- 1Faculty of Pharmacy, Middle East University, Amman, Jordan
- 2College of Pharmacy, Alnoor University, Nineveh, Iraq
- 3College of MLT, Ahl Al Bayt University, Karbala, Iraq
- 4Marwadi University Research Center, Department of Pharmaceutical Sciences, Faculty of Health Sciences, Marwadi University, Rajkot, Gujarat, India
- 5Management and Science University, Shah Alam, Selangor, Malaysia
- 6Department of Endocrinology, National Institute of Medical Sciences, NIMS University Rajasthan, Jaipur, India
- 7Centre for Research Impact and Outcome, Chitkara University Institute of Engineering and Technology, Chitkara University, Rajpura, Punjab, India
- 8Department of Public Health and Healthcare Management, Samarkand State Medical University, Samarkand, Uzbekistan
- 9College of Nursing, National University of Science and Technology, Nasiriyah, Iraq
- 10Pharmacy College, Al-Farahidi University, Baghdad, Iraq
- 11Department of Pharmacy, Al-Zahrawi University College, Karbala, Iraq
- 12Gilgamesh Ahliya University, Baghdad, Iraq
Background: This meta-analysis evaluates the effects of omega-3 supplementation on metabolic, inflammatory, and oxidative stress in pregnancy women by synthesizing findings from randomized controlled trials (RCTs), as existing evidence remains inconclusive.
Methods: A systematic search was conducted using PubMed, Scopus, and Web of Science until July 2024. Random-effects models were applied to estimate each outcome's standardized mean difference (SMD) and 95% confidence intervals (CI).
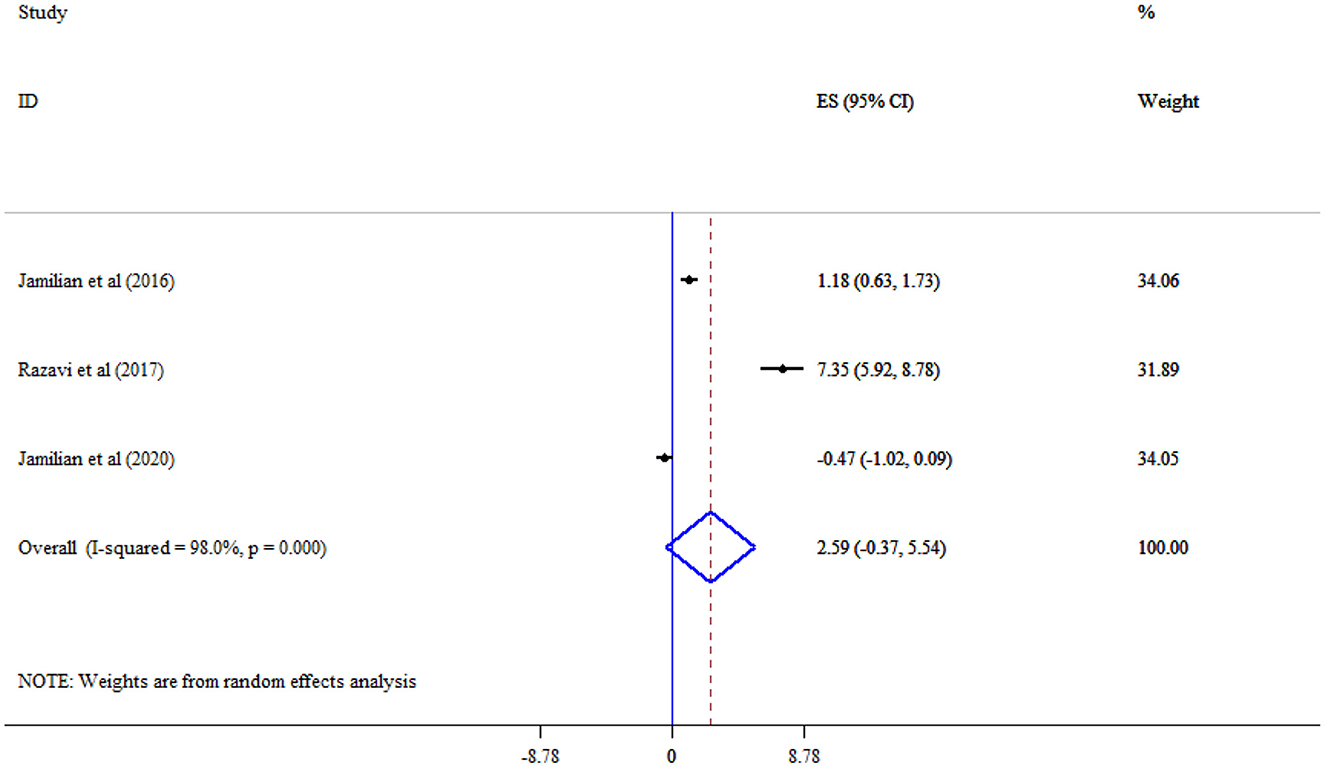
Results: A total of 14 studies were included in the meta-analysis. The duration of omega-3 supplementation ranged from 6 to 29 weeks. Omega-3 supplementation did not have a significant effect on FBS (SMD = −0.74, 95% CI: −1.94, 0.45), and insulin (SMD = −0.76, 95% CI: −1.77, 0.24), TC (SMD = 0.11, 95% CI: −0.20, 0.42), and LDL-C (SMD = 0.32, 95% CI: −0.17, 0.81), IL-6 (SMD = 2.12, 95% CI: −0.56, 4.80), MDA (SMD = −1.67, 95% CI: −3.39, 0.05), and TAC (SMD = 2.59, 95% CI: −0.37, 5.54). However, triglyceride (SMD = −0.96, 95% CI: −1.77, −0.16) and CRP (SMD = −0.98, 95% CI: −1.86, −0.11) significantly decreased, and HDL-C cholesterol levels significantly increased (SMD = 0.72, 95% CI: 0.21, 1.22) following omega-3 supplementation.
Conclusion: This study suggests omega-3 supplementation may improve lipid profiles and reduce inflammatory biomarkers during pregnancy. However, the presence of heterogeneity across trials highlights the need for further well-conducted studies. Thus, findings should be interpreted with caution.
Introduction
Pregnant women undergo substantial metabolic adaptations—progressive insulin resistance, dyslipidemia, heightened inflammation, and oxidative stress—particularly pronounced in gestational diabetes mellitus (GDM), elevating risks of macrosomia, preeclampsia, and long-term metabolic sequelae in both mother and offspring (1). Nutritional strategies that target these disturbances are of significant clinical interest. Omega-3 polyunsaturated fatty acids (PUFAs), notably EPA and DHA, exhibit insulin-sensitizing, lipid-regulating, anti-inflammatory, and antioxidant properties in general populations with type 2 diabetes and metabolic syndrome (2–4).
Although existing RCT evidence in pregnant populations is limited, a focused systematic review and meta-analysis of trials up to 2019 demonstrated that omega-3 supplementation significantly improved fasting plasma glucose, HOMA-IR, and reduced high-sensitivity C-reactive protein (hs-CRP) in women with GDM, while effects on nitric oxide, lipid fractions, IL-6, and malondialdehyde were not significant (5). Another meta-analysis also found reductions in fasting insulin, triglycerides, VLDL-cholesterol, modest HDL-cholesterol increases, and CRP declines (6). RCTs involving omega-3 plus vitamin E co-supplementation in GDM women also report significant increases in total antioxidant capacity (TAC), nitric oxide (NO), and reductions in malondialdehyde (MDA). However, effects on glutathione were inconsistent (7). A network meta-analysis further confirmed that omega-3 with vitamin E outperformed placebo in improving oxidative stress markers (TAC and MDA) in GDM pregnancies (8–10). In summary, while individual trials yield heterogeneous findings, pooled data suggest that omega-3 supplementation—especially when combined with antioxidants—can beneficially modulate glycemic indices, lipid metabolism, inflammation, and redox balance in pregnant women with GDM. Yet, dosage, intervention duration, and outcome selection inconsistencies highlight the imperative for a comprehensive systematic review and meta-analysis. Accordingly, this study aims to quantify the effects of omega-3 PUFAs on glycemic, lipid, inflammatory, and oxidative stress biomarkers in pregnant women, providing evidence-informed insights for clinical nutrition guidelines.
Materials and methods
This study was performed following the PRISMA (Preferred Reporting Items of Systematic Reviews and Meta-Analysis) statement guidelines (11). The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database.
Search strategy
A thorough literature review was conducted using Web of Science, Scopus, and PubMed, including all records up to July 2024. The search terms included various combinations of keywords related to pregnancy, gestation, omega-3 fatty acids (e.g., Omega-3 Fatty Acid, n-3 Oil, n-3 Fatty Acids, n-3 Polyunsaturated Fatty Acids, Omega-3 Polyunsaturated Fatty Acid), and metabolic outcomes (e.g., lipid, total cholesterol (TC), high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], triglycerides [TG], fasting blood glucose, inflammation, and oxidative stress) and intervention, controlled trial, randomized controlled trial, clinical trial, RCT (see Supplementary Table 1 for specific search terms). Additionally, we examined the reference lists of existing systematic reviews and meta-analyses to ensure comprehensive coverage. No language restrictions were applied. Our search methodology was based on the Cochrane PICO framework: (1) Participants—pregnant women at any stage of gestation or age; (2) Intervention—omega-3 supplementation; (3) Comparator –placebo, no intervention, or lower doses of the same supplement; and (4) Outcomes—metabolic and biochemical measures such as lipid profile (total cholesterol, HDL-C, LDL-C, triglycerides), fasting glucose, inflammatory markers, and oxidative stress indicators.
Study selection
We included randomized controlled trials and other controlled study designs involving pregnant women of any age, body mass index, or pregnancy-related health conditions such as gestational diabetes, preeclampsia, or pregnancy-induced hypertension. The eligible interventions included omega-3 fatty acids (EPA, DHA, or ALA) alone or with common pregnancy supplements like vitamin E, D, or folic acid. Studies were excluded if they involved infants, children, or adolescents; were published in languages other than English; were animal or in vitro experiments; or were secondary sources such as systematic reviews, meta-analyses, editorials, conference abstracts, gray literature, or book chapters.
Data extraction
Two separate researchers reviewed and selected studies to reduce bias and maintain uniformity. Disagreements were settled through discussion, with the principal investigator acting as a tiebreaker for unresolved issues—a standard approach in systematic reviews. Studies that appeared potentially relevant were obtained in full and evaluated independently by both researchers, with exclusion reasons recorded in line with PRISMA standards. When key outcome data were absent or inconsistently reported, we emailed the authors to obtain the needed information. If no response was received or the data remained inaccessible, the outcomes were omitted or marked as missing.
We gathered the specified data using standardized forms in all the studies included. This data encompassed the first author's name, publication year, location of the study, type of trial (either parallel or crossover), characteristics of participants such as gestational age and health condition, the number of participants, length of the intervention, the form and amount of omega-3 used, details on any additional supplements, and the outcomes measured—including glycemic, lipid, inflammatory, and oxidative stress markers. When feasible, two researchers independently extracted the data and verified it against each other's work to confirm its accuracy and completeness.
Assessment of the quality of studies
Two reviewers independently assessed the methodological quality of the included randomized controlled trials. Any disagreements between reviewers were resolved through discussion until consensus was reached. The evaluation was conducted using the Cochrane Collaboration's Risk of Bias tool (RoB 2) (12).
Quantitative data synthesis and statistical analysis
The mean outcome change was determined by subtracting the final follow-up measurement from the baseline. To compute the standard deviation of this mean change, the following formula was applied: SD = Square root of [(SD pre-treatment)2 + (SD post-treatment)2 – (2R × SD pre-treatment × SD post-treatment)], assuming a correlation coefficient (R) of 0.5 (13). Study heterogeneity was assessed using Cochran's Q test and I2 test, with values ≥50% (or p < 0.05) indicating significant heterogeneity, where the random effects model was applied for reporting (14). Subgroup analyses were performed based on various covariates to investigate possible reasons for heterogeneity across combined studies. When fewer than 10 studies reported outcomes, formal assessments for publication bias, such as funnel plots and Egger's test, were not conducted, following established standards, because these methods lack sufficient statistical power with small sample sizes (15). A meta-regression analysis examined how study-level covariates—such as maternal overweight or obesity, GDM or preeclampsia status, and the risk-of-bias score—might influence the effect estimates. A sensitivity analysis using the leave-one-out approach was conducted to evaluate how each study influences the overall effect size estimate (16). Statistical significance was defined as P < 0.05.
Results
Study selection process
The study selection process is described in Figure 1, following the PRISMA 2020 standards for systematic reviews. A total of 3,742 records were initially identified. After removing 1,863 duplicates, 1,879 unique entries were subjected to title and abstract screening. Of these, 34 full-text articles were assessed for eligibility based on specific inclusion and exclusion criteria. In the end, 14 studies had enough data to be included in the meta-analysis (7, 9, 10, 17–27).
Study characteristics
The 14 RCTs included in the meta-analysis were conducted across diverse geographic settings: seven in Iran; two in the USA; and one each in Chile, Finland, Germany, Norway, and Spain (Table 1). Participants were enrolled between 10 and 29 weeks of gestation, representing early and mid-pregnancy time points. Omega-3 supplementation doses ranged from 300 to 4,000 mg per day, administered as fish oil preparations (containing DHA, EPA, or both) or flaxseed oil formulations rich in ALA. The study populations encompassed healthy pregnant women, women with overweight or obesity, and those diagnosed with GDM. Supplementation durations varied substantially, spanning 6–29 weeks, and covered interventions initiated from early pregnancy through to late gestation.
Risk of bias assessment
Table 2 details the risk of bias evaluation for the included randomized controlled trials. Among the 14 RCTs included, most showed low risk of bias for randomization, deviation from intended interventions, missing outcome data and outcome measurement. However, selective reporting was a concern in 13 studies due to lack of existing protocols or pre-registration. In total, 13 trials were assessed as having some concerns due to reporting limitations, while one study was assessed as being at low risk of bias. No studies were classified as high risk, indicating generally strong methodology with minor concerns about transparency of reporting.
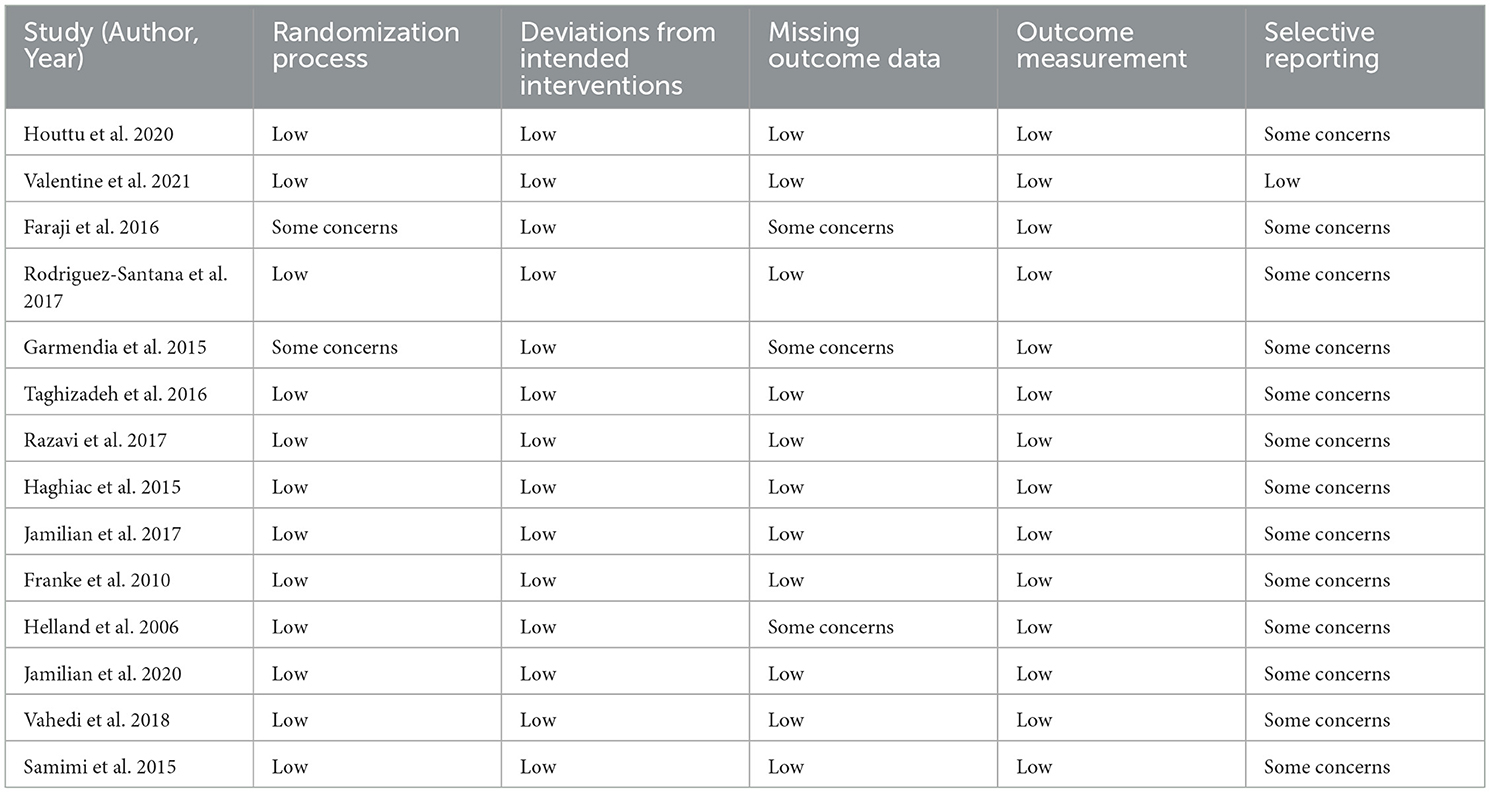
Table 2. Summarizes the Risk of Bias (RoB 2) assessment for the included randomized controlled trials.
Effect of omega-3 supplementation on glycemic indices
FBS (Seven studies, SMD = −0.74, 95% CI: −1.94, 0.45; p = 0.224; I2 = 98.0%, p < 0.001) (Figure 2), and insulin (Six studies, SMD = −0.76, 95% CI: −1.77, 0.24; p = 0.136; I2 = 96.2%, p < 0.001) (Figure 3) level did not significantly decrease following omega-3 supplementation with substantial heterogeneity. Subgroup analysis revealed the most substantial effects in patients with GDM and gestational age >20 weeks (Table 3).
Effect of omega-3 supplementation on lipid profile
The analysis revealed that omega-3 supplementation significantly improved TG (Eight studies, SMD = −0.96, 95% CI: −1.77, −0.16; p = 0.019; I2 = 97.2%, p < 0.001) (Figure 4), but not TC (Eight studies, SMD = 0.11, 95% CI: −0.20, 0.42; p = 0.478; I2 = 83.0%, p < 0.001) (Figure 5), and LDL-C (Six studies, SMD = 0.32, 95% CI: −0.17, 0.81; p = 0.195; I2 = 87.1%, p < 0.001) (Figure 6) levels. A significant increase in HDL-C (Seven studies, SMD = 0.72, 95% CI: 0.21, 1.22; p = 0.006; I2 = 92.4%, p < 0.001) levels was observed following omega-3 supplementation (Figure 7). Subgroup analysis revealed the most substantial effects in patients with GDM, in a sample size of ≥60 and intervention duration < 15 weeks (Table 3).
Effect of omega-3 supplementation on inflammatory biomarkers
Omega-3 supplementation significantly reduced CRP (Five studies, SMD = −0.98, 95% CI: −1.86, −0.11; p = 0.028; I2 = 94.8%, p < 0.001) (Figure 8), but not IL-6 (Three studies, SMD = 2.12, 95% CI: −0.56, 4.80; p = 0.120; I2 = 97.8%, p < 0.001) levels (Figure 9).
Effect of omega-3 supplementation on oxidative stress
Omega-3 supplementation did not significantly improve MDA (Three studies, SMD = −1.67, 95% CI: −3.39, 0.05; p = 0.056; I2 = 95.3%, p < 0.001) (Figure 10), and TAC (Three studies, SMD = 2.59, 95% CI: −0.37, 5.54; p = 0.087; I2 = 98.0%, p < 0.001) levels (Figure 11).
Sensitivity analysis and meta-regression
The overall results were reinforced by sensitivity analysis, which showed significance at p < 0.05. Meta-regression analysis demonstrated no linear relationship between effect size and covariates (p > 0.05).
Discussion
This systematic review and meta-analysis evaluated the impact of omega-3 supplementation on maternal glycemic indices, lipid profiles, inflammation, and oxidative stress during pregnancy, drawing on RCTs conducted up to mid-2024. While significant benefits were observed in lipid and inflammatory outcomes, effects on glycemic and oxidative markers were inconclusive. Subgroup analyses were conducted to investigate potential sources of heterogeneity across studies assessing the effects of omega-3 supplementation on FBS, insulin, and TG levels during pregnancy. Notably, the intervention effects appeared to vary according to gestational age at the start of supplementation, intervention duration, sample size, and participants' health conditions.
For FBS, a greater reduction was observed in studies initiating supplementation after 20 weeks of gestation and among women with GDM, while studies involving participants with obesity showed an increase in FBS. Similarly, for insulin, reductions were more prominent in subgroups with later gestational initiation and GDM. A statistically significant decrease in insulin was also found in studies with smaller sample sizes (< 60 participants), where heterogeneity was minimal (I2 = 6.0%).
Regarding TG, more pronounced reductions were observed in studies with shorter intervention durations (< 15 weeks), larger sample sizes (≥60), and participants with obesity. However, these findings should be interpreted cautiously due to the limited number of studies within several subgroups and the generally high heterogeneity between studies. Overall, these subgroup findings suggest that the effects of omega-3 supplementation during pregnancy may be influenced by maternal health status, timing of intervention, and study design characteristics. While exploratory, these analyses provide insights that may guide future research and targeted clinical applications.
Glycemic indices
Although our meta-analysis found non-significant trends toward reductions in fasting blood glucose and insulin levels, high heterogeneity suggests that effects may be context-dependent. Previous meta-analyses in gestational diabetes mellitus (GDM) populations have demonstrated modest but statistically significant reductions in fasting plasma glucose and HOMA-IR, along with cuts in hs-CRP (28, 29). However, such effects appear inconsistent across studies, possibly due to differences in baseline insulin resistance, dosage of omega-3, and duration of supplementation.
Lipid profile
Omega-3 supplementation was associated with a robust reduction in triglycerides and a significant increase in HDL-C, echoing findings from non-pregnant cohorts and pregnant populations. These favorable shifts can be attributed to established mechanisms whereby EPA and DHA inhibit triglyceride synthesis and enhance reverse cholesterol transport (30–32). The lack of significant changes in total and LDL cholesterol may reflect the dual effects of DHA, raising LDL particle size even while modestly affecting LDL levels, as well as variability in dosages and duration across trials.
Inflammatory markers and oxidative stress
A significant reduction in CRP was observed, consistent with prior evidence in GDM and broader populations. Conversely, IL-6 levels did not change, likely due to limited sample sizes and heterogeneity. Omega-3s may reduce inflammation via suppression of NF-κB signaling, modulation of eicosanoid pathways, and enhanced production of resolvins and protectins (33, 34).
Although our pooled estimates for MDA and TAC were non-significant, individual studies—particularly those combining omega-3 with antioxidant co-supplements—showed improvements in oxidative stress markers. Observational data, such as the TIDES cohort, indicated that third-trimester omega-3 intake was associated with lower maternal urinary markers of oxidative stress (e.g., 8-iso-PGF2α decreased by ~10%) (35, 36). The discrepancies may reflect limited statistical power, variability in biomarkers measured, and dosage-dependent antioxidant effects.
Clinical and research implications
Our findings support the potential role of omega-3 supplementation in improving lipid and inflammatory markers in pregnant women, particularly those with GDM. Given that maternal hypertriglyceridemia and inflammation are risk factors for adverse obstetric outcomes, omega-3 could be a helpful adjunct in clinical nutrition strategies. However, the lack of consistent glycemic and oxidative effects highlights the need for future RCTs with larger sample sizes, standardized dosing (e.g.,≥ 1 g/day EPA + DHA), longer durations, and precise biomarker selection. The combination of omega-3 with antioxidants may further enhance outcomes and warrants investigation.
Strengths and limitations
The strengths of this meta-analysis include comprehensive literature coverage across multiple databases, rigorous use of Cochrane and PRISMA methodologies, and use of subgroup and meta-regression techniques to explore moderators. Furthermore, due to the limited number of studies, we could not perform subgroup analyses comparing different dosages, duration of supplementation, or timing within pregnancy trimesters.
Conclusion
Omega-3 PUFA supplementation during pregnancy appears to confer significant reductions in triglyceride and CRP levels and increases in HDL-C, while evidence for glycemic and oxidative outcomes remains inconclusive. Future high-quality, well-powered RCTs are needed to define optimal dosing, timing, and target populations—especially among those with GDM—to elucidate the metabolic benefits of omega-3 in pregnancy fully.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
MJS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ZS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MSa: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SK: Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. MSr: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – original draft, Writing – review & editing. DB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. JR: Conceptualization, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. WT: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. MA: Conceptualization, Formal analysis, Investigation, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. MJ: Conceptualization, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. AH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Authors are grateful to the Researchers Supporting Project (ANUI2024M111), Alnoor University, Mosul, Iraq.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1639906/full#supplementary-material
References
1. Phoswa WN, Khaliq OP. The role of oxidative stress in hypertensive disorders of pregnancy (preeclampsia, gestational hypertension) and metabolic disorder of pregnancy (gestational diabetes mellitus). Oxid Med Cell Longev. (2021) 2021:5581570. doi: 10.1155/2021/5581570
2. Abdissa D. Purposeful review to identify the benefits, mechanism of action and practical considerations of omega-3 polyunsaturated fatty acid supplementation for the management of diabetes mellitus. Nutr Diet Suppl. (2021) 2021:53–65. doi: 10.2147/NDS.S298870
3. Flachs P, Rossmeisl M, Kopecky J. The effect of n-3 fatty acids on glucose homeostasis and insulin sensitivity. Physiol Res. (2014) 63:S93. doi: 10.33549/physiolres.932715
4. Musazadeh V, Tandorost A, Zarezadeh M, Jafarzadeh J, Ghavami Z, Jamilian P, et al. Can omega-3 fatty acids and vitamin E co-supplementation affect obesity indices? Int J Vitamin Nutr Res. (2022) 93:471–80. doi: 10.1024/0300-9831/a000757
5. Gao L, Lin L, Shan N, Ren CY, Long X, Sun YH, et al. The impact of omega-3 fatty acid supplementation on glycemic control in patients with gestational diabetes: a systematic review and meta-analysis of randomized controlled studies. J Matern Fetal Neonatal Med. (2020) 33:1767–73. doi: 10.1080/14767058.2018.1526916
6. Natto ZS, Yaghmoor W, Alshaeri HK, Van Dyke TE. Omega-3 fatty acids effects on inflammatory biomarkers and lipid profiles among diabetic and cardiovascular disease patients: a systematic review and meta-analysis. Sci Rep. (2019) 9:18867. doi: 10.1038/s41598-019-54535-x
7. Jamilian M, Hashemi Dizaji S, Bahmani F, Taghizadeh M, Memarzadeh MR, Karamali M, et al. A randomized controlled clinical trial investigating the effects of omega-3 fatty acids and vitamin e co-supplementation on biomarkers of oxidative Stress, inflammation and pregnancy outcomes in gestational diabetes. Can J Diabetes. (2017) 41:143–9. doi: 10.1016/j.jcjd.2016.09.004
8. Chatzakis C, Sotiriadis A, Tsakmaki E, Papagianni M, Paltoglou G, Dinas K, et al. The effect of dietary supplements on oxidative stress in pregnant women with gestational diabetes mellitus: a network meta-analysis. Nutrients. (2021) 13:2284. doi: 10.3390/nu13072284
9. Vahedi L, Ostadrahimi A, Edalati-Fard F, Aslani H, Farshbaf-Khalili A. Is fish oil supplementation effective on maternal serum FBS, oral glucose tolerance test, hemoglobin and hematocrit in low risk pregnant women? A triple-blind randomized controlled trial Journal of Complementary and Integrative. Medicine. (2018) 15:20180010. doi: 10.1515/jcim-2018-0010
10. Samimi M, Jamilian M, Asemi Z, Esmaillzadeh A. Effects of omega-3 fatty acid supplementation on insulin metabolism and lipid profiles in gestational diabetes: randomized, double-blind, placebo-controlled trial. Clin Nutr. (2015) 34:388–93. doi: 10.1016/j.clnu.2014.06.005
11. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (2021) 372:n160. doi: 10.1136/bmj.n160
12. Flemyng E, Moore TH, Boutron I, Higgins JP, Hróbjartsson A, Nejstgaard CH, et al. Using Risk of Bias 2 to assess results from randomised controlled trials: guidance from Cochrane. BMJ Evid-Based Med. (2023) 28:260–6. doi: 10.1136/bmjebm-2022-112102
13. Higgins JP. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0. 1. The Cochrane Collaboration (2008). Available online at: http://www.cochrane-handbook.org
14. Chen D-GD, Peace KE. Heterogeneity in Meta-Analysis, in Applied Meta-Analysis with R and Stata. Chapman and Hall;CRC (2021). p. 155–88. doi: 10.1201/9780429061240-6
15. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane (2021).
16. Mathur MB, VanderWeele TJ. Sensitivity analysis for publication bias in meta-analyses. J R Stat Soc Ser C: Appl Stat. (2020) 69:1091–119. doi: 10.1111/rssc.12440
17. Houttu N, Mokkala K, Koivuniemi E, Pellonperä O, Juhila J, Sorsa T, et al. The impacts of fish oil and/or probiotic intervention on low-grade inflammation, IGFBP-1 and MMP-8 in pregnancy: a randomized, placebo-controlled, double-blind clinical trial. Biomolecules. (2020) 11:5. doi: 10.3390/biom11010005
18. Valentine CJ, Khan AQ, Brown AR, Sands SA, Defranco EA, Gajewski BJ, et al. Higher-dose DHA supplementation modulates immune responses in pregnancy and is associated with decreased preterm birth. Nutrients. (2021) 13:4248. doi: 10.3390/nu13124248
19. Faraji I, Ostadrahimi A, Farshbaf-Khalili A, Aslani H. The impact of supplementation with fish oil on lipid profile of pregnant mothers: a randomized controlled trial. CJMB (2016) 3:100–6.
20. Rodriguez-Santana Y, Ochoa JJ, Lara-Villoslada F, Kajarabille N, Saavedra-Santana P, Hurtado JA, et al. Cytokine distribution in mothers and breastfed children after omega-3 LCPUFAs supplementation during the last trimester of pregnancy and the lactation period: a randomized, controlled trial. Prostagland Leukotr Essential Fatty Acids. (2017) 126:32–8. doi: 10.1016/j.plefa.2017.09.006
21. Garmendia ML, Corvalán C, Casanello P, Araya M, Flores M, Bravo A, et al. Effectiveness on maternal and offspring metabolic control of a home-based dietary counseling intervention and DHA supplementation in obese/overweight pregnant women (MIGHT study): a randomized controlled trial—Study protocol. Contemp Clin Trials. (2018) 70:35–40. doi: 10.1016/j.cct.2018.05.007
22. Taghizadeh M, Jamilian M, Mazloomi M, Sanami M, Asemi Z. A randomized-controlled clinical trial investigating the effect of omega-3 fatty acids and vitamin E co-supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes. J Clin Lipidol. (2016) 10:386–93. doi: 10.1016/j.jacl.2015.12.017
23. Razavi M, Jamilian M, Samimi M, Afshar Ebrahimi F, Taghizadeh M, Bekhradi R, et al. The effects of vitamin D and omega-3 fatty acids co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in patients with gestational diabetes. Nutr Metab. (2017) 14:80. doi: 10.1186/s12986-017-0236-9
24. Haghiac M, Yang XH, Presley L, Smith S, Dettelback S, Minium J, et al. Dietary omega-3 fatty acid supplementation reduces inflammation in obese pregnant women: a randomized double-blind controlled clinical trial. PloS ONE. (2015) 10:e0137309. doi: 10.1371/journal.pone.0137309
25. Franke C, Demmelmair H, Decsi T, Campoy C, Cruz M, Molina-Font JA, et al. Influence of fish oil or folate supplementation on the time course of plasma redox markers during pregnancy. Br J Nutr. (2010) 103:1648–56. doi: 10.1017/S0007114509993746
26. Helland IB, Saugstad OD, Saarem K, Van Houwelingen AC, Nylander G, Drevon CA. Supplementation of n-3 fatty acids during pregnancy and lactation reduces maternal plasma lipid levels and provides DHA to the infants. J Mater-Fetal Neonat Med. (2006) 19:397–406. doi: 10.1080/14767050600738396
27. Jamilian M, Tabassi Z, Reiner Ž, Panahandeh I, Naderi F, Aghadavod E, et al. The effects of n-3 fatty acids from flaxseed oil on genetic and metabolic profiles in patients with gestational diabetes mellitus: a randomised, double-blind, placebo-controlled trial. Br J Nutr. (2020) 123:792–9. doi: 10.1017/S0007114519003416
28. Amirani E, Asemi Z, Asbaghi O, Milajerdi A, Reiner Ž, Mansournia MA, et al. The effects of omega-3 fatty acids supplementation on metabolic status in pregnant women: a systematic review and meta-analysis of randomized controlled trials. J Diabetes Metab Disord. (2020) 19:1685–99. doi: 10.1007/s40200-020-00558-5
29. Liu W, Gao M, Yang S, Sun C, Bi Y, Li Y, et al. Effects of omega-3 supplementation on glucose and lipid metabolism in patients with gestational diabetes: a meta-analysis of randomized controlled trials. J Diabetes Complic. (2023) 37:108451. doi: 10.1016/j.jdiacomp.2023.108451
30. Pizzini A, Lunger L, Demetz E, Hilbe R, Weiss G, Ebenbichler C, et al. The role of omega-3 fatty acids in reverse cholesterol transport: a review. Nutrients. (2017) 9:1099. doi: 10.3390/nu9101099
31. Farhangi MA, Dehghan P, Musazadeh V, Kavyani M, Maleki P. Effectiveness of omega-3 and prebiotics on adiponectin, leptin, liver enzymes lipid profile and anthropometric indices in patients with non-alcoholic fatty liver disease: a randomized controlled trial. J Funct Foods. (2022) 92:105074. doi: 10.1016/j.jff.2022.105074
32. Musazadeh V, Mahmoudinezhad M, Pam P, Brazandeh S, Faramarzi F, Mohammadpour Y, et al. Omega-3 supplementation and cardiometabolic risk factors in obese/overweight children and adolescents: a GRADE assessed systematic review and meta-analysis. Nutr Metab. (2025) 22:78. doi: 10.1186/s12986-025-00952-x
33. Kang JX, Weylandt KH. Modulation of inflammatory cytokines by omega-3 fatty acids. Subcell Biochem. (2008) 49:133–43. doi: 10.1007/978-1-4020-8831-5_5
34. Weylandt KH, Chiu CY, Gomolka B, Waechter SF, Wiedenmann B. Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation. Prostagland Other Lipid Mediat. (2012) 97:73–82. doi: 10.1016/j.prostaglandins.2012.01.005
35. Sley EG, Rosen EM, van 't Erve TJ, Sathyanarayana S, Barrett ES, Nguyen RHN, et al. Omega-3 fatty acid supplement use and oxidative stress levels in pregnancy. PLoS ONE. (2020) 15:e0240244. doi: 10.1371/journal.pone.0240244
36. Shen X, Génard-Walton M, Williams PL, Ford JB, Souter I, Allan Y, et al. Effect modification of serum omega-3 fatty acids on the associations between urinary phthalate biomarkers mixture and pregnancy outcomes among women seeking fertility care. Environ Health Perspect. (2025) 133:067005. doi: 10.1289/EHP15942
Keywords: omega-3, pregnancy, lipid, inflammation, oxidative stress
Citation: Saadh MJ, Sabah Ghnim Z, Salih Mahdi M, Baldaniya L, Karim SA, Srivastava M, Bhanot D, Rizaev J, Taher WM, Alwan M, Jawad MJ and Hamad AK (2025) The effect of omega-3 supplementation on metabolic, inflammatory and oxidative stress biomarkers in pregnant women: a systematic review and meta-analysis. Front. Nutr. 12:1639906. doi: 10.3389/fnut.2025.1639906
Received: 02 June 2025; Accepted: 25 August 2025;
Published: 22 September 2025.
Edited by:
Amber Farooqui, Omega Laboratories Inc., CanadaReviewed by:
Jiajie Yu, Sichuan University, ChinaEstela Godínez, Instituto Nacional de Perinatología (INPER), Mexico
Copyright © 2025 Saadh, Sabah Ghnim, Salih Mahdi, Baldaniya, Karim, Srivastava, Bhanot, Rizaev, Taher, Alwan, Jawad and Hamad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zahraa Sabah Ghnim, c2FiYWguemFocmExOTk0QGdtYWlsLmNvbQ==
 Mohamed J. Saadh1
Mohamed J. Saadh1 Zahraa Sabah Ghnim
Zahraa Sabah Ghnim