- 1Yunnan Tobacco Company Baoshan Company, Baoshan, China
- 2Marine Agriculture Research Center, Tobacco Research Institute of Chinese Academy of Agricultural Sciences, Qingdao, China
- 3National Technical Innovation Center for Comprehensive Utilization of Saline-Alkali Land, Dongying, China
The yield of agricultural wastes is increasing year by year, and composting is a common and effective strategy. However, the influence of marine-derived carbohydrates such as chitosan oligosaccharide (COS) and Enteromorpha prolifera polysaccharides (EPP)—on composting efficiency and microbial dynamics remains poorly understood. Here, we conducted composting experiments using agricultural wastes (cow manure, rice husks, and tomato residues) as substrates, and systematically evaluated the effects of chitosan oligosaccharide (COS) and Enteromorpha prolifera polysaccharides (EPP) supplementation through physicochemical analysis and high-throughput sequencing. These additives not only advanced the thermophilic phase and facilitated compost maturation but also reshaped the composition of the bacterial community in compost piles. By providing suitable microenvironments, the carbohydrates-containing group increased the abundance of thermophilic bacteria such as Ureibacillus, Geobacillus, and Ammoniibacillus, facilitating the degradation and utilization of organic matter. During which, the organic matters loss rates of COS and EPP were 26.61 and 12.66% higher than CK, respectively. By increasing the abundance of Cellvibrio and Flavobacterium in the cooling phase, carbohydrates-containing additives are expected to promote the conversion of recalcitrant lignocellulosic fibers. Additionally, PICRUSt 2 predictions revealed that carbohydrates addition increased the gene abundance related to amino carbohydrates and nucleotide carbohydrates metabolism, fructose and mannose metabolism, galactose metabolism, and butanoate metabolism in the initial and thermophilic phases, thereby facilitating carbohydrate metabolism. In summary, the addition of carbohydrates-containing additives enhanced the maturity and fertility of compost products and exerted significant regulatory effects on the composition and function of the bacterial community during composting.
1 Introduction
The accelerated advancement of agricultural modernization, coupled with rising living standards, has significantly increased the generation of agricultural waste, including crop residues and livestock manure. Current estimates indicate China produces approximately 97 million tons of crop straw and 3.8 billion tons of livestock and poultry manure annually (Ge et al., 2020; Sun et al., 2021). Improper management of these organic wastes may lead to substantial environmental degradation and public health risks (Li et al., 2019). Composting is the current ideal treatment (Wang et al., 2018), which is a complex process that involves the utilization of various microorganisms to drive the conversion of biodegradable organic materials into stable humus-like substances (Cesaro et al., 2015). And the resulting compost products, such as agricultural fertilizers or soil amendments, can not only enhance crop quality but also improve soil structure, increasing its capacity to retain water, heat, air, and nutrients (Wu et al., 2022).
Microorganisms (e.g., bacteria) drive the biological decomposition and play a vital role in the composting process (Wu et al., 2017). For example, bacteria accelerate compost maturation by promoting the breakdown of recalcitrant organic compounds and facilitating nutrient conversion through elevated metabolic activity and extracellular enzyme secretion (Liu X. et al., 2023). Microbial community dynamics during composting exhibit successional patterns that are both shaped by and reciprocally influence key physicochemical parameters, ultimately determining composting efficiency (Meng et al., 2019; Zhong et al., 2020; Hernandez-Lara et al., 2022). Furthermore, microbial co-occurrence patterns can disentangle complex microbial communities and delineate the underlying ecological processes (Banerjee et al., 2016). Network analysis identifies keystone microorganisms that shape community structure and function through node-link interactions (Ai et al., 2023). Monitoring the dynamic changes in microbial community structure, co-occurrence network and metabolic functions during composting is crucial for unraveling the mechanisms of material transformation and enhancing the quality of compost products (Li D. et al., 2023).
However, the long composting time, nitrogen loss, incomplete organic matter decomposition, and low humification degree directly reduce the application value of compost products (Wang et al., 2016; Chen et al., 2023). Several approaches have been proposed to improve the composting process as well as the quality of the finished products, including the addition of physical, chemical and microbial additives (Wu et al., 2019; Zhou et al., 2019; Duan et al., 2020; Wang et al., 2021; Li S. et al., 2023). In recent years, marine carbohydrates, e.g., chitosan oligosaccharides (COS) and Enteromorpha prolifera polysaccharides (EPP), which have been widely used in food, medical and livestock and poultry industry (Li et al., 2018; Wang W.-W. et al., 2022; Kim et al., 2024; Li et al., 2024). COS are degradation products obtained from chitin or chitosan in shrimp shells or crab shells through chemical or enzymatic hydrolysis. Previous studies have revealed that COS can not only promote plant growth, enhance their nitrogen fixation capacity and nutrient absorption efficiency but also significantly optimize soil microbial community structures, leading to a substantial increase in the abundance of beneficial bacteria such as Talaromyces (Guo et al., 2012; Salachna et al., 2017; Mukhtar Ahmed et al., 2020). EPP are a water-soluble sulfated heteropolysaccharide extracted from Enteromorpha prolifera, which have been proven to act as a biostimulant, promoting seed germination, plant growth, and inducing plant defense responses (Battacharyya et al., 2015; Shukla et al., 2016; Mzibra et al., 2018). In addition, EPP could promote root colonization of plant growth-promoting rhizobacteria by serving as an environmental signal for bacterial biofilm formation (Chu et al., 2023). Furthermore, both COS and EPP, being carbon sources to be metabolized by bacteria easily, serve to bolster microbial activity and expedite the decomposition of organic matter. Given the potential role of COS and EPP in regulating microbial structure directly and indirectly, we speculate that the application of COS and EPP to compost would reshape bacterial community, and then impact compost performance. However, it remains unclear how COS and EPP, as compost additives, affect the microbial community characteristics, co-occurrence network and their metabolic functions, as well as composting performance during composting.
Therefore, in this study, composting experiments was conducted with cow manure, rice husks, and tomato residues as substrates, with COS and EPP as additives. The main objectives of this study were to: (1) investigate the effects of COS and EPP on the physicochemical properties of compost; (2) explore the influence of COS and EPP on bacterial community succession during composting using high-throughput sequencing technology, and to analyze the correlation between physicochemical properties and bacterial community dynamics; (3) to identify the core bacteria and interaction of key bacterial community in composting with co-occurrence network analysis; (4) analyze the impact of COS and EPP on the metabolic functionalities of bacterial community through PICRUSt 2 functional prediction.
2 Materials and methods
2.1 Experimental design and sampling
The husks of rice (Oryza sativa L.) were provided by Beisenmiao Energy Company. Cow manures were obtained from the local dairy farm in the Jimo District, Qingdao, Shandong Province, China. Tomato residues were collected from a conventional tomato farm located in Jimo District, Qingdao. COS and EPP were provided by Qingdao Seawin Biotech Group. The three components were thoroughly mixed at a wet weight ratio of 5:1:2, and their main physicochemical properties are summarized in Supplementary Table S1. The composting process employed a 60 L airtight stainless steel composting reactor, equipped with a device capable of automatically recording temperature and constructed with double-layer insulated stainless steel. The experiment adopted a bottom ventilation design, with a ventilation device connected to the bottom of the composting reactor. To ensure effective ventilation and oxygen supply, a stainless steel porous sieve plate was placed at the bottom to support the composting materials. Three treatments were set up: (1) rice husks + cow manure + tomato residues (CK); (2) rice husks + cow manure + tomato residues + 0.02% chitosan oligosaccharide (COS); (3) rice husks + cow manure + tomato residues + 0.02% Enteromorpha prolifera polysaccharide (EPP). Samples were collected after each turning of the compost pile to ensure uniformity. At each sampling time point, three samples were collected from three different vertical positions and then thoroughly mixed together. Samples were treated following Xie’s protocol (Xie et al., 2024). All samples were ground, sieved, thoroughly mixed, and divided into two parts. One sample was stored at 4°C for physicochemical property analysis, while the remaining sample was frozen at −80°C for microbiological analysis. Samples collected on days 0, 2, 8, and 20 were selected to represent the initial, thermophilic, cooling, and maturation stages, respectively.
2.2 Physicochemical analyses
The compost temperature was automatically recorded using a temperature sensor at the center of the compost pile, with a time interval of 1 h. Fresh compost samples were mixed with deionized water at a ratio of 1:10 (w/v) and oscillated at 200 rpm for 30 min. pH and electrical conductivity (EC) were measured with a digital pH meter (pHS-3C, China) and a conductivity meter (DS-307A, China), respectively. The water extract and pak choi seeds were incubated in a constant temperature incubator at 25°C in darkness for 48 h to determine the germination index (GI). The dried sample weighing 1.00 g was transferred to a crucible and then placed in a muffle furnace, where it was incinerated at 550°C for 6 h until a constant weight was achieved. The difference in mass before and after incineration, divided by the mass of the sample before incineration, represented the organic matter content (OM). The sample to be tested reacted with ammonium molybdate to form yellow phosphomolybdate, and the concentration of phosphate ions was determined by measuring the absorbance of the solution. The total carbon (TC) and nitrogen (TN) content of the dried sample were determined using an elemental analyzer (ThermoFisher FlashSmart, USA), and the carbon/nitrogen ratio (C/N) was calculated. Ammonium nitrogen (NH4+–N) and nitrate nitrogen (NO3−–N) in the compost samples were extracted using a 2 mol/L KCl solution and analyzed using a segmented flow analyzer (Skeleton San++, France). Unfortunately, due to the low concentration of NO3−–N, it was not measurable, and therefore not included in the subsequent analysis.
2.3 DNA extraction and high-throughput sequencing
Total microbial DNA was extracted using the FastDNA® Spin Kit for Soil (MP Biomedicals, Solon, USA). A NanoDrop 2000 ultraviolet–visible spectrophotometer was used to measure DNA concentration and purity. The V3–V4 region of the bacterial 16S rRNA gene was amplified using primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The amplified products were subjected to high-throughput sequencing on the Illumina Miseq PE 300 platform by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China).
2.4 Data analysis
All samples for analysis were triplicated and all data was analyzed and visualized in Excel 2019 and OriginPro 2021. One-way analysis of variance (ANOVA) and post-hoc multiple comparison tests were conducted using SPSS 25.0 (SPSS for Windows, version 25.0, USA). Differences with a p < 0.05 were considered statistically significant. Based on the MGISEQ bioinformatics platform, sequences were clustered into Operational Taxonomic Units (OTUs) with a 97% similarity threshold. OTU sequences were then annotated against the Silva138/16s_bacteria database. To visually display differences in bacterial community composition among different composting treatments and over time, non-metric multidimensional scaling (NMDS) statistical analysis and plotting were conducted using R (version 3.3.1). Redundancy analysis (db-RDA) based on Bray-Curtis distance matrix was employed to investigate the relationship between bacterial community composition in compost and environmental factors. Spearman correlation analysis was conducted using R (version 3.3.1) with the pheatmap package to reveal the correlation between environmental parameters and the abundance of bacterial community composition (Top 30 genes). PICRUSt 2 was utilized for predicting functional gene profiles based on bacterial 16S rRNA data using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. The network analysis was performed to explore the co-occurring relationships between the members of bacterial communities using the “WGCNA” and “igraph” R packages. The interactive platform “Gephi” was used to identify the modules (ecological clusters) of microbial taxa.
3 Results and discussion
3.1 Changes in physicochemical properties during composting
Temperature, as one of the crucial indicators in composting, determines the succession of microbial communities and the degradation process of organic matter (Wei et al., 2014). In this study, CK, COS, and EPP reached peak temperatures on the 3rd, 1st, and 2nd days, respectively, with peak temperatures of 57.96°C, 65.12°C, and 63.15°C (Figure 1A). This finding suggested that the addition of marine carbohydrate led to an advance in the thermophilic phase and an increase in peak temperature, especially COS (Zhao et al., 2022). pH is a critical parameter affecting the composting process, influencing microbial nutrient uptake and altering their activity (Cao et al., 2023). As illustrated in Figure 1B, the pH values of all treatments exhibited an initial increase followed by a decrease trend, reaching their peaks on the 2nd day. The pH increase was likely attributed to microbial decomposition of organic nitrogen and reduction of NH4+–N, while the subsequent decrease may have been caused by rapid microbial degradation of organic matter, resulting in the accumulation of organic and inorganic acids (Jiang et al., 2015). After composting, the final pH values were 7.78 ± 0.07, 8.22 ± 0.06, and 8.06 ± 0.14, respectively, all within the optimal pH range (5.5–8.5) (Wang Z. et al., 2023). The EC of each treatment exhibited a continuous upward trend, reaching peak values at the end of composting, all exceeding 4 ms·cm−1 (Figure 1C), which was related with the ongoing decomposition of organic matter (Liu J. et al., 2023). The GI reflects the phytotoxicity of compost (Cao et al., 2023). As GI values indicated, marine carbohydrates promoted compost maturity, with final values of 104.82 ± 20.83%, 156.12 ± 16.02%, and 119.47 ± 15.48% in CK, COS, and EPP, respectively (Figure 1D), all meeting the security requirements (>80%). The loss rates of organic matters (OMs) for COS and EPP were 51.41 and 37.46%, respectively, higher than 24.80% in CK (Figure 1E). It was speculated that carbohydrates provided nutrients to promote microbial metabolism, thereby increasing the degradation rate of organic matter (Zhang et al., 2013). After composting, the content of PO43− decreased slightly. Compared with CK, the addition of marine carbohydrates increased the phosphorus content in the compost, possibly because the carbon-containing additives directly drove the phosphorus decarbonating microorganisms to contribute microbial biomass P (Chen P. et al., 2022) (Figure 1F). Compared to the initial stage, the TN concentration of CK, COS, and EPP in the mature stage increased by 3.46, 91.74, and 96.84%, respectively, resulting in a higher TN of final compost in COS and EPP than in CK (Figure 1G). These results indicated that the addition of marine carbohydrates increased the nutrient content of the compost (Zhou et al., 2019). In the initial stage of composting, the decrease rate of NH4+-N in the carbohydrates-added group was faster than that in the CK group, which was due to the faster heating rate in the carbohydrates-added group, resulting in more intense NH3 volatilization. Notably, the CK group exhibited a distinct NH₄+–N variation pattern: continuous decline until day 2 followed by stabilization through day 8, then rebounding by day 20—contrasting sharply with the carbohydrates-added group pattern of initial rapid decrease followed by slight recovery. This divergence likely stemmed from microbial community restructuring induced by carbohydrate supplementation, thereby promoting NH₄+–N accumulation (Szliszka et al., 2009) (Figure 1H). Ultimately, all treatments achieved comparable NH₄+–N reduction levels by day 20. The C/N ratio in composting is an important indicator affecting the nutrient requirements of microorganisms (Cao et al., 2023). C/N < 20 can be considered as the standard for compost maturity (Chen et al., 2021). At the end of composting, the C/N of CK, COS and EPP decreased to 27.29, 11.40, and 15.37, respectively, and all the carbohydrates groups met the requirements of decomposition (Figure 1I).
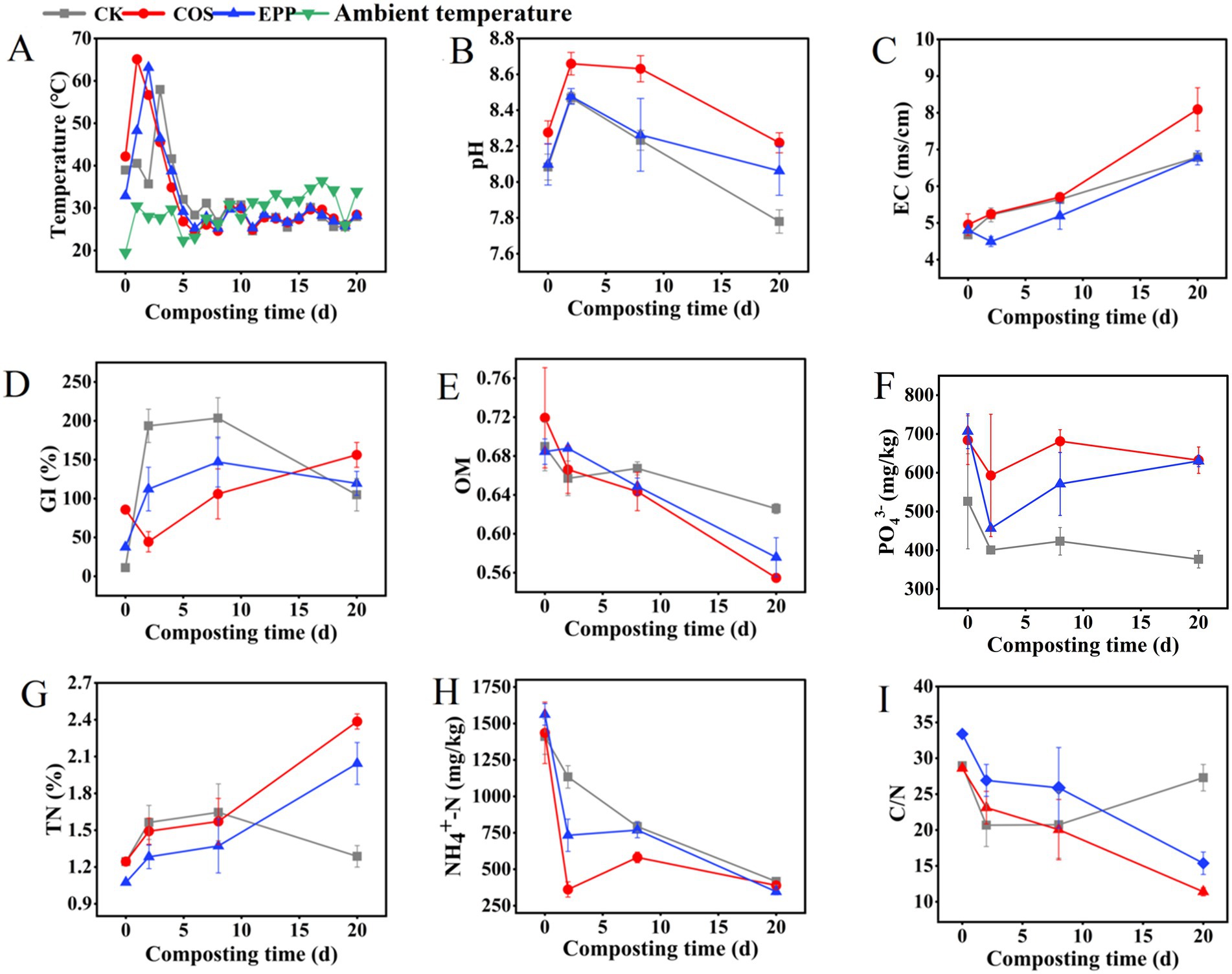
Figure 1. Physicochemical properties in different treatments during composting. (A) Temperature, (B) pH, (C) EC, (D) germination index (GI), (E) organic matter content, (F) phosphate content, (G) total nitrogen (TN), (H) NH4+–N, and (I) C/N. CK, rice husks + cow manure + tomato residues; COS, rice husks + cow manure + tomato residues + 0.02% chitosan oligosaccharide; EPP, rice husks + cow manure + tomato residues + 0.02% Enteromorpha prolifera polysaccharides. Error bars represent standard deviations of triplicate measurements.
3.2 Bacterial diversity and community composition during composting
Bacteria, being the most abundant microorganisms during composting, exhibit rapid growth and play a key role in the degradation of nutrients such as carbohydrates and proteins. Differences in bacterial diversity among different treatments at different stages were observed. The Chao 1 index reflects the richness of bacterial community, while the Shannon index indicates the diversity of bacterial community. Higher values signify greater richness or diversity (Liu et al., 2017; Zhong et al., 2020). As shown in Figure 2A, the richness of bacterial community in the composting process exhibited a trend of initially decreasing and then increasing, and the initial decline may be attributed to a sharp rise in temperature during the first 2 days (Wang Y. et al., 2022). Meanwhile, bacterial diversity showed a continuous increase, indicating microbial adaptation to the environment throughout the composting process, ultimately reaching its highest level in mature compost (Xu et al., 2021; Chen Y. et al., 2022) (Figure 2B). Notably, EPP outperformed COS in promoting microbial richness and diversity, potentially because of COS’ antimicrobial activity, though the distinction diminished by day 20 (Liu et al., 2018; Phil et al., 2018; Yin et al., 2020).
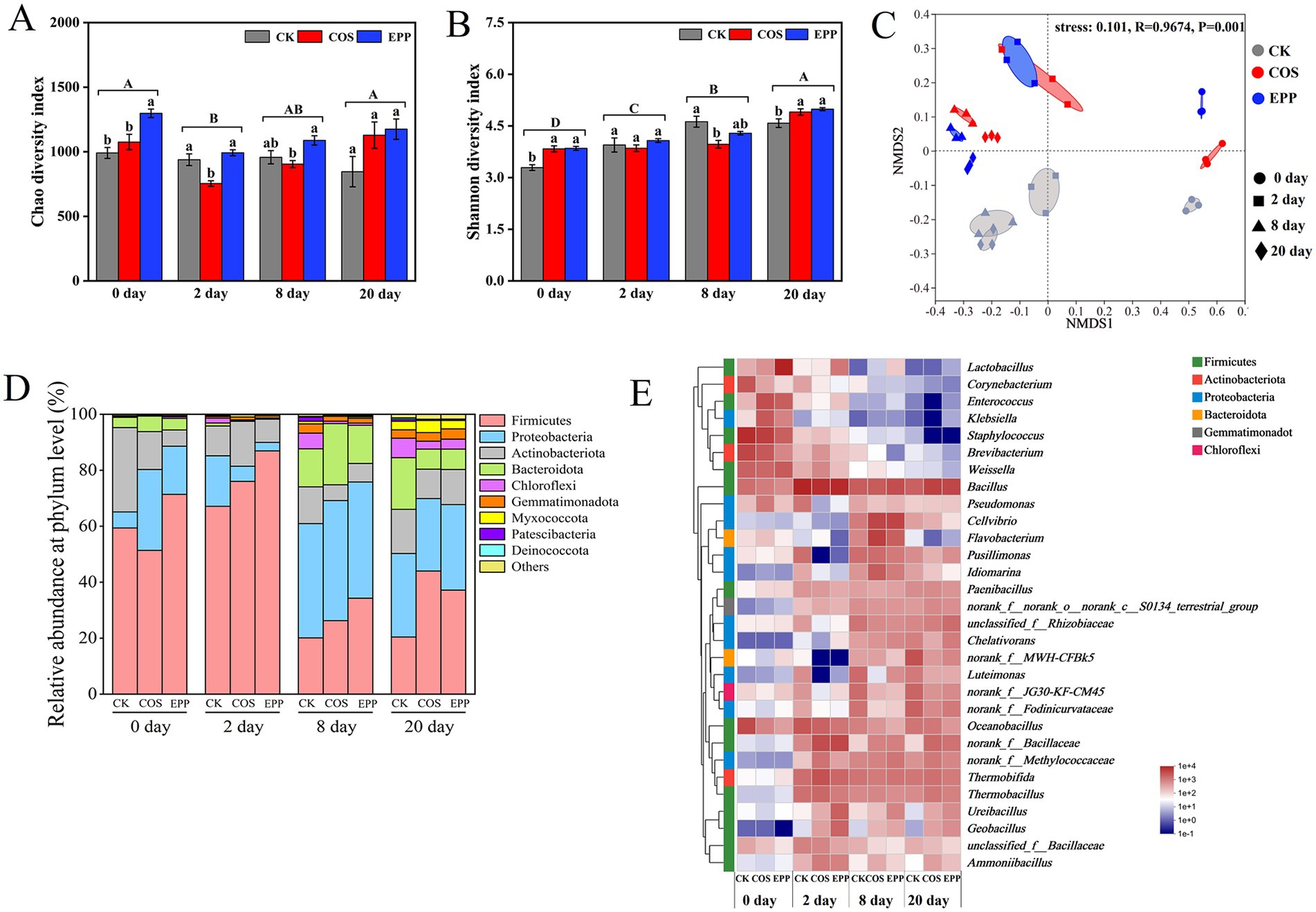
Figure 2. Changes in bacterial diversity index and community composition in different treatments during composting. (A) Chao 1 diversity index, (B) Shannon diversity index. Bars without shared lowercase and uppercase letters indicate significant differences among different additive treatments at each composting time and among different composting time according to Tukey’s HSD. (C) Non-metric multidimensional scaling (NMDS) analysis of microbial communities based on bray–curtis dissimilarity. (D) Phylum level and (E) genus level. CK, rice husks + cow manure + tomato residues; COS, rice husks + cow manure + tomato residues + 0.02% chitosan oligosaccharide; EPP, rice husks + cow manure + tomato residues + 0.02% Enteromorpha prolifera polysaccharides.
Non-metric multidimensional scaling (NMDS) was conducted to analyze β diversity, and the results were shown in Figure 2C. It could be observed that the although composting stage was the primary factor (Han et al., 2022), additives also significantly influenced the bacterial community composition. During composting, the phyla Firmicutes, Proteobacteria, Actinobacteriota, and Bacteroidota are important dominant bacteria in the composting system (Figure 2D). These phyla were widely utilized in agricultural waste treatment (Liu et al., 2017; Wang et al., 2018; Wang G. Y. et al., 2022). During the initial stage, the abundance of Firmicutes was highest, with an increase during the thermophilic phase and a subsequent decrease during the cooling and maturation phases. This pattern was attributed to the predominance of thermophilic bacteria within the Firmicutes phylum, such as the genera Bacillus, Thermobacillus, and Ureibacillus (Zhong et al., 2018) (Figure 2E). These bacteria have the capability to produce heat-resistant endospores and efficiently degrade large molecular organic compounds like cellulose and polysaccharides (Li et al., 2019; Awasthi et al., 2020; Bello et al., 2020). The abundance of Proteobacteria and Bacteroidota was inhibited at high temperatures, resulting in a decrease in abundance, followed by an increase during the cooling and maturation phases. Actinobacteriota exhibited less fluctuation in abundance with temperature changes, suggesting their tolerance to the high temperatures in the composting process. They worked synergistically with Firmicutes in cellulose degradation (He et al., 2022). In addition, except for the initial stage, the relative abundance of Firmicutes in the CK group was lower than in the carbohydrates-containing group (Supplementary Figure S1). This might be due to the incorporation of carbohydrates-containing additives, which changed microbial activity by increasing the amount of organic compounds in the heap (Zhong et al., 2018; Bello et al., 2020).
At the genus level (Figure 2E), there were differences in the dominant bacterial genera and their relative abundance among treatments at the initial phase. The dominant genera in the CK group included Staphylococcus (27.99%), Brevibacterium (14.33%), Oceanobacillus (12.35%), Weissella (6.67%), and Bacillus (4.79%); In group COS, the dominant bacterial genera included Staphylococcus (17.99%), Enterococcus (11.24%), Brevibacterium (8.59%), Weissella (5.96%), and Bacillus (3.31%); In group EPP, the dominant bacterial genera were Lactobacillus (37.04%), Weissella (8.15%), Enterococcus (7.20%), Staphylococcus (7.14%), and Brevibacterium (2.49%). It was worth emphasizing that Lactobacillus was capable of organic matter degradation and promoted the initiation of composting (Xu et al., 2022). In summary, the addition of carbohydrates-containing additives could alter the composition of initial bacterial communities, indicating a meaningful additive significance (Wang Y. et al., 2022). In the thermophilic phase, the dominant genera in each treatment were Bacillus (25.67–34.63%), Thermobifida (4.84–10.44%), Thermobacillus (3.30–5.69%), and Oceanobacillus (4.01–8.98%). Oceanobacillus has strong protein hydrolysis ability and participates in the humification of organic matter (Zhang et al., 2020). In addition, there were also relatively high abundances of norank _f_ Bacillaceae in groups COS and EPP. During the cooling phase, compared with CK group, the carbohydrates-added group had more Cellvibrio (belonging to the Proteobacteria phylum) and Flavobacterium (belonging to the Bacteroidetes phylum). Both Cellvibrio and Flavobacterium have excellent abilities to utilize polysaccharides, converting recalcitrant lignocellulose into stable humic substances. The addition of carbohydrates-containing additives provided them with a suitable growth environment (He et al., 2022; Wang C. et al., 2022; Wang S. P. et al., 2022). In the maturation stage, the diversity of dominant bacteria decreased, and the abundance of each bacterial taxon tended to be uniform.
Interestingly, the abundance of Ureibacillus, Geobacillus, and Ammoniibacillus increased in the thermophilic stage, with the abundance in the carbohydrates-added group significantly greater than that of CK group. The thermophilic bacterium Ureibacillus is one of the main microbial groups responsible for organic matter degradation under high-temperature conditions, and it has been reported in most high-temperature phases of composting trials (Li et al., 2020; Wang Y. et al., 2022). Geobacillus, like Ureibacillus, is also a thermophilic bacterium that dominates during the high-temperature phase of composting and can prolong this phase (Sarkar et al., 2010; Xu et al., 2022). Ammoniibacillus, another thermophilic bacterium, relies on NH4+–N as its sole nitrogen source for growth (Sakai et al., 2015; Qiu et al., 2021). In other words, the higher the abundance of Ammoniibacillus, the higher the utilization rate of corresponding NH4+–N and the less nitrogen loss (Wang N. et al., 2022). The above taxa belong to the thermophilic bacteria of Firmicutes and are closely related to the degradation and utilization of organic matter. The observed results might be attributed to the provision of a more suitable microenvironment for their growth by the addition of carbohydrates-containing additives (Wang et al., 2021; Wang Y. et al., 2022). In summary, the application of carbohydrates additives increased the abundance of microorganisms that could effectively degrade organic matter in compost, thereby promoting the maturity of compost.
3.3 Drivers of microbial communities during composting
Changes of environmental factors drive bacterial community structure succession during composting (Ge et al., 2020). The relationship among samples, environmental factors, and bacterial community was explored using distance-based redundancy analysis (db-RDA). As shown in Figure 3A, in the CK treatment, only two environmental factors, EC (R2 = 0.9527, p = 0.001) and NH4+–N (R2 = 0.8901, p = 0.001), showed significant correlations with bacterial community structure. By contrast, in the COS treatment, eight environmental factors, pH (R2 = 0.8001, p = 0.002), EC (R2 = 0.8769, p = 0.001), OM (R2 = 0.6555, p = 0.002), GI (R2 = 0.6347, p = 0.02), TC (R2 = 0.6681, p = 0.015), TN (R2 = 0.861, p = 0.001), C/N (R2 = 0.7644, p = 0.002), and NH4+–N (R2 = 0.7099, p = 0.008) were significantly correlated with bacterial community structure (Figure 3B). For the EPP treatment, five environmental factors, EC (R2 = 0.7443, p = 0.011), OM (R2 = 0.8027, p = 0.001), TN (R2 = 0.6261, p = 0.017), C/N (R2 = 0.5112, p = 0.044), and NH4+–N (R2 = 0.6507, p = 0.019) exhibited significant correlations with bacterial community structure (Figure 3C). These findings suggested that carbohydrates additives could significantly enhance the correlation between bacterial community and environmental factors. Carbohydrate amendment induces bacterial population shifts, altering community structure and function, thereby modulating the system’s environmental responsiveness. Therefore, the observed correlations suggest that carbohydrates additives can play a key role in modulating the relationship between bacterial communities and their surroundings.
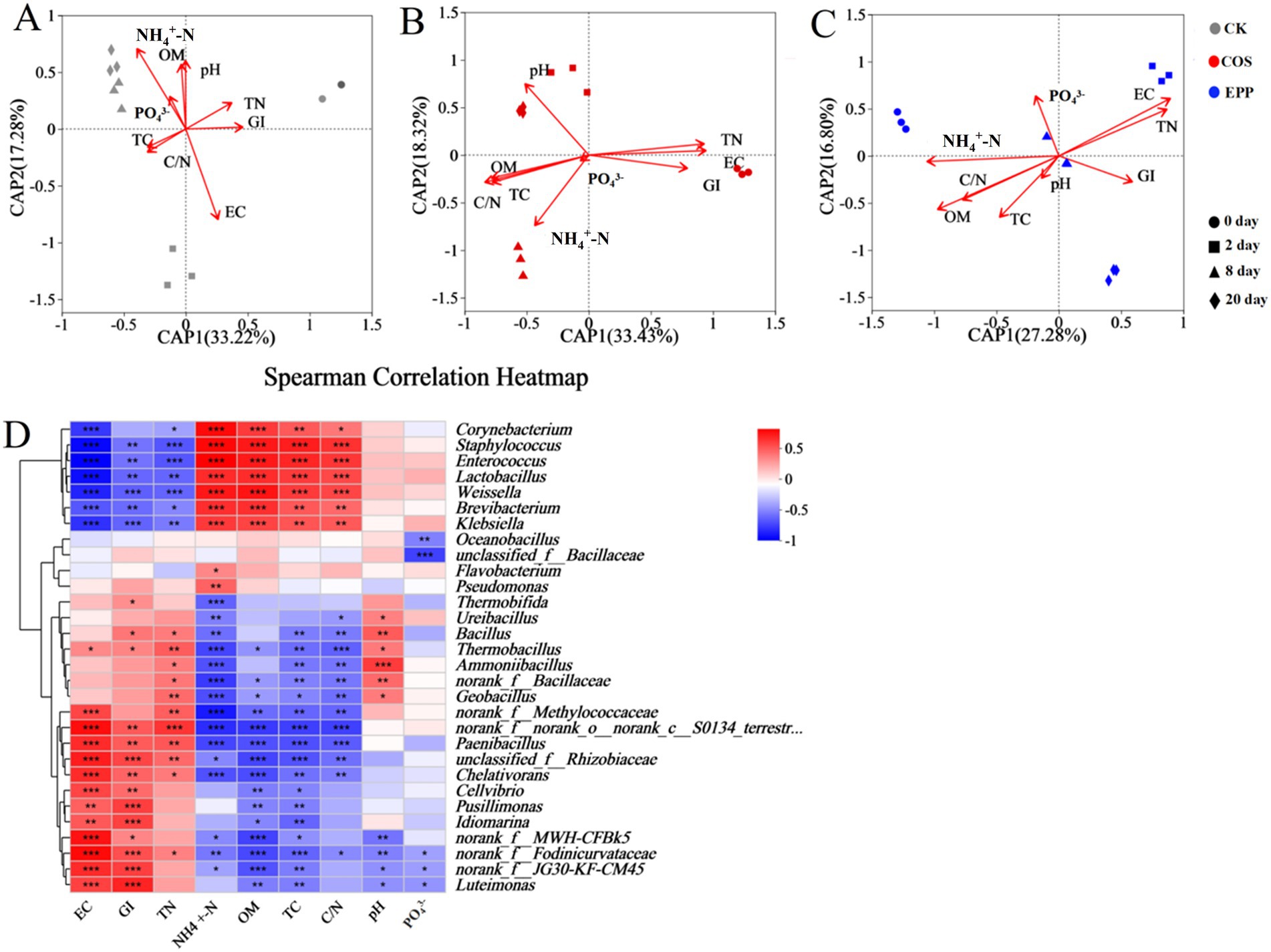
Figure 3. Db-RDA of the bacterial community in CK (A), COS (B) and EPP (C) during composting. (D) Spearman’s correlation heatmap between compost physicochemical properties and top 30 genera. The value of p < 0.05 is marked with “*,” p < 0.01 is marked with “**,” and p < 0.001 is marked with “***.” CK, rice husks + cow manure + tomato residues; COS, rice husks + cow manure + tomato residues + 0.02% chitosan oligosaccharide; EPP, rice husks + cow manure + tomato residues + 0.02% Enteromorpha prolifera polysaccharides.
The Spearman correlation analysis reflected the relationship between dominant bacteria genera and environmental factors (Figure 3D). It could be seen that the relative abundance of Ureibacillus, Geobacillus, and Ammoniibacillus in the carbohydrates-added group compared with that in the CK group was mainly positively correlated with pH and TN. The Spearman correlation heatmap broadly reflected two clusters of taxa: the first cluster included Corynebacterium, Staphylococcus, Enterococcus, Lactobacillus, Weissella, Brevibacterium, and Klebsiella, which were mainly pathogenic bacteria, lactic acid bacteria, and thermosensitive bacteria (Lasaridi et al., 2006; Wang et al., 2014; Tran et al., 2019; Liu et al., 2021; Shangguan et al., 2022; Shi et al., 2022). Their abundance was negatively correlated with EC, GI, and TN, and positively correlated with NH4+–N, OM, TC, and C/N. The second cluster included norank_f__Methylococcaceae, Paenibacillus, unclassified_f__Rhizobiaceae, Chelativorans, Cellvibrio, Pusillimonas, and Luteimonas, which were mainly associated with the degradation of cellulose, lignin, nitrogen-containing compounds, and organic matter (Yin et al., 2017; Wang Y. et al., 2023; Wongkiew et al., 2023; Xie et al., 2023). The correlation of their abundance with environmental factors was exactly opposite to that of the first cluster of genera. This suggested that the bacteria in the first cluster primarily relied on NH4+–N, OM, and TC for nutrition and were responsible for TN degradation, while TN primarily supported the nutrition of bacteria in the second cluster and promoted the transformation of NH4+–N and the humification of OM. Combining the relative abundance heatmap (Figure 2E), it was observed that the bacteria belonging to the first cluster decreased continuously as composting progresses, while those in the second cluster increased. This indicated that significant succession of bacterial community occurred during composting, and the abundance of dominant genera was closely related to environmental factors.
3.4 Bacterial co-occurrence network patterns during composting
The addition of marine polysaccharides affected the co-occurrence network patterns of bacteria during composting (Figure 4; Table 1). Compared to CK, the addition of COS reduced the total number of nodes, total number of edges, and avgD, but increased the avgCC, GD, and proportion of positive edges. The addition of EPP reduced the total number of edges, avgD, and proportion of positive edges, but increased the total number of nodes, avgCC, and GD. In summary, the addition of carbohydrates decreased the total number of edges and avgD, and increased GD, indicating that the addition of carbohydrates weakened the overall associations among bacteria and reduced network complexity, which was also directly reflected in the graph density data. However, the higher avgCC suggested that the addition of carbohydrates could enhance the connections within certain bacterial communities (Li G. et al., 2023).
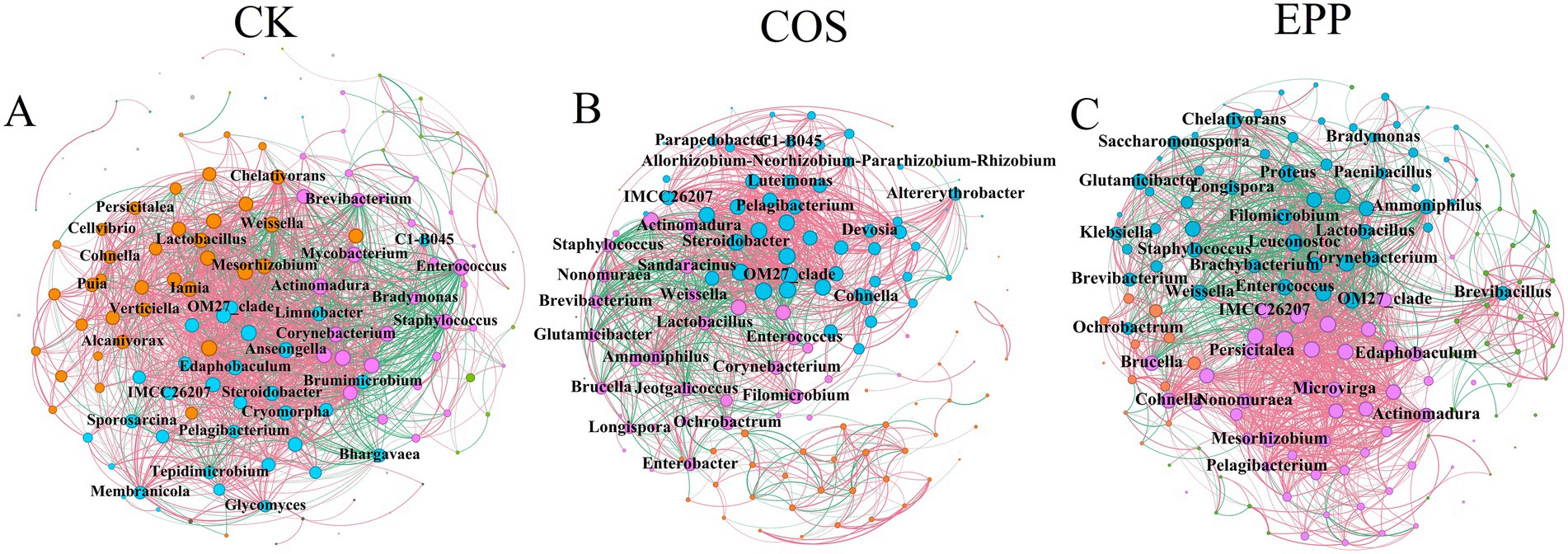
Figure 4. Molecular ecological network analysis showing the bacterial co-occurrence pattern during composting. (A–C) The bacterial co-occurrence network of different treatments. The colors of the nodes are assigned according to the module. The size of each node is proportional to the degree. Edge thickness is proportional to the weight of each correlation. A red edge indicates a positive interaction between two individual nodes, whereas a green edge indicates a negative interaction. Only nodes whose degree is greater than the average degree of corresponding processing are shown. CK, rice husks + cow manure + tomato residues; COS, rice husks + cow manure + tomato residues + 0.02% chitosan oligosaccharide; EPP, rice husks + cow manure + tomato residues + 0.02% Enteromorpha prolifera polysaccharides.
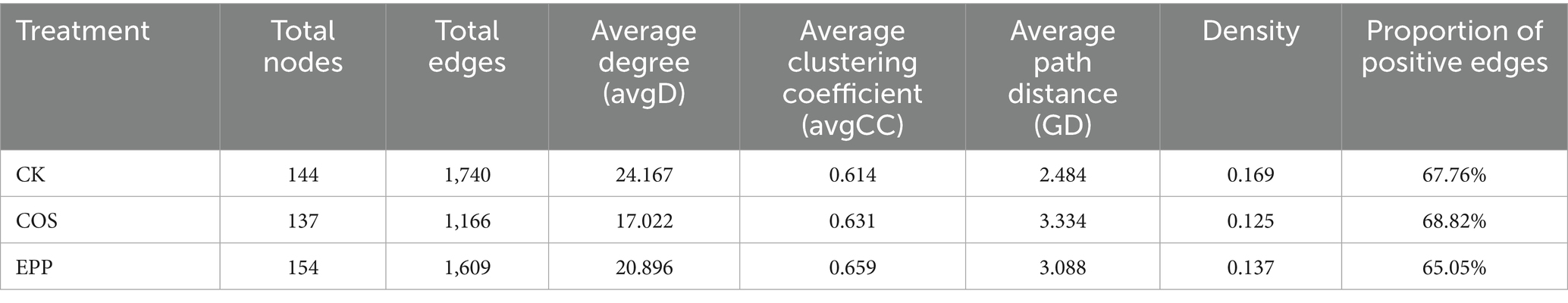
Table 1. Key topological features of soil microbial communities under different fertilization treatments.
Keystone taxa are highly connected taxa in microbial communities, which have special and important roles, and their removal can cause drastic changes in community structure and function. The keystone taxa from the highest connectome were Mesorhizobium (Lin et al., 2023), Steroidobacter (Krishnan et al., 2017), Edaphobaculum, Anseongella (Wang Y. et al., 2023), Actinomadura (Wang Y. et al., 2023) with degrees of 61, 59, 59, 59, 59 in the CK treatment. The COS treatment keystone taxa composed of Actinomadura, Steroidobacter, Ammoniphilus, Cohnella (Rastogi et al., 2010) and Luteimonas (Yang et al., 2023; Liu N. et al., 2024) with 47, 46, 37, 37, 37 degrees. The EPP treatment keystone taxa composed of Leuconostoc (Kot et al., 2014), Persicitalea, Corynebacterium (Ahmed et al., 2023), Enterococcus (Liu S. et al., 2024) and Proteus (Shi et al., 2022) with 54, 52, 51, 51, 50 degrees.
Although taxonomic composition of keystone taxa differed in different treatments, the critical bacteria remain those involved in the degradation of organic matter and nitrogen cycling. These potential core bacteria played a crucial role in the maturation of compost, underscoring the importance of enhancing indigenous functional bacteria in compost. Interestingly, the first bacterial cluster mentioned earlier (e.g., Corynebacterium, Enterococcus, Staphylococcus, Lactobacillus, Weissella, and Brevibacterium) exhibited high degree, demonstrating significant linking roles (Supplementary Table S2). These bacterial groups exhibited close interactions with other bacteria during the composting process, performing their main functional roles before gradually disappearing. Considering that this bacterial group included some potential pathogens or antibiotic resistance genes hosts, their disappearance after compost maturation provided a safeguard for compost safety (Wei et al., 2018; Wang B. et al., 2022).
3.5 Prediction of microbial metabolic functions during composting
The changes in bacterial community often lead to alterations in bacterial metabolic functions (Zhou et al., 2019). Bacterial metabolic function was predicted by PICRUSt 2 based on KEGG pathway database. As shown in Figure 5A, most of the predicted functional genes were assigned to metabolic (76.22–78.19%), genetic information processing (6.50–7.23%), environmental information processing (5.89–7.64%), and cellular process (3.71–5.01%) pathways in different composting processes.
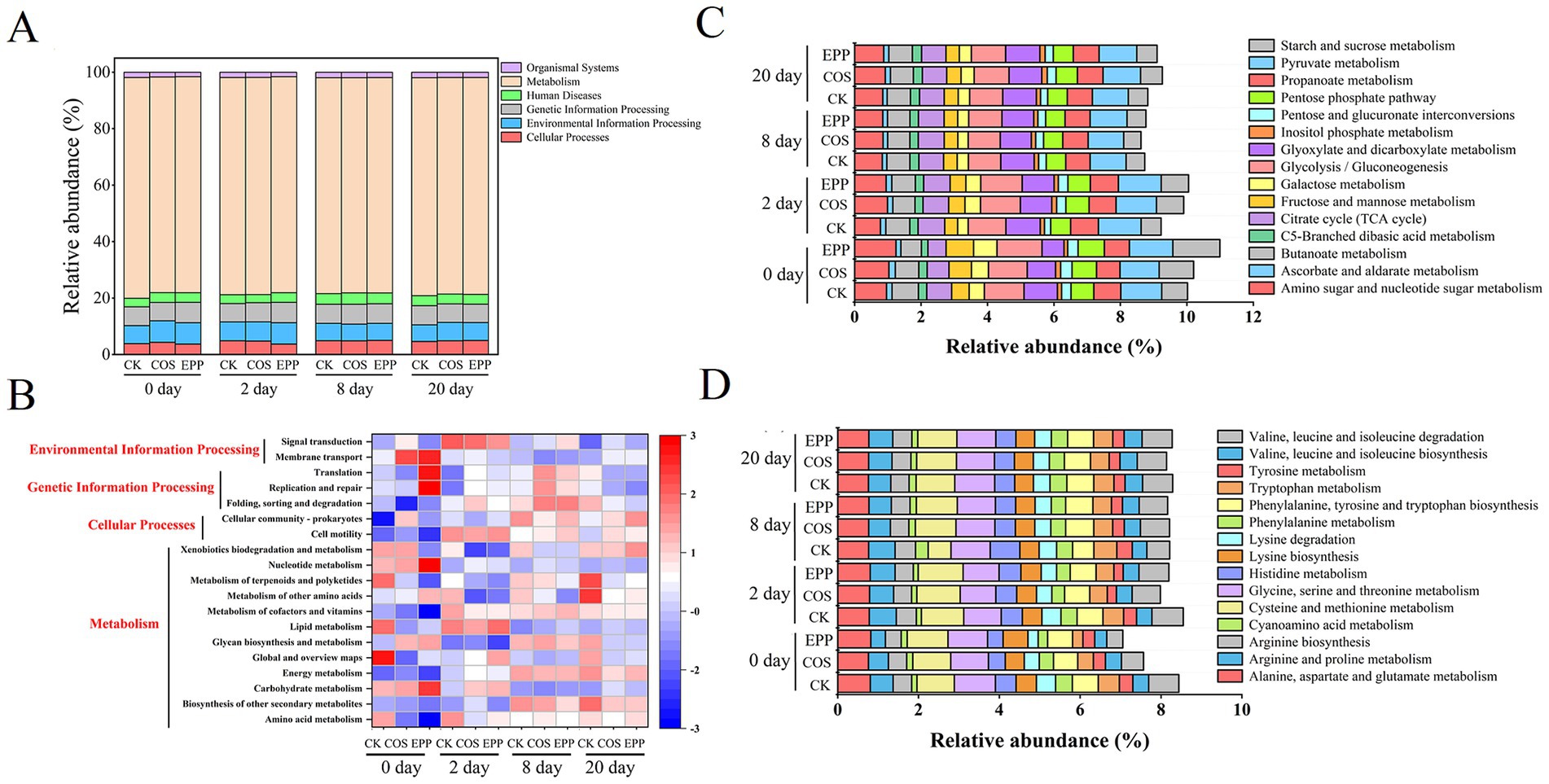
Figure 5. Variation of bacterial function profiles in different treatments during composting analyzed by PICRUSt2. (A) Relative abundance of KEGG primary function. (B) Heatmap showing the changes of bacterial metabolic functions at KEGG level 2. And changes in abundance of carbohydrate metabolism (C) and amino acid metabolism (D) at each step during composting. CK, rice husks + cow manure + tomato residues; COS, rice husks + cow manure + tomato residues + 0.02% chitosan oligosaccharide; EPP, rice husks + cow manure + tomato residues + 0.02% Enteromorpha prolifera polysaccharides.
As shown in Figure 5B, among the pathways with relative abundances exceeding 1%, the second-level KEGG orthology function predictions encompassed 12 metabolic pathways, 2 environmental information processing pathways, 3 genetic information processing pathways, and 2 cellular processes pathways. The major metabolic pathways included global and overview maps (39.33–40.18%), carbohydrate metabolism (8.62–10.99%), and amino acid metabolism (6.90–8.42%).
Carbohydrate metabolism primarily provides substrates for microbial reproduction and the formation of humic substances through the degradation of cellulose and hemicellulose (Toledo et al., 2017; Yin et al., 2019). It was easy to predict that the addition of marine carbohydrates significantly promoted carbohydrate metabolism. As expected, the gene abundance related to carbohydrate metabolism was higher in group EPP than in group COS, that was, polysaccharides had a more pronounced effect than oligosaccharides in promoting carbohydrate metabolism (Figure 5B).
As illustrated in Figure 5C, the addition of carbohydrates augmented the gene abundance related to amino carbohydrates and nucleotide carbohydrates metabolism, fructose and mannose metabolism, galactose metabolism, and butanoate metabolism during the initial and thermophilic stages of composting. These substrates are readily degradable and can be rapidly consumed during the early stages of composting (Zhong et al., 2020). By modulating the gene abundance associated with the metabolism of arginine, proline, lysine, phenylalanine, and tryptophan, among others, carbohydrates were speculated to reduce bacterial protein synthesis and activity in the amino acid metabolism pathway (Figure 5D) (Zhou et al., 2019).
4 Conclusion
The present study revealed that COS and EPP had significant effects on the physicochemical properties of cow manure and rice husk compost, which could accelerate compost decomposition and promote bacterial community succession, and enhance the correlation of bacterial communities with environmental factors. Specifically, chitosan oligosaccharides outperform Enteromorpha prolifera polysaccharides in terms of temperature, TN concentration and GI. EPP exhibited higher influence on microbial diversity and metabolic function. However, they did not contribute to enhancing the complexity of the co-occurrence network. This study provides a theoretical basis for the utilization of chitosan oligosaccharides and Enteromorpha prolifera polysaccharides as compost additives to ameliorate agricultural waste composting.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
XYi: Formal analysis, Methodology, Writing – original draft. SC: Formal analysis, Validation, Writing – original draft, Writing – review & editing. GS: Conceptualization, Supervision, Writing – review & editing. GZ: Investigation, Software, Writing – review & editing. GM: Methodology, Supervision, Writing – original draft. SG: Methodology, Software, Writing – original draft. HY: Formal analysis, Visualization, Writing – review & editing. XYo: Conceptualization, Resources, Writing – review & editing. YL: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported financially by the Major Science and Technology Program Project of Yunnan Branch of China National Tobacco Corporation (contract number 2023530000241017).
Acknowledgments
We would like to thank all persons in our lab for offering the experimental help.
Conflict of interest
XYi, GS, GZ, GM, and SG were employed by Yunnan Tobacco Company Baoshan Company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2025.1563421/full#supplementary-material
References
Ahmed, I., Zhang, Y., Sun, P., and Zhang, B. (2023). Co-occurrence pattern of ARGs and N-functional genes in the aerobic composting system with initial elevated temperature. J. Environ. Manag. 343:118073. doi: 10.1016/j.jenvman.2023.118073
Ai, Z., Deng, Y., Li, X., Zhang, J., Liu, H., Xu, H., et al. (2023). Effect of plant-soil feedback on soil microbial co-occurrence network depends on the stage of secondary succession. Rhizosphere 27:100733. doi: 10.1016/j.rhisph.2023.100733
Awasthi, M. K., Duan, Y., Liu, T., Awasthi, S. K., and Zhang, Z. (2020). Relevance of biochar to influence the bacterial succession during pig manure composting. Bioresour. Technol. 304:122962. doi: 10.1016/j.biortech.2020.122962
Banerjee, S., Baah-Acheamfour, M., Carlyle, C. N., Bissett, A., Richardson, A. E., Siddique, T., et al. (2016). Determinants of bacterial communities in Canadian agroforestry systems. Environ. Microbiol. 18, 1805–1816. doi: 10.1111/1462-2920.12986
Battacharyya, D., Babgohari, M. Z., Rathor, P., and Prithiviraj, B. (2015). Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 196, 39–48. doi: 10.1016/j.scienta.2015.09.012
Bello, A., Han, Y., Zhu, H. F., Deng, L. T., Yang, W., Meng, Q. X., et al. (2020). Microbial community composition, co-occurrence network pattern and nitrogen transformation genera response to biochar addition in cattle manure-maize straw composting. Sci. Total Environ. 721:137759. doi: 10.1016/j.scitotenv.2020.137759
Cao, Z. L., Deng, F., Wang, R. X., Li, J. B., Liu, X. F., and Li, D. (2023). Bioaugmentation on humification during co-composting of corn straw and biogas slurry. Bioresour. Technol. 374:128756. doi: 10.1016/j.biortech.2023.128756
Cesaro, A., Belgiorno, V., and Guida, M. (2015). Compost from organic solid waste: quality assessment and European regulations for its sustainable use. Resour. Conserv. Recycl. 94, 72–79. doi: 10.1016/j.resconrec.2014.11.003
Chen, L., Chen, Y., Li, Y., Liu, Y., Jiang, H., Li, H., et al. (2023). Improving the humification by additives during composting: a review. Waste Manag. 158, 93–106. doi: 10.1016/j.wasman.2022.12.040
Chen, Z., Fu, Q., Cao, Y., Wen, Q., and Wu, Y. (2021). Effects of lime amendment on the organic substances changes, antibiotics removal, and heavy metals speciation transformation during swine manure composting. Chemosphere 262:128342. doi: 10.1016/j.chemosphere.2020.128342
Chen, Y., Tang, P., Li, Y., Chen, L., Jiang, H., Liu, Y., et al. (2022). Effect of attapulgite on heavy metals passivation and microbial community during co-composting of river sediment with agricultural wastes. Chemosphere 299:134347. doi: 10.1016/j.chemosphere.2022.134347
Chen, P., Zheng, X., and Cheng, W. (2022). Biochar combined with ferrous sulfate reduces nitrogen and carbon losses during agricultural waste composting and enhances microbial diversity. Process. Saf. Environ. Prot. 162, 531–542. doi: 10.1016/j.psep.2022.04.042
Chu, D., Xu, Y., Ding, P., Du, X., Wang, S., Yuan, Y., et al. (2023). Study on the promotion of flue-cured tobacco growth by Enteromorpha prolifera polysaccharide cooperating with Bacillus amyloliquefaciens CAS02. Acta Tabac. Sin. 29, 76–84. doi: 10.16472/j.chinatobacco.2022.184
Duan, M., Zhang, Y., Zhou, B., Qin, Z., Wu, J., Wang, Q., et al. (2020). Effects of Bacillus subtilis on carbon components and microbial functional metabolism during cow manure–straw composting. Bioresour. Technol. 303:122868. doi: 10.1016/j.biortech.2020.122868
Ge, M., Zhou, H., Shen, Y., Meng, H., Li, R., Zhou, J., et al. (2020). Effect of aeration rates on enzymatic activity and bacterial community succession during cattle manure composting. Bioresour. Technol. 304:122928. doi: 10.1016/j.biortech.2020.122928
Guo, W., Yin, H., Ye, Z., Zhao, X., Yuan, J., and Du, Y. (2012). A comparison study on the interactions of two oligosaccharides with tobacco cells by time-resolved fluorometric method. Carbohydr. Polym. 90, 491–495. doi: 10.1016/j.carbpol.2012.05.070
Han, W., Chen, S., Tan, X., Li, X., Pan, H., Ma, P., et al. (2022). Microbial community succession in response to sludge composting efficiency and heavy metal detoxification during municipal sludge composting. Front. Microbiol. 13:1015949. doi: 10.3389/fmicb.2022.1015949
He, J., Zhu, N., Xu, Y., Wang, L., Zheng, J., and Li, X. (2022). The microbial mechanisms of enhanced humification by inoculation with Phanerochaete chrysosporium and Trichoderma longibrachiatum during biogas residues composting. Bioresour. Technol. 351:126973. doi: 10.1016/j.biortech.2022.126973
Hernandez-Lara, A., Ros, M., Cuartero, J., Angeles Bustamante, M., Moral, R., Javier Andreu-Rodriguez, F., et al. (2022). Bacterial and fungal community dynamics during different stages of agro-industrial waste composting and its relationship with compost suppressiveness. Sci. Total Environ. 805:150330. doi: 10.1016/j.scitotenv.2021.150330
Jiang, J. S., Liu, X. L., Huang, Y. M., and Huang, H. (2015). Inoculation with nitrogen turnover bacterial agent appropriately increasing nitrogen and promoting maturity in pig manure composting. Waste Manag. 39, 78–85. doi: 10.1016/j.wasman.2015.02.025
Kim, M., Song, C. Y., Lee, J. S., Ahn, Y. R., Choi, J., Lee, S. H., et al. (2024). Exosome isolation using chitosan oligosaccharide lactate-1-pyrenecarboxylic acid-based self-assembled magnetic nanoclusters. Adv. Healthc. Mater. 13:e2303782. doi: 10.1002/adhm.202303782
Kot, W., Neve, H., Heller, K. J., and Vogensen, F. K. (2014). Bacteriophages of leuconostoc, oenococcus, and weissella. Front. Microbiol. 5:186. doi: 10.3389/fmicb.2014.00186
Krishnan, Y., Bong, C. P. C., Azman, N. F., Zakaria, Z., Othman, N. A., Abdullah, N., et al. (2017). Co-composting of palm empty fruit bunch and palm oil mill effluent: microbial diversity and potential mitigation of greenhouse gas emission. J. Clean. Prod. 146, 94–100. doi: 10.1016/j.jclepro.2016.08.118
Lasaridi, K., Protopapa, I., Kotsou, M., Pilidis, G., Manios, T., and Kyriacou, A. (2006). Quality assessment of composts in the Greek market: the need for standards and quality assurance. J. Environ. Manag. 80, 58–65. doi: 10.1016/j.jenvman.2005.08.011
Li, S., Gu, X., Li, H., Li, M., Liu, Z., Xu, Z., et al. (2023). Effects of phosphorus-containing additives on carbon transformation during pig manure composting. Environ. Technol. Innovation 32:103290. doi: 10.1016/j.eti.2023.103290
Li, J., Jiang, F., Chi, Z., Han, D., Yu, L., and Liu, C. (2018). Development of Enteromorpha prolifera polysaccharide-based nanoparticles for delivery of curcumin to cancer cells. Int. J. Biol. Macromol. 112, 413–421. doi: 10.1016/j.ijbiomac.2018.02.002
Li, C., Li, H., Yao, T., Su, M., Ran, F., Han, B., et al. (2019). Microbial inoculation influences bacterial community succession and physicochemical characteristics during pig manure composting with corn straw. Bioresour. Technol. 289:121653. doi: 10.1016/j.biortech.2019.121653
Li, Y. C., Liu, Y. D., Yong, X. Y., Wu, X. Y., Jia, H. H., Wong, J. W. C., et al. (2020). Odor emission and microbial community succession during biogas residue composting covered with a molecular membrane. Bioresour. Technol. 297:122518. doi: 10.1016/j.biortech.2019.122518
Li, G., Niu, W., Ma, L., Du, Y., Zhang, Q., Sun, J., et al. (2023). Legacy effects of wheat season organic fertilizer addition on microbial co-occurrence networks, soil function, and yield of the subsequent maize season in a wheat-maize rotation system. J. Environ. Manag. 347:119160. doi: 10.1016/j.jenvman.2023.119160
Li, D., Wang, H., Ding, J., Zhou, Y., Jia, Y., Fan, S., et al. (2023). Comparative study on aerobic compost performance, microbial communities and metabolic functions between human feces and cattle manure composting. Environ. Technol. Innovation 31:103230. doi: 10.1016/j.eti.2023.103230
Li, Y., Zheng, L., Mustafa, G., Shao, Z., Liu, H., Li, Y., et al. (2024). Enhancing post-harvest quality of tomato fruits with chitosan oligosaccharide-zinc oxide nanocomposites: a study on biocompatibility, quality improvement, and carotenoid enhancement. Food Chem. 454:139685. doi: 10.1016/j.foodchem.2024.139685
Lin, X., Al-Dhabi, N. A., Li, F., Wang, N., Peng, H., Chen, A., et al. (2023). Relative contribution of ammonia-oxidizing bacteria and denitrifying fungi to N2O production during rice straw composting with biochar and biogas residue amendments. Bioresour. Technol. 390:129891. doi: 10.1016/j.biortech.2023.129891
Liu, N., Liu, Z., Wang, K., Zhao, J., Fang, J., Liu, G., et al. (2024). Comparison analysis of microbial agent and different compost material on microbial community and nitrogen transformation genes dynamic changes during pig manure compost. Bioresour. Technol. 395:130359. doi: 10.1016/j.biortech.2024.130359
Liu, X., Rong, X., Yang, J., Li, H., Hu, W., Yang, Y., et al. (2023). Community succession of microbial populations related to CNPS biological transformations regulates product maturity during cow-manure-driven composting. Bioresour. Technol. 369:128493. doi: 10.1016/j.biortech.2022.128493
Liu, J., Shen, Y., Ding, J., Luo, W., Zhou, H., Cheng, H., et al. (2023). High oil content inhibits humification in food waste composting by affecting microbial community succession and organic matter degradation. Bioresour. Technol. 376:128832. doi: 10.1016/j.biortech.2023.128832
Liu, X., Xia, W., Jiang, Q., Yu, P., and Yue, L. (2018). Chitosan oligosaccharide-N-chlorokojic acid mannich base polymer as a potential antibacterial material. Carbohydr. Polym. 182, 225–234. doi: 10.1016/j.carbpol.2017.11.019
Liu, N., Xu, L., Han, L. J., Huang, G. Q., and Ciric, L. (2021). Microbiological safety and antibiotic resistance risks at a sustainable farm under large-scale open-air composting and composting toilet systems. J. Hazard. Mater. 401:123391. doi: 10.1016/j.jhazmat.2020.123391
Liu, S., Zeng, J.-L., Cheng, Z.-W., He, J.-L., Pang, Y.-L., Liao, X.-D., et al. (2024). Evaluation of compost quality and the environmental effects of semipermeable membrane composting with poultry manure using sawdust or mushroom residue as the bulking agent*. J. Environ. Manag. 353:120162. doi: 10.1016/j.jenvman.2024.120162
Liu, N., Zhou, J., Han, L., Ma, S., Sun, X., and Huang, G. (2017). Role and multi-scale characterization of bamboo biochar during poultry manure aerobic composting. Bioresour. Technol. 241, 190–199. doi: 10.1016/j.biortech.2017.03.144
Meng, Q., Yang, W., Men, M., Bello, A., Xu, X., Xu, B., et al. (2019). Microbial community succession and response to environmental variables during cow manure and corn straw composting. Front. Microbiol. 10:529. doi: 10.3389/fmicb.2019.00529
Mukhtar Ahmed, K. B., Khan, M. M. A., Siddiqui, H., and Jahan, A. (2020). Chitosan and its oligosaccharides, a promising option for sustainable crop production- a review. Carbohydr. Polym. 227:115331. doi: 10.1016/j.carbpol.2019.115331
Mzibra, A., Aasfar, A., El Arroussi, H., Khouloud, M., Dhiba, D., Kadmiri, I. M., et al. (2018). Polysaccharides extracted from Moroccan seaweed: a promising source of tomato plant growth promoters. J. Appl. Phycol. 30, 2953–2962. doi: 10.1007/s10811-018-1421-6
Phil, L., Naveed, M., Mohammad, I. S., Bo, L., and Bin, D. (2018). Chitooligosaccharide: an evaluation of physicochemical and biological properties with the proposition for determination of thermal degradation products. Biomed. Pharmacother. 102, 438–451. doi: 10.1016/j.biopha.2018.03.108
Qiu, Z., Li, M., Song, L., Wang, C., Yang, S., Yan, Z., et al. (2021). Study on nitrogen-retaining microbial agent to reduce nitrogen loss during chicken manure composting and nitrogen transformation mechanism. J. Clean. Prod. 285:124813. doi: 10.1016/j.jclepro.2020.124813
Rastogi, G., Bhalla, A., Adhikari, A., Bischoff, K. M., Hughes, S. R., Christopher, L. P., et al. (2010). Characterization of thermostable cellulases produced by Bacillus and Geobacillus strains. Bioresour. Technol. 101, 8798–8806. doi: 10.1016/j.biortech.2010.06.001
Sakai, M., Deguchi, D., Hosoda, A., Kawauchi, T., and Ikenaga, M. (2015). Ammoniibacillus agariperforans gen. nov., sp. nov., a thermophilic, agar-degrading bacterium isolated from compost. Int. J. Syst. Evol. Microbiol. 65, 570–577. doi: 10.1099/ijs.0.067843-0
Salachna, P., Grzeszczuk, M., and Sobol, M. (2017). Effects of Chitooligosaccharide coating combined with selected ionic polymers on the stimulation of Ornithogalum saundersiae growth. Molecules 22:1903. doi: 10.3390/molecules22111903
Sarkar, S., Banerjee, R., Chanda, S., Das, P., Ganguly, S., and Pal, S. (2010). Effectiveness of inoculation with isolated Geobacillus strains in the thermophilic stage of vegetable waste composting. Bioresour. Technol. 101, 2892–2895. doi: 10.1016/j.biortech.2009.11.095
Shangguan, H., Fu, T., Shen, C., Mi, H., Wei, J., Tang, J., et al. (2022). In situ generated oxygen distribution causes maturity differentiation during electrolytic oxygen aerobic composting. Sci. Total Environ. 850:157939. doi: 10.1016/j.scitotenv.2022.157939
Shi, W., Dong, Q., Saleem, M., Wu, X., Wang, N., Ding, S., et al. (2022). Microbial-based detonation and processing of vegetable waste for high quality compost production at low temperatures. J. Clean. Prod. 369:133276. doi: 10.1016/j.jclepro.2022.133276
Shukla, P. S., Borza, T., Critchley, A. T., and Prithiviraj, B. (2016). Carrageenans from red seaweeds as promoters of growth and elicitors of defense response in plants. Front. Mar. Sci. 3:81. doi: 10.3389/fmars.2016.00081
Sun, H., Wang, E., Li, X., Cui, X., Guo, J., and Dong, R. (2021). Potential biomethane production from crop residues in China: contributions to carbon neutrality. Renew. Sust. Energ. Rev. 148:111360. doi: 10.1016/j.rser.2021.111360
Szliszka, E., Czuba, Z. P., Domino, M., Mazur, B., Zydowicz, G., and Krol, W. (2009). Ethanolic extract of propolis (EEP) enhances the apoptosis- inducing potential of TRAIL in cancer cells. Molecules 14, 738–754. doi: 10.3390/molecules14020738
Toledo, M., Gutiérrez, M. C., Siles, J. A., García-Olmo, J., and Martín, M. A. (2017). Chemometric analysis and NIR spectroscopy to evaluate odorous impact during the composting of different raw materials. J. Clean. Prod. 167, 154–162. doi: 10.1016/j.jclepro.2017.08.163
Tran, Q. N. M., Mimoto, H., Koyama, M., and Nakasaki, K. (2019). Lactic acid bacteria modulate organic acid production during early stages of food waste composting. Sci. Total Environ. 687, 341–347. doi: 10.1016/j.scitotenv.2019.06.113
Wang, Q., Awasthi, M. K., Ren, X., Zhao, J., Li, R., Wang, Z., et al. (2018). Combining biochar, zeolite and wood vinegar for composting of pig manure: the effect on greenhouse gas emission and nitrogen conservation. Waste Manag. 74, 221–230. doi: 10.1016/j.wasman.2018.01.015
Wang, C., Guo, X. H., Deng, H., Dong, D., Tu, Q. P., and Wu, W. X. (2014). New insights into the structure and dynamics of actinomycetal community during manure composting. Appl. Microbiol. Biotechnol. 98, 3327–3337. doi: 10.1007/s00253-013-5424-6
Wang, N., Huang, D. D., Shao, M. S., Sun, R., and Xu, Q. Y. (2022). Use of activated carbon to reduce ammonia emissions and accelerate humification in composting digestate from food waste. Bioresour. Technol. 347:126701. doi: 10.1016/j.biortech.2022.126701
Wang, W.-W., Ru, M., Wu, X., and Qu, M.-R. (2022). Physiological functions of Enteromorpha prolifera polysaccharide and its applications in animal production. Chin. J. Anim. Nutr. 34, 5489–5499. doi: 10.3969/j.issn.1006-267x.2022.09.005
Wang, Y., Tang, Y., and Yuan, Z. (2022). Improving food waste composting efficiency with mature compost addition. Bioresour. Technol. 349:126830. doi: 10.1016/j.biortech.2022.126830
Wang, Q., Wang, Z., Awasthi, M. K., Jiang, Y., Li, R., Ren, X., et al. (2016). Evaluation of medical stone amendment for the reduction of nitrogen loss and bioavailability of heavy metals during pig manure composting. Bioresour. Technol. 220, 297–304. doi: 10.1016/j.biortech.2016.08.081
Wang, S. P., Wang, L., Sun, Z. Y., Wang, S. T., Shen, C. H., Tang, Y. Q., et al. (2021). Biochar addition reduces nitrogen loss and accelerates composting process by affecting the core microbial community during distilled grain waste composting. Bioresour. Technol. 337:125492. doi: 10.1016/j.biortech.2021.125492
Wang, S. P., Wang, L., Sun, Z. Y., Wang, S. T., Yuan, H. W., An, M. Z., et al. (2022). Effect of distillery sewage sludge addition on performance and bacterial community dynamics during distilled grain waste composting. Bioresour. Technol. 345:126486. doi: 10.1016/j.biortech.2021.126486
Wang, B., Wang, Y., Wei, Y., Chen, W., Ding, G., Zhan, Y., et al. (2022). Impact of inoculation and turning for full-scale composting on core bacterial community and their co-occurrence compared by network analysis. Bioresour. Technol. 345:126417. doi: 10.1016/j.biortech.2021.126417
Wang, Y., Wang, J., Wu, X., Zhao, R., Zhang, Z., Zhu, J., et al. (2023). Synergetic effect and mechanism of elementary sulphur, MgSO4 and KH2PO4 progressive reinforcement on pig manure composting nitrogen retention. Environ. Pollut. 331:121934. doi: 10.1016/j.envpol.2023.121934
Wang, C., Wu, M. H., Peng, C. H., Yan, F. F., Jia, Y. X., Li, X., et al. (2022). Bacterial dynamics and functions driven by a novel microbial agent to promote kitchen waste composting and reduce environmental burden. J. Clean. Prod. 337:130491. doi: 10.1016/j.jclepro.2022.130491
Wang, Z., Xu, Y. L., Yang, T., Liu, Y. Q., Zheng, T. T., and Zheng, C. L. (2023). Effects of biochar carried microbial agent on compost quality, greenhouse gas emission and bacterial community during sheep manure composting. Biochar 5:3. doi: 10.1007/s42773-022-00202-w
Wang, G. Y., Yang, Y., Kong, Y. L., Ma, R. N., Yuan, J., and Li, G. X. (2022). Key factors affecting seed germination in phytotoxicity tests during sheep manure composting with carbon additives. J. Hazard. Mater. 421:126809. doi: 10.1016/j.jhazmat.2021.126809
Wei, L., Shutao, W., Jin, Z., and Tong, X. (2014). Biochar influences the microbial community structure during tomato stalk composting with chicken manure. Bioresour. Technol. 154, 148–154. doi: 10.1016/j.biortech.2013.12.022
Wei, Y., Zhao, Y., Shi, M., Cao, Z., Lu, Q., Yang, T., et al. (2018). Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour. Technol. 247, 190–199. doi: 10.1016/j.biortech.2017.09.092
Wongkiew, S., Polprasert, C., Noophan, P., Koottatep, T., Kanokkantapong, V., Surendra, K. C., et al. (2023). Effects of vermicompost leachate on nitrogen, phosphorus, and microbiome in a food waste bioponic system. J. Environ. Manag. 339:117860. doi: 10.1016/j.jenvman.2023.117860
Wu, J., He, S., Li, G., Zhao, Z., Wei, Y., Lin, Z., et al. (2019). Reducing ammonia and greenhouse gas emission with adding high levels of superphosphate fertilizer during composting. Environ. Sci. Pollut. Res. 26, 30921–30929. doi: 10.1007/s11356-019-06209-4
Wu, X., Wang, J., Yu, Z., Amanze, C., Shen, L., Wu, X., et al. (2022). Impact of bamboo sphere amendment on composting performance and microbial community succession in food waste composting. J. Environ. Manag. 303:114144. doi: 10.1016/j.jenvman.2021.114144
Wu, J., Zhao, Y., Qi, H., Zhao, X., Yang, T., Du, Y., et al. (2017). Identifying the key factors that affect the formation of humic substance during different materials composting. Bioresour. Technol. 244, 1193–1196. doi: 10.1016/j.biortech.2017.08.100
Xie, J., Gu, J., Wang, X. J., Hu, T., Sun, W., Song, Z. L., et al. (2023). Response characteristics of denitrifying bacteria and denitrifying functional genes to woody peat during pig manure composting. Bioresour. Technol. 374:128801. doi: 10.1016/j.biortech.2023.128801
Xie, C. H., Wang, X., Zhang, B. Q., Liu, J. T., Zhang, P., Shen, G. C., et al. (2024). Co-composting of tail vegetable with flue-cured tobacco leaves: analysis of nitrogen transformation and estimation as a seed germination agent for halophyte. Front. Microbiol. 15:1433092. doi: 10.3389/fmicb.2024.1433092
Xu, Z., Xu, W., Zhang, L., Ma, Y., Li, Y., Li, G., et al. (2021). Bacterial dynamics and functions driven by bulking agents to mitigate gaseous emissions in kitchen waste composting. Bioresour. Technol. 332:125028. doi: 10.1016/j.biortech.2021.125028
Xu, M., Yang, M., Sun, H., Meng, J., Li, Y., Gao, M., et al. (2022). Role of multistage inoculation on the co-composting of food waste and biogas residue. Bioresour. Technol. 361:127681. doi: 10.1016/j.biortech.2022.127681
Yang, X., Li, R., Li, Y., Mazarji, M., Wang, J., Zhang, X., et al. (2023). Composting pig manure with nano-zero-valent iron amendment: insights into the carbon cycle and balance. Bioresour. Technol. 371:128615. doi: 10.1016/j.biortech.2023.128615
Yin, N., Du, R., Zhao, F., Han, Y., and Zhou, Z. (2020). Characterization of antibacterial bacterial cellulose composite membranes modified with chitosan or chitooligosaccharide. Carbohydr. Polym. 229:115520. doi: 10.1016/j.carbpol.2019.115520
Yin, Y. N., Gu, J., Wang, X. J., Song, W., Zhang, K. Y., Zhang, X., et al. (2017). Effects of chromium(III) on enzyme activities and bacterial communities during swine manure composting. Bioresour. Technol. 243, 693–699. doi: 10.1016/j.biortech.2017.06.169
Yin, Y. A., Gu, J., Wang, X. J., Zhang, Y. J., Zheng, W., Chen, R., et al. (2019). Effects of rhamnolipid and Tween-80 on cellulase activities and metabolic functions of the bacterial community during chicken manure composting. Bioresour. Technol. 288:121507. doi: 10.1016/j.biortech.2019.121507
Zhang, C., Gao, Z., Shi, W., Li, L., Tian, R., Huang, J., et al. (2020). Material conversion, microbial community composition and metabolic functional succession during green soybean hull composting. Bioresour. Technol. 316:123823. doi: 10.1016/j.biortech.2020.123823
Zhang, L., Sun, X., Tian, Y., and Gong, X. (2013). Effects of brown sugar and calcium superphosphate on the secondary fermentation of green waste. Bioresour. Technol. 131, 68–75. doi: 10.1016/j.biortech.2012.10.059
Zhao, X., Xu, K., Wang, J., Wang, Z., Pan, R., Wang, Q., et al. (2022). Potential of biochar integrated manganese sulfate for promoting pig manure compost humification and its biological mechanism. Bioresour. Technol. 357:127350. doi: 10.1016/j.biortech.2022.127350
Zhong, X. Z., Li, X. X., Zeng, Y., Wang, S. P., Sun, Z. Y., and Tang, Y. Q. (2020). Dynamic change of bacterial community during dairy manure composting process revealed by high-throughput sequencing and advanced bioinformatics tools. Bioresour. Technol. 306:123091. doi: 10.1016/j.biortech.2020.123091
Zhong, X.-Z., Ma, S.-C., Wang, S.-P., Wang, T.-T., Sun, Z.-Y., Tang, Y.-Q., et al. (2018). A comparative study of composting the solid fraction of dairy manure with or without bulking material: performance and microbial community dynamics. Bioresour. Technol. 247, 443–452. doi: 10.1016/j.biortech.2017.09.116
Keywords: agricultural wastes, composting performance, bacterial community, metabolic function, co-occurrence network
Citation: Yin X, Chen S, Shen G, Zhang G, Meng G, Gao S, Yao H, You X and Li Y (2025) Chitosan oligosaccharide and Enteromorpha prolifera polysaccharides as additives affect the bacterial community and optimize the compost performance. Front. Sustain. Food Syst. 9:1563421. doi: 10.3389/fsufs.2025.1563421
Edited by:
Inga Grinfelde, Latvia University of Agriculture, LatviaReviewed by:
Xiaomeng Chen, Northeast Agricultural University, ChinaCatia Francisco, Federal University of the Southern Frontier, Brazil
Copyright © 2025 Yin, Chen, Shen, Zhang, Meng, Gao, Yao, You and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Yao, eWFvaHVpQGNhYXMuY24=; Xiangwei You, eW91eGlhbmd3ZWlAY2Fhcy5jbg==
†These authors have contributed equally to this work and share first authorship
 Xingsheng Yin1†
Xingsheng Yin1† Shutong Chen
Shutong Chen Hui Yao
Hui Yao Xiangwei You
Xiangwei You Yiqiang Li
Yiqiang Li