Abstract
Farmland biodiversity has undergone drastic declines due to agricultural intensification during the last decades. To prevent further biodiversity loss, the maintenance and restoration of non-productive habitats is essential. Woodlots, small patches of woody vegetation in agricultural landscapes, are one such habitat that are currently subsidized by the European Union’s Common Agricultural Policy (EU’s CAP). For effective implementation, however, it is necessary to assess what habitat characteristics are the most beneficial for biodiversity. Our study performs such an assessment using birds as model organisms. Specifically, we related characteristics of various woodlots to (I) the species richness of all birds, and (II) the species richness of both forest and farmland birds–groups with different ecological requirements. For this purpose, we counted birds (27 farmland and 26 forest species) and measured habitat characteristics (describing vegetation structure, diversity and nativeness) and spatial characteristics (area, shape and isolation) in 82 mid-field woodlots (0.76–1.25 ha, average 0.46 ha) in the Czech Republic (Central Europe). After accounting for the effects of spatial characteristics, overall bird species richness increased with vegetation height and woody plant species richness. In addition, richness showed a non-linear decrease with the cover of an invasive tree, the Black Locust Robinia pseudoaccacia. Interestingly, forest bird species richness was related to the same habitat characteristics as the overall bird species richness. By contrast, farmland bird species richness was positively related to the diversity of woodlot microhabitats, which reflects mainly enrichment by non-forest sites such as grassland or sparse shrubs. Our results indicate that the ecological performance of habitat characteristics (and not only the woodlot area) is important for the restoration of bird diversity in woodlots, and as such should be taken into consideration by the EU’s CAP subsidy system. Moreover, if woodlot management aims to maximize the overall bird diversity—a common practice in biodiversity conservation—our results show that current practices may favor widespread forest bird species, but not the farmland birds that are more threatened in Europe. To manage the woodlot habitat for farmland birds, we suggest that microhabitat heterogeneity should be maximized.
Introduction
During the last decades, farmland has undergone drastic changes due to ongoing agricultural intensification (Foley et al., 2011). These changes have caused a rapid decline of farmland biodiversity and threatened the ecosystem services important for global food production (such as pollination or soil functioning) over the long-term (Stoate et al., 2001, 2009). This is particularly the case in Europe, where agricultural landscapes currently account for about half of the area of the continent (Food and Agriculture Organization of the United Nations, 2014). Moreover, a large proportion of European biodiversity is associated with farmland habitat due a long co-evolution of biota and agriculture (Batáry et al., 2015). Thus, preventing further losses of biodiversity and sustaining the ecosystem services of agricultural landscapes has become one of the most urgent challenges the European Union (EU) currently faces (Stoate et al., 2009). In order to address this issue, the EU’s Common Agricultural Policy (CAP) has incorporated various environmental measures, but their effectiveness varies considerably (Batáry et al., 2015; Gamero et al., 2017). Of the various measures in place, the restoration of non-productive habitats was found to be the most promising in promoting biodiversity and ecosystem services (Batáry et al., 2015; European Commission, 2017). Nevertheless, their conservation potential remains still largely untapped, as the system is designed to subsidize non-productive habitats according to just their area, without taking their other characteristics or future management into account (European Commission, 2017).
This is also the case of woodlots, small non-productive patches of woody vegetation, which are subsidized within both pillars of the CAP to some extent (“tree clusters” up to an area of 0.3 ha in the case of Ecological Focus Areas) (Pe’er et al., 2017). However, even though woodlots could serve as key refuges for farmland biodiversity, the impacts of their ecological characteristics on biodiversity remain largely unknown. According to ecological theory, habitat quality is an important aspect for habitat selection decisions of particular species (Guisan and Thuiller, 2005), suggesting that this aspect of woodlots is an overlooked but crucial predictor of their ecological performance.
To fill this critical knowledge gap, here we focused on investigating different aspects of the habitat of woodlots in relation to the species richness of the organisms they host. We used birds as model organisms since they are currently one of the groups most severely affected by agricultural intensification (Reif and Hanzelka, 2020). Moreover, they often serve as state of nature indicators reflecting conditions at large spatial scales and higher trophic levels (Fraixedas et al., 2020). Previous research has shown that woodlots are inhabited primarily by two bird groups with largely opposite habitat requirements: forest and farmland birds (Bellamy et al., 1996; Fuller et al., 2001, 2004). However, previous studies focused on effects of woodlot characteristics have mostly considered larger woodland patches (0.02–30 ha in Bellamy et al., 1996 and Hinsley et al., 1995; 0.3–302 ha in Lorenzetti and Battisti, 2006; 0.7–14.5 in McCollin, 1993), and investigated them rather from the perspective of forest fragmentation, focusing mainly on the effects of woodlot spatial characteristics − size, isolation or shape (McCollin, 1993; Hinsley et al., 1995; Lorenzetti and Battisti, 2006). The importance of habitat characteristics has generally not been recognized (but see Bellamy et al., 1996 and Mason, 2001). Since there has been little effort to study woodlots as a specific habitat for farmland biodiversity, their importance for the farmland birds remains to be assessed.
In this study, we surveyed birds in 82 woodlots scattered over 3,000 km2 of farmland in the Czech Republic, central Europe. These woodlots showed high variability in their habitat characteristics, which we sorted into three groups: 1) the structure of woodland vegetation (i.e., tree height and the density of the tree and shrub layers), 2) the habitat diversity (i.e. the diversity of microhabitats and richness of woody plant species in the woodlot), and 3) the nativeness of woodlot vegetation (i.e., the proportion of coniferous trees, which are not native in woodlots in the study area, and the proportion of the exotic Black Locust Robinia pseudoaccacia). The aim of our study was to discover which of these characteristics are linked to overall bird species richness, as well as forest and farmland bird species richness separately (Table 1).
TABLE 1
| Explanatory variables (units) | Abbreviation | Characterization | Expectation | Justification | References | ||
|---|---|---|---|---|---|---|---|
| HABITAT CHARACTERISTICS | STRUCTURE | Tree height (m) | HEIGHT | Maximum height of tree vegetation | ↗ | More available habitat can support more species | Sparks et al., (1996); Hinsley and Bellamy, (2000) |
| Shrub layer density (%) | DENSE_SHRUB | Percentage of a woodland patch covered by shrubs (up to 4 m in height) | ↗ | More available habitat can support more species | Doherty and Grubb, (2000); Hinsley and Bellamy, (2000) | ||
| Tree layer density (%) | DENSE_TREE | Percentage of woodland habitat covered by tree foliage (higher than 4 m) | ↗ | More available habitat can support more species | Bellamy et al., (1996); Hinsley and Bellamy, (2000) | ||
| DIVERSITY | Woody plant species richness | PLANTS | Total number of tree and shrub species in the woodlot | ↗ | More diverse breeding and feeding resources provide more opportunities for species’ coexistence | Green et al., (1994); Ampoorter et al., (2020) | |
| Microhabitat diversity | HABITAT_DIV | Shannon diversity index of five microhabitat types | ↗ | More microhabitats provide more opportunities for species’ coexistence | Mason, (2001); Fuller et al., (2004) | ||
| • Grassland | |||||||
| • Wetland | |||||||
| • Sparse shrubland (= walk-through shrubs) | |||||||
| • Dense shrubland (= impassable shrubs) | |||||||
| • Woodland (= vegetation with trees > 4 m) | |||||||
| NATIVENESS | Proportion of Black Locust (%) | ROBINIA | Proportion of the Black Locust Robinia pseudoacacia in the tree canopy | ↘ | Exotic tree suppressing native plants (allelopathy) and insects (phytophagous species are not adapted to its leaves) resulting in more homogenous habitat and less food sources for birds | Reif, Hanzelka, et al., (2016); Štrobl et al., (2019) | |
| Proportion of coniferous (%) | CONIFERS | Proportion of coniferous vegetation in the tree canopy | ↘ | Not naturally occurring in study area and thus birds adapted to their stands may be missing | Reif, Hanzelka, et al., (2016); Štrobl et al., (2019) | ||
| SPATIAL CHARACTERISTICS | Area (ha) | AREA | Total area of the woodlot | ↗ | Larger woodlots have a higher carrying capacity for species richness | Mason, (2001); Lorenzetti and Battisti, (2006) | |
| Shape | SHAPE | Woodlot perimeter divided by the perimeter of a circle with the same area | ↗ | More complex shapes provide more ecotones and thus more opportunities for species’ coexistence | Hinsley et al., (1995); Bellamy et al., (1996) | ||
| Isolation | ISOLATION | The 1st axis of the principal component analysis ran on five isolation parameters | ↘ | Due to dispersal limitation, more isolated woodlots are less likely to be occupied | McCollin, (1993); Bennet et al. (2004) | ||
| • Woodlot distances to the nearest forest | |||||||
| • Woodlot distances to the nearest urban area | |||||||
| • Relative coverage of forests in a 1,000 m buffer | |||||||
| • Relative coverage of urban areas in a 1,000 m buffer | |||||||
| • Cumulative hedgerow length in a 1,000 m buffer | |||||||
Variables describing habitat and spatial characteristics of woodlots and their expected relationships with bird species richness (↗ stands for positive and ↘ for negative relationship).
Study Area and Methods
Study Area and Woodlot Selection
The study was carried out in central Bohemia, the Czech Republic, in a lowland area of circa 3,000 km2 (Figure 1). This area is dominated by an intensive agriculture landscape (covering about 70% of the area) with a considerable proportion of human settlements and industrial areas (20%), and a small amount of forests (10%) (Ložek et al., 2003).
FIGURE 1
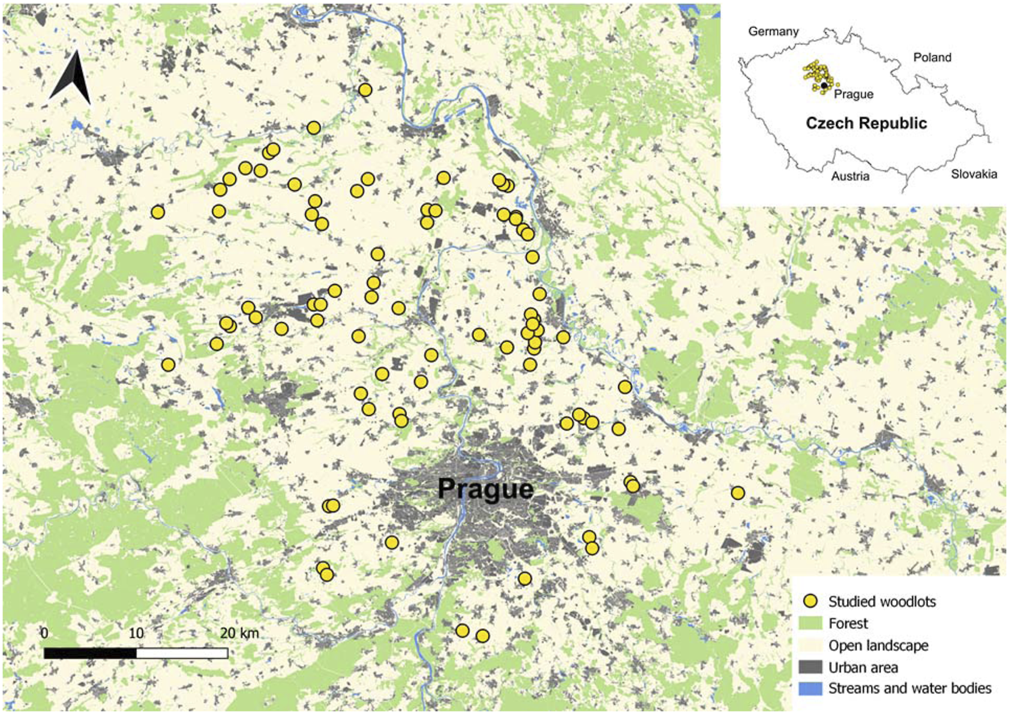
Studied woodlots (yellow dots) on a map showing the main landcover types in the Czech Republic. Inset shows the position of the study area within the Czech Republic.
For the purposes of this study, we defined a mid-field woodlot as a woody patch smaller than 1.25 ha, approximately circular or rectangular in shape, with continuous tree vegetation higher than 4 m on at least part of its area (i.e., we did not consider patches of small shrubs or herbaceous vegetation). Such a definition corresponds to the typical character of mid-field woody vegetation in the region, and avoids the inclusion of forests. Moreover, we considered only woodlots fully surrounded by intensively cultivated agricultural land with no other woody vegetation or urban areas present within 100 m of each woodlot. In addition, these selection rules aimed to minimize the variability in woodlots’ spatial characteristics (area, shape and isolation) since our primary research interests were the effects of habitat characteristics. We considered all woodlots in the study area meeting these criteria, with the resulting dataset containing 82 woodlots (Figure 1, Supplementary Table S1). Woodlots were surrounded by large homogenous agricultural blocks composed of fields of cereals, oilseed rape and maize, and less frequently by other crops. Distance between the nearest neighboring woodlots varied from 360 m to 11.5 km (average 2 km).
Woodlot Characteristics
Woodlots were characterized by several variables reflecting their structure, diversity and nativeness (see Table 1). The structure of the continuous woody vegetation of each woodlot was expressed using three variables: the tree height was the maximal height of the tree canopy; the shrub layer density (vegetation up to 4 m above the ground) and tree layer density (higher than 4 m) were estimated as the percentages of the area covered by the respective vegetation. For describing habitat diversity, the total number of tree and shrub species was used as a measure of woody plant species richness; the microhabitat diversity was assessed by estimating the proportions of five microhabitat types (grassland, wetland, sparse shrubland, dense shrubland and woodland) and calculating the Shannon diversity index. Finally, for describing the nativeness of vegetation, we estimated the proportion of Black Locust (an exotic tree species of North American origin) and the proportion of conifers in the tree canopy. (Although the coniferous trees present are a part of the native Czech flora, they are not natural vegetation in the study area and were all planted by humans.) Because the woodlots were relatively small, all characteristics were measured over the whole area, without using any survey plots.
In addition, we used aerial photographs (scale 1:1,000) and QGIS 2.18 (QGIS Development Team, 2019) to estimate the area, shape and isolation of each woodlot. The area of the woodlots varied from 0.076 to 1.25 ha (average 0.46 ha). The shape was expressed as the ratio of a woodlot perimeter divided by the perimeter of a hypothetical circular woodlot with the same area–the lower the ratio, the closer the shape is to circular (Bellamy et al., 1996). This ratio varied from 1.004 to 1.627 (average 1.189) in our woodlots. To express the isolation of each woodlot, we used the first axis of a principal component analysis (PCA) ran using the R-package “vegan” (Oksanen et al., 2019) with five isolation parameters: a woodlot distance to the nearest areas of forest and urban habitat, the relative coverage of these habitats in a 1,000 m buffer around a given woodlot, and the cumulative hedgerow length in the same buffer. All these habitat types can host some of the bird species observed in our woodlots (Šťastný and Hudec, 2011) and it is thus necessary to take them into account. The PCA showed a gradient from the least to the most isolated woodlots (Supplementary Figure S1).
Bird Survey
Birds were counted in 2017 (in 12 woodlots) and 2018 (in the remaining 70 woodlots). Each woodlot was visited twice per breeding season in a given year (in the second half of April and in the second half of June) to cover both early- and late-breeding species. During each visit, the whole area of each woodlot was explored systematically by a slow walk and all birds detected either visually or acoustically were recorded except for individuals flying over the woodlot. All surveys were conducted at the time of the highest bird activity from6:00 to 10:00 under favorable weather conditions (i.e. no rain or strong wind). The time devoted for a single visit of a woodlot was 5–30 min depending on its area, so that small and large woodlots were given approximately the same effort per unit area.
All nocturnal species were excluded from further analysis because the field technique was not suitable for their detection. The Common Pheasant Phasianus colchicus and the Grey Partridge Perdixperdix were excluded as well because both species are bred in captivity and occasionally released by hunters, and thus their abundance may not represent local environmental conditions.
For each woodlot, we expressed the species richness of all birds (hereafter called “overall bird species richness”), forest birds (“forest bird species richness”) and farmland birds (“farmland bird species richness”) as the total number of species in the respective groups across both visits. To correct the data for sample size bias, we also calculated a rarefied version of these variables (using the R-package iNEXT; Hsieh et al., 2020). In addition, we calculated the Shannon diversity index for all birds, forest birds and farmland birds separately, taking the numbers of recorded individuals (as a maximum count of each species across both visits) into account. To categorize species as forest or farmland, we followed the classification of Reif et al. (2010), who performed a multivariate analysis of birds’ habitat preferences based on breeding bird monitoring data of country-wide coverage (for recorded species and their categorization see Supplementary Table S2).
Statistical Analyses
Before building statistical models, we assessed the collinearity between all explanatory variables using Pearson’s correlation coefficient and the variance inflation factor (VIF) with the R package “usdm” (Naimi et al., 2014). We did not detect any signs of collinearity in the data (Supplementary Table S3). Exploratory searching for possible non-linear relationships revealed non-linearity for the proportion of Black Locust. We applied generalized linear modelling in two distinctive steps to uncover both the more general effects of the spatial variables, vegetation structure, diversity and nativeness as well as the specific effect of each explanatory variable.
Firstly, for each of the nine response variables (i.e., species richness, rarefied species richness and the Shannon index for all birds, forest birds and farmland birds) we composed six generalized linear models (GLMs): a null model, a model containing solely spatial variables, one model for each of the three general habitat characteristics (i.e., vegetation structure, diversity and nativeness) that also included the spatial variables, and a full model including all ten variables (i.e., tree height, tree layer density, shrub layer density, woody plant species richness, microhabitat diversity, the proportion of Black Locust, the proportion of conifers, area, shape and isolation). The area was log-transformed and the proportion of Black Locust was modeled using b-splines to accommodate the non-linear relationship using the R package “splines” (Perperoglou et al., 2019). The rarefied species richness was log-transformed. For modeling of species richness variables, we used the Poisson distribution and log link function; for modeling rarefied species richness and the Shannon diversity index we used the Gaussian distribution and identity link function.
Secondly, for each response variable, each of the full GLMs was processed in the “MuMIn” R package (Bartoń, 2019) to assess all possible combinations of explanatory variables’ main effects using the Akaike Information Criterion corrected for small sample sizes (AICc). We did not consider interactive effects because they were not justified by the hypotheses and the sample size did not enable such complex models. To avoid model overfitting given the sample size of 83 woodlots, the maximum number of explanatory variables included in a single model was set to eight following the recommendation of Burnhan and Anderson (2002). The variable of woodlot area was included in every candidate model in order to fully account for the species-area relationship (Rosenzweig, 1995). For each response variable, the top set of models with ∆AICc < 4 were used for inference as recommended by recent reviews on model selection (e.g., Harrison et al., 2018). For this purpose, we performed conditional model averaging of the parameter estimates across the top models.
We then plotted the relationships between the respective response variables and their predictors estimated by the single best-supported model for each response variable. In the main manuscript, we present plots with the original values of the response variable (Figure 2), but the individual study sites cannot be visualized in such plots; plots showing the study sites are shown as partial residual plots in the Supplementary Online Material (Supplementary Figure S2). For each of the top models, we checked for the possible presence of spatial autocorrelation in residuals using smoothed nonparametric functions (spline.correlog function from the R package “ncf”; Bjornstad, 2019) with 95% confidence intervals computed using a bootstrap with 1,000 replications. No significant autocorrelation was indicated in any model (results not shown). All analyses were run using R 3.6.0 (R Core Team, 2019).
FIGURE 2
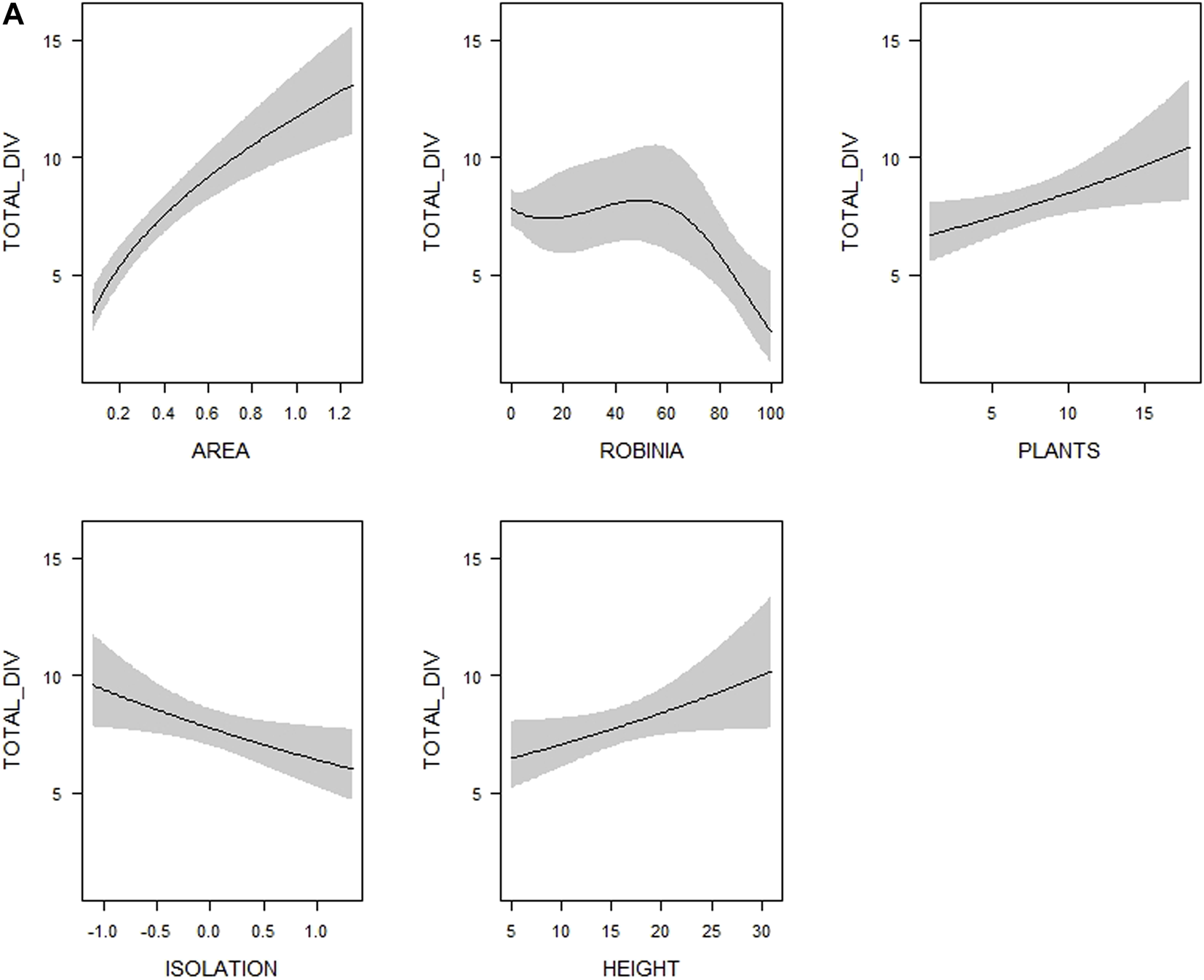
Visualization of the relationships between (A) overall bird species richness (TOTAL_DIV–number of all bird species), (B) forest bird species richness (FOREST_DIV–number of forest bird species) and (C) farmland bird species richness (FARMLAND_DIV–number of farmland bird species), respectively, and woodlot characteristics (see Table 1 for their definitions and abbreviations) included in the most supported generalized linear models (see Supplementary Table S4). Shaded areas correspond to 95% confidence intervals. The estimates from the best model for each response variable are shown.
Results
In 82 surveyed woodlots we detected 53 bird species; 26 of them were classified as forest birds and 27 as farmland birds. On average, one woodlot hosted seven bird species (range 0–18 species), five forest bird species (0–12) and three farmland bird species (0–10). The most frequently recorded species were the Common Blackbird Turdus merula, the Yellowhammer Emberiza citrinella, the Great Tit Parus major, the Eurasian Blackcap Sylvia atricapilla and the Common Chaffinch Fringilla coelebs. For the complete list of recorded species see Supplementary Table S2.
Zero deviance explained by the respective null models, i.e. the models without predictors containing solely the intercept, indicated that our focal explanatory variables were important predictors of bird species richness in woodlots (Table 2). Indeed, spatial characteristics models, i.e. the models containing woodlots’ area, shape and isolation, but not their habitat characteristics, improved the explained deviance considerably and led to a marked decrease in AICc compared to the null models (Table 2). These patterns were observed for overall bird species richness, as well as for the species richness of forest and farmland birds (Table 2). Moreover, in all these bird groups, the spatial characteristics model was not the best performing model, indicating that habitat characteristics play an important role in explaining the variation of bird species richness across woodlots (Table 2). However, the roles of respective habitat characteristics differed among the bird groups.
TABLE 2
| All birds | Forest birds | Farmland birds | ||||
|---|---|---|---|---|---|---|
| Deviance | AICc | Deviance | AICc | Deviance | AICc | |
| (A) | ||||||
| Null model | 0.000 | 486.848 | 0.000 | 396.731 | 0.000 | 347.985 |
| Spatial variables model | 56.652 | 389.671 | 47.811 | 329.200 | 32.790 | 319.352 |
| Spatial variables and habitat structure variables model | 58.146 | 393.933 | 49.896 | 332.968 | 33.632 | 325.444 |
| Spatial variables and habitat diversity variables model | 59.934 | 388.269 | 54.824 | 322.946 | 42.984 | 313.039 |
| Spatial variables and habitat nativeness variables model | 64.380 | 384.986 | 54.761 | 327.898 | 37.315 | 323.960 |
| Full model | 69.811 | 388.430 | 63.693 | 327.453 | 50.103 | 323.651 |
| (B) | ||||||
| Null model | 0.000 | 206.503 | 0.000 | 216.964 | 0.000 | 180.920 |
| Spatial variables model | 43.114 | 166.882 | 47.222 | 171.198 | 27.612 | 161.061 |
| Spatial variables and habitat structure variables model | 46.348 | 169.265 | 52.062 | 170.493 | 31.136 | 164.151 |
| Spatial variables and habitat diversity variables model | 45.323 | 168.359 | 52.056 | 168.044 | 36.700 | 154.784 |
| Spatial variables and habitat nativeness variables model | 53.147 | 160.681 | 53.231 | 170.996 | 32.743 | 164.743 |
| Full model | 59.445 | 162.6128 | 61.708 | 168.3664 | 44.609 | 162.5954 |
| (C) | ||||||
| Null model | 0.000 | 168.368 | 0.000 | 169.346 | 0.000 | 157.822 |
| Spatial variables model | 50.708 | 116.999 | 51.210 | 117.137 | 34.125 | 130.231 |
| Spatial variables and habitat structure variables model | 52.249 | 121.577 | 55.286 | 117.167 | 36.162 | 134.838 |
| Spatial variables and habitat diversity variables model | 54.633 | 114.917 | 56.550 | 112.356 | 47.486 | 116.368 |
| Spatial variables and habitat nativeness variables model | 62.108 | 105.140 | 56.832 | 116.808 | 39.812 | 132.539 |
| Full model | 67.759 | 105.667 | 65.713 | 111.6894 | 54.170 | 123.960 |
Explained deviance and AICc of six generalized linear models explaining (A) species richness, (B) rarified species richness, and (C) the Shannon diversity index for all, forest and farmland bird species. For specific variables categorized as spatial, habitat structure, diversity and nativeness see Table 1.
FIGURE 2

(Continued)
FIGURE 2
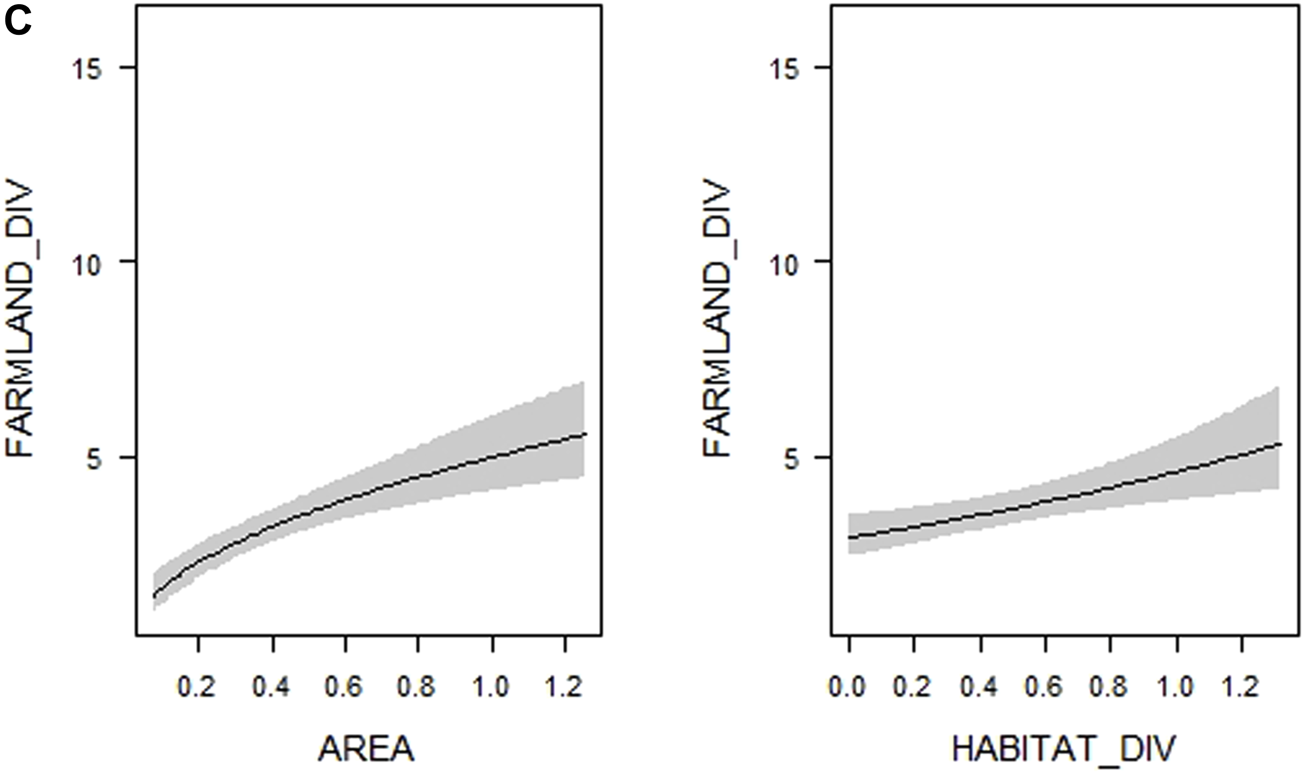
(Continued)
All Birds
Overall bird species richness was best explained by the model containing the spatial characteristics together with the variables describing the nativeness of the woodlot vegetation–represented by the proportions of Black Locust and coniferous trees (Table 2). When we used the variables describing vegetation structure (tree height, tree layer density and shrub layer density) instead of the nativeness variables, model performance decreased and was lower than in the spatial characteristics model (Table 2). The performance of the model containing the diversity variables (microhabitat diversity and woody plant species richness) was similar to the spatial characteristics model (Table 2). Multimodel inference of the top models (22 out of 502 candidate models meeting the ∆AICc < 4, Supplementary Table S4) uncovered the importance of particular explanatory variables: the proportion of Black Locust, woody plant species richness, tree height and isolation (together with the woodlot’s area included in all models by default). These variables were also the only explanatory variables with 95% confidence intervals not overlapping zero (Table 3). The overall bird species richness was positively related to tree height and woody plant species richness. More isolated woodlots and woodlots with a higher coverage of Black Locust hosted a lower number of bird species (Figure 2A, Supplementary Figure S2A). In case of Black Locust, this negative effect was non-linear and bird species richness tended to drop after it reached about 60% of the woodlot cover (see Figure 2A). Results of the multimodel inference were similar in case of the overall bird species richness adjusted by the rarefaction, showing qualitatively the same effects of the Black Locust cover and tree height (Supplementary Table S5). These two variables were also the only ones related to the Shannon index of overall bird diversity according to the confidence intervals (Supplementary Table S5).
TABLE 3
| Explanatory variable | Coefficient | 2.5% CL | 97.5% CL | Variable importance (sum of Akaike weights) | No of modelsa | |
|---|---|---|---|---|---|---|
| (A) | ||||||
| HABITAT CHARACTERISTICS | HEIGHT | 0.01853 | 0.000 | 0.037 | 0.74 | 14 |
| DENSE_SHRUB | 0.00052 | −0.002 | 0.003 | 0.11 | 3 | |
| DENSE_TREE | 0.00014 | −0.004 | 0.004 | 0.10 | 3 | |
| PLANTS | 0.02519 | 0.002 | 0.048 | 0.90 | 19 | |
| HABITAT_DIV | 0.14934 | −0.076 | 0.374 | 0.35 | 8 | |
| bs (ROBINIA)1b | −0.23820 | −1.094 | 0.617 | 1.00 | 22 | |
| bs (ROBINIA)2b | 0.77265 | −0.368 | 1.913 | |||
| bs(ROBINIA)3b | −1.06876 | −1.777 | -0.361 | |||
| CONIFERS | 0.00061 | −0.003 | 0.004 | 0.11 | 3 | |
| SPATIAL CHARACTERISTICS | log(AREA) | 0.49283 | 0.345 | 0.641 | 1.00 | 22 |
| SHAPE | 0.08564 | −0.582 | 0.754 | 0.10 | 3 | |
| ISOLATION | −0.18194 | −0.350 | −0.014 | 0.90 | 19 | |
| (B) | ||||||
| HABITAT CHARACTERISTICS | HEIGHT | 0.02749 | 0.003 | 0.052 | 0.89 | 12 |
| DENSE_SHRUB | −0.00015 | −0.004 | 0.004 | 0.10 | 2 | |
| DENSE_TREE | −0.00026 | −0.006 | 0.006 | 0.11 | 2 | |
| PLANTS | 0.04481 | 0.014 | 0.076 | 1.00 | 14 | |
| HABITAT_DIV | −0.22276 | −0.544 | 0.098 | 0.43 | 7 | |
| bs (ROBINIA)1b | −0.41523 | −1.589 | 0.759 | 0.90 | 12 | |
| bs (ROBINIA)2b | 1.08258 | −0.569 | 2.734 | |||
| bs(ROBINIA)3b | −1.39184 | −2.491 | −0.293 | |||
| CONIFERS | 0.00043 | -0.004 | 0.005 | 0.11 | 2 | |
| SPATIAL CHARACTERISTICS | log(AREA) | 0.53662 | 0.337 | 0.736 | 1.00 | 14 |
| SHAPE | 0.10486 | −0.802 | 1.012 | 0.11 | 2 | |
| ISOLATION | −0.35234 | −0.585 | −0.120 | 1.00 | 14 | |
| (C) | ||||||
| HABITAT CHARACTERISTICS | HEIGHT | 0.01387 | −0.012 | 0.040 | 0.29 | 10 |
| DENSE_SHRUB | 0.00116 | −0.003 | 0.005 | 0.13 | 5 | |
| DENSE_TREE | 0.00351 | −0.002 | 0.009 | 0.33 | 10 | |
| PLANTS | 0.00876 | −0.023 | 0.041 | 0.15 | 6 | |
| HABITAT_DIV | 0.49820 | 0.203 | 0.793 | 1.00 | 30 | |
| bs (ROBINIA)1b | 0.18575 | −1.033 | 1.405 | 0.05 | 2 | |
| bs (ROBINIA)2b | 0.50743 | −1.098 | 2.113 | |||
| bs (ROBINIA)3b | -0.73644 | −1.661 | 0.189 | |||
| CONIFERS | 0.00236 | −0.003 | 0.008 | 0.23 | 9 | |
| SPATIAL CHARACTERISTICS | log(AREA) | 0.46346 | 0.276 | 0.651 | 1.00 | 30 |
| SHAPE | −0.04929 | −1.077 | 0.978 | 0.10 | 4 | |
| ISOLATION | 0.07542 | −0.167 | 0.318 | 0.17 | 7 | |
Relationships of (A) overall bird species richness, (B) forest bird species richness and (C) farmland bird species richness to woodlot characteristics (see Table 1 for their definitions and abbreviations) estimated by generalized linear models. The model-averaged coefficients together with their confidence limits (CL) across the top models (defined by ∆AICc < 4; see Supplementary Table S4) are shown. Variables with confidence limits not including zero are in bold. For results for rarefied species richness and the Shannon index see Supplementary Table S5.
Number of models containing a given variable, see Supplementary Table S4.
Fitted as a nonlinear relationship using b-splines (see Methods section for more details).
Forest Birds
The species richness of forest birds was best explained by the model containing both spatial and diversity variables (Table 2), while the model containing both spatial and vegetation structure variables was the worst performing (Table 2). Adding the nativeness variables into the spatial variables model did not considerably improve its performance (Table 2). In the multimodel inference, 14 models met the ∆AICc < 4 threshold (Supplementary Table S4). The important explanatory variables were the same as in the case of overall species richness: tree height, woody plant species richness, the proportion of Black Locust and isolation (Table 3). The 95% confidence intervals of these variables did not overlap zero (Table 3). The direction and shape of their effects were the same as in the case of overall species richness, i.e. more species were found in woodlots with higher trees, a higher number of woody plant species, a lower coverage of Black Locust and lower isolation (Figure 2B, Supplementary Figure S2B). Using the rarified richness and Shannon diversity index as respective response variables provided the same patterns (Supplementary Table S5).
Farmland Birds
In the case of farmland bird species richness, the model containing both diversity and spatial variables was the only one outperforming the model with solely spatial variables (Table 2). According to multimodel inference, the top models (30 models which met the ∆AICc < 4 threshold) explained 42.5–48% of the deviance, considerably less than top models for overall and forest bird species richness, which explained 56.2–69.6% (Supplementary Table S4). Only one variable–microhabitat diversity–was of considerable importance, and its 95% confidence interval did not overlap zero (Table 3). More farmland bird species were recorded in woodlots with higher microhabitat diversity (Figure 2C, Supplementary Figure S2C). The same variable was the only one supported by the model selection procedure in the case of rarified farmland bird richness (Supplementary Table S5). However, the results somewhat differed in the analysis of the Shannon index of farmland bird diversity. In addition to microhabitat diversity, both the tree layer density and the proportion of coniferous vegetation had slightly positive effects on values of this index, with their lower confidence limits only slightly overlapping zero (Supplementary Table S5).
Discussion
Mid-field woodlots are small non-productive elements in agricultural landscapes that may serve as important biodiversity refuges (e.g., Tryjanowski et al., 2014; Štrobl et al., 2019; Pustkowiak et al., 2021). However, their role as distinctive landscape features has been largely overlooked (but see Gottschalk et al., 2010; Aue et al., 2014), since previous studies have included mostly all semi-natural landscape features together at the regional scale (Billeter et al., 2008; Doxa et al., 2010; Sasaki et al., 2020) or considered larger woodland patches from the perspective of forest habitat fragmentation (McCollin, 1993; Bellamy et al., 1996; Doherty and Grubb, 2000; Bennett et al., 2004; Lorenzetti and Battisti, 2006). Such studies mainly focused on the effects of area, shape and isolation (e.g. Hofmeister et al., 2017) for the purposes of forest bird conservation (McCollin, 1993; Lorenzetti and Battisti, 2006). If the effects of some habitat characteristics were tested, they mostly proved insignificant (McCollin, 1993; Hinsley et al., 1995; Bellamy et al., 1996; Mason, 2001; Lorenzetti and Battisti, 2006) most likely due to strong area effects driven by the large ranges of woodlot sizes in studied samples.
As a consequence, the importance of woodlot habitat characteristics for supporting farmland biodiversity has remained insufficiently explored (Vanhinsbergh et al., 2002). Our results begin to address this issue, showing that habitat characteristics, namely vegetation structure, diversity and nativeness, shape bird species richness and the proportions of forest vs. farmland birds in woodlots. A comparison of the performance of models containing different groups of habitat variables indicated that overall bird species richness was best explained by nativeness variables, whereas forest and farmland bird richness was better explained by diversity variables (see Table 2). However, each variable group contained both good and poor predictors of bird species richness, so it is necessary to focus on the individual habitat characteristics instead on their groups. In this respect, the importance of different habitat characteristics varied among bird groups: overall and forest bird species richness increased with the height of trees and with the species richness of woody plants and decreased with the increasing proportion of a non-native tree, the Black Locust. In contrast, farmland bird species richness was positively related only to the diversity of microhabitats. These results are comparable to some extent to those obtained by research focused on linear landscape features–hedgerows–where the positive effects of vegetation height, plant species richness and microhabitat diversity, as well as vegetation density on overall bird species richness have been observed (for example Green et al., 1994; Sparks et al., 1996; Hinsley and Bellamy, 2000).
In total, we recorded 53 bird species in 82 woodlots with areas up to 1.25 ha. This number of species is comparable to the bird species richness of woodlots studied in the United Kingdom. For example, the same number of species was recorded in sixteen woodlots (McCollin, 1993), whereas Mason (2001) recorded 46 species in 35 wood forest fragments, and Bellamy et al. (1996) found 64 species breeding in 151 fragments (in all cases without considering predators, owls, or feathered game). Besides the considerably wider range areas (which probably leads to a wider scale of habitat types), these studies also did not limit the selection of woodlots according to their isolation from different habitats, while we specifically focused on isolated ones. Therefore, a number of species that were missing in our woodlots were recorded in those studies–for instance, water birds such as the Mute Swan (Cygnus olor), Mallard (Anas platyrhynchos) and Common Moorhen (Gallinula chloropus); synanthropic species such as the House Sparrow (Passer domesticus) and Western Jackdaw (Corvus monedula), or species dependent on larger forest areas such as the Stock Dove (Columba oenas), Coal Tit (Parus ater), and Eurasian Treecreeper (Certhia familiaris). On the other hand, we recorded 12 species not reported in the studies from the United Kingdom including the Eurasian Wryneck (Jynx torquilla), Red-backed Shrike (Lanius collurio), Great Grey Shrike (Lanius excubitor), Common Grasshopper Warbler (Locustella naevia) and Western Yellow Wagtail (Motacilla flava). It is possible that our woodlots contained a higher proportion of grasslands, providing habitat for the Yellow Wagtail, and sparse shrubs preferred by the Common Grasshopper Warbler. Moreover, some of the above mentioned species, such as shrikes and the Eurasian Wryneck, are still commonly breeding in Central European landscapes, but are rare or even absent in the United Kingdom (Keller et al., 2020).
Habitat Characteristics
The only habitat characteristic related to farmland bird species richness was microhabitat diversity. This characteristic expressed the proportion of each habitat in a woodlot, reflecting its enrichment by sparsely vegetated habitats to a large extent. The strong and positive relationship with the number of farmland birds implies that many such species need sparsely vegetated non-productive habitats even within our very small woodlots, most likely for foraging and nesting (Bellamy et al., 1996; Bennett et al., 2004). In addition, this pattern could reflect the importance of a heterogeneous habitat mosaic for some farmland birds. For example, some bunting species such as the Corn Bunting Emberiza calandra need a high proportion of grasslands in their territory for foraging but also need high trees suitable as song posts (Altewischer et al., 2015). Similarly, the Red-backed Shrike Lanius collurio uses sparse shrubs for its sit-and-wait foraging strategy, but also requires dense shrubs for nesting and cover from predators (Ceresa et al., 2012). The Song Thrush Turdus philomelos requires a habitat mosaic providing song posts and nesting opportunities on trees and shrubs and simultaneously foraging sites in grassy patches for gleaning insects on the ground (Peach et al., 2004).
We found a positive linear relationship between the maximal height of woodlot tree vegetation and the species richness of all birds as well as forest birds. Higher vegetation is related to a higher volume of available habitat, and due to the presence of multiple vegetation layers also to a wider supply of different habitat niches, and thus can support more species (Sparks et al., 1996). Furthermore, height is supposed to positively correlate with the age of trees and may also reflect the presence of dead trees and cavities (Guby and Dobbertin, 1996). As our results indicate, such mature vegetation may be beneficial for many forest bird species such as woodpeckers, which forage and/or nest in the tree layer (Lorenzetti and Battisti, 2006). In addition, higher and older tree vegetation may attract larger-bodied species (e.g. corvids and birds of prey) for perching (Hinsley and Bellamy, 2000) and their presence can thus increase the total number of bird species recorded in a woodlot.
A similar positive effect of tree height has previously been reported for overall bird species richness in hedgerows (Hinsley and Bellamy, 2000), but not in studies focusing on woodland fragments (e.g., Nol et al., 2005). The reason for the absence of this effect may be due to the focus on forest fragmentation of those studies, which considered much larger woodlots where the tree height contribution to the overall habitat amount and dimensionality was presumably small. This implies that vegetation structure for birds may be more important in smaller woodlots, indicating the need for the appropriate management of such habitats.
Bird species richness in our study also increased with woody plant species diversity (see also Osborne, 1984; Green et al., 1994), and as in the case of tree vegetation height this overall positive effect was driven by forest birds. Generally, more woody plant species create more diverse breeding and feeding resources for birds and thus provide more opportunities for their coexistence (Ampoorter et al., 2020). In addition, a higher diversity of woody plants may provide food supply for birds over a larger part of the year (Arnold, 1983). Forest birds probably respond to this resource provision more strongly because they largely depend on woody vegetation, whereas farmland birds may partly satisfy their needs in open landscapes (Bellamy et al., 1996) and thus they are not limited by the woody plant composition within woodlots.
Finally, the proportion of an invasive exotic tree, the Black Locust, had a negative effect on both overall and forest bird species richness. The shape of this relationship was nonlinear: species richness stagnated up to ca 60% of Black Locust cover and then steeply decreased (see Figure 2). This non-linear response of bird species richness to Black Locust cover was also found in central European forests (Kroftová and Reif, 2017). It seems that there may be a certain threshold proportion of this exotic tree, over which bird species richness becomes limiting, possibly due to the homogenization of the tree stand composition. In addition, Black Locust hosts fewer insect species and changes the composition of the insect community, which may limit the food supply for birds (Kadlec et al., 2018; Štrobl et al., 2019). This is particularly likely to be important for the specialized insectivorous species foraging on trees (Reif, Hanzelka, et al., 2016) that correspond to the forest species in our study.
Spatial Characteristics
In addition to the habitat characteristics that were of our primary research interest, the area and isolation of woodlots were also related to bird species richness. Indeed, they explained a higher proportion of the variability in bird species richness than the habitat characteristics (see Table 2). But this was largely driven by area effects, with area positively affecting overall, forest as well as farmland bird species richness. Even though the variation of woodlot area was kept as small as possible, this was to be expected, considering that area is the main driver of species richness in habitat fragments at the landscape scale (De Camargo et al., 2018). In contrast, increasing isolation (a composite measure expressing the connection of a given woodlot with various types of landscape features and land cover classes in its surroundings including woodlands, hedgerows and urban areas) had a negative effect only on overall species richness and on forest birds, whereas no effect was found on farmland birds. These results are consistent with previous findings from forest fragmentation studies in England, where forest bird species richness decreased with isolation, whereas so called “edge species”, largely corresponding to our farmland birds, tended to occupy more isolated fragments (McCollin, 1993; Bellamy et al., 1996; Bennett et al., 2004). This suggests that small mid-field woodlots may be a suboptimal habitat for forest birds (Loman, 2003; Nol et al., 2005) but a suitable habitat for many farmland birds. As woodlots have been found to possibly function as ecological traps for some bird species under some circumstances (Loman, 2003), another question is whether this also applies to those in our study area. In addition, future studies should go beyond the species richness we focused on here and test whether the bird populations occupying woodlots are viable long-term.
Forest Birds Drive Overall Species Richness
Interestingly, even though the total number of forest and farmland bird species recorded in woodlots was almost equal, the variability in bird species richness was explained by exactly the same set of characteristics for overall species richness and for the species richness of forest birds, suggesting that forest bird distribution accounts for overall species richness variation across woodlots. This can be explained by the relative commonness of forest bird species because spatial patterns in species richness are formed mainly by common species rather than rare ones (Lennon et al., 2004). Indeed, forest species found in our woodlots are rather widespread generalists, such as the Great Tit, Eurasian Blackcap, Chaffinch and Common Chiffchaff Phylloscopus collybita (Reif, Hořák, et al., 2016), whose requirements for a minimum area of habitat can be satisfied even in our small and highly isolated forest patches. On the other hand, farmland birds tend to be more rare and specialized species (Reif, Hořák, et al., 2016) deviating from the overall richness pattern. Even though some of these species may not use only woodlots exclusively but also take advantage of surrounding agricultural lands (Bellamy et al., 1996), woodlots represent an indispensable part of their territories (Pustkowiak et al., 2021). This invokes an important message for conservation practice. The development of measures for woodlot habitat management should be not based on findings arising from overall species richness, but rather be specifically customized in respect to the needs of farmland birds, assuming these species are intended to benefit from such management. Future research should assess how to compensate for the trade-offs between the demands of both groups and maximize bird species richness at the landscape scale (Simberloff, 2001).
At the same time, our classification of species as forest or farmland birds may mask subtle ecological preferences because species habitat selection usually follows a continual gradient rather than discrete categories (Knick et al., 2008). On the other hand, some kind of categorization is necessary to infer general insights, and our classification was based on the best available objective data, i.e. a multivariate analysis of bird habitat preferences based on country-wide breeding bird monitoring taking potential regional variations into account (Reif et al., 2010). This makes us confident that the observed differences between forest and farmland birds in relation to woodlot habitat characteristics reflect their genuine ecological requirements.
Rarified Species Richness and the Shannon Diversity Index
Patterns provided by rarified species richness were very similar to those obtained by the analysis of raw species richness. We suggest that this similarity is driven by including woodlot area into all models. Because the rarefaction analysis corrects for unequal sample size (Gotelli and Colwell, 2001) and the sample size, in terms of the number of species or individuals sampled, is strongly influenced by woodlot area, we have indeed taken the woodlot area into account when analyzing the raw species richness. Moreover, the similarity of the raw and rarified richness results suggests that our sampling was adequate for the purposes of our study provided that the differences in woodlot area were carefully considered.
Although the Shannon diversity index also provided very similar findings to those obtained for the species richness, the pattern for farmland birds shows that this index increases not only with higher microhabitat diversity (as was observed for species richness) but also tended to increase with increasing tree layer density and the higher proportion of coniferous trees. The Shannon diversity index reflects the dominance of individual species together with their number (Jost, 2006). We suggest that higher values of this index in woodlots with a denser tree layer and more coniferous trees may result from the preference of some farmland bird species for these habitats, such as Eurasian Tree Sparrow or the Yellowhammer. Such a preference may be too weak to affect the raw species richness but could be reflected in the Shannon index.
Conclusion
Our study shows that besides the spatial characteristics of woodlots (i.e., area, isolation and shape) previously studied from a forest fragmentation perspective (for example McCollin, 1993; Bellamy et al., 1996; Doherty and Grubb, 2000), various characteristics of local habitat are important predictors of woodlot bird species richness. Moreover, we show that woodlots should be not considered as a sort of small forest with impoverished biodiversity. Instead, they represent a specific farmland habitat that is important for specific farmland species.
Our findings thus allow the formulation of clear recommendations for woodlot management. However, it should be kept in mind that our findings concern a single study area and a limited spectrum of variables, so our recommendations should be always considered in respect to local circumstances. Further studies in different areas are needed to corroborate our results; for instance, we can imagine that woodlots located in High Nature Value farmland, i.e. not surrounded by intensively managed large arable fields, may show different relationships to bird species richness and diversity (Doxa et al., 2010). In addition, recommendations for woodlot management strongly depend on the species being targeted. If the aim is to improve the habitat for overall species richness, management should support high trees of diverse woody plant species. These trees should be native, but the inclusion of non-native Black Locust may not necessarily be harmful if its coverage remains relatively low. A reduction of woodlot isolation would also bring more bird species. However, these guidelines would improve conditions specifically for common generalist forest birds. Since populations of such generalist forest birds are increasing in Central Europe and the amount of their preferred forest habitats has been expanding due to both intentional and spontaneous afforestation (Schulze et al., 2019), we do not find these species an appropriate target of woodlot habitat management. Instead, we recommend focusing on improving the habitat characteristics beneficial for farmland birds, whose populations are declining at an unprecedented rate, at least in Central Europe (Reif and Vermouzek, 2019). Moreover, those species using woodlots as a breeding habitat in farmland have only a few alternatives (Rajmonová and Reif, 2018). Thus, woodlots should be managed to increase the diversity of various microhabitats, such as dense and sparse shrubs, grasslands or marshes. Although it may be possible to reconcile the different needs of forest and farmland birds in individual woodlots (e.g. increasing the proportion of open areas for farmland birds may be offset by tree maturation for forest birds that benefit from increasing tree height), we suggest that accommodating these diverse management targets for overall (and forest) bird species richness and for farmland birds is not feasible within individual woodlots due to their small size. Such reconciliation can be realized at the landscape scale, however, with a heterogeneous agricultural landscape including a mosaic of woodlots with different habitat characteristics. To target forest birds, several woodlots situated into clusters can be used to lower the effects of isolation (Loman and Von Schantz, 1991).
Statements
Data availability statement
Dataset used for the analyses is available as Table S1 in Supplementary Online Material. Raw data from bird counts are available upon request to the authors.
Author contributions
JR conceived the idea; LD and JR designed the study; LD and JR-S. carried out the fieldwork; LD analysed the data with inputs from LK and JR; LD led writing with contributions from all co-authors. All authors gave final approval for publication.
Funding
This work was supported by the Charles University, Prague (projects GAUK 70120 and PRIMUS/17/SCI/16). Beleco had no role in designing the research, in the results of the analyzes and in interpreting the results.
Acknowledgments
D. Romportl helped us with drawing Figure 1. Comments of three reviewers greatly improved the manuscript. D. W. Hardekopf kindly corrected the English.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.816255/full#supplementary-material
References
1
AltewischerA.BuschewskiU.EhrkeC.FröhlichJ.GärtnerA.GieseP.et al (2015). Habitat Preferences of Male Corn Buntings Emberiza Calandrain North-Eastern Germany. Acta Ornithologica50, 1–10. 10.3161/00016454AO2015.50.1.001
2
AmpoorterE.BarbaroL.JactelH.BaetenL.BobergJ.CarnolM.et al (2020). Tree Diversity Is Key for Promoting the Diversity and Abundance of Forest‐Associated Taxa in Europe. Oikos129, 133–146. 10.1111/oik.06290
3
ArnoldG. W. (1983). The Influence of Ditch and Hedgerow Structure, Length of Hedgerows, and Area of woodland and Garden on Bird Numbers on farmland. J. Appl. Ecol.20, 731–750. 10.2307/2403123
4
AueB.DiekötterT.GottschalkT. K.WoltersV.HotesS. (2014). How High Nature Value (HNV) farmland Is Related to Bird Diversity in Agro-Ecosystems - Towards a Versatile Tool for Biodiversity Monitoring and Conservation Planning. Agric. Ecosyst. Environ.194, 58–64. 10.1016/J.AGEE.2014.04.012
5
BartońK. (2019). MuMIn: Multi-Model Inference. Available online at: https://cran.r-project.org/package=MuMIn.
6
BatáryP.DicksL. V.KleijnD.SutherlandW. J. (2015). The Role of Agri‐Environment Schemes in Conservation and Environmental Management. Conservation Biol.29, 1006–1016. 10.1111/cobi.12536
7
BellamyP. E.HinsleyS. A.NewtonI. (1996). Factors Influencing Bird Species Numbers in Small Woods in South-East England. J. Appl. Ecol.33, 249–262. 10.2307/2404747
8
BennettA. F.HinsleyS. A.BellamyP. E.SwetnamR. D.Mac NallyR. (2004). Do Regional Gradients in Land-Use Influence Richness, Composition and Turnover of Bird Assemblages in Small Woods?Biol. Conservation119, 191–206. 10.1016/j.biocon.2003.11.003
9
BilleterR.LiiraJ.BaileyD.BugterR.ArensP.AugensteinI.et al (2008). Indicators for Biodiversity in Agricultural Landscapes: a Pan-European Study. J. Appl. Ecol.45, 141–150. 10.1111/j.1365-2664.2007.01393.x
10
BjornstadO. N. (2019). Ncf: Spatial Covariance Functions. Available online at: https://cran.r-project.org/package=ncf.
11
BurnhanK. P.AndersonD. R. (2002). Model Selection and Multimodel Inference. 2nd ed.New York: Springer.
12
CeresaF.BoglianiG.PedriniP.BrambillaM. (2012). The Importance of Key Marginal Habitat Features for Birds in Farmland: An Assessment of Habitat Preferences of Red-Backed Shrikes Lanius Collurioin the Italian Alps. Bird Study59, 327–334. 10.1080/00063657.2012.676623
13
De CamargoR. X.Boucher-LalondeV.CurrieD. J. (2018). At the Landscape Level, Birds Respond Strongly to Habitat Amount but Weakly to Fragmentation. Divers. Distrib.24, 629–639. 10.1111/ddi.12706
14
DohertyP. F.GrubbT. C. (2000). Habitat and Landscape Correlates of Presence, Density, and Species Richness of Birds Wintering in Forest Fragments in Ohio. Wilson Bull.112, 388–394. 10.1676/0043-5643(2000)112[0388:halcop]2.0.co;2
15
DoxaA.BasY.ParacchiniM. L.PointereauP.TerresJ.-M.JiguetF. (2010). Low-Intensity Agriculture Increases Farmland Bird Abundances in France. J. Appl. Ecol.47, 1348–1356. 10.1111/J.1365-2664.2010.01869.X
16
European Commission (2017). Report from the Commission to the European Parliament and the Council on the Implementation of the Ecological Focus Area Obligation Under the Green Direct Payment Scheme. Brussels. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52017DC0152&from=EN. (Accessed February 1, 2020).
17
FoleyJ. A.RamankuttyN.BraumanK. A.CassidyE. S.GerberJ. S.JohnstonM.et al (2011). Solutions for a Cultivated Planet. Nature478, 337–342. 10.1038/nature10452
18
Food and Agriculture Organization of the United Nations (2014). FAO Statistical Yearbook 2014: Europe and Central Asia Food and Agriculture. Budapest: Food and Agriculture Organization of the United Nations.
19
FraixedasS.LindénA.PihaM.CabezaM.GregoryR.LehikoinenA. (2020). A State-Of-The-Art Review on Birds as Indicators of Biodiversity: Advances, Challenges, and Future Directions. Ecol. Indicators118, 106728. 10.1016/j.ecolind.2020.106728
20
FullerR. J.ChamberlainD. E.BurtonN. H. K.GoughS. J. (2001). Distributions of Birds in Lowland Agricultural Landscapes of England and Wales: How Distinctive Are Bird Communities of Hedgerows and Woodland?Agric. Ecosyst. Environ.84, 79–92. 10.1016/s0167-8809(00)00194-8
21
FullerR. J.HinsleyS. A.SwetnamR. D. (2004). The Relevance of Non-farmland Habitats, Uncropped Areas and Habitat Diversity to the Conservation of Farmland Birds. Ibis (Lond. 1859)146, 22–31. 10.1111/j.1474-919X.2004.00357.x
22
GameroA.BrotonsL.BrunnerA.FoppenR.FornasariL.GregoryR. D.et al (2017). Tracking Progress Toward EU Biodiversity Strategy Targets: EU Policy Effects in Preserving its Common Farmland Birds. Conservation Lett.10, 395–402. 10.1111/conl.12292
23
GotelliN. J.ColwellR. K. (2001). Quantifying Biodiversity: Procedures and Pitfalls in the Measurement and Comparison of Species Richness. Ecol. Lett.4, 379–391. 10.1046/j.1461-0248.2001.00230.x
24
GottschalkT. K.DittrichR.DiekötterT.SheridanP.WoltersV.EkschmittK. (2010). Modelling Land-Use Sustainability Using farmland Birds as Indicators. Ecol. Indicators10, 15–23. 10.1016/J.ECOLIND.2009.05.008
25
GreenR. E.OsborneP. E.SearsE. J. (1994). The Distribution of Passerine Birds in Hedgerows During the Breeding Season in Relation to Characteristics of the Hedgerow and Adjacent Farmland. J. Appl. Ecol.31, 677–692. 10.2307/2404158
26
GubyN. A. B.DobbertinM. (1996). Quantitative Estimates of Coarse Woody Debris and Standing Dead Trees in Selected Swiss Forests. Glob. Ecol. Biogeogr. Lett.5, 327–341. 10.2307/2997588
27
GuisanA.ThuillerW. (2005). Predicting Species Distribution: Offering More Than Simple Habitat Models. Ecol. Lett.8, 993–1009. 10.1111/j.1461-0248.2005.00792.x
28
HarrisonX. A.DonaldsonL.Correa-CanoM. E.EvansJ.FisherD. N.GoodwinC. E. D.et al (2018). A Brief Introduction to Mixed Effects Modelling and Multi-Model Inference in Ecology. PeerJ6, e4794. 10.7717/peerj.4794
29
HinsleyS. A.BellamyP. E.NewtonI.SparksT. H. (1995). Habitat and Landscape Factors Influencing the Presence of Individual Breeding Bird Species in woodland Fragments. J. Avian Biol.26, 94–104. 10.2307/3677057
30
HinsleyS. A.BellamyP. E. (2000). The Influence of Hedge Structure, Management and Landscape Context on the Value of Hedgerows to Birds: A Review. J. Environ. Manage.60, 33–49. 10.1006/jema.2000.0360
31
HofmeisterJ.HošekJ.BrabecM.KočvaraR. (2017). Spatial Distribution of Bird Communities in Small Forest Fragments in Central Europe in Relation to Distance to the Forest Edge, Fragment Size and Type of Forest. For. Ecol. Manage.401, 255–263. 10.1016/j.foreco.2017.07.005
32
HsiehT. C.MaK. H.ChaoA. (2020). iNEXT: Interpolation and Extrapolation for Species Diversity. R package version 2.0.20. Available online at: http://chao.stat.nthu.edu.tw/wordpress/software_download/.
33
JostL. (2006). Entropy and Diversity. Oikos113, 363–375. 10.1111/j.2006.0030-1299.14714.x
34
KadlecT.ŠtroblM.HanzelkaJ.HejdaM.ReifJ. (2018). Differences in the Community Composition of Nocturnal Lepidoptera Between Native and Invaded Forests Are Linked to the Habitat Structure. Biodivers. Conserv.27, 2661–2680. 10.1007/s10531-018-1560-8
35
KellerV.HerrandoS.VorisekP.FranchM. (2020). In European Breeding Bird Atlas 2: Distribution, Abundance and Change.KellerV.HerrandoS.VoříšekP.FranchM.KipsonM.MilanesiP.. Editors (Barcelona: European Bird Census Council & Lynx Edicions).
36
KnickS. T.RotenberryJ. T.LeuM. (2008). Habitat, Topographical, and Geographical Components Structuring Shrubsteppe Bird Communities. Ecography31, 389–400. 10.1111/J.0906-7590.2008.05391.x
37
KroftováM.ReifJ. (2017). Management Implications of Bird Responses to Variation in Non-native/native Tree Ratios within Central European Forest Stands. For. Ecol. Manage.391, 330–337. 10.1016/j.foreco.2017.02.034
38
LennonJ. J.KoleffP.GreenwoodJ. J. D.GastonK. J. (2004). Contribution of Rarity and Commonness to Patterns of Species Richness. Ecol. Lett.7, 81–87. 10.1046/j.1461-0248.2004.00548.x
39
LomanJ.SchantzT. (1991). Birds in a Farmland-More Species in Small Than in Large Habitat Island. Conservation Biol.5, 176–188. 10.1111/j.1523-1739.1991.tb00122.x
40
LomanJ. (2003). Small Habitat Islands Are Inferior Breeding Habitats But Are Used by Some Great Tits – Competition or Ignorance?Biodivers. Conserv.12, 1467–1479. 10.1023/A:1023629810919
41
LorenzettiE.BattistiC. (2006). Area as Component of Habitat Fragmentation: Corroborating its Role in Breeding Bird Communities and Guilds of Oak Wood Fragments in Central Italy. Rev. D’écologie – La Terre La Vie61, 53–68.
42
LožekV.CílekV.KubíkováJ. (2003). Střední Čechy: Příroda, Člověk, Krajina. 1st ed. Praha: Středočeský kraj v produkci nakladatelství Dokořán.
43
MasonC. F. (2001). Woodland Area, Species Turnover and the Conservation of Bird Assemblages in Lowland England. Biodivers. Conserv.10, 495–510. 10.1023/A:1016606410892
44
McCollinD. (1993). Avian Distribution Patterns in a Fragmented Wooded Landscape (North Humberside, U.K.): The Role of Between-Patch and Within-Patch Structure. Glob. Ecol. Biogeogr. Lett.3, 48–62. 10.2307/2997459
45
NaimiB.HammN. A. S.GroenT. A.SkidmoreA. K.ToxopeusA. G. (2014). Where Is Positional Uncertainty a Problem for Species Distribution Modelling?Ecography37, 191–203. 10.1111/j.1600-0587.2013.00205.x
46
NolE.FrancisC. M.BurkeD. M. (2005). Using Distance from Putative Source Woodlots to Predict Occurrence of Forest Birds in Putative Sinks. Conservation Biol.19, 836–844. 10.1111/j.1523-1739.2005.00367.x
47
OksanenJ.BlanchetG. F.FriendlyM.KindtR.LegendreP.McGlinnD.et al (2019). Vegan: Community Ecology Package. R package version 2.5-5. Available online at: https://cran.r-project.org/package=vegan.
48
OsborneP. (1984). Bird Numbers and Habitat Characteristics in Farmland Hedgerows. J. Appl. Ecol.21, 63–82. 10.2307/2403037
49
Pe'erG.ZinngrebeY.HauckJ.SchindlerS.DittrichA.ZinggS.et al (2017). Adding Some Green to the Greening: Improving the EU's Ecological Focus Areas for Biodiversity and Farmers. Conservation Lett.10, 517–530. 10.1111/conl.12333
50
PeachW. J.DennyM.CottonP. A.HillI. F.GruarD.BarrittD.et al (2004). Habitat Selection by Song Thrushes in Stable and Declining farmland Populations. J. Appl. Ecol.41, 275–293. 10.1111/j.0021-8901.2004.00892.x
51
PerperoglouA.SauerbreiW.AbrahamowiczM.SchmidM. (2019). A Review of Spline Function Procedures in R. BMC Med. Res. Methodol.19, 1–16. 10.1186/s12874-019-0666-3
52
PustkowiakS.KwiecińskiZ.LendaM.ŻmihorskiM.RosinZ. M.TryjanowskiP.et al (2021). Small Things Are Important: The Value of Singular Point Elements for Birds in Agricultural Landscapes. Biol. Rev.96, 1386–1403. 10.1111/BRV.12707
53
QGIS Development Team (2019). QGIS Geographic Information System.
54
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. http://qgis.osgeo.org
55
RajmonováL.ReifJ. (2018). Significance of Non-Forest Woody Vegetation for Birds in Farmland. Sylvia54, 3–24.
56
ReifJ.HanzelkaJ. (2020). Continent‐wide Gradients in Open‐habitat Insectivorous Bird Declines Track Spatial Patterns in Agricultural Intensity Across Europe. Glob. Ecol. Biogeogr.29, 1988–2013. 10.1111/geb.13170
57
ReifJ.HanzelkaJ.KadlecT.ŠtroblM.HejdaM. (2016a). Conservation Implications of Cascading Effects Among Groups of Organisms: The Alien Tree Robinia Pseudacacia in the Czech Republic as a Case Study. Biol. Conservation198, 50–59. 10.1016/j.biocon.2016.04.003
58
ReifJ.HořákD.KrištínA.KopsováL.DevictorV. (2016b). Linking Habitat Specialization with Species' Traits in European Birds. Oikos125, 405–413. 10.1111/oik.02276
59
ReifJ.JiguetF.ŠťastnýK. (2010). Habitat Specialization of Birds in the Czech Republic: Comparison of Objective Measures with Expert Opinion. Bird Study57, 197–212. 10.1080/00063650903477046
60
ReifJ.VermouzekZ. (2019). Collapse of Farmland Bird Populations in an Eastern European Country Following its EU Accession. Conservation Lett.12, e12585–8. 10.1111/conl.12585
61
RosenzweigM. L. (1995). Species Diversity in Space and Time. New York: Cambridge University Press.
62
SasakiK.HotesS.KadoyaT.YoshiokaA.WoltersV. (2020). Landscape Associations of farmland Bird Diversity in Germany and Japan. Glob. Ecol. Conservation21, e00891. 10.1016/J.GECCO.2019.E00891
63
SchulzeE. D.CravenD.DursoA. M.ReifJ.GuderleM.KroiherF.et al (2019). Positive Association Between Forest Management, Environmental Change, and forest Bird Abundance. For. Ecosyst.6. 10.1186/s40663-019-0160-8
64
SimberloffD. (2001). Management of Boreal Forest Biodiversity - A View from the outside. Scand. J. For. Res.16, 105–118. 10.1080/028275801300090726
65
SparksT. H.ParishT.HinsleyS. A. (1996). Breeding Birds in Field Boundaries in an Agricultural Landscape. Agric. Ecosyst. Environ.60, 1–8. 10.1016/S0167-8809(96)01067-5
66
ŠťastnýK.HudecK. (2011). Fauna of the Czech Republic. 2nd ed, Birds 3. Prague: Academia.
67
StoateC.BáldiA.BejaP.BoatmanN. D.HerzonI.van DoornA.et al (2009). Ecological Impacts of Early 21st Century Agricultural Change in Europe - A Review. J. Environ. Manage.91, 22–46. 10.1016/j.jenvman.2009.07.005
68
StoateC.BoatmanN. D.BorralhoR. J.CarvalhoC. R.SnooG. R. d.EdenP. (2001). Ecological Impacts of Arable Intensification in Europe. J. Environ. Manage.63, 337–365. 10.1006/jema.2001.0473
69
ŠtroblM.SaskaP.SeidlM.KocianM.TajovskýK.ŘezáčM.et al (2019). Impact of an Invasive Tree on Arthropod Assemblages in Woodlots Isolated within an Intensive Agricultural Landscape. Divers. Distrib.25, 1800–1813. 10.1111/ddi.12981
70
TryjanowskiP.SparksT. H.JerzakL.RosinZ. M.SkórkaP. (2014). A Paradox for Conservation: Electricity Pylons May Benefit Avian Diversity in Intensive Farmland. Conservation Lett.7, 34–40. 10.1111/conl.12022
71
VanhinsberghD.GoughS.FullerR. J.BrierleyE. D. R. (2002). Summer and Winter Bird Communities in Recently Established Farm Woodlands in Lowland England. Agric. Ecosyst. Environ.92, 123–136. 10.1016/S0167-8809(01)00301-2
Summary
Keywords
farmland birds, greening measures, habitat quality, non-productive habitat, non-native plants, species richness, woodlot
Citation
Dvořáková L, Kuczyński L, Rivas-Salvador J and Reif J (2022) Habitat Characteristics Supporting Bird Species Richness in Mid-Field Woodlots. Front. Environ. Sci. 10:816255. doi: 10.3389/fenvs.2022.816255
Received
16 November 2021
Accepted
14 March 2022
Published
26 April 2022
Volume
10 - 2022
Edited by
Attila D. Sándor, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania
Reviewed by
Leonardo Chapa-Vargas, Instituto Potosino de Investigación Científica y Tecnológica (IPICYT), Mexico
Stefan Hotes, Chuo University, Japan
Yuichi Yamaura, Forestry and Forest Products Research Institute, Japan
Updates
Copyright
© 2022 Dvořáková, Kuczyński, Rivas-Salvador and Reif.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lenka Dvořáková, Lenka.rajmonova@seznam.cz
This article was submitted to Conservation and Restoration Ecology, a section of the journal Frontiers in Environmental Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.