- Laboratory of Agronomy, Department of Crop Science, Agricultural University of Athens, Athens, Greece
Weeds pose a major threat to world agriculture by reducing detrimentally crop yield and quality. However, at the same time, weeds are major interacting components of the agroecosystems. Abundance and diversity of weeds vary significantly among the several communities. In order to evaluate each community's structure and the interactions among them, several population indices are used as key tools. In parallel, various cultivation and land management strategies, such as tillage and fertilization, are commonly used in terms of integrated weed management. Estimating the response of weed species on those practices is crucial for both biodiversity maintenance and alternative weed control methods. Many experiments have confirmed the fundamental role of tillage intensity and nutrition supply in weed species' abundance and diversity. For instance, in some studies, the abundance of perennial weeds was doubled under reduced tillage intensity. In addition, higher values of Shannon-Weiner and Pielou indices were reported in the PK fertilization treatment compared to the control and NK fertilization treatments. The objective of this paper is to provide a brief overview of the key results of these experiments and summarize the part of the literature related to the effect of tillage systems and fertilization on weed species abundance and diversity. Such knowledge could contribute to the sound design and implementation of integrated weed management programs which in turn may lead to a decrease in the density of serious and noxious weeds and an increase in the overall balance of agroecosystems.
Introduction
Since the beginning of crop production weeds have represented a serious constraint to worldwide agriculture, as when left uncontrolled they can cause over 80% yield loss (Cousens and Mortimer, 1995). Agricultural practice has demonstrated that the basic principles of integrated management used for insect control also ought to be adopted for weed control (Labrada, 2003). Opposed to more pest management tactics, establishing integrated weed management programs focused on diverse weed species with different life history attributes can be particularly complicated. As a result, stable systems that stand in need of ecological awareness outside the scope of individual species are deemed necessary (Mortensen et al., 2000).
A community can be described as a group of interacting species populations occurring together in space (Stroud et al., 2015). Abundance is defined as “the measure of the number or frequency of individuals of the same species,” whereas diversity demonstrates the “number of species present (species richness) and their abundance (species evenness) in an area or in a community” (Booth et al., 2003). The correlation between species diversity and community stability stresses the need to preserve the greatest richness among biological communities (Rafferty, 2011). The biodiversity of weed communities in a cropland can be influenced by tillage systems and fertilization treatments. In addition, the influence of environmental conditions should also be taken into account (Fried et al., 2008; Pinke et al., 2012). When studying a weed community, the choice of the appropriate indices is crucial. Changes in crop management are likely to act as filters on the weed community by removing or limiting species that lack specific traits or combinations of them (Storkey et al., 2010). Weed abundance and diversity should be intensively studied as key aspects of both biodiversity conservation within agroecosystems (Marshall et al., 2003; Gibson et al., 2006; Power, 2010; Rassam et al., 2011) and integrated weed management (Maxwell and O'Donovan, 2007; Smith et al., 2010; Travlos, I., 2013).
Each agricultural practice has a higher or lower potential to influence the abundance and diversity of weed species in a crop field. Both positive and negative effects of reduced tillage intensity on weed diversity have been reported. In general, conservation tillage systems usually increase weed abundance (Travlos and Economou, 2010; Melander et al., 2013), while the trends regarding weed diversity are less clear and they are usually dependent on the basis (long-term or mid-term) of the relevant evaluations (Hernandez Plaza et al., 2011; Bilalis et al., 2012b; Santín-Montanyá et al., 2013). In many cases, annual broadleaf species tend to be more abundant in frequently disturbed conventional tillage systems (Streit et al., 2003), while perennial weeds are favored by the absence of disturbance (Buhler, 1995). However, in vegetable fields in California, reduced tillage increased the density of weeds like the annual broadleaf Capsella bursa-pastoris in the top 15 cm of soil compared to conventional tillage (Fennimore and Jackson, 2003).
Differentiation in the concentration and availability of plant nutrients may also affect weed populations (Murphy and Lemerle, 2006). Several studies have reported that fertilization has a strong impact on weed species' abundance and diversity (Banks et al., 1976; Nie et al., 2009; Cheimona et al., 2016). In particular, quite a few experiments have demonstrated that high level of nitrogen (N) fertilization results in decrease of weed species richness (Pyšek and Lepš, 1991; Inouye and Tilman, 1995) and change of the weed species composition, since specific species are favored (Mahn, 1988; Gu et al., 2007; Huang et al., 2013). Moreover, Storkey et al. (2010) found that several rare and endangered weed species were further reduced due to the added nitrogen. Previous studies have shown that the abundance of several weeds was also greatly influenced by added phosphorus (P) (Blackshaw et al., 2004), while dandelion abundance was greatly dependent on potassium (K) fertilization (Tilman et al., 1999).
The traditional approach to the study of weeds is to examine their control or management rather than study their effect on the community. However, the main objective of this paper is to display some of the changes in weed abundance, diversity and community composition, under conservation and conventional tillage systems and different fertilization patterns, as they are expressed by means of various indices. Despite the space limitations of this condensed review that make the detailed presentation of all the relative studies exceptionally difficult, this paper aims to improve our ability to understand species' responses to human-induced land-use change.
Estimating Species Abundance and Diversity
Species abundance describes the number of individuals on the same sample plot (Kent, 2012). There are quite a few different indices (frequency, density, cover and biomass) for measuring abundance depending among the others on the target species, the habitat type, the aim of the study and the economic resources (Kraehmer, 2016). Frequency, usually expressed as a percentage, is “the proportion of sampling units (e.g. quadrats) that contains the target species” (Booth et al., 2003). It is an easy and quick method which is considerably affected by the size and the shape of the sampling units.
Frequency is a useful index for monitoring and comparing plant community changes over time (Bonham, 2013). Frequency reflects both a species' presence or absence and how much it is distributed within a community. Relative frequency, also expressed as a percentage, is the degree of dispersion of target species in the sampling unit in relation to the number of all the species occurred.
Density measures the number of target species per given area (e.g. square meter or hectare). Brix and Andreasen (2000) proposed the McCullagh model for prediction of the mean weed density from the frequency data by calibration.
The ability of individuals to be separated is a prerequisite for measuring the density of each species. However, individuals of weed species are often difficult to be distinguished, especially in early growth stages. In addition, age, growth stage, biotype or environment may affect the morphological appearance or at least particular morphological traits of the same weed species (Booth et al., 2010; Travlos and Giannopolitis, 2010; Travlos, I. S., 2013). Consequently, in many cases it is preferable to use frequency and density measurements in uniform size vegetation in order to avoid underestimation of individuals' abundance. In general, relative density expresses the numerical strength of a target species in relation to the total number of individuals of all the species occurred.
Furthermore, coverage and biomass are used instead of frequency and density when size or weight differentiation of individuals is needed. Coverage is “the percentage of the surface area of the sample plot covered by a given species” and consists a commonly used measure of abundance because it is not limited by the size or distribution of individuals (Floyd and Anderson, 1987). It is usually estimated visually from the above by using several scales for cover classes (Krajina, 1933; Evans and Dahl, 1955; Daubenmire, 1959; Barkman et al., 1964; Braun-Blanquet, 1964). However, visual under- or overestimations may occur (Kercher et al., 2003). Precise measurements of vegetation coverage are available by using various software and different applications. In addition, total vegetation coverage is feasible to be measured, based on various vegetation indices such as NDVI, NDRE or NGRDI (Travlos et al., 2017; Van Evert et al., 2017). The influence of plant distribution should be also taken into consideration on the estimation of a population's density, cover and frequency. Bilalis et al. (2009) introduced the MDR index as an indicator of weed volume for each weed species with the following equation:
Biomass is expressed as “the dry-matter weight of individual species per unit area.” It is a reliable and accurate index; however, it is a method requiring time and destructive sampling. Biomass is determined by collecting the above-ground and/or the below-ground part of a plant species. When collecting, the plant can also be divided into roots, stems, leaves and reproductive structures.
Species diversity, usually described by two basic constituents (richness and evenness), represents the different species within a community. Species richness is the number of species present, whereas evenness reveals how the community is dominated by the existing species or whether these species are represented by approximately equal numbers (Nkoa et al., 2015). Species diversity is divided in the within community diversity [α- (alpha-) diversity] and between community diversity [β- (beta-) diversity]. The most widely used indices for the estimation of α-diversity are the following: Margalef, Shannon-Weiner and the Simpson. However, measuring β-diversity gives the opportunity to determine differences among the communities as well as the effect of putative environmental factors on species composition (Booth et al., 2003). The most widely used indices for the estimation of β-diversity are the following three: Whittaker's statistic, Jaccard's, and Sorensen and Steinhaus' coefficients of similarity. In Table 1, the main differences among them are presented; however, this review paper will focus on α-diversity comparison studies.
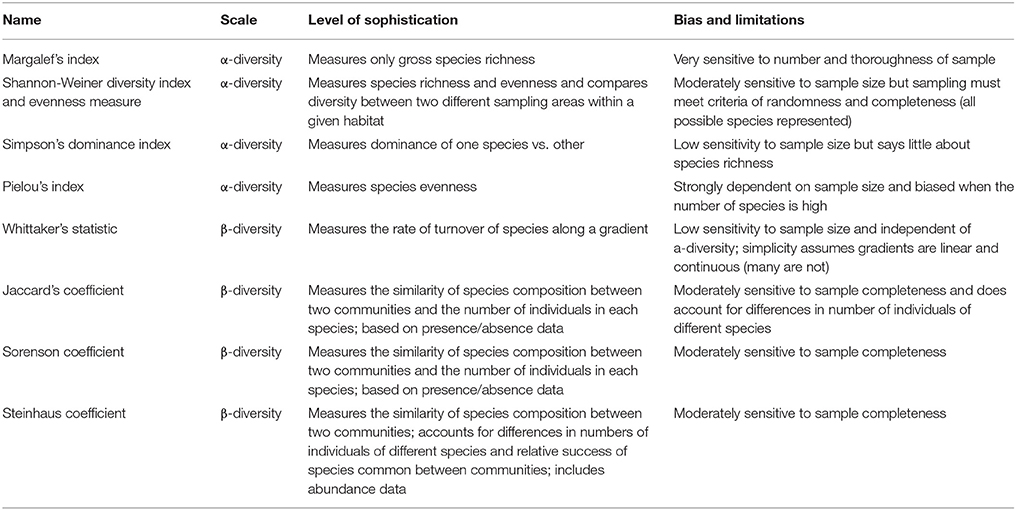
Table 1. Comparing the scale, sophistication, biases and limitations of diversity measures discussed above (modified from Booth et al., 2003, 2010).
The Margalef diversity index (Margalef, 1958) (DMg) can be easily determined by the following equation:
where S is the number of species, and N is the total number of individuals in the sample. As previously commented by Magurran (2004), this index is highly sensitive to sample size although it is intended as a counterbalance to sampling effects. However, in another study it was mentioned that if a density data set is inserted in place of absolute numbers, a sub estimation of the index may occur (Gamito, 2010).
The Shannon-Weiner diversity index (Shannon and Weaver, 1963) (H′) is calculated by the following equation:
where pi is the proportion of individuals belonging to the ith species and Sis the total number of species. The values of this index range between 1.5 and 3.5 (MacDonald, 2003).
The evenness of a community can be represented by the index (J′) that Pielou (1969) proposed with the following equation:
where H′ is the number derived from the Shannon diversity index and H′max is the maximum possible value of H' (if every species was equally present), equal to:
where S is the total number of species.
The Simpson index was introduced by Simpson (1949) in order to measure the degree of dominance of individuals weed species, according to the following equation:
where pi is the proportion of individuals belonging to the ith species and S is the total number of species.
A separate measure of evenness can be calculated by dividing the reciprocal form of the Simpson index by the number of species in the sample (Smith and Wilson, 1996).
The values for that measure range from 0 to 1.
There are several methods for measuring β-diversity. Herein, only some of the most frequently used examples of the relative indices will be presented. One of the best methods is called Whittaker's statistic (βW) (Whittaker, 1960) and is calculated by the following equation:
where γ is the number of species in an entire study area and α is the number of species per plot within the study area. This measure examines the difference among areas of α-diversity in relation to the total species richness (γ-diversity) (Kraehmer, 2016).
There are more than 20 binary similarity measures in the literature (Cheetham and Hazel, 1969) and they have been reviewed by Clifford and Stephenson (1975), and Romesburg (1984). Below, two of the indices that are most frequently used are presented
Jaccard's Index of similarity (Jaccard, 1908) is given as follows:
where α is the total number of species present in both samples, b is the number of species present only in the one sample and c is the number of species in the second sample.
Sørensen's index is calculated by means of the following equation:
where c is the number of species shared by both plots and α is the mean number of species in each plot. Binary measures are appropriate for data sets where variables can only take the values “1” or “0,” such as presence/absence data sets.
Steinhaus coefficient (SA) is another common index:
where W is the sum of the lower of the two abundances of each species of the community, A the total number of individuals in population A and B the total number of individuals in population B (Nkoa et al., 2015). This asymmetric coefficient also accounts for the quantitative differences in species' communities. If applied to binary data, this is equivalent to the Sørensen coefficient. The one-complement of this coefficient is the popular Bray-Curtis dissimilarity measure (Bray and Curtis, 1957).
It is apparent that the selection of the appropriate index has to be carefully performed by the researchers in order to meet the requirements of each specific study and particular requirements. In studies where rare and abundant weed species are expected to be equally significant, diversity could be appropriately measured by the Shannon-Weiner index. However, when dominant species are supposed to be more significant, Simpson index could be more suitable (Morris et al., 2014).
Tillage Effect on Weed Abundance and Diversity
Tillage systems are often but not solely classified to several types depending on the amount of residues left on the soil surface (Magdoff and van Es, 2000). Particularly, conventional tillage includes all tillage treatments that leave less than 15% of crop residues on the soil surface after planting the next crop, or less than 1,100 kg/ha of small grain residues throughout a critical erosion period. In general, such tillage techniques involve plowing or intensive tillage (Koller, 2003). Conservation tillage retains an amount of about 30% or greater of soil surface covered by crop residues and it includes four main types: mulch tillage, ridge tillage, zone tillage, and no-tillage (Carter, 2005).
Crop management strongly affects the abundance and diversity of weeds and changes in tillage are likely to have a clear effect on the community structure (Nichols et al., 2015). Such changes in tillage may result in weed species shifts (Coffman and Frank, 1992; Buhler et al., 1994; Swanton et al., 1999; Tuesca et al., 2001; Bilalis et al., 2003). In parallel, some studies revealed a direct correlation between the presence of specific weed species and the tillage system (Buhler, 1992). In the study of Thomas et al. (2017) perennial species such as Cirsium arvense and Sonchus arvensis were associated with reduced- and zero-tillage systems, while annual species were associated with a range of tillage systems. The study carried out by Grey et al. (2017) showed that tillage alone can effectively control the potential invasive napiergrass (Pennisetum purpureum) within a range from 12 to 33%. However, invasive weeds are inclined to recover rapidly when tillage is interrupted (Sheley et al., 2011). Shifts in plant communities are usually described or quantified by means of the various existing abundance and diversity indices.
Armengot et al. (2016) observed an increasing trend in weed richness under reduced tillage compared with conventional tillage. However, crop type was recognized as the main driver of the shifts in the functional composition of weed communities. Armengot et al. (2015) also found that total weed coverage was higher under reduced tillage, though this result was not consistent fordifferent crops. In particular, average abundance of perennials almost doubled overtime under reduced tillage, while yields did not show any difference between the different treatments. Higher weed abundance and density under conservation tillage have been also confirmed by other studies (Peigné et al., 2007; Gruber and Claupein, 2009). Cardina et al. (2002) reported that in moldboard plow plots the densities of Amaranthus retroflexus and Veronica arvensis were both lower compared to no-tillage plots.
Kakabouki et al. (2015) indicated that weed biomass and density in quinoa crop were influenced by the different fertilization and tillage treatments with tillage effects being species dependent. Similarly, total weed coverage and perennial coverage in reduced tillage treatments were two to three times greater compared with conventional treatments (Sans et al., 2011). Weed biomass in barley showed also higher rates in the conservation compared to the conventional tillage treatment (Vakali et al., 2011). On the contrary, other researchers like Demjanová et al. (2009) suggested that mouldboard plowing was connected with significantly lower weed biomass compared to reduced soil tillage.
Santín-Montanyá et al. (2013) reported that the abundance, diversity and evenness of the weed community in a wheat field, were greatly increased in no-tillage systems. Mulugeta et al. (2001) also confirmed that species richness was higher in long-term no-tilled fields than in tilled or short-term no-tilled fields. Furthermore, it is noted that less important weeds often become dominant after a period of no-tillage in which weed seeds are retained near the soil surface (Soane et al., 2012). Counter results were found by Shrestha et al. (2002), revealing higher weed densities in the conventional tillage than in the no-tillage systems, while in other studies perennial weeds and overwintering weed species increased with reduced tillage compared to plowing in autumn or spring (Tørresen et al., 2003).
On the other hand, Amuri et al. (2010) suggested no-tillage and high residues' level as contributors to the suppression of many weed species in terms of crop management. Consistent with that outcome is the study that Bilalis et al. (2012a) carried out which highlights the suppressive role of wheat residues in weed abundance under no-tillage conditions in an organic flax crop. In particular, the highest density of competitive weeds was mentioned under minimum and conventional tillage systems, whereas the lowest density was found in no-tillage plots. Similarly, the lowest population of weeds like Echinochloa colona and Digera arvensis was found in zero tillage-raised bed conditions over a four-year study (Sepat et al., 2017). With regard to weed species diversity, the percentage of rare species was enhanced under no-tillage treatment (Gill and Arshad, 1995).
While current knowledge suggests that weed community composition will change in response to different tillage systems, the alterations in weed diversity of the community remain less clear. Bilalis et al. (2001) used both Simpson's and Shannon-Weiner's indices to verify the impact of three different tillage amendments on shifts in weed flora in a 3-year crop-rotation treatment. In all crops, apart from cotton, significant differences were found among the tillage systems. Three annual species prevailed in the conventional and minimum tillage systems (Sinapis arvensis, Solanum nigrum and Tribulus terrestris), while one perennial species (Malva sp.) prevailed in the no-tillage system. Mas and Verdú (2003) reported that the highest values of Shannon-Wiener diversity index were noted under no-till conditions.
Sans et al. (2011) suggested that tillage had no significant impacts on species diversity in wheat and spelt crops. However, low evenness values and high dominance of Stellaria media demonstrated a decrease in weed diversity in reduced tillage plots of sunflower. On the contrary, Shannon's diversity and evenness indices were higher under the conservation than in the conventional tillage system (Dorado and Lopez-Fando, 2006). Conservation tillage systems resulted in increased weed diversity compared with conventional mouldboard plow-based tillage systems. Some species, such as Capsella bursa-pastoris and Torilis nodosa, were dominant in the reduced tillage systems (no tillage, no-tillage with paraplow and minimum tillage), while two different weed species (Polygonum aviculare and Phalaris paradoxa) were the dominant ones in the conventional system.
In addition, Menalled et al. (2001) reported that aboveground weed biomass, species density, and diversity lowest values were obtained under conventional tillage system, intermediate values under no-tillage system, and highest values under low-input and organic systems. Moreover, it was observed that annual grass species, such as Digitaria sanguinalis and Panicum dichotomiforum dominated the no-tillage system. It is noteworthy to mention that different diversity pattern with regards to tillage among crops suggests that other agronomic practices and environmental factors may interact in a complex way with tillage and affect the weed diversity within communities (Légère et al., 2005). A summary of the above-mentioned responses of weed abundance and diversity to different tillage is given in Table 2.
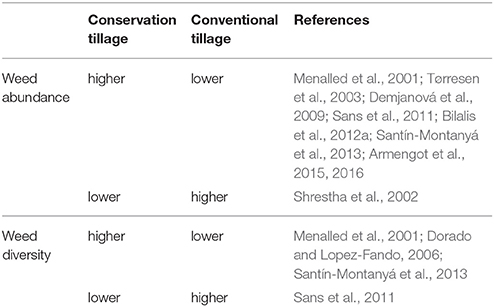
Table 2. Indicative response of conservation and conventional tillage practices on weed abundance and diversity.
Fertilization Effect on Weed Abundance and Diversity
Fertilization affects soil fertility and nutrient uptake, thus resulting inincreasing agricultural yields, as well as in modifications of weed communities (Allan et al., 2015). Weed communities tend to be more diversified in low than in high input systems (Gough et al., 2000; Suding et al., 2005; Bilalis et al., 2010), while Santín-Montanyá et al. (2013) did not observeany significant effect of fertilization on weed abundance and diversity. The kind of fertility inputs varies between conventionally and organically-managed systems and weed species richness and abundance are considered to be strongly related to organic compared with conventional farming (Hyvönen et al., 2003). In many cases, organic farming usually increases species richness, having on average 30% higher species richness than conventional farming systems (Bengtsson et al., 2005) and also favors the existence of habitats for rare weed species (van Elsen, 2000). Finally, it has been proposed that at higher nitrogen level of soils, invasive annual grasses can be reproduced better compared to native species (Vasquez et al., 2008).
Blackshaw et al. (2005) confirmed that compost tends to contain relatively high numbers of weed seeds which can contribute to the soil seed bank. In addition, Sweeney et al. (2008) reported that weeds' biomass was higher with added nitrogen, whereas Berner et al. (2008) suggested that slower release of nitrogen by solid farmyard manures appears to benefit weeds more than early nitrogen demanding crops. At the same time, soil N fertility was considered to have effects on weed seed mortality for some specific weed species, such as Abutilon theophrasti, Ambrosia trifida, and Eriochloa villosa (Davis, 2017). On the other hand, Kakabouki et al. (2015) mentioned an increase in total weed density and biomass under either manure application or inorganic fertilization.
Cover crops and intercropping are considered to be practices that improve soil fertility with adverse effects on weed communities (Bilalis et al., 2009; Travlos, 2010). As mentioned by Bilalis et al. (2012a), differences in nitrogen availability posed a significant effect on weed density of many competitive weeds with the lowest weed density found in the plots treated with compost. Similarly, the lowest value of Shannon's index was also found in the same plots.
It is reported that increased nutrients level increased the dry matter of weeds (Mohammaddoust-e-Chamanadad et al., 2006). In particular, Pyšek and Lepš (1991) noted that a high rate of nitrogen fertilization enhanced tall and erect weed species, an outcome which is in full agreement with the hypothesis of light limitation in weed communities. Blackshaw and Brandt (2008) reported that the competitiveness of Lolium persicum (low N-responsive) was not influenced by nitrogen added. On the contrary, the competitive ability of Amaranthus retroflexus (high N responsive) increased with higher levels of nitrogen. In the case of Avena ludoviciana the added N increased the negative effect on wheat grain yield (Lack et al., 2011).
Previous studies had shown that soil enrichment significantly reduced the richness of native species in grassy woodland ecosystems (McIntyre and Martin, 2001). In addition, species richness was negatively correlated with phosphorus, and species evenness was negatively correlated with the ratio of organic carbon to total nitrogen in soil according to the study of Ma (2005). Blackshaw and Brandt (2009) also mentioned that P-responsive species were more competitive as added P increased. On the contrary, Freyman et al. (1989) mentioned that P had a small effect and K had no effect on weed communities without any significant interactions.
Hyvönen and Salonen (2002) suggested that cropping without herbicide application and with lower nitrogen supply resulted in greater weed species diversity than conventional cropping with a higher nitrogen supply and herbicides did. In another study, Digitaria ischaemum was found to be the dominant species under NK and non-fertilized treatments, Cyperus rotundus dominated under phosphorus PK treatment, while more weed species and higher Shannon' s diversity values were detected in the balanced fertilization treatment (Yin et al., 2006). Wan et al. (2012) evaluated the influence of different fertilization on weed diversity in rice paddy fields. Five fertilization treatments (no fertilization or NOF, PK, NP, NK, and NPK) were applied and according to the results the following models were occurred: PK > NOF > NK > NP > NPK, PK > NOF > NK > NP > NPK, NPK > NP > NK > NOF > PK and PK > NOF > NK > NP > NPK for species richness, species diversity, dominance and evenness of community, respectively.
Than et al. (2017) studied the effect of different fertilizer treatments on weed densities and richness indices. The results showed that the N and P fertilizer application had a more significant impact on weed community compared to the K application. In another study, the growth responses of common crops and weeds with addition of composted poultry manure (CPM) were compared (Little et al., 2015). The results indicated that weed growth response to CPM was not explained by K or N added (with the exception of velvetleaf). In another study, Ugen et al. (2002) evaluated dry bean's competitiveness against the following annual weeds: Solanum nigrum, Bidens pilosa and Galinsoga parviflora. The results revealed that the weed nutrient uptake and growth was increased with N and P application, whereas the relative competitiveness of bean was increased further to K application.
Tang et al. (2014) reported that the density and diversity of weed community was enhanced after PK fertilization. The highest values of Shannon-Weiner and Pielou indices were obtained in the PK treatment compared to the control and NK treatments, while in the latter treatments the Simpson index was lower compared with the NP and NPK treatments. The same authors reported that the PK treatment favored weed density, shoot biomass and diversity compared to N plus P fertilizer treatments. The number of weed species in the N1/2PK treatment was the same as in the PK treatment (Tang et al., 2013).
Conclusions
Weed community composition and structure can be greatly influenced by different management practices, such as tillage, and fertilization. Weed species abundance and diversity can be incorporated into numerous population indices. This article cites only a few of the most commonly used indices. Choosing the accurate index with regard to the aims and context of each study should be a priority for species' abundance and diversity estimations. In general, conservation of tillage systems seems to be associated with higher weed richness and diversity, as the elimination of tillage creates more enhancing conditions for some weed species. However, there are cases where reduced tillage systems led to less diverse weed communities compared to more intensive tillage systems.
Similarly, various results about the influence of different fertilization patterns were shown in the carried out experiments. There is a common trend that weed abundance and richness are positively affected by organic farming. Furthermore, diversity of weed species seems to be enhanced under low-input conditions, while low N fertilization level enhanced the effective control of weeds. On the other hand, demands of weeds on nutrients are quite often proved to be species dependant. It is noteworthy that, at field level, predicting weed flora species responses to management filters, such as tillage or fertilization, remains a difficult task due to the environmental conditions which vary in time and space.
This overview of the numerous experiments that determine the effect of tillage systems and fertilization on the composition and abundance of weed species in crop fields can be helpful in understanding how particular weed species increase or decrease, in terms of numbers and diversity, and how crop management can contribute to the suppression of weeds. Another aim of this review is to raise awareness on the importance of conserving weeds biodiversity as an integral part of balanced agroecosystems. Further research is essential in order to understand the complex relationships of weed species and how they are affected by different tillage amendments and fertilization patterns.
Author Contributions
IT, NC, IR, and DB reviewed the literature and equally contributed to the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Allan, E., Manning, P., Alt, F., Binkenstein, J., Blaser, S., Blüthgen, N., et al. (2015). Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett. 18, 834–843. doi: 10.1111/ele.12469
Amuri, N., Brye, K. R., Gbur, E. E., Oliver, D., and Kelley, J. (2010). Weed populations as affected by residue management practices in a wheat–soybean double-crop production system. Weed Sci. 58, 234–243. doi: 10.1614/WS-09-088.1
Armengot, L., Berner, A., Blanco-Moreno, J. M., Mäder, P., and Sans, F. X. (2015). Long-term feasibility of reduced tillage in organic farming. Agron. Sustain. Develop. 35, 339–346. doi: 10.1007/s13593-014-0249-y
Armengot, L., Blanco-Moreno, J., Bàrberi, P., Bocci, G., Carlesi, S., Aendekerk, R., et al. (2016). Tillage as a driver of change in weed communities: a functional perspective. Agric. Ecosyst. Environ. 222, 276–285. doi: 10.1016/j.agee.2016.02.021
Banks, P., Santelmann, P., and Tucker, B. (1976). Influence of long-term soil fertility treatments on weed species in winter wheat. Agron. J. 68, 825–827. doi: 10.2134/agronj1976.00021962006800050037x
Barkman, J. J., Doing, H., and Segal, S. (1964). Kritische bemerkungen und vorschläge zur quantitativen vegetations analyse. Acta Botanica Neerlandica 13, 394–419. doi: 10.1111/j.1438-8677.1964.tb00164.x
Bengtsson, J., Ahnstr, M. J., and Weibull, A.-C. (2005). The effects of organic agriculture on biodiversity and abundance: a meta-analysis. J. Appl. Ecol. 42, 261–269. doi: 10.1111/j.1365-2664.2005.01005.x
Berner, A., Hildermann, I., Fliessbach, A., Pfiffner, L., Niggli, U., and Mäder, P. (2008). Crop yield and soil fertility response to reduced tillage under organic management. Soil Tillage Res. 101, 89–96. doi: 10.1016/j.still.2008.07.012
Bilalis, D., Efthimiadis, P., and Sidiras, N. (2001). Effect of three tillage Systems on weed flora in a 3-year rotation with four crops. J. Agron. Crop Sci. 186, 135–141. doi: 10.1046/j.1439-037X.2001.00458.x
Bilalis, D., Karkanis, A., and Efthimiadou, A. (2009). Effects of two legume crops, for organic green manure, on weed flora, under Mediterranean conditions: competitive ability of five winter season weed species. Afr. J. Agric. Res. 4, 1431–1441. Available online at: https://www.academicjournals.org/AJAR
Bilalis, D., Karkanis, A., Pantelia, A., Patsiali, S., Konstantas, A., and Efthimiadou, A. (2012a). Weed populations are affected by tillage systems and fertilization practices in organic flax (‘Linum usitatissimum’ L.) crop. Aust. J. Crop Sci. 6, 157-163. Available online at: https://www.cropj.com/
Bilalis, D., Papastylianou, P., Konstantas, A., Patsiali, S., Karkanis, A., and Efthimiadou, A. (2010). Weed-suppressive effects of maize–legume intercropping in organic farming. Int. J. Pest Manag. 56, 173–181. doi: 10.1080/09670870903304471
Bilalis, D., Sidiras, N., Economou, G., and Vakali, C. (2003). Effect of different levels of wheat straw soil surface coverage on weed flora in Vicia faba crops. J. Agron. Crop Sci. 189, 233–241. doi: 10.1046/j.1439-037X.2003.00029.x
Bilalis, D., Triantafyllidis, V., Karkanis, A., Efthimiadou, A., and Kakabouki, I. (2012b). The effect of tillage system and rimsulfuron application on weed flora, arbuscular mycorrhizal (AM) root colonization and yield of maize (Zea mays L.). Notulae Botanicae Horti Agrobotanici Cluj-Napoca 40, 73–79. doi: 10.15835/nbha4028218
Blackshaw, R. E., and Brandt, R. N. (2008). Nitrogen fertilizer rate effects on weed competitiveness is species dependent. Weed Sci. 56, 743–747. doi: 10.1614/WS-08-065.1
Blackshaw, R. E., and Brandt, R. N. (2009). Phosphorus fertilizer effects on the competition between wheat and several weed species. Weed Biol. Manag. 9, 46–53. doi: 10.1111/j.1445-6664.2008.00317.x
Blackshaw, R. E., Brandt, R. N., Janzen, H. H., and Entz, T. (2004). Weed species response to phosphorus fertilization. Weed Sci. 52, 406–412. doi: 10.1614/WS-03-122R
Blackshaw, R., Molnar, L., and Larney, F. (2005). Fertilizer, manure and compost effects on weed growth and competition with winter wheat in western Canada. Crop Protect. 24, 971–980. doi: 10.1016/j.cropro.2005.01.021
Booth, B. D., Murphy, S. D., and Swanton, C. J. (2003). Weed Ecology in Natural and Agricultural Systems. Wallingford, CT: CABI Publishing.
Booth, B. D., Murphy, S. D., and Swanton, C. J. (2010). Invasive Plant Ecology in Natural and Agricultural Systems. Wallingford, CT: CABI.
Bray, J. R., and Curtis, J. T. (1957). An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 27, 325–349. doi: 10.2307/1942268
Brix, A., and Andreasen, C. (2000). The relation between densities and frequencies of weeds in arable fields. J. Agric. Biol. Environ. Stat. 5, 372–386. doi: 10.2307/1400460
Buhler, D. D. (1992). Population dynamics and control of annual weeds in corn (Zea mays) as influenced by tillage systems. Weed Sci. 40, 241–248. doi: 10.1017/S0043174500057295
Buhler, D. D. (1995). Influence of tillage systems on weed population dynamics and management in corn and soybean in the central USA. Crop Sci. 35, 1247–1258. doi: 10.2135/cropsci1995.0011183X003500050001x
Buhler, D. D., Stoltenberg, D. E., Becker, R. L., and Gunsolus, J. L. (1994). Perennial weed populations after 14 years of variable tillage and cropping practices. Weed Sci. 42, 205–209.
Cardina, J., Herms, C. P., and Doohan, D. J. (2002). Crop rotation and tillage system effects on weed seedbanks. Weed Sci. 50, 448–460. doi: 10.1614/0043-1745(2002)050[0448:CRATSE]2.0.CO;2
Carter, M. R. (2005). “Conservation tillage,” in Encyclopedia of Soils in the Environment, ed D. Hillel (Oxford: Elsevier), 306–311.
Cheetham, A. H., and Hazel, J. E. (1969). Binary (presence absence) similarity coefficients. J. Paleont. 43, 1130–1136.
Cheimona, N., Angeli, C., Panagiotou, E., Tzanidaki, A., Drontza, C., Travlos, I., et al. (2016). Effect of different types of fertilization on weed flora in processed tomato crop. Agric. Agric. Sci. Procedia 10, 26–31. doi: 10.1016/j.aaspro.2016.09.005
Clifford, H. T., and Stephenson, W. (1975). An Introduction to Numerical Classification. New York, NY: Academic Press.
Coffman, C. B., and Frank, J. R. (1992). Corn-weed Interactions with long-term conservation tillage management. Agron. J. 84, 17–21. doi: 10.2134/agronj1992.00021962008400010004x
Cousens, R., and Mortimer, M. (1995). Dynamics of Weed Populations. Cambridge, UK: Cambridge University Press.
Davis, A. S. (2017). Nitrogen fertilizer and crop residue effects on seed mortality and germination of eight annual weed species. Weed Sci. 55, 123–128. doi: 10.1614/WS-06-133.1
Demjanová, E., Macák, M., Dalovic, I., Majernik, F., Tyr, S., and Smatana, S. (2009). Effects of tillage systems and crop rotation on weed density, weed species composition and weed biomass in maize. Agron.Res. 7, 785–792. Available online at: http://agronomy.emu.ee/
Dorado, J., and Lopez-Fando, C. (2006). The effect of tillage system and use of a paraplow on weed flora in a semiarid soil from central Spain. Weed Res. 46, 424–431. doi: 10.1111/j.1365-3180.2006.00526.x
Evans, F. C., and Dahl, E. (1955). The vegetational structure of an abandoned field in southeastern Michigan and its relation to environmental factors. Ecology 36, 685–706. doi: 10.2307/1931307
Fennimore, S. A., and Jackson, L. E. (2003). Organic amendment and tillage effects on vegetable field weed emergence and seedbanks. Weed Technol. 17, 42–50. doi: 10.1614/0890-037X(2003)017[0042:OAATEO]2.0.CO;2
Floyd, D. A., and Anderson, J. E. (1987). A comparison of three methods for estimating plant cover. J. Ecol. 75, 221–228. doi: 10.2307/2260547
Freyman, S., Kowalenko, C., and Hall, J. (1989). Effect of nitrogen, phosphorus and potassium on weed emergence and subsequent weed communities in south coastal British Columbia. Canadian J. Plant Sci. 69, 1001–1010. doi: 10.4141/cjps89-121
Fried, G., Norton, L. R., and Reboud, X. (2008). Environmental and management factors determining weed species composition and diversity in France. Agric. Ecosyst. Environ. 128, 68–76. doi: 10.1016/j.agee.2008.05.003
Gamito, S. (2010). Caution is needed when applying Margalef diversity index. Ecol. Indic. 10, 550–551. doi: 10.1016/j.ecolind.2009.07.006
Gibson, R., Nelson, I., Hopkins, G., Hamlett, B., and Memmott, J. (2006). Pollinator webs, plant communities and the conservation of rare plants: arable weeds as a case study. J. Appl. Ecol. 43, 246–257. doi: 10.1111/j.1365-2664.2006.01130.x
Gill, K. S., and Arshad, M. A. (1995). Weed flora in the early growth period of spring crops under conventional, reduced, and zero tillage systems on a clay soil in northern Alberta, Canada. Soil and Tillage Res. 33, 65–79. doi: 10.1016/0167-1987(94)00429-I
Gough, L., Osenberg, C. W., Gross, K. L., and Collins, S. L. (2000). Fertilization effects on species density and primary productivity in herbaceous plant communities. Oikos 89, 428–439. doi: 10.1034/j.1600-0706.2000.890302.x
Grey, T. L., Webster, T. M., Li, X., Anderson, W., and Cutts, G. S. (2017). Evaluation of control of napiergrass (Pennisetum purpureum) with tillage and herbicides. Invasive Plant Sci. Manag. 8, 393–400. doi: 10.1614/IPSM-D-15-00012.1
Gruber, S., and Claupein, W. (2009). Effect of tillage intensity on weed infestation in organic farming. Soil Tillage Res. 105, 104–111. doi: 10.1016/j.still.2009.06.001
Gu, Q. Z., Yang, X. Y., Sun, B. H., Zhang, S. L., and Tong, Y. A. (2007). Weed biodiversity in winter wheat field of loess soil under different fertilization regimes. Chin. J. Appl. Ecol. 18, 1038–1042.
Hernandez Plaza, E., Kozak, M., Navarrete, L., and Gonzalez-Andujar, J. L. (2011). Tillage system did not affect weed diversity in a 23-year experiment in Mediterranean dryland. Agric. Ecosyst. Environ. 140, 102–105. doi: 10.1016/j.agee.2010.11.016
Huang, S., Pan, X., Sun, Y., Zhang, Y., Hang, X., Yu, X., et al. (2013). Effects of long-term fertilization on the weed growth and community composition in a double-rice ecosystem during the fallow period. Weed Biol. Manag. 13, 10–18. doi: 10.1111/wbm.12004
Hyvönen, T., Ketoja, E., Salonen, J., Jalli, H., and Tiainen, J. (2003). Weed species diversity and community composition in organic and conventional cropping of spring cereals. Agric. Ecosyst. Environ. 97, 131–149. doi: 10.1016/S0167-8809(03)00117-8
Hyvönen, T., and Salonen, J. (2002). Weed species diversity and community composition in cropping practices at two intensity levels–a six-year experiment. Plant Ecol. 159, 73–81. doi: 10.1023/A:1015580722191
Inouye, R. S., and Tilman, D. (1995). Convergence and divergence of old-field vegetation after 11 yr of nitrogen addition. Ecology 76, 1872–1887. doi: 10.2307/1940720
Jaccard, P. (1908). Nouvelles recherches sur la distribution floral. Bull. Soc. Vaudoise Sci. Nat. 44, 223–270. doi: 10.5169/seals-268384
Kakabouki, I., Karkanis, A., Travlos, I. S., Hela, D., Papastylianou, P., Wu, H., et al. (2015). Weed flora and seed yield in quinoa crop (Chenopodium quinoa Willd.) as affected by tillage systems and fertilization practices. Int. J. Pest Manag. 61, 228–234. doi: 10.1080/09670874.2015.1042413
Kercher, S. M., Frieswyk, C. B., and Zedler, J. B. (2003). Effects of sampling teams and estimation methods on the assessment of plant cover. J. Veg. Sci. 14, 899–906. doi: 10.1111/j.1654-1103.2003.tb02223.x
Koller, K. (2003). “Techniques of soil tillage,” in Soil Tillage in Agroecosystems, ed A. E. Titi (Boca Raton, FL: CRC Press), 1–25.
Krajina, V. J. (1933). Die Pflanzengesellshaften des Mlynica-Tales in den Vysoke Tatra (Hohe Tatra). Botanisches Centralblatt 51, 1–224.
Labrada, R. (2003). Present Trends in Weed Management. Rome: FAO Plant Production and Protection Paper (FAO).
Lack, S., Parchami, P., and Modhej, A. (2011). Study the effects of nitrogen levels and wild oat (Avena ludoviciana L.) densities on grain yield and agronomic nitrogen efficiency of wheat (Triticum aestivum L.). Adv. Environ. Biol. 5, 2445–2451. Available online at: http://www.aensiweb.com/AEB/
Légère, A., Stevenson, F. C., and Benoit, D. L. (2005). Diversity and assembly of weed communities: contrasting responses across cropping systems. Weed Res. 45, 303–315. doi: 10.1111/j.1365-3180.2005.00459.x
Little, N. G., Mohler, C. L., Ketterings, Q. M., and DiTommaso, A. (2015). Effects of organic nutrient amendments on weed and crop growth. Weed Sci. 63, 710–722. doi: 10.1614/WS-D-14-00151.1
Ma, M. (2005). Species richness vs evenness: independent relationship and different responses to edaphic factors. Oikos 111, 192–198. doi: 10.1111/j.0030-1299.2005.13049.x
MacDonald, G. (2003). Biogeography: Introduction to Space, Time, and Life. Hoboken, NJ: Blackwell Publishing.
Magdoff, F., and van Es, H. (2000). Building Soils for Better Crops. Beltsville: Sustainable Agriculture Network
Mahn, E.-G. (1988). Changes in the structure of weed communities affected by agro-chemicals: what role does nitrogen play? Ecol. Bull. 39, 71–73.
Marshall, E., Brown, V., Boatman, N., Lutman, P., Squire, G., and Ward, L. (2003). The role of weeds in supporting biological diversity within crop fields. Weed Res. 43, 77–89. doi: 10.1046/j.1365-3180.2003.00326.x
Mas, M. T., and Verdú, A. M. (2003). Tillage system effects on weed communities in a 4-year crop rotation under Mediterranean dryland conditions. Soil Tillage Res. 74, 15–24. doi: 10.1016/S0167-1987(03)00079-5
Maxwell, B., and O'Donovan, J. (2007). Understanding weed–crop interactions to manage weed problems. Non-Chemical Weed Management: Principles, Concepts and Technology. Oxfordshire: CAB International, 17–33.
McIntyre, S., and Martin, T. G. (2001). Biophysical and human influences on plant species richness in grasslands: comparing variegated landscapes in subtropical and temperate regions. Austral Ecol. 26, 233–245. doi: 10.1046/j.1442-9993.2001.01108.x
Melander, B., Munier-Jolain, N., Charles, R., Wirth, J., Schwarz, J., van der Weide, R., et al. (2013). European perspectives on the adoption of nonchemical weed management in reduced-tillage systems for arable crops. Weed Technol. 27, 231–240. doi: 10.1614/WT-D-12-00066.1
Menalled, F. D., Gross, K. L., and Hammond, M. (2001). Weed aboveground and seedbank community responses to agricultural management systems. Ecol. Appl. 11, 1586–1601. doi: 10.1890/1051-0761(2001)011[1586:WAASCR]2.0.CO;2
Mohammaddoust-e-Chamanadad, H., Tulikov, A., and Baghestani, M. (2006). Effect of long-term fertilizer application and crop rotation on the infestation of fields by weeds. Pak. J. Weed Sci. Res. 12, 221–234. doi: 10.28941/pjwsr
Morris, E. K., Caruso, T., Buscot, F., Fischer, M., Hancock, C., Maier, T. S., et al. (2014). Choosing and using diversity indices: insights for ecological applications from the German Biodiversity Exploratories. Ecol. Evol. 4, 3514–3524. doi: 10.1002/ece3.1155
Mortensen, D. A., Bastiaans, L., and Sattin, L. (2000). The role of ecology in the development of weed management systems: an outlook. Weed Res. 40, 49–62. doi: 10.1046/j.1365-3180.2000.00174.x
Mulugeta, D., Stoltenberg, D. E., and Boerboom, C. M. (2001). Weed species–area relationships as influenced by tillage. Weed Sci. 49, 217–223. doi: 10.1614/0043-1745(2001)049[0217:WSARAI]2.0.CO;2
Murphy, C. E., and Lemerle, D. (2006). Continuous cropping systems and weed selection. Euphytica 148, 61–73. doi: 10.1007/s10681-006-5941-9
Nichols, V., Verhulst, N., Cox, R., and Govaerts, B. (2015). Weed dynamics and conservation agriculture principles: a review. Field Crops Res. 183, 56–68. doi: 10.1016/j.fcr.2015.07.012
Nie, J., Yin, L. C., Liao, Y. L., Zheng, S. X., and Xie, J. (2009). Weed community composition after 26 years of fertilization of late rice. Weed Sci. 57, 256–260. doi: 10.1614/WS-08-106.1
Nkoa, R., Owen, M. D. K., and Swanton, C. J. (2015). Weed abundance, distribution, diversity, and community analyses. Weed Sci. 63, 64–90. doi: 10.1614/WS-D-13-00075.1
Peigné, J., Ball, B. C., Roger-Estrade, J., and David, C. (2007). Is conservation tillage suitable for organic farming? A review. Soil Use Manag. 23, 129–144. doi: 10.1111/j.1475-2743.2006.00082.x
Pinke, G., Karácsony, P., Czúcz, B., Botta-Dukát, Z., and Lengyel, A. (2012). The influence of environment, management and site context on species composition of summer arable weed vegetation in Hungary. Appl. Veg. Sci. 15, 136–144. doi: 10.1111/j.1654-109X.2011.01158.x
Power, A. G. (2010). Ecosystem services and agriculture: tradeoffs and synergies. Philos. Trans. R. Soc. B Biol. Sci. 365, 2959–2971. doi: 10.1098/rstb.2010.0143
Pyšek, P., and Lepš, J. (1991). Response of a weed community to nitrogen fertilization: a multivariate analysis. J. Veg. Sci. 2, 237–244. doi: 10.2307/3235956
Rassam, G., Latifi, N., Soltani, A., and Kamkar, B. (2011). Impact of crop management on weed species diversity and community composition of winter wheat fields in Iran. Weed Biol. Manag. 11, 83–90. doi: 10.1111/j.1445-6664.2011.00407.x
Romesburg, H. C. (1984). Cluster Analysis for Researchers. Belmont, CA: Lifetime Learning Publications.
Sans, F. X., Berner, A., Armengot, L., and Mäder, P. (2011). Tillage effects on weed communities in an organic winter wheat–sunflower–spelt cropping sequence. Weed Res. 51, 413–421. doi: 10.1111/j.1365-3180.2011.00859.x
Santín-Montanyá, M. I., Martín-Lammerding, D., Walter, I., Zambrana, E., and Tenorio, J. L. (2013). Effects of tillage, crop systems and fertilization on weed abundance and diversity in 4-year dry land winter wheat. Eur. J. Agron. 48, 43–49. doi: 10.1016/j.eja.2013.02.006
Sepat, S., Thierfelder, C., Sharma, A. R., Pavuluri, K., Kumar, D., Iquebal, M. A., et al. (2017). Effects of weed control strategy on weed dynamics, soybean productivity and profitability under conservation agriculture in India. Field Crops Res. 210, 61–70. doi: 10.1016/j.fcr.2017.05.017
Shannon, C. E., and Weaver, W. (1963). The Mathematical Theory of Communication. Urbana, IL: University of Illinois Press.
Sheley, R., James, J., Rinella, M., Blumenthal, D., DiTomaso, J., and Briske, D. (2011). “Invasive plant management on anticipated conservation benefits: a scientific assessment,” Conservation Benefits of Rangeland Practices—Assessment, Recommendations, and Knowledge Gaps, ed D. D. Briske (Lawrence: Allen Press), 291–335.
Shrestha, A., Knezevic, S., Roy, R., Ball-coelho, B., and Swanton, C. (2002). Effect of tillage, cover crop and crop rotation on the composition of weed flora in a sandy soil. Weed Res. 42, 76–87. doi: 10.1046/j.1365-3180.2002.00264.x
Smith, B., and Wilson, J. B. (1996). A consumer's guide to evenness indices. Oikos 76, 70–82. doi: 10.2307/3545749
Smith, R. G., Mortensen, D. A., and Ryan, M. (2010). A new hypothesis for the functional role of diversity in mediating resource pools and weed–crop competition in agroecosystems. Weed Res. 50, 37–48. doi: 10.1111/j.1365-3180.2009.00745.x
Soane, B. D., Ball, B. C., Arvidsson, J., Basch, G., Moreno, F., and Roger-Estrade, J. (2012). No-till in northern, western and south-western Europe: A review of problems and opportunities for crop production and the environment. Soil Tillage Res. 118, 66–87. doi: 10.1016/j.still.2011.10.015
Storkey, J., Moss, S. R., and Cussans, J. W. (2010). Using assembly theory to explain changes in a weed flora in response to agricultural intensification. Weed Sci. 58, 39–46. doi: 10.1614/WS-09-096.1
Streit, B., Rieger, S., Stamp, P., and Richner, W. (2003). Weed populations in winter wheat as affected by crop sequence, intensity of tillage and time of herbicide application in a cool and humid climate. Weed Res. 43, 20–32. doi: 10.1046/j.1365-3180.2003.00310.x
Stroud, J. T., Bush, M. R., Ladd, M. C., Nowicki, R. J., Shantz, A. A., and Sweatman, J. (2015). Is a community still a community? Reviewing definitions of key terms in community ecology. Ecol. Evol. 5, 4757–4765. doi: 10.1002/ece3.1651
Suding, K. N., Collins, S. L., Gough, L., Clark, C., Cleland, E. E., Gross, K. L., et al. (2005). Functional-and abundance-based mechanisms explain diversity loss due to N fertilization. Proc. Natl. Acad. Sci. U.S.A. 102, 4387–4392. doi: 10.1073/pnas.0408648102
Swanton, C. J., Shrestha, A., Roy, R. C., Ball-Coelho, B. R., and Knezevic, S. Z. (1999). Effect of tillage systems, N, and cover crop on the composition of weed flora. Weed Sci. 47, 454–461.
Sweeney, A. E., Renner, K. A., Laboski, C., and Davis, A. (2008). Effect of fertilizer nitrogen on weed emergence and growth. Weed Sci. 56, 714–721. doi: 10.1614/WS-07-096.1
Tang, L., Cheng, C., Wan, K., Li, R., Wang, D., Tao, Y., et al. (2014). Impact of fertilizing pattern on the biodiversity of a weed community and wheat growth. PLoS ONE 9:e84370. doi: 10.1371/journal.pone.0084370
Tang, L., Wan, K., Cheng, C., Li, R., Wang, D., Pan, J., et al. (2013). Effect of fertilization patterns on the assemblage of weed communities in an upland winter wheat field. J. Plant Ecol. 7, 39–50. doi: 10.1093/jpe/rtt018
Than, N. N., Zhang, S., Sun, B., Yi, H., and Yang, X. (2017). Long-term diverse fertilizer management on weed species and communities in winter wheat field. Am. J. Plant Sci. 8, 1790-1800. doi: 10.4236/ajps.2017.88122
Thomas, A. G., Derksen, D. A., Blackshaw, R. E., Van Acker, R. C., Légère, A., Watson, P. R., et al. (2017). A multistudy approach to understanding weed population shifts in medium- to long-term tillage systems. Weed Sci. 52, 874–880. doi: 10.1614/WS-04-010R1
Tilman, E. A., Tilman, D., Crawley, M. J., and Johnston, A. (1999). Biological weed control via nutrient competition: potassium limitation of dandelions. Ecol. Appl. 9, 103–111. doi: 10.1890/1051-0761(1999)009[0103:BWCVNC]2.0.CO;2
Tørresen, K. S., Skuterud, R., Tandsaether, H., and Hagemo, M. B. (2003). Long-term experiments with reduced tillage in spring cereals. I. Effects on weed flora, weed seedbank and grain yield. Crop Protect. 22, 185–200. doi: 10.1016/S0261-2194(02)00145-X
Travlos, I. (2013). “Weeds in perennial crops as an unexpected tool of integrated crop management,” in Weeds and their Ecological Functions, ed A. Taab (Hauppauge, NY: Nova Science Publishers), 97–113.
Travlos, I. S. (2010). “Legumes as cover crops or components of intercropping systems and their effects on weed populations and crop productivity,” in Advances in Food Science and Technology, ed A. J. Greco (Hauppauge, NY: Nova Science Publishers), 151–164.
Travlos, I. S. (2013). Competition between ACCase-inhibitor resistant and susceptible sterile wild oat (Avena sterilis) Biotypes. Weed Sci. 61, 26–31. doi: 10.1614/WS-D-12-00065.1
Travlos, I. S., and Economou, G. (2010). “Effects of no-till farming on global weed related problems and weed communities of greece and the ongoing challenges of integrated weed and crop management,” in No-Till Farming: Effects on Soil, Pros and Cons and Potential, ed E. T. Nardali (Hauppauge, NY: Nova Science Publishers), 145–157.
Travlos, I. S., and Giannopolitis, C. N. (2010). Assessment of distribution and diversity of Avena sterilis L. and Avena fatua L. in cereal crops of Greece based on a 3-year survey and selected morphological traits. Genet. Resour. Crop Evolut. 57, 337-341. doi: 10.1007/s10722-010-9535-y
Travlos, I., Mikroulis, A., Anastasiou, E., Fountas, S., Bilalis, D., Tsiropoulos, Z., et al. (2017). The use of RGB cameras in defining crop development in legumes. Adv. Anim. Biosci. Precis. Agric. 8, 224–228.
Tuesca, D., Puricelli, E., and Papa, J. (2001). A long-term study of weed flora shifts in different tillage systems. Weed Res. 41, 369–382. doi: 10.1046/j.1365-3180.2001.00245.x
Ugen, M. A., Wien, H. C., and Wortmann, C. S. (2002). Dry bean competitiveness with annual weeds as affected by soil nutrient availability. Weed Sci. 50, 530–535. doi: 10.1614/0043-1745(2002)050[0530:DBCWAW]2.0.CO;2
Van Evert, F. K., Fountas, S., Jakotevic, D., Crnojevic, V., Travlos, I., and Kempenaar, C. (2017). Big data for weed control and crop protection. Weed Res. 57, 218–233. doi: 10.1111/wre.12255
Vakali, C., Zaller, J. G., and Köpke, U. (2011). Reduced tillage effects on soil properties and growth of cereals and associated weeds under organic farming. Soil Tillage Res. 111, 133–141. doi: 10.1016/j.still.2010.09.003
van Elsen, T. (2000). Species diversity as a task for organic agriculture in Europe. Agric. Ecosyst. Environ. 77, 101–109. doi: 10.1016/S0167-8809(99)00096-1
Vasquez, E., Sheley, R., and Svejcar, T. (2008). Creating invasion resistant soils via nitrogen management. Invasive Plant Sci. Manag. 1, 304–314. doi: 10.1614/IPSM-07-059.1
Wan, K., Tao, Y., Li, R., Pan, J., Tang, L., and Chen, F. (2012). Influences of long-term different types of fertilization on weed community biodiversity in rice paddy fields. Weed Biol. Manag. 12, 12–21. doi: 10.1111/j.1445-6664.2011.00430.x
Whittaker, R. H. (1960). Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 30, 279–338. doi: 10.2307/1943563
Keywords: abundance, diversity, weed flora, tillage, fertilization
Citation: Travlos IS, Cheimona N, Roussis I and Bilalis DJ (2018) Weed-Species Abundance and Diversity Indices in Relation to Tillage Systems and Fertilization. Front. Environ. Sci. 6:11. doi: 10.3389/fenvs.2018.00011
Received: 05 January 2018; Accepted: 20 February 2018;
Published: 03 April 2018.
Edited by:
Sudhakar Srivastava, Banaras Hindu University, IndiaReviewed by:
Leonardo Bianco de Carvalho, Universidade Estadual Paulista Júlio de Mesquita Filho (UNESP), BrazilGerassimos G. Peteinatos, University of Hohenheim, Germany
Copyright © 2018 Travlos, Cheimona, Roussis and Bilalis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ilias S. Travlos, dHJhdmxvc0BhdWEuZ3I=
 Ilias S. Travlos
Ilias S. Travlos Nikolina Cheimona
Nikolina Cheimona