- Department of Food Science and Technology, National Institute of Food Technology Entrepreneurship and Management, Sonipat, India
Introduction: Polyunsaturated fatty acids (PUFAs) are highly susceptible to oxidative deterioration, limiting their application in functional food systems. Synthetic antioxidants such as TBHQ are widely used to delay lipid oxidation, but their long-term consumption raises health concerns. This study investigated the use of rosemary extract (RE) as a natural antioxidant to enhance oxidative stability of omega-3 fatty acid rich margarine developed with structured lipids derived from perilla seed oil (PeO).
Methods: Structured lipid margarine (SLM) was developed using enzymatic interesterification of PeO, and RE was incorporated at concentrations of 500, 1000, 1500, and 2000 ppm. Samples were subjected to accelerated storage conditions using the Schaal oven test. Oxidative stability was evaluated by measuring peroxide value, acid value, p-Anisidine value, TOTOX index, conjugated dienes, conjugated trienes, and fatty acid composition. Antioxidant efficacy was compared with tert-butylhydroquinone (TBHQ, 200 ppm). Data were further analyzed by Principal Component Analysis (PCA).
Results: Incorporation of RE significantly delayed lipid oxidation in SLM, with reductions of about 40% in peroxide values compared to control samples at 1500 ppm. It reduced TOTOX index and the levels of conjugated dienes and trienes, indicating improved protection against primary and secondary oxidation. The p-Anisidine value showed reductions of 21.7%, 21.6%, 26.9%, 37.7%, and 29.3% for SLM and SLM with RE at 500, 1000, 1500, and 2000 ppm, respectively, compared to PeO. Antioxidant activity was concentration dependent, with 1500 ppm RE showing optimal stabilization. PCA confirmed that RE at 1500 ppm was comparable to TBHQ in enhancing stability.
Discussion and conclusion: The study demonstrates effectiveness of RE as natural antioxidant capable of stabilizing PUFA rich structured lipids systems. At 1500 ppm, RE provided oxidative protection equivalent to TBHQ, with the upside of being sustainable, plant-derived alternative. The combined effect of enzymatic interesterification and natural antioxidant supplementation offers a dual strategy to improve the stability of functional fat based products, supporting RE as a promising substitute for synthetic preservatives in the food industry.
1 Introduction
The oxidative stability of lipids is a significant challenge for food manufacturers, particularly in products containing unsaturated fatty acids. Lipid oxidation causes the degradation of lipids, thereby resulting in rancidity, off-flavors, and loss of nutritional value of the final product. To address this concern, techniques such as antioxidant addition along with physical modification technologies including blending and interesterification have been explored (Mishra et al., 2022). Antioxidants play a crucial role in mitigating oxidative deterioration by scavenging free radicals, chelating metal ions, and inhibiting the formation of peroxides. Traditionally, synthetic antioxidants, such as TBHQ (tert-butylhydroquinone), BHA (butylated hydroxyanisole) and BHT (butylated hydroxytoluene) have been used to extend the shelf life of lipids due to their high antioxidant potency and low cost. However, they are linked to negative health implications including cancer, mutations, and blood clotting when subjected to long term consumption (Rashid et al., 2022; Sharma et al., 2019). Furthermore, the growing consumer demand for natural food products has led to increased interest from industries and researchers towards plant-based antioxidants. Rosemary extract (RE) is one such antioxidant, which is known for its potent efficacy in inhibiting lipid oxidation (Kum et al., 2025). The extract contains a wide range of bioactive compounds, including phenolics like carnosol and carnosic acid, as well as flavonoids and rosmarinic acid, which exhibit potent free radical scavenging activity (Couto et al., 2012). Moreover, RE has shown notable health benefits including reduction in inflammation, memory improvement, and cardiovascular function (Veenstra and Johnson, 2021).
Margarine is a widely consumed fat that serves as a substitute to butter in various bakery and culinary applications. It is usually formulated by partial hydrogenation of plant-based oils to achieve the desirable textural and functional properties (Li et al., 2018). However, this process leads to the development of trans-fats, which have been associated to the risk of developing cardiovascular diseases, cancer, atherosclerosis and other fatal disorders (Marangoni et al., 2020; Nagpal et al., 2021; Shin et al., 2021). Additionally, it lacks or contains negligible amount of PUFA (polyunsaturated fatty acid), especially essential omega-3 fatty acid. The consumption of PUFAs and omega-3 fatty acids is associated with reduced risk of cardiovascular diseases, improved cognitive function, and anti-inflammatory effects. Thus, lipid modification technologies such as blending and interesterification have garnered attention in the recent years for the development of healthier margarine formulations (Dhiman et al., 2024; Li et al., 2018). These modification techniques have been employed to enhance the stability as well as applicability of PUFA rich oils in the food industry.
Oil blending is a widely employed technique for enhancing oxidative and thermal stability. It is also cost-effective and facilitates the improvement of nutritional properties. Nevertheless, oil blends exhibit non-uniformity and phase separation, which limits their applicability due to poor techno-functional properties (Dhiman et al., 2025). This can be addressed by interesterification, which is another lipid modification approach involving the redistribution of fatty acids within or between the triglyceride species. Interesterification is categorized into chemical and enzymatic process, depending on the type of catalyst used. Enzymatic interesterification is preferred due to its mild processing conditions, high substrate specificity, reduced formation of undesirable by-products, and improved environmental sustainability (Jadhav and Annapure, 2021). This method has been extensively utilized for the development of structured lipids for applications in margarine, cocoa butter alternatives, and functional fat replacers (de Oliveira et al., 2021; Dhiman et al., 2024; Dong et al., 2023; Ma et al., 2019). It also facilitates the formulation of fats with an increased amount of PUFAs, which are found in negligible amounts in commercial margarines. The intake of PUFAs has been associated with various health benefits, including anti-inflammatory effects, cardiovascular protection, and neurological development (Wang et al., 2024). They aid in reduction of high-density lipoprotein (HDL) cholesterol while regulating triglyceride levels and blood pressure. Additionally, their regular consumption leads to a lower risk of non-communicable diseases such as cancer, osteoarthritis, and autoimmune disorders (Kapoor et al., 2021). They have also been reported to enhance metabolic health by improving insulin sensitivity and reducing the potential of metabolic disorders.
Perilla seed oil (PeO) is known for its high PUFA content (75%), of which about 60% is omega-3 fatty acid, ALA (Dhyani et al., 2022). It is extracted from the seeds of Perilla frutescens, a plant widely cultivated in East Asian countries, particularly China, Korea, and Japan (Kaur et al., 2024). Owing to its high ALA content, PeO has been extensively studied for its potential health benefits, including anti-inflammatory, neuroprotective, and cardioprotective effects. Despite its nutritional advantages, the application of perilla seed oil in food formulations poses significant challenges due to its oxidative instability (Aldamarany et al., 2023; Kaur et al., 2024).
In this study, the shelf life of trans-fat free omega-3 fatty acid rich margarine developed through the enzymatic interesterification of perilla seed oil was assessed. The oxidative stability of the formulated margarine was evaluated with and without the incorporation of RE at different concentrations. To assess the impact of RE on oxidative stability, an accelerated storage experiment was conducted using the Schaal oven test, a widely used method for determining lipid oxidation under elevated temperature conditions (Pokhrel et al., 2024). The study aims to determine the efficacy of RE in enhancing the oxidative stability of omega-3-rich margarine and its potential as a natural alternative to synthetic antioxidants. The present study introduces a novel approach by developing a trans-fat-free, omega-3-rich margarine using PeO, an underutilized but rich source of α-linolenic acid. The oil was incorporated into a structured lipid system by enzymatic interesterification, a sustainable alternative to chemical hydrogenation used to enhance the physical, chemical, and functional properties of the solid fat. The margarine was further stabilized using RE, a natural plant-based antioxidant, which provided oxidative stability comparable to synthetic additives. This combination of structured lipids and a natural antioxidant incorporates the innovation and distinguishes the formulation from existing commercial products.
2 Materials and methods
Perilla seeds utilized in the study were procured from Meghalaya farmers through Sudasha Foods Private Limited. All the chemicals, solvents, and gases used were of analytical grade. Standards for tocopherol, fatty acid and antioxidant analysis were procured from Sigma Aldrich. Rosemary extract was purchased from Veda oils, Delhi, India and the oil samples were stored at 4° C in tightly sealed amber bottles.
2.1 Preparation of margarine
The margarine was developed from the structured lipid (SL) using method given by Dhiman et al. (2024). Blended oil was initially prepared by blending the PeO and palm stearin (PS) in 50:50 ratio on a magnetic stirrer at 55° C and 150 rpm for 15 min. The SL was initially developed by incorporating immobilized TLIM at a concentration of 6.2% by weight. The interesterification reaction was carried out at 54° C for 5.4 h, after which the enzyme was recovered using a 0.45 μm PTFE membrane filter. This enzyme was subsequently rinsed with hexane, dried at 40° C for 6 h, and preserved for further use. Margarine was then formulated using these structured lipid systems. The lipid phase consisted of 80% SL and 0.5% soy lecithin. The aqueous phase contained 18% distilled water and 1.5% table salt. Both phases were heated to 60° C separately and then thoroughly mixed for 5 min to form an emulsion. Artificial butter flavour was added to the emulsion, which was then crystallized for 15 min using an ice-cream maker. The resulting margarine was refrigerated overnight. For tempering, the developed margarines were held at room temperature for 4 h and then vigorously blended to achieve a smooth, crystal-free consistency (Pande et al., 2013).
2.2 Rosemary extract incorporation
To evaluate the effect of RE on SLM, it was added at four distinct concentrations, i.e., 500, 1,000, 1,500, and 2000 ppm. A 200 ppm concentration of tertiary butylhydroquinone (TBHQ) was also included as a positive control, as it is the maximum permitted level for edible oils and fats as specified by the international and national safety standards, including regulations by FDA, European Food Safety Authority and FSSAI [Food Safety and Standards Authority of India (FSSAI), 2011; U.S. Food and Drug Administration (FDA), 2018; European Commission, 2008]. The chosen range for RE concentrations (500–2000 ppm) was based on relevant literature and pre-trial experiments confirming RE’s effectiveness as an antioxidant (Cordeiro et al., 2013; Kum et al., 2025; Yeşilsu and Özyurt, 2019; Yıldırım and Yorulmaz, 2018). Also, RE is classified as Generally Recognized as Safe (GRAS), and its use in fats and oils is regulated by international food authorities. According to the European Food Safety Authority (EFSA) (2008) and the U.S. Food and Drug Administration (FDA) (2018), rosemary extract is permitted within specified limits, typically up to 2000 ppm depending on the application. For preparation, both RE and TBHQ were precisely weighed and dissolved into the lipid samples using a magnetic stirrer at 300 rpm for 30 min at 50° C.
2.3 Volatile organic profile of rosemary extract
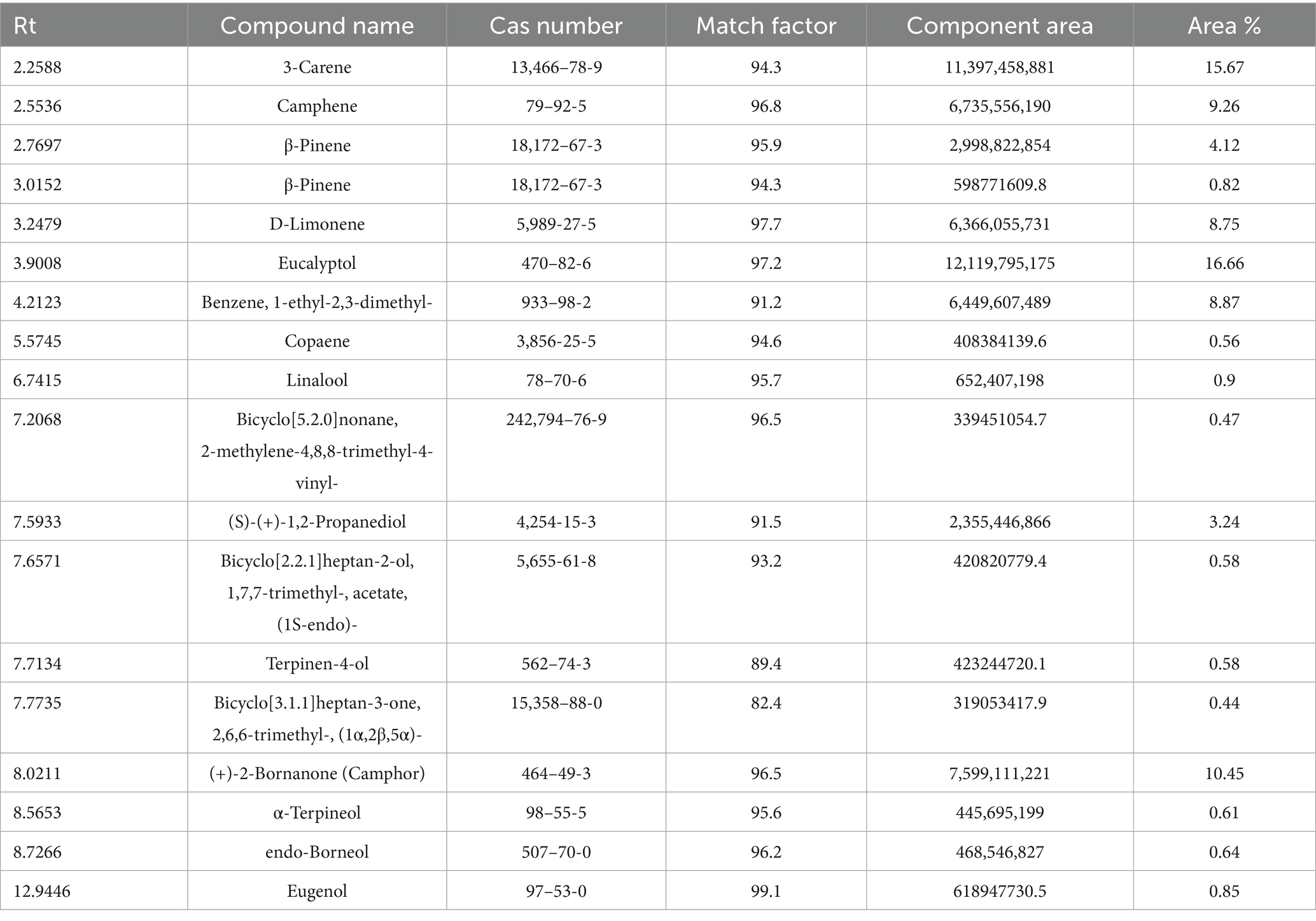
Volatile organic compounds in RE were identified using Headspace Gas Chromatography–Mass Spectrometry (HSGC-MS) method on Agilent 7010B GC/TQ system equipped with HP-88 GC column. A precisely weighed 200 mg sample was incubated at 120° C for 20 min with agitation to collect volatiles. Nitrogen was used as a carrier gas at a 1 mL/min flow rate with a 10:1 split ratio. The GC oven temperature was ramped from 50 to 250° C at rate of 10° C/min, holding at 230° C for 10 min (Sharma et al., 2021). Compounds were identified by comparing retention times and mass spectra to the NIST Mass Spectral Library.
2.4 Schaal oven test
The oxidative stability test of the samples was carried out at by keeping 300 mL of each sample at 60° C in an oven over a period of 21 days (Baştürk et al., 2018; Moczkowska et al., 2020; Wang et al., 2018). The physical and oxidative analysis of the samples was performed every 3 days while the fatty acids profile, and tocopherol content was determined at the start (0th day) and end (21st day) of the storage study.
2.5 Oxidative indices
2.5.1 Induction period
The oxidative stability index of the samples was evaluated on the initial day in terms of induction period (IP) at 110° C using Rancimat (Metrohm 892, Herisau, Switzerland). 3 g of oil sample was precisely weighed in the reaction vessel and subjected to heating with air flow of 20 L/h (Li et al., 2019).
2.5.2 Primary oxidation
The peroxide value (PV) and acid value (AV) of the samples were assessed every 3 days using AOCS methods Cd 9–53 and Ca 5a-40, respectively. The solid fat was melted and accurately weighed. In order to determine AV, 50 mL of neutralized ethyl alcohol was initially added to the sample, which was further subjected to boiling. This mixture was titrated against 0.1 N KOH solution after the addition of phenolphthalein reagent. For determining the PV, hydroperoxides formed in the fat sample due to oxidation were reacted with a saturated KI solution, which was further titrated against 0.01 M sodium thiosulfate solution.
2.5.3 Secondary oxidation
The p-Anisidine value (pAV), conjugated dienes (CD) and conjugated trienes (CT) of the samples were evaluated after every third day by spectrophotometric measurements at 530 nm, 232 nm and 268 mm, respectively, using a UV–Vis spectrophotometer (UV–Vis-1800 spectrophotometer). The analyses were performed in accordance with standard IS methods (Bureau of Indian Standards, 2021).
2.5.4 TOTOX
TOTOX value was used to determine the overall oxidative stability of the samples. It was computed by using the following equation combining PV and p-AV.
2.6 Color
The color of the fat samples was evaluated in terms of L, a* and b* values, representing lightness, redness and yellowness, respectively, using a CR400 Minolta colorimeter (Konica Minolta Inc., Tokyo, Japan). Triplicate value of all the samples were taken.
2.7 Fatty acid composition
The fatty acid profile of the lipid samples was determined in accordance with AOCS official method 996.06. They were initially trans esterified to FAMEs using BF3 and further subjected to gas chromatography (GC) coupled with a flame ionized detector (FID) using an Agilent 7890B. Separation was achieved using a DB capillary column (100 mm x 0.25 m x 0.2 m). The injection volume was set at 1 μL with a split ratio of 100:1. Nitrogen served as a carrier gas at a flow rate of 1.5 mL/min and column pressure of 20 psi. Both the injector and detector temperature were maintained at 280° C, while the hydrogen and oxygen flow rate were set at 30 mL/min and 300 mL/min, respectively. The oven temperature program began at 100° C for 4 min, followed by a ramp of 4° C/min to 240° C, which was held for 25 min. Identification and quantification of the fatty acids in the samples were performed by comparing the retention times to those of reference standard.
2.8 Antioxidant capacity
Polar compounds were extracted from the fat samples through liquid–liquid extraction using 80% (v/v) methanol solution. Briefly, 1 g of sample was diluted in 3 mL of methanolic solution and vortexed for a minute (Pan et al., 2019). Following this, the mixture was centrifuged at 6000 rpm for 5 min, and the supernatant was collected. The process was repeated thrice and the collected supernatants were combined and made up to a final volume of 10 mL by the addition of methanol solution.
2.8.1 DPPH radical scavenging activity
The free radical scavenging capacity of the samples was determined using the DPPH assay. A DPPH reagent solution (0.1 mM) was prepared by dissolving 4.9 mg of DPPH in 50 mL of 80% methanol in an amber volumetric flask. 0.5 mL of each sample extract was reacted with 2 mL of DPPH reagent and incubated in the dark at 25° C for 30 min followed by spectrophotometric measurements at 517 nm (Pointner et al., 2024; Samani, 2017). Results were quantified as μm TE (Trolox equivalent)/Kg using a Trolox standard curve (Brand-Williams et al., 1995).
2.8.2 Total phenolic compounds (TPC)
The TPC of the margarine samples was evaluated using the FC (Folic-Ciocalteu) method. Absorbance measurements were recorded at 765 nm with a UV–Visible spectrophotometer and the results were expressed as milligrams of gallic acid equivalent (mg GAE)/kg of the sample. The quantification was based on a standard curve with a gallic acid solution (0.02–0.1 mg/mL) (Polavarapu et al., 2011).
2.8.3 Total tocopherol content
Total tocopherol content (TTC) was determined following the methodology described by Singh et al. (2024). HPLC with a Shimadzu TF-10AXL fluorescence detector was used for detection, with excitation and emission wavelengths set at 294 nm and 329 nm, respectively. Separation was carried out on a Spinco nucleosil C18 column (100–5, 5 μm, 4.6 mm x 250 mm) with a mobile phase of 3.85% tetrahydrofuran in n-heptane at a flow rate of 1 mL/min (Dhiman et al., 2024). 100 mg of precisely weighed sample was dissolved in n-heptane and 20 μL of aliquot was injected into the system. Data acquisition was performed using Lab Solution software.
2.9 Statistical analysis
All analyses were performed in triplicate. Mean values and standard deviations were calculated, and significant differences (p < 0.05) were identified using Tukey’s test in SPSS. Furthermore, principal component analysis (PCA) was conducted using Origin Pro software to compare samples based on oxidative parameters.
3 Result and discussion
3.1 Volatile organic profile of rosemary extract
The HSGC-MS analysis of the RE demonstarted a complex profile of volatile organic compounds, with eucalyptol (1,8-cineole, 16.66%), 3-carene (15.67%), camphene (9.26%), D-limonene (8.75%), camphor [(+)-2-bornanone, 10.45%], and benzene, 1-ethyl-2,3-dimethyl (8.87%) as the predominant constituents (Table 1). Several other terpenoids and phenolic compounds, such as linalool (0.90%), α-terpineol (0.61%), terpinen-4-ol (0.58%), endo-borneol (0.64%), and eugenol (0.85%), were present in lower concentrations, contributing to the extract’s overall potential bioactivity. This aligns with recent literature reporting 1,8-cineole, camphor, camphene, and borneol as major volatiles in rosemary essential oils and extracts. Minor compounds such as linalool, α-terpineol, and eugenol, though present in smaller amounts, have been detected in rosemary and are known for their antioxidant and antimicrobial properties (Luca et al., 2023; Mena et al., 2016). These compounds are not only responsible for the characteristic aroma of RE but also significantly contribute to its antioxidant and antimicrobial properties. The presence of both hydrocarbon and oxygenated volatiles suggests RE’s synergistic role in stabilizing lipid systems by scavenging free radicals and delaying oxidative degradation. These volatiles suggest the functional potential of RE as a natural preservative in food applications, particularly in PUFA-rich formulations.
3.2 Oxidative indices
3.2.1 Induction period
The initial oxidative stability of the developed lipid systems was evaluated using the Rancimat method, which provides an accelerated measure of the oxidative stability by determining the induction period (IP) or oxidative stability index (OSI) under standardized conditions of elevated temperature and constant air flow (Ghosh et al., 2019). The OSI is a widely used parameter to predict the relative shelf life of lipid-based products by assessing their resistance to thermo-oxidative degradation. The incorporation of both the natural (RE) and synthetic (TBHQ) antioxidants significantly enhanced the IP values (Figure 1). PeO, characterized by high proportion of PUFAs exhibited lowest IP values (1.96 h). This can be attributed to the high susceptibility of PUFAs to oxidation resulting from the multiple double bonds in their structure, which are prone to attack by reactive oxygen species, leading to peroxidation (Machado et al., 2023). In contrast to this, the SLM possessed significantly higher stability with IP of 4.40 h. This observation aligns with previous studies suggesting that lipid modification by incorporation of saturated fat fractions like palm stearin, palm olein, and coconut oil through interesterification or blending significantly improves the oxidative stability of the lipid systems (Boukandoul et al., 2019; de Oliveira et al., 2021; Pang et al., 2019).
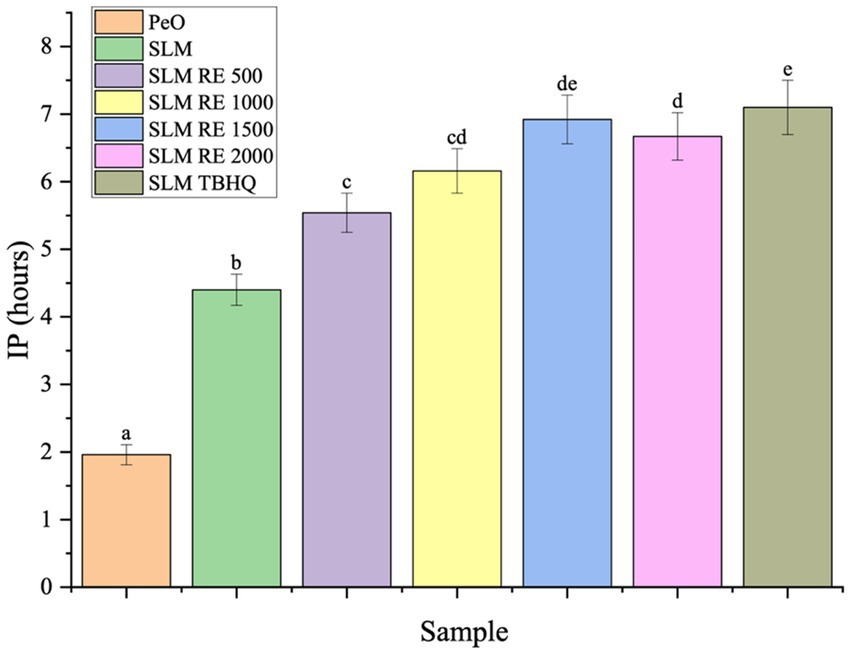
Figure 1. IP values of SL based margarine added with RE at different concentrations. Means with different lowercase superscripts signify sample-wise statistical difference (p < 0.05).
The incorporation of RE to SLM demonstrated a significant, concentration dependent increase on the oxidative stability. This can be attributed to the radical scavenging and metal chelating activity of the phenolic compounds present in RE, namely, carnosic acid and rosmarinic acid. The OSI values escalated from 5.54 h (500 ppm RE) to 6.16 h (1,000 ppm RE), reaching a maximum protective effect at 1500 ppm RE (6.92 h). Notably, a further increase to 2000 ppm RE (6.67 h) resulted in a non-significant decrease in the IP value as compared to the 1,500 ppm concentration. The highest IP values were observed for SLM incorporated with TBHQ. However, statistical analysis (p < 0.05) revealed no significant difference between the stability imparted by TBHQ (200 ppm) and that achieved with RE at 1500 ppm and 2000 ppm. This suggests that RE has promising potential as a natural antioxidant for preserving PUFA-rich oils.
3.2.2 Peroxide value
The degree of formation of primary oxidation products (hydroperoxides) was evaluated by monitoring the PV over the storage period of 21 days as illustrated in Figure 2. The PV of all the samples increased with storage time, indicating a progressive lipid oxidation, PeO exhibited the highest susceptibility to oxidation with PV values rising from 3.46 to 26.74 meqO2/Kg by day 21. In contrast to PeO, the increase observed in SLM was not as prominent, indicating higher oxidative stability of SLM, thereby suggesting the effectiveness of enzymatic interesterification technique for enhancing the oxidative stability and functional properties, and hence the applicability of PUFA rich oils. The SLM samples incorporated with RE had significantly lower PVs throughout the storage period, demonstrating improved oxidation stability. SLM with 500 ppm RE had final PV of 24.25 meqO2/Kg, showing a 19.05% reduction from the control SLM without any antioxidant, which further decreased at higher RE concentrations. Overall, a 38.25, 43.32 and 42.9% decrease in PV was observed at 1000, 1500 and 2000 ppm RE, respectively. Notably, the lowest oxidative susceptibility was achieved at 1500 ppm RE suggesting it be the optimal concentration beyond which no further antioxidant effect was observed with RE addition. SLM added with TBHQ demonstrated no significant differences in the PV when compared to that with RE at 1500 and 2000 ppm. Shelf life of the margarine formulations was estimated using established oxidative quality limits, with peroxide value (PV) maintained below 10 meq O₂/kg and acid value (AV) below 4 mg KOH/g, in accordance with guidelines (European Commission, 2001; Food Safety and Standards Authority of India, 2021). The antioxidant effect of RE can be attributed to its high phenolic and bioactive concentration, which aids in scavenging free radicals and chelating pro-oxidant metals, thereby preventing lipid oxidation (Tohma and Turan, 2015; Veenstra and Johnson, 2021). However, no further increase in the protective effect was observed on increasing the concentration of RE above 1,500 ppm, which can be attributed to the pro-oxidant activity of antioxidants at higher concentrations, a phenomenon observed in other studies as well (Dhiman et al., 2025; Martin-Rubio et al., 2018).
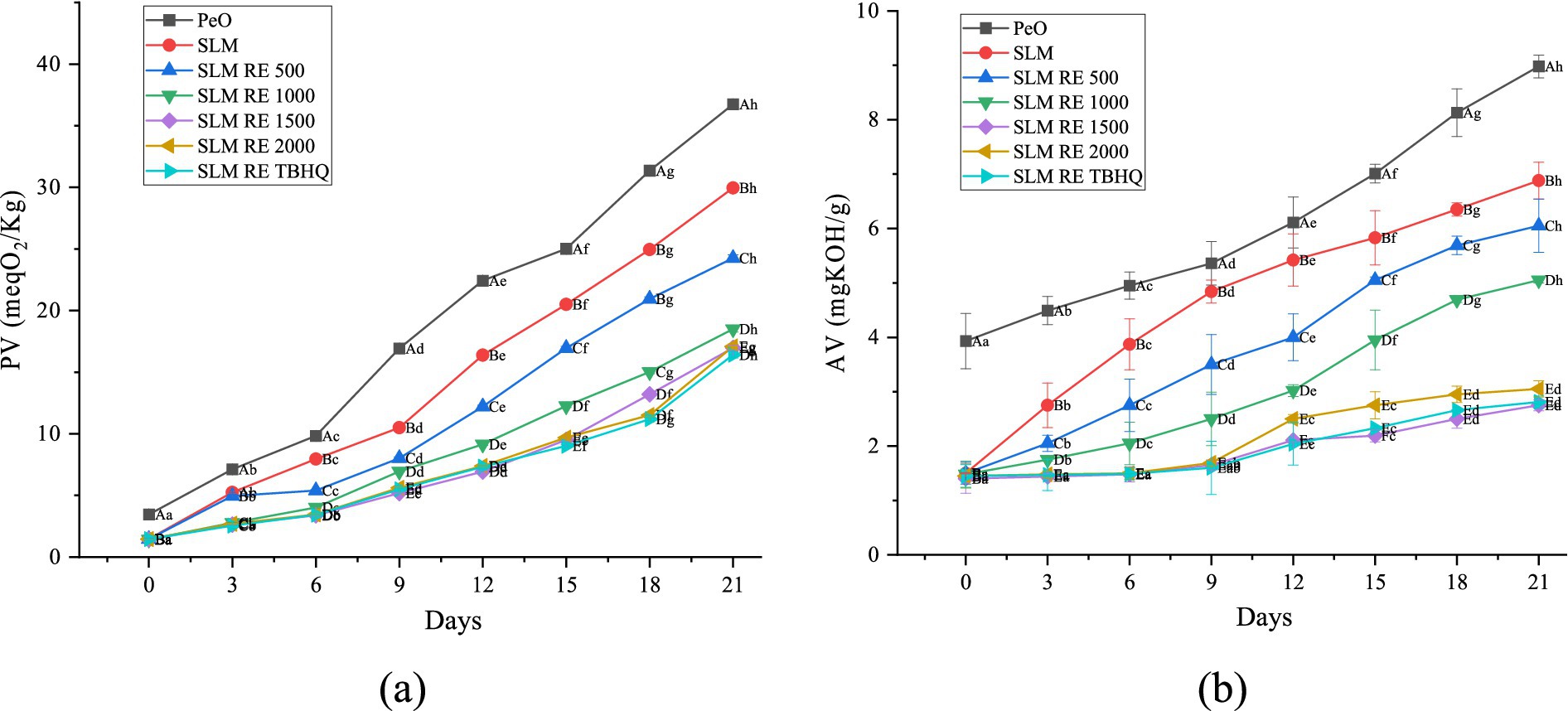
Figure 2. Changes in primary oxidation markers during the storage study. (a) PV; (b) AV. Mean values with different lowercase superscripts signify statistical difference sample-wise (p < 0.05) and means with different upper-case superscripts signify statistical difference day-wise (p < 0.05).
3.2.3 Acid value
Acid value is one the most important parameter for evaluating the quality of fats and oils. The value is generally elevated on exposure to heat, light or other harsh conditions during storage due to the hydrolysis reaction. This leads to the degradation in quality of oil, which is often accompanied by a decline in smoke point (Ramroudi et al., 2022). Similar to the trend observed for peroxide value, the highest AV was reported for PeO, owing to its high unsaturated fatty acid content which makes it susceptible to oxidation due to presence of multiple double bonds in its structure. As storage time increased, the AV rose across all samples. This increase can be attributed to the breakdown of TAG and a decrease in the extracts’ ability to prevent hydrolysis. Notably, SLM exhibited significantly lower AV (p < 0.05) as compared to PeO (Tohma and Turan, 2015). The changes in acid value of SLM and SLM supplemented with varying levels of RE over 21 days of accelerated storage has been depicted in Figure 2b. The PeO control exhibited the highest increase in AV, rising from an initial value of about 3.5 mg KOH/kg to about 9 mg KOH/g. This significant rise (p < 0.05) suggests the high susceptibility of PeO to oxidative degradation under accelerated conditions. The SLM was found to be more stable than PeO, however, it showed a continuous increase in AV, reaching up to 7 mg KOH/g by the end of storage study. These results are consistent with previous studies, which report that structured lipids and polyunsaturated oils are particularly prone to hydrolytic and oxidative deterioration during storage (Golmakani et al., 2020; Kumari Singh et al., 2024). The addition of RE at increasing concentrations lead to a concentration dependent protective effect against rise in acid value. SLM incorporated with 1,500 ppm RE showed the highest improvement over that without antioxidant, resulting in significantly lower AV throughout the study, with final values of 2.5–2.8 mg KOH/g. Notably, lowest AV was reported for RE incorporated at 1500 ppm, suggesting it to be optimal concentration for oxidative stability. SLM RE 2000 ppm did not provide further significant benefit, indicating a stagnation in antioxidant efficacy at higher doses. The synthetic antioxidant TBHQ (SLM RE TBHQ) performed comparably to the higher concentrations of rosemary extract, maintaining AVs below 3 mg KOH/kg throughout the storage period. This demonstrates that RE, at appropriate concentrations, can be as effective as TBHQ in inhibiting oxidative degradation in structured lipids (see Figure 3).
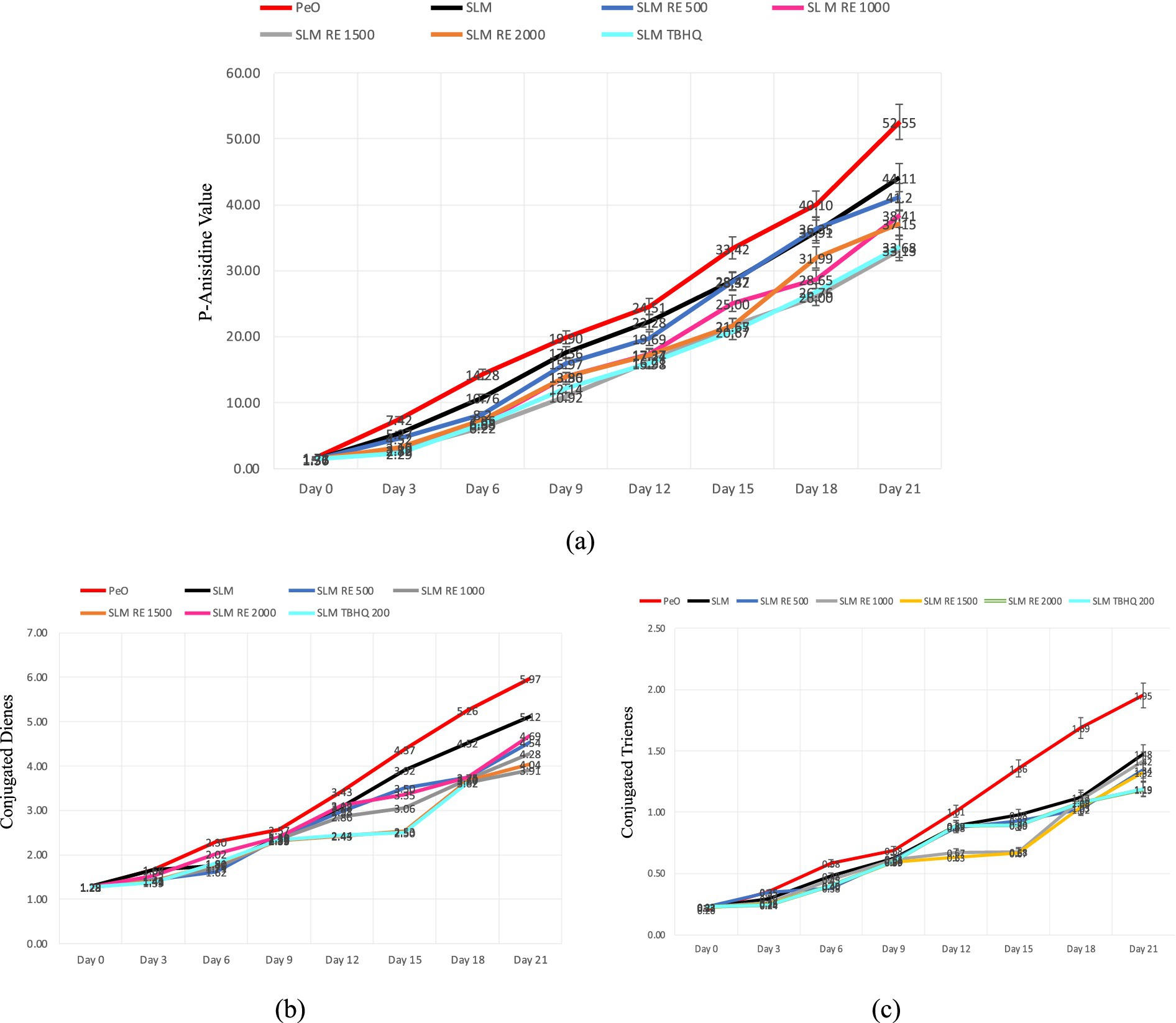
Figure 3. Changes in secondary oxidation markers of margarine throughout the accelerated storage period; (a–c) depict p-AV, CD and CT values, respectively.
3.3 Secondary oxidation
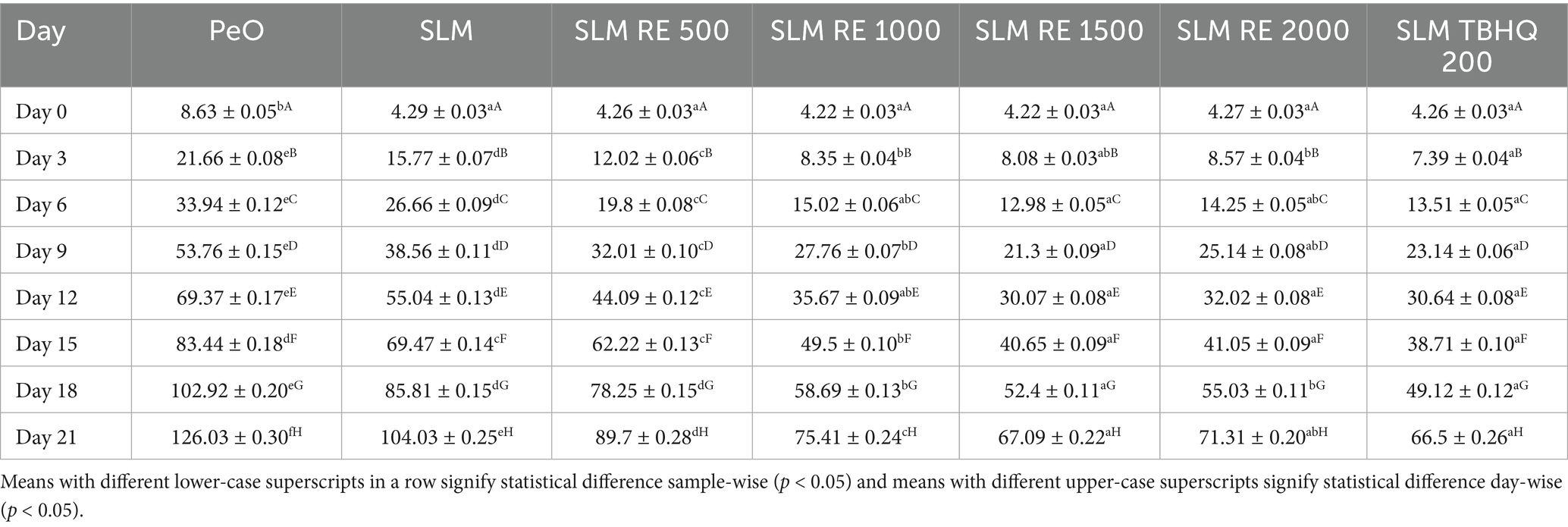
The degree of secondary oxidation in the samples was evaluated through p-AV, CD, and CT over 21 days of storage period under accelerated conditions. These parameters indicate the extent of oxidative degradation beyond primary oxidation, measuring the level of aldehydes and conjugated compounds formation, which in turn are responsible for imparting rancid flavours to food products. The p-AV showed a consistent increase across all samples with storage time, indicating the formation of aldehydes as secondary oxidation products. PeO exhibited most rapid and significant rise, with values reaching to a maximum of 52.55 by the end of the storage period. Conversely, SLM showed a comparatively lower increase, with maximum value of 44.11. This indicates the improvement of oxidative stability resulting from the interesterification reaction. Amongst the samples treated with antioxidants, SLM TBHQ and SLM RE 1500 exhibited the best oxidative resistance, with final pAVs of 33.68 and 33.13, respectively. Notably, RE at 1500 ppm provided comparable protection to synthetic antioxidant TBHQ, indicating its efficacy as a natural alternative. Compared to PeO by the end of the storage period, p-AV was reduced by 21.7, 21.6, 26.9, 37.7, and 29.3%, in SLM, and SLM incorporated with RE at 500, 1000, 1,500, and 2000 ppm, respectively. The addition of TBHQ resulted in a 36% reduction in p-AV compared to PeO at the end of study.
Both CD and CT values exhibited a rising trend throughout the storage study. PeO, due to high content of PUFAs and absence of antioxidant intervention, showed the highest degree of secondary oxidation with a value reaching 5.97 for CD and 1.954 for CT on day 21. In contrast, the SLM without added antioxidants displayed lower values, indicating a 14.2 and 24.4% reduction, respectively for CD and CT, which can be attributed to the oxidative stability imparted by enzymatic interesterification. SLM values containing antioxidant showed enhanced oxidative stability than both PeO and SLM in mitigating oxidation. SLM with TBHQ (3.91 for CD and 1.19 for CT) and SLM RE 1500 (4.04 for CD and 1.321 for CT) were most effective, with CD reductions of 34.5 and 32.3%, and CT reductions of 39.1 and 32.4%, respectively, compared to PeO. Samples with RE at 1000 ppm (4.28 for CD, 1.418 for CT) and 2000 ppm (4.69 for CD, 1.186 for CT) also showed significant protection, though the marginal improvement between 1,500 and 2000 ppm suggests a stagnation beyond which the antioxidant effect does not increase. SLM RE 500 showed moderate protective effect reducing CD and CT by 23.9 and 31.2%, respectively.
3.4 TOTOX
The TOTOX value represents the combined effect of primary and secondary oxidation products. In this study a progressive increase in TOTOX values for all samples was observed during the Schaal oven accelerated storage period, indicating a continuous oxidative degradation over time (Table 2). PeO showed the highest values starting at 8.63 and reaching 126.03 by Day 21, thereby indicating its high susceptibility to oxidation due to its PUFA content. In contrast, the SLM and its antioxidant-enriched samples demonstrated significantly lower TOTOX values throughout the storage. On Day 21, SLM without antioxidants exhibited a value of 104.03, while incorporation of RE at increasing concentrations resulted in an increased resistance to oxidation. SLM RE 1500 ppm showed the highest oxidative stability closely aligned to SL TBHQ. Notably, RE supplemented at 1000 ppm and 2000 ppm also provided significant protection, however, similar to p-AV no significant improvement was observed after 1,500 ppm, indicating a stagnant effect.
3.5 Colour
A constant decreasing trend was observed for the L* values of all samples during the accelerated storage study (Figure 4). Owing to the high susceptibility to oxidation due to the PeO’s fatty acid composition, which is rich in PUFA, it showed the most significant darkening due to extensive oxidative degradation, with value reducing from 71.92 to 66.30. In contrast to this, the SLM samples incorporated with antioxidants exhibited a better colour retention, indicating a protective effect against pigment deterioration. The a* values on the other hand exhibited an increase during the storage period indicating reduced greenness, most notably in PeO. Antioxidant enriched samples, particularly SLM RE 1500 and TBHQ, maintained more stable colour values, suggesting slower pigment breakdown. A reducing trend in b* values was observed in all samples, demonstrating a decline in yellowness, due to the degradation of In carotenoids during storage. The reduction again was most significant in PeO, while SLM RE 1500, RE 2000, and TBHQ showed higher b* values on Day 21 suggesting the protective effect of antioxidant addition.
3.6 Fatty acid profile
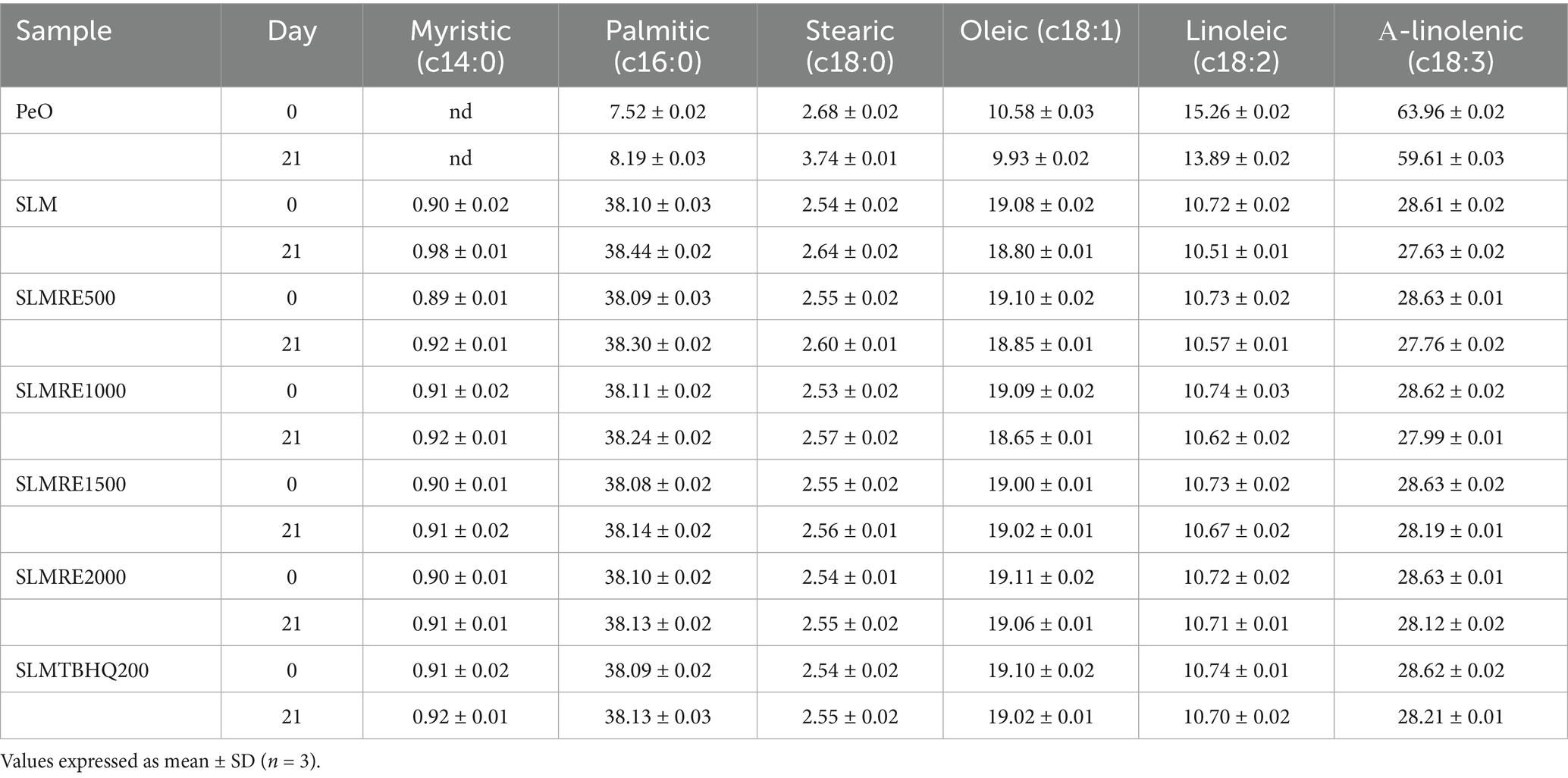
The accelerated storage conditions led to a significant changes in the fatty acids profile of the lipid samples. This change was based on both the lipid modification (enzymatic interesterification) as well as the antioxidant supplementation. The fatty acid composition of PeO, SLM, and SLM enriched with RE or TBHQ was analysed at day 0 and day 21 of accelerated oxidation study (Table 3). A substantial oxidative degradation of PUFA was observed in PeO, with about 7.22% reduction observed from initial to final day. These findings suggest the PUFA’s susceptibility to oxidative degradation in the absence of structural modifications or antioxidants (Mane et al., 2024). Compared to SLM systems, PeO showed the highest PUFA loss, indicating the limitations of using it in functional foods without stabilization. This loss of PUFA is consistent with their high susceptibility to oxidative degradation during storage, as previously reported for edible oils rich in omega-3 and omega-6 FAs (Kum et al., 2025; Kumari Singh et al., 2024). The SLM sample without the addition of any antioxidants showed a higher omega-3 fatty acid degradation, corresponding to about 3.5% reduction in linolenic acid. These changes were accompanied by slight increase in palmitic and stearic acid, suggesting the early onset of lipid oxidation. The improved oxidative stability compared to PeO can be attributed to the modified physical structure of the lipid matrix. The addition of RE was found to be effective in mitigating the FA loses due to the Schaal oven storage conditions. The SLM added with RE at 1500, and 2000 ppm showed minimal ALA degradation by only 1.5 and 1.8%, respectively, over 21 days. This concentration dependent protective effect of RE aligns with recent findings demonstrating its efficacy as a natural antioxidant in preserving PUFA content and delaying lipid oxidation in edible oils (Wang et al., 2018). The synthetic antioxidant, TBHQ provided similar protection, with minimal changes in FA composition, in line with recent comparative studies on the effectiveness of natural and synthetic antioxidants (Mishra et al., 2022).
3.7 Antioxidant capacity
The antioxidant activity demonstrated both a time and concentration dependent decrease in all samples (Figure 5). PeO exhibited a relatively high initial DPPH value owing to its inherent high concentration of bioactive compounds, however, it also showed a rapid decline in the absence of added antioxidants due to high PUFA content. In contrast, samples containing the RE at concentrations of 1,500 ppm and 2000 ppm along with the synthetic antioxidant, TBHQ exhibited significantly higher initial radical scavenging capacities compared to SLM. The addition of RE showed a concentration dependent effect on the preservation of radical scavenging activity; Notably, by Day 21, SLM RE 1500 exhibited statistically comparable radical scavenging activity to SLM TBHQ 200 verifying its efficiency as a natural antioxidant. Previous studies have reported the enhancement of antioxidant capacity and in turn the oxidative stability in oils and other products on addition of antioxidants (He et al., 2023; Kumari Singh et al., 2024; Wang et al., 2018).
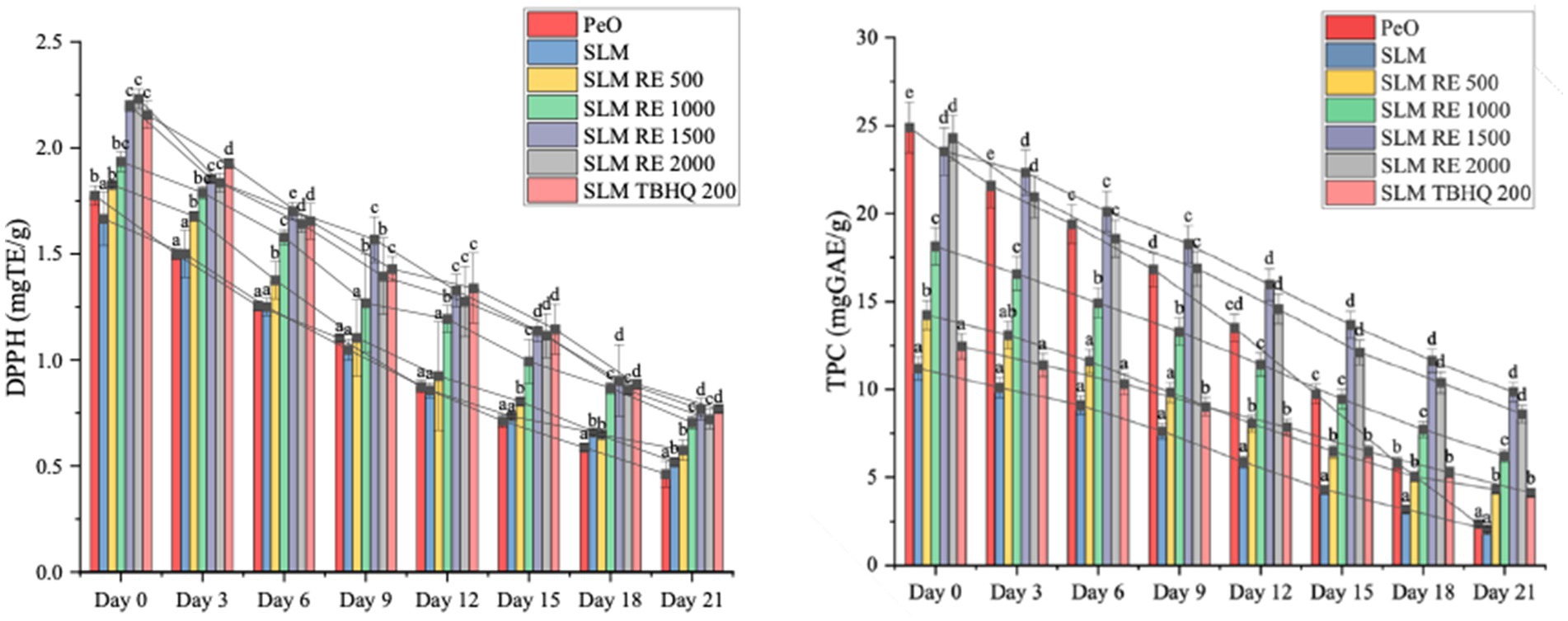
Figure 5. Antioxidant values of margarines during the accelerated storage study. Means different lowercase superscripts signify statistical difference sample-wise (p < 0.05).
The TPC analysis also showed a consistent decline in phenolic compounds across all samples over the 21 days of storage reflecting their utilization in preventing oxidative deterioration. Similar to DPPH, PeO was found to have a high initial value, indicating the presence of naturally occurring bioactive compounds but its sharp deline by Day 21 highlighted the degradation of these compounds in the absence of added antioxidants. In contrast, SLM RE 1500 and SLM RE 2000 had the highest initial TPC values, that can be attributed to the high content of phenolic compounds present in RE. Notably, by Day 21, SLM RE 1500 retained the highest phenolic content amongst these samples. In contrast, SLM TBHQ 200 exhibited a comparatively low initial TPC and retained similarly low levels by Day 21. The TPC of TBHQ is relatively less due to absence of phenolic compounds in it, however it retained due to its potent antioxidant capacity, also observed in DPPH assay.
3.8 Total tocopherol content
Tocopherols are naturally found in most plant oils, and serve as important antioxidants that help prevent these oils from oxidizing (Guo et al., 2023; Martin-Rubio et al., 2018; Pan et al., 2019). The highest tocopherol content was observed in the PeO control on the initial day (482.67 ± 18.56 mg/kg), followed by significantly lower values in SLM and treated samples, indicating antioxidant loss due to enzymatic interesterification (Figure 6). This was also due to the addition of palm stearin, lacking bioactive compounds and antioxidants. Consequently, blending PeO with palm stearin for interesterification led to an overall decrease in tocopherol content in the SLM samples (Dhiman et al., 2024; Singh et al., 2023). Apart from this, the interesterification reaction conditions also contribute to this tocopherol loss. After 21 days, a significant decline in tocopherol levels was observed in the samples, with the most significant reduction in the PeO control corresponding to a 77.15% loss. This sharp decline indicates the high oxidative susceptibility of PeO rich in linolenic acid. In contrast, SLM samples treated with antioxidants exhibited significantly better tocopherol retention. Among them, SLM RE 1500, SLM RE 2000, and SLM 200 TBHQ showed the highest tocopherol content at the end of the study, with only about 45 to 50% reduction from their initial values. The effectiveness of natural antioxidant RE was concentration-dependent, with higher levels providing greater protection comparable to TBHQ. The sample with 1,500 ppm RE also demonstrated highest tocopherol retention, further supporting the potential of RE as a natural alternative to synthetic antioxidants. These findings indicate that antioxidant incorporation, especially at higher concentrations, plays a significant role in mitigating tocopherol degradation during storage, thereby enhancing the oxidative stability of omega-3 rich lipid systems.
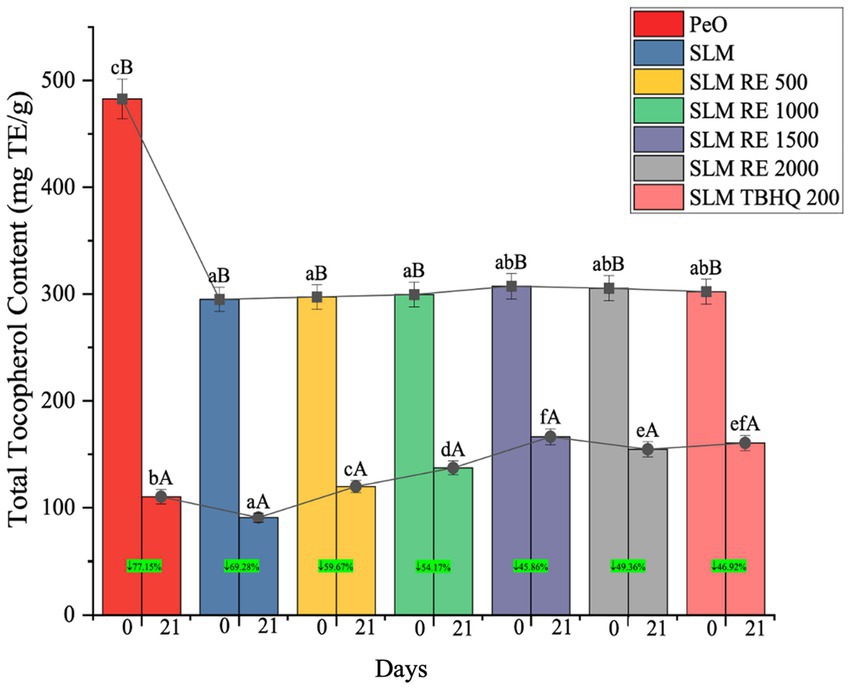
Figure 6. Changes in tocopherol values of margarines samples during the storage study with % reduction from day 0 to day 21 for each sample highlighted in green colour.
3.9 Principal component analysis
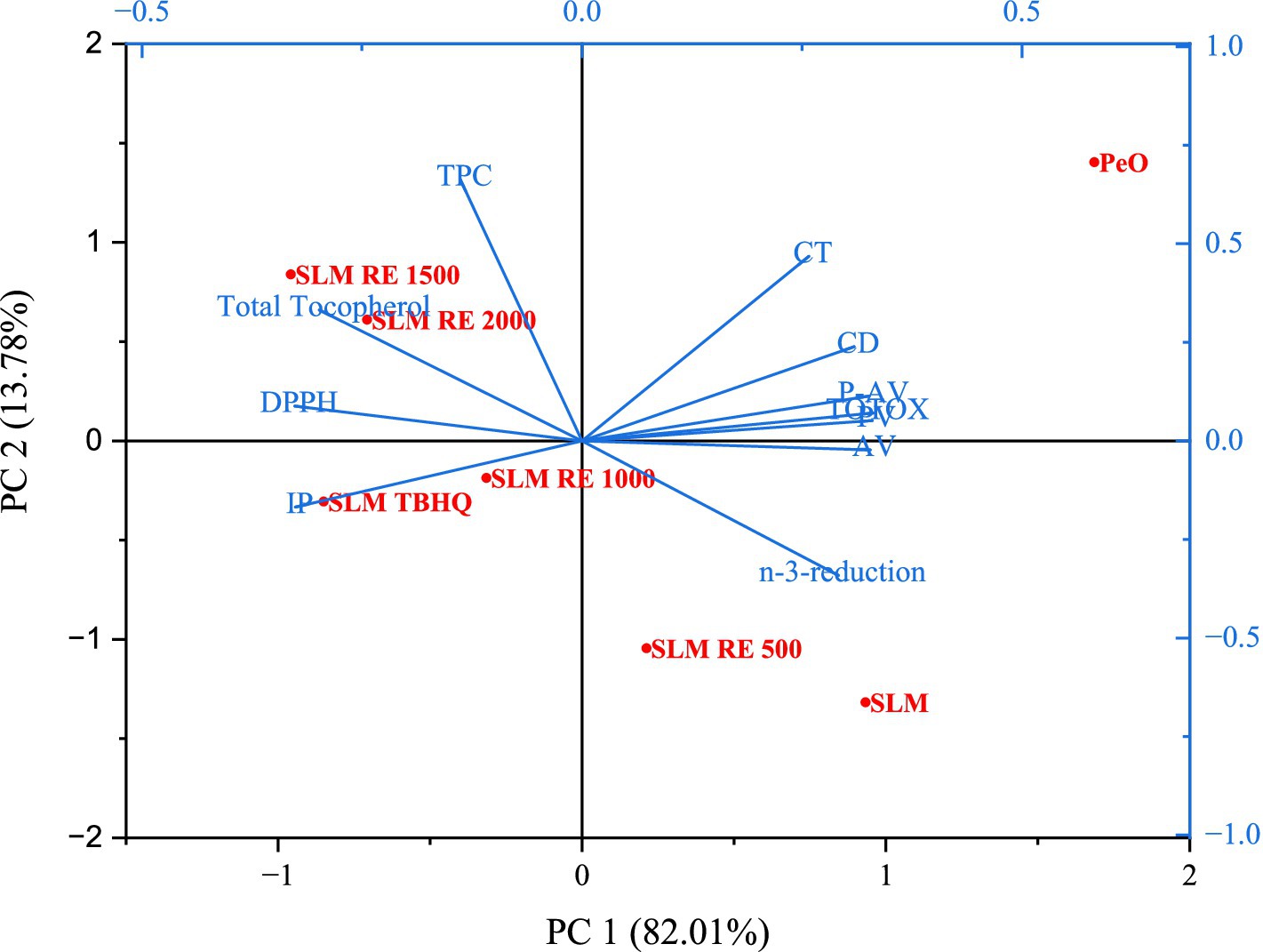
The Principal Component Analysis (PCA) biplot provided a comprehensive multivariate depiction of the oxidative stability and antioxidant properties of the lipid systems analyzed in the study, subjected to different treatments. The first two principal components, PC 1 and PC2 explained a cumulative of 95.79% of total variance (82.01% by PC1 and 13.78% by PC2), indicating that majority of the variability in the dataset was explained in two dimensions. Amongst the loading vectors for individual variables, a distinct separation was observed between oxidative deterioration markers and antioxidant attributes. Oxidation indices including PV, AV, p-AV, TOTOX value, CD, and CT were clustered together in the positive quadrant of PC1, suggesting their mutual positive correlation and shared contribution to lipid oxidation. In contrast, variables representing antioxidant capacity, i.e., DPPH radical scavenging activity, TPC, and total tocopherol content were observed to be in the opposite direction, indicative of a negative correlation with oxidation parameters. The variable n-3 reduction, representing the loss of omega-3 fatty acids was aligned with oxidative parameters, thus validating that oxidative deterioration is directly linked to the degradation of PUFA. The position of the samples in the biplot further illustrates the effect of RE and TBHQ on the lipid systems. PeO, characterized by high unsaturation and absence of antioxidant or any lipid modification was found to be close to the oxidation parameters, indicating its high susceptibility to oxidative degradation. The control structured lipid, SLM, although slightly improved, remained on the oxidative side of the plot. However, with the addition of rosemary extract, a significant variation was observed based on the concentration of RE. The samples progressively shifted away from the oxidative markers and towards the antioxidant-based variables. Also, the minute shift between TBHQ and RE at 1500 and 2000 ppm is mainly due to low antioxidant capacity of TBHQ in terms of TPC and tocopherol content. SLM RE 1500 clustered closely with SLM RE 2000 and SLM TBHQ, suggesting that at RE at 1500 ppm concentration showed similar efficacy in enhancing oxidative stability and radical scavenging activity as the highest concentration (RE 2000 ppm) and TBHQ (Gurkan and Hayaloglu, 2023). These results validate the potential of RE as a sustainable and natural alternative for enhancing the shelf life and nutritional quality of PUFA-rich structured lipid-based margarine formulations (see Figure 7).
4 Conclusion
This study demonstrates the effectiveness of rosemary extract as a potential natural antioxidant for improving the oxidative stability and shelf life of omega-3 fatty acid rich margarine formulated using perilla seed oil based structured lipid systems. Among all the concentrations, rosemary extract at 1500 ppm exhibited significant reduction in the lipid oxidation and retention of polyunsaturated fatty acids, thereby maintaining the nutritional value of the PUFA rich margarine. The antioxidant performance of rosemary extract was comparable to that of the synthetic antioxidant (TBHQ), indicating its potential as a sustainable alternative for lipid stabilization in food products.
Additionally, lipid modification through enzymatic interesterification of perilla seed oil with palm stearin significantly enhanced the oxidative stability of PeO. The extrapolation of accelerated oxidation data indicates that the shelf life of the margarine formulations at ambient conditions is approximately three months for PeO, six months for the control SLM, seven to eight months for samples containing 500 and 1,000 ppm RE and about nine months for formulations enriched with 1,500 and 2000 ppm RE, with stability comparable to that of TBHQ. The combination of enzymatic interesterification and rosemary extract incorporation resulted in a synergistic effect, offering enhanced protection against the generation of primary and secondary oxidation products while minimizing the degradation of omega-3 fatty acids. This not only enhanced the physicochemical quality of the margarine but also aligned with the growing demand for natural, sustainable food preservation strategies. In conclusion, the findings of the study demonstrate the potential of green technologies like enzymatic interesterification and addition of plant-based antioxidants for developing functional lipid systems with extended shelf life and enhanced nutritional properties. Future studies can be aimed on the study of sensory attributes of the product.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Writing – original draft, Writing – review & editing. RC: Conceptualization, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. AD and RC gratitude to National Institute of Food Technology Entrepreneurship and Management, Kundli, Sonipat (Haryana) for providing infrastructural and funding support. The authors are grateful to University Grant Commission (UGC), New Delhi to provide financial support.
Acknowledgments
The authors acknowledge the Novozymes, India for providing the immobilised lipase Lipozyme TL-IM enzyme used in this study. NIFTEM-K communication number: NIFTEM-P-2025-63.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aldamarany, W. A. S., Taocui, H., Liling, D., Mei, H., Yi, Z., and Zhong, G. (2023). Perilla, sunflower, and tea seed oils as potential dietary supplements with anti-obesity effects by modulating the gut microbiota composition in mice fed a high-fat diet. Eur. J. Nutr. 62, 2509–2525. doi: 10.1007/s00394-023-03155-3
Baştürk, A., Ceylan, M. M., Çavuş, M., Boran, G., and Javidipour, I. (2018). Effects of some herbal extracts on oxidative stability of corn oil under accelerated oxidation conditions in comparison with some commonly used antioxidants. LWT 89, 358–364. doi: 10.1016/j.lwt.2017.11.005
Boukandoul, S., Santos, C. S., Casal, S., and Zaidi, F. (2019). Oxidation delay of sunflower oil under frying by moringa oil addition: more than just a blend. J. Sci. Food Agric. 99, 5483–5490. doi: 10.1002/jsfa.9809
Brand-Williams, W., Cuvelier, M. E., and Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 28, 25–30. doi: 10.1016/S0023-6438(95)80008-5
Bureau of Indian Standards. (2021). IS 548: part 1: sec 1: 2021: method of sampling and test for oils and fats part i sampling, physical and chemical tests section 1 sampling. Available online at: http://archive.org/details/gov.in.is.548.1.1.2021
Cordeiro, A. M. T. M., Medeiros, M. L., Santos, N. A., Soledade, L. E. B., Pontes, L. F. B. L., Souza, A. L., et al. (2013). Rosemary (Rosmarinus officinalis L.) extract: Thermal study and evaluation of the antioxidant effect on vegetable oils. Journal of Thermal Analysis and Calorimetry, 113, 889–895. doi: 10.1007/s10973-012-2778-4
Couto, R. O., Conceição, E. C., Chaul, L. T., Oliveira, E. M. S., Martins, F. S., Bara, M. T. F., et al. (2012). Spray-dried rosemary extracts: physicochemical and antioxidant properties. Food Chem. 131, 99–105. doi: 10.1016/j.foodchem.2011.08.036
de Oliveira, P. D., da Silva, D. A., Pires, W. P., Bezerra, C. V., da Silva, L. H. M., and da Cruz Rodrigues, A. M. (2021). Enzymatic interesterification effect on the physicochemical and technological properties of cupuassu seed fat and inaja pulp oil blends. Food Res. Int. 145:110384. doi: 10.1016/j.foodres.2021.110384
Dhiman, A., Chopra, R., Homroy, S., and Chand, M. (2025). Perilla seed oil-based omega-3 fatty acid rich designer lipid enriched with rosemary extract: a sustainable approach to enhance oxidative stability and functional properties. Discov. Food 5:134. doi: 10.1007/s44187-025-00388-5
Dhiman, A., Chopra, R., Singh, P. K., Singh, A., and Homroy, S. (2024). Enzymatic interesterification of perilla seed oil and palm stearin: a sustainable approach to develop a novel zero-trans-fat margarine rich in omega-3 fatty acids. J. Food Sci. 5:134. doi: 10.1111/1750-3841.17483
Dhyani, A., Singh, P., Chopra, R., and Garg, M. (2022). Enhancement of oxidative stability of Perilla seed oil by blending it with other vegetable oils. J. Oleo Sci. 71, 1135–1144. doi: 10.5650/jos.ess22013
Dong, S., Zhou, Y., Sun, S., and Chen, X. (2023). Preparation of a novel healthy tiger nut oil-based margarine fat with low trans and saturated fatty acids. Food Chem. 427:136731. doi: 10.1016/j.foodchem.2023.136731
European Commission. (2001). Commission Directive 2001/15/EC of 15 February 2001 on substances that may be added for specific nutritional purposes in foods for particular nutritional uses. Official Journal of the European Communities. Available at: https://eur-lex.europa.eu/eli/dir/2001/15/oj/eng
European Commission. (2008). Regulation (EC) no 1333/2008 on food additives. Annex II, E319: tertiary butylhydroquinone (TBHQ). Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02008R1333-20170612
Food Safety and Standards Authority of India (FSSAI). (2011). Food safety and standards (food products standards and food additives) regulations. Available online at: https://www.fssai.gov.in/cms/food-safety-and-standards-regulations.php (Accessed August 10, 2025).
Food Safety and Standards Authority of India. (2021). Food Safety and Standards (Food Products Standards and Food Additives) Regulations, 2011: Appendix A – List of Food Additives. FSSAI.
Ghosh, M., Upadhyay, R., Mahato, D. K., and Mishra, H. N. (2019). Kinetics of lipid oxidation in omega fatty acids rich blends of sunflower and sesame oils using Rancimat. Food Chem. 272, 471–477. doi: 10.1016/j.foodchem.2018.08.072
Golmakani, M.-T., Soltani, A., Hosseini, S. M. H., and Keramat, M. (2020). Improving the oxidation kinetics of linseed oil using the blending approach. J. Food Proc. Preserv. 44:e14964. doi: 10.1111/jfpp.14964
Guo, M., Yang, L., Li, X., Tang, H., Li, X., Xue, Y., et al. (2023). Antioxidant efficacy of rosemary extract in improving the oxidative stability of rapeseed oil during storage. Foods 12:3583. doi: 10.3390/foods12193583
Gurkan, H., and Hayaloglu, A. A. (2023). Changes in volatiles and essential oil composition of three organs (leaf, stem and flower) of purple basil (Ocimum basilicum L.) by GC–MS combined with multivariate statistical approach. Food Chem. Adv. 2:100292. doi: 10.1016/j.focha.2023.100292
He, J., Hadidi, M., Yang, S., Khan, M. R., Zhang, W., and Cong, X. (2023). Natural food preservation with ginger essential oil: biological properties and delivery systems. Food Res. Int. 173:113221. doi: 10.1016/j.foodres.2023.113221
Jadhav, H. B., and Annapure, U. (2021). Designer lipids -synthesis and application – a review. Trends Food Sci. Technol. 116, 884–902. doi: 10.1016/j.tifs.2021.08.020
Kapoor, B., Kapoor, D., Gautam, S., Singh, R., and Bhardwaj, S. (2021). Dietary polyunsaturated fatty acids (PUFAs): uses and potential health benefits. Curr. Nutr. Rep. 10, 232–242. doi: 10.1007/s13668-021-00363-3
Kaur, S., Seem, K., Ali, A., Jaiswal, S., Gumachanamardi, P., Kaur, G., et al. (2024). A comprehensive review on nutritional, nutraceutical, and industrial perspectives of perilla (Perilla frutscens L.) seeds – an orphan oilseed crop. Heliyon 10:e33281. doi: 10.1016/j.heliyon.2024.e33281
Kum, S.-R. M., Djikeng, F. T., Ufuan, A. A., Ninying, V.-T. Z. S., and Tiencheu, B. (2025). Uses of rosemary (Salvia rosmarinus) extract as natural preservative in peanut oil, African walnut oil and their 60:40 and 50:50 blends during accelerated storage. Food Human. 4:100507. doi: 10.1016/j.foohum.2025.100507
Kumari Singh, P., Chopra, R., Garg, M., Chauhan, K., Singh, N., Homroy, S., et al. (2024). Shelf life enhancement of structured lipids rich in Omega-3 fatty acids using rosemary extract: a sustainable approach. ACS Omega 9, 31359–31372. doi: 10.1021/acsomega.3c09584
Li, X., Li, Y., Yang, F., Liu, R., Zhao, C., Jin, Q., et al. (2019). Oxidation degree of soybean oil at induction time point under Rancimat test condition: theoretical derivation and experimental observation. Food Res. Int. 120, 756–762. doi: 10.1016/j.foodres.2018.11.036
Li, Y., Zhao, J., Xie, X., Zhang, Z., Zhang, N., and Wang, Y. (2018). A low trans margarine fat analog to beef tallow for healthier formulations: optimization of enzymatic interesterification using soybean oil and fully hydrogenated palm oil. Food Chem. 255, 405–413. doi: 10.1016/j.foodchem.2018.02.086
Luca, S. V., Zengin, G., Sinan, K. I., Korona-Glowniak, I., Minceva, M., Skalicka-Woźniak, K., et al. (2023). Value-added compounds with antimicrobial, antioxidant, and enzyme-inhibitory effects from post-distillation and post-supercritical CO2 extraction by-products of rosemary. Antioxidants 12:244. doi: 10.3390/antiox12020244
Ma, X., Hu, Z., Mao, J., Xu, Y., Zhu, X., and Xiong, H. (2019). Synthesis of cocoa butter substitutes from Cinnamomum camphora seed oil and fully hydrogenated palm oil by enzymatic interesterification. J. Food Sci. Technol. 56, 835–845. doi: 10.1007/s13197-018-3543-x
Machado, M., Rodriguez-Alcalá, L. M., Gomes, A. M., and Pintado, M. (2023). Vegetable oils oxidation: mechanisms, consequences and protective strategies. Food Rev. Intl. 39, 4180–4197. doi: 10.1080/87559129.2022.2026378
Mane, S., Kumari, P., Singh, A., Taneja, N. K., and Chopra, R. (2024). Amelioration for oxidative stability and bioavailability of N-3 PUFA enriched microalgae oil: an overview. Crit. Rev. Food Sci. Nutr. 64, 2579–2600. doi: 10.1080/10408398.2022.2124505
Marangoni, A. G., Van Duynhoven, J. P. M., Acevedo, N. C., Nicholson, R. A., and Patel, A. R. (2020). Advances in our understanding of the structure and functionality of edible fats and fat mimetics. Soft Matter 16, 289–306. doi: 10.1039/C9SM01704F
Martin-Rubio, A. S., Sopelana, P., Ibargoitia, M. L., and Guillén, M. D. (2018). Prooxidant effect of α-tocopherol on soybean oil. Global monitoring of its oxidation process under accelerated storage conditions by 1H nuclear magnetic resonance. Food Chem. 245, 312–323. doi: 10.1016/j.foodchem.2017.10.098
Mena, P., Cirlini, M., Tassotti, M., Herrlinger, K. A., Dall’Asta, C., and Del Rio, D. (2016). Phytochemical profiling of flavonoids, phenolic acids, Terpenoids, and volatile fraction of a rosemary (Rosmarinus officinalis L.) extract. Molecules 21:1576. doi: 10.3390/molecules21111576
Mishra, S. K., Belur, P. D., and Iyyaswami, R. (2022). Comparison of efficacy of various natural and synthetic antioxidants in stabilizing the fish oil. J. Food Proc. Preserv. 46:e16970. doi: 10.1111/jfpp.16970
Moczkowska, M., Karp, S., Horbanczuk, O. K., Hanula, M., Wyrwisz, J., and Kurek, M. A. (2020). Effect of rosemary extract addition on oxidative stability and quality of hemp seed oil. Food Bioprod. Process. 124, 33–47. doi: 10.1016/j.fbp.2020.08.002
Nagpal, T., Sahu, J. K., Khare, S. K., Bashir, K., and Jan, K. (2021). Trans fatty acids in food: a review on dietary intake, health impact, regulations and alternatives. J. Food Sci. 86, 5159–5174. doi: 10.1111/1750-3841.15977
Pan, F., Wen, B., Wang, X., Ma, X., Zhao, J., Liu, C., et al. (2019). Effect of the chemical refining process on perilla seed oil composition and oxidative stability. J. Food Proc. Preserv. 43:e14094. doi: 10.1111/jfpp.14094
Pande, G., Akoh, C. C., and Shewfelt, R. L. (2013). Utilization of enzymatically interesterified cottonseed oil and palm stearin-based structured lipid in the production of trans-free margarine. Biocatal. Agric. Biotechnol. 2, 76–84. doi: 10.1016/j.bcab.2012.08.005
Pang, M., Ge, Y., Cao, L., Cheng, J., and Jiang, S. (2019). Physicochemical properties, crystallization behavior and oxidative stabilities of enzymatic Interesterified fats of beef tallow, palm stearin and Camellia oil blends. J. Oleo Sci. 68, 131–139. doi: 10.5650/jos.ess18201
Pointner, T., Rauh, K., Auñon-Lopez, A., Kostadinović Veličkovska, S., Mitrev, S., Arsov, E., et al. (2024). Comprehensive analysis of oxidative stability and nutritional values of germinated linseed and sunflower seed oil. Food Chem. 454:139790. doi: 10.1016/j.foodchem.2024.139790
Pokhrel, K., Kouřimská, L., Rudolf, O., and Tilami, S. K. (2024). Oxidative stability of crude oils relative to tocol content from eight oat cultivars: comparing the Schaal oven and Rancimat tests. J. Food Compos. Anal. 126:105918. doi: 10.1016/j.jfca.2023.105918
Polavarapu, S., Oliver, C. M., Ajlouni, S., and Augustin, M. A. (2011). Physicochemical characterisation and oxidative stability of fish oil and fish oil–extra virgin olive oil microencapsulated by sugar beet pectin. Food Chem. 127, 1694–1705. doi: 10.1016/j.foodchem.2011.02.044
Ramroudi, F., Yasini Ardakani, S. A., Dehghani-tafti, A., and Khalili Sadrabad, E. (2022). Investigation of the physicochemical properties of vegetable oils blended with sesame oil and their oxidative stability during frying. Int. J. Food Sci. 2022:e3165512. doi: 10.1155/2022/3165512
Rashid, R., Masoodi, F. A., Wani, S. M., Manzoor, S., and Gull, A. (2022). Ultrasound assisted extraction of bioactive compounds from pomegranate peel, their nanoencapsulation and application for improvement in shelf life extension of edible oils. Food Chem. 385:132608. doi: 10.1016/j.foodchem.2022.132608
Samani, M. A. (2017). Antioxidant activity and properties of walnut Brown seed coat extract. J. Glob. Pharma Technol. 8, 26–30.
Sharma, A., Bhardwaj, G., and Cannoo, D. S. (2021). Antioxidant potential, GC/MS and headspace GC/MS analysis of essential oils isolated from the roots, stems and aerial parts of Nepeta leucophylla. Biocatal. Agric. Biotechnol. 32:101950. doi: 10.1016/j.bcab.2021.101950
Sharma, S., Cheng, S.-F., Bhattacharya, B., and Chakkaravarthi, S. (2019). Efficacy of free and encapsulated natural antioxidants in oxidative stability of edible oil: special emphasis on nanoemulsion-based encapsulation. Trends Food Sci. Technol. 91, 305–318. doi: 10.1016/j.tifs.2019.07.030
Shin, D.-M., Yune, J. H., Kim, T.-K., Kim, Y. J., Kwon, H. C., Kim, D. H., et al. (2021). Physicochemical properties and oxidative stability of duck fat-added margarine for reducing the use of fully hydrogenated soybean oil. Food Chem. 363:130260. doi: 10.1016/j.foodchem.2021.130260
Singh, P., Chopra, R., Dhiman, A., Chuahan, K., and Garg, M. (2023). Development of omega-3-rich structured lipids using perilla seed oil and palm olein: optimization and characterization. Biomass Convers. Biorefinery 14, 23857–23871. doi: 10.1007/s13399-023-04422-3
Singh, P. K., Chopra, R., Garg, M., Chauhan, K., and Agarwal, A. (2024). Stability of perilla seed oil based PUFA-rich structured lipids using enzymatic interesterification: a thermo-oxidative kinetic study. Ind. Crop. Prod. 209:118029. doi: 10.1016/j.indcrop.2024.118029
Tohma, S., and Turan, S. (2015). Rosemary plant (Rosmarinus officinalis L.), solvent extract and essential oil can be used to extend the usage life of hazelnut oil during deep frying. Eur. J. Lipid Sci. Technol. 117, 1978–1990. doi: 10.1002/ejlt.201400382
U.S. Food and Drug Administration (FDA). (2018). Food additive status list. CFR title 21, §172.185 tertiary Butylhydroquinone. Available online at: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-172/section-172.185
Veenstra, J. P., and Johnson, J. J. (2021). Rosemary (Salvia rosmarinus): health-promoting benefits and food preservative properties. Int. J. Nutr. 6, 1–10.
Wang, Y.-Z., Fu, S.-G., Wang, S.-Y., Yang, D.-J., Wu, Y.-H. S., and Chen, Y.-C. (2018). Effects of a natural antioxidant, polyphenol-rich rosemary (Rosmarinus officinalis L.) extract, on lipid stability of plant-derived omega-3 fatty-acid rich oil. LWT 89, 210–216. doi: 10.1016/j.lwt.2017.10.055
Wang, Y., Wang, Y., Shehzad, Q., Su, Y., Xu, L., Yu, L., et al. (2024). Does omega-3 PUFAs supplementation improve metabolic syndrome and related cardiovascular diseases? A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 64, 9455–9482. doi: 10.1080/10408398.2023.2212817
Yeşilsu, A. F., and Özyurt, G. (2019). Oxidative stability of microencapsulated fish oil with rosemary, thyme and laurel extracts: A kinetic assessment. Journal of Food Engineering, 240, 171–182. doi: 10.1016/j.jfoodeng.2018.07.021
Keywords: PUFA, Rosmarinus officinalis L., preservation, shelf-life, enzymatic interesterification
Citation: Dhiman A and Chopra R (2025) Rosemary extract as a natural antioxidant in structured lipid systems: a sustainable approach to enhance stability of perilla seed oil based omega-3 fatty acid rich margarine. Front. Sustain. Food Syst. 9:1654058. doi: 10.3389/fsufs.2025.1654058
Edited by:
Prasanth Kumar Sasidharan Pillai, University of Minnesota Twin Cities, United StatesReviewed by:
Utai Klinkesorn, Kasetsart University, ThailandAdeyemi Adeyanju, Landmark University, Nigeria
Copyright © 2025 Dhiman and Chopra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajni Chopra, cmFqbmljaG9wcmEubmlmdGVtQGdtYWlsLmNvbQ==
 Aishwarya Dhiman
Aishwarya Dhiman Rajni Chopra
Rajni Chopra