Abstract
Haptic feedback, or tactile perception, is presented by many authors as a technology that can greatly impact biomedical fields, such as minimally invasive surgeries. Laparoscopic interventions are considered the gold standard for many surgical interventions, providing recognized benefits, such as reduced recovery time and mortality rate. In addition to this, the advances in robotic engineering in the last few years have contributed to the increase in the number of robotic and tele-operated interventions, providing surgeons with fewer hand tremors and increased depth perception during surgery. However, currently, both techniques are totally or partially devoid of haptic feedback. This added to the fact that the skill acquisition process to be able to use these technologies shows a pronounced learning curve, has propelled biomedical engineers to aim to develop safe and realistic training programs using simulators to address surgical apprentices’ needs in safe environments for the patients. This review aims to present and summarize some of the latest engineering advances reported in the current literature related to the development of haptic feedback systems in surgical simulators and robotic surgical systems, as well as highlight the benefits that these technologies provide in medical settings for surgical training and preoperative rehearsal.
1 Introduction
Nowadays, laparoscopic surgery is a minimally invasive procedure considered the gold standard approach in many surgical interventions due to the many advantages that the technique provides, such as reduced recovery time and lower mortality rates (Kawka et al., 2023). However, laparoscopic training shows a pronounced learning curve that enhances the need for safe training programs for the patients that are also effective for the surgical apprentices outside the operating room (Kim et al., 2021; Braga et al., 2012). This surgical technique requires a deep understanding of laparoscopic instrumentation and a training period with diverse experiences and scenarios so that the trainee can be aware of the possible complications and learn how to prevent and treat them (Tang et al., 2017). The acquisition of these skills is challenged by the restricted working hours that limit the process of teaching and learning minimally invasive techniques (Sparn et al., 2024).
Following the example of the aviation industry, in which simulations are used to achieve the eminent technical skills required, along with a very small margin of error, technological advances in computer-aided simulations are also being applied to laparoscopic training (Vitish-Sharma et al., 2011). The creation of virtual environments provides the opportunity to recreate tailored and risky surgical situations without real-life repercussions, so surgeons can mitigate skill decay over time and trainees can improve according to their personalized needs and increase their confidence in their surgical skills (Nassar et al., 2021; Lohre et al., 2021; Mao et al., 2021). Considering that a hundred cases may be required for appropriate learning of the most complex procedures, virtual and computer-based simulations provided by biomedical engineers may be a good source of unlimited training cases (Susmitha et al., 2015).
In addition to this, laparoscopic surgery can benefit greatly from simulations since surgical instrumentation differs from the one used in conventional open surgery. Furthermore, psychomotor practice of complex maneuvers is required before the actual surgery to prove sufficient surgical competence to participate in human interventions, where margins of error are very small (Cardoso et al., 2023). Some of the required dexterities include aspects such as appropriate depth perception, hand-eye coordination, or bimanual manipulation (Vitish-Sharma et al., 2011; Sinha et al., 2017). For these reasons, the usage of these simulators would optimize the training time inside the operating room, where once the technical side is assimilated, the teachings can be more focused on decision-making training and intraoperative complications treatment (Matwala et al., 2024; Selva Raj et al., 2024).
Current laparoscopic simulators, such as the LapMentor™ (Simbionix USA Corp. Cleveland, OH) provide basic skill training, procedural tasks, simulation of full procedures, and feedback upon completion regarding parameters such as efficiency, accuracy rate, or safety parameters. Simulators usually provide performance curves that can be used to optimize training by tracking improvements and tackling specific weak points (Olivas-Alanis et al., 2020; Atesok et al., 2017).
Robotic surgery, on the other hand, provides high accuracy when performing repetitive tasks, with the additional advantage of telepresence, where a master console controls the slave robot that executes the command (Biswas et al., 2023). Surgical robots are composed of articulated instrumentation that accurately reproduces surgeons’ movements, overcoming the limitations imposed by the long and rigid instruments used in laparoscopic surgery. In addition to this, they provide relevant benefits, such as precise movements without tremors or improved visualization, which have contributed to their increased use over the years (Meli et al., 2017; Goldberg and Guthart, 2024; Mertz, 2022).
The ongoing engineering developments and applications of surgical robots are reflected in the percentage of robotic surgeries performed in the last years, which went from 1.8% in 2012 to 15% in 2020 (Sheetz et al., 2020). The most prevalent robotic surgical system is the da Vinci robot (Intuitive Surgical Inc.), created in 2000 with four different generations developed over the last 20 years, and the first to have FDA approval for surgical applications (Hamdi et al., 2024).
Other robot-assisted surgical systems are: the Arthrobot, developed in 1983 and considered the first robot to assist a surgical procedure in history (The Medical Post, 1985); the Puma robot, developed in 1985, used to perform a brain biopsy (Kwoh et al., 1988) and a transurethral prostate resection (Davies et al., 1991); and the ROBODOC, developed in 1991, and involved in assisting in implant positioning (Taylor et al., 1999).
However, although in laparoscopic surgery, the tactile perception, or “haptic feedback,” is severely limited by the interaction between laparoscopic instruments and the patient, in robotic surgery, the telemanipulation and the physical isolation of the surgeon from the patient worsen even more this sensory loss since direct contact between these is nonexistent (Meccariello et al., 2016). Therefore, the lack of haptic feedback is currently the main limitation of robotic surgical systems, especially since it is considered a key element to increase performance in a wide variety of tasks, such as robotic catheter insertion, palpation, or microneedle positioning (Meli et al., 2017; Najafi et al., 2023). In addition to this, the lack of haptic feedback also leads to excessive force application when using robotic systems for inexperienced surgeons (Jourdes et al., 2022).
Haptic feedback can be divided into kinesthetic and cutaneous. While the first one is related to the forces applied to joints and muscles, the latter is focused on tactile sensations associated with the skin. Tactile haptics refers to the stimulation of tactile sensing through haptic devices, which evokes the real feeling when touching an object (Selim et al., 2024; Abiri et al., 2019a).
Under the term “haptics”, several magnitudes are included, such as pressure, forces, texture, temperature, or vibrations (Shi and Shen, 2024). In humans, the sense of touch requires a combined activation of both tactile and kinesthetic force feedback through mechanoreceptors in skin and muscles, respectively. However, contrary to what happens in open surgery, in minimally invasive interventions, sensory perception is limited to the interaction between the tissues and the instrumentation used (Jourdes et al., 2022). While in open surgery surgeons rely on their fingertips’ sensations, in minimally invasive surgery all the sensory feedback comes from the tip of the tool that the surgeon uses.
Most minimally invasive devices lack haptic feedback, and physicians deal with an absent touch perception that difficult crucial tasks, such as tissue manipulation (Selim et al., 2024; Abiri et al., 2019a) since surgeons make use of their sense of touch to locate hidden structures and to distinguish abnormal tissues based on their altered mechanical properties in comparison to the healthy adjacent ones. Many researchers have focused on developing tactile feedback systems using vibrational or pneumatic stimuli to activate skin mechanoreceptors to improve surgical performance (Abiri et al., 2019a). Therefore, it is critical to dispose of devices capable of delivering haptic feedback during training, in the case of simulators, and intraoperatively, in the case of robotic surgeries (Abiri et al., 2019b).
Furthermore, considering that tactile sensations are essential in surgical fields, the archetype of a surgical simulator should provide an immersive experience, with the same stimuli and sensations that are encountered in the operating room (Di Vece et al., 2021). Current simulators come with some degree of feedback for the trainee. This feedback can be augmented feedback, referring to the information or total score that the users receive according to their technique (patterns, incorrect movements…), or intrinsic feedback, related to the sensorial stimulation that the trainees experience while using the simulator (haptics, audiovisual…) (Nassar et al., 2021). In addition to this, the main components responsible for haptic feedback are sensors and actuators. While sensors act by detecting the forces that the user applies to the tissues through the instrumentation, actuators transmit this information to surgeons’ hands (Abiri et al., 2019b).
Surgeons usually compensate for this lack of haptic feedback by increasing their training level and experience, and by focusing on intraoperative visual cues. However, this may lead to longer interventions and increased risk of complications than if haptic feedback were perceived similarly to in open surgery (Pinzon et al., 2016). Considering that the perception of an object’s mechanical properties requires a combination of visual and haptic information, motor task performance can be greatly improved by integrating haptic feedback that complements the already existing visual information (Pinzon et al., 2016).
In light of the above, this review aims to provide insights into the benefits and limitations of laparoscopic simulators and robotic surgery, the recent bioengineering developments in haptic feedback integrations, and their potential impact on training and procedure outcomes.
2 Benefits of laparoscopic simulators in medical training
Several authors have explored the utility of simulators for medical training. For instance, the impact of computer simulators on surgical skills was evaluated on a 3-week training program on a LapMentor™ simulator that included residents and medical students with basic to no laparoscopic experience (Kim et al., 2009). The authors showed that residents with some laparoscopic background initially benefited the most from the program, with faster acquisition and accuracy of the learned techniques, evaluated according to each task’s total transit time and accuracy. However, in the last stages of the interventional program, medical students improved significantly to almost reach the residents’ level of proficiency (Kim et al., 2009). These findings seem to indicate that, regardless of initial laparoscopic experience, computer-based simulators can help in a substantial acquisition of surgical techniques within a short time frame.
In addition to this, another study carried out a 4-week training intervention on 21 surgical residents using a LapMentor™ simulator (Andreatta et al., 2006). Once the program was completed, they evaluated participants’ improvement by putting into practice some laparoscopic skills in male pigs. The author reported a more accurate and precise 30° camera navigation in comparison to the control group as well as better ambidexterity abilities. The clinical consequences of this improved performance were less peripheral organ injury and decreased rates of untargeted electrocautery damage.
Important aspects of these simulators are their predictive validity, which refers to how reliably the real-life proficiency can be predicted according to the surgeon’s score and performance on the simulator, and their construct validity, which appropriately distinguishes experienced from inexperienced surgeons according to their score on the simulator. These parameters were evaluated by some authors, who correlated motion analysis data obtained from the LapMentor™ with the outcome of video assessments and the surgeon’s experience. They reported that the LapMentor™ distinguishes novices from experienced laparoscopic surgeons, and also that those with accurate performances on the simulator also executed safe laparoscopic procedures (Matsuda et al., 2012).
Some authors compared the efficiency of the LapMentor™ in comparison to a box trainer [Large Body MITS (TRLCD05)]. After 3 h of training for each group, the authors described an increased safe performance in both groups, which was higher in the group trained with the LapMentor™ simulator. The authors evaluated path length, tissue handling, and how the trainees were able to maintain the surgical instruments within the field of vision, and suggested that a combination of both methods may lead to a reduction in the learning curve and better laparoscopic training (Vitish-Sharma et al., 2011). In addition to this, simulators provide additional benefits lacking in box trainers, such as personalized feedback or complex procedure simulations (Våpenstad et al., 2013).
A more broad-ranging study was performed of three different simulators: LapSim® (Surgical Science Sweden AB, Gothen ® burg, Sweden), LAP Mentor III® (Simbionix, Tel Aviv, ® Israel), and LaparoS® (VirtaMed AG, Zurich, Switzer land). The authors showed faster task completion and a reduced path length in tasks such as bimanual handling, clip application, and tissue dissection. Moreover, they also reported that remarkable improvements can be achieved in virtual laparoscopic training regardless of the type of simulator employed (Sparn et al., 2024).
3 Value of haptic feedback technology in simulators for surgical training
In addition to appropriate training, touch sensations are also extremely important for surgical performance. It has been reported in the literature that better performance and higher learning rates are observed in trainees who were exposed to haptic feedback during their training stages than those who were not trained using haptic feedback. This improvement is especially remarkable in the early stages of learning (Zhou et al., 2012).
Since the haptic feedback in current simulators is not yet well developed, most of the laparoscopic learning process is spent on adapting to the loss of physical cues and their replacement with visual ones (Trute et al., 2024). Surgeons are already so used to this sensory substitution that some studies reported that when experienced surgeons undergo laparoscopic training without haptic feedback, their performance does not seem to be greatly affected. This is a consequence of their vast experience, which allows them to replace the haptic feedback with visual cues learned during their careers (Pinzon et al., 2016).
Nevertheless, many bioengineering studies are working on mimicking tactile feelings using haptic devices and on simulating as realistically as possible both the appearance and the interactions between the different instruments and the tissues involved (Table 1).
TABLE 1
| Reference | Year | Region | Participants | Engineering device | Instruments | Haptic type | Tasks | Measurements |
|---|---|---|---|---|---|---|---|---|
| Zhang et al. (2023) | 2023 | Canada | 10 novices | Phantom Omni (Senseable Technologies) | Grasper Laparoscopic scissors |
Unspecified | Pattern-cutting tasks inside a training box | • Total task time • Total grasping time • Total scissor time • Total scissor cutting time • Number of haptic feedback instances • Cutting accuracy |
| Våpenstad et al. (2013) | 2013 | Norway | 20 surgeons | LapSim (Surgical Science AB) | Xitact IHP Xitact ITP |
Force | Fine dissection Lifting and grasping |
Questionnaires related to the perception of the handles |
| Zhou et al. (2012) | 2012 | United States | 20 novices | ProMIS MIST-VR |
Laparoscopic tools | Unspecified | Suturing and knot-tying | • Time to task completion • Instrument path • Instrument smoothness • Errors |
| Di Vece et al. (2021) | 2021 | Italy United States |
14 urologists and students | CHAI3D OpenHaptics™ |
Veress Needle controlled by a haptic device stylus | Tactile | Access the abdominal cavity twice | • Insertion error • Duration of the task • Number of mistakes |
| Alleblas et al. (2019) | 2019 | Netherlands | 11 surgeons | Force Reflecting Operation Instrument (FROI) | Laparoscopic grasper | Unspecified | 10 partial bowel resections 6 hemihysterectomies 2 ovariectomies 6 partial ureter dissections |
Questionnaire (NASA Task Load Index) |
| Alleblas et al. (2016) | 2016 | Netherlands | 279 gynecologists, general surgeons, urologists, pediatric surgeons and medical technicians | Laparoscopic tools | Scissors In-line handles Pistols |
Unspecified | Obtain expert opinions regarding handle designs and expectations towards haptic feedback instruments | Questionnaires assessing: • Handgrip assessment • Haptic feedback |
| Botden et al. (2008) | 2008 | United Arab Emirates Netherlands |
45 surgical residents | SimSurgery Simulator Box trainer |
Xitact HTP instrument ports | None | Two-handed stitch with traction Realistic surgeon’s knot Realistic interrupted suture Realistic free knot |
Parameters of the final laparoscopic knot: • Position of the needle in the holder • Running needle through suturing pad • Taking proper bites of the suturing pad, during suturing • Throwing thread around a holder • Pulling tight of the thread • Quality (strength) of knot • Global evaluation of performance |
| Quesada et al. (2018) | 2018 | Spain France |
— | OpenHaptics Toolkit | Laparoscopic grasper | Visual Force |
Tearing of soft tissues | Computing times |
| Kim et al. (2013) | 2013 | South Korea | — | Custom-designed device | Conventional laparoscopic instruments | Force | Gallbladder removal | Computation time |
| Wu et al. (2022) | 2022 | China | — | Geomagic Touch |
Coagulation hook Grasping forces Titanium clamps |
Force | Clipping procedure Disjunction of vessels |
The time cost of each step |
| Hamza-Lup et al. (2012) | 2012 | Romania United States |
— | Phantom Omni (Senseable Technologies) | Tools for palpation (e.g., Babcock grasper, Maryland grasping forceps) | Force | Liver palpation | • Questionnaires • Forces applied to the liver |
| WU et al. (2021) | 2021 | China | — | Geomagic Touch |
Forceps Scissors |
Visual Force |
Hepatic parenchymal removal | • Time cost of the simulation cycle • Geometrical information |
| Boutin et al. (2024) | 2024 | Canada | 30 surgical residents | SemseGlove NOVA haptic gloves | Surgical drill | Vibrotactile Force |
External ventricular drain placement | • Questionnaire • Speed and accuracy of the procedure |
| Heredia-Pérez et al. (2019) | 2019 | Mexico Japan |
5 expert neurosurgeons 11 surgical residents |
PHANTOM PREMIUM 1.0, Geomagic | Forceps | Force | Removal of transsphenoidal tumor | • Colission point report • % healthy and tumor tissue removed • Total time of task completion • Path of virtual tools • Frequency of grasping action • Frequency of foot pedal activation |
| Lin et al. (2014) | 2014 | China | 9 experienced surgeons 16 novices |
Omega.6 (Force Dimension) | Saw Other tools for craniomaxillofacial surgery |
Force | Bone-sawing procedure | • Operative time • Haptic forces of the process • Feed velocity • Acceleration of the tool |
| Heimann et al. (2021) | 2021 | Germany United States |
— | TouchX (3D Systems) | Forceps Syringe Straight instruments (scalpel, cotton tip) Special tool for implant release |
Visual | Insertion and refill of an eye implant for intravitreal drug delivery | — |
Relevant studies included in this review about haptic feedback in simulators.
To achieve this, some authors focused their efforts on gathering information about this tissue-tool interaction and how it is perceived by surgeons to develop an accurate model. They evaluated surgeons’ perception of tissue stiffness when using laparoscopic instruments and compared their subjective opinions with laboratory measurements (Pinzon et al., 2016). The authors reported that the bigger the amount of tissue held in the grasper, the more accurate their stiffness assessment. Moreover, they also proposed four parameters responsible for the differentiation among several tissue types, which are the mass of the tissue, the mass of the tissue held by the laparoscopic instruments, tissue stiffness, and the degree of attachment to the abdominal wall (Pinzon et al., 2016).
Regarding the value of haptic feedback, previous work proposed suturing as a surgical task that benefits greatly from the tactile sensation of the tissue tension and suture thread tightness. Since the interactions between the different elements (needle, tissue, thread, instruments…) were so important, trainees did not benefit from the available simulations at that time without any haptic feedback, and participants reported a preferred use of box trainers instead (Botden et al., 2008).
However, a posterior study showed that haptic feedback is more relevant in some force-related tasks than in orientation-based procedures that require precise gestures and instrument control, such as suturing or knot-tying (Zhou et al., 2012). This reinforces the results of a recent publication, in which haptic feedback is presented as especially relevant for some crucial laparoscopic tasks, such as the abdominal insertion of the needle (Di Vece et al., 2021). This procedure heavily relies on tactile perception as the needle is inserted across the layers of the abdominal wall. The authors developed a simulator called OpenHapticsTM to evaluate the combined benefit of the combination of virtual simulations and haptic feedback for an appropriate insertion of the Veress needle without internal organ damage (Figure 1) (Di Vece et al., 2021). In this line of thought, some authors also explored the influence of force feedback on the amount of exerted force during dissection tasks using a master-slave device. They found a decrease in the applied forces when haptic feedback was present (Pinzon et al., 2016).
FIGURE 1

Qualitative features of two simulation platforms, the OpenHapticsTM and the CHAI3D (upper panel). Boxplots showing performance of surgeons and students at two different attempts, using the simulation platforms (lower panel). Image modified from Di Vece et al. (2021). (a) Seperate groups (surgeons and students). (b) Merged groups.
Considering the importance of haptic feedback, to further enhance training using devices with this property, a very recent work aimed to develop a dual-user simulator and achieve haptic feedback transfer from one user (e.g., an experienced surgeon) to another (e.g., a trainee), and guide novices’ hand movement based on the maneuvers executed by the expert surgeon. The authors of this work reported improved learning when haptic feedback between users was involved, and when trainees were assisted in simple executive tasks with laparoscopic tools, although further studies are required for more complex procedures (Figure 2) (Zhang et al., 2023).
FIGURE 2
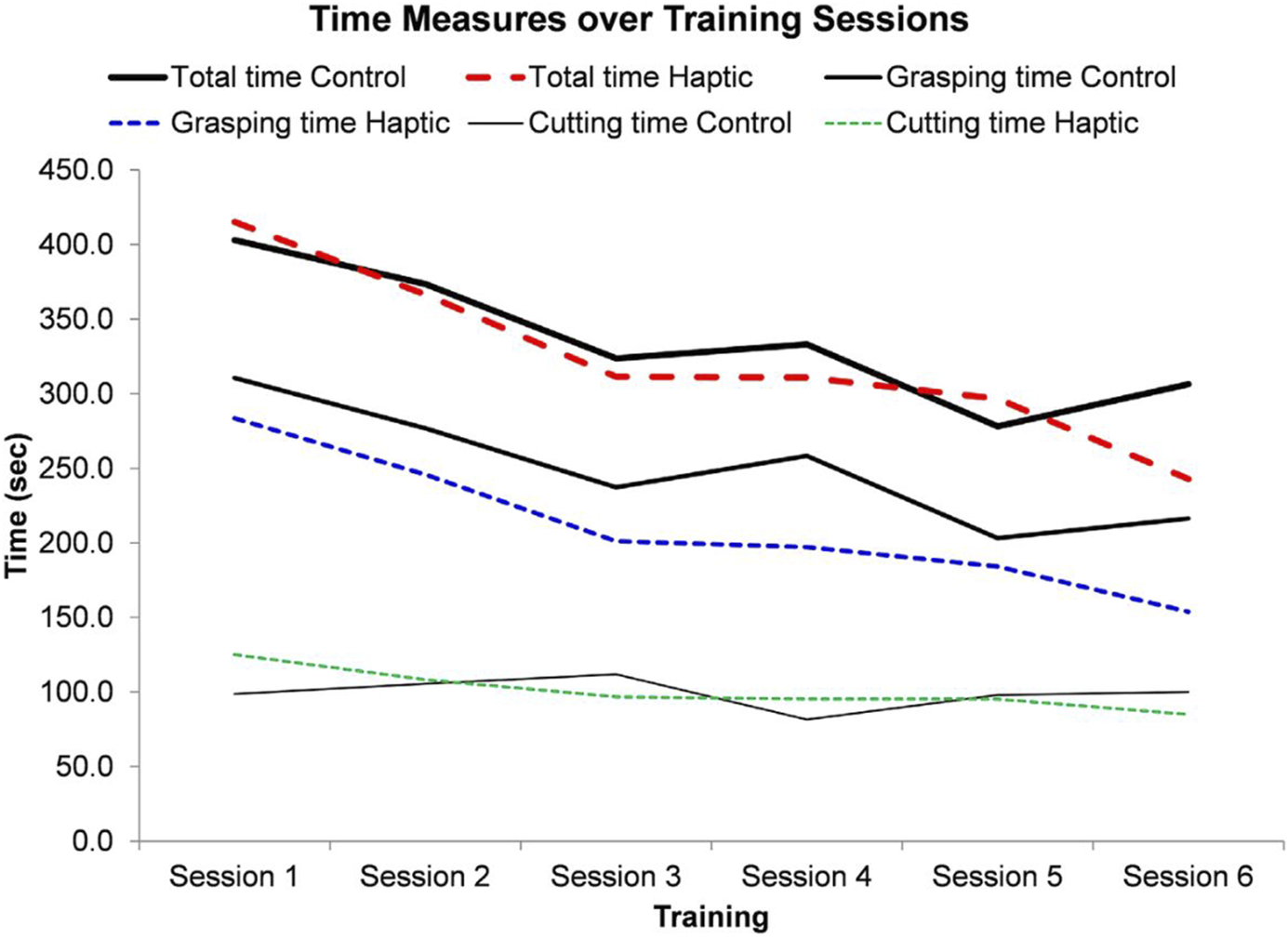
Time measurements over six training sessions. Image obtained from Zhang et al. (2023).
In addition to this, recent improvements are being made in the development of new haptic laparoscopic tools. For instance, a haptic laparoscopic grasper called the Force Reflecting Operation Instrument (FROI) was created using optical fiber Bragg grating technology and provides some resistance at the level of the tool trigger, as well as an audio warning and a mechanical brake that is activated when excessive forces are exerted over the tissue (Alleblas et al., 2019).
In this line of thought, some authors have previously explored the ergonomic aspects of laparoscopic handle design. They reported a considerable number of physical complaints from experienced surgeons associated with the use of laparoscopic instruments (i.e., the standardized size of the tools), which can lead to excessive force application and unfavorable postures (Alleblas et al., 2016). This highlights the importance of ergonomically designed handles that may optimize the implementation of laparoscopic tools with haptic feedback.
The value and significance of simulators have been enhanced in the last years, after the COVID-19 pandemic. A few years ago, the Royal College of Surgeons of England (RCSE) published a report after the pandemic about how technology may help to identify trainees’ needs and enhance surgical training, especially in those situations (i.e., a pandemic) where the number of surgical interventions is reduced to the bare essentials and no real practice is possible. The RCSE reported that virtual solutions may help assess surgical competence and involvement in surgical training. Furthermore, they stated that although haptic feedback may increase the applicability of simulation technologies, further research is still required for its optimization (Adebayo et al., 2022).
In light of the above, it is evident that laparoscopic simulators are a potent tool to refine surgical competence outside of the operating room and achieve a proper transfer of the learned skills to real-life clinical scenarios. However, the application of this technology in clinical frameworks may be cost-prohibitive, and open centralized training centers may help to overcome this limitation. In addition to this, the implementation of haptic feedback in simulation technologies is challenged by the need for realistic modeling of the elements involved in a surgical intervention (i.e., tools, organs) and the interactions between them, as well as the computational costs that these calculations require (Jourdes et al., 2022). Moreover, tissue simulations are often based on laboratory measurements from cadaveric samples, which makes the biomechanical properties simulated (Young’s modulus and Poisson’s ratio) unrealistic, and require more in vivo measurement approaches.
4 Robotic surgery and haptic feedback integration
Robotic surgery arose as a technology aimed at overcoming some of the limitations of laparoscopic surgery, such as hand tremors, poor depth perception, or the impossibility of telepresence. However, while laparoscopic techniques provide surgeons with some extent of haptic feedback, robotic surgery suffers from a complete loss of it. In addition to this, robotic surgical systems are more complex and entail some costs related to their acquisition and maintenance (Bergholz et al., 2023), as well as others associated with disposable supplies, such as trocars or drapes (Feldstein et al., 2019). This makes robotic surgery considerably more expensive than conventional minimally invasive surgery (Hamdi et al., 2024; El Rassi and El Rassi, 2020). The technical complexity, the economic costs, and the need to modify current instrumentation are the main limitations of haptic feedback integration with current robotic technologies in surgical settings (Hamdi et al., 2024). Table 2 shows the most relevant works on robotic surgery and haptic feedback included in this section.
TABLE 2
| Reference | Year | Region | Participants | Engineering device | Instruments | Haptic type | Tasks | Measurements |
|---|---|---|---|---|---|---|---|---|
| Hamdi et al. (2024) | 2024 | Saudi Arabia Japan |
— | A custom robotic master-slave | Custom robotic finger | Vibrotactile | Interact with 3 surfaces of different materials | Joint motion from: • metacarpophalangeal master-slave • proximal interphalangeal master-slave • wrist master-slave |
| Golahmadi et al. (2021) | 2014 | Australia | 20 subjects | A robotic master-slave system (PRAMiSS) | Camera Phantom Desktop (Sensable Technologies) Laparoscopic instrument |
Visual Force |
Vision feedback for tissue stiffness characterization Force feedback for tissue stiffness characterization Vision and force feedback for tissue characterization Direct exploration of tissue |
Questionnaires (True/False answers) |
| Ouyang et al. (2021) | 2021 | Germany | 31 inexperienced subjects | A robotic master-slave system (FLEXMIN) | Intracorporeal single-port instrument | Force | Precise moving and positioning of the instrument tip | • Applied touching forces • Total times |
| Ueda et al. (2023) | 2021 | China United States |
15 novices | Vinci Robotic Surgical System (Intuitive Surgical, Sunnyvale, CA) |
Surgical forceps | Visual Bio-inspired |
Phantom palpation to locate a tumor | • Accuracy of the localization • Mean force • Time to completion • Tumor contact time |
| Abiri et al. (2019b) | 2019 | United States | 15 novices | Vinci IS 1200 surgical system | da Vinci Fenestrated Bipolar forceps | Force Pneumatic Kinesthetic Hybrid Kinesthetic-Tactile |
Single handed peg-transfer tasks | • Average grip force • Peak grip force • Number of faults |
| Di Vece et al. (2021) | 2019 | United States | 19 subjects (task 1) 14 subjects (task 2) |
Da Vinci robot (Intuitive Surgical, Sunnyvale, CA) | da Vinci Fenestrated Bipolar forceps | Force Vibrotactile |
Detection of tubular structure Detection of discrete tumor |
• Correct localization • False localization • Time of completion |
| Meccariello et al. (2016) | 2016 | Italy Saudi Arabia |
25 surgeons (Otolaryngology, Thoracic Surgery, General Surgery, Urology and Gynecology) | Vinci Si Robotic Surgical System (Intuitive Surgical, Sunnyvale, CA) |
Maryland forceps A monopolar rounded tip |
Visual | Identify consistency or softness of tissue Locate a small metallic clip |
Questionnaire |
| Jourdes et al. (2022) | 2022 | France | 1 robotic surgeon | Vinci Xi surgical system (Intuitive Surgical) Simulator |
Needle Scissors Grasper |
Visual | Knot-tying and suturing tasks | • Thread behavior tests • Stress of tissue • Stress on rigid objects |
Relevant studies included in this review about haptic feedback in robotic surgery.
The lack of haptic feedback in robotic surgery, along with the inherent capacity of surgical robots to exert strong compressive and shear forces, has led to an increased risk of surgical mistakes during blunt dissection tasks and intraoperative tissue injuries. In the absence of haptic feedback, previous studies have reported that forces of only 1.25 N can cause tissue damage (Abiri et al., 2019a). In this regard, some authors have presented design frameworks aimed at providing an adjustable and constant force to ensure safe tissue maneuvering during minimally invasive robotic interventions in the absence of haptic feedback (Cheng et al., 2023; Sun and Lueth, 2023).
In addition to this, the importance of haptic feedback in tissue handling was also evaluated in a study in which haptic feedback was incorporated in a Parallel Robot Assisted MIS System (PRAMISS) that measured tissue interaction forces at the tooltip and achieved a proper attenuation if these were excessive (Moradi Dalvand et al., 2014). Moreover, authors of a recent evaluation reported 22.7% more force application by novices than experienced surgeons. They also showed that trainees benefit from feedback mechanisms, leading to a 47.9% decrease in the exerted forces (Golahmadi et al., 2021).
In a similar study, authors developed a robotic master-slave system called FLEXMIN to evaluate the effect that tactile perception has on the forces applied using the surgical robot. In this work, the authors also reported a significant reduction in the exerted forces when haptic feedback was present (Figure 3A) (Miller et al., 2021).
FIGURE 3

(A) Graphs showing force exertion (left), and error rates (right) with and without haptic feedback. Image obtained from Miller et al. (2021). (B) Performance in terms of average and peak grip force under different feedback situations: no feedback (NF), tactile, kinesthetic, and hybrid feedback. Image obtained from Abiri et al. (2019a). (C) Stress gradients representation on thread and tissue when excessive force is applied. Image obtained from Jourdes et al. (2022).
Considering that the actuators transmit information to surgeons’ hands according to what the sensor in the robotic instruments detects, the conversion process of the information from the sensor to the actuator is the main factor to address to provide natural haptic feedback that consists of more than just vibration or pressure. Some authors aimed to develop bioinspired algorithms that convert the information received by the sensors in a similar way that rapidly adapting type 1 neurons and Pacinian corpuscles do in the skin to increase performance. With this approach, they achieved less force exertion and better localization of soft tumors (Ouyang et al., 2021).
In addition to this, other recent works have been reported progressing towards the development of haptic sensations in robotic surgery. For instance, some authors are developing a wearable glove (Figure 4A) with robotic surgical fingers that use vibrational amplitude differences to help surgeons distinguish between hard, firm, and soft surfaces (Hamdi et al., 2024). Additionally, they also report a good correlation between the surgeon’s and the robotic finger’s movements (Figure 4B). On the other hand, other studies propose alternative mechanical feedback approaches, such as pneumatic balloons for the da Vinci robot that stimulate surgeons’ mechanoreceptors (Abiri et al., 2019b). This is an example of how some authors are also exploring the incorporation of analog or hybrid haptic solutions to avoid adding complexity to the already complex robotic devices. Additionally, these hybrid implementations (Abiri et al., 2019b; Ueda et al., 2023) are potentially less expensive than fully digital haptic systems, which is one of the main limitations of the acquisition of surgical robots.
FIGURE 4
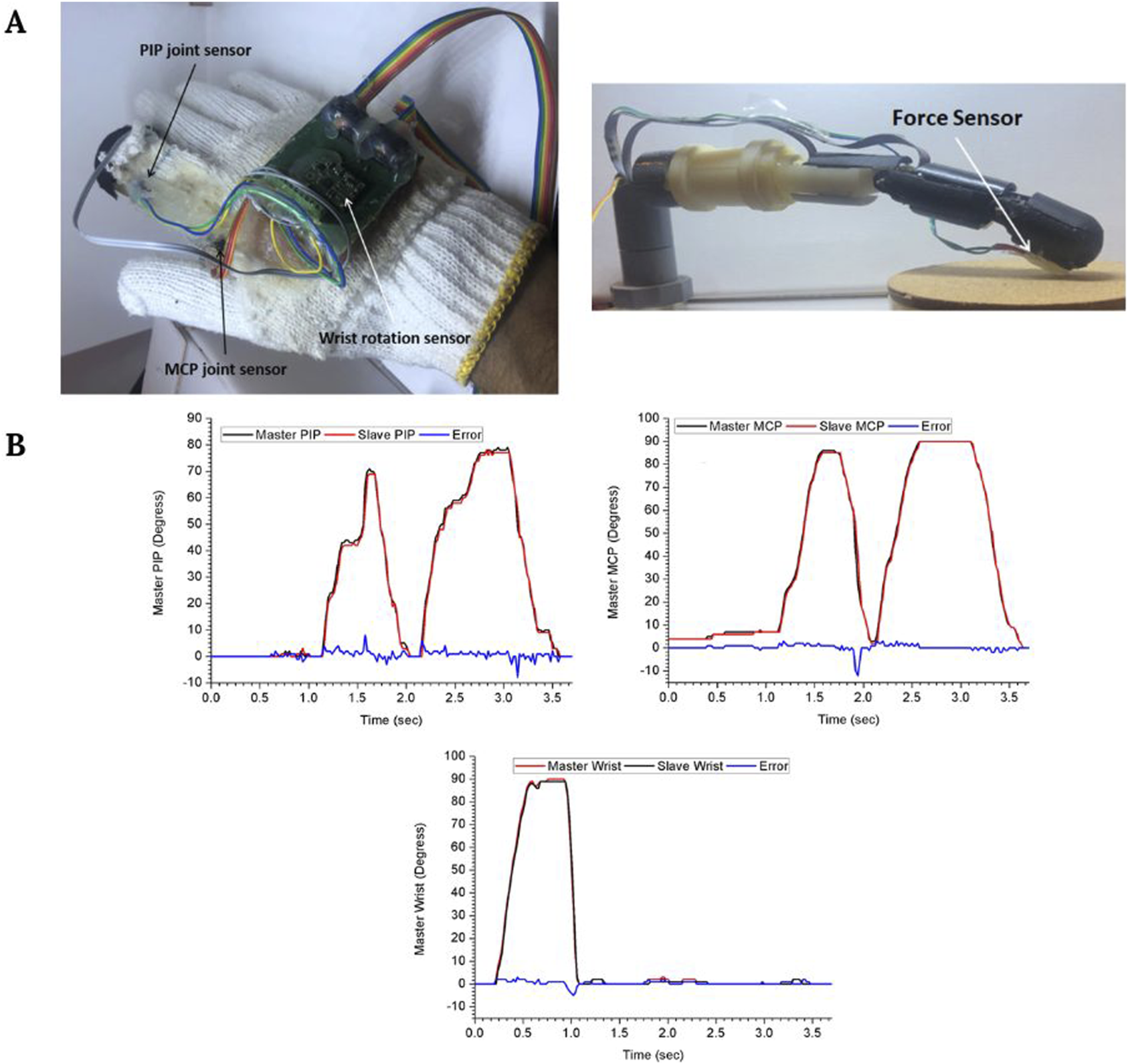
(A) Master glove and robotic finger. (B) Master and slave motion at the proximal interphalangeal (PIP) joint, the metacarpophalangeal (MCP) joint, and the wrist. Figure modified from Hamdi et al. (2024).
However, despite the extensive research focused on the development of haptic feedback in robotic surgery in the last few years, current technologies still fail to replicate the natural touch feelings. This is a consequence of the current unimodal technologies, which focus on a single modality of feedback (tactile or kinesthetic) (Amirabdollahian et al., 2018). This approach is not well interpreted by the human brain, which is accustomed to receiving and integrating sensory signals from both pathways.
Therefore, multimodal haptic feedback systems that target skin and muscle mechanoreceptors constitute a promising approach to the current haptic feedback challenge in minimally invasive surgeries, both robotic and laparoscopic. Nevertheless, this line of action has not been widely explored, due to the technical limitations associated with the integration of several sensing and feedback modalities. Some authors explored this approach by developing a multimodal haptic feedback system that combined tactile and kinesthetic force feedback. In this study, the authors evaluated this multimodal system through the performance of two-handed peg transfer tasks and recorded the users’ grip force. With this multimodal system, authors reported greater benefits, especially in novice surgeons, in comparison with single modality systems. Among the observed advantages, authors described 50% less grip force than when haptic feedback is absent (Figure 3B) (Abiri et al., 2019a).
Additionally, the introduction of haptic feedback systems in robotic surgery is limited due to the requirements for modification in robotic instrumentation. Some authors have proposed employing multimodal haptic feedback sensors as add-ons to robotic tools, therefore allowing some degree of versatility and compatibility with several robotic systems available on the market. They evaluated the ability of this approach to accurately discriminate soft tissues and discern underlying structures through force and vibrotactile feedback and found that multimodal haptic feedback significantly increased the effectiveness of artificial palpation devices (Abiri et al., 2019b).
Since haptic feedback in robotic surgery is still far from developed, many studies have focused on understanding how robotic surgeons rely on visual cues to evaluate and interpret the surgical field, and how their expertise also may impact the interpretation of this visual information and therefore, the surgical outcome, when haptic feedback is nonexistent (Green et al., 2022; Hagen et al., 2008). In addition to this, some authors have also performed some comparisons between visual and haptic feedback in virtual reality environments, where users were found to perceive better the interactions when both modalities coexist, although haptic feedback alone was more effective than visual feedback alone (Gibbs et al., 2022). On the other hand, a different study performing a similar comparison using a robotic arm found that visual feedback modalities reduced the most the grasping force during object-grasping tasks (Haruna et al., 2021). Therefore, appropriate and comprehensive communication of visual information is key to robotic surgery training so that future surgeons can interpret visual signals of force application when working with insensitive surgical robots (Miller et al., 2021).
In addition to this, when haptic feedback is not possible, sensory substitution arises as a compensatory approach to provide surgeons with a different sensory modality to represent, for instance, the force applied by the surgical instruments (Meccariello et al., 2016), through graphical or audio feedback (Okamura, 2009). An example of this is a work in which, to replace haptic feedback in simulators, some authors developed a training approach in which as the users perform tasks related to suturing and knot-tying, they receive color-based feedback according to the stress fields computation performed in real-time (Figure 3C). This approach may help trainees acclimate to the lack of tactile feedback and prevent excessive force application (Jourdes et al., 2022). However, for other authors, sensory substitution is counterintuitive and unnatural, may worsen the learning curve, and does not provide any information related to hidden structures in the subsurface (Miller et al., 2021).
To sum up, future studies are still needed to tackle some important limitations of haptic feedback implementation in robotic surgery. One of them is the standardization and magnification of the force feedback perceived through the device (Pinzon et al., 2016). In addition to this, there is a pressing need for feedback systems that encode information in a way that emulates the human nervous system. A stable implementation should also be achieved without time delays and communication latency during processing and transmission tasks to minimize the risk of instability of the haptic loop and compromise the surgery, especially for intercontinental applications.
5 Case studies of novel haptic feedback developments for simulations and surgical robots
5.1 Abdominal surgery
Nowadays, the core problem that prevents current simulation technologies from adopting haptic feedback is the feedback rates, which need to be extremely fast (from 500 to 1 kHz) to achieve a realistic feeling. This implies a huge computational cost since nonlinear mechanics equations must be solved around 1,000 times per second. In surgical simulations, the cutting steps that require big changes in the mesh topology are especially hard to represent. For this reason, some authors have focused on the simulation of interventions for the cutting steps that are minimal, such as laparoscopic cholecystectomies, where only one step is properly considered as cutting, and where tissue tearing is a more frequent procedure. In a study, authors reported the development of a novel algorithm for simulating the tearing of the fat tissue that usually takes most of the intervention time, with reduced computation times, and is compatible with haptic implementations (Quesada et al., 2018).
There are three basic procedures in laparoscopic cholecystectomy: 1) Calot’s triangle dissection, 2) cystic artery clipping, and 3) gallbladder separation. Some authors used finite element methods to simulate the step related to gallbladder removal that consists of the separation from the liver by burning the surrounding connective tissue (Figure 5A) (Kim et al., 2013). On the other hand, another study went further and tried to simulate all three of them. Their approach facilitates both soft deformation and haptic rendering and uses a position-based dynamics method that overcomes the real-time limitation posed by the high computational cost of finite element methods, but with shortcomings related to the graphics and unrealistic visual results (Wu et al., 2022).
FIGURE 5
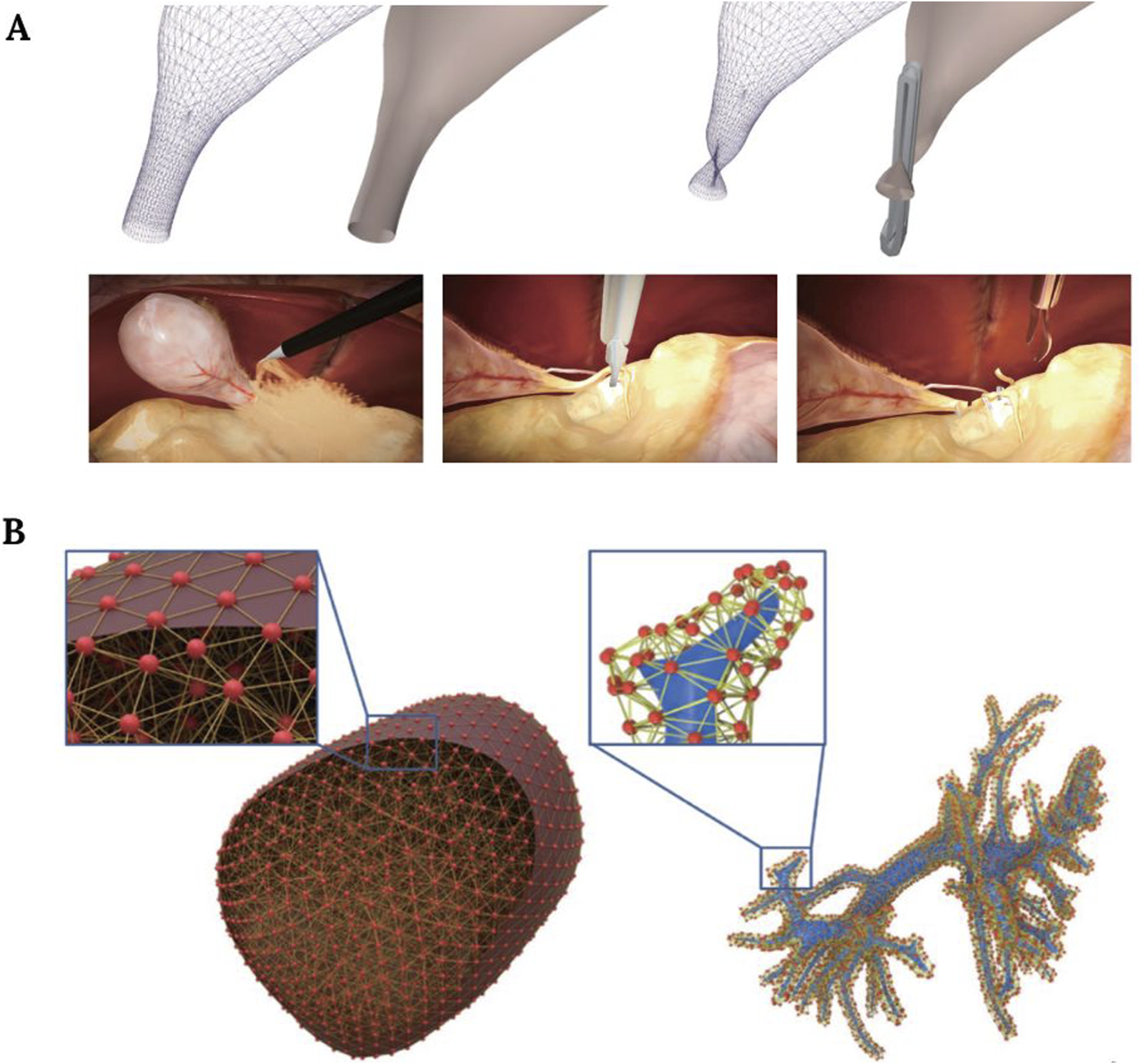
(A) Simulation of the clipping procedure during a cholecystectomy. Image modified from Wu et al. (2022). (B) Liver model illustration, showing the mesh and physical particles of the liver surface (left), and intrahepatic vessels (right). Image obtained from Wu et al. (2021).
In liver surgery, preoperative palpation is an important task that can reveal multiple pathological conditions. With this in mind, some authors presented a simulation system in which these palpation skills can be learned, implementing a force feedback hardware interface and a deformable liver model (Hamza-Lup et al., 2012). A similar attempt was reported by authors who tried to simulate liver parenchymal transection (Figure 5B) (Wu et al., 2021).
In robotic interventions, the Senhance Surgical System (SSS) is an alternative to the da Vinci robot. It has been tested for robotic cholecystectomies, and some authors report good haptic force feedback perception. The SSS also offers additional benefits, such as eye-tracking camera control and high configuration versatility (Aggarwal et al., 2020; Melling et al., 2019).
In addition to this, some authors are working on novel robotic systems. For instance, a recently published study presented a surgical-assisting robot (Riverfield Inc., Japan) with bimanual haptic devices placed in the surgeon’s console. The device was tested in animal models to evaluate its impact on force reduction in cases with a high risk of intestinal damage (Ota et al., 2024).
5.2 Brain surgery
In brain surgery, the placement of a ventricular drain to relieve intracranial pressure is a common procedure that must be mastered by neurosurgical trainees. To help with this task, some authors developed a simulator combined with haptic gloves, the SenseGlove NOVA gloves, which incorporated vibrotactile feedback. The feedback intensity increased as the skull was penetrated by the surgical drill, and then dropped to indicate that the drill must be stopped immediately (Boutin et al., 2024).
Moreover, other complex surgical brain interventions also benefit from virtual simulators, such as the resection of pituitary tumors through a transsphenoidal procedure. A study reported the development of an intuitive simulator with haptic feedback that helped achieve finer movements with less undesired contact with healthy tissue (Heredia-Pérez et al., 2019).
In addition to this, during real-life brain surgery, it is of utmost importance to distinguish between tumor tissue and normal brain tissue according to stiffness differences. For this purpose, some authors developed a haptic forceps prototype and aimed to determine their clinical utility on mouse models of brain tumors (Figure 6) (Ezaki et al., 2024).
FIGURE 6
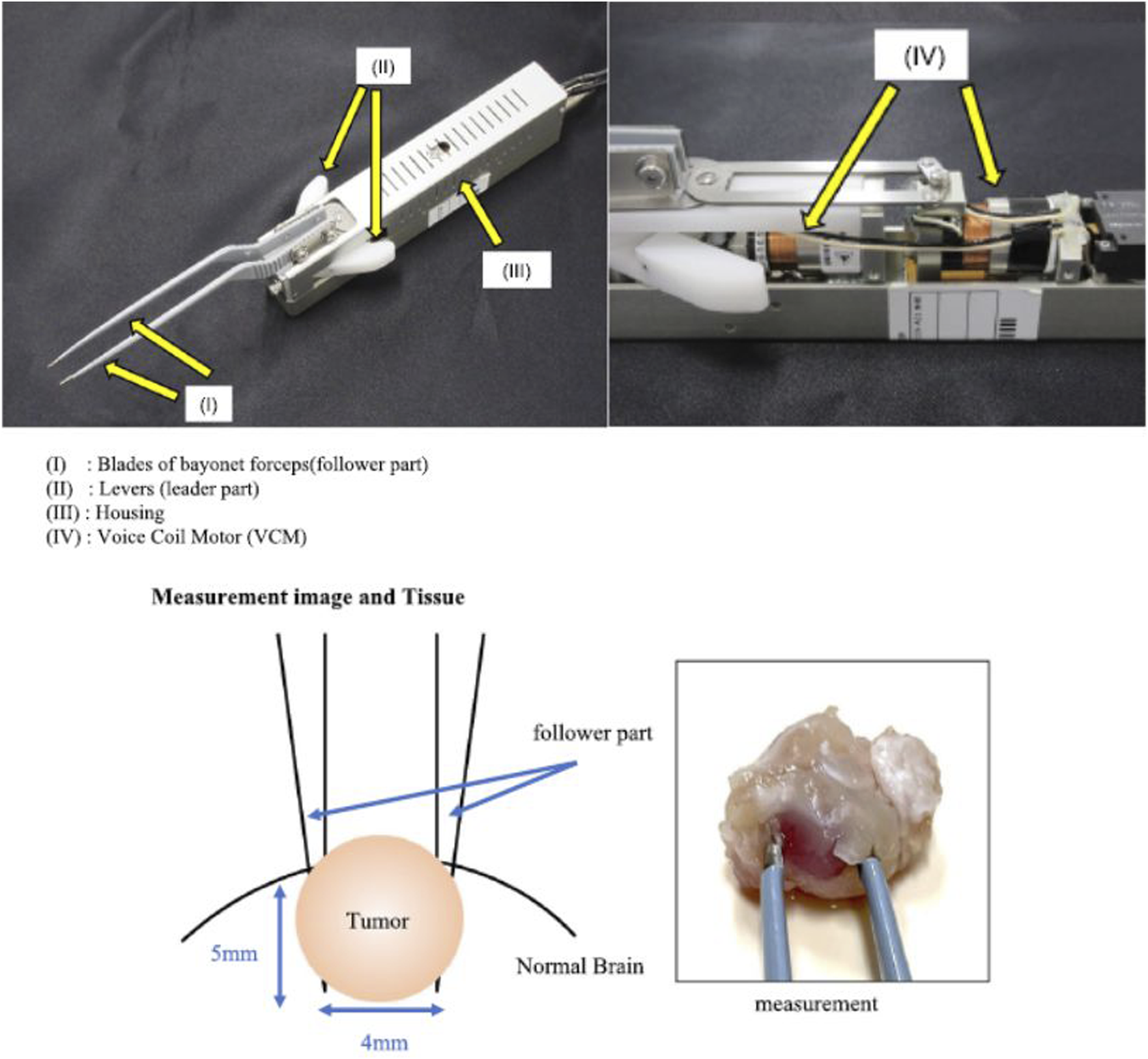
Surgical forceps with haptic technology for brain tissue stiffness measurement. Image modified from Ezaki et al. (2024).
5.3 Bone surgery
In bone surgery, bone sawing in maxillofacial surgery is a complex procedure where surgeons must be sensitive to the force they apply in the operating room. Due to the relevance of this procedure, some authors aimed to develop a haptic simulation platform where bone-sawing skills can be assessed and validated. They employed multi-point collision detection methods to simulate tool-bone interactions, and considered bone density (cortical and trabecular), feed velocity, and spindle speed to simulate the haptic feedback (Lin et al., 2014).
On the other hand, in robotic spine surgery, a recently published study reported the development of a promising surgical drill that integrates a haptic interface (Figure 7A). The authors performed an evaluation test on female pigs to detect the penetration of the posterior lamina. They found that in the absence of haptic feedback, the reaction time until surgeons perceived that they had penetrated the lamina with the drill was 0.10–0.22 s, while in the presence of haptic feedback, this reaction time was reduced to 0.01–0.02 s (Figure 7B). Therefore, the integration of these haptic technologies may provide more safety and accuracy in spinal robotic surgeries (Yamanouchi et al., 2023).
FIGURE 7
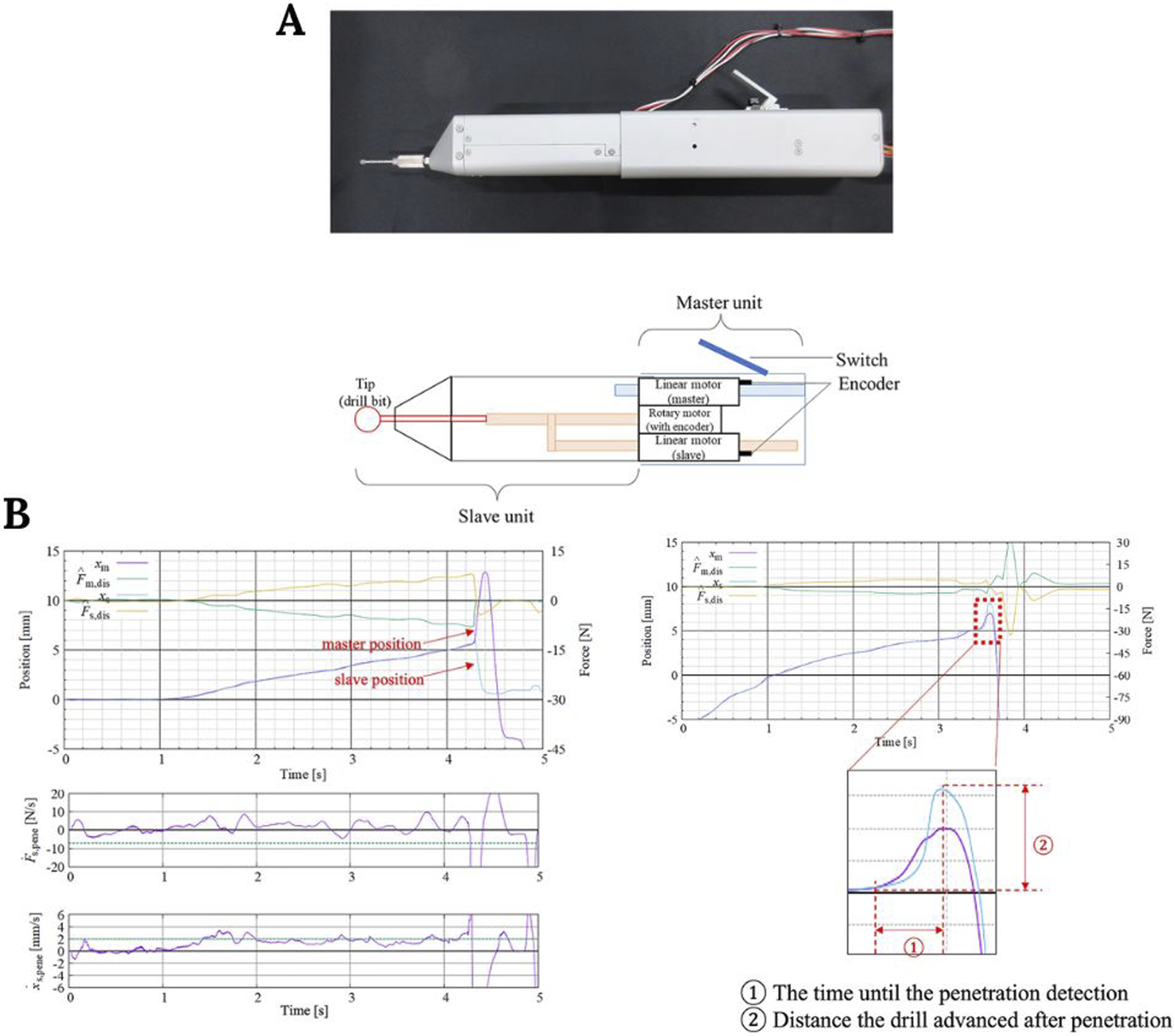
(A) Surgical grill and control unit representation. (B) Plots showing the time required for penetration detection and the distance traveled during that time. Image modified from Yamanouchi et al. (2023).
5.4 Cardiovascular surgery
In cardiovascular surgery, controlling the amount of pressure exerted on the blood vessels is key for a successful intervention. For this reason, cardiovascular interventions constitute another category in which robotic approaches may be beneficial. For instance, some authors developed a master-slave robot for vascular interventions where catheters and guidewire insertions are needed. They aimed to create a steerable catheter with haptic feedback to minimize the risk of vessel damage and the time of radiation exposure (Woo et al., 2019).
5.5 Ophthalmological surgery
Intraocular surgery is one of the disciplines of microsurgery and requires precise motor skills for safe outcomes since many small and delicate structures are involved. To master some fine maneuvers, some authors developed a virtual reality simulator in which surgeons were able to train tasks such as the insertion and refill of an eye implant for intravitreal drug delivery. The system was also equipped with haptic resistance during the implant insertion (Heimann et al., 2021).
Moreover, the absence of tremors in robot surgery is a factor extremely beneficial in these interventions. Some authors developed an eye surgery robot that would allow for to safe performance of micromanipulations. The incorporation of haptic feedback using the Phantom Premium devices (Sensible Technologies, 3D Systems) made it more intuitive and ergonomic (Barthel et al., 2015).
6 Future directions of haptic feedback systems in robotic surgery
Despite the great advances that have been witnessed in the field of haptic feedback devices, there are still many areas that require further development and research. For instance, some authors support the idea that coupling haptic systems and current surgical robots may lead to diminished surgical performance and heightened physical fatigue during interventions. In this regard, ergonomics is a concern when the surgical team is presented with large devices that might interfere with usual hand movements, either because of their dimensions or their weight (Colan et al., 2024).
Furthermore, in some clinical applications in which the appropriate distinction between the different layers of tissue is needed, such as needle insertions, current challenges rely on the integration of haptic feedback systems for the identification of changes in force measurements with reduced time delay and less risk of tissue damage (Selim et al., 2024).
In addition, further research is still required to recreate tactile sensations more accurately through haptic feedback systems. Multimodal approaches combining kinaesthetic and tactile feedback seem promising, but the determination of the best combination of these modalities is still a challenge to be tackled in the future to achieve a natural palpation feeling (Abiri et al., 2019b; Colan et al., 2024). All these technological advances should be coupled with training programs to teach and improve robotic surgical skills among residents and surgeons.
Recent artificial intelligence (AI) developments can also be applied to haptic feedback systems. The integration of AI models with robotic surgical systems would not only improve efficiency and accuracy by being involved in tasks such as the identification of anatomical structures or path planning through tasks, but also magnify the user’s sense of touch according to the mechanical properties of the tissues (Kella et al., 2025; Sun et al., 2022). In addition to this, some authors are currently working on machine learning models that can also be used to interpolate and infer the magnitude of haptic forces according to measured deformations (Sun and Martius, 2019) and achieve stable haptic rendering in real time for teleoperation applications (Sun et al., 2024).
7 Conclusion
Laparoscopic simulators constitute an interesting resource for medical training and preoperative rehearsal. Current bioengineering approaches aim to improve training programs and surgical outcomes, especially with the integration of haptic feedback systems, contributing to more realistic interaction and training. Although novel studies are being carried out to develop haptic feedback integrations for both laparoscopic simulators and surgical robots, further research is still needed to achieve bioinspired artificial palpation that is both affordable and realistic.
Statements
Author contributions
IL-A: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review and editing. MR: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review and editing. AP-E: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – review and editing. JR-M: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – review and editing. IE-S: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – review and editing. SP-A: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – review and editing. RJ-R: Conceptualization, Data curation, Formal Analysis, Methodology, Supervision, Validation, Writing – review and editing. JP-R: Conceptualization, Data curation, Formal Analysis, Methodology, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Ministry of Health and Consumer Affairs. Reference: EXC-2023-05.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abiri A. Juo Y. Y. Tao A. Askari S. J. Pensa J. Bisley J. W. et al (2019b). Artificial palpation in robotic surgery using haptic feedback. Surg. Endosc.33 (4), 1252–1259. 10.1007/s00464-018-6405-8
2
Abiri A. Pensa J. Tao A. Ma J. Juo Y. Y. Askari S. J. et al (2019a). Multi-modal haptic feedback for grip force reduction in robotic surgery. Sci. Rep.9 (1), 5016. 10.1038/s41598-019-40821-1
3
Adebayo O. Aldoori J. Allum W. Aruparayil N. Badran A. Winter Beatty J. et al (2022). Future of surgery: technology enhanced surgical training: report of the FOS:TEST commission.
4
Aggarwal R. Winter Beatty J. Kinross J. von Roon A. Darzi A. Purkayastha S. (2020). Initial experience with a new robotic surgical system for cholecystectomy. Surg. Innov.27 (2), 136–142. 10.1177/1553350619890736
5
Alleblas C. C. J. Vleugels M. P. H. Nieboer T. E. (2016). Ergonomics of laparoscopic graspers and the importance of haptic feedback: the surgeons’ perspective. Gynecol. Surg.13 (4), 379–384. 10.1007/s10397-016-0959-z
6
Alleblas C. C. J. Vleugels M. P. H. Stommel M. W. J. Nieboer T. E. (2019). Performance of a haptic feedback grasper in laparoscopic surgery: a randomized pilot comparison with conventional graspers in a porcine model. Surg. Innov.26 (5), 573–580. 10.1177/1553350619848551
7
Amirabdollahian F. Livatino S. Vahedi B. Gudipati R. Sheen P. Gawrie-Mohan S. et al (2018). Prevalence of haptic feedback in robot-mediated surgery: a systematic review of literature. J. Robot. Surg.12 (1), 11–25. 10.1007/s11701-017-0763-4
8
Andreatta P. B. Woodrum D. T. Birkmeyer J. D. Yellamanchilli R. K. Doherty G. M. Gauger P. G. et al (2006). Laparoscopic skills are improved with LapMentor™ Training. Ann. Surg.243 (6), 854–863. 10.1097/01.sla.0000219641.79092.e5
9
Atesok K. Satava R. M. Marsh J. L. Hurwitz S. R. (2017). Measuring surgical skills in simulation-based training. J. Am. Acad. Orthop. Surg.25 (10), 665–672. 10.5435/jaaos-d-16-00253
10
Barthel A. Trematerra D. Nasseri M. A. Zapp D. Lohmann C. P. Knoll A. et al (2015). “Haptic interface for robot-assisted ophthalmic surgery,” 2015 37th annual international conference of the IEEE engineering in medicine and biology society (EMBC). (IEEE), 4906–4909.
11
Bergholz M. Ferle M. Weber B. M. (2023). The benefits of haptic feedback in robot assisted surgery and their moderators: a meta-analysis. Sci. Rep.13 (1), 19215. 10.1038/s41598-023-46641-8
12
Biswas P. Sikander S. Kulkarni P. (2023). Recent advances in robot-assisted surgical systems. Biomed. Eng. Adv.6, 100109. 10.1016/j.bea.2023.100109
13
Botden S. M. B. I. Torab F. Buzink S. N. Jakimowicz J. J. (2008). The importance of haptic feedback in laparoscopic suturing training and the additive value of virtual reality simulation. Surg. Endosc.22 (5), 1214–1222. 10.1007/s00464-007-9589-x
14
Boutin J. Kamoonpuri J. Faieghi R. Chung J. de Ribaupierre S. Eagleson R. (2024). Smart haptic gloves for virtual reality surgery simulation: a pilot study on external ventricular drain training. Front. Robot. AI10, 1273631. 10.3389/frobt.2023.1273631
15
Braga M. Ridolfi C. Balzano G. Castoldi R. Pecorelli N. Di Carlo V. (2012). Learning curve for laparoscopic distal pancreatectomy in a high-volume hospital. Updat. Surg.64 (3), 179–183. 10.1007/s13304-012-0163-2
16
Cardoso S. A. Suyambu J. Iqbal J. Cortes Jaimes D. C. Amin A. Sikto J. T. et al (2023). Exploring the role of simulation training in improving surgical skills among residents: a narrative review. Cureus15, e44654. 10.7759/cureus.44654
17
Cheng Z. Savarimuthu T. R. Foong S. Tan U. X. (2023). Design of adjustable constant force/torque mechanisms for medical applications. J. Mech. Robot.15 (2). 10.1115/1.4054638
18
Colan J. Davila A. Hasegawa Y. (2024). Tactile feedback in robot-assisted minimally invasive surgery: a systematic review. Int. J. Med. Robotics Comput. Assisted Surg.20 (6), e70019. 10.1002/rcs.70019
19
Davies B. L. Hibberd R. D. Ng W. S. Timoney A. G. Wickham J. E. A. (1991). The development of a surgeon robot for prostatectomies. Proc. Inst. Mech. Eng. H.205 (1), 35–38. 10.1243/pime_proc_1991_205_259_02
20
Di Vece C. Luciano C. De Momi E. (2021). Psychomotor skills development for Veress needle placement using a virtual reality and haptics-based simulator. Int. J. Comput. Assist. Radiol. Surg.16 (4), 639–647. 10.1007/s11548-021-02341-0
21
El Rassi I. El Rassi J. M. (2020). A review of haptic feedback in tele-operated robotic surgery. J. Med. Eng. Technol.44 (5), 247–254. 10.1080/03091902.2020.1772391
22
Ezaki T. Kishima K. Shibao S. Matsunaga T. Pareira E. S. Kitamura Y. et al (2024). Development of microsurgical forceps equipped with haptic technology for in situ differentiation of brain tumors during microsurgery. Sci. Rep.14 (1), 21430. 10.1038/s41598-024-72326-x
23
Feldstein J. Schwander B. Roberts M. Coussons H. (2019). Cost of ownership assessment for a da Vinci robot based on US real-world data. Int. J. Med. Robotics Comput. Assisted Surg.15 (5), e2023. 10.1002/rcs.2023
24
Gibbs J. K. Gillies M. Pan X. (2022). A comparison of the effects of haptic and visual feedback on presence in virtual reality. Int. J. Hum. Comput. Stud.157, 102717. Available online at: https://www.sciencedirect.com/science/article/pii/S107158192100135X?via%3Dihub. (Accessed April 30, 2025). 10.1016/j.ijhcs.2021.102717
25
Golahmadi A. K. Khan D. Z. Mylonas G. P. Marcus H. J. (2021). Tool-tissue forces in surgery: a systematic review. Ann. Med. and Surg.65, 102268. 10.1016/j.amsu.2021.102268
26
Goldberg K. Guthart G. (2024). Augmented dexterity: how robots can enhance human surgical skills. Sci. Robot.9 (95), eadr5247. 10.1126/scirobotics.adr5247
27
Green C. A. Lin J. Higgins R. O’Sullivan P. S. Huang E. (2022). Expertise in perception during robotic surgery (ExPeRtS): what we see and what we say. Am. J. Surg.224 (3), 908–913. 10.1016/j.amjsurg.2022.05.006
28
Hagen M. E. Meehan J. J. Inan I. Morel P. (2008). Visual clues act as a substitute for haptic feedback in robotic surgery. Surg. Endosc.22 (6), 1505–1508. 10.1007/s00464-007-9683-0
29
Hamdi J. T. Munshi S. Azam S. Omer A. (2024). Development of a master–slave 3D printed robotic surgical finger with haptic feedback. J. Robot. Surg.18 (1), 43. 10.1007/s11701-024-01819-8
30
Hamza-Lup F. G. Crenguta M. B. Seitan A. (2012). Haptic simulator for liver diagnostics through palpation, 173. Netherlands: IOS Press, 156–160.
31
Haruna M. Noboru K. Ogino M. Koike-Akino T. (2021). Comparison of three feedback modalities for haptics sensation in remote machine manipulation. IEEE Robot. Autom. Lett.6 (3), 5040–5047. 10.1109/lra.2021.3070301
32
Heimann F. Barteselli G. Brand A. Dingeldey A. Godard L. Hochstetter H. et al (2021). A custom virtual reality training solution for ophthalmologic surgical clinical trials. Adv. Simul.6 (1), 12. 10.1186/s41077-021-00167-z
33
Heredia-Pérez S. A. Harada K. Padilla-Castañeda M. A. Marques-Marinho M. Márquez-Flores J. A. Mitsuishi M. (2019). Virtual reality simulation of robotic transsphenoidal brain tumor resection: evaluating dynamic motion scaling in a master‐slave system. Int. J. Med. Robotics Comput. Assisted Surg.15 (1), e1953. 10.1002/rcs.1953
34
Jourdes F. Valentin B. Allard J. Duriez C. Seeliger B. (2022). Visual haptic feedback for training of robotic suturing. Front. Robot. AI9, 800232. 10.3389/frobt.2022.800232
35
Kawka M. Fong Y. Gall T. M. H. (2023). Laparoscopic versus robotic abdominal and pelvic surgery: a systematic review of randomised controlled trials. Surg. Endosc.37 (9), 6672–6681. 10.1007/s00464-023-10275-8
36
Kella D. Varshitha E. Bhargavi C. Rao P. B. (2025). Artificial intelligence: transforming the future of robotic surgery. Int. J. Innov. Sci. Res. Technol., 1155–1161. 10.38124/ijisrt/25mar997
37
Kim S. Yoon Y. S. Han H. S. Cho J. Y. Choi Y. Lee B. (2021). Evaluation of a single surgeon’s learning curve of laparoscopic pancreaticoduodenectomy: risk-adjusted cumulative summation analysis. Surg. Endosc.35 (6), 2870–2878. 10.1007/s00464-020-07724-z
38
Kim T. H. Ha J. M. Cho J. W. You Y. C. Sung G. T. (2009). Assessment of the laparoscopic training validity of a virtual reality simulator LAP mentor TM. Korean J. Urol.50 (10), 989. 10.4111/kju.2009.50.10.989
39
Kim Y. Chang D. Kim J. Park S. (2013). Gallbladder removal simulation for laparoscopic surgery training: a hybrid modeling method. J. Comput. Sci. Technol.28 (3), 499–507. 10.1007/s11390-013-1351-3
40
Kwoh Y. S. Hou J. Jonckheere E. A. Hayati S. (1988). A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans. Biomed. Eng.35 (2), 153–160. 10.1109/10.1354
41
Lin Y. Wang X. Wu F. Chen X. Wang C. Shen G. (2014). Development and validation of a surgical training simulator with haptic feedback for learning bone-sawing skill. J. Biomed. Inf.48, 122–129. 10.1016/j.jbi.2013.12.010
42
Lohre R. Warner J. J. P. Morrey B. R. Athwal G. S. Morrey M. E. Mazzocca A. D. et al (2021). Mitigating surgical skill decay in orthopaedics using virtual simulation learning. JAAOS Glob. Res. Rev.5 (10). 10.5435/jaaosglobal-d-21-00193
43
Mao R. Q. Lan L. Kay J. Lohre R. Ayeni O. R. Goel D. P. et al (2021). Immersive virtual reality for surgical training: a systematic review. J. Surg. Res.268, 40–58. 10.1016/j.jss.2021.06.045
44
Matsuda T. McDougall E. M. Ono Y. Hattori R. Baba S. Iwamura M. et al (2012). Positive correlation between motion analysis data on the LapMentor virtual reality laparoscopic surgical simulator and the results from videotape assessment of real laparoscopic surgeries. J. Endourol.26 (11), 1506–1511. 10.1089/end.2012.0183
45
Matwala K. Shakir T. Bhan C. Chand M. (2024). The surgical metaverse. Cirugía Española102, S61–S65. 10.1016/j.ciresp.2023.10.004
46
Meccariello G. Faedi F. AlGhamdi S. Montevecchi F. Firinu E. Zanotti C. et al (2016). An experimental study about haptic feedback in robotic surgery: may visual feedback substitute tactile feedback?J. Robot. Surg.10 (1), 57–61. 10.1007/s11701-015-0541-0
47
Meli L. Pacchierotti C. Prattichizzo D. (2017). Experimental evaluation of magnified haptic feedback for robot-assisted needle insertion and palpation. Int. J. Med. Robotics Comput. Assisted Surg.13 (4). 10.1002/rcs.1809
48
Melling N. Barr J. Schmitz R. Polonski A. Miro J. Ghadban T. et al (2019). Robotic cholecystectomy: first experience with the new Senhance robotic system. J. Robot. Surg.13 (3), 495–500. 10.1007/s11701-018-0877-3
49
Mertz L. (2022). Robots to improve surgery for all. IEEE Pulse13 (6), 6–11. 10.1109/mpuls.2022.3227808
50
Miller J. Braun M. Bilz J. Matich S. Neupert C. Kunert W. et al (2021). Impact of haptic feedback on applied intracorporeal forces using a novel surgical robotic system—a randomized cross-over study with novices in an experimental setup. Surg. Endosc.35 (7), 3554–3563. 10.1007/s00464-020-07818-8
51
Moradi Dalvand M. Shirinzadeh B. Nahavandi S. Smith J. (2014). Effects of realistic force feedback in a robotic assisted minimally invasive surgery system. Minim. Invasive Ther. and Allied Technol.23 (3), 127–135. 10.3109/13645706.2013.867886
52
Najafi G. Kreiser K. Abdelaziz MEMK Hamady M. S. (2023). Current state of robotics in interventional radiology. Cardiovasc Interv. Radiol.46 (5), 549–561. 10.1007/s00270-023-03421-1
53
Nassar A. K. Al-Manaseer F. Knowlton L. M. Tuma F. (2021). Virtual reality (VR) as a simulation modality for technical skills acquisition. Ann. Med. Surg.71, 102945. 10.1016/j.amsu.2021.102945
54
Okamura A. M. (2009). Haptic feedback in robot-assisted minimally invasive surgery. Curr. Opin. Urol.19 (1), 102–107. 10.1097/mou.0b013e32831a478c
55
Olivas-Alanis L. H. Calzada-Briseño R. A. Segura-Ibarra V. Vázquez E. V. Diaz-Elizondo J. A. Flores-Villalba E. et al (2020). LAPKaans: tool-motion tracking and gripping force-sensing modular smart laparoscopic training system. Sensors20 (23), 6937. 10.3390/s20236937
56
Ota M. Oki E. Nakanoko T. Tanaka Y. Toyota S. Hu Q. et al (2024). Field experiment of a telesurgery system using a surgical robot with haptic feedback. Surg. Today54 (4), 375–381. 10.1007/s00595-023-02732-7
57
Ouyang Q. Wu J. Sun S. Pensa J. Abiri A. Dutson E. et al (2021). Bio-inspired haptic feedback for artificial palpation in robotic surgery. IEEE Trans. Biomed. Eng.68 (10), 3184–3193. 10.1109/tbme.2021.3076094
58
Pinzon D. Byrns S. Zheng B. (2016). Prevailing trends in haptic feedback simulation for minimally invasive surgery. Surg. Innov.23 (4), 415–421. 10.1177/1553350616628680
59
Quesada C. Alfaro I. González D. Chinesta F. Cueto E. (2018). Haptic simulation of tissue tearing during surgery. Int. J. Numer. Method Biomed. Eng.34 (3). 10.1002/cnm.2926
60
Selim M. Dresscher D. Abayazid M. (2024). A comprehensive review of haptic feedback in minimally invasive robotic liver surgery: advancements and challenges. Int. J. Med. Robotics Comput. Assisted Surg.20 (1), e2605. 10.1002/rcs.2605
61
Selva Raj D. R. Kumar S. Nallathamby K. Raj K. Hristova M. (2024). A systematic review of the learning curves of novices and trainees to achieve proficiency in laparoscopic skills: virtual reality simulator versus box trainer. Cureus16, e72923. 10.7759/cureus.72923
62
Sheetz K. H. Claflin J. Dimick J. B. (2020). Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw. Open3 (1), e1918911. 10.1001/jamanetworkopen.2019.18911
63
Shi Y. Shen G. (2024). Haptic sensing and feedback techniques toward virtual reality. Research7, 0333. 10.34133/research.0333
64
Sinha R. Raje S. Rao G. (2017). Three-dimensional laparoscopy: principles and practice. J. Minim. Access Surg.13 (3), 165. 10.4103/0972-9941.181761
65
Sparn M. B. Teixeira H. Chatziisaak D. Schmied B. Hahnloser D. Bischofberger S. (2024). Virtual reality simulation training in laparoscopic surgery – does it really matter, what simulator to use? Results of a cross-sectional study. BMC Med. Educ.24 (1), 589. 10.1186/s12909-024-05574-0
66
Sun H. Martius G. (2019). Machine learning for haptics: inferring multi-contact stimulation from sparse sensor configuration. Front. Neurorobot13, 51. 10.3389/fnbot.2019.00051
67
Sun Y. Lueth T. C. (2023). Safe manipulation in robotic surgery using compliant constant-force mechanism. IEEE Trans. Med. Robot. Bionics.5 (3), 486–495. 10.1109/tmrb.2023.3237924
68
Sun Y. Meng F. Yang D. Xiong M. Xu X. (2024). Low-cost modeling and haptic rendering for membrane-like deformable objects in model-mediated teleoperation. IEEE Access12, 141198–141210. 10.1109/access.2024.3467706
69
Sun Z. Zhu M. Shan X. Lee C. (2022). Augmented tactile-perception and haptic-feedback rings as human-machine interfaces aiming for immersive interactions. Nat. Commun.13 (1), 5224. 10.1038/s41467-022-32745-8
70
Susmitha W. K. Mathew G. Devasahayam S. R. Perakath B. Velusamy S. K. (2015). Factors influencing forces during laparoscopic pinching: towards the design of virtual simulator. Int. J. Surg.18, 211–215. 10.1016/j.ijsu.2015.04.078
71
Tang J. Xu L. He L. Guan S. Ming X. Liu Q. (2017). Virtual laparoscopic training system based on VCH model. J. Med. Syst.41 (4), 58. 10.1007/s10916-017-0702-y
72
Taylor R. H. Joskowicz L. Williamson B. Guéziec A. Kalvin A. Kazanzides P. et al (1999). Computer-integrated revision total hip replacement surgery: concept and preliminary results. Med. Image Anal.3 (3), 301–319. 10.1016/s1361-8415(99)80026-7
73
The Medical Post (1985). World’s first surgical robot in B.C. Available online at: https://www.brianday.ca/imagez/1051_28738.pdf (Accessed November 12, 2024).
74
Trute R. J. Alijani A. Erden M. S. (2024). Visual cues of soft-tissue behaviour in minimal-invasive and robotic surgery. J. Robot. Surg.18 (1), 401. 10.1007/s11701-024-02150-y
75
Ueda Y. Miyahara S. Tokuishi K. Nakajima H. Waseda R. Shiraishi T. et al (2023). Impact of a pneumatic surgical robot with haptic feedback function on surgical manipulation. Sci. Rep.13 (1), 22615. 10.1038/s41598-023-49876-7
76
Våpenstad C. Hofstad E. F. Langø T. Mårvik R. Chmarra M. K. (2013). Perceiving haptic feedback in virtual reality simulators. Surg. Endosc.27 (7), 2391–2397. 10.1007/s00464-012-2745-y
77
Vitish-Sharma P. Knowles J. Patel B. (2011). Acquisition of fundamental laparoscopic skills: is a box really as good as a virtual reality trainer?Int. J. Surg.9 (8), 659–661. 10.1016/j.ijsu.2011.08.009
78
Woo J. Song H. S. Cha H. J. Yi B. J. (2019). Advantage of steerable catheter and haptic feedback for a 5-DOF vascular intervention robot system. Appl. Sci.9 (20), 4305. 10.3390/app9204305
79
Wu H. Ye F. Gao Y. Cong Y. Hao A. (2022). Real-time laparoscopic cholecystectomy simulation using a particle-based physical system. Complex Syst. Model. Simul.2 (2), 186–196. 10.23919/csms.2022.0009
80
Wu H. Yu H. Ye F. Sun J. Gao Y. Tan K. et al (2021). Interactive hepatic parenchymal transection simulation with haptic feedback. Virtual Real. and Intelligent Hardw.3 (5), 383–396. 10.1016/j.vrih.2021.09.003
81
Yamanouchi K. Takano S. Mima Y. Matsunaga T. Ohnishi K. Matsumoto M. et al (2023). Validation of a surgical drill with a haptic interface in spine surgery. Sci. Rep.13 (1), 598. 10.1038/s41598-023-27467-w
82
Zhang Y. Wang O. Wang Y. Tavakoli M. Zheng B. (2023). Enhancing skill learning with dual-user haptic feedback: insights from a task-specific approach. Front. Robot. AI. 10, 1286282. 10.3389/frobt.2023.1286282
83
Zhou M. Tse S. Derevianko A. Jones D. B. Schwaitzberg S. D. Cao C. G. L. (2012). Effect of haptic feedback in laparoscopic surgery skill acquisition. Surg. Endosc.26 (4), 1128–1134. 10.1007/s00464-011-2011-8
Summary
Keywords
biomedical engineering, 3D modeling, haptic feedback, haptic technologies, robotic surgery, surgical simulations, surgical training
Citation
Laga Boul-Atarass I, Rubio Manzanares Dorado M, Padillo-Eguía A, Racero-Moreno J, Eguía-Salinas I, Pereira-Arenas S, Jiménez-Rodríguez RM and Padillo-Ruiz J (2025) Role of haptic feedback technologies and novel engineering developments for surgical training and robot-assisted surgery. Front. Robot. AI 12:1567955. doi: 10.3389/frobt.2025.1567955
Received
03 February 2025
Accepted
05 May 2025
Published
04 June 2025
Volume
12 - 2025
Edited by
Pasqualino Sirignano, Sapienza University of Rome, Italy
Reviewed by
Raza Qazi, University of Colorado Boulder, United States
Yilun Sun, Technical University of Munich, Germany
Updates
Copyright
© 2025 Laga Boul-Atarass, Rubio Manzanares Dorado, Padillo-Eguía, Racero-Moreno, Eguía-Salinas, Pereira-Arenas, Jiménez-Rodríguez and Padillo-Ruiz.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mercedes Rubio Manzanares Dorado, mercedesrmd@gmail.com
†These authors have contributed equally to this work
ORCID: Mercedes Rubio Manzanares, orcid.org/0000-0002-0433-1881; Andrés Padillo-Eguía, orcid.org/0009-0009-7653-7748; Jesus Racero-Moreno, orcid.org/0000-0002-7482-0744; Ignacio Eguía-Salinas, orcid.org/0000-0003-3969-9958; Sheila Pereira-Arenas, orcid.org/0000-0002-5009-5699; Rosa María Jiménez-Rodríguez, orcid.org/0000-0001-8587-8768; Javier Padillo-Ruiz, orcid.org/0000-0002-1220-0822
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.