- 1Department of Human Nutrition and Health, Lilongwe University of Agriculture and Natural Resources, Lilongwe, Malawi
- 2Department of Crops and Soil Science, Lilongwe University of Agriculture and Natural Resources, Lilongwe, Malawi
- 3Department of Food Science and Technology, Lilongwe University of Agriculture and Natural Resources, Lilongwe, Malawi
Introduction: Pigeon pea (Cajanus cajan) is a vital protein source in southern Malawi, yet the relationship between seed coat characteristics and cooking quality remains poorly understood. This study evaluated the influence of variety and seed coat thickness on physicochemical and cooking properties of six pigeon pea varieties: Kachangu (00040), Mwaiwathualimi (ICEAP00557), Chitedze 1 (ICEAP 01514/15), Mthawajuni, Sauma (ICP 9145), and ICPL 93026.
Method and material: Seeds were obtained from ICRISAT and ChitedzeResearch Station and analyzed for water absorption, cooking time, and splitting behavior in both whole and dehulled forms.
Results: Variety significantly influenced cooking time (p < 0.001), water absorption during soaking and cooking (p < 0.001), and splitting tendency (p < 0.001). Cooking times ranged from 97 to 193 min for whole seeds versus 26–54 min for dehulled samples. Seed coat removal enhanced water absorption and reduced cooking time by 66% across all varieties. Kachangu and Mwaiwathualimi exhibited intense splitting, while thin-coated varieties showed higher aromatic intensity. Surprisingly, seed coat thickness did not significantly affect physical characteristics, water absorption, or cooking time. However, seed coat presence was the critical determinant of cooking performance.
Discussion: This study demonstrates that varietal selection combined with dehulling represents a strategic intervention for optimizing pigeon pea utilization, while enhancing nutritional accessibility and supporting sustainable food systems in resource-constrained environments. Results provide valuable insights for breeding programs targeting improved cooking quality and utilization of pigeon pea varieties for sub-Saharan Africa.
1 Introduction
Pigeon pea (Cajanus cajan) represents a critical yet underexploited protein source in Malawi, where cultivation spans 196,516 hectares in the southern region, yielding 216,716 tons annually (Yohane et al., 2021). Despite this production capacity, utilization remains geographically restricted, with consumption limited primarily to indigenous communities in southern Malawi (Zhang et al., 2022). This regional constraint is particularly concerning given Malawi’s widespread protein-energy malnutrition, where 37% of children under five exhibits stunting due to inadequate protein intake (Majili, 2023).
The primary barrier to expanded pigeon pea utilization is extended cooking time (170–210 min), which significantly exceeds that of other pulses (Ukomadu et al., 2023; Majili et al., 2020). In Malawi’s rural context, where 85% of the population depends on biomass fuels, prolonged cooking times impose substantial socioeconomic burdens (Barzehkar, 2024). Women, who bear primary responsibility for food preparation, face increased time allocation to cooking activities, reducing availability for other productive activities. Moreover, escalating firewood costs due to deforestation make energy-intensive food preparation economically prohibitive for resource-poor households (Vermeulen et al., 2020).
Malawian pigeon pea varieties exhibit seed coat thickness ranging from 40 to 60 μm, which contributes to thermal resistance during cooking (Mulenga et al., 2021). However, the relationship between seed coat morphology and cooking behavior remains poorly characterized for local varieties. While dehulling has demonstrated cooking time reductions in other regions (74–116 min to 28–43 min for whole versus dehulled seeds), no study has been conducted for Malawian varieties (Ravichandran and Upadhyay, 2022; Syed, 2019). Additionally, dehulling effectively reduces concentrations of antinutritional factors, including trypsin inhibitors, hemagglutinins, and phytate, which can limit protein bioavailability and overall nutritional utilization (Sherpa, 2023). This processing method simultaneously improves palatability and digestibility, making pulses more accessible to diverse consumer populations (Gu et al., 2023).
Although several pigeon pea varieties have been released in Malawi, research on their cooking properties remains limited. This study examined newly released, medium-maturing, high-yielding cultivars, focusing on how seed coat thickness and varietal differences affect physicochemical and cooking characteristics. The findings will inform processing strategies and varietal selection, ultimately enhancing utilization and nutritional accessibility of pigeon peas in Malawi.
2 Materials and methods
2.1 Sample preparation and study site
Five kilograms each of pigeon pea (Cajanus cajan L.) varieties (Mwaiwathualimi (ICEAP00557), ICEAP01514/15, Kachangu (ICEAP 00040), Sauma (ICP9145), Mthawajuni and ICPL 93026) grown during the 2012/2013 season at Chitedze Research Station and ICRISAT in Lilongwe Malawi were obtained. Samples were cleaned to remove dirt, shriveled and broken grains followed by packing in ziplock bags and stored at 4 °C until further use. Dhal was processed by blanching method (Yadav et al., 2021) where pigeon pea grains were soaked for 30 min in a Liter of boiled water (97.1 °C). After the 30 min of soaking, the water was drained, and the pigeon peas were lightly pounded using mortar and pestle in order to remove the seedcoats. The pounded pigeon peas were washed in clean water to separate and the split-dehulled pigeon peas were sundried (Tiwari et al., 2020).
The study was done at Lilongwe University of Agriculture and Natural Resource (Bunda college campus) in Foods laboratory. Proximate analysis, calcium and phytate analyses were done at Chancellor College in Zomba.
2.2 Experimental design
The study employed a completely randomized block design, with experiments conducted in duplicate and replicated three times. Variety and seed coat served as independent variables, while the treatments consisted of whole and dehulled pigeon peas. The dependent variables measured were physicochemical and cooking properties.
2.3 Physical properties of whole and dehulled pigeon peas
2.3.1 Determination of seed color, seed coat thickness and seed size
Color was determined using a Chroma meter CR- 400 (Konica Monolta Inc) measured values expressed as L*, a*, b* where L* = lightness, a* = redness, and b* = yellowness. The grains were then cut using a razor blade to separate the seed coat and a digital Venier calliper with 0.01 mm accuracy was used to measure seed coat thickness, grain length, breadth, and thickness of 30 randomly selected individual grains per variety (Kaliniewicz et al., 2022).
The overall size of the pigeon pea grains was expressed as weight of 100 seeds per variety weighed using an Ohaus (0.0001 g) top loading balance.
2.3.2 Bulk density of whole pigeon peas
Seed bulk density was determined according to Solanki et al. (2019). One hundred seeds were weighed and transferred into 100 mL measuring cylinder containing 50 mL of tap water at room temperature. The seeds were allowed to soak for 10 min for equilibration and the volume of water displaced were recorded. The mass and volume were used to calculate density (g/cm3).
2.4 Proximate composition of whole and dehulled pigeon peas
2.4.1 Moisture content determination
Moisture content of pigeon pea seeds was determined according to method described by Ajibola et al. (2003) (Equation 1). Thirty grams of pigeon pea seeds were weighed (M0) into pre dried moisture tins (1 h, 103 °C) that were cooled in a desiccator. The samples were dried for 72 h in a hot air oven (Mummert universal oven, Buchen Bach, Germany) at 103 °C. The dried samples were cooled in desiccator and weighed (M1). Moisture content was calculated as follows:
Where: MO = Weight of pre dried samples; M1 = Weight of dried samples.
2.4.2 Determination of protein
Determination of protein was done using Kjeldhal method (Magomya et al., 2014) (Equation 2). Total nitrogen was determined and multiplied with a factor 6.25 to obtain protein content. One gram of sample was mixed with 10mls of concentrated H2SO4 in a digestion flask. A tablet of selenium catalyst was added to digest the sample before it was heated under fume hood until a clear solution was obtained. The digest was diluted with 100mls of deionized water in volumetric flask and used for analysis. Ten milliliters of the digest were mixed with equal volume of 45% NaOH solution in a Kjeldhal distillation apparatus (UDK129, Velp Scientific, via Stazione, 1,620,865 Usmate (MB) Italy). The mixture was distilled into 10mls of 4% boric acid containing 3 drops of mixed indicator, 9 bromocresol green and methyl red. A total of 50 mL of distillate was collected and titrated with standardized 0.1 N Hydrochloric acid until the first appearance of pink color. The volume used was recorded to the nearest 0.05 mL. The protein content was calculated using the formula below:
Where: A = volume (ml) of 0.2 N HCL used sample titration; B = volume (ml) of 0.2 N HCL used blank titration; N = Normality of HCL; W = weight (g) of sample;14.007 = atomic weight of nitrogen; 6.25 = the protein nitrogen conversation.
2.4.3 Ash determination
Determination of ashes was done by the furnaces incineration gravimetric method (Thiex et al., 2012) (Equation 3). Five grams of the processed samples was measured into a previously weighed porcelain crucible. The samples were burnt to ashes in the muffle furnace (Carbolite, AAF11/7, serial number4/02/1208, Derbyshire, UK) at 550 °C for 5 h. When completely burnt to ashes, samples were cooled in desiccator and weighed. The weight of ash obtained was calculated by difference and expressed as a percentage of the weight of sample analyzed as:
Where: W1 = weight of crucible; W2 = weight of crucible +sample; W3 = weight of crucible + ash.
2.4.4 Crude fat
The solvent extraction gravimetric method AOAC Method 920.39c Nielsen (2010) was used for the determination of crude fat (Equation 4). One gram of the sample was wrapped in a filter paper and was placed in a thimble. The thimble was place in a Soxhlet reflux flask and mounted into a weighed extraction flask containing ether. The end of reflux was connected to water. The extractor was filled with ether up to a quarter of the volume of the collection flask. Extraction was done on a hot plate for 16 h after that ether was collected. The remaining ether in the flask was evaporated in a preheated oven (100 °C). The flask was cooled in a desiccator and weighed (W2). Crude fat was calculated using the formula below:
Where: W1 = Weight of flask (g); W2 = Weight of flask after extraction (g).
2.4.5 Crude fiber
The Weende method [(AOAC, 2000) was used to determine crude fiber; Möller, 2014 (Equation 5)]. Two grams of processed sample were boiled in 150 mL of 1.25% H2SO4 solution for 30 min. Samples were washed in several portion of hot water (60 °C) and then were allowed to drain dry before being transferred to a weighed crucible where they were dried in the oven at 105 °C to constant weight. Samples were taken to muffle furnace (Carbolite, AAF11/7, serial number 4/02/1208) where they were burnt until only ash was left. The weight of fiber was obtained and expressed as a percentage of weight of sample analyzed using this formula:
Where: W2 = weight of crucible and sample after boiling, washing and drying; W3 = weight of crucible + sample and ash.
2.4.6 Carbohydrate content
Carbohydrate content was determined by difference. It was calculated using the formula below as described by FAO (1998) (Abiola et al., 2019):
2.4.7 Determination of calcium and phytate content
Calcium (Ca) content was determined using Microwave plasma Atomic Emission Spectrophotometer (MP-AES 4100, Agilent Technologies, Santa Clara, California, United States) at 393.366 nm (Kuhn et al., 2020). Phytic acid was determined using the method by Marolt and Kolar (2020). Molar Phytic acid: Calcium ratio was calculated using the following formula:
The mole of phytate and mineral were determined by: Weight of the phytate and calcium/ their atomic weight.
2.5 Soaking and cooking properties of whole and dehulled pigeon peas
2.5.1 Water absorption during soaking
Ten grams seeds of each pigeon pea variety (whole and dehulled) were soaked in 50 mL deionized water at 22 °C for 1, 2, 3, 4, 5, and 6 h. At the specified time, excess water was drained, and the pigeon pea grains were blotted dry using absorbent paper to remove adherent water and weighed (Upretee et al., 2024).
WAS (g/kg−1) = weight of sample after soaking- weight of the sample before soaking/ Weight of the sample before soaking.
2.5.2 Water absorption during cooking
Ten grams of pigeon pea (whole seeds and dhal) was placed Erlenmeyer flasks containing 50 mL deionized water. The flasks were placed in a heavy aluminum pan containing 1,500 mL of deionized water. The pan was tightly covered and brought to boil allowing 5 min for heating up. The whole pigeon peas were boiled for 15, 30, 45, 60, 75 and 90 min while dehulled seeds, were boiled for 5, 10, 15, 20, 25, 30 min. At each designated time, 2 flasks per pigeon pea variety (Whole and Dehulled) were removed and excess water was drained, cooled to room temperature for 1 h, blotted dry to remove excess water and weighed (Heydari Foroushani, 2022).
WAC = Weight of the sample after cooking – weight of sample before cooking/ Weight of the sample before cooking.
2.5.3 Splitting during cooking of pigeon peas
Splitting of seeds during cooking was determined according to the method described by Munthali et al. (2022). The pigeon pea seeds with split seed coats and cotyledons were counted as splits. The degree was calculated as follows:
2.5.4 Cooking time
Cooking time was determined according to Mattson method (Mattson 1946) and later modified by Varriano- Marston and Jackson (1981) using Mattson cooking device (Sabadoti et al., 2020). The apparatus had a cooking rack (Mattson cooker,) with 25 rods 49.8 g each and 25 cylindrical holes (nine-millimeter diameter) where seeds were placed. The piercing tip of each rod was placed in contact with the surface of the seed. The Mattson cooker was placed into a stainless-steel pot containing about 1,500 mL of boiling deionized water. When the pigeon pea seed was sufficiently tender, the piercing tip penetrated the cooked seed, and the rods dropped through the hole in the saddle (Munthali et al., 2022). The cooking time was recorded when 20 rods (80%) had fallen through the cooked seeds.
2.6 Statistical analysis
Physicochemical properties were done in duplicate and were replicated three times in order to generate the data. The effect of seedcoat thickness on physicochemical and proximate composition were analyzed using generalised least model two-way ANOVA with seedcoat thickness and variety as factors in IBM SPSS software version 20.0. When the F-value was significant, differences between means were determined using the Least Significant Difference (LSD) test at 5% level of significance. A statistical assumption for a study was that the measurements taken for these characteristics were normally distributed within each sample group. Additionally, it was assumed that the observations were independent and that the variance of these measurements was equal across the whole and dehulled groups.
3 Results and discussion
3.1 Grain physical properties for whole and dehulled pigeon peas
3.1.1 Color characteristics
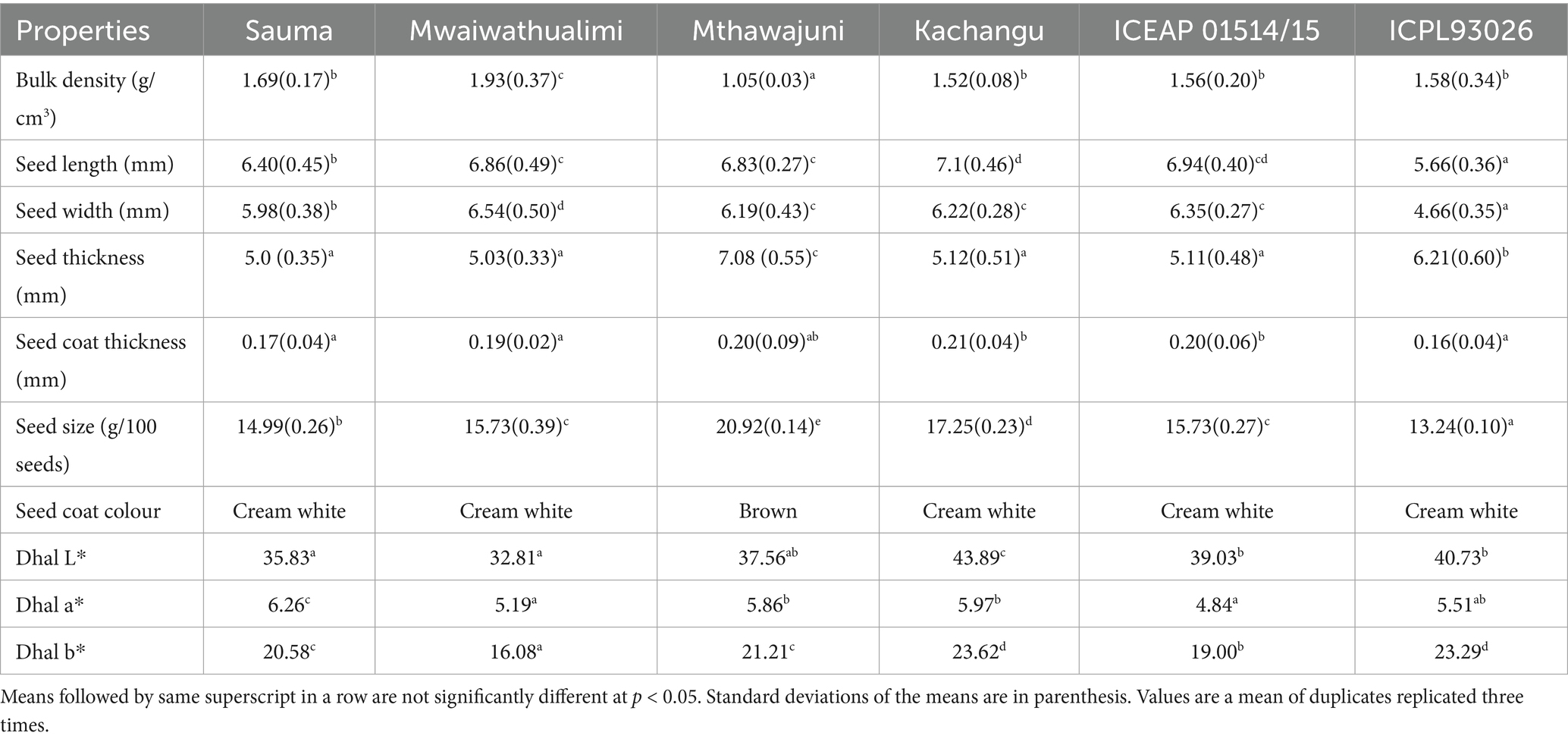
The whole Mthawajuni variety exhibited distinct brown coloration (L* = 29.43, a* = 12.02, b* = 10.63), while other varieties displayed cream white coloration (Table 1). The dark pigmentation in Mthawajuni is attributed to elevated tannin content, as darker coloration in uncooked legumes correlates positively with tannin concentration (Khazaei and Vandenberg, 2020). The L* value of 29.43 indicates relatively dark pigmentation, considering this parameter ranges from 0 (black) to 100 (white). The positive a* value (12.02) confirms a red shift on the red-green axis.
Tannins are polyphenolic compounds that form complexes with carbohydrates, proteins, and mineral ions through hydrogen bonding, exhibiting stronger affinity for proteins than carbohydrates due to interactions with carbonyl peptide oxygen atoms (Ojo, 2022; Bule et al., 2020). These tannin-protein complexes reduce protein digestibility and amino acid bioavailability, contributing to growth depression and increased fecal nitrogen excretion (Choi and Kim, 2020). Additionally, tannins impair nutrient absorption by chelating essential minerals (iron, zinc, copper) and contribute to astringency and bitterness, potentially affecting consumer acceptance (Cosme et al., 2025).
Dhal produced from Kachangu and ICPL 93026 exhibited brighter yellow coloration compared to other varieties, while Mwaiwathualimi dhal displayed duller yellow pigmentation. This yellow coloration is primarily attributed to carotenoids, including violaxanthin and other xanthophylls, which serve multiple physiological functions including photoprotection, antioxidant activity, and visual attraction for pollinators and seed dispersers (Giusti et al., 2023; Swami et al., 2020; Takemura et al., 2021). β-carotene, a vitamin A precursor, has been reported in pigeon peas at concentrations ranging from 23.31 to 469.6 mg/100 g (Bamidele and Akanbi, 2015; Kunyanga et al., 2013) supporting their nutritional value as carotenoid sources.
3.1.2 Morphological characteristics
Significant inter varietal differences were observed in seed dimensions (p < 0.001). Mthawajuni produced significantly larger seeds than other varieties, while ICPL 93026 exhibited the smallest seed size (Table 1). Seed size is critical for mechanized dhal processing, where grains are screened based on dimensions to separate foreign materials (Jeevarathinam and Chelladurai, 2020). Varietal differences in seed size reflect genetic factors and environmental conditions, with favorable growing conditions potentially increasing seed mass (Vanniarajan et al., 2023).
Kachangu grains were significantly longer than other varieties (p < 0.001), while Mthawajuni demonstrated greater thickness. Seed coat thickness varied significantly among varieties, with ICPL 93026 and Sauma exhibiting thinner coats compared to Kachangu, Mthawajuni, and ICEAP 01514/15. Seed coat thickness influences water absorption capabilities, as amorphous cells in thinner coats facilitate water uptake, whereas palisade cells in thicker coats present greater resistance to water penetration (Abati et al., 2022).
Bulk density differed significantly among varieties (p = 0.016), with Mthawajuni exhibiting the lowest density and Mwaiwathualimi the highest (Table 1). Lower bulk density correlates with larger intercellular spaces in cotyledons, while higher density indicates tighter cellular packing (Abati et al., 2022).
3.1.3 Proximate composition
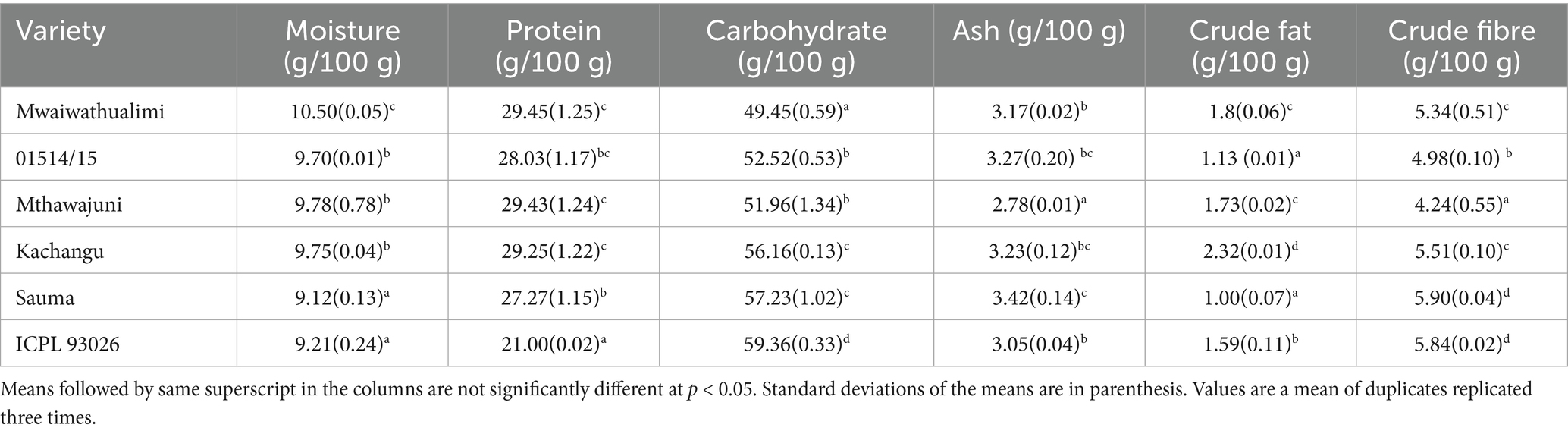
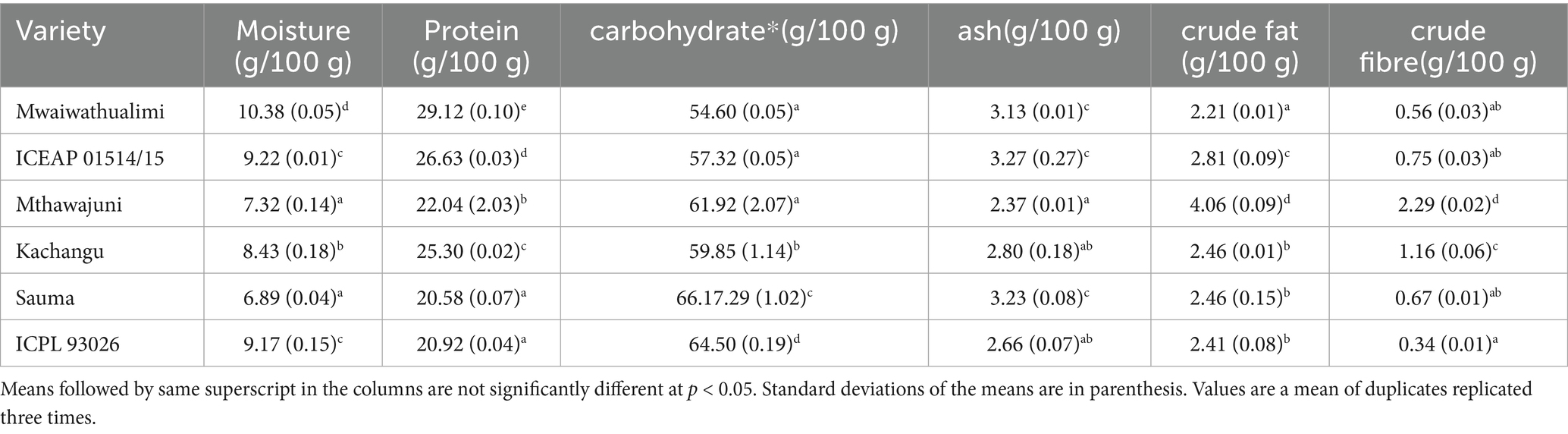
Moisture content ranged from 9.12 to 10.5% in whole seeds and 6.89 to 10.48% in dehulled samples (Table 2), falling within typical ranges for dry legumes (5–16%). These low moisture levels suggest extended shelf life potential (Ziegler et al., 2021). Protein content varied significantly among varieties, with Mwaiwathualimi and Mthawajuni exhibiting substantially higher concentrations than other cultivars (p < 0.001). Dehulling did not significantly affect protein content across varieties (p = 0.053), suggesting that protein is distributed relatively uniformly throughout the seed. The elevated protein content of pigeon pea is nutritionally significant in addressing protein-energy malnutrition.
Carbohydrate content was consistently higher in whole seeds compared to their dehulled counterparts across all varieties. The dehulling process resulted in a significant increase in total carbohydrates (p = 0.004), likely due to the removal of carbohydrate-rich seed coat components (Table 2). Carbohydrates are classified into enzymatically digestible forms (absorbed in the small intestine) and unavailable carbohydrates. Available carbohydrates include monosaccharides, disaccharides, and starch, which serve as energy sources for growth. Unavailable carbohydrates comprise of oligosaccharides and dietary fiber (Vieira et al., 2025). Dietary fiber is nutritionally important as it reduces blood lipid levels and helps prevent or treat cardiovascular diseases. Oligosaccharides are responsible for flatulence after fermentation by colonic bacteria, producing gas containing carbon dioxide, hydrogen, and methane. Flatulence problems are commonly associated with pulse consumption (He et al., 2022).
Crude fat content in whole seeds ranged from 1.00 ± 0.70 to 2.34 ± 0.01 g/100 g for Sauma and Kachangu, respectively. For dehulled seeds, significant differences were observed among Kachangu, Sauma, and ICPL 93026 varieties. Mthawajuni exhibited the highest crude fat content (4.06 ± 0.09 g/100 g), while Mwaiwathualimi had the lowest. Interestingly, crude fat content was higher in dehulled seeds compared to whole seeds, contrasting with findings reported from a research done in India (Jawalekar et al., 2020). Since lipids in legumes are primarily stored in cotyledons, this observation suggests that dehulling concentrates the fat content on a unit weight basis. The fat content aligns with established knowledge that legumes are not significant fat sources, except for oil seeds such as groundnut and soybean (Khrisanapant et al., 2019).
As expected, crude fiber content was substantially elevated in whole pigeon peas relative to dehulled samples, reflecting the contribution of the fibrous seed coat. The major components of crude fiber are cellulose and hemicellulose, predominantly concentrated in the seed coat. Theoretically, seed coat thickness should influence crude fiber content differences among pigeon pea cultivars, as thicker seed coats typically contain higher fiber content (Serna Saldívar and Hernández, 2020). Interestingly, seed coat thickness showed no significant correlation with fiber content (r = −0.628, p = 0.182), indicating that fiber concentration is independent of seedcoat thickness and may be influenced by other structural or compositional factors (Zakaria et al., 2024).
Ash content, an indicator of total mineral concentration (Bayero et al., 2019), varied considerably among varieties and processing treatments. Whole Mthawajuni seeds exhibited the lowest ash content, while whole Sauma and dehulled ICEAP01514/15 demonstrated the highest values. The elevated ash content in these samples suggests enhanced mineral density, which may have nutritional implications for these specific varieties (Table 3).
3.1.4 Calcium and phytic acid content
ICPL 93026 demonstrated the highest calcium content among all varieties (Table 4). Elevated calcium levels contribute to seed coat integrity and reduced splitting during thermal processing, while promoting calcium pectate formation that restricts cell separation, potentially resulting in firmer cooked texture. Higher calcium concentration in the seed coat maintains structural integrity and results in fewer splits during thermal processing of legumes. Additionally, elevated calcium content contributes to calcium pectate formation, which restricts cell separation and results in firmer cooked bean texture (Chen et al., 2023).
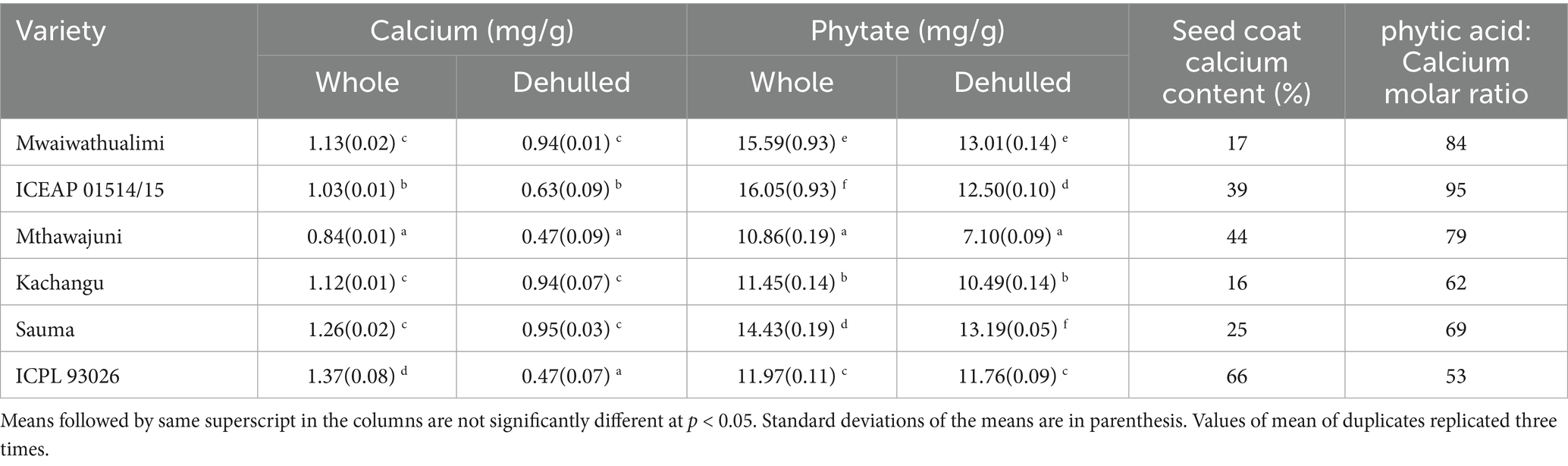
Table 4. Calcium, phytate composition, seed coat calcium content and phytic acid: calcium molar ratio of whole and dehulled pigeon peas.
Phytate content was significantly higher in whole compared to dehulled grains across all varieties (p = 0.001), except ICPL 93026. The phytic acid:calcium ratio was lowest in ICPL 93026 (53:1) and highest in ICEAP01514/15 (95:1). Lower ratios are associated with firmer cooked texture due to calcium pectate formation restricting cell separation, while higher ratios indicate greater calcium binding by phytate. Phytate in legumes is primarily located in cotyledons, and dehulling can increase phytic acid content on a unit weight basis. The observed differences may be attributed to varietal characteristics. A low phytic acid and calcium ratio is associated with harder cooked texture in legumes, where calcium pectate formation restricts cell separation, resulting in a grainy texture (Mwangwela et al., 2021). This low ratio signifies reduced calcium binding to phytic acid, allowing free calcium migration and calcium pectate formation that restricts cell separation, ultimately leading to longer cooking times and increased cooked bean hardness. Mwangwela et al. (2021) reported a negative correlation between phytic acid:calcium ratio and cooking time in beans meaning that the higher the ratio, the less the cooking time (Mwangwela et al., 2021).
3.2 Water absorption during soaking
Water absorption mechanisms during soaking were primarily influenced by variety (p < 0.001), while seed coat thickness and the variety-seed coat interaction showed no significant effects. This suggests that individual varietal characteristics, rather than physical seed coat properties, determine water absorption capabilities.
Among whole seeds, Mthawajuni demonstrated the highest water uptake capacity, achieving rapid initial absorption within the first hour and maintaining superior hydration throughout the 6-h soaking period (1,000 g kg−1). Four varieties (Mwaiwathualimi, Kachangu, Mthawajuni, and ICEAP93026) exhibited biphasic absorption patterns characterized by accelerated initial uptake followed by equilibrium after 5 h (Figure 1). This hydration behavior aligns with established mechanisms whereby water imbibition in legumes is governed by seed physical characteristics and macromolecular composition, particularly protein content (Wiraguna, 2022).
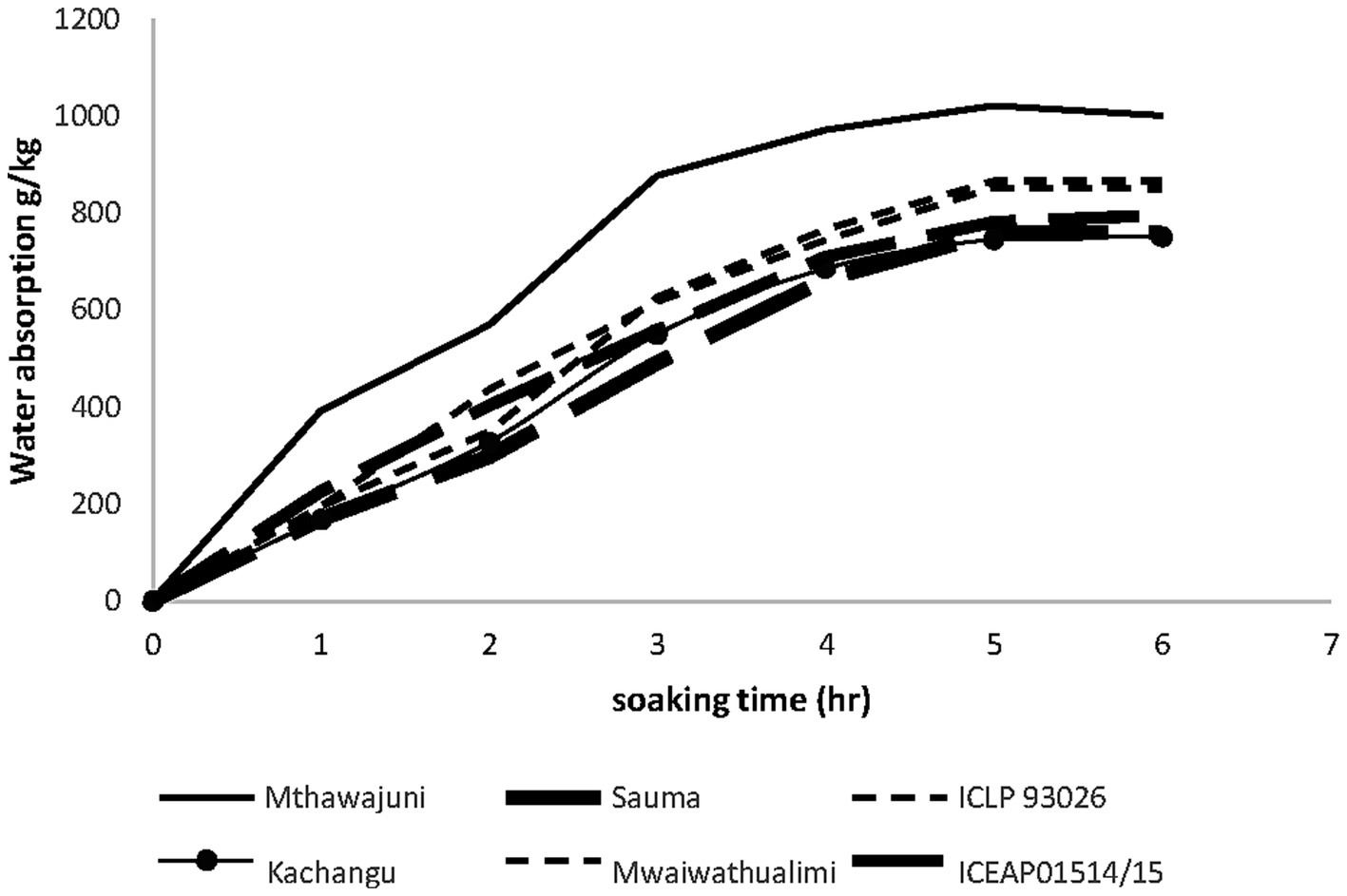
Figure 1. Water absorption during 6 h of soaking of whole seed pigeon pea varieties at room temperature.
The superior water absorption in Mthawajuni can be attributed to two complementary factors, elevated protein content, which enhances hydrophilic interactions, and reduced bulk density, which facilitates rapid initial water penetration into capillary spaces and intercellular voids. Conversely, Mwaiwathualimi’s higher bulk density resulted in restricted initial water uptake due to compact particle arrangement, though its elevated protein content subsequently promoted sustained absorption throughout the 5-h period before declining in the final hour.
Dehulled pigeon peas exhibited markedly different hydration kinetics, reaching saturation within 2–3 h before plateauing (Figure 2). This equilibrium phase likely represents saturation of intercellular spaces and maximum hydration of hydrophilic biomolecules, including proteins, starch, and pectic substances (Coffigniez et al., 2019).
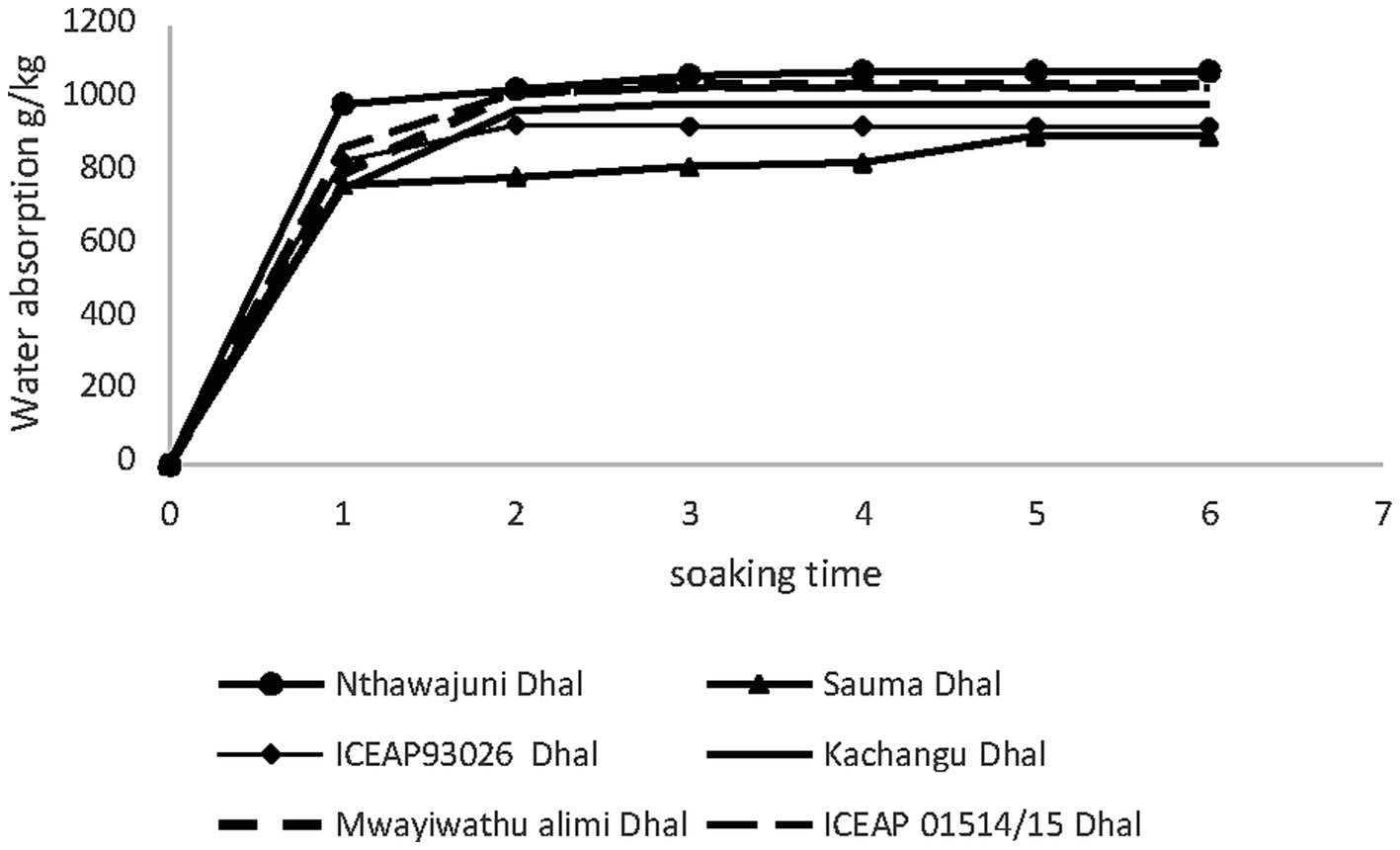
Figure 2. Water absorption during soaking of dehulled pigeon pea varieties at different time intervals.
A distinct temporal divergence in water absorption patterns was observed between processing treatments. Whole seeds required 5–6 h to achieve saturation, while dehulled seeds reached equilibrium in 2–3 h. This acceleration in dehulled samples directly reflects the removal of the seed coat barrier, which substantially enhances water penetration rates by eliminating the primary diffusion-limiting layer (Makinde and Abolarin, 2020).
3.3 Water absorption during cooking
Water absorption during cooking was significantly influenced by variety (p < 0.001), demonstrating difference in mechanism profiles across cultivars and processing treatments (Table 5).
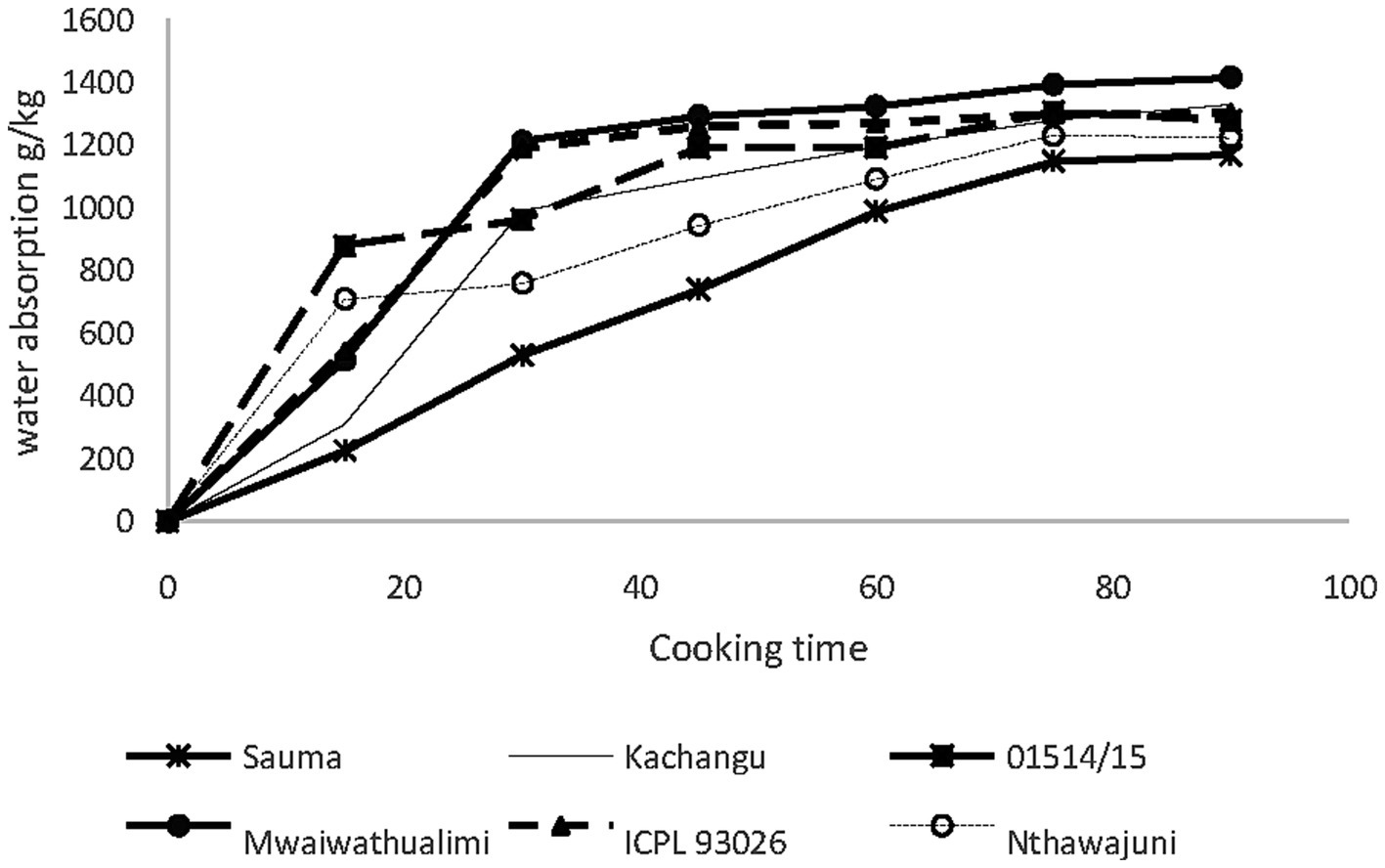
In whole seed cooking trials (Figure 3), water uptake exhibited a pattern with three distinct phases. During the initial 15-min period, ICEAP 01514/15 demonstrated superior water absorption compared to other varieties, while all cultivars showed rapid initial uptake which is attributed to water infiltration through hilum capillaries and surface pores (Devkota et al., 2022). The second phase (15–30 min) was characterized by elevated absorption in Kachangu, Mwaiwathualimi, and ICPL 93026, this happens with the onset of starch gelatinization and protein denaturation processes. Subsequently, water uptake rates declined progressively until reaching equilibrium at 75 min, indicating complete hydration of gelatinized starch and denatured protein matrices.
The enhanced water absorption capacity observed in Kachangu and Mwaiwathualimi during cooking correlates with their elevated protein content, consistent with established relationships between protein concentration and hydration capacity in legume seeds (Upretee et al., 2024). This supports the mechanistic role of proteins as primary hydrophilic macromolecules enhance water uptake capabilities.
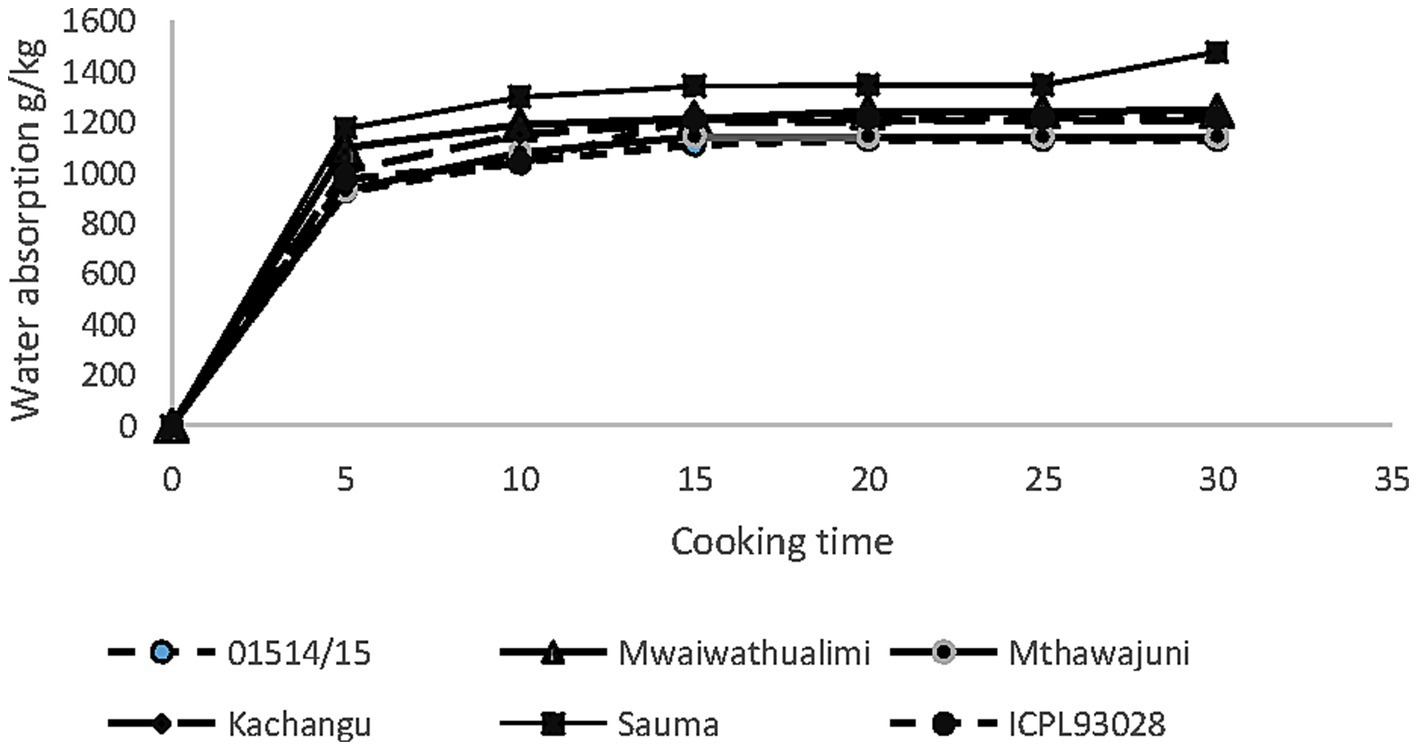
Dehulled pigeon peas exhibited markedly accelerated hydration capabilities, with pronounced water uptake occurring within the first 5 min of cooking (Figure 4). This rapid absorption directly reflects the elimination of the seed coat diffusion barrier, allowing unimpeded water access to cotyledonary tissues. Water absorption in dehulled samples reached saturation within 10–15 min, representing a 5-fold reduction in equilibration time compared to whole seeds (Figure 5).
The accelerated saturation in dehulled cotyledons can be attributed to the combined effects of barrier removal and enhanced heat-mediated molecular transformations, including carbohydrate gelatinization and protein thermal dissociation, which collectively promote increased water binding capacity (Li, 2022). This processing induced modification of tissue and macromolecular structure altering the hydration behavior during thermal treatment.
3.4 Cooking time for whole and dehulled pigeon peas
Cooking time was significantly influenced by variety, while seed coat thickness showed no effect, and no variety-seed coat thickness interaction was detected. This indicates that genetic factors governing seed composition and structure, rather than physical seed coat dimensions, primarily determine cooking behavior.
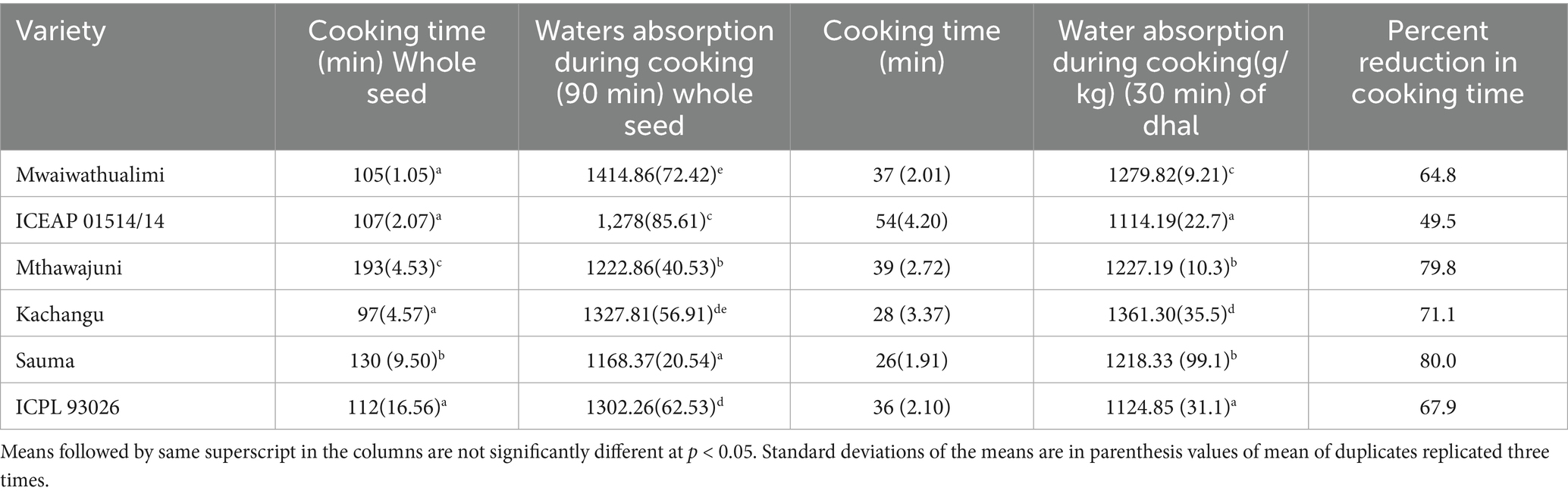
Cooking quality, defined as the achievement of consumer-acceptable tenderness in dry legume seeds, represents a critical functional property given the characteristically prolonged cooking times required for most dried legumes to attain optimal softness and palatability (Chigwedere et al., 2019). Four varieties (Kachangu, Mwaiwathualimi, ICEAP93026, and ICEAP01514/15) demonstrated statistically equivalent cooking times that were significantly shorter than other cultivars (Table 5).
The rapid-cooking phenotype in these varieties can be attributed to several complementary mechanisms. Enhanced water uptake capacity, a well-established predictor of reduced cooking time (Jeevarathinam and Chelladurai, 2020), facilitates efficient heat transfer and subsequent macromolecular transformations. Additionally, elevated phytic acid/calcium ratios may contribute to accelerated cooking by isolating calcium ions, thereby reducing calcium migration to the middle lamella and preventing excessive calcium pectate formation (Mwangwela et al., 2021).
The cooking process involves sequential biochemical transformations critical to seed softening. Initial starch gelatinization, facilitated by absorbed water acting as a heat transfer medium within cotyledonary tissues (Huang et al., 2024), must lead to protein denaturation to prevent competitive water binding that could restrict starch granule swelling (Awuchi et al., 2019). Varieties exhibiting prolonged cooking times typically contain elevated levels of insoluble dietary fiber, which result in cellular resistance to thermolysis (Hamad, 2021), and may accumulate hydrophobic compounds derived from phenolic oxidation in seed coats, reducing water permeability (Li et al., 2020). Furthermore, dense cotyledonary cellular matrix with limited intercellular spaces impedes water diffusion and heat transfer, contributing to extended cooking time requirements (Perera et al., 2023).
Dehulling dramatically improved cooking efficiency, reducing cooking times by 49.5–80% (mean reduction: 66%) compared to whole seeds. This substantial improvement reflects the elimination of the primary water diffusion barrier, as seed coat removal fundamentally alters hydration mechanism during thermal processing (Munthali et al., 2022). The seed coat constitutes the principal hindrance to water imbibition, controlling permeability rates through its physico-chemical properties (Upretee et al., 2024). Beyond reducing cooking time, dehulling provides additional processing benefits including tannin reduction and enhanced digestibility (Wu et al., 2024), making it a valuable post-harvest intervention for improving both functional and nutritional quality of pigeon pea.
3.5 Seed coat splitting during cooking of whole pigeon peas
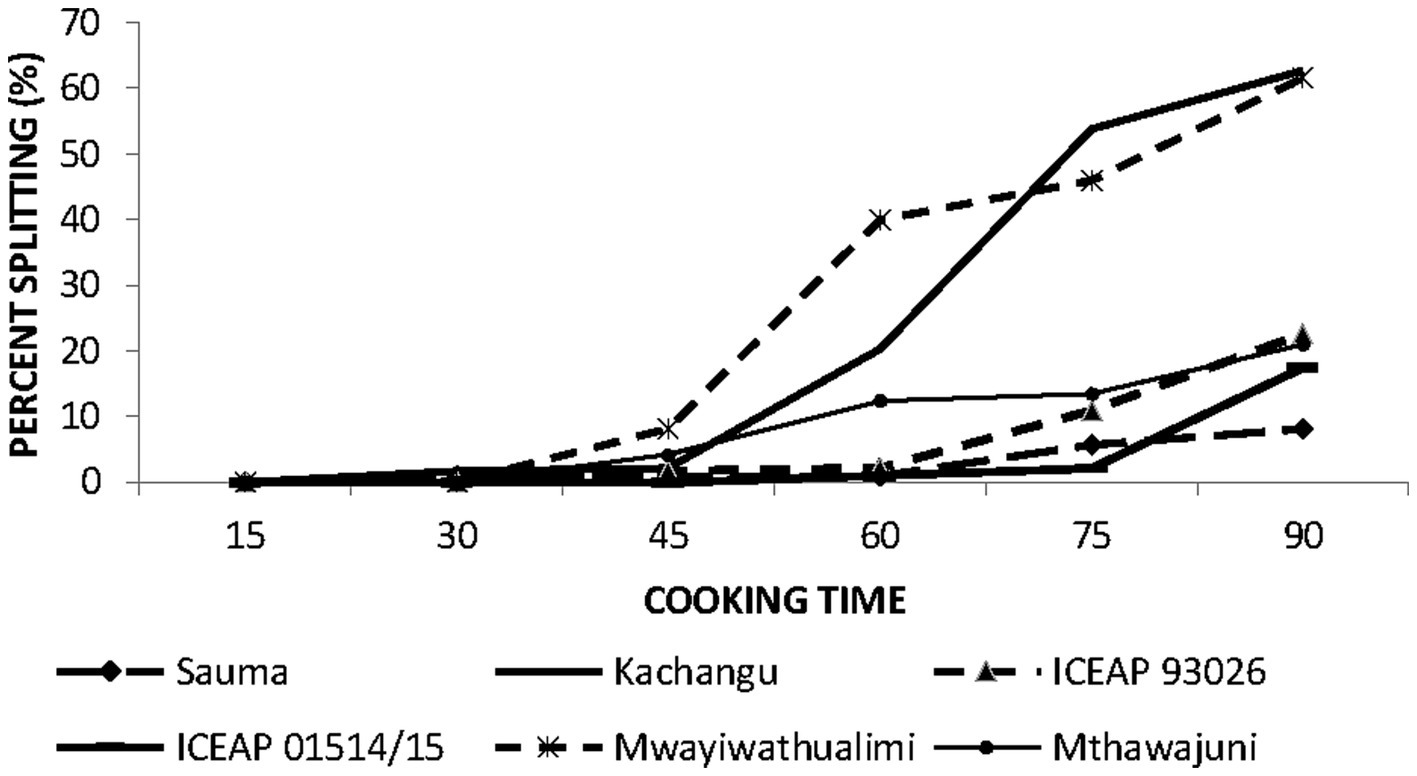
Seed coat integrity during thermal processing represents a critical quality parameter affecting consumer acceptance of cooked legumes. Split formation was not observed until 45 min of cooking, after which splitting incidence varied significantly among varieties.
At 90 min of cooking, Mwaiwathualimi and Kachangu exhibited the highest splitting frequencies compared to other cultivars. A positive relationship was observed between bulk density, water absorption capacity, and splitting susceptibility. Dense seed varieties, demonstrated by Mwaiwathualimi, undergo substantial volumetric expansion during hydration, generating internal mechanical stress that enhances cotyledon splitting (Mwangwela et al., 2021). The pronounced splitting in Kachangu may be attributed to the combined effects of high final water uptake and variety-specific structural characteristics that compromise seed coat integrity under thermal stress.
Conversely, mineral composition of the seed coat plays a protective role in maintaining structural integrity. ICPL 93026, containing elevated seed coat calcium concentrations (1.360 ± 0.00 mg g−1), demonstrated superior resistance to splitting. This protective effect is mechanistically explained by calcium-pectin cross linking, which forms a robust polysaccharide network that reinforces seed coat structure and enhances resistance to thermal-induced mechanical failure (Nayagam and Rajan, 2021). High concentrations of calcium, sodium, and iron collectively contribute to seed coat stability by strengthening intercellular matrix interactions.
The significance of splitting resistance extends beyond processing considerations, as visual appearance of both raw and cooked legumes constitutes a primary determinant of consumer acceptance (Asiimwe et al., 2024). Therefore, varieties exhibiting low splitting tendencies during cooking possess enhanced market value and processing utility, making this trait an important selection criterion in cultivar development programs.
3.5.1 Strengths and limitations
This study strength relies on its study design, controlled experimental design with appropriate replication across treatments. Such an approach enhances the reliability and internal validity of the findings. Nonetheless, the study has notable limitations. It is restricted to six pigeon pea varieties derived from a single growing season and location, which constrains the external validity and limits the generalizability of results across broader environmental conditions and genetic backgrounds. Furthermore, the absence of sensory consumer evaluation data reduces its applicability to practical adoption. Future research should extend varietal and environmental coverage and incorporate sensory analyses, while also investigating the mechanism behind the observed lack of correlation between seed coat thickness and cooking properties.
4 Conclusion
This study demonstrates that varietal selection and processing methods significantly influence pigeon pea functional properties, with critical implications for food processing and nutritional accessibility. While variety primarily determined cooking characteristics, seed coat thickness showed no correlation with cooking behavior, indicating that biochemical rather than morphological factors influence thermal processing.
Dehulling achieved substantial cooking time reductions (66% average, from 97–193 min to 26–54 min), representing a breakthrough for both industrial processing and household preparation. This translates into reduced energy consumption, supporting sustainable food practices and improving economic viability of pigeon pea products.
These findings support dehulling as a strategic intervention optimizing pigeon pea utilization within sustainable dietary frameworks. By reducing cooking energy while maintaining nutrition, dehulled pigeon peas serve as accessible protein sources in resource-constrained environments, supporting global plant-based protein initiatives.
Integrating appropriate varietal selection with strategic processing emerges as a pathway toward enhancing pigeon pea contributions to food security and sustainable food systems. Future research should focus on energy-efficient dehulling technologies and shelf-life characteristics of processed products. Though the hydration behavior of the pigeon pea aligns with the established mechanisms in literature for legumes, this area may need to be further investigated with respect to the specific macromolecular content of the pigeon pea aside from protein.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TM: Investigation, Validation, Data curation, Software, Writing – review & editing, Methodology, Visualization, Resources, Conceptualization, Project administration, Writing – original draft, Funding acquisition, Formal analysis, Supervision. VK: Supervision, Writing – review & editing, Conceptualization, Funding acquisition, Project administration, Visualization, Methodology, Resources, Validation. AM: Conceptualization, Writing – review & editing, Funding acquisition, Supervision, Project administration, Validation, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by United States Agency for International Development (USAID).
Acknowledgments
The authors acknowledge students from Home Economics Department who took part in sensory evaluation during the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abati, J., Zucareli, C., Brzezinski, C. R., Lopes, I. O. N., Krzyzanowski, F. C., Moraes, L. A. C., et al. (2022). Water absorption and storage tolerance of soybean seeds with contrasting seed coat characteristics. Acta Sci. Agron. 44:e53096. doi: 10.4025/actasciagron.v44i1.53096
Abiola, T., Akinyode, O., and Folami, I. (2019). Nutritional profile, protein quality and the biological value of raw and cooked pigeon pea (Cajanus cajan) seeds. EC Nutr. 14, 218–226.
Ajibola, O., Aviara, N., and Ajetumobi, O. (2003). Sorption equilibrium and thermodynamic properties of cowpea (Vigna unguiculata). J. Food Eng. 58, 317–324. doi: 10.1016/S0260-8774(02)00394-1
AOAC. (2000). Official methods of analysis. 17th Edn, The association of official analytical chemists, Gaithersburg, MD, USA.
Asiimwe, R., Katungi, E., Marimo, P., Mukankusi, C., Rubyogo, J. C., and Anthony, V. (2024). Evaluating consumer preferences for reduced cooking time, taste and colour of beans in rural and urban communities in Uganda. Agric. Food Secur. 13:19. doi: 10.1186/s40066-024-00466-4
Awuchi, C.-G., Igwe, V.-S., and Echeta, C.-K. (2019). The functional properties of foods and flours. Int. J. Adv. Acad. Res. 5, 139–160.
Bamidele, O., and Akanbi, C. (2015). Effect of gamma irradiation on amino acids profile, minerals and some vitamins content in pigeon pea (Cajanus cajan) flour. Br. J. Appl. Sci. Technol. 5, 90–98. doi: 10.9734/BJAST/2015/10245
Barzehkar, S. (2024). Exploring the dynamics between deforestation and energy use for cooking in Tanzania. Norway: Norwegian University of Life Sciences.
Bayero, A., Datti, M., Yahya, S., Oduah, A. T., Salihu, L., Lado, U. A., et al. (2019). Proximate composition and the mineral contents of soya beans (Glycine max) available in Kano state, Nigeria. Chem. J. 10, 62–65.
Bule, M., Khan, F., Nisar, M. F., and Niaz, K. (2020). Tannins (hydrolysable tannins, condensed tannins, phlorotannins, flavono-ellagitannins). Recent Adv. Nat. Prod. Anal. 1, 132–146.
Chen, D., Bernaerts, T., Debon, S., Oduah, C. O., Zhu, L., Wallecan, J., et al. (2023). Novel insights into the role of the pectin-cation-phytate mechanism in ageing induced cooking texture changes of red haricot beans through a texture-based classification and in situ cell wall associated mineral quantification. Food Res. Int. 163:112216. doi: 10.1016/j.foodres.2022.112216
Chigwedere, C. M., Njoroge, D. M., van Loey, A. M., and Hendrickx, M. E. (2019). Understanding the relations among the storage, soaking, and cooking behavior of pulses: a scientific basis for innovations in sustainable foods for the future. Compr. Rev. Food Sci. Food Saf. 18, 1135–1165. doi: 10.1111/1541-4337.12461
Choi, J., and Kim, W. K. (2020). Dietary application of tannins as a potential mitigation strategy for current challenges in poultry production: a review. Animals 10:2389. doi: 10.3390/ani10122389
Coffigniez, F., Briffaz, A., Mestres, C., Akissoé, L., Bohuon, P., and el Maâtaoui, M. (2019). Impact of soaking process on the microstructure of cowpea seeds in relation to solid losses and water absorption. Food Res. Int. 119, 268–275. doi: 10.1016/j.foodres.2019.02.010
Cosme, F., Aires, A., Pinto, T., Oliveira, I., Vilela, A., and Gonçalves, B. (2025). A comprehensive review of bioactive tannins in foods and beverages: functional properties, health benefits, and sensory qualities. Molecules 30:800. doi: 10.3390/molecules30040800
Devkota, L., He, L., Midgley, J., and Haritos, V. S. (2022). Effect of seed coat microstructure and lipid composition on the hydration behavior and kinetics of two red bean (Phaseolus vulgaris L.) varieties. J. Food Sci. 87, 528–542. doi: 10.1111/1750-3841.16030
Giusti, M. M., Miyagusuku-Cruzado, G., and Wallace, T. C. (2023). Flavonoids as natural pigments. In: T. Bechtold, A. P. Manian, and T. Pham Handbook of natural colorants. New York: John Wiley and Sons, pp. 371–390.
Gu, J., Bk, A., Wu, H., Lu, P., Nawaz, M. A., Barrow, C. J., et al. (2023). Impact of processing and storage on protein digestibility and bioavailability of legumes. Food Rev. Intl. 39, 4697–4724. doi: 10.1080/87559129.2022.2039690
Hamad, A. M. (2021). Evaluation of dietary fiber and the effect on physicochemical properties of foods. Int. J. Sci. Res. Sci. Technol. 8, 421–433. doi: 10.32628/IJSRST218385
Heydari Foroushani, M. M. (2022). Investigation of lentil seed behavior under microwave and microwave-infrared thermal treatments and their impact on modifying the physico-chemical and functional properties. University of Saskatchewan.
He, Y., Wang, B., Wen, L., Wang, F., Yu, H., Chen, D., et al. (2022). Effects of dietary fiber on human health. Food Sci. Human Wellness 11, 1–10. doi: 10.1016/j.fshw.2021.07.001
Huang, P.-H., Chiu, C. S., Lu, W. C., Shao, H., and Li, P. H. (2024). Study on activation energy and water adsorption behavior of adzuki beans under different soaking and cooking processing. J. Agric. Food Res. 18:101313. doi: 10.1016/j.jafr.2024.101313
Jawalekar, K. V., Baishya, S., Rathi, S., Gade, K., Jagtap, M., Ithape, D. M., et al. (2020). Assessment of nutritional composition in prominent Cajanus cajan germplasm in India. Int. J. Curr. Microbiol. App. Sci. 9, 387–397. doi: 10.20546/ijcmas.2020.912.049
Jeevarathinam, G., and Chelladurai, V. (2020). Pigeon pea. Pulses: processing and product development. Berlin: Springer, 275–296.
Kaliniewicz, Z., Choszcz, D., and Lipiński, A. (2022). Determination of seed volume based on selected seed dimensions. Appl. Sci. 12:9198. doi: 10.3390/app12189198
Khazaei, H., and Vandenberg, A. (2020). Seed mineral composition and protein content of faba beans (Vicia faba L.) with contrasting tannin contents. Agronomy 10:511. doi: 10.3390/agronomy10040511
Khrisanapant, P., Kebede, B., Leong, S. Y., and Oey, I. (2019). A comprehensive characterisation of volatile and fatty acid profiles of legume seeds. Foods 8:651. doi: 10.3390/foods8120651
Kuhn, R., Jensch, R., Bryant, I. M., Fischer, T., Liebsch, S., and Martienssen, M. (2020). Rapid sample clean-up procedure for aminophosphonate determination by LC/MS analysis. Talanta 208:120454. doi: 10.1016/j.talanta.2019.120454
Kunyanga, C. N., Imungi, J. K., and Vellingiri, V. (2013). Nutritional evaluation of indigenous foods with potential food-based solution to alleviate hunger and malnutrition in Kenya. J. Appl. Biosci. 67, 5277–5288. doi: 10.4314/jab.v67i0.95049
Li, C. (2022). Recent progress in understanding starch gelatinization-an important property determining food quality. Carbohydr. Polym. 293:119735. doi: 10.1016/j.carbpol.2022.119735
Li, P., Li, Y., Wang, L., Zhang, H., Qi, X., and Qian, H. (2020). Study on water absorption kinetics of black beans during soaking. J. Food Eng. 283:110030. doi: 10.1016/j.jfoodeng.2020.110030
Magomya, A., Kubmarawa, D., Ndahi, J. A., and Yebpella, G. G. (2014). Determination of plant proteins via the Kjeldahl method and amino acid analysis: a comparative study. Int. J. Sci. Technol. Res. 3, 68–72.
Majili, Z. (2023). Pigeon peas: an opportunity for improving nutrient content of food consumed among resource-poor households to ensure sustainable health. Tanzania J. Agric. Sci. 22, 358–372.
Majili, Z. S., Nyaruhucha, C., Kulwa, K., Mutabazi, K., Rybak, C., and Sieber, S. (2020). Preferences and consumption of pigeon peas among rural households as determinants for developing diversified products for sustainable health. Sustainability 12:6130. doi: 10.3390/su12156130
Makinde, F., and Abolarin, O. (2020). Effect of post-dehulling treatments on anti-nutritional and functional properties of cowpea (Vigna unguiculata) flour. J. Appl. Sci. Environ. Manag. 24, 1641–1647. doi: 10.4314/jasem.v24i9.23
Marolt, G., and Kolar, M. (2020). Analytical methods for determination of phytic acid and other inositol phosphates: a review. Molecules 26:174. doi: 10.3390/molecules26010174
Mulenga, H., Mwangwela, A. M., Kampanje-Phiri, J., and Mtimuni, B. (2021). Influence of gendered roles on legume utilization and improved child dietary intake in Malawi. Afr. J. Food Agric. Nutr. Dev. 21, 17764–17786. doi: 10.18697/ajfand.98.18205
Munthali, J., Nkhata, S. G., Masamba, K., Mguntha, T., Fungo, R., and Chirwa, R. (2022). Soaking beans for 12 h reduces split percent and cooking time regardless of type of water used for cooking. Heliyon 8:e10561. doi: 10.1016/j.heliyon.2022.e10561
Mwangwela, A., Mwachumu, M., and Banda, I. (2021). Cooking characteristics and consumer acceptability of bio-fortified beans.
Nayagam, J. R., and Rajan, R. (2021). Calcium oxalate crystals as raw food antinutrient: a review. J. Pharm. Res. Int 33, 295–301. doi: 10.9734/jpri/2021/v33i41B32368
Ojo, M. A. (2022). Tannins in foods: nutritional implications and processing effects of hydrothermal techniques on underutilized hard-to-cook legume seeds–a review. Prevent. Nutr. Food Sci. 27, 14–19. doi: 10.3746/pnf.2022.27.1.14
Perera, D., Devkota, L., Garnier, G., Panozzo, J., and Dhital, S. (2023). Hard-to-cook phenomenon in common legumes: chemistry, mechanisms and utilisation. Food Chem. 415:135743. doi: 10.1016/j.foodchem.2023.135743
Ravichandran, C., and Upadhyay, A. (2022). “Processing of pulses” in Agro-processing and food engineering: Operational and application aspects. eds. H. K. Sharma and N. Kumar (Berlin: Springer), 455–481.
Sabadoti, V. D., Miano, A. C., and Augusto, P. E. D. (2020). Automation of a Mattson bean cooker: a simple and a low-cost approach. J. Food Proc. Preserv. 44:e14769. doi: 10.1111/jfpp.14769
Serna Saldívar, S. O., and Hernández, D. S. (2020). “Dietary fiber in cereals, legumes, pseudocereals and other seeds” in Science and technology of fibers in food systems. ed. S. O. Serna Saldívar (Berlin: Springer), 87–122.
Sherpa, P. (2023). Effect of processing on phytochemicals present in horsegram (Macrotyloma uniflorum).
Solanki, C., Saha, D., and Singh, R. (2019). Moisture dependent physical properties of dehusked unsplitted pigeon pea. J. Food Saf. Food Qual. 70, 117–123. doi: 10.31083/0003-925X-70-117
Swami, S. B., Ghgare, S. N., Swami, S. S., Shinde, K. J., Kalse, S. B., and Pardeshi, I. L. (2020). Natural pigments from plant sources: a review. Pharma Innov. J. 9, 566–574.
Syed, R. (2019). A study of health promoting components in pigeon pea and its application in food models. Nashville: Tennessee State University.
Takemura, M., Sahara, T., and Misawa, N. (2021). Violaxanthin: natural function and occurrence, biosynthesis, and heterologous production. Appl. Microbiol. Biotechnol. 105, 6133–6142. doi: 10.1007/s00253-021-11452-2
Thiex, N., Novotny, L., and Crawford, A. (2012). Determination of ash in animal feed: AOAC official method 942.05 revisited. J. AOAC Int. 95, 1392–1397. doi: 10.5740/jaoacint.12-129
Tiwari, B. K., Gowen, A., and McKenna, B. (2020). Pulse foods: processing, quality and nutraceutical applications. Cambridge, MA: Academic Press.
Ukomadu, J., Odogwu, B., and Agbagwa, I. (2023). The culinary traits: cooking time and canning quality of pulses. Agroscience 22, 43–54. doi: 10.4314/as.v22i1.7
Upretee, P., Bandara, M. S., and Tanino, K. K. (2024). The role of seed characteristics on water uptake preceding germination. Seeds 3, 559–574. doi: 10.3390/seeds3040038
Vanniarajan, C., Magudeeswari, P., Gowthami, R., Indhu, S. M., Ramya, K. R., Monisha, K., et al. (2023). Assessment of genetic variability and traits association in pigeonpea [Cajanus cajan (L.) Millsp.] germplasm. Legum. Res. 46, 1280–1287. doi: 10.18805/LR-4442
Varriano- Marston, E., and Jackson, G. M. (1981). Hard to cook phenomenon in beans: Structural changes during storage and imbibition. J. Food Sci. 46, 1379–1385. doi: 10.1111/j.1365-2621.1981tb04179.x
Vermeulen, S. J., Park, T., Khoury, C. K., and Béné, C. (2020). Changing diets and the transformation of the global food system. Ann. N. Y. Acad. Sci. 1478, 3–17. doi: 10.1111/nyas.14446
Vieira, B. M., Silva, G. N., and Silva, M. I. (2025). “Nutritional elements I: nutrients, proteins, carbohydrates, and lipids” in Fundamentals of drug and non-drug interactions: Physiopathological perspectives and clinical approaches. ed. B. M. Vieira (Berlin: Springer), 35–56.
Wiraguna, E. (2022). Adaptation of legume seeds to waterlogging at germination. Crops 2, 111–119. doi: 10.3390/crops2020009
Wu, J., Zhou, Q., Zhou, C., Cheng, K. W., and Wang, M. (2024). Strategies to promote the dietary use of pigeon pea (Cajanus cajan L.) for human nutrition and health. Food Front. 5, 1014–1030. doi: 10.1002/fft2.381
Yadav, D. N., Tashir, S., Guru, P. N., Yadav, D. K., and Vashwakama, R. K. (2021). “Technological advancements in processing of legumes and pulses” in Advances in cereals processing technologies. ed. D. N. Yadav (Boca Raton, FL: CRC Press), 79–107.
Yohane, E. N., Shimelis, H., Laing, M., Shayanowako, A., Mathew, I., and Chintu, J. M. (2021). Pigeonpea production constraints and farmers’ trait preferences in Malawi: implications for variety design. S. Afr. J. Plant Soil 38, 326–337. doi: 10.1080/02571862.2021.1925760
Zakaria, F. R., Lioe, H. N., and Setiawan, R. D. (2024). Dietary fibre profiling of various edible parts of winged bean (Psophocarpus tetragonolobus L.): pods, whole seeds, endosperms, seed coats, and cooked seeds. Bioact. Carbohydr. Diet. Fibre 32:100425. doi: 10.1016/j.bcdf.2024.100425
Zhang, X., Zhang, L., Pu, Y., Sun, M., Zhao, Y., Zhang, D., et al. (2022). Global, regional, and national burden of protein–energy malnutrition: a systematic analysis for the global burden of disease study. Nutrients 14:2592. doi: 10.3390/nu14132592
Keywords: pigeon peas, cooking, soaking, seed coat, dehulling, proximate composition, calcium and phytate content
Citation: Machinjili TT, Kabambe V and Mwangwela AM (2025) Physicochemical and cooking properties of six pigeon pea (Cajanus cajan L) genotypes from Malawi. Front. Sustain. Food Syst. 9:1613117. doi: 10.3389/fsufs.2025.1613117
Edited by:
Oluwatoyin Oluwole, Federal Institute of Industrial Research Oshodi, NigeriaReviewed by:
Waseem Khalid, Government College University, PakistanMuhammad Zubair Khalid, Government College University, Pakistan
Copyright © 2025 Machinjili, Kabambe and Mwangwela. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Agnes M. Mwangwela, YW13YW5nd2VsYUBsdWFuYXIuYWMubXc=
†Present address: Vernon Kabambe, Malawi Adventist University, Ntcheu, Malawi
 Tamara Tumasile Machinjili
Tamara Tumasile Machinjili Vernon Kabambe2†
Vernon Kabambe2† Agnes M. Mwangwela
Agnes M. Mwangwela