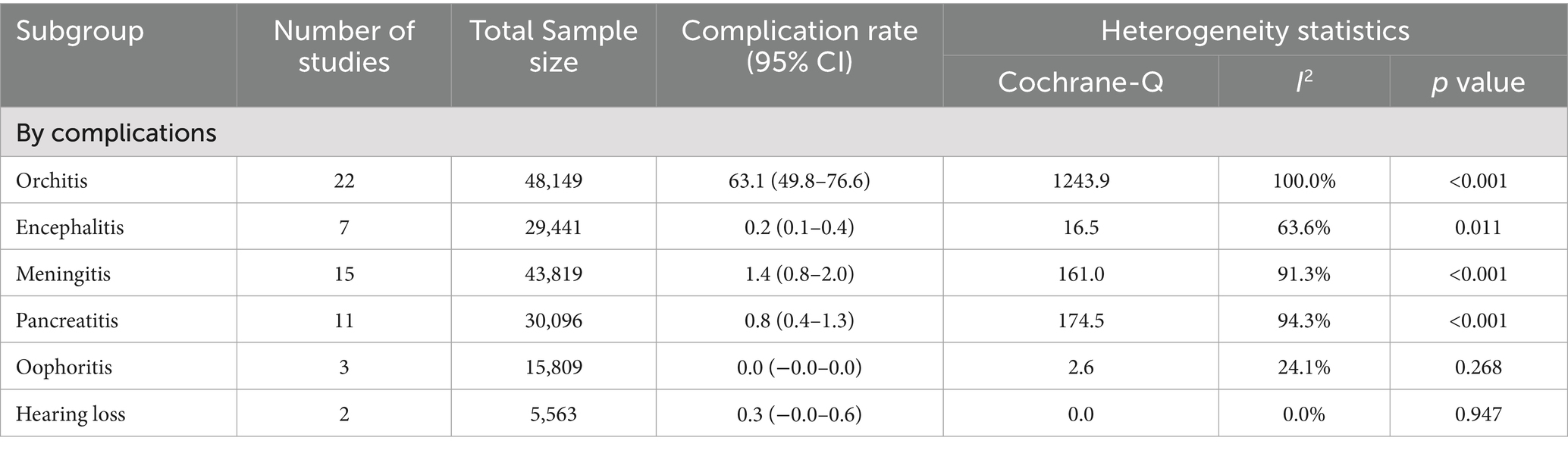
The pooled complication rate was 10.3% (95% CI: 5.7–14.9), with substantial heterogeneity (p < 0.001; I2 = 99.7%). A forest plot displaying the pooled complication rate is shown in Figure 2. Orchitis was the most common complication, with a pooled prevalence of 63.1% (95% CI: 49.8–76.6) across 22 studies. Other complications included encephalitis (0.2, 95% CI: 0.1–0.4), oophoritis, and hearing loss, which were infrequently reported. The full breakdown of complication rates is shown in Table 4.
ARs of mumps outbreaks globally
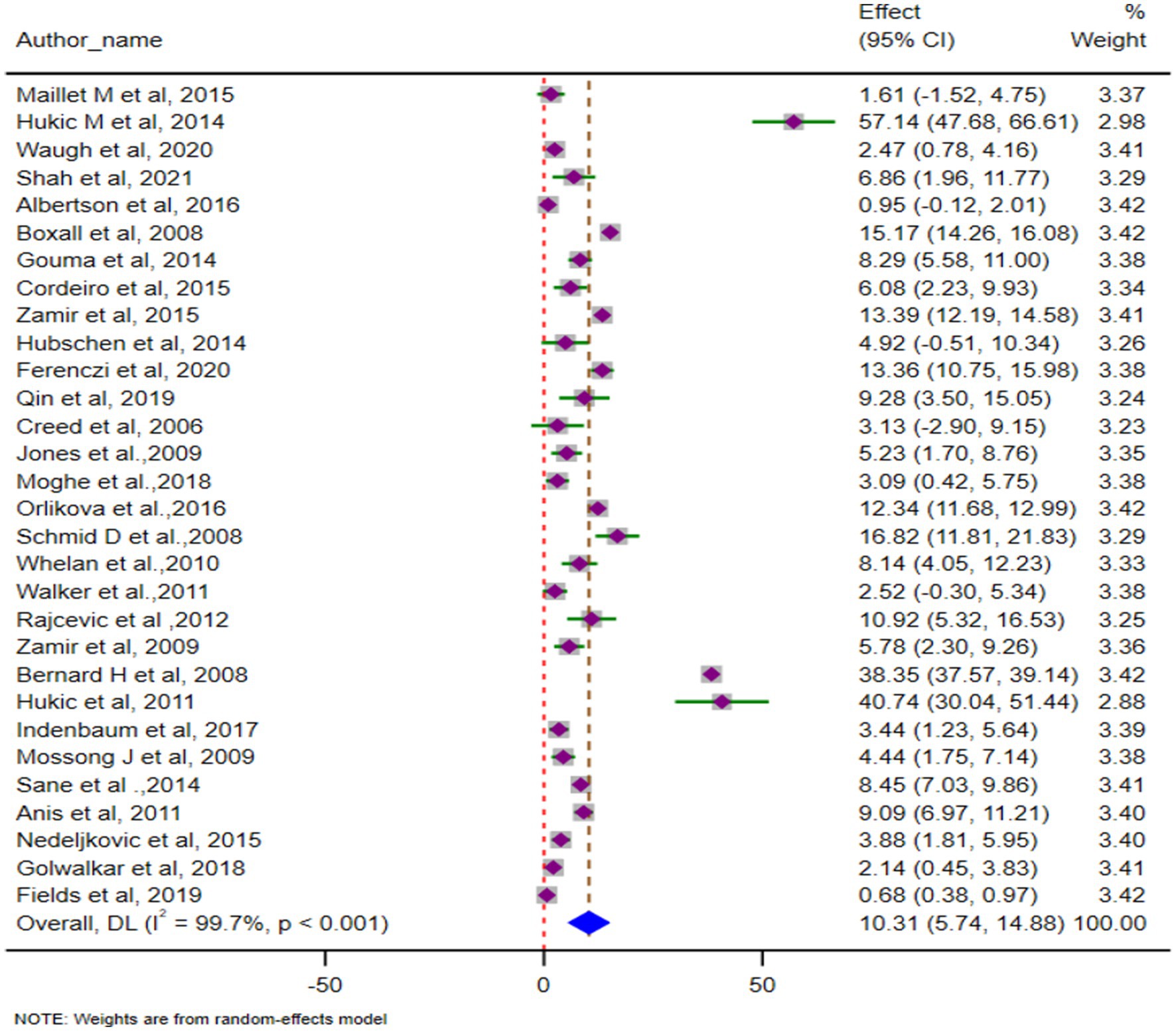
The pooled AR, based on 30 studies involving 50,643 cases, was 14.52% (95% CI: 12.91–16.11). Heterogeneity was significant (Cochrane Q = 10,238.2, p < 0.001; I2 = 99.7%). The forest plot of pooled ARs is shown in Figure 3. We have also depicted the global trend of mumps outbreak from the year 2004 to 2024. It is visually depicted in Figure 4.
Subgroup analysis for AR determinants
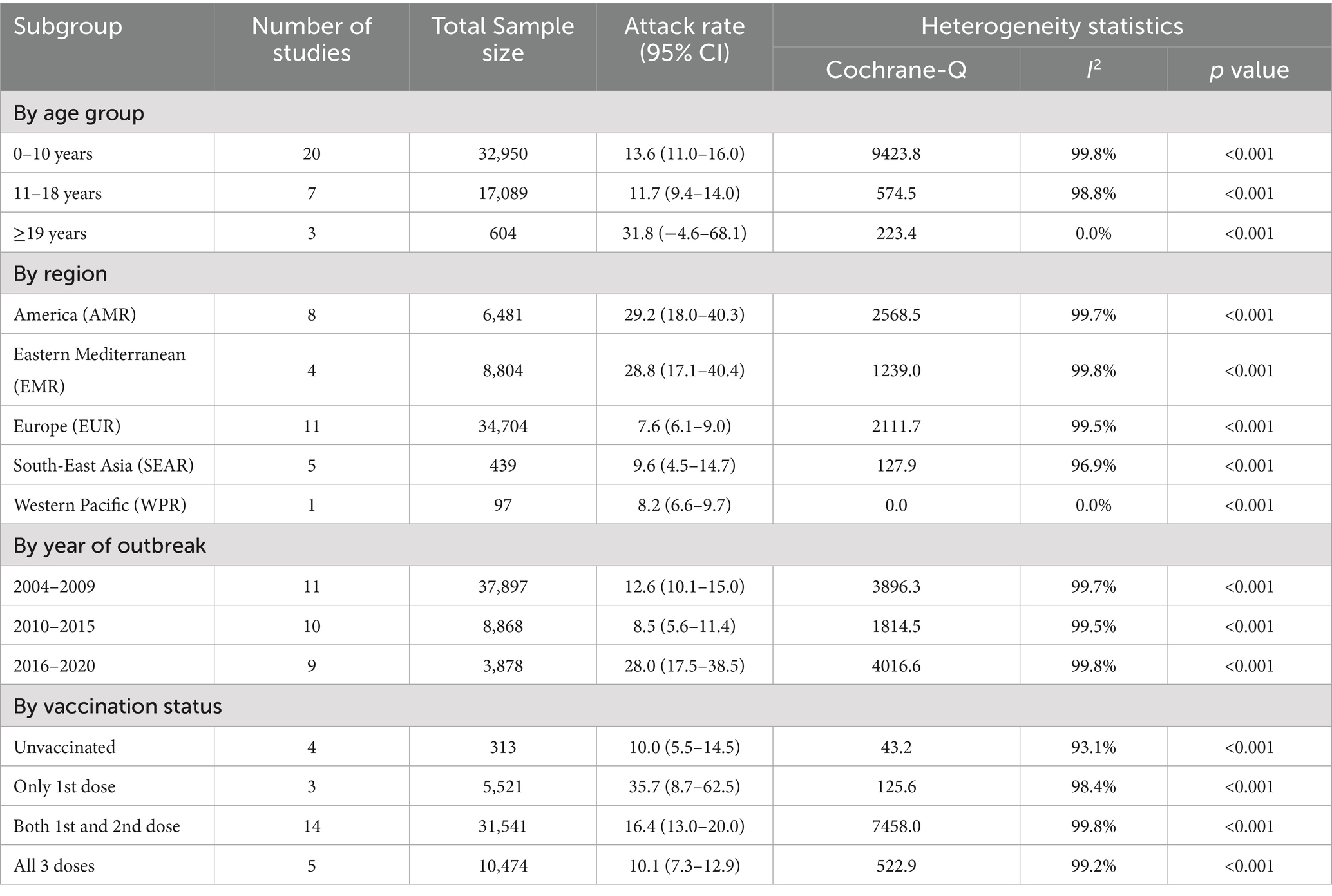
Subgroup analysis for determinants of AR shows that adults (≥19 years) had the highest AR (31.8, 95% CI: 4.6–68.1, I2 = 0.0%), while children aged 0–10 years had an AR of 13.6% (95% CI: 11.0–16.0, I2 = 99.8%). AMR (29.2, 95% CI: 18.0–40.3, I2 = 99.7%) and EMR (28.8, 95% CI: 17.1–40.4, I2 = 99.8%) reported the highest ARs, while EUR (7.6%) and WPR (8.2%) exhibited lower rates with significant heterogeneity. Outbreaks reported between 2016 and 2020 showed a higher AR (28.0, 95% CI: 17.5–38.5) compared to earlier periods (8.5–12.6%). Individuals with only one vaccine dose had the highest AR (35.7, 95% CI: 8.7–62.5), while those with three doses showed significantly lower rates (10.1, 95% CI: 7.3–12.9). The detailed description is given in Table 5.
Association between various covariates with AR
A random-effects meta-regression with restricted maximum likelihood (REML) method was conducted to investigate the influence of study-level predictors on AR across 30 studies. The results indicated substantial residual heterogeneity, with a tau-squared of 2.50 and I2 of 99.80%. These metrics suggest that nearly all the variability in effect sizes across studies was due to true heterogeneity rather than sampling error. Despite this, the meta-regression model explained 0.00% of the between-study variance (R2 = 0.00%) and was not statistically significant overall (p = 0.58). None of the included covariates showed a statistically significant association with the AR. The detail description is provided in the Table 6.

Table 6. Multivariable meta-regression analysis for assessing the association between AR and various covariates.
Leave-one-out sensitivity analysis
To assess the influence of individual studies on the overall pooled AR and complication rate leave-one-out sensitivity analysis was performed. The sensitivity analysis demonstrated the robustness of the finding, showing that no single study had a strong influence on the pooled estimate of AR and complication rate when results were computed excluding one study at time. The pooled AR remained stable, ranging from 12.03% (when Indenbaum et al., was removed) (16) to 16.24% (when Orlikova et al., was removed) (83), confirming the reliability of the overall result. However, the pooled complication rate also remains stable ranging from 8.76% (when Bernard H et al., is removed) (89) to a high of 10.66% (when Albertson et al., is removed) (66). The detailed description for both this estimate is provided in the Table 7.

Table 7. Influence of individual studies on pooled AR and complication rate as determined by leave-one-out sensitivity analysis.
Quality assessment and publication bias
Quality assessment using JBI tools showed that 96% of studies were high quality (scores >70%) (95), with only two studies rated as having moderate risk (50–69%) (28, 57). No studies were excluded based on quality assessment. A detailed quality assessment is provided in Supplementary Table 5. Funnel plot analysis and Egger’s regression test showed minimal publication bias, with no significant asymmetry detected Supplementary Figure 1.
Discussion
This systematic review and meta-analysis assessed mumps outbreaks globally over the last two decades (2004–2024). We included 47 studies with 71,174 cases from 21 countries. The pooled AR of mumps outbreaks was 14.5%, while the overall complication rate was 10.3%, with orchitis being the most common complication. Peaks in outbreak activity were observed during 2004–2009 (38.3%) and 2016–2020 (34.6%), with most outbreaks occurring in the EUR (44.7%) and AMR (25.5%). Children were the most affected demographic, constituting 66.7% of cases, and 57.4% of cases occurred in individuals who had received two doses of the MMR vaccine.
The pooled AR of 14.5% varied significantly across age groups, regions, and vaccination statuses. Adults exhibited the highest AR, likely due to waning immunity and reduced natural exposure to mumps. The AR in the AMR (29.2%) was significantly higher than in the EUR (7.6%) and SEAR (9.6%), reflecting disparities in vaccination schedules, outbreak settings, and demographic characteristics. Similar findings were reported in a U. S. study by Clemmons et al. (102), which documented comparable ARs during outbreaks. In contrast, studies from China (76) and the Netherlands (33) reported lower ARs, ranging from 8.2 to 9.5%, suggesting that regional variations in vaccination coverage, waning immunity, and population density influence outbreak dynamics (103). These findings highlight the importance of regional monitoring and tailoring vaccination strategies to local epidemiological contexts.
A critical finding of this review is the high proportion of mumps cases among vaccinated individuals, particularly those with two doses of the MMR vaccine (57.4%) which likely reflects the widespread adoption of the two-dose schedule in many countries. The AR was highest among individuals with a single dose (35.7%) and lowest among those with three doses (10.1%), demonstrating the protective effect of booster doses. Studies such as Nelson et al. (104) and Cardemil et al. (105) corroborate these results, showing that a third dose reduces ARs by 60 to 78% compared to two doses. Despite this, the resurgence of outbreaks among fully vaccinated individuals underscores challenges related to waning immunity and evolving viral strains. Evidence indicates that MMR vaccine effectiveness decreases substantially over time, from 82% within 5 years of a single dose to 41% after 10 years (106). Similar declines are seen with two doses, highlighting the necessity of booster doses, especially in high-risk populations (78, 107). The best possible reason for increased risk after a single dose among vaccinated individuals is the waning of immunity and variability of vaccine effectiveness depending upon the age of vaccination and the time since vaccination (9).
The pooled complication rate of 10.3% aligns with findings from similar settings, with orchitis being the most common complication (63.1%). Complications such as encephalitis and hearing loss were rare but remain significant due to their potential long-term impact. Studies from the United Kingdom (108) and Korea (109) reported complication rates ranging from 5.3 to 16%, with variability influenced by vaccination coverage and diagnostic practices. Orchitis, particularly in post-pubertal males, is a notable concern due to its association with testicular atrophy and infertility (110). These findings emphasize the critical role of vaccination in reducing the severity and prevalence of complications. However, the resurgence of outbreaks in highly vaccinated populations raises concerns about herd immunity, underscoring the need for booster doses to mitigate severe outcomes.
Temporal trends revealed cyclical surges in mumps outbreaks approximately every 5–10 years, consistent with previous studies (111, 112). The absence of published evidence of mumps outbreaks beyond 2020 in the studies included in this review could be attributed to several factors, particularly those associated with the global COVID-19 pandemic and its aftermath. During the COVID-19 pandemic, public health priorities shifted dramatically, focusing almost exclusively on managing SARS-CoV-2 transmission and its associated burden on healthcare systems. This shift likely diverted resources, attention, and surveillance capabilities away from other infectious diseases, including mumps. For instance, in 2021, the overall notification rate was 0.4 cases per 100,000 population by European Union Member States. This was substantially lower than the notification rates observed in the previous 4 years, which ranged from 1.7 to 4.2 cases per 100,000 (113). This decline may reflect underreporting rather than an actual reduction in mumps incidence, as healthcare systems were overwhelmed, and routine disease surveillance was disrupted. Although data beyond 2020 were not included in our meta-analysis, but the reemergence of cases with the relaxation of restrictions underscores the enduring challenges of mumps control. For example, the United States reported 328 cases in 2024, reflecting the resurgence of outbreaks post-pandemic (114).
The geographical analysis demonstrated that mumps outbreaks are truly global, with substantial variation across WHO regions (115). EUR and AMR reported the majority of outbreaks, reflecting robust surveillance systems and higher reporting rates (102, 116). In contrast, outbreaks in SEAR and the EMR were less frequently reported, potentially due to limited surveillance or underreporting (117–119). The absence of outbreak data from the African region more likely reflects the under-reporting and limitations in surveillance systems rather than the actual absence of outbreaks. A recent report has highlighted persistent gaps in vaccine coverage, high numbers of “zero-dose” children, and incomplete vaccine preventable disease surveillance in the African Region, all of which may contribute to under-ascertainment of mumps cases (120, 121). These findings underscore the need for strengthening surveillance systems and ensuring equitable vaccine access in resource-limited settings.
Global differences in the establishment and development of mumps vaccination programs have an impact on vaccine policy and outbreak trends. Due to declining immunity and decreased natural boosting, mumps outbreaks among vaccinated adolescents and young adults are becoming more frequent in nations with high incomes that include the US, Canada, Israel, and parts of Europe where two-dose MMR vaccination has been in place for decades. As a result, during outbreaks, a third MMR dose is recommended for high-risk groups (29, 105, 122). The introduction of a standard primary doses is a higher priority in LMICs, such as India, where the mumps vaccination is still not part of national schedules (123). After repeated outbreaks, China added a second dose in 2008 after initially implementing a one-dose schedule (76). These differences underscore that booster dose recommendations are context dependent.
According to recent data, the number of mumps cases worldwide has increased since the COVID-19 pandemic (124). Many vaccine-preventable diseases, including mumps, saw a significant decrease in incidence after 2020. This may be due to lack of reported data on mumps outbreaks after the year 2020 which may be explained by multiple factors related to COVID-19 pandemic. Firstly, non-pharmaceutical interventions (NPIs) implemented during this period to control and prevent the SARS-CoV-2 transmission, including the use of masks, school closures, and social distancing, these all factors substantially reduced the circulation of several respiratory and close-contact viruses, and likely suppressed mumps transmission as well (11, 125). Additionally, the challenges faced in routine childhood immunisation and health services during the COVID-19 pandemic have been documented globally, which may have altered both susceptibility and case detection (126). Finally, with delayed reporting and reduced notification of vaccine-preventable diseases during 2020–21 adversely affected the surveillance systems as well (127). Collectively, all these factors may have contributed to the observed decline in reported mumps outbreaks during this period. However, several nations have reported an upsurge in the incidence of mumps since these restrictions were relaxed. For instance, the incidence rate increased from 4.4 per million in 2021 to 11.3 per million in 2023 in the WHO European Region, and from 0.6 to 18.7 per million in Northeast Asia during the same time-period (124). These patterns resemble those seen in other diseases that can be prevented, like pertussis, and they point to a possible rebound effect brought on by immunity gaps that could have waned during the pandemic. This resurgence emphasises how crucial it is to maintain ongoing surveillance and have strong vaccination campaigns to stop outbreaks in the future.
The genetic evolution of the mumps virus, specifically the emergence and predominance of genotype G strains, has raised concerns about the effectiveness of existing vaccines derived from the Jeryl Lynn strain (genotype A) (128). While the Jeryl Lynn-based vaccine has significantly reduced mumps incidence, recent outbreaks in highly vaccinated populations suggest that antigenic differences between vaccine and circulating strains may compromise vaccine-induced immunity (128, 129). Studies have identified amino acid variations in key antigenic sites, such as the hemagglutinin-neuraminidase (HN) protein, between genotype A and genotype G strains, potentially affecting neutralizing antibody responses (130). In the Netherlands, outbreaks predominantly caused by genotype G have been documented among vaccinated individuals, indicating possible immune escape due to these antigenic differences (128).
Future research should focus on understanding the genetic evolution of mumps virus strains and its impact on vaccine efficacy. Longitudinal studies evaluating antibody persistence post-vaccination would provide crucial data for refining booster dose recommendations. Improved global surveillance systems and data-sharing initiatives are essential for strengthening outbreak preparedness and response. By integrating these strategies, public health systems can reduce the global burden of mumps, protect vulnerable populations, and improve overall immunization efforts.
This study has several strengths. As the first meta-analysis on mumps outbreaks in two decades, it includes data from diverse geographical regions, offering a global perspective on mumps epidemiology. The use of standardized inclusion criteria and rigorous quality assessment ensures the reliability of findings. However, there are limitations to consider. High heterogeneity was observed in many pooled estimates, likely due to variability in study designs, diagnostic methods, and reporting standards. The reliance on original studies may also have introduced publication bias, despite minimal asymmetry observed in funnel plots and Egger’s tests. During our regional analysis although our aim was to include data from the entire American region, only studies from the USA and Canada were identified and met the inclusion criteria. Therefore, the findings may not be generalizable to the entire region. Another potential limitation is the underreporting of the outbreak most commonly in low-resource settings for example from the African region. Weak surveillance infrastructure, limited laboratory capacity, and competing health priorities may have resulted in missed or unreported outbreaks, particularly in LMIC countries (131). Consequently, the global pooled estimates derived in this review may disproportionately reflect data from high- and middle-income countries with more robust surveillance systems, limiting their generalizability to low-resource settings.
Conclusion
A pooled AR of 14.5% and a complication rate of 10.3% were observed in global mumps outbreaks over the past two decades, with adults exhibiting the highest AR of 31.8%. Booster doses significantly reduced ARs to 10.1%. Incorporating a third MMR booster dose into vaccination schedules should be considered, particularly for high-risk groups, to mitigate outbreak severity and reduce complications worldwide. Such recommendations are primarily applicable to countries with long-standing two-dose coverage and documented waning immunity such as US, Australia, Canada, Israel and Part of Europe. In LMIC countries that have not yet introduced mumps vaccination, such as India, priority should be given to implementing and strengthening routine two-dose immunization programs before considering booster strategies. While the pooled estimates provide important insights into the global burden of mumps outbreaks, the high heterogeneity observed across studies indicates that the results should be interpreted with caution. The robustness of the findings in sensitivity analyses suggests that they are not driven by any single study but highlight the urgent need for more standardized reporting and improved surveillance systems.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
RA: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. TR: Conceptualization, Data curation, Investigation, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DS: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. PC: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. MS: Conceptualization, Data curation, Investigation, Resources, Software, Supervision, Validation, Writing – review & editing. DK: Conceptualization, Investigation, Resources, Software, Supervision, Validation, Writing – review & editing. SM: Methodology, Supervision, Validation, Visualization, Writing – review & editing. AK: Formal analysis, Methodology, Validation, Visualization, Writing – review & editing. SK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing. SP: Conceptualization, Data curation, Methodology, Resources, Software, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors are grateful to the Department of Health Research, Ministry of Health and Family Welfare, Government of India, for establishing the Model Rural Health Research Unit (MRHRU), Namkum.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1711759/full#supplementary-material
Abbreviations
AR, Attack rate; CDC, Centers for Disease Control and Prevention; CI, Confidence Interval; JBI, Joanna Briggs Institute; MeSH, Medical Subject Heading; MMR, Measles, Mumps, and Rubella vaccine; NPIs, Non pharmaceutical Interventions; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; WHO, World Health Organization.
References
1. Rima, B, Balkema-Buschmann, A, Dundon, WG, Duprex, P, Easton, A, Fouchier, R, et al. ICTV virus taxonomy profile: Paramyxoviridae. J Gen Virol. (2019) 100:1593–4. doi: 10.1099/jgv.0.001328,
2. Latner, DR, and Hickman, CJ. Remembering Mumps. PLoS Pathog. (2015) 11:e1004791. doi: 10.1371/journal.ppat.1004791,
3. Betakova, T, Svetlikova, D, and Gocnik, M. Overview of measles and mumps vaccine: origin, present, and future of vaccine production. Acta Virol. (2013) 57:91–6. doi: 10.4149/av_2013_02_91,
4. Australian Government Department of Health. The Australian immunisation handbook, 10th edition. (2025). Available online at: https://immunisationhandbook.health.gov.au/ (Accessed April 14, 2025).
5. Hardt, K, Bonanni, P, King, S, Santos, JI, El-Hodhod, M, Zimet, GD, et al. Vaccine strategies: optimising outcomes. Vaccine. (2016) 34:6691–9. doi: 10.1016/j.vaccine.2016.10.078,
6. World Health Organization. Vaccination schedule for mumps. (2025). Available online at: https://immunizationdata.who.int/global?topic=&location= (Accessed April 25, 2025).
7. Nature. Mumps is rising in some nations - but a fresh dose of vaccine might help. Nature. (2024) 634:757. doi: 10.1038/d41586-024-03352-y,
8. Yang, T, Wang, Y, Zhao, Q, Guo, X, Yu, S, Zhao, Z, et al. Age-specific transmission dynamic of mumps: a long-term large-scale modeling study in Jilin province, China. Front Public Health. (2022) 10:968702. doi: 10.3389/fpubh.2022.968702,
9. Masters, NB, Leung, J, Tappe, J, Marin, M, Crooke, SN, Bankamp, B, et al. Disease description. (2025). Available online at: https://www.cdc.gov/surv-manual/php/table-of-contents/chapter-9-mumps.html (Accessed April 25, 2025).
10. World Health Organization. Mumps reported cases and incidence. (2023). Available online at: https://immunizationdata.who.int/global/wiise-detail-page/mumps-reported-cases-and-incidence?CODE=Global&YEAR= (Accessed April 25, 2025).
11. Mumps. Annual surveillance epidemiological report for 2022. (2025). Available online at: https://www.ecdc.europa.eu/sites/default/files/documents/mumps-annual-epidemiological-report-for-2022.pdf (Accessed April 25, 2025).
12. Ozawa, S, Portnoy, A, Getaneh, H, Clark, S, Knoll, M, Bishai, D, et al. Modeling the economic burden of adult vaccine-preventable diseases in the United States. Health Aff. (2016) 35:2124–32. doi: 10.1377/hlthaff.2016.0462,
13. Anis, E, Grotto, I, Moerman, L, Warshavsky, B, Slater, PE, and Lev, B. Mumps outbreak in Israel's highly vaccinated society: are two doses enough? Epidemiol Infect. (2012) 140:439–46. doi: 10.1017/S095026881100063X,
14. Principi, N, and Esposito, S. Mumps outbreaks: a problem in need of solutions. J Infect. (2018) 76:503–6. doi: 10.1016/j.jinf.2018.03.002,
15. Azimaqin, N, Peng, Z, Ren, X, Wei, Y, and Liu, X. Vaccine failure, seasonality and demographic changes associate with mumps outbreaks in Jiangsu Province, China: age-structured mathematical modelling study. J Theor Biol. (2022) 544:111125. doi: 10.1016/j.jtbi.2022.111125,
16. Lam, E, Rosen, JB, and Zucker, JR. Mumps: an update on outbreaks, vaccine efficacy, and genomic diversity. Clin Microbiol Rev. (2020) 33:10–128. doi: 10.1128/CMR.00151-19,
17. Indenbaum, V, Hübschen, JM, Stein-Zamir, C, Mendelson, E, Sofer, D, Hindiyeh, M, et al. Ongoing mumps outbreak in Israel, January to august 2017. Euro Surveill. (2017) 22:30605. doi: 10.2807/1560-7917.ES.2017.22.35.30605,
18. López-Perea, N, Masa-Calles, J, Torres de Mier, MV, Fernández-García, A, Echevarría, JE, De Ory, F, et al. Shift within age-groups of mumps incidence, hospitalizations and severe complications in a highly vaccinated population Spain, 1998-2014. Vaccine. (2017) 35:4339–45. doi: 10.1016/j.vaccine.2017.06.075,
19. Davison, P, Morris, J, and Haddad, LM. Mumps (nursing), continuing education activity. (2025). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK534785/ (Accessed April 25, 2025).
20. Abu Bashar, MD, Khan, IA, and Sridevi, G. Recent surge in mumps cases in India: need for urgent remedial measures. Indian Pediatr. (2024) 61:370–4. doi: 10.1007/s13312-024-3162-8,
21. Yin, Z, Wen, T, Fang, Q, Zheng, C, Gong, X, Li, J, et al. Assessment of mumps-containing vaccine effectiveness by dose during 2006 to 2020 in Quzhou, China. Hum Vaccin Immunother. (2022) 18:2086774. doi: 10.1080/21645515.2022.2086774,
22. Deeks, SL, Lim, GH, Simpson, MA, Gagné, L, Gubbay, J, Kristjanson, E, et al. An assessment of mumps vaccine effectiveness by dose during an outbreak in Canada. CMAJ. (2011) 183:1014–20. doi: 10.1503/cmaj.101371,
23. Moon, JY, Jung, J, and Huh, K. Universal measles-mumps-rubella vaccination to new recruits and the incidence of mumps in the military. Vaccine. (2017) 35:3913–6. doi: 10.1016/j.vaccine.2017.06.025,
24. National Health Society. MMR (measles, mumps and rubella) vaccine. (2025). Available online at: https://www.nhs.uk/vaccinations/mmr-vaccine/ (Accessed April 27, 2025).
25. Vygen, S, Fischer, A, Meurice, L, Njoya, IM, Gregoris, M, Ndiaye, B, et al. Waning immunity against mumps in vaccinated young adults, France 2013. Euro Surveill. (2016) 21:30156. doi: 10.2807/1560-7917.ES.2016.21.10.30156,
26. Hamami, D, Cameron, R, Pollock, KG, and Shankland, C. Waning immunity is associated with periodic large outbreaks of mumps: a mathematical modeling study of Scottish data. Front Physiol. (2017) 8:233. doi: 10.3389/fphys.2017.00233,
27. Kennedy, RB, Ovsyannikova, IG, Thomas, A, Larrabee, BR, Rubin, S, and Poland, GA. Differential durability of immune responses to measles and mumps following MMR vaccination. Vaccine. (2019) 37:1775–84. doi: 10.1016/j.vaccine.2019.02.030,
28. Marlow, M, Haber, P, Hickman, C, and Patel, M. Mumps epidemiology and prevention of vaccine-preventable diseases. Washington: Public Health Foundation, pp. 225–238. (2021).
29. Marin, M. Recommendation of the advisory committee on immunization practices for use of a third dose of mumps virus–containing vaccine in persons at increased risk for mumps during an outbreak. MMWR Morb Mortal Wkly Rep. (2018) 67:33–8. doi: 10.15585/mmwr.mm6701a7
30. European Centre for Disease Prevention and Control (ECDC). Mumps - Annual epidemiological report for 2022. (2024). Available online at: https://www.ecdc.europa.eu/sites/default/files/documents/mumps-annual-epidemiological-report-for-2022.pdf (Accessed April 28, 2025).
31. Chen, Y, Aldridge, T, Ferraro, C, and Khaw, FM. COVID-19 outbreak rates and infection attack rates associated with the workplace: a descriptive epidemiological study. BMJ Open. (2022) 12:e055643. doi: 10.1136/bmjopen-2021-055643,
32. Kaaijk, P, Emmelot, ME, Kerkhof, J, van Els, CA, Meiring, HD, de Wit, J, et al. Genetic analysis reveals differences in CD8+ T cell epitope regions that may impact cross-reactivity of vaccine-induced T cells against wild-type mumps viruses. Vaccine. (2021) 9:699. doi: 10.3390/vaccines9070699,
33. Van Boven, M, Backer, JA, Veldhuijzen, I, Gomme, J, van Binnendijk, R, and Kaaijk, P. Estimation of the infection attack rate of mumps in an outbreak among college students using paired serology. Epidemics. (2024) 46:100751. doi: 10.1016/j.epidem.2024.100751,
34. Brockhoff, HJ, Mollema, L, Sonder, GJ, Postema, CA, van Binnendijk, RS, Kohl, RH, et al. Mumps outbreak in a highly vaccinated student population, the Netherlands, 2004. Vaccine. (2010) 28:2932–6. doi: 10.1016/j.vaccine.2010.02.020,
35. Greenland, K, Whelan, J, Fanoy, E, Borgert, M, Hulshof, K, Yap, KB, et al. Mumps outbreak among vaccinated university students associated with a large party, the Netherlands, 2010. Vaccine. (2012) 30:4676–80. doi: 10.1016/j.vaccine.2012.04.083,
36. PROSPERO. International prospective register of systematic reviews. (2025). Available online at: https://www.crd.york.ac.uk/prospero/ (Accessed April 28, 2025).
37. Page, MJ, Moher, D, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160,
38. Stern, C. Q is for question…. JBI Database Syst Rev Implement Rep. (2015) 13:1–2. doi: 10.11124/jbisrir-2015-2610,
39. WHO. Vaccine preventable diseases surveillance standards. (2018). Available online at: https://www.who.int/docs/default-source/immunization/vpd_surveillance/vpd-surveillance-standards-publication/who-surveillancevaccinepreventable-13-mumps-r2.pdf?sfvrsn=a3f182d_5 (Accessed April 28, 2025).
40. Borchardt, SM, Rao, P, and Dworkin, MS. Compliance with exclusion requirements to prevent mumps transmission. Emerg Infect Dis. (2007) 13:1617–8. doi: 10.3201/eid1310.070117,
41. Hatchette, TF, Mahony, JB, Chong, S, and LeBlanc, JJ. Difficulty with mumps diagnosis: what is the contribution of mumps mimickers? J Clin Virol. (2009) 46:381–3. doi: 10.1016/j.jcv.2009.09.024,
42. Yoo, JW, Tae, BS, Chang, HK, Song, MS, Cheon, J, Park, JY, et al. Epidemiology of mumps, mumps complications, and mumps orchitis in Korea using the National Health Insurance Service database. Investig Clin Urol. (2023) 64:412–7. doi: 10.4111/icu.20230064,
43. Rayyan. Intelligent systematic review. (2025). Available online at: https://new.rayyan.ai/ (Accessed May 8, 2025).
44. R Version. The R project for statistical computing. (2025). Available online at: https://www.r-project.org/ (Accessed May 8, 2025).
45. Li, M, Liu, Y, Yan, T, Xue, C, Zhu, X, Yuan, D, et al. Epidemiological characteristics of mumps from 2004 to 2020 in Jiangsu, China: a flexible spatial and spatiotemporal analysis. Epidemiol Infect. (2022) 150:e86. doi: 10.1017/S095026882200067X,
46. Baiee, HA, and Hatif, W. Epidemiological characteristics of mumps outbreak in the south districts of Babylon province during the years 2016-2017. Med J Babylon. (2017) 14:585–92. Available online at: https://iasj.rdd.edu.iq/journals/uploads/2024/12/09/0e8df8e2dc3f87fe0e504b1cfbde9b2f.pdf
47. WHO. Classification and standards of country groupings. (2025). Available online at: https://www.who.int/observatories/global-observatory-on-health-research-and-development/classifications-and-standards/country-groupings (Accessed May 15, 2025).
48. QGIS. Spatial without compromise. (2025). Available online at: https://www.qgis.org (Accessed May 15, 2025).
49. Wang, T, Wang, J, Rao, J, Han, Y, Luo, Z, Jia, L, et al. Meta-analysis of the effects of ambient temperature and relative humidity on the risk of mumps. Sci Rep. (2022) 12:6440. doi: 10.1038/s41598-022-10138-7,
50. Takkouche, B, Cadarso-Suarez, C, and Spiegelman, D. Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol. (1999) 150:206–15. doi: 10.1093/oxfordjournals.aje.a009981,
51. Deeks, JJ, Higgins, JP, and Altman, DG. Analysing data and undertaking meta-analyses. in: JP Higgins and S Green. Cochrane handbook for systematic reviews of interventions. London: Cochrane Statistical Methods Group, pp. 241–284 (2019).
52. Okumura, S, Suzuki, Y, and Takeuchi, I. Quick sensitivity analysis for incremental data modification and its application to leave-one-out CV in linear classification problems. In Proceedings of the 21st ACM SIGKDD International Conference on Knowledge Discovery and Data Mining (pp. 885–894). (2015).
53. Tanriver-Ayder, E, Faes, C, Van De Casteele, T, McCann, SK, and Macleod, MR. Comparison of commonly used methods in random effects meta-analysis: application to preclinical data in drug discovery research. BMJ Open Sci. (2021) 5:e100074. doi: 10.1136/bmjos-2020-100074,
54. Egger, M, Smith, GD, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629,
55. JBI. JBI critical appraisal tools for cross sectional studies. (2025). Available online at: https://jbi.global/sites/default/files/202007/Checklist_for_Analytical_Cross_Sectional_Studies.pdf (Accessed May 22, 2025).
56. JBI. JBI critical appraisal tools for cohort studies. (2025). Available online at: https://jbi.global/sites/default/files/2020-08/Checklist_for_Cohort_Studies.pdf (Accessed May 22, 2025).
57. JBI. JBI critical appraisal tools for case control studies. (2025). https://jbi.global/sites/default/files/2020-08/Checklist_for_Case_Control_Studies.pdf (Accessed May 22, 2025).
58. Walker, J, Adegbija, O, Smoll, N, Khan, A, Whicker, J, Carroll, H, et al. Epidemiology of mumps outbreaks and the impact of an additional dose of MMR vaccine for outbreak control in regional Queensland, Australia 2017-2018. Commun Dis Intell (2018). (2021) 1:45. doi: 10.33321/cdi.2021.45.67,
59. Maillet, M, Bouvat, E, Robert, N, Baccard-Longère, M, Morel-Baccard, C, Morand, P, et al. Mumps outbreak and laboratory diagnosis. J Clin Virol. (2015) 62:14–9. doi: 10.1016/j.jcv.2014.11.004,
60. Hukic, M, Hajdarpasic, A, Ravlija, J, Ler, Z, Baljic, R, Dedeic Ljubovic, A, et al. Mumps outbreak in the Federation of Bosnia and Herzegovina with large cohorts of susceptibles and genetically diverse strains of genotype G, Bosnia and Herzegovina, December 2010 to September 2012. Euro Surveill. (2014) 19:879. doi: 10.2807/1560-7917.es2014.19.33.20879,
61. Tan, KE, Anderson, M, Krajden, M, Petric, M, Mak, A, and Naus, M. Mumps virus detection during an outbreak in a highly unvaccinated population in British Columbia. Can J Public Health. (2011) 102:47–50. doi: 10.1007/BF03404876,
62. Schulte, J, Short, K, and Persse, D. Management and control issues related to two mumps outbreaks in Houston: future implications. Clin Infect Dis. (2023) 76:e1416–20. doi: 10.1093/cid/ciac650,
63. Waugh, CJ, Willocks, LJ, Templeton, K, and Stevenson, J. Recurrent outbreaks of mumps in Lothian and the impact of waning immunity. Epidemiol Infect. (2020) 148:e131. doi: 10.1017/S0950268820001296,
64. Tilavat, SM, Vaidya, SR, and Hamde, VS. Mumps outbreak in a tribal population from the union territory of Dadra and Nagar Haveli, India. J Med Virol. (2017) 89:2064–8. doi: 10.1002/jmv.24860,
65. Shah, AA, Bodewes, R, Reijnen, L, Boelsums, T, Weller, CM, Fanoy, EB, et al. Outbreaks of mumps genotype G viruses in the Netherlands between October 2019 and march 2020: clusters associated with multiple introductions. BMC Infect Dis. (2021) 21:1–9. doi: 10.1186/s12879-021-06702-7,
66. Albertson, JP. Mumps outbreak at a university and recommendation for a third dose of measles-mumps-rubella vaccine—Illinois, 2015–2016. MMWR Morb Mortal Wkly Rep. (2016) 65:731–4. doi: 10.15585/mmwr.mm6529a2,
67. Boxall, N, Kubinyiova, M, Príkazský, V, Beneš, C, and Castkova, J. An increase in the number of mumps cases in the Czech Republic, 2005-2006. Euro Surveill. (2008) 13:18842. doi: 10.2807/ese.13.16.18842-en
68. Gouma, S, Sane, J, Gijselaar, D, Cremer, J, Hahné, S, Koopmans, M, et al. Two major mumps genotype G variants dominated recent mumps outbreaks in the Netherlands (2009–2012). J Gen Virol. (2014) 95:1074–82. doi: 10.1099/vir.0.062943-0,
69. Cordeiro, E, Ferreira, M, Rodrigues, F, Palminha, P, Vinagre, E, and Pimentel, JP. Mumps outbreak among highly vaccinated teenagers and children in the central region of Portugal, 2012-2013. Acta Med Port. (2015) 28:435–41. doi: 10.20344/amp.5756,
70. Vaidya, SR, Tilavat, SM, Hamde, VS, and Bhattad, DR. Outbreak of mumps virus genotype G infection in tribal individuals during 2016–17 in India. Microbiol Immunol. (2018) 62:517–23. doi: 10.1111/1348-0421.12606,
71. Marx, GE. Mumps outbreak in a Marshallese community—Denver metropolitan area, Colorado, 2016–2017. MMWR Morb Mortal Wkly Rep. (2018) 67:1143–6. doi: 10.15585/mmwr.mm6741a2,
72. Aasheim, ET, Inns, T, Trindall, A, Emmett, L, Brown, KE, Williams, CJ, et al. Outbreak of mumps in a school setting, United Kingdom, 2013. Hum Vaccin Immunother. (2014) 10:2446–9. doi: 10.4161/hv.29484,
73. Zamir, CS, Schroeder, H, Shoob, H, Abramson, N, and Zentner, G. Characteristics of a large mumps outbreak: clinical severity, complications and association with vaccination status of mumps outbreak cases. Hum Vaccin Immunother. (2015) 11:1413–7. doi: 10.1080/21645515.2015.1021522,
74. Hübschen, JM, Vilivong, K, Souvannaso, C, Black, AP, Lütteke, N, Samountry, B, et al. High prevalence of mumps in Lao People's Democratic Republic. Clin Microbiol Infect. (2014) 20:O664–71. doi: 10.1111/1469-0691.12586,
75. Ferenczi, A, Gee, S, Cotter, S, and Kelleher, K. Ongoing mumps outbreak among adolescents and young adults, Ireland, august 2018 to January 2020. Euro Surveill. (2020) 25:2000047. doi: 10.2807/1560-7917.ES.2020.25.4.2000047,
76. Qin, W, Wang, Y, Yang, T, Xu, XK, Meng, XM, Zhao, CJ, et al. Outbreak of mumps in a student population with high vaccination coverage in China: time for two-dose vaccination. Hum Vaccin Immunother. (2019) 15:2106–11. doi: 10.1080/21645515.2019.1581526,
77. Watson-Creed, G, Saunders, A, Scott, J, Lowe, L, Pettipas, J, and Hatchette, TF. Two successive outbreaks of mumps in Nova Scotia among vaccinated adolescents and young adults. CMAJ. (2006) 175:483–8. doi: 10.1503/cmaj.060660,
78. Kutty, PK, McLean, HQ, Lawler, J, Schulte, C, Hudson, JM, Blog, D, et al. Risk factors for transmission of mumps in a highly vaccinated population in Orange County, NY, 2009–2010. Pediatr Infect Dis J. (2014) 33:121–5. doi: 10.1097/INF.0000000000000020,
79. Paul, S, Mahajan, PB, Sahoo, J, Bhatia, V, and Subba, SH. Investigating mumps outbreak in Odisha, India: an opportunity to assess the health system by utilizing the essential public health services framework. Am J Trop Med Hyg. (2017) 96:1215–21. doi: 10.4269/ajtmh.15-0593,
80. Cohen, C, White, JM, Savage, EJ, Glynn, JR, Choi, Y, Andrews, N, et al. Vaccine effectiveness estimates, 2004–2005 mumps outbreak, England. Emerg Infect Dis. (2007) 13:12–7. doi: 10.3201/eid1301.060649,
81. Bangor-Jones, RD, Dowse, GK, Giele, CM, van Buynder, PG, Hodge, MM, and Whitty, MM. A prolonged mumps outbreak among highly vaccinated aboriginal people in the Kimberley region of Western Australia. Med J Aust. (2009) 191:398–401. doi: 10.5694/j.1326-5377.2009.tb02850.x,
82. Moghe, CS, Goel, P, Singh, J, Nayak, NR, Dhuria, M, Jain, R, et al. Mumps outbreak investigation in Jaisalmer, Rajasthan, India, June-September 2016. J Med Virol. (2019) 91:347–50. doi: 10.1002/jmv.25324,
83. Orlíková, H, Malý, M, Lexová, P, Šebestová, H, Limberková, R, Jurzykowská, L, et al. Protective effect of vaccination against mumps complications, Czech Republic, 2007–2012. BMC Public Health. (2016) 16:1–20. doi: 10.1186/s12889-016-2958-4,
84. Schmid, D, Holzmann, H, Alfery, C, Wallenko, H, Popow-Kraupp, TH, and Allerberger, F. Mumps outbreak in young adults following a festival in Austria, 2006. Euro Surveill. (2008) 13:11–2. doi: 10.2807/ese.13.07.08042-en,
85. Whelan, J, Van Binnendijk, R, Greenland, K, Fanoy, E, Khargi, M, Yap, K, et al. Ongoing mumps outbreak in a student population with high vaccination coverage, Netherlands, 2010. Euro Surveill. (2010) 15:19554. doi: 10.2807/ese.15.17.19554-en,
86. Walker, J, Huc, S, Sinka, K, Tissington, A, and Oates, K. Ongoing outbreak of mumps infection in Oban, Scotland, November 2010 to January 2011. Euro Surveill. (2011) 16:19803. doi: 10.2807/ese.16.08.19803-en
87. Rajčević, S, Šeguljev, Z, Petrovic, V, Medić, S, Nedelijković, J, Milošević, V, et al. Ongoing mumps outbreak in Novi Sad, the autonomous province of Vojvodina, Serbia, January to April 2012. Eurosurveillance. (2012) 17:20169. doi: 10.2807/ese.17.19.20169-en,
88. Stein-Zamir, C, Shoob, H, Abramson, N, Tallen-Gozani, E, Sokolov, I, and Zentner, G. Mumps outbreak in Jerusalem affecting mainly male adolescents. Eurosurveillance. (2009) 14:19440. doi: 10.2807/ese.14.50.19440-en,
89. Bernard, H, Schwarz, NG, Melnic, A, Bucov, V, Caterinciuc, N, Pebody, RG, et al. Mumps outbreak ongoing since October 2007 in the Republic of Moldova. Euro Surveill. (2008) 13:3–4. doi: 10.2807/ese.13.13.08079-en,
90. Hukic, M, Ravlija, J, Ljubovic, AD, Moro, A, Arapcic, S, Muller, CP, et al. Ongoing large mumps outbreak in the Federation of Bosnia and Herzegovina, Bosnia and Herzegovina, December 2010 to July 2011. Euro Surveill. (2011) 16:19959. doi: 10.2807/ese.16.35.19959-en,
91. Raut, CG, Sinha, DP, Jayaprakash, H, Hanumiah, H, and Manjunatha, MJ. Mumps disease outbreak in Davangere district of Karnataka, India. Indian J Med Microbiol. (2015) 33:378–82. doi: 10.4103/0255-0857.158558,
92. Mossong, J, Bonert, C, Weicherding, P, Opp, M, Reichert, P, Even, J, et al. Mumps outbreak among the military in Luxembourg in 2008: epidemiology and evaluation of control measures. Eurosurveillance. (2009) 14:19121. doi: 10.2807/ese.14.07.19121-en,
93. Patel, LN, Arciuolo, RJ, Fu, J, Giancotti, FR, Zucker, JR, Rakeman, JL, et al. Mumps outbreak among a highly vaccinated university community—new York City, January–April 2014. Clin Infect Dis. (2017) 64:408–12. doi: 10.1093/cid/ciw762,
94. Sane, J, Gouma, S, Koopmans, M, de Melker, H, Swaan, C, van Binnendijk, R, et al. Epidemic of mumps among vaccinated persons, the Netherlands, 2009–2012. Emerg Infect Dis. (2014) 20:643–8. doi: 10.3201/eid2004.131681,
95. Walkty, A, Van Caeseele, P, Hilderman, T, Buchan, S, Weiss, E, Sloane, M, et al. Mumps in prison: description of an outbreak in Manitoba, Canada. Can J Public Health. (2011) 102:341–4. doi: 10.1007/BF03404173,
96. Saboui, M, and Squires, SG. Oral health in Canada: mumps outbreaks across Canada, 2016 to 2018. Can Commun Dis Rep. (2020) 46:427–31. doi: 10.14745/ccdr.v46i1112a10,
97. McKay, SL. Notes from the field: mumps outbreak in a recently vaccinated population—Kosrae, Federated States of Micronesia, august–December 2017. MMWR Morb Mortal Wkly Rep. (2019) 68:95–6. doi: 10.15585/mmwr.mm6804a5,
98. Tiffany, A. Notes from the field: mumps outbreak Alaska, may 2017–July. MMWR Morb Mortal Wkly Rep. (2018) 67:940–1. doi: 10.15585/mmwr.mm6733a6
99. Nedeljkovic, J, Kovačević-Jovanović, V, Milošević, V, Šeguljev, Z, Petrovic, V, Muller, CP, et al. A mumps outbreak in Vojvodina, Serbia, in 2012 underlines the need for additional vaccination opportunities for young adults. PLoS One. (2015) 10:e0139815. doi: 10.1371/journal.pone.0139815,
100. Golwalkar, M. Mumps outbreaks at four universities—Indiana, 2016. MMWR Morb Mortal Wkly Rep. (2018) 67:793–7. doi: 10.15585/mmwr.mm6729a1,
101. Fields, VS, Safi, H, Waters, C, Dillaha, J, Capelle, L, Riklon, S, et al. Mumps in a highly vaccinated Marshallese community in Arkansas, USA: an outbreak report. Lancet Infect Dis. (2019) 19:185–92. doi: 10.1016/S1473-3099(18)30607-8,
102. Clemmons, NS, Redd, SB, Gastañaduy, PA, Marin, M, Patel, M, and Fiebelkorn, AP. Characteristics of large mumps outbreaks in the United States, July 2010–December 2015. Clin Infect Dis. (2019) 68:1684–90. doi: 10.1093/cid/ciy779,
103. Livingston, KA, Rosen, JB, Zucker, JR, and Zimmerman, CM. Mumps vaccine effectiveness and risk factors for disease in households during an outbreak in new York City. Vaccine. (2014) 32:369–74. doi: 10.1016/j.vaccine.2013.11.021,
104. Nelson, GE, Aguon, A, Valencia, E, Oliva, R, Guerrero, ML, Reyes, R, et al. Epidemiology of a mumps outbreak in a highly vaccinated island population and use of a third dose of measles–mumps–rubella vaccine for outbreak control—Guam 2009 to 2010. Pediatr Infect Dis J. (2013) 32:374–80. doi: 10.1097/INF.0b013e318279f593,
105. Cardemil, CV, Dahl, RM, James, L, Wannemuehler, K, Gary, HE, Shah, M, et al. Effectiveness of a third dose of MMR vaccine for mumps outbreak control. N Engl J Med. (2017) 377:947–56. doi: 10.1056/NEJMoa1703309,
106. Ma, C, Liu, Y, Tang, J, Jia, H, Qin, W, Su, Y, et al. Assessment of mumps-containing vaccine effectiveness during an outbreak: importance to introduce the 2-dose schedule for China. Hum Vaccin Immunother. (2018) 14:1392–7. doi: 10.1080/21645515.2018.1428508,
107. Huang, AS, Cortese, MM, Curns, AT, Bitsko, RH, Jordan, HT, Soud, F, et al. Risk factors for mumps at a university with a large mumps outbreak. Public Health Rep. (2009) 124:419–26. doi: 10.1177/003335490912400311,
108. Willocks, LJ, Guerendiain, D, Austin, HI, Morrison, KE, Cameron, RL, Templeton, KE, et al. An outbreak of mumps with genetic strain variation in a highly vaccinated student population in Scotland. Epidemiol Infect. (2017) 145:3219–25. doi: 10.1017/S0950268817002102,
109. Rhie, K, Park, HK, Kim, YS, Yeom, JS, Park, JS, Seo, JH, et al. Factors associated with mumps meningitis and the possible impact of vaccination. Korean J Pediatr. (2016) 59:24–9. doi: 10.3345/kjp.2016.59.1.24,
110. Abdullah, SM. Relationship between post-pubertal mumps infection in males with infertility and its effect on the result of seminal fluid analysis and occurrence of immunological infertility. Diyala J Med. 19:118–28. doi: 10.26505/DJM.19025500814
111. Barskey, AE, Glasser, JW, and LeBaron, CW. Mumps resurgences in the United States: a historical perspective on unexpected elements. Vaccine. (2009) 27:6186–95. doi: 10.1016/j.vaccine.2009.06.109,
112. Ramanathan, R, Voigt, EA, Kennedy, RB, and Poland, G. Knowledge gaps persist and hinder progress in eliminating mumps. Vaccine. (2018) 36:3721–6. doi: 10.1016/j.vaccine.2018.05.067,
113. Surveillance Report. Annual Epidemiological Report for 2021. (2025). Available online at: https://www.ecdc.europa.eu/sites/default/files/documents/AER-Mumps-2021.pdf (Accessed June 10, 2025).
114. Centres for Disease Control (CDC), Mumps cases and outbreaks. (2025). Available online at: https://www.cdc.gov/mumps/outbreaks/index.html (Accessed June 10, 2025).
115. Puca, C, and Trent, M. Using the surveillance tool EpiWATCH to rapidly detect global mumps outbreaks. Global Biosecur. (2020) 1:54. doi: 10.31646/gbio.54
116. Deal, A, Halliday, R, Crawshaw, AF, Hayward, SE, Burnard, A, Rustage, K, et al. Migration and outbreaks of vaccine-preventable disease in Europe: a systematic review. Lancet Infect Dis. (2021) 21:e387–98. doi: 10.1016/S1473-3099(21)00193-6,
117. Sulaiman, FB, Yanti, NS, Lesmanawati, DA, Trent, M, Chughtai, AA, and MacIntyre, CR. Language specific gaps in identifying early epidemic signals–a case study of the Malay language. Global Biosecur. (2019) 1:1. doi: 10.31646/gbio.33
118. Mair, L, Relan, P, Hamilton, DO, Al-Noman, A, and O'Dempsey, T. Lessons learned from an under-reported mumps epidemic among Rohingya refugees in Cox's Bazar District, Bangladesh. Trans R Soc Trop Med Hyg. (2020) 114:635–8. doi: 10.1093/trstmh/traa048,
119. Buliva, E, Elhakim, M, Tran Minh, NN, Elkholy, A, Mala, P, Abubakar, A, et al. Emerging and reemerging diseases in the World Health Organization (WHO) eastern Mediterranean region—progress, challenges, and WHO initiatives. Front Public Health. (2017) 5:276. doi: 10.3389/fpubh.2017.00276,
120. Sinumvayo, JP, Munezero, PC, Tope, AT, Adeyemo, RO, Bale, MI, Mutsaka-Makuvaza, MJ, et al. Vaccination and vaccine-preventable diseases in Africa. Sci Afr. (2024) 24:e02199. doi: 10.1016/j.sciaf.2024.e02199
121. United Nations Children's Fund (UNICEF). UNICEF Immunization Data. (2023). Available online at: https://www.unicef.org/esa/press-releases/new-unicef-report-shows-127-million-children-africa-missed-out-one-or-more (Accessed June 14, 2025).
122. Lewnard, JA, and Grad, YH. Vaccine waning and mumps re-emergence in the United States. Sci Transl Med. (2018) 10:eaao5945. doi: 10.1126/scitranslmed.aao5945,
123. Ministry of Health and Family Welfare (MoHFW), Government of India. Universal Immunisation Programme (UIP). (2025). Available online at: https://www.mohfw.gov.in/?q=Major-Programmes/universal-immunization-programme-uip (Accessed June 14, 2025).
124. World Health Organization (WHO). The Global Health Observatory. Available online at: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/mumps---number-of-reported-cases (Accessed June 20, 2025).
125. Ye, Q, and Liu, H. Impact of non-pharmaceutical interventions during the COVID-19 pandemic on common childhood respiratory viruses–an epidemiological study based on hospital data. Microbes Infect. (2022) 24:104911. doi: 10.1016/j.micinf.2021.104911,
126. Causey, K, Fullman, N, Sorensen, RJ, Galles, NC, Zheng, P, Aravkin, A, et al. Estimating global and regional disruptions to routine childhood vaccine coverage during the COVID-19 pandemic in 2020: a modelling study. Lancet. (2021) 398:522–34. doi: 10.1016/S0140-6736(21)01337-4,
127. Ristić, M, Vuković, V, Rajčević, S, Koprivica, M, Agbaba, N, and Petrović, V. Mumps epidemiology in the Autonomous Province of Vojvodina, Serbia: long-term trends, immunization gaps, and conditions favoring future outbreaks. Vaccine. (2025) 13:839. doi: 10.3390/vaccines13080839,
128. Bodewes, R, Reijnen, L, Kerkhof, J, Cremer, J, Schmitz, D, van Binnendijk, R, et al. Molecular epidemiology of mumps viruses in the Netherlands, 2017-2019. PLoS One. (2020) 15:e0233143. doi: 10.1371/journal.pone.0233143,
129. Shaikh, S, Carpenter, M, Lin, L, Frost, JR, McLachlan, E, Stein, D, et al. Serologic cross-reactivity between the mumps virus vaccine genotype a strain and the circulating genotype G strain. Viruses. (2024) 16:1434. doi: 10.3390/v16091434,
130. Gouma, S, Vermeire, T, Van Gucht, S, Martens, L, Hutse, V, Cremer, J, et al. Differences in antigenic sites and other functional regions between genotype a and G mumps virus surface proteins. Sci Rep. (2018) 8:13337. doi: 10.1038/s41598-018-31630-z,
Keywords: attack rate, MMR vaccine, mumps, outbreak, vaccination
Citation: Agrawal R, Rehman T, Sinha D, Chakraborty P, Sakthivel M, Kumar D, Mitra S, Karumathil A, Kanungo S and Pati S (2025) Epidemiological trends and determinants of mumps outbreaks: a systematic review and meta-analysis. Front. Public Health. 13:1711759. doi: 10.3389/fpubh.2025.1711759
Edited by:
Andrew Omame, York University, CanadaReviewed by:
Tarun Kumar Suvvari, Squad Medicine and Research (SMR), IndiaFrank Mbulinyingi Msafiri, Muhimbili University of Health and Allied Sciences, Tanzania
Copyright © 2025 Agrawal, Rehman, Sinha, Chakraborty, Sakthivel, Kumar, Mitra, Karumathil, Kanungo and Pati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanghamitra Pati, ZHJzYW5naGFtaXRyYTEyQGdtYWlsLmNvbQ==; Tanveer Rehman, ZHJ0YW52ZWVycmVobWFuQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
‡ORCID: Ritik Agrawal, orcid.org/0000-0001-5345-1559
Tanveer Rehman, orcid.org/0000-0003-2377-4394
Deepti Sinha, orcid.org/0009-0000-6115-5581
Poulomee Chakraborty, orcid.org/0009-0003-7160-1321
Manikandanesan Sakthivel, orcid.org/0000-0002-5438-3970
Dewesh Kumar, orcid.org/0000-0001-5356-801X
Srijeeta Mitra, orcid.org/0009-0005-3430-0428
Afeeq Karumathil, orcid.org/0009-0006-8464-3792
Srikanta Kanungo, orcid.org/0000-0001-5647-0122
Sanghamitra Pati, orcid.org/0000-0002-7717-5592
 Ritik Agrawal1,2†‡
Ritik Agrawal1,2†‡ Tanveer Rehman
Tanveer Rehman Srikanta Kanungo
Srikanta Kanungo Sanghamitra Pati
Sanghamitra Pati