- 1Department of Soil, Plant and Food Sciences, University of Bari, Bari, Italy
- 2Institute for Sustainable Plant Protection, CNR, Bari, Italy
- 3Department of Environmental Biology, “Sapienza” University of Rome, Rome, Italy
Introduction: Ensuring crop health is essential to meet the growing demand for food and effectively managing root-knot nematodes (RKNs) can help maximize agricultural output. Indeed, plant diseases caused by RKNs belonging to the genus Meloidogyne, among the most harmful pests of horticultural crops, lead to severe productivity and economic losses.
Methods: This study aimed at assessing the effectiveness of wasted bread, brewers’ spent grain, and spent coffee grounds, native or bioprocessed, to suppress RKN Meloidogyne incognita, when used as soil amendments. Bioprocessing included the use of enzymatic treatments and fermentation with selected lactic acid bacteria or an incubation with compost tea. The matrices were incorporated to infested soil at doses corresponding to 3,000 kg ha−1 organic carbon. Biomasses were characterized for their main physico-chemical and biochemical properties. Then plant growth and soil infestation were monitored to assess the biomasses potential as amendments.
Results: The different nature of the biomasses resulted in a supplementation in total nitrogen, phosphorous, organic acids and phenolic compounds strictly dependent on the food residue used and the bioprocessing employed. Although all the amendments significantly suppressed the multiplication of M. incognita and gall formation on tomato roots (up to 98 and 79%, respectively), the strongest suppressive effect was achieved by using bioprocessed brewers’ spent grain, resulting in performances comparable to synthetic nematicides (79 eggs and juveniles g−1 roots).
Discussion: The nematocidal effect was ascribed to phenolic compounds and organic acids produced during lactic acid bacteria fermentation. Bioprocessed brewers’ spent grain also resulted in the best growth effect on tomato plants.
1 Introduction
Feeding 9 billion people is one of the challenges the food system is going to face in the next few decades. However, doing so while dealing with the aftermath of the always more frequent natural disasters resulting from climate change, pandemics, and conflicts is certainly not an easy task. It has become clearer that preventing the further weakening of the food systems and ensuring food security, requires an efficient use of agricultural resources, especially land, water, energy, and nutrients (Wang, 2022). For instance, the conversion of food industry waste and by-products into soil conditioners represents a potential valorization strategy for these residues, but could also enhance nutrient inputs and soil fertility.
Indeed, soil amendments, which besides stimulating plant growth, can also provide antagonistic microorganisms or compounds that inhibit the development of plant diseases in crops, are considered a promising tool for developing a more sustainable primary production (Garbowski et al., 2023). Among plant diseases, those caused by root-knot nematodes (RKNs) belonging to the genus Meloidogyne, among the most harmful pests of horticultural crops, lead to severe yield losses (Rawal, 2020). Four species (Meloidogyne incognita, M. javanica, M. arenaria, and M. hapla) are primarily responsible for reduction up to 73.3% in tomato production, causing roughly 125 billion dollars in annual losses worldwide (Rawal, 2020). The parasitic life cycle of M. incognita includes several phases. Matured female lay eggs on the surface of the root. First stage juvenile (J1), after the embryogenesis molts within the egg to the infective juvenile of the second stage (J2). Then, J2 penetrates host plant roots, moving between cortical tissue and cells, and gets into the plant vascular cylinder becoming sedentary. J2 provokes the dedifferentiation of surrounding cells into multinucleated and enlarged giant cells that act as a nutrient source to the nematode, which then goes through three molts, juvenile third stage (J3), fourth stage (J4) and adult (Subedi et al., 2020). RKNs infections appear with stunted or slow growth as well as other symptoms like oval patches, root galling, chlorosis, and premature wilting (Rawal, 2020). Additional crop damage can also derive from RKNs synergism with important soil-borne pathogens such as Fusarium spp., Verticillium spp., Pyrenochaeta lycopersici and viruses that penetrate nematode root lesions (Thligene et al., 2019). For decades, the control of RKNs was effectively based on soil treatments with synthetic nematicides, mainly fumigants. However, due to the environmental and health concerns raised by these products, as well as their negative impact on soil physical and chemical characteristics and microbial diversity, the search for new sustainable control strategies has become necessary (Bonanomi et al., 2020).
Over the years, numerous studies evaluated biological technologies, also including soil organic amendments, as a sustainable alternative to the common chemical nematicides. For instance, a recent meta-analysis (Peiris et al., 2020) demonstrated that the application of organic amendments including plant residues, composts, and animal manures generally results in a significant reduction of RKNs population and damage levels by approximately 63% compared to untreated controls. This reduction seems to occur regardless of amendment type, application rates, RKNs species and crop. Nevertheless, plant-based derivatives seem to generate the best RKNs reduction (up to 79%) effect compared to animal manures and composts (Peiris et al., 2020). The mechanisms behind nematode suppression after amendment incorporation in soil have not been fully elucidated, though it is generally ascribed to the release of nematotoxic compounds, either pre-existing or formed during biomasses decomposition, as well as to the development of a nematode-antagonist microflora on the incorporated feeding substrate (Moosavi, 2022).
In this framework, this study aimed at assessing the effectiveness of food industry waste and by-products, used as amendments, in suppressing RKN M. incognita on tomato plants. Three matrices, resulting from the production process of different food industries were evaluated: (i) wasted bread (WB), surplus from the sandwich production, (ii) brewers’ spent grain (BSG), by-product of the beer brewing industry, and (iii) spent coffee grounds (SCG), residues of the coffee brewing process. SCG and BSG are a source of value-added compounds such as fatty acids, phenolic compounds, lignin, cellulose, hemicellulose, and other polysaccharides (Chetrariu and Dabija, 2020; Pérez-Burillo et al., 2022) which can display toxicity to infective juveniles of the RKN M. incognita (D’Addabbo et al., 2013; Zhang et al., 2012). Moreover, all biomasses also have a discrete amount of nitrogen, generally reported as a prerequisite for nematode-suppressive soil organic amendments (Oka and Yermiyahu, 2002). However, to date, no other study has ever evaluated the effect of BSG and WB on nematode suppression, nor the possibility of using bioprocessing technologies to improve the efficiency of the amendments. Hence, based on the lack of previous literature data on nematode suppression of the above-described biomasses, a pot trial was carried out to assess the effects of soil organic amendments, based on SCG, BSG and WB, on RKNs infestation. Biomasses, used either native or bioprocessed with lactic acid bacteria or compost tea (CT), were characterized for their main physico-chemical and biochemical properties. Then plant growth and soil infestation were monitored to assess the biomasses potential as amendments.
2 Materials and methods
2.1 Raw materials and bioprocessing
BSG and WB were kindly provided by an industrial brewery (Peroni Srl, Bari, Italy) and an industrial bakery (Vallefiorita Srl, Ostuni, Italy), respectively, whereas SCG was collected from various coffee bars in Bari. The biomasses were used native or after bioprocessing (bBSG, bWB, and bSCG, respectively). More specifically, BSG was subjected to an enzymatic treatment with a commercial xylanase (DEPOL™ 761P, Biocatalysts, Chicago, IL, USA) and fermentation with a selected strain of Lactiplantibacillus plantarum PU1, as previously reported by Cacace et al. (2022a). WB bioprocessing included an enzymatic treatment with amylase Veron® Mac and fermentation with Lactiplantibacillus plantarum H64, according to the protocol described by Cacace et al. (2022b). SCG was bioprocessed with a CT extracted from a mature green waste compost, using a compost/water ratio of 1:5 and a 6-h extraction time. The CT had the following main characteristics: pH 8.4 ± 0.4, electrical conductivity (EC) 2,800 ± 200 μS cm−1, organic carbon (OC) content 3.9 ± 0.5 g L−1, total nitrogen (TN) 0.5 ± 0.2 g L−1, and available phosphorus (AP) 368 ± 2.6 mg L−1. The SCG/CT ratio was 1:2.5 and the SCG was soaked and aerated in CT for about 24 h at room temperature prior to soil amendment.
2.2 Characterization of biomasses
All biomasses were analyzed, before and after bioprocessing, for pHH2O, EC, moisture and AP content according to the methods of Trinchera et al. (2006). Moisture, expressed as percentage of the initial weight, was determined by drying samples at 105°C overnight. EC was measured in sample/water extracts (1:10 w/v) after shaking for 30 min using a Hanna Edge® EC instrument. AP content was determined spectrophotometrically on acid digested samples, TN content was determined by the Kjeldahl method, while OC was determined by the Springer-Klee method (Ciavatta et al., 1989).
Bioprocessing of the biomasses was monitored by measuring the pH and total titratable acidity (TTA) before and after incubation. The pH was determined using a pHmeter (Model 507, Crison, Milan, Italy) with a food penetration probe, while the TTA was measured according to the AACC method 02-31.01 (2010) and expressed as the amount (mL) of 0.1 M NaOH necessary to reach a pH of 8.4. Organic acids were determined on biomasses water/salt-soluble extracts (WSE). WSE were prepared according to the method originally described by Osborne and modified by Weiss et al. (1993) using 50 mM Tris–HCl (pH 8.8) for 1 h at 4°C. After centrifugation, the supernatants were used to determine lactic and acetic acids using the K-DLATE and K-ACET Megazyme kits (Megazyme International Ireland Limited, Bray, Ireland), respectively, following the manufacturer’s instructions. Total phenols content (TPC) was determined, before and after bioprocessing, using the Folin–Ciocalteu method, according to the method of Slinkard and Singleton (1977) using gallic acid as standard.
2.3 Soil sampling and characterization
Soil used in experiment was collected from a table grape vineyard located at Trani (province of Barletta-Andria-Trani, Apulia Region). The soil was thoroughly mixed in a concrete mixer and then the absence of phytoparasitic nematodes was checked. An extraction from four random samples according to the Coolen’s flotation and centrifugation method (Coolen, 1979), considered one of the most common extraction methods (Viaene et al., 2021), was performed and the presence of nematode specimens was identified morphologically under a microscope.
The soil was chemically characterized both before and at the end of the experiment, according to the conventional analytical methods described by Sparks et al. (1996). The particle size distribution was determined using the pipette method, while soil texture was identified according to the USDA soil textural classification system (Soil Survey Staff, 2014). The pHH2O and pHKCl were measured suspending 10 g of soil in 25 mL of high purity water (Milli-Q Element system, Millipore, Molsheim, France) and 1 M KCl solution, respectively, while EC was measured in filtrates with a soil-to-water ratio of 1:2. Total carbonates were analyzed using the calcimeter method while TN and OC contents were determined using the Kjeldahl and Walkley-Black methods, respectively. The AP was extracted with a 0.5 M NaHCO3 solution and determined spectrophotometrically at 650 nm, according to the Olsen method (1954) considered a standard operating procedure by FAO (2021).
2.4 Experiment set-up
An Italian population of M. incognita was previously reared for 2 months on tomato cv. Regina di Fasano in a greenhouse at 25 ± 2°C. The infested tomato roots were finely minced and thoroughly mixed before taking four random 10 g samples of root on which the density of eggs and juveniles (J2s) of M. incognita per gram of root was assessed. Nematode eggs and J2s were extracted by shaking the root samples for 3 min in a 1% sodium hypochlorite aqueous solution, according to the Hussey and Barker’s method (1973) recently reported by Viaene et al. (2021).
The extracted eggs and J2s were recovered onto a 20-μm sieve and counted under a microscope. Proper amounts of this inoculum were added to the non-sterilized experimental soil to reach an initial density of nematode population of about 7 eggs and J2s mL−1 soil (about 7,000 eggs and J2s per pot), similar to that used by Corbett et al. (2011). The homogeneous distribution of the root inoculum in the soil was ensured by mixing for 10 min in a concrete mixer.
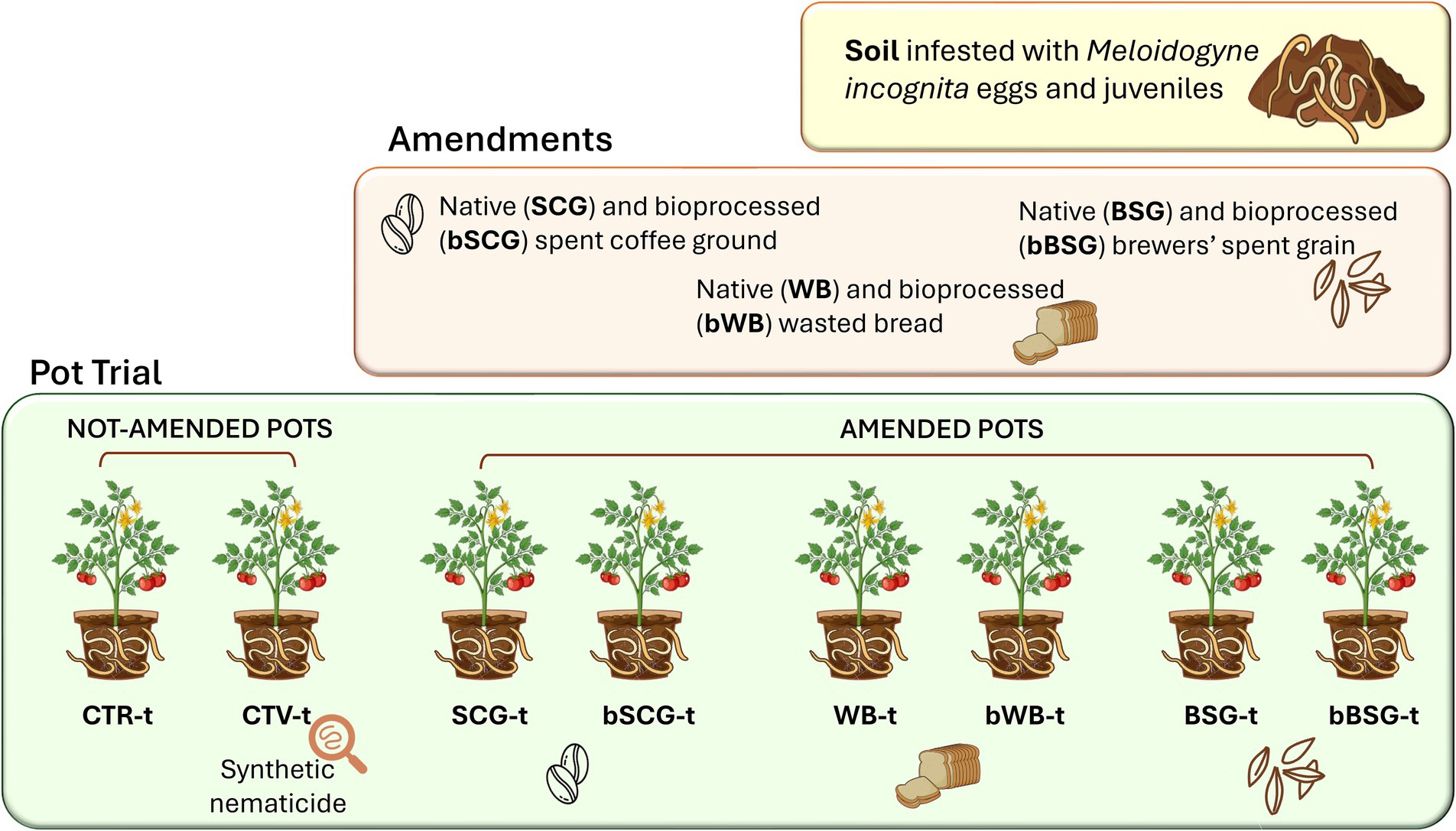
The infested soil was then amended with SCG, BSG or WB, native or bioprocessed, added at an amount corresponding to 3,000 kg of OC ha−1, according to the dry OC content of each biomass, respectively, generating the following treatments: SCG-t, BSG-t, WB-t, bSCG-t, bBSG-t, and bWB-t (Figure 1).
The amended soil was then poured into 1 L clay pots (having 14 cm diameter), providing eight replicates of each treatment, and including soil non-treated (CTR-t) and treated (CTV-t) with a commercial formulation of the nematicide Fluopyram (Velum Prime®, Bayer Crop Science, Milan, Italy) as controls. The synthetic nematicide was added at a dose of 0.5 L ha−1 the day after tomato transplantation and 2 weeks later. The pots were placed in a completely randomized block design on the benches of a greenhouse maintained at 25 ± 2°C. One month-old tomato seedlings cv. Regina di Fasano were transplanted into each pot 2 weeks after the amendments.
A SPAD-502 chlorophyll meter (Konica Minolta, Japan) was used to verify the effects of the treatments on plant health status during the trial. The tomato plants were kept in the greenhouse for 2 months, after which they were uprooted, recording the height and dry weight of the aerial parts and roots of each plant. The root gall index (RGI) was estimated according to a 0–10 scale, where 0 = no galls; 1–4 = galling of secondary roots only, 5–10 = galling of primary laterals and tap root, with 5 equals to 50% of roots galled and 10 the maximum nematode infestation possible (Bridge and Page, 1980). The number of eggs and J2s per gram of roots was assessed by processing a 10 g root sample from each tomato plant using the Hussey and Barker’s method (1973). Moreover, to assess the effect of the treatments on plant growth, at the end of the trial, plant height, dry weight of aerial part and dry weight of roots were determined.
2.5 Statistical analysis
Experimental results were tested against the normal distribution of variables (Shapiro-Wilk test) and the homogeneity of variance (Bartlett test) using the software R studio version 4.1.3 (The R Foundation for Statistical Computing, Vienna, Austria). Data normally distributed and with homogeneity of variances were subjected to an ANOVA and HSD test. Data not normally distributed were subjected to the Levene test and a non-parametric ANOVA analysis (Kruskal-Wallis test) and the Dunn test. Data were analyzed through principal component analysis (PCA), using the software GraphPad Prism 7.0 (GraphPad Software, Inc., San Diego, CA, USA). Correlations within soil, plant, and roots features (p < 0.05), assessed based on Pearson’s bivariate correlation, were determined with GraphPad Prism 7.0.
3 Results
3.1 Biomasses characterization
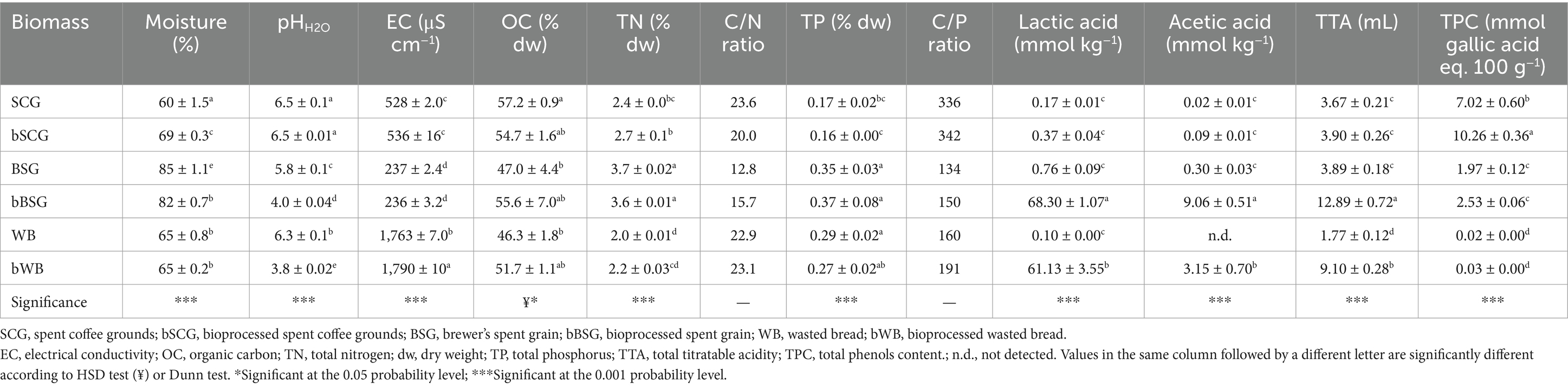
After bioprocessing, bBSG and bWB showed significant acidification compared to native biomasses, indeed, the pH decreased by 1.8 and 2.5 units, reaching values of 4 and 3.8, respectively. On the contrary, bioprocessing with CT did not influence the pH of SCG (Table 1). Lactic and acetic acids were detected in traces in WB, BSG, SCG and bSCG, while their content was significantly higher in bWB and bBSG, reaching 61 and 68 mmol kg−1 in bWB and bBSG, respectively. Whereas acetic acid was found in concentrations of 3.15 and 9.06 mmol kg−1 in bWB and bBSG, respectively. Accordingly, TTA values were significantly higher in bWB and bBSG (9.10 and 12.89 mL NaOH 0.1 N, respectively) compared to the other biomasses. bSCG showed the highest TPC (Table 1), followed by SCG with roughly 30% less TPC. BSG and bBSG had lower TPC compared to SCG and bSCG, although not statistically different from each other, and, finally, WB and bWB showed only traces of total phenols (Table 1).
Among native biomasses, WB showed the highest EC values, which further increased after bioprocessing, while the lowest values were found for BSG and bBSG. OC content of bWB and bBSG was slightly higher in comparison to the corresponding non-bioprocessed biomasses, whereas the contrary occurred for SCG. TN content, instead, was not affected by bioprocessing in any of the three raw biomasses, showing the highest values in BSG (Table 1).
3.2 Soil characterization
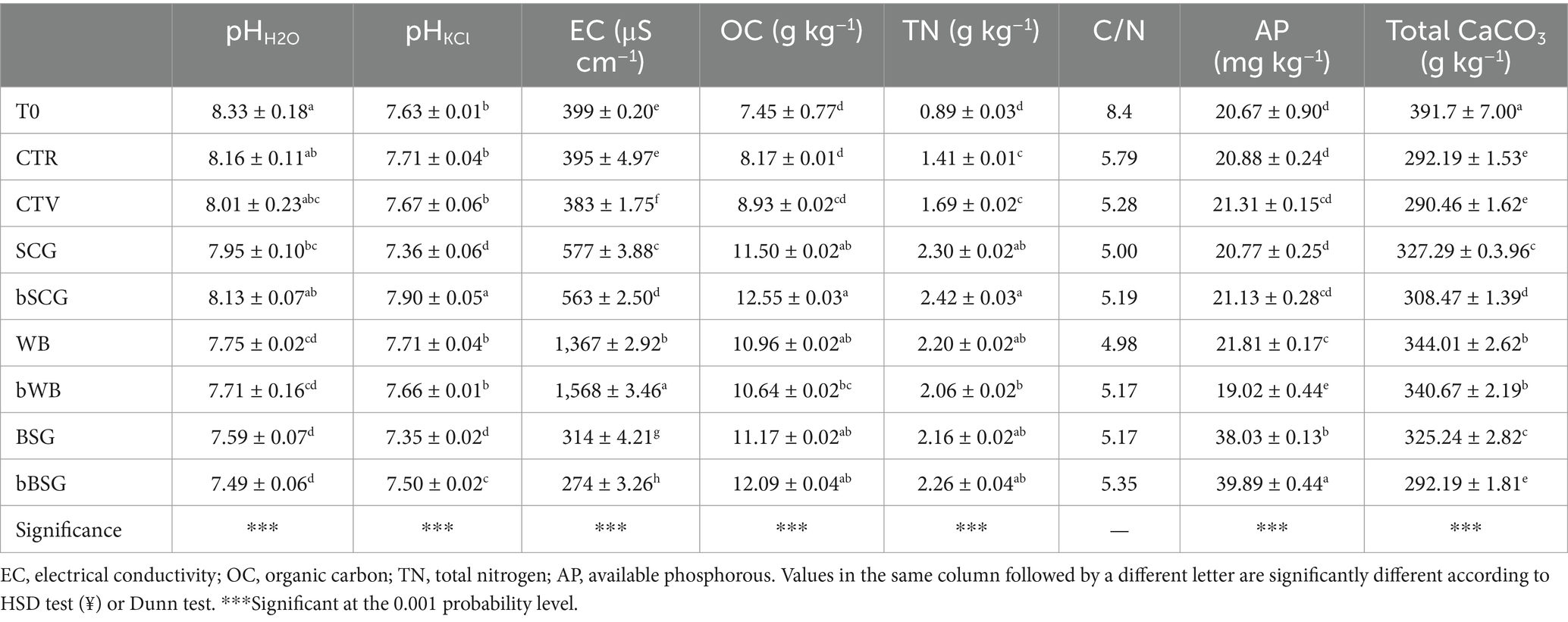
The soil at the beginning of the trial (T0) was typical of the Mediterranean basin, that is, alkaline, relatively rich in total CaCO3, and poor in OC (<1%) and TN. The texture was loam, the EC was relatively low, with a good supply of AP. As a result of the supplementation, its physical and chemical properties changed after the trial (Table 2). Indeed, while the pHH2O of the CTV-t, CTR-t and bSCG-t soils was quite similar to T0, all other soils showed a significant (p < 0.01) decrease in pH. The highest pHKCl value was recorded for bSCG-t, followed by CTR-t, CTV-t, WB-t and bWB-t with values similar to T0 (p > 0.001), while the lowest values were recorded for bBSG-t, BSG-t and SCG-t. Soils amended with bWB and WB showed the highest EC values among all treatments. Overall, all amended soils had a higher OC and TN content than T0, CTR-t and CTV-t, while AP resulted significantly higher in BSG- and bBSG-amended soils compared to the other soils. Moreover, soils at the end of the trial showed significantly lower values of total carbonates than T0.
3.3 Effects of treatments on plant growth and Meloidogyne incognita suppression
As expected, plants from CTR-t had the worst growth parameters, whereas those from soil amended with bBSG or WB were significantly taller and heavier and presented significantly larger root biomass (Figure 2) compared to the other treatments. Plants cultivated with the other amendments showed virtually no statistical differences between growth parameters. Spad values showed slight differences (p < 0.01) between treatments only 23 days after transplantation, with CTR-t that showed the highest value, followed by plants from soil amended with bWB. However, no significant differences were observed for Spad values of the different treatments until the end of the experiment.
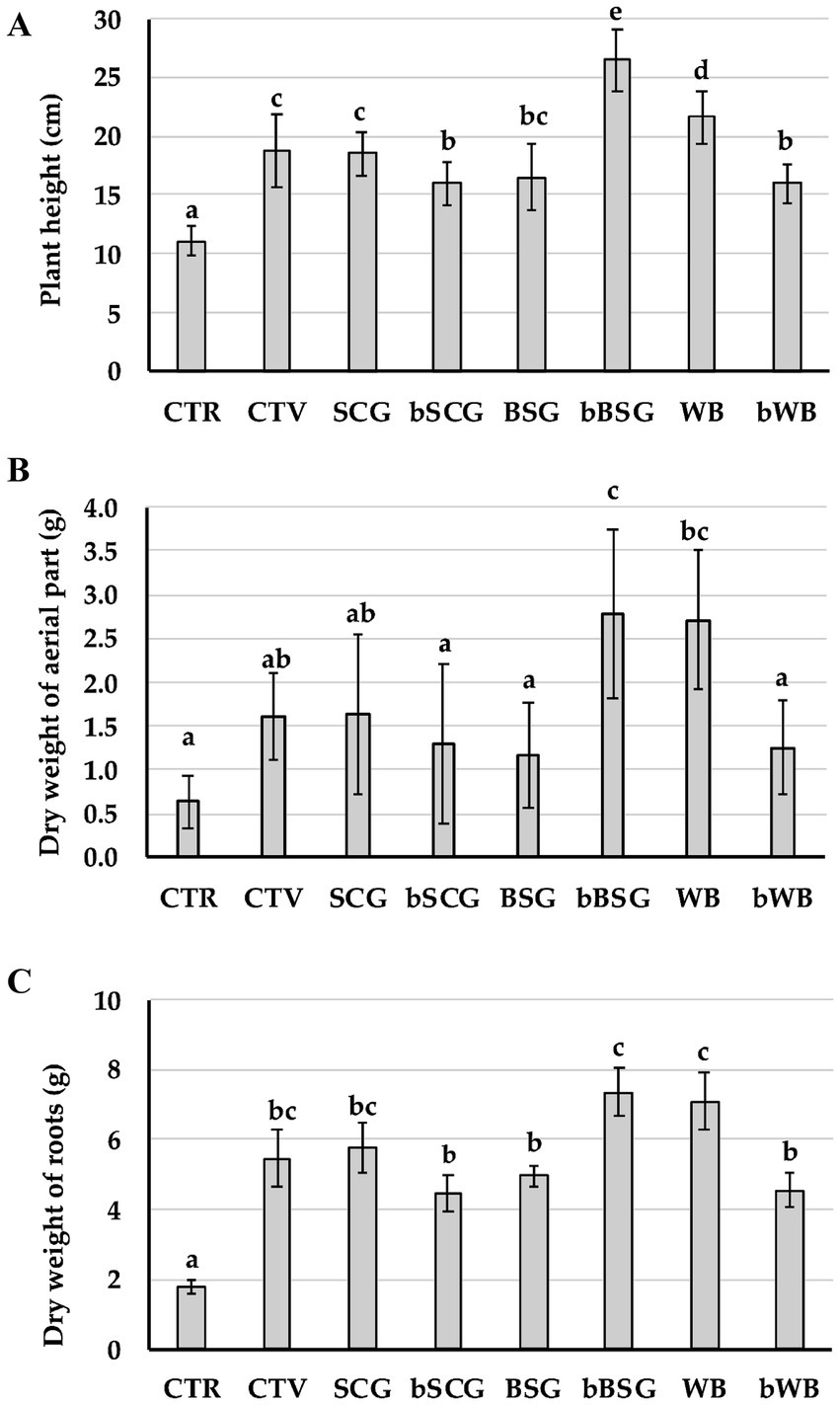
Figure 2. Biometric features of tomato plants at the end of the trial. (A) Plant height; (B) dry weight of aerial part; (C) dry weight of roots. Bars with different letters are significantly different (p ≤ 0.001) according to Dunn test. CTR-t: untreated infested soil; CTV-t: infested soil, treated with a synthetic nematicide; SCG-t: infested soil, treated with spent coffee grounds; bSCG-t: infested soil, treated with bioprocessed spent coffee grounds; BSG-t: infested soil, treated with brewer’s spent grain; bBSG-t: infested soil, treated with bioprocessed spent grain; WB-t: infested soil, treated with wasted bread; bWB-t: infested soil, treated with bioprocessed wasted bread.
All tested treatments significantly reduced gall formation on tomato roots and M. incognita multiplication (Figure 3) compared to the non-treated control, with no differences between the amended treatments and CTV-t. The lowest multiplication of nematodes occurred in soil amended with bBSG, with only 79 eggs and J2s g−1 roots and a 1.0 RGI, while the highest number of eggs and J2s per gram of root, 1,619, was recorded on tomato roots from soil added with SCG. All other treatments resulted in a similar suppressive effect on the RKNs, ranging from 379 to 474 eggs and J2s g−1 roots and 1.1–1.2 RGI values.
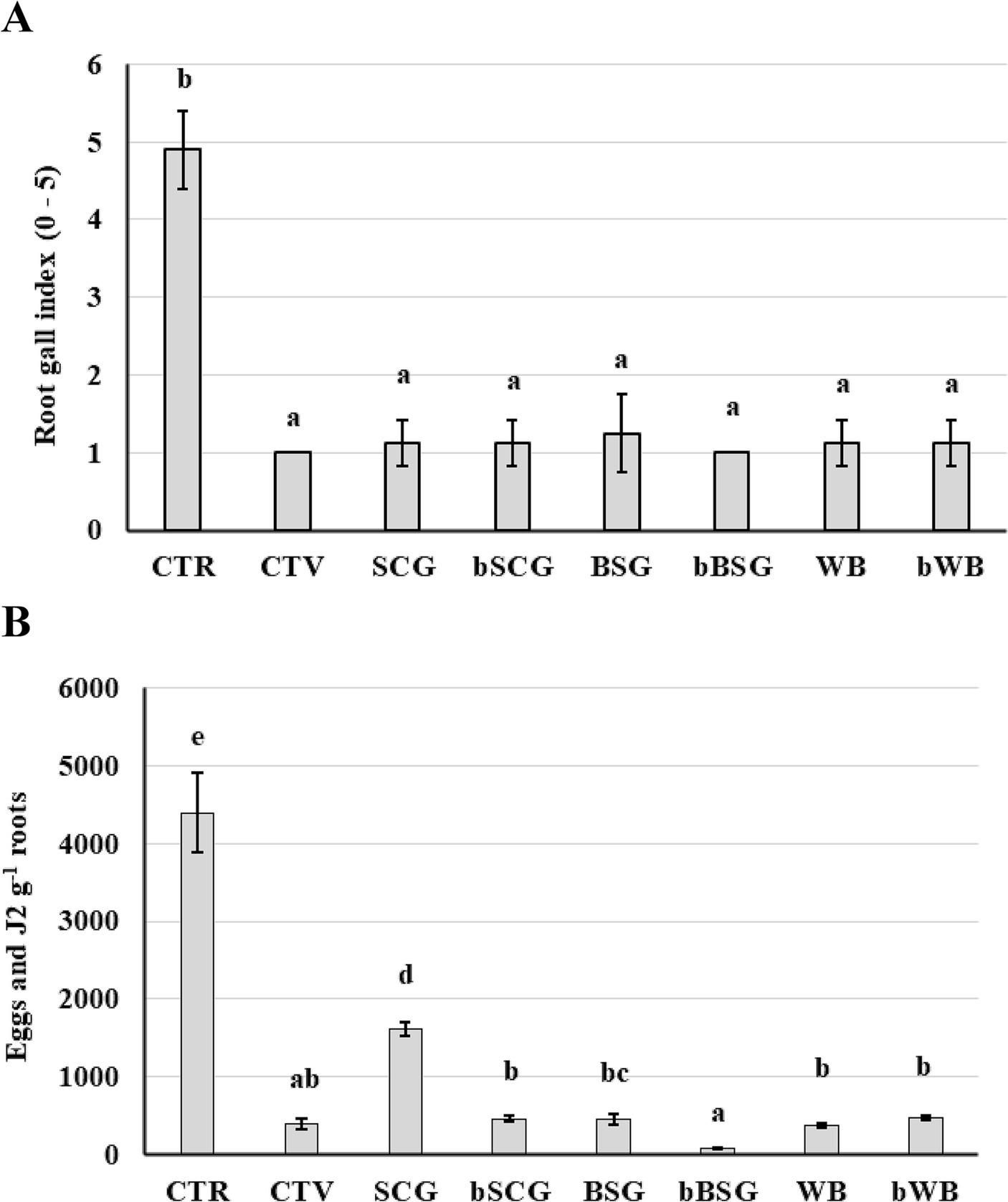
Figure 3. Effect of treatments on the infestation of the root-knot nematode Meloidogyne incognita on tomato cv. “Regina di Fasano.” (A) Root gall index; (B) Eggs and J2 g−1 roots. Bars with different letters are significantly different (p ≤ 0.001) according to Dunn test. CTR-t: untreated infested soil; CTV-t: infested soil, treated with a synthetic nematicide; SCG-t: infested soil, treated with spent coffee grounds; bSCG-t: infested soil, treated with bioprocessed spent coffee grounds; BSG-t: infested soil, treated with brewer’s spent grain; bBSG-t: infested soil, treated with bioprocessed spent grain; WB-t: infested soil, treated with wasted bread; bWB-t: infested soil, treated with bioprocessed wasted bread.
Data collected from soil physico-chemical features, plant growth and roots infestation were subjected to a principal component analysis as showed in Figure 4. The first and second factors explained 45.95 and 21.08% of the total variance, respectively. Factor 1 clearly separated the treatments amended with native and bioprocessed WB and BSG, whose soils were more acidic and characterized by high TN content and plant growth was more pronounced. Indeed, a negative and significant correlation was found between pH and plant height (r = −0.58), as well as pH and plant dry weight (r = −0.48). Similarly, TTA positively correlated with plant growth parameters (Figure 5). Not amended soils (CTR-t and CTV-t) grouped on the lower left side of the PCA graph (Figure 4) because of the lower OC and TN content compared to amended pots and, especially for CTR-t, higher M. incognita infestation. In fact, OC and TN negatively correlated with J2s count (r = −0.46 and −0.55, respectively, p < 0.05) and RGI (r = −0.52 and −0.65, respectively, p < 0.05).
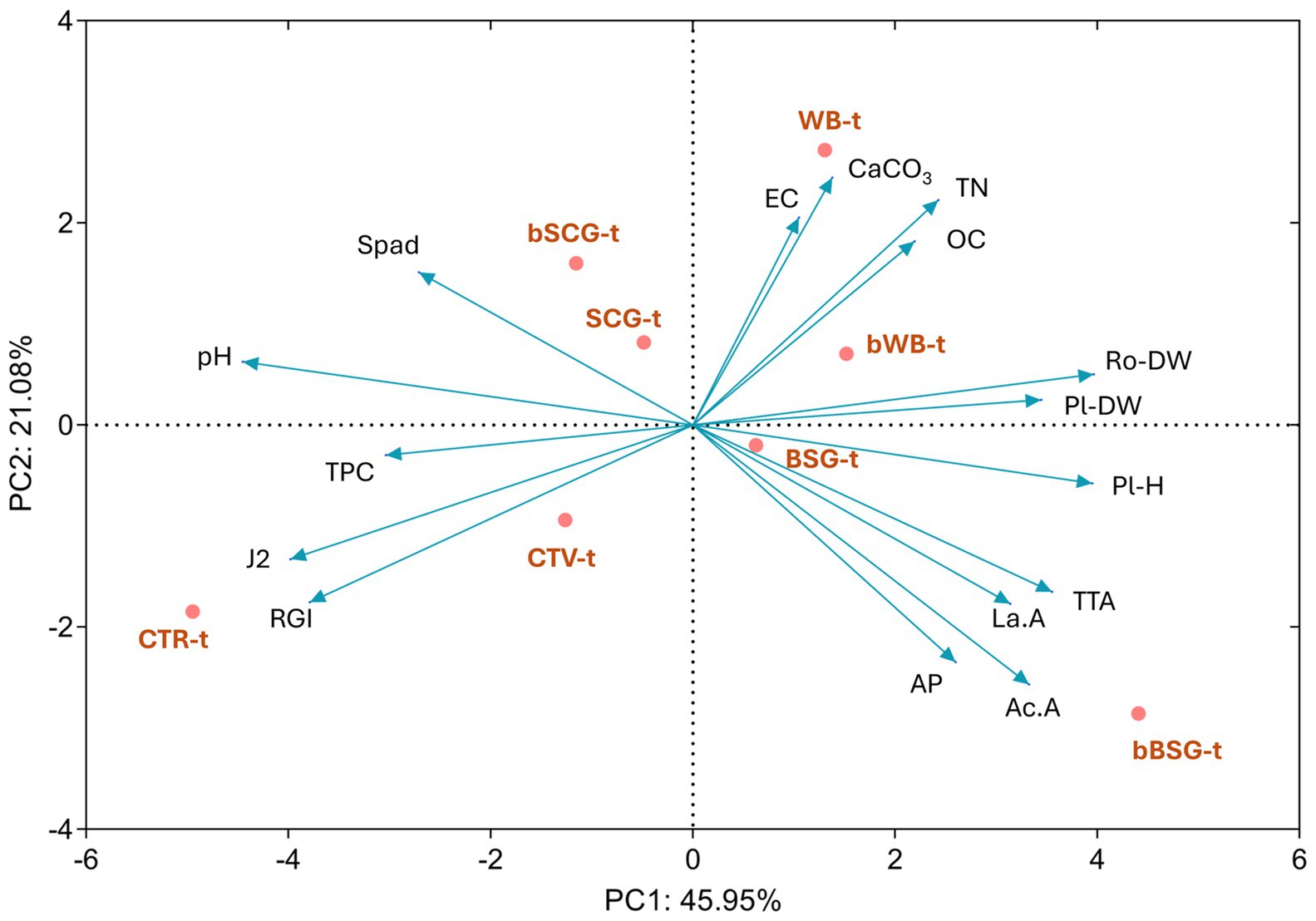
Figure 4. Principal component analysis (PCA) of soil physic-chemical features, plant growth and roots infestation. CTR-t: untreated infested soil; CTV-t: infested soil, treated with a synthetic nematicide; SCG-t: infested soil, treated with spent coffee grounds; bSCG-t: infested soil, treated with bioprocessed spent coffee grounds; BSG-t: infested soil, treated with brewer’s spent grain; bBSG-t: infested soil, treated with bioprocessed spent grain; WB-t: infested soil, treated with wasted bread; bWB-t: infested soil, treated with bioprocessed wasted bread. Soil features: EC, electrical conductivity; OC, organic carbon; TN, total nitrogen; AP, available phosphorous; CaCO3, total carbonates; La.A, lactic acid; Ac.A, acetic acid; TPC, total phenolic compounds; TTA, total titratable acidity. Roots features: RGI, root gall index; J2, juveniles; Ro-DW, roots dry weight. Plant features: Pl-DW, plant dry weight; Pl-H, plant height; Spad, chlorophyll spad units.
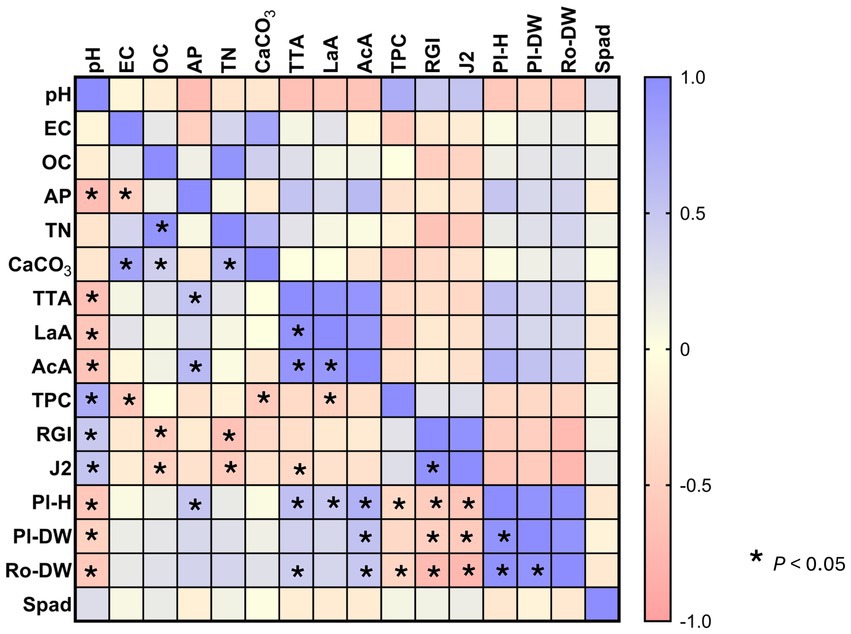
Figure 5. Pearson correlation within the parameters collected from soil, roots and plants characterization. Soil features: EC, electrical conductivity; OC, organic carbon; TN, total nitrogen; AP, available phosphorous; CaCO3, total carbonates; La.A, lactic acid; Ac.A, acetic acid; TPC, total phenolic compounds; TTA, total titratable acidity. Roots features: RGI, root gall index; J2, juveniles; Ro-DW, roots dry weight. Plant features: Pl-DW, plant dry weight; Pl-H, plant height; Spad, chlorophyll spad units. Asterisks indicate strong correlations with p < 0.05. Scale bar colors describe the type of correlation: 1 indicates a perfect positive correlation (liliac), and −1 indicates a perfect negative correlation (peachy).
4 Discussion
Among vegetables, tomato, the second most important fresh vegetable globally, is the most susceptible to diseases among which infestation of root-knot nematodes of the Meloidogyne genus is increasingly alarming (Gutierrez, 2018; Rawal, 2020). The infestation reduces the ability of crops to absorb water and nutrients, which can lead to stunted growth and lower productivity. RKNs management is of paramount importance due to its significant impact on crop health and yield, especially considering that with the global population growing at a steady rate, the demand for food production is higher than ever. Currently, the control of pathogenic nematodes is achieved through the application of chemical nematicides that act on specific biological processes (e.g., respiration, transmission of nerve impulses, steroid metabolism) (Sikder and Vestergård, 2020). However, the detrimental impact of nematicides on beneficial soil organisms and the emergence of nematode strains that have developed a high level of resistance to chemical nematicides, is forcing researchers to seek better and more effective strategies to control plant-parasitic nematodes in agriculture (Gamalero and Glick, 2020). For this reason, the application of biocontrol agents, botanicals essential oils and growing resistant cultivars is also being explored (Shahid et al., 2024; Subedi et al., 2020; Yaseen and Mukhtar, 2024).
Based on the above considerations, this study aimed at evaluating the suppressive effect on RKNs of food industry residues, when used as soil amendment in tomato cultivation. Specifically, spent coffee ground, wasted bread and brewers’ spent grain were used either native or after bioprocessing treatments. WB and BSG were subjected to an enzymatic treatment with amylase and xylanase, respectively, and fermentation with selected lactic acid bacteria strains as already reported in Cacace et al. (2022a, 2022b), whereas SCG was treated for 24 h with compost tea. Indeed, although it was reported that SCG can improve soil chemical and physical properties, even at low concentrations it can inhibit plant growth due to its ability to stimulate microbial growth in the soil, consequently causing a competition for soil nitrogen between soil microorganisms and plant roots, as well as to the presence of phytotoxic compounds like polyphenols (Pérez-Burillo et al., 2022). However, these compounds have chelating properties, generating a reserve of microelements (Co, Fe, K, Mg, Mn, and Zn) in soil far superior to that of commercial products which makes their complete elimination not recommended. Although results on the subject are contradictory, it appears that composting, vermicomposting, pyrolysis, or a combination of these techniques can mitigate these issues (Pérez-Burillo et al., 2022). For this reason, in this study, SCG was treated with compost tea, a liquid organic formulate obtained using the aqueous extraction of composted material, which along with macro- and micro-nutrients, is characterized by a microbial consortium (protists, fungi, oomycetes, yeasts, actinomycetes and bacteria), useful to plants, due to their suppressive and/or growth-promoting properties (Pilla et al., 2023).
Bioprocessing, besides generating metabolites which could promote plant growth or suppress RKNs, is critical to achieve a higher microbial stability, as it prevents the proliferation of bacteria and molds potentially spoiling the biomasses, still rich of water and nutrients. The significant impact of bioprocessing on biomasses shelf-life is an aspect particularly appealing in view of their potential large-scale application. In accordance with our previous studies (Cacace et al., 2022a, 2022b), as consequence of lactic acid bacteria metabolism and the enzymatic activity, bioprocessing of WB and BSG generated copious amount of lactic and acetic acids which determined lower pH and higher TTA in bWB and bBSG compared to the corresponding native biomasses. An increase in total phenolic compounds was also observed after BSG and SCG bioprocessing (Table 1). Indeed, while it was demonstrated that in bBSG the xylanase treatment and, to a lower extent, fermentation with L. plantarum are essential to liberate ferulic acid and its derivatives otherwise bound to the cell wall polysaccharides (Verni et al., 2020), it is possible that something similar happened in bSCG. A combination of the metabolic activity of the microbial consortium provided by CT, as well as phenolic compounds contained in CT (Pilla et al., 2023) are likely responsible for the higher phenolic content observed in bSCG compared to SCG.
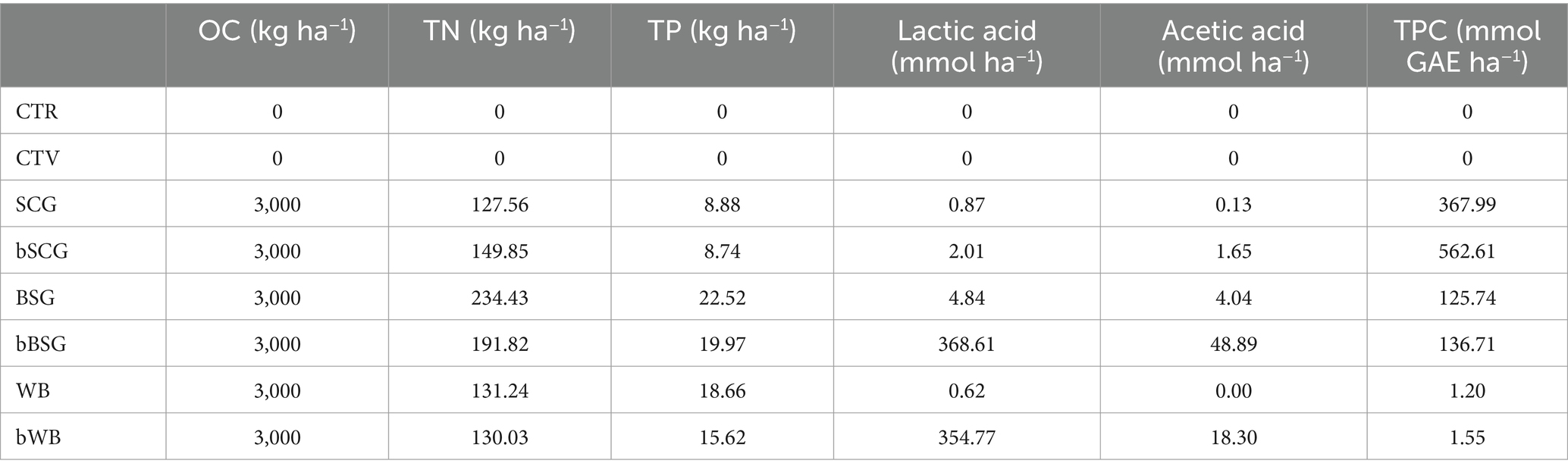
For the pot trial, biomasses were added to soil infested with M. incognita at an amount corresponding to 3,000 kg of OC ha−1. The choice of such an amount was motivated by good local agricultural practices according to which a maximum of 25 tons per hectare of a typical soil conditioners (e.g., compost, manure) should be applied. Since soil conditioners have an average moisture content of about 50% and an organic carbon content of 20–25% on dry weight, about 3,000 kg of carbon per hectare are applied. Clearly, the different nature of the biomasses determined a supplementation in nitrogen, phosphorous, organic acids and phenolic compounds strictly dependent on the food residue used and the bioprocessing employed (Table 3), which in turn, affected plant growth and nematode suppression.
The mechanisms by which organic soil amendments can suppress nematode are generally related to the release of nematotoxic organic compounds or, indirectly, to the development of soil microbial populations antagonistic or parasitic to phyto-parasitic nematodes (Peiris et al., 2020). SCG was found to contain high levels of potentially nematotoxic organic compounds such as fatty acids, phenolic acids and tannins (Campos-Vega et al., 2015). Indeed, caffeic and chlorogenic acids as well as tannins are known to be toxic for several nematode species (D’Addabbo et al., 2013; Zhang et al., 2012). The nematocidal effect of SGC was first proposed by an old study of Mian and Rodriguez-Kabana (1982), which documented the efficacy of soil amendments with SCG against M. arenaria on squash (Cucurbita pepo L.) at doses much higher than those applied in our experiment. Whereas, more recently, soil treatment with a SGC water extract was found to have a significant suppression of M. incognita infestation on potted tomato (Thligene et al., 2019). The same study also documented the lack of nematocidal activity of BSG aqueous extract, as it was not able to reduce the final population of M. incognita in comparison with untreated control. Apart from the latter, no other study has ever reported the suppressive effect of BSG or WB on phyto-parasitic nematodes, nor the possibility to subject them to a bioprocessing protocol before their application.
In this study, although all biomasses significantly suppressed the multiplication of Meloidogyne incognita and gall formation on tomato roots (up to 98 and 79%, respectively), the greatest suppressive effect was found in bBSG-t. The stronger nematocidal effect of bBSG compared to BSG may be due to the bioprocessing itself which determined the synthesis of organic acids and, to a lesser extent, the release of hydroxycinnamic acids into free form (Verni et al., 2020). As a matter of fact, the presence of organic acids, main metabolites of carbohydrate fermentation by lactic acid bacteria should not be overlooked. Indeed, the positive correlation between pH and RGI (r = 0.47, p < 0.05) and J2s (r = 0.52, p < 0.05) indicates that the acidification highly suppressed the infestation. Bioprocessed amendments, and to a lesser extent BSG and WB, contained discrete amount of lactic and acetic acids, comparable to those previously reported (Cacace et al., 2022a, 2022b). Indeed, the nematocidal effect of several organic acids against eight plant parasitic nematode species, including Meloidogyne spp. was studied by some authors (Abdel-Rahman et al., 2008; Seo and Kim, 2014). Abdel-Rahman et al. (2008) found that lactic acid has an 88% mortality rate thus being considered a moderately active compound among all those tested, whereas Seo and Kim (2014) specifically focused on the effect of lactic and acetic acids, used alone or in mixture, on root-knot M. incognita. They found that 100% nematode mortality can be achieved with 1% of acids, alone or combined, though at lower percentages of addition more effective results are obtained when the acids are mixed. Hence a combination of multiple factors, (i) the higher nitrogen contribution to soil, common to all biomasses (Table 2); (ii) lactic and acetic acids produced during fermentation, as well as (iii) the high phenolic compounds content further enhanced by the bioprocessing might be the reason behind the highest suppressive effect on M. incognita infestation of all amendments, but particularly bBSG.
Apart from a more effective control of RKNs infestation, amendments (especially bBSG) were responsible for the higher plant growth, comparable or higher than CTV-t. As previously hypothesized (Cacace et al., 2022a), and confirmed by the correlation analysis, the stimulation can be mostly ascribed to the organic acids produced during fermentation. Indeed, previous findings (Macias-Benitez et al., 2020) revealed that organic acids not only influence soil physicochemical properties, and contribute to nutrients solubilization (Sposito, 2016), but also induce modification in the soil microbiota, thereby promoting the growth of microorganisms involved in soil degradation and fertility (Macias-Benitez et al., 2020). In this study, a positive and significant correlation was found between plant height and lactic (r = 0.50) and acetic acid (r = 0.68). Moreover, acetic acid, which had the highest concentration (49 mmol ha−1, Table 3) in soils amended with bBSG also positively correlated with plant and roots dry weight thus explaining the best performances recorded for bBSG-t. Conversely, when wasted bread was used as amendment, despite the nematocidal effect was quite similar between WB-t and bWB-t, the effect of bioprocessing on plant growth was not as pronounced, confirming the results obtained in a previous study on escarole plants (Cacace et al., 2022b).
Since chlorosis, a condition by which leaves produce insufficient chlorophyll, is one of the possible symptoms of RKNs infestation (Rawal, 2020), Spad values were also monitored, yet on average no differences were observed among treatments. Indeed, no significant correlations were found between Spad values and any of the other parameter considered (Figure 5).
5 Conclusion
Ensuring crops health is essential to meet the growing demand for food and achieve food security; and the effective management of RKNs can help maximize agricultural output, thereby supporting efforts to sustainably feed the expanding population. All the biomasses investigated in this study provided an effective suppression of M. incognita infestation on tomato, though the strongest suppressive effect was achieved by using bSCG and bBSG, resulting in performances comparable to synthetic nematicides, the latter also determined the best growth effect on tomato plants. On the other hand, WB bioprocessing should be optimized to further promote plant growth. Moreover, the potential synergistic effect among different amendments, as well as their long-term effects on nematode populations, soil health and plant growth could be explored in further studies.
The findings of this study suggest that the food residues examined may play a role in the sustainable management of RKNs. Their use as soil amendments could also provide nutrients, contributing to soil fertility thus rendering additional fertilizers unnecessary. Provided that the preliminary standardization of the raw materials through bioprocessing is necessary to ensure consistent and predictable effects on nematode infestation and crop growth, it should also be noted that their use as organic amendments could provide a cheap and environmentally sustainable alternative for their disposal. Overall, the approach used in this study is unique in its kind. Indeed, to date, commercial formulations of plant growth promoting microorganisms and compost-based organic amendments, can be found in the market. However, to the best of our knowledge, there are no organic amendments, obtained fermenting food industry waste and by-products, integrating the benefits of organic biomass (e.g., supply of nutrients to the soil) with those derived from growth promoting microorganisms. The amendments developed in this study, not only provide nutrients to the soil as a common compost or waste biomass could, they have multiple effects, and the bioprocessing, causing a partial decomposition and mineralization of the biomasses, as well as stimulating plant growth and responses to diseases, plays a pivotal role. Hence, although spent coffee grounds were previously used as soil improvers and for their nematocidal effect, the bioprocessed biomasses developed in this study (especially bBSG), which showed more potential than the native once, are a promising solution to current challenges.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
CCa: Formal analysis, Writing – original draft. TD'A: Formal analysis, Methodology, Resources, Writing – original draft. MV: Data curation, Formal analysis, Writing – review & editing. CR: Conceptualization, Funding acquisition, Writing – review & editing. MS: Resources, Writing – original draft. GB: Conceptualization, Writing – original draft. PV: Formal analysis, Writing – original draft. FM: Data curation, Formal analysis, Writing – original draft. AT: Formal analysis, Writing – original draft. CCo: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was carried out within the Agritech National Research Center and received funding from the European Union Next-GenerationEU [PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)-MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4-D.D. 1032 17/06/2022, CN00000022]. This manuscript reflects only the authors’ views and opinions, neither the European Union nor the European Commission can be considered responsible for them.
Acknowledgments
Authors acknowledge Mr. Fabio Catalano, from IPSP-CNR, for his technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
AACC (2010). Approved methods of the American Association of Cereal Chemistry. 11th Edn. St. Paul, Minnesota, U.S.A.: AACC.
Abdel-Rahman, F. H., Clark, S., and Saleh, M. A. (2008). Natural organic compounds as alternative to methyl bromide for nematodes control. J. Environ. Health Part B 43, 680–685. doi: 10.1080/03601230802388751
Bonanomi, G., De Filippis, F., Zotti, M., Idbella, M., Cesarano, G., Al-Rowaily, S., et al. (2020). Repeated applications of organic amendments promote beneficial microbiota, improve soil fertility and increase crop yield. Appl. Soil Ecol. 156:103714. doi: 10.1016/j.apsoil.2020.103714
Bridge, J., and Page, S. L. J. (1980). Estimation of root-knot nematode infestation levels on roots using a rating chart. Int. J. Pest Manag. 26, 296–298. doi: 10.1080/09670878009414416
Cacace, C., Cocozza, C., Traversa, A., Coda, R., Rizzello, C. G., Pontonio, E., et al. (2022a). Potential of native and bioprocessed brewers' spent grains as organic soil amendments. Front. Sustain. Food Syst. 6:1010890. doi: 10.3389/fsufs.2022.1010890
Cacace, C., Rizzello, C. G., Brunetti, G., Verni, M., and Cocozza, C. (2022b). Reuse of wasted bread as soil amendment: bioprocessing, effects on alkaline soil and escarole (Cichorium endivia) production. Food Secur. 11:189. doi: 10.3390/foods11020189
Campos-Vega, R., Loarca-Piña, G., Vergara-Castañeda, H. A., and Oomah, B. D. (2015). Spent coffee grounds: a review on current research and future prospects. Trends Food Sci. Technol. 45, 24–36. doi: 10.1016/j.tifs.2015.04.012
Chetrariu, A., and Dabija, A. (2020). Brewer’s spent grains: possibilities of valorization, a review. Appl. Sci. 10:5619. doi: 10.3390/app10165619
Ciavatta, C., Antisari, L. V., and Sequi, P. (1989). Determination of organic carbon in soils and fertilizers. Commun. Soil Sci. Plant Anal. 20, 759–773. doi: 10.1080/00103628909368115
Coolen, W. A. (1979). “Methods for the extraction of Meloidogyne spp., and other nematodes from roots and soil” in Root-knot nematodes Meloidogyne species systematics, biology and control. eds. F. Lamberti and C. E. Taylor, vol. 1979 (London, UK: Academic Press), 317–329.
Corbett, B. P., Jia, L., Sayler, R. J., Arevalo-Soliz, L. M., and Goggin, F. (2011). The effects of root-knot nematode infection and mi-mediated nematode resistance in tomato on plant fitness. J. Nematol. 43:82.
D’Addabbo, T., Carbonara, T., Argentieri, M. P., Radicci, V., Leonetti, P., Villanova, L., et al. (2013). Nematicidal potential of Artemisia annua and its main metabolites. Eur. J. Plant Pathol. 137, 295–304. doi: 10.1007/s10658-013-0240-5
Gamalero, E., and Glick, B. R. (2020). The use of plant growth-promoting bacteria to prevent nematode damage to plants. Biology 9:381. doi: 10.3390/biology9110381
Garbowski, T., Bar-Michalczyk, D., Charazińska, S., Grabowska-Polanowska, B., Kowalczyk, A., and Lochyński, P. (2023). An overview of natural soil amendments in agriculture. Soil Till. Res. 225:105462. doi: 10.1016/j.still.2022.105462
Gutierrez, E. E. V. (2018). An overview of recent studies of tomato (Solanum lycopersicum spp) from a social, biochemical and genetic perspective on quality parameters. Basic Microbiol. 50, 211–217.
Hussey, R. S., and Barker, K. R. (1973). A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Dis. Rep. 57, 1025–1028.
Macias-Benitez, S., Garcia-Martinez, A. M., Caballero Jimenez, P., Gonzalez, J. M., Tejada Moral, M., and Parrado Rubio, J. (2020). Rhizospheric organic acids as biostimulants: monitoring feedbacks on soil microorganisms and biochemical properties. Front. Plant Sci. 11:633. doi: 10.3389/fpls.2020.00633
Mian, I. H., and Rodriguez-Kabana, R. (1982). Organic amendments with high tannin and phenolic contents for control of Meloidogyne arenaria in infested soil. Nematropica 2, 221–234.
Moosavi, M. R. (2022). “Potential of soil amendment with organic matters in controlling phytonematodes” in New and future developments in microbial biotechnology and bioengineering (Amsterdam, Netherlands: Elsevier), 315–344.
Oka, Y., and Yermiyahu, U. (2002). Suppressive effects of composts against the root-knot nematode Meloidogyne javanica on tomato. Nematology 4, 891–898. doi: 10.1163/156854102321122502
Olsen, S. R. (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate, vol. 939. Washington, DC, USA: US Department of Agriculture.
Peiris, P. U. S., Li, Y., Brown, P., and Xu, C. (2020). Efficacy of organic amendments to control Meloidogyne spp. in crops: a systematic review and meta-analysis. J. Soils Sediments 20, 1584–1598. doi: 10.1007/s11368-019-02498-x
Pérez-Burillo, S., Cervera-Mata, A., Fernández-Arteaga, A., Pastoriza, S., Rufián-Henares, J. Á., and Delgado, G. (2022). Why should we be concerned with the use of spent coffee grounds as an organic amendment of soils? A narrative review. Agronomy 12:2771. doi: 10.3390/agronomy12112771
Pilla, N., Tranchida-Lombardo, V., Gabrielli, P., Aguzzi, A., Caputo, M., Lucarini, M., et al. (2023). Effect of compost tea in horticulture. Horticulturae 9:984. doi: 10.3390/horticulturae9090984
Rawal, S. (2020). A review on root-knot nematode infestation and its management practices through different approaches in tomato. Trop. Agroecosyst. 1, 92–96. doi: 10.26480/taec.02.2020.92.96
Seo, Y., and Kim, Y. H. (2014). Control of Meloidogyne incognita using mixtures of organic acids. Plant Pathol. J. 30, 450–455. doi: 10.5423/PPJ.NT.07.2014.0062
Shahid, M., Gowen, S. R., Burhan, M., Niaz, M. Z., Anwar-ul-Haq, M., and Mehmood, K. (2024). Differential responses of Meloidogyne spp. to Pasteuria isolates over crop cycles. Plant Prot. 8, 257–267. doi: 10.33804/pp.008.02.5192
Sikder, M. M., and Vestergård, M. (2020). Impacts of root metabolites on soil nematodes. Front. Plant Sci. 10:1792. doi: 10.3389/fpls.2019.01792
Slinkard, K., and Singleton, V. L. (1977). Total phenol analysis: automation and comparison with manual methods. Am. J. Enol. Vitic. 28, 49–55. doi: 10.5344/ajev.1977.28.1.49
Soil Survey Staff (2014). Keys to soil taxonomy. 12th Edn. Washington, DC, USA: USDA-Natural Resources Conservation Service.
Sparks, D. L., Page, A. L., Helmke, P. A., Loeppert, R. H., Soltanpour, P. N., Tabatabai, M. A., et al. (1996). Methods of soil analysis. Part 3. Chemical methods, vol. 5. Madison: SSSA.
Subedi, S., Thapa, B., and Shrestha, J. (2020). Root-knot nematode (Meloidogyne incognita) and its management: a review. J. Agric. Nat. Res. 3, 21–31. doi: 10.3126/janr.v3i2.32298
Thligene, N., Mezzapesa, G. N., Mondelli, D., Trani, A., Veronico, P., Melillo, M. T., et al. (2019). Effect of coffee silver skin and brewers’ spent grain in the control of root-knot nematodes. Helminthologia 56, 30–41. doi: 10.2478/helm-2018-0038
Trinchera, L., Leita, P., and Sequi, P. (2006). Metodi di analisi per i fertilizzanti. Roma: Ministero delle Politiche Agricole Alimentari e Forestali.
Verni, M., Pontonio, E., Krona, A., Jacob, S., Pinto, D., Rinaldi, F., et al. (2020). Bioprocessing of brewers’ spent grain enhances its antioxidant activity: characterization of phenolic compounds and bioactive peptides. Front. Microbiol. 11:1831. doi: 10.3389/fmicb.2020.01831
Viaene, N., Hallmann, J., and Molendijk, L. (2021). “Methods for nematode extraction” in Techniques for work with plant and soil nematodes. eds. R. N. Perry, D. J. Hunt, and S. A. Subbotin (Wallingford, UK; Boston, MA, USA: CABI), 12–41.
Wang, X. (2022). Managing land carrying capacity: key to achieving sustainable production systems for food security. Land 11:484. doi: 10.3390/land11040484
Weiss, W., Vogelmeier, C., and Görg, A. (1993). Electrophoretic characterization of wheat grain allergens from different cultivars involved in bakers' asthma. Electrophoresis 14, 805–816. doi: 10.1002/elps.11501401126
Yaseen, I., and Mukhtar, T. (2024). Impact of sequential and concurrent inoculations of Meloidogyne incognita and Fusarium oxysporum f. sp. vasinfectum on the growth performance of diverse okra cultivars. Plant Prot. 8, 303–313. doi: 10.33804/pp.008.02.5186
Keywords: root-knot nematode, soil amendments, spent coffee ground, brewer’s spent grain, wasted bread, bioprocessing
Citation: Cacace C, D’Addabbo T, Verni M, Rizzello CG, Spagnuolo M, Brunetti G, Veronico P, De Mastro F, Traversa A and Cocozza C (2025) Short-term effect of native and bioprocessed food residues on the suppression of root-knot Meloidogyne incognita in tomato plants. Front. Sustain. Food Syst. 9:1477959. doi: 10.3389/fsufs.2025.1477959
Edited by:
Maria Pilar Bernal, Spanish National Research Council (CSIC), SpainReviewed by:
Tariq Mukhtar, Pir Mehr Ali Shah Arid Agriculture University, PakistanFatma Abdel Mohsen Mostafa, Mansoura University, Egypt
Copyright © 2025 Cacace, D’Addabbo, Verni, Rizzello, Spagnuolo, Brunetti, Veronico, De Mastro, Traversa and Cocozza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michela Verni, bWljaGVsYS52ZXJuaUB1bmlyb21hMS5pdA==
 Claudio Cacace
Claudio Cacace Trifone D’Addabbo
Trifone D’Addabbo Michela Verni
Michela Verni Carlo Giuseppe Rizzello
Carlo Giuseppe Rizzello Matteo Spagnuolo
Matteo Spagnuolo Gennaro Brunetti
Gennaro Brunetti Pasqua Veronico
Pasqua Veronico Francesco De Mastro
Francesco De Mastro Andreina Traversa
Andreina Traversa Claudio Cocozza
Claudio Cocozza