Abstract
Phytonutrients are widely recognized for providing protective human health benefits. Among the phytonutrients, epidemiological and experimental studies show that dietary organosulfur compounds (OSC) play a significant role in preventing various human pathological progressions, including chronic inflammation, by decreasing inflammatory mediators such as nitric oxide (NO), prostaglandin (PG)E2, interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and IL-17, which are all typical hallmarks of inflammation. Evidence supports OSC in reducing the expression of these markers, thereby attenuating chronic inflammatory processes. Nuclear factor-kappa B (NF-κB) is a key regulating factor during inflammation, and novel evidence shows that OSC downregulates this transcriptional factor, thus contributing to the anti-inflammatory response. In vitro and in vivo studies show that inflammation is mechanistically linked with acute and chronic pathological conditions including cancer, diabetes, obesity, neural dysfunction, etc. Furthermore, a considerable number of experiments have demonstrated that the anti-inflammatory properties of OSC occur in a dose-dependent manner. These experiments also highlight indirect mechanisms as well as potent co-functions for protective roles as antioxidants, and in providing chemoprotection and neuroprotection. In this brief review, we provided an overview of the anti-inflammatory effects of OSC and elucidated probable mechanisms that are associated with inflammation and chronic disorders.
Introduction
We are obtaining a growing understanding of how bioactive compounds in plants are helpful agents to protect human health. These compounds are also referred to as nutraceuticals, or phytonutrients. These compounds are usually present in cereals (grains), pulses (legumes), vegetables, fruits, and other plant foods. Having a diet rich in plant foods, therefore, ensures the consumption of millions of phytonutrients, and provides health-protective benefits. Regularly consuming fruits and vegetables is linked with reducing the risk of chronic diseases including cardiovascular diseases (1), obesity (2), hypertension, type 2 diabetes mellitus, stroke, cancer, and chronic inflammatory bowel diseases (IBD), etc., (3). This risk reduction is purported to be from fruits, vegetables, whole grains, and other plant-based foods containing significant amounts of phytonutrients that exert potential attributes beyond those obtained from basic nutrition. Dietary phytonutrients are very diverse, and are as such classified as phenolics, alkaloids, nitrogen-containing compounds, organosulfur compounds (OSCs), phytosterols, and carotenoids (4). Each class is divided into further classes, but herein within the scope of this review, only the OSC group will be discussed. The OSC group includes isothiocyanates, indoles, allylic sulfur compounds, and sulforaphane (SFN) (5). Figure 1 listed some basic generic structure of some organosulfur compounds. These compounds are widely known for their unique medicinal properties and health benefits, i.e., they act as antioxidants to scavenge free radicals (6), and possess antimicrobial and anti-inflammatory properties (7). Numerous health benefits of these compounds are evidenced against chronic diseases by e.g., their cardioprotective effects of reducing low-density lipoproteins (LDL), and anti-carcinogenic effects by detoxifying carcinogens or toxicants (8). Vegetables in the Allium and Brassica (Cruciferous) genus, i.e., onion, garlic, broccoli, cabbage, cauliflower, etc., are good sources of OSCs. These are widely consumed by the general population, with well-documented benefits (9, 10).
Figure 1
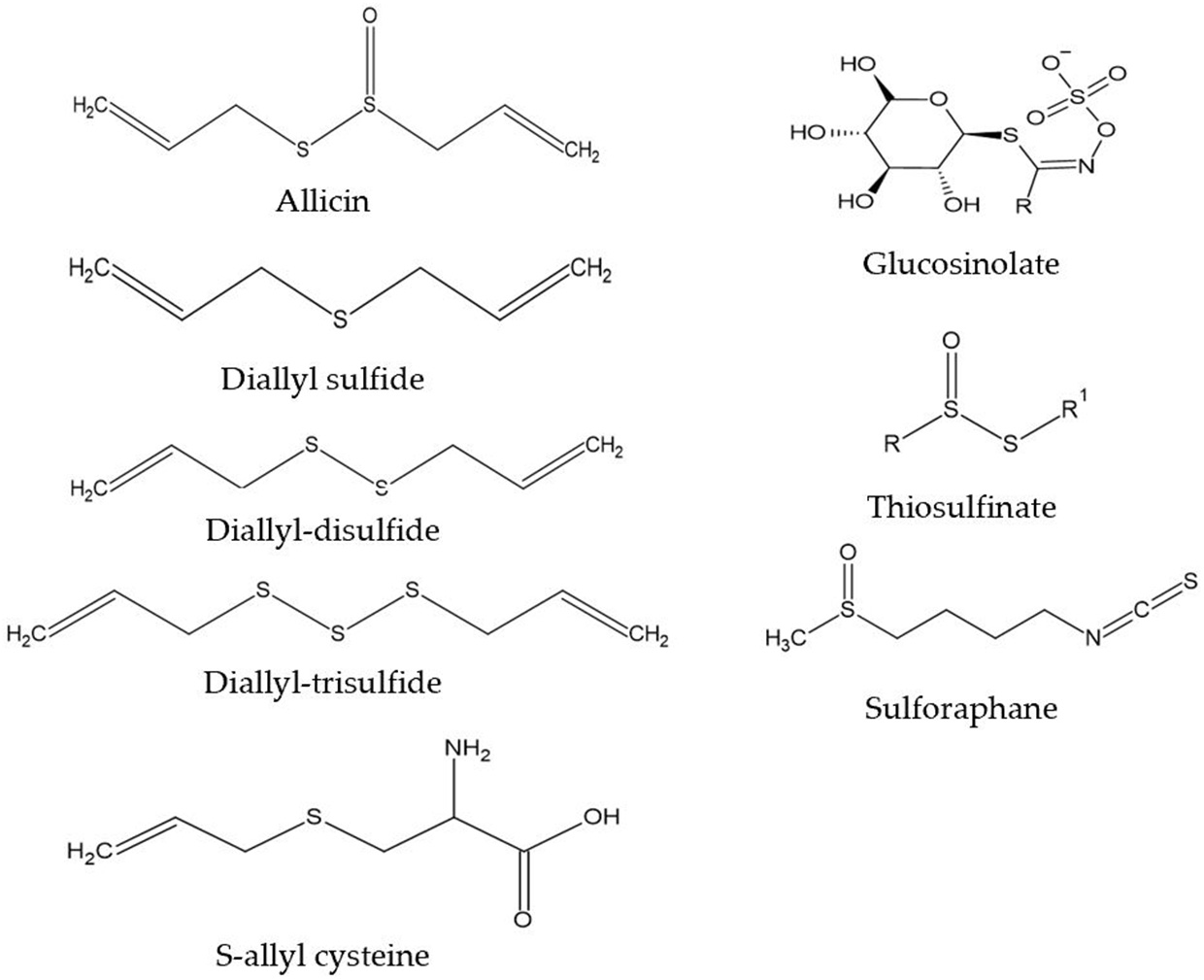
Generic structure of some organosulfur compounds.
Vegetables of the Allium genus can be easily identified by their typical pungent flavor and aroma, due to the presence of oil-soluble or volatile sulfur compounds (diallyl sulfides) (11). However, some less odorous OSCs, i.e., S-allyl cysteine (SAC) or S-allyl mercapto cysteine (SAMC) found in aqueous extracts of garlic are present in vegetables of the Allium genus and are water-soluble in nature (12). When garlic is cut or crushed into pieces, OSC is naturally activated within a short period of time (4). Also, crushed raw garlic contains high concentrations of allicin, and it is allicin that is responsible for most of the potential pharmacological activities (13). The strong flavor of onion is identified due to some intermediary sulfur compounds, namely thiosulfinates, thiosulfonates, and mono-, di-, and tri-sulfides (14). Glucosinolates (GST) are natural plant compounds in Brassica (Cruciferous) genus, also known as precursors of isothiocyanates. While cellular disruption by the enzyme myrosinase is caused by chewing, food preparation or damage by insects causes the hydrolysis of GST into several bioactive compounds i.e., isothiocyanates, thiocyanates, and others (15). SFN is an important dietary isothiocyanate that is a hydrolytic product from glucoraphanin (4-methyl-sulfinyl butyl glucosinolate). A high concentration of glucoraphanin is available in broccoli, around 0.8 to 21.7 μmol/g of dry weight (DW) (16). In broccoli sprouts, the SFN concentration (1,153 mg/100 g DW) is about ten times greater than mature broccoli (44–171 mg/100 g DW) (17). SFN is an indirect antioxidant, that induces immunomodulatory, anti-inflammatory, anti-microbial, cardioprotective, chemopreventive, and neuroprotective effects (18–23).
Inflammation is a physiological state that occurs when our body is exposed to harmful endogenous or exogenous materials, injury or trauma (24). It is mainly a protective response, that is essential for survival. The regular inflammatory response involves the accumulation of white blood cells, especially neutrophils, macrophages, and plasma proteins in sites of injury or foreign particle location, to selectively eliminate cells and to restore homeostasis (24, 25). Consequently, secondary responses are also often visible such as redness, pain, fever, and swelling (24). Such an inflammatory response is called acute inflammation, but if it lasts for prolonged periods, it is then termed chronic, or homeostatic inflammation. Further, activating the innate immune system can also induce a more chronic inflammatory response (26). Therefore, regulating both metabolic and immune responses is essential to maintain central homeostasis, because a deviation may occur in normal immune responses if there is any chronic disturbance in metabolic homeostasis by endogenous or exogenous inducers (27). This is followed by a disrupted metabolic function that results in over- or undernutrition (28). This kind of metabolically triggered chronic inflammation is termed metaflammation, also known as low-grade inflammation, that is associated with many pathophysiologies (28), including obesity, type 2 diabetes and cardiovascular diseases (28, 29). Therefore, attention should be paid to OSC as they exert physiological effects against both acute and chronic inflammation, not only in vitro, but also in humans (30). Subsequently in this review, we will describe the principles of anti-inflammatory regulation by OSC, and the potential for OSC to contribute to alleviating common metabolic disorders.
Effects of OSC in Preventing Acute and Chronic Inflammation
Average Consumption and Bioavailability of OSC
Ensuring good nutrition is essential to live a disease-free healthy life. According to a survey conducted by the Japanese government in 2011–13, cruciferous vegetable consumption topped a list among all commonly consumed vegetables, made up of around 21% of purchased volume for cabbage, onion, and broccoli (31). However, other statistics from a population-based cohort study in Spain revealed that the average intake of cruciferous vegetables among Spanish people was 11.3 g/day, the lowest consumption compared with other European countries (32). Some studies propose that the bioavailability and metabolic rate of oil-soluble OSC in garlic, like allicin and other allicin-derived compounds, is rapid, and absorbed intestinally; however, the administration of a high dose (60 mg) of pure allicin, or a lower dose of 25 g from crushed garlic did not appear in human blood, urine or feces within 48 h of consumption (33). On the other hand, SAC is a stable water-soluble OSC in garlic, that can be absorbed easily in the gastrointestinal tract within 30–60 min and detected in liver, kidney, and plasma after oral intake in animals (34). In urine it was identified as a metabolite of N-acetyl-SAC, transformed by N-acetyltransferase. The bioavailability of SAC is 98.2% in rats, 103.0% in mice and 87.2% in dogs (34). On the contrary, the bioavailability of cruciferous vegetables is measured by analyzing their metabolites and conjugated products excreted through urine. For example, the bioavailability of SFN derived from broccoli is determined by the excretion of GSH conjugates excreted through urine within 24 h post-ingestion, which indicates the transport of bioactive compounds throughout the body (35, 36). Thus, the bioavailability of OSC can differ according to the origin and dose (37).
Inflammation Induced by Exogenous Stimuli
Inflammation is an important part of our body's immune response. In the case of acute injury, neutrophils play a crucial role in enhancing the inflammatory response by producing cytokines, whilst neutrophils are considered as both inflammatory effectors and immunoregulatory cells (38). Exogenous stimulation can be caused by mechanical, physical, chemical or biological stimulation, or a combination of these stimuli (39). Researchers in the field of sports science have revealed that exercise has both inflammatory and anti-inflammatory effects (40). In 1994, it was hypothesized that unaccustomed exercise causes muscle damage mainly by metabolic and mechanical stressors (41). In fact, an influx of inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-8, and IL-12 are secreted due to inflammation, whilst neutrophils and macrophages also accumulate in the inflamed body part or organ (42–44). Furthermore, during exercise, the movement of electrons through the mitochondrial electron transport system results in increased levels of peroxides, superoxide radicals, their by-products, and other ROS (45). Some studies suggest that dietary immunostimulants i.e., antioxidants, polyphenols, etc., can positively improve exercise-induced changes in immune function (46–48). Therefore, evidence suggests that OSC can modulate our immune system, and are able to ensure protection against inflammation and oxidative stress (19, 49–51). A couple of experimental studies with animal models have been conducted with SFN obtained from Brassicaceae vegetables, to examine its protective effect against muscle inflammation. It was revealed that SFN could reduce exhaustive exercise-induced muscle damage as well as modulate the muscle redox environment by inducing transcription factor NF-E2-related factor 2 (Nrf2)-dependent phase 2 enzymes (52). Also, SFN has been shown to reduce muscle inflammation by acting as an indirect antioxidant and anti-inflammatory compounds, by activating the Nrf2-induced inhibition of nuclear factor-kappa B (NF-κB) signaling pathway (53). Regardless of whether OSC comes from Allium or Brassica genus, it seems to serve the same role against inflammation (54).
Inflammation Induced by Endogenous Stimuli
Endogenous inducers of inflammation are responses produced by stressed, malfunctioning or dead cells, damaged tissues, plasma, or the extracellular matrix. Based on the nature and location of inflammation in our body, a common mechanism is followed by all inducers, which is summarized in the following process. Firstly, detrimental stimulation is recognized by cell surface pattern recognition receptors (PRRs); secondly, inflammatory pathways are activated; thirdly, inflammatory mediators are released; and finally, inflammatory cells are recruited (55). At the initial point of inflammation, PRRs recognize molecules of pathogens and damaged cells by orientating pathogen-associated molecular patterns (PAMPs) and/or damage-associated molecular patterns (DAMPs) from endogenous stress. Therefore, activation of inflammasomes [a group of cytosolic protein containing nucleotide-binding oligomerization domain (NOD)-containing protein 2-like receptors (NLRs)] throughout the innate immune response promotes the production of pro-inflammatory cytokines (56). Furthermore, it is necessary to ensure a balance of expression between pro- and anti-inflammatory cytokines to maintain regular cell and organ function (57). Chronic dysregulation of an increased volume of pro-inflammatory cytokines may lead to atherosclerosis, type 2 diabetes and other chronic diseases (28, 58). Thereby, high circulating inflammatory substances are released from adipose tissues at the onset of metabolic disorders, which is termed low-grade systemic inflammation (59). One of the possible mechanisms of OSC against carcinogenesis could be due to the xenobiotic mechanism of phase 1 and phase 2 enzymes (60). According to previous research, an association has been found between elevated levels of circulating inflammatory indicators such as IL-6 and TNF-α, and the development of chronic diseased conditions, e.g., type 2 diabetes, atherosclerosis, and cardiovascular diseases, or low-grade systemic inflammation (61–63). Moreover, circulating acute-phase protein, C-reactive protein (CRP), is also elevated alongside IL-6 and TNF-α (64), which further highlights the risks of developing low-grade systemic inflammation. Several investigations have been performed to identify the role of OSC against inflammation. One such study has used human embryonic kidney cell line 293 (HEK293) with sulfur fertilized garlic powder extracts (GPE, the sulfur amount rose to 190 mmol/kg), to determine lipopolysaccharide (LPS, at a final concentration 10 μg/L, 100 mg/L GPE)-induced inflammation (63). Results were compared between unfertilized and fertilized GPE and evidenced that fertilized GPE could significantly reduce NF-κB, IL-1β, and TNF-α, and LPS-induced liberation of anti-inflammatory IL-10 was not affected by fertilized GPE (65). The aging process of garlic produces odorless sulfur compounds, including the conversion of unstable allicin into a stable and more beneficial one, with a substantially increased amount of S-allyl cysteine and S-allyl mercapto cysteine. Furthermore, S-allyl compounds are higher in commercially produced aged garlic compared with fresh garlic (66). As an example, a 6-week double-blind, randomized, placebo-controlled nutritional intervention study was conducted using 51 healthy adult obese human subjects supplemented daily with aged garlic extract in capsule form, or placebo. The subjects were instructed to consume three capsules (3.6 g aged garlic extract/day) with food throughout the study timeline. Besides other biomarkers, supplementation leads to a decrease in serum IL-6 and TNF-α concentrations below those at baseline (67). This exemplifies how aged garlic supplementation might prevent the progression of obesity-induced inflammation.
Influence of OSC on Transcriptional Regulation of Pro-inflammatory Mediators
Toll-like receptors (TLR) are highly conserved PRRs expressed in immune cells (i.e., macrophages). They recognize inflamed areas and activate gene transcription factor NF-κB, the pivotal transcription factor with five subunits (p50, p52, p65, RelB, and cRel) that subsequently regulate more than 400 different genes, including inflammatory cytokines and pro-inflammatory enzymes cyclooxygenase (COX)-2, and 5-lipoxygenase (LOX) (68, 69). Furthermore, at the site of inflammation, the release of pro-inflammatory cytokines and the accumulation of ROS are also regulated by the transcriptional activation of NF-κB through the phosphorylation of IκB (Figure 2). Activated NF-κB with attached subunits p65 and p50 translocate into the nucleus from the cytoplasm to induce pro-inflammatory genes (70). The production of pro-inflammatory cytokines may upregulate the expression of inducible nitric oxide synthase (iNOS), which leads to the increased production of nitric oxide (NO) (71). COX-2 is another rate-limiting enzyme and is responsible for the production of the prostaglandin E2 (PGE2). Lee et al. (72) performed an experiment on garlic-derived OSC (ajoene) cultured with LPS activated RAW 264.7 macrophages. OSC suppressed NO and PGE2 production, and the mRNA expression of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α. Additionally, LPS challenged or activated macrophages displayed repression of NF-κB (72). Another study elucidated synergistically that SFN in combination with other bioactive compounds such as phenethyl isothiocyanate and curcumin could work more effectively against inflammation by reducing iNOS and COX-2 protein expression, and NO, PGE2, TNF-α, and IL-1 production (73). SAC, a potential cholesterol-lowering compound present in aged garlic extract, has roles in preventing oxidized LDL (ox-LDL)-induced cell damage by reducing intracellular glutathione (GSH) depletion, and TNF-α or H2O2-induced NF-κB activation (74). Also, it was observed that elevated levels of ox-LDL are one of the major reasons for atherosclerosis (75). Allyl sulfides, isothiocyanates, and indoles are the most studied OSCs. Garlic and onion are also considered as natural hypolipidemic spices, and lower serum cholesterol levels are induced if applied in appropriate doses and durations (76–78). In addition, OSC possesses antioxidant effects by acting as an inducer of phase 2 enzymes, named as the antioxidant response element (ARE) (12). In an animal model of neurodegeneration, it was evidenced that SFN could modulate the transcription factor Nrf2-dependent phase 2 enzymes to reach the central nervous system (CNS) by crossing the blood-brain barrier (BBB), thereby showing neuroprotective effects (79). As such, to some extent, administering OSC rich foods in the diet may downregulate the expression of NF-κB, and inhibit the production of pro-inflammatory mediators.
Figure 2
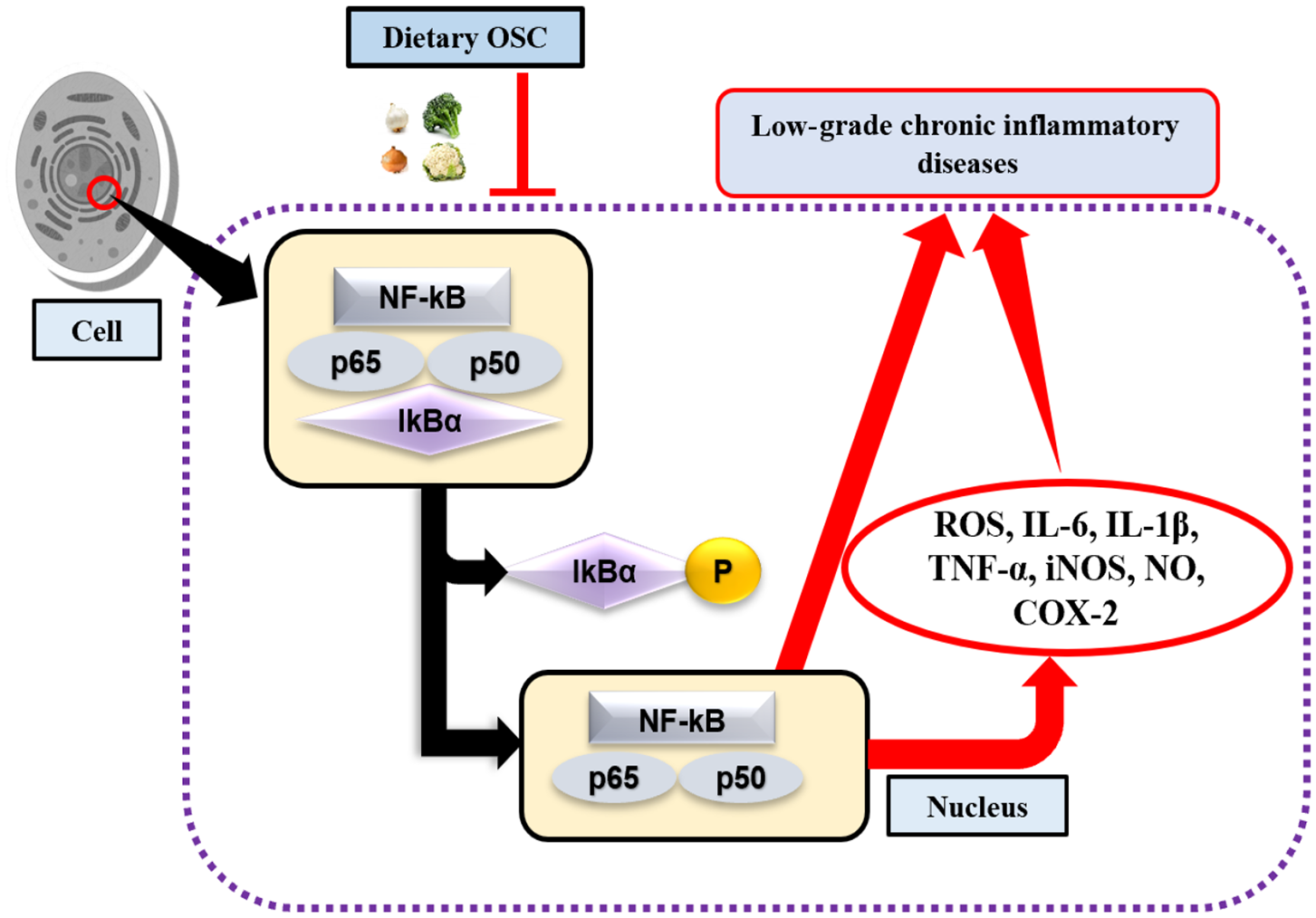
OSC in activation of NF-κB signaling pathway. Lack of dietary OSC may activate the transcription factor NF-κB through the phosphorylation of IκB complex at the site of inflammation. NF-κB along with the subunits (p65 and p50) enters into the nucleus, thereby induces production of ROS, pro-inflammatory cytokines (IL-6, IL-1β, TNF-α) and enzymes (iNOS, COX-2), while their chronic production leads to increase risk of low-grade chronic inflammatory diseases.
Chronic activation of inflammasomes, as well as the production of pro-inflammatory cytokines induces inflammatory cascades, that further lead to the genesis of chronic inflammatory disease. Among the cytokines, IL-1β, and IL-18 are particularly produced actively by interacting with TLRs (80). During chronic inflammation, the production of the major pro-inflammatory enzymes, iNOS and COX-2, increase and contribute toward developing cardiovascular diseases (81). According to previous research, SFN suppresses the expression of pro-inflammatory enzymes by blocking the mitogen-activated protein kinase (MAPK) signaling cascade, thus showing preventive effects against chronic inflammation (82). This mechanism, to some extent, is supportive to protect against neurodegenerative disorders and supports anti-inflammasome properties (80). In response to pathogens, macrophages undergo a metabolic shift, and exhibit the Wargburg effect (as traditionally associated with cancer cells) (83). OCS has been shown to influence cancer cell metabolism through inhibiting the activity of histone deacetylases (HDACs) and histone acetyltransferases (HATs), thereby inducing an epigenetic mechanism to normalize cancer cell metabolism (84).
Microglia cells of the CNS also release pro-inflammatory cytokines and mediators which cause neural damage (85). SFN can protect neuro-inflammation or inflammation in the brain by attenuating such inflammatory markers, as modeled by LPS-stimulated microglial cells in vitro (86). A considerable number of studies have identified that inflammation is associated with oxidative stress and pathophysiological domains of depression, and there are some depression-induced neuropsychiatric disorders that can be identified by an increase in serum levels of circulating inflammatory biomarkers (87, 88). Though depression is a multifaceted disorder, inflammatory biomarkers could provide useful clinical information for initial treatment. The transcription factor Nrf2 is associated with the protection against depression or oxidative stress through anti-inflammatory mechanisms. For example, SFN was administered as an antidepressant in an LPS-challenged inflammatory model of depression (89). To examine the anti-inflammatory effects of SFN, iNOS expression was measured in the hippocampus of the mice and was shown to be significantly reduced, compared with LPS-inducing iNOS by 60% (89). To summarize, this anti-inflammatory action of SFN in conjunction with its antioxidant function could introduce a novel therapeutic treatment for diseases. Links already exist, for example, with chronic obstructive pulmonary diseases (COPD) (90), with great potentials for future examination of the links between the anti-inflammatory properties of OSC, and the progression of chronic disease prevention. However, besides the anti-inflammatory properties, the effects of SFN on inflammasomes are also being studied, with reports emerging of SFN reducing the expression of the cytokines IL-1β and NOD-like receptors (NLRP3 and NLRC4), but not for AIM2 (absent in melanoma 2) (91). Similar effects have also been observed from the sulfur extract derived from garlic and onion in inhibiting the NLRP3 inflammasomes activation (92). Therefore, OSC may not be an ideal modulator for the inactivation of inflammasomes, rather than inhibiting other inflammatory regulators.
Experimental Evidence of OSC-Mediated Anti-Inflammatory Effects, and Their Associated Mechanisms
Anti-inflammatory Effects of OSC on Chronically Induced Low-Grade Systemic Inflammation
For decades, research has been conducted into the regulation and/or suppression of pro-inflammatory mediators through dietary sources in the context of low-grade systemic inflammation, where a series of pro-inflammatory markers are released into the circulation. Table 1 represents a summary of some key pieces of evidence surrounding the anti-inflammatory effects of different OSCs. In an in vitro analysis, Heiss et al. (94) proposed that the anti-inflammatory effects of SFN could also be used as a drug for cancer chemo-prevention, through the transcriptional down-regulation of NF-κB, as well as attenuating iNOS and COX-2 expression, and the secretion of TNF-α. Lee et al. (96) demonstrated that diallyl sulfide (DAS) extracted from garlic oil, a principal flavoring compound, can reduce joint-inflammation induced by monosodium urate (MSU) crystals and IL-1β, in both in vivo and in vitro models. They also showed that the pathway of action involves downregulating COX-2 expression as well as PGE2, which is attributed to the attenuation of NF-κB activation. The anti-inflammatory effects of garlic sulfur compounds have also been shown to prevent atherosclerosis by inhibiting the production of NO by suppressing iNOS mRNA, as demonstrated in an LPS-stimulated macrophage cell line RAW264.7 (98). Furthermore, remarkable anti-inflammatory effects of OSC can be observed if applied in an appropriate dose and concentration. For example, compared to the OSC, DAS (1–10 μM), diallyl disulfide (DADS, 0.1–0.2 μM) and allyl methyl sulfide (AMS, 2–20 μM) derived from garlic oil against endotoxicity, induced diverse effects on both pro- and anti-inflammatory cytokines, in a concentration-dependent manner. In this study, a strong correlation was also found between DAS-induced suppression of NO, PGE2 and the decreased production of cytokines (102). If applied in a high concentration (~200 mg/kg body weight), an imbalance between chemical stress and response capacity leads to contradictory results along with adverse side effects, i.e., acute pulmonary edema, toxicity to the heart, brain, liver, and other organs (103). One study was performed with both low (50 mg/kg) and high (500 mg/kg) doses of garlic extract, and concluded that intraperitoneal administration of high-dose garlic extract causes damage to the lungs and liver in rats (104). Similarly, low (50 mg/kg) and high (500 mg/kg) amounts of aqueous onion extract were administered both orally and intraperitoneally, and resulted in damaging effects to the lung and liver with intraperitoneal application compared with oral application, including a 25% mortality rate in the treatment group (105).
Table 1
| Compound name, source | Experimental model; cell line/animal type | Dose | Key observations of inflammatory mediators | Proposed mechanism of anti-inflammatory effects | References |
|---|---|---|---|---|---|
| DATS, Garlic | LPS induced model; RAW 264.7 macrophages | LPS 100 ng/mL DATS 0, 10, 20, 30, 40 μM |
NO, and iNOS protein expression was inhibited and PIC IL-1β and TNF-α reduced the expression at the transcriptional level. | Suppressed DNA binding activity and nuclear translocation of NF-κB p65, as well as inhibiting IκB degradation. | (93) |
| SFN, Broccoli | LPS induced model; RAW 264.7 macrophages | LPS 500 ng/mL SFN 10 or 20 μM |
Expression of TNF-α, iNOS and COX-2 protein was inhibited. Also, iNOS mRNA expression downregulated. | Inhibition of NF-κB DNA binding and of transactivation of κB-dependent genes. | (94) |
| SFN, Broccoli | LPS induced model; RAW 264.7 macrophages | LPS 50 ng/mL SFN 20 μM |
Inhibited COX-2 protein and mRNA expression. | Modulation of core promoter elements (NF-κB, C/EBP, CREB, and AP-1) in the COX-2 transcriptional regulation. | (95) |
| SFN, Broccoli | LPS induced model; RAW 264.7 macrophages | LPS 1 μg/mL SFN 10 or 20 μM |
Reduced PIC, iNOS and NO expression. | Activation of Nrf2/HO-1 signal transduction pathway. | (71) |
| DAS, Garlic | MSU crystals and IL-1β: HIG-82 synovial cell | MSU crystals 2 mg/mL, IL-1β 10 ng/mL DAS 20 mM |
COX-2 and PGE2 gene expression were inhibited by the presence of DAS. | Prevent the activation of NF-κB. | (96) |
| Ajoene, Garlic | LPS induced model; RAW 264.7 macrophages | LPS 1 μg/mL Ajoene 20 μM |
Inhibited production of iNOS, COX-2 at the transcriptional level, and expression of TNF-α, IL-1β, and IL-6 mRNA. | Prevent the activation of NF-κB and decreased the phosphorylation of p38 and ERK. | (72) |
| Allicin and ajoene, Garlic | LPS induced model; RAW 264.7 macrophages | LPS 1 μg/mL Ajoene 10 μM, Allicin 50 μM |
Reduced expression of iNOS in activated macrophages. | The inhibitory effect is mediated via reduction of iNOS mRNA expression rather than the effect on the iNOS enzymatic activity. | (97) |
| SAC, Garlic | LPS induced model; RAW 264.7 macrophages | LPS 1 ng/mL SAC 20 μM |
Inhibited production of NO by suppressing iNOS mRNA and protein expression. | Suppressed activation of NF-κB. | (98) |
| DAS, DADS, and AMS, Garlic | LPS induced model; RAW 264.7 macrophages | LPS 330 ng/mL DAS 1-10 μM; DADS 0.1-0.2 μM; AMS 2-20 μM |
PIC TNF-α, IL-1β, IL-6, and AIC IL-10 concentration were inhibited by DAS. DADS increased the production of PIC IL-1β and IL-6 and decreased AIC IL-10 production. AMS suppressed TNF-α, NO and enhanced AIC IL-10. | Mediated by reducing inflammatory enzymes (iNOS and COX-2) activity. | (99) |
| GE, Garlic | LPS stimulated model; PBMCs | LPS 1 μg/mL GE 1-100 μg/mL |
Monocyte cytokine and their percentage of producing TNF-α, IL-6, and IL-8 inhibited and IL-10 increased significantly. | Mediated by inhibiting Th1 and inflammatory cytokines production. | (100) |
| SFN, Broccoli | LPS induced model; Endothelial cell line ECV304 | LPS 500 ng/mL SFN 5-20 μM |
Suppressed expression of COX2 and iNOS protein. | Mediated by suppressing the phosphorylation of ERK1/2, JNK, and p38. | (82) |
| SFN, Broccoli | CCI induced neuropathic pain model; Male C57 BL/6J mice | SFN 0.1-100 mg/kg by i.p. injection Control vehicle: Saline |
Attenuated mRNA and protein expression of COX-2, iNOS and mRNA expression of TNF-α, IL-1β, IL-6. Also elevated IL-10 mRNA expression. | Reduced inflammatory cytokines expression by inhibiting inflammatory enzymes (iNOS, COX-2) expression. | (101) |
| SFN, Broccoli | LPS induced ALI mice model; Male BALB/c mice | SFN 50 mg/kg by i.p. injection Control vehicle: PBS LPS 25 μg in 50 μL PBS |
Decreased NO, COX-2 (PGE2) and NF-κB, IL-6, and TNF-αprotein expression in comparison to the control group. | Reduced ALI injury through the Nrf2/ARE pathway. | (20) |
| SFN, Broccoli | Dystrophin deficient mdx mouse model; C57BL/10ScSn-Dmdmdx/NJU mice |
SFN 2 mg/kg by gavage Control vehicle: corn oil |
Reduced mRNA expression of TNF-α, IL-1β, IL-6, NF-κB in muscle cells. Inverse correlation observed between TNF-α mRNA, and Nrf2 protein expression. | Increased expression of HO-1 and Nrf2, which decreased the expression of NF-κB, and phosphorylated IκB. | (53) |
Research elucidating the in vitro and in vivo anti-inflammatory effects of organosulfur compounds (OSCs).
DATS, diallyl trisulfide; PIC, pro-inflammatory cytokines; MSU, monosodium urate; SAC, S-allyl cysteine; AIC, anti-inflammatory cytokines; PBMCs, peripheral blood mononuclear cells; Th1, T-helper-1; GE, garlic extract; CCI, chronic constriction injury; i.p., intraperitoneal; PBS, phosphate buffer saline; ALI, acute lung injury.
An increased concentration of circulating inflammatory components (in plasma and urine) has also been reported for type 2 diabetes (106). Treatment with OSC (AMS) showed a reduction in the protein expression of pro-inflammatory cytokines in streptozotocin (STZ)-induced experimental rats (107). Moreover, extracts of OSC showed significant effects in reducing the risks of cancers (108), especially prostate cancer, through suppressing inflammation and altering cell signaling pathways (109, 110). Based on our knowledge of their molecular mechanisms and biological activity, these OSC could be metabolized inside our body in multiple ways. Chronic inflammation of the digestive tract or the pathogenesis of inflammatory bowel diseases (IBD) also secrete mucosal cytokines (111). IBD occurs due to an imbalance in bacterial composition and disturbance in microbial functionality. Human microbiota releases beneficial metabolites i.e., short chain fatty acids (SCFA) notably acetate, butyrate and propionate (112). Besides that, SCFA can interact with neutrophils at the site of inflammation, and regulates production of inflammatory cytokines besides minimizing the harmful effects of ROS (113, 114). Some OSCs like isothiocyanates and garlic showed potential epigenetic activities in the modification of bacterial DNA or genes (115). Garlic as a source of prebiotics also has significant influence on creating a nutritional niche in GI tract. A study was conducted with IBD patients, examining the use of garlic extract as a therapeutic agent. Human whole blood cells and peripheral blood mononuclear cells (PBMC) were treated with various concentrations of garlic extract and showed immunosuppressive activity by significantly decreasing the amount of IFN-γ, TNF-α, and IL-2 produced by T cells (100).
Anti-inflammatory Effects of OSC Through Multiple Signaling Pathways
DAS is a potential natural phytonutrients from garlic and may prevent oxidative stress-induced inflammation of the airway (116). For example, an in vitro model was performed examining DAS (7.5 μM) treatment against TNF-α (10 ng/mL) exposure. Inflammation was inhibited by DAS through the downstream regulation of NF-κB and activator protein-1 (AP-1), and the ROS-induced expression of P13K/Akt signals (116). SFN in broccoli and cabbage also exert significant roles in downregulating pro-inflammatory cytokines and up-regulating anti-inflammatory cytokines, by interfering with the NF-κB or Nrf2 pathway. To some extent, the activation of the Nrf2/ARE pathway is also associated with the reduction of inflammation through repressing p38 MAPK (117), whilst COX-2 and iNOS expression can also be manipulated by SFN through the p38 MAPK-dependent pathway (118). Moreover, some other inflammatory genes, i.e., monocyte chemoattractant protein (MCP)-1, vascular cell adhesion molecule (VCAM)-1 and TNF-α expression can also be influenced by the p38 MAPK pathway (119, 120). Recently, an interesting result was obtained from a study conducted to examine the effects of SFN administration on reducing muscle inflammation in a dystrophin-deficient mdx mouse model. Administering SFN reduced muscle inflammation by the Nrf2-induced inhibition of NF-κB (p65) mRNA, and reduced the expression of pro-inflammatory cytokines (53). To this end, we can conclude that OSC is acting as a potential anti-inflammatory compound and actively induces effects in a dose-dependent manner.
Discussion
Phytonutrients in our diet play emerging roles in preventing inflammation and modulating metabolic pathways associated with developing chronic disorders (121). Existing data to date suggest that worldwide, heart disease, stroke, and COPD are the top three leading causes of death (122). A proper diet with a balance of phytonutrients may lower the risk of such diseases (121). Depending on their biosynthesis and biogenesis, food-derived bioactive compounds are influencing human health to prevent and/or remodel the genesis of chronic metabolic disorders. In humans, a considerable amount of IL-6 (15–35%) is also released from adipose tissue by T-cells and macrophages (25, 123), and there are various mechanisms by which inflammation can be reduced. Naturally occurring OSCs are recognized for their anti-inflammatory and medicinal purposes, as well as for inducing diverse health benefits. On the contrary, some well-controlled studies have displayed no preventative effects of applying various forms of OSC (124, 125). Therefore, it is mandatory to ensure our understanding and examination of the efficacy and the mechanisms of action of such compounds. Oxidative stress can lead to tissue injury and inflammation by releasing inflammatory cytokines and mediators, which can also act as secondary messengers to induce the functions of NF-κB (126). Moreover, the active functional subunits of NF-κB are both p50 and p65, and the inactivation of NF-κB is carried out by binding SFN to the thiol groups of the subunits. Therefore, the presence of OSC in the diet can directly or indirectly down-regulate the activity of NF-κB to mitigate inflammatory markers (127). In this regard, adding OSC to our diet may also help reduce the pathological expression of these biomarkers, thereby providing protective support against chronic diseases (25). Despite the inverse relationship between OSC and inflammation, some studies reported opposing biological effects. For example, a concentrated dose of garlic powder (200 mg/mL) has been reported to cause significant injury in the liver (128). Moreover, the beneficial effect of garlic may be lost after chronic administration of concentrated dose (2,000 mg/kg), and this could further cause significant mortality due to myocardial injury (129). It is important to consider the optimal supplementation regime for OSC, to induce the desired effects. Concurrently, knowing the bioavailability of the selected OSC is crucial, based on how it is absorbed, metabolized and utilized by the body.
Concluding Remark
It can be summarized that inflammation is associated with chronic disease in many ways. In addition, OSC may have an important role in preventing inflammation, as well as chronic diseases. Here, we have discussed the probable signaling pathways relating to how a reduction in inflammatory biomarkers could provide probable protection for human health against chronic diseases. Although a limited number of animal studies were evidenced, the results seem convincing, whilst in vivo and in vitro trials are being carried out to identify appropriate and feasible applications for phytonutrients, and to promote these as medicines against inflammation. Furthermore, advanced research is required to build a more thorough understanding of the anti-inflammatory functions of OSC.
Statements
Author contributions
RR: conceptualization, writing, and original draft preparation. KS and SM: review and editing. LR: critical evaluation and editing.
Funding
This work was partly supported by the Waseda University Grant for Special Research Projects 2019Q-056.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Hung HC Joshipura KJ Jiang R Hu FB Hunter D Smith-Warner SA et al . Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. (2004) 96:1577–84. 10.1093/jnci/djh296
2.
He K Hu FB Colditz GA Manson JE Willett WC Liu S . Changes in intake of fruits and vegetables in relation to risk of obesity and weight gain among middle-aged women. Int J Obes Relat Metab Disord. (2004) 28:1569–74. 10.1038/sj.ijo.0802795
3.
Boeing H Bechthold A Bub A Ellinger S Haller D Kroke A et al . Critical review: vegetables and fruit in the prevention of chronic diseases. Eur J Nutr. (2012) 51:637–63. 10.1007/s00394-012-0380-y
4.
Garrow JS James WPT Ralph A . Phytoprotectants. Human Nutrition and Dietetics. Edinburgh: Churchill Livingstone (2001). p. 417–25.
5.
Liu RH . Health benefits of phytochemicals in whole foods. In: TempleNJWilsonTJacobsJDR, editors. Nutritional Health: Strategies for Disease Prevention. Totowa, NJ: Humana Press (2012). p. 293–310. 10.1007/978-1-61779-894-8_13
6.
Kim S Kim DB Jin W Park J Yoon W Lee Y et al . Comparative studies of bioactive organosulphur compounds and antioxidant activities in garlic (Allium sativum L.), elephant garlic (Allium ampeloprasum L.) and onion (Allium cepa L.). Nat Prod Res. (2018) 32:1193–7. 10.1080/14786419.2017.1323211
7.
Putnik P Gabric D Roohinejad S Barba FJ Granato D Mallikarjunan K et al . An overview of organosulfur compounds from Allium spp.: from processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. (2019) 276:680–91. 10.1016/j.foodchem.2018.10.068
8.
Kris-Etherton PM Hecker KD Bonanome A Coval SM Binkoski AE Hilpert KF et al . Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. (2002) 113(Suppl. 9B):71s−88s. 10.1016/S0002-9343(01)00995-0
9.
Garrow JS James WPT Ralph A . Human Nutrition and Dietetics. Edinburgh: Churchill Livingstone (2001).
10.
Cartea González ME Velasco Pazos P . Glucosinolates in Brassica foods: bioavailability in food and significance for human health. Phytochem Rev. (2008) 7:213–29. 10.1007/s11101-007-9072-2
11.
Itakura Y Ichikawa M Mori Y Okino R Udayama M Morita T . How to distinguish garlic from the other Allium vegetables. J Nutr. (2001) 131:963s−7s. 10.1093/jn/131.3.963S
12.
Iciek M Kwiecien I Wlodek L . Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen. (2009) 50:247–65. 10.1002/em.20474
13.
Lawson LD Hunsaker SM . Allicin Bioavailability and Bioequivalence from garlic supplements and garlic foods. Nutrients. (2018) 10:812. 10.3390/nu10070812
14.
Liguori L Califano R Albanese D Raimo F Crescitelli A Di Matteo M . Chemical composition and antioxidant properties of five white onion (Allium cepa L.) landraces. J Food Qual. (2017) 2017:6873651. 10.1155/2017/6873651
15.
Cabello-Hurtado F Gicquel M Esnault M-A . Evaluation of the antioxidant potential of cauliflower (Brassica oleracea) from a glucosinolate content perspective. Food Chem. (2012) 132:1003–9. 10.1016/j.foodchem.2011.11.086
16.
Kushad MM Brown AF Kurilich AC Juvik JA Klein BP Wallig MA et al . Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agric Food Chem. (1999) 47:1541–8. 10.1021/jf980985s
17.
Gupta U . Brassica Vegetables. What's new about crop plants: novel discoveries of the 21st century. Enfield, NH: Science Publishers (2011) 379–93.
18.
Yeh CT Yen GC . Chemopreventive functions of sulforaphane: a potent inducer of antioxidant enzymes and apoptosis. J Funct Foods. (2009) 1:23–32. 10.1016/j.jff.2008.09.002
19.
Thejass P Kuttan G . Immunomodulatory activity of Sulforaphane, a naturally occurring isothiocyanate from broccoli (Brassica oleracea). Phytomedicine. (2007) 14:538–45. 10.1016/j.phymed.2006.09.013
20.
Qi T Xu F Yan X Li S Li H . Sulforaphane exerts anti-inflammatory effects against lipopolysaccharide-induced acute lung injury in mice through the Nrf2/ARE pathway. Int J Mol Med. (2016) 37:182–8. 10.3892/ijmm.2015.2396
21.
Angeloni C Malaguti M Rizzo B Barbalace MC Fabbri D Hrelia S . Neuroprotective effect of sulforaphane against methylglyoxal cytotoxicity. Chem Res Toxicol. (2015) 28:1234–45. 10.1021/acs.chemrestox.5b00067
22.
Piao CS Gao S Lee GH Kim DS Park BH Chae SW et al . Sulforaphane protects ischemic injury of hearts through antioxidant pathway and mitochondrial K(ATP) channels. Pharmacol Res. (2010) 61:342–8. 10.1016/j.phrs.2009.11.009
23.
Romeo L Iori R Rollin P Bramanti P Mazzon E . Isothiocyanates: an overview of their antimicrobial activity against human infections. Molecules. (2018) 23:624. 10.3390/molecules23030624
24.
Kumar V Abbas AK Fausto N Aster JC . Robins and Cortan Pathologic Basis of Disease. Inflammation and Repair. 9th ed.Philadelphia, PA: Elsevier health sciences (2014). p. 69–112.
25.
Patel H Patel V . Inflammation and metabolic syndrome-an overview. Curr Res Nutr Food Sci J. (2015) 3:263–8. 10.12944/CRNFSJ.3.3.10
26.
Nijhuis J Rensen SS Slaats Y van Dielen FM Buurman WA Greve JW . Neutrophil activation in morbid obesity, chronic activation of acute inflammation. Obesity. (2009) 17:2014–8. 10.1038/oby.2009.113
27.
Medzhitov R . Origin and physiological roles of inflammation. Nature. (2008) 454:428–35. 10.1038/nature07201
28.
Hotamisligil GS . Inflammation and metabolic disorders. Nature. (2006) 444:860–7. 10.1038/nature05485
29.
Esser N Legrand-Poels S Piette J Scheen AJ Paquot N . Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabet Res Clin Pract. (2014) 105:141–50. 10.1016/j.diabres.2014.04.006
30.
Sahu SC . Dual role of organosulfur compounds in foods: a review. J Environ Sci Health. (2002) 20:61–76. 10.1081/GNC-120005388
31.
What Vegetable is Most Consumed in Japan. (2014). Available online at: http://nbakki.hatenablog.com/entry/2014/03/31/124219 (accessed March 20, 2019).
32.
Agudo A Ibanez R Amiano P Ardanaz E Barricarte A Berenguer A et al . Consumption of cruciferous vegetables and glucosinolates in a Spanish adult population. Eur J Clin Nutr. (2008) 62:324–31. 10.1038/sj.ejcn.1602750
33.
Lawson LD Wang ZJ . Allicin and allicin-derived garlic compounds increase breath acetone through allyl methyl sulfide: use in measuring allicin bioavailability. J Agric Food Chem. (2005) 53:1974–83. 10.1021/jf048323s
34.
Nagae S Ushijima M Hatono S Imai J Kasuga S Matsuura H et al . Pharmacokinetics of the garlic compound S-allylcysteine. Planta Medica. (1994) 60:214–7. 10.1055/s-2006-959461
35.
Kassahun K Davis M Hu P Martin B Baillie T . Biotransformation of the naturally occurring isothiocyanate sulforaphane in the rat: identification of phase I metabolites and glutathione conjugates. Chem Res Toxicol. (1997) 10:1228–33. 10.1021/tx970080t
36.
Egner PA Chen JG Wang JB Wu Y Sun Y Lu JH et al . Bioavailability of Sulforaphane from two broccoli sprout beverages: results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev Res. (2011) 4:384–95. 10.1158/1940-6207.CAPR-10-0296
37.
Shuruq A Noura A Ghada A Alammari G Kavita MS Maha AT et al . Role of phytochemicals in health and nutrition. BAOJ Nutr. (2017) 3:28. 10.24947/baojn/3/2/00128
38.
Simon HU . Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev. (2003) 193:101–10. 10.1034/j.1600-065X.2003.00038.x
39.
Pathophysiology of inflammation Budapest, Hungary. (2016). Available online at: http://semmelweis.hu/oralbiologia/files/2016/02/2016_Pathophysiology-of-inflammation.pdf (accessed June 12, 2019).
40.
Petersen AM Pedersen BK . The anti-inflammatory effect of exercise. J Appl Physiol. (2005) 98:1154–62. 10.1152/japplphysiol.00164.2004
41.
Pyne DB . Exercise-induced muscle damage and inflammation: a review. Aust J Sci Med Sport. (1994) 26:49–58.
42.
Suzuki K . Cytokine response to exercise and its modulation. Antioxidants. (2018) 7:17. 10.3390/antiox7010017
43.
Suzuki K Totsuka M Nakaji S Yamada M Kudoh S Liu Q et al . Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J Appl Physiol. (1999) 87:1360–7. 10.1152/jappl.1999.87.4.1360
44.
Suzuki K . Characterization of exercise-induced cytokine release, the impacts on the body, the mechanisms and modulations. Int J Sports Exerc Med. (2019) 5:122. 10.23937/2469-5718/1510122
45.
Powers SK Jackson MJ . Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. (2008) 88:1243–76. 10.1152/physrev.00031.2007
46.
Gleeson M Nieman DC Pedersen BK . Exercise, nutrition and immune function. J Sports Sci. (2004) 22:115–25. 10.1080/0264041031000140590
47.
Suzuki K . Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules. (2019) 9:223. 10.3390/biom9060223
48.
Ruhee R Ma S Suzuki K . Protective Effects of sulforaphane on exercise-induced organ damage via inducing antioxidant defense responses. Antioxidants. (2020) 9:136. 10.3390/antiox9020136
49.
Vazquez-Prieto MA Miatello RM . Organosulfur compounds and cardiovascular disease. Mol Aspects Med. (2010) 31:540–5. 10.1016/j.mam.2010.09.009
50.
Arreola R Quintero-Fabian S Lopez-Roa RI Flores-Gutierrez EO Reyes-Grajeda JP Carrera-Quintanar L et al . Immunomodulation and anti-inflammatory effects of garlic compounds. J Immunol Res. (2015) 2015:401630. 10.1155/2015/401630
51.
Kuttan G . Immunomodulatory effect of some naturally occuring sulphur-containing compounds. J Ethnopharmacol. (2000) 72:93–9. 10.1016/S0378-8741(00)00211-7
52.
Malaguti M Angeloni C Garatachea N Baldini M Leoncini E Collado PS et al . Sulforaphane treatment protects skeletal muscle against damage induced by exhaustive exercise in rats. J Appl Physiol. (2009) 107:1028–36. 10.1152/japplphysiol.00293.2009
53.
Sun CC Li SJ Yang CL Xue RL Xi YY Wang L et al . Sulforaphane attenuates muscle inflammation in dystrophin-deficient Mdx mice via Nrf2-mediated inhibition of NF-κB signaling pathway. J Biol Chem. (2015) 290:17784–95. 10.1074/jbc.M115.655019
54.
Goncharov N Orekhov AN Voitenko N Ukolov A Jenkins R Avdonin P . Organosulfur compounds as nutraceuticals. Nutraceuticals. (2016) 2016:555–68. 10.1016/B978-0-12-802147-7.00041-3
55.
Chen L Deng H Cui H Fang J Zuo Z Deng J et al . Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. (2018) 9:7204–18. 10.18632/oncotarget.23208
56.
Hoffman HM Broderick L . The role of the inflammasome in patients with autoinflammatory diseases. J Allergy Clin Immunol. (2016) 138:3–14. 10.1016/j.jaci.2016.05.001
57.
Dinarello CA . Proinflammatory cytokines. Chest. (2000) 118:503–8. 10.1378/chest.118.2.503
58.
Libby P . Inflammation in atherosclerosis. Arterioscl Throm Vasc Biol. (2012) 32:2045–51. 10.1161/ATVBAHA.108.179705
59.
Kang YE Kim JM Joung KH Lee JH You BR Choi MJ et al . The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS ONE. (2016) 11:e0154003. 10.1371/journal.pone.0154003
60.
Moriarty RM Naithani R Surve B . Organosulfur compounds in cancer chemoprevention. Mini Rev Med Chem. (2007) 7:827–38. 10.2174/138955707781387939
61.
Vozarova B Weyer C Hanson K Tataranni PA Bogardus C Pratley RE . Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res. (2001) 9:414–7. 10.1038/oby.2001.54
62.
Yudkin JS Kumari M Humphries SE Mohamed-Ali V . Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link?Atherosclerosis. (2000) 148:209–14. 10.1016/S0021-9150(99)00463-3
63.
Pratley RE Wilson C Bogardus C . Relation of the white blood cell count to obesity and insulin resistance: effect of race and gender. Obes Res. (1995) 3:563–71. 10.1002/j.1550-8528.1995.tb00191.x
64.
Maachi M Pieroni L Bruckert E Jardel C Fellahi S Hainque B et al . Systemic low-grade inflammation is related to both circulating and adipose tissue TNFalpha, leptin and IL-6 levels in obese women. Int J Obes Relat Metab Disord. (2004) 28:993–7. 10.1038/sj.ijo.0802718
65.
Keiss HP Dirsch VM Hartung T Haffner T Trueman L Auger J et al . Garlic (Allium sativum L.) modulates cytokine expression in lipopolysaccharide-activated human blood thereby inhibiting NF-kappaB activity. J Nutr. (2003) 133:2171–5. 10.1093/jn/133.7.2171
66.
Amagase H Petesch BL Matsuura H Kasuga S Itakura Y . Intake of garlic and its bioactive components. J Nutr. (2001) 131:955s−62s. 10.1093/jn/131.3.955S
67.
Xu C Mathews AE Rodrigues C Eudy BJ Rowe CA O'Donoughue A et al . Aged garlic extract supplementation modifies inflammation and immunity of adults with obesity: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr ESPEN. (2018) 24:148–55. 10.1016/j.clnesp.2017.11.010
68.
Ma S Suzuki K . Toll-like receptor 4: target of lipotoxicity and exercise-Induced anti-inflammatory effect. Ann Nutr Food Sci. (2018) 2:1027.
69.
Ahn KS Aggarwal BB . Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann N Y Acad Sci. (2005) 1056:218–33. 10.1196/annals.1352.026
70.
Mittal M Siddiqui MR Tran K Reddy SP Malik AB . Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. (2014) 20:1126–67. 10.1089/ars.2012.5149
71.
Ruhee RT Ma S Suzuki K . Sulforaphane protects cells against lipopolysaccharide-stimulated inflammation in murine macrophages. Antioxidants. (2019) 8:577. 10.3390/antiox8120577
72.
Lee DY Li H Lim HJ Lee HJ Jeon R Ryu JH . Anti-inflammatory activity of sulfur-containing compounds from garlic. J Med Food. (2012) 15:992–9. 10.1089/jmf.2012.2275
73.
Cheung KL Khor TO Kong AN . Synergistic effect of combination of phenethyl isothiocyanate and sulforaphane or curcumin and sulforaphane in the inhibition of inflammation. Pharm Res. (2009) 26:224–31. 10.1007/s11095-008-9734-9
74.
Ide N Lau BH . Garlic compounds minimize intracellular oxidative stress and inhibit nuclear factor-kappa b activation. J Nutr. (2001) 131:1020s−6s. 10.1093/jn/131.3.1020S
75.
Li D Mehta JL . Oxidized LDL, a critical factor in atherogenesis. Cardiovasc Res. (2005) 68:353–4. 10.1016/j.cardiores.2005.09.009
76.
Kleijnen J Knipschild P ter Riet G . Garlic, onions and cardiovascular risk factors. A review of the evidence from human experiments with emphasis on commercially available preparations. Br J Clin Pharmacol. (1989) 28:535–44. 10.1111/j.1365-2125.1989.tb03539.x
77.
Liu L Yeh YY . Water-soluble organosulfur compounds of garlic inhibit fatty acid and triglyceride syntheses in cultured rat hepatocytes. Lipids. (2001) 36:395–400. 10.1007/s11745-001-0734-4
78.
Babu PS Srinivasan K . Influence of dietary capsaicin and onion on the metabolic abnormalities associated with streptozotocin induced diabetes mellitus. Mol Cell Biochem. (1997) 175:49–57. 10.1023/A:1006881027166
79.
Jazwa A Rojo AI Innamorato NG Hesse M Fernandez-Ruiz J Cuadrado A . Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid Redox Signal. (2011) 14:2347–60. 10.1089/ars.2010.3731
80.
Ahn H Kwon HM Lee E Kim P-H Jeung E-B Lee G-S . Role of inflammasome regulation on immune modulators. J Biomed Res. (2018) 32:401–10. 10.7555/JBR.32.20170120
81.
Baker CS Hall RJ Evans TJ Pomerance A Maclouf J Creminon C et al . Cyclooxygenase-2 is widely expressed in atherosclerotic lesions affecting native and transplanted human coronary arteries and colocalizes with inducible nitric oxide synthase and nitrotyrosine particularly in macrophages. Arterioscl Throm Vasc Biol. (1999) 19:646–55. 10.1161/01.ATV.19.3.646
82.
Shan Y Zhao R Geng W Lin N Wang X Du X et al . Protective effect of sulforaphane on human vascular endothelial cells against lipopolysaccharide-induced inflammatory damage. Cardiovasc Toxicol. (2010) 10:139–45. 10.1007/s12012-010-9072-0
83.
Kelly B O'Neill LAJ . Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. (2015) 25:771–84. 10.1038/cr.2015.68
84.
Vahid F Zand H Nosrat-Mirshekarlou E Najafi R Hekmatdoost A . The role dietary of bioactive compounds on the regulation of histone acetylases and deacetylases: a review. Gene. (2015) 562:8–15. 10.1016/j.gene.2015.02.045
85.
Hanisch UK . Microglia as a source and target of cytokines. Glia. (2002) 40:140–55. 10.1002/glia.10161
86.
Brandenburg LO Kipp M Lucius R Pufe T Wruck CJ . Sulforaphane suppresses LPS-induced inflammation in primary rat microglia. Inflamm Res. (2010) 59:443–50. 10.1007/s00011-009-0116-5
87.
Raison CL Capuron L Miller AH . Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. (2006) 27:24–31. 10.1016/j.it.2005.11.006
88.
Miller AH Maletic V Raison CL . Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. (2009) 65:732–41. 10.1016/j.biopsych.2008.11.029
89.
Martin-de-Saavedra MD Budni J Cunha MP Gomez-Rangel V Lorrio S Del Barrio L et al . Nrf2 participates in depressive disorders through an anti-inflammatory mechanism. Psychoneuroendocrinology. (2013) 38:2010–22. 10.1016/j.psyneuen.2013.03.020
90.
Barnes PJ . New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discov. (2013) 12:543–59. 10.1038/nrd4025
91.
Lee J Ahn H Hong EJ An BS Jeung EB Lee GS . Sulforaphane attenuates activation of NLRP3 and NLRC4 inflammasomes but not AIM2 inflammasome. Cell Immunol. (2016) 306–7:53–60. 10.1016/j.cellimm.2016.07.007
92.
Ahn H Kim J Lee MJ Kim YJ Cho YW Lee GS . Methylsulfonylmethane inhibits NLRP3 inflammasome activation. Cytokine. (2015) 71:223–31. 10.1016/j.cyto.2014.11.001
93.
Lee HH Han MH Hwang HJ Kim GY Moon SK Hyun JW et al . Diallyl trisulfide exerts anti-inflammatory effects in lipopolysaccharide-stimulated RAW 264.7 macrophages by suppressing the Toll-like receptor 4/nuclear factor-kappaB pathway. Int J Mol Med. (2015) 35:487–95. 10.3892/ijmm.2014.2036
94.
Heiss E Herhaus C Klimo K Bartsch H Gerhauser C . Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. (2001) 276:32008–15. 10.1074/jbc.M104794200
95.
Woo KJ Kwon TK . Sulforaphane suppresses lipopolysaccharide-induced cyclooxygenase-2 (COX-2) expression through the modulation of multiple targets in COX-2 gene promoter. Int Immunopharm. (2007) 7:1776–83. 10.1016/j.intimp.2007.09.018
96.
Lee HS Lee CH Tsai HC Salter DM . Inhibition of cyclooxygenase 2 expression by diallyl sulfide on joint inflammation induced by urate crystal and IL-1beta. Osteoarthr Cartel. (2009) 17:91–9. 10.1016/j.joca.2008.05.010
97.
Dirsch VM Kiemer AK Wagner H Vollmar AM . Effect of allicin and ajoene, two compounds of garlic, on inducible nitric oxide synthase. Atherosclerosis. (1998) 139:333–9. 10.1016/S0021-9150(98)00094-X
98.
Kim KM Chun SB Koo MS Choi WJ Kim TW Kwon YG et al . Differential regulation of NO availability from macrophages and endothelial cells by the garlic component S-allyl cysteine. Free Radic Biol Med. (2001) 30:747–56. 10.1016/S0891-5849(01)00460-9
99.
Chang HP Chen YH . Differential effects of organosulfur compounds from garlic oil on nitric oxide and prostaglandin E2 in stimulated macrophages. Nutrition. (2005) 21:530–6. 10.1016/j.nut.2004.07.018
100.
Hodge G Hodge S Han P . Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry. (2002) 48:209–15. 10.1002/cyto.10133
101.
Wang C Wang C . Anti-nociceptive and anti-inflammatory actions of sulforaphane in chronic constriction injury-induced neuropathic pain mice. Inflammopharmacology. (2017) 25:99–106. 10.1007/s10787-016-0307-y
102.
Chang HP Huang SY Chen YH . Modulation of cytokine secretion by garlic oil derivatives is associated with suppressed nitric oxide production in stimulated macrophages. J Agric Food Chem. (2005) 53:2530–4. 10.1021/jf048601n
103.
Banerjee SK Mukherjee PK Maulik SK . Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. (2003) 17:97–106. 10.1002/ptr.1281
104.
Alnaqeeb MA Thomson M Bordia T Ali M . Histopathological effects of garlic on liver and lung of rats. Toxicol Lett. (1996) 85:157–64. 10.1016/0378-4274(96)03658-2
105.
Thomson M Alnaqeeb MA Bordia T Al-Hassan JM Afzal M Ali M . Effects of aqueous extract of onion on the liver and lung of rats. J Ethnopharmacol. (1998) 61:91–9. 10.1016/S0378-8741(98)00004-X
106.
Mora C Navarro JF . Inflammation and pathogenesis of diabetic nephropathy. Metabolism. (2004) 53:265–6. 10.1016/j.metabol.2003.11.005
107.
Sujithra K Srinivasan S Indumathi D Vinothkumar V . Allyl methyl sulfide, an organosulfur compound alleviates hyperglycemia mediated hepatic oxidative stress and inflammation in streptozotocin - induced experimental rats. Biomed Pharmacother. (2018) 107:292–302. 10.1016/j.biopha.2018.07.162
108.
Georgia S Catherine HK . The immunomodulation and anti-inflammatory effects of garlic organosulfur compounds in cancer chemoprevention. Anti-Cancer Agents Med Chem. (2014) 14:233–40. 10.2174/18715206113136660370
109.
Zhou XF Ding ZS Liu NB . Allium vegetables and risk of prostate cancer: evidence from 132,192 subjects. Asian Pac J Cancer Prev. (2013) 14:4131–4. 10.7314/APJCP.2013.14.7.4131
110.
Borkowska A Knap N Antosiewicz J . Diallyl trisulfide is more cytotoxic to prostate cancer cells PC-3 than to noncancerous epithelial cell line PNT1A: a possible role of p66Shc signaling axis. Nutr Cancer. (2013) 65:711–7. 10.1080/01635581.2013.789115
111.
Fujino S Andoh A Bamba S Ogawa A Hata K Araki Y et al . Increased expression of interleukin 17 in inflammatory bowel disease. Gut. (2003) 52:65–70. 10.1136/gut.52.1.65
112.
Celiberto LS Graef FA Healey GR Bosman ES Jacobson K Sly LM et al . Inflammatory bowel disease and immunonutrition: novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology. (2018) 155:36–52. 10.1111/imm.12939
113.
Liu Q Shimoyama T Suzuki K Umeda T Nakaji S Sugawara K . Effect of sodium butyrate on reactive oxygen species generation by human neutrophils. Scand J Gastroenterol. (2001) 36:744–50. 10.1080/003655201300192012
114.
Correa-Oliveira R Fachi JL Vieira A Sato FT Vinolo MA . Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol. (2016) 5:e73. 10.1038/cti.2016.17
115.
Hullar MA Fu BC . Diet, the gut microbiome, and epigenetics. Cancer J. (2014) 20:170–5. 10.1097/PPO.0000000000000053
116.
Ho CY Weng CJ Jhang JJ Cheng YT Huang SM Yen GC . Diallyl sulfide as a potential dietary agent to reduce TNF-α- and histamine-induced proinflammatory responses in A7r5 cells. Mol Nutr Food Res. (2014) 58:1069–78. 10.1002/mnfr.201300617
117.
Shan Y Wang X Wang W He C Bao Y . p38 MAPK plays a distinct role in sulforaphane-induced up-regulation of ARE-dependent enzymes and down-regulation of COX-2 in human bladder cancer cells. Oncol Rep. (2010) 23:1133–8. 10.3892/or_00000742
118.
Shan Y Wu K Wang W Wang S Lin N Zhao R et al . Sulforaphane down-regulates COX-2 expression by activating p38 and inhibiting NF-kappaB-DNA-binding activity in human bladder T24 cells. Int J Oncol. (2009) 34:1129–34. 10.3892/ijo_00000240
119.
Xu W Yan M Lu L Sun L Theze J Zheng Z et al . The p38 MAPK pathway is involved in the IL-2 induction of TNF-beta gene via the EBS element. Biochem Biophys Res Commun. (2001) 289:979–86. 10.1006/bbrc.2001.6069
120.
Pietersma A Tilly BC Gaestel M de Jong N Lee JC Koster JF et al . p38 mitogen activated protein kinase regulates endothelial VCAM-1 expression at the post-transcriptional level. Biochem Biophys Res Commun. (1997) 230:44–8. 10.1006/bbrc.1996.5886
121.
Craig WJ . Phytochemicals: guardians of our health. J Am Diet Assoc. (1997) 97(10 Suppl. 2):S199–204. 10.1016/S0002-8223(97)00765-7
122.
WHO . The Top 10 Causes of Death. (2018). Available online at: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed December 02, 2020).
123.
Ouchi N Parker JL Lugus JJ Walsh K . Adipokines in inflammation and metabolic disease. Nat Rev Immunol. (2011) 11:85–97. 10.1038/nri2921
124.
Isaacsohn JL Moser M Stein EA Dudley K Davey JA Liskov E et al . Garlic powder and plasma lipids and lipoproteins: a multicenter, randomized, placebo-controlled trial. Arch Intern Med. (1998) 158:1189–94. 10.1001/archinte.158.11.1189
125.
Byrne DJ Neil HA Vallance DT Winder AF . A pilot study of garlic consumption shows no significant effect on markers of oxidation or sub-fraction composition of low-density lipoprotein including lipoprotein(a) after allowance for non-compliance and the placebo effect. Clin Chim Acta. (1999) 285:21–33. 10.1016/S0009-8981(99)00063-7
126.
Grimm S Baeuerle PA . The inducible transcription factor NF-kappa B: structure-function relationship of its protein subunits. Biochem J. (1993) 290:297–308. 10.1042/bj2900297
127.
Mithen R . Sulphur-containing compounds. In: CrozierACliffordMNAshiharaH, editors. Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet. Oxford: Blackwell Publishing Ltd. (2008). p. 25–46. 10.1002/9780470988558.ch2
128.
Egen-Schwind C Eckard R Kemper F . Metabolism of garlic constituents in the isolated perfused rat liver. Planta Med. (1992) 58:301–5. 10.1055/s-2006-961471
129.
Banerjee SK Maulik M Mancahanda SC Dinda AK Gupta SK Maulik SK . Dose-dependent induction of endogenous antioxidants in rat heart by chronic administration of garlic. Life Sci. (2002) 70:1509–18. 10.1016/S0024-3205(01)01514-4
Summary
Keywords
organosulfur compounds, inflammation, chronic diseases, cytokines, NF-κB
Citation
Ruhee RT, Roberts LA, Ma S and Suzuki K (2020) Organosulfur Compounds: A Review of Their Anti-inflammatory Effects in Human Health. Front. Nutr. 7:64. doi: 10.3389/fnut.2020.00064
Received
19 August 2019
Accepted
20 April 2020
Published
02 June 2020
Volume
7 - 2020
Edited by
Brandt D. Pence, University of Memphis, United States
Reviewed by
Mourad Aribi, University of Abou Bekr Belkaïd, Algeria; Wasaporn Chanput, Kasetsart University, Thailand
Updates
Copyright
© 2020 Ruhee, Roberts, Ma and Suzuki.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katsuhiko Suzuki katsu.suzu@waseda.jp
This article was submitted to Nutritional Immunology, a section of the journal Frontiers in Nutrition
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.