- Department of Pharmacy, Sri Shanmugha College of Pharmacy, Sankari, Salem, Tamil Nadu, India
Lyotropic liquid crystalline (LLC) nanoparticles have gained significant attention as drug delivery systems owing to their unique self-assembly properties, biocompatibility, and ability to encapsulate both hydrophilic and hydrophobic drugs. This chapter explores recent advances in LLC formulations, focusing on their structural classification, physicochemical properties, and applications in controlled-drug delivery. Various mesophases, including lamellar, cubic, and hexagonal structures, have been discussed, highlighting their roles in controlled release. A comparative analysis reveals that cubic phases offer superior structural stability for sustained release, while hexagonal phases excel in high-viscosity applications, though their complex preparation limits scalability. In addition, key characterization techniques such as small-angle X-ray scattering, differential scanning calorimetry, and rheology are examined to offer insights into their stability and performance. Furthermore, the development of in situ gelling precursor systems and their applications in oral, transdermal, ocular, nasal, injectable, and periodontal drug delivery have been explored. The incorporation of stimuli-responsive materials into LLC systems enhances their adaptability to personalized medicine and advanced therapeutic strategies. Despite these advancements, challenges such as scalability, long-term stability, and clinical translation remain unresolved. This chapter highlights the potential of LLC nanoparticles to revolutionize modern drug delivery by improving bioavailability, therapeutic efficacy and patient compliance. Future research should focus on optimizing formulation strategies and exploring novel biomaterials to expand the clinical utility of LLC-based drug delivery systems.
1 Introduction
LLCs, or lyotropic liquid crystals, exhibit highly ordered internal structures and self-assemble into lamellar, hexagonal, and cubic phases. These structures are ideal for drug delivery because they can encapsulate and release substances of various sizes and polarities. The specific LLC phase influences the properties of the encapsulated drug, allowing for customized release profiles, such as sustained or targeted delivery (Baldha et al., 2025). Lyotropic liquid crystals are increasingly being recognized for their potential in advanced drug delivery systems. These materials are excellent carriers due to their unique properties, which include stimuli-responsive drug delivery and sustained release patterns. They can respond to environmental changes such as temperature or pH, ensuring that drugs are delivered precisely where and when they are needed. This adaptability not only improves the efficiency of drug delivery but also enhances patient outcomes by reducing side effects and improving the therapeutic index of drugs. As research progresses, lyotropic liquid crystals are poised to revolutionize how medications are administered, offering a promising future in personalized medicine (Baldha et al., 2025; Govindan et al., 2024). LLC structures, abundant in biological systems like cellular membranes, enable innovative drug delivery applications due to their biocompatibility and structural versatility (Behera et al., 2024).
These systems respond to environmental stimuli (e.g., pH, temperature, light, ultrasound, electromagnetic fields, concentration gradients), enabling controlled drug release tailored to specific conditions (Zhao et al., 2022). For instance, cubic phases provide a tortuous diffusion pathway, enabling prolonged release compared to lamellar phases, which are less viscous and facilitate faster drug release. This method opens new possibilities for modifying the pharmacokinetic profiles of drugs and potentially improving their efficacy and safety. The ability to control drug release is particularly beneficial in treating diseases for which controlled dosing is crucial. The durability of active ingredients when encapsulated forms a strong foundation for drug delivery systems (Rahman et al., 2020). The sol-gel strategy allows precise control over the microstructure of LLC systems, enabling the design of tailored carriers that respond dynamically to environmental changes. This adaptability is critical for the development of smart delivery systems that can release drugs in a controlled manner, improve their therapeutic efficacy, and minimize side effects. However, the high viscosity of cubic and hexagonal phases can complicate administration, posing challenges for patient-friendly delivery methods. Ongoing research in this field is promising for the advancement of personalized medicine, in which treatments can be fine-tuned to individual patient needs, optimizing outcomes, and enhancing patient compliance (AbuAlrob et al., 2025; Shukla and Kumar, 2025).
The amphiphilic nature of polar lipids plays a crucial role in their ability to self-assemble into unique structures that exhibit polymorphism owing to their interactions with water molecules. Amphiphilic molecules have both hydrophilic (water-attracting) and hydrophobic (water-repelling) components, allowing them to arrange themselves in ways that minimize energy, usually by forming distinct phases (Manna et al., 2024). When exposed to water, these lipids can be organized into various liquid crystalline phases, each with distinct structural characteristics. The main types of lipid polymorphisms include the lamellar phase, where lipids form bilayer sheets; the cubic phase, characterized by a complex three-dimensional network; and the hexagonal phase, where lipids are arranged into cylindrical structures. These phases are significant in fields such as drug delivery and nanotechnology because they offer diverse properties and functionalities based on their structure (Sahu et al., 2021). Different phase types offer unique advantages: lamellar phases are simple to produce but lack durability, cubic phases provide excellent encapsulation but require complex characterization, and hexagonal phases have high viscosity but are challenging to scale up. Figure 1 illustrates the phase transitions of lyotropic liquid crystalline systems, depicting the structural evolution from lamellar to unorganized and bicontinuous phases as a function of surfactant concentration and solvent conditions. This schematic highlights the dynamic interplay between molecular organization and environmental factors, supporting the design of tailored drug delivery systems.
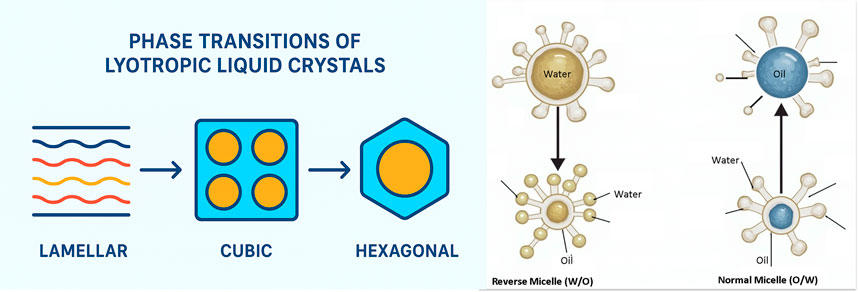
Figure 1. Schematic showing phase transitions of lyotropic liquid crystals from lamellar to cubic and hexagonal structures.
1.1 Classification of liquid crystalline phases
LLCs are categorized based on the specific arrangement and behavior of amphiphilic molecules within a solvent. Figure 2 provides a schematic representation of lyotropic liquid crystalline phases, including nematic, smectic, and columnar arrangements, illustrating their molecular orientations and structural order. This figure aids in understanding the structural diversity of LLCs for drug delivery applications (Blanco-Fernández et al., 2023). The primary categories include nematic, smectic, and columnar phases. Nematic LLCs are characterized by molecules oriented parallel to each other, allowing fluidity similar to that of conventional liquids (Blanco-Fernández et al., 2023). Smectic LLCs, on the other hand, exhibit a more ordered structure in which molecules form distinct layers, each maintaining a degree of fluidity. Columnar LLCs arrange amphiphilic molecules into cylindrical structures often forming hexagonal lattices. These phases are influenced by the concentration of amphiphilic molecules and nature of the solvent, which can lead to diverse applications in fields such as drug delivery, biosensors, and advanced materials. Compared to nematic phases, smectic and columnar phases (Figure 3) illustrates the structural differences between smectic and columnar phases, highlighting their layered and cylindrical organizations, respectively, which contribute to their suitability for controlled drug release offer greater structural order, making them more suitable for controlled drug release, though their higher viscosity can limit injectability.
Figure 2. Illustration of nematic, smectic, and columnar lyotropic phases based on molecular orientation and order.
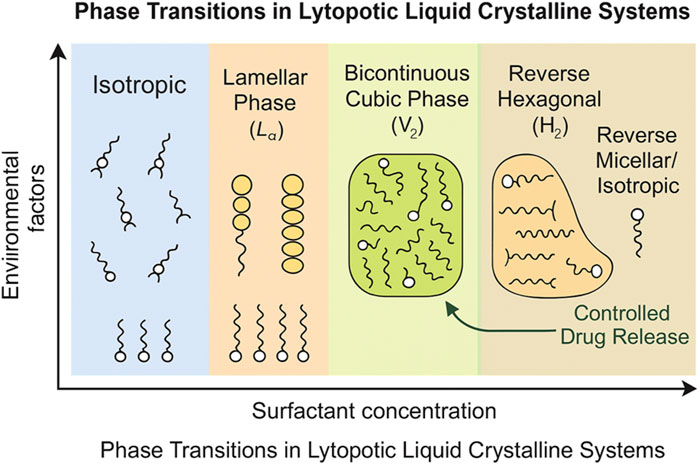
Figure 3. Comparison of nanoscale drug delivery systems including liposomes, niosomes, nanocapsules, and cubosomes.
Emerging advanced techniques in drug delivery include surface modification with various ligands to enhance targeting, stimuli-responsive LLCs for on-demand drug release, and combination therapies using multifunctional LLCs for synergistic effects. These innovations aim to improve targeted and site-specific drug delivery, increasing therapeutic efficacy and minimizing side effects (Priya et al., 2024). Table 1 presents the different types of lyotropic liquid crystalline nanoparticles, along with their key components and primary applications in drug delivery. Each formulation was categorized based on its structural composition, including cubosomes, hexosomes, micellar cubosomes, emulsified microemulsions, LLC gels, and liquid crystalline nanoparticles (LCNPs). The components listed for each formulation included lipids, surfactants, and other amphiphilic molecules, which facilitated the self-assembly of these nanostructures. These applications highlight their role in encapsulating poorly soluble therapeutics, enhancing bioavailability, enabling controlled drug release, and providing targeted delivery. Cubosomes, for instance, excel in encapsulating hydrophobic drugs due to their bicontinuous structure, but their complex preparation limits large-scale production compared to simpler micellar systems.
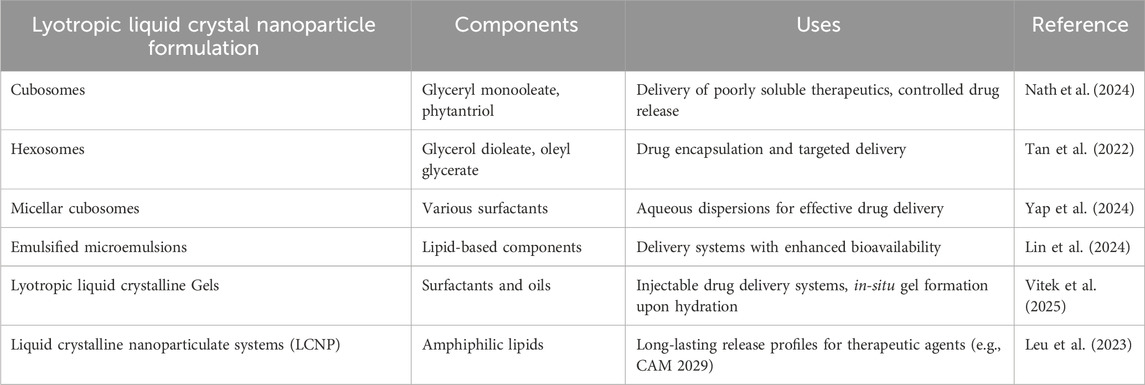
Table 1. Overview of various lyotropic liquid crystal nanoparticle formulations used in drug delivery.
The choice of the LLC phase is determined by a combination of the physicochemical properties of the drug (e.g., solubility, stability, and molecular size) and its therapeutic application. For instance, lamellar phases are preferred for transdermal and skin hydration therapies because of their structural resemblance to stratum corneum lipids. Cubic phases, with their large water channels and tortuous paths, are suited for the sustained delivery of hydrophilic drugs via ocular and injectable routes. Hexagonal phases, particularly H2, are ideal for depot formulations or lipophilic drugs in which prolonged retention is desired. Comparison of lamellar, cubic, and hexagonal lyotropic liquid crystalline phases for drug delivery applications illustrated in Table 2. Moreover, the disease condition (e.g., chronic vs acute), desired release kinetics, and administration route should inform the formulation strategy. Employing computational modeling and phase diagrams helps predict phase behavior, thereby improving formulation efficiency and targeting precision.
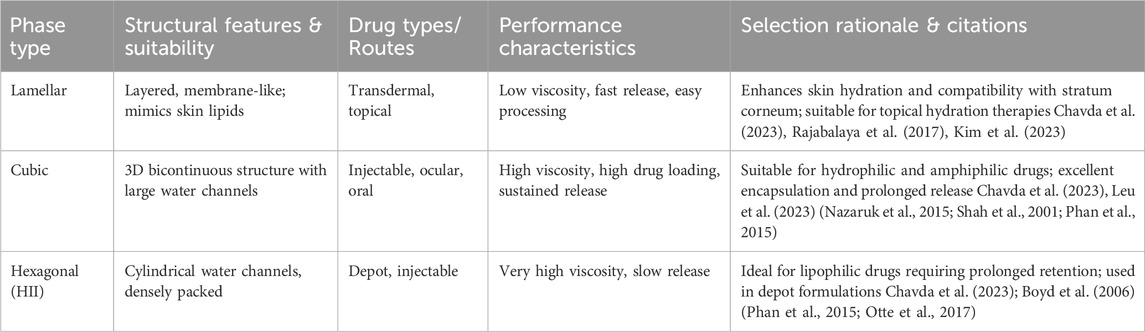
Table 2. Comparison of lamellar, cubic, and hexagonal lyotropic liquid crystalline phases for drug delivery applications.
1.1.1 The lamellar liquid crystalline phase
The lamellar phase is an intriguing structural arrangement of lipid bilayers characterized by one-dimensional layering with interspersed water layers. This unique organization allows the bilayers to slide past each other in a manner restricted to right angles relative to the plane of the layers, contributing to its lower viscosity compared to other mesophases, such as hexagonal and cubic (Gu et al., 2023). Within the lamellar phase, further classification is based on the degree of molecular ordering, resulting in three distinct subclasses: fluid lamellar (Lα), lamellar gel (Lβ), and crystalline lamellar (Lc) phases. The Lα phase is the least ordered, with melted and fluid-like alkyl chains, offering flexibility within the bilayer structure. In contrast, the Lβ phase is more ordered, resembling a gel-like state, whereas the Lc phase is highly structured, akin to a crystalline arrangement. Each phase exhibits unique properties and behaviors that are influenced by molecular interactions and thermal conditions (Kuwabara et al., 2024). The Lα phase’s fluidity makes it ideal for transdermal delivery, but its lower stability compared to cubic phases limits its use in sustained-release applications. Figure 4 depicts the structural organization of lamellar liquid crystalline phases, illustrating the bilayer arrangements of Lα, Lβ, and Lc subclasses. This figure emphasizes the varying degrees of molecular ordering and their implications for drug delivery, particularly in transdermal applications.
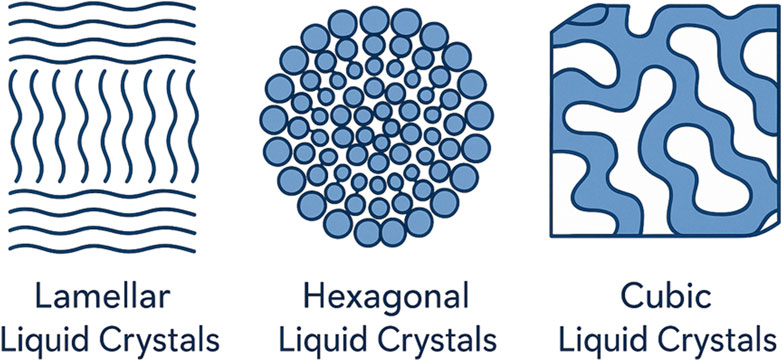
Figure 4. Structural arrangement of Lα, Lβ, and Lc lamellar phases highlighting differences in molecular ordering.
1.1.2 The cubic liquid crystalline phase
Cubic liquid crystalline phases captivate researchers due to their sophisticated molecular structures, which are classified into bicontinuous and micellar types. These phases are characterized by unique symmetrical space groups such as Ia3d (Q230) and Pn3m (Q224). Cubic phase structures are particularly notable for their curved three-dimensional lipid bilayers interspersed with water channels, which contribute to their distinct properties (Sparavigna, 2023). The elastic energy of the curvature of these bilayers plays a critical role in the stability of cubic phases, which is influenced by the composition of the system. Owing to the absence of shear planes, these structures exhibited sticky and viscous behavior. The cubic phases can be further classified into three mesomorphic structures: Schwartz double diamond lattice (D, Pn3m, Q224), Schwartz primitive cubic phase (P, Im3m, Q229), and Schoen gyroid lattice (G, Ia3d, Q230). The capacity and size of the water channels increase from the G to D to the P phase, with the diameters of these structures ranging from 4 to 20 nm, allowing them to effectively incorporate water-soluble molecules (Rani et al., 2024). The bicontinuous cubic phase’s large water channels enable high drug-loading capacity, but its high viscosity complicates processing and administration compared to lamellar phases.
This elaborate configuration serves as a striking example of the detailed geometry commonly seen in liquid crystalline structures. In the Im3m configuration, the orthogonal network of water channels is notable for its symmetry and effectiveness in linking the unit cells, resulting in a strong and stable cubic structure. The P-minimal surface acted as a partition, adding to the structural integrity and unique characteristics of the network. Conversely, the bilayer configuration of the D structure permits the formation of a diamond lattice, which is distinctive for its tetrahedral angle of 109.5°, enhancing its three-dimensional connectivity (Priya et al., 2024). The G-surface, with its left- and right-handed helically positioned channels, adds another layer of complexity, demonstrating the diversity of the possible arrangements within these systems. Such structural intricacies have significant implications in fields such as materials science and biochemistry, where understanding the spatial organization of molecules can lead to advances in technology and medicine (Král et al., 2024).
1.1.3 The hexagonal liquid crystalline phase
The hexagonal mesophase represents a captivating structural pattern that arises from the self-assembly process of surfactant molecules. In this phase, surfactants are organized into a hexagonally packed structure, creating long-range order in two dimensions. This mesophase can manifest in two distinct forms: the normal hexagonal phase (H1) and the reverse or inverted hexagonal phase (H2) (Smaisim et al., 2023). In the H1 phase, the hydrophobic tails of the surfactant molecules form an inner core with the polar head groups oriented outward, thus creating continuous water layers. Conversely, in the H2 phase, the core was composed of hydrophilic polar head groups with alkyl chains forming a continuous network. The viscosity of these hexagonal structures is especially higher than that of the lamellar phase. The H2 phase’s high viscosity makes it suitable for depot formulations, but its complex self-assembly process hinders scalability compared to simpler lamellar systems. Recently, a novel lyotropic liquid crystalline phase was discovered, exhibiting a three-dimensional hexagonal close-packed arrangement of inverse micelles, identified with space-group symmetry (P63/mmc). This discovery opens new avenues for exploring the dynamic properties and potential applications of these mesophases (Uranga Wassermann et al., 2024; Pal et al., 2023). Figure 5 illustrates the structural organization of hexagonal liquid crystalline phases, depicting the normal (H1) and reverse (H2) hexagonal arrangements, along with their cross-sectional views. This figure highlights the molecular packing and high viscosity of hexagonal phases, supporting their application in depot drug delivery systems (Pal et al., 2023; Smaisim et al., 2023).

Figure 5. Representation of normal (H1) and reverse (H2) hexagonal phases showing cylindrical packing of surfactants.
1.2 Composition of lyotropic liquid crystalline gelling system
LLC gels are captivating materials that form through the combination of water, surfactants, and oils, resulting in the self-organization into structured phases. The surfactants in these gels are amphiphilic, indicating that they have both hydrophilic (water-attracting) and lipophilic (oil-attracting) properties (Karakasidis et al., 2025). This dual nature is crucial for the formation of various structures at different concentrations. Initially, at low concentrations, the surfactants formed micelles and spherical aggregates that stabilized the solution. As the concentration increases, these micelles transform into more complex structures, such as cylindrical or plate-like aggregates, leading to the formation of diverse mesophases such as cubic, hexagonal, and lamellar phases. The formation of these phases is significantly influenced by factors such as the surfactant concentration, temperature, and specific molecular structure of the surfactants used (Aguirre-Ramírez et al., 2021). Commonly studied amphiphilic materials include glycerol monooleate and phytantriol, which have shown great potential for the formation of stable and versatile liquid crystalline gels. Glycerol monooleate-based gels offer excellent biocompatibility but are prone to oxidation, whereas phytantriol provides greater chemical stability but is less flexible in phase transitions (Milak and Zimmer, 2015). Phase diagrams are often used to understand and predict the behavior and structure of these systems, offering insights into their practical applications in various fields such as drug delivery and materials science (Kumar et al., 2021).
1.3 Methods for preparation of liquid crystalline gels
Liquid crystalline gels represent an innovative approach to drug delivery systems, leveraging their unique structural properties for enhanced stability and controlled release. The preparation of these gels is straightforward and energy-efficient, involving the combination of aqueous and lipid phases through vortexing or ultrasonication. Their thermodynamic stability ensures longevity without phase separation, making them ideal for long-term storage (Jacob et al., 2024). The process begins with the integration of the drug into the appropriate phase: hydrophobic drugs are mixed with surfactants before dissolving in water, whereas hydrophilic drugs are first dissolved in the aqueous phase. After centrifugation to remove excess water, the mixture was allowed to reach equilibrium by using either a tube roller or standing alone. This careful preparation process creates a strong medium that is ideal for the efficient delivery of various pharmaceutical compounds (Islam et al., 2024). While vortexing is cost-effective, ultrasonication provides more uniform particle sizes, though both methods struggle with scaling up for industrial production.
1.3.1 Characterization of liquid crystalline gels
Characterization of LLC gels is critical for elucidating their structural, thermal, and mechanical properties, which govern their efficacy in drug delivery systems. This section categorizes key characterization techniques into three groups: Microscopic Methods, Spectroscopic Techniques, and Rheological Analyses. These methods offer complementary insights into phase behavior, stability, and functionality, facilitating the optimization of LLC gels for pharmaceutical applications.
1.3.1.1 Microscopic methods
1.3.1.1.1 Small-angle X-Ray scattering (SAXS)
Small-angle X-ray scattering (SAXS) is a precise technique for determining the three-dimensional structure and long-range order of LLC phases. By analyzing X-ray diffraction patterns, SAXS reveals the symmetry, lattice parameters, and spatial arrangement of lipid molecules, including hydrocarbon chain spacing and lipid layer thickness. The small-angle region provides data on phase symmetry, while the wide-angle region details molecular packing. Despite its high precision, SAXS requires specialized equipment, limiting accessibility compared to rheology (Masime et al., 2025; Lu et al., 2025).
1.3.1.1.2 Freeze-fracture electron microscopy (FFEM)
Freeze-fracture electron microscopy (FFEM) visualizes the nanoscale morphology and self-assembly of LLC gels, capturing high-resolution images of coexisting phases, such as vesicular and micellar structures. Combined with rheological data, FFEM enhances understanding of mechanical properties and phase stability. However, FFEM is labor-intensive and less quantitative than SAXS (Dombrowski et al., 2024).
1.3.1.2 Spectroscopic techniques
1.3.1.2.1 Differential scanning calorimetry (DSC)
Differential scanning calorimetry (DSC) measures heat flow during phase transitions, providing insights into the thermal stability and behavior of LLC gels. It identifies transitions between lamellar, cubic, or hexagonal phases and characterizes water molecule interactions within surfactant aggregates. DSC is cost-effective and widely available but less sensitive to microstructural changes than SAXS (Abdulnaby et al., 2024).
1.3.1.2.2 Nuclear magnetic resonance spectroscopy (NMR)
Nuclear magnetic resonance (NMR) provides detailed molecular insights into LLC phase structures by analyzing nuclear responses to magnetic fields. NMR diffusion measurements assess polar and apolar domain stability, while Raman spectroscopy probes vibrational modes to study phase transitions. These techniques offer comprehensive data but are time-consuming and costly compared to DSC (Li et al., 2022).
1.3.1.2.3 Low frequency dielectric spectroscopy
Dielectric spectroscopy examines dielectric properties of LLC gels using an oscillating electric field (10–3–106 Hz), identifying mesophases and drug-mesophase interactions. It provides insights into molecular dynamics but requires complex data interpretation compared to DSC [41, 42].
1.3.1.3 Rheological analyses
1.3.1.3.1 Rheology
Rheological measurements characterize the viscoelastic properties of LLC gels, influencing drug diffusion and release. Bicontinuous cubic phases exhibit rigidity, reversed hexagonal phases show moderate viscoelasticity, and lamellar phases display plasticity with a defined yield value. Parameters like storage modulus (G′) and loss modulus (G″) reveal structural dynamics under shear stress. Rheology is critical for optimizing injectability, though cubic phases’ rigidity may hinder syringeability compared to lamellar phases (Özkaynak et al., 2024).
1.4 Development of an in-situ gelling lyotropic liquid crystalline precursor system
The development of an in-situ gelling lyotropic liquid crystalline precursor system represents a significant advancement in drug delivery and biomedical applications. This innovative system is designed to undergo a phase transition from liquid to gel upon contact with physiological fluids, ensuring the localized and controlled release of therapeutic agents. Lyotropic liquid crystals are particularly advantageous because of their ability to form highly ordered structures, such as micellar cubic, hexagonal, or lamellar phases, which can encapsulate and protect active pharmaceutical ingredients (Shete et al., 2021). The precursor system typically comprises biocompatible and biodegradable materials responsive to stimuli. By fine-tuning the composition and properties of these systems, researchers can enhance the drug solubility, stability, and bioavailability, thereby offering a versatile platform for controlled drug delivery. In-situ gelling systems excel in site-specific delivery but face challenges in achieving consistent gelation kinetics across diverse physiological conditions. This technology holds promise for applications in areas such as oncology, ophthalmology, and wound healing, where precision and efficiency are paramount (Omenogor and Adeniran, 2024).
1.5 Application of LLC gels and in-situ gelling lyotropic liquid crystalline precursor system
1.5.1 Oral drug delivery
The unique structure of these inverse hexagonal gels allows encapsulation of poorly soluble drugs, improving their dissolution and stability in the gastrointestinal environment. This structure consists of a lipid-based matrix that forms a network of channels, facilitating improved drug dispersion and sustained release. Moreover, the bioadhesive properties of these gels ensure prolonged retention at the absorption site in the gastrointestinal tract, thereby enhancing drug uptake. By utilizing oleyl-glycerate-based gels, researchers aim to overcome the limitations associated with traditional oral drug delivery systems, offering a promising approach to increasing the therapeutic efficacy of drugs with poor solubility and absorption (Vrettos et al., 2021). Hexagonal gels outperform micelles in bioadhesion but are less effective for drugs requiring rapid release due to their slower diffusion rates.
LLCs offer distinct advantages as oral formulations targeting intestinal lymphatic transport. These systems utilize micelle compositions to form structures that enhance drug absorption and transport in the intestines. Key strategies for improving lymphatic transport include the use of lipid-based LLCs to promote chylomicron secretion and the design of LLC nanoparticles with ligands that target M cells. A novel bottom-up fabrication method enables precise control over the LLC bead size, determining the drug release rate by managing the diffusional interfacial surface. The LLC beads, embedded in a gelatin-chitosan coacervate to prevent coalescence, were also formulated to be pH-responsive. This design reduces premature drug release in the acidic gastric environment and enhances drug release at higher pH levels. The effectiveness of this approach was demonstrated using celecoxib, a model drug with low water solubility, which showed increased release rates at elevated pH levels (Dinh and Yan, 2023; Goldmünz et al., 2025).
1.5.2 Skin drug delivery
The innovative formulation of a mixture of TPGS, isopropyl myristate, and propylene glycol in a water-based system is a promising vehicle for delivering quercetin into the skin. This specific combination, formed in a lamellar phase, ensures the preservation of the antioxidant properties of quercetin, making it a valuable candidate for both cosmetic and pharmaceutical applications. The structural similarity of these lamellar systems to the skin enhances drug penetration, allowing gradual release of quercetin and reducing systemic exposure. Additionally, the inclusion of water in these mesophases aids in hydrating the tissues, further improving their penetration capabilities (Brito et al., 2024). Liquid crystalline precursor systems offer strong payload stability and mechanical resilience, making them highly effective for drug delivery, especially in tissue regeneration applications. Lamellar systems are superior for skin hydration but less effective for deep tissue penetration compared to cubosomes.
This study explored the potential of LLCs as temperature-sensitive transdermal drug delivery systems, specifically for doxorubicin (DOX) in the treatment of breast cancer. This highlights how LLC structures transform with temperature, from cubic to hexagonal to micellar, allowing controlled drug release. Incorporating paeonol and adjusting the composition helped fine-tune the phase transition temperatures to near human body levels. In vitro tests showed that LLCs were temperature-sensitive, had low toxicity, and improved DOX permeability and deposition in the skin. Confocal laser microscopy and cytotoxicity studies confirmed enhanced drug delivery and significantly increased activity against MCF-7 cell lines compared to DOX solutions, demonstrating the promising potential of LLCs in drug delivery applications (Liu et al., 2021; Shafie et al., 2025).
1.5.3 Ocular drug delivery
Innovations in ocular drug delivery have aimed to improve the effectiveness and safety of treatments for glaucoma. A promising advancement is the development of biofilm-like lipid liquid crystal (LC) gels with cubic and hexagonal matrices. Patel et al. (2022) demonstrated that these gels have been shown to enhance the in vivo residence time of drugs such as pilocarpine nitrate when applied topically to the eye (Patel et al., 2022; Adwan et al., 2024). In studies conducted in rabbits, these gels demonstrated superior performance compared with traditional eye drops, maintaining the medication’s presence longer in the eye and potentially increasing its therapeutic efficacy. Additionally, drug-loaded liquid crystal gels have been designed to boost drug effectiveness while minimizing systemic absorption, thereby reducing the potential side effects. Cubic gels excel in prolonging residence time but may cause discomfort due to their viscosity compared to lamellar gels. This innovative approach holds promise for more effective and patient-friendly ocular treatment (Malode et al., 2025).
LLCs, particularly those formed from mesogen-like sodium dodecyl sulfate (SDS), display distinct phases that influence their bulk and micro viscosities, which are crucial for applications in energy transfer and drug delivery. LLCs have emerged as promising agents for ocular drug delivery, offering improved targeting and efficacy compared to conventional liposomes. This review explores the photophysical behavior of coumarin 6 (C6), a laser dye and ophthalmic drug, in various LLC phases. The interaction of C6 with LLCs modulates fluorescence, enhancing Förster resonance energy transfer (FRET) with N-doped carbon nanoparticles, which serve as photosensitizers. This dynamic can lead to the generation of reactive oxygen species, which is beneficial in therapeutic contexts. Advanced analytical techniques, including AI-driven modeling, are pivotal in predicting LLC phase behavior and optimizing their use in biosensing and diagnostics. Overall, LLCs have transformative potential in ocular health, providing innovative solutions for drug delivery and diagnostic advancements (Chatterjee et al., 2024; Adwan et al., 2024).
1.5.4 Nasal drug delivery
Nasal drug delivery is an innovative and effective method of administering medications directly through the nasal cavity. This route offers several advantages, such as rapid absorption and onset of action owing to the rich vascularization of the nasal mucosa. It is particularly beneficial for drugs that require rapid systemic effects, such as pain relievers, or treatments for conditions such as migraine and allergic rhinitis. Moreover, nasal delivery bypasses the gastrointestinal tract, reducing the risk of degradation by stomach acids and first-pass metabolism in the liver, which can enhance the bioavailability of certain drugs (Tafazzoli Mehrjardi et al., 2025). Additionally, it is more convenient and less invasive than injections, thereby improving patient compliance. However, challenges remain, including ensuring drug formulation stability and addressing the potential irritation of nasal passages. LLC gels enhance nasal retention but may irritate mucosa compared to simpler aqueous formulations. Overall, nasal drug delivery is a promising area of research and development in pharmaceutical science (Xia et al., 2025).
The development of an Almotriptan malate (ALM)-loaded cubosomal in situ gel designed for enhanced permeation through the blood–brain barrier via the intranasal route, promising rapid relief from migraine pain. Cubosomes were crafted with Pluronic F127 and glyceryl monooleate using a Box–Behnken design and optimized using desirability functions. High-resolution transmission electron microscopy confirmed the morphology, while differential scanning calorimetry confirmed drug encapsulation. The optimized formulation, with a particle size of 177.15 nm, exhibited a 90.69% drug release in vitro over 5 h and a 2.52-fold enhancement in ex vivo permeation compared to plain gels. Histopathology confirmed non-toxicity to the nasal mucosa, and short-term stability was demonstrated at room temperature. This approach highlights the potential for improved migraine treatment with enhanced patient compliance through rapid and effective delivery (Xia et al., 2025; Desai et al., 2023).
1.5.5 Injectable drug delivery
Injectable drug delivery involves the administration of medications directly into the body through a needle and syringe, typically targeting a vein, muscle, or tissue. This approach is especially advantageous for delivering drugs that require rapid absorption and precise dosing, or are ineffective when taken orally because of degradation of the digestive system. Common forms of injectable delivery include intravenous (IV), intramuscular (IM), and subcutaneous (SC) injections, each with varying rates of drug absorption and effects. Injectable delivery is widely used in the clinical settings for vaccines, insulin for diabetes management, pain relief, and antibiotics. Recent advancements in this field have focused on improving patient comfort, reducing pain, and developing smart delivery systems that can provide the controlled release of medication over time. Cubic LLC systems provide sustained release for injectables but require specialized syringes due to high viscosity, unlike lamellar systems. As technology evolves, the future of injectable drug delivery may see innovations such as microneedle patches and minimally invasive techniques, further enhancing patient compliance and therapeutic outcomes (Erstad and Barletta, 2022).
Postoperative analgesia, an in situ forming gel (ISFG) based on a lyotropic liquid crystal has been developed to deliver bupivacaine hydrochloride (BUP) for long-acting pain relief. The BUP-ISFG transforms into a gel upon exposure to physiological fluids, undergoing a lamellar-hexagonal-cubic phase transition. This transition facilitates low viscosity for easy injection and sustained drug release owing to the unique nanostructures of the gel phases. In vivo studies have shown that BUP-ISFG provides persistent analgesic effects with steadier plasma BUP concentrations than traditional injections, offering a promising solution for reducing the frequency of injections and improving patient compliance while effectively managing postoperative pain (Mei et al., 2018).
1.6 Factor affecting drug delivery systems
The effectiveness of a drug delivery system is influenced by various factors, each of which plays a crucial role in the successful administration and absorption of medication. Physicochemical properties of a drug, such as its solubility and stability, significantly affect its absorption and distribution in the body. Additionally, the route of administration (oral, intravenous, transdermal, or inhalation) affects the speed and efficiency of delivery. The design of the delivery system itself, including the use of carriers such as nanoparticles or liposomes, can enhance targeting and reduce side effects. Patient-specific factors, such as age, weight, and overall health, also play a critical role as they can influence metabolism and the body’s response to the drug. Furthermore, environmental conditions and storage can affect drug potency and efficacy. In LLC systems, the complexity of formulation and the stability of phases are crucial. While cubic phases provide strong encapsulation, they present more challenges in scalability compared to liposomes. Understanding and optimizing these factors are essential for developing effective and reliable drug delivery systems (Zhang et al., 2022).
1.7 Translational challenges and clinical readiness of LLC-Based systems
LLC nanocarriers have shown considerable promise in controlled and targeted drug delivery, several critical challenges must be addressed to enable their successful clinical translation. A major concern is toxicity and biocompatibility of the nanomaterials. Although many LLC systems utilize biocompatible lipids, such as glyceryl monooleate and phytantriol, their interactions with biological membranes, long-term accumulation, and degradation byproducts require rigorous evaluation through in vivo toxicity and immunogenicity studies (Zhai et al., 2019; Chavda et al., 2023; Waheed and Aqil, 2021; Tiboni et al., 2023). Moreover, scalability and manufacturing consistency are significant obstacles. The production of LLC-based systems, especially high-viscosity cubic and hexagonal phases, often involves complex and energy-intensive techniques, such as high-pressure homogenization or ultrasonication. Ensuring batch-to-batch reproducibility while maintaining structural integrity and drug-loading efficiency is essential for industrial applications.
Another major bottleneck is the regulatory approval process. LLC-based formulations fall under the category of complex drug delivery systems, and the regulatory frameworks for such nanosystems remain underdeveloped. Demonstrating physicochemical stability, pharmacokinetics, biodistribution, and therapeutic efficacy in a reproducible and clinically relevant manner is crucial for regulatory approval (Madheswaran et al., 2019; Chavda et al., 2023). In addition, in vivo behavior, including biodistribution, drug release kinetics, metabolism, and clearance, remains insufficiently characterized for many LLC systems. This hinders predictions of therapeutic performance and safety profiles. Therefore, extensive preclinical and clinical studies are needed to bridge the gap between laboratory research and clinical applications (Leu et al., 2023). Future efforts should prioritize the development of standardized manufacturing protocols, toxicity evaluation frameworks, and regulatory guidance tailored to LLC-based drug delivery (Tiboni et al., 2023). Collaboration between academic researchers, industry stakeholders, and regulatory bodies will be vital in overcoming these translational hurdles and realizing the full therapeutic potential of LLC systems.
1.8 Reproducibility and regulatory considerations in LLC systems
The reproducibility of LLC-based formulations remains a major concern because of their sensitivity to preparation conditions, such as lipid composition, temperature, and hydration levels. Minor deviations in processing can lead to phase transitions or instability, thereby affecting the drug release kinetics and bioavailability. Establishing robust standard operating procedures (SOPs) and validated analytical methods is essential for ensuring batch-to-batch consistency. From a regulatory standpoint, LLCs fall under complex nanostructured drug delivery systems, and their evaluation often lacks harmonized guidelines. Agencies such as the FDA and EMA require comprehensive data on physicochemical stability, critical quality attributes (CQA), and performance metrics. Regulatory bottlenecks include classification ambiguity (drug, device, or combination), scalability challenges, and insufficient long-term toxicological data requirements. Collaborative efforts among academia, industry, and regulatory bodies are vital for developing fit-for-purpose evaluation protocols and accelerating the clinical translation of LLC technologies.
2 Conclusion
LLC nanoparticles represent a promising frontier in advanced drug delivery owing to their unique structural versatility, biocompatibility, and ability to encapsulate a wide range of therapeutic agents. The key findings emphasize that cubic phases provide excellent structural stability and sustained drug release, whereas lamellar and hexagonal phases offer advantages in terms of ease of processing and depot formulations, respectively. In situ gelling systems have expanded the application potential of LLCs for localized and controlled delivery, particularly in ocular, injectable, and transdermal therapies. However, several challenges remain, including the scalability of high-viscosity formulations, inconsistent gelation under physiological conditions, and limited regulatory clarity for their clinical adoption. To advance the field, future research should focus on developing shear-thinning LLC systems for easier administration, utilizing artificial intelligence to model phase transitions and optimize formulations, exploring novel biodegradable and stimuli-responsive materials, and prioritizing comprehensive in vivo and clinical evaluations. By addressing these limitations and leveraging emerging technologies, LLC-based systems can evolve into smart, patient-personalized platforms for precision delivery.
Author contributions
PS: Writing – original draft, Writing – review and editing, Visualization, Validation, Conceptualization. SK: Conceptualization, Writing – review and editing, Writing – original draft, Project administration, Visualization, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdulnaby, H. M., Elkashef, I., Ibrahim, S., and Labeeb, A. M. (2024). Synthesis of silver nanoparticles with different decoration forms dispersed in nematic liquid crystals. Egypt. J. Chem. 67 (2), 601–613. doi:10.21608/ejchem.2023.211205.8009
AbuAlrob, M. A., Itbaisha, A., and Mesraoua, B. (2025). Unlocking new frontiers in epilepsy through AI: from seizure prediction to personalized medicine. Epilepsy Behav. 166, 110327. doi:10.1016/j.yebeh.2025.110327
Adwan, S., Qasmieh, M., Al-Akayleh, F., and Ali Agha, A. S. A. (2024). Recent advances in ocular drug delivery: insights into lyotropic liquid crystals. Pharmaceuticals 17 (10), 1315. doi:10.3390/ph17101315
Aguirre-Ramírez, M., Silva-Jiménez, H., Banat, I. M., and Díaz De Rienzo, M. A. (2021). Surfactants: physicochemical interactions with biological macromolecules. Biotechnol. Lett. 43 (3), 523–535. doi:10.1007/s10529-020-03054-1
Baldha, R., Chakraborthy, G. S., and Rathod, S. (2025). Current status and future prospects of lyotropic liquid crystals as a nanocarrier delivery system for the treatment of cancer. AAPS PharmSciTech 26 (2), 58. doi:10.1208/s12249-025-03058-y
A. Behera, A. K. Nayak, R. K. Mohapatra, and A. A. Rabaan (2024). Smart micro- and nanomaterials for pharmaceutical applications (CRC Press). doi:10.1201/9781003468431
Blanco-Fernández, G., Blanco-Fernandez, B., Fernández-Ferreiro, A., and Otero-Espinar, F. J. (2023). Lipidic lyotropic liquid crystals: insights on biomedical applications. Adv. Colloid Interface Sci. 313, 102867. doi:10.1016/j.cis.2023.102867
Boyd, B., Whittaker, D., Khoo, S., and Davey, G. (2006). Lyotropic liquid crystalline phases formed from glycerate surfactants as sustained release drug delivery systems. Int. J. Pharm. 309 (1–2), 218–226. doi:10.1016/j.ijpharm.2005.11.033
Brito, S., Baek, M., and Bin, B. H. (2024). Skin structure, physiology, and pathology in topical and transdermal drug delivery. Pharmaceutics 16 (11), 1403. doi:10.3390/pharmaceutics16111403
Chatterjee, A., Joy, A., and Purkayastha, P. (2024). Microviscosity-assisted disaggregation of a model ophthalmic drug and FRET-controlled singlet oxygen generation in lyotropic liquid crystals. Langmuir 40 (8), 4321–4332. doi:10.1021/acs.langmuir.3c03588
Chavda, V., Dyawnapelly, S., Dawre, S., Ferreira-Faria, I., Bezbaruah, R., Gogoi, N., et al. (2023). Lyotropic liquid crystalline phases: drug delivery and biomedical applications. Int. J. Pharm. 641, 123546. doi:10.1016/j.ijpharm.2023.123546
Desai, G. N., Dandagi, P. M., and Kazi, T. M. (2023). Nanosized intranasal delivery of novel self-assembled cubic liquid crystals: formulation and evaluation. J. Pharm. Innov. 18 (3), 934–951. doi:10.1007/s12247-022-09695-1
Dinh, L., and Yan, B. (2023). Oral drug delivery via intestinal lymphatic transport utilizing lipid-based lyotropic liquid crystals. Liquids 3 (4), 456–468. doi:10.3390/liquids3040029
Dombrowski, M., Herbst, M., Preisig, N., Giesselmann, F., and Stubenrauch, C. (2024). Time dependence of gel formation in lyotropic nematic liquid crystals: from hours to weeks. Gels 10, 261. doi:10.3390/gels10040261
Erstad, B. L., and Barletta, J. F. (2022). Implications of obesity for drug administration and absorption from subcutaneous and intramuscular injections: a primer. Am. J. Health Syst. Pharm. 79 (15), 1236–1244. doi:10.1093/ajhp/zxac058
Goldmünz, E. Y., Aserin, A., Pal, A., Shimon, D., Ottaviani, M. F., and Garti, N. (2025). pH-sensitive lyotropic liquid crystal beads designed for oral zero-order extended drug release. Int. J. Pharm. 674, 125412. doi:10.1016/j.ijpharm.2025.125412
Govindan, I., Paul, A., Rama, A., Kailas, A. A., Abutwaibe, K. A., Annadurai, T., et al. (2024). Mesogenic architectures for advanced drug delivery: interrogating lyotropic and thermotropic liquid crystals. AAPS PharmSciTech 26 (1), 6. doi:10.1208/s12249-024-02985-6
Gu, S., Zhang, L., de Campo, L., O’Dell, L. A., Wang, D., Wang, G., et al. (2023). Lyotropic liquid crystal (LLC)-templated nanofiltration membranes by precisely administering LLC/substrate interfacial structure. Membranes 13 (6), 549. doi:10.3390/membranes13060549
Islam, M. A., Hasan, M., Rahman, M., Mobarak, M. H., Mimona, M. A., and Hossain, N. (2024). Advances and significances of carbon nanotube applications: a comprehensive review. Eur. Polym. J. 2024, 113443. doi:10.1016/j.eurpolymj.2024.113443
Jacob, S., Kather, F. S., Boddu, S. H., Shah, J., and Nair, A. B. (2024). Innovations in nanoemulsion technology: enhancing drug delivery for oral, parenteral, and ophthalmic applications. Pharmaceutics 16 (10), 1333. doi:10.3390/pharmaceutics16101333
Karakasidis, T., Kalogianni, E. P., Kontogiorgos, V., and Ritzoulis, C. (2025). Emulsification properties and interfacial behavior of okra proteins. Food Biophys. 20 (1), 47. doi:10.1007/s11483-025-09938-x
Kim, S., Seo, M., Lim, H., and Hong, J. (2023). Lipid-based liquid crystalline phases for biocompatible and versatile drug delivery. Yakhak Hoeji 67 (3), 137–144. doi:10.17480/psk.2023.67.3.137
Král, P., Chan, H., Vuković, L., Raj, S., Sen, S., Han, Y., et al. (2024). Simulation methods for self-assembling nanoparticles. Prog. Mater. Sci. 142, 101225. doi:10.1016/j.pmatsci.2023.101225
Kumar, H., Sureshkumar, A., Badduri, N., and Jain, V. (2021). A review on lyotropic liquid crystals and its potential applications. Nanosci. Nanotechnol. Asia 11 (6), e070921191688–63. doi:10.2174/2210681211666210204114532
Kuwabara, H., Ogura, T., Tsuchiya, K., Akamatsu, M., Arakawa, K., Sakai, K., et al. (2024). Structural analysis of interfacial films of oil/water emulsions prepared by emulsification via lamellar gels. J. Oleo Sci. 73 (12), 1467–1477. doi:10.5650/jos.ess24181
Leu, J. S., Teoh, J. J., Ling, A. L., Chong, J., Loo, Y. S., Mat Azmi, I. D., et al. (2023). Recent advances in the development of liquid crystalline nanoparticles as drug delivery systems. Pharmaceutics 15 (5), 1421. doi:10.3390/pharmaceutics15051421
Li, G. W., Wang, X. J., Lei, X., Liu, N., and Wu, Z. Q. (2022). Self-assembly of helical polymers and oligomers to create liquid crystalline alignment for anisotropic NMR parameters. Macromol. Rapid Commun. 43 (14), 2100898. doi:10.1002/marc.202100898
Lin, S. Y., Chen, T. T., Farag, M. A., Teng, H., and Cao, H. (2024). A modified self-micro emulsifying liposome for bioavailability enhancement of quercetin and its biological effects. Food Biosci. 62, 105352. doi:10.1016/j.fbio.2024.105352
Liu, J., Cheng, R., Heimann, K., Wang, Z., Wang, J., and Liu, F. (2021). Temperature-sensitive lyotropic liquid crystals as systems for transdermal drug delivery. J. Mol. Liq. 326, 115310. doi:10.1016/j.molliq.2021.115310
Lu, S. G., Sindhu, K. K., and Rowley, J. P. (2025). Changes in employment and practice locations among radiation oncologists: 2015-2023. Int. J. Radiat. Oncol. Biol. Phys. 122 (5), 1095–1101. doi:10.1016/j.ijrobp.2025.02.036
Madheswaran, T., Kandasamy, M., Bose, R., and Karuppagounder, V. (2019). Current potential and challenges in the advances of liquid crystalline nanoparticles as drug delivery systems. Drug Discov. Today 24 (7), 1405–1412. doi:10.1016/j.drudis.2019.05.004
Malode, T. D., Pillare, S. B., Shahi, A. K., Lade, S. N., Taksande, J. B., and Umekar, M. J. (2025). Cubosomes as versatile nanocarriers: insights into composition, mechanisms, and therapeutic applications. Biomed. Mater. Devices 2025, 1–22. doi:10.1007/s44174-025-00416-z
Manna, K., Roy, A., Dey, S., Ghosh, S., Pradhan, P., and Pal, S. (2024). A review on the culmination of rational development of stimuli-responsive polymeric micelles as vehicles for site-specific hydrophobic therapeutics. J. Macromol. Sci. A 61 (6), 349–375. doi:10.1080/10601325.2024.2335277
Masime, J. O., Ndangili, P. M., and Lalah, J. O. (2025). “X-ray techniques in analytical chemistry,” in X-ray techniques in analytical chemistry (Elsevier).
Mei, L., Xie, Y., Huang, Y., Wang, B., Chen, J., Quan, G., et al. (2018). Injectable in situ forming gel based on lyotropic liquid crystal for persistent postoperative analgesia. Acta Biomater. 67, 99–110. doi:10.1016/j.actbio.2017.11.057
Milak, S., and Zimmer, A. (2015). Glycerol monooleate liquid crystalline phases used in drug delivery systems. Int. J. Pharm. 478 (2), 569–587. doi:10.1016/j.ijpharm.2014.11.072
Nath, A. G., Dubey, P., Kumar, A., Vaiphei, K. K., Rosenholm, J. M., Bansal, K. K., et al. (2024). Recent advances in the use of cubosomes as drug carriers with special emphasis on topical applications. J. Lipids 2024 (1), 2683466. doi:10.1155/2024/2683466
Nazaruk, E., Miszta, P., Filipek, S., Górecka, E., Landau, E. M., and Bilewicz, R. (2015). Lyotropic cubic phases for drug delivery: diffusion and sustained release from the mesophase evaluated by electrochemical methods. Langmuir 31 (46), 12753–12761. doi:10.1021/acs.langmuir.5b0324
Omenogor, C. E., and Adeniran, A. A. (2024). Advancing precision healthcare: the integration of nanotechnology, millimeter wave sensing, laser technology, fibre Bragg grating, and deep learning models. Int. J. Res. Publ. Rev. 5 (6), 639–657. doi:10.55248/gengpi.5.0924.2421
Otte, I., Detsch, F., Gütlein, A., Scholl, M., Kiese, R., Appelhans, T., et al. (2017). Seasonality of stable isotope composition of atmospheric water input at the southern slopes of Mt. Kilimanjaro, Tanzania. Hydrol. Process. 31 (22), 3932–3947. doi:10.1002/hyp.11311
Özkaynak, M. U., Kocaaga, B., Dönmez, K. B., Dağlar, S., Türker, Y., Karatepe, N., et al. (2024). Understanding the role of water in the lyotropic liquid crystalline mesophase of high-performance flexible supercapacitor electrolytes using a rheological approach. J. Mol. Liq. 394, 123705. doi:10.1016/j.molliq.2023.123705
Pal, A., Jaju, S. J., and Kumaran, V. (2023). The relationship between structure and rheology in a three-dimensional sheared lamellar mesophase. Soft Matter 19 (28), 5262–5287. doi:10.1039/d3sm00455d
Patel, K. D., Silva, L. B., Park, Y., Shakouri, T., Keskin-Erdogan, Z., Sawadkar, P., et al. (2022). Recent advances in drug delivery systems for glaucoma treatment. Mater. Today Nano 18, 100178. doi:10.1016/j.mtnano.2022.100178
Phan, S., Salentinig, S., Gilbert, E., Darwish, T. A., Hawley, A., Nixon-Luke, R., et al. (2015). Disposition and crystallization of saturated fatty acid in mixed micelles of relevance to lipid digestion. J. Colloid Interface Sc. 449, 160–166. doi:10.1016/j.jcis.2014.11.026
Priya, S., Desai, V. M., and Singhvi, G. (2024). Tailoring lyotropic liquid crystals for skin barrier penetration: exploring composition and structure–function relationships. Appl. Phys. Rev. 11 (3), 031307. doi:10.1063/5.0204909
Rahman, H. S., Othman, H. H., Hammadi, N. I., Yeap, S. K., Amin, K. M., Abdul Samad, N., et al. (2020). Novel drug delivery systems for loading of natural plant extracts and their biomedical applications. Int. J. Nanomedicine 15, 2439–2483. doi:10.2147/ijn.s227805
Rajabalaya, R., Musa, M., Kifli, N., and David, S. (2017). Oral and transdermal drug delivery systems: role of lipid-based lyotropic liquid crystals. Drug Des. devel. Ther. 11, 393–406. doi:10.2147/DDDT.S103505
Rani, A., Verma, R., Kumar, M., Tiwari, A., Tiwari, V., Bhatt, S., et al. (2024). Nanosuspension as a novel nanovehicle for drug delivery: a recent update on patents and therapeutic applications. Curr. Nanomed. 14 (2), 88–98. doi:10.2174/0124681873270131231023082115
Sahu, T., Ratre, Y. K., Chauhan, S., Bhaskar, L. V. K. S., Nair, M. P., and Verma, H. K. (2021). Nanotechnology-based drug delivery system: current strategies and emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 63, 102487. doi:10.1016/j.jddst.2021.102487
Shafie, M. A. A., Mohamed, H. B., and Mekkawy, A. I. (2025). Transdermal delivery of doxorubicin-loaded lyotropic liquid crystals formulations for enhanced drug skin deposition and cytotoxicity against MCF-7 breast cancer cells. J. Drug Deliv. Sci. Technol. 111, 107170. doi:10.1016/j.jddst.2025.107170
Shah, J. (2001). Cubic phase gels as drug delivery systems. Adv. Drug Deliv. Rev. 47 (2-3) 229–250. doi:10.1016/s0169-409x(01)00108-9
Shete, A., Nadaf, S., Doijad, R., and Killedar, S. (2021). Liquid crystals: characteristics, types of phases and applications in drug delivery. Pharm. Chem. J. 55 (2), 106–118. doi:10.1007/s11094-021-02396-y
Shukla, S., and Kumar, B. (2025). “Next generation healthcare: leveraging AI for personalized diagnosis, treatment, and monitoring,” in Artificial intelligence and cybersecurity in healthcare, 147–171.
Smaisim, G. F., Mohammed, K. J., Hadrawi, S. K., Koten, H., and Kianfar, E. (2023). Properties and application of nanostructure in liquid crystals: review. BioNanoScience 13 (2), 819–839. doi:10.1007/s12668-023-01082-5
Sparavigna, A. C. (2023). Role of lyotropic liquid crystals in templating mesosilica materials. Int. J. Sci. 12 (7), 7–40. doi:10.18483/ijsci.2691
Tafazzoli Mehrjardi, S., Tafaghodi, M., Malek, S., Yari, D., Mohammadpour, A. H., Kamali, H., et al. (2025). Intranasal delivery of cetrorelix via lipid liquid crystal nanoparticles: characterization and pharmacokinetic studies in rats. AAPS PharmSciTech 26 (6), 176. doi:10.1208/s12249-025-03169-6
Tan, C., Hosseini, S. F., and Jafari, S. M. (2022). Cubosomes and hexosomes as novel nanocarriers for bioactive compounds. J. Agric. Food Chem. 70 (5), 1423–1437. doi:10.1021/acs.jafc.1c06747
Tiboni, M., Astolfi, P., Verboni, M., Benedetti, S., Giorgini, E., Notarstefano, V., et al. (2023). The influence of mannose-based esters on the mesophase behaviour of lyotropic liquid crystalline nanosystems as drug delivery vectors. Colloids Surf. B Biointerfaces 232, 113596. doi:10.1016/j.colsurfb.2023.113596
Uranga Wassermann, M. V., Soulé, E. R., and Balbuena, C. (2024). Exploring mesophase formation: structural characterization approaches in a soft sphere model. J. Mol. Liq. 411, 125713. doi:10.1016/j.molliq.2024.125713
Vitek, M., Zvonar Pobirk, A., Roškar, R., and Matjaž, M. G. (2025). Exploiting the potential of in situ forming liquid crystals: development and in vitro performance of long-acting depots for peptide drug thymosin alpha 1 subcutaneous administration. Drug Deliv. 32 (1), 2460708. doi:10.1080/10717544.2025.2460708
Vrettos, N. N., Roberts, C. J., and Zhu, Z. (2021). Gastroretentive technologies in tandem with controlled-release strategies: a potent answer to oral drug bioavailability and patient compliance implications. Pharmaceutics 13 (10), 1591. doi:10.3390/pharmaceutics13101591
Waheed, A., and Aqil, M. (2021). Lyotropic liquid crystalline nanoparticles: scaffolds for delivery of myriad therapeutics and diagnostics. J. Mol. Liq. 338, 116919. doi:10.1016/j.molliq.2021.116919
Xia, X., Zhong, Z., Wei, C., Wang, G., Zhao, Z., He, J., et al. (2025). Lyotropic liquid crystalline-based nasal spray for improved Parkinson's treatment: enhanced superior nasal tract deposition and antioxidation strategy. Adv. Funct. Mater. 35 (1), 2411426. doi:10.1002/adfm.202411426
Yap, S. L., Yu, H., Li, S., Drummond, C. J., Conn, C. E., and Tran, N. (2024). Cell interactions with lipid nanoparticles possessing different internal nanostructures: liposomes, bicontinuous cubosomes, hexosomes, and discontinuous micellar cubosomes. J. Colloid Interface Sci. 656, 409–423. doi:10.1016/j.jcis.2023.11.059
Zhai, L., Lauing, K. L., Chang, A. L., Dey, M., Qian, J., Cheng, Y., et al. (2014). The role of IDO in brain tumor immunotherapy. J. Neurooncol. 123 (3), 395–403. doi:10.1007/s11060-014-1687-8
Zhang, Y. B., Xu, D., Bai, L., Zhou, Y. M., Zhang, H., and Cui, Y. L. (2022). A review of non-invasive drug delivery through respiratory routes. Pharmaceutics 14 (9), 1974. doi:10.3390/pharmaceutics14091974
Keywords: bioavailability, drug delivery system, lyotropic liquid crystals, liquid crystalline phase, nanoparticle
Citation: Subash P and Khute S (2025) Recent advances in lyotropic liquid crystal nanoparticle formulations for drug delivery systems. Front. Soft Matter 5:1658466. doi: 10.3389/frsfm.2025.1658466
Received: 02 July 2025; Accepted: 25 August 2025;
Published: 11 September 2025.
Edited by:
Alejandro D. Rey, McGill University, CanadaReviewed by:
Linda Reven, McGill University, CanadaDana Grecov, University of British Columbia, Canada
Copyright © 2025 Subash and Khute. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sulekha Khute, c3VsZWtoYWtodW50ZUBnbWFpbC5jb20=
†ORCID: Paranthaman Subash, orcid.org/0000-0003-8060-1591; Sulekha Khute, orcid.org/0000-0003-0197-2541
 Paranthaman Subash
Paranthaman Subash Sulekha Khute
Sulekha Khute