- 1Department of Biological Sciences, College of Science, University of Jeddah, Jeddah, Saudi Arabia
- 2Department of Food Science and Human Nutrition, College of Agriculture and Food, Qassim University, Buraydah, Saudi Arabia
Background: This study was designed to extract and evaluate the physicochemical properties of gelatin recovered from chicken feet of different ages and its effect on sensory evaluation of the produced yogurt.
Methods: Fresh chicken feet of 12, 14 and 16 weeks old supplied by Al-watania poultry company (Saudi Arabia) were used to extract gelatin in this study.
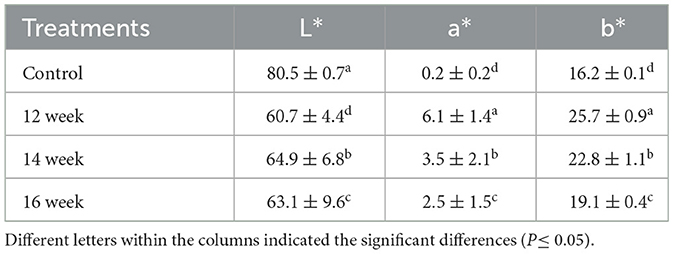
Results: The highest gelatin yield (3.4%) was obtained from chicken feet aged 16 weeks. No significant differences (p ≥ 0.05) were observed in both pH (6.21 to 6.34) and WHC (0.90% to 0.92%) of the gelatin. The highest gel strength value was 138.3 Bloom, which was observed in the gelatin extracted from chicken feet of 12- weeks age. The values of the melting point ranged from 29.3 to 33.9 and significantly (p ≥ 0.05) different. The control sample had the highest lightness value (L* 80.5), followed by gelatin from chickens aged 14 weeks (L* 64.9), 15 weeks (L* 63.1), and 12 weeks (L* 60.7). The highest yellowness (b* 25.7) was found in the gelatin from 12-week-old chicken, while the highest redness (a* 6.1) was also observed in the 12-week-old, followed by the 14-week-old.
Conclusion: Chicken leg gelatin is a natural alternative to gelatin extracted from other sources that many people may not agree to consume. Chicken gelatin has many properties and benefits, which makes it a preferred ingredient in many industries such as yogurt manufacturers, because its molecules work to form a weak gelatinous network structure, which prevents the separation of whey. The yogurt incorporated with gelatin achieved overall acceptability scores exceeding 8 (ranging from 8.5 to 8.7), indicating the successful application of gelatin in food systems.
1 Introduction
1.1 Gelatin and its functional properties
Gelatin is a protein derived from collagen, which is abundant in the connective tissues of all animals, including bones, cartilage, skin, and muscle tendons (Ahmad et al., 2023; Jongjareonrak et al., 2010). It can be obtained by partial hydrolysis/thermal denaturation (Siburian et al., 2020). Gelatin has several critical functional properties (Ghorani et al., 2020; Alipal et al., 2021). In addition to its role as a protein, gelatin is used in a variety of food industries due to its functional properties such as high water-binding capacity, emulsifying power, foaming ability, viscosity enhancement, elasticity, and formation of a protective layer around food (Luo et al., 2022). It has distinct properties when compared to other gel-forming polymers such as pectin and starch (Choe and Kim, 2018). When heated, gelatin transforms into a gel and can melt and solidify repeatedly without fracture (Torrejon et al., 2022).
1.2 Gelatin uses and applications
Technically, it may be used as a component to improve the flexibility, thickness, texture, and stability of food products, particularly in the manufacture of dairy, meat products and confections (Ahmad et al., 2023, Zhang et al., 2024), It is also used for packaging and film production, which provides substantial benefits in industrial applications. It is also utilized as a unique functional material in pharmaceutical, medicinal, and cosmetic products (Milano et al., 2023; Muñoz et al., 2004; Ahmad Anuar et al., 2023). Gelatin is not a complete protein because it lacks tryptophan one of the 10 necessary amino acids required (Mikhailov, 2023).
1.3 Gelatin from chicken feet
Many research works have been dedicated recently with the objectives of extracting and studying the properties of gelatin from chicken feet (Bagal-Kestwal et al., 2019; Mokrejš et al., 2019; Abedinia et al., 2020; Fatima et al., 2022; Aidat et al., 2023; Goudie et al., 2023; Harini et al., 2023; Sedaghat and Mohsenzadeh, 2022; Rather et al., 2022; Lu et al., 2022; Lamers et al., 2024; Usman et al., 2024; Al-Gheethi et al., 2021; Zambuto et al., 2024). Waste from slaughterhouses pollutes the environment and has negative effects on human health (Arshad, 2023; Ragasri and Sabumon, 2023; Wang et al., 2024). It is very important to treat the slaughterhouse waste by recycling and turning them into secondary materials that can be used (Philipp et al., 2021; Chowdhury et al., 2022; Ungureanu et al., 2024). It can be used in various food products such as yogurt, beverages, bakery, meat, and dairy products as thickening, binding, stabilizing and emulsifying agent (Usman et al., 2023; Gao et al., 2024). Saudi Arabia has a significant poultry industry, leading to abundant chicken feet as a byproduct. However, utilizing these chicken feet for gelatin extraction can reduce waste and create an alternative gelatin product.
1.4 Properties of chicken feet gelatin
The physicochemical properties of gelatin extracted from these chicken feet could vary based on chicken ages and the yogurt containing chicken feet gelatin will have enhanced sensory attributes properties such as taste, texture, color, and overall quality of the developed yogurt. Therefore, the objective of this research was to investigate the physicochemical properties of gelatin extracted from chicken feet of varying ages from a local poultry slaughterhouse in Saudi Arabia. Additionally, the study aimed to evaluate the impact of the extracted gelatin on the sensory properties of the developed yogurt.
2 Materials and methods
2.1 Materials
Fresh chicken feet of the ROSS strain were obtained from slaughterhouse of Al-watania Poultry Company, Buraidah, Qassim, Saudi Arabia and transported to the meat laboratory, Faculty of Agriculture and Food, Qassim University for gelatin extraction purpose. Chicken feet from the mentioned strain at three different ages (12, 14, and 16 weeks) which are produced and available in Al watania Poultry Compony, Saudi Arabia were chosen for extraction. Bovine standard gelatin, hydrochloric acid, sodium hydroxide and acetic acid were purchased from Sigma-Aldrich, Saudi Arabia.
2.2 Sample preparation
Chicken feet were prepared at the Meat Laboratory, College of Agriculture and Food, Qassim University according to the slightly modified method of Potti and Fahad (2017). The samples of chicken feet were first cleaned by removing the skin and fat. The samples were immersed in boiling water at 100°C for 40 min and then dried in an oven for 18 h at 50°C. Alkaline pretreatment was performed using sodium hydroxide solution (NaOH) with a concentration of 0.2% (wt/vol) to remove the non-collagenous material and mineral ions and dried in the oven. The dried samples were soaked in hydrochloric acid solution with a concentration of 89 0.2% (wt/vol at room temperature (27 ± 1°C). The soaking solution was changed every 3 days for 9 days.
2.3 Extraction of gelatin
Gelatin extraction was carried out according to the method of Ab Rahim et al. (2021). The pre-treated chicken feet were cut into small pieces and were immersed in distilled water at a temperature of 70°C in a ratio of 1:9 (w/v) in a shaking water bath and shaken for 90 min. This process was repeated three times until the chicken feet became soft and ready for extraction. After that, the samples were soaked in acetic acid at a concentration of 0.2% (v/v) for 40 min. The resulting acidic extract was then dried and washed with distilled water until the pH became neutral. The extract was filtered using two layers of cheese cloth, pour it sanitized trays individually and dried in the oven at 105°C for 24 h. The dried gelatin obtained was ground individually using electric grinder model Moulinex, AR110027, France. The powders of the final gelatin of the different ages were obtained.
2.4 Gelatin yield
Gelatin yield (GY) was calculated using the following equation according to the method of Balaji Wamanrao and Tanaji (2022) according to Equation 1 below:
2.5 pH measurement
The gelatin solution was prepared individually by dissolving the gelatin powder in the ratio of 1:10 gelatin: distilled water (w/v). A pre-calibrated pH meter (pH meter HI 2211 – pH Meter HANNA) was used for pH measurement.
2.6 Gel strength
The gel strength was determined using the method of Hirbo et al. (2023). Gelatin solution was prepared by mixing the dried gelatin with distilled water. The mixture was left at room temperature for 30 min, pre-heated at a temperature of 65°C for 20 min until the gelatin was completely dissolved. The samples were stored at a refrigerator adjusted at a temperature of 4°C for 16 ± 2 h. To determine the gel strength the gelatin sample was transferred to a cylindrical mold measuring 3 cm in diameter and 2.5 cm in height to assess gel strength. A texture analyzer (Stable Micro System, Surrey, UK) with a 5 kg load cell, a crosshead speed of 1 mm/s, and a 1.27 cm diameter flat-faced cylindrical teflon plunger was used to evaluate gel strength. When the plunger penetrated 4 mm into the gelatin gels, the maximum force (in grams) was recorded.
2.7 Gel viscosity
The gel viscosity was measured according to the modified method. Gelatin powder was dissolved in distilled water in a ratio of 1:10 (W:V) and heated to 60°C. The viscosity was measured using a Brookfield digital viscometer model RVDV- I with spindle No. 1 at a speed of 60 rpm and a temperature of 40 ± 1°C.
2.8 Melting point (MP)
The melting point was measured according to the modified method of Choi and Regenstein (2000). The gelatin solution used in gel viscosity test was used in this measurement. It was placed in a screw cap test tubes. The samples were tightly closed and stored in the refrigerator at 7°C for 16 to 18 h. After that, the sample was transferred to a water bath at 10°C and placed upside down inside the water bath so that the temperatures were controlled. The heating process was carried out in stages by increasing the temperature by 1°C per min, and the melting point (MP) of the extracted gelatin was obtained by averaging the temperatures between the starting and ending melting points as follows (Equation 2):
2.9 Water holding capacity (WHC)
Modified method described by Warner (2014) was used to determine the WHC of the gelatin. One gram of the extracted gelatin was placed in a centrifuge tube using a device (SIGMA 3K30) and centrifuged at a speed of 920 rpm for 10 min. The percentage of centrifuged water was calculated by subtracting the weight of gelatin after the centrifugation process from the first basic weight (1.0 g), then the result was calculated as a percentage. The WHC values were calculated according to the following Equation 3:
2.10 Color measurement
The gelatin solution was prepared and cooled at 10 ± 1°C for 18 h. The color of the gelatin was determined according to Kim et al. (2020). The colorimeter (Hunter lab, model 16, Minolta Ltd., Japan) was used in the measurement. The device was calibrated using a white plate; L* = +97.83, a* = −0.43, and b* = +1.98). The following colors values were obtained (Lightness L*, redness a* and yellowness b*) which represent the mean intensity of lightness, redness, and yellowness, respectively.
2.11 Proximate analysis
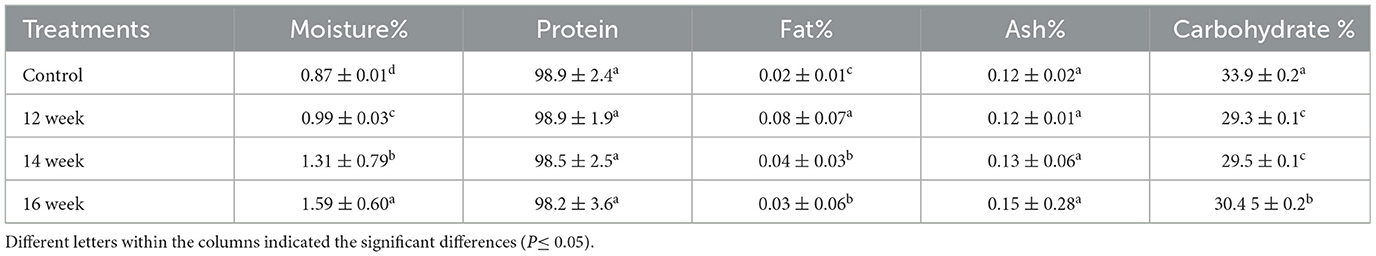
The proximate analysis values (moisture, protein, fat and ash) of the extracted gelatin were determined according to the method of AOAC (Horwitz and Latimer, 2000) and carbohydrates were obtained by subtraction from 100.
2.12 Preparation of the yogurt for sensory evaluation
The yogurt samples were prepared according to the modified published method of Arioui et al. (2018). Fresh cow milk was used in yogurt preparation. The total soluble solids and fats were maintained to be 15 and 3%, respectively. The milk was homogenized and heated to 90°C for 3 min for pasteurization. Once cooled to 45°C, the gelatin powder was incorporated individually in percentages of 0%, 1%, 2%, 3% into the pasteurized milk and stirred until completely dissolved in the milk. The starter [(Streptococcus thermophilus (YC-X16) and Lactobacillus bulgaricus (C H N- 1 1), CHR HANSEN Denmark)] was added the incorporated milk at a percentage of 3%. The treated milk samples were poured into yogurt cups and distributed randomly using different three-digit numbers and kept into incubation room set at 45°C for 4 h. The yogurt samples incorporated with the gelatin were then cooled to 4°C overnight for sensory evaluation.
2.13 Sensory evaluation studies
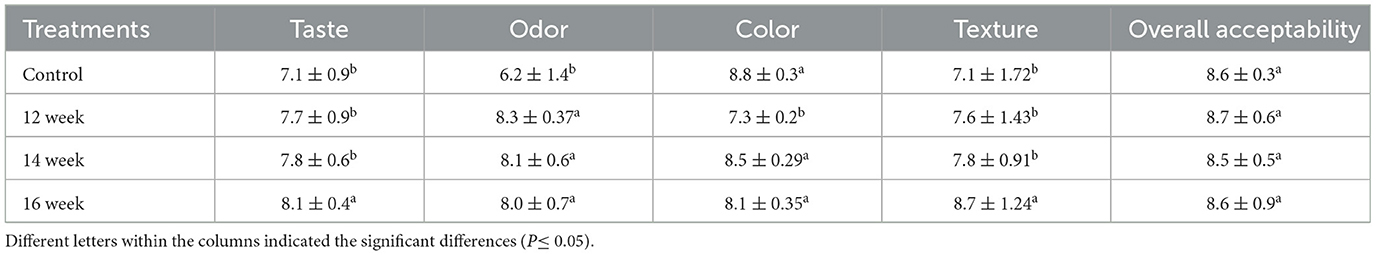
The prepared yogurt samples were evaluated the following day by 25 untrained faculty members and students from the College of Agriculture and Food, Qassim University, KSA, who regularly consume yogurt. This evaluation followed the guidelines and protocols established by the university's ethical committee of the university (details provided in the declarations). The yogurt samples were evaluated to the following attributes: taste, smell, color, texture, and overall acceptability. The panelists were asked to indicate how much they liked or disliked the prepared yogurt using a hedonic scale [1 = dislike extremely, 9 = like extremely]. The scores were obtained and statistically analyzed.
2.14 Statistical analysis
The data obtained from 3 replicates were statistically analyzed. The statistical analysis was performed using Two–way analysis of variance (ANOVA) followed by Duncan's Multiple Range Test with P ≤ 0.05 significance level. Minitab Statistical Software version 17 was used for data analysis.
3 Results and discussion
3.1 Gelatin yield
Figure 1 shows the yield of the gelatin. The percentage was 3.4%, obtained from the chicken feet of 16 weeks, followed by 2.7%, obtained from chicken feet of 14 weeks and 2.2%, obtained in the chicken feet of 12 weeks' age. It was noticed that the yield of the gelatin obtained increased as the age of the chicken increased. This result suggests a correlation between the yield of the gelatin and the age of the chicken extracted from their feet which means that older chicken feet produce higher gelatin yield. This finding agreed with the earlier finding observed by Rana et al. (2024). They found that older chickens (42 days old) yielded more gelatin compared to younger ones (21 and 35 days old). Specifically, the gelatin yields on a dry weight basis were 0.132% for 21-day-old chickens, 0.182% for 35-day-old chickens, and 0.215% for 42-day-old chickens. Older chicken develops tougher connective tissues which may be more abundant and denser offering more collagen to convert into gelatin when the extraction condition is optimized. However, the acid treatment and the temperature used in gelatin extraction process in this study may also affect the yield of gelatin. The difference in gelatin yield might be due to the different collagen contents in raw materials (Rao and Poonia, 2023). The variation in gelatin yield may result from varying amounts of collagen in the raw materials (Rao and Poonia, 2023). Sinthusamran et al. (2014) claimed that the gelatin yield was influenced by the raw material composition and the extraction conditions. However, Aykin-Dinçer et al. (2017) and Zou et al. (2024) stated that the different gelatin yields may result from variations in the length of the polypeptide chains caused by pretreatment, pH, temperature, and extraction time. Fatima et al. (2022) found that a high yield of 7.5% can be obtained from chicken feet if the extraction conditions are optimized with pretreatment of the chicken feet with acetic acid in a concentration of 4.2% and an extraction temperature of 66°C for 4.2 h. In another study Usman et al. (2024) investigated gelatin extraction condition in native chicken feet gelatin using 1:3 (w/v) of distilled water at 55°C for 6 h. They found that the yield obtained from native and broiler chicken feet using 10% acetic acid was found to be 7.93 and 7.06%, respectively, on a dry basis.
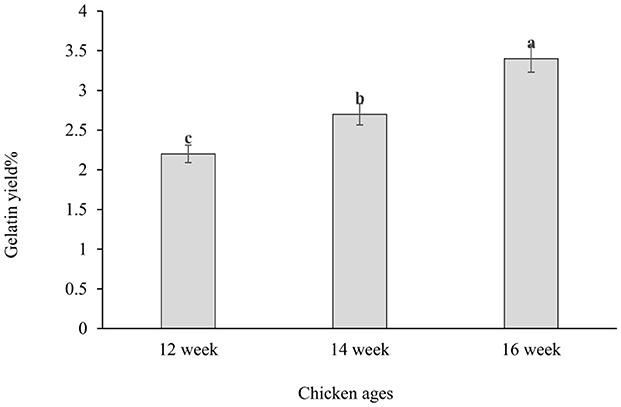
Figure 1. Gelatin yield extracted from chicken feet of different ages. The data with the lowercase subscription letters (a, b, c) are significantly (P ≤ 0.05) different.
3.2 pH measurement
Figure 2 shows the pH values which range from 6.21 to 6.34. This result suggested that pH value of the gelatin was not influenced by NaOH pretreatments during extraction process (p ≥ 0.05). This finding disagreed with the finding of Saenmuang et al. (2019). They found that the pH of gelatin extracted from black-bone chicken feet and skin were acidic ranging from 3.71 to 4.81. Furthermore, the washing technique is essential for eliminating acid and/or alkaline residues. In addition, the washing procedure is an important step in removing acid and/or alkaline residues. The pH levels of chicken gelatins were previously reported by Kim et al. (2012) and Widyasari and Rawdkuen (2014). In those investigations, the pH of chicken gelatins was found to be close to neutral, ranging from 6.1 to 6.8 which is in a good agreement with finding in this study. The control gelatin's pH dropped from 6.5 to 6.2 in the treated samples. The same observation was reported by Taufik (2010) who notied that immersing the feet in a mixture of sodium hydroxide and acetic acid and repeating the process helps decrease the acidity of the gelatin.
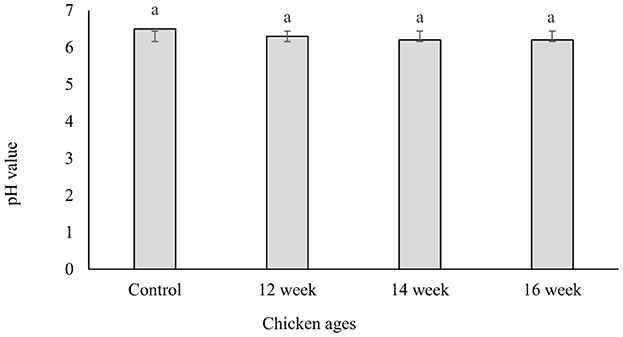
Figure 2. pH values of gelatin extracted from chicken feet of different ages. The data with the lowercase subscription letters "a" above the bars are not significantly (P ≥ 0.05) different.
3.3 Gel strength
The gel strength values of the gelatin are presented in Table 1. Saenmuang et al. (2019) reported that gelatin is generally divided into three categories: high bloom (200–300 g), medium bloom (100–200 g), and low bloom (50–100 g) at 6.67 percent (Bloom value).The values of gel strength in this study are significantly (P ≤ 0.05) different and were ranged from 111.2 to 138.3 g, classifying as medium bloom gelatin. However, this result agreed with Santana et al. (2020) who reported that gel strength of chicken feet gelatin had gel strength value of 119.1 g bloom. The control sample had the highest value of 138.3 bloom followed by 130.2, 120.8, and 111.2 g, in gelatin extracted from chicken feet aged 16, 14, and 12 weeks, respectively. The chicken feet from the oldest chickens exhibited the highest gel strength values. It was reported that gelatin with medium bloom has moderate gel-forming ability and is commonly used in thickening food products such as yogurt (Supavititpatana et al., 2008), cariating a strong and flexible gels (Bahar and Kusumawati, 2021). According to Calvarro et al. (2016) this variation in the bloom strength may be due to chicken feet pretreatment and hydroxyproline and proline content in chicken feet, because hydroxyline and proline are major components of collagen and play key roles in stabilizing the protein structure (Revert-Ros et al., 2024).
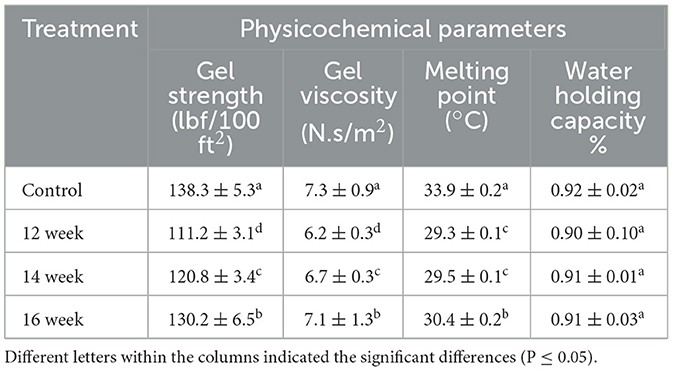
Table 1. Physicochemical parameters measured in the gelatin extracted from different chicken strain feet.
3.4 Gel viscosity
The viscosity values are present in Table 1. The values range from 6.2 to 7.3 cP. This result agreed with viscosity value reported (Rafieian et al., 2015). They found that most gelatin viscosity values ranged from 6 to 7 cP. Mirzapour-Kouhdasht et al. (2019) claimed that high viscosity of gelatin may be due to the formation of random chain gelatin molecules because of the breakdown of hydrogen linkage from the triple helix structure. However, low viscosity (1-2 cP) the gelatin solution is quite thin, and gelatin derived from poultry with low viscosity may form softer or weaker gels when incorporated in food systems (Ashrafi et al., 2023). It was observed that physical properties in gelatin such as viscosity affect the properties of the gel, especially at the point of gel formation (Kurt et al., 2024). It was reported that gelatin with viscosity values ranged from 6 to 8 cP in gelatin solutions formed thicker, denser and stronger gels (Rather et al., 2022), making it suitable for application as thickener ingredient for many food products such as yogurt (Kurt et al., 2022).
3.5 Melting point
Table 1 shows the melting point of gelatin for each age. The average values of the melting point range from 29.3 to 33.9 °C. The values are significantly (P ≤ 0.05) different. This finding is coincided with that reported by Goudie et al. (2023). They reported that typical melting point of gelatin ranges between 30°C and 35°C. The highest value of the melting point was observed in control (33.9°C) followed by 30.4°C which recorded in the gelatin obtained from the chicken feet of 16 weeks' age. Zhang et al. (2020) reported that the variation in the melting point of gelatin depends on the exact method of extraction and the gelatin concentration. However, there was no significant (P ≥ 0.05) difference in the melting points values of the gelatin extracted from the chicken feet of 12- and 14-weeks' age, respectively. Avallone et al. (2021) reported that gelatin melts when exposed to body or room temperature. This fact supports the finding in this research regarding the values of melting point.
3.6 Water holding capacity (WHC)
Table 1 shows the WHC of the gelatin extracted from different chicken strain feet. The values range from 0.90% to 0.92%, there was no significant (P ≥ 0.05) difference in the WHC. This finding is in a good agreement with that of Rasli and Sarbon (2015), but it was observed that the ability to retain water increased from 0.90 to 0.91% with increasing chicken ages. Higher WHC however, indicates that the gelatin can absorb and hold more water (Fairuza and Amertaningtyas, 2024; Khalesi et al., 2024). Another explanation for the high WHC of the gelatin may be the interaction between protein molecules and gelatin polysaccharides, which may form compounds with positively charged protein bonds in the gel, which increase the structure of the protein gel, leading to a high hydrophilicity of the gel and a higher WHC (Derkach et al., 2022).
3.7 Color measurement
Table 2 shows that all the obtained color values differ significantly (P ≤ 0.05). The control sample had the lightest gelatin (L*80.5), whereas the gelatin extracted from chickens at 14, 15, and 12 years old yielded 64.9, 63.1, and 60.7, respectively. The gelatin extracted from the feet of 12 weeks age had the greatest redness value (a* 6.1) followed by 14 weeks age, 16 weeks age and control. The yellowness values showed a similar pattern. It was reported that the color values of gelatin depend on the extraction and degree of drying, as the color has no effect on the functional properties of the gelatin, but it influences its acceptance by the consumer (Rather et al., 2022). It was reported that a higher L* value indicates lighter, more transparent gelatin. Du et al. (2013) found that gelatin derived from chicken head exhibited higher L* and b* values, but lower a* values compared to gelatin extracted from turkey heads. Similarly, Chakka et al. (2017) observed increased L* and b* values alongside reduced a* values under varying acid concentrations. The type of raw material and the extraction conditions significantly influence the color characteristics of gelatin (Du et al., 2013). However, a positive b* value reflects yellow tones, which may be expected in chicken-derived gelatin.
3.8 Proximate composition
Table 3 presents the results of the proximate analysis of gelatin. The moisture content values were significantly different (P ≤ 0.05), with higher moisture levels observed in older samples. The control sample exhibited the lowest moisture content at 0.87%. The moisture content of the extracted gelatin ranged from 0.99% to 1.59%. The protein content of the extracted gelatin in this study exceeded 98%, which is higher than the protein percentage reported in the study by Widyasari and Rawdkuen (2014), where the protein content of chicken feet was found to be 90.06%. However, these findings disagree with those from previous studies on gelatins extracted from normal chicken waste, which reported protein levels ranging from 80% to 90% (Rafieian et al., 2015; Sarbon et al., 2013; Widyasari and Rawdkuen, 2014). Additionally, Almeida and Lannes (2013) observed that the protein content in chicken by-products was only 84.96%. The ash content ranged from 0.12% to 0.15%. These values were highly dependent on the age of the chickens; as the age of the chickens increased, the ash content also increased, although no significant differences were observed (P ≥ 0.05). These findings disagreed with those reported by Usman et al. (2024), who found that the ash content in native and broiler chicken feet gelatins was 2.06 and 1.85%, respectively.
3.9 Sensory evaluation studies
Table 4 shows the sensory evaluation attributes of the yogurt prepared using the extracted gelatin. There were significant (P ≤ 0.05) differences in the attributes evaluated. The control had the lowest values for both texture and odor attributes. The scores were 7.1 and 6.2, respectively. No significant (P ≤ 0.05) differences observed between control yogurt and samples prepared using 1 and 2% of the extracted gelatin in terms of taste, color, texture and overall acceptability. The yogurt prepared using 3% of the gelatin showed the highest score values for taste, texture and overall acceptability of 8.5–8.7. This finding agreed with that of Guo et al. (2022) who reported the sensory evaluation properties of yogurt supplemented with glycyrrhiza polysaccharide as potential replacement for gelatin in concentration of 0.1% and found that the highest total acceptance scores of 8.5. This finding suggested that adding the appropriate percentage of gelatin was sufficient to improve yogurt's sensory qualities. It has been noted that some food systems based on gelatin enhance the sensory quality of dairy products. This might be mostly because the final products fortified with gelatin have better flavors and textures/mouthfeel (Ares et al., 2007; Mudgil et al., 2018; Riantiningtyas et al., 2021; Mohammadnezhad and Farmani, 2022; Nami et al., 2023).
4 Conclusion
In conclusion, extraction yield, color, gel strength, melting point, water holding capacity and viscosity are important properties for applications of gelatin, with the latter 4 properties being the main criteria to determine the quality of gelatin. The quality properties of gelatin extracted from the feet of ROSS strain chickens were significantly influenced by the age of the chickens studied and the experiment's condition. Considering the results, it can be concluded that the gelatin extracted from ROSS strain chickens' feet could be utilized as thickening food grade to develop yogurt and enhance its sensory evaluation properties.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because the research was conducted by the following guidelines of the Committee of Research Ethics Institutional Review Board (IRB), Qassim University, constructed according to the decision no. 344859 dated 9/8/1437H – 16/05/2016. Ethics approval and consent to participate No animal or human study was involved in this work; however, for sensory evaluation study, the participants (all above 18 years old) gave their consent to participate. Proper protocols approved by the Ethical Committee were followed during the sensory evaluation. Further, the research was carried out following the guidelines provided by the Qassim University Ethics committee (Dr. Osama Mohamed, Dr. Abdullah Saleh, Dr. Ibrahim Saheh, Dr. Saad Ali, Dr. Fahad Saleh, Dr. Abdulrahman Mohamed, Dr. Abduaziz Abdulmohsen, Dr. Fahd Muqrin) constituted by the Qassim University, KSA. The Committee of Research Ethics Institutional Review Board (IRB), Qassim University approved the study.
Author contributions
AM: Supervision, Writing – original draft, Methodology, Validation, Conceptualization, Writing – review & editing. MA: Writing – original draft, Data curation, Methodology, Formal analysis, Supervision, Investigation, Resources, Writing – review & editing. RH: Project administration, Conceptualization, Writing – review & editing, Writing – original draft, Funding acquisition. IA: Resources, Methodology, Formal analysis, Data curation, Investigation, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the University of Jeddah, Jeddah, Saudi Arabia, under Grant no. (UJ-24-DR-20680-1). Herefore, the authors thank the University of Jeddah for its technical and financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fsufs.2025.1702713.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ab Rahim, H., Ahmad, H., and Ab Rahim, M. H. (2021). Extraction of gelatin from different parts of Gallus Gallus Domesticus. Curr. Sci. Techno. 1, 50–55. doi: 10.15282/cst.v1i1.6447
Abedinia, A., Nafchi, A. M., Sharifi, M., Ghalambor, P., Oladzadabbasabadi, N., Ariffin, F., et al. (2020). Poultry gelatin: characteristics, developments, challenges, and future outlooks as a sustainable alternative for mammalian gelatin. Trends Food Sci. Techno. 104, 14–26. doi: 10.1016/j.tifs.2020.08.001
Ahmad Anuar, N. A., Tukiran, N. A., and Jamaludin, M. A. (2023). Gelatin in halal pharmaceutical products. Malaysia J. Syar. Law 11, 64–78. doi: 10.33102/mjsl.vol11no1.344
Ahmad, M. I., LI, Y., Pan, J., Liu, F., Dai, H., Fu, Y., et al. (2023). Collagen and gelatin: Structure, properties, and applications in food industry. Inter. J. Bio. Macromol. 254:128037. doi: 10.1016/j.ijbiomac.2023.128037
Aidat, O., Belkacemi, L., Belalia, M., Khairi, Z. M., and Barhoum, H. S. (2023). Physicochemical, rheological, and textural properties of gelatin extracted from chicken by-products (feet-heads) blend and application. Int. J. Gastron. Food Sci. 32:100708. doi: 10.1016/j.ijgfs.2023.100708
Al-Gheethi, A., Ma, N. L., Rupani, P. F., Sultana, N., Yaakob, M. A., Mohamed, R. M. S. R., et al. (2021). Biowastes of slaughterhouses and wet markets: an overview of waste management for disease prevention. J. Environ. Sci. Eng. 1–4. doi: 10.1007/s11356-021-16629-w
Alipal, J., Pu'ad, N. M., Lee, T., Nayan, N., Sahari, N., Basri, H., et al. (2021). A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 42, 240–250. doi: 10.1016/j.matpr.2020.12.922
Almeida, P. F., and Lannes, S. C. D. S. (2013). Extraction and physicochemical characterization of gelatin from chicken by-product. J. Food Process. Eng. 36, 824–833. doi: 10.1111/jfpe.12051
Ares, G., GonCAlvez, D., Perez, C., Reolon, G., Segura, N., Lema, P., et al. (2007). Influence of gelatin and starch on the instrumental and sensory texture of stirred yogurt. Int. J. Dairy Technol. 60, 263–269. doi: 10.1111/j.1471-0307.2007.00346.x
Arioui, F., Saada, D. A., and Cheriguene, A. (2018). Functional properties of bovine bone gelatin and impact on physicochemical, microbiological and organoleptic quality of set yoghurt. Biotechnology 17, 1–11. doi: 10.3923/biotech.2018.1.11
Arshad, M. (2023). Climate Changes Mitigation and Sustainable Bioenergy Harvest Through Animal Waste: Sustainable Environmental Implications of Animal Waste. London: Springer Nature. doi: 10.1007/978-3-031-26224-1
Ashrafi, A., Babapour, H., Johari, S., Alimohammadi, F., Teymori, F., Nafchi, A. M., et al. (2023). Application of poultry gelatin to enhance the physicochemical, mechanical, and rheological properties of fish gelatin as alternative mammalian gelatin films for food packaging. Foods 12:670. doi: 10.3390/foods12030670
Avallone, P. R., Raccone, E., Costanzo, S., Delmonte, M., Sarrica, A., Pasquino, R., et al. (2021). Gelation kinetics of aqueous gelatin solutions in isothermal conditions via rheological tools. Food Hydrocoll. 111:106248. doi: 10.1016/j.foodhyd.2020.106248
Aykin-Dinçer, E., Koç, A., and Erbaş, M. (2017). Extraction and physicochemical characterization of broiler (Gallus gallus domesticus) skin gelatin compared to commercial bovine gelatin. Poult. Sci. 96, 4124–4131. doi: 10.3382/ps/pex237
Bagal-Kestwal, D. R., Pan, M. H., and Chiang, B. H. (2019). “Properties and applications of gelatin, pectin, and carrageenan gels,” in Bio Monomers for Green Polymeric Composite Materials (Hoboken, NJ: Wiley), 117–140.
Bahar, A., and Kusumawati, N. (2021). Comparison of the physico-chemical properties of type,-B., halal gelatin from bovine and goat skin material. IOP Conference Series: Earth, and Environmental, Science, IOP Publishing 012033. doi: 10.1088/1755-1315/709/1/012033
Balaji Wamanrao, K., and Tanaji, G. K. (2022). Impact of diferent extraction conditions on yield, physicochemical and functional characteristics of gelatin from Labeo rohita swim bladder. Food Sci. Biotech. 31, 1277–1287. doi: 10.1007/s10068-022-01121-z
Calvarro, J. M. D., Pérez-palacios, T., and Ruiz, J. (2016). Modification of gelatin functionality for culinary applications by using transglutaminase. Int. J. Gastron. Food Sci. 5, 27–32. doi: 10.1016/j.ijgfs.2016.11.001
Chakka, A. K., Muhammed, A., Sakhare, P., and Bhaskar, N. (2017). Poultry processing waste as an alternative source for mammalian gelatin: extraction and characterization of gelatin from chicken feet using food grade acids. Waste Biomass Valoriz. 8, 2583–2593. doi: 10.1007/s12649-016-9756-1
Choe, J., and Kim, H. (2018). Effects of chicken feet gelatin extracted at different temperatures and wheat fiber with different particle sizes on the physicochemical properties of gels. Poult. Sci. 97, 1082–1088. doi: 10.3382/ps/pex381
Choi, S. S., and Regenstein, J. (2000). Physicochemical and sensory characteristics of fish gelatin. J. Food Sci. 65, 194–199. doi: 10.1111/j.1365-2621.2000.tb15978.x
Chowdhury, M. W., Nabi, M. N., Arefin, M. A., Rashid, F., Islam, M. T., Gudimetla, P., et al. (2022). Recycling slaughterhouse wastes into potential energy and hydrogen sources: an approach for the future sustainable energy. Bioresour. Technol. Rep. 19:101133. doi: 10.1016/j.biteb.2022.101133
Derkach, S. R., Voron'ko, N. G., and Kuchina, Y. A. (2022). Intermolecular interactions in the formation of polysaccharide-gelatin complexes: a spectroscopic study. Polymers 14:2777. doi: 10.3390/polym14142777
Du, L., Khiari, Z., Pietrasik, Z., and Betti, M. (2013). Physicochemical and functional properties of gelatins extracted from turkey and chicken heads. Poultry Sci. 92, 2463–2474. doi: 10.3382/ps.2013-03161
Fairuza, T. A., and Amertaningtyas, D. (2024). The effect of gelatin on water holding capacity, water activity, water content, and randement of chicken-liver meatball. Bio Web Conf. doi: 10.1051/bioconf/20248800016
Fatima, S., Mir, M. I., Khan, M. R., Sayyed, R., Mehnaz, S., Abbas, S., et al. (2022). The optimization of gelatin extraction from chicken feet and the development of gelatin based active packaging for the shelf-life extension of fresh grapes. Sustainability 14:7881. doi: 10.3390/su14137881
Gao, Y., Liu, R., and Liang, H. (2024). Food hydrocolloids: structure, properties, and applications. Foods 13:1077. doi: 10.3390/foods13071077
Ghorani, B., Emadzadeh, B., Rezaeinia, H., and Russell, S. J. (2020). Improvements in gelatin cold water solubility after electrospinning and associated physicochemical, functional and rheological properties. Food Hydrocoll. 104:105740. doi: 10.1016/j.foodhyd.2020.105740
Goudie, K., Mccreath, S., Parkinson, J., Davidson, C., and Liggat, J. (2023). Investigation of the influence of pH on the properties and morphology of gelatin hydrogels. J. Polymer Sci. 61, 2316–2332. doi: 10.1002/pol.20230141
Guo, D., Yin, X., Cheng, H., Ye, X., and Chen, J. (2022). Evaluating physicochemical and sensory properties of functional yogurt supplemented with glycyrrhiza polysaccharide as potential replacement for gelatin. Agri. 12:1289. doi: 10.3390/agriculture12091289
Harini, N., Mousa Atoum, M. F., Aji Wulandari, S. T., Wahyudi, V. A., Jan, A., and Iqrar, I. (2023). Extraction, characterization, amino acid profile of halal gelatin from Kampong and broiler chicken feet skin. Jordan J. Bio. Sci. 16. doi: 10.54319/jjbs/160301
Hirbo, H. G., Kuse, K. A., and Alemu, A. D. (2023). Extraction and characterization of gelatin from Nile Tilapia (Oreochromis Niloticus) skin wastes generated in Arba Minch local fish processing industry and its value addition as stabilizer on yoghurt. Available online at: https://www.researchsquare.com/article/rs-2882193/v1 (accessed July 23, 2022).
Horwitz, W., and Latimer, G. (2000). Association of official analytical chemists. Gaithersburg, MD, USA.
Jongjareonrak, A., Rawdkuen, S., Chaijan, M., Benjakul, S., Osako, K., and Tanaka, M. (2010). Chemical compositions and characterisation of skin gelatin from farmed giant catfish (Pangasianodon gigas). LWT-Food Sci. Techno. 43, 161–165. doi: 10.1016/j.lwt.2009.06.012
Khalesi, M., Glenn-Davi, K., Mohammadi, N., and Fitzgerald, R. J. (2024). Key factors influencing gelation in plant vs. animal proteins: a comparative mini-review. Gels 10:575. doi: 10.3390/gels10090575
Kim, H.-W., Song, D.-H., Choi, Y.-S., Kim, H.-Y., Hwang, K.-E., Park, J.-H., et al. (2012). Effects of soaking pH and extraction temperature on the physicochemical properties of chicken skin gelatin. Korean J. Food Sci. Anim. Resour. 32, 316–322. doi: 10.5851/kosfa.2012.32.3.316
Kim, T.-K., Ham, Y.-K., Shin, D.-M., Kim, H.-W., Jang, H. W., Kim, Y.-B., et al. (2020). Extraction of crude gelatin from duck skin: effects of heating methods on gelatin yield. Poult. Sci. 99, 590–596. doi: 10.3382/ps/pez519
Kurt, A., Bursa, K., and Toker, O. S. (2022). Gummy candies production with natural sugar source: effect of molasses types and gelatin ratios. Food Sci. Techno. Inter. 28, 118–127. doi: 10.1177/1082013221993566
Kurt, A., Toker, O. S., Akbulut, M., Coklar, H., Ozmen, D., Ozcan, Y., et al. (2024). Textural, rheological, and structural properties of turkey and chicken gelatins from mechanical deboning residues. Food Sci. Nutr. 12, 4143–4150. doi: 10.1002/fsn3.4143
Lamers, D. L., Rigueto, C. V. T., Krein, D. D. C., Loss, R. A., Dettmer, A., and Gutterres, M. (2024). Sequential extraction and characterization of gelatin from turkey (Meleagris gallopavo) feet. Polymer Bull. 1–17. doi: 10.1007/s00289-024-05283-0
Lu, Y., Luo, Q., Chu, Y., Tao, N., Deng, S., Wang, L., et al. (2022). Application of gelatin in food packaging: a review. Polymers 14:436. doi: 10.3390/polym14030436
Luo, Q., Hossen, M. A., Zeng, Y., Dai, J., Li, S., Qin, W., et al. (2022). Gelatin-based composite films and their application in food packaging: a review. J. Food Eng. 313:110762. doi: 10.1016/j.jfoodeng.2021.110762
Mikhailov, O. V. (2023). Gelatin as it is: history and modernity. Inter. J. Molec. Sci. 24:3583. doi: 10.3390/ijms24043583
Milano, F., Masi, A., Madaghiele, M., Sannino, A., Salvatore, L., and Gallo, N. (2023). Current trends in gelatin-based drug delivery systems. Pharmaceutics 15:1499. doi: 10.3390/pharmaceutics15051499
Mirzapour-Kouhdasht, A., Sabzipour, F., Taghizadeh, M. S., and Moosavi-nasab, M. (2019). Physicochemical, rheological, and molecular characterization of colloidal gelatin produced from Common carp by-products using microwave and ultrasound-assisted extraction. J. Text. Stud. 50, 416–425. doi: 10.1111/jtxs.12408
Mohammadnezhad, S., and Farmani, J. (2022). Rheological and functional characterization of gelatin and fat extracted from chicken skin for application in food technology. Food Sci. Nutr. 10, 1908–1920. doi: 10.1002/fsn3.2807
Mokrejš, P., Mrázek, P., Gál, R., and Pavlačková, J. (2019). Biotechnological preparation of gelatines from chicken feet. Polymers 11:1060. doi: 10.3390/polym11061060
Mudgil, P., Jumah, B., Ahmad, M., Hamed, F., and Maqsood, S. (2018). Rheological, micro-structural and sensorial properties of camel milk yogurt as influenced by gelatin. LWT 98, 646–653. doi: 10.1016/j.lwt.2018.09.008
Muñoz, J., Freile-Pelegrin, Y., and Robledo, D. (2004). Mariculture of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) colour strains in tropical waters of Yucat?n, M?xico. Aquaculture 239, 161–177. doi: 10.1016/j.aquaculture.2004.05.043
Nami, B., Tofighi, M., Molaveisi, M., Mahmoodan, A., and Dehnad, D. (2023). Gelatin-maltodextrin microcapsules as carriers of vitamin D3 improve textural properties of synbiotic yogurt and extend its probiotics survival. Food Biosci. 53:102524. doi: 10.1016/j.fbio.2023.102524
Philipp, M., Masmoudi Jabri, K., Wellmann, J., Akrout, H., Bousselmi, L., and Geißen, S.-U. (2021). Slaughterhouse wastewater treatment: a review on recycling and reuse possibilities. Water 13:3175. doi: 10.3390/w13223175
Potti, R. B., and Fahad, M. O. (2017). Extraction and characterization of collagen from broiler chicken feet (Gallus gallus domesticus)-biomolecules from poultry waste. J. Pure Appl. Microbiol. 11, 315–322. doi: 10.22207/JPAM.11.1.39
Rafieian, F., Keramat, J., and Shahedi, M. (2015). Physicochemical properties of gelatin extracted from chicken deboner residue. LWT-Food Sci. Techno. 64, 1370–1375. doi: 10.1016/j.lwt.2015.04.050
Ragasri, S., and Sabumon, P. (2023). A critical review on slaughterhouse waste management and framing sustainable practices in managing slaughterhouse waste in India. J. Envir. Manag. 327:116823. doi: 10.1016/j.jenvman.2022.116823
Rana, J., Keshri, O., Rahman, C. F., and Kumar, V. (2024). Extraction and evaluation of collagen as biomaterial from chicken shank. Indian J. Anim. Res. 1:8. doi: 10.18805/IJAR.B-5502
Rao, V., and Poonia, A. (2023). Protein characteristics, amino acid profile, health benefits and methods of extraction and isolation of proteins from some pseudocereals—a review. Food Prod. Process. Nutr. 5:37. doi: 10.1186/s43014-023-00154-z
Rasli, H., and Sarbon, N. (2015). Effects of different drying methods on the rheological, functional and structural properties of chicken skin gelatin compared to bovine gelatin. Inter. Food Res. J. 22:584.
Rather, J. A., Akhter, N., Ashraf, Q. S., Mir, S. A., Makroo, H. A., Majid, D., et al. (2022). A comprehensive review on gelatin: Understanding impact of the sources, extraction methods, and modifications on potential packaging applications. Food Packag Shelf Life 34:100945. doi: 10.1016/j.fpsl.2022.100945
Revert-Ros, F., Ventura, I., Prieto-ruiz, J. A., Hernández-Andreu, J. M., and Revert, F. (2024). The versatility of collagen in pharmacology: targeting collagen, targeting with collagen. Int. J. Mol. Sci. 25:6523. doi: 10.3390/ijms25126523
Riantiningtyas, R. R., Sager, V. F., Chow, C. Y., Thybo, C. D., Bredie, W. L., and Ahrn,é, L. (2021). 3D printing of a high protein yoghurt-based gel: effect of protein enrichment and gelatine on physical and sensory properties. Food Res. Inter. 147:110517. doi: 10.1016/j.foodres.2021.110517
Saenmuang, S., Phothiset, S., and Chumnanka, C. (2019). Extraction and characterization of gelatin from black-bone chicken by-products. Food Sci. Biotechnol. 29, 469–478. doi: 10.1007/s10068-019-00696-4
Santana, J. C., Gardim, R. B., Almeida, P. F., Borini, G. B., Quispe, A. P., Llanos, S. A., et al. (2020). Valorization of chicken feet by-product of the poultry industry: High qualities of gelatin and biofilm from extraction of collagen. Polymers 12:529. doi: 10.3390/polym12030529
Sarbon, N. M., Badii, F., and Howell, N. K. (2013). Preparation and characterisation of chicken skin gelatin as an alternative to mammalian gelatin. Food Hydrocolloids 30, 143–151. doi: 10.1016/j.foodhyd.2012.05.009
Sedaghat, N., and Mohsenzadeh, M. (2022). Effect of edible chicken feet gelatin green walnut husk extracted coating on chemical, physical, sensory properties and storage time of refrigerated temperature rainbow trout fillets (5.0 ± 1.0° C). J. Food Sci. Technol. 19, 37–56.
Siburian, W. Z., Rochima, E., Andriani, Y., and Praseptiangga, D. (2020). Fish gelatin (definition, manufacture, analysis of quality characteristics, and application): a review. Int. J. Fish Aquat. Stud. 8, 90–95.
Sinthusamran, S., Benjakul, S., and Kishimura, H. (2014). Characteristics and gel properties of gelatin from skin of seabass (Lates calcarifer) as influenced by extraction conditions. Food Chem. 152, 276–284. doi: 10.1016/j.foodchem.2013.11.109
Supavititpatana, P., Wirjantoro, T. I., Apichartsrangkoon, A., and Raviyan, P. (2008). Addition of gelatin enhanced gelation of corn–milk yoghurt. Food Chem. 106, 211–216. doi: 10.1016/j.foodchem.2007.05.058
Taufik, M. (2010). Effect of broiler age and extraction temperature on characteristic chicken feet skin gelatin. International Seminar on Tropical Animal Production (ISTAP) 2010. 649–656.
Torrejon, V. M., Song, J., Yu, Z., and Hang, S. (2022). Gelatin-based cellular solids: fabrication, structure and properties. J. Cell. Plastics. 58, 797–858. doi: 10.1177/0021955X221087602
Ungureanu, N., Vlăduţ, N. V., Biriş, S. Ş., Gheorghiţă, N. E., Ionescu, M., Milea, O. E., et al. (2024). Management of waste and by–products from meat industry. Acta Technica Corvin-Bull. Eng. 17, 49–58.
Usman, M., Ishaq, A., Regenstein, J. M., Sahar, A., Aadil, R. M., Sameen, A., et al. (2023). Valorization of animal by-products for gelatin extraction using conventional and green technologies: a comprehensive review. Biomass Conver. Bioref. 104, 1–13. doi: 10.1007/s13399-023-04547-5
Usman, M., Sahar, A., Aadil, R. M., and Shahid, M. (2024). Extraction and physicochemical characterization of native and broiler chicken feet gelatin. J. Sci. Food Agri. 104, 8939–8944. doi: 10.1002/jsfa.13720
Wang, S., Wei, Z., and Wang, L. (2024). Improving slaughterhouse byproducts utilization via anaerobic digestion, composting, and rendering. Renew. Sustain. Energy Rev. 189:113881. doi: 10.1016/j.rser.2023.113881
Warner, R. (2014). “Measurement Of Meat Quality Measurements of Water-holding Capacity and Color,” in Objective and Subjective in Encyclopedia of Meat Sciences, eds. C. Devine and M. Dikeman (Oxford, UK Academic Press) doi: 10.1016/B978-0-12-384731-7.00210-5
Widyasari, R., and Rawdkuen, S. (2014). Extraction and characterization of gelatin from chicken feet by acid and ultrasound assisted extraction. Food Appl. Biosci. J. 2, 85–97.
Zambuto, S. G., Kolluru, S. S., Ferchichi, E., Rudewick, H. F., Fodera, D. M., Myers, K. M., et al. (2024). Evaluation of gelatin bloom strength on gelatin methacryloyl hydrogel properties. J. Mech. Behav. Biomed. Mater. 154:106509. doi: 10.1016/j.jmbbm.2024.106509
Zhang, W., Li, M., Chen, J., Chen, Y., Liu, C., and Wu, X. (2024). A review of modified gelatin: physicochemical properties, modification methods, and applications in the food field. J. Agri. Food Chem. 72, 20705–20721. doi: 10.1021/acs.jafc.4c03194
Zhang, X., Xu, S., Shen, L., and Li, G. (2020). Factors affecting thermal stability of collagen from the aspects of extraction, processing and modification. J. Leather Sci. Eng. 2, 1–29. doi: 10.1186/s42825-020-00033-0
Zou, Y., Chen, X., Lan, Y., Yang, J., Yang, B., Ma, J., et al. (2024). Find alternative for bovine and porcine gelatin: Study on physicochemical, rheological properties and water-holding capacity of chicken lungs gelatin by ultrasound treatment. Ultrason Sonochem. 109:107004. doi: 10.1016/j.ultsonch.2024.107004
Keywords: gelatin yield, ross chicken strain, gel viscosity, water holding capacity, sensory evaluation
Citation: Hussein RH, Abd Elgadir M, Mariod AA and Alnughaymishi I (2025) Extraction and physicochemical properties of gelatin from chicken feet of different ages and its effect on sensory evaluation of the developed yogurt. Front. Sustain. Food Syst. 9:1614286. doi: 10.3389/fsufs.2025.1614286
Received: 18 April 2025; Accepted: 02 June 2025;
Published: 25 June 2025; Corrected: 21 October 2025.
Edited by:
B. N. Dar, Islamic University of Science and Technology, IndiaReviewed by:
José Armando Ulloa, Autonomous University of Nayarit, MexicoOmaima Aidat, Université Abdelhamid Ibn Badis Mostaganem, Algeria
Copyright © 2025 Hussein, Abd Elgadir, Mariod and Alnughaymishi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdalbasit A. Mariod, YWFsbmFkaWZAdWouZWR1LnNh
†ORCID: Abdalbasit A. Mariod orcid.org/0000-0003-3237-7948
 Rasha Hamed Hussein1
Rasha Hamed Hussein1 M. Abd Elgadir
M. Abd Elgadir Abdalbasit A. Mariod
Abdalbasit A. Mariod