- 1School of Agricultural Sciences, Faculty of Agriculture and Natural Sciences, University of Mpumalanga, Nelspruit, South Africa
- 2Department of Animal Science, Faculty of Natural and Agricultural Sciences, North-West University, Mafikeng, South Africa
- 3Food Security and Safety Focus Area, Faculty of Natural and Agricultural Sciences, North-West University, Mafikeng, South Africa
Heavy dependence on soybeans for non-ruminant diets is unsustainable in regions where poor growing conditions limit production. Across much of the Global South, local supply rarely meets demand, forcing costly imports that erode feed profitability. Indigenous pulses such as cowpeas, well-adapted to local climates and soils, offer a practical alternative or complement to soybean protein. Cowpeas are rich in protein and contain unique bioactive compounds with antimicrobial, antioxidant, anti-inflammatory, anticancer, and immunostimulatory potential. However, their nutritional quality remains inferior to soybeans due to anti-nutritional factors (ANFs) and an imbalanced amino acid profile, particularly a deficiency in sulfur-containing amino acids like methionine and cysteine. Additionally, like many legumes, cowpeas also exhibit relatively low protein digestibility, which further limits their direct use in non-ruminant diets. To unlock the potential of cowpeas as a sustainable feed ingredient, effective valorization strategies are essential. Techniques such as solid-state fermentation, sprouting, soaking, roasting, boiling, dehulling, extrusion, hot-air drying, and enzymatic treatments have been explored to enhance nutritional value. These methods aim to reduce ANFs, improve amino acid balance, and increase protein digestibility. This systematic review synthesizes current research on the mechanisms and efficacy of cowpea valorization techniques, with a particular focus on their capacity to achieve nutritional and functional parity with soybean meal in non-ruminant diets. By critically evaluating the impact of these approaches, the review provides a foundation for optimizing cowpea utilization in animal feeding systems. Such advancements could contribute significantly to climate-resilient, economically viable, and nutrition-secure food systems in the Global South.
1 Introduction
Soybeans have long dominated global legume production, particularly as a protein source for animal nutrition, because of their high protein content (44–48%) and well-established international supply chains. Well adapted to the major producing regions of South America and North America, soybeans are cultivated and traded at a scale that enables them to meet protein demands worldwide. In 2023, global soybean output reached 398.2 million tons, with Argentina, Brazil, and the United States accounting for most production and consumption (Volkova and Smolyaninova, 2023). Despite this success, soybean cultivation is often poorly aligned with the agro-ecological realities of many regions in the Global South. High yields typically require substantial inputs of fertilizer, irrigation, and pesticides, which limit both environmental sustainability and affordability in resource-constrained settings. In many low- and middle-income countries, domestic production shortfalls also necessitate costly imports, placing additional pressure on local food and feed systems (Gbenle et al., 2025). These constraints have intensified the search for alternative, regionally adapted protein sources.
One promising candidate is cowpea (Vigna unguiculata), an indigenous, climate-resilient legume that thrives in marginal environments with minimal external inputs. Widely cultivated in Africa, Asia, and Latin America, and especially important in sub-Saharan Africa, where Nigeria and Niger contribute nearly half of global production (Anele et al., 2010; Maila and Tseke, 2024), cowpeas are increasingly viewed as a strategic crop for sustainable food and feed security in the Global South. Nutritionally, cowpeas contain 17.4–31.7% protein (predominantly globulins), 50–60% carbohydrates, about 1% fat, and appreciable dietary fiber, vitamins, minerals, and bioactive compounds such as flavonoids, lignins, and phenolic acids with antioxidant and anti-inflammatory properties (Tzanova et al., 2023; Santos et al., 2020). Their amino-acid profile compares favorably with soybeans in lysine, leucine, and arginine, yet they remain deficient in the sulfur-containing amino acids methionine and cysteine (Lubisi et al., 2023; Kur et al., 2013).
Several factors still limit the wider use of cowpeas in non-ruminant diets. Competition with human consumption, limited breeding investment, and the presence of antinutritional factors (ANFs), including phytic acid, oxalates, tannins, lectins, saponins, and amylase and protease inhibitors, impair nutrient bioavailability and reduce protein digestibility, ultimately constraining animal growth performance (Verni et al., 2019). Protein digestibility, the proportion of dietary protein broken down into absorbable amino acids, is especially affected by these compounds and by structural features of the seed coat (Santos-Sánchez et al., 2024). Although molecular breeding for low-ANF cultivars is possible, it is often costly and may compromise yield or nutrient content. To overcome these challenges, a variety of valorization techniques, including fermentation, germination, soaking, thermal processing, dehulling, and enzymatic treatments, have been investigated for their ability to reduce ANFs, enhance amino-acid availability, and improve protein digestibility.
This systematic review critically evaluates those techniques and the mechanisms by which they enhance the nutritional and functional value of cowpeas for non-ruminant feeding. Specifically, it assesses how different interventions reduce ANFs, improve amino acid profiles, and promote efficient protein utilization. By synthesizing current evidence, the review advances the case for cowpeas as a sustainable, locally adapted alternative to soybean meal, thereby supporting more resilient food systems and improved nutritional security across the Global South.
2 Methodology
A systematic literature search was performed in ScienceDirect, Google Scholar, Taylor & Francis Online, Scopus, and Wiley Online Library. Five keyword combinations guided the strategy:
i. amino acids AND antinutritional factors AND functional properties AND legumes AND valorization techniques,
ii. the same terms with cowpeas specified,
iii. the core terms plus mechanical treatment AND thermal treatment,
iv. the core terms plus sprouting, fermentation, AND enzymatic treatment, and
v. valorization AND economic implications AND environmental implications AND legumes.
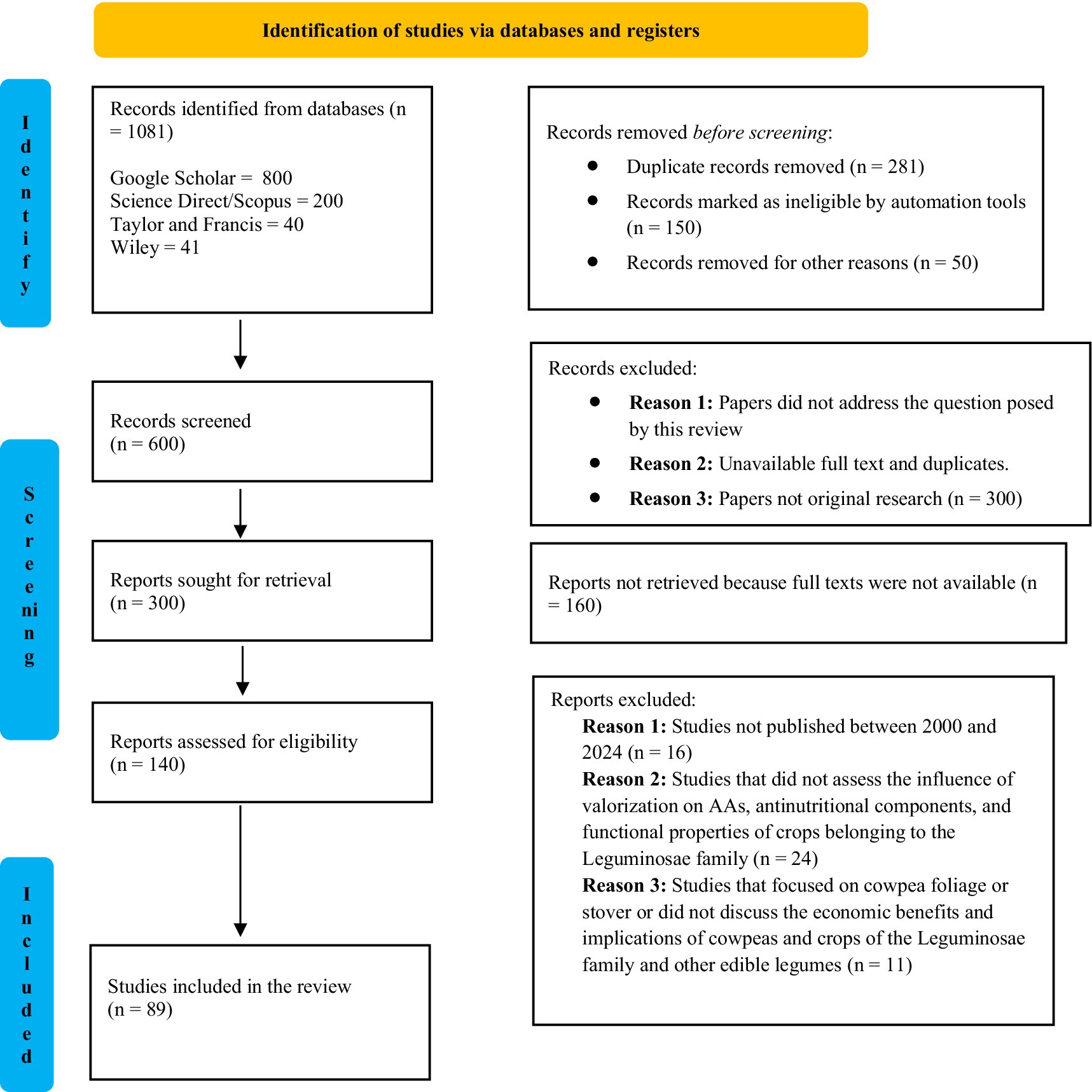
A total of 1,081 records were identified through database searches and subjected to a multi-stage PRISMA screening. We included only peer-reviewed studies published between 2000 and 2024 that examined the effects of valorization techniques, such as fermentation, soaking, germination, dehulling, or thermal processing, on the nutritional and functional properties of cowpeas or other edible legumes. Studies were excluded if they (i) focused on cowpea foliage or stover rather than seed, (ii) lacked a clear valorization intervention, or (iii) did not report outcomes related to protein content, amino-acid composition, or key functional properties. After applying these criteria, 89 studies met the inclusion requirements and were incorporated into this review. The article selection and screening process, including inclusion and exclusion steps, is illustrated in Figure 1.
Only peer-reviewed articles published between 2000 and 2024 that examined the effects of valorization techniques, fermentation, soaking, germination, dehulling, or thermal processing, on the nutritional or functional properties of cowpeas or other edible legumes were included. Studies were excluded if they focused on foliage or stover, lacked a valorization intervention, or failed to report outcomes on protein content, amino-acid composition, or functional properties. Eighty-nine studies met these criteria.
3 Comparative nutritional profile of cowpeas and soybeans
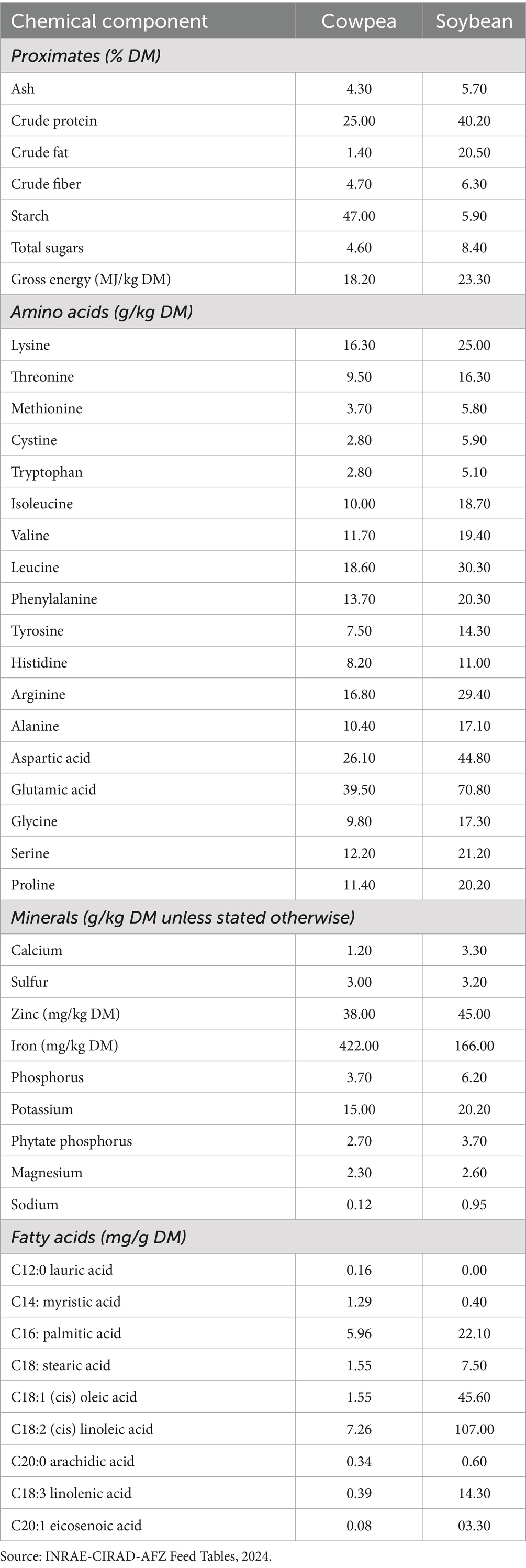
Soybeans and cowpeas differ markedly in their macronutrient profiles (Table 1). Soybeans are a true oilseed legume, supplying about 40% crude protein and 20% fat, which together yield a high gross energy content of 23 MJ/kg DM. In contrast, cowpeas provide only 25% protein and 1–2% fat, resulting in a lower energy density of about 18 MJ/kg DM. Cowpeas compensate with a much higher starch content (~47% DM vs. <6% in soybeans), making them closer to a cereal–legume hybrid in energy contribution. The amino acid balance reinforces soybeans’ reputation as a benchmark protein source. Essential amino acids, including lysine, leucine, isoleucine, and valine, are consistently higher in soybeans. Most importantly, the sulfur-containing amino acids methionine and cystine total only 6.4 g/kg in cowpeas compared with 11.8 g/kg in soybeans, underscoring the need for methionine supplementation or targeted processing when cowpeas are used in diets of non-ruminants. Mineral profiles show a different pattern. Cowpeas provide more than double the iron of soybeans (~422 vs. 166 mg/kg DM), an advantage in regions where iron deficiency is prevalent. Soybeans, however, are richer in calcium and phosphorus and slightly higher in zinc and potassium. Fatty-acid composition reflects the overall lipid contrast. Soybeans are a rich source of polyunsaturated fats, especially linoleic (~107 mg/g) and linolenic acids (~14 mg/g), whereas cowpeas contain only trace amounts of these essential fatty acids. In summary, soybeans offer a concentrated, balanced protein and energy source with valuable unsaturated oils, while cowpeas provide lower-cost, climate-resilient protein with exceptional iron and starch content but require valorization and amino-acid balancing to substitute effectively for soybean meal in non-ruminant feed systems.
4 Nutritional limitations in cowpeas
Cowpeas, like most underutilized legumes, contain various ANFs that adversely affect feed intake, digestibility, and nutrient bioavailability in non-ruminant animals (Silva et al., 2023). These include tannins, phytic acid, oxalates, amylase inhibitors, chymotrypsin inhibitors, saponins, and oligosaccharides such as raffinose, verbascose, and stachyose (Kur et al., 2013; Gautheron et al., 2024; Table 1). These secondary metabolites serve protective roles in plants against pests and pathogens (Salim et al., 2023) but are detrimental when included in non-ruminant diets. In addition, the amino acid profile of cowpeas is inferior to that of soybeans (Lubisi et al., 2023). These limitations and their impact on the nutritional value of cowpeas are briefly discussed below.
4.1 Phytic acid
Phytic acid is the principal storage form of phosphorus in legumes, accounting for 50–85% of their total phosphorus content. It is predominantly localized within protein body globoids in the cotyledons. Phytic acid strongly chelates essential minerals such as iron, zinc, calcium, and magnesium, forming insoluble phytate complexes that reduce mineral bioavailability in the gastrointestinal tract (Emkani et al., 2023; Kumar et al., 2021). Additionally, it impairs nutrient digestion by binding directly to digestive enzymes or by sequestering metal cofactors required for enzymatic activity, thereby inhibiting protein and lipid breakdown (Akissoé et al., 2021). As shown in Table 2, cowpeas contain approximately 836 mg/100 g of phytic acid (Abebe and Alemayehu, 2022), which is lower than the 1076.2 mg/100 g reported for soybeans. In non-ruminant animals, the absence of endogenous phytase to hydrolyze phytate further limits the nutritional utilization of legumes such as cowpeas (Simion, 2018).
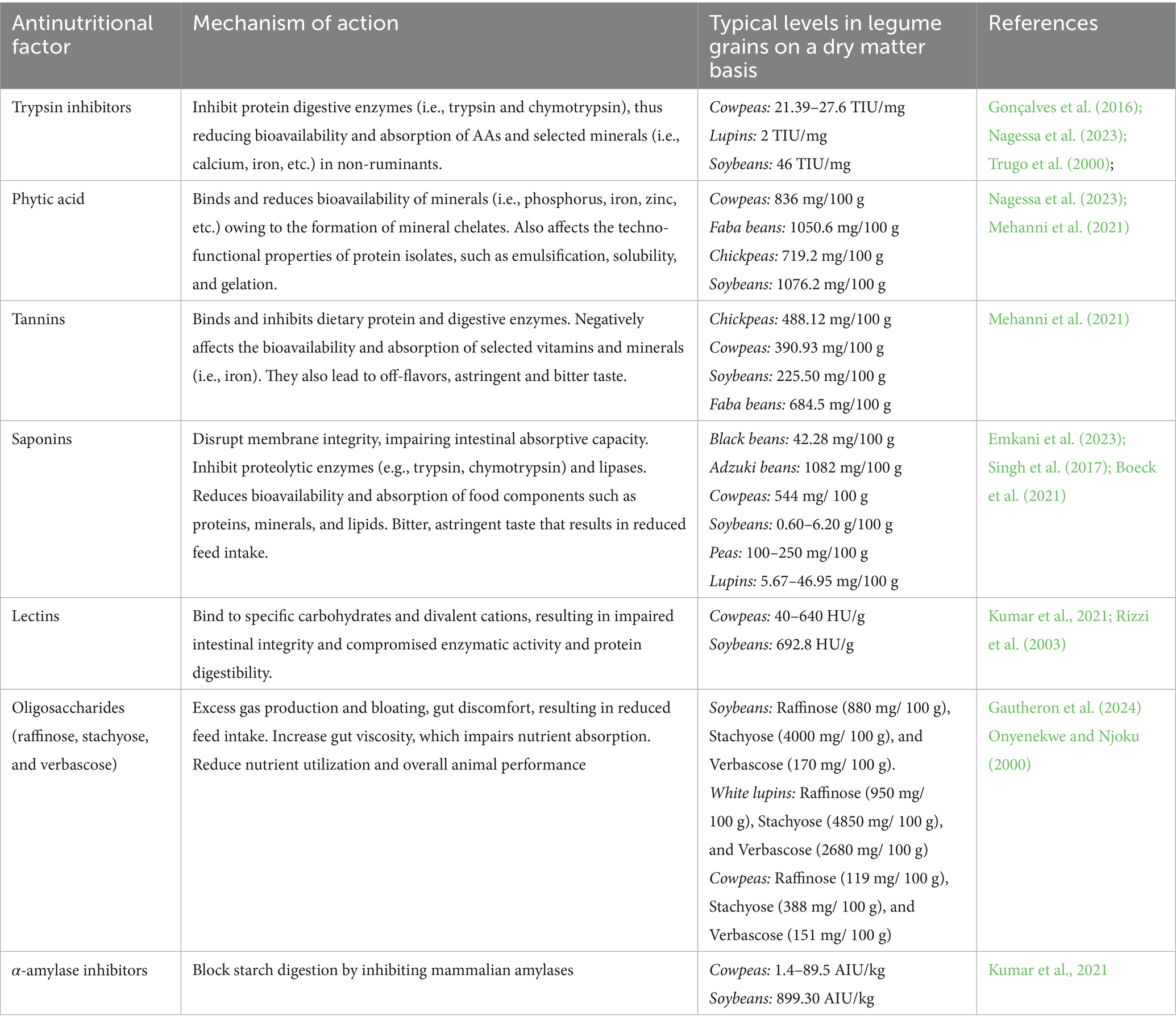
Table 2. Antinutritional factors common in indigenous legume food crops and their mechanisms of action.
4.2 Tannins
Hydrolysable tannins consist of a central polyol, typically D-glucose, esterified with phenolic acids, while condensed tannins (proanthocyanidins) are polymers of flavonoids such as catechins, gallocatechins, and epicatechins (Singh et al., 2017; Salim et al., 2023). Tannins reduce nutrient utilization by forming complexes with proteins, carbohydrates, and digestive enzymes, thereby impairing protein and starch digestibility (Khattab and Arntfield, 2009). They are broadly classified into hydrolysable and condensed tannins. In cowpeas, condensed tannins predominate and are known to reduce feed palatability and nutrient absorption by inhibiting key enzymes like trypsin and amylase (Rehman and Shah, 2005). As shown in Table 1, tannin content is higher in cowpeas (390.93 mg/100 g) than in soybeans (225.50 mg/100 g).
4.3 Trypsin inhibitors
Trypsin inhibitors are low-molecular-weight proteins that inactivate digestive enzymes such as trypsin, chymotrypsin, and elastase, thereby impairing proteolysis and reducing AA absorption (Emkani et al., 2023; Salim et al., 2023). The two principal types, Kunitz and Bowman–Birk inhibitors, are well-characterized in legumes (Pedrosa et al., 2021). Their antinutritional effects are primarily linked to growth suppression due to reduced protein digestibility in the gastrointestinal tract (Feng et al., 2007; Khattab and Arntfield, 2009). Trypsin inhibitor activity is higher in soybeans (46.00 TIU/mg) than in cowpeas (21.39 TIU/mg), indicating a greater inhibitory potential in soybeans (Table 2).
4.4 α-galactosides
The raffinose-family oligosaccharides (RFOs) such as raffinose, stachyose, and verbascose are prominent in cowpeas and are indigestible by monogastrics due to the absence of α-galactosidase (Pedrosa et al., 2021). Their fermentation by gut microbiota leads to excess gas production and flatulence, reducing the acceptance of cowpea-based diets (Ofuya, 2006; Gautheron et al., 2024). As shown in Table 2, the concentrations of raffinose, stachyose, and verbascose in soybeans are approximately 880, 4000, and 170 mg per 100 g, respectively, compared to 119, 388, and 151 mg per 100 g in cowpeas. These values indicate that soybeans contain substantially higher levels of α-galactosides than cowpeas.
4.5 Saponins
Saponins are glycosidic compounds with a triterpene or spirostane aglycone and sugar moieties. They impart a bitter taste, reduce palatability, and can irritate the gastrointestinal tract, thereby decreasing feed intake (Veer et al., 2021; Kumar et al., 2021). Saponins also interfere with nutrient and enzyme activity by forming complexes with digestive enzymes and trace elements such as zinc (Salim et al., 2023). In cowpeas, saponin levels average about 544 mg/100 g (Table 2).
4.6 α-amylase inhibitors
Proteinaceous α-amylase inhibitors in cowpeas block starch digestion by inhibiting mammalian amylases, though they are ineffective against microbial amylases (Salim et al., 2023). This disrupts energy metabolism by reducing starch utilization, potentially impairing animal growth and performance (Shi et al., 2017; Veer et al., 2021). The α-amylase inhibitory activity of cowpeas and soybeans was reported as 1.4–89.5 and 899.30 AIU/kg, respectively. This clearly shows that soybeans have a higher α-amylase inhibitory activity compared to cowpeas (Table 2). Interestingly, the comparatively low α-amylase inhibitor activity in cowpeas may be advantageous for human consumption, as it reduces the risk of excessive inhibition of starch digestion and associated gastrointestinal discomfort.
4.7 Lectins
Lectins (phytohemagglutinins) are carbohydrate-binding glycoproteins that disrupt intestinal integrity by adhering to epithelial cells, thereby facilitating the translocation of pathogens across the gut barrier (Salim et al., 2023). They also impair nutrient utilization by forming complexes with divalent cations such as calcium and iron, which can inhibit enzymatic activity and reduce protein digestibility (Kumar et al., 2021; Veer et al., 2021). As shown in Table 2, hemagglutinin activity ranges from 40 to 640 HU/g in cowpeas and is approximately 692.8 HU/g in soybeans, indicating that both legumes contain appreciable levels of lectins, with soybeans exhibiting slightly higher activity.
4.8 Amino acid deficiencies
Despite their high protein content, cowpeas are deficient in sulfur-containing essential amino acids (EAAs), particularly methionine and cysteine (Menssen et al., 2017; Lubisi et al., 2023). While cowpeas are rich in lysine, leucine, arginine, and tryptophan, the absence of sufficient methionine and cysteine restricts their use in non-ruminant diets. Methionine plays critical roles in protein synthesis, lipid metabolism, and the regulation of antioxidant enzymes (e.g., methionine sulfoxide reductase; Martínez et al., 2017). It is also a precursor for compounds such as cysteine, creatine, and carnitine. Cysteine is central to protein structure, redox regulation via glutathione, and cellular signaling pathways (Muthuraman et al., 2021). Deficiencies in these AAs can impair growth, immune function, and physiological processes in monogastrics.
Overall, the cumulative effect of ANFs and amino acid imbalance in cowpeas limits their direct inclusion in non-ruminant diets. While ANFs disrupt gut integrity, hinder enzyme activity, and reduce nutrient digestibility, resulting in endogenous nutrient losses (Lubisi et al., 2023), a deficiency of sulfur-containing amino acids further impairs growth and physiological performance in non-ruminant animals. Therefore, effective valorization strategies are essential to enhance cowpea protein quality and mitigate antinutritional effects. Techniques such as solid-state fermentation (SSF), sprouting, soaking, roasting, boiling, dehulling, extrusion, drying, and enzymatic treatment have demonstrated efficacy in reducing ANFs and improving amino acid profiles in legume grains (Dueñas et al., 2016; Emkani et al., 2023). These valorization techniques hold promise for improving the nutritional and functional parity of cowpeas relative to soybean meal and are discussed next.
5 Valorization techniques for cowpeas
Building on the nutritional limitations outlined above, this section reviews the principal valorization techniques that can enhance the protein quality and functional properties of cowpeas. These methods, including fermentation, sprouting, thermal and mechanical processing, and enzymatic treatments, are evaluated for their capacity to reduce antinutritional factors, improve amino acid balance, and increase protein digestibility.
5.1 Fermentation
Fermentation uses selected microorganisms to break down complex compounds, improving nutrient availability, reducing ANFs, and enhancing the functional properties of legumes. It also extends shelf life and improves sensory quality, making it a promising valorization strategy for underutilized crops such as cowpeas (Emkani et al., 2023). Although cowpea-specific data remain limited, extensive evidence from lentils, marama beans, soybeans, and other pulses (Table 3) provides a strong rationale for its application. Fermentation relies on GRAS-certified lactic acid bacteria (LAB), yeasts, and fungi (e.g., Lactobacillus, Rhizopus, Aspergillus, Pleurotus). These microbes secrete enzymes, cellulases, amylases, tannases, and proteases that hydrolyze macronutrients and phytochemicals, thereby lowering phytate and tannin levels, improving mineral bioavailability, and increasing free amino acids and bioactive peptides (Verni et al., 2019; Asensio-Grau et al., 2020). Fermentation is typically carried out as solid-state (SSF), submerged (SmF), or anaerobic (AF) processes, each offering distinct advantages.
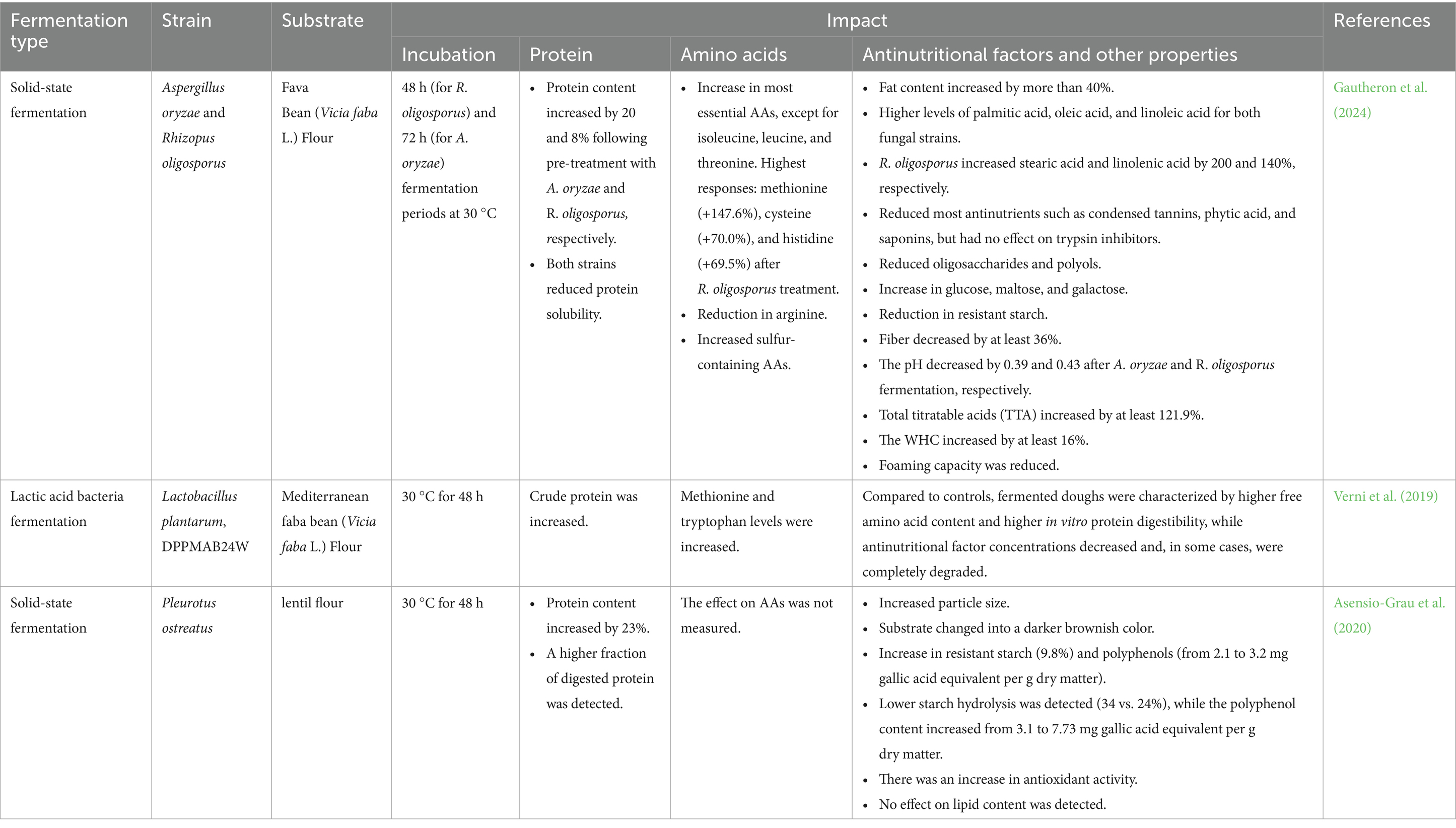
Table 3. Impact of fermentation on amino acid profiles, protein content, antinutritional factors, and other properties of different legume grains.
5.1.1 Solid-state fermentation
In SSF, fungi grow on moist solids with limited free water, producing enzymes that degrade ANFs and convert carbohydrates to microbial protein. Across legumes, SSF raises protein content (up to ~20% in lentils and ~9% in soybeans) and enriches sulfur amino acids such as methionine and cysteine (Asensio-Grau et al., 2020; Thakur et al., 2022; Gautheron et al., 2024). It also improves mineral bioavailability (e.g., iron and zinc) and functional traits such as water-holding capacity (WHC) and oil-absorption capacity (OAC). Fungal strains such as Aspergillus oryzae, A. sojae, Rhizopus oligosporus, and Pleurotus ostreatus, effective in related pulses, are likely strong candidates for cowpea SSF.
5.1.2 Submerged fermentation
SmF provides a controlled aqueous environment for LAB and fungi. It consistently reduces phytates (50–70%), tannins (~80%), and oxalates (~60%), while improving protein solubility, emulsification, and WHC/OAC (Benjamin et al., 2021; Batbayar et al., 2023). Protein gains of 20–30% have been reported in pea and other pulses (Emkani et al., 2021). Careful timing is essential, as prolonged fermentation can lead to nutrient losses.
5.1.3 Anaerobic fermentation
Anaerobic fermentation, driven primarily by LAB under oxygen-limited conditions, enhances essential amino acids, including methionine and tryptophan, lowers pH, and inhibits spoilage organisms (Verni et al., 2019; Arshad et al., 2023). It effectively degrades raffinose-family oligosaccharides and other ANFs while improving mineral bioavailability and protein digestibility. Genotype-specific responses highlight the need to tailor AF conditions for cowpea varieties.
5.1.4 Implications for cowpea valorization
Each method offers unique benefits for cowpea processing. Solid-state fermentation provides the most robust antinutrient reduction and protein enrichment, ideal for high-protein feed. Anaerobic fermentation is especially promising for boosting sulfur-containing amino acids, directly addressing cowpea’s key nutritional limitation. Submerged fermentation excels when improved solubility and emulsifying properties are desired for food or protein-isolate applications. Method selection should match the intended end use, and combined or sequential approaches (e.g., sprouting followed by SSF) may yield synergistic gains. Although cowpea-specific studies are scarce, the strong parallels with other pulses indicate that optimized fermentation can significantly narrow the nutritional gap with soybean meal.
5.2 Sprouting treatments
Sprouting, also referred to as germination, is a cost-effective and biologically driven valorization strategy that involves the activation of the embryonic axis in viable seeds under controlled environmental conditions, typically involving optimal moisture, temperature, and aeration (Sibian et al., 2017). This process is initiated through imbibition, whereby the seed absorbs water, triggering enzymatic and metabolic changes that culminate in visible morphological changes such as radicle protrusion and the loosening or rupture of the seed coat. When applied to legume grains intended for food or feed, these biochemical transformations can enhance nutritional quality, improve digestibility and functional properties, and concurrently reduce ANFs (Atudorei et al., 2021) as shown in Table 4. While relatively underexplored in cowpeas, available evidence from cowpeas and related legumes demonstrates consistent improvements across multiple quality indicators.
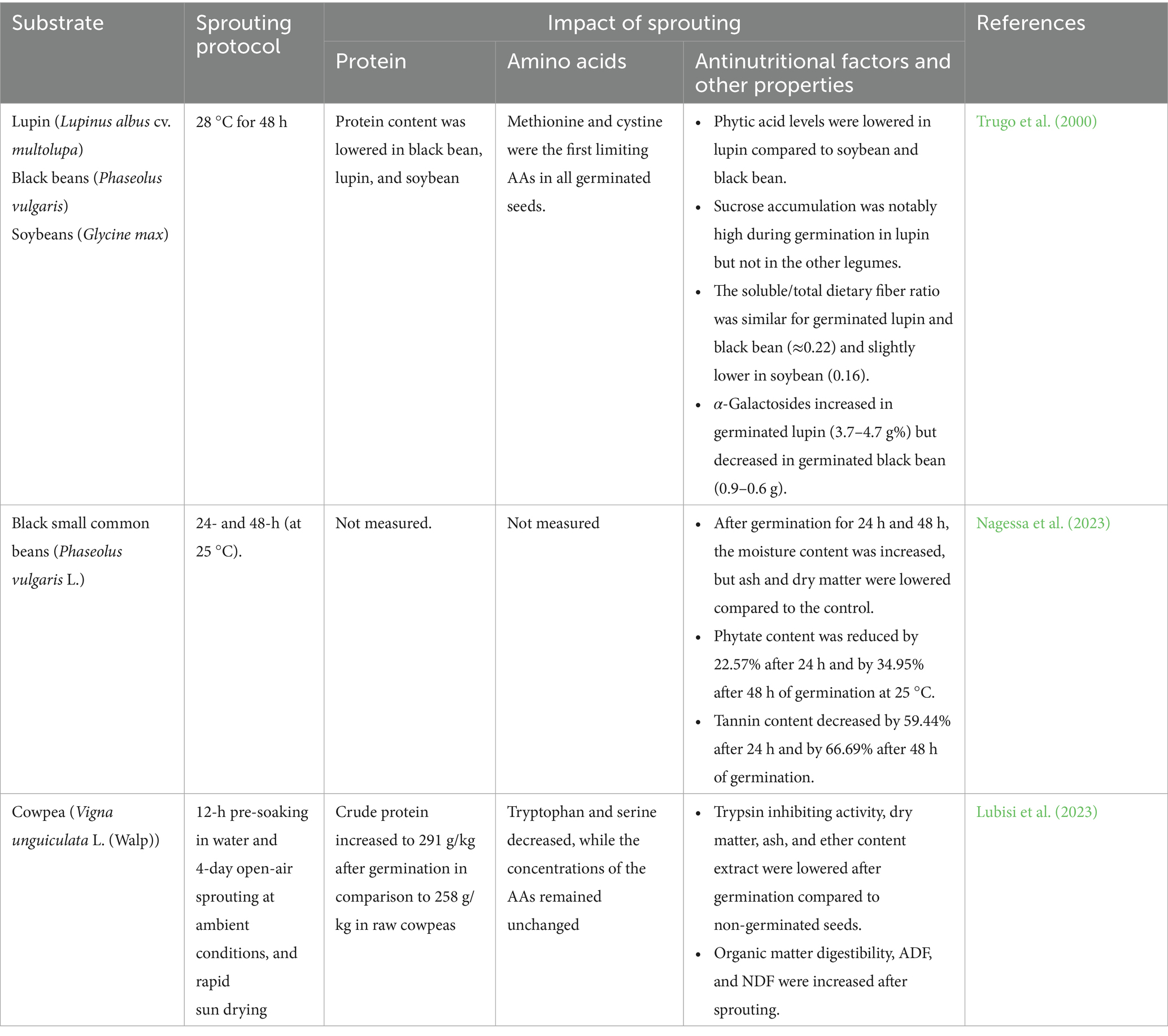
Table 4. Impact of sprouting on amino acid profiles, protein content, antinutritional factors, and other properties of different legume grains.
Sprouting is widely reported to increase crude protein, EAAs, and vitamin content. In cowpeas, Lubisi et al. (2023) documented an increase in crude protein from 258 g/kg to 291 g/kg post-germination. Amino acid profiling revealed improvements in most AAs, although tryptophan and serine declined. In grass pea, Arshad et al. (2023) found that protein content increased from 22.6 to 30.7%, and fiber rose from 15.1 to 19.4%, while carbohydrate content dropped from 59.1 to 46.0%, suggesting nutrient concentration due to storage reserve mobilization. Sprouting also boosts micronutrient density. El-Safy et al. (2013) reported increased iron, zinc, sodium, and magnesium levels following sprouting in lentils, chickpeas, and faba beans. Sprouted legumes were also richer in riboflavin, thiamine, choline, pantothenic acid, and vitamin C (El-Safy et al., 2013; Trugo et al., 2000). These improvements are attributed to enzyme activation (e.g., phytase, amylase, protease, lipase), which degrades storage compounds, liberates micronutrients, and supports enhanced protein digestibility (Mubarak, 2005; Arshad et al., 2023). With regards to ANFs, Nagessa et al. (2023) demonstrated that phytate levels declined by 22.6 and 34.9%, and tannins by 59.4 and 66.7% after 24 and 48 h of black bean germination. Similarly, Mubarak (2005) reported a 91% reduction in phytic acid, alongside reductions in stachyose, raffinose, trypsin inhibitors, and haemagglutinins in mung beans. In pigeon pea, trypsin inhibitors decreased from 573.1 to 308.8 UTI/g, a 46% reduction (Wisaniyasa et al., 2015). In El-Safy et al. (2013) study, prolonged germination (4 days) further reduced ANFs compared to 2-day treatments. In finger millet and kidney beans, Mbithi-Mwikya et al. (2000) noted increases in sulfur-containing AAs with only minimal lysine loss in kidney beans.
Sprouting alters several techno-functional properties, some positively and others negatively. Ghavidel and Prakash (2006) observed increases in WAC and OAC in cowpeas. Similarly, Wisaniyasa et al. (2015) reported 15.7 and 14.8% increases in WAC and OAC of pigeon peas. Benítez et al. (2013) observed a reduction in resistant starch and total fiber, improving starch availability. They also noted enhancements in gelation properties, WAC, and OAC, though emulsification and foaming capacities were reduced, likely due to proteolytic degradation affecting surface-active proteins. These changes in WHC, OAC, and gelation properties are largely driven by the enzymatic remodeling of seed macromolecules during germination (Ghavidel and Prakash, 2006). Activation of endogenous proteases and amylases partially hydrolyzes storage proteins and starch, exposing additional polar and hydrophobic sites that enhance water and oil binding (Wisaniyasa et al., 2015). Concurrent degradation of cell-wall polysaccharides and the loosening of seed microstructure further improve swelling and dispersibility, which together account for the observed improvements in texture-related properties (Benítez et al., 2013).
The reported nutritional and functional effects of sprouting are strongly influenced by species, germination time, soaking regimes, light exposure, and temperature. For instance, Trugo et al. (2000) observed divergent protein retention in black beans, lupins, and soybeans when germination was combined with heat treatment. Germination under light vs. darkness (El-Safy et al., 2013) or different soaking temperatures (Wisaniyasa et al., 2015) yielded distinct effects on swelling, digestibility, and protein solubility. Lysine loss in kidney beans but not in finger millet (Mbithi-Mwikya et al., 2000) illustrates genotype-dependent responses.
The information in Table 4 indicates that sprouting is a biologically driven valorization strategy that consistently improves the nutritional, bioactive, and functional profile of legumes, including cowpeas. It enhances protein content and digestibility, amino acid profiles, and mineral bioavailability, while significantly reducing ANFs such as phytates, tannins, and enzyme inhibitors. These effects are underpinned by the activation of endogenous enzymes and compositional remodeling during germination. Although impacts on functional properties such as WAC and emulsification are sometimes mixed, sprouting remains a potent and accessible method for improving cowpea value. However, optimization must consider species-specific and condition-dependent outcomes to ensure maximal nutritional and functional gains.
While only a few studies have directly examined sprouting in cowpea, the available evidence, such as the increases in crude protein and mineral content and the reductions in trypsin inhibitors and phytate reported by Lubisi et al. (2023), indicates clear nutritional benefits. Extrapolating from related legumes, sprouting of cowpea seeds is also likely to enhance bioactive compound release, improve amino-acid availability, and increase mineral bioaccessibility through activation of endogenous phytases and proteases. These changes could expand cowpea’s potential in both animal feed and human food applications by improving digestibility, flavor, and functional properties of cowpea-based ingredients. Future research focusing on the optimization of germination time, temperature, and light exposure for cowpea is, therefore, warranted.
5.3 Thermal processing treatments
The thermal processing techniques, such as dry roasting, boiling, toasting, and microwaving, have gained prominence due to their practicality and proven ability to enhance the nutritional value and shelf life of legume grains used in food and feed systems. These methods are particularly beneficial in regions with short growing seasons or limited access to advanced storage infrastructure, as they facilitate microbial inactivation, moisture reduction, and the prevention of insect infestation (Cerma and Yu, 2023; Irondi et al., 2019). In low-resource settings, thermal treatments allow seasonal legume harvests to be preserved and utilized throughout the year, particularly as animal feed. Heat treatments, however, can also compromise the bioavailability of certain heat-sensitive nutrients. For instance, Irakli et al. (2020) reported reductions in vitamin E during rice bran stabilization via infrared radiation, dry heating, and microwaving, likely due to oxidative degradation. Similarly, Asunni et al. (2024) observed a decline in total mineral content in African locust bean (Parkia biglobosa) when irradiation was combined with cooking, attributed to leaching of soluble minerals into the cooking water.
5.3.1 Dry roasting
Dry roasting transfers heat via conduction, convection, and radiation, using electrical or gas-based heat sources. Typical roasting temperatures can reach up to 200 °C, with residence times ranging from minutes to hours, depending on the grain type and desired effect (Yu et al., 2002). While effective for reducing moisture and microbial load, roasting can significantly reduce thermolabile bioactives. Irondi et al. (2019) found that roasting whole chickpea (Vigna unguiculata) pulses at 150 °C and 180 °C resulted in the complete loss of apigenin, kaempferol, and catechin, and the disappearance of gallic acid at 180 °C, likely due to heat-induced oxidation and thermal degradation of phenolic compounds.
Dry roasting is widely employed in soybean processing to inactivate antinutritional factors such as trypsin inhibitors and lectins. However, prolonged or high-temperature roasting can reduce protein quality by inducing Maillard reactions and cross-linking of amino acids, which lowers protein digestibility and the availability of essential amino acids such as lysine. Similar trade-offs are likely in cowpeas, underscoring the importance of optimizing roasting temperature and duration to maximize antinutrient reduction while preserving protein integrity.
5.3.2 Boiling
Boiling is widely used for legume detoxification. Trugo et al. (2000) demonstrated that boiling germinated lupin, soybean, and black beans for 20 min completely inactivated trypsin inhibitors without altering phytic acid levels or macronutrient composition. However, effects on low-molecular-weight sugars varied. Notably, the sugar digestibility ratio doubled in boiled germinated black beans, while true protein digestibility improved only in soybeans. Net protein utilization increased by 20% in germinated lupin and soybean following boiling. Conversely, Yadav et al. (2018) reported that boiling cowpea seeds for 90 min reduced total phenolic content and antioxidant capacity in two cultivars, suggesting that prolonged boiling can compromise certain beneficial phytochemicals.
5.3.3 Microwave and comparative heat treatments
Cerma and Yu (2023) investigated the effect of dry heat (oven at 100 °C for 60 min), wet heat (autoclaving at 120 °C for 60 min), and microwave irradiation (3 min at 900 W) on newly developed cool-season chickpeas intended for ruminants. Microwave-treated chickpeas exhibited the highest dry matter content (93.5%) compared to dry heat (92.6%) and autoclaved (90.6%) samples, indicating lower water retention and better potential for long-term storage. Dry heat treatment yielded the highest soluble crude protein (SCP) content (14.2%), while microwave and autoclave treatments yielded lower values (7.8 and 3.1%, respectively). High SCP levels are less desirable in ruminants due to the risk of excess ammonia production from rapid rumen degradation. Autoclaved chickpeas also had the highest neutral detergent insoluble crude protein (NDICP, 5.7% DM), suggesting the formation of heat-damaged proteins possibly bound to fiber, in contrast to dry heat (1.4%) and microwave (1.6%) treatments. While thermal treatments effectively reduce antinutritional factors (e.g., trypsin inhibitors and certain phenolics) and improve energy and protein digestibility, their application requires careful optimization. Excessive heating can trigger Maillard reactions and protein cross-linking, which may reduce amino acid availability and overall protein digestibility (Alonso et al., 2000). Moreover, some functional or bioactive compounds, such as vitamins, phenolics, and flavonoids, may be partially or entirely degraded under high heat.
Overall, thermal processing techniques such as roasting, boiling, and microwaving hold promise for improving the feed value of cowpeas by reducing moisture, microbial load, and antinutritional compounds while enhancing shelf life and nutrient digestibility. However, the effectiveness and impact of each technique vary depending on the specific method, temperature, and duration applied. Although these treatments improve protein utilization and energy availability, particularly in large-scale non-ruminant production systems, they may also lead to losses of heat-sensitive micronutrients and bioactive compounds. Thus, balancing nutritional gains with the preservation of heat-labile nutrients is critical. Further research is warranted to refine thermal protocols for cowpeas, with the goal of maximizing their nutritional and functional value without compromising their bio-efficacy. The choice of thermal treatment for cowpea should be guided by the intended application. Dry roasting is well-suited for animal-feed ingredients where maximum inactivation of trypsin inhibitors is desired, but it must be carefully controlled to prevent Maillard reactions and lysine loss that can reduce protein quality. Boiling effectively eliminates enzyme inhibitors and lectins and is appropriate for human food uses, although prolonged boiling can leach heat-sensitive vitamins and minerals. Microwave and other rapid-heat methods provide efficient moisture reduction and microbial control with minimal nutrient loss, making them attractive for cowpea flours and ready-to-use protein products. Because cowpeas are consumed both as food and as feed, each method can be optimal in different contexts, and processing parameters should be tailored to balance antinutrient reduction with nutrient retention.
5.4 Mechanical processing
Mechanical processing involves the application of physical force or machinery to cut, separate, or reshape food components, and is widely used to improve the quality, digestibility, and functionality of legume grains (Abd El-Hady and Habiba, 2003). Among the most common mechanical processing techniques applied to legumes are dehulling and extrusion (Alonso et al., 2000). Of these, extrusion, a high-temperature, short-time processing method involving the passage of material through a die using heat, pressure, and moisture, has been extensively adopted for its capacity to enhance the nutritional and functional attributes of feed ingredients (Osen et al., 2015). Extrusion modifies legume matrices through the gelatinization of starch, denaturation of proteins, and depolymerization of structural polysaccharides such as cellulose, hemicellulose, and lignin. These transformations enhance nutrient accessibility and promote digestibility. For example, Alonso et al. (2000) demonstrated that extrusion increased the WHC and water solubility index (WSI) of peas and kidney beans, while reducing their OAC. This reduction in OAC is attributed to protein denaturation and starch gelatinization, which reduce the porosity of the extrudate and limit oil-binding sites (Kesselly et al., 2023). Extrusion has also been shown to significantly alter pasting properties, as observed by Lopes et al. (2012), due to disrupted starch granules and enhanced enzyme accessibility (Mitrus et al., 2023). Martin et al. (2021) reported that the enhanced WHC and WSI of extruded legumes are directly linked to structural modifications that increase solubility and improve functionality in feed formulations.
Another benefit of extrusion is its ability to reduce ANFs. Abd El-Hady and Habiba (2003) and Lopes et al. (2012) found that soaking followed by extrusion markedly decreased levels of trypsin inhibitors, α-amylase inhibitors, and haemagglutinins in peas, chickpeas, faba, and kidney beans. Similarly, Pasqualone et al. (2020) reported the inactivation of ANFs through starch gelatinization and protein denaturation. However, nutrient losses may occur: Jeunink and Cheftel (1979) found that lysine residues in soybeans and field peas became chemically unavailable post-extrusion, potentially compromising protein quality.
Extrusion also influences protein conformation. Jiang et al. (2024) and Osen et al. (2015) noted that extrusion enhances protein solubility by disrupting structural bonds. However, under high-moisture extrusion, solubility may decrease due to protein aggregation, disulfide bond formation, and non-covalent interactions. This was supported by Osen et al. (2015), who observed reduced solubility in pea protein isolates subjected to high-moisture conditions. Additionally, Gall et al. (2005) reported that heat-induced extrusion increased the hydrolysates of legumin and convicilin, while reducing albumin, possibly due to protein aggregation and cross-linking via disulfide bridges. Importantly, extrusion outcomes are strongly influenced by processing conditions. Alonso et al. (2000) demonstrated that the protein solubility of extruded peas and kidney beans increased when treated with chemical buffers such as mercaptoethanol (2-ME) or sodium dodecyl sulfate (SDS). In contrast, solubility declined in samples not treated with these buffers, underscoring the impact of extrusion-induced protein aggregation and the need for post-processing modification.
In summary, extrusion presents a promising mechanical valorization strategy for cowpeas by reducing ANFs, improving digestibility, and enhancing functional properties such as water solubility capacity and WHC. These improvements stem from thermo-mechanical disruption of cellular structures and macromolecules. However, extrusion conditions, particularly moisture, temperature, and residence time, must be carefully optimized to maximize nutritional benefits while minimizing undesirable changes, such as reduced amino acid availability or protein insolubility. As such, extrusion can be effectively integrated into cowpea-based feed processing systems if formulation and processing parameters are tailored to preserve nutrient integrity and functional value.
5.5 Enzymatic treatments
Enzymatic hydrolysis has emerged as a targeted and adaptable approach to valorize legume proteins, with demonstrated improvements in protein recovery, functional properties, and bioactivity as shown in Table 5. For instance, Perović et al. (2022) achieved ~90% protein recovery from defatted chickpeas using arabinofuranosidase and cellulase+xylanase, outperforming conventional alkaline extraction by >25%. This was attributed to cell wall polysaccharide degradation, which also improved WHC, OAC, emulsifying activity, foaming capacity, and antioxidant activity. Protease-assisted hydrolysis using enzymes such as pepsin, trypsin, alcalase, and flavourzyme has further enhanced protein solubility and emulsification in faba beans, cowpeas, lentils, and pigeon peas (Eckert et al., 2019; Segura-Campos et al., 2012; Xu et al., 2021). Alcalase hydrolysates, for example, exhibited superior antioxidant and oxygen radical absorbance capacity, while bromelain-treated samples showed improved DPPH and NO radical scavenging. Sequential enzyme systems have demonstrated synergistic benefits. For example, pepsin–pancreatin hydrolysates of lima beans yielded low-molecular-weight peptides with ACE inhibitory activity and enhanced functional attributes (Polanco-Lugo et al., 2014). Similarly, Betancur-Ancona et al. (2014) reported that alcalase–flavourzyme combinations improved nitrogen solubility and foaming/emulsifying properties in French beans.
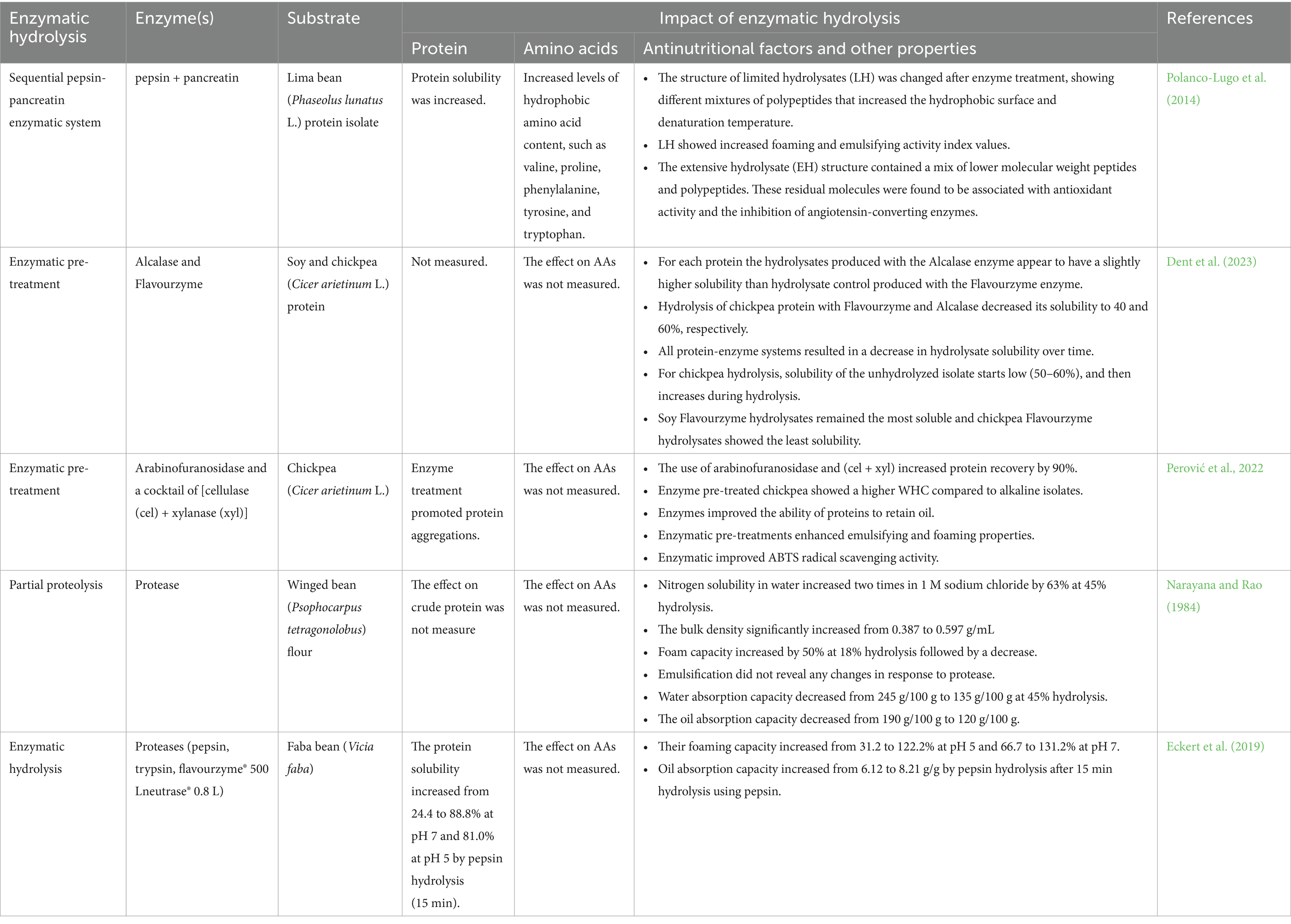
Table 5. Impact of enzymatic hydrolysis on amino acid profiles, protein content, antinutritional factors, and other properties of different legume grains.
However, limitations remain, for example, enzyme–substrate specificity can lead to reduced solubility, as seen in chickpea hydrolysates (Dent et al., 2023), and hydrolysis beyond 5% DH may compromise IVPD and essential amino acid profiles (Goertzen et al., 2020). Furthermore, inhibitory effects on pancreatic lipase (Moreno et al., 2020) raise caution regarding unintended bioactivity. Overall, enzymatic valorization offers a potent avenue to enhance cowpea utilization by improving protein yield, techno-functionality, and health-related properties. Yet, outcomes are highly enzyme- and genotype-dependent, necessitating optimized and potentially combined processing strategies for broader feed and food applications.
The evidence presented in this section suggests that no single valorization method is universally superior for cowpea; the optimal approach depends on the intended application. Fermentation, particularly solid-state for protein enrichment or anaerobic for methionine enhancement, offers the most comprehensive reduction of antinutritional factors and is well-suited for high-protein feed or functional food ingredients. Sprouting is inexpensive and biologically driven, making it attractive for small-scale or household applications where improved mineral bioavailability and moderate protein gains are desired. Thermal treatments such as roasting or boiling provide rapid antinutrient inactivation and are practical for both feed manufacturing and human food preparation, although careful control is needed to avoid losses of heat-sensitive amino acids. Mechanical methods like extrusion excel when improved texture, solubility, and shelf life are priorities for feed pellets or protein concentrates, while enzymatic treatments allow precise modification of protein functionality and bioactive peptide release for specialized food or nutraceutical products. Because cowpea is used across diverse feed and food systems, tailoring the method, or combining complementary techniques, to match the target product will yield the greatest nutritional and economic benefits.
5.6 Summary and outlook
Valorization methods, including fermentation, sprouting, thermal and mechanical treatments, and enzymatic hydrolysis, consistently enhance the functional quality of cowpeas while reducing antinutritional factors. Fermentation, particularly solid-state and anaerobic approaches, improves protein digestibility, enriches sulfur-containing amino acids such as methionine, and increases water-holding and oil-absorption capacities, making fermented cowpea flours suitable for high-protein feed and functional foods. Sprouting offers a low-cost route to higher protein content, greater mineral bioavailability, and better gelation and binding, desirable for bakery and snack applications. Thermal processes such as roasting or microwave heating effectively inactivate trypsin inhibitors and lectins and improve texture and shelf life, though excessive heat can diminish heat-labile amino acids. Mechanical methods like extrusion enhance dispersibility, solubility, and shelf stability for pelleted feeds and protein concentrates, while enzymatic hydrolysis precisely tailors protein solubility and generates bioactive peptides for nutraceutical or premium food ingredients.
The optimal technique depends on the intended product. Solid-state fermentation or dry roasting is well-suited to animal-feed formulations that require maximum antinutrient reduction, whereas sprouting or controlled enzymatic hydrolysis better preserves delicate nutrients and texture for human foods. Combinations, such as sprouting followed by extrusion, can further improve nutritional quality and techno-functional performance. Among these options, enzymatic hydrolysis and extrusion show particular promise for narrowing the functional gap between cowpea and soybean meal, enhancing solubility, emulsification, foaming, and hydration properties that enable broader use in industrial feed and food systems. However, variability across cowpea genotypes, enzyme systems, and processing conditions complicates standardization and scalability. In vivo feeding trials and economic assessments, especially for enzyme-based methods, remain limited, restricting confident translation from laboratory findings to practical applications.
In summary, functional parity between valorized cowpeas and conventional protein sources appears achievable through optimized processing. Future research should prioritize genotype-specific protocols, a deeper understanding of protein structure–function relationships, validation through animal feeding trials, and integration of enzymatic, thermal, and bioprocessing strategies to maximize synergistic benefits for sustainable feed and food development.
6 Economic and environmental implications of valorized cowpeas
Valorized cowpeas offer compelling economic and environmental advantages as alternative protein sources in non-ruminant feed (Table 6). From an economic standpoint, reliance on soybean meal exposes feed manufacturers to volatile international markets due to its integration into global commodity supply chains (Boerema et al., 2016; Kuzhkuzha et al., 2021). This volatility disproportionately affects regions that are dependent on imports for livestock feed. In contrast, cowpeas are widely cultivated across Africa, Asia, and Latin America, providing a locally available and comparatively price-stable protein source (Obour et al., 2025). Agronomically, cowpeas are well adapted to low-input systems, being drought-tolerant, nitrogen-fixing, and resilient in nutrient-poor soils, which makes them highly suitable for resource-limited smallholder production systems (Singh, 2020). However, unlike soybean meal, which is marketed in a processed, ready-to-use form, cowpeas require post-harvest valorization to enhance their suitability for non-ruminant feeding. The costs and technical requirements associated with valorization vary by method. Low-input strategies such as soaking, sprouting, or basic fermentation can be implemented at household or community levels with minimal infrastructure (Nwagboso et al., 2024). In contrast, advanced techniques such as extrusion or enzyme-assisted processing entail higher capital investment, skilled labor, and access to processing infrastructure (Kebede and Bekeko, 2020). Despite this, the decentralization potential of cowpea valorization presents an opportunity to promote rural agro-industrial development, improve local feed autonomy, and create value chains anchored in local production systems (Ariong, 2024).
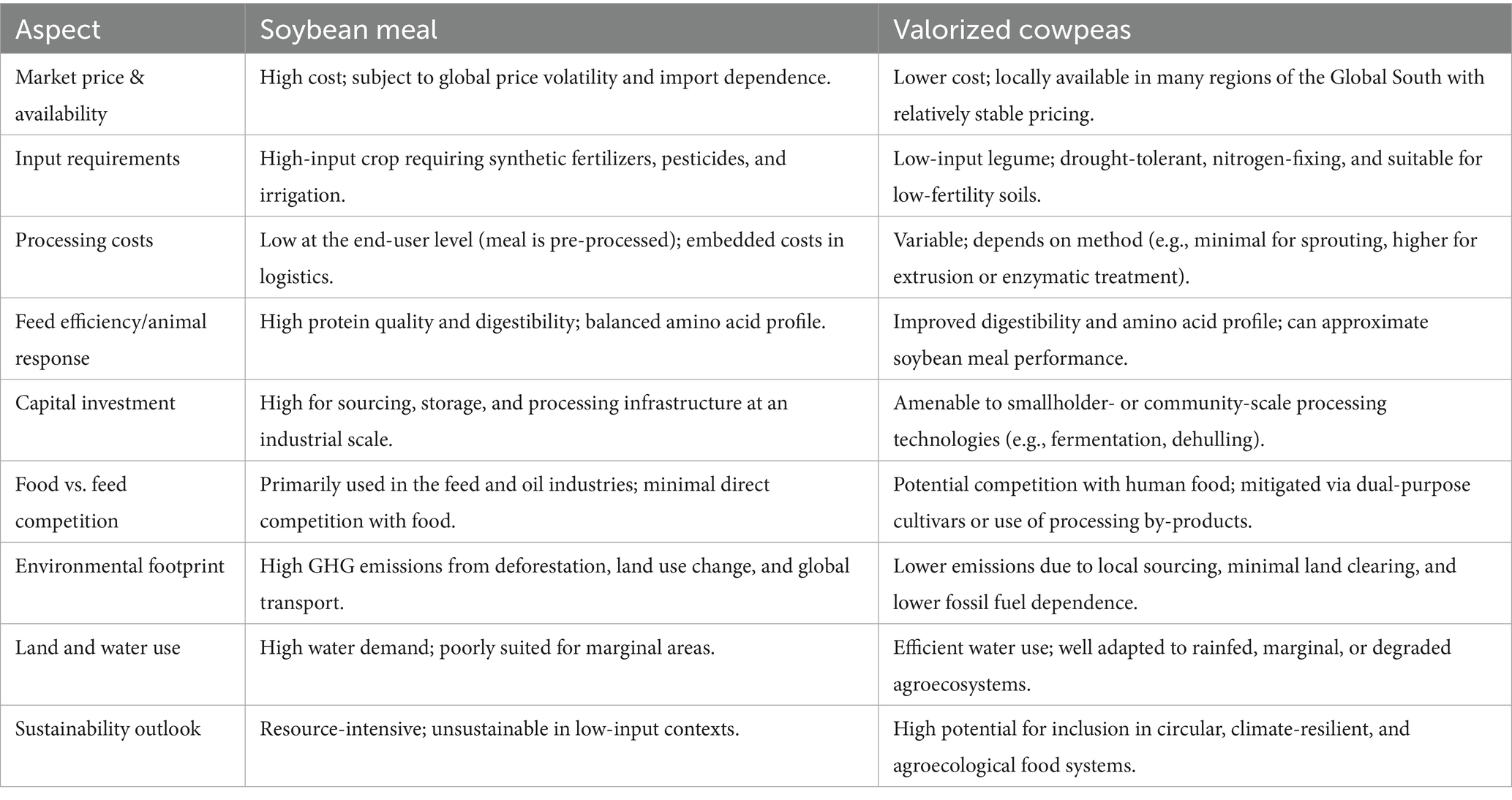
Table 6. Economic and environmental implications of using valorized cowpeas as a replacement for soybean meal.
Environmentally, the substitution of soybean meal with valorized cowpeas holds promise for reducing the ecological footprint of feed production. Soybean cultivation, particularly in Latin America, is associated with deforestation, greenhouse gas emissions, water use, and intensive agrochemical application (Boerema et al., 2016). By contrast, cowpeas are well-suited to rainfed agriculture and require minimal external inputs. Their ability to fix atmospheric nitrogen further enhances soil fertility, reducing dependence on synthetic fertilizers (Obour et al., 2025). These attributes position cowpeas as a low-emission, climate-resilient alternative within sustainable and regenerative agriculture frameworks (Singh, 2020). Nevertheless, one challenge that requires consideration is the food vs. feed dynamic. In many regions, cowpeas serve as staple food crops, and their diversion into animal feed could raise concerns over food security (Singh et al., 2003; Omokanye et al., 2003). This trade-off can be addressed through the development and use of dual-purpose cultivars or the valorization of by-products such as damaged seeds, husks, and milling residues, which are less suitable for direct human consumption but still nutritionally relevant for animal feeding.
In conclusion, valorized cowpeas present a viable and sustainable alternative to soybean meal, with the potential to enhance feed security, reduce environmental impacts, and stimulate local value addition, particularly in the Global South. To realize these benefits at scale, further research is needed to: (1) optimize low-cost, context-specific valorization methods; (2) assess long-term animal performance across species and production systems; and (3) develop processing infrastructure and institutional support systems that can facilitate widespread adoption. Economic modeling and life cycle assessments will also be critical in quantifying trade-offs and guiding investment in sustainable legume-based feed systems.
7 Recommendations
7.1 Research and development
Further studies are needed to optimize valorization protocols and assess their efficacy across diverse cowpea cultivars and non-ruminant species. In particular, controlled feeding trials should be conducted to evaluate the effects of valorized cowpeas on growth performance, nutrient utilization, gut health, and product quality in broilers, pigs, and other monogastrics. The development of multi-enzyme blends specifically targeted at cowpea ANFs, along with investigations into synergistic processing combinations (e.g., soaking followed by fermentation or enzyme treatment), should be prioritized. Additionally, the bio-efficacy of cowpea-derived bioactive compounds in promoting animal health and productivity deserves greater research attention.
7.2 Policy and practice
To support the mainstreaming of cowpeas in animal nutrition, public and private stakeholders should invest in the development and dissemination of low-cost, scalable valorization technologies suited to rural and peri-urban feed processing contexts. Breeding programs should prioritize cowpea varieties with improved protein content, reduced ANFs, and higher digestibility. National extension services and feed industry stakeholders should promote awareness and knowledge transfer to enable smallholder farmers and feed manufacturers to adopt cowpea-inclusive diets. Moreover, policy frameworks should incentivize the use of locally produced feed ingredients to reduce import dependency and enhance feed sovereignty.
7.3 Sustainability and food system integration
Cowpea valorization aligns with broader goals of building climate-smart, nutrition-sensitive, and economically resilient food systems. Efforts to integrate cowpeas into animal feeding strategies should be embedded within national food security and agricultural sustainability agendas. Interdisciplinary collaboration among crop scientists, animal nutritionists, food technologists, and policymakers will be essential to realize the full potential of cowpeas as a strategic feed resource. With targeted investment, innovation, and coordinated action, cowpea valorization could play a transformative role in enhancing protein self-sufficiency and strengthening sustainable livestock production in the Global South.
8 Conclusion
This review highlights cowpeas as a sustainable, locally adapted alternative to soybean meal for non-ruminant feeding systems in the Global South. Soybeans remain the protein benchmark but require high inputs and costly imports, whereas cowpeas thrive in low-input, marginal environments. Their wider use is constrained by antinutritional factors, low protein digestibility, and deficiencies in sulfur-containing amino acids such as methionine and cysteine. Valorization methods, including soaking, dehulling, thermal processing, germination, fermentation, extrusion, and enzyme supplementation, can reduce antinutritional factors, enhance amino-acid availability, and improve protein digestibility. Solid-state fermentation, extrusion, and enzyme treatments show the greatest promise, though no single technique achieves full nutritional parity with soybean meal. Integrated, optimized combinations tailored to animal species and local conditions offer the best prospects.
Scaling these approaches can lower feed costs, reduce reliance on imported soy, and strengthen livestock resilience. Incorporating cowpea processing into circular agriculture, supporting breeding for high-protein, low-antinutrient varieties, and investing in cooperative-level processing infrastructure would accelerate adoption. With strategic research, policy support, and public–private partnerships, valorized cowpeas could become a cornerstone of sustainable feed systems and enhance food and feed sovereignty across the Global South.
Author contributions
TM: Writing – review & editing, Conceptualization, Supervision, Investigation, Methodology, Software, Funding acquisition, Project administration, Resources, Visualization, Formal analysis, Writing – original draft, Data curation, Validation. VM: Resources, Methodology, Validation, Conceptualization, Writing – review & editing, Formal analysis, Data curation, Investigation, Visualization, Writing – original draft, Funding acquisition, Project administration, Software, Supervision. GM: Methodology, Validation, Data curation, Visualization, Investigation, Writing – review & editing, Funding acquisition, Formal analysis, Resources, Writing – original draft, Conceptualization, Project administration, Software, Supervision. SD: Resources, Visualization, Formal analysis, Funding acquisition, Project administration, Writing – original draft, Validation, Data curation, Investigation, Conceptualization, Methodology, Supervision, Writing – review & editing, Software. CM: Resources, Funding acquisition, Visualization, Conceptualization, Project administration, Formal analysis, Validation, Writing – review & editing, Supervision, Data curation, Writing – original draft, Investigation, Software, Methodology.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to express their heartfelt gratitude to the University of Mpumalanga for providing financial support for publishing this systematic review paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. During the preparation of this work, the authors used generative AI to improve readability and language use in some parts of the paper. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd El-Hady, E. A., and Habiba, R. A. (2003). Effect of soaking and extrusion conditions on antinutrients and protein digestibility of legume seeds. LWT Food Sci. Technol. 36, 285–293. doi: 10.1016/S0023-6438(02)00217-7
Abebe, B. K., and Alemayehu, M. T. (2022). A review of the nutritional use of cowpea (Vigna unguiculata L. Walp) for human and animal diets. J. Agri. Food Res 10:e100383. doi: 10.1016/j.jafr.2022.100383
Akissoé, L., Mdodé, Y. E., Hemery, Y. M., Donadjé, B. V., Icard-Verniére, C., and Hounhouigan, D. J. (2021). Impact of traditional processing on proximate composition, folate, mineral, phytate, and alpha-galacto-oligosaccharide contents of two West African cowpea (Vigna unguiculata L. Walp) based doughnuts. J. Food Compost. Anal. 96:e10375. doi: 10.1016/j.jfca.2020.103753
Alonso, R., Orue, E., Zabalza, M. J., Grant, G., and Marzo, F. (2000). Effect of extrusion cooking on structure and functional properties of pea and kidney bean proteins. J. Sci. Food Agric. 80, 397–403. doi: 10.1002/1097-0010(200002)80:3<397:AID-JSFA542>3.0.CO;2-3
Anele, U. Y., Arigbede, O. M., Südekum, K. H., Ike, K. A., Oni, A. O., and Olanite, J. A. (2010). Effects of processed cowpea (Vigna unguiculata L. Walp) haulms as a feed supplement on voluntary intake, utilization and blood profile of west African dwarf sheep fed a basal diet of Pennisetum purpureum in the dry season. Anim. Feed Sci. Technol. 159, 10–17. doi: 10.1016/j.anifeedsci.2010.05.004
Ariong, T. (2024). Profitability analysis of cowpeas production among the smallholder farmers in Arapai sub county. Abstract retrieved from Soroti district Busitema University. (Accession No. BU/UP/2021/0197)
Arshad, N., Akhtar, S., Ismail, T., Saeed, W., Qamar, M., Özogul, F., et al. (2023). The comparative effect of lactic acid fermentation and germination on the levels of neurotoxin, anti-nutrients, and nutritional attributes of sweet blue pea (Lathyrus sativus L.). Foods 12:2851. doi: 10.3390/foods12152851
Asensio-Grau, A., Calvo-Lerma, J., Heredia, A., and Andrés, A. (2020). Enhancing the nutritional profile and digestibility of lentil flour by solid state fermentation with Pleurotus ostreatus. Food Funct. 11, 7905–7912. doi: 10.1039/D0FO01527J
Asunni, A. O., Fagbemi, S. A., Oyinloye, A. M., Gowon, C. B., and Enujiugha, V. N. (2024). Amino acid profile and physicochemical properties of African locust bean (Parkia biglobosa) seeds as affected by combined irradiation and cooking. Int. J. Environ. Agric. Biotechnol. 9, 153–164. doi: 10.22161/ijeab.91.16
Atudorei, D., Stroe, S. G., and Codină, G. G. (2021). Impact of germination on the microstructural and physicochemical properties of different legume types. Plants 10:592. Go to original source. Plants. 10(3), 592. doi: 10.3390/plants10030592
Batbayar, B., Nickerson, Y. K. M. T., Korber, N. D. R., and Tanaka, T. (2023). Solid-state and submerged fermentation effects on functional properties of pea protein-enriched flour. Cereal Chem. 100, 1092–1105. doi: 10.1002/cche.10691
Benítez, V., Cantera, S., Aguilera, Y., Mollá, E., Esteban, R. M., and Díaz, M. F. (2013). Impact of germination on starch, dietary fiber and physicochemical properties in non-conventional legumes. Food Res Intern, 50, 64–69. doi: 10.1016/j.foodres.2012.09.044
Benjamin, Y. N. Z., Serge, E. A. G., Martial-Didier, A. K., Phares, A. K. S., and Kablan, T. (2021). Effect of fermentation on the biochemical composition of soybean (Glycine max L.) and red bean (Vigna unguiculata L.) consumed in the city of Daloa (Côte d’Ivoire). Int. J. Curr. Microbiol. App. Sci. 10, 578–583. doi: 10.20546/ijcmas.2021.1010.068
Betancur-Ancona, D., Sosa-Espinoza, T., Ruiz-Ruiz, J., Segura-Campos, M., and Chel-Guerrero, L. (2014). Enzymatic hydrolysis of hard-to-cook bean (Phaseolus vulgaris L.) protein concentrates and its effects on biological and functional properties. Int. J. Food Sci. Technol. 49, 2–8. doi: 10.1111/ijfs.12267
Boeck, T., Sahin, A. W., Zannini, E., and Arendt, E. K. (2021). Nutritional properties and health aspects of pulses and their use in plant-based yogurt alternatives. Compr. Rev. Food Sci. Food Saf. 20, 3858–3880. doi: 10.1111/1541-4337.12778
Boerema, A., Peeters, A., Swolfs, S., Vandevenne, F., Jacobs, S., Staes, J., et al. (2016). Soybean trade: balancing environmental and socio-economic impacts of an intercontinental market. PLoS One 11:e0155222. doi: 10.1371/journal.pone.0155222
Cerma, L., and Yu, P. (2023). Effect of thermal processing methods on structural, physicochemical and nutritional characteristics of cool-season chickpeas in ruminant systems. Anim. Feed Sci. Technol. 303:115698. doi: 10.1016/j.anifeedsci.2023.115698
Dent, T., Campanella, O., and Maleky, F. (2023). Enzymatic hydrolysis of soy and chickpea protein with Alcalase and Flavourzyme and formation of hydrogen bond mediated insoluble aggregates. Curr. Res. Food Sci. 6:100487. doi: 10.1016/j.crfs.2023.100487
Dueñas, M., Sarmento, T., Aguilera, Y., Benitez, V., Mollá, E., and Esteban, R. M. (2016). Impact of cooking and germination on phenolic composition and dietary fibre fractions in dark beans (Phaseolus vulgaris L.) and lentils (Lens culinaris L.). LWT Food Sci. Technol. 66, 72–78. doi: 10.1016/j.lwt.2015.10.025
Eckert, E., Han, J., Swallow, K., Tian, Z., Jarpa-Parra, M., and Chen, L. (2019). Effects of enzymatic hydrolysis and ultrafiltration on physicochemical and functional properties of faba bean protein. Cereal Chem. 96:725741. doi: 10.1002/cche.10169
El-Safy, F., Salem, R. H. A., and Mukhtar, Y. Y. (2013). The impact of soaking and germination on chemical composition, carbohydrate fractions, digestibility, antinutritional factors and minerals content of some legumes and cereals grain seeds. Alexandria Sci. Exchange J. 34, 499–513. doi: 10.21608/asejaiqjsae.2013.3112
Emkani, M., Moundanga, S., Oliete, B., and Saurel, R. (2023). Protein composition and nutritional aspects of pea protein fractions obtained by a modified isoelectric precipitation method using fermentation. Front. Nutr. 10:1284413. doi: 10.3389/fnut.2023.1284413
Emkani, M., Oliete, B., and Saurel, R. (2021). Pea protein extraction assisted by lactic fermentation: impact on protein profile and thermal properties. Foods. 10:549. doi: 10.3390/foods10030549
Feng, J., Liu, X., Xu, Z. R., Lu, Y. P., and Liu, Y. Y. (2007). The effect of aspergillus oryzae fermented soybean meal on growth performance, digestibility of dietary components and activities of intestinal enzymes in weaned piglets. Anim. Feed Sci. Technol. 134, 295–303. doi: 10.1016/j.anifeedsci.2006.10.004
Gall, K. M., Séve, J. G. B., and Quillien, L. (2005). Effects of grinding and thermal treatments on hydrolysis susceptibility of pea proteins (Pisum sativum L.). J. Agric. Food Chem. 53, 3057–3064. doi: 10.1021/jf040314w
Gautheron, O., Nyhan, L., Torreiro, M. G., Tlais, A. Z. A., Cappello, C., and Gobbetti, M. (2024). Exploring the impact of solid-state fermentation on fava bean flour: a comparative study of aspergillus oryzae and Rhizopus oligosporus. Foods 13:2922. doi: 10.3390/foods13182922
Gbenle, J., Mert, M., Phasha, N. N., Madibana, M. J., Manyeula, F., Bamidele, O. P., et al. (2025). Fungal-mediated solid-state fermentation ameliorates antinutritional factors but does not improve in vitro digestibility of marama (Tylosema esculentum) beans. Future Foods 11:100664. doi: 10.1016/j.fufo.2025.100664
Ghavidel, R. A., and Prakash, J. (2006). Effect of germination and dehulling on functional properties of legume flours. J. Sci. Food Agric. 86, 1189–1195. doi: 10.1016/j.foodchem.2024.139265
Goertzen, A. D., House, J. D., Nickerson, M. T., and Tanaka, T. (2020). The impact of enzymatic hydrolysis using three enzymes on the nutritional properties of a chickpea protein isolate. Cereal Chem. 98, 275–284. doi: 10.1002/cche.10361
Gonçalves, A., Goufo, P., Barros, A., Domínguez-Perles, R., Trindade, H., and Rosa, E. A. (2016). Cowpea (Vigna unguiculata L. Walp), a renewed multipurpose crop for a more sustainable Agri-food system: nutritional advantages and constraints. J. Sci. Food Agric. 96, 2941–2951. doi: 10.1002/jsfa.7644
Irakli, M., Lazaridou, A., and Biliaderis, C. G. (2020). Comparative evaluation of the nutritional, antinutritional, functional, and bioactivity attributes of rice bran stabilized by different heat treatments. Foods 10:57. doi: 10.3390/foods10010057
Irondi, E. A., Ogunsanmi, A. O., Ahmad, R. S., Ajani, E. O., Adegoke, B. M., and Boligon, A. A. (2019). Effect of roasting on phenolics composition, enzymes inhibitory and antioxidant properties of cowpea pulses. J. Food Meas. Charact. 13, 1489–1496. doi: 10.1007/s11694-019-00064-0
Jeunink, J., and Cheftel, J. C. (1979). Chemical and physicochemical changes in field bean and soybean proteins texturized by extrusion. J. Food Sci. 44, 1322–1325. doi: 10.1111/j.1365-2621.1979.tb06430.x
Jiang, W., Feng, J., Yang, X., and Li, L. (2024). Structure of pea protein-based complexes on high-moisture extrusion: raw materials and extrusion zones. Lwt 194:115823. doi: 10.1016/j.lwt.2024.115823
Kebede, E., and Bekeko, Z. (2020). Expounding the production and importance of cowpea (Vigna unguiculata (L.) Walp.) in Ethiopia. Cogent Food Agric. 6:1769805. doi: 10.1080/23311932.2020.1769805
Kesselly, S. R., Mugabi, R., and Byaruhanga, Y. B. (2023). Effect of soaking and extrusion on functional and pasting properties of cowpeas flour. Sci. Afr. 19:e01532. doi: 10.1016/j.sciaf.2022.e01532
Khattab, R. Y., and Arntfield, S. D. (2009). Nutritional quality of legume seeds as affected by some physical treatments 2. Antinutritional factors. LWT - Food Sci. Technol. 42, 1113–1118.
Kumar, Y., Basu, S., Goswam, D., Devi, M., Shivhare, U. S., and Vishwakarma, R. K. (2021). Anti-nutritional compounds in pulses: implications and alleviation methods. Legume Sci. 4:111. doi: 10.1002/leg3.111
Kur, A. T. Y., AbdelAtti, K. A., Dousa, B. M., Elagib, H. A. A., Malik, H. E. E., and Elamin, K. M. (2013). Effect of treated cowpea seeds on broiler chicken. Glob. J. Anim. Sci. Res. 1, 58–65.
Kuzhkuzha, M. D., Girei, A. A., and Ogezi, E. (2021). Benefits and constraints of cowpea production in the Western agricultural zone of Nasarawa state, Nigeria. Direct Res. J. Agric. Food Sci. 9, 425–432. doi: 10.26765/DRJAFS
Lopes, L. C. M., de Aleluia Batista, K., and Fernandes, K. F. (2012). Functional, biochemical and pasting properties of extruded bean (Phaseolus vulgaris) cotyledons. Int. J. Food Sci. Technol. 47, 1859–1865. doi: 10.1111/j.1365-2621.2012.03042.x
Lubisi, M. W., Baloyi, J. J., and Fushai, F. (2023). Nutrient digestibility and nitrogen balance in different pig breeds fed raw, sprouted, or roasted (Vigna unguiculata) diets. Trop. Anim. Health Prod. 55:334. doi: 10.1007/s11250-023-03742-w
Maila, M. Y., and Tseke, P. E. (2024). Influence of blanching time on the phytochemical and nutritive value of cowpea (Vigna unguiculata L. Walp) leafy vegetable. Int. J. Food Sci. 2024:9095035. doi: 10.1155/2024/9095035
Martin, A., Naumann, S., Osen, R., Karbstein, H. P., and Emin, M. A. (2021). Extrusion processing of rapeseed press cake-starch blends: effect of starch type and treatment temperature on protein, fiber and starch solubility. Foods 10:1160. doi: 10.3390/foods10061160
Martínez, Y., Li, X., Liu, G., Bin, P., Yan, W., and Más, D. (2017). The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 49, 2091–2098. doi: 10.1007/s00726-017-2494-2
Mbithi-Mwikya, S., Ooghe, W., Van Camp, J., Ngundi, D., and Huyghebaert, A. (2000). Amino acid profiles after sprouting, autoclaving, and lactic acid fermentation of finger millet (Eleusine coracan) and kidney beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 48, 3081–3085. doi: 10.1021/jf0002140
Mehanni, A., Sorour, M., Abd El-Galel, H., and Ahmed, W. K. (2021). Soaking and germination procedures actually impact polyphenols, tannins, and phytate contents in some Egyptian pulses. SVU-Int. J. Agric. Sci. 3, 63–72. doi: 10.21608/svuijas.2021.89539.1135
Menssen, M., Linde, M., Omondi, E. O., Abukutsa-Onyango, M., Dinssa, F. F., and Winkelmann, T. (2017). Genetic and morphological diversity of cowpea (Vigna unguiculata (L.) Walp.) entries from East Africa. Sci. Hortic. 226, 268–276. doi: 10.1016/j.scienta.2017.08.003
Mitrus, M., Wójtowicz, A., Oniszczuk, T., Combrzyński, M., Bouasla, A., Kocira, S., et al. (2023). Application of extrusion-cooking for processing of white and red bean to create specific functional properties. Appl. Sci. 13:1671. doi: 10.3390/app13031671
Moreno, C., Mojica, L., de Mejía, E. G., Ruiz, R. M. C., and Luna-Vital, D. A. (2020). Combinations of legume protein hydrolysates synergistically inhibit biological markers associated with adipogenesis. Foods. 9:1678. doi: 10.3390/foods9111678
Mubarak, A. E. (2005). Nutritional composition and antinutritional factors of mung bean seeds (Phaseolus aureus) as affected by some home traditional processes. Food Chem. 89.489-495. doi: 10.1016/j.foodchem.2004.01.007
Muthuraman, A., Ramesh, M., Shaikh, S. A., Aswinprakash, S., and Jagadeesh, D. (2021). Physiological and pathophysiological role of cysteine metabolism in human metabolic syndrome. Drug Metab. Lett. 14, 177–192. doi: 10.2174/1872312814666211210111820
Nagessa, W. B., Chambal, B., and Macuamule, C. (2023). Effects of processing methods on phytate and tannin content of black small common beans (Phaseolus vulgaris L.) cultivated in Mozambique. Cogent Food Agric. 9:e2289713. doi: 10.1080/23311932.2023.2289713
Narayana, K., and Rao, M. N. (1984). Effect of partial proteolysis on the functional properties of winged bean (Psophocarpus tetragonolobus) flour. J. Food Sci. 49, 944–947. doi: 10.1111/j.1365-2621.1984.tb13247.x
Nwagboso, C., Andam, K. S., Amare, M., Bamiwuye, T., and Fasoranti, A. (2024). The economic importance of cowpea in Nigeria trends and implications for achieving Agri-food system transformation. Intl. Food Policy Res. Inst. 9, 425–432. doi: 10.26765/DRJAFS98201857
Obour, A. K., Faye, A., Akplo, T. M., Stewart, Z. P., Min, D., Prasad, P. V., et al. (2025). Economic value of dual-purpose cowpea as affected by variety, fertilizer, and environment. Agrosystems, Geosci. Environ. 8:e70045. doi: 10.1002/agg2.70045
Ofuya, Z. M. (2006). The effect of cowpea oligosaccharides on gas production in adult rats. Asian J. Plant Sci. 5, 590–597. doi: 10.3923/ajps.2006.590.597
Omokanye, A. T., Onifade, O. S., Amodu, J. T., Balogun, R. O., and Kallah, M. S. (2003). Evaluation of dual-purpose cowpea (Vigna unguiculata (L.) Walp.) varieties for grain and fodder production at Shika, Nigeria. Tropicultura 21, 42–46.
Onyenekwe, P. C., and Njoku, G. C. (2000). Effect of cowpea (Vigna unguiculata) processing methods on flatus causing oligosaccharides. Nutr. Res. 20, 349–358. doi: 10.1016/S0271-5317(00)00128-7
Osen, R., Toelstede, S., Eisner, P., and Schweiggert-Weisz, U. (2015). Effect of high moisture extrusion cooking on protein–protein interactions of pea (Pisum sativum L.) protein isolates. Int. J. Food Sci. Technol. 50, 1390–1396. doi: 10.1111/ijfs.12783
Pasqualone, A., Costantini, M., Coldea, T. E., and Summo, C. (2020). Use of legumes in extrusion cooking: a review. Foods 9:958. doi: 10.3390/foods9070958
Pedrosa, M. M., Guillamón, E., and Arribas, C. (2021). Autoclaved and extruded legumes as a source of bioactive phytochemicals: a review. Foods. 10:379. doi: 10.3390/foods10020379
Perović, M. N., Pajin, B. S., and Antov, M. G. (2022). The effect of enzymatic pretreatment of chickpea on functional properties and antioxidant activity of alkaline protein isolate. Food Chem. 374:131809. doi: 10.1016/j.foodchem.2021.131809
Polanco-Lugo, E., Dávila-Ortiz, G., Betancur-Ancona, D. A., and Chel-Guerrero, L. A. (2014). Effects of sequential enzymatic hydrolysis on structural, bioactive and functional properties of Phaseolus lunatus protein isolate. Food Sci. Technol. 34, 441–448. doi: 10.1590/1678-457x.6349
Rehman, Z., and Shah, W. H. (2005). Thermal heat processing effects on antinutrients, protein and starch digestibility of food legumes. Food Chem. 91, 327–331. doi: 10.1016/j.foodchem.2004.06.019
Rizzi, C., Galeoto, L., Zoccatelli, G., Vincenzi, S., Chignola, R., and Peruffo, A. D. (2003). Food Res. Int. 36, 815–821. doi: 10.1016/S0963-9969(03)00076-0
Salim, R., Nehvi, I. B., Mir, R. A., Tyagi, A., Ali, S., and Bhat, O. M. (2023). A review on anti-nutritional factors: unraveling the natural gateways to human health. Front. Nutr. 10. doi: 10.3389/fnut.2023.1215873
Santos, R., Carvalho, M., Rosa, E., Carnide, V., and Castro, I. (2020). Root and agro- morphological traits performance in cowpea under drought stress. Agronomy 10:e1604. doi: 10.3390/agronomy10101604
Santos-Sánchez, G., Miralles, B., Brodkorb, A., Dupont, D., Egger, L., and Recio, I. (2024). Current advances for in vitro protein digestibility. Front Nutr. 11:e1404538. doi: 10.3389/fnut.2024.1404538
Segura-Campos, M. R., Espinosa-García, L., Chel-Guerrero, L. A., and Betancur-Ancona, D. A. (2012). Effect of enzymatic hydrolysis on solubility, hydrophobicity, and in vivo digestibility in cowpea (Vigna unguiculata). Int. J. Food Prop. 15, 770–780. doi: 10.1080/10942912.2010.501469
Sibian, M. S., Saxena, D. C., and Riar, C. S. (2017). Effect of germination on chemical, functional and nutritional characteristics of wheat, brown rice and triticale: A comparative study. J. Sci. Food Agric. 97, 4643–4651. doi: 10.1002/jsfa.8336
Shi, L., Mu, K., Arntfield, S. D., and Nickerson, M. T. (2017). Changes in levels of enzyme inhibitors during soaking and cooking for pulses available in Canada. J. Food Sci. Technol. 54, 1014–1022. doi: 10.1007/s13197-017-2519-6
Silva, I. C. V., Damasceno-Silva, K. J., Hashimoto, J. M., Carvalho, C. W. P. D., Ascheri, J. L. R., and Galdeano, M. C. (2023). Effect of different processing conditions to obtain expanded extruded based on cowpea. Braz. J. Food Technol. 26:e2022052. doi: 10.1590/1981-6723.05222
Simion, T. (2018). Breeding cowpea Vigna unguiculata L. Walp for quality traits. Ann. Rev. Res. 3:e555609. doi: 10.19080/ARR.2018.03.555609
Singh, B. (2020). Cowpea: The food legume of the 21st century. Wisconsin: Crop Science Society of America.
Singh, B. B., Ajeigbe, H. A., Tarawali, S. A., Fernandez-Rivera, S., and Abubakar, M. (2003). Improving the production and utilization of cowpea as food and fodder. Field Crop Res. 84, 169–177. doi: 10.1016/S0378-4290(03)00148-5
Singh, B., Singh, J. P., Kaur, A., and Singh, N. (2017). Phenolic composition and antioxidant potential of grain legume seeds: a review. Food Res. Int. 101, 1–16. doi: 10.1016/j.foodres.2017.09.026
Thakur, P., Kumar, K., Ahmed, N., Yadav, A. N., Kumar, S., Rizvi, Q. U. E. H., et al. (2022). Impact of diverse processing treatments on nutritional and anti-nutritional characteristics of soybean (Glycine max L.). J. Appl. Biol. Biotechnol. 10, 97–105. doi: 10.7324/JABB.2022.100313
Trugo, L. C., Donangelo, C. M., Trugo, N. M. F., and Bach Knudsen, K. E. (2000). Effect of heat treatment on nutritional quality of germinated legume seeds. J. Agric. Food Chem. 48, 2082–2086. doi: 10.1021/jf9913920
Tzanova, M. T., Stoilova, T. D., Todorova, M. H., Memdueva, N. Y., Gerdzhikova, M. A., and Grozeva, N. H. (2023). Antioxidant potentials of different genotypes of cowpea (Vigna unguiculata L. Walp.) cultivated in Bulgaria, southern Europe. Agronomy 13:1684. doi: 10.3390/agronomy13071684
Veer, S. J., Pawar, V. S., and Kambale, R. E. (2021). Antinutritional factors in foods. Pharma Innov. J. 10, 01–04. doi: 10.22271/tpi
Verni, M., De Mastro, G., De Cillis, F., Gobbetti, M., and Rizzello, C. G. (2019). Lactic acid bacteria fermentation to exploit the nutritional potential of Mediterranean faba bean local biotypes. Food Res. Int. 125:e108571. doi: 10.1016/j.foodres.2019.108571
Volkova, E., and Smolyaninova, N. (2023). Analysis of world trends in soybean production. BIO Web Conf. 141:01026. doi: 10.1051/bioconf/202414101026
Wisaniyasa, N. W., Suter, I. K., Marsono, Y., and Putra, I. K. (2015). Germination effect on functional properties and antitrypsin activities of pigeon pea (Cajanus cajan (L.) Millsp.) sprout flour. Food Sci Quality Manag 43, 9–83.
Xu, X., Qiao, Y., Shi, B., and Dia, V. P. (2021). Alcalase and bromelain hydrolysis affected physicochemical and functional properties and biological activities of legume proteins. Food Struct. 27:100178. doi: 10.1016/j.foostr.2021.100178
Yadav, N., Kaur, D., Malaviya, R., Singh, M., Fatima, M., and Singh, L. (2018). Effect of thermal and non-thermal processing on antioxidant potential of cowpea seeds. Int. J. Food Prop. 21, 437–451. doi: 10.1080/10942912.2018.1431659
Keywords: amino acids, antinutritional factors, cowpeas, orphan pulses, soybeans, valorization techniques
Citation: Mashiloane T, Mlambo V, Mhlongo G, Dibakoane SR and Mnisi CM (2025) Cowpeas vs. soybeans: Can valorization bridge the nutritional gap for sustainable animal feeding systems in the Global South? Front. Sustain. Food Syst. 9:1657018. doi: 10.3389/fsufs.2025.1657018
Edited by:
Abena Boakye, Kwame Nkrumah University of Science and Technology, GhanaReviewed by:
Zachary Shea, Virginia Tech, United StatesSamuel Tonyemevor, Kwame Nkrumah University of Science and Technology, Ghana
Copyright © 2025 Mashiloane, Mlambo, Mhlongo, Dibakoane and Mnisi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Victor Mlambo, VmljdG9yLk1sYW1ib0B1bXAuYWMuemE=
 Thabang Mashiloane1,2
Thabang Mashiloane1,2 Victor Mlambo
Victor Mlambo Caven M. Mnisi
Caven M. Mnisi