- 1Taizhou Academy of Agricultural Sciences, Taizhou, China
- 2State Key Laboratory of Vegetable Bio-breeding, Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing, China
Sweet cherry (Prunus avium L.) is one of the fruits that are widely acclaimed around the world. However, its fruits are prone to cracking from onset of color to full maturity, especially in cherry-producing regions where rain events are common near harvest. Cracked cherries have an unpleasant appearance, as well as susceptible to invasion by fungal pathogens, therefore dramatically depreciated, incurring considerable economic losses to growers, quite dampening their planting enthusiasm, subsequently restricting the advancement of sweet cherry industry. The incidence and severity of fruit cracking in sweet cherry are affected by genotypic, environmental, as well as agronomic factors. This review provides an overview of the causes, testing methods, and mitigation strategies related to fruit cracking in sweet cherry. Based on recent research advances, this review proposes the perspectives that developing crack resistant varieties is as a promising strategy to mitigate fruit cracking in sweet cherry.
1 Introduction
Sweet cherry (Punus avium L.) is gaining popularity worldwide owing to the vibrant color, unique flavor, as well as high nutritional value (e.g., rich in minerals, proteins, vitamins, carotenoids, and ascorbic acid) and highly praised by growers for its enormous economic return (Blanco et al., 2021; Gutierrez-Jara et al., 2021; Ricardo-Rodrigues et al., 2023; Wang et al., 2023; Bal, 2024; Sajid et al., 2024). Therefore, sweet cherry is now among the fruit trees cultivated globally with the greatest spread (Blanco et al., 2021; Magri et al., 2023). At present, the cultivation of sweet cherries has been an economically important planting industry in more than 70 countries (Yang et al., 2022), such as Turkey (Yılmaz et al., 2023), Chile (Time et al., 2021), the United States (Trouillas et al., 2011), Italy (Waqas et al., 2023), Uzbekistan (Shin and Ji, 2021), Iran (Soltani Nejad et al., 2024), Spain (Giné-Bordonaba et al., 2017), New Zealand (Marroni et al., 2023), Canada (Larrabee et al., 2021), Romania (Pereira et al., 2020), and China (Zhang et al., 2020). However, some factors are seriously hindering further development of sweet cherry industry, the predominant risk is fruit cracking from onset of color to maturity (Figure 1), particularly in the agricultural locations where frequent rain events occur close to harvest (Michailidis et al., 2020; Schumann and Knoche, 2020; Crump et al., 2022).
As shown in Figure 2, cracked sweet cherries are notably susceptible to the entry of fungus rot (e.g., Monilinia spp., Alternaria spp., and Botrytis spp.). These cracked fruits therefore have completely lost their commercial value for fresh markets because of the undesirable sensory attributes and microbiological contamination (Hauschildt et al., 2020; Brinkmann et al., 2022; Deltedesco et al., 2023; Waqas et al., 2023). Under unfavorable meteorological conditions like untimely rainfalls, more than 80% of output may be unmarketable due to fruit cracking, on occasion, the percentage of cracked fruits among some cracking-susceptible genotypes might reach approximately 90% of all mature fruits (Wójcik et al., 2013; Quero-García et al., 2021; Rombolà et al., 2023). However, a study revealed that, given the expense of local labor, harvesting becomes financially unfeasible when the cracked fruit accounts for over 25% of the total yield (Wójcik et al., 2013; Winkler et al., 2020; Crump et al., 2022). Obviously, fruit cracking leads to substantial losses for producers, eventually diminishing their incentive to planting sweet cherries (Goncalves et al., 2023). For instance, rain-induced fruit cracking poses a major deterrent to the prosperity and investment attraction of the sweet cherry industry in the United Kingdom (Wermund et al., 2005).
When categorized based on size, fruit cracks of sweet cherry can be divided into two types: (1) macrocracks, which can extend deep to the epidermal and hypodermal cell layers and can be identified through direct observation, and (2) microcracks, which are typically invisible to the naked eye (Correia et al., 2018; Goncalves et al., 2023). In general, fruit cracks occur in three different areas, including stylar scar region (i.e., calyx end), stem cavity region (i.e., stem end), and cheek region (i.e., fruit side) (Simon, 2006; Correia et al., 2018; Quero-García et al., 2021; Goncalves et al., 2023). Thereinto, the cracks in the stylar scar region and stem cavity region are frequently shallow cracks, which normally appear when cherries are not fully ripe, might be acceptable by a group of customers, provided there is no accompanying fungal infection, while the cracks in the cheek region tend to penetrate deeply into the fruit’s pulp, which are generally deemed unacceptable by consumers (Goncalves et al., 2023).
Systematic research into the phenomenon of sweet cherry cracking was first initiated more than ninety years ago (i.e., the early1930s) (Wermund et al., 2005; Michailidis et al., 2021). Over recent decades, this issue has captured the considerable attention from the scientific community. Up to now, global scientists have been tirelessly and diligently researching etiological factors of this physiological disorder, along with developing effective preventative countermeasures as well as breeding cracking-tolerant cultivars to improve the status quo. This paper presents an overview of advances in the understanding of cherry cracking. Furthermore, it offers viewpoints in light of current studies with the intention of directing further exploration and advancement in area of fruit cracking in sweet cherry.
2 Cracking causes
2.1 Cracking hypotheses
Currently, two predominant hypotheses have been proposed to elucidate the mechanism behind rain-induced fruit cracking: “critical turgor” hypothesis and “zipper” hypothesis (Schumann and Knoche, 2020).
The “critical turgor” hypothesis envisions fruits as thin-walled, pressurized vessels filled with a solution replete with osmotically active carbohydrates. This substantial negative osmotic potential of the solution drives water absorption from the environment. As the fruit absorbs water, its volume and surface area expand, thereby increasing the internal pressure, known as “turgor”. The hypothesis posits that cracking occurs when the fruit’s turgor pressure and/or the strain on its skin exceed a predetermined critical threshold (Measham et al., 2009).
The “zipper” hypothesis describes a multi-stage process for rain-induced fruit cracking. Initially, microcracks emerge as a result of cuticular strain, which arises from the downregulation of cutin and wax synthesis and deposition during the fruit’s early growth phase (Knoche et al., 2004; Peschel and Knoche, 2005; Alkio et al., 2012), coupled with subsequent exposure to moisture (Knoche and Peschel, 2006). This is followed by localized water penetration through these microcracks (Winkler et al., 2016). In the third phase, individual mesocarp cells rupture, releasing malic acid into the cell-wall-free spaces, damaging neighboring cells, including those of the epidermis (Winkler et al., 2015; Grimm et al., 2019). The fourth phase witnesses a complete loss of the already minimal turgor pressure, leading to cell wall expansion (Grimm and Knoche, 2015). Finally, in the concluding stage, the expanded cell walls reduce their stiffness, along with the fracture tension and adhesiveness between cells, culminating in the detachment of adjacent cells (Brüggenwirth and Knoche, 2017).
Besides the two hypotheses mentioned above, Koumanov (2015) proposed a novel perspective on fruit cracking, suggesting that it might be a consequence of the fruit’s skin contracting due to rapid cooling events triggered by rainfall or a significant temperature decrease, rather than the expansion of the fruit’s flesh.
2.2 Environmental factors
2.2.1 Rainfall
Fruit cracking in sweet cherry primarily occurs during and soon after rainfall events (Simon, 2006). Some previous studies have demonstrated that the cracking of sweet cherries is related to increased internal turgor pressure due to water uptake by the fruits, which may occur externally across the surface of the fruit (osmotic uptake), or internally through the vascular system of the fruit stem (Measham et al., 2009). However, Winkler et al. proposed that rain-induced cracking in sweet cherry is caused by surface wetness rather than water uptake, and both the percentage of wet surface area and wetness duration have a strong positive association with the frequency of cracking (Winkler et al., 2016; Winkler et al., 2020).
2.2.2 Non-precipitation related high atmospheric humidity
Fruit cracking in sweet cherry may occur even without substantial precipitation accumulation before harvest, possibly due to the cherry cultivation site’s proximity to rivers or other water areas, leading to a frequent morning dew and elevated relative humidity levels (Quero-García et al., 2021; Rombolà et al., 2023).
2.2.3 High temperature
Under high temperature conditions, the transpiration rate of sweet cherry tree significantly increases, which intensify the uptake of water by the trees. As an integral component of tree, the fruits absorb more water than they can effectively manage, leading to an increased internal pressure. This excess pressure can cause the fruit skin to crack. Therefore, high temperatures exacerbate the issue of fruit cracking in sweet cherries (Simon, 2006; Stone et al., 2022; Goncalves et al., 2023).
2.3 Genotypic factors
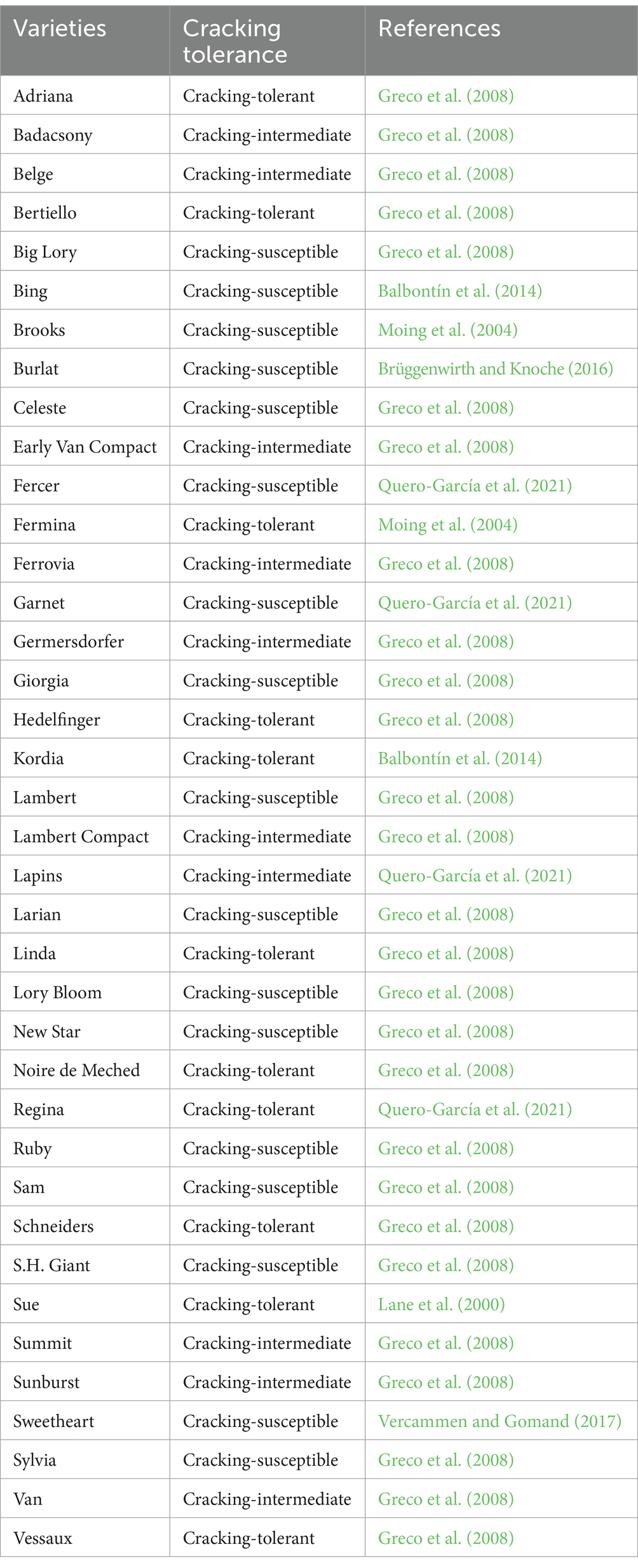
The varietal susceptibility to cracking in sweet cherry is varied, which may be related to the fruit shapes (Simon, 2006), flesh texture (Simon, 2006), skin thickness (Ruiz-Aracil et al., 2024), metabolite content (Michailidis et al., 2020), intra- and epicuticular wax content (Pereira et al., 2020), along with the rootstocks used (Sekse, 1995; Simon et al., 2004). Table 1 lists some sweet cherry varieties along with their cracking tolerance.
2.3.1 Fruit shapes
Varieties with a kidney-shaped or heart-shaped fruit have a deeper stem cavity, which may retain rain drops for an extended period, this prolonged contact facilitates greater water uptake through the fruit’s surface, thereby increasing the risk of fruit cracking in sweet cherry (Simon, 2006).
2.3.2 Flesh texture
Sweet cherry varieties with firm flesh are more likely to suffer from fruit cracking compared to those with softer flesh because firmer fruits are more prone to localized bursting of the skin (Simon, 2006; Pereira et al., 2020). Conversely, findings from another investigation revealed no substantial correlation between cracking incidence as visually observed on harvested fruit and their measured firmness (Measham et al., 2009).
2.3.3 Skin thickness
Sweet cherry varieties with thinner skins are typically more prone to cracking (Moing et al., 2004; Ruiz-Aracil et al., 2024).
2.3.4 Metabolite content
Variations in the levels of certain metabolites, including fucose and taxifolin, have been found to have a significant correlation with the cracking susceptibility across different sweet cherry cultivars (Michailidis et al., 2020).
2.3.5 Intra- and epicuticular wax content
When intra- and epicuticular wax content is diminished, the fruit’s skin becomes less robust and more susceptible to the damaging effects of water uptake and transpiration, leading to an increased risk of cracking, thus sweet cherries with lower intra- and epicuticular wax content are more prone to cracking (Pereira et al., 2020).
2.3.6 Rootstocks
Except for the own variety properties of scions, interestingly, the susceptibility to fruit cracking in sweet cherry can be modulated by the rootstocks (Sekse, 1995; Simon et al., 2004). Rootstocks have a significant impact on the tree form. In compact trees, the cherries are better shielded from damage caused by rain-induced cracking (Goncalves et al., 2023). In addition, cherry fruits grown on the “Colt” rootstock demonstrated a significant decrease in cracking under field conditions, regardless of whether they were protected by rain covers or not (Cline et al., 1995).
2.4 Fruit developmental stage
Sweet cherry exhibits a distinctive double-sigmoid growth pattern, characterized by three distinct phases. Initially, the fruit undergoes a phase of swift mesocarp cell division, indicative of a pronounced expansion rate. The second phase involves embryo development and endocarp hardening, with slow fruit growth. In the third phase, fruit growth restarts, the color of the fruit changes, and the final fruit size is reached. During the initial phase, the fruit’s expansion is complemented by substantial cuticle membrane production, thus minimizing skin tension. In stark contrast, during the third phase of fruit development, the amount of cuticle membrane per unit area decreases, resulting in the formation of microscopic cracks in the cuticle, increasing the fruit’s susceptibility to cracking (Peschel and Knoche, 2005; Balbontín et al., 2018).
2.5 Crop load
Sweet cherries with firm flesh are more susceptible to cracking compared to those with a softer texture (Simon, 2006). Previous study indicated that for a given variety, fruits from heavily cropping trees are less firm than fruits from lightly cropping trees (Simon et al., 2004). Consequently, sweet cherries from trees with high crop loads are less likely to experience cracking than those from trees with low crop loads (Simon, 2006), and similar results were also observed in other studies (Measham et al., 2014; Bound et al., 2017; Correia et al., 2018; Blanco et al., 2022).
3 Testing methods
Establishment of reliable methods for assessing the susceptibility of fruit cracking is crucial for the selection and breeding of cracking-resistant fruit varieties. There three standardized protocol for testing cracking susceptibility in vitro: (1) the Christensen method, (2) the Waterfall method, and (3) the intrinsic cracking susceptibility method (Correia et al., 2018; Michailidis et al., 2020).
Currently, the Christensen method is the primary protocol used to evaluate the cracking sensitivity of sweet cherry cultivars owing to its brevity, efficiency, and adaptability for automated processes. In detail, the Christensen method involves immersing 50 defect-free fruits in 2 L containers filled with distilled water at a temperature of 20 ± 1°C for 6 h. Any fruits that develop cracks are removed, counted, and the remaining uncracked fruits are then re-incubated. At intervals of 2, 4, and 6 h, the fruits are inspected for the presence of macroscopic cracks. The cracking index (CI) is calculated using the formula: CI = ((5a + 3b + c) ∗ 100)/250, where a, b, and c represent the number of cracked cherries after 2, 4, and 6 h of immersion, respectively (Pereira et al., 2020).
However, the Christensen method falls short in accurately simulating the actual field conditions where the entire fruit skin is not subjected to uniform water pressure. To address this limitation, the Waterfall method has been developed as an alternative or complementary approach. This method effectively simulates the water pressure on the fruit surface, as well as the absorption of water by the fruit skin during rainfall (Michailidis et al., 2020).
The intrinsic cracking susceptibility method involves the water uptake (in mg) at 50% fruit cracking (WU50), which is an indirect measurement of the extensibility of the fruit skin and inversely related to the cracking susceptibility according to: WU50 = R*T50, with R the mean rate of water uptake (mg h−1) and T50 (h) the time to 50% cracking (Correia et al., 2018).
In practice, employing the Christensen method in conjunction with the other two methods may provide a more accurate assessment of the vulnerability to fruit cracking in sweet cherry.
4 Mitigation strategies
For most cherry growers worldwide, fruit cracking is a formidable problem, in order to minimize its negative impacts to a great extent, various attempts have been made previously. Some preventative countermeasures will be introduced below.
4.1 Appropriate site selection
Researchers concur that selecting appropriate sites to establish orchards is paramount in mitigating rain-induced cracking. The ideal location of sweet cherry orchards should be with little or without rain incidence at or near harvest time (Simon, 2006).
4.2 Utilization of protective covers
Excessive rainfalls are identified as one of the principal causes of cherry cracking since it frequently coincides with the fruit-ripening season (Lang et al., 2016; Ruiz-Aracil et al., 2024). Climate change Forecasts indicate a potential rise in the frequency of heavy rainfall events, which could lead to a heightened incidence of cherry cracking (Correia et al., 2018; Ruiz-Aracil et al., 2023). As an effective remedy against rain-induced cracking, protective covers (e.g., retractable roofs, high tunnels, rain-shelters, and net covers) are widely employed for cherry cultivation, for instance, the implementation of net covers has significantly reduced the fruit cracking index by a remarkable 40% (Lang, 2009; Thomidis and Exadaktylou, 2013; Lang et al., 2016; Blanco et al., 2021; Schumann et al., 2022; Goncalves et al., 2023). A protective cover for sweet cherry cultivation in Taizhou, Zhejiang Province, located in the southeast region of China, is depicted in Figure 3. Despite significantly decreasing the occurrence and intensity of fruit cracking, production capital expenditures of sweet cherry are also remarkably raised owing to installation of rain covering equipment (Correia et al., 2018; Schumann et al., 2022). Besides higher implementation costs, elevated ambient temperature and air humidity in the relatively closed rain covering systems may contribute to damage on both leaves and fruits, thereby increasing the risk of diseases and having negative effects on color development in sweet cherries (Simon, 2006). In addition, it is crucial to initiate using rain cover protection approximately three weeks before harvest, as this coincides with the period when sweet cherries begin to change color and become increasingly vulnerable to damage from rain (Cline et al., 1995; Simon, 2006).

Figure 3. A protective cover for sweet cherry cultivation in Taizhou, Zhejiang Province, the southeast region of China.
4.3 Rainwater removal by air blast sprayer or helicopters
Additionally, the implementation of techniques such as utilizing the crosswind from an orchard air-blast sprayer or the downdraft generated by helicopters to promptly disperse rainwater after rainfall has proven effective in preventing fruit cracking. These methods efficiently reduce the moisture on the fruit surface, mitigating the risk of damage due to water penetration (Correia et al., 2018). Air-blast sprayer crosswinds have been successfully employed to effectively clear rainwater from the upper canopy regions in both Y-trellised and vertically structured orchards (Zhou et al., 2016). Moreover, an innovative in-field sensing system has been developed that meticulously monitors the wetness within the canopy and the micro-climate conditions for canopy structures. This advanced system is designed to assist growers in making informed decisions regarding the timing and extent of rainwater removal from cherry canopies, preventing cracking damage (Zhou et al., 2017).
4.4 Irrigation management
A recent study revealed that deficit irrigation at 70% crop evapotranspiration markedly decreased cracking ratio of sweet cherries from 27.11 to 8.33% (Blanco et al., 2022).
4.5 Planting cultivars with different ripening times
Planting cultivars with different ripening times presents a strategic approach to circumvent the periods with high precipitation levels near harvest time, significantly reducing the incidence of fruit cracking in sweet cherry (Simon, 2006; Pereira et al., 2020; Goncalves et al., 2023; Ruiz-Aracil et al., 2024).
4.6 Exogenous compound sprays
4.6.1 Calcium salts
During rainfall, the most commonly adopted approach to achieve cracking reduction in cherry orchards without rain covers is the application of calcium salts (e.g., calcium chloride, calcium nitrate, and calcium hydroxide) solutions (Wermund et al., 2005; Simon, 2006; Correia et al., 2018; Rombolà et al., 2023). The swelling of cell walls in the epidermis of sweet cherries reduces the adhesiveness between cells, making them more prone to cracking (Schumann et al., 2019). A recent study elucidated that calcium treatments decrease cherry cracking susceptibility by decreasing swelling (Schumann et al., 2022). However, calcium treatments may be ineffective occasionally, some studies revealed that the effectiveness of this initiative is influenced by a multitude of factors, including air temperature, precipitation levels during the maturation phase of fruits, the timing and method of calcium application, the chemical characteristics of the calcium-containing substance, variety, and the rootstock used (Wójcik et al., 2013; Kafle et al., 2016; Winkler and Knoche, 2019, 2021; Matteo et al., 2022).
To determine the optimal re-application rates and mitigate fruit cracking in sweet cherries, it’s essential to evaluate the washout rates of calcium-based mineral sprays. A study was conducted on “Selah”, “Skeena”, and “Rainer” sweet cherry cultivars under field conditions, measuring the washout rates involved applying 0.5–1% solutions of calcium hydroxide and calcium nitrate to both the fruit surface and leaf samples. Simulating varying rainfall intensities of 2.5, 5, and 10 mm. The results showed that these sprays notably decreased the incidence of cracking. Specifically, at a 5 mm rainfall level, there was a 50% washout rate, indicating the need for reapplication before the subsequent rainfall event to maintain the effectiveness of calcium treatments (Kafle et al., 2016).
Besides, while reducing fruit cracking in sweet cherry, application of calcium salts may result in a reduction of fruit size and soluble solids concentration, simultaneously left unsightly residue on the fruit surface (Wermund et al., 2005; Wójcik et al., 2013; Measham et al., 2017; Rombolà et al., 2023).
4.6.2 Sodium silicate
Improving the cell wall characteristics of the epidermal layer, such as its extensibility and stability, as well as maintaining the integrity of fruit cuticles through agricultural practices, could significantly result in the mitigation of fruit cracking (Rombolà et al., 2023). Silicon, recognized as a beneficial element, exerts its positive influence primarily through its substantial accumulation in plant tissues. This accumulation bolsters the tissues’ strength and rigidity, thereby enhancing their overall structural integrity (Ma and Yamaji, 2006). Furthermore, silicon contributes to the formation of a protective barrier on fruit surfaces, which effectively impedes the penetration of water, thus safeguarding the fruits from potential cracking damage (Rombolà et al., 2023). A more recent study reported that when cherries are exposed to favorable conditions for fruit cracking, application of 2.3 g/L sodium silicate three times a week from fruit onset of color to approximately one week before harvest, can efficiently decrease the proportion of cracked fruits to a comparable or greater degree than applying with 5.0 g/L calcium chloride (Rombolà et al., 2023).
4.6.3 Abscisic acid
The suppression of cutin and wax synthesis can lead to the formation of microcracks on the surface of sweet cherry fruits. These initial microcracks have the potential to escalate into more pronounced macrocracks as the fruit matures (Knoche et al., 2004; Peschel and Knoche, 2005; Alkio et al., 2012). Previous reports suggest that abscisic acid stimulates the expression of genes involved in the biosynthesis and transportation of cutin and waxes (Yeats and Rose, 2013; Martin et al., 2017). In practice, exogenous application of abscisic acid at a concentration of 0.1 mM through foliar spray has been demonstrated to significantly decrease the occurrence of cracking in “Bing” cherry fruits (Balbontín et al., 2018). Foliar application of abscisic acid at a concentration of 10 μM, along with calcium chloride at a rate of 5 g kg−1 has been shown to significantly mitigate the incidence of fruit cracking in both “Skeena” and “Sweetheart” cherry cultivars (Correia et al., 2020a,b).
4.6.4 Gibberellic acid
In some investigations, exogenous application of gibberellic acid has been proven to markedly reduce the incidence of cracking in some cherry cultivars (Usenik et al., 2005; Suran et al., 2016). However, other studies have indicated that the foliar application of gibberellic acid may have no impact (Horvitz et al., 2003), or in some cases, it could even exacerbate the issue of fruit cracking in sweet cherry (Cline and Trought, 2007; Correia et al., 2020a,b).
4.6.5 Methyl jasmonate
Foliar sprays of 0.4 mM methyl jasmonate has shown to notably lower the frequency of cracking in “Bing” cherry fruits (Balbontín et al., 2018). Furthermore, preharvest foliar application of 0.5 mM methyl jasmonate, at a rate of 3 L per tree, along with the inclusion of 1 mL L−1 of Tween 20 as a surfactant, has shown to significantly reduce the incidence of cracking in various cherry varieties, including “Prime Giant”, “Early Lory”, “Staccato”, and “Sweetheart” (Ruiz-Aracil et al., 2023).
4.6.6 Putrescine
Foliar applications of putrescine (3 L per tree) at concentrations of 1 mM, with the addition of 1 mL L−1 of Tween 20 as a surfactant, can effectively alleviate the prevalence of cracking during ripening in both “Prime Giant” and “Sweetheart” sweet cherry cultivars (Ruiz-Aracil et al., 2024).
4.6.7 Glycine betaine
A recent study has shown that the application of glycine betaine can successfully mitigate cherry cracking. By foliar application of glycine betaine at a concentration of 1 mL L−1, in conjunction with calcium chloride at a rate of 5 g kg−1, the incidence of cracking in “Sweetheart” cherry cultivars was significantly reduced when compared to the untreated control group (Correia et al., 2020a,b).
4.6.8 Seaweed extract
Preharvest application of extract derived from seaweed, such as Ascophyllum nodosum, has been shown to effectively reduce the cracking index in both “Skeena” and “Sweetheart” cherry varieties (Correia et al., 2015).
4.6.9 Other spray with complex formulations
Application of sprays based on calcium hydroxide and silicic acid, enriched with boron, iron, and zinc, to “Sweetheart”, “Sumtare”, and “Grace Star” sweet cherry cultivars after fruit set and prior to stone hardening, resulted in a 50% reduction in the fruit cracking index (Correia et al., 2018).
4.6.10 Edible coatings
Edible coatings offer a potent solution to mitigate rain-induced cherry cracking (Jung et al., 2016; Yang et al., 2022; Chen et al., 2024). Edible coatings must meet several stringent requirements, including: (1) superior wetting capabilities to ensure complete adhesion to the surface of cherries; (2) excellent surface hydrophobicity to enable water to roll off easily; (3) robust barrier properties to shield the fruit from rainwater; and (4) the absence of any inhibitory or toxic effects that may impede fruit development (Chen et al., 2024).
A previous study reported a notable decrease in cherry cracking by application of cellulose nanofiber based hydrophobic coatings, which contains 0.5% cellulose nanofiber (w/w), 0.5% potassium sorbate (w/w), 0.1% glycerol (w/w), along with surfactant mixture (1:1 ratio of Tween 80 and Span 80) at 0.1 or 0.2% (w/w), and did not have detrimental effect on fruit growth and quality (Jung et al., 2016). Gutierrez-Jara et al. unveiled the development of an innovative electro-sprayed nanoemulsion film, formulated with a sodium alginate and ethanol solution, which significantly reduced the cracking index of sweet cherries from 60 to 12% in laboratory settings (Gutierrez-Jara et al., 2021). Yang et al. prepared a novel edible coating, which includes cold-water fish gelatin (CFG), cellulose nanocrystals (CNC), chitosan hydrochloride-sodium alginate nanoparticles (CA), is further enhanced with magnetic field (MF) treatment, resulting in the CFG–CNC–CA (MF) coating. This advanced coating has demonstrated significant efficacy in reducing the cracking of cherries. When applied, the total cracking index was lowered from 43.3 to 11.8%, and the overall cracking ratio was similarly reduced from 34.8 to 11.1%, in comparison to the untreated control group (Yang et al., 2022). Recently, Chen et al. developed an innovative edible coating, which is composed of chitosan hydrochloride (CHC), phosphorylated zein nanoparticles (PZNP), along with cellulose nanocrystals (CNC), and field trials revealed that applying this coating leads to a significant reduction in cherry cracking, decreasing the cracking ratio from 34.6 to 15.8% compared to the control group (Chen et al., 2024).
4.7 Breeding novel cracking-tolerant cultivars
Despite various agronomic techniques being suggested, the most environmentally friendly solution to mitigate fruit cracking in sweet cherry is still to breed cracking-tolerant cultivars (Quero-García et al., 2021), because the vulnerability to cracking in sweet cherries is highly cultivar-dependent (Ruiz-Aracil et al., 2024). As a highly complicated physiological disorder, fruit cracking is not only regulated by a variety of environmental conditions, but also genetically controlled by multiple genes (Balbontín et al., 2014; Michailidis et al., 2021; Quero-García et al., 2021).
The identification of genes related to cracking resistance of sweet cherry is a pivotal goal for the majority of breeding programs (Balbontín et al., 2013). In the past several years, scientists have discovered that cracking phenotypes of sweet cherry are strongly linked to genes encoding enzymes associated with pectin metabolism, including, endotransglycosylase (XET), polygalacturonase (PG), and β-galactosidase (β-Gal), as well as the genes encoding expansions (PaEXP1) (Balbontín et al., 2013; Balbontín et al., 2014; Giné-Bordonaba et al., 2017; Breia et al., 2020; Michailidis et al., 2021). In addition, some genes, PaATT1, PaWINA, PaWINB, PaLipase, PaLTG1, PaLCR, PaGPAT4/8, PaLACS2, PaLACS1, PaCER1, wax synthase (PaWS), and 3-ketoacyl-CoA synthase (PaKCS6), have been recognized for their roles in biosynthesis of cuticular wax previously and are potentially linked to the development of fruit cracking in sweet cherry (Alkio et al., 2012; Alkio et al., 2014; Balbontín et al., 2014; Correia et al., 2018). Furthermore, a recent study involved in integrated profiling of metabolomics and transcriptomics of two cultivars with varied cracking tolerance has revealed genotype- and tissue-specific metabolic pathways, including the tricarboxylic acid cycle, cell enlargement, ethanol biosynthesis, as well as plant defense, are likely to contribute to the rain-induced cherry cracking, which might pave the way for the exploration of novel candidate genes that may confer tolerance to fruit cracking in sweet cherry (Michailidis et al., 2021).
5 Research perspectives and conclusion
Fruit cracking in sweet cherry is a multi-factorial event, to date, while some studies have explored, the corresponding mechanisms are not completely disentangled, specifically, genetic studies are scarce. Given the present trend, future investigations ought to concentrate on deciphering the genetic mechanisms, including: (1) further identification of cracking-related genes; (2) searching for genetic pathways that manipulate the expression levels of enzymes affecting fruit cracking during the maturation process of sweet cherry; (3) analyzing the metabolomes of sweet cherry cultivars at various developmental stages and determining how metabolic alterations relate to changes in the genome and proteome. All above-mentioned endeavors may provide new insights for breeding at the molecular level and are crucial for effectively modifying cracking characteristic in breeding, thereby accelerating the improvement of cracking resistance in sweet cherry cultivars.
More than 600 sweet cherry varieties are cultivated worldwide, according to statistics (Yang et al., 2022). While no variety was completely resistant to cracking, several varieties exhibit a notable level of resistance, for example, past findings in combination with breeders’ experience indicated that some sweet cherry cultivars—“Cristalina”, “Fermina”, “Hedelfingen”, “Kordia”, “Regina”, “Summersun”, and “Sue”—are relatively resistant to cracking (Lane et al., 2000; Moing et al., 2004; Wermund et al., 2005; Balbontín et al., 2014; Giné-Bordonaba et al., 2017; Correia et al., 2018; Michailidis et al., 2021; Quero-García et al., 2021). Using these valuable germplasm resources is crucial for the future of breeding sweet cherries with tolerance to cracking.
In sum, there are numerous conundrums that need to be explored and developed further related to fruit cracking in sweet cherry, effectively tackling these challenges requires collaboration between agricultural experts, coupled with the full utilization of existing plant resources.
Author contributions
JX: Conceptualization, Data curation, Supervision, Visualization, Writing – original draft, Writing – review & editing. LC: Data curation, Writing – review & editing. JD: Data curation, Writing – review & editing. LJ: Data curation, Writing – review & editing. LH: Data curation, Funding acquisition, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by the Science and Technology Plan Project of Taizhou (22nya14; 23nya10), and 2024–2026 Fruit Industry Technology Project of Zhejiang Province (2024QYGP08).
Acknowledgments
The authors would like to thank Lin Chai, Heng Wang, and Jiaqi Zou for their assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alkio, M., Jonas, U., Declercq, M., Van Nocker, S., and Knoche, M. (2014). Transcriptional dynamics of the developing sweet cherry (Prunus avium L.) fruit: sequencing, annotation and expression profiling of exocarp-associated genes. Hortic. Res. 1:11. doi: 10.1038/hortres.2014.11
Alkio, M., Jonas, U., Sprink, T., van Nocker, S., and Knoche, M. (2012). Identification of putative candidate genes involved in cuticle formation in Prunus avium (sweet cherry) fruit. Ann. Bot. 110, 101–112. doi: 10.1093/aob/mcs087
Bal, E. (2024). Impact of chitosan-melatonin composite coating on postharvest quality of sweet cherry. Appl. Fruit Sci. 66, 763–770. doi: 10.1007/s10341-023-00994-6
Balbontín, C., Ayala, H., Bastías, R. M., Tapia, G., Ellena, M., Torres, C., et al. (2013). Cracking in sweet cherries: a comprehensive review from a physiological, molecular, and genomic perspective. Chil. J. Agric. Res. 73, 66–72. doi: 10.4067/S0718-58392013000100010
Balbontín, C., Ayala, H., Rubilar, J., Cote, J., and Figueroa, C. R. (2014). Transcriptional analysis of cell wall and cuticle related genes during fruit development of two sweet cherry cultivars with contrasting levels of cracking tolerance. Chil. J. Agric. Res. 74, 162–169. doi: 10.4067/S0718-58392014000200006
Balbontín, C., Gutiérrez, C., Wolff, M., and Figueroa, C. R. (2018). Effect of abscisic acid and methyl jasmonate preharvest applications on fruit quality and cracking tolerance of sweet cherry. Chil. J. Agric. Res. 78, 438–446. doi: 10.4067/S0718-58392018000300438
Blanco, V., Blaya-Ros, P. J., Torres-Sánchez, R., and Domingo, R. (2022). Irrigation and crop load management lessen rain-induced cherry cracking. Plan. Theory 11:3249. doi: 10.3390/plants11233249
Blanco, V., Zoffoli, J. P., and Ayala, M. (2021). Influence of high tunnel microclimate on fruit quality and calcium concentration in ‘Santina’ sweet cherries in a Mediterranean climate. Agronomy 11:1186. doi: 10.3390/agronomy11061186
Bound, S. A., Close, D. C., Measham, P. F., and Whiting, M. D. (2017). Regulating crop load of ‘sweetheart’ and ‘Van’ sweet cherry for optimal quality and reduced risk of cracking. Acta Hortic. 16, 91–96. doi: 10.17660/ActaHortic.2017.1161.16
Breia, R., Mósca, A. F., Conde, A., Correia, S., Conde, C., Noronha, H., et al. (2020). Sweet cherry (Prunus avium L.) PaPIP1;4 is a functional aquaporin upregulated by pre-harvest calcium treatments that prevent cracking. Int. J. Mol. Sci. 21:3017. doi: 10.3390/ijms21083017
Brinkmann, T., Kuhnke, F., Grimm, E., and Knoche, M. (2022). Sweet cherry flesh cells burst in non-random clusters along minor veins. Planta 255:100. doi: 10.1007/s00425-022-03882-7
Brüggenwirth, M., and Knoche, M. (2016). Mechanical properties of skins of sweet cherry fruit of differing susceptibilities to cracking. J. Am. Soc. Hortic. Sci. 141, 162–168. doi: 10.21273/JASHS.141.2.162
Brüggenwirth, M., and Knoche, M. (2017). Cell wall swelling, fracture mode, and the mechanical properties of cherry fruit skins are closely related. Planta 245, 765–777. doi: 10.1007/s00425-016-2639-7
Chen, C., Zhang, S., Cheng, X., Ren, Y., Qian, Y., Zhang, C., et al. (2024). Reducing cherry rain-cracking: enhanced wetting and barrier properties of chitosan hydrochloride-based coating with dual nanoparticles. Int. J. Biol. Macromol. 268:131660. doi: 10.1016/j.ijbiomac.2024.131660
Cline, J. A., Meland, M., Sekse, L., and Webster, A. D. (1995). Rain cracking of sweet cherries: II. Influence of rain covers and rootstocks on cracking and fruit quality. Acta Agric. Scand. B. Soil Plant Sci. 45, 224–230. doi: 10.1080/09064719509413108
Cline, J. A., and Trought, M. (2007). Effect of gibberellic acid on fruit cracking and quality of Bing and Sam sweet cherries. Can. J. Plant Sci. 87, 545–550. doi: 10.4141/P06-132
Correia, S., Oliveira, I., Queirós, F., Ribeiro, C., Ferreira, L., Luzio, A., et al. (2015). Preharvest application of seaweed based biostimulant reduced cherry (Prunus avium L.) cracking. Procedia Environ. Sci. 29, 251–252. doi: 10.1016/j.proenv.2015.07.187
Correia, S., Queiros, F., Ferreira, H., Morais, M. C., Afonso, S., Silva, A. P., et al. (2020a). Foliar application of calcium and growth regulators modulate sweet cherry (Prunus avium L.) tree performance. Plan. Theory 9:410. doi: 10.3390/plants9040410
Correia, S., Santos, M., Glinska, S., Gapinska, M., Matos, M., Carnide, V., et al. (2020b). Effects of exogenous compound sprays on cherry cracking: skin properties and gene expression. J. Sci. Food Agric. 100, 2911–2921. doi: 10.1002/jsfa.10318
Correia, S., Schouten, R., Silva, A. P., and Gonçalves, B. (2018). Sweet cherry fruit cracking mechanisms and prevention strategies: a review. Sci. Hortic. 240, 369–377. doi: 10.1016/j.scienta.2018.06.042
Crump, W. W., Peace, C., Zhang, Z., and McCord, P. (2022). Detection of breeding-relevant fruit cracking and fruit firmness quantitative trait loci in sweet cherry via pedigree-based and genome-wide association approaches. Front. Plant Sci. 13:823250. doi: 10.3389/fpls.2022.823250
Deltedesco, E., Oettl, S., and Spitaler, U. (2023). First report of brown rot caused by Monilinia polystroma on sweet cherry and almond in Italy. Plant Dis. 107:2252. doi: 10.1094/PDIS-10-22-2482-PDN
Giné-Bordonaba, J., Echeverria, G., Ubach, D., Aguiló-Aguayo, I., López, M. L., and Larrigaudière, C. (2017). Biochemical and physiological changes during fruit development and ripening of two sweet cherry varieties with different levels of cracking tolerance. Plant Physiol. Biochem. 111, 216–225. doi: 10.1016/j.plaphy.2016.12.002
Goncalves, B., Silva, V., Bacelar, E., Guedes, F., Ribeiro, C., Silva, A. P., et al. (2023). Orchard net covers improve resistance to cherry cracking disorder. Food Secur. 12:543. doi: 10.3390/foods12030543
Greco, P., Palasciano, M., Mariani, R., Pacifico, A., and Godini, A. (2008). Susceptibility to cracking of thirty sweet cherry cultivars. Acta Hortic. 795, 379–382. doi: 10.17660/ActaHortic.2008.795.56
Grimm, E., Hahn, J., Pflugfelder, D., Schmidt, M. J., van Dusschoten, D., and Knoche, M. (2019). Localized bursting of mesocarp cells triggers catastrophic fruit cracking. Hortic. Res. 6:79. doi: 10.1038/s41438-019-0161-3
Grimm, E., and Knoche, M. (2015). Sweet cherry skin has a less negative osmotic potential than the flesh. J. Am. Soc. Hortic. Sci. 140, 472–479. doi: 10.21273/JASHS.140.5.472
Gutierrez-Jara, C., Bilbao-Sainz, C., McHugh, T., Chiou, B. S., Williams, T., Villalobos-Carvajal, R., et al. (2021). Effect of cross-linked alginate/oil nanoemulsion coating on cracking and quality parameters of sweet cherries. Food Secur. 10:449. doi: 10.3390/foods10020449
Hauschildt, M., Steinkellner, S., and Weber, R. W. S. (2020). Grey mould populations in northern German sweet cherry and plum orchards: selection of fungicide-resistant Botrytis cinerea strains over sensitive B. Pseudocinerea by fungicide treatments. Eur. J. Plant Pathol. 157, 615–623. doi: 10.1007/s10658-020-02026-5
Horvitz, S., Godoy, C., López Camelo, A. F., and Yommi, A. (2003). Application of gibberellic acid to ‘sweetheart’ sweet cherries: effects on fruit quality at harvest and during cold storage. Acta Hortic. 628, 311–316. doi: 10.17660/ActaHortic.2003.628.37
Jung, J., Deng, Z., Simonsen, J., Bastías, R. M., and Zhao, Y. (2016). Development and preliminary field validation of water-resistant cellulose nanofiber based coatings with high surface adhesion and elasticity for reducing cherry rain-cracking. Sci. Hortic. 200, 161–169. doi: 10.1016/j.scienta.2016.01.016
Kafle, G. K., Khot, L. R., Zhou, J., Bahlol, H. Y., and Si, Y. (2016). Towards precision spray applications to prevent rain-induced sweet cherry cracking: understanding calcium washout due to rain and fruit cracking susceptibility. Sci. Hortic. 203, 152–157. doi: 10.1016/j.scienta.2016.03.027
Knoche, M., Beyer, M., Peschel, S., Oparlakov, B., and Bukovac, M. J. (2004). Changes in strain and deposition of cuticle in developing sweet cherry fruit. Physiol. Plant. 120, 667–677. doi: 10.1111/j.0031-9317.2004.0285.x
Knoche, M., and Peschel, S. (2006). Water on the surface aggravates microscopic cracking of the sweet cherry fruit cuticle. J. Am. Soc. Hortic. Sci. 131, 192–200. doi: 10.21273/jashs.131.2.192
Koumanov, K. S. (2015). On the mechanisms of the sweet cherry (Prunus avium L.) fruit cracking: swelling or shrinking? Sci. Hortic. 184, 169–170. doi: 10.1016/j.scienta.2015.01.002
Lane, W. D., Meheriuk, M., and McKenzie, D. L. (2000). Fruit cracking of a susceptible, an intermediate, and a resistant sweet cherry cultivar. HortScience 35, 239–242. doi: 10.21273/hortsci.35.2.239
Lang, G. A. (2009). High tunnel tree fruit production: the final frontier? HortTechnology 19, 50–55. doi: 10.21273/HORTTECH.19.1.50
Lang, G. A., Sage, L., and Wilkinson, T. (2016). Ten years of studies on systems to modify sweet cherry production environments: retractable roofs, high tunnels, and rain-shelters. Acta Hortic. 12, 83–90. doi: 10.17660/ActaHortic.2016.1130.12
Larrabee, M. M., Voegel, T. M., and Nelson, L. M. (2021). A duplex droplet digital PCR assay for quantification of Alternaria spp. and Botrytis cinerea on sweet cherry at different growth stages. Can. J. Plant Pathol. 43, 734–748. doi: 10.1080/07060661.2021.1913646
Ma, J. F., and Yamaji, N. (2006). Silicon uptake and accumulation in higher plants. Trends Plant Sci. 11, 392–397. doi: 10.1016/j.tplants.2006.06.007
Magri, A., Malorni, L., Cozzolino, R., Adiletta, G., Siano, F., Picariello, G., et al. (2023). Agronomic, physicochemical, aromatic and sensory characterization of four sweet cherry accessions of the Campania region. Plan. Theory 12:610. doi: 10.3390/plants12030610
Marroni, M. V., Casonato, S., Visnovsky, S. B., Pitman, A. R., Beresford, R. M., and Jones, E. E. (2023). Genetic characterization and prevalence of Pseudomonas syringae strains from sweet cherry orchards in New Zealand. Plant Pathol. 72, 1673–1686. doi: 10.1111/ppa.13775
Martin, L. B. B., Romero, P., Fich, E. A., Domozych, D. S., and Rose, J. K. C. (2017). Cuticle biosynthesis in tomato leaves is developmentally regulated by abscisic acid. Plant Physiol. 174, 1384–1398. doi: 10.1104/pp.17.00387
Matteo, M., Zoffoli, J. P., and Ayala, M. (2022). Calcium sprays and crop load reduction increase fruit quality and postharvest storage in sweet cherry (Prunus avium L.). Agronomy 12:829. doi: 10.3390/agronomy12040829
Measham, P. F., Bound, S. A., Gracie, A. J., and Wilson, S. J. (2009). Incidence and type of cracking in sweet cherry (Prunus avium L.) are affected by genotype and season. Crop Pasture Sci. 60, 1002–1008. doi: 10.1071/CP08410
Measham, P. F., Gracie, A. J., Wilson, S. J., and Bound, S. A. (2014). An alternative view of rain-induced cracking of sweet cherries (Prunus avium L). Acta Hortic. 1020, 217–222. doi: 10.17660/ActaHortic.2014.1020.31
Measham, P. F., Richardson, A., and Townsend, A. (2017). Calcium application and impacts on cherry fruit quality. Acta Hortic. 60, 375–382. doi: 10.17660/ActaHortic.2017.1161.60
Michailidis, M., Karagiannis, E., Bazakos, C., Tanou, G., Ganopoulos, I., and Molassiotis, A. (2021). Genotype- and tissue-specific metabolic networks and hub genes involved in water-induced distinct sweet cherry fruit cracking phenotypes. Comp. Struct. Biotechnol. J. 19, 5406–5420. doi: 10.1016/j.csbj.2021.09.030
Michailidis, M., Karagiannis, E., Tanou, G., Sarrou, E., Karamanoli, K., Lazaridou, A., et al. (2020). Sweet cherry fruit cracking: follow-up testing methods and cultivar-metabolic screening. Plant Methods 16:51. doi: 10.1186/s13007-020-00593-6
Moing, A., Renaud, C., Christmann, H., Fouilhaux, L., Tauzin, Y., Zanetto, A., et al. (2004). Is there a relation between changes in osmolarity of cherry fruit flesh or skin and fruit cracking susceptibility? J. Am. Soc. Hortic. Sci. 129, 635–641. doi: 10.21273/JASHS.129.5.0635
Pereira, S., Silva, V., Bacelar, E., Guedes, F., Silva, A. P., Ribeiro, C., et al. (2020). Cracking in sweet cherry cultivars early Bigi and Lapins: correlation with quality attributes. Plan. Theory 9:1557. doi: 10.3390/plants9111557
Peschel, S., and Knoche, M. (2005). Characterization of microcracks in the cuticle of developing sweet cherry fruit. J. Am. Soc. Hortic. Sci. 130, 487–495. doi: 10.21273/JASHS.130.4.487
Quero-García, J., Letourmy, P., Campoy, J. A., Branchereau, C., Malchev, S., Barreneche, T., et al. (2021). Multi-year analyses on three populations reveal the first stable QTLs for tolerance to rain-induced fruit cracking in sweet cherry (Prunus avium L.). Hortic. Res. 8:136. doi: 10.1038/s41438-021-00571-6
Ricardo-Rodrigues, S., Laranjo, M., and Agulheiro-Santos, A. C. (2023). Methods for quality evaluation of sweet cherry. J. Sci. Food Agric. 103, 463–478. doi: 10.1002/jsfa.12144
Rombolà, A. D., Quartieri, M., Rodríguez-Declet, A., Minnocci, A., Sebastiani, L., and Sorrenti, G. (2023). Canopy-applied silicon is an effective strategy for reducing sweet cherry cracking. Hortic. Environ. Biotechnol. 64, 371–378. doi: 10.1007/s13580-022-00486-8
Ruiz-Aracil, M. C., Valverde, J. M., Beltrà, A., Carrión-Antolí, A., Lorente-Mento, J. M., Nicolás-Almansa, M., et al. (2024). Putrescine increases frost tolerance and effectively mitigates sweet cherry (Prunus avium L.) cracking: a study of four different growing cycles. Agronomy 14:23. doi: 10.3390/agronomy14010023
Ruiz-Aracil, M. C., Valverde, J. M., Lorente-Mento, J. M., Carrión-Antolí, A., Castillo, S., Martínez-Romero, D., et al. (2023). Sweet cherry (Prunus avium L.) cracking during development on the tree and at harvest: the impact of methyl jasmonate on four different growing seasons. Agriculture 13:1244. doi: 10.3390/agriculture13061244
Sajid, M., Basit, A., Shah, S. T., Khan, A., Ullah, I., Bilal, M., et al. (2024). Enhancing the quality and fruit yield of sweet cherry (Prunus avium) cultivars by foliar application of boron. Applied Fruit Science 66, 485–494. doi: 10.1007/s10341-023-01023-2
Schumann, C., and Knoche, M. (2020). Swelling of cell walls in mature sweet cherry fruit: factors and mechanisms. Planta 251:65. doi: 10.1007/s00425-020-03352-y
Schumann, C., Winkler, A., Brüggenwirth, M., Köpcke, K., and Knoche, M. (2019). Crack initiation and propagation in sweet cherry skin: a simple chain reaction causes the crack to ‘run’. PLoS One 14:e219794. doi: 10.1371/journal.pone.0219794
Schumann, C., Winkler, A., and Knoche, M. (2022). Calcium decreases cell wall swelling in sweet cherry fruit. Sci. Rep. 12:16496. doi: 10.1038/s41598-022-20266-9
Sekse, L. (1995). Fruit cracking in sweet cherries (Prunus avium L.) some physiological aspects—a mini review. Sci. Hortic. 63, 135–141. doi: 10.1016/0304-4238(95)00806-5
Shin, S., and Ji, S. (2021). Consumers’ willingness to purchase imported cherries towards sustainable market: evidence from the republic of Korea. Sustain. For. 13:5420. doi: 10.3390/su13105420
Simon, G. (2006). Review on rain induced fruit cracking of sweet cherries (Prunus avium L.), its causes and the possibilities of prevention. Int. J. Horticult. Sci. 12, 27–35. doi: 10.31421/IJHS/12/3/654
Simon, G., Hrotkó, K., and Magyar, L. (2004). Fruit quality of sweet cherry cultivars grafted on four different rootstocks. Acta Hortic. 10, 366–370. doi: 10.31421/IJHS/10/3/490
Soltani Nejad, M., Najafabadi, N. S., Aghighi, S., Zargar, M., Bayat, M., and Pakina, E. (2024). Green synthesis of silver nanoparticles by sweet cherry and its application against cherry spot disease. Heliyon 10:e31508. doi: 10.1016/j.heliyon.2024.e31508
Stone, C. H., Close, D. C., Bound, S. A., and Corkrey, R. (2022). Orchard microclimate, tree water uptake and sweet cherry fruit quality under protected cropping. Front. Plant Sci. 13:993817. doi: 10.3389/fpls.2022.993817
Suran, P., Vávra, R., and Zeleny, L. (2016). Effectiveness of potential products to reduce rain cracking of cherry fruit. Acta Hortic. 1137, 183–186. doi: 10.17660/ActaHortic.2016.1137.26
Thomidis, T., and Exadaktylou, E. (2013). Effect of a plastic rain shield on fruit cracking and cherry diseases in Greek orchards. Crop Prot. 52, 125–129. doi: 10.1016/j.cropro.2013.05.022
Time, A., Ponce, C., Kuhn, N., Arellano, M., Sagredo, B., Donoso, J. M., et al. (2021). Canopy spraying of abscisic acid to improve fruit quality of different sweet cherry cultivars. Agronomy 11:1947. doi: 10.3390/agronomy11101947
Trouillas, F. P., Peduto, F., Lorber, J. D., Sosnowski, M. R., Grant, J., Coates, W. W., et al. (2011). Calosphaeria canker of sweet cherry caused by Calosphaeria pulchella in California and South Australia. Plant Dis. 96, 648–658. doi: 10.1094/PDIS-03-11-0237
Usenik, V., Kastelec, D., and Štampar, F. (2005). Physicochemical changes of sweet cherry fruits related to application of gibberellic acid. Food Chem. 90, 663–671. doi: 10.1016/j.foodchem.2004.04.027
Vercammen, J., and Gomand, A. (2017). Testing of sweet cherry cultivars in Belgium. Acta Hortic. 1161, 249–254. doi: 10.17660/ActaHortic.2017.1161.40
Wang, Y., Xiao, Y., Sun, Y., Zhang, X., Du, B., Turupu, M., et al. (2023). Two B-box proteins, PavBBX6/9, positively regulate light-induced anthocyanin accumulation in sweet cherry. Plant Physiol. 192, 2030–2048. doi: 10.1093/plphys/kiad137
Waqas, M., Prencipe, S., Guarnaccia, V., and Spadaro, D. (2023). Molecular characterization and pathogenicity of Alternaria spp. associated with black rot of sweet cherries in Italy. J. Fungi 9:992. doi: 10.3390/jof9100992
Wermund, U., Holland, A., and Reardon, S. (2005). Cracking susceptibility of sweet cherries in the United Kingdom in relation to calcium application and covering systems. Acta Hortic. 667, 475–482. doi: 10.17660/ActaHortic.2005.667.69
Winkler, A., Blumenberg, I., Schürmann, L., and Knoche, M. (2020). Rain cracking in sweet cherries is caused by surface wetness, not by water uptake. Sci. Hortic. 269:109400. doi: 10.1016/j.scienta.2020.109400
Winkler, A., and Knoche, M. (2019). Calcium and the physiology of sweet cherries: a review. Sci. Hortic. 245, 107–115. doi: 10.1016/j.scienta.2018.10.012
Winkler, A., and Knoche, M. (2021). Calcium uptake through skins of sweet cherry fruit: effects of different calcium salts and surfactants. Sci. Hortic. 276:109761. doi: 10.1016/j.scienta.2020.109761
Winkler, A., Ossenbrink, M., and Knoche, M. (2015). Malic acid promotes cracking of sweet cherry fruit. J. Am. Soc. Hortic. Sci. 140, 280–287. doi: 10.21273/JASHS.140.3.280
Winkler, A., Peschel, S., Kohrs, K., and Knoche, M. (2016). Rain cracking in sweet cherries is not due to excess water uptake but to localized skin phenomena. J. Am. Soc. Hortic. Sci. 141, 653–660. doi: 10.21273/JASHS03937-16
Wójcik, P., Akgül, H., Demirtaş, İ., Sarısu, C., Aksu, M., and Gubbuk, H. (2013). Effect of preharvest sprays of calcium chloride and sucrose on cracking and quality of ‘Burlat’ sweet cherry fruit. J. Plant Nutr. 36, 1453–1465. doi: 10.1080/01904167.2013.793714
Yang, S., Zhang, S., Qu, Z., Xiu, T., Hu, Y., Chen, C., et al. (2022). Reducing cherry rain-cracking: development and characterization of cold-water fish gelatin films reinforced by dual rod-spherical nanoscale structures formed under magnetic fields. Food Hydrocoll. 128:107579. doi: 10.1016/j.foodhyd.2022.107579
Yeats, T. H., and Rose, J. K. C. (2013). The formation and function of plant cuticles. Plant Physiol. 163, 5–20. doi: 10.1104/pp.113.222737
Yılmaz, H., Dalgıç, A., and Özkara, P. (2023). Research on comparison of sweet cherry farms in terms of adopting good agricultural practices (GAPs): evidence from Turkey. Erwerbs-obstbau 65, 453–459. doi: 10.1007/s10341-023-00849-0
Zhang, X., Wang, X., Xing, S., Ma, Y., and Wang, X. (2020). Multi-sensors enabled dynamic monitoring and quality assessment system (DMQAS) of sweet cherry in express logistics. Food Secur. 9:602. doi: 10.3390/foods9050602
Zhou, J., Khot, L. R., Bahlol, H. Y., Kafle, G. K., Peters, T., Whiting, M. D., et al. (2017). In-field sensing for crop protection: efficacy of air-blast sprayer generated crosswind in rainwater removal from cherry canopies. Crop Prot. 91, 27–33. doi: 10.1016/j.cropro.2016.09.010
Keywords: Prunus avium L., cracking mechanisms, testing methods, orchard managements, mitigation strategies
Citation: Xu J, Chen L, Dong J, Jiang L and Hong L (2025) Overview of fruit cracking in sweet cherry (Prunus avium L.): causes, testing methods, mitigation strategies, and research perspectives. Front. Sustain. Food Syst. 9:1534778. doi: 10.3389/fsufs.2025.1534778
Edited by:
Vuk M. Maksimović, University of Belgrade, SerbiaReviewed by:
Pedro Martinez-Gomez, Spanish National Research Council (CSIC), SpainZhilang Qiu, Guizhou Medical University, China
Copyright © 2025 Xu, Chen, Dong, Jiang and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Hong, ODUwOTgzNzEwQHFxLmNvbQ==
†ORCID: Jingcheng Xu, orcid.org/0009-0004-9127-8514
 Jingcheng Xu
Jingcheng Xu Linghui Chen1
Linghui Chen1