Abstract
Data from pre-clinical and clinical studies form part of an integrated assessment of the tobacco harm reduction (THR) potential of novel products that may act as cigarette alternatives for adult smokers. We report data from pre-clinical (emissions chemistry and in vitro toxicology) and clinical (nicotine pharmacokinetics and subjective effects) studies conducted with the iSENZIA™ heated herbal system (HHS; PULZE™ 2.0 device with iSENZIA™ sticks), which utilizes electronic heating of a tea-based substrate to generate an inhalable nicotine-containing aerosol. The aerosols from the iSENZIA™ HHS contained significantly lower levels, by up to 99.8%, of the nine World Health Organization Study Group on Tobacco Product Regulation (WHO TobReg) analytes compared with 1R6F reference cigarette smoke and elicited significantly lower in vitro cytotoxicity, genotoxicity, and mutagenicity responses. The clinical study demonstrated that the iSENZIA™ HHS delivers satisfactory levels of nicotine to users and has lower abuse liability than cigarettes. Overall, our data suggest that iSENZIA™ has the potential to offer substantially reduced toxicant exposure, as well as a reduction in toxicity, compared to cigarettes, while delivering satisfactory levels of nicotine. These findings support the THR potential of the iSENZIA™ HHS as a reduced-risk, acceptable alternative product for adult smokers.
Introduction
Smoking is a cause of serious diseases in smokers, including lung cancer, cardiovascular disease, and emphysema. Cigarette smoking is reported as the world’s leading cause of preventable deaths (International Agency fo r Research on Cancer, 2007; US Department of Health and Human Services, 2014; World Health Organization, 2011; World Health Organization, 2023), responsible for more than 7 million deaths per year globally (World Health Organization, 2023). The greatest risk of smoking-related diseases comes from burning tobacco and inhaling smoke containing approximately 7,000 chemicals. One component of cigarette smoke is nicotine. While nicotine is an addictive chemical and not risk-free, it is not thought to be directly responsible for the harmful effects of cigarette smoking (Abrams et al., 2018; Gottlieb and Zeller, 2017; Royal College of Physicians, 2016). A number of chemicals identified in cigarette smoke (Perfetti and Rodgman, 2011; Toll et al., 2024) have been termed harmful and potentially harmful constituents (HPHCs) and are linked to cardiovascular and respiratory diseases, lung cancer, and reproductive/developmental toxicity (Food and Drug Administration, 2012). The best possible action for smokers to reduce or eliminate exposure to HPHCs and other toxicants, and therefore to reduce disease risk, is to stop smoking (US Department of Health and Human Services, 2014). However, although a large proportion of smokers report intending to take this course of action or begin a quit attempt each year, only a small fraction of smokers manage to successfully quit smoking (Babb et al., 2017; Centers for Disease Control and Prevention, 2021; Centers for Disease Control and Prevention, 2022; Pierce, 2022).
For those smokers who are unwilling or uninterested in quitting, a tobacco harm reduction (THR) approach to reducing the harms associated with smoking has been proposed as beneficial. The THR concept of reducing exposure to harmful toxicants as a means of reducing morbidity and mortality was first highlighted by the US Institute of Medicine (IoM) in 2001 (Institute of Medicine, 2001; Stratton et al., 2001) in their ‘Clearing the Smoke’ report. In this report, the IoM defined THR as “minimizing harms and decreasing total morbidity and mortality, without completely eliminating tobacco and nicotine use,” and suggested that while a significant proportion of individuals will continue to use tobacco, THR can be achieved by decreasing the risk associated with tobacco use, by decreasing the users’ consumption, or by decreasing the prevalence of use (Institute of Medicine, 2001). This gives rise to the concept that both the individual- and population-level health impacts of smoking can be reduced by novel nicotine and tobacco products that deliver nicotine without exposing the user to the HPHCs and other toxicants responsible for smoking-related diseases. In recent years, there has been increased support for a toxicant exposure reduction approach to THR, and a number of global public health authorities now advocate for this approach (Royal College of Physicians, 2016; Health Canada, 2022; New Zealand Ministry of Health, 2020; Office for Health Improvement and Disparities, 2022), while other global health authorities have been called upon to make THR a central strategy in promoting public health (Beaglehole and Bonita, 2024; Warner, 2019; Yach, 2022).
One alternative form of nicotine-containing product for adult smokers who would otherwise continue to smoke is heated tobacco products (HTPs), which electrically heat a tobacco-based substrate in a controlled manner to produce a nicotine-containing aerosol (Eaton et al., 2018; Jaccard et al., 2017; Schaller J. P. et al., 2016; Cordery et al., 2024; Smith et al., 2016). Because this heating occurs at temperatures that are significantly lower than those that cause combustion in cigarettes, the levels of HPHCs and other toxicants are significantly lower in HTP aerosols than the levels found in cigarette smoke (Chapman et al., 2023a; Forster et al., 2018; Malt et al., 2022; Poynton et al., 2017; Szparaga et al., 2021). This results in reduced toxicant exposure among smokers who exclusively switch to using HTPs (Gale et al., 2021a; Gale et al., 2021b; Gale et al., 2019; Gale et al., 2022; Haziza et al., 2020a; Haziza et al., 2016; Haziza et al., 2017; Lüdicke et al., 2018a). Furthermore, data from a number of pre-clinical and clinical studies conducted to date support the potentially reduced risk profile and THR potential of HTPs (Cordery et al., 2024). An emerging category of heated nicotine delivery products for adult smokers who would otherwise continue to smoke is heated herbal products (HHPs). Instead of heating tobacco, HHPs electronically heat a non-tobacco, nicotine-containing substrate, at temperatures lower than those which allow combustion, to produce an aerosol containing nicotine. A tobacco-derived nicotine salt, compliant with pharmaceutical-grade quality standards, is added to the substrate of the HHP. To date, however, unlike HTPs, there are no published studies assessing HHPs. In this article, we describe the findings from a suite of pre-clinical (emissions chemistry and in vitro toxicology) studies, and a clinical study assessing nicotine pharmacokinetics and subjective effects, conducted with a novel heated herbal system (HHS), iSENZIA™, which is comprised of the PULZE™ 2.0 device and iSENZIA™ sticks. The data arising from these multidisciplinary studies provide the first evidence of the THR potential of the iSENZIA™ HHS and contribute to the growing body of evidence for this novel product category.
Results
Pre-clinical studies
Emissions chemistry assessment
In the aerosols generated from the iSENZIA™ HHS with Forest Berry and Summer Watermelon sticks, six of the nine World Health Organization (WHO) Study Group on Tobacco Product Regulation (TobReg 9) analytes were below the limits of quantification (<LOQ; Supplementary Table S1): N′-nitrosonornicotine (NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), benzo[a]pyrene (B[a]P), formaldehyde, 1,3-butadiene, and benzene. When comparing iSENZIA™ Forest Berry and iSENZIA™ Summer Watermelon aerosol analyte levels with those in 1R6F reference cigarette smoke, substantial reductions in all analytes were observed in the HHS aerosols on a per-puff and per-stick basis (Figure 1; Supplementary Table S1). For both varieties of iSENZIA™ HHS sticks assessed, percentage reductions in WHO TobReg 9 analyte levels for analytes that were >LOQ ranged from approximately 96.3% for acetaldehyde to approximately 98.8% for acrolein (Supplementary Table S1). The iSENZIA™ HHS aerosol nicotine yield was also substantially lower than that of 1R6F reference cigarette smoke by approximately 65% (Forest Berry) and 67% (Summer Watermelon). Aerosol collected mass (ACM) was lower for the iSENZIA™ HHS aerosols than total particulate matter (TPM) in 1R6F reference cigarette whole smoke by approximately 18% for both flavor variants (Supplementary Table S1).
FIGURE 1
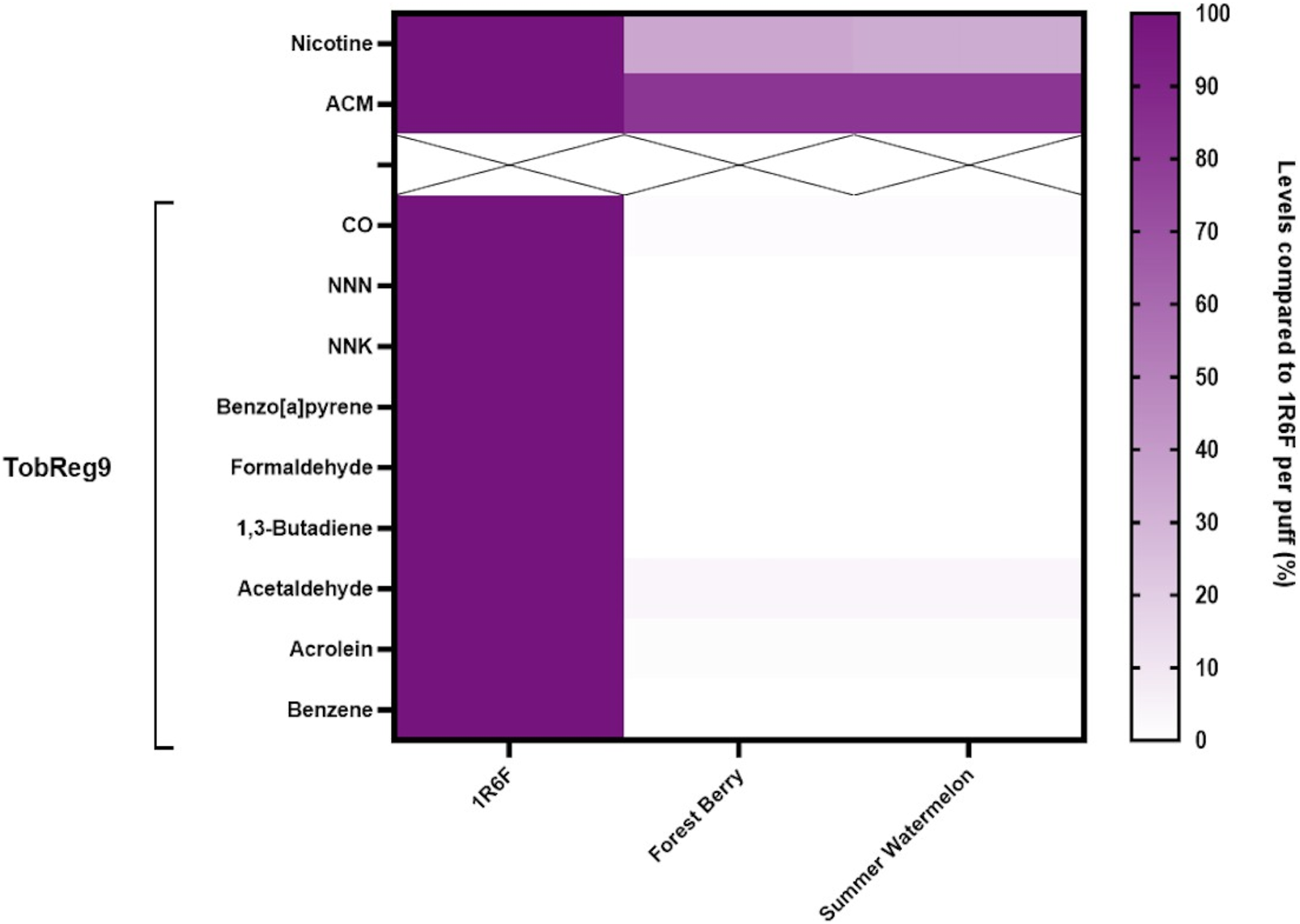
Heatmap of analytes in iSENZIA™ HHS Forest Berry and Summer Watermelon aerosol relative to 1R6F reference cigarette smoke. Data are presented as analyte levels in the iSENZIA™ Forest Berry or iSENZIA™ Summer Watermelon aerosol. Analyte levels are presented on a per-puff basis normalized to analyte levels in 1R6F reference cigarette smoke. 1R6F reference cigarettes were smoked on a rotary smoking machine according to the ISO 20778:2018 smoking regimen (55 mL puff volume, 2-s puff duration, 30-s puff interval, bell-shaped puff profile, filter vents blocked). The iSENZIA™ HHS was used on a linear smoking machine according to a modified version of ISO 20778:2018 (55 mL puff volume, 2-s puff duration, 30-s puff interval, bell-shaped puff profile, with no requirement for filter vent blocking). Abbreviations: ACM, aerosol collected mass; CO, carbon monoxide; HHS, heated herbal system; NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NNN, N-nitrosonornicotine; TPM, total particulate matter; WHO TobReg 9, World Health Organization Study Group on Tobacco Product Regulation proposal of toxicants mandated for lowering in cigarette smoke (Burns et al., 2008).
In vitro toxicology
Neutral red uptake cytotoxicity assay
Cytotoxicity assessments using the neutral red uptake (NRU) assay were conducted in Beas-2b cells following exposure to whole smoke/aerosol at the air–liquid interface (ALI). Cytotoxicity was observed (>IC50 achieved) following exposure to 1R6F reference cigarette whole smoke or iSENZIA™ HHS whole aerosols, and there were clear dose responses to all three test articles (Figure 2). The number of puffs required to induce IC50 was, on average, 50-fold and 35-fold higher for the iSENZIA™ HHS Forest Berry and Summer Watermelon aerosols, respectively, than for the 1R6F reference cigarette smoke (Figure 2).
FIGURE 2
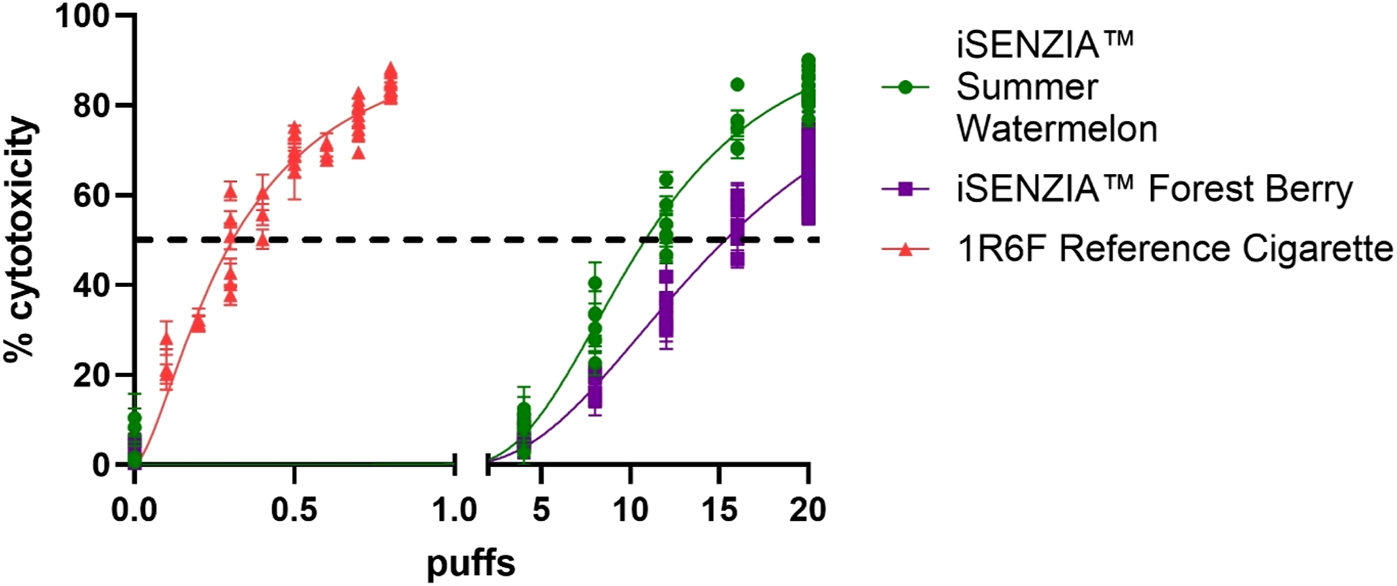
Cytotoxicity assessment of iSENZIA™ HHS Forest Berry and Summer Watermelon aerosol relative to 1R6F reference cigarette smoke. Percentage cytotoxicity induced in the neutral red uptake assay in Beas-2B cells following exposure to increasing puff numbers (log scale) of 1R6F reference cigarette whole smoke or iSENZIA™ HHS whole aerosol. Fifty percent cytotoxicity compared to the negative control (IC50) is marked with the black dotted line. Data shown are the mean +/− standard deviation with n = 3 (biological replicates). Generation of both cigarette smoke and iSENZIA™ HHS whole aerosol was performed in accordance with the ISO 20778:2018 smoking regimen. Abbreviation: HHS, heated herbal system.
Ames bacterial reverse mutation mutagenicity assay
Ames tests were conducted in two bacterial strains, TA98 and TA100, both with and without S9 metabolic activation. In either strain, both in the presence and absence of S9, 1R6F reference cigarette smoke induced significant increases in revertant colonies with increasing numbers of puffs (Figures 3A,B). This occurred over a lower range of exposure levels (puff numbers) than for either of the iSENZIA™ HHS variant aerosols, which did not induce significant increases in the number of revertant colonies in either bacterial strain, with and without S9, under the test conditions even at high puff numbers (Figures 3A,B). Overall, the assay was negative for both of the iSENZIA™ HHS variant aerosols.
FIGURE 3
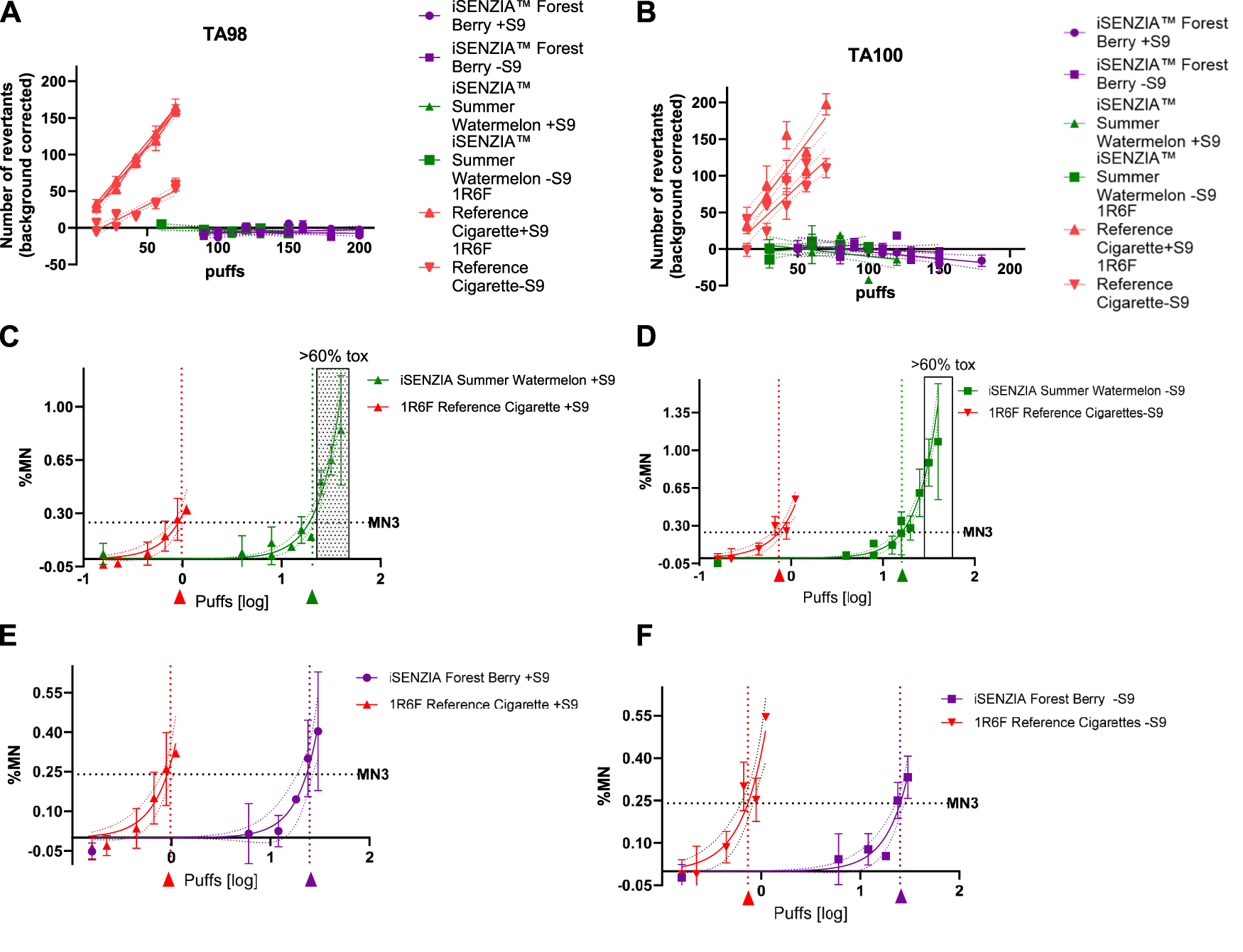
Mutagenicity and genotoxicity assessments of the iSENZIA™ HHS Forest Berry and Summer Watermelon aerosol relative to 1R6F reference cigarette smoke. Panels A and B show the average (background corrected) number of revertant colonies per plate in an Ames bacterial reverse mutation assay for TA98 (A) or TA100 (B) strains exposed to increasing numbers of puffs of 1R6F whole smoke or iSENZIA™ HHS whole aerosol, +/− S9 metabolic activation. n = 3 (technical replicates); error bars represent the standard deviation. Linear regression analysis was applied to the data (trendlines as indicated), and the slope was calculated. For the Ames assay, generation of fresh smoke from 1R6F reference cigarettes was performed according to the ISO3308:2012 smoking regimen, while for the iSENZIA™ HHS, ISO 20778:2018, without requiring vent blocking, was used to generate the aerosol. Background levels of spontaneous revertants for the Ames assay were: TA98 + S9: 33; TA98-S9: 22; TA100 + S9: 115; and TA100-S9: 100. (C–F) show the background subtracted micronucleus frequency in an in vitro micronucleus (IVM) assay in V79 cells following exposure to increasing puffs (log scale) of 1R6F whole smoke or HHS whole aerosol, +/− S9 metabolic activation. ECMN3 (number of puffs necessary to reach a three-fold increase over background micronucleus frequencies) analysis was carried out using nonlinear regression analysis (solid lines for each test article) to determine the puff-based concentration required to induce micronucleus frequencies three-fold over background levels for each test article. The dotted lines parallel to the y-axis intersect the ECMN3 values on the x-axis at the corresponding puff-based dose levels for 1R6F (red arrow) and the iSENZIA™ HHS variant (Forest Berry = purple arrow, Summer Watermelon = green arrow). The grey area indicates toxicity levels above 60%. n = 2 (biological replicates); error bars represent the standard deviation. For the IVM assay, generation of both cigarette smoke and HHS aerosol was performed in accordance with the ISO 20778:2018 smoking regimen. Historical background levels for the IVM assay were: V79-S9: 0.12%; V79 + S9: 0.14%. Abbreviation: HHS, heated herbal system.
In Vitro micronucleus genotoxicity assay
The genotoxic activity of whole smoke/aerosol was assessed using in vitro micronucleus (IVM) assay in V79 cells, both with and without S9 metabolic activation. Significant responses were observed at substantially lower exposure levels (puff numbers) for the 1R6F reference cigarette smoke compared with either of the iSENZIA™ HHS aerosols, both with and without S9 activation (Figures 3C–F). Due to technical limitations, a short-term treatment in the absence of S9 was not applicable in parallel. As indicated by calculations of the number of puffs necessary to reach a three-fold increase over background micronucleus frequencies (ECMN3), 1R6F reference cigarette smoke exhibited substantially higher potency than either the iSENZIA™ HHS Forest Berry or Summer Watermelon aerosols, with ECMN3 values that were 24.8 fold and 21.4 fold higher, respectively, for the 1R6F reference cigarette smoke with S9 activation and 34.7 fold and 21.9 fold higher, respectively, for the 1R6F reference cigarette smoke without S9 activation (Figures 3C–F). Overall, the results for the assay were positive for both of the iSENZIA™ HHS variant aerosols and for the 1R6F reference cigarette.
Clinical study
Subject demographics
Brief demographic details of the 25 subjects in the safety population are provided in Supplementary Table S2 for each randomization sequence and overall. Forty percent of subjects were male, and all subjects were White and not of Hispanic/Latino origin. No major differences in demographics were observed between the randomization sequence groups, although the mean age of the subjects and number of years smoking in one of the product sequence groups was higher than the other four groups (Supplementary Table S2). Of the 25 subjects, all smoked non-mentholated cigarettes as their usual brand.
Nicotine pharmacokinetics
Prior to the start of the controlled puffing sessions and following 12 h of abstinence from the use of any tobacco- or nicotine-containing products, the mean uncorrected plasma nicotine concentration was 1.06 ng/mL (standard deviation [SD] 0.756 ng/mL). During use of any of the study products in the controlled use sessions (puffs taken at 30-s intervals with puffs 3 s in duration), plasma nicotine levels rose rapidly (Figure 4). On average, during the controlled use session, subjects took 9.3 puffs on each of the iSENZIA™ stick variants and 10.3 puffs on their usual brand cigarettes (Supplementary Table S3).
FIGURE 4
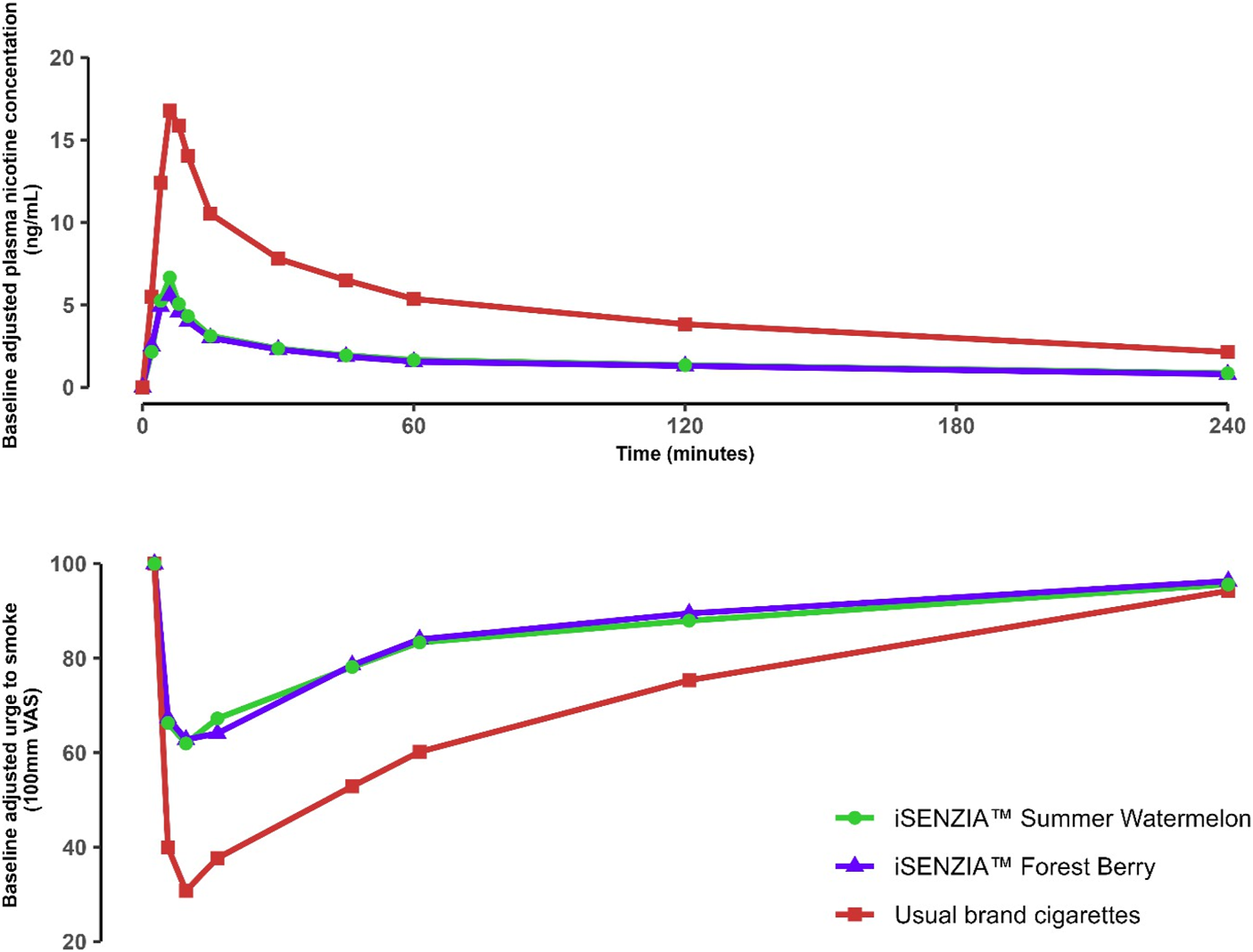
Baseline-adjusted plasma nicotine concentrations (upper) and urge to smoke (lower) over time following controlled product use in the outcomes population of the clinical study. N = 25 in each case. Data points are arithmetic means for each product at each time point. Error bars have been removed for clarity; for variability estimates, refer to Tables 2, 4. Abbreviation: VAS, visual analog scale.
The mean maximum plasma nicotine concentration (Cmax) and area under the plasma nicotine concentration-time curve (AUC; the parameters AUC0–90, AUC0–240, and AUCt were derived) values were highest for usual brand cigarettes and lower for each of the iSENZIA™ HHS flavor variants (Table 1). Statistical analyses showed that Cmax and AUCt values for iSENZIA™ HHS Forest Berry and Summer Watermelon variants were significantly lower than for subjects’ usual brand cigarettes but not significantly different from one another (Supplementary Table S4).
TABLE 1
| Variable | Cmax (ng/mL) | AUC0–90 (ng*min/mL) | AUC0–240 (ng*min/mL) | AUCt (ng*min/mL) | Tmax (minutes) | |
|---|---|---|---|---|---|---|
| Study product | n | 25 | 25 | 25 | 25 | 25 |
| iSENZIA™ HHS Forest Berry | Geometric mean | 5.296 | 186.7 | 342.8 | 343.0 | NC |
| Geometric CV (%) | 53.9 | 36.4 | 36.9 | 36.9 | NC | |
| Mean | 6.0 | 197.8 | 363.7 | 363.9 | 6.47 | |
| SD | 3.28 | 65.95 | 123.73 | 123.83 | 2.319 | |
| Median | 4.912 | 196.3 | 358.7 | 358.8 | 6.033 | |
| Range | 1.99, 16.7 | 107, 314 | 193, 561 | 193, 561 | 4.02, 13.75 | |
| iSENZIA™ HHS Summer Watermelon | Geometric mean | 6.164 | 194.8 | 357.1 | 357.3 | NC |
| Geometric CV (%) | 51.3 | 38.9 | 39.2 | 39.2 | NC | |
| Mean | 6.87 | 208.3 | 382.2 | 382.5 | 6.46 | |
| SD | 3.24 | 78.45 | 144.94 | 144.95 | 2.240 | |
| Median | 6.247 | 178.3 | 372.7 | 373.5 | 6.067 | |
| Range | 2.43, 14.8 | 97.9, 407 | 172, 736 | 172, 736 | 3.98, 15.32 | |
| Usual brand cigarettes | Geometric mean | 16.44 | 620.6 | 1077 | 1078 | NC |
| Geometric CV (%) | 51.9 | 35.9 | 35.5 | 35.5 | NC | |
| Mean | 18.5 | 658.7 | 1143.0 | 1144.0 | 7.1 | |
| SD | 10.05 | 242.87 | 427.68 | 427.87 | 1.66 | |
| Median | 16.48 | 628.6 | 1039 | 1042 | 6.350 | |
| Range | 5.38, 51.1 | 317, 1330 | 616, 2200 | 617, 2210 | 4.02, 10.25 |
Baseline-adjusted plasma nicotine pharmacokinetic parameters following controlled product use in the outcomes population of the clinical study.
Abbreviations: AUC0–90 and AUC0–240, area under the plasma nicotine concentration-time curve from zero to 90 min and 240 min minutes, respectively; AUCt, area under the plasma nicotine concentration-time curve from zero to the time of the last measurable non-zero concentration; Cmax, maximum plasma nicotine concentration; CV, coefficient of variation; HHS, heated herbal system; n, number of observations used in the analysis; NC, not calculated; SD, standard deviation; Tmax, time of the maximum plasma nicotine concentration.
Among the iSENZIA™ HHS flavor variants, the median time taken to reach the maximum plasma nicotine concentration (Tmax; Table 1) was highest for subjects’ usual brand cigarettes (6.350 min), and marginally lower for the iSENZIA™ HHS Forest Berry (6.033 min) and Summer Watermelon (6.067 min). Median Tmax for subjects’ usual brand cigarettes was 6.250 min (Table 1). Statistical analyses of differences in Tmax between the study products showed that these were not statistically significant for any of the comparisons (Supplementary Table S5).
Subjective effects
The urge to smoke was assessed prior to study product use and at various time points after product use using a single-item questionnaire. Subjects responded to the question “Right now, how much would you like to smoke a cigarette?” by providing responses on a 100 mm visual analog scale (VAS) ranging from “Not at all” to “A great deal.” Mean baseline VAS scores ranged from 90.0 to 92.9 across all products. Use of all study products elicited robust reductions in urge to smoke (Figure 4), which were greater for subjects’ usual brand cigarettes (Figure 4). The mean maximum change in urge to smoke (Emax) was highest for subjects’ usual brand cigarettes (69.2 ± 28.38) and approximately 36%–38% lower for the iSENZIA™ HHS flavor variants (Table 2). Emax was not significantly different between the iSENZIA™ HHS flavor variants, but the differences between the iSENZIA™ HHS flavor variants and usual brand cigarettes were significant (Supplementary Table S6). The time of the maximum change in urge to smoke (TEmax) was highest for iSENZIA™ HHS Forest Berry, intermediate for iSENZIA™ HHS Summer Watermelon, and lowest for usual brand cigarettes (Table 2). Mean AUEC0–240 (area under the effect-time curve from zero to 240 min) was highest for subjects’ usual brand cigarettes, and significantly lower for both iSENZIA™ HHS flavor variants (Table 2; Supplementary Table S6). However, no statistically significant differences in AUEC0–240 were seen between the individual iSENZIA™ variants.
TABLE 2
|
Parameter |
n |
iSENZIA™ HHS Forest Berry | iSENZIA™ HHS Summer Watermelon | Usual brand cigarettes |
|---|---|---|---|---|
| 25 | 25 | 25 | ||
| Emax | Mean ± SD | 43.0 ± 31.70 | 44.3 ± 28.87 | 69.2 ± 28.38 |
| Median | 38.0 | 41.0 | 80.0 | |
| 95% CI | 29.9, 56.0 | 32.4, 56.2 | 57.5, 80.9 | |
| TEmax | Mean ± SD | 21.6 ± 47.62 | 14.1 ± 17.52 | 11.1 ± 12.93 |
| Median | 7.100 | 7.050 | 7.217 | |
| 95% CI | 1.92, 41.24 | 6.83, 21.29 | 5.71, 16.39 | |
| AUEC0–240 | Mean ± SD | 3369 ± 3720.2 | 3228 ± 3892.8 | 6083 ± 6037.4 |
| Median | 2444 | 2010 | 6579 | |
| 95% CI | 1833, 4905 | 1622, 4835 | 3591, 8575 |
Summary of urge to smoke parameters following controlled product use in the outcome population of the clinical study.
Abbreviations: AUEC0–240, area under the effect-time curve from zero to 240 min minutes; CI, confidential interval; Emax, maximum value of the difference between pre- and post-use; HHS, heated herbal system; n, number of observations; SD, standard deviation; TEmax, time of the maximum difference between pre- and post-use.
Product evaluation scale factor scores are presented in Table 3. On a scale of 1–7, with 1 being “not at all” and 7 being “extremely,” overall mean product evaluation scores for iSENZIA™ HHS Forest Berry and Summer Watermelon in the domain of satisfaction were 3.5 ± 1.61 and 3.7 ± 1.55, respectively, with a higher score of 5.8 ± 1.31 for subjects’ usual brand cigarettes. Similarly, in the domains of psychological reward, relief, ease of use, comfort using in public, and concerns about dependence, mean values were highest for subjects’ usual brand cigarettes and marginally lower, and comparable, for each of the iSENZIA™ HHS flavor variants (Table 3). For aversion, mean values were similar across all study products.
TABLE 3
|
Subscale |
n |
iSENZIA™ HHS Forest Berry | iSENZIA™ HHS Summer Watermelon | Usual brand cigarettes |
|---|---|---|---|---|
| 25 | 25 | 25 | ||
| Satisfaction | Mean ± SD | 3.5 ± 1.61 | 3.7 ± 1.55 | 5.8 ± 1.31 |
| Median | 3.3 | 3.5 | 6.0 | |
| 95% CI | 2.80, 4.13 | 3.02, 4.30 | 5.21, 6.29 | |
| Psychological reward | Mean ± SD | 2.9 ± 1.37 | 3.4 ± 1.30 | 5.2 ± 1.19 |
| Median | 2.80 | 3.20 | 5.00 | |
| 95% CI | 2.38, 3.51 | 2.85, 3.92 | 4.68, 5.67 | |
| Aversion | Mean ± SD | 1.3 ± 0.41 | 1.3 ± 0.70 | 1.4 ± 0.78 |
| Median | 1.00 | 1.00 | 1.30 | |
| 95% CI | 1.17, 1.50 | 1.04, 1.62 | 1.12, 1.76 | |
| Relief | Mean ± SD | 3.1 ± 1.43 | 3.3 ± 1.42 | 5.4 ± 1.23 |
| Median | 2.80 | 3.00 | 5.60 | |
| 95% CI | 2.48, 3.66 | 2.72, 3.89 | 4.86, 5.88 | |
| Was it easy to use? | Mean ± SD | 5.6 ± 1.58 | 6.0 ± 1.12 | 6.4 ± 1.04 |
| Median | 6.0 | 6.0 | 7.0 | |
| 95% CI | 4.9, 6.2 | 5.5, 6.5 | 5.9, 6.8 | |
| Comfortable using the product in public? | Mean ± SD | 5.3 ± 1.34 | 5.4 ± 1.58 | 6.2 ± 1.27 |
| Median | 5.0 | 6.0 | 7.0 | |
| 95% CI | 4.7, 5.8 | 4.7, 6.1 | 5.7, 6.8 | |
| Concerned you would become dependent? | Mean ± SD | 2.4 ± 1.61 | 2.2 ± 1.59 | 3.6 ± 2.12 |
| Median | 2.0 | 1.0 | 4.0 | |
| 95% CI | 1.7, 3.1 | 1.6, 2.9 | 2.7, 4.5 |
Product evaluation scale factor scores by study product in the outcome population of the clinical study.
Subscale scores in the domains of satisfaction (items 1, 2, 3, and 12), psychological reward (items 4, 5, 6, 7, and 8), aversion (items 9, 10, 16, and 18), and relief (items 11, 13, 14, 15, and reversed for 20) were generated from the individual items as described previously (Hatsukami et al., 2013). Abbreviations: CI, confidence intervals; HHS, heated herbal system; n, number of observations; SD, standard deviation.
Regarding intent to use the product again, 52% and 44% of subjects expressed positive likelihood (assessed as a rating on the VAS between the midpoint and “Extremely”) of buying and using the iSENZIA™ HHS Forest Berry and Summer Watermelon, respectively, compared with 76% of subjects who expressed positive likelihood of using their usual brand cigarettes again (Table 4). Overall, the mean raw VAS scores for intent to use the product again were 43.4 and 44.4 (0 = definitely would not and 100 = definitely would) for iSENZIA™ HHS Forest Berry and Summer Watermelon, respectively, with a higher mean score of 71.2 for subjects’ usual brand cigarettes.
TABLE 4
|
Bipolar score category |
Sex |
iSENZIA™ HHS Forest Berry | iSENZIA™ HHS Summer Watermelon | Usual brand cigarettes |
|---|---|---|---|---|
| 25 | 25 | 25 | ||
| −50 to <0 | Male | 8 (32%) | 8 (32%) | 3 (12%) |
| Female | 4 (16%) | 6 (24%) | 3 (12%) | |
| Overall | 12 (48%) | 14 (56%) | 6 (24%) | |
| 0 | Male | 0 (0%) | 0 (0%) | 0 (0%) |
| Female | 0 (0%) | 0 (0%) | 0 (0%) | |
| Overall | 0 (0%) | 0 (0%) | 0 (0%) | |
| >0 to 50 | Male | 7 (28%) | 7 (28%) | 12 (48%) |
| Female | 6 (24%) | 4 (16%) | 7 (28%) | |
| Overall | 13 (52%) | 11 (44%) | 19 (76%) |
Intent to use iSENZIA™ heated herbal systems (HHS) compared to usual brand cigarettes in the outcomes population of the clinical study.
Intent to use was measured using a 100-point visual analog scale (VAS), where 0 indicated “definitely would not use again” and 100 indicated “definitely would use again.” A positive likelihood of use was defined as a score above the midpoint (>50). The table includes the frequency of intent to use VAS, bipolar scores stratified by sex and product, and the percentage of subjects expressing intent to use. Abbreviations: HHS, heated herbal system.
Safety
There were no serious adverse events (SAEs) reported in this study, and no subjects discontinued due to adverse events (AEs). Overall, AEs were infrequently reported in this study, with AEs reported by five (20%) and three (12%) subjects associated with the use of iSENZIA™ HHS Forest Berry and Summer Watermelon, respectively. Mild dizziness was the most frequently reported AE, with eight (32%) subjects reporting dizziness events, including five (20%) subjects following use of their usual brand cigarettes, three (12%) subjects following iSENZIA™ HHS Summer Watermelon use, and two (8%) subjects following iSENZIA™ HHS Forest Berry use. Mild nausea was reported by two (8%) subjects following iSENZIA™ HHS Summer Watermelon use and one (4%) subject following either iSENZIA™ HHS Forest Berry or usual brand cigarette use.
Catheter site pain was reported three times by three (13%) subjects, and the remaining AEs (constipation, dizziness, and neck pain) were reported by one (4%) subject each. The constipation and dizziness events were moderate in severity, and the catheter site pain and neck pain events were mild. The investigator considered all AEs to be unlikely related or unrelated to the study product.
Mean vital signs (heart rate and blood pressure) remained within normal limits at all study time points, with minimal change from baseline. There were no individual clinically significant vital sign findings in this study.
Discussion
In this article, we describe the findings from a suite of pre-clinical and clinical studies assessing the iSENZIA™ HHS with two flavor variants. The HHS heats a non-tobacco, nicotine-containing tea-based substrate to produce an inhalable aerosol. Our main pre-clinical findings were that the iSENZIA™ HHS aerosols did not contain six of the WHO TobReg 9 analytes at levels above their respective limits of quantification. Those that were detectable were found at levels substantially lower than the levels found in 1R6F reference cigarette smoke, by up to approximately 99.8%. These aerosol toxicant reductions translated into significant reductions in biological activity in three toxicological assays assessing cytotoxicity, mutagenicity, and genotoxicity compared to 1R6F cigarette smoke. In a clinical study among adult cigarette smokers, both flavor variants of the iSENZIA™ HHS delivered nicotine and elicited subjective effects such as satisfaction, psychological reward, and relief, but to a lesser degree than subjects’ usual brand cigarettes. This is overall suggestive that the iSENZIA™ HHS may offer a harm-reduced, acceptable, and satisfying alternative to cigarettes among smokers with a lower abuse liability.
Our studies are the first to report aerosol chemistry and in vitro toxicology of an HHP, and as such, we cannot corroborate our findings with those of other studies in the literature. However, the pre-clinical study findings are consistent with those in the literature for HTPs, which similarly use electrical heating of consumable sticks but with a tobacco-based substrate. Similar to the iSENZIA™ HHS, the aerosol generated from HTPs contains either lower levels of, or an absence of, HPHCs and other toxicants when compared to cigarette smoke (Chapman et al., 2023a; Forster et al., 2018; Jaccard et al., 2018; Mallock et al., 2018; Farsalinos et al., 2018; Schaller J.-P. et al., 2016; Hashizume et al., 2023). For those toxicants present in HTP aerosols, the percentage reductions compared with reference cigarette smoke are comparable to those reported here for the iSENZIA™ HHS (Chapman et al., 2023a; Schaller J.-P. et al., 2016; Hashizume et al., 2023). Of particular importance, the very low level of CO in both the iSENZIA™ HHS and HTP aerosols is indicative of a lack of combustion during heating of both the tea- and the tobacco-based consumable sticks (Chapman et al., 2023a; Schaller J.-P. et al., 2016; Hashizume et al., 2023). More generally, the toxicant reductions common to both product types are indicative of the potential for reduced exposure among smokers switching to exclusive use of either product type, which has previously been demonstrated for HTPs in clinical biomarker studies (Gale et al., 2021a; Gale et al., 2021b; Gale et al., 2019; Gale et al., 2022; Haziza et al., 2020a; Haziza et al., 2016; Haziza et al., 2017; Lüdicke et al., 2018a). Perhaps of note regarding differences in toxicant levels between the iSENZIA™ HHS and HTP aerosols is the absence of tobacco-specific nitrosamines (TSNAs: NNN and NNK) in the iSENZIA™ HHS aerosol. TSNAs are present at low levels in aerosols from some HTPs (Jaccard et al., 2017; Chapman et al., 2023a; Forster et al., 2018; Schaller J.-P. et al., 2016; Hashizume et al., 2023), although these levels are much lower than those in cigarette smoke. Similarly, B[a]P, formaldehyde, 1,3-butadiene, and benzene were below their respective limits of quantification in iSENZIA™ HHS aerosols, but some or all of these toxicants have been reported at low levels in some HTP aerosols (Chapman et al., 2023a; Forster et al., 2018; Mallock et al., 2018; Farsalinos et al., 2018; Schaller J.-P. et al., 2016; Hashizume et al., 2023). The percentage reductions in acrolein and acetaldehyde in the iSENZIA™ HHS aerosols compared to reference cigarette smoke appear greater than the percentage reductions reported for both IQOS and a prototype HTP (Farsalinos et al., 2018; Schaller J.-P. et al., 2016; Hashizume et al., 2023). To summarize for iSENZIA™ HHS aerosol: Both HHS products deliver non-quantifiable amounts of six of nine priority compounds (NNN, NNK, BaP, 1,3-butadiene, benzene, and formaldehyde) in aerosol under the stated puffing conditions conditions. The remaining three substances of the TobReg 9 analytes (CO, acetaldehyde, and acrolein) could be quantified but are still reduced by >96% on a per-puff basis compared to the reference cigarette 1R6F. We acknowledge that only a limited number of analytes were presented here. A broader range of analytes, combined with non-targeted analysis of the aerosols, is required to fully characterize the chemistry of HHP aerosols and will form the basis of future publications.
The iSENZIA™ HHS aerosol toxicant reductions relative to 1R6F cigarette smoke resulted in reduced biological activity of the iSENZIA™ HHS whole aerosol when assessed using assays to detect potential cytotoxic, mutagenic, and genotoxic effects, which concurs with findings from in vitro toxicological studies assessing HTP aerosols (Chapman et al., 2023a; Schaller J.-P. et al., 2016; Hashizume et al., 2023; Keyser et al., 2024; Chapman et al., 2023b; Wang et al., 2021; Thorne et al., 2020; Thorne et al., 2018). This suggests that iSENZIA™ HHS use is potentially less harmful to users than cigarette smoking and provides initial evidence of its THR potential. While additional studies are required to confirm the risk reduction potential, it is notable that the reductions in aerosol toxicants and in vitro biological activity, described above for HTPs, which were either similar or lesser in magnitude than our findings for the iSENZIA™ HHS, led to reduced in vivo toxicological responses (Titz et al., 2020; Phillips et al., 2018; Titz et al., 2018; Kogel et al., 2016; Wong et al., 2016). As well as the reductions in biomarkers of exposure described above, clinical evidence from studies assessing the impact of exclusive switching to HTP use is also associated with favorable changes in a number of biomarkers of potential harm (Gale et al., 2022; Haziza et al., 2020b; Lüdicke et al., 2019; Lüdicke et al., 2018b). Overall, while further studies are required, our findings support the idea that, at the level of the individual smoker, the iSENZIA™ HHS has THR potential.
THR is based on the principle that the individual- and population-level harms associated with cigarette smoking can be reduced by providing adult smokers with smoking alternatives that deliver nicotine but with levels of HPHCs and other toxicants that are either reduced or absent (Institute of Medicine, 2001; Stratton et al., 2001). The pre-clinical study findings described above provide evidence of the potential for exposure reductions among smokers who fully switch to using the iSENZIA™ HHS, and therefore the potential for reduced human health impact compared to cigarette smoking. Our clinical study findings additionally suggest that adult smokers are likely to find the iSENZIA™ HHS to be an acceptable and satisfying alternative to cigarettes, which is an important determinant of whether a given product will support smokers, who would otherwise continue to smoke, to transition away from cigarette smoking. Two major components thought to contribute to acceptability are nicotine delivery and the elicitation of positive subjective effects such as satisfaction, reward, and relief from smoking urges and withdrawal symptoms; both factors were demonstrated in the clinical study. Overall, as suggested for other smoking alternatives such as HTPs and e-cigarettes, these attributes can contribute to the uptake of alternatives to cigarette smoking, such as the iSENZIA™ HHS, among adult smokers, its continued use, and the prevention of relapse back to cigarette smoking (Fearon, 2022; Goldenson et al., 2021; Hajek et al., 2020; Phillips-Waller et al., 2021a; Phillips-Waller et al., 2021b; Hughes, 2007; Lechner et al., 2018; Shiffman and Kirchner, 2009; West, 2017).
It is important to consider other factors that may determine the overall THR potential of smoking alternatives. Abuse liability, also termed dependence potential, is a multifactorial measure that provides an estimation of the likelihood that use of a tobacco product will give rise to dependence on its use. Abuse liability can be determined by considering findings from nicotine pharmacokinetic and subjective effect assessments, which together constitute an assessment of abuse liability (Fearon, 2022; Vansickel et al., 2022). The THR potential of novel nicotine and tobacco products is proposed to be maximized when appeal and abuse liability are high and when toxicity/harmfulness are low (Abrams et al., 2018). Novel products that possess at least some degree of abuse liability may be beneficial because this allows the novel product to compete with cigarettes and support transitioning away from smoking (Abrams et al., 2018; Fearon, 2022; Ashley et al., 2020; Cahn et al., 2021). However, high abuse liability may lead to unintended consequences because the novel product may pose an initiation or addiction risk among non-users of nicotine products, particularly among susceptible populations such as youth and young adults (Ashley et al., 2020; Cahn et al., 2021). In this regard, optimizing abuse liability may be regarded as a balancing act between maximizing uptake and exclusive use among the intended audience, that is, adult cigarette smokers, while minimizing use among those for whom use is not intended (never smokers, ex-smokers, and youth). Cigarettes have the highest abuse liability of any tobacco products due to their ability to rapidly and efficiently deliver nicotine into the blood of smokers and their elicitation of strong subjective effects, while forms of nicotine delivery systems that deliver nicotine more slowly and less substantially have a lower abuse liability (Abrams et al., 2018; Gottlieb and Zeller, 2017; Carter et al., 2009). We concluded from our clinical study findings that the iSENZIA™ HHS has a lower abuse liability than cigarettes because, although both flavor variants of the iSENZIA™ HHS robustly delivered nicotine and elicited positive subjective effects among users, the magnitudes of these effects were lower than those observed when subjects smoked their usual brand cigarettes. Whilst this should not be interpreted as suggesting that iSENZIA™ HHS use is not addictive, it is suggestive that its use will likely have lower positive reinforcing effects. In the context of population-level THR, this finding supports that while the iSENZIA™ HHS may be an acceptable alternative to cigarette smoking that is satisfying to adult smokers and therefore may support transitioning away from smoking, the lower abuse liability relative to cigarettes suggests that iSENZIA™ likely presents less of an initiation/addiction risk among non-users of nicotine. Overall, this potential generation of an “off-ramp” away from cigarette smoking, while minimizing the “on-ramp” to initiating tobacco/nicotine use, may enhance the contribution the iSENZIA™ HHS can make to THR strategies.
The results presented in this article should be considered in the context of several limitations. First, our studies only assessed a single type of HHP, and our data may not be extrapolated to other HHPs that may either use different types and compositions of non-tobacco substrates or heat via different means and temperatures. We also only assessed two of the available iSENZIA™ HHS flavor variants. Second, our pre-clinical studies generated aerosol from the iSENZIA™ HHS using a single puffing regimen, and this may not be indicative of puffing patterns of users in the real world. However, to date, no data are available concerning puffing topography among users of HHPs. Furthermore, our pre-clinical analytical chemistry and toxicology studies were limited to a small number of endpoints and further chemical and biological characterization of the iSENZIA™ HHS aerosol is necessary in order to fully understand the potential human health impact of iSENZIA™ HHS use, and human exposure and/or biological effects studies would also be informative in this regard. Third, our clinical study has a number of limitations, including that we only assessed nicotine pharmacokinetics and subjective effects profiles. While the findings from these studies support the conclusion that iSENZIA™ HHS use may provide an acceptable alternative to smoking cigarettes, no data were presented concerning its actual ability to help smokers transition away from cigarette smoking. Additionally, and similar to the pre-clinical limitation described earlier, the clinical study assessed the use of the iSENZIA™ HHS in a clinical laboratory environment using a controlled puffing regimen, and also at a single point in time with only a very brief familiarization period. While this experimental approach allows us to estimate the relative abuse liability of the iSENZIA™ HHS compared to cigarettes, absolute abuse liability in real-world settings may differ from that reported in the clinical study.
In conclusion, our pre-clinical studies provide evidence that the aerosol from the iSENZIA™ HHS is fundamentally different from cigarette smoke and contains significantly lower levels of HPHCs and NNN, all of which were mandated for lowering in cigarette smoke in the WHO TobReg proposal. These lower levels of toxicants give rise to a significantly reduced biological impact in a number of in vitro toxicological assays. Because the iSENZIA™ HHS delivered satisfactory levels of nicotine to users and generated positive subjective effects and reduced urges to smoke in a clinical study, it may provide a suitable alternative to smoking for adults who would otherwise continue to smoke. The abuse liability of the iSENZIA™ HHS is likely to be lower than that of cigarettes, which may help to limit its ability to facilitate initiation among non-users of tobacco and nicotine products. Overall, considering the totality of data presented, the iSENZIA™ HHS is a potentially reduced exposure and risk alternative to cigarettes that may play a meaningful role in THR by generating an “off-ramp” away from cigarette smoking for adult smokers while minimizing the “on-ramp” to initiation of tobacco and/or nicotine use. Further studies, however, must be conducted to fully understand the potential human health impact of iSENZIA™ HHS use and its contribution to THR. These studies should assess mechanistic toxicological impacts, broader clinical assessments reflecting real-world usage, and long-term behavioral outcomes.
Methods
Products assessed in pre-clinical and clinical studies
iSENZIA™ heated herbal system
The iSENZIA™ HHS, which comprises the PULZE™ 2.0 heating device and iSENZIA™ sticks (see Supplementary Figure S1), generates an inhalable aerosol by heating the sticks using an electrically powered heating device. Once switched on, the PULZE™ 2.0 heating device is operational for 5 min, which allows users to take approximately 10 puffs from a single iSENZIA™ stick. The device can be operated in two different user-selected modes (Intense and Mild modes) in which the device heats to temperatures below combustion. In the studies described in this article, the iSENZIA™ HHS was used in the Intense mode.
The iSENZIA™ sticks contain a portion of substrate that is tea-based and contains other components, including cellulose, nicotine, a binder, and a variety of flavor ingredients. Two flavored iSENZIA™ consumables (used with the PULZE™ 2.0 heating device) were assessed in the pre-clinical and clinical studies described: iSENZIA™ Forest Berry and iSENZIA™ Summer Watermelon. These flavors are released into the tea-based substrate prior to heating by squeezing a capsule (“crushball;” see Supplementary Figure S1) that contains the flavoring, as per the standard procedure and according to the study protocol. The tea-based substrate in each of the iSENZIA™ sticks had a target specification of 2.7 mg of nicotine per stick.
Cigarettes
The 1R6F reference cigarette was used as a comparator product in the pre-clinical analytical chemistry and in vitro toxicology studies (CTRP, 2023). These cigarettes were stored frozen and unopened in their original packaging until conditioned. In the clinical study, all subjects provided their usual brand of cigarette for use as a comparator product.
Pre-clinical studies
Emissions chemistry
Cigarette smoke and iSENZIA™ HHS aerosol generation
The 1R6F reference cigarettes were smoked on a rotary smoking machines (RM20D, RM 400A2, and RM 20H2, Borgwaldt (Körber), Hamburg, Germany) according to the ISO 20778:2018 smoking regimen (55 mL puff volume, 2-s puff duration, 30-s puff interval, bell-shaped puff profile, filter vents blocked). The iSENZIA™ HHS was used on a linear smoking machine (LM4C Borgwaldt (Körber), Hamburg, Germany) according to a modified version of ISO 20778:2018 (55 mL puff volume, 2-s puff duration, 30-s puff interval, bell-shaped puff profile, with no filter vent blocking). The filter vent must be blocked if there is a possibility that the vent could be covered while smoking. As iSENZIA™ HHS does not have ventilated filters, filter blocking was not required during testing. The aerosol was trapped on either a Cambridge filter pad in a gas collection bag (e.g., Tedlar bag) or an impinger using 2,4-Dinitrophenylhydrazine (DNPH) solution, depending on the targeted analytes.
Individual analytes assessed were those nine listed in the WHO TobReg proposal (Burns et al., 2008), plus aerosol-collected mass (ACM)/total particulate matter (TPM) and nicotine. To determine ACM and TPM, iSENZIA™ HHS whole aerosol or 1R6F reference cigarette whole smoke was trapped on a Cambridge filter pad. The mass of the filter pad, including the holder, was determined before and after use, and the mass of the collected matter per iSENZIA™ HHS stick and per 1R6F reference cigarette was deemed to be the ACM and TPM, respectively.
For the extraction and measurement of TSNAs, Cambridge filter pads (92 mm diameter) following machine smoking of 1R6F reference cigarettes were transferred to a 100-mL Erlenmeyer flask containing 40 mL of extraction solution (1:1 methanol: water, containing deuterated internal standard compounds). Cambridge filter pads (44 mm diameter) following machine use of the iSENZIA™ HHS were transferred to a 50-mL Erlenmeyer flask containing 20 mL of extraction solution. The flasks were shaken using an automated shaker for 60 min at ∼180 rpm. Following this extraction period, aliquots of the extraction solutions were membrane filtered into vials for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis to determine levels of NNN and NNK. For the high-performance liquid chromatography (HPLC), an Agilent 1290 system was used with a Gemini 3 µm C18 column (150 mm × 4.6 mm, 110 Å) (55°C), injection volume: 5 μL, flow rate: 800 µL/min. The eluent gradient was applied as follows: 0 min, 40% A (0.05% acetic acid in water):60% B (0.05% acetic acid in methanol); 4 min, 40% A:60% B; 5 min, 90% A:10% B; 6 min, 90% A:10% B. An API 6500 QTRAP (SCIEX, Framingham, MA, United States) was used for the MS/MS step: Turbo Spray ion source, ESI positive ionization mode; 4,500 V; 500°C; nebulizing gas pressure, 70 psi; turbo heater gas pressure, 70 psi; dwell time 60 ms. Multiple reaction monitoring (MRM) quantification was performed using NNN-d4 and NNK-d4 (purity >98%; TRC, ON, Canada).
For measurement of B[a]P, the Cambridge filter pads were transferred to 100 mL Erlenmeyer flasks and extracted for 20 min with 30 mL cyclohexane containing the internal standard, B[a]P-d12 (Dr. Ehrenstorfer, LGC). Aliquots of 15 mL were then reduced to a volume of 3 mL under reduced pressure using a Turbovap apparatus. The samples were cleaned using an NH2-Phase solid phase extraction (SPE) column with 500 mg of sorbent (Phenomenex, CA, United States), reduced to a final volume of 0.5 mL, and then analyzed using gas chromatography-mass spectroscopy (GC-MS). For the GC-MS, a Thermo Trace 1310 GC system with a Thermo TSQ 8000 Evo MS detector was used along with a J&W DB-17 ms GC-capillary (30 m, 0.25 mm, 0.25 µm). The following GC temperature program was applied: initial temperature: 120°C, hold for 1 min, ramp 30°C/min to 310°C, hold for 20 min. A flow rate of 1 mL/min (helium) was set; injector temperature: 300°C; injection volume: 1 µL splitless; MS source temperature: 300°C; transfer line temperature: 290°C. Detection mode: selected ion monitoring, B[a]P: 252.1, 250.1 and B[a]P-d12: 264.1, 260.1.
For carbonyl quantification, the unfiltered mainstream smoke/aerosol was guided through two impingers containing 35 mL 2,4-dinitrophenylhydrazine (DNPH) solution (>99.9% purity, Merck). Following smoking, the contents of the two impingers were combined and transferred to Erlenmeyer flasks. Here, the carbonyl derivatization reaction was carried out for 30 min, following which time aliquots were taken and stabilized using Trizma base solution (Sigma Aldrich, Germany) and membrane filtered prior to analysis via high-performance liquid chromatography with diode-array detection (HPLC-DAD). For this, an Agilent 1100 Series HPLC-DAD was used with an RP C18 column (125 Å, 150 mm × 4.6 mm, 3 µm) (Phenomenex, CA, United States); sample injection volume, 20 μL; column oven temperature, 40°C; flowrate, 1.2 mL/min; 365 nm detector. The eluent gradient was applied as follows: 0 min, 60% A (acetonitrile:tetrahydrofuran:isopropanol:H2O 30:10:1:59): 40% B (acetonitrile:H2O 65:35): 0% C (acetonitrile); 12 min, 60% A: 40% B: 0% C; 15 min, 60% A: 40% B: 0% C; 21 min, 0% A: 100% B: 0%C; 22.2 min, 0% A: 0% B: 100% C; 25.2 min, 0% A: 0% B: 100% C; 28.2 min, 100% A: 0% B: 0% C; 34.2 min, 100% A: 0% B: 0% C. For nicotine, the particulate phase of the HHS aerosol was trapped on a Cambridge filter pad, and the filter pad was extracted with propan-2-ol. An aliquot was analyzed using a GC-flame ionization detector (FID).
For the gas phase analytes, the vapor phase of the HHS aerosol was collected in a Tedlar bag located after the Cambridge filter pad. Vapor phases containing the volatiles selected for analysis were injected directly into a GC 7890 A (Agilent Technologies) with a DB 624 UI (60 m, 0.25 mm, 1.4 µm) column and an MS 5975 C detector (Agilent Technologies) with a Loop Filling Manager 205 (Teutner Analysentechnik GmbH, Germany). GC oven temperature: 40°C for 6 min, heating rate 20°C/minute, 230°C for 5 min; run time, 20.5 min. The injector temperature was 180°C; split, 1:20; constant flow rate, 1.5 mL/min, and the valve box temperature was 180°C. The MS source temperature was 230°C; MS Quad, 150°C; transfer line, 230°C; detection mode: selected ion monitoring. The sample (vapor phase) was separated by gas chromatography (GC), and selected gas phase compounds were quantified by mass spectrometry (MS). Identification of the single components was carried out using the retention time and their specific masses. For CO, the vapor phase of the aerosol was also collected after passing through a Cambridge filter pad. The CO content was determined using a calibrated non-dispersive infrared analyzer (NDIR).
Where appropriate, limits of quantification for the analytes were as follows: NNN, 5 ng; NNK, 5 ng; B[a]P, 2.5 ng; formaldehyde, 1.58 µg; 1,3-butadiene, 0.6 µg; acetaldehyde, 10.5 µg; acrolein, 2.63 µg; benzene, 0.27 µg. All values are per stick or per cigarette.
In Vitro toxicology
Cigarette smoke and iSENZIA™ HHS aerosol generation
Prior to the generation of cigarette smoke, the 1R6F reference cigarettes were conditioned according to ISO 3402:1999 (ISO 3402:1999, 1999), while the iSENZIA™ HHS sticks were not conditioned. For the NRU and IVM assays, the fresh smoke/aerosol was generated using the Smoke Aerosol Exposure In Vitro System (SAEIVS; Burghart Tabaktechnik, Wedel, Germany) (Wieczorek et al., 2023). Generation of both cigarette smoke and aerosol was performed in accordance with the ISO 20778:2018 smoking regimen (55 mL puff volume, 2-s puff duration, 30-s puff interval, bell-shaped puff profile) (ISO 20778:2018, 2018). For the Ames assay, bacterial cultures were exposed to the whole smoke/aerosol using a Smoking Robot VC 10-S (Vitrocell Systems GmbH, Waldkirch, Germany). Generation of fresh smoke from 1R6F reference cigarettes (CTRP, 2023) was performed according to the ISO3308:2012 smoking regimen (35 mL puff volume, 2 s puff duration, 60 s puff interval, bell-shaped puff profile) (International Organization for Standardization, 2012) while for the iSENZIA™ HHS, ISO 20778:2018 (ISO 20778:2018, 2018) without vent blocking was used to generate the aerosol.
Neutral red uptake cytotoxicity assay
Cytotoxicity of the whole smoke/aerosols was measured using the NRU assay with Beas-2B human bronchial epithelial cells (ECACC Catalogue No. 95102433). Cell stocks were stored in liquid nitrogen until use, and cultures were checked for the absence of mycoplasma. The Beas-2B cells were cultured in Epithelial Cell Growth Medium (Promocell #C-21060) with Supplement Mix (Promocell #C-39165) added. Only cells between 3 and 20 passages after thawing were used for the experiments. Prior to whole smoke/aerosol exposure, 100 μL of cell suspension was seeded at a cell density of 0.5 × 104 cells/mL into the inner 60 wells of 96-well round-bottomed collagen I-coated plates and incubated at 37°C and 5% CO2 for 20 ± 3 h. The collagen I-coated plates were prepared by adding 25 μL of collagen I solution (20% PureCol® EZ Gel, 2% 1 M HEPES buffer, and 78% cell culture medium; final collagen I concentration, 0.1%) to each well of the 96-well plate. Directly prior to exposure, medium was removed from the cells by suction and reverse plate centrifugation for 10 s at 70 × g to guarantee complete and homogenous removal of medium from the wells for the ALI exposures. Cells were then exposed at the ALI to increasing puff numbers of fresh whole smoke/aerosol using the SAEIVS according to the puffing regimens described above. The final exposure range for the positive control of 1R6F smoke (diluted either 1:15 or 1:20 with air) was 0–0.8 puffs (corrected for dilutions) and 0–20 puffs for the test item HHP aerosols (undiluted). Cells were exposed for not longer than 15 min, and following exposure, 200 μL of fresh medium was added to each well and cells were incubated for 65 ± 2 h. Following this incubation period, the incubation medium was replaced by 200 μL fresh medium containing neutral red dye for 3 h, during which time the dye was taken up by viable cells. After washing and lysing of the cells, absorbance (540 nm) in the wells was then measured using a Tecan Sunrise plate reader, with absorbance directly proportional to the number of live cells present. From the absorbance values, the mean relative cell viability compared to the values measured for control wells (0 puffs of smoke/aerosol) was calculated for each test concentration. Three replicates were performed on different days. Each day, two 96-well multiwell plates were exposed, each with six wells exposed to the same number of pulls.
Ames bacterial reverse mutation genotoxicity assay
Mutagenic potential was determined using the in vitro bacterial reverse mutation test (Ames assay) with Salmonella Typhimurium strains TA98 and TA100 (+/−S9; Trinova Biochem GmbH, Giessen, Germany) in compliance with the Organisation for Economic Co-operation and Development (OECD) Test Guideline 471 (Organisation for Economic Co-operation and Development, 2020). Nutrient broth No. 2 (OXOID) cultures were prepared by inoculating 40 mL of medium with 0.7 mL of 6 h pre-culture in a 100-mL Erlenmeyer flask. These were incubated overnight at 37°C and shaken at 120 rpm. Following this, the respective bacterial suspensions were pooled into 120 mL suspensions (3 × 40 mL) and centrifuged at 1800 × g for 10 min. The supernatant medium was removed, and cells were resuspended in 12 mL of Dulbecco’s Ca2+- and Mg2+-free PBS (DPBS). A 10-mL sample of this suspension was added to a glass tube, which was inserted into an impinger connected to the Vitrocell VC 10-S Smoking Robot. Exposure to the test article smoke/aerosols was carried out at room temperature and protected from direct light.
The bacterial suspensions were exposed to increasing puff numbers of undiluted fresh whole smoke/aerosol. The final exposure range for the 1R6F smoke was 70 puffs, and it was 120–200 puffs for the HHS aerosols. During exposure, 350 μL of bacterial suspension was taken at regular intervals. For each repeat (Petri dish), 50 μL of the suspension was added to sterile 15 mL tubes, followed by 0.5 mL of 5% S9 mix or 0.5 mL 0.2 M phosphate buffer, followed by 2 mL of top agar (45°C). This mixture was then poured onto Vogel–Bonner agar plates (3 plates +S9, three plates −S9 per biological replicate), and the top agar was distributed by tilting/rotating. When the top agar solidified, plates were inverted and incubated at 37°C for 48 h. Following this, the total number of revertant colonies per plate was counted automatically using the Synbiosis ProtoCOL SR Automatic Colony Counter (Meintrup-DWS). Validity of the results was checked against the following criteria: mean negative control counts fell within the historical range, positive controls (the smoke of 1R6F research cigarette) induced clear increases in revertant colonies (+/−S9), no more than 5% of plates were lost due to contamination/other unforeseen circumstances. Any observed toxic effects (pinpoint colonies or reduction of revertant number) were excluded from the analysis. For each test item, two biological replicates (independent smoking sessions) were set up, each with three technical replicates per defined number of puffs.
In Vitro micronucleus genotoxicity assay
These assays were carried out in accordance with OECD Test Guideline 487 (Organisation for Economic Co-operation and Development, 2023) with the exception that only long-term experiments in the absence of S9 and short-term experiments in the presence of S9 as the most relevant were conducted. Due to technical limitations, a short-term treatment in the absence of S9 was not applicable in parallel. V79 Chinese hamster lung fibroblast cell (ECACC 86041102) stocks were stored in liquid nitrogen until use, and cultures were checked to confirm the absence of mycoplasma. Only cells between 3 and 20 passages after thawing were used for the experiments. Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. Prior to exposure, 400 μL of the medium was added to each well of a 24-well plate, and polycarbonate Transwell inserts (0.4-μm pore membrane; 140620, Nunc) were added to these. Aliquots (250 μL) of V79 cell suspension were seeded at a density of 1 × 105 cells/mL into each insert, and then the plates were incubated at 37°C and 5% CO2 for 20 ± 2 h. Directly prior to whole smoke/aerosol exposure, the inserts were transferred to fresh plates containing 250 μL of medium per well supplemented with HEPES buffer (final concentration, 20 mM), and the apical medium was removed. Whole smoke/aerosol exposures were subsequently carried out at the ALI, and cells were exposed to increasing puff numbers of whole smoke (diluted) or aerosol (undiluted), achieved using the sliding plate cover within the SAEIVS exposure chamber. The exposure range for the (Whole iSENZIA™ HHS aerosol was 0–30 puffs (+/-S9) (iSENZIA™ Forest Berry) and 0–40 puffs (iSENZIA™ Summer Watermelon) without dilution. For the 1R6F smoke, 0 to 1.11 puffs (−/+S9) (corrected for dilution of 1:9 with air) were applied.
Exposures were no longer than 20 min. Based on toxicity values, an adaptation of puff numbers from 20 to 40 puffs was applied in the replicate test for iSENZIA™ Summer Watermelon. For iSENZIA™ Forest Berry, the puff number of 30 showed good toxicity results in both replicate tests. Following exposure, inserts were transferred to plates containing 400 μL of fresh basal medium. For metabolic activation of whole smoke/aerosol components, S9 mix containing an S9 fraction derived from Aroclor 1254-treated male Sprague–Dawley rats (10% v/v S9 fraction, 90% v/v REGENSYS A, 1.3 mM NADP; Trinova Biochem GmbH) was added to the medium (final concentration, 1% S9), and 175 μL of this added to each insert immediately following exposure. Following a 3-h incubation, the S9 mix was removed from the cells, and 175 μL fresh medium was added. Cells were then incubated for a 20 ± 2 h recovery period. For exposed cultures without metabolic activation, 175 μL of fresh medium was added into the inserts immediately following exposure, and cells were incubated for 20 ± 2 h.
Following this incubation period, cells were detached from the inserts using Accutase® and counted using the Scepter™ Cell Counter (Millipore) to determine cell density for microscope slide preparation and cell counts for relative cell count (RCC), relative population doubling (RPD, used as measure for the assessment), and relative increase in cell count (RICC) calculation to assess treatment-induced toxicity. Cell suspensions were fixed to slides by spinning at 380 × g for 5 min using a cytospin and applying further spin cycles for drying. Fixative solution was then applied to the slides (methanol:glacial acetic acid:37% formaldehyde:water 150:18.5:1:30.5), followed by one rinse in methanol, and slides were allowed to air dry. Prior to slide analysis, cells were stained with 1 μg/mL DAPI in mounting medium (VECTASHIELD, H-1000). Slides were analyzed using the automated Metafer system with a Z2 microscope (Zeiss). In each of the two tests performed per test condition, two replicate preparations per dose level with at least 1,000 cells each were evaluated for the occurrence of micronuclei (MN). That is, in sum more than 2,000 cells per dose level were evaluated using pre-programmed Metafer parameters (Metafer version 3.14.2), based on the criteria described previously (Chapman et al., 2023a; Fenech, 1993). For each test article, two biological replicates, each with two technical replicates were carried out for each of the +/−S9 treatments.
Statistical analyses
Typical dose–response curves for the neutral red uptake cytotoxicity assay follow a symmetrical sigmoidal shape and the effective concentration IC50 is defined as the concentration that causes a response halfway between minimum and maximum response. Thus, the IC50 describes the test substance concentration needed to achieve a 50% growth inhibition. The statistical evaluation and determination of the IC50 were carried out with the software GraphPad Prism 8.4.3. Based on the basal and maximum response, as well as the Hill slope, a nonlinear regression model calculates the IC50 value with the associated confidence intervals. The Hill slope describes the steepness of the curve and is calculated individually for each data set.
The Ames assay was considered valid if the mean negative control colony count fell within the normal/historical range and the positive control induced a clear increase in revertant numbers, confirming the sensitivity of the assay and the metabolic activity of S9 preparation. If the validity criteria were met, the test article was considered to be mutagenic if:
1. It produces a two-fold increase in the number of induced revertants compared to the negative control (not-exposed bacteria suspension)
2. Revertant numbers of three or more of the test substance concentrations are significantly higher than the negative control
3. Linear dose-response was observed. This would provide stronger evidence of mutagenic activity
4. The positive responses described above were reproducible
The analysis was performed using the statistical software GraphPad Prism version 8.4.3. In case of results with positive slope in non-threshold model and Dunnett’s test (P value < 0.05), the tests were repeated. A test substance is deemed mutagenic if the effect is confirmed in two replicates.
The micronucleus values obtained by the different test article concentrations were compared pairwise to those from the corresponding negative controls using chi-square analysis for a qualitative analysis. A Cochran–Armitage trend test was performed to check for a dose-response relationship. Criteria to consider a result positive were: 1. A reproducible, dose-dependent increase in MN frequencies should be obtained; 2. The increase should reach statistical significance compared to the study’s negative control and the lab’s historical negative control; 3. The toxicity associated with the statistically significant increase should not exceed 60%. For a reproducible positive response, the quantitative determination of the effective dose necessary to reach the 3-fold increase in micronuclei over background frequencies was performed (ECMN3) on a “per-puff” basis. That is, the higher the ECMN3, the lower the genotoxic potential. For the calculation of the ECMN3, the historical background frequency was subtracted from the single raw data, and a nonlinear regression was applied. According to guidelines, MN values associated with toxicities above 60% were not considered for the qualitative assessment. However, in case of a positive result, MN data associated with toxicities >60% were included for the quantitative assessment to ensure the best curve fit and ECMN3 calculation.
Clinical study
Overall study design
This open-label, randomized, crossover, clinical study was designed to assess nicotine pharmacokinetics and subjective effects among cigarette smokers using the iSENZIA™ HHS. The study received a favorable opinion (equivalent to an ethics approval) from the Office for Research Ethics Committees Northern Ireland (ORECNI) Health and Social Care Research Ethics Committee A (reference number 23-NI-0099) prior to study commencement. The study was performed in accordance with ethical principles set forth in the Declaration of Helsinki and was compliant with the principles and requirements of International Council for Harmonisation (ICH) E6 (R2) guidelines for Good Clinical Practice (GCP), the European Union Clinical Trials Directive, and applicable local regulatory requirements. The study was performed at a single clinical site in Belfast, Northern Ireland (United Kingdom) and was registered in the ClinicalTrials.gov repository (NCT06093659). Twenty-five (25) male and female healthy adult smokers participated in this study. Subjects attended the clinic site on two separate occasions: a screening visit and a 6-day confinement period (Supplementary Figure S2). After completing the study, a follow-up telephone call was conducted for all subjects no longer than 1 week after the end of their confinement period. All subjects provided written informed consent prior to the commencement of any study procedures, including screening assessments.
Study subjects
In total, 67 subjects were screened for participation in this study; 42 subjects either did not meet screening criteria (inclusion and exclusion criteria detailed below) or were not needed for study participation (i.e., they were study reserves but were not required to take part in the study). A total of 25 subjects were enrolled, and all subjects completed the study.
Inclusion criteria were that subjects were healthy adults aged 21–65 years inclusive at screening; self-reported smoking at least 10 manufactured combustible (menthol or non-menthol) cigarettes per day (CPD) for at least 12 months prior to screening; had a urine cotinine ≥500 ng/mL at screening; had an exhaled carbon monoxide (eCO) level >10 parts per million (ppm) at screening; if female and of childbearing potential, were using at least one approved form of contraception; if female and of non-childbearing potential, had undergone a sterilization procedure at least 6 months prior to check-in or was postmenopausal with amenorrhea (verified by measuring follicle-stimulating hormone (FSH) levels) for at least 1 year prior to check-in; if a non-vasectomized male, agreed to use a condom with spermicide or abstain from intercourse for duration of the study and extending up to 90 days post-study; if male, agreed not to donate sperm for duration of the study and extending up to 90 days post-study; was willing comply with the requirements of the study, including a willingness to use the study HHP; and provided voluntary consent to participate in this study, which was documented by signing of the signed informed consent form.
Subjects were not allowed to enter the study if any exclusion criteria were met. The main exclusion criteria were a history or presence of clinically significant disease or disorder that, in the opinion of the investigator, would have jeopardized the safety of the subject or impacted the validity of the study results; had a clinically significant abnormal finding on the physical examination, medical history, vital signs, electrocardiogram, or clinical laboratory results; had an acute illness (e.g., upper respiratory tract infection, viral infection) requiring treatment within 14 days prior to check-in; systolic blood pressure (BP) < 90 mmHg or >150 mmHg, diastolic BP < 40 mmHg or >95 mmHg, or heart rate (HR) < 40 bpm or >99 bpm at screening; estimated creatinine clearance (using the Cockcroft Gault equation) < 70 mL/min at screening; used medications known to interact with cytochrome P450 2A6 within 3 months prior to check-in and throughout the study; used inhalers to treat any medical condition within 3 months prior to check-in and throughout the study; used prescription or over-the-counter bronchodilator medication (e.g., inhaled or oral β-agonists) for treatment of any illness within 12 months prior to check-in and throughout the study; was allergic to or could not tolerate flavoring agents used in any of the study products; had used any prescription smoking cessation treatments, including, but not limited to, varenicline (Chantix®) or bupropion (Zyban®) within 3 months prior to check-in; was planning to quit smoking during the study or within the next 3 months or was postponing a quit attempt in order to participate in the study; or had donated blood or blood products (including plasma), had significant blood loss, or received whole blood or a blood product transfusion within 90 days prior to check-in.
Randomization
Subjects who completed the study screening assessments were assigned a unique randomization identification number. Subsequently, each subject, based on the identification number, was assigned to use the study products according to one of five product sequences with five subjects per sequence. Randomization sequences were prepared by Celerion, Inc.
Study procedures
This study was a randomized, open-label, crossover, confinement study with 25 male and female cigarette smokers. The study assessed five test products and a cigarette comparator and evaluated nicotine pharmacokinetics, subjective effects, puffing topography, and product safety. Of the five test products, data regarding two HTP products assessed are not described in this article. An overview of the study design is presented in Supplementary Figure S2.
At Visit 1 (screening), which took place within 28 days prior to study procedures on Day −1, subjects underwent numerous assessments to check their eligibility to participate in the study, to review their health status, and to assess their nicotine consumption habits. Screening procedures included a physical examination (including oral cavity and oropharynx), vital signs, electrocardiogram, body mass index (BMI), clinical laboratory tests (hematology, serum chemistry, and urinalysis), serology, urine/saliva drugs of abuse screen, urine/breath alcohol, cotinine screen, eCO, and pregnancy and FSH tests (for females as appropriate). If requested, subjects were offered smoking cessation advice and contact information for a smoking cessation support service.
Visit 2 was a 6-day confinement period. subjects who successfully completed the screening procedures and met all the inclusion criteria and none of the exclusion criteria were eligible to check-in to the clinical site for the confinement period. Subjects checked into the clinic on Day −1 and remained at the clinic until Day 5 for daily study product use, nicotine pharmacokinetics sampling, subjective questionnaire assessments, puffing topography assessments, and safety assessments. On Day −1, following eligibility confirmation, subjects undertook a familiarization session with the study products and the questionnaires. The site’s clinical team explained how the iSENZIA™ HHS stick was to be used. Subjects had the opportunity to see the products/devices and packaging and participated in a product trial in which they took five puffs on a single iSENZIA™ HHS stick of a flavor of their own choosing. An explanation of how the questionnaires were to be administered to the subjects was given. After the familiarization session and completion of check-in procedures, subjects were allowed to smoke their own cigarettes ad libitum but abstained from the use of any tobacco- or nicotine-containing products for at least 12 h prior to the start of the controlled product use session on the morning of Day 1. In the morning of Day 1, after pre-use assessments and confirmation of eligibility, the subjects were randomized to one of the five product sequences and then provided a single product of the study product in the sequence to which they had been randomized. On Days 1 through 5, subjects used the assigned study product under controlled conditions (i.e., completely used a single iSENZIA™ HHS stick or smoked a single cigarette), with puffs taken at 30-s intervals and puffs 3 s in duration. Blood samples for nicotine assessment were collected 5 min prior to initiating product use and at 2 min, 4 min, 6 min, 8 min, 10 min, 15 min, 30 min, 45 min, 60 min, 120 min, and 240 min following the start of study product use. Subjective effects questionnaires were administered to the subjects at defined intervals throughout the day to assess the subjects’ urge to smoke, intent to use, and product evaluation. The Urge to Smoke questionnaire was administered 10 min before product use (Time −10), and at 4 min, 8 min, 15 min, 45 min, 60 min, 120 min, and 240 min post-use. Intent to Use and Product Evaluation questionnaires were administered at the 240-min time point. When coinciding with a blood draw, the Urge to Smoke questionnaire was administered approximately 30 s prior to the blood draw. All other questionnaires were completed within approximately 2 min following the final scheduled blood draw at 240 min. Safety was also monitored throughout the day. For all subjects, meals and snacks were provided at the appropriate times during confinement at the clinic site. Each meal and/or snack served at the site was standardized, similar in caloric content and composition, and taken at approximately the same time on each day. When confined at the clinic site, subjects were required to fast from all food and drink except water between meals and snacks.
On Days 1 through 5, following the 4-h pharmacokinetic blood collection period, subjects started a 4-h ad libitum product use session (no limits on cigarette or HHS stick consumption) with the same study product as that used during the morning controlled use session. Puffing topography assessments were made at this time but are not reported in this manuscript, and no blood samples were taken in this period for nicotine pharmacokinetic analyses. After completion of the ad libitum use session, subjects were allowed to smoke their own cigarettes ad libitum until at least 12 h prior to the start of the morning controlled product use session scheduled on the following day. On Day 5, following completion of study assessments, subjects were allowed to smoke their own cigarettes. They left the clinic after completing all final check-out requirements.
A follow-up telephone call (Visit 3) was made by the clinic to contact all subjects using their standard procedures approximately 7 days after the final product use to determine if any adverse events (AEs) had occurred since the last study visit.
Nicotine pharmacokinetics
To determine blood plasma nicotine concentrations during and after use of the study products, blood samples (approximately 4 mL) were collected either by direct venipuncture or through an indwelling venous catheter at the time points described above. Blood samples were drawn into dipotassium ethylenediaminetetraacetic acid (K2EDTA) vacutainer tubes, and the plasma fraction was separated off by centrifugation and pipetting. Plasma nicotine was analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) at Celerion Bioanalytical Services (Lincoln, Nebraska, United States) using a validated analytical method with appropriate quality controls according to the Food and Drug Administration (FDA) Guidance for Industry (Title 21 Code of Federal Regulations Part 58). Sample processing was completed by a non-tobacco user. The lower limit of quantification of plasma nicotine using the analytical method was 0.2 ng/mL.
Subjective effect assessments
The Intent to Use (100-mm visual analog scale (VAS)), Urge to Smoke (VAS), and Product Evaluation Scale (PES; 7-point scale; Hatsukami et al., 2013) questionnaires were completed using a Veeva ePro computerized tablet device (Veeva, Pleasanton, CA, United States). All relevant software and staff training specific to the electronic questionnaires were provided by Celerion, Inc. Any electronic device used met all regulatory requirements, including FDA 21 CFR Part 11. The Urge to Smoke questionnaire collected subjects’ responses to the question “Right now, how much would you like to smoke a cigarette?” with responses ranging from “Not at all” (0) to “A great deal” (100). Responses were collected at Time 0 (pre-product use) and at 4 min, 8 min, 15 min, 45 min, 60 min, 120 min, and 240 min relative to the start of product use on Days 1–5. The Intent to Use questionnaire collected subjects’ responses to the question “If available, how likely are you to buy your assigned study product in the future?” with responses ranging from “Not at all” (0) to “Extremely” (100). Responses were collected at 240 min following the start of study product use on Days 1–5. The 21-item PES questionnaire was completed at 240 min following the start of study product use on each of Days 1–5. PES subscale scores in the domains of satisfaction (items 1, 2, 3, and 12), psychological reward (items 4, 5, 6, 7, and 8), aversion (items 9, 10, 16, and 18), and relief (items 11, 13, 14, 15, and reversed for 20), were generated from the individual items as described previously (Hatsukami et al., 2013). Items 17, 19, and 21 were summarized as individual item scores.
Statistical analyses
The safety population comprised all subjects who successfully completed eligibility requirements after checking in to the clinic site and used at least one study product. The outcome population was a subset of the safety population and consisted of subjects who used a study product and had evaluable nicotine pharmacokinetics or subjective effect data. This population was used in the summary and analysis of all data presented in this article.
Due to this being the first study to assess the nicotine pharmacokinetics and subjective effects of the PULZE™ 2.0 heated device used with the iSENZIA™ consumables, no sample size calculations could be performed. However, a sample size of 25 subjects was deemed adequate to meet the study objectives, and this is in line with similar HTP nicotine pharmacokinetic study designs in the literature (e.g., Phillips-Waller et al., 2021a; Maloney et al., 2020; Picavet et al., 2016; McDermott et al., 2023).
Demographics
Descriptive statistics are reported for continuous variables (age, weight, height, and BMI), and frequency counts were tabulated for categorical demographic variables (sex, ethnicity, and race). Descriptive statistics are also provided for smoking history variables (cigarettes smoked per day and number of years smoking).
Nicotine pharmacokinetics
Unadjusted plasma nicotine concentrations that were below the limit of quantitation (BLQ) were set to one-half of the lower limit of quantitation (LLOQ) for the calculation of descriptive statistics. Individual plasma nicotine concentrations were adjusted for baseline nicotine levels (“baseline-adjusted”), and all pharmacokinetic parameters were calculated based on the adjusted concentrations. Baseline adjustment was performed by subtraction of the pre-existing nicotine concentration from each nicotine concentration obtained after test product administration in that period/day for each subject using the following equation:where Ct is the adjusted concentration at time t, Ct unadjusted is the observed concentration at time t, C0 is the pre-product use concentration (−5 min), Kel = , t½ is 2 h (approximate nicotine half-life), t is the actual sampling time since product administration, and t1 is the actual sampling time since the time of the pre-product use sample. After correction for pre-product use values, negative values were assigned a value of zero, and all other values obtained were reported as is, even if these values were BLQ.
SAS® software (Version 9.4) was used for data presentation and summarization, including descriptive statistics, statistical analyses, summary tables, graphs, and data listings. Descriptive statistics were generated for plasma nicotine concentrations and nicotine pharmacokinetic parameters by study product for all subjects, including sample size (n), arithmetic mean (mean), SD, coefficient of variation (CV%), standard error of the mean (SEM), minimum, median, and maximum at each nominal time point. In addition, geometric mean and geometric CV% are provided for the Cmax (maximum plasma nicotine concentration) and AUC (area under the plasma nicotine concentration-time curve) parameters. Mean concentration–time profiles are presented on linear scales. Missing data were treated as missing, and no imputation was conducted.
A linear mixed-effects model for analysis of variance (ANOVA) was performed on the natural log-transformed pharmacokinetic parameters Cmax and AUCt following the morning product use session on each of Days 1–5. The model included sequence, product, and study period as fixed effects and subject-nested-within-sequence as a random effect. Geometric least-squares means (LSMs) and 95% intervals (CIs) are provided for the pharmacokinetic parameters Cmax and AUCt by study product. Geometric LSM ratios, 95% CIs of the geometric LSM ratios, and p-values are provided for the comparisons of Cmax and AUCt. The comparisons of interest included each of the products compared to each other. These statistical analyses were performed using SAS® PROC MIXED.
A non-parametric analysis (Wilcoxon signed rank test) was performed for the comparisons of Tmax between each of the study products. The Hodges–Lehman estimate median difference and 95% CI of the difference are presented for each comparison. The CIs were constructed using Walsh averages and the appropriate quantile of the Wilcoxon signed rank test statistic. Tmax was not log-transformed for these analyses.
Subjective effects
Urge to smoke
The derived parameters Emax, TEmax, and AUEC0–240 were listed by subject and summarized by product using descriptive statistics, including n, mean, SD, CV%, SEM, minimum, Q1, median, Q3, maximum, and 95% CI. A linear mixed-effects model for ANOVA was used to compare urge to smoke parameters without data transformation; the model includes product sequence, period, and product as fixed effects and subject-nested-within-sequence as a random effect. LSM and 95% CIs are provided for Emax and AUEC0–240 by study product. LSM differences, 95% CIs of the LSM difference, and p-values are provided for the product comparisons for Emax and AUEC0–240. The comparisons of interest included each of the products compared to each other. These statistical analyses were performed using SAS® PROC MIXED.
Product evaluation scale
Descriptive statistics for the composite (satisfaction, psychological reward, aversion, and relief) and individual (ease of use, comfort using in public, and dependence concerns) PES factor scores (Hatsukami et al., 2013) are summarized by study product.
Intent to use
Descriptive statistics for the VAS raw score and bipolar score are summarized for each study product. A frequency count table is presented for the categories of the bipolar scores. Bipolar scores were calculated by subtracting 50 from the original VAS score, then categorizing into three categories: −50 to < 0, 0, and > 0 to 50.
Safety assessments
Safety was monitored through physical examination (symptom-driven), vital signs measurements, electrocardiograms, and clinical laboratory tests (serum chemistry, hematology, and urinalysis). Adverse event information was also collected throughout the study. Adverse events (including SAEs) were recorded from the start of the first product used until the end-of-study telephone call. Severity/intensity were graded as mild, moderate, or severe, and AEs were also assessed as unlikely, possibly, or probably related to the study product by the investigator.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Office for Research Ethics Committees Northern Ireland (ORECNI). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
A-FM-L: Conceptualization, Investigation, Project administration, Writing – review and editing. IF: Methodology, Writing – original draft. MS: Conceptualization, Formal Analysis, Investigation, Project administration, Resources, Supervision, Visualization, Writing – review and editing. TA: Project administration, Validation, Writing – review and editing. FC: Formal Analysis, Writing – review and editing. ES: Data curation, Formal Analysis, Investigation, Methodology, Writing – review and editing. RW: Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing – review and editing. SP: Data curation, Formal Analysis, Investigation, Methodology, Writing – review and editing. OD: Data curation, Investigation, Methodology, Supervision, Writing – review and editing. OK: Conceptualization, Writing – review and editing. MB: Formal Analysis, Investigation, Validation, Visualization, Writing – review and editing. LS: Conceptualization, Funding acquisition, Writing – review and editing. TN: Conceptualization, Funding acquisition, Resources, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank Celerion (Belfast, United Kingdom) for conducting the clinical study and the LabNetwork of Reemtsma Cigarettenfabriken GmbH, a subsidiary of Imperial Brands PLC. The authors would also like to thank the internal Imperial Brands PLC Reading Committee for their critical review of the manuscript.
Conflict of interest
AML, MS, TA, FC, OK, MB, and LS were employed by Imperial Brands PLC. IF is the Director of WhatIF? Consulting Ltd and an independent consultant to tobacco and nicotine product manufacturers, including Imperial Brands PLC, contract research organizations, and pharmaceutical companies, to provide scientific support for clinical and behavioural studies and general regulatory support. IF is also a retained Scientific Advisory Board member of Qnovia, LLC, platform pharmaceutical company exploring the use of a combination nicotine replacement therapy drug product for smoking cessation, and a Non-Executive Director of Advanced Inhalation Rituals, a global manufacturer of shisha tobacco products. IF holds shares in British American Tobacco p.l.c. Authors ETS, RW, SP, OD, and TN were employed by Reemtsma Cigarettenfabriken GmbH, An Imperial Brands PLC company, Hamburg, Germany.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ftox.2025.1589480/full#supplementary-material
References
1
Abrams D. B. Glasser A. M. Villanti A. C. Pearson J. L. Rose S. Niaura R. S. (2018). Managing nicotine without smoke to save lives now: evidence for harm minimization. Prev. Med.117, 88–97. 10.1016/j.ypmed.2018.06.010
2
Ashley D. L. Spears C. A. Weaver S. R. Huang J. Eriksen M. P. (2020). E-cigarettes: how can they help smokers quit without addicting a new generation?Prev. Med.140, 106145. 10.1016/j.ypmed.2020.106145
3
Babb S. Malarcher A. Schauer G. Asman K. Jamal A. (2017). Quitting smoking among adults - United States, 2000-2015. MMWR Morb. Mortal. Wkly. Rep.65, 1457–1464. 10.15585/mmwr.mm6552a1
4
Beaglehole R. Bonita R. (2024). Harnessing tobacco harm reduction. Lancet403, 512–514. 10.1016/S0140-6736(24)00140-5
5
Burns D. M. Dybing E. Gray N. Hecht S. Anderson C. Sanner T. et al (2008). Mandated lowering of toxicants in cigarette smoke: a description of the World Health Organization TobReg proposal. Tob. Control17, 132–141. 10.1136/tc.2007.024158
6
Cahn Z. Drope J. Douglas C. E. Henson R. Berg C. J. Ashley D. L. et al (2021). Applying the population health standard to the regulation of electronic nicotine delivery systems. Nicotine Tob. Res.23, 780–789. 10.1093/ntr/ntaa190
7
Carter L. P. Stitzer M. L. Henningfield J. E. O'Connor R. J. Cummings K. M. Hatsukami D. K. (2009). Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiol. Biomarkers Prev.18, 3241–3262. 10.1158/1055-9965.Epi-09-0948
8
Centers for Disease Control and Prevention (2021). Smoking and tobacco use. Fast facts. Available online at: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm.
9
Centers for Disease Control and Prevention (2022). Smoking cessation: fast facts. Available online at: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/smoking-cessation-fast-facts/index.html.
10
Chapman F. Pour S. J. Wieczorek R. Trelles Sticken E. Budde J. Röwer K. et al (2023b). Twenty-eight day repeated exposure of human 3D bronchial epithelial model to heated tobacco aerosols indicates decreased toxicological responses compared to cigarette smoke. Front. Toxicol.5, 1076752. 10.3389/ftox.2023.1076752
11
Chapman F. Sticken E. T. Wieczorek R. Pour S. J. Dethloff O. Budde J. et al (2023a). Multiple endpoint in vitro toxicity assessment of a prototype heated tobacco product indicates substantially reduced effects compared to those of combustible cigarette. Toxicol Vitro86, 105510. 10.1016/j.tiv.2022.105510
12
Cordery S. Thompson K. Stevenson M. Simms L. Chapman F. Grandolfo E. et al (2024). The product science of electrically heated tobacco products: an updated narrative review of the scientific literature. Cureus16, e61223. 10.7759/cureus.61223
13
CTRP (2023). University of Kentucky center for tobacco reference products. 1R6F Cigarettes. Available online at: https://ctrp.uky.edu/products/gallery/Reference%20Cigarettes/detail/937.
14
Eaton D. Jakaj B. Forster M. Nicol J. Mavropoulou E. Scott K. et al (2018). Assessment of tobacco heating product THP1.0. Part 2: product design, operation and thermophysical characterisation. Regul. Toxicol. Pharmacol.93, 4–13. 10.1016/j.yrtph.2017.09.009
15
Farsalinos K. E. Yannovits N. Sarri T. Voudris V. Poulas K. Leischow S. J. (2018). Carbonyl emissions from a novel heated tobacco product (IQOS): comparison with an e-cigarette and a tobacco cigarette. Addiction113, 2099–2106. 10.1111/add.14365
16
Fearon I. M. (2022). Human abuse liability assessment of e-cigarettes: why, what and how?Drug Test. Anal.15, 1211–1221. 10.1002/dta.3251
17
Fenech M. (1993). The cytokinesis-block micronucleus technique and its application to genotoxicity studies in human populations. Environ. Health Perspect.101 (Suppl. 3), 101–107. 10.1289/ehp.93101s3101
18
Food and Drug Administration (2012). in Federal register (ed food and drug administration).
19
Forster M. Fiebelkorn S. Yurteri C. Mariner D. Liu C. Wright C. et al (2018). Assessment of novel tobacco heating product THP1.0. Part 3: comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regul. Toxicol. Pharmacol.93, 14–33. 10.1016/j.yrtph.2017.10.006
20
Gale N. McEwan M. Camacho O. M. Hardie G. Murphy J. Proctor C. J. (2021a). Changes in biomarkers of exposure on switching from a conventional cigarette to the glo tobacco heating product: a randomized, controlled ambulatory study. Nicotine Tob. Res.23, 584–591. 10.1093/ntr/ntaa135
21
Gale N. McEwan M. Camacho O. M. Hardie G. Proctor C. J. Murphy J. (2021b). Changes in biomarkers after 180 days of tobacco heating product use: a randomised trial. Intern Emerg. Med.16, 2201–2212. 10.1007/s11739-021-02798-6
22
Gale N. McEwan M. Eldridge A. C. Fearon I. M. Sherwood N. Bowen E. et al (2019). Changes in biomarkers of exposure on switching from a conventional cigarette to tobacco heating products: a randomized, controlled study in healthy Japanese subjects. Nicotine Tob. Res.21, 1220–1227. 10.1093/ntr/nty104
23
Gale N. McEwan M. Hardie G. Proctor C. J. Murphy J. (2022). Changes in biomarkers of exposure and biomarkers of potential harm after 360 days in smokers who either continue to smoke, switch to a tobacco heating product or quit smoking. Intern Emerg. Med.17, 2017–2030. 10.1007/s11739-022-03062-1
24
Goldenson N. I. Fearon I. M. Buchhalter A. R. Henningfield J. E. (2021). An open- label, randomized, controlled, crossover study to assess nicotine pharmacokinetics and subjective effects of the JUUL system with three nicotine concentrations relative to combustible cigarettes in adult smokers. Nicotine Tob. Res.23, 947–955. 10.1093/ntr/ntab001
25
Gottlieb S. Zeller M. (2017). A nicotine-focused framework for public health. N. Engl. J. Med.377, 1111–1114. 10.1056/NEJMp1707409
26
Hajek P. Pittaccio K. Pesola F. Myers Smith K. Phillips-Waller A. Przulj D. (2020). Nicotine delivery and users' reactions to Juul compared with cigarettes and other e-cigarette products. Addiction115, 1141–1148. 10.1111/add.14936
27
Hashizume T. Ishikawa S. Matsumura K. Ito S. Fukushima T. (2023). Chemical and in vitro toxicological comparison of emissions from a heated tobacco product and the 1R6F reference cigarette. Toxicol. Rep.10, 281–292. 10.1016/j.toxrep.2023.02.005
28
Hatsukami D. K. Zhang Y. O'Connor R. J. Severson H. H. (2013). Subjective responses to oral tobacco products: scale validation. Nicotine Tob. Res.15, 1259–1264. 10.1093/ntr/nts265
29
Haziza C. de La Bourdonnaye G. Donelli A. Poux V. Skiada D. Weitkunat R. et al (2020a). Reduction in exposure to selected harmful and potentially harmful constituents approaching those observed upon smoking abstinence in smokers switching to the menthol tobacco heating system 2.2 for 3 Months (Part 1). Nicotine Tob. Res.22, 539–548. 10.1093/ntr/ntz013
30
Haziza C. de La Bourdonnaye G. Donelli A. Skiada D. Poux V. Weitkunat R. et al (2020b). Favorable changes in biomarkers of potential harm to reduce the adverse health effects of smoking in smokers switching to the menthol tobacco heating system 2.2 for 3 Months (Part 2). Nicotine Tob. Res.22, 549–559. 10.1093/ntr/ntz084
31
Haziza C. de La Bourdonnaye G. Skiada D. Ancerewicz J. Baker G. Picavet P. et al (2016). Evaluation of the Tobacco Heating System 2.2. Part 8: 5-Day randomized reduced exposure clinical study in Poland. Regul. Toxicol. Pharmacol.81 (Suppl. 2), S139-S150–s150. 10.1016/j.yrtph.2016.11.003
32
Haziza C. de La Bourdonnaye G. Skiada D. Ancerewicz J. Baker G. Picavet P. et al (2017). Biomarker of exposure level data set in smokers switching from conventional cigarettes to Tobacco Heating System 2.2, continuing smoking or abstaining from smoking for 5 days. Data Brief.10, 283–293. 10.1016/j.dib.2016.11.047
33
Health Canada (2022). Vaping and quitting smoking. Available online at: https://www.canada.ca/en/health-canada/services/smoking-tobacco/vaping/smokers.html.
34
Hughes J. R. (2007). Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob. Res.9, 315–327. 10.1080/14622200701188919
35
Institute of Medicine (2001). Clearing the smoke - assessing the science base for tobacco harm reduction. Washington, DC: The National Academies Press.
36
International Agency for Research on Cancer (2007). IARC handbooks of cancer prevention, vol. 11, reversal of risk after quitting smoking. Lyon, France: IARC.
37
International Organization for Standardization (2012). in International organization for standardization (Geneva: International Organization for Standardization).
38
ISO 3402:1999 (1999). ISO 3402:1999 - tobacco and tobacco products - atmosphere for conditioning and testing. ICS, 65 (65), 160.
39
ISO 20778:2018 (2018). ISO 20778:2018- cigarettes - routine analytical cigarette smoking machine - definitions and standard conditions with an intense smoking regime. ICS. 65 (65), 160.
40
Jaccard G. Kondylis A. Gunduz I. Pijnenburg J. Belushkin M. (2018). Investigation and comparison of the transfer of TSNA from tobacco to cigarette mainstream smoke and to the aerosol of a heated tobacco product, THS2.2. Regul. Toxicol. Pharmacol.97, 103–109. 10.1016/j.yrtph.2018.06.011
41
Jaccard G. Tafin Djoko D. Moennikes O. Jeannet C. Kondylis A. Belushkin M. (2017). Comparative assessment of HPHC yields in the Tobacco Heating System THS2.2 and commercial cigarettes. Regul. Toxicol. Pharmacol.90, 1–8. 10.1016/j.yrtph.2017.08.006
42
Keyser B. M. Leverette R. McRae R. Wertman J. Shutsky T. Jordan K. et al (2024). In vitro toxicological evaluation of glo menthol and non-menthol heated tobacco products. Toxicology504, 153801. 10.1016/j.tox.2024.153801
43
Kogel U. Titz B. Schlage W. K. Nury C. Martin F. Oviedo A. et al (2016). Evaluation of the Tobacco Heating System 2.2. Part 7: systems toxicological assessment of a mentholated version revealed reduced cellular and molecular exposure effects compared with mentholated and non-mentholated cigarette smoke. Regul. Toxicol. Pharmacol.81 (Suppl. 2), S123-S138–s138. 10.1016/j.yrtph.2016.11.001
44
Lechner W. V. L Gunn R. Minto A. Philip N. S. Brown R. A. Uebelacker L. A. et al (2018). Effects of negative affect, urge to smoke, and working memory performance (n-back) on nicotine dependence. Subst. Use Misuse53, 1177–1183. 10.1080/10826084.2017.1400569
45
Lüdicke F. Ansari S. M. Lama N. Blanc N. Bosilkovska M. Donelli A. et al (2019). Effects of switching to a heat-not-burn tobacco product on biologically relevant biomarkers to assess a candidate modified risk tobacco product: a randomized trial. Cancer Epidemiol. Biomarkers Prev.28, 1934–1943. 10.1158/1055-9965.Epi-18-0915
46
Lüdicke F. Picavet P. Baker G. Haziza C. Poux V. Lama N. et al (2018a). Effects of switching to the tobacco heating system 2.2 menthol, smoking abstinence, or continued cigarette smoking on biomarkers of exposure: a randomized, controlled, open-label, multicenter study in sequential confinement and ambulatory settings (Part 1). Nicotine Tob. Res.20, 161–172. 10.1093/ntr/ntw287
47
Lüdicke F. Picavet P. Baker G. Haziza C. Poux V. Lama N. et al (2018b). Effects of switching to the menthol tobacco heating system 2.2, smoking abstinence, or continued cigarette smoking on clinically relevant risk markers: a randomized, controlled, open-label, multicenter study in sequential confinement and ambulatory settings (Part 2). Nicotine Tob. Res.20, 173–182. 10.1093/ntr/ntx028
48
Mallock N. Böss L. Burk R. Danziger M. Welsch T. Hahn H. et al (2018). Levels of selected analytes in the emissions of “heat not burn” tobacco products that are relevant to assess human health risks. Arch. Toxicol.92, 2145–2149. 10.1007/s00204-018-2215-y
49
Maloney S. Eversole A. Crabtree M. Soule E. Eissenberg T. Breland A. (2020). Acute effects of JUUL and IQOS in cigarette smokers. Tob. Control30, 449–452. 10.1136/tobaccocontrol-2019-055475
50
Malt L. Thompson K. Mason E. Walele T. Nahde T. O'Connell G. (2022). The product science of electrically heated tobacco products: a narrative review of the scientific literature. F1000 Res.11, 121. 10.12688/f1000research.74718.1
51
McDermott S. Reichmann K. Mason E. Fearon I. M. O'Connell G. Nahde T. (2023). An assessment of nicotine pharmacokinetics and subjective effects of the pulze heated tobacco system compared with cigarettes. Sci. Rep.13, 9037. 10.1038/s41598-023-36259-1
52
New Zealand Ministry of Health (2020). Position statement on vaping. Available online at: https://www.health.govt.nz/our-work/preventative-health-wellness/tobacco-control/vaping-smokefree-environments-and-regulated-products/position-statement-vaping.
53
Office for Health Improvement and Disparities (2022). Nicotine vaping in England: an evidence update including health risks and perceptions. Available online at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1107701/Nicotine-vaping-in-England-2022-report.pdf.
54
Organisation for Economic Co-operation and Development. Test No. 471: bacterial reverse mutation test. (2020).
55
Organisation for Economic Co-operation and Development. Test No. 487: in vitro mammalian cell micronucleus test. (2023).
56
Perfetti T. Rodgman A. (2011). The complexity of tobacco and tobacco smoke. Beiträge zur Tab. Int.24, 215–232. 10.2478/cttr-2013-0902
57
Phillips B. W. Schlage W. K. Titz B. Kogel U. Sciuscio D. Martin F. et al (2018). A 90-day OECD TG 413 rat inhalation study with systems toxicology endpoints demonstrates reduced exposure effects of the aerosol from the carbon heated tobacco product version 1.2 (CHTP1.2) compared with cigarette smoke. I. Inhalation exposure, clinical pathology and histopathology. Food Chem. Toxicol.116, 388–413. 10.1016/j.fct.2018.04.015
58
Phillips-Waller A. Przulj D. Pesola F. Smith K. M. Hajek P. (2021a). Nicotine delivery and user ratings of IQOS heated tobacco system compared with cigarettes, juul, and refillable E-cigarettes. Nicotine Tob. Res.23, 1889–1894. 10.1093/ntr/ntab094
59
Phillips-Waller A. Przulj D. Smith K. M. Pesola F. Hajek P. (2021b). Nicotine delivery and user reactions to Juul EU (20 mg/ml) compared with Juul US (59 mg/ml), cigarettes and other e-cigarette products. Psychopharmacol. Berl.238, 825–831. 10.1007/s00213-020-05734-2
60
Picavet P. Haziza C. Lama N. Weitkunat R. Lüdicke F. (2016). Comparison of the pharmacokinetics of nicotine following single and ad libitum use of a tobacco heating system or combustible cigarettes. Nicotine Tob. Res.18, 557–563. 10.1093/ntr/ntv220
61
Pierce J. P. (2022). Quitting smoking by age 35 Years—a goal for reducing mortality. JAMA Netw. Open5, e2231487. 10.1001/jamanetworkopen.2022.31487
62
Poynton S. Sutton J. Goodall S. Margham J. Forster M. Scott K. et al (2017). A novel hybrid tobacco product that delivers a tobacco flavour note with vapour aerosol (Part 1): product operation and preliminary aerosol chemistry assessment. Food Chem. Toxicol.106, 522–532. 10.1016/j.fct.2017.05.022
63
Royal College of Physicians (2016). Nicotine without smoke. Tobacco harm reduction. A report by the tobacco advisory group of the royal College of Physicians. London, United Kingdom: Royal College of Physicians.
64
Schaller J.-P. Keller D. Poget L. Pratte P. Kaelin E. McHugh D. et al (2016b). Evaluation of the Tobacco Heating System 2.2. Part 2: chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul. Toxicol. Pharmacol.81, S27–S47. 10.1016/j.yrtph.2016.10.001
65
Schaller J. P. Pijnenburg J. P. M. Ajithkumar A. Tricker A. R. (2016a). Evaluation of the Tobacco Heating System 2.2. Part 3: influence of the tobacco blend on the formation of harmful and potentially harmful constituents of the Tobacco Heating System 2.2 aerosol. Regul. Toxicol. Pharmacol.81 (Suppl. 2), S48-S58–s58. 10.1016/j.yrtph.2016.10.016
66
Shiffman S. Kirchner T. R. (2009). Cigarette-by-cigarette satisfaction during ad libitum smoking. J. Abnorm Psychol.118, 348–359. 10.1037/a0015620
67
Smith M. R. Clark B. Lüdicke F. Schaller J. P. Vanscheeuwijck P. Hoeng J. et al (2016). Evaluation of the tobacco heating system 2.2. Part 1: description of the system and the scientific assessment program. Regul. Toxicol. Pharmacol.81 (Suppl. 2), S17-S26–s26. 10.1016/j.yrtph.2016.07.006
68
Stratton K. Shetty P. Wallace R. Bondurant S. (2001). Clearing the smoke: the science base for tobacco harm reduction-executive summary. Tob. Control10, 189–195. 10.1136/tc.10.2.189
69
Szparaga M. Świercz R. Stępnik M. (2021). Review of data on chemical content in an aerosol resulting from heating a tobacco or a solution used in e-cigarettes and in the smoke generated from the reference cigarettes. Toxicol. Mech. Methods31, 323–333. 10.1080/15376516.2021.1884922
70
Thorne D. Breheny D. Proctor C. Gaca M. (2018). Assessment of novel tobacco heating product THP1.0. Part 7: comparative in vitro toxicological evaluation. Regul. Toxicol. Pharmacol.93, 71–83. 10.1016/j.yrtph.2017.08.017
71
Thorne D. Whitwell J. Clements J. Walker P. Breheny D. Gaca M. (2020). The genotoxicological assessment of a tobacco heating product relative to cigarette smoke using the in vitro micronucleus assay. Toxicol. Rep.7, 1010–1019. 10.1016/j.toxrep.2020.08.013
72
Titz B. Kogel U. Martin F. Schlage W. K. Xiang Y. Nury C. et al (2018). A 90-day OECD TG 413 rat inhalation study with systems toxicology endpoints demonstrates reduced exposure effects of the aerosol from the carbon heated tobacco product version 1.2 (CHTP1.2) compared with cigarette smoke. II. Systems toxicology assessment. Food Chem. Toxicol.115, 284–301. 10.1016/j.fct.2018.02.058
73
Titz B. Sewer A. Luettich K. Wong E. T. Guedj E. Nury C. et al (2020). Respiratory effects of exposure to aerosol from the candidate modified-risk tobacco product THS 2.2 in an 18-month systems toxicology study with A/J mice. Toxicol. Sci.178, 138–158. 10.1093/toxsci/kfaa132
74
Toll B. A. Smith T. T. King B. A. (2024). Nicotine e-cigarettes: considerations for healthcare providers. Nat. Med.30, 1513–1514. 10.1038/s41591-024-02926-7
75
US Department of Health and Human Services (2014). The health consequences of smoking: 50 Years of progress: a report of the surgeon general. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
76
Vansickel A. Baxter S. Sherwood N. Kong M. Campbell L. (2022). Human abuse liability assessment of tobacco and nicotine products: approaches for meeting current regulatory recommendations. Nicotine Tob. Res.24, 295–305. 10.1093/ntr/ntab183
77
Wang H. Chen H. Huang L. Li X. Wang L. Li S. et al (2021). In vitro toxicological evaluation of a tobacco heating product THP COO and 3R4F research reference cigarette on human lung cancer cells. Toxicol Vitro74, 105173. 10.1016/j.tiv.2021.105173
78
Warner K. E. (2019). How to think—not feel—about tobacco harm reduction. Nicotine and Tob. Res.21, 1299–1309. 10.1093/ntr/nty084
79
West R. (2017). Tobacco smoking: health impact, prevalence, correlates and interventions. Psychol. and Health32, 1018–1036. 10.1080/08870446.2017.1325890
80
Wieczorek R. Trelles Sticken E. Pour S. J. Chapman F. Röwer K. Otte S. et al (2023). Characterisation of a smoke/aerosol exposure in vitro system (SAEIVS) for delivery of complex mixtures directly to cells at the air-liquid interface. J. Appl. Toxicol.43, 1050–1063. 10.1002/jat.4442
81
Wong E. T. Kogel U. Veljkovic E. Martin F. Xiang Y. Boue S. et al (2016). Evaluation of the Tobacco Heating System 2.2. Part 4: 90-day OECD 413 rat inhalation study with systems toxicology endpoints demonstrates reduced exposure effects compared with cigarette smoke. Regul. Toxicol. Pharmacol.81 (Suppl. 2), S59-S81–s81. 10.1016/j.yrtph.2016.10.015
82
World Health Organization (2011). WHO report on the global tobacco epidemic: warning about the dangers of tobacco. Geneva, Switzerland: World Health Organization.
83
World Health Organization (2023). Tobacco. Available online at: https://www.who.int/news-room/fact-sheets/detail/tobacco.
84
Yach D. (2022). Tobacco harm reduction matters. Lancet399, 1864–1865. 10.1016/S0140-6736(22)00834-0
Summary
Keywords
cigarette smoking, tobacco harm reduction, heated herbal product, analytical chemistry, toxicology, abuse liability
Citation
Marinas-Lacasta A-F, Fearon IM, Stevenson M, Abusalem T, Chapman F, Trelles Sticken E, Wieczorek R, Pour SJ, Dethloff O, Komini O, Brown M, Simms L and Nahde T (2025) Assessment of heated herbal products’ tobacco harm reduction potential: pre‐clinical and clinical studies. Front. Toxicol. 7:1589480. doi: 10.3389/ftox.2025.1589480
Received
12 March 2025
Accepted
20 June 2025
Published
11 August 2025
Volume
7 - 2025
Edited by
Rosalia Emma, Kore University of Enna, Italy
Reviewed by
Israr Ahmad, University of Alabama at Birmingham, United States
Brian Keyser, Reynolds American, United States
Updates
Copyright
© 2025 Marinas-Lacasta, Fearon, Stevenson, Abusalem, Chapman, Trelles Sticken, Wieczorek, Pour, Dethloff, Komini, Brown, Simms and Nahde.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew Stevenson, matthew.stevenson@impbrands.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.